

Abhay R. Satoskar, MD, PhD
Ohio State University
Columbus, Ohio, USA
Gary L. Simon, MD, PhD
Th e George Washington University
Washington DC, USA
Peter J. Hotez, MD, PhD
Th e George Washington University
Washington DC, USA
Moriya Tsuji, MD, PhD
Th e Rockefeller University
New York, New York, USA
Medical Parasitology
Austin, Texas
U.S.A.
v a d e m e c u m
L A N D E S
B
I
O
S
C
I
E
N
C
E

VADEMECUM
Parasitology
LANDES BIOSCIENCE
Austin, Texas USA
Copyright ©2009 Landes Bioscience
All rights reserved.
No part of this book may be reproduced or transmitted in any form or by any means,
electronic or mechanical, including photocopy, recording, or any information storage
and retrieval system, without permission in writing from the publisher.
Printed in the USA.
Please address all inquiries to the Publisher:
Landes Bioscience, 1002 West Avenue, Austin, Texas 78701, USA
Phone: 512/ 637 6050; FAX: 512/ 637 6079
ISBN: 978-1-57059-695-7
Library of Congress Cataloging-in-Publication Data
Medical parasitology / [edited by] Abhay R. Satoskar ... [et al.].
p. ; cm.
Includes bibliographical references and index.
ISBN 978-1-57059-695-7
1. Medical parasitology. I. Satoskar, Abhay R.
[DNLM: 1. Parasitic Diseases. WC 695 M489 2009]
QR251.M426 2009
616.9'6--dc22
2009035449
While the authors, editors, sponsor and publisher believe that drug selection and dosage and
the specifi cations and usage of equipment and devices, as set forth in this book, are in accord
with current recommend ations and practice at the time of publication, they make no warranty,
expressed or implied, with respect to material described in this book. In view of the ongoing
research, equipment development, changes in governmental regulations and the rapid accumula-
tion of information relating to the biomedical sciences, the reader is urged to carefully review and
evaluate the information provided herein.

Dedications
To Anjali, Sanika and Monika for their support —Abhay R. Satoskar
To Vicki, Jason and Jessica for their support —Gary L. Simon
To Ann, Matthew, Emily, Rachel, and Daniel —Peter J. Hotez
To Yukiko for her invaluable support —Moriya Tsuji


About the Editors...
ABHAY R. SATOSKAR is Associate Professor of Microbiology at the Ohio
State University, Columbus. Main research interests include parasitology and
development of immunotherapeutic strategies for treating parasitic diseases.
He is a member of numerous national and international scientifi c organiza-
tions including American Association of Immunologists and American Society
of Tropical Medicine and Hygiene. He has served as a consultant for several
organizations including NIH (USA), National Research Foundation (South
Africa), Wellcome Trust (UK) and Sheikh Hamadan Foundation (UAE).
He holds visiting faculty appointments in institutions in India and Mexico.
Abhay Satoskar received his medical degree (MB, BS and MD) from Seth
G. S. Medical College and King Edward VII Memorial Hospital affi liated to
University of Bombay, India. He received his doctoral degree (PhD) from
University of Strathclyde, Glasgow.

About the Editors...
GARY L. SIMON is the Walter G. Ross Professor of Medicine and
Director of the Division of Infectious Diseases at Th e George Washington
University School of Medicine. He is also Vice-Chairman of the Department
of Medicine. Dr. Simon is also Professor of Microbiology, Tropical Medicine
and Immunology and Professor of Biochemistry and Molecular Biology. His
research interests are in the diagnosis and treatment of HIV infection and
its complications. He is especially interested in the interaction between HIV
and diseases of sub-Saharan Africa, notably tuberculosis.
Dr. Simon is a native of Brooklyn, New York, but grew up in the Wash-
ington, DC metropolitan area. He obtained his undergraduate degree in
chemistry from the University of Maryland and a PhD degree in physical
chemistry from the University of Wisconsin. He returned to the University
of Maryland where he received his MD degree and did his internal medicine
residency. He did his infectious disease training at Tuft s-New England Medi-
cal Center in Boston.

About the Editors...
PETER J. HOTEZ is Distinguished Research Professor and the Walter G. Ross
Professor and Chair of the Department of Microbiology, Immunology and Tropi-
cal Medicine at Th e George Washington University, where his major research
and academic interest is in the area of vaccine development for neglected tropical
diseases and their control. Prof. Hotez is also the President of the Sabin Vaccine
Institute, a non-profi t medical research and advocacy organization. Th rough the
Institute, Dr. Hotez founded the Human Hookworm Vaccine Initiative, a product
development partnership supported by the Bill and Melinda Gates Foundation,
to develop a recombinant vaccine for human hookworm disease, and the Global
Network for Tropical Neglected Diseases Control, a new partnership formed to
facilitate the control of neglected tropical diseases in developing countries. He is
also the Founding Editor-in-Chief of PLoS Tropical Neglected Diseases.
Dr. Hotez is a native of Hartford, Connecticut. He obtained his BA degree in
Molecular Biophysics Phi Beta Kappa from Yale University (1980) and his MD
and PhD from the medical scientist-training program at Weill Cornell Medical
College and Th e Rockefeller University.

About the Editors...
MORIYA TSUJI is Aaron Diamond Associate Professor and Staff Investiga-
tor, HIV and Malaria Vaccine Program at the Aaron Diamond AIDS Research
Center, Th e Rockefeller University, New York. He is also Adjunct Associate
Professor in the Department of Medical Parasitology at New York University
School of Medicine. He is a member of various national and international
scientifi c organizations, including Faculty of 1000 Biology, United States-Israel
Binational Science Foundation, the Center for Scientifi c Review at the National
Institute of Health of the United States Department of Health and Human
Services, the Science Programme at the Wellcome Trust of the United Kingdom,
the French Microbiology Program at the French Ministry of Research and New
Technologies, and the Board of Experts for the Italian Ministry for University
and Research. He is also an editorial board member of the journal Virology:
Research and Treatment. His major research interests are (i) recombinant viral
vaccines against microbial infections, (ii) identifi cation of novel glycolipid-based
adjuvants for HIV and malaria vaccines, and (iii) the protective role of CD1
molecules in HIV/malaria infection. Moriya Tsuji received his MD in 1983 from
Th e Jikei University School of Medicine, Tokyo, Japan, and in 1987 earned his
PhD in Immunology from the University of Tokyo, Faculty of Medicine.
Contents
Preface
.......................................................................xxi
Section I. Nematodes
1.
Enterobiasis.................................................................. 2
Janine R. Danko
2.
Trichuriasis
.................................................................. 8
Rohit Modak
3.
Ascariasis
................................................................... 14
Afsoon D. Roberts
4.
Hookworm
................................................................. 21
David J. Diemert
5.
Strongyloidiasis
.......................................................... 31
Gary L. Simon
6.
Trichinellosis
.............................................................. 39
Matthew W. Carroll
7.
Onchocercosis
............................................................ 45
Christopher M. Cirino
8.
Loiasis
........................................................................ 53
Murliya Gowda
9.
Dracunculiasis
............................................................ 58
David M. Parenti
10. Cutaneous Larva Migrans: “Th e Creeping Eruption” ... 63
Ann M. Labriola
11. Baylisascariasis and Toxocariasis ................................. 67
Erin Elizabeth Dainty and Cynthia Livingstone Gibert
12. Lymphatic Filariasis ................................................... 76
Subash Babu and Th omas B. Nutman
Section II. Trematodes
13. Clonorchiasis and Opisthorchiasis .............................. 86
John Cmar
14. Liver Fluke: Fasciola hepatica ...................................... 92
Michelle Paulson
15. Paragonimiasis ........................................................... 98
Angelike Liappis
16. Intestinal Trematode Infections ................................ 104
Sharon H. Wu, Peter J. Hotez and Th addeus K. Graczyk
17. Schistosomiasis: Schistosoma japonicum ................... 111
Edsel Maurice T. Salvana and Charles H. King
18. Schistosomiasis: Schistosoma mansoni ...................... 118
Wafa Alnassir and Charles H. King
19. Schistosomiasis: Schistosoma haematobium .............. 129
Vijay Khiani and Charles H. King
Section III. Cestodes
20. Taeniasis and Cyticercosis ........................................ 138
Hannah Cummings, Luis I. Terrazas and Abhay R. Satoskar
21. Hydatid Disease ....................................................... 146
Hannah Cummings, Miriam Rodriguez-Sosa
and Abhay R. Satoskar
Section IV. Protozoans
22. American Trypanosomiasis (Chagas Disease) ........... 154
Bradford S. McGwire and David M. Engman
23. African Trypanosomiasis .......................................... 161
Guy Caljon, Patrick De Baetselier and Stefan Magez
24. Visceral Leishmaniasis (Kala-Azar) ........................... 171
Ambar Haleem and Mary E. Wilson
25. Cutaneous Leishmaniasis
.......................................... 182
Claudio M. Lezama-Davila, John R. David
and Abhay R. Satoskar
26. Toxoplasmosis .......................................................... 190
Sandhya Vasan and Moriya Tsuji
27. Giardiasis ................................................................. 195
Photini Sinnis
28. Amebiasis
................................................................. 206
Daniel J. Eichinger
29. Cryptosporidiosis .................................................... 214
Gerasimos J. Zaharatos
30. Trichomoniasis
......................................................... 222
Raymond M. Johnson
31. Pneumocystis Pneumonia .......................................... 227
Allen B. Clarkson, Jr. and Salim Merali
32. Malaria
..................................................................... 237
Moriya Tsuji and Kevin C. Kain
Section V. Arthropods
33. Clinically Relevant Arthropods ................................ 250
Sam R. Telford III
Appendix
.................................................................. 261
Drugs for Parasitic Infections .......................................................261
Safety of Antiparasitic Drugs .......................................................284
Manufacturers of Drugs Used to Treat Parasitic Infections ...287
Index
........................................................................ 291

Editors
Abhay R. Satoskar, MD, PhD
Department of Microbiology
and
Department of Molecular Virology, Immunology
and Medical Genetics
Ohio State University
Columbus, Ohio, USA
Email: satoskar.2@osu.edu
Chapters 20, 21, 25
Gary L. Simon, MD, PhD
Department of Medicine
and
Department of Microbiology, Immunology
and Tropical Medicine
and
Department of Biochemistry and Molecular Biology
Division of Infectious Diseases
Th e George Washington University
Washington DC, USA
Email: gsimon@mfa.gwu.edu
Chapters 5
Peter J. Hotez, MD, PhD
Department of Microbiology, Immunology
and Tropical Medicine
Th e George Washington University
Washington DC, USA
Email: mtmpjh@gwumc.edu
Chapter 16
Moriya Tsuji, MD, PhD
HIV and Malaria Vaccine Program
Th e Aaron Diamond AIDS Research Center
Th e Rockefeller University
New York, New York, USA
Email: mtsuji@adarc.org
Chapters 26, 32

Wafa Alnassir, MD
Department of Medicine
Division of Infectious Diseases
University Hospitals of Cleveland
Cleveland, Ohio, USA
Email: wafanassirali@yahoo.com
Chapter 18
Subash Babu, PhD
Helminth Immunology Section
Laboratory of Parasitic Diseases
National Institutes of Health
Bethesda, Maryland, USA
Email: sbabu@niaid.nih.gov
Chapter 12
Guy Caljon, PhD
Unit of Cellular and Molecular
Immunology
Department of Molecular and Cellular
Interactions
VIB, Vrije Universiteit Brussel
Brussels, Belgium
Email: gucaljon@vub.ac.be
Chapter 23
Matthew W. Carroll, MD
Division of Infectious Diseases
Th e George Washington University
School of Medicine
Washington DC, USA
Email: mcarroll@gwu.edu
Chapter 6
Christopher M. Cirino, DO, MPH
Division of Infectious Diseases
Th e George Washington University
School of Medicine
Washington DC, USA
Email: ccirino710@hotmail.com
Chapter 7
Allen B. Clarkson, Jr, PhD
Department of Medical Parasitology
New York University
School of Medicine
New York, New York, USA
Email: clarka01@med.nyu.edu
Chapter 31
John Cmar, MD
Department of Medicine
Divisions of Infectious Diseases
and Internal Medicine
Sinai Hospital of Baltimore
Baltimore, Maryland, USA
Email: doc.operon@gmail.com
Chapter 13
Hannah Cummings, BS
Department of Microbiology
Ohio State University
Columbus, Ohio, USA
Email: cummings.123@osu.edu
Chapters 20, 21
Erin Elizabeth Dainty, MD
Department of Obstetrics
and Gynecology
University of Pennsylvania
Philadelphia, Pennsylvania, USA
Email: erin.dainty@uphs.upenn.edu
Chapter 11
Janine R. Danko, MD, MPH
Department of Infectious Diseases
Uniformed Services University
of the Health Sciences
Naval Medical Research Center
Bethesda, Maryland, USA
Email: janine.danko@med.navy.mil
Chapter 1
Contributors
John R. David, MD
Department of Immunology
and Infectious Diseases
Harvard School of Public Health
Boston, Massachusetts, USA
Email: jdavid@hsph.harvard.edu
Chapter 25
Patrick De Baetselier, PhD
Unit of Cellular and Molecular
Immunology
Department of Molecular and Cellular
Interactions
VIB, Vrije Universiteit Brussel
Brussels, Belgium
Email: pdebaets@vub.ac.be
Chapter 23
David J. Diemert, MD
Human Hookworm Vaccine Initiative
Albert B. Sabin Vaccine Institute
Washington DC, USA
Email: david.diemert@sabin.org
Chapter 4
Daniel J. Eichinger, PhD
Department of Medical Parasitology
New York University
School of Medicine
New York, New York, USA
Email: eichid01@med.nyu.edu
Chapter 28
David M. Engman, MD, PhD
Departments of Pathology
and Microbiology-Immunology
Northwestern University
Chicago, Illinois, USA
Email: d-engman@northwestern.edu
Chapter 22
Cynthia Livingstone Gibert, MD
Department of Medicine
Division of Infectious Diseases
Th e George Washington University
Washington VA Medical Center
Washington DC, USA
Email: cynthia.gibert@med.va.gov
Chapter 11
Murliya Gowda, MD
Infectious Disease Consultants (IDC)
Fairfax, Virginia, USA
Email: pgowda2000@yahoo.com
Chapter 8
Th addeus K. Graczyk, MSc, PhD
Department of Environmental
Health Sciences
Division of Environmental
Health Engineering
Johns Hopkins Bloomberg
School of Public Health
Baltimore, Maryland, USA
Email: tgraczyk@jhsph.edu
Chapter 16
Ambar Haleem, MD
Department of Internal Medicine
University of Iowa
Iowa City, Iowa, USA
Email: ambar-haleem@uiowa.edu
Chapter 24
Raymond M. Johnson, MD, PhD
Department of Medicine
Indiana University School of Medicine
Indianapolis, Indiana, USA
Email: raymjohn@iupui.edu
Chapter 30
Kevin C. Kain, MD, FRCPC
Department of Medicine
University of Toronto
Department of Global Health
McLaughlin Center for Molecular
Medicine
and
Center for Travel and Tropical
Medicine
Toronto General Hospital
Toronto, Ontario, Canada
Email: kevin.kain@uhn.on.ca
Chapter 32
Vijay Khiani, MD
Department of Medicine
University Hospitals of Cleveland
Cleveland, Ohio, USA
Email: vijay.khiani@gmail.com
Chapter 19
Charles H. King, MD, FACP
Center for Global Health and Diseases
Case Western Reserve University
School of Medicine
Cleveland, Ohio, USA
Email: chk@cwru.edu
Chapters 17-19
Ann M. Labriola, MD
Department of Medicine
Division of Infectious Diseases
Th e George Washington University
Washington VA Medical Center
Washington DC, USA
Email: ann.labriola@va.gov
Chapter 10
Claudio M. Lezama-Davila, PhD
Department of Microbiology
and
Department of Molecular Virology,
Immunology and Medical Genetics
Ohio State University
Columbus, Ohio, USA
Email: lezama-davila.1@osu.edu
Chapter 25
Angelike Liappis, MD
Departments of Medicine
and Microbiology, Immunology
and Tropical Medicine
Division of Infectious Diseases
Th e George Washington University
Washington DC, USA
Email: mtmapl@gwumc.edu
Chapter 15
Stefan Magez, PhD
Unit of Cellular and Molecular
Immunology
Department of Molecular and Cellular
Interactions
VIB, Vrije Universiteit Brussel
Brussels, Belgium
Email: stemagez@vub.ac.be
Chapter 23
Bradford S. McGwire, MD, PhD
Division of Infectious Diseases
and
Center for Microbial Interface Biology
Ohio State University
Columbus, Ohio, USA
Email: brad.mcgwire@osumc.edu
Chapter 22
Salim Melari, PhD
Department of Biochemistry
Fels Institute for Cancer Research
and Molecular Biology
Temple University School of Medicine
Philadelphia, Pennsylvania, USA
Email: salim.merali@temple.edu
Chapter 31
Rohit Modak, MD, MBA
Division of Infectious Diseases
Th e George Washington University
Medical Center
Washington DC, USA
Email: Rohitmodak@yahoo.com
Chapter 2
Th omas B. Nutman, MD
Helminth Immunology Section
Laboratory of Parasitic Diseases
National Institutes of Health
Bethesda, Maryland, USA
Email: tnutman@niaid.nih.gov
Chapter 12
David M. Parenti, MD, MSc
Department of Medicine
and
Department of Microbiology,
Immunology and Tropical Medicine
Division of Infectious Diseases
Th e George Washington University
Washington DC, USA
Email: dparenti@mfa.gwu.edu
Chapter 9
Michelle Paulson, MD
National Institute of Allergy
and Infectious Diseases
National Institutes of Health
Bethesda, Maryland, USA
Email: paulsonm@niaid.nih.gov
Chapter 14
Afsoon D. Roberts, MD
Department of Medicine
and
Department of Microbiology,
Immunology and Tropical Medicine
Division of Infectious Diseases
Th e George Washington University
School of Medicine
Washington DC, USA
Email: aroberts@mfa.gwu.edu
Chapter 3
Miriam Rodriguez-Sosa, PhD
Unidad de Biomedicina
FES-Iztacala
Universidad Nacional Autómonia
de México
México
Email: rodriguezm@campus.iztacala.
unam.mx
Chapter 21
Edsel Maurice T. Salvana, MD
Department of Medicine
Division of Infectious Diseases
University Hospitals of Cleveland
Cleveland, Ohio, USA
Email: edsel.salvana@case.edu
Chapter 17
Photini Sinnis, MD
Department of Medicine
and
Department of Medical Parasitology
New York University School of
Medicine
New York, New York, USA
Email: photini.sinnis@med.nyu.edu
Chapter 27
Sam R. Telford, III, SD, MS
Department of Biomedical Sciences
Infectious Diseases
Tuft s University School
of Veterinary Medicine
Graft on, Massachusetts, USA
Email: sam.telford@tuft s.edu
Chapter 33
Luis I. Terrazas, PhD
Unidad de Biomedicina
FES-Iztacala
Universidad Nacional Autónoma
de México
México
Email: literrazas@campus.iztacala.
unam.mx
Chapter 20
Sandhya Vasan, MD
Th e Aaron Diamond AIDS
Research Center
Th e Rockefeller University
New York, New York, USA
Email: svasan@adarc.org
Chapter 26
Mary E. Wilson, MD, PhD
Departments of Internal Medicine,
Microbiology and Epidemiology
Iowa City VA Medical Center
University of Iowa
Iowa City, Iowa, USA
Email: mary-wilson@uiowa.edu
Chapter 24
Sharon H. Wu, MS
Department of Microbiology,
Immunology and Tropical Medicine
Th e George Washington University
Washington DC, USA
Email: sharonwu@gwu.edu
Chapter 16
Gerasimos J. Zaharatos, MD
Division of Infectious Diseases,
Department of Medicine
and
Department of Microbiology
Jewish General Hospital
McGill University
Montreal, Quebec, Canada
Email: gerasimos.zaharatos@mcgill.ca
Chapter 29


Preface
Infections caused by parasites are still a major global health problem.
Although parasitic infections are responsible for a signifi cant morbidity and
mortality in the developing countries, they are also prevalent in the developed
countries. Early diagnosis and treatment of a parasitic infection is not only
critical for preventing morbidity and mortality individually but also for reduc-
ing the risk of spread of infection in the community. Th is concise book gives
an overview of critical facts for clinical and laboratory diagnosis, treatment
and prevention of parasitic diseases which are common in humans and which
are most likely to be encountered in a clinical practice. Th is book is a perfect
companion for primary care physicians, residents, nurse practitioners, medical
students, paramedics, other public health care personnel and as well as travel-
ers. Th e editors would like to thank all the authors for their expertise and their
outstanding contributions. We would also like to thank Dr. Ronald Landes
and all other staff of Landes Bioscience who has worked tirelessly to make this
magnifi cent book possible.
Abhay R. Satoskar, MD, PhD
Gary Simon, MD, PhD
Moriya Tsuji, MD, PhD
Peter J. Hotez, MD, PhD


S
ECTION
I
Nematodes

Chapter 1
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Enterobiasis
Janine R. Danko
Background
Enterobius vermicularis, commonly referred to as pinworm, has the largest
geographical distribution of any helminth. Discovered by Linnaeus in 1758, it was
originally named Oxyuris vermicularis and the disease was referred to as oxyuriasis
for many years. It is believed to be the oldest parasite described and was recently
discovered in ancient Egyptian mummifi ed human remains as well as in DNA
samples from ancient human coprolite remains from North and South America.
Enterobius is one of the most prevalent nematodes in the United States and in
Western Europe. At one time, in the United States there are an estimated 42 million
infected individuals. It is found worldwide in both temperate and tropical areas.
Prevalence is highest among the 5-10 year-old age group and infection is uncom-
mon in children less than two years old. Enterobiasis has been reported in every
socioeconomic level; however spread is much more likely within families of infected
individuals, or in institutions such as child care centers, orphanages, hospitals and
mental institutions. Humans are the only natural host for the parasite.
Infection is facilitated by factors including overcrowding, wearing soiled cloth-
ing, lack of adequate bathing and poor hand hygiene, especially among young
school-aged children. Infestation follows ingestion of eggs which usually reach
the mouth on soiled hands or contaminated food. Transmission occurs via direct
anus to mouth spread from an infected person or via airborne eggs that are in the
environment such as contaminated clothing or bed linen. Th e migration of worms
out of the gastrointestinal tract to the anus can cause local perianal irritation and
pruritus. Scratching leads to contamination of fi ngers, especially under fi ngernails
and contributes to autoinfection. Finger sucking and nail biting may be sources of
recurrent infection in children. Spread within families is common. E. vermicularis
may be transmitted through sexual activity, especially via oral and anal sex.
When swallowed via contaminated hands, food or water, the eggs hatch releasing
larvae (Fig. 1.1). Th e larvae develop in the upper small intestine and mature in 5 to
6 weeks without undergoing any further migration into other body cavities (i.e.,
lungs). Both male and female forms exist. Th e smaller male is 2-5 mm in length and
0.3 mm in diameter whereas the female is 8-13 mm long and up to 0.6 mm in di-
ameter (Fig. 1.2). Copulation occurs in the distal small bowel and the adult females
settle in the large intestine where they can survive for up to 13 weeks (males live for
approximately 7 weeks). Th e adult female can produce approximately 11,000 eggs.
A gravid female can migrate out through the anus to lay her eggs. Th is phenomenon
usually occurs at night and is thought to be secondary to the drop in host body
3
Enterobiasis
1
temperature at this time. Th e eggs embyonate and become infective within 6 hours
of deposition. In cool, humid climates the larvae can remain infective for nearly 2
weeks, but under warm, dry conditions, they begin to lose their infectivity within
2 days. Most infected persons harbor a few to several hundred adult worms.
Disease Signs and Symptoms
Th e majority of enterobiasis cases are asymptomatic; however the most common
symptom is perianal or perineal pruritus. Th is varies from mild itching to acute
pain. Symptoms tend to be most troublesome at night and, as a result, infected
individuals oft en report sleep disturbances, restlessness and insomnia. Th e most
common complication of infection is secondary bacterial infection of excoriated
skin. Folliculitis has been seen in adults with enterobiasis.
Gravid female worms can migrate from the anus into the female genital tract.
Vaginal infections can lead to vulvitis, serous discharge and pelvic pain. Th ere are
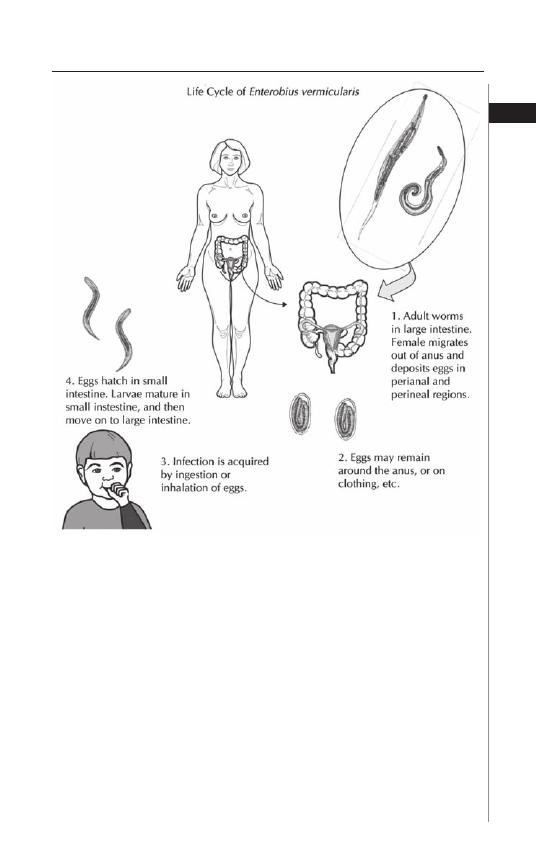
Figure 1.1. Life-cycle of Enterobius vermicularis. Reproduced from: Nappi
AJ, Vass E, eds. Parasites of Medical Importance. Austin: Landes Bioscience,
2002:84.

4
Medical Parasitology
1
numerous reports of granulomas in the vaginal wall, uterus, ovary and pelvic peri-
toneum caused by E. vermicularis dead worms or eggs. Pre-pubertal and adolescent
girls with E. vermicularis infection can develop vulvovaginitis. Scratching may lead
to introital colonization with colonic bacteria and thus may increase susceptibility
to urinary tract infections.
Although ectopic lesions due to E. vermicularis are rare, pinworms can also
migrate to other internal organs, such as the appendix, the prostate gland, lungs
or liver, the latter being a result of egg embolization from the colon via the portal
venous system. Within the colonic mucosa or submucosa granulomas can be
uncomfortable and may mimic other diseases such as carcinoma of the colon or
Crohn’s disease. E. vermicularis has been found in the lumen of uninfl amed ap-
pendices in patients who have been operated on for acute appendicitis. Although
eosinophilic colitis has been described with enterobiasis, eosinophilia is uncommon
in infected individuals.
Diagnosis
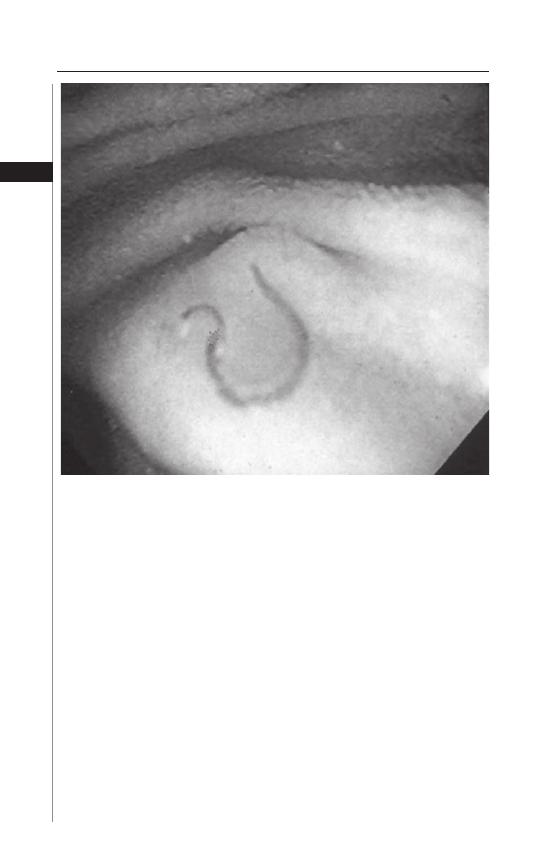
Th e diagnosis of E. vermicularis infestation rests on the recognition of dead
adult worms or the characteristic ova. In the perianal region, the adult female
worm may be visualized as a small white “piece of thread”. Th e most successful
diagnostic method is the “Scotch tape” or “cellophane tape” method (Fig. 1.3).
Th is is best done immediately aft er arising in the morning before the individual
defecates or bathes. Th e buttocks are spread and a small piece of transparent or
cellulose acetate tape is pressed against the anal or perianal skin several times.
Th e strip is then transferred to a microscope slide with the adhesive side down.
Th e worms are white and transparent and the skin is transversely striated. Th e
egg is also colorless, measures 50-54 × 20-27 mm and has a characteristic shape,
Figure 1.2. Enterobius vermicularis.

5
Enterobiasis
1
fl attened on one side. Examination of a single specimen detects approximately
50% of infections; when this is done on three consecutive mornings sensitivity
rises to 90%. Parija et al. found a higher sensitivity if lacto-phenol cotton blue
stain was used in detecting eggs aft er the tape test was performed. Six consecu-
tive negative swabs on separate days are necessary to exclude the diagnosis. Stool
examination for eggs is usually not helpful, as only 5-15% of infected persons
will have positive results. Rarely, E. vermicularis eggs have been found in cervi-
cal specimens (done for routine Papanicolaou smears), in the urine sediment,
or the worms have been seen during colonoscopy. Serologic tests specifi c for E.
vermicularis are not available.
Treatment
E. vermicularis is susceptible to several anthelmintic therapies, with a cure rate
of >90%. Mebendazole (100 mg), albendazole (400 mg), or pyrantel pamoate
(11 mg/kg of base) given as a single dose and then repeated aft er 14 days are all
eff ective regimens. Mebendazole or albendazole are preferred because they have
relatively few side eff ects. Th eir mode of action involves inhibition of the micro-
tubule function in adult worms and glycogen depletion. For children less than 2,
200 mg should be administered. Although equally eff ective, pyrantel pamoate is
associated with more side eff ects including gastrointestinal distress, neurotoxicity
and transient increases in liver enzymes. Both mebendazole and albendazole are
category C drugs, thus contraindicated in pregnancy although an Israeli study by
Diav-Citrin et al of 192 pregnant women exposed to mebendazole, failed to reveal
an increase in the number of malformations or spontaneous abortions compared
to the general population.
Persons with eosinophilic colitis should be treated for
three successive days with mebendazole (100 mg twice daily). Experience with
Figure 1.3. Enterobius vermicularis captured on scotch tape.

6
Medical Parasitology
1
mebendazole or albendazole with ectopic enterobiasis is limited; persons who
present with pelvic pain, those who have salpingitis, tuboovarian abscesses or
painful perianal granulomas or signs or symptoms of appendicitis oft en proceed
to surgery. In most reported cases, the antiparasitic agent is given aft er surgery
when the diagnosis of pinworm has been established. Conservative therapy with
local or systemic antibiotics is usually appropriate for perianal abscesses due to
enterobiasis. Ivermectin has effi cacy against pinworm but is generally not used for
this indication and is not approved for enterobiasis in the United States. Overall,
prognosis with treatment is excellent. Because pinworm is easily spread throughout
households, the entire family of the infected person should be treated. All bedding
and clothing should be thoroughly washed. Th e same rule should be applied to
institutions when an outbreak of pinworm is discovered.
Prevention and Prophylaxis
Th ere are no eff ective prevention or prophylaxis strategies available. Although
mass screening campaigns and remediation for parasite infection is costly, treatment
of pinworm infection improves the quality of life for children. Th e medications,
coupled with improvements in sanitation, especially in rural areas can provide a
cost-eff ective way at treating this nematode infection. Measures to prevent rein-
fection and spread including clipping fi ngernails, bathing regularly and frequent
hand washing, especially aft er bowel movements. Routine laundering of clothes
and linen is adequate to disinfect them. House cleaning should include vacuum-
ing around beds, curtains and other potentially contaminated areas to eliminate
other environmental eggs if possible. Health education about route of infection,
especially autoinfection and these prevention tactics should always be incorporated
into any treatment strategy.
Disclaimer
Th e views expressed in this chapter are those of the author and do not neces-
sarily refl ect the offi cial policy or position of the Department of the US Navy, the
Department of Defense or the US Government.
I am a military service member (or employee of the US Government). Th is
work was prepared as part of my offi cial duties. Title 17 USC §105 provides that
‘Copyright protection under this title is not available for any work of the United
States Government.’ Title 17 USC §101 defi nes a US Government work as a work
prepared by a military service member or employee of the US Government as part
of that person’s offi cial duties. —Janine R. Danko
Suggested Reading
1. Al-Rufaie HK, Rix GH, Perez Clemente MP et al. Pinworms and postmenopausal
bleeding. J Clin Path 1998; 51:401-2.
2. Arca MJ, Gates RL, Groner JL et al. Clinical manifestations of appendiceal
pinworms in children: an institutional experience and a review of the literature.
Pediatr Surg Int 2004; 20:372-5.
3. Beaver PC, Kriz JJ, Lau TJ. Pulmonary nodule caused by Enterobium vermicularis.
Am J Trop Med Hyg 1973; 22:711-13.
4. Bundy D, Cooper E. In: Strickland GT, ed. Hunter’s Tropical Medicine and
Emerging Infectious Diseases, 8th Edition. Philadelphia: W.B. Saunders Company,
2000.

7
Enterobiasis
1
5. Diav-Citrin O, Shechtman S, Arnon J et al. Pregnancy outcome aft er gestational
exposure to mebendazole: a prospective controlled cohort study. Am J Obstet
Gynecol 2003; 188:282-5.
6. Fernandez-Flores A, Dajil S. Enterobiasis mimicking Crohn’s disease. Indian J
Gastroenterol 2004; 23:149-50.
7. Georgiev VS. Chemotherapy of enterobiasis. Exp Opin Pharmacother 2001;
2:267-75.
8. Goncalves ML, Araujo A, Ferreira LF. Human intestinal parasites in the past: New
fi ndings and a review. Mem Inst Oswaldo Cruz 2003; 98:103-18.
9. Herrstrom P, Fristrom A, Karlsson A et al. Enterobius vermicularis and fi nger
sucking in young Swedish children. Scand. J Prim Healthcare 1997; 115:146-8.
10. Little MD, Cuello CJ, D’Allessandra A . Granuloma of the liver due to Enterobius
vermicularis: report of a case. Am J Trop Med Hyg 1973; 22:567-9.
11. Liu LX, Chi J, Upton MP. Eosinophilic colitis associated with larvae of the pin-
worm Enterobius vermicularis. Lancet 1995; 346:410-12.
12. Neva FA, Brown HW. Basic Clinical P, 6th Edition. Norwalk: Appleton and Lange,
1994.
13. Parija SC, Sheeladevi C, Shivaprakash MR et al. Evaluation of lacto-phenol
cotton blue stain for detection of eggs of Enterobius vermicularis in perianal surface
samples. Trop Doctor 2001; 31:214-5.
14. Petro M, Iavu K, Minocha A. Unusual endoscopic and microscopic view of
E. vermicularis: a case report with a review of the literature. South Med Jrnl 2005;
98:927-9.
15. Smolyakov R, Talalay B, Yanai-Inbar I et al. Enterobius vermicularis infection of
the female genital tract: a report of three cases and review of the literature. Eur J
Obstet Gynecol Reproduct Biol 2003; 107:220-2.
16. Sung J, Lin R, Huang L et al. Pinworm control and risk factors of pinworm
infection among primary-school chdilren in Taiwan. Am J Trop Med Hyg 2001;
65:558-62.
17. Tornieporth NG, Disko R, Brandis A et al. Ectopic enterobiasis: a case report and
review. J Infect 1992; 24:87-90.
18. Wagner ED, Eby WC. Pinworm prevalence in California elementary school
children and diagnostic methods. Am J Trop Med Hyg 1983; 32:998-1001.

Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Trichuriasis
Rohit Modak
Background
Trichuris trichiura is an intestinal nematode aff ecting an estimated 795
million persons worldwide. Also known as whipworm due to its characteristic
shape, Trichuris can be classifi ed as a soil-transmitted helminth because its life cycle
mandates embryonic development of its eggs or larvae in the soil. It is the second
most common nematode found in humans, behind Ascaris.
Trichuriasis is more common in areas with tropical weather such as Asia,
Sub-Sarahan Africa and the Americas, particularly in impoverished regions of
the Caribbean. It is also more common in poor rural communities and areas that
lack proper sanitary facilities with easily contaminated food and water. A large
number of individuals who are infected actually harbor fewer than 20 worms
and are asymptomatic; those with a larger burden of infection (greater than 200
worms) are most likely to develop clinical disease. School age children tend to be
most heavily infected.
Th ere is no reservoir host for Trichuris. Transmission occurs when contaminated
soil reaches the food, drink, or hands of a person and is subsequently ingested.
Th erefore, poor sanitary conditions is a major risk factor. It is noteworthy that
patients are oft en coinfected with other soil-transmitted helminths like Ascaris
and hookworm due to similar transmission modalities.
Life Cycle
Adult female worms shed between 3,000 to 20,000 eggs per day, which are
passed with the stool. In the soil, the eggs develop into a 2-cell stage, an advance
cleavage stage and then embryonate. It is the embryonated egg that is actually
infectious. Environmental factors such as high humidity and warm temperature
quicken the development of the embryo. Th is helps explain the geographic
predilection for tropical environments. Under optimal conditions, embryonic
development occurs between 15-30 days. Infection begins when these embryo-
nated eggs are ingested.
Th e eggs fi rst hatch in the small intestine and release larvae that penetrate the
columnar epithelium and situate themselves just above the lamina propria. Aft er
four molts, an immature adult emerges and is passively carried to the large intestine.
Here, it re-embeds itself into the colonic columnar cells, usually in the cecum and
ascending colon. Heavier burdens of infection spread to the transverse colon and
rectum. Th e worm creates a syncytial tunnel between the mouths of crypts; it is
here that the narrow anterior portion is threaded into the mucosa and its thicker
C
HAPTER
2
posterior end protrudes into the lumen, allowing its eggs to escape. Maturation
and mating occur here as well.
Th e pinkish gray adult worm is approximately 30-50 mm in length, with the
female generally being slightly larger than the male. Th e nutritional requirements of
Trichuris are unclear; unlike hookworm however, it does not appear that Trichuris is
dependent on its host’s blood. Eggs are fi rst detectable in the feces of those infected
about 60-90 days following ingestion of the embryonated eggs. Th e life span of an
adult worm is about one to three years. Unlike Ascaris and hookworm, there is no
migratory phase through the lung.
9
Trichuriasis
2
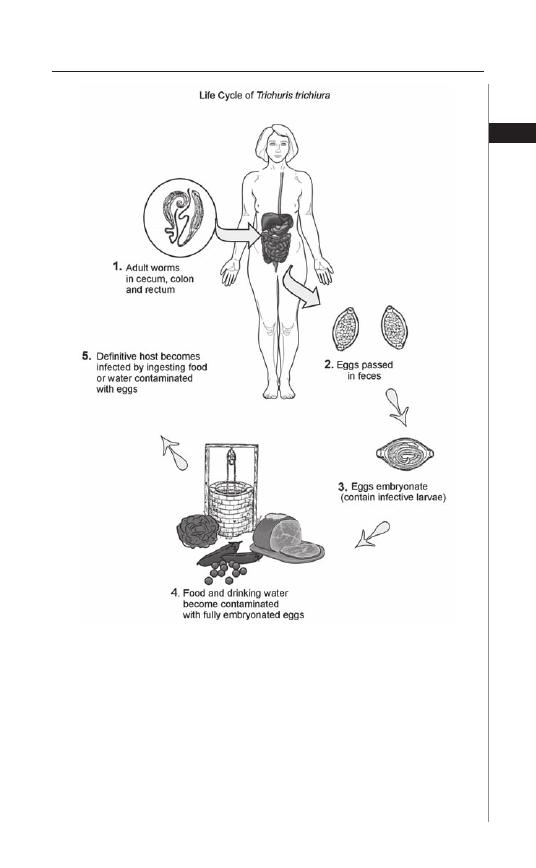
Figure 2.1. Life Cycle of Trichuris Trichura. Reproduced from: Nappi AJ, Vass E,
eds. Parasites of Medical Importance. Austin: Landes Bioscience, 2002:73.

Disease Signs and Symptoms
Frequently, infection with Trichuris is asymptomatic or results only in peripheral
eosinophilia. Clinical disease most oft en occurs in children, as it is this population
that tends to be most heavily-infected and presents as Trichuris colitis. In fact, this is
the most common and major disease entity associated with infection. Acutely, some
patients will develop Trichuris dysentery syndrome, characterized by abdominal
pain and diarrhea with blood and mucus. With severe dysentery, children develop
weight loss and become emaciated. Anemia is common and results from both
mucosal bleeding secondary to capillary damage and chronic infl ammation. Th e
anemia of trichuriasis is not as severe as that seen with hookworm. Trichuris infec-
tion of the rectum can lead to mucosal swelling. In that case, tenesmus is common
and if prolonged can lead to rectal prolapse, especially in children. Adult worms
can be seen on the prolapsed mucosa.
Chronic trichuriasis oft en mimics infl ammatory bowel disease. Physical symp-
toms include chronic malnutrition, short stature and fi nger clubbing. Th ese symp-
toms are oft en alleviated with appropriate anthelminthic treatment. Rapid growth
spurts have been reported in children following deworming with an anthelminthic
agent. Defi cits in the cognitive and intellectual development of children have also
been reported in association with trichuriasis.
Host Response
Infection with Trichuris results in a low-grade infl ammatory response that
is characterized by eosinophilic infi ltration of the submucosa. Th ere is an active
humoral immune response to Trichuris infection, but it is not fully protective.
Like hookworm infections, anthelminthic therapy in endemic areas provides only
10
Medical Parasitology
2
Figure 2.2. Egg of Trichuris trichiura. Reproduced from: Centers for Disease
Control and Prevention (CDC) (http://www.dpd.cdc.gov/DPDx/).

transient relief and reexposure to contaminated soil leads to reinfection. Th e T-cell
immune response to Trichuris infection is primarily a Th 2 response. Th is suggests
that trichuriasis, like other nematode infections, has modest immunomodulatory
eff ects.
Diagnosis
Infection can be diagnosed by microscopic identifi cation of Trichuris eggs in
feces. Th e eggs are quite characteristic, with a barrel or lemon shape, thick shell
and a clear plug at each end.
Because the level of egg output is high (200 eggs/g feces per worm pair), a
simple fecal smear is usually suffi cient for diagnosis. However in light infections,
a concentration procedure is recommended.
Trichuriasis can also be diagnosed by identifying the worm itself on the mucosa
of a prolapsed rectum or during colonoscopy. Th e female of the species is generally
longer, while the male has a more rounded appearance.
Because of the frequency of co-infections, a search for other protozoa, specifi -
cally Ascaris and hookworm should be considered. Charcot-Leyden crystals in the
stool in the absence of eggs in the stool should lead to further stool examinations
for T. trichuria. Although infl ammatory bowel disease is oft en in the diff erential,
the sedimentation rate (ESR) is generally not elevated in trichuriasis and the degree
of infl ammation evident on colonoscopic examination is much less than that seen
with Crohn’s disease or ulcerative colitis.
Treatment
Benzimidazoles are the drugs of choice in treating trichuriasis. Th eir anthelm-
inthic activity is primarily due to their ability to inhibit microtubule polymeriza-
tion by binding to beta-tubulin, a protein unique to invertebrates. A single dose
of albendazole has been suggested for treatment; however, despite the appeal of
adequate single dose therapy, clinical studies have shown a cure rate of less than 25
percent. Longer duration of therapy, resulting in higher cure rates, is recommended
for heavier burdens of infection. High cure rates are diffi cult to establish because
of the constant re-expsoure to the organisms. Mebendazole at a dose of 100 mg
twice daily for three days is also eff ective, with cure rates of almost 90 percent. In
some countries, pyrantel-oxantel is used for treatment, with the oxantel compo-
nent having activity against Trichuris and the pyrantel component having activity
against Ascaris and hookworm.
Albendazole and mebendazole are generally well tolerated when given at doses
used to treat trichuriasis, even in pediatric populations. Adverse eff ects include
transient abdominal pain, diarrhea, nausea and dizziness. With long-term use,
reported toxicities include bone marrow suppression, alopecia and hepatotox-
icity. Both drugs are not recommended in pregnancy, as they have been shown to
be teratogenic and embryotoxic in laboratory rats. However, albendazole should
be considered in pregnant women in the second or third trimester when the
potential benefi t outweighs the risks to the fetus. Although these drugs have not
been studied in very young children, the World Health Organization (WHO) has
recommended that both agents may be used for treatment in patients as young as
12 months, albeit at reduced dosages.
11
Trichuriasis
2

Prevention and Prophylaxis
Drinking clean water, properly cleaning and cooking food, hand washing
and wearing shoes are the most eff ective means of preventing soil-transmitted
helminth infections. Adequately sanitizing areas in which trichuriasis is prevalent
is extremely problematic; these communities oft en lack the resources needed for
such a substantial undertaking.
Direct exposure to sunlight for greater than 12 hours or temperatures exceed-
ing 40 degrees C in excess of 1 hour kills the embryo within the egg, but under
optimal conditions of moisture and shade in the warm tropical and subtropical soil,
Trichuris eggs can remain viable for months. Th ere is relative resistance to chemical
disinfectants and eggs can survive for prolonged periods even in treated sewage.
Th erefore, proper disposal of sewage is vital to control this infection. In areas of the
world where human feces is used as fertilizer, this is practically impossible.
Because the prevalence of trichuriasis has been estimated to be up to 80% in
some communities and can frequently be asymptomatic, the WHO advocates
empiric treatment of soil-transmitted helminths by administering anthelminthic
drugs to populations at risk. Specifi cally, WHO recommends periodic treatment
of school-aged children, the population in whom the burden of infection is great-
est. Th e goal of therapy is to maintain the individual worm burden at a level less
than that needed to cause signifi cant morbidity or mortality. Th is strategy has
been used successfully in preventing and reversing malnutrition, iron-defi ciency
anemia, stunted growth and poor school performance. Th is is in large part due to
the effi cacy and broad spectrum activity of a single dose of anthelminthic drugs
like albendazole. Because reinfection is a common problem as long as poor sanitary
conditions remain, it is proposed that single dose therapy be given at regular inter-
vals (1-3 times per year). Th e WHO hopes that by 2010, 75% of all school-aged
children at risk for heavy infection will have received treatment.
Major challenges to controlling the infection include continued poor sanitary
conditions. Additionally, the use of benzimidazole drugs at regular intervals may
lead to the emergence of drug resistance. Resistance has been documented in live-
stock and suspected in humans. Since the single dose regimen is not ideal (although
the most feasible), continued monitoring and screening is necessary.
Suggested Reading
1. Adams VJ, Lombard CJ, Dhansay MA et al. Effi cacy of albendazole against the
whipworm Trichuris trichiura: a randomised, controlled trial. S Afr Med J 2004;
94:972-6.
2. Albonico M, Crompton DW, Savioli L. Control strategies for intestinal nematode
infections. Adv Parasito 1999; 42:277-341.
3. Albonico M, Bickle Q, Haji HJ et al. Evaluation of the effi cacy of pyrantel-oxantel
for the treatment of soil-transmitted nematode infections. Trans R Soc Trop Med
Hyg 2002; 96:685-90.
4. Belding D. Textbook of Parasitology. New York: Appleton-Century-Crofts,
1965:397-8.
5. Cooper ES. Bundy DAP: Trichuris is not trivial. Parasitol Today 1988; 4:301-5.
6. De Silva N. Impact of mass chemotherapy on the morbidity due to soil-transmitted
nematodes. Acta Tropica 2003; 86:197-214.
7. de Silva NR, Brooker S, Hotez PJ et al. Soil-transmitted helminth infections:
updating the global picture. Trends Parasitol 2003; 19:547-51.
12
Medical Parasitology
2

13
Trichuriasis
2
8. Despommier DD, Gwadz RW, Hotez PJ et al, eds. Parasitic Diseases, 5th Edition.
New York: Apple Trees Productions, LLC, 2005:110-5.
9. Division of Parasitic Diseases, National Centers for Infectious Diseases, Centers
for Disease Control and Prevention, Atlanta, GA: [http://www.dpd.cdc.
gov/DPDx/].
10. Geerts S, Gryseels B. Anthelminthic resistance in human helminthes: a review.
Trop Med Int Health 2001; 6:915-21.
11. Gilman RH, Chong YH, Davis C et al. Th e adverse consequences of heavy Trichuris
infection. Trans R Soc Trop Med Hyg 1983; 77:432-8.
12. Lin AT, Lin HH, Chen CL. Colonoscopic diagnosis of whip-worm infection.
Hepato-Gastroenterol 1998; 45:2105-9.
13. Legesse M, Erko B, Medhin G. Comparative effi cacy of albendazole and three
brands of mebendazole in the treatment of ascariasis and trichuriasis. East Afr
Med J 2004; 81:134-8.
14. MacDonald TT, Choy MY, Spencer J et al. Histopathology and immunohisto-
chemistry of the caecum in children with the Trichuris dysentery syndrome. J Clin
Pathol 1991; 44:194-9.
15. Maguire J. Intestinal Nematodes. Mandell, Douglas and Bennett’s Principles
and Practice of Infectious Diseases, 6th Edition. Philadelphia: Elsevier,
2005:3263-4.
16. Montresor A, Awasthi S, Crompton DWT. Use of benzimadazoles in children
younger than 24 months for the treatment of soil-transmitted helminthiases. Acta
Tropica 2003; 86:223-32.
17. Sirivichayakul C, Pojjaroen-Anant C, Wisetsing P et al. Th e eff ectiveness of 3, 5,
or 7 days of albendazole for the treatment of Trichuris trichiura infection. Ann
Trop Med Parasitol 2003; 97:847-53.

C
HAPTER
3
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Ascariasis
Afsoon D. Roberts
Introduction
Ascariasis, a soil-transmitted infection, is the most common human helminthic
infection. Current estimates indicate that more than 1.4 billion people are infected
worldwide. In the United States, there are an estimated 4 million people infected,
primarily in the southeastern states and among immigrants. Th e etiologic agent,
Ascaris lumbricoides, an intestinal roundworm, is the largest nematode to infect
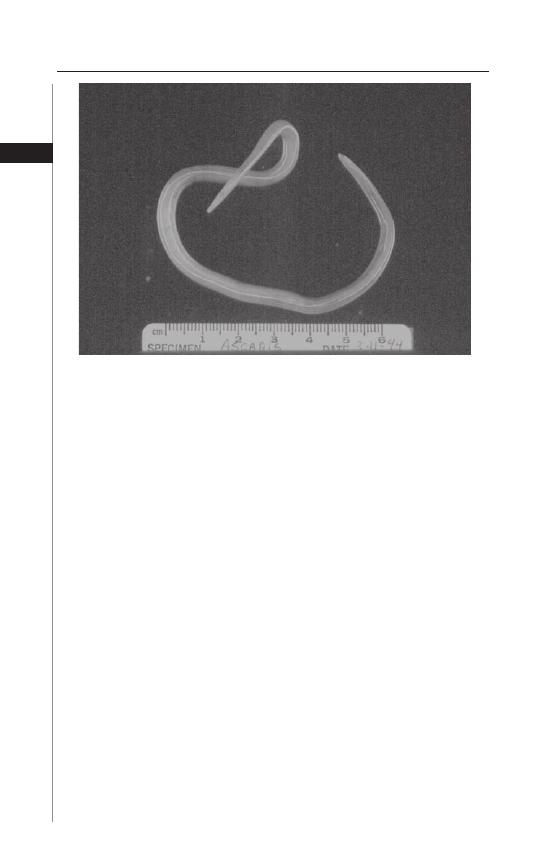
humans. Th e adult worm lives in the small intestine and can grow to a length of
more than 30 cm. Th e female worms are larger than the males. Important fac-
tors associated with an increased prevalence of disease include socio-economic
status, defecation practices and cultural diff erences relating to personal and food
hygiene as well as housing and sewage systems. Most infections are subclinical;
more severe complications occur in children who tend to suff er from the highest
worm burdens.
Epidemiology and Transmission
Th ere are a number of factors that contribute to the high frequency of infec-
tion with Ascaris lumbricoides. Th ese include its ubiquitous distribution, the high
number of eggs produced by the fecund female parasite and the hardy nature of the
eggs which enables them to survive unfavorable conditions. Th e eggs can survive
in the absence of oxygen, live for 2 years at 5-10º C and be unaff ected by dessica-
tion for 2 to 3 weeks. In favorable conditions of moist, sandy soil, they can survive
for up to 6 years, even in freezing winter conditions. Th e greatest prevalence of
disease is in tropical regions, where environmental conditions support year round
transmission of infection. In dry climates, transmission is seasonal and occurs most
frequently during the rainy months.
Ascariasis is transmitted primarily by ingestion of contaminated food or water.
Although infection occurs in all age groups, it is most common in preschoolers and
young children. Sub-optimal sanitation is an important factor, leading to increased
soil and water contamination. In the United States, improvements in sanitation and
waste management have led to a dramatic reduction in the prevalence of disease.
Recently, patterns of variation in the ribosomal RNA of Ascaris worms isolated
in North America were compared to those of worms and pigs from other worldwide
locations. Although repeats of specifi c restriction sites were found in most parasites
from humans and pigs in North America, they were rarely found in parasites from
elsewhere. Th is evidence suggests that perhaps human infections in North America
may be related to Ascaris suum.
15
Ascariasis
3
Life Cycle
Th e adult ascaris worms reside in the lumen of the small intestine where
they feed on predigested food (Fig. 3.1). Th eir life span ranges from 10 to 24
months. Th e adult worms are covered with a tough shell composed of collagens
and lipids. Th is outer covering helps protect them from being digested by in-
testinal hydrolases. Th ey also produce protease inhibitors that help to prevent
digestion by the host.
Th e adult female worm can produce 200,000 eggs per day (Fig. 3.2). Th e eggs
that pass out of the adult worm are fertilized, but not embryonated. Once the
eggs exit the host via feces, embryonation occurs in the soil and the embryonated
eggs are subsequently ingested. Th ere is a mucopolysaccharide on the surface that
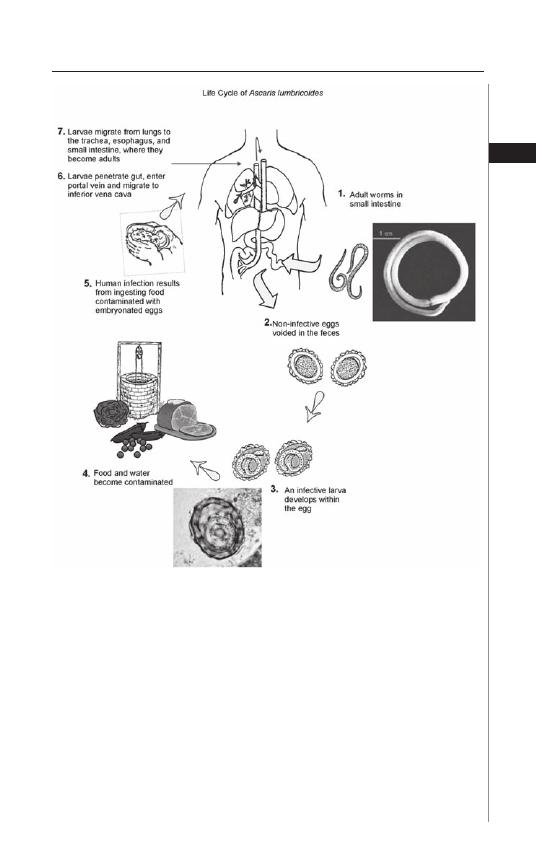
Figure 3.1. Life Cycle of Ascaris lumbricoides. Reproduced from: Nappi AJ,
Vass E, eds. Parasites of Medical Importance. Austin: Landes Bioscience,
2002:82.
16
Medical Parasitology
3
promotes adhesion of the eggs to environmental surfaces. Within the embryonated
egg, the fi rst stage larva develops into the second stage larva. Th is second stage larva
is stimulated to hatch by the presence of both the alkaline conditions in the small
intestine and the solubilization of its outer layer by bile salts.
Th e hatched parasite that now resides in the lumen of the intestine penetrates
the intestinal wall and is carried to the liver through the portal circulation. It then
travels via the blood stream to the heart and lungs by the pulmonary circulation.
Th e larva molts twice, enlarges and breaks into the alveoli of the lung. Th ey then
pass up through the bronchi and into the trachea, are swallowed and reach the
small intestine once again. Within the small intestine, the parasites molt twice
more and mature into adult worms. Th e adult worms mate, although egg produc-
tion may precede mating.
Clinical Manifestations
Although most individuals infected with Ascaris lumbricoides are essentially
asymptomatic, the burden of symptomatic infection is relatively high as a result
of the high prevalence of infection on a worldwide basis. Symptomatic disease is
usually related to either the larval migration stage and manifests as pulmonary
disease, or to the intestinal stage of the adult worm.
Th e pulmonary manifestations of ascariasis occur during transpulmonary
migration of the organisms and are directly related to the concentration of
larvae. Th us, symptoms are more pronounced with higher burdens of migratory
worms. Th e transpulmonary migration of helminth larvae is responsible for the
development of a transient eosinophilic pneumonitis characteristic of Loeffl er’s
syndrome with peripheral eosinophilia, eosinophilic infi ltrates and elevated se-
rum IgE concentrations. Symptoms usually develop 9-12 days aft er ingestion of
the eggs, while the larvae reside in the lung. Aff ected individuals oft en develop
Figure 3.2. Ascaris lumbricoides.

17
Ascariasis
3
bronchospasm, dyspnea and wheezing. Fever, a persistent, nonproductive cough
and, at times chest pain, can also occur. Hepatomegaly may also be present. In
some areas of the world such as Saudi Arabia where transmission of infection is
related to the time of the year, seasonal pneumonitis has been described.
Th e diagnosis of Ascaris-related pneumonitis is suspected in the correct clinical
setting by the presence of infi ltrates on chest X-ray which tend to be migratory and
usually completely clear aft er several weeks. Th e pulmonary infi ltrates are usually
round, several millimeters to centimeters in size, bilateral and diff use.
Among the more serious complications of Ascaris infection is intestinal obstruc-
tion. Th is occurs when a large number of worms are present in the small intestine
and is usually seen in children with heavy worm burdens. Th ese patients present
with nausea, vomiting, colicky abdominal pain and abdominal distention. In this
condition worms may be passed via vomitus or stools. In endemic areas, 5-35%
of all cases of intestinal obstruction can be attributable to ascariasis. Th e adult
worms can also perforate the intestine leading to peritonitis. Ascaris infection can
be complicated by intussusception, appendicitis and appendicular perforation due
to worms entering the appendix.
A potenital consequence of the intestinal phase of the infection relates to the
eff ect it may have on the nutritional health of the host. Children heavily infected
with Ascaris have been shown to exhibit impaired digestion and absorption of
proteins and steatorrhea. Heavy infections have been associated with stunted
growth and a reduction in cognitive function. However, the role of Ascaris in these
defi ciencies is not clearly defi ned. Some of these studies were done in developing
countries where additional nutritional factors cannot be excluded. Th ere is also
a high incidence of co-infection with other parasites that can aff ect growth and
nutritional status. Interestingly, a controlled study done in the southern United
States failed to demonstrate signifi cant diff erences in the nutritional status of
Ascaris infected and uninfected individuals.
Hepatobiliary and Pancreatic Symptoms
Hepatobiliary symptoms have been reported in patients with Ascariasis and
are due to the migration of adult worms into the biliary tree. Aff ected individuals
can experience biliary colic, jaundice, ascending cholangitis, acalculous cholecys-
titis and perforation of the bile duct. Pancreatitis may develop as a result of an
obstruction of the pancreatic duct. Hepatic abscesses have also been reported.
Sandouk et al studied 300 patients in Syria who had biliary or pancreatic involve-
ment. Ninety-eight percent of the patients presented with abdominal pain, 16%
developed ascending cholangitis, 4% developed pancreatitis and 1% developed
obstructive jaundice. Both ultrasonography, as well as endoscopic retrograde
cholangiopancreatography (ERCP) have been used as diagnostic tools for biliary
or pancreatic ascariasis. In Sandouk’s study extraction of the worms endoscopi-
cally resulted in resolution of symptoms.
Diagnosis
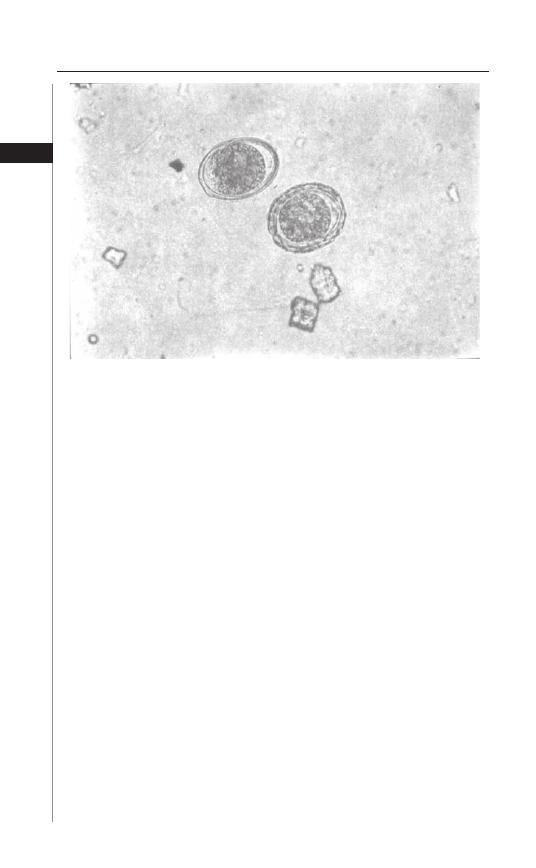
Th e diagnosis of ascariasis is made through microscopic examination of stool
specimens. Ascaris eggs are easily recognized, although if very few eggs are present
the diagnosis may be easily missed (Fig. 3.3). Techniques for concentrating the stool
18
Medical Parasitology
3
specimen will increase the yield of diagnosis through microscopy. Occasionally an
adult worm is passed via rectum. Eosinophilia may be present, especially during
the larval migration through the lungs. In very heavily infected individuals a plain
X-ray of the abdomen may sometimes reveal a mass of worms.
Treatment
Both albendazole and mebendazole are effective therapies for ascariasis.
Mebendazole can be prescribed as 100 mg BID for 3 days or 500 mg as a single
dose. Th e adverse eff ects of the drug include gastrointestinal symptoms, headache
and rarely leukopenia. Albendazole is prescribed as a single dose of 400 mg.
Albendazole’s side eff ect profi le is similar to mebendazole.
Th e drug piperazine citrate is an alternative therapeutic option, but it is not
widely available and has been withdrawn from the market in some developed
countries as other less toxic and more eff ective therapy is available. However, in
cases of intestinal or biliary obstruction it can be quite useful as it paralyses the
worms, allowing them to be expelled by peristalsis. It is dosed as 50-75 mg/kg QD,
up to a maximum of 3.5 g for 2 days. It can be administered as piperazine syrup
via a naso-gastric tube.
Finally, pyrantel pamoate can be used at a single dose of 11 mg/kg, up to a maxi-
mum dose of 1 g. Th is drug can be used in pregnancy. Th e side eff ects of pyrantel
pamoate include headache, fever, rash and gastrointestinal symptoms. It has been
reported to be up to 90% eff ective in treating the infection.
Th ese medications are all active against the adult worm and are not active against
larval stage. Th us, reevaluation of infected individuals is recommended following
therapy. Family members should also be screened as infection is common among
other members of a household. Treatment does not protect against reinfection.
Figure 3.3. Ascaris lumbricoides egg.

19
Ascariasis
3
Prevention
Given the high prevalence of infection with Ascaris lumbricoides and the
potential health and educational benefi ts of treating the infection in children,
the World Health Organization (WHO) has recommended global deworming
measures aimed at school children. Th e goal of recent helminth control programs
has been to recommend periodic mass treatment where the prevalence of infection
in school aged children is greater than 50%. Th e current goal is to treat infected
individual 2 to 3 times a year with either mebendazole or albendazole. Integrated
control programs combining medical treatment with improvements in sanitation
and health education are needed for eff ective long-term control.
Suggested Reading
1. Ali M, Khan AN. Sonography of hepatobiliary ascariasis. J Clin Ultrasound 1996;
24:235-41.
2. Anderson TJ. Ascaris infections in human from North America: Molecular
evidence for cross infection. Parasitology 1995; 110:215-29.
3. Bean WJ. Recognition of ascariasis by routine chest or abdomen roentgenograms.
Am J Roentgenol Rad Th er Nucl Med 1965; 94:379.
4. Blumenthal DS, Schultz AG. Incidence of intestinal obstruction in children
infected by Ascaris lumbricoides. Am J Trop Med Hyg 1974; 24:801.
5. Blumenthal DS, Schultz MG. Eff ect of Ascaris infection on nutritional status in
children. Am J Trop Med Hyg 1976; 25:682.
6. Chevarria AP, Schwartzwelder JC et al. Mebendazole, an eff ective broad spectrum
anti-heminthic. Am J Trop Med Hyg 1973; 22:592-5.
7. Crampton DWT, Nesheim MC, Pawlowski ZS, eds. Ascariasis and Its Public
Health Signifi cance. London: Taylor and Francis, 1985.
8. De Silva NR, Guyatt HL, Bundy DA. Morbidity and mortality due to
Ascaris-induced intestinal obstruction. Trans R Soc Trop Med Hyg 1997;
91:31-6.
9. DeSilva NR, Chan MS, Bundy DA. Morbidity and mortality due to ascariasis:
Re-estimation and sensitivity analysis of global numbers at risk. Trop Med Int
Health 1997; 2:519-28.
10. Despommier DD, Gwadz RW, Hotez PJ et al, eds. Parasitic Diseases, 5th edition.
New York: Apple Tree Productions, 2005:115-20.
11. Gelpi AP, Mustafa A. Seosonal pneumonitis with eosinophilia: A study of larval
ascariasis in Saudi Arabia. Am J Trop Med Hyg 1967; 16:646.
12. Jones JE. Parasites in Kentucky: the past seven decades. J KY Med Assoc 1983;
81:621.
13. Khuroo MS. Ascariasis. Gastroenterol Clin North Am 1996; 25:553-77.
14. Khuroo MS. Hepato-biliary and pancreatic ascariasis. Indian J Gastroenterol 2001;
20:28.
15. Loeffl er W. Transient lung infi ltrations with blood eosinophilia. Int Arch Allergy
Appl Immunol 1956; 8:54.
16. Mandell GL, Bennett JE, Dolin R, eds. Principles and Practices of Infectious
Disease, 5th edition. Philadelphia: Churchill Livingstone, 2000:2941.
17. Norhayati M, Oothuman P, Azizi O et al. Effi cacy of single dose albendazole on the
prevalence and intensity of infection of soil-transmitted helminths in Orang Asli
children in Malaysia. Southeast Asian J Trop Med Public Health 1997; 28:563.
18. O’Lorcain, Holland CU. Th e public health importance of Ascaris lumbricoides.
Parasitology 2000; 121:S51-71.
19. Phills JA, Harold AJ, Whiteman GV et al. Pulmonary infi ltrates, asthma, eosino-
philia due to Ascaris suum infestation in man. N Engl J Med 1972; 286:965.

20
Medical Parasitology
3
20. Reeder MM. Th e radiographic and ultrasound evaluation of ascariasis of the
gastrointestinal, biliary and respiratory tract. Semin Roentgenol 1998; 33:57.
21. Sandou F, Haff ar S, Zada M et al. Pancreatic-biliary ascariasis: Experience of 300
cases. Am J Gastroenterol 1997; 92:2264-7.
22. Sinniah B. Daily egg production of Ascaris lumbricoides: Th e distribution of eggs
in the feces and the variability of egg counts. Parasitology 1982; 84:167.
23. Stephenson LS. Th e contribution of Ascaris lumbricoides to malnutrition in
children. Parasitolgy 1980; 81:221-33.
24. Warren KS, Mahmoud AA. Algorithems in the diagnosis and management of
exotic diseases, xxii ascariasis and toxocariaisis. J Infec Dis 1977; 135:868.
25. WHO Health of school children: Treatment of intestinal helminths and schistoso-
miasis (WHO/Schisto/95.112; WHO/CDS/95.1). World Health Organisation
1995.

C
HAPTER
4
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Hookworm
David J. Diemert
Introduction
Human hookworm infection is a soil-transmitted helminth infection caused
primarily by the nematode parasites Necator americanus and Ancylostoma duo-
denale. It is one of the most important parasitic infections worldwide, ranking
second only to malaria in terms of its impact on child and maternal health. An
estimated 576 million people are chronically infected with hookworm and an-
other 3.2 billion are at risk, with the largest number of affl icted individuals living
in impoverished rural areas of sub-Saharan Africa, southeast Asia and tropical re-
gions of the Americas. N. americanus is the most widespread hookworm globally,
whereas A. duodenale is more geographically restricted in distribution. Although
hookworm infection does not directly account for substantial mortality, its
greater health impact is in the form of chronic anemia and protein malnutrition
as well as impaired physical and intellectual development in children.
Humans may also be incidentally infected by the zoonotic hookworms
Ancylostoma caninum, Ancylostoma braziliensis and Uncinaria stenocephala,
which can cause self-limited dermatological lesions in the form of cutaneous
larva migrans. Additionally, Ancylostoma ceylanicum, normally a hookworm
infecting cats, has been reported to cause hookworm disease in humans espe-
cially in Asia, whereas A. caninum has been implicated as a cause of eosinophilic
enteritis in Australia.
Life Cycle
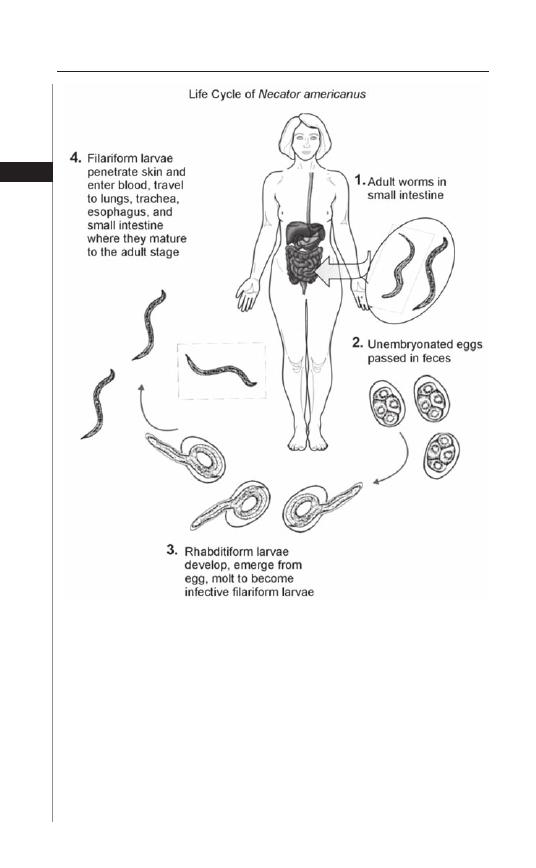
Hookworm transmission occurs when third-stage infective fi lariform larvae
come into contact with skin (Fig. 4.1). Hookworm larvae have the ability to actively
penetrate the cutaneous tissues, most oft en those of the hands, feet, arms and legs
due to exposure and usually through hair follicles or abraded skin. Following skin
penetration, the larvae enter subcutaneous venules and lymphatics to gain access
to the host’s aff erent circulation. Ultimately, they enter the pulmonary capillaries
where they penetrate into the alveolar spaces, ascend the brachial tree to the trachea,
traverse the epiglottis into the pharynx and are swallowed into the gastrointestinal
tract. Larvae undergo two molts in the lumen of the intestine before developing into
egg-laying adults approximately fi ve to nine weeks aft er skin penetration. Although
generally one centimeter in length, adult worms exhibit considerable variation in
size and female worms are usually larger than males (Fig. 4.2).
Adult Necator and Ancylostoma hookworms parasitize the proximal portion of
the human small intestine where they can live for several years, although diff erences
22
Medical Parasitology
4
exist between the life spans of the two species: A. duodenale survive for on average
one year in the human intestine whereas N. americanus generally live for three to
fi ve years (Fig. 4.3). Adult hookworms attach onto the mucosa of the small intestine
by means of cutting teeth in the case of A. duodenale or a rounded cutting plate in
the case of N. americanus. Aft er attachment, digestive enzymes are secreted that
enable the parasite to burrow into the tissues of the submucosa where they derive
nourishment from eating villous tissue and sucking blood into their digestive tracts.
Hemoglobinases within the hookworm digestive canal enable digestion of human
hemoglobin, which is a primary nutrient source of the parasite.
Figure 4.1. Life cycle of the hookworm, Necator americanus. Reproduced
from: Nappi AJ, Vass E, eds. Parasites of Medical Importance. Austin: Landes
Bioscience, 2002:80.

23
Hookworm
4
Humans are considered the only major defi nitive host for these two parasites and
there are no intermediate or reservoir hosts; in addition, hookworms do not reproduce
within the host. Aft er mating in the host intestinal tract, each female adult worm
produces thousands of eggs per day which then exit the body in feces. A. duodenale
female worms lay approximately 28,000 eggs daily, while the output from N. ameri-
canus worms is considerably less, averaging around 10,000 a day. N. americanus and
A. duodenale hookworm eggs hatch in warm, moist soil, giving rise to rhabditiform
larvae that grow and develop, feeding on organic material and bacteria. Aft er about
seven days, the larvae cease feeding and molt twice to become infective third-stage
fi lariform larvae. Th ird-stage larvae are nonfeeding but motile organisms that seek out
higher ground such as the tips of grass blades to increase the chance of contact with
human skin and thereby complete the life cycle. Filariform larvae can survive for up
to approximately two weeks if an appropriate host is not encountered.
A. duodenale larvae can also be orally infective and have been conjectured to
infect infants during breast feeding.
Epidemiology and Burden of Disease
Human hookworm infections are widely distributed throughout the trop-
ics and sub-tropics (Fig. 4.4). N. americanus is the most prevalent hookworm
worldwide, with the highest rates of infection in sub-Saharan Africa, the tropical
Figure 4.2. Adult male Ancylostoma duodenale hookworm. Reproduced
with permission from: Despommier DD, Gwadz RW, Hotez PJ, Knirsch CA.
Parasitic Diseases. New York: Apple Trees Productions, 2005:121.
24
Medical Parasitology
4
regions of the Americas, south China and southeast Asia, whereas A. duodenale
is more focally endemic in parts of India, China, sub-Saharan Africa, North
Africa and a few regions of the Americas. Climate is an important determinant
of hookworm transmission, with adequate moisture and warm temperature
essential for larval development in the soil. An equally important determinant
of infection is poverty and the associated lack of sanitation and supply of clean
water. In such conditions, other helminth species are frequently co-endemic,
with emerging evidence that individuals infected with multiple diff erent types
of helminths (most commonly the triad of Ascaris lumbricoides, hookworm
and Trichuris trichiura) are predisposed to developing even heavier intensity
infections than those who harbor single-species infections. Because morbidity
from hookworm infections and the rate of transmission are directly related to
the number of worms harbored within the host, the intensity of infection is the
primary epidemiological parameter used to describe hookworm infection as
measured by the number of eggs per gram of feces.
While prevalence in endemic areas increases markedly with age in young chil-
dren and reaches a plateau by around an age of ten years, intensity of infection
Figure 4.3. Adult hookworm, diagnosed by endoscopy. Reproduced with per-
mission from: Despommier DD, Gwadz RW, Hotez PJ, Knirsch CA. Parasitic
Diseases. New York: Apple Trees Productions, 2005:123.

25
Hookworm
4
rises at a slower rate during childhood, reaching a plateau by around 20 years and
then increasing again from age 60 years onward. Controversy persists whether
such age-dependency refl ects changes in exposure, acquired immunity, or a com-
bination of both. Although heavy hookworm infections also occur in childhood,
it is common for prevalence and intensity to remain high into adulthood, even
among the elderly. Hookworm infections are oft en referred to as “overdispersed” in
endemic communities, such that the majority of worms are harbored by a minority
of individuals in an endemic area. Th ere is also evidence of familial and household
aggregation of hookworm infection, although the relative importance of genetics
over the shared household environment is debated.
Although diffi cult to ascertain, it is estimated that worldwide approximately
65,000 deaths occur annually due to hookworm infection. However, hookworm
causes far more disability than death. Th e global burden of hookworm infec-
tions is typically assessed by estimating the number of Disability Adjusted Life
Years (DALYs) lost. According to the World Health Organization, hookworm
accounts for the loss of 22 million DALYs annually, which is almost two-thirds
that of malaria or measles. Th is estimate refl ects the long-term consequences of
hookworm-associated malnutrition, anemia and delays in cognitive development,
especially in children.
Recent data support the high disease burden estimates of hookworm infection
and highlight its importance as a maternal and child health threat. For example,
studies in Africa and Asia show that between one-third and one-half of moderate
to severe anemia among pregnant women can be attributable to hookworm and
recent evidence from interventional studies further suggest that administration of
anthelmintics antenatally can substantially improve maternal hemoglobin as well
as birth weight and neonatal and infant survival. In addition to pregnant women,
hookworm contributes to moderate and severe anemia among both preschool and
primary school-aged children.
Figure 4.4. Prevalence of hookworm infection worldwide. From: Hotez et
al, Public Library of Science.

26
Medical Parasitology
4
Clinical Manifestations
Th e clinical features of hookworm infection can be separated into the acute
manifestations associated with larval migration through the skin and other tis-
sues and the acute and chronic manifestations resulting from parasitism of the
gastrointestinal tract by adult worms.
Migrating hookworm larvae provoke reactions in many of the tissues through
which they pass, including several cutaneous syndromes that result from
skin-penetrating larvae. Repeated exposure to N. americanus and A. duodenale
fi lariform larvae can result in a hypersensitivity reaction known as “ground itch”,
a pruritic local erythematous and papular rash that appears most commonly on
the hands and feet. In contrast, when zoonotic hookworm larvae (typically A.
braziliensis,A. caninum or U. stenocephala) penetrate the skin, usually aft er direct
contact between skin and soil or sandy beaches contaminated with animal feces,
they produce cutaneous larva migrans, most oft en on the feet, buttocks and
abdomen. Since these zoonotic larvae are unable to complete their life cycle in
the human host, they eventually die aft er causing a typical clinical syndrome of
erythematous linear tracts with a serpiginous appearance and intense pruritus.
Th ese tracts can elongate by several centimeters a day; larvae can migrate for up
to one year, but the lesions usually heal spontaneously within weeks to months
although secondary pyogenic infection may occur at these sites as well as those
of ground itch.
One to two weeks following skin invasion, hookworm larvae travel through
the vasculature and enter the lungs, where they can uncommonly result in
pneumonitis. Th e pulmonary symptoms that may develop are usually mild and
transient, consisting of a dry cough, sore throat, wheezing and slight fever. Th e
pulmonary symptoms are more pronounced and of longer duration with A.
duodenale than with N. americanus infection. Acute symptomatic disease may
also result from oral ingestion of A. duodenale larvae, referred to as the Wakana
syndrome, which is characterized by nausea, vomiting, pharyngeal irritation,
cough, dyspnea and hoarseness.
In hookworm infection, the appearance of eosinophilia coincides with the
development of adult hookworms in the intestine. Th e major pathology of hook-
worm infection, however, results from the intestinal blood loss that results from
adult parasite invasion and attachment to the mucosa and submucosa of the small
intestine. Usually only moderate and high intensity hookworm infections in the
gastrointestinal tract produce clinical manifestations, with the highest intensity
infections occurring most oft en in children, although even in low intensity infec-
tions, initial symptoms may include dyspepsia, nausea and epigastric distress. A.
duodenale may also result in acute enteritis with uncontrollable diarrhea and foul
stools that may last indefi nitely.
In general, the precise numerical threshold at which worms cause disease
has not been established since this is highly dependent on the underlying nutri-
tional status of the host. Chronic hookworm disease occurs when the blood loss
due to infection exceeds the nutritional reserves of the host, thus resulting in
iron-defi ciency anemia. It has been estimated that the presence of more than 40
adult worms in the small intestine is suffi cient to reduce host hemoglobin levels
below 11 g per deciliter, although the exact number depends on several factors

27
Hookworm
4
including the species of hookworm and host iron reserves. Worm-for-worm, A.
duodenale causes more blood loss than N. americanus: whereas each N. americanus
worm produces a daily blood loss of 0.03 to 0.1 ml, the corresponding fi gure for
A. duodenale is between 0.15 and 0.26 ml.
Th e clinical manifestations of chronic hookworm disease resemble those of
iron defi ciency anemia due to other etiologies, while the protein loss from heavy
hookworm infection can result in hypoproteinemia and anasarca. Th e anemia and
protein malnutrition that results from chronic intestinal parasitism cause long-term
impairments in childhood physical, intellectual and cognitive development. As
iron defi ciency anemia develops and worsens, an infected individual may have
weakness, palpitations, fainting, dizziness, dyspnea, mental apathy and headache.
Uncommonly, there may be constipation or diarrhea with occult blood in the
stools or frank melena, especially in children; there may also be an urge to eat soil
(pica). Overwhelming hookworm infection may cause listlessness, coma and even
death, especially in infants under one year of age. Because children and women of
reproductive age have reduced iron reserves, they are considered populations that
are at particular risk for hookworm disease. As noted above, the severe iron defi -
ciency anemia that may arise from hookworm disease during pregnancy can result
in adverse consequences for the mother, her unborn fetus and the neonate.
Zoonotic infection with the dog hookworm A. caninum has been reported as
a cause of eosinophilic enteritis in Australia, although considering the ubiquitous
worldwide distribution of this parasite, unrecognized clinical disease due to this
worm may be more widespread than previously recognized. Reported clinical
features of this syndrome include abdominal pain, diarrhea, abdominal bloating,
weight loss and rectal bleeding. A. ceylanicum, while normally a hookworm that
parasitizes cats, has been reported as a cause of chronic intestinal infection in
humans living in Asia.
Th e most common manifestations of hookworm infection seen by the average
healthcare practitioner in developed countries are cutaneous larva migrans in
returning travelers and chronic intestinal infection with resulting anemia and
peripheral eosinophilia in immigrants and long-term expatriate residents or
military personnel returning from long-term postings in endemic areas.
Diagnosis
Diagnosis of established hookworm infections is made primarily by means
of microscopic identifi cation of characteristic eggs in the stool (Fig. 4.5). In an
infected human, a single adult female hookworm will produce thousands of eggs
per day. Because hookworm infections will oft en not present with specifi c signs
and symptoms, the clinician typically requires some index of suspicion, such as
local epidemiology or country of origin or travel, to request a fecal examination
for ova and parasites.
Hookworm eggs are colorless and have a single thin hyaline shell with blunted
ends, ranging in size from 55-75
μ
m by 36-40
μ
m. Several sensitive egg concen-
tration techniques, such as the formalin-ethyl acetate sedimentation method, can
be used to detect even light infections. Where concentration procedures are not
available, a direct wet mount examination of the specimen is adequate for detecting
moderate to heavy infections. A single stool sample is oft en suffi cient to diagnose
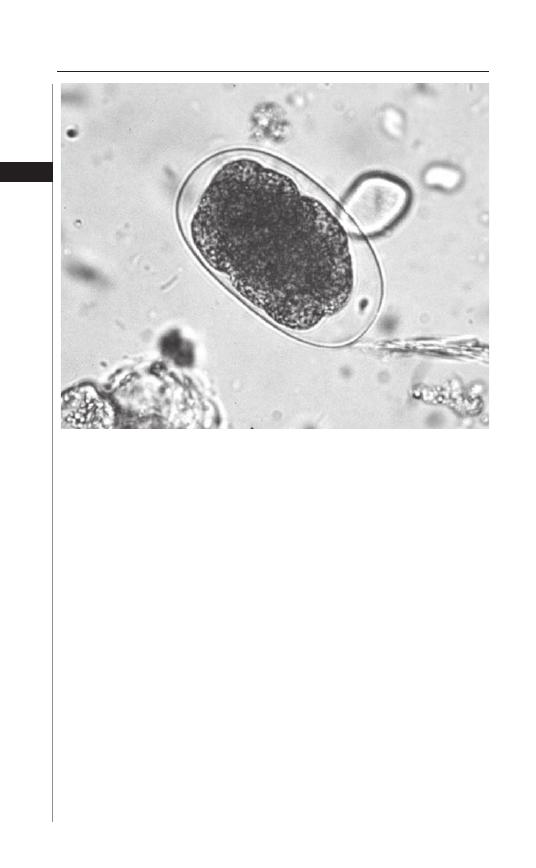
28
Medical Parasitology
4
hookworm infection, with diagnostic yield not appreciably increased by examining
further specimens. Although examination of the eggs cannot distinguish between
N. americanus and A. duodenale, this is not clinically relevant. Diff erentiation be-
tween the two species can be made by either rearing fi lariform larvae from a fecal
sample smeared on a moist fi lter paper strip for fi ve to seven days (the Harada-Mori
technique) or by recovering the adult worms following treatment and examining
for such features as the mouthparts.
Besides microscopic examination of feces, eosinophilia is a common fi nding in
persistent infection and also during the phase of migration of larvae through the
lungs. A chest radiograph will usually be negative during the pulmonary phase of
larval migration, although sputum examination may reveal erythrocytes, eosino-
phils and rarely migrating larvae.
Dermatological infection with the zoonotic hookworms A. caninum, A. braziliensis
and U. stenocephala is primarily diagnosed clinically, although eosinophilia and
elevated serum IgE occur in between 20% to 40% of patients with cutaneous larva
migrans. Since larvae do not migrate to the gastrointestinal tract to develop into
adult worms, fecal examination will be negative in these cases.
Treatment
Th e goal of treatment for N. americanus and A. duodenale infections is to
eliminate adult worms from the gastrointestinal tract. Th e most common drugs
Figure 4.5. Fertilized, embryonated hookworm egg. Reproduced with per-
mission from: Despommier DD, Gwadz RW, Hotez PJ, Knirsch CA. Parasitic
Diseases. New York: Apple Trees Productions, 2005:123.

29
Hookworm
4
used for the treatment of hookworm infections worldwide are members of the ben-
zimidazole anthelmintic class of drugs, of which mebendazole and albendazole are
the two principle members. Th e benzimidazoles bind to hookworm
β
-tubulin and
inhibit microtubule polymerization, which causes death of adult worms through a
process that can take several days. Although both albendazole and mebendazole are
considered broad-spectrum anthelmintic agents, there are important therapeutic
diff erences that aff ect their use in clinical practice. For hookworm, a single 500 mg
dose of mebendazole oft en achieves a low cure rate, with a higher effi cacy with a
single, 400 mg dose of albendazole, although multiple doses of the benzimidazoles
are oft en required.
Systemic toxicity, such as hepatotoxicity and bone marrow suppression, is rare
for the benzidimazoles in the doses used to treat hookworm infections. However,
transient abdominal pain, diarrhea, nausea, dizziness and headache can com-
monly occur. Uncommon adverse reactions include alopecia, elevated hepatic
transaminases and rarely, leukopenia. Because the benzidimazoles are embryotoxic
and teratogenic in pregnant rats, there are concerns regarding their use in children
younger than one year of age and during pregnancy. Overall, the experience with
these drugs in children under the age of six years is limited, although data from
published studies indicate that they are probably safe. Both pyrantel pamoate and
levamisole are considered alternative drugs for the treatment of hookworm and
they are administered by body weight.
Cutaneous larva migrans should be treated empirically with either albendazole
400 mg daily for three days, or ivermectin 200 mcg/kg/d for 1-2 days; topical
thiabendazole (10% to 15%) has also been used to treat this manifestation of
hookworm infection in the past. Mebendazole is poorly absorbed from the gas-
trointestinal tract and therefore does not attain high tissue levels necessary to kill
larvae in the skin and treat this condition.
Prevention and Control
In the past, eff orts at hookworm control in endemic areas have focused on
behaviour modifi cation, such as encouraging the use of proper footwear and im-
proving the disposal of human feces. Unfortunately, these eff orts largely met with
failure due to such reasons as the continued high rate of occupational exposure
to hookworm during agricultural work, the ability of N. americanus larvae to
penetrate the skin of any part of the body and the use of human feces as fertilizer
for crops. Instead, recent control eff orts have focused on the use of anthelmintic
chemotherapy as a useful tool for large-scale morbidity reduction in endemic com-
munities throughout the world. Annual mass administration of benzidimazoles
to school-aged children reduces and maintains the adult worm burden below the
threshold associated with disease. Th e benefi ts of regular deworming in this age
group include improvements in iron stores, growth and physical fi tness, cognitive
performance and school attendance. In preschool children, studies have demon-
strated improved nutritional indicators such as wasting malnutrition, stunting
and appetite. Treated children experience improved scores on motor and language
testing in their early development. In addition to children, pregnant women and
their newborns in endemic areas, if treated once or twice during pregnancy, achieve
signifi cant improvement of maternal anaemia and benefi t from a reduction of

30
Medical Parasitology
4
low-birth weight and infant mortality at six months. In areas where hookworm
infections are endemic, anthelmintic treatment is recommended during pregnancy
except in the fi rst trimester.
However, periodic anthelmintic administration is not expected to lead to
complete elimination of hookworm. Reasons include the variable effi cacy of ben-
zimidazoles against hookworm, rapid reinfection following treatment especially
in areas of high transmission where this can occur within four to 12 months, the
diminished effi cacy of the benzimidazoles with frequent and repeated use and the
possibility drug resistance may develop in time, as has been observed in veterinary
medicine. Development of sensitive tools for the early detection of anthelmintic
resistance is underway, with special attention being given to in vitro tests and
molecular biology techniques that could be adaptable to fi eld conditions. Since
no new anthelmintic drugs will be entering the marketplace any time soon, the
effi cacy of currently available products must be preserved. Furthermore, these
concerns have prompted interest in developing alternative tools for hookworm
control, such as antihookworm vaccines. Vaccination to prevent high intensity
hookworm infection would alleviate much of the global public health impact of
this widespread infection.
Suggested Reading
1. Bethony J, Brooker S, Albonico M et al. Soil-transmitted helminth infections: asca-
riasis, trichuriasis and hookworm. Th is is a thorough review of the soil-transmitted
helminth infections, including hookworm. Lancet 2006; 367:1521-32.
2. Brooker S, Bethony J, Hotez PJ. Human hookworm infection in the 21st Century.
Adv Parasitol 2004; 58:197-288.
3. Hotez P, Brooker S, Bethony J et al. Hookworm Infection. N Engl J Med 2004;
351:799-807. An excellent review.
4. Chan MS, Bradley M, Bundy DA. Transmission patterns and the epidemiology
of hookworm infection. Int J Epidemiol 1997; 26:1392-1400.
5. Brooker S, Bethony JM, Rodrigues L et al. Epidemiological, immunological and
practical considerations in developing and evaluating a human hookworm vaccine.
Expert Rev Vaccines 2005; 4:35-50.
6. Jelinek T, Maiwald H, Nothdurft HD et al. Cutaneous larva migrans in travelers:
synopsis of histories, symptoms and treatment of 98 patients. Clin Infect Dis 1994;
19:1062-6.
7. Crompton DW. Th e public health importance of hookworm disease. Parasitology
2000; 121:S39-S50.

C
HAPTER
5
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Strongyloidiasis
Gary L. Simon
Background, Epidemiology
Strongyloidiasis is an intestinal infection caused by a parasitic nematode
that has a widespread distribution throughout the tropics and subtropics.
Th e number of individuals infected with this nematode is unknown, but esti-
mates range from 30 million to 100 million. Infection in the United States is
relatively uncommon and the available prevalence data dates back more than
two decades. Among the populations that have been shown to be at increased
risk are immigrants from developing countries, veterans who have served in
endemic areas, especially among those who were prisoners of war, and residents
of Appalachia. Epidemiologic studies in rural Kentucky have revealed preva-
lence rates of 3-4%. A much lower prevalence is found in urban centers in the
Southeast. Evidence of strongyloidiasis was noted in only 0.4% of stool samples
in Charleston, West Virginia and New Orleans, Louisiana. On the other hand,
a large study in New York City found a prevalence of 1%, presumably due to
the large Central American immigrant population. Asymptomatic infection is
common in Latin America, Southeast Asia and sub-Saharan Africa. Although
less common, infection still occurs in Europe, most notably in the southern and
eastern portions of the continent.
Strongyloides may persist in the host for decades. Evidence of infection
for more than 40 years has been documented among British soldiers who were
prisoners of war in the Far East during World War II. Th ere is no endemic focus
of strongyloides in the United Kingdom so that the original infection must have
occurred during their incarceration in Th ailand or Burma. Among 2,072 former
prisoners who were studied between 1968 and 2002, 12% had strongyloides
infection. Th ere was a strong association between being held in captivity along
the infamous Th ai-Burma railway and the presence of strongyloides in stool
specimens. Among 248 individuals with strongyloides, 70% had the typical
larva currens rash and 68% had eosinophilia.
Strongyloides infection is not limited to human hosts. Dogs and nonhu-
man primates are susceptible to infection and this may play an important role
in transmission. Th ere have been a number of outbreaks of strongyloidiasis
among animal handlers. Human-to-human transmission has also been described
among homosexual men and in day care centers and mental institutions. Most
infections, however, are related to exposure to soil that has been contaminated
with fecal material that contained Strongyloides stercoralis larvae.
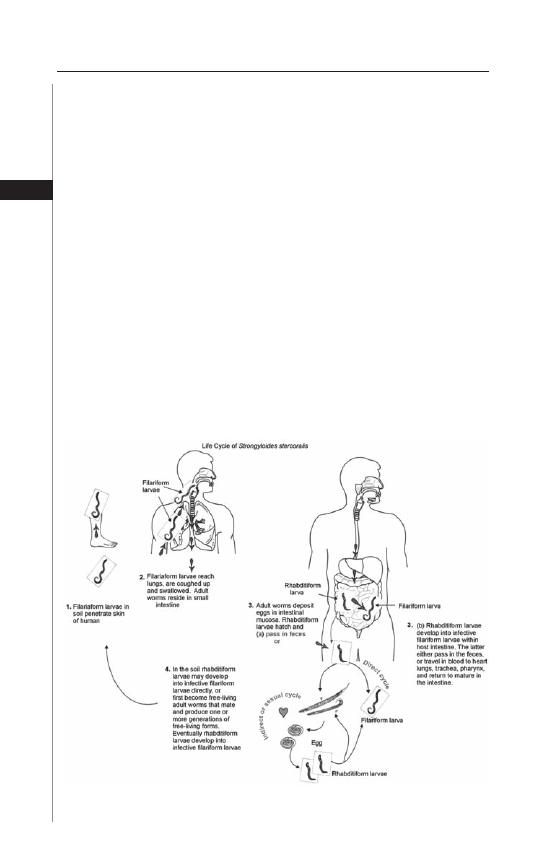
32
Medical Parasitology
5
Causative Agent
Th e vast majority of infections are caused by S. stercoralis. Th ere is another spe-
cies, S. fuelleborni, which infects infants in Papua, New Guinea and sub-Saharan
Africa. Th ese children develop a “swollen belly syndrome” that is characterized by
generalized edema and respiratory distress. Th ere is a signifi cant mortality associ-
ated with this condition. In a study done in Democratic Republic of Congo, Africa,
34% of 76 children less than 7 months of age were infected with this organism and,
in one mother, the organism was found in breast milk suggesting transmammary
passage as a means of infection.
Life Cycle
Strongyloides stercoralis exists in both a free living form in the soil and as an
intestinal parasite (Fig. 5.1). Th e parasitic females are 2.2 mm in length, semi-
transparent and colorless and lie embedded within the mucosal epithelium of the
proximal small intestine where they deposit their eggs. A single female worm will
produce up to 50 eggs per day. Th ere is no parasitic male and reproduction is by
parthenogenesis.
Th e embryonated eggs hatch within the mucosa and emerge into the lumen of the
small bowel as noninfectious rhabditiform larvae (Fig. 5.2). Th e rhabditiform larvae
are excreted in the stool and, in a warm, humid environment, mature and become
free-living adult male and female worms. Th e adult worms mate and the female
produces embryonated eggs (Fig. 5.3) that, aft er several molts, ultimately become
Figure 5.1. Life Cycle of Strongyloides. Adapted from Nappi AJ, Vass E, eds.
Parasites of Medical Importance. Austin: Landes Bioscience, 2002:77.
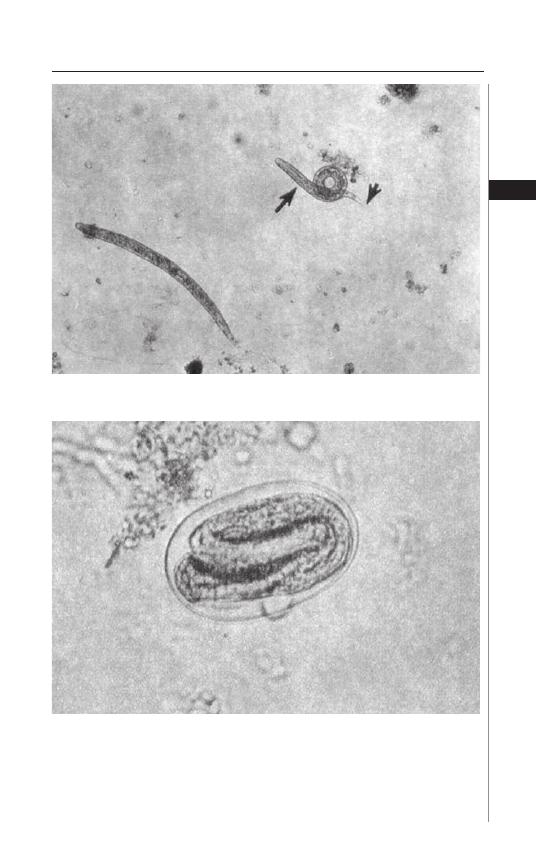
33
Strongyloidiasis
5
Figure 5.2. Rhabditiform larvae of S. stercoralis.
Figure 5.3. Ova of S. stercoralis.
infectious fi lariform larvae (Fig. 5.4). Alternatively, there may be several free-living
cycles which precede the development of the fi lariform larvae. Th e fi lariform larvae
are long and slender whereas the rhabditiform larvae are shorter and thicker. Th e
fi lariform larvae penetrate intact skin leading to the parasitic phase of infection.
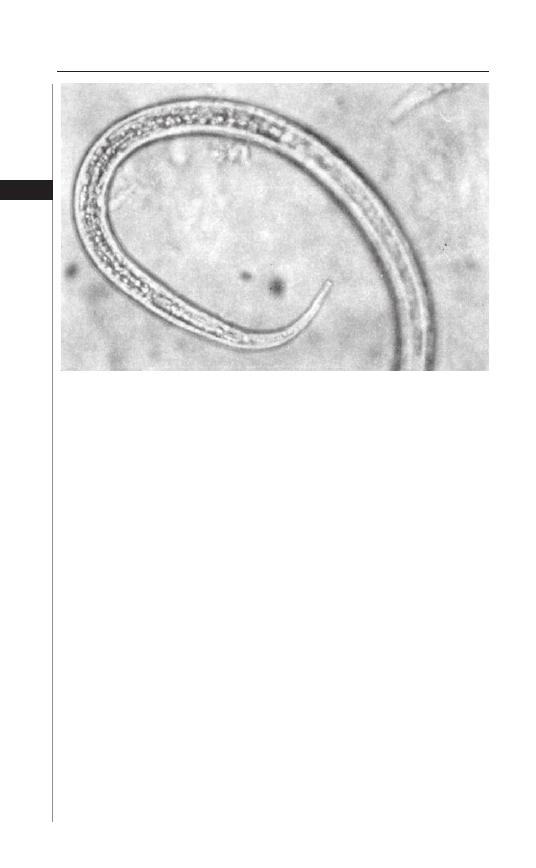
34
Medical Parasitology
5
Aft er penetrating the skin, the fi lariform larvae migrate to the right side of the
heart, either through the lymphatics or the venules and then to the lungs via the
pulmonary circulation. In the lungs the larvae penetrate the alveoli, migrate up the
tracheobronchial tree and are swallowed. In the proximal small intestine the larvae
molt, become adult female worms and lodge in submucosal tissues. Egg production
begins in 25-30 days aft er initially penetrating the skin.
Autoinfection, Hyperinfection
One of the more unusual features of strongyloidiasis is the concept of autoinfection.
In the distal colon or moist environment of the perianal region rhabditiform larvae can
undergo transformation to fi lariform larvae. Th ese infectious forms may penetrate the
colonic mucosa or perianal skin and “reinfect” the host. Th is process of autoinfection
may be quite common and accounts for the persistence of this organism in the host for
decades. In fact, as a result of this autoinfection process, S. stercoralis can actually increase
its numbers in the same individual without additional environmental exposures.
A more severe form of autoinfection is the hyperinfection syndrome in which
large numbers of rhabditiform larvae transform to fi lariform larvae, penetrate the
colonic mucosa and cause severe disease. Th is occurs in debilitated, malnourished
or immunosuppressed individuals. Administration of prednisone is a common
risk factor and cases have been reported following treatment of asthma with glu-
cocorticosteroids. Other risk factors include impaired gut motility, protein-calorie
malnutrition, alcoholism, hypochlorhydria, lymphoma, organ transplantation and
lepromatous leprosy. Human T-cell lymphotropic virus Type 1 (HTLV-1) has also
been associated with severe strongyloides infection. Th e most severe form of the
hyperinfection syndrome is disseminated infection in which larvae are found in
other organs including the liver, kidney and central nervous system.
Figure 5.4. Posterior end of fi lariform larva of S. stercoralis.

35
Strongyloidiasis
5
Immune Response
Eosinophils, mast cells and T-cell mediated immune function appear to play a role
in resistance to strongyloides. Eosinophilia is common in controlled chronic strongy-
loides infection whereas eosinophils are absent in patients with the hyperinfection
syndrome. Eosinophils, when in close proximity to a helminth, can kill the organism
through exocytosis of granular contents, eosinophil major basic protein and eosinophil
cationic protein. Eosinophils have IgE receptors on their surface and their cytotoxic
eff ects may be mediated through an IgE-antibody dependant cellular cytotoxicity.
Mast cells have several functions in the host defense against helminths. Th ey
promote peristalsis and mucus production which aid in expelling the parasite,
they produce IL-4 and they attract eosinophils. Release of mast cell granules is an
important gastrointestinal infl ammatory stimulus.
Th e immunosuppressive character of those conditions which predispose to
hyperinfection has indicated that T-cell mediated immunity is an important com-
ponent of the host response to Strongyloides. Th us, although initially predicted to
be an AIDS-related disease, it was rather surprising to fi nd very little evidence of
excessive numbers of advanced strongyloidiasis among HIV-infected individuals.
One possible explanation for these fi ndings may be the distinguishing features of
the TH-1 and TH-2 helper lymphocyte response.
TH-1 cells are primarily associated with the cellular immune response and the
production of proinfl ammatory cytokines such as IL-2, IL-12 and
γ
-interferon. Th e
predominant defect in HIV-infected individuals is in the TH-1 mediated infl am-
matory response. On the other hand, control of helminthic infections is more a
function of the TH-2 response which stimulates the production of cytokines such
as IL-4, IL-5 and IL-10. Interleukin-4 and IL-5 promote IgE synthesis; IL-5 regu-
lates eosinophil migration and activation. Both IL-4 and IL-10 are antagonists of
the TH-1 response, thus promoting the shift to a TH-2 humoral response. It is the
absence of an eff ective TH-2 response that appears to be one of the major factors
in promoting development of the hyperinfection syndrome.
Corticosteroids promote the hyperinfection syndrome through a variety of
mechanisms. Corticosteroids inhibit the proliferation of eosinophils, induce
apoptosis of eosinophils and T-lymphocytes and inhibit the transcription of IL-4
and IL-5. Corticosteroids may also have direct eff ects on the worms themselves.
Th ey may stimulate female worms to increase larval output and promote molting
of rhabditiform larvae into the invasive fi lariform larvae.
Clinical Findings
Th e clinical manifestations of uncomplicated strongyloidiasis are cutaneous,
pulmonary and gastrointestinal. Following penetration of the skin, there may be a
localized, erythematous, papular, pruritic eruption. Migrating larvae may produce
a serpiginous urticarial rash, larva currens (Fig. 5.5), that can progress as fast as
10 cm/hr. Th is is frequently seen on the buttocks, perineum and thighs and may
represent autoinfection. Within a few days of initial infection, pulmonary symp-
toms such as cough, wheezing or shortness of breath may develop as well as fl eeting
pulmonary infi ltrates and eosinophilia. With the development of gastrointestinal
involvement there may be epigastric discomfort suggestive of peptic ulcer disease,
bloating, nausea and diarrhea. Rarely, more severe infections have been associated
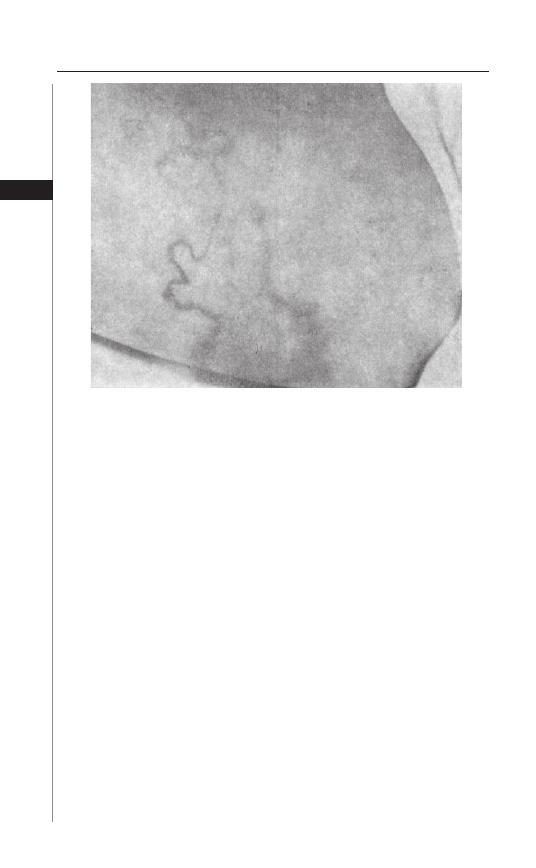
36
Medical Parasitology
5
with malabsorption and protein-calorie malnutrition. However, in many chroni-
cally infected individuals, symptoms resolve with the appearance of larvae in the
stool, although most have persistent eosinophilia.
Radiologic fi ndings are quite variable ranging from no abnormalities to duode-
nal dilatation and thickened mucosal folds. In severe cases, there may be nodular
intraluminal defects secondary to granuloma formation as well as loss of mucosal
architecture and luminal narrowing secondary to fi brosis.
Th e nonspecifi c symptoms of chronic strongyloidiasis may lead to endoscopic
evaluation for consideration of peptic ulcer disease, especially in urban centers.
Endoscopic fi ndings consist of mucosal hyperemia and white punctate lesions
in the duodenum. Histologic examination reveals larvae and adult worms in the
duodenal crypts, submucosa and lamina propria.
Th e large numbers of organisms in the intestines and lungs in patients with
the hyperinfection syndrome result in symptoms that are in sharp contrast to the
oft en relatively benign nature of chronic strongyloidiasis. Abdominal pain, nausea,
vomiting and profuse diarrhea are common. An ileus may be present and bowel
edema leading to intestinal obstruction has been described. Duodenal ulceration
and hemorrhage, perforation and peritonitis are rare complications. Pulmonary
fi ndings include cough, hemoptysis, shortness of breath, diff use pulmonary infi l-
trates and even respiratory failure. Larvae are oft en found in the sputum of patients
with the hyperinfection syndrome. Patients with the hyperinfection syndrome may
present with Gram-negative sepsis as a result of colonic bacteria entering the host
either through intestinal perforation resulting from the invasion of fi lariform larvae,
or on the back or in the gut of the larvae. In patients with bacteremia, respiratory
failure may ensue as a result of the direct eff ects of larvae migrating through the
Figure 5.5. Larva currens.

37
Strongyloidiasis
5
lungs, accompanying Gram-negative pneumonia or from the development of
sepsis-mediated adult respiratory distress syndrome. Meningitis is frequently seen
in patients with disseminated infection. Pneumonia, peritonitis and infection of
other organs may also occur in patients with disseminated infection. Most patients
with severe hyperinfection or disseminated infection do not survive their illness.
Diagnosis
Th e diagnosis of strongyloidiasis can be established by identifi cation of the lar-
vae in the stool. Th is is oft en quite diffi cult, even for the experienced parasitologist,
because of the low number of larvae produced on a daily basis by an adult worm.
Furthermore, release of larvae is intermittent so that even careful examination of
a single stool specimen may not reveal the organism. Th e sensitivity for fi nding
S. stercoralis in a single stool specimen from an infected individual has been esti-
mated to be only 30%. Multiple microscopic examinations using large volumes of
stool concentrated by a sedimentation method are oft en necessary. Frequently, the
only clue to the diagnosis is the presence of unexplained eosinophilia.
A variety of methods have been developed to increase the likelihood of fi nding
larvae in a stool specimen. One method employs an agar culture on which a stool
sample is placed on nutrient agar and then incubated for several days. As the motile
larvae crawl over the agar, they carry bacteria with them leaving visible tracks. In one
large study, this technique was found to be 96% sensitive in determining the pres-
ence of strongyloides. Unfortunately, it is rather laborious and time-consuming and
is not usually employed in routine clinical laboratories. Most clinical laboratories
utilize a lugol-iodine staining technique of a stool sample that has been subjected
to a sedimentation procedure in order to concentrate the larvae.
Examination of specimens obtained directly from the duodenum has been used in
the past. Both duodenal aspiration, especially in children and the string test have been
utilized with positive results. Th e latter employs a procedure in which a gelatin capsule
with a string attached is swallowed and then retrieved aft er several hours. Th e string is
then stripped of mucus and examined microscopically for the presence of larvae. In many
developed areas, these tests have been replaced by esophagogastroduodenoscopy.
Serologic tests have been developed that can aid in the diagnosis of strongy-
loidiasis. An enzyme-linked immunoassy is available from the Centers for Disease
Control (Atlanta, GA) which has a sensitivity of 95%. Specifi city is less as a result
of cross-reactivity with other helminth infections. An immediate hypersensitivity
type of skin test has been developed which has a sensitivity of 82-100%, but has
signifi cant cross-reactivity with fi larial infections.
Treatment
Th e goal of treatment of strongyloidiasis is eradication of the organism. Unlike
hookworm, where simply reducing worm burden is effi cacious, the process of autoin-
fection will result in prolonged infection, potentially increasing worm burden over time
and the risk of hyperinfection in anyone with residual organisms. Th e drug of choice
for treatment of strongyloidiasis is ivermectin given orally at a dose of 200
μ
g/kg once
daily for 2 days. Th iabendazole is equally eff ective at a dose of 25 mg/kg twice daily
for 3 days, but has a much greater incidence of side eff ects including nausea, vomiting,
foul taste and foul smelling urine. Albendazole has also been used, but is less eff ective
than either thiabendazole or ivermectin, and requires 7 days of treatment.

38
Medical Parasitology
5
Hyperinfection syndrome may be treated with either thiabendazole or ivermec-
tin. Th erapy should be continued for at least 2 weeks, an autoinfection cycle, aft er
larvae can no longer be detected. Either drug can be administered by nasogastric
tube or per rectum in patients unable to tolerate oral medications. If possible, im-
munosuppressive therapy should be discontinued. In those patients in whom the
immunosuppressive condition cannot be altered, it may be prudent to repeat a
brief course of therapy every few weeks. Preemptive therapy should be considered
in individuals from endemic areas who are going to undergo immunosuppressive
therapy, especially if eosinophilia is present.
Suggested Reading
1. Ashford RW, Vince JD, Gratten MJ et al. Strongyloides infection associated with acute
infantile disease in Papua, New Guinea. Trans R Soc Trop Med Hyg 1978; 72:554.
2. Barnes PJ. Corticosteroids, IgE and atopy. J Clin Invest; 2001; 107:265-6.
3. Berkmen YM, Rabinowitz J. Gastrointestinal manifestations of the Strongyloidiasis.
Amer J Roentg, Rad Th er, Nuc Med 1972; 115:306-11.
4. Brown RC, Girardeau MHF. Trans-mammary passage of Strongyloides spp. Larvae
in the human host. Amer J Trop Med Hyg 1977; 26:215-9.
5. Concha R, Harrington, W Jr et al. Intestinal Strongyloidiasis: recognition, man-
agement and determinants of outcome. J Clin Gastro 2005; 39:203-11.
6. Despommier DD, Gwadz RW, Hotez PJ et al. Strongyloides stercoralis. In:
Parasitic Diseases. New York: Apple Tree Productions, 2005:129-34.
7. Gatti S, Lopes R, Cevini C. Intestinal parasitic infections in an institution for the
mentally retarded. Ann Trop Med Parasitol 2000; 94:453-60.
8. Genta RM. Dysregulation of strongyloidiasis: a new hypothesis. Clin Microbiol
Rev 1992; 5:345-55.
9. Genta RM. Global Prevalence of Strongyloidiasis: Critical review with epidemiologic
insights into the prevention of disseminated disease. Rev Inf Dis 1989; 11:755-67.
10. Georgi JR, Sprinkle CL. A case of human strongyloidiasis apparently contracted
from asymptomatic colony dogs. Amer J Trop Med Hyg 1974; 23:899-901.
11. Gill GV, Welch E, Bailey JW et al. Chronic strongyloides stercoralis infection if
former british far east prisoners of war. Q uar J Med 2004; 97:789-95.
12. Goka AK, Rolston DD, Mathan VI et al. Diagnosis of Strongyloides and hook-
worm infections: comparison of faecal and duodenal fl uid microscopy. Trans R
Soc Trop Med Hyg 1990; 84:829-31.
13. Grove DI. Human Strongyloidiasis. Adv Parasitol 1996; 38:251-309.
14. Lindo JF, Conway DJ, Atkins NS et al. Prospective evaluation of enzyme-linked
immunosorbent assay and immunoblot methods for the diagnosis of endemic
Strongyloides stercoralis infection. Amer J Trop Med Hyg 1994; 51:175-9.
15. Neva FA, Gamm AA, Maxwell C et al. Skin test antigens for immediate hypersen-
sitivity prepared from infective larvae of Strongyloides stercoralis. Amer J Trop
Med Hyg 2001; 65:567-72.
16. Overstreet K, Chem J, Wang J et al. Endoscopic and histopathologic fi ndings
of Strongyloides stercoralis in a patient with AIDS. Gastrointest Endos 2003;
58:928-31.
17. Salazar SA, Berk SH, Howe D et al. Ivermectin vs. Th iabendazole in the treatment
of strongyloidiasis. Infect Med 1994; 50-9.
18. Sato Y, Kobayashi J, Toma H et al. Effi cacy of stool examination for detection of
Stronyloides infection. Amer J Trop Med Hyg 1995; 53:248-50.
19. Siddiqui AA, Berk SL. Diagnosis of Strongyloides stercoralis infection. Clin Inf
Dis 2001; 33:1040-7.
20. Zaha O, Hirata T, Kinjo F et al. Strongyloidiasis—progress in diagnosis and treat-
ment. Intern Med 2000; 39:695-700

C
HAPTER
6
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Trichinellosis
Matthew W. Carroll
Background
Epidemiology
Trichinella spp. are tissue nematodes of nearly worldwide distribution. Only the
Australian mainland and Puerto Rico remain trichinella free. Members of the genus
Trichinella are able to infect a wide range domestic and wild (sylvatic) animals and
cause the clinical syndrome trichinellosis, oft en referred to as trichinosis, in humans.
Trichinellosis is rare in the United States; fewer than 100 cases are reported annu-
ally. Th e disease has become more common in Eastern Europe and Asia. Th is is due
to a breakdown in governmental inspection of pork, lack of education regarding
the cooking of pork and ongoing feeding of raw garbage to swine. Pigs will also
eat rats and mice that may be infected, participate in pig cannibalism and eat fe-
cal matter which can lead to ingestion of live adult worms. On a worldwide basis,
pigs represent the most frequently identifi ed vehicle of trichinellosis, although a
variety of other animals have been recognized as transmitting this illness. In the
United States, nearly one-half of the cases are due to consumption of wild game.
Bear meat, cougar and wild boar have been implicated as vehicles of trichinellosis.
In Russia, more than 90% of cases were traced to ingestion of bear or wild boar
meat. Horse meat is a common vehicle in France and Italy.
Causative Agents
Trichinellosis is most commonly caused by the consumption of raw or un-
dercooked pork products infected with Trichinella spiralis. However, there are a
number of other Trichinella spp. that have been associated with human infection.
In North America both T. murelli and T. nativa have been recognized as causes
of sylvatic (wild) trichinellosis. Trichinella murelli is most commonly acquired
following ingestion of wild bear meat. Trichinella nativa tends to have an arctic
or subarctic distribution and is found in polar bears, walrus, seals, wolves and sled
dogs. Other species of Trichinella have been reported to cause human illness. In
Europe, T. brivoti has been recognized as a cause of sylvatic disease in wild boar
and red fox. Th ere are two Trichinella species that do not encyst in muscle. T.
pseudospiralis has been described in birds of prey as well as pigs and wild game. T.
paupae, which is endemic to New Guinea, infects domestic and wild swine. Human
disease is common among native hunters in New Guinea who oft en consume
undercooked game meat.
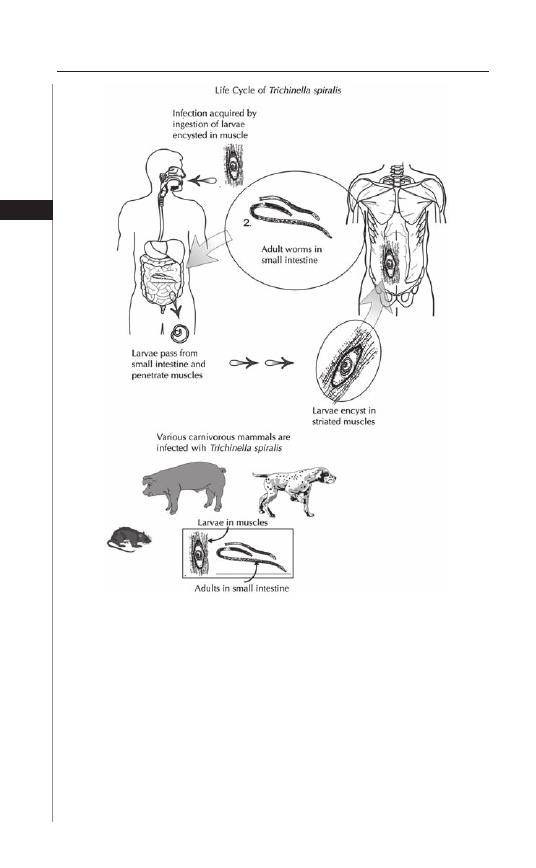
40
Medical Parasitology
6
Life Cycle
Infection with Trichinella spp. occurs when raw or undercooked meat con-
taining the nurse-cell larva complex in ingested (Fig. 6.1). Larvae are released by
acid-pepsin digestion in the stomach then enter the small intestine where they
penetrate the columnar epithelium at the base of a villous. Th ey are obligate
intracellular organisms and localize in the intestinal epithelial cells. Within the
intestine the larvae molt 4 times during a 30-hour period and develop into adults.
Th e adult female measures 3 × 0.036 mm, the male is smaller, 1.5 × 0.030 mm.
Aft er copulation, which occurs within the intestinal epithelium, the female worms
expel newborn larvae. Th is occurs 4-7 days aft er infection. Females survive 4-16
weeks and, during that time, may give birth to 1,500 larvae.
Figure 6.1. Life Cycle of Trichinella spiralis. Reproduced from: Nappi AJ, Vass E,
eds. Parasites of Medical Importance. Austin: Landes Bioscience, 2002:75.

41
Trichinellosis
6
Newborn larvae measure 0.08 mm long by 7
μ
m in diameter and possess a
unique sword-like stylet. Th is enables the larvae to cut an entry hole in the lamina
propria and enter the mesenteric lymphatics or capillaries. Th e newborn larvae
subsequently make their way to the arterial circulation and disseminate throughout
the host. Th e larvae are capable of entering any cell type but will only survive in
striated muscle. Once within the skeletal muscle the Trichinella larva induce the
myocyte to transform into a new cell type, a nurse cell, which sustains the life of the
larva (Fig. 6.2). During this metamorphosis the muscle cell switches to anaerobic
respiration and, for most species of Trichinella, develops into a cyst of collagen
and hyaline. Th ese larva-containing cysts can persist for many years although most
calcify and die within a few months.
Pathogenesis and Host Response
Th e development of adult worms in the intestinal epithelium leads to an
immune-mediated infl ammatory response in an eff ort to expel the organism. IgE is
increased and, pathologically, there is an infl ammatory infi ltrate, rich in eosinophils
and mast cells. Eosinophils elaborate major basic protein and toxic oxygen species
that help clear the parasite but also cause tissue damage. Mast cells produce a mol-
ecule, mast cell protease-1 (MCP-1), that is also toxic to the organism. Knock-out
mice that lack the gene encoding MCP-1 have delayed expulsion of the worms from
the GI tract as well as increased numbers of encysted larvae. Interleukin-5, which
inhibits eosinophil apoptosis, is an integral part of this process. Genetically altered
“knockout mice” that lack this gene also have delayed clearance of worms.
As larvae enter cells in various tissues during the parenteral phase of infection,
there is widespread infl ammation. Th e resulting pathology is proportionate to the
extent of infection. Cell death is frequent and with heavy infections there is edema,
proteinuria and organ toxicity including the development of central nervous system
disorders and cardiomyopathies.
Figure 6.2. Trichinella cysts in muscle.

42
Medical Parasitology
6
Trichinella species tend to persist within head and neck muscles including
the tongue, laryngeal muscles, masseter muscles, as well as respiratory muscles,
especially the intercostals and diaphragm.
Clinical Features
Ingestion of larvae is characterized by abdominal pain, secretory diarrhea, nausea
and vomiting during the fi rst week of infection. Occasionally, constipation may be
present. Parasite inoculum is a major factor in disease severity and, in many cases,
infected individuals may be asymptomatic. On the other hand, in patients with very
heavy worm loads, severe enteritis can occur leading to severe dehydration.
Th e parenteral phase, which occurs approximately one week aft er infection, is
accompanied by systemic symptoms; fever, oft en 40-41˚C, myalgias and muscle
tenderness. Petechial hemorrhages may appear in the mucous membranes, con-
junctivae and subungal skin and, rarely, an urticarial rash occurs. Periorbital edema
is a classic fi nding that has been reported to occur in 77% of those infected. Th is
can be so severe that the infected individual is unrecognizable. Th is is thought to
be a result of a generalized allergic reaction and typically lasts 1 to 2 weeks. Its
resolution is heralded by spontaneous diuresis.
Muscle pain and swelling are the most prominent features of trichinellosis.
Muscles become stiff , hard, tender and edematous and oft en appear hypertro-
phic. Change in voice is commonly seen with involvement of laryngeal muscles.
Pharyngeal, lingual and masseter involvement may make eating and swallowing
diffi cult. Diplopia may occur when the extraocular muscles are aff ected. Th ese
symptoms usually peak 2-3 weeks aft er infection and then resolve. Laboratory
abnormalities include a modest leukocytosis with eosinophila which can be as high
as 50%, as well as a mild elevation of creatine kinase and other muscle enzymes.
Other Trichinella species may exhibit diff erent symptoms. Trichinella nativa
tends to produce a prolonged diarrheal illness with a paucity of systemic and my-
algic complaints, whereas Th ai patients with T. pseudospiralis infection may have
severe myalgias for up to 4 months.
Involvement of tissues other than muscle can lead to a wide variety of com-
plications. Central nervous system involvement has been reported in 10-25% of
patients. Most commonly, patients with neurotrichinosis present with meningo-
encephalitic signs. Paralysis, delirium, psychosis and peripheral neuropathies have
been described.
Cardiac involvement may also occur in patients with trichinellosis.
Pericardial effusions were found in nearly 10% of patients in one study.
Myocarditis is uncommon, but in patients with severe infection it can be lethal.
Electrocardiographic abnormalities may also be found including arrhythmias
and heart blocks.
Lethal infection due to Trichinella is uncommon, but deaths have been re-
ported, mostly in patients with severe worm burdens. Besides myocarditis, central
nervous system involvement and pneumonia may lead to a fatal outcome. Sepsis
and death can occur in patients who develop bacteremia with enteric organisms
following penetration of larvae from the GI tract into the bloodstream. A sudden
reduction in eosinophils in a patient with severe trichinellosis may herald death
or indicate superimposed bacterial infection.

43
Trichinellosis
6
Diagnosis
In the United States trichinellosis is quite rare. Since its symptoms mimic com-
mon diseases such as viral gastroenteritis or food poisoning, many clinicians fail
to consider it when formulating a diff erential diagnosis. Sporadic cases oft en go
undetected and thus the true prevalence of this disease is oft en underestimated.
Trichinellosis should be considered in the presence of fever, myalgias, periorbital
edema and eosinophilia; and an epidemiologic history of consumption of raw or
undercooked meat. Muscle biopsy is the “gold standard” for the diagnosis of trichinel-
losis. Typically a 1-4 cubic millimeter piece of muscle is pressed between two slides
and viewed under the microscope for the presence of larvae. Th is is positive in heavy
infections. Organisms such as T. psuedospiralis and T. papaue that do not encyst, as
well as other Trichinella species if examined prior to encysting, may be missed since
they resemble muscle tissue. Alternatively, the muscle can be digested in 1% HCl-1%
pepsin at 37º C for 1 hour aft er which the larvae are released from nurse cells and
are much easier to identify. Th is process oft en destroys young larvae making it more
diffi cult to diagnose early infection. Routine histological examination may reveal a
basophilic reaction in muscle tissue as well as demonstrating the presence of larvae.
Specifi c immunodiagnostic tests are also available. Several tests designed to look
for the presence of larvae group 1 antigens are available. Th ese include indirect he-
maggutination, bentonite fl occulation, indirect immunofl ouresence and, the most
sensitive test, an enzyme linked immunoabsorbant assay (ELISA). Th ese tests are quite
specifi c for trichinellosis, but may be insensitive during the fi rst few weeks of infection.
Seroconversion usually occurs between the third and fourth week of infection; the
ELISA may be positive aft er 12 days. By day 50 virtually 100% of infected individuals
will have IgG antibody directed against the infecting organism. Tests for the detection
of larval antigens are also available and indicate active infection. Cross-reactions oc-
cur with both antigen and antibody assays, typically in individuals with autoimmune
disease or patients infected with parasites that exhibit cross reactivity.
Newer molecular biologic techniques can be used for both diagnosis and to
distinguish between species of Trichinella. Polymerase chain reaction (PCR)
assays are able to determine the species of Trichinella with as few as one larva.
Diff erences in 5S rRNA can be used to distinguish between Trichinella spp. that
are morphologically identical.
Treatment
Th ere is no specifi c anthelminthic therapy that is eff ective for the treatment
of trichinellosis. Benzimidazoles, mebendazole and albendazole, are active only
against the adult worm. When used shortly aft er ingestion, they will reduce
worm burden. Albendazole is given at a dose of 400 mg twice daily for 8-14 days.
Mebendazole 200-400 mg three times a day for 3 days then 400-500 mg three times
a day for ten days is also eff ective. Th e initial dose of mebendazole and albendazole is
lower to prevent a Mazzoti reaction, an anaphylactic type reaction due to antigenic
simulation following the death of the adult worms aft er treatment.
Treatment of the parenteral stage of the disease is directed towards symptomatic
relief. In general, mild disease does not require treatment. Treatment of moderate to
severe disease can decrease morbidity and, possibly, mortality. In such patients, corti-
costeroids have been given to reduce tissue damage. Th ese drugs may be lifesaving in

44
Medical Parasitology
6
patients with myocarditis or neurotrichinellosis. Prednisolone at doses of 40 to 60 mg
per day are typically used. However, this will slow the clearance of adult worms from
the gut and should not be given for a prolonged period. Analgesics and antipyretics
such as NSAIDs and acetaminophen provide symptomatic relief.
Prevention
Eff ective prevention of trichinellosis requires proper animal husbandry practices as
well as adequate cooking of meat. Feeding swine only cooked scraps and controlling the
population of infected rodents will help to prevent disease. Freezing pork usually kills T.
spiralis eff ectively. However, wild game infected with T. nativa are resistant to freezing.
Cooking pork to when it is no longer pink (170˚F; 77˚C) exceeds the thermal death
point of Trichinella spp. Game meat is much darker and it is necessary to use a meat ther-
mometer to determine if it has been adequately cooked. Microwave cooking oft en leaves
“cold spots”, so this cooking method should not be used with possibly infected meat.
Suggested Reading
1. Centers for Disease Control. Horsemeat associated Trichinosis—France;
MMWR1986; 35:291-2,297-8.
2. Bruschi F, Murell KD. New aspects of human Trichinellosis: Th e impact of new
Trichinella Species. Postgrad Med J 2002; 78:15-22.
3. Capo V, Despommier DD. Clinical aspects of infection with Trichinella spp. Clin
Microbiol Rev 1996; 9(1):47-54.
4. Despommier DD, Gwadz RW, Hotez PJ et al, eds. Parasitic Diseases, 5th Edition.
New York: Apple Tree Production LLC, 2002:135-42.
5. Escalante M, Romarís F, Rodríguez M. Evaluation of Trichinella spiralis group 1 antigens
for the serodiagnosis of human trichinellosis. J Clin Microbio 2004; 42:4060-6.
6. Knight PA, Wright SH, Lawrence CE. Delayed expulsion of the nematode T.
spiralis in mice lacking the mucosal mast cell-specifi c granule chymase, mouse
mast cell protease-1. J Exp Med 2000; 192:1849-56.
7. Pérez-Martín JE, Serrano FJ, Reina D et al. Sylvatic trichinellosis in Southwestern
Spain. J Wildl Dis 2000; 36:531-4.
8. Pozio E, La Rosa G. Trichinella murelli n.sp: eitiologic agent of sylvatic trichinel-
losis in temprate areas of North America. J Parisitology 2000; 86:134-9.
9. Jongwutiwes S, Chantachum N, Kraivichian P et al. First outbreak of human
trichinellosis caused by Trichinella pseudospiralis. Clin Inf Dis 1998; 26:111-5.
10. Ranque S, Faugère B, Pozio E et al. Trichinella pseudospiralis outbreak in France.
Emerg Infect Dis 2000; 6:1-7.
11. Rehmet S, Sinn G, Robstad O et al. Trichinellosis Outbreaks—Northrhine-Westfalia,
Germany, 1988-1999. MMWR 1999; 48:488-92.
12. Rombout YB, Bosch S, Van Der Giessen JW. Detection and identifi cation of eight
trichinella genotypes by reverse line blot hybidization. J Clin Microbiol 2001;
39:642-6.
13. Rotolo R, Garcia R, Habib A et al. Epidemiologic notes and reports common source
outbreaks of thicinosis—New York City, Rhode Island. MMWR1982; 31:161-4.
14. Roy S, Lopez A, Schantz P. Trichinellosis survey—United States, 1997-2001;
MMWR 52:1-8.
15. Centers for Disease Control and Prevention. Trichinellosis associated with bear
meat in New York and Tennessee—2003; MMWR 2004; 53:606-9.
16. Centers for Disease Control and Prevention. Outbreak of Trichinellosis associated
with eating cougar jerky—Idaho. MMWR 1996; 45:205-6.
17. Watt G, Saisorn S, Jongsakul K et al. Blinded, placebo controlled trial of antipara-
sitic drugs for Trichinosis myositis. J Infect Dis 2000; 182:371-4.
18. Zarnke RL, Worley DE, Ver Hoef JM et al. Trichinella sp. in wolves from the
interior of Alaska. J Wildl Dis 1999; 35:94-7.

C
HAPTER
7
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Onchocercosis
Christopher M. Cirino
Background
Onchocercosis (river blindness) is caused by the fi larial nematode Onchocerca vol-
vulus and transmitted by several species of blackfl ies in the genus Simulium. Th e dis-
ease represents a leading infectious cause of blindness and visual impairment, second
to Chlamydia trachomatis. An estimated 17.7 million people are infected worldwide,
with 270,000 blind and 500,000 with severe visual impairment as a result.
Onchocercosis is distributed primarily in Africa between the tropics of Cancer
and Capricorn, while smaller foci exist in Central and Latin America (Guatemala,
Mexico, Brazil, Ecuador, Venezuela and Columbia) as well as in the Arabian pen-
insula (Yemen and Saudi Arabia). Nigeria alone accounts for more than one-third
of the global prevalence of disease. In these areas, the disease usually is found along
rapid-fl owing rivers and streams, where blackfl ies lay their eggs. Consequently,
prior to eradication eff orts, whole areas of arable land had been uninhabitable
because of the disease.
Life Cycle
Th e life cycle of O. volvulus, consists of developmental stages in the blackfl y
vector and the defi nitive human host (Fig. 7.1). When an infected female black
fl y takes a bloodmeal, infective larvae (larval stage 3 (L3)) penetrate a break in
the skin and migrate into connective tissues. Aft er several stages of molting, the
larvae develop into adult male (4 cm × 0.2 mm) and female worms (50 cm × 0.4
mm) (macrofi lariae, Fig. 7.2) and localize in fi brous subcutaneous nodules, known
as onchocercomas. Within these nodules female worms release 1,300-1,900
microfi lariae daily for as long as 14 years. Microfi lariae (250 to 300
μ
m in size)
migrate into the subcutaneous tissues via the lymphatic vessels. Th ese microfi lariae
may survive for 6 to 30 months, but the majority do not complete the life cycle.
When a blackfl y bites an infected human, microfi lariae (L1) are ingested with the
bloodmeal. Th ey develop into infective larvae (L3) in the fl y and migrate to the
insect’s mouth within a few weeks.
Microfi lariae released from onchocercomas migrate through the subcutane-
ous tissues, eliciting only a minimal infl ammatory response. A greater reaction
occurs with microfi larial death and degeneration in the tissue, which is exacer-
bated by onchocercosis chemotherapy. Both B- and T-cell mediated responses
occur in response to fi larial products with antibody production, involvement of
macrophages and recruitment of neutrophils and eosinophils. Additionally, mac-
rophages, dendritic cells and T-cells have been detected in onchocercomata.
46
Medical Parasitology
7
Th e mechanism by which O. volvulus and other fi larial nematodes are able
to evade the host immune system is under intensive investigation and has been
reviewed elsewhere. Th ree immune manifestations of onchocerciasis have been
described: the generalized form, the hyperreactive form and the putatively immune
individuals (PI). Implicated are fi larial-derived molecules, T-helper subset popula-
tions, cytokine and immunoglobulin regulation, host genetic diff erences between
various forms and a host response to the onchocercal endosymbiont Wolbachia.
An association with high skin and blood worm loads in fi larial infections was
made with a decreased response of T-helper cell Type 2 (Th 2) and increases
in the immunosuppressive cytokines interleukin-10 and transforming growth
factor-
β
. Unlike generalized onchocerciasis, the hyperreactive form (sowda) is
associated with a heightened T-helper 2 (Th 2) which is eosinophil predominant
Figure 7.1. Life cycle of Onchocerca volvulus. Adapted from: Nappi AJ,
Vass E, eds. Parasites of Medical Importance. Austin: Landes Bioscience,
2002:93.
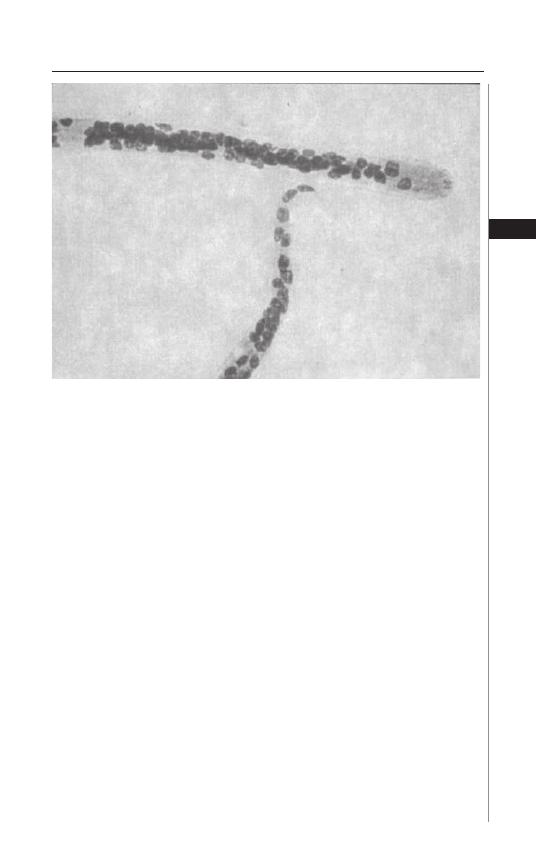
47
Onchocercosis
7
in the setting of minimal microfi laridermia. Levels of IgG1, IgG3 as well as IgE
are elevated in this disease manifestation. Sowda has been found to have a genetic
basis with particular major histocompatibility complex alleles and a mutation in
the interleukin-13 gene associated with other hyperreactive states (asthma, atopy).
Th ose putatively immune have been found to have higher interferon gamma and
interleukin-5 levels and a mixed Th 1 and Th 2 subset response.
Endobacteria of the genus Wolbachia have been identifi ed as essential for worm
fertility in many fi larial nematodes, including Onchocerca volvulus. Th e release
of Wolbachia molecules aft er microfi lariae death may contribute directly to the
pathogenesis of skin lesions and visual impairment (e.g., keratitis), the severity of
posttreatment reactions and the persistence of infection in the host. In a murine
model, the infl ammatory response following intracorneal injection of fi larial ex-
tracts was contingent on the presence of Wolbachia, with only a minimal response
seen from extracts of worms pretreated with doxycycline (Wolbachia negative).
Th ere is evidence of an innate immune response in the cornea with neutrophil
activation mediated by host cell toll-like receptors 2 and 4 (TLR2, TLR4) to an
endotoxin-like surface protein and Wolbachia surface protein (WSP) of this bac-
teria. Th is may contribute to a shift in T-helper subset balance, which allows the
parasite to persist in the host through decreased immune activation.
Disease Signs and Symptoms
Largely, the immune response to microfi lariae death and degeneration leads to
the clinical fi ndings of the disease, including pruritis, acute and chronic dermatitis
and visual impairment and blindness. Th e earliest and most common symptom
of onchocerciasis is pruritis, which may be severe. Acute or chronic lymphedema,
Figure 7.2. Onchocerca volvulus. Obtained with kind permission from Dr.
Abhay Satoskar.

48
Medical Parasitology
7
inguinal lymph node swelling and loss of elasticity resulting in an adenolymphocele,
or “hanging groin”, have been described in some individuals as a result of microfi lariae
migration. A classifi cation scheme was developed for the myriad skin manifestations
of onchocercosis:
Acute Papular Onchodermatitis (APOD)
APOD presents as a scattered, pruritic, papular eruption, which may be
transient and associated with erythema or swelling. Areas aff ected are predomi-
nantly the trunk and limbs. APOD is generally seen in younger populations in
hyperendemic areas but may be seen in returning travelers who have acquired
the infection.
Chronic Papular Onchodermatitis (CPOD)
CPOD consists of larger, scattered papules over the waist, buttocks and
shoulders, which may be hyperpigmented. Pruritis is oft en severe and the resulting
excoriation can predispose to secondary infections. CPOD is the most common
manifestation of onchocerciasis in hyperendemic areas.
Lichenifi ed Onchodermatitis (LOD)
Lichenifi ed onchodermatitis represents a more localized hyper-reactive form of
CPOD. Th e lesions are hyperpigmented plaques, which are usually asymmetrical,
involving one limb with generally less numbers of microfi lariae and onchocercomas.
Regional lymphadenopathy is seen in these chronic lesions. LOD is referred to as “sow-
dah”, Arabic for “black”, in southern Saudi Arabia and Yemen, where it is endemic.
Atrophy (ATR)
Th e skin aft er prolonged infection becomes wrinkled, thin and atrophic, with
loss of elasticity and hair. It most commonly occurs on the buttocks and limbs.
Depigmentation (DPM)
Depigmentation is a result of longstanding infl ammation and scarring from
onchocercosis infection. It is most commonly seen pretibially and has a patchy
distribution, which has been referred to as “leopard skin”.
Onchocercomas
Onchocercomas are nodules composed of coiled adult female and male worms
that average 3 cm in diameter. Th ey are usually found on bony prominences such
as the pelvis, chest wall, head or limbs. Th e variation in location of these nodules
between Central and South American and Africa appears to be related to the
biting habits of the vector. In the Americas, Simulium ochraceum have a tendency
to bite on the upper part of the body, whereas in Africa, Simulium damnosum
typically bite below the waste line. Isolated onchocercomas caused by zoonotic
species of Onchocercoca (usually Onchocerca gutturosa, a cattle nematode) have
also been reported.
Visual Impairment and Blindness
Th e risk of impaired vision and blindness is associated with the proximity of
the onchocercoma to the eyes, such as with head nodules, as well as disease burden.

49
Onchocercosis
7
Moreover, blindness appears to be geographically associated with the onchocer-
cal strains in the West African savannahs as opposed to rainforest areas where
more severe skin disease is present. Microfi larial migration from the conjunctiva
into the cornea likely plays a role in the pathogenesis of ocular disease. Disease
involvement in the eye may be present in the anterior and/or posterior segment.
Anterior disease causes punctate keratitis, identifi ed as small (0.5 mm), discrete
areas of the superfi cial corneal stroma (snowfl akes opacities), which are generally
reversible. Other fi ndings such as limbitis, chemosis, conjunctival injection and
epiphora may accompany punctate keratitis. Iris and ciliary body involvement may
lead to anterior uveitis, iridocyclitis and ultimately sclerosing keratitis, leading to
severely impaired vision or complete blindness. With extensive infl ammation and
synechiae formation, seclusion- and occlusion- pupillae, secondary cataracts and
glaucoma may also ensue. Posterior disease is caused typically by microfi lariae that
have entered the retina along posterior ciliary vessels, leading to chorioretinitis.
Posterior disease can result in optic neuritis and lead to optic atrophy as a result
of recurrent episodes of acute infl ammation.
Other Disease Manifestations
Systemic signs and symptoms have been described in onchocercosis infections.
Low body weight and diff use musculoskeletal pain may occur. Growth arrest in
severe disease, known as the Nakalonga syndrome, has been described in western
Uganda. Onchocercosis has been linked to infertility, amenorrhea and spontane-
ous abortion.
Diagnosis
Presumptive diagnosis of onchocercosis usually can be made in a patient present-
ing with a travel history to an endemic area and the clinical syndrome of pruritis,
dermatitis, ocular fi ndings and skin nodules. Th e standard method of diagnosis of
onchocerciasis involves skin snippings to identify microfi laria or the identifi cation
of macrofi lariae in extirpated onchocercoma. Th e probability that skin snips will
detect microfi laria relates to the parasite burden and the amount of samples taken.
Sensitivities range from 80-100%, but lower sensitivities have been noted in areas
with long-term ivermectin programs. Skin nodules are more likely to be seen in the
setting of greater parasite burden, and the macrofi lariae may be directly visualized
following removal and examination of a nodule.
Indirect diagnosis may be made through the use of provocation methods,
such as the Mazzotti reaction, originally described following the administration
of 6 mg of oral diethylcarbamazine (DEC), characterized by fever, pruritis and
is potentially sight threatening. More favorable is a topical application of DEC,
referred to as the DEC-patch assay, which produces a local reaction with a raised,
pustular rash in those harboring Onchocerca. Th e sensitivity of this method ranges
from 30% to 92%, with specifi cities of 80 to 95%. Th e strength of the reaction was
independent of the level of microfi laridermia, suggesting that this is a qualitative
method. Such methods may play a greater role in screening endemic areas for
recrudescence of infection.
Th e use of serologic studies and more recently polymerase chain reaction
(PCR) methods have received particular attention because these methods are more

50
Medical Parasitology
7
sensitive and potentially less invasive than skin snips. Using ELISA and various
onchocercal antigen “cocktails” have achieved a sensitivity ranging from 70-96%
with a specifi city of 98-100%. However, these serologic methods are unable to
discern active from past infection, as well as cross-reactivity with other parasites.
PCR methods detect the parasite DNA sequence O-150, which is only found
in O. volvulus and have shown a nearly 100% sensitivity and specifi city when used
on skin snips. Skin abrasion methods may reduce invasiveness and aff ord similar
sensitivity. Newer PCR methods, such as an antigen detection dipstick, are even less
invasive and are useful in the setting of lower parasitemia and disease prevalence. A
urine dipstick assay demonstrated a sensitivity and specifi city near 100%.
Treatment: Current Th erapy and Alternatives
Identifi cation and removal of skin nodules can be curative in patients with a
minimal exposure history, but its role is otherwise unclear. Nodulectomy in pa-
tients with head onchocercomata and evidence of ocular disease may reduce the
likelihood of blindness and is therefore recommended.
Ivermectin
Ivermectin (Mectizan), released in 1987, is the most widely used treatment
for onchocercosis. It primarily functions as a microfi laricide. A single dose of
ivermectin clears the skin of microfi lariae and suppresses further microfi laridermia
for several months. However, since the macrofi lariae are not very susceptible to
ivermectin, the microfi larial burden is eventually replaced and aft er several months
to a year, repeat treatment is necessary. Th is has formed the crux of onchocercosis
mass treatment programs with ivermectin. Ivermectin leads to improvement in
skin lesions and pruritis and retards the progression of anterior and posterior eye
disease. Because high dose ivermectin has been associated with visual symptoms,
it is usually safely dosed at 150 micrograms/kg, and repeated every 6-12 months
until asymptomatic. A mild Mazzotti reaction may occur in 10-20% of patients
receiving the fi rst doses of ivermectin, but can be more severe in patients with
higher microfi larial counts. Most reactions require only supportive care.
Diethylcarbamazine (DEC)
Diethylacarbamazine, a piperazine derivative, has microfilaricide activity
against O. volvulus, but its use has gone into disfavor, because of the potentially
sight-threatening infl ammatory reaction that is induced from rapidly killing
microfi lariae. Ocular complications reported in the treatment of onchocercosis
infection with DEC are constriction of visual fi elds, damage to the optic nerves,
chorioretinitis, anterior uveitis and punctate keratitis. Additionally, DEC was
shown to induce encephalopathy in patients co-infected with Loa loa who had
very high microfi larial burdens.
Doxycycline and Wolbachia Endobacteria
Wolbachia species are Rickettsial bacteria that have been identifi ed as “obligatory
symbionts” presumably for fertility in many fi larial species, in that they are required
for all stages of embryogenesis. Targeting Wolbachia with doxycycline therapy dosed
at 100 mg daily for 6 weeks demonstrated potent activity against embryogenesis of
Onchocerca. In one study of bovine onchocercosis (O. ochengi), it even demonstrated

51
Onchocercosis
7
partial macrofi laricide activity. Although doxycycline is contraindicated in some
patient populations (children and pregnant or breastfeeding women), it may have a
future role as prophylaxis, such as for those leaving an endemic area aft er a long stay,
or aid in eradication eff orts through more prolonged clearance of microfi lariae.
Th ere is yet to be developed an eff ective pharmacologic treatment for mac-
rofi lariae, which may survive for more than a decade, mandating long-term
maintenance of ivermectin treatment programs. Suramin has activity against the
macrofi laria of onchocerciasis, but has limited applicability due to its toxicity
and intravenous administration route. Ivermectin may actually have some mac-
rofi laricidal properties. Recent studies have shown it may exhibit macrofi larial
activity, particularly if given at 3-monthly dosing regimens. Doxycycline’s activity
against Wolbachia endosymbionts in Onchocerca may play a potential role as a
macrofi laride, as demonstrated in a bovine models.
Although an eff ective vaccine has yet to be discovered, animal models have
suggested that eff ective immunity can be mounted against stage 3 larvae (L3) and
microfi lariae (Mf ).
Prevention and Prophylaxis
Strategies toward eradication of onchocerciasis focus on the interruption of the
onchocercal life cycle, whether through large scale vector control or microfi laricide
treatment. Th e Onchocerciasis Control Programme (OCP) began in 1974 and
was comprised of vector control in seven (eventually 11) West African countries
with hyperendemic onchocercosis. Th ese measures were intensifi ed to include
weekly, aerial larvicide spraying in a wide area of 700,000 km
2
. Infection control
models which focused on the identifi cation of the Community Microfi larial Load
(CMFL) through skin snip sampling indicated that the OCP was successful, allay-
ing concerns that migration of black fl ies from outside areas would hamper eff orts.
A micro simulation model formed in collaboration with the Erasmus University of
Rotterdam, ONCHOSIM, predicted that a 14 year span of a satisfactory vector
control program would control onchocerciasis infection without recrudescence.
Vector control was discontinued aft er 14 years in 1989 and epidemiologic studies
almost a decade later demonstrated that the onchocercosis threat was largely eradi-
cated except for small residual foci. Th e OCP concluded in 2002, aft er more than
25 years of activity. As testimony to its success, ocular disease from onchocercosis,
widespread prior to the OCP, has not been observed.
With the donation of ivermectin by Merck in 1987, other programs were estab-
lished in endemic areas and focused on mass distribution of ivermectin (Ivermectin
Distribution Program (IDP)) with assistance from nongovermental development
organizations (NDGOs), in Latin America and in Africa.
However, the potential of onchocercosis eradication in some countries remains
unclear. Th e conclusion of an expert panel at the Conference on Eradication of
Onchocerciasis which convened in Atlanta Georgia in 2002 was that the disease
was not eradicable in Africa with the current methods due to several major barriers:
the unlikelihood that ivermectin as a single intervention will interrupt transmission;
programmatic challenges in an extensive endemic area with mobile vectors and in-
fected humans; poor health infrastructure and political instability in these countries;
Loa loa co-infections in many onchocerciasis endemic areas; and inadequate funds

52
Medical Parasitology
7
and political support to expand the coverage to hypoendemic areas. Recrudescence
has occurred when programs were disrupted from civil unrest. For the Americas
and possibly Yemen, onchocerciasis transmission may be interrupted with current
programs due to vector characteristics and/or geographic isolation of foci.
Suggested Reading
1. Bianco AE. Onchocerciasis-river blindness. In: Macpherson CNL, Craig PS,
eds. Parasitic Helminths and Zoonoses in Africa. London: Unwin Hyman Press,
1991:128-203.
2. Brattig NW, Bazzocchi C, Kirschning CJ et al. Th e major surface protein of
wolbachia endosymbionts in fi larial nematodes elicits immune responses through
TLR2 and TLR4. J Immunol 2004; 173:437-45.
3. Burnham G. Onchocerciasis. Lancet 1998; 351:1341-46.
4. Burnham G, Mebrahtu T. Review: Th e delivery of ivermectin (Mectizan). Trop
Med Int Health 2004; 9:A26-A44 SUPPL.
5. Cooper PJ, Nutman TB. Onchocerciasis. Current Treatment Options in Infectious
Diseases 2002; 4:327-35.
6. Dadzie Y, Neira M, Hopkins DR. Final Report of the Conference on the eradica-
bility of Onchocerciasis 2002.
7. Duke BOL. Evidence for macrofi laricidal activity of ivermectin against female
Onchocerca volvulus: further analysis of a clinical trial in the Republic of
Cameroon indicating two distinct killing mechanisms. Parasitology 2005;
130:447-53.
8. Gilbert J, Nfon CK, Makepeace BL et al. Antiobiotic chemotherapy of onchocer-
ciasis: in a bovine model, killing of adult parasites requires sustained depletion of
endosymbiotic bacteria (Wolbachia species). J Infect Dis 2005; 192:1483-93.
9. Hise AG, Gillette-Ferguson I, Pearlman E. Th e role of emdosymbiotic Wolbachia
bacteria in fi larial disease. Cell Microbiol 2004; 6:97-104.
10. Hoerauf A, Brattig N. Resistance and susceptibility in human onchocerciasis—
beyond Th 1 vs Th 2. Trends Parasitol 2002; 18:25-31.
11. Hougard JM, Alley ES, Yameogo L et al. Eliminating onchocerciasis aft er 14 years
of vector control: a proved strategy. J Infect Dis 2001; 184:497-503.
12. Murdoch ME, Hay RJ, Mackenzie CD et al. A Clinical classifi cation and grading
system of cutaneous changes in onchocerciasis. Br J Dermatol 1993; 129:260-9.
13. Murdoch ME, Asuzu MC, Hagan M et al. Onchocerciasis: the clinical and
epidemiological burden of skin disease in Africa. Ann Trop Med Parasitol 2002;
9(3):283-96.
14. Paisier AP, van Oortmarssen GJ, Remme J et al. Th e risk and dynamics of oncho-
cerciasis recrudescence aft er cessation of vector control. Bull World Health Organ
1991; 69:169-78.
15. Rodger FC. Th e movement of microfi lariae of Onchocerca volvulus in the human
eye from lid to retina. Trans Roy Soc Trop Med Hyg 1959; 53:138-41.
16. World Health Organization. Onchocerciasis (river blindness). Report from the
fourteenth inter American conference on onchocerciasis, Atlanta, Georgia, United
States. [Congresses] Weekly Epidemiological Record 2005; 80:257-60.
17. World Health Organization. Onchocerciasis and its control. Report of a WHO
Expert Committee on Onchocerciasis Control 1995; 852:1-103.
18. Zimmerman PA, Guderian RH, Araujo E et al. Polymerase Chain Reaction-based
diagnosis of Onchocerca volvulus infection: improved detection of patients with
onchocerciasis. J Infect Dis 1994; 169:686-9.

C
HAPTER
8
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Loiasis
Murliya Gowda
Introduction
Loa loa, a fi larial nematode, is the causative organism of Loiasis. More commonly
known as the African eye worm, infection with this organism is characterized by
transient swelling of subcutaneous tissues, known as Calabar swellings, which occur
as the adult worms migrate.
Epidemiology
Loa loa is endemic to the central and western regions of Africa. In these
areas as many as 13 million people are aff ected. In endemic areas, up to 30%
of long-term inhabitants can be infected. Th e vector, the Chrysops fl y, breeds
in mud near shaded water sources of the rain forest. Rubber tree plantations
are especially prone to infestation with Chrysops. Th e fl ies are typically more
common during the rainy season and are attracted to movement, smoke, dark
skin and clothing.
Th ere are no nonhuman reservoirs of L. loa. Th ere is a form of Loa that is
seen in nonhuman primates, but, in contrast to human L. loa infection, the
organism tends to have a nocturnal periodicity and the fl ies that transmit this
infection bite at night.
Life Cycle
Th e mango fl y, or tabanid fl y, which belong to the genus Chrysops, transmits L.
loa. Chrysops silacea and Chrysops dimidiata are the primary vectors. Th e female
fl y, which feeds during daylight hours, acquires the organism when it ingests a
microfi lariae-containing blood meal from an infected individual. Th e microfi -
lariae, which are sheathed and contain three or more nuclei in the caudad end
(Fig. 8.1), maintain a diurnal rhythm such that they remain in the capillaries of
the lung and other organs at night, but during the day, circulate in the peripheral
blood and are thus available for ingestion by the day-feeding fl ies. Aft er the mi-
crofi lariae are ingested, they penetrate the wall of the stomach, and enter the fat
body. Over the course of the next 8-12 days, the microfi lariae increase in length,
mature to the infective larval form and then migrate to the fl y’s mouth. When
the Chrysops fl y takes its next meal, the larvae are released into the host. Over the
course of the next 1 to 4 years, the adult worms develop within the subcutaneous
tissues of the host. Th e female adult worms are typically larger, measuring 0.5 mm
in width and 50 to 70 mm in length whereas the male worms are 0.4 mm wide
and 30 to 35 mm long. Th e adult worms reproduce and deposit microfi lariae in
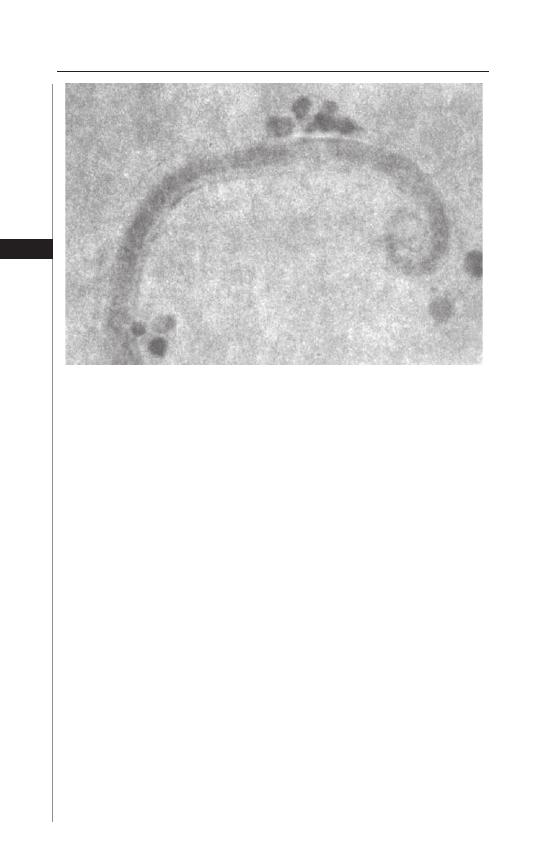
54
Medical Parasitology
8
the peripheral blood during the day thus completing the cycle. Adult worms can
survive for up to 17 years in host tissues. Th e transparent adult worms can be seen
migrating underneath the conjunctiva and through subcutaneous tissues causing
migratory swelling and angioedema known as “Calabar swellings.”
Immune Response
Immunological responses to parasite infection can be divided into three
groups. Individuals who have high levels of microfi lariae in the blood, but have
weak responses to the parasite, characterize the fi rst group. A second group in-
cludes individuals who develop the Calabar swellings and angioedema. Secretions
from the adult worms and microfi larial antigens trigger host IgE responses and
IL-5 production, resulting in host infl ammatory responses and eosinophilia. Th e
third subset of patients, typically live in hyperendemic areas and have neither
microfi lariae nor Calabar swellings. Th is group is thought to have protective
immunity. Native individuals with L. loa infection tend to be asymptomatic
whereas the hypersensitivity reactions are more common in expatriates and
long-term visitors to the endemic areas.
Clinical Manifestations
Infection with L. loa can cause a broad range of symptoms from swelling in
the face, extremities, and periorbital areas to more serious complications such as
renal failure, central nervous system involvement. People infected with L. loa may
not present with symptoms for years. Infected individuals may develop pain and
pruritis along the path of the traveling adult worms. An area of nonpitting edema
(Calabar of fugitive swelling) may soon develop along the migratory path of the
worm and can last from two days to several weeks. Th e swellings are believed to
Figure 8.1. Loa loa microfi laria in blood.

55
Loiasis
8
be a hypersensitivity reaction secondary to antigenic substances released by the
parasite. Repeated episodes of swelling or angioedema are common. Typically,
the extremities, ankles, wrists and the periorbital areas are aff ected.
Eye involvement is the most well known clinical manifestation of L. loa infec-
tion. Movement of the adult worm under the conjunctiva causes conjunctivitis,
as well as pruritis, pain and edema of the eyelid. Occasionally, the transparent
adult worm can be seen migrating under the conjunctiva.
Rare ocular complications include macular deterioration and retinal artery
occlusion. Other infrequent complications of loiasis infection include lymph-
adenitis, pulmonary infi ltrates, hydroceles and joint involvement. Occasionally,
intense swelling can cause nerve compression and subsequent neuropathies. Th e
median nerve is the most common site of neural involvement and can lead to the
development of carpal tunnel syndrome. With diethylcarbamazine therapy, the
dying organism can activate host infl ammatory responses, worsening swelling
and nerve compression, thereby intensifying neurological symptoms. Another
uncommon manifestation of loiasis is endomyocardial fibrosis, which has
been epidemiologically linked to regions where L. loa is prevalent. One study
describes a patient with loiasis and biopsy proven endomyocardial fi brosis who
was treated with diethylcarbamazine with a subsequent decrease in eosinophilia
and antifi larial antibodies.
More serious complications involving the kidneys may also occur in loiasis.
Renal disease may worsen with diethylcarbamazine treatment, but are typi-
cally not permanent. Damage to the glomeruli as a result of immune complex
deposition or direct injury from the renal fi ltration of microfi lariae can lead to
proteinuria and hematuria. Rarely, microfi lariae are evident in the urine.
Central nervous system involvement, particularly meningoencephalitis, can
occur in individuals with a high microfi larial burden; typically greater than 2,500
microfi laria/ml. Symptoms such as headaches can progress to meningoencepha-
litis or death. Seizures have also been reported. Microfi lariae can be found in the
cerebrospinal fl uid in individuals with a high organism burden. Furthermore,
treatment with diethylcarbamazine can exacerbate CNS symptoms. Neurological
symptoms have also been described in patients who were treated for onchocer-
ciasis with ivermectin, but were also co-infected with L. loa.
Diagnosis
Loiasis should be considered as a potential diagnosis in individuals from en-
demic regions of Africa who have swelling in the face, extremities, and periorbital
areas. Eosinophilia should also raise the index of suspicion for this diagnosis.
Elevated IgE levels are commonly observed in symptomatic patients and may
be detected despite a paucity of microfi lariae in peripheral blood samples. Th e
diagnosis is established by detection of the microfi lariae from a daytime sample
of peripheral blood on Giemsa or Wright’s stain. Characteristically, the microfi -
lariae are sheathed and have three or more terminal nuclei (Fig. 8.1). In patients
with low-level parasitemia, concentration methods such as microfi ltration can
be used to improve diagnostic sensitivity. Extraction of the adult worm from the
conjunctiva or subcutaneous tissues is also diagnostic.

56
Medical Parasitology
8
In the past, serological testing was infrequently used due to cross reactivity
with other fi larial antigens, especially in endemic populations in whom the
incidence of antifi larial antibodies may be quite high. Indeed, in hyperendemic
regions, up to 95% of population have developed antibodies to L. loa antigens
by the age of two. However, in travelers to endemic areas who have unexplained
eosinophilia, but no detectable microfi lariae on peripheral smear examination,
the presence of antifi larial antibodies may be suffi cient evidence to warrant
therapy for loiasis. More specifi c serological tests are available through research
institutions. IgG4 anti-Loa antibodies have been used as a marker of infection,
and a PCR testing of blood has also been developed.
Treatment, Prevention and Control
Diethylcarbamazine is the treatment of choice for loiasis. It is eff ective against
both adult worms and microfi lariae. Multiple courses diethylcarbamazine are
frequently required to completely eradicate infection since recurrences have
been documented several years aft er initial treatment.
Administration of diethylcarbamazine is associated with a variety of adverse
eff ects which are believed to be secondary to antigens released from dying mi-
crofi lariae. Severity of symptoms is related to organism burden. Serious renal
and central nervous system complications tend to occur with higher organism
burden. Milder adverse eff ects include fever, arthralgias and Calabar swellings,
which tend to occur during the fi rst few days of therapy. To reduce the incidence
of treatment-induced complications, a test dose of 1 mg/kg is given, followed
by escalating doses (1 mg/kg three times a day on day 2 and 1-2 mg/kg three
times a day on day 3), until a maximum dose of 8-10 mg/kg/d is tolerated. A
21-day course of diethylcarbamazine at 8-10 mg/kg/d is standard treatment.
Alternatively, some clinicians recommend 50 mg on day 1, 50 mg three times a
day on day 2, 100 mg three times a day on day 3, and 8 mg/kg/d in three doses
on days 4 through 14.
Antihistamine and corticosteroid therapy can also be administered to mini-
mize symptoms. Prior to initiating diethylcarbamazine, ivermectin or albenda-
zole can be used to reduce the microfi laria or adult worm burden respectively. In
patients with a high organism levels, cytopheresis has been suggested as a means
of reducing microfi laria burden prior to treatment with diethylcarbamazine.
Adult worms in the eye must be removed surgically.
In those areas with extremely high prevalence, mass treatment with diethyl-
carbamazine may help to reduce transmission to uninfected individuals. Periodic
chemotherapy of populations at risk has been utilized in sub-Saharan Africa,
but the side eff ects of diethylcarbamazine may limit its usefulness in this setting.
Albendazole or ivermectin may be more useful alternatives for such unmonitored
therapy, although the latter is associated with encephalopathy in individuals with
extremely high levels of microfi laremia.
Prevention methods include the use of insecticides and clearing forests in
order to control the vector population. Insect repellants and protective clothing
also provide added protection. In endemic areas, diethylcarbamazine prophylaxis
is recommended for visitors. A weekly dose of 300 mg is suffi cient and over a
course of two years was not associated with serious side eff ects.

57
Loiasis
8
Selected Readings
1. Akue JP, Egwang TG, Devaney E. High levels of parasite-specifi c IgG4 in the ab-
sence of microfi laremia in Loa loa infection. Trop Med Parasitol 1994; 45:246.
2. Boussinesq M, Gardon J, Gardon-Wendel N et al. Clinical picture, epidemiology
and outcome of Loa-associated serious adverse events related to mass ivermectin
treatment of onchocerciasis in Cameroon. Filaria J 2003; 2:S4.
3. Carme B, Boulesteix J, Boutes H et al. Five cases of encephalitis during treatment
of loiasis with diethylcarbamazine. Am J Trop Med Hyg 1991; 44:684-90.
4. Loiasis. In: Conner DH, Neafi e RC, Meyers, WM, eds. Pathology of Tropical
and Extraordinary Disease, Volume 2. Washington DC: Armed Forces Institute
of Pathology, 1976:356-9.
5. Th e Filariae. In: Cupp EW, Jung RC, Beaver PC, eds. Clinical Parasitology, 9th
Edition. Philadelphia: Lea & Ferbiger 1984:350-1,377-800.
6. Loa loa. In: Despommier DD, Gwadz RW, Hotez PJ, Knirsch CA, eds. Parasitic
Diseases, 4th edition. New York: Apple Tree Productions, LLC 2000:143-6.
7. Horse fl ies, deer fl ies and snipe fl ies. In: James MT, Harwood RF, eds. Herm’s
Medical Entomology, 6th Edition. Toronto: Macmillan Company, 1969:228-9.
8. Klion AD, Nutman TB. Loiasis and mansonella infections. In: Guerrant RL.
Walker DH, Weller PF, eds. Tropical Infectious Disease Principles, Pathogens and
Practice. Vol 2. Philadelphia: Churchill Livingstone1999:861-72.
9. Klion AD, Otteson EA, Nutman TB. Eff ectiveness of diethylcarbamazine in treat-
ing loiasis acquired by expatriate visitors to endemic regions: long term follow-up.
J Infect Dis 1994; 169: 604-10.
10. Mackenzie CD, Geary TG, Gerlach JA. Possible pathogenic pathways in the
adverse clinical events seen following ivermectin administration to onchocerciasis
patients. Filarial J 2003; 2:S5.
11. Nutman TB, Miller KD, Mulligan M et al. Diethylcarbamazine prophylaxis for
human loiasis. Results of a double blind study. N Engl J Med 1988; 319:752-6.
12. Nutman TB, Miller KD, Mulligan M et al. Loa loa infection in temporary residents
of endemic regions: recognition of a hyperresponsive syndrome with characteristic
clinical manifestations. J Infect Dis 1986; 154:10-8.
13. Nutman TB, Zimmermann PA, Kubofcik J et al. Elisa-based detection of PCR
products. A universally applicable approach to the diagnosis of fi larial and other
infections. Parisitol Today 1994; 10:239-43.
14. Ottenson EA. Filarial infections. Infect Dis Clin North Amer 1993; 7:619-33.
15. Loiasis. In: Strickland GT, ed. Hunter’s Tropical Medicine and Emerging Infectious
Diseases, 8th Edition. Philadelphia: W.B. Saunders, 2000:754-6.

C
HAPTER
9
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Dracunculiasis
David M. Parenti
Dracunculiasis (also know as dracontiasis) is caused by the “guinea worm”
Dracunculus medinensis. It has been described in humans since antiquity with
references to this infection being noted in the Bible and ancient Greek and Roman
texts. A calcifi ed adult worm has also been noted by X-ray in an Egyptian mummy
and there are descriptions of the disease in ancient papyrus texts. It is transmitted
by ingestion of a fresh water copepod (Mesocyclops, Metacyclops, Th ermocyclops)
containing infective larvae and is endemic only in areas where this intermediate
host is found. Transmission occurs in underdeveloped regions with limited access
to a safe water supply. Debilitation as a result of guinea worm infection is common,
resulting in chronic pain, acute or chronic infection, impaired joint mobility and
occasionally tetanus. Short term and long term disability from dracunculiasis leads
to lost work days and decreased economic productivity.
Phylogenetically Dracunculus is related to the helminths in the Order Spirurida,
which also contains the fi lariae. It is felt to be primarily a human parasite; but other
Dracunculus species may infect humans and other animals. Recently ribosomal 18S
rRNA sequencing has been able to distinguish at least some of these species and
a worm identical to the human parasite has been obtained from a dog in Ghana.
Th ere is no clear evidence that animals are important reservoir hosts.
Epidemiology
Two important conditions need to be satisfi ed for transmission of Dracunculus
to occur: emergent guinea worm lesions discharging larvae need to be in contact
with drinking water and the intermediate copepod host needs to be present in
the water supply. Contamination of step wells, cisterns and ponds is common in
endemic areas. Transmission in ponds is highest just before the rainy season, when
the density of copepods may be the highest.
Dracunculus infections were once distributed throughout equatorial Africa
from the northwest (Senegal, Burkina Faso, Ghana, Nigeria) to Sudan and Uganda.
It was also endemic in Iran, Afghanistan, Pakistan, India and the southernmost
new Soviet republics. An estimated 3 million cases occurred worldwide in 1986.
Transmission oft en occurs in remote areas with limited water supplies, or where
larval contamination can easily occur. Although transmission does not occur in
the United States, rare importations have occurred.
More recently, following the establishment of the WHO global eradication
campaign, endemic regions have been limited to those listed in Table 9.1.

59
Dracunculiasis
9
Life Cycle
Th e copepod is the intermediate host for Dracunculus and measures 1-3 mm in
size. Following ingestion of an infected copepod the third stage infective larva is
released. Whether the presence of reduced gastric pH is important in this process
is unclear. Th e larva then penetrates through the intestinal mucosa and migrates
to connective tissues, especially in the abdominal wall and thoracic wall and re-
troperitoneum, where it develops into an adult male or female worm.
Th e adult female worms are cylindroidal, 1-2 mm in width and up to 800 mm
in length; the males much smaller at about 40 mm in length. Th e female is likely
fertilized in this site before migration begins to the subcutaneous tissues. By 8-9
months aft er ingestion the uterus of the female worm becomes fi lled with eggs
which then develop into fi rst stage larvae. Full maturation takes up to 12 months,
contributing to its seasonal endemicity. Th e gravid worm, containing 1-3 million
fi rst stage larvae, then embarks on a period of subcutaneous migration eventually
taking it to the surface.
Aft er migration the anterior end of the worm approaches the dermis, where
fi rst a papule and then a blister is formed. Th e lesions occur predominately on the
distal lower extremities. On contact with fresh water the blister ruptures and fi rst
stage larvae (rhabditoid) are released as the uterus prolapses either through the
mouth or body wall. Th e motile larvae are 15-25
μ
m × 500-750
μ
m in size and
are subsequently ingested by the copepods. Th ey penetrate through the intestinal
wall, perhaps through use of a dorsal tooth that has been described and develop
in the coelomic cavity. Th e larvae molt twice in the copepod over a period of 2-4
weeks and remain dormant as third stage (infective) larvae. Human infection again
takes place aft er ingestion of infected copepods.
Clinical Manifestations
Initial ingestion of the infected copepod does not usually provoke any
symptoms although urticarial reactions sometimes occur. Clinical disease is
a direct result of the adult female worm which migrates in the subcutaneous
tissue until stopping to discharge the larvae through the skin. Th e individual
may notice a palpable or migrating worm in up to one-third of cases. Others
Table 9.1. Number of cases of Dracunculiaisis January-September 2005
(CDC)
Sudan 5,008
Ghana 2,936
Mali 475
Nigeria 116
Niger 66
Togo 58
Ethiopia 37
Burkina Faso
25
Cote d’Ivoire
9
Uganda 6
Benin 1

60
Medical Parasitology
9
may complain of allergic symptomatology prior to worm emergence: urticaria,
infra-orbital edema, fever, dyspnea. Th e majority present with local signs of
emergent worms.
Emergent lesions are primarily located in the lower extremities (> 90%), which
are also the body areas most likely to come in contact with fresh water and thus
allow completion of the life cycle. Worms may also emerge from the upper extrem-
ity, trunk, or head. Errant worms have appeared rarely in unusual locations such
as the epidural space, testicle, orbit and eyelid.
Each individual usually has 1-3 worms emerge, but rarely multiple lesions may
occur. Th e blister is usually painful and 1-3 cm in size and may also be pruritic.
Aft er emergence an ulcer forms and this remains an open wound until the worm
is expelled. Th e dying worm provokes a brisk infl ammatory response which may
lead to abscess formation. Abscesses may be seen in up to 10% of non-emergent
worms. If the female emerges near or in a joint, usually the knee or ankle, a frank
arthritis may ensue and larvae may be recovered from joint fl uid. Nonemergent
worms remain in the tissues to be contained by the host response and to become
calcifi ed.
Secondary infection is also a common complication of dracunculiasis (in up
to 50% of cases) and a cause of signifi cant morbidity. Infections may be caused
by skin fl ora such as staphylococci or streptococci or enteric organisms such as
Escherichia coli. Chronic skin ulcers of the extremities are an ideal portal of entry
for Clostridium tetani and clinical tetanus is a not infrequent complication in
endemic areas. In one study in Nigeria guinea worm lesions were the third most
common portal of entry for the development of tetanus.
Diagnosis
Aft er the appearance of the skin ulcer and worm, the diagnosis can be certain.
Discharged material from the gravid uterus will reveal larvae characteristic of
Dracunculus. Characteristic calcifi cations can also be identifi ed in areas where the
worms have died and calcifi ed and represent a “sarcophagus around a long departed
parasite”. Th ese are most common in the lower extremities where they lie along
tissue planes in a linear fashion; but have also been noted in the upper extremities
and in the trunk where they may have a coiled appearance.
In prepatent infection immunodiagnosis with ELISA or dot-ELISA may
be useful, but there may also be cross reactivity with other helminths such as
Wuchereria and Onchocerca. Eosinophilia may be present, particularly just prior
to emergence. Th e diff erential diagnosis of dracunculiasis includes other migra-
tory helminths such as Ancylostoma braziliense (cutaneous larval migrans) and
Stronglyoides (larva curens). Nodular lesions reminiscent of unerupted worms may
be seen with adult worms of Onchocerca, the tapeworm Spirometra (sparganosis)
and cutaneous myiasis. Extracted adult worms of Onchocerca have been confused
with Dracunculus in the Central African Republic, although they are smaller in
diameter (0.3 mm vs 1-2mm).
Treatment
Treatment of dracunculiasis is generally focused on mechanical removal of the
worm and treatment of secondary infection. Th e traditional method has been to

61
Dracunculiasis
9
wind the protruding worm on a stick and with gentle traction to remove several
inches at a time. Extraction of the worm may occasionally be diffi cult. Incomplete
removal or breakage of the worm with spillage of contents can lead to a more
signifi cant infl ammatory response and appears to increase the risk of secondary
infection. At least one study has suggested that removal of unerupted worms might
be more easily accomplished with less disability. In this study in India 161 patients
had surgical removal of worms prior to eruption, with an improvement in average
time of disability from 3 weeks to 1 week.
Several antiparasitic agents have been studied in the treatment of dracuncu-
liasis in humans and in animal models, including thiabendazole, mebendazole,
niridazole, metronidazole and ivermectin, with little evidence for a cidal eff ect on
the adult worms. A variety of treatment outcomes have been evaluated including
time to worm expulsion, prevention of emergence of new lesions and ulcer healing.
It has been suggested that metronidazole and some of the anthelminthics may
modulate the infl ammatory response and facilitate worm removal. Ivermectin
has been tested in a single-blind placebo-controlled trial of 400 adults at risk
for guinea worm infection. Th ere was no eff ect on migration of the worms or
prevention of emergent lesions. In a randomized, single blind, controlled trial
topical antimicrobials have been shown to reduce the rate of secondary infection
and improve wound healing.
Prevention
Because of the lack of eff ective anthelminthic agents, mass chemotherapy
programs for treatment or prevention have not been feasible. Th erefore the focus
has been on elimination of the copepod intermediate host and establishment of
safe supplies of potable water. Improved water sanitation can be accomplished by
establishing a piped in water supply, construction of bore or tube wells, or other
measures to prevent contamination. Implementation of individual or communal
nylon or cloth fi lters (100-200
μ
m pore size) has also been eff ective in preventing
transmission. Eradication of the intermediate host through water treatment with
cyclopiscides such as the organophosphate temephos has also been used in many
eradication programs.
Dracunculiasis is an ideal candidate for eradication: the disease is easy to
diagnose, the adult worms have a limited lifespan, it has only a human host, it is
prevalent in a limited geographic area, the intermediate host is not mobile and
treatment of the intermediate host is relatively inexpensive. Eradication programs
have included intensive cases fi nding, health education programs, utilization of
nylon or cloth fi lters and application of cyclopiscides.
Th e campaign for global eradication of dracunculiasis began in 1980 as part
of the International Drinking-Water Supply and Sanitation Decade (CDC) and
was expanded by the WHO in 1986. Substantial progress has been made with a
98% decline in cases worldwide. Dracunculiasis eradication has been accomplished
in Pakistan (1994) and India (1997). Th ose countries remaining with continued
transmission are confi ned to Africa. Although many countries have continued to
show a decline in cases during 2005, an increased number of cases has been noted
in Mali and Ethiopia. Control measures in the Sudan have been particularly dif-
fi cult because of sociopolitical unrest.

62
Medical Parasitology
9
Suggested Reading
1. Beaver PC, Jung RC, Cupp EW. Th e Spirurida: Dracunculus and others. Clinical
Parasitology, 9th Edition. Philadelphia: Lea & Febiger, 1984:335-40.
2. Belcher DW, Wunapa FK, Ward WB. Failure of thiabendazole and metronidazole
in the treatment and suppression of guinea worm disease. Am J Trop Med Hyg
1975; 24:444-46.
3. Bimi L, Freeman AR, Eberhard ML et al. Diff erentiating Dracunculus medinensis
from D. insignis, by the sequence analysis of the 18S rRNA gene. Ann Trop Med
Parasitol 2005; 99:511-17.
4. Bloch P, Simonsen PE, Weiss N, Nutman TB. Th e signifi cance of guinea worm
infection in the immunological diagnosis of onchocerciasis and brancroft ian fi lari-
asis. Trans R Soc Trop Med Hyg 1998; 92:518-21.
5. Cairncross S, Muller R, Zagaria N. Dracunculiasis (Guinea worm disease) and the
eradication initiative. Clin Microbiol Rev 2002; 15:223-346.
6. CDC. Imported Dracunculiasis—United States, 1995 and 1997. MMWR 1998;
47:209-11.
7. CDC/WHO. Guinea Worm Wrap-Up #157, 10/26/05. http://www.cdc.gov/
ncidod/dpd/parasites/dracunculiasis/wrapup/157.pdf
8. Cockshott P, Middlemiss H. Clinical Radiology in the Tropics. Edinburgh:
Churchill Livingstone, 1979: 5-6.
9. Cox FEG. History of human parasitology. Clin Microbiol Rev 2002; 15:595-612.
10. Drugs for parasitic infections. Th e Medical Letter 2002:1-12.
11. Eberhard ML, Melemoko G, Zee AK et al. Misidentifi cation of Onchocerca
volvulus as guinea worm. Ann Trop Med Parasitol 2001; 95:821-26.
12. Hopkins DR, Azam M. Eradication of dracunculiasis from Pakistan. Lancet 1995;
346:621-24.
13. Hours M, Cairncross S. Long-term disability due to guinea worm disease. Trans
R Soc Trop Med Hyg 1994; 8: 559-60.
14. Issaka-Tinorgah A, Magnussen P, Bloch P, Yakubu A. Lack of eff ect of ivermectin
on prepatient guinea-worm: a single blind, placebo-controlled trial. Trans R Soc
Trop Med Hyg 1994; 88:346-48.
15. Kaul SM, Sharma RS, Verghese T. Monitoring the effi cacy of temephos application
and use of fi ne mesh nylon strainers by examination of drinking water containers
in guinea worm endemic villages. J Commun Dis 1992; 24:159-63.
16. Magnusssen P, Yakubu A, Bloch P. Th e eff ect of antibiotic- and hydrocorti-
sone-containing ointments in prevention secondary infections in guinea worm
disease. Am J Trop Med Hyg 1994; 51:797-79.
17. Muller R. Dracunculus and dracunculiasis. Adv Parasitol 1971; 9:73-151.
18. Neafi e RC, Connor DH, Meyers WM. Dracunculiasis. In: Binford CH, Connor
DH, eds. Pathology of Tropical and Extraordinary Diseases. Washington, DC:
AFIP, 1976:397-401.
19. Rohde JE, Sharma BL, Patton H et al. Surgical extraction of guinea worm: dis-
ability reduction and contribution to disease control. Am J Trop Med Hyg 1993;
48:71-76.
20. Sullivan JJ, Long EG. Synthetic-fi bre fi lters for preventing dracunculiasis: 100
versus 200 micrometres pore size. Trans R Soc Trop Med Hyg 1988; 82:465-66.
21. WHO. Weekly Epidemiological Record. 5/13/2005; 80: 165-76.

C
HAPTER
10
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Cutaneous Larva Migrans:
“The Creeping Eruption”
Ann M. Labriola
Background
Cutaneous larva migrans (CLM), frequently termed “creeping eruption,” is
a parasitic skin infection that is caused by the fi lariform larvae of various animal
hookworm nematodes. CLM has a worldwide distribution wherever humans
have had skin contact with soil contaminated with infected animal feces. Th e
disease most commonly occurs in subtropical and tropical regions, but may
also occur in temperate climates particularly during the summer months and
during rainy seasons. In the United States, CLM is seen in individuals living in
the southeastern states, Florida and the Gulf Coast states, in travelers returning
from sandy beaches and in military personnel returning from tropical postings.
Children develop the disease by walking barefoot in sandy areas or playing in dirt
or sandboxes that contain infected animal feces. Electricians, plumbers, utility
workers and pest exterminators who have contact with soil under houses or at
construction sites are at risk, as are fi sherman, hunters, farmers and gardeners
who handle contaminated soil.
Causative Agents
Ancylostoma braziliense, a hookworm of wild and domestic dogs and cats is the
most commonly identifi ed etiologic agent of CLM. A. braziliense is distributed
throughout the tropics and subtropics, especially on the warm sandy beaches of
the southeastern and Gulf Coast states, the Caribbean and southeast Asia where
dogs and cats are permitted to defecate. Ancylostoma caninum (dog hookworm),
Ancylostoma ceylanicum (dog and cat hookworm) and Ancylostoma tubaeforme
(cat hookworm) may also produce cutaneous lesions. Whereas the skin lesions
of A. braziliense may persist for months, the skin lesions that are seen with other
Ancylostoma species resolve within several weeks.
A number of other nematodes have been associated with CLM-like lesions.
Strongyloides species such as S. papillosus, S. westeri, S. stercoralis, S. procyonis
and S. myopotami, nematodes found in the small intestine of mammals, migrate
more quickly in human skin than hookworm larva to form skin eruptions called
larva currens (“racing larva”). Gnathostoma species, a dog and cat roundworm
found in Southeast Asia and Dirofi laria repens, a fi larial nematode of dogs,

64
Medical Parasitology
10
cats and wild carnivores, can travel through the skin and cause dermatitis,
swelling and subcutaneous nodules. Uncinaria stenocephala (dog hookworm)
and Bunostomum phlebotomum (cattle hookworm) may also cause cutaneous
disease. Ancylostoma duodenale and Necator americanus, human hookworms,
that, upon larval migration in the skin, produce a pruritic dermatitis (“ground
itch”) similar to CLM of animal hookworms. Free-living nematodes such as
Peloderma strongyloides have also been described as a rare cause of cutaneous
infection in humans.
Life Cycle
Unlike the classical hookworm life cycle, the cycle of the organisms that are
typically associated with CLM is much abbreviated. A. braziliense third stage
infective larvae (L3) enter the epidermis and migrate laterally in epidermis,
generally unable to penetrate the basement membrane of the epidermal dermal
junction. Humans are thus incidental dead-end hosts.
Disease Signs and Symptoms
Th e most common portals of entry by the larvae are the exposed areas of
the body such as the dorsum of the feet, lower legs, arms and hands. Th e but-
tocks, thighs and abdomen also may be involved, probably due to lying directly
on contaminated sand. CLM also occurs in the interdigital spaces of the toes,
the anogenital region, knees and rarely on the face. Symptoms usually occur
within the fi rst hours of larval infection, although, occasionally, the larvae may
lie dormant for several weeks. Th e fi rst sign of infection is a stinging, tingling
or burning sensation at the site of larval entry, followed by the development of
an edematous, erythematous pruritic papule. Th e larvae migrate laterally from
the papule and form raised 2-4 mm wide serpinginous tracks. Th ese tracks mark
the migratory route of the larvae and may advance from a few millimeters to
several centimeters each day, hence the name “creeping eruption.” As they burrow,
the larvae produce hydrolytic enzymes that provoke the intense infl ammatory
reaction that characterizes CLM. In heavy infections, an individual may have
hundreds of tracts. Severe infl ammatory reactions may cause such an intense
pruritis that individuals are unable to sleep, develop anorexia and even become
psychotic. Secondary bacterial infections such as impetigo or cellulitis from
scratching the skin are common complications.
CLM is a usually a self-limited disease. Th e larvae, unable to complete their
life cycle, die in the epidermis within several weeks to months if left untreated.
Th ere are reports of larvae that have migrated for over one year. Th e skin
lesions ulitmately resolve although there may be scarring. Rarely larva may
migrate past the dermis to cause myositis, pneumonitis (Loffl er’s syndrome)
or an eosinophilic enteritis (viz., A. caninum) of the small intestine. A pruritic
folliculitis is another uncommon form of CLM that presents with serpiginous
tracks interspersed with papules and pustules confi ned to a particular area of
the body, usually the buttocks.

65
Cutaneous Larva Migrans
10
Diagnosis
Th e diagnosis of CLM is based on a history of exposure and the characteristic
clinical appearance of the skin eruption. Th ere may be a delay in diagnosis in chronic
cases due to superimposed allergic dermatitis, secondary bacterial infection or
lack of recognition of CLM by health care personnel. Peripheral eosinophilia and
increased IgE levels are found in a minority of patients. Skin biopsies are usually
not eff ective at establishing the diagnosis. Histologic examination usually reveals
only an eosinophilic infi ltrate or a nonspecifi c infl ammatory response, although,
occasionally, a skin biopsy taken at the leading edge of the track may contain a
larva trapped in a follicular canal, stratum cornea or dermis.
Th e diff erential diagnosis of CLM should also include cercarial dermatitis
(“swimmer’s itch”), migratory myiasis, scabies, jelly fi sh sting, photoallergic der-
matitis, epidermal dermatophytosis, erythema chronicum migrans of Lyme disease
and photoallergic dermatitis. Vesicular lesions may be mistaken for viral infections
or phytophotodermatitis.
Treatment
Oral albendazole (400 mg daily for 1-3 days) or ivermectin (200
μ
g/kg daily
for 1-2 days) are the drugs of choice for the treatment of CLM. Oral thiabendazole
is less eff ective than either albendazole or ivermectin. Whereas the cure rate with
oral thiabendazole was 87% aft er 3-4 consecutive days of treatment, a single dose of
ivermectin was eff ective in 81-100% of patients. Most studies of albendazole have
shown cure rates 92-100%. A single randomized study of single dose ivermectin
versus albendazole revealed cure rates of 100% for ivermectin with no relapses com-
pared to a 90% cure rate with albendazole. However, half of the albendazole-treated
patients relapsed. With treatment, symptoms of pruritus improve within 24-48
hours and the skin lesions usually resolve within one week. Th iabendazole is poorly
tolerated compared to either albendazole or ivermectin. Side eff ects of thiabenda-
zole include nausea, vomiting, headache and giddiness.
Treatment with topical thiabendazole is eff ective in treating patients with a
few lesions. Th is approach avoids systemic side eff ects, but is less convenient and
requires multiple applications. Cryotherapy or surgical excision is painful and
not very eff ective because the larvae may be several centimeters beyond the end
of the visible track. Its use is limited to pregnant women because of the uncertain
teratogenic potential of ivermectin or albendazole.
Prevention and Prophylaxis
CLM can be prevented by avoiding skin contact with soil contaminated with
infected animal feces. In geographic areas where the infection is endemic, one
should avoid touching soil with bare hands, walking barefoot or lying directly on
moist, warm, shady soil or warm, dry, sandy beaches protected from tidal move-
ment. Beaches should also be kept free of dogs and cats. Pets should be examined
and treated when infected with parasites and should receive periodic prophylaxis.
Pet feces should be disposed of in a sanitary manner and sandboxes should be
kept covered.

66
Medical Parasitology
10
Suggested Reading
1. Albanese G, Venturi C. Albendazole: A new drug for human parasitoses. Dermatol
Clin 2003; 21:283-90.
2. Blackwell V, Vega-Lopez F. Cutaneous larva migrans: clinical features and man-
agement of 44 cases presenting in the returning traveler. Br J Dermatol 2001;
145:434-7.
3. Bouchaud O, Houzé H, Schiemann R et al. Cutaneous larva migrans in travelers:
A prospective study, with assessment of therapy with ivermectin. Clin Infect Dis
2000; 31:493-8.
4. Caumes E. Treatment of cutaneous larva migrans. Clin Infect Dis 2000;
30.5:811-4.
5. Caumes E, Ly F, Bricaire F. Cutaneous larva migrans with folliculitis: report of
seven cases and review of the literature. Br J Dermatol 2002; 146:314-6.
6. Davies HD, Sakuls P, Keystone JS. Creeping eruption. A review of clinical pre-
sentation and management of 60 cases presenting to a tropical disease unit. Arch
Dermatol 1993; 129:588-91.
7. Despommier DD, Gwadz RW, Hotez PJ et al. Parasitic Diseases. Clinical
Parasitology; A Practical Approach, 4th ed. Philadelphia: Saunders, 1997.
8. Douglass MC. Cutaneous larva migrans. eMedicine.com. http://www.emedicine.
com/derm/topic91.htm. Accessed 2005.
9. Edelglass JW, Douglass MC, Stiefl er R et al. Cutaneous larva migrans in north-
ern climates. A souvenir of your dream vacation. J Am Acad Dermatol 1982;
7.3:353-8.
10. Guill M, Odom R. Larva migrans complicated by Loffl er’s syndrome. Arch
Dermatol 1978; 114:1525-6.
11. Herbener D, Borak J. Cutaneous larva migrans in northern climates. Am J Emerg
Med 1988; 6.5:462-4[Medline].
12. Hotez PJ, Brooker S, Bethony JM et al. Current concepts: hookworm infection.
N Engl J Med 2004; 351:799-807.
13. Jelinek T, Maiwald H, Nothdurft HD et al. Cutaneous larva migrans in travelers:
synopsis of histories, symptoms and treatment of 98 patients. Clin Infect Dis 1994;
19.6:1062-6.
14. Jones CC, Rosen T, Greenberg C. Cutaneous larva migrans due to pelodera
strongyloides. Cutis 1991; 48.2:123-6.
15. Kelsey DS. Enteric nematodes of lower animals transmitted to humans: zoonoses,
(monogram online). In: Baron S, ed. Medical Microbiology, 4th ed. New York:
Churchill Livingston; 1996. http://www.ncbi.nlm.nih.gov/books/bv.fcgi?call=bv.
View..ShowTOC&rid=mmed.TOC&depth=10. Accessed 2005.
16. Kwon IH, Kim HS, Lee JH et al. A serologically diagnosed human case of cuta-
neous larva migrans caused by ancylostoma caninum. Korean J Parasitol 2003;
41:233-7.
17. Meyers WM, Neafi e RC. Creeping eruption. In: Binford CH, Connor DH, eds.
Pathology of Tropical and Extraordinary Diseases, 2nd Edition. Armed Forces
Institute of Pathology, 1976:437-9.
18. Richey TK, Gentry RH, Fitzpatrick JE et al. Persistent cutaneous larva migrans
due to ancylostoma species. South Med J 1996; 89.6:609-11.
19. Van den Enden E, Stevens A, Van Gompel A. Treatment of cutaneous larva migrans.
N Engl J Med 1998; 339.17:1246-7.
20. Wang J. Cutaneous larva migrans. eMedicine.com. 16 March 2005. http://www.
emedicine.com/ped/topic1278.htm. Accessed 2005.

Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Baylisascariasis and Toxocariasis
Erin Elizabeth Dainty and Cynthia Livingstone Gibert
Baylisascariasis
Background
Causative Agent
Baylisascariasis is caused by the nematode parasite Baylisascaris procyonis. Th e
North American raccoon (Procyon lotor) is the defi nitive host for B. procyonis. B.
procyonis is also recognized as one of the most common causes of visceral larva
migrans in animals, causing infection in over 100 species other than raccoons.
Humans are infected as accidental intermediate hosts.
Geographical Distribution/Epidemiology
Raccoons are native to the Americas from Canada to Panama and have become
increasingly concentrated in urban areas in recent years. B. procyonis is endemic
in raccoons in most regions of the United States, with a higher prevalence on the
West Coast, Midwest and Northeastern regions.
While the geographic distribution of the North American raccoon has been
well established, the distribution of B. procyonis itself is not fully known. Th is
uncertainty is due to the large abundance of eggs shed by raccoons that survive
in the environment for extended periods of time and the large number of species
that B. procyonis can potentially infect as accidental hosts.
Life Cycle and Mode of Transmission
Raccoons are infected by B. procyonis in one of two ways, depending on the age
of the raccoon. Juvenile raccoons are typically infected by the ingestion of eggs in
the environment. Younger raccoons have a higher prevalence of infection and have
typically been shown to harbor a larger parasite burden. By contrast, adult raccoons
are usually infected through ingesting larvae in the fl esh of paratenic hosts, such as
squirrels and rodents. In raccoons, infection with B. procyonis rarely causes clinical
symptoms, as the adult worms are confi ned to the small intestine.
Once ingested, larvae migrate to the small intestine of the raccoon and remain
in the lumen as they mature into adult worms. Maturation typically takes 1 to 2
months, depending upon the larval stage at the time of ingestion. Adult female
worms have an extremely high fecundity rate, producing between 115,000 to
877,000 eggs/worm/d. An infected raccoon can shed as many as 45,000,000 eggs
daily. An infectious dose of eggs is estimated to be less than 5,000. It takes 2 to 4
weeks for eggs to become infectious aft er being shed.
C
HAPTER
11
Communities of raccoons habitually defecate in discrete areas termed “latrines.”
Th is serves to harbor and accumulate increasingly large numbers of B. procyonis
eggs and becomes the main means of transmission to intermediate hosts.
Humans are infected by ingesting B. procyonis eggs, typically at the sites of rac-
coon defecation. Pica and geophagia are risk factors for infection and are common
in children under two years of age. Aft er ingestion, larvae penetrate the intestinal
mucosa and migrate rapidly to the liver and then to the lungs through the portal
circulatory system. In the lungs, the larvae gain access to the pulmonary veins and
are then distributed through the systemic circulation to the tissues. Larvae do not
mature into adult worms in human hosts.
Clinical Features
In humans, baylisascariasis results in death or severe neurologic sequelae. To
date, 13 confi rmed cases of baylisascariasis in humans have been reported in the
literature. In all cases, the victims were either young children or young adults with
severe developmental disabilities. Disease results in neural larva migrans (NLM),
ocular larva migrans (OLM) and visceral larva migrans (VLM). B. procyonis is
distinct from other common parasites causing larva migrans by its propensity for
continued larval growth in intermediate hosts, invasion of the central nervous
system and its aggressive migration through host tissues.
B. procyonis is relatively unique among the zoonotic helminthes in its ability
to cause neural larva migrans in addition to ocular and visceral larva migrans.
It is estimated that 5 to 7% of ingested B. procyonis eggs travel to the central
nervous system (CNS). When CNS involvement occurs, it most commonly
presents as acute eosinophilic meningoencephalitis. Case reports in children
have documented clinical signs including, sudden onset of lethargy, irritabil-
ity, loss of motor coordination, weakness, generalized ataxia, stupor, coma,
opisthotonus and death. Clinical symptoms may occur as early as 2 to 4 weeks
post infection.
B. procyonis is the primary cause of the large nematode variant of diff use uni-
lateral subacute neuroretinitis (DUSN). Th is neuroretinitis is characterized by
infl ammatory and degenerative changes involving the retina and optic disk.
Based on the above data, the diagnosis of baylisascariasis should be considered in
the setting of eosinophilia, both peripherally and in the CNS, DUSN, neurologic
signs and a history of possible environmental exposure to raccoon feces.
Diagnosis
Laboratory Tests/Microscopy
Eosinophilic pleocytosis in the cerebral spinal fl uid in combination with
peripheral eosinophilia is highly suggestive of parasitic infection. Because of the
prolonged, aberrant migration of B. procyonis larva, eosinophilic infl ammation can
be quite marked (28% or higher).
Defi nitive diagnosis is through morphologic identifi cation of larvae in tissue
sections, which is usually only accomplished at autopsy. Because B. procyonis
does not establish an intestinal infection in humans, eggs will never be observed
in feces.
68
Medical Parasitology
11

Radiographic
Central nervous system infection with B. procyonis has been noted to cause
diff use, periventricular white matter changes on MRI, though this fi nding is not
pathognomonic. Global atrophy as well as capsular and brain stem changes have
been noted as late radiographic fi ndings.
Molecular Diagnostic
Serologic testing for anti-bp antibodies in the CSF and serum by enzyme-linked
immunosorbent assay (ELISA) or immunofl uorescent antibody (IFA) has become
the most important defi nitive diagnostic modality. Th is antibody assay has not
been shown to have cross-reactivity with antibodies toward other ascarids, such as
Toxocara species. In the United States, serologic testing is only available through the
Department of Veterinary Pathobiology at Purdue University in West Lafayette, IN
(phone 765-494-7558). B. procyonis infection also causes an elevation in isohemaglu-
tinins due to a cross-reaction of larval proteins with human blood group antigens.
Treatment
Neural larva migrans has a universally poor prognosis. Th is is due in large
part to failure of medical therapy when initiated at the onset of clinical signs and
symptoms. Albendazole should be administered at a dose of 25 mg/kg for 20 days
to a child with known exposure to raccoon stool. Ivermectin, mebendazole and
thiabendazole can also be considered. Th eoretically, the most promising anthel-
minthic agent is albendazole because of its ability to cross the blood-brain barrier.
Whether albendazole would have any benefi cial eff ect in treating CNS disease
is unknown. In the past, corticosteroids have been administered to decrease the
deleterious eff ects of the host infl ammatory response to the migrating larvae. When
a motile larva is found in the retina in a patient with ocular larva migrans, laser
photocoagulation is curative.
Prevention and Prophylaxis
It is likely that B. procyonis will become a more signifi cant pathogen for humans
as the geographic distribution of the North American raccoon continues to expand
and encroach upon urban human environments. Due to the relatively poor effi cacy
of treatment, preventative strategies are increasingly important. When possible,
raccoons should be prevented from frequenting areas of human habitation. If
an area is known to be contaminated by raccoon feces, the preferred method of
disinfection is with direct fl ames.
Education of the public regarding the dangers of raccoon contact is paramount.
Children should be closely monitored for ingestion of soil while playing outside.
If ingestion of raccoon feces is suspected in a child, prophylactic treatment should
consist of immediate administration of albendazole (25-50 mg/kg/d × 20 d; or
400 mg twice a day × 10 days).
Suggested Reading
1. Chronic and Recurrent Meningitis. Harrison’s Online. McGraw-Hill Company,
2004-2005. www.accessmedicine.com. A comprehensive review of meningitis.
2. Gavin PJ, Kazacos KR, Shulman ST. Baylisascariasis. Clin Microbiol Rev 2005;
18:703-18. Th is is an excellent review.
69
Baylisascariasis and Toxocariasis
11
3. Gavin PJ, Shulman ST. Raccoon roundworm (Baylisascaris procyonis). Pediatr
Infect Dis 2003; 22:651-2.
4. Murray WJ, Kazacos KR. Raccoon roundworm encephalitis. Clin Infect Dis 2004;
39:1484-92.
5. Nash TE. Visceral larva migrans and other unusual helminth infections.
In: Mandell GM, Bennett JE, Dolin R, eds. Principles and Practice of
Infectious Diseases, 6th Edition. Philadelphia: Elsevier Churchill Livingstone
2005:3293-300. A comprehensive review of unusual helmith infections.
6. Page LK, Swihart RK, Kazacos KR. Implications of raccoon latrines in the epizo-
otiology of baylisascaris. J Wildl Dis 1999; 35:474-80.
7. Park SY, Glaser C, Murray WJ et al. Raccoon roundworm (Baylisascaris procyonis)
encephalitis: case report and fi eld investigation. Pediatrics 2000; 106.http://www.
pediatrics.org/cgi/content/full/106/4-e56.
8. Roussere GP, Murray WJ, Raudenbush CB et al. Raccoon roundworm eggs near
homes and risk for larva migrans disease, California communities. Emerg Infect
Dis 2003; 9:1516-22.
9. Rowley HA, Uht RM, Kazacos KR et al. Radiologic-pathologic fi ndings in rac-
coon roundworm (Baylisascaris procyonis) encephalitis. Amv J Neuroradiol 2000;
21:415-20.
10. Sorvillo F, Ash LR, Berlin OG et al. Baylisascaris procyonis: An emerging helmin-
thic zoonosis. Emerg Infect Dis 2002; 8:355-59.
11. Wise ME, Sorvillo FJ, Shafi r SC et al. Severe and fatal central nervous system
disease in humans caused by Baylisascaris procyonis, the common roundworm of
raccoons: A review of current literature. Microbes Infect 2005; 7:317-23.
Toxocariasis
Many animal parasites are capable of infecting humans but rarely do so. Some
helminths, however, infect humans more frequently and cause distinctive clinical
syndromes. In the human host, especially children, the tissue migrating larvae of
roundworms, Toxocara canis and T. cati, can cause serious complications includ-
ing visceral larva migrans and ocular larva migrans. Originally toxocariasis was
thought to be an uncommon pediatric infection. With improved serologic test-
ing, toxocariasis is now known to be the most prevalent helminthic zoonosis in
industrialized countries.
Background
Geographical Distribution and Epidemiology
Worldwide, toxocariasis is one of the most commonly reported zoonotic
infections. Toxocara infect most domestic and many feral dogs and cats as well as
foxes. In humans, infection is far more common in children than adults. Children
usually acquire the infection through the ingestion of soil contaminated with
embryonated Toxocara eggs.
Exposure is most likely to occur in playgrounds and sandboxes contaminated
with cat and dog feces. Th ere is evidence that the infection may also be acquired
from direct contact with infected dogs, given the fact that the egg density on dog
hair may be higher than that in soil.
Between 2% and 8% of healthy adults in Western countries in urban areas have
serologic evidence of prior infection. Th e seroprevalence in children is much higher,
ranging from 4 to 31% in developed countries to as high as 92.8% in rural areas in
tropical countries. In a recent sero-epidemiological study of T. canis in schoolchildren
70
Medical Parasitology
11
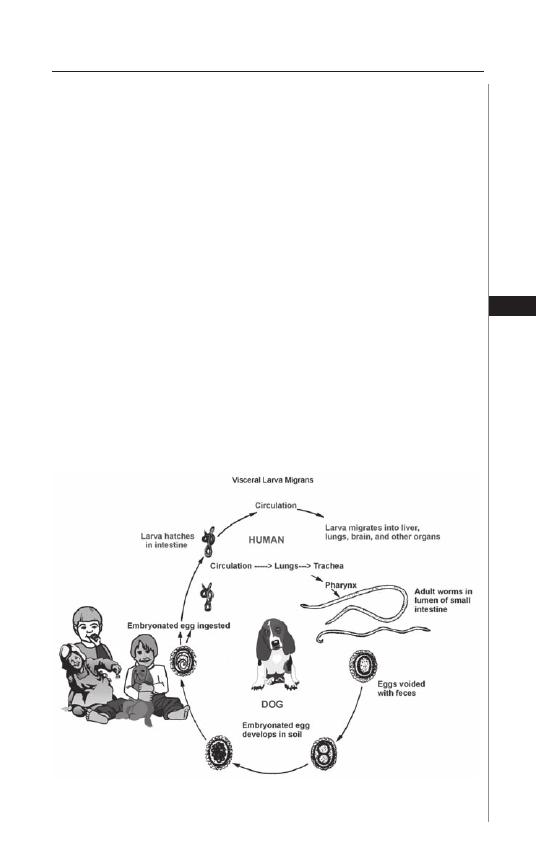
in a mountainous region of Taiwan, 57.5% of children were seropositive. Th e two
most signifi cant risk factors for infection were living in household with dogs or play-
ing in soil. In a similar study in Sorocaba, Brazil, 38.3% of children living in poorer
outskirts of the city were infected compared to 11.1% in the central city. Overall,
infection is most common in children with geographia or pica and exposure to pup-
pies. Several studies have shown higher rates of infection in mentally retarded adults
and children. In Israel, 8.5% of institutionalized mentally retarded adults had serologic
evidence of infection. Several studies of mentally retarded children have found rates
of infection from 10.6 to 20 percent. Although infection with Toxocara is common
in cats, Toxocara cati is under recognized as a zoonotic infection.
Life Cycle and Mode of Transmission
In humans, Toxocara worms have a life cycle similar to that of Ascaris
lumbricoides (Fig. 11.1). In the both animals and the aberrant host, ingestion
of embryonated eggs initiates infection. Dogs and cats can also acquire the
infection by eating earthworms or other paratenic hosts carrying embryonated
eggs. Following ingestion, the eggs hatch in the small intestine to release larvae
which penetrate the intestinal wall. The larvae then migrate via the bloodstream
to the liver, lung and trachea. In the definitive host, particularly in dogs less
than 6 months old, the larvae complete the life cycle after they are coughed
up and swallowed, returning to the gastrointestinal tract. The larvae develop
into the adult stage in the small intestine 60 to 90 days after hatching. Mating
occurs in the intestine. Female worms may produce up to 200,000 eggs per
day (Fig. 11.2). These nonembryonated eggs are then excreted into the soil
71
Baylisascariasis and Toxocariasis
11
Figure 11.1. Life Cycle of Toxocara canis. Reproduced from: Nappi AJ, Vass E,
eds. Parasites of Medical Importance. Austin: Landes Bioscience, 2002:86.

where embryonation occurs within one to two weeks. Embryonated eggs can
survive in the soil from days to months depending on the soil temperature. In
pregnant female dogs and cats, dormant larvae, activated by hormonal stimuli,
may develop and migrate transplacentally. Puppies infected in utero or trans-
mammarily also shed eggs.
In humans, ingested eggs hatch in the small intestine and penetrate the in-
testinal wall in the same way as in the defi nitive host, but the larvae, unable to
mature, migrate through the body for a prolonged period. Infective larvae, which
do not mature into adult worms in humans, can persist for years aft er becoming
encapsulated within granulomas. Hatched larvae have been isolated from the eye,
liver, heart and brain. Besides ingestion of soil contaminated with embryonated
eggs, infection can also occur aft er consumption of raw or undercooked meat in-
fected with Toxocara larvae, as well as by ingestion of vegetables and salads grown
in contaminated soil.
Clinical Features
Th e severity of disease and degree of host response vary dependent upon the
tissue invaded by the migrating larvae, the number of larvae and the age of the
host. Th e death of the juvenile larvae in tissue, particularly the lung, liver and
brain, evokes an infl ammatory response with marked eosinophilia, producing the
symptoms of visceral larva migrans (VLM).
In the eye damage to the retina from the migrating larvae, ocular larva migrans
(OLM), results in a granulomatous reaction and impaired vision. OLM is thought
to occur mostly in children not previously sensitized to toxocariasis while VLM
occurs following repeated exposure to migrating larvae.
72
Medical Parasitology
11
Figure 11.2. Toxocara canis egg.Reproduced from: Centers for Disease
Control and Prevention (CDC) (http://www.dpd.cdc.gov/dpdx/).

Visceral Larva Migrans
VLM occurs primarily in young and preschool children. Most infections are
asymptomatic, but fulminant disease and death do occur. Children come to medical
attention with unexplained prolonged fever, cough, hepatosplenomegaly, wheeze
and eosinophilia.
Other clinical signs and symptoms include lymphadenopathy, skin lesions,
pruritus, anemia, failure to thrive, decreased appetite, nausea, vomiting, headache
and pneumonia, as well as behavioral and sleep disturbances. In children with
toxocariasis, pica or geographia are common. Myocarditis, respiratory failure or
seizures may complicate overwhelming infection. Other less frequently reported
complications included multiple ecchymoses with eosinophilia, pyogenic liver
abscess, urticaria or prurigo, Henoch-Schönlein purpura, nephrotic syndrome,
secondary thrombocytosis and eosinophilic arthritis. Th ere is a report of VLM
mimicking lymphoma with hilar and mediastinal lymphadenopathy and another
of systemic vasculitis with lymphocytic temporal arteritis. Both of these two com-
plications occurred in patients over 60 years of age.
Toxocariasis has been suggested to be an environmental risk factor for asthma
among children living in urban areas, but supportive evidence for this hypothesis
is lacking. Central nervous system involvement is a rare complication reported
more frequently in adults. Toxocariasis should be considered as a causative agent
in patients with eosinophilic meningoencephalitis or meningitis. T. canis has been
reported to cause epileptic seizures, particularly late-onset partial epilepsy.
Ocular Larva Migrans
Ocular toxocariasis occurs primarily in young adults and older children who
present with unilateral visual loss over days to weeks. OLM follows entrapment of
a larva in the eye causing an intense eosinophilic infl ammatory reaction. Posterior
pole granuloma is the most common form of OLM in children between 6 and 14
years and causes decreased vision. Peripheral granuloma is usually seen at an older
age in association with macular heterotropia, strabismus and decreased vision.
Endophthalmitis occurs in younger children aged 2 to 9 years in whom there is
marked visual impairment with evidence of vitritis and anterior uveitis. Retinal
detachment may be seen on fundoscopic examination. OLM should be included
in the diff erential diagnosis of any child with leukocoria.
Diagnosis
Th e presence of eosinophilia in a child with unexplained fever, abdominal
pain, hepatosplenomegaly and multisystem illness raises the possibility of VLM,
especially if there is a history of geographia or pica and contact with puppies.
For a child with unilateral visual loss and strabismus, a diagnosis of OLM must
be excluded. In OLM the blood eosinophil count is frequently not elevated.
Leukocytosis, hypergammaglobulinemia, increased anti-Toxocara IgE serum con-
centration and elevated isohemagglutinin titers to A and B blood group antigens
may be present in VLM.
Th e diagnosis of both VLM and OLM is usually based on serologic tests.
However, serologic tests do not reliably distinguish between recent and past infec-
tion. Th e most frequently performed test is the enzyme-linked immunosorbent assay
(ELISA) which uses Toxocara excretory-secretory antigens of the second-stage larvae.
73
Baylisascariasis and Toxocariasis
11
Th is test is suffi ciently specifi c to be the best indirect diagnostic assay. At a titer of
greater than 1:32 the sensitivity of this test for diagnosing VLM is about 78%. Th e
ELISA is less reliable for the diagnosis of OLM. Th e presence of elevated vitreous
and aqueous fl uid titers relative to serum titers supports the diagnosis of OLM.
Microscopy
A defi nitive diagnosis of VLM is based upon the visualization of the larvae in
infected tissue such as lung, liver, or brain. In OLM the larvae may be seen in the
enucleated eye.
Molecular Diagnostics and Radiographic
In OLM, ultrasound biomicroscopy has been used to detect the morphologic
changes of peripheral vitreoretinal toxocariasis.
Ultrasonographic fi ndings of hepatic toxocariasis complicating VLM may
include ill-defi ned focal lesions, hepatosplenomegaly, the presence of biliary sludge
and dilatation and periportal lymphadenopathy. If present, follow-up CT scan or
MR imaging should be considered following treatment.
In patients with cerebral granulomatous toxocariasis, multiple subcortical, corti-
cal, or white matter lesions that were hypoattenuating on CT scan, hyperintense on
T2-weighted MR images and homogeneously enhancing have been reported. Th ese
fi ndings are nonspecifi c. It has been suggested, nonetheless, that serial MR imaging
may be used to monitor the course of disease treated with anthelminthic therapy.
Treatment
Th e decision to treat toxocariasis is made based on the type of infection and
severity of clinical symptoms. Most patients do not require treatment.
Current Th erapy and Alternative Th erapies
Visceral Larva Migrans
Albendazole, in doses of 10 mg/kg or 400 mg, both given twice daily for 5
days is the treatment of choice for toxocariasis. Mebendazole is a second-line
therapy since it is not absorbed outside of the gastrointestinal tract. Th ese two
agents have fewer side eff ects than thiabendazole and diethylcarbamazine, both
of which require treatment for one to three weeks. Injury to the parasite may
cause a more intense infl ammatory response with worsening of symptoms for
which antihistamines and corticosteroids may be of benefi t.
Ocular Larva Migrans
Th e goal of therapy for the treatment of OLM is to decrease the severity of the
infl ammatory response. If given within the fi rst four weeks, systemic or intraocular
corticosteroids are the most eff ective intervention. Th ere are reports of treatment
with albendazole and corticosteroids although the ocular penetration of albendazole
and other benzimidazoles has not been established. Diethlycarbamazine (DEC) may
be the preferred agent for ocular disease, but the activity of DEC may be inhibited
by corticosteroids. Accordingly, they should not be co-administered. If the migrat-
ing larva can be visualized, laser photocoagulation is eff ective and will destroy the
organism. Treatment of chronic ocular infection, infection for more than 8 weeks, is
problematic and should be managed by an experienced ophthalmologist.
74
Medical Parasitology
11

Follow-Up aft er Treatment
Within a week of treatment there is usually a rise in eosinophilia accompanied
by improvement in clinical parameters. By 4 weeks post-therapy, eosinophilia has
usually resolved and the antitoxocara IgE has become negative.
Prevention and Control
Exposure to toxocariasis occurs primarily in overcrowded urban areas where
children are in close contact with dogs and cats. Public education and control ef-
forts should be directed at limiting exposure of children to soil contaminated with
Toxocara eggs in public parks, playgrounds, sandboxes, home gardens and other
areas where children congregate. Dogs and cats should be restricted from entering
public areas where children play. Dog owners should clean up aft er their pets have
defecated and have their pets wormed regularly. Children should wash their hands
aft er playing in a park or coming in close contact with dogs, especially puppies and
cats. Children with geographia or pica require medical evaluation.
Suggested Reading
1. Glickman LT, Schnatz PM. Epidemiology and pathogenesis of zoonotic toxoca-
riasis. Epidemiol Rev 1981; 3:230-50.
2. Wolfe A, Wright IP. Human toxocariasis and direct contact with dogs. Vet Rec
2003; 152:419-22.
3. Fan Ck, Liao CW, Kao TC et al. Sero-epidemiology of Toxocara canis infection
among aboriginal schoolchildren in the mountainous areas of northeastern Taiwan.
Ann Trop Med Parasitol 2005; 99:593-600.
4. Coelho LM, Silva MV, Dini CY et al. Human toxocariasis: a seroepidemiological survey
in schoolchildren in Sorocaba, Brazil. Mem Inst Oswaldo Cruz 2004; 99:553-7.
5. Kaplan M, Kalkan A, Hosoglu S et al. Th e frequency of Toxocara infection in
mental retarded children. Mem Inst Oswaldo Cruz 2004; 99:121-5.
6. Huminer D, Symon K, Groskopf I et al. Seroepidemiologic study of toxocariasis
and strongyloidiasis in institutionalized mentally retarded adults. Am J Trop Med
Hyg 1992; 46:278-81.
7. Gillespie SH. Migrating worms. In: Cohen J, Powderly W, eds. Infectious Diseases,
2nd Edition. Oxford: Elsevier Inc., 2004:1633-5. A comprehensive review, includ-
ing the pathogenesis.
8. Beaver PC. Th e nature of visceral larva migrans. J Parasitol 1969; 55:3-12.
9. Buijs J, Borsboom G, van Gemund JJ et al. Toxocara seroprevalence in 5-year-old elemen-
tary schoolchildren: relation with allergic asthma. Am J Epidemiol 1994; 140:839-47.
10. Sharghi N, Schantz PM, Caramico L et al. Environmental exposure to Toxocara as a
possible risk factor for asthma: a clinic-based case-control study. Clin Infect Dis 2001;
32:111-6. Th e association of Toxocara infection with asthma is refuted in this study.
11. Nicoletti A, Bartoloni A, Reggio A et al. Epilepsy, cysticercosis and toxocariasis: a pop-
ulation-based case-control study in rural Bolivia. Neurology 2002; 58:1256-61.
12. Hemang P, Goldstein D. Pediatric uveitis. Pediatr Clin N Am 2003; 50:125-36. A
comprehensive review of pediatric uveitis including toxocariasis and other infec-
tious pathogens.
13. Good B, Holland CV, Taylor MRH et al. Ocular toxocariasis in schoolchildren.
Clin Infect Dis 2004; 39:173-8.
14. Gillespie SH, Bidwell D, Voller A et al. Diagnosis of human toxocariasis by antigen
capture enzyme linked immunoabsorbent assay. J Clin Pathol 1993; 46:551-4.
15. Xinou E, Lefk opoulos A, Gelagoti M et al. CT and MRI imaging fi ndings in
cerebral toxocaral disease. Am J Neuroadiol 2003; 24:714-8.
16. Strucher D, Schubarthi P, Gualzata M et al. Th iabendazole vs. albendazole in treat-
ment of toxocariasis: a clinical trial. Ann Trop Med Parasitol 1989; 83:473-8.
75
Baylisascariasis and Toxocariasis
11

C
HAPTER
12
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Lymphatic Filariasis
Subash Babu and Thomas B. Nutman
Background
Th e term “lymphatic fi lariasis” encompasses infection with three closely related
nematode worms—Wuchereria bancroft i, Brugia malayi and Brugia timori. All
three parasites are transmitted by the bites of infective mosquitoes and have quite
similar life cycles in humans (Fig. 12.1) with the adult worms living in the aff erent
lymphatic vessels while their off spring, the microfi lariae, circulate in the peripheral
blood and are available to infect mosquito vectors when they feed. Th ough not fatal,
the disease is responsible for considerable suff ering, deformity and disability and
is the second leading parasitic cause of disability with DALYs (disability-adjusted
life years) estimated to be 5.549 million.
Lymphatic fi lariasis is a global health problem. At a recent estimate, it has
been determined that over 2 billion people are at risk and at least 129 million
people actually infected. W. bancroft i accounts for nearly 90% of these cases. W.
bancroft i has the widest geographical distribution and is present in Africa, Asia,
the Caribbean, Latin America and many islands of the Western and South Pacifi c
Ocean. B. malayi is geographically more restricted, being found in Southwest
India, China, Indonesia, Malaysia, Korea, the Philippines and Vietnam. B. timori
is found in Timor, Flores, Alor, Roti and Southeast Indonesia.
Humans are the defi nitive host and mosquitoes are the intermediate hosts of
W. bancroft i and Brugia spp. Th e life cycle of fi larial parasites involves four larval
stages and an adult stage. Infection begins with the deposition of infective stage
larvae (L3) on the skin near the site of puncture during a mosquito bite. Th e larvae
then pass through the puncture wound and reach the lymphatic system. Within
the lymphatics and lymph nodes, the L3 larvae undergo molting and development
to form L4 larvae. Th is takes about 7-10 days for both W. bancroft i and B. malayi.
Th e L4 larvae undergo a subsequent molting/developmental step to form adult
worms. Th is occurs about 4-6 weeks aft er L3 entry in the case of B. malayi and
aft er several months in the case of W. bancroft i. Th e adult worms take permanent
residence in aff erent lymphatics or the cortical sinuses of lymph nodes and generate
microscopic live progeny called “microfi lariae”. Th e female worms can give birth
to as many as 50,000 microfi lariae per day, which fi nd their way into the blood
circulation from the lymphatics. Th e adult worms are estimated to survive for a
period of 5-10 years although longer durations have been recorded.
Th e major vectors of W. bancroft i are culicine mosquitoes in most urban and
semi-urban areas, anophelines in the more rural areas of Africa and elsewhere and
Aedes species in many of the endemic Pacifi c islands. For the Brugian parasites,
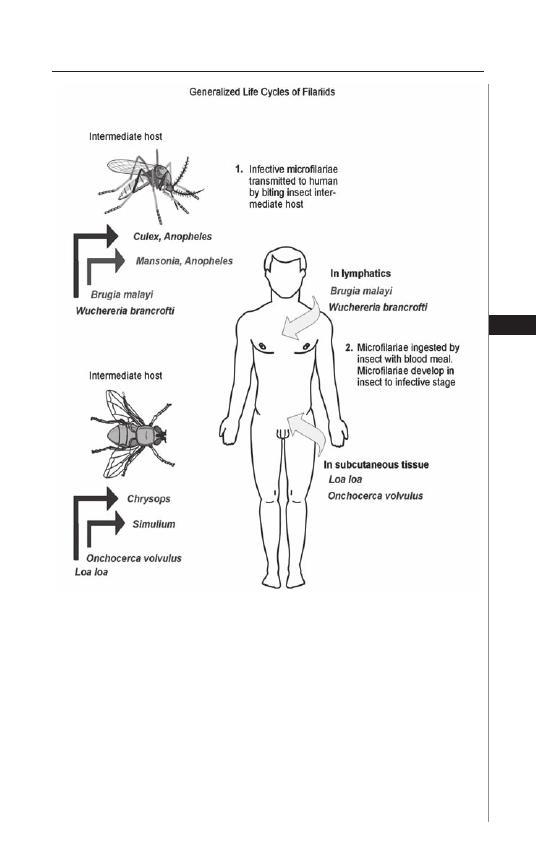
77
Lymphatic Filariasis
12
Mansonia species serve as the main vector, but in some areas anopheline mosqui-
toes can transmit infection as well. Culex quinquefasciatus is the most important
vector of W. bancroft i and is responsible for more than half of all lymphatic
fi larial infections. Th e microfi lariae of W. bancroft i and B. malayi, for the large
part, exhibit a phenomenon called nocturnal periodicity, i.e., they appear in
larger numbers in the peripheral circulation at night and retreat during the day.
Subperiodic or nonperiodic W. bancroft i and B. malayi are also found in certain
parts of the world.
Filarial nematodes belong to the phylum Nematoda, class Secernentea, and
superfamily Filarioidea. Adult W. bancroft i and B. malayi worms are long, slender,
tapered and cylindrical worms. Th e males (4 cm × 0.1 mm for W. bancroft i and
Figure 12.1. Generalized life cycle of fi lariids. Adapted from: Nappi AJ, Vass E,
eds. Parasite of Medical Importance. Austin: Landes Bioscience, 2002:93.
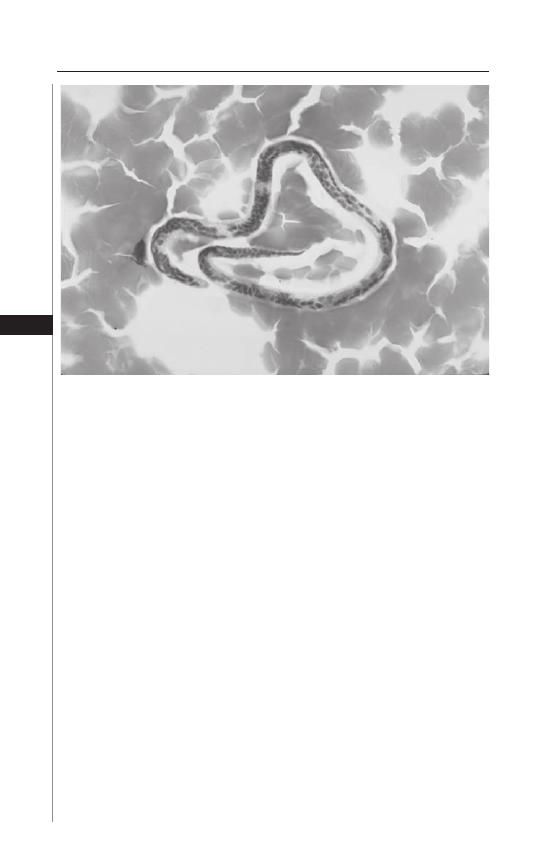
78
Medical Parasitology
12
3.5 cm × 0.1 mm for B. malayi) are strikingly smaller than the females (6-10 cm ×
0.2-0.3 mm for W. bancroft i and 5-6 cm × 0.1 mm for B. malayi). Th e microfi lariae
of W. bancroft i are ensheathed and measure about 245-300
μ
m by 7.5-10
μ
m (Fig.
12.2). Th e microfi lariae of B. malayi are ensheathed and measure 175-230
μ
m by
5-6
μ
m. Most pathogenic human fi larial parasites are infected with a bacterial en-
dosymbiont called Wolbachia. It is an alpha-proteobacteria, related to Rickettsia,
Erlichia and Anaplasma and is maternally inherited. It has been detected in all
life cycle stages of the parasite and found to be essential for adult worm viability,
normal fertility and larval development.
Clinical Manifestations
Lymphatic fi lariasis can manifest itself in a variety of clinical and subclinical
conditions.
Subclinical (or asymptomatic) microfi laremia: In areas endemic for lymphatic
fi lariasis, many individuals exhibit no symptoms of fi larial infection and yet, on
routine blood examinations, demonstrate the presence of signifi cant numbers
of parasites. Th ese individuals are carriers of infection (and the reservoir for
ongoing transmission) and have commonly been referred to as asymptomatic
microfi laremics. Th e parasite burdens in these individuals can reach dramatically
high numbers, exceeding 10,000 microfi lariae in 1 ml of blood. With the advent
of newer imaging techniques, it has become apparent that virtually all persons
with microfi laremia have some degree of subclinical disease. Th ese include pro-
found changes such as marked dilatation and tortuosity of lymph vessels with
collateral channeling and increased fl ow and abnormal patterns of lymph fl ow, a
considerable degree of scrotal lymphangiectasia and microscopic hematuria and/
Figure 12.2. Microfi laria of W.bancrofti in a blood fi lm stained with Giemsa.
The stain reveals the nuclei and the sheath.

79
Lymphatic Filariasis
12
or proteinuria (indicative of low-grade renal damage). Th us, while apparently free
of overt symptomatology, the asymptomatic microfi laremic individuals clearly
are subject to subtle pathological changes.
Acute Clinical Disease
Th e acute manifestations of lymphatic fi lariasis are characterized by recurrent
attacks of fever associated with the infl ammation of lymph nodes (lymphadenitis)
and lymphatics (lymphangitis). In brugian fi lariasis, episodes of fever, lymph-
adenitis and lymphangitis are common, while bancroft ian fi lariasis present more
insidiously with fewer overt acute episodes. Th e lymph nodes commonly involved
are the inguinal, axillary and epitrochlear nodes and, in addition, the lymphatic
system of the male genitals are frequently aff ected in W. bancroft i infection leading
to funiculitis, epididymitis and/or orchitis.
It has been proposed that there are at least two distinct mechanisms involved in
the pathogenesis of acute attacks. Th e more classical is acute fi larial adenolymphan-
gitis, which is felt to refl ect an immune-mediated infl ammatory response to dead
or dying adult worms. Th e striking manifestation is a distinct well-circumscribed
nodule or cord along with lymphadenitis and retrograde lymphangitis. Funiculo-
epididymoorchitis is the usual presenting feature when the attacks involve the
male genitalia. Fever is not usually present, but pain and tenderness at the aff ected
site are common.
Th e other has been termed acute dermatotolymphangitis, a process character-
ized by development of a plaque-like lesion of cutaneous or subcutaneous infl am-
mation and accompanied by ascending lymphangitis and regional lymphadenitis.
Th ere may or may not be edema of the aff ected limbs. Th ese pathological features
are accompanied by systemic signs of infl ammation including fever and chills. Th is
manifestation is thought to result primarily from bacterial and fungal superinfec-
tions of the aff ected limbs.
Manifestations of Chronic Infection
Th e chronic sequelae of fi lariasis are postulated to develop approximately 10 to
15 years aft er initial infection. In Bancroft ian fi lariasis, the main clinical features
are hydrocele, lymphedema, elephantiasis and chyluria. Th e manifestations in
descending order of occurrence are hydrocele and swelling of the testis, followed
by elephantiasis of the entire lower limb, the scrotum, the entire arm, the vulva
and the breast. In Brugian fi lariasis, the leg below the knee and the arm below the
elbow are commonly involved but rarely the genitals. Lymphedema can be classifi ed
or graded, a scheme proven very useful in clinical trials:
Grade 1
Pitting edema reversible on limb elevation.
Grade 2
Pitting/nonpitting edema not reversible on limb elevation and normal skin.
Grade 3
Nonpitting edema of the limb, not reversible on elevation with skin
thickening.

80
Medical Parasitology
12
Grade 4
Nonpitting edema with fi brotic and verrucous skin changes (elephantiasis)
(Fig. 12.3A).
In men, scrotal hydrocele is the most common chronic clinical manifestation
of bancroft ian fi lariasis (Fig. 12.3B). Hydroceles are due to accumulation of
edematous fl uid in the cavity of the tunica vaginalis testis. Chronic epididymitis
and funiculitis can also occur. Chyloceles can also occur. Th e prevalence of chyluria
(excretion of chyle, a milky white fl uid in the urine) is very low.
Tropical Pulmonary Eosinophilia
Tropical pulmonary eosinophilia (TPE) is a distinct syndrome that develops
in some individuals infected with W. bancroft i and B.malayi. Th is syndrome aff ects
males and females at a ratio of 4:1, oft en during the third decade of life. Th e majority
of cases have been reported from India, Pakistan, Sri Lanka, Brazil, Guyana and
Southeast Asia. Th e main clinical features include paroxysmal cough and wheez-
ing that are usually nocturnal (and probably related to the nocturnal periodicity
of microfi lariae), weight loss, low-grade fever, adenopathy and pronounced blood
eosinophilia (>3000 eosinophils/
μ
L). Chest X-rays may be normal but gener-
ally show increased bronchovascular markings; diff use miliary lesions or mottled
opacities may be present in the middle and lower lung fi elds. Tests of pulmonary
function show restrictive abnormalities in most cases and obstructive defects in
half. Total serum IgE levels (10,000 to 100,000 ng/mL) and antifi larial antibody
titers are characteristically elevated.
Other Manifestations
Lymphatic fi lariasis has been associated with a variety of renal abnormalities
including hematuria, proteinuria, nephrotic syndrome and glomerulonephritis.
Circulating immune complexes containing fi larial antigens have been implicated
in the renal damage. Lymphatic fi lariasis may also present as a mono-arthritis of
the knee or ankle joint.
Uninfected, but exposed individuals (asymptomatic amicrofi laremia or endemic
normals): In endemic areas, a proportion of the population remains uninfected
despite exposure the parasite to the same degree as the rest of the population. Th is
group has been termed endemic normal. Th e incidence of endemic normals in a
population ranges from 0% to 50% in diff erent endemic areas.
Diagnosis
Th e traditional method of diagnosing lymphatic fi larial infections has been
the detection of microfi lariae in the peripheral blood collected during the night
in areas of nocturnal periodicity and during the day in areas of subperiodic
lymphatic fi lariae. Th e simplest method is a thick blood fi lm of capillary blood
stained with Giemsa stain, its disadvantage being poor sensitivity. Th e sensitivity
of detection can be augmented by the use of concentration techniques such as the
Knott’s concentration method in which 1 ml of whole blood is added to 9 ml of
a 2% formalin solution, centrifuged and the sediment examined for microfi lariae.
Another widely used concentration technique is the membrane fi ltration technique
whereby 1-5 ml of blood is passed through a 3 or 5
μ
M polycarbonate membrane
which retains the microfi lariae.
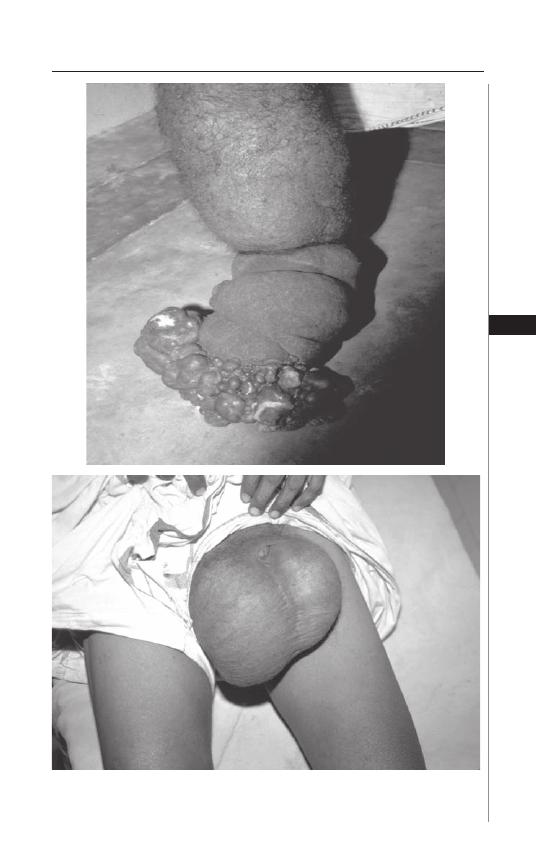
81
Lymphatic Filariasis
12
Figure 12.3. Chronic manifestations of lymphatic fi lariasis. A) Elephantiasis
of the lower limb with verrucous and fi brotic skin changes. B) Hydrocele in
a male patient.
A
B

82
Medical Parasitology
12
For bancroft ian fi lariasis, assays for the detection of circulating parasite anti-
gens have been developed based on one of two well-characterized monoclonal
antibodies, Og4C3 or AD12. Th e commercial Og4C3 ELISA (Trop Bio Og4C3
Antigen test, produced by Trop Bio Pty Ltd) has a sensitivity approaching 100%
and specifi city of 99-100%. Th e ICT fi larial antigen test (Binax) is a rapid format
card test with a sensitivity of 96-100% and specifi city of 95-100%. It utilizes capil-
lary or venous blood and is simple enough for fi eld use.
Antibody-based assays for diagnosing fi larial infection have typically used
crude parasite extract and have suff ered from poor specifi city. Improvements
have been made by the use of detection of antifi larial IgG4 antibodies in that they
are produced in relative abundance during chronic infection. IgG4 antibodies
correlate well with the intensity and duration of fi larial exposure and the level
of microfi laremia; these IgG4 antibodies also have very little cross-reactivity to
nonfi larial helminths. In addition, IgG4 antibodies are also useful in the diagnosis
of Brugian infections; indeed a diagnostic dipstick test has been used in areas
endemic for Brugian fi lariasis based on a recombinant Brugian antigen.
PCR-based methods have been developed for the detection of W. bancroft i
DNA in blood, plasma, paraffi n embedded tissue sections, sputum, urine and in
infected mosquitoes; and for B. malayi DNA in blood and in mosquitoes.
Finally, the examination of scrotum and breast using ultrasonography in con-
junction with pulse wave Doppler techniques can identify motile adult worms
within the lymphatics. Th e adult worms exhibit a characteristic pattern of move-
ment known as the fi larial dance sign and the location of these adult worm nests
remains remarkably stable. On rare occasions, living adult worms reside in the
lymphatics of inguinal crural, axillary and epitrochlear lymph nodes.
Treatment
Diethylcarbamazine (DEC, 6 mg/kg in three divided doses daily for 12 days),
which has both macro- and microfi laricidal properties, remains the treatment
of choice for the individual with active lymphatic fi lariasis (microfi laremia, an-
tigen positivity, or adult worms on ultrasound), although albendazole (400 mg
twice daily for 21 days) and ivermectin (400
μ
g/kg) also have activity against
microfi lariae.
In persons with chronic manifestations of lymphatic fi lariasis, treatment regi-
mens that emphasize hygiene, prevention of secondary bacterial infections and
physiotherapy have gained wide acceptance for morbidity control. Hydroceles can
be drained repeatedly or managed surgically. In patients with chronic manifesta-
tions of lymphatic fi lariasis, drug treatment should be reserved for individuals with
evidence of active infection as therapy has been associated with clinical improve-
ment and, in some, reversal of lymphedema.
Th e recommended course of DEC treatment (12 days; total dose, 72 mg/kg)
has remained standard for many years; however, data indicate that single-dose
DEC treatment with 6 mg/kg may be equally effi cacious. Th e 12-day course
provides more rapid short-term microfi larial suppression. Regimens that utilize
single-dose DEC or ivermectin or combinations of single doses of albendazole
and either DEC or ivermectin have all been demonstrated to have a sustained
microfi laricidal eff ect.

83
Lymphatic Filariasis
12
In the past few years, the use of antibiotics such as doxycycline, which have an
eff ect on Wolbachia, to treat lymphatic fi lariasis has been investigated. Preliminary
data suggest that 200 mg of doxycycline for 8 weeks is eff ective in eliminating
microfi laremia.
Prevention and Control
DEC has the ability of killing developing forms of fi larial parasites and has been
shown to be useful as a prophylactic agent in humans. Like those integrated control
programs used for onchocerciasis, long term microfi larial suppression using mass,
annual distribution of single dose combinations of albendazole with either DEC
or ivermectin is underway in many parts of the world. Th ese strategies have as their
basis the microfi larial suppression of >1 year using single dose combinations of
albendazole/ivermectin or albendazole/DEC. An added benefi t of these combina-
tions is their secondary salutary eff ects on gastrointestinal helminth infections.
Vector control measures such as the use of insecticides, the use of polystyrene
beads in infested pits, the use of Bacillus sphaericus as a larvicide, the use of larvi-
vorous fi sh and the use of insecticide treated bednets have all been advocated as
adjunct measure for control of fi lariasis.
Lymphatic filariasis is one of six potentially eradicable diseases (WHO
International Task Force 1993) and the development of a global program to
eliminate fi lariasis (GPELF) came about following a resolution by the WHO
Assembly in 1997. Th e principal aims of the program are to interrupt transmis-
sion of infection and to alleviate and/or prevent disability. Th e recent advances
in immunology, molecular biology and imaging technology have helped pave the
way for the realization of the goal to eliminate of lymphatic fi lariasis.
Suggested Reading
1. Adebajo AO. Rheumatic manifestations of tropical diseases. Curr Opin Rheumatol
1996; 8:85-9.
2. Amaral F, Dreyer G, Figueredo-Silva J et al. Live adult worms detected by
ultrasonography in human Bancroft ian fi lariasis. Am J Trop Med Hyg 1994;
50:753-757.
3. Dreyer G, Amaral F, Noroes J et al. Ultrasonographic evidence for stability of
adult worm location in bancroft ian fi lariasis. Trans R Soc Trop Med Hyg 1994;
88:558.
4. Dreyer G, Medeiros Z, Netto MJ et al. Acute attacks in the extremities of persons
living in an area endemic for bancroft ian fi lariasis: diff erentiation of two syndromes.
Trans R Soc Trop Med Hyg 1999a; 93:413-417.
5. Dreyer G, Santos A, Noroes J et al. Proposed panel of diagnostic criteria, includ-
ing the use of ultrasound, to refi ne the concept of ‘endemic normals’ in lymphatic
fi lariasis. Trop Med Int Health 1999b; 4:575-579.
6. Eberhard ML, Lammie PJ. Laboratory diagnosis of fi lariasis. Clin Lab Med 1991;
11:977-1010.
7. Horton J, Witt C, Ottesen EA et al. An analysis of the safety of the single dose, two
drug regimens used in programmes to eliminate lymphatic fi lariasis. Parasitology
2000; 121 Suppl:S147-160.
8. Kazura J, Greenberg J, Perry R et al. Comparison of single-dose diethylcarbamazine
and ivermectin for treatment of bancroft ian fi lariasis in Papua New Guinea. Am
J Trop Med Hyg 1993; 49:804-811.

84
Medical Parasitology
12
9. Kimura E, Spears GF, Singh KI et al. Long-term effi cacy of single-dose mass treat-
ment with diethylcarbamazine citrate against diurnally subperiodic Wuchereria
bancroft i: eight years’ experience in Samoa. Bull World Health Organ 1992;
70:769-776.
10. Lagraulet J. Current status of fi lariasis in the Marquises and diff erent epidemiologi-
cal aspects. Bull Soc Pathol Exot Filiales 1973; 66:311-320.
11. Maxwell CA, Mohammed K, Kisumku U et al. Can vector control play a useful
supplementary role against bancroft ian fi lariasis? Bull World Health Organ 1999;
77:138-143.
12. Melrose WD. Lymphatic fi lariasis: new insights into an old disease. Int J Parasitol
2002; 32:947-960.
13. Michael E, Bundy DA, Grenfell BT. Re-assessing the global prevalence and distri-
bution of lymphatic fi lariasis. Parasitology 1996; 112 (Pt 4):409-428.
14. Molyneux DH, Neira M, Liese B et al. Lymphatic fi lariasis: setting the scene for
elimination. Trans R Soc Trop Med Hyg 2000; 94:589-591.
15. More SJ, Copeman DB. A highly specifi c and sensitive monoclonal antibody-based
ELISA for the detection of circulating antigen in bancroft ian fi lariasis. Trop Med
Parasitol 1990; 41:403-406.
16. Ong RK, Doyle RL. Tropical pulmonary eosinophilia. Chest 1998; 113:1673-1679.
17. Ottesen EA, Nutman TB. Tropical pulmonary eosinophilia. Annu Rev Med 1992;
43:417-424.
18. Ottesen EA. Th e global programme to eliminate lymphatic fi lariasis. Trop Med
Int Health 2000; 5:591-594.
19. Pani SP, Krishnamoorthy K, Rao AS et al. Clinical manifestations in malayan
fi lariasis infection with special reference to lymphoedema grading. Indian J Med
Res 1990; 91:200-207.
20. Pani SP, Srividya A. Clinical manifestations of bancroft ian fi lariasis with special
reference to lymphoedema grading. Indian J Med Res 1995; 102:114-118.
21. Partono F. Th e spectrum of disease in lymphatic fi lariasis. Ciba Found Symp 1987;
127:15-31.
22. Partono F, Dennis DT, Atmosoedjono S et al. Th e microfi laria of Brugia timori:
morphologic description with comparison to Brugia malayi of Indonesia. J Parasitol
1977; 63:540-546.
23. Rahmah N, Taniawati S, Shenoy RK et al. Specifi city and sensitivity of a rapid
dipstick test (Brugia Rapid) in the detection of Brugia malayi infection. Trans R
Soc Trop Med Hyg 2001; 95:601-604.
24. Taylor MJ, Hoerauf A. Wolbachia bacteria of fi larial nematodes. Parasitol Today
1999; 11:437-442.
25. Taylor MJ, Makunde WH, McGarry HF et al. Macrofi laricidal activity aft er
doxycycline treatment of Wuchereria bancroft i: a double-blind, randomised
placebo-controlled trial. Lancet 2005; 365:2067-2068.
26. Vanamail P, Subramaniam S, Das PK et al. Estimation of age-specifi c rates of ac-
quisition and loss of Wuchereria bancroft i infection. Trans R Soc Trop Med Hyg
1989; 83:689-693.
27. Weil GJ, Jain DC, Santhanam S et al. A monoclonal antibody-based enzyme im-
munoassay for detecting parasite antigenemia in bancroft ian fi lariasis. J Infect Dis
1987; 156:350-355.
28. WHO. Lymphatic fi lariasis. World Health Organ Tech Rep Ser 1992; 821:1-71.
29. WHO. Lymphatic fi lariasis. Weekly Epidemiological Record 2003; 78:171-179.

S
ECTION
II
Trematodes

C
HAPTER
13
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Clonorchiasis and Opisthorchiasis
John Cmar
Background
Causative Agents
The class Trematoda contains 13 species of parasitic flatworms that cause
biliary tract disease in humans. Residing in the family Opisthorchiidae, the
three most common are Clonorchis (formerly Opisthorchis) sinensis, Opisthorchis
felineus and Opisthorchis viverrini. Adults of these species are dorso-ventrally
flattened lancet-shaped hermaphroditic worms and typically reach 10-25 mm
in length and 3-5 mm in width (Fig. 13.1). While similar in appearance, these
species can be distinguished morphologically by the shape and appearance of
the testes, as well as the arrangement of the vitelline glands. The eggs are 30
μ
m by 15
μ
m in size, ovoid and yellowish brown in color, with a well-developed
operculum. In contrast to the adult forms, it is difficult to distinguish these
species from each other on the basis of egg morphology.
Humans are among the piscivorous mammals that are the definitive hosts
in whom the C. sinensis and Opisthorchis species undergo sexual reproduction.
The flukes reproduce asexually in several species of snails, which are the first
intermediate hosts. Various freshwater fish and crustaceans serve as the sec-
ond intermediate hosts. The geographic distribution of human clonorchiasis
is related to both the areas of population of the aforementioned snails and
marine animals, as well as the local culinary and hygiene habits concerning
said animals.
Geographical Distribution/Epidemiology
Th e 1994 report of the World Health Organization and the International
Agency for Research on Cancer estimated the global number of C. sinensis infec-
tions to be 7 million; however, a series of more recent studies places the number
at closer to 35 million, with over 15 million of those infected living in China.
In addition, Japan, Taiwan, Hong Kong, Korea and Vietnam are also primary
endemic countries for this infection. Prevalence rates within endemic regions
can vary widely with local culinary customs and sanitation; various Chinese
provinces have described prevalence ranging from <1 to 57 percent.
Th e two Opisthorchis species under consideration have diff erent geographic
distributions. O. felineus is endemic to Southeast Asia and Central and Eastern

87
Clonorchiasis and Opisthorchiasis
13
Europe, especially in Siberia and other former territories of the Soviet Union. Over
16 million people worldwide are thought to be infected, with prevalence rates of 40
to 95%. O. viverrini occurs primarily in Th ailand, Laos and Kampuchea. Estimates
of infection are 10 million people worldwide, with 24 to 90 percent prevalence in
Th ailand and 40 to 80 percent in Laos.
While these organisms may not have a major impact in non-endemic countries,
clonorchiasis and opisthorchiasis can still occur there by one of several means.
Travelers to an endemic area may return infected, acquiring the parasite from eat-
ing improperly prepared freshwater fi sh, or via the fecal-oral route in areas of poor
sanitation. Immigrants from endemic countries to non-endemic countries can bring
the disease with them; early studies of clinical clonorchiasis in Asian immigrants
to North America described prevalence rates of up to 28%. Finally, freshwater
fi sh and shrimp that are improperly pickled, dried, or salted for importation by
non-endemic countries can harbor living encysted organisms and bring disease to
those who have never visited an endemic region.
Th e adult fl ukes of C. sinensis and Opisthorchis species have a lifespan of up
to 30 years in the bile ducts of humans, during which time immunity does not
Figure 13.1. Adult Clonorchis sinensis, a hepatic fl uke. Reproduced from:
Nappi AJ, Vass E, eds. Parasites of Medical Importance. Austin: Landes
Bioscience, 2002:44.
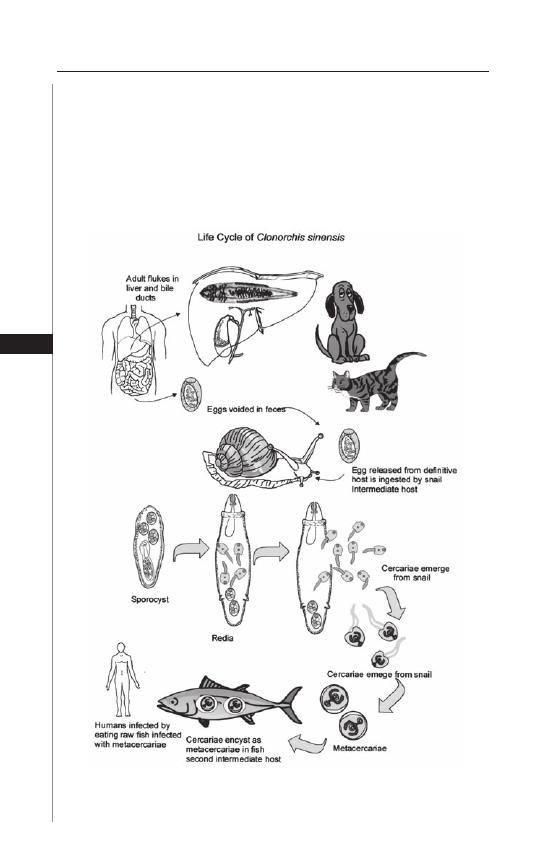
88
Medical Parasitology
13
develop. As such, cumulative infections can occur, resulting in increased intensity
of infection and greater worm burden. Th us, symptomatology is most common in
older adults who may be far removed from their initial exposure.
Mode of Transmission
Clonorchiasis and opisthorchiasis are perpetuated in the following cycle
(Fig. 13.2): Adult fl ukes, residing in the biliary tracts of a defi nitive host, produce
eggs, which pass with bile into the feces and are ultimately expelled into a fresh-
water environment. Th e eggs hatch into miracidia and infect snails of the Bithynia
Figure 13.2. Life cycle of Clonorchis sinensis. Reproduced from: Nappi AJ,
Vass E, eds. Parasites of Medical Importance. Austin: Landes Bioscience,
2002:45.

89
Clonorchiasis and Opisthorchiasis
13
and Parafossarulus geni, which serve as the fi rst intermediate hosts. Th e miracidia
multiply in the snail and develop into cercariae.
Aft er 4-6 weeks of gestation, the cercariae are expelled back into the fresh-
water environment, where they infect a second intermediate host. Th ese are
typically fi sh of the family Cyprinidae, but at least 113 species of freshwater
fi sh from 13 families have been identifi ed. Th e cercariae penetrate under the
scales of the second intermediate host, where they then encyst in the muscle
and form metacercariae. In this stage, the parasite lies dormant until ingested
by the defi nitive host.
In addition to humans, naturally occurring defi nitive hosts include dogs, pigs,
cats (both domestic and wild), martens, badgers, minks, weasels and rats. Once
ingested, the metacercariae excyst in the duodenum or jejunum and migrate
through the ampulla of Vater to adhere to the common bile duct. Following
the epithelial lining of the biliary tree, they proceed into the intrahepatic ducts,
typically the smaller branches in the left lobe. Other less common sites of
residence include the gallbladder, pancreatic duct and, very rarely, the stomach.
Once their fi nal destination is reached, they mature into egg-producing adults
worms within 4 weeks.
Disease Signs and Symptoms
Symptomatology of active clonorchiasis and opisthorchiasis, whether in an
acute or chronic stage of infection, is dependent on the burden of adult worms in
the biliary tree. So-called “light” infections, classifi ed by either <10,000 eggs per
gram of stool, or <100 adult worms, rarely cause symptoms. “Heavy” infections,
which are more likely to cause symptoms, occur in only 10% of cases.
While O. felineus is the most likely of the three species under discussion to
result in symptomatic acute infection, symptomatology in the acute phase is un-
common. Th e onset is usually 1-3 weeks aft er the ingestion of metacercariae and
can persist for 2-4 weeks prior to resolution. Symptoms include fever, malaise,
arthralgia/myalgia and anorexia, as well as abdominal pain and urticaria. Clinical
signs are usually limited to tender hepatomegaly and lymphadenopathy. At this
stage, peripheral eosinophilia is common and as symptoms resolve, eggs become
detectible in the stool.
Aft er infection has become established, further symptoms and complications
can develop by a number of mechanisms. Mechanical obstruction of the biliary
tract by adult worms results in intermittent but recurring symptoms, especially in
those with “heavy” infection, including anorexia, weight loss, fatigue, abdominal
pain, diarrhea and dyspepsia. Additionally, persistent irritation and damage to the
biliary epithelium results in both intimal desquamation and proliferation, which
eventually results in fi brotic, as well as hyperplastic and dysplastic, alterations in
architecture. Immune-mediated tissue damage occurs locally, characterized by
the periductal infi ltration of lymphocytes and eosinophils. Ductal dilatation and
stricture formation, pigment stone generation and hepatocellular fi brosis develop
and the gallbladder becomes distended with stones. Th e ultimate serious sequelae
of this include recurrent cholangitis, cholangiohepatitis and pancreatitis.
Th e most dire complication of clonorchiasis and opisthorchiasis is cholangiocarcino-
ma. While the specifi c carcinogenic mechanism is uncertain, postulated means include

90
Medical Parasitology
13
both intrinsic nitrosation and nitric oxide formation, as well as enzymatic activation.
Weight loss and epigastric pain are the primary complaints, with ascities, jaundice and
a palpable abdominal mass notable on exam. Overall survival is 6.5 months.
Diagnosis
Laboratory Tests, Microscopy
Classically, the diagnosis of clonorchiasis and opisthorchiasis is made by
demonstrating eggs in the stool of infected hosts. Th ey are typically not present
until 4 weeks aft er establishment of infections, and in light infections may require
specimen concentration to be detected. As the eggs of C. sinensis are diffi cult
to distinguish from Opisthorchis spp., diff erentiation of species usually requires
examination of expired adult fl ukes following therapy.
Biliary or duodenal fl uid, sampled by endoscopic retrograde cholangiopancre-
atography (ERCP) or needle aspiration, can also be found to demonstrate eggs
or adult worms.
Other Tests
Serum IgE levels may be elevated in liver fl uke infection. Peripheral eosinophilia
can be observed, but does not typically exceed 10-20% of the total WBC count.
Alkaline phosphatase levels can be elevated in advanced liver disease, but other liver
associated enzymes, such as the transaminases, are usually within normal limits.
Radiologic tests demonstrate the hepatic and biliary sequelae of chronic infection.
Asymptomatic disease is associated with no radiologic abnormalities. Ultrasound can
demonstrate nonshadowing echogenic foci within bile ducts that represent fl uke ag-
gregates. Other nonspecifi c changes by ultrasound can include hepatomegaly, ductal
fi brosis and infl ammation, as well as gallbladder irregularities and sludge. However,
such changes are only seen in 50% of patients with active chronic disease. CT scanning
is reportedly more sensitive, especially for detecting relapsing cholangitis.
Cholangiography can show multiple fi ndings, depending on the stage of infec-
tion and worm burden. Multiple saccular or cystic dilatations of the intrahepatic
bile ducts can appear as a “mulberry sign.” Th e “arrow-head sign” is the rapid
tapering of the intrahepatic bile ducts to the periphery. Portal and periportal
fi brosis can result in a decrease in the number of intrahepatic radicles visualized.
Individual adult worms can be seen as fi lamentous, wavy and elliptical fi lling de-
fects. Duct wall irregularities, varying from small indentations—which, in series,
can give rise to a “scalloped” appearance—to hemispherical fi lling defects.
Molecular Diagnosis
Th ere are no widely available ELISA tests for liver fl ukes currently, although several
are under investigation. Concerns surrounding one ELISA for O. viverrini include the
inability of a positive result to distinguish between current and prior infection, as
well as possible cross-reactivity with other parasitic infections. Another monoclonal
antibody-based dot-ELISA has been demonstrated to have 100% sensitivity and
specifi city for O. viverrini, using purifi ed antigen from that organism. An immu-
noblot assay for C. sinensis-specifi c excretory-secretory antigens has been shown
to have 92% sensitivity in active infection.

91
Clonorchiasis and Opisthorchiasis
13
PCR is also being investigated as a diagnostic modality for O. viverrini in stool.
It has been described to have a sensitivity correlated with the amount of egg burden
present, 100% for >1000 eggs per gram to 50% for <200 eggs per gram.
Treatment
Th e treatment of choice is praziquantel, 75 mg/kg divided into three doses
given for 2 days. Th is has been demonstrated to have a nearly 100% cure rate,
except for very heavy infections, for which 2 days of therapy can achieve similar
success. Typically, eggs will disappear from the stool within 1 week aft er treatment,
although clinical symptoms may take months to resolve due to residual damage to
the biliary tract from the adult worms.
Side eff ects of treatment can be signifi cant and include nausea, vomiting, head-
ache, dizziness and insomnia. In an attempt to reduce the severity of adverse reactions,
a lower dose regimen of 25 mg/kg/d for 3 days was investigated, but was found to
have only an eradication rate of 29%. Similarly, single-dose praziquantel at 40 mg is
oft en used in the setting of mass therapy for whole communities, both for convenience
and for reduced side eff ects, but has only a 25% eradication rate.
Albendazole is an alternative to praziquantel therapy and is typically given 10
mg/kg/d for 7 days. It has been described as having eradication rates from 90-100%.
While it does have a less severe side eff ect profi le, its signifi cantly longer therapy
course and higher pill burden can make it less appealing.
With treatment, prognosis is good in light infections. Heavy infections, especially
those that are long-standing, can occasionally end in death due to complications.
Surgery is reserved for severe sequelae of clonorchiasis, such as emergent cholecystec-
tomy with cholangitis, or palliative choledochojejunostomy in the setting of obstruc-
tive jaundice. Urgent biliary decompression may be required for acute cholangitis.
When cholangiocarcinoma is suspected, endoscopic biopsies can be taken. Secondary
bacterial infections can occur and should be managed with appropriate antibiotics.
Prevention and Prophylaxis
Proper preparation of freshwater fi sh, such as cooking or freezing, destroys
the metacercariae and prevents infection. As fecal-oral inoculation can occur,
transmission can also be reduced by strict hygiene measures. Th ere is no role for
chemoprophylaxis.
Suggested Reading
1. Kaewkes S. Taxonomy and biology of liver fl ukes. Acta Tropica 2003; 88:177-86.
2. Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Dis 2005;
11:1507-14.
3. Leder K, Weller P. Liver fl ukes: Clonorchiasis and opisthorchiasis. UpToDate.com.
4. Lun ZR, Gasser RB, Lai DH et al. Clonorchiasis: A key foodborn zoonosis in
China. Lancet Infect Dis 2005; 5:31-41.
5. Mairiang E, Mairiang P. Clinical manifestation of opisthorchiasis and treatment.
Acta Tropica 2003; 88:221-7.
6. Reddy DN, Kumar YR. Endoscopic diagnosis and management of biliary parasitosis.
UpToDate.com.
7. Rim HJ. Clonorchiasis: An update. J Helminthol 2005; 79:269-81.
8. Upatham ES, Viyanant V. Opisthoschis viverrini and opisthorchiasis: A historical
review and future perspective. Acta Tropica 2003; 88:171-6.

C
HAPTER
14
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Liver Fluke: Fasciola hepatica
Michelle Paulson
Background
Geographical Distribution/Epidemiology
Fascioliasis, also known as sheep liver fl uke infection, was once primarily
thought of as a veterinary problem. Now it is emerging as a signifi cant human
parasitic disease throughout the world. It has been estimated that up to 17 mil-
lion people are infected and that another 180 million are at risk. Th e pathogen
is widespread, causing infections in Europe, Central and South America, Mexico,
the Middle East and Asia. Areas with particularly high prevalence include the
Altiplano region of Bolivia, the Mantaro Valley in Peru, the Abis area along the
Nile River basis in Egypt and the Gilan province in Iran. Other rural areas may
also have a high disease burden, but do not have the means to diagnose or report
it. Contrary to expectation, human disease does not correlate to the areas where
the most livestock are infected. Rather, it more closely correlates with the popula-
tion of the intermediate host, the Lymnaeidae family of snails. In France, disease
has primarily been associated with consumption of contaminated watercress. Th e
United Kingdom has reported fascioliasis from vegetables. It has also been de-
scribed within the United States, although the predominant threat is to livestock,
rather than humans. Because of increasing commerce between countries, the risk
of limited outbreaks within the United States exists.
In endemic areas children are most commonly aff ected. In one study of Peruvian
children, disease was associated with consumption of alfalfa juice. It is also postu-
lated that children are left to tend to animal herds, thereby increasing their length of
exposure to infectious sources. Oft en a history of eating potentially contaminated
vegetables cannot be elicited, suggesting that water contamination is likely.
Causative Agents
Fascioliasis is caused by a leaf-shaped trematode, Fasciola hepatica. Th e adult
is brown and fl at with average size of 2.5 × 1 cm. Th e eggs are yellow-brown, oval
and measure 140 × 75
μ
m.
While termed the sheep liver fl uke, it typically infects sheep, goats and cattle.
In Bolivia, pigs and donkeys are also effi cient reservoirs. In Corsica, the fl ukes
have been isolated from black rats. Snails from the Lymnaeidae family are found
in many diff erent geographical areas, demonstrating an ability to adapt to a broad
spectrum of environments, from the Andes Mountains to the French countryside.
Humans obtain the disease by eating contaminated water plants, in particular

93
Liver Fluke: Fasciola hepatica
14
watercress (Nasturtium offi cinale), or by ingestion of contaminated water or foods
prepared in that water.
Mode of Transmission
Th e human cycle begins when encysted metacercariae adhere to water plants,
resulting in infection if the vegetation is not washed carefully prior to consump-
tion. Once inside the small bowel, the metacercariae excyst and the larvae emerge.
Th ey travel through the lumen of the bowel, penetrate into the peritoneum, and
invade the liver capsule. At this point, the larvae mature into adults, which gradually
migrate into the biliary tract. Th e hermaphroditic fl ukes dwell in the biliary tree
and produce eggs that may be passed in the stool. Th e eggs hatch in fresh water
and the new miracidia infect snails, in particular those of the family Lymnaeidae,
which are the intermediate host. Th e miracidia mature within the snails to form
cercariae. Th ey are released from snails in water, where they can attach to water-
borne plants such as watercress. Th e cycle begins again when the contaminated
vegetation or water are consumed.
Disease Signs and Symptoms
Fascioliasis occurs in two stages, which diff er in symptoms based on the migra-
tion of F. hepatica through various organs.
Th e fi rst stage, or prepatent or larval period, is marked clinically by abdominal
pain, fever, weight loss and urticaria. Eosinophilia and elevations in liver trans-
aminase enzymes may occur. A small portion of patients also experience cough
and chest discomfort. Th is stage corresponds to the ingestion of the organism
and penetration through the intestinal wall to the liver. Th e fi rst stage can last for
several months. Egg production during this time is minimal.
Th e second stage, referred to as the patent or biliary period, represents the
maturation of larvae into adult fl ukes that pass into the biliary ducts. Symptoms
during this phase are oft en subtle, vague and even asymptomatic. Patients may
develop intermittent right upper quadrant pain, which can mimic cholecystitis.
Eosinophilia may still be present. During this phase, ova are released and may be
found on careful, repeated stool examination. See Figure 14.1.
Complications from chronic disease include anemia, cholangitis and biliary
obstruction. Subcapsular liver hematomas and hemoperitoneum are also reported.
Cases of invasion into inguinal lymph nodes have been described. Other ectopic
sites include subcutaneous skin, brain and eyes. Th ere is no known potential for
malignancy of the biliary tract associated with chronic infection.
Diagnosis
Fascioliasis should be suspected in patients with abdominal pain and fever,
in the setting of abnormal biliary tract fi ndings on radiological studies. In the
acute phase, eosinophilia provides a clue. Later in the disease, symptoms may
only occur intermittently and are oft en vague, making the diagnosis more dif-
fi cult. Abnormal liver function tests and elevated total bilirubin level may or
may not be present. Coprologic analysis is limited, especially in the early stages
of the disease, but is very specifi c when eggs are found. Even in later stages,
multiple stool examinations may be necessary to increase sensitivity. Indirect
hemagglutination tests were previously used to determine titers. Th e tests most
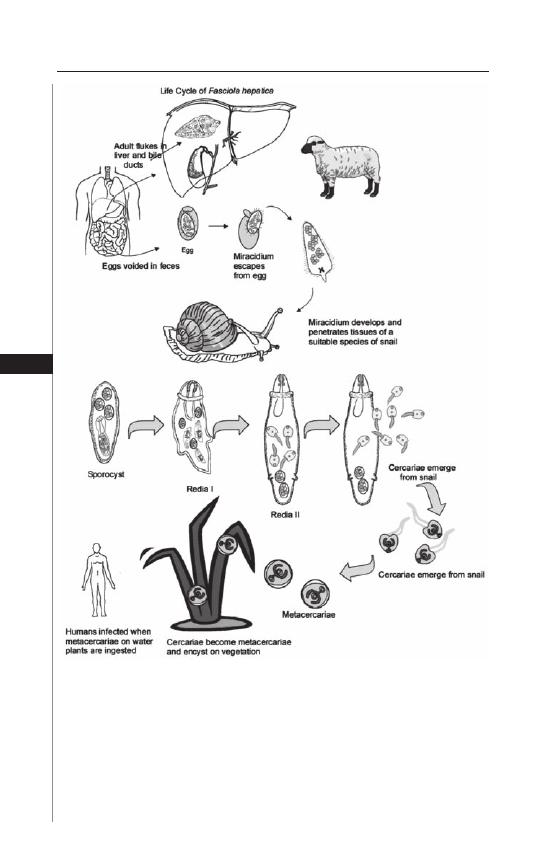
94
Medical Parasitology
14
commonly used to confi rm the diagnosis are ELISAs, which detect various F.
hepatica antigens. Earlier developed ELISAs utilized excretory-secretory antigens
from adult organisms or coproantigens. One study described a sensitivity of 100%
for detection of acute infection through use of excretory-secretory antigens.
In chronic infections, however, the sensitivity was only 70%. Th e antigen tests
reverted to normal generally within six months. Th e greatest drawback to these
Figure 14.1. Life cycle of Fasciola hepatica. Reproduced from: Nappi AJ, Vass E,
eds. Parasites of Medical Importance. Austin: Landes Bioscience, 2002:43.

95
Liver Fluke: Fasciola hepatica
14
tests is cross-reactivity with other parasitic infections. More recently the cathepsin
L1 (CL1) proteinase was found to react with serum IgG4 and was incorporated
into an ELISA. Th is improved detection when compared to coprologic testing
alone. Another ELISA under development uses the purifi ed protein Fas2, which
is an adult F. hepatica cysteine proteinase.
Radiological studies are useful in aiding diagnosis. Computerized tomography
and ultrasound can assist in assessing biliary invasion. Common fi ndings include
thickening of the bile duct wall and biliary dilation. On ultrasound, mobile fl ukes
may be demonstrated, but the more characteristic lesion is a crescent-shape in the
biliary tract. A negative ultrasound examination does not exclude the possibility of
infection. By CT, lesions are characterized as small, multiple hypodensities less than
10 mm in size. Th ey can project in a tunneled, branching pattern. Larger abscesses
have also been reported. Endoscopic retrograde cholangiography is not necessary
for diagnosis in most cases but can be used to extract fl ukes from the bile ducts when
they cause signifi cant obstruction. Liver biopsy is not routinely indicated but may
show eosinophils, histiocytes, granulomas and in some cases, even eggs.
Treatment
Triclabendazole, a benzimidazole, is the fi rst line treatment for F. hepatica. It
has an active sulfoxide metabolite with can inhibit fl uke microtubules and protein
synthesis. Th erefore, it is a good drug to target both immature and mature forms
of the trematode. Its primary use stems from treatment of fascioliasis in sheep
and cattle beginning in 1983. It subsequently was used for human disease in 1989
during an epidemic in Iran. In 1997 it was offi cially listed on the World Health
Organization’s list of essential drugs. Currently, triclabendazole is the drug of
choice selected by the Center for Disease Control and Prevention. Th e recom-
mended dose is 10 mg/kg as a single dose. For more severe disease, an additional
dose taken 12 hours later may be necessary. One study of Cuban patients who had
previously been treated with anthelminthic medications showed that two doses
of 10 mg/kg taken over 1 day eliminated egg excretion in 71 of 77 patients. Th e
main side eff ect noted was abdominal discomfort, which was attributed to the
expulsion of the organism through the bile ducts. Th is occurred 2-7 days aft er
taking triclabendazole. Other reported side eff ects include dizziness, headache
and fever. In another study, 134 Egyptian patients with disease, as established by
egg count, were treated with triclabendazole. An initial dose provided cure in
86.6% of those infected and a subsequent dose in those who remained positive
increased the number cured to 93.9%.
Nitazoxanide, an inhibitor of pyruvate:ferredoxin oxidoreductase enzyme,
has also shown to be eff ective in small trials and case reports. One randomized,
placebo controlled study demonstrated successful parasite elimination in 18 of 30
adults (60%) and 14 of 35 children (40%), whereas only 1 of 8 adults and 0 of 8
children were cured with placebo alone. Th e adult dose used in this trial was 500
mg taken twice a day with meals for 7 days. Most side eff ects reported were mild
and included abdominal pain, diarrhea and headaches.
Bithionol, at a dose of 30-50 mg/kg on alternate days for 10-15 doses is an
alternative choice, but carries signifi cant side eff ects such as nausea, vomiting,
abdominal pain and urticaria.

96
Medical Parasitology
14
Praziquantel, which is used in other fl uke infections, is not eff ective in fascio-
liasis. Other medications used to treat parasitic infections such as metronidazole
and albendazole are also not effi cacious.
Larger therapy trials need to be undertaken to better establish drug effi cacy of
current regimens, while development of new antifasciola medications continues.
Treatment success can be based on several endpoints: lack of further detection
of fecal eggs, antigen reversion to negative, absence of radiological evidence of
persistent infection and resolution of symptoms. ELISA testing may take up to a
year or more to normalize.
Prevention and Prophylaxis
Early detection is one strategy to prevent chronic fascioliasis. Despite its world-
wide prevalence, it is oft en goes unsuspected in nonendemic areas. It is therefore
important to develop a simple, cost eff ective method for rapid diagnosis. In endemic
regions public health measures have been instituted to raise awareness of symptoms
that can aid in earlier detection. Health care workers themselves need to be bet-
ter trained to recognize disease. Other measures have attempted to better defi ne
the population at risk and not limit disease potential to only persons who report
consumption of watercress; frequently this history cannot be elicited.
Another approach to disease prevention focuses on improving sanitation.
Improvement of inspection and transport of vegetation can further help to reduce
risk. Educating the public on the proper ways of cooking and cleaning vegetation
also decreases disease. Better sanitation, in particular decreasing outdoor defecation
and thus shedding of viable eggs, will also be key in limiting fascioliasis.
Governments need to establish guidelines with the assistance of medical
specialists for routine treatment of livestock. Safe means of controlling snail
populations also need to be explored. Th e Egyptian Ministry of Health and
Population launched a program targeting school children from high prevalence
areas, screening 36,000 children and subsequently treating 1280. Th e prevalence
of the disease then fell from 5.6 to 1.2%. Similar programs need to be explored
further to evaluate their feasibility in other endemic regions.
Suggested Reading
1. Arjona R, Riancho JA, Aguado JM et al. Fascioliasis in developed countries: a
review of classic and aberrant forms of the disease. Medicine 1995; 74:13-23.
2. Curtale F, Hassanein Y, Savioli L. Control of human fascioliasis by selective chemo-
therapy: design, cost and eff ect of the fi rst public health, school-based intervention
implemented in endemic areas of the Nile Delta, Egypt. Trans Royal Soc of Trop
Med Hyg 2005; 99:599-609.
3. El-Morshedy H, Farghaly A, Sharaf A et al. Triclabendazole in the treatment
of human fascioliasis: a community-based study. East Mediterr Health J 1999;
5:888-94.
4. Espino AM, Diaz A, Perez A et al. Dynamics of antigenemia and coproantigens
during a human Fasciola hepatica outbreak. J Clin Mic 1998; 36:2723-6.
5. Espinoza JR, Timoteo O, Herrera-Velit P. Fas-2 ELISA in the detection of human
infection by Fasciola hepatica. J Helminth 2005; 79:235-40.
6. Farag HF. Human fascioliasis in some countries of the Eastern Mediterranean
Region. East Mediterr Health J 1998; 4:156-60.

97
Liver Fluke: Fasciola hepatica
14
7. Favennec L, Jave Ortiz J, Gargala G et al. Double-blind, randomized, placebo-con-
trolled study of nitazoxanide in the treatment of fascioliasis in adults and children
from northern Peru. Aliment Pharmacol Th er 2003; 17:265-70.
8. Graham CS, Brodie SB, Weller PF. Imported Fasciola hepatica infection in the
United States and treatment with triclabendazole. Clin Inf Dis 2001; 33:1-6.
9. Harris NL, McNeely WF, Shepard JO et al. Case 12-2002, Weekly clinicopatho-
logical exercises. NEJM 2002; 346:1232-9.
10. Keiser J, Utzinger J. Emerging foodborne trematodiasis. EID 2005; 10:1507-14.
11. Mas-Coma MS, Esteban JG, Bargues MD. Epidemiology of human fascioliasis: a
review and proposed new classifi cation. Bull WHO 1999; 77:340-6.
12. Millán JC, Mull R, Freise S et al. Th e effi cacy and tolerability of triclabendazole
in Cuban patients with latent and chronic Fasciola hepatica infection. Am J Trop
Med Hyg 2000; 63:264-9.
13. O’Neill SM, Parkinson M, Dowd AJ et al. Short report: immunodiagnosis of
human fascioliasis using recombinant Fasciola hepatica cathepsin L1 cysteine
proteinase. Am J Trop Med Hyg 1999; 60:749-51.
14. Rossignol JF, Abaza H, Friedman H. Successful treatment of human fascioliasis
with nitazoxanide. Trans Royal Soc of Trop Med Hyg 1998; 92:103-4.
15. Saba R, Korkmaz M, Inan D et al. Human fascioliasis. Clin Microbiol Infect 2004;
10:385-7.
16. Sezgin O, Altintas E, Disibeyaz S et al. Hepatobiliary fascioliasis: clinical and
radiologic features and endoscopic management. J Clin Gastroenterol 2004;
38:285-91.
17. Shehab AY, Hassan EM, Abou Basha LM et al. Detection of circulating E/S an-
tigens in the sera of patients with fascioliasis by IELISA: a tool of serodiagnosis
and assessment of cure. Trop Med Int Health 1999; 4:686-90.
18. Talaie H, Emami H, Yadegarinia D et al. Randomized trial of a single, double and
triple dose of 10 mg/kg of a human formulation of triclabendazole in patients with
fascioliasis. Clin Exp Pharm Phys 2004; 31:777-82.
19. Trueba G, Guerrero T, Fornasini M et al. Detection of Fasciola hepatica infection
in a community located in the Ecuadorian Andes. Am J Trop Med Hyg 2000;
62:518.

C
HAPTER
15
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Paragonimiasis
Angelike Liappis
Introduction
Paragonimiasis is a parasitic disease acquired when trematodes of the genus
Paragonimus are accidentally consumed by a human host. Th e adult Paragonimus
(or lung fl uke) commonly localizes to the bronchioles aft er ingestion. While
pulmonary involvement is the most common clinical complication of infection,
the disease is oft en asymptomatic. When manifested by pleuritic chest pain and
hemoptysis, pulmonary paragonimiasis may mimic the clinical presentation of
tuberculosis in countries where both diseases are endemic. A careful epidemiologic
history and a high clinical suspicion for diagnosis are necessary when considering
paragonimiasis in a patient with suspected pulmonary disease.
Geographic Distribution and Epidemiology
Paragonimus is a parasite of mammalian carnivores and omnivores whose diet
includes fresh-water crustaceans (crabs, crayfi sh). First described in tigers more
than a century ago, a variety of wild and domesticated carnivores have been found
to harbor Paragonimus species worldwide.
With a geographic range which spans most of Asia and parts of Africa,
Paragonimus westermani is the most commonly identifi ed lung fl uke. Globally, a
quarter of the more than 50 known species of this parasite have been associated
with human disease. Regional endemic species causing clinical infection include
P. mexicanus (Central America), P. skrjabini (China), P. miyazakii ( Japan), P.
uterobilateralis (West Africa) and P. kellicotti (North America).
Humans acquire the parasite from the ingestion of uncooked or insuffi ciently
cooked fresh-water crustaceans or, rarely, through the fl esh of mammalian reser-
voirs which harbor immature fl ukes. In experimental models, feeding Paragonimus
metacercariae to rats results in the development of mature fl ukes in the lung, but
immature, underdeveloped parasites in the muscles. Clinical human disease has
been associated with the consumption of undercooked wild boar in Asia.
In North America, P. kellicotti is a rare cause of human infection. Th e majority
of paragonimiasis diagnosed in the United States is attributable to exposures to
imported, high risk food items or to those patients traveling from countries where
the local, endemic Paragonimus species are associated with human disease and have
had the proper epidemiologic exposure history.
In countries where the parasite is endemic, social customs and regional prepara-
tion techniques have been implicated as factors which increase the risk of human
infection. Raw crab and/or crayfi sh preparations eaten locally or frozen for export
are potential sources of exposure. In some cultural settings, the consumption of

99
Paragonimiasis
15
pickled or raw seafood is a traditional food preparation technique. Infections
among families and other groups who routinely share meals have been described.
Clinical paragonimiasis has been linked to several traditional dishes, including a
raw seafood salad served in the Americas known as seviche and drunken crab (raw
crabmeat marinated in wine or alcohol) common to several Asian countries.
Th e infection rate of freshwater crabs, collected from regional waterways or
sold in local markets, are as high as 6 to 12% in Africa and Asia, respectively.
Paragonimiasis is estimated to infect several million people worldwide, with pro-
jections of those at risk of acquiring disease reaching 200 million.
Pathogenesis
Adult worms (approximately 0.8-1.6 cm in length × 0.4-0.8 cm in width) de-
velop in the pulmonary bronchioles, where they can encapsulate (Fig. 15.1). Aft er
approximately 6 weeks, dark brown, operculate eggs (80-120
μ
m × 50-60
μ
m) are
produced. Th e parasite eggs pass from stool and respiratory secretions into fresh-
water and develop into miracidia, passing through two intermediate invertebrate
hosts. Over several weeks to months, the parasite matures in freshwater snails to
become cercaria which are either eaten or penetrate the secondary, crustacean
hosts. Th e cercaria mature within the muscles and viscera of a crayfi sh or crab into
metacercariae over 6-8 weeks. Th e cycle is completed when these infective meta-
cercariae are consumed by the mammalian host and penetrate the intestinal wall,
migrating through the peritoneum into the lungs and other organs.
Migration and maturation in the lungs of the defi nitive host may take several
weeks. Th e parasite localizes to the bronchioles where it may cause an acute pneu-
monitis, associated with hemorrhage and an eosinophilic infi ltrate. Adult fl ukes
mature within the lumen of the bronchioles or may encyst in the lung parenchyma.
Infection can persist for several years aft er a host leaves an endemic area.
Paragonimus has evolved a number of mechanisms to evade host defense. Th e
parasites elaborate several excretory-secretory products (ESP) including a number
of cysteine proteinases and, in a recent report, copper/zinc superoxide dismutase.
Superoxide dismutase is constitutively expressed during maturation and protects
the parasite from toxic cellular enzymes elaborated by the host. Paragonimus ESPs
also modulate other aspects of the host infl ammatory response. ESPs have been
found to regulate the survival of eosinophils, to play a role in the degradation of
host antiparasitic immunoglobulin, to regulate migration and tissue penetration,
to facilitate encystment within the lung and other tissues and to modulate the
production of chemokines such as interleukin-8.
Clinical Presentation and Diagnostic Evaluation
Paragonimiasis may present with a range of clinical fi ndings. Th e majority of
patients infected will have few clinical symptoms. Th e most common fi nding may
be a nonspecifi c elevation of the peripheral eosinophil count. In one report, 80% of
patients with paragonimiasis had eosinophilia detected on peripheral blood smear.
More severe disease is associated with higher organism burden. Symptomatic
infection is a manifestation of infl ammatory, oft en hemorrhagic lesions produced
as the organisms migrate from the bowel, traverse the peritoneal cavity and lodge in
the lungs and/or other organs. Th e local infl ammatory reaction includes eosinophilic
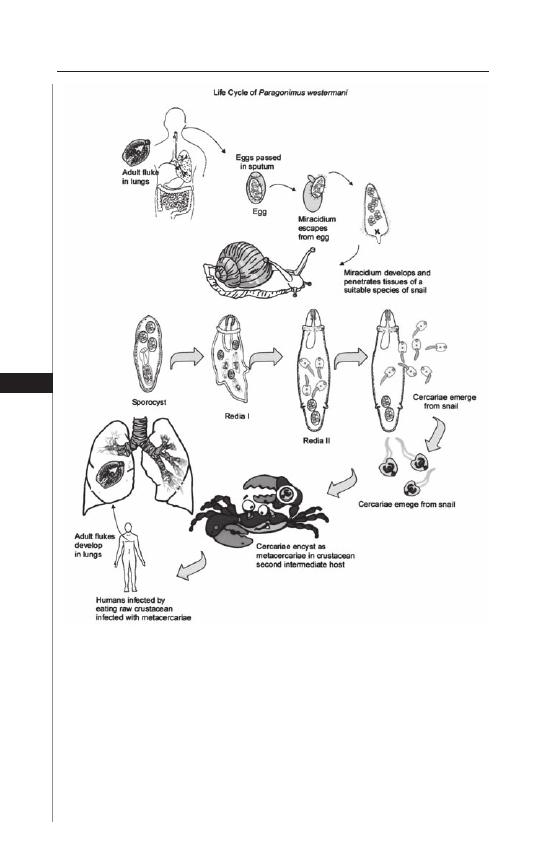
100
Medical Parasitology
15
exudates and, in some cases, a granulomatous reaction to the eggs. Examination of
expectorated sputum under light microscopy should be obtained for the presence of
the brown, operculate eggs as well as for eosinophils and Charcot-Leyden crystals.
When secretions are swallowed, eggs may also be found in the stool of infected
patients. Both sputum and stool should be sent for microscopic evaluation when
pulmonary paragonimiasis is suspected.
Hemoptysis and cough are the most common clinical symptoms of pulmonary
paragonimiasis. In countries where both paragonimiasis and tuberculosis are
Figure 15.1. Life Cycle of Paragonimus westermani. Reproduced from:
Nappi AJ, Vass E, eds. Parasites of Medical Importance. Austin: Landes
Bioscience, 2002:47.

101
Paragonimiasis
15
endemic, it is not uncommon for patients to be treated empirically for tubercu-
losis based on their similar clinical presentations. Th e parasitic diagnosis is oft en
obtained aft er a more thorough inspection of the sputum and/or the stools are
sent for examination. In addition to mimicking tuberculosis, pulmonary paragoni-
miasis can be mistaken for bronchiectasis or other respiratory diseases that may
present with increased sputum production and/or pleuritic chest pain. A careful
epidemiologic history and high clinical suspicion for diagnosis are necessary when
considering this disease in a patient with pulmonary disease.
Examination of pleural fl uid may also demonstrate eosinophilia and the eggs
on cytologic examination. Other pleural fl uid indicators mimic bacterial infection
with low pH, low glucose (<10 mg/dl) and elevated protein and lactate dehy-
drogenase, suggesting an exudative process. Th e parasite and the eggs have been
identifi ed on microscopic examination of bronchoalveolar lavage and fi ne needle
aspiration of pulmonary lesions. In rare cases, the adult worms may be seen in the
sputum or expectorated with coughing aft er the initiation of therapy.
Th e organism’s migration through the peritoneal cavity may lead to intra-
abdominal seeding. Clinical cases of hepatic, omental and retroperitoneal collec-
tions have been described. Eggs reaching the circulation or fl uke migrations outside
the pulmonary cavity may direct disease to distal sites, leading to ectopic foci of
infection. Th e immature fl ukes of P. skrjabini, native to China, have been associ-
ated with cutaneous involvement known as trematode larval migrans. Unlike the
other species of Paragonimus, this particular species is associated with migratory
subcutaneous nodules and, only rarely, pulmonary disease.
Worldwide, other focal skin infections have been reported, as well as in-
traocular, pericardial or brain involvement with P. westermani and a variety of
other Paragonimus species. Cerebral involvement is one of the most common
extrapulmonary sites. When aff ecting the brain, paragonimiasis may present
as a mass lesion with or without seizure, chronic meningeal irritation or acute
meningitis; it is associated with a high mortality due to the higher incidence of
intracranial hemorrhage.
Th e diagnosis of paragonimiasis may be aided by radiologic imaging; however
the fi ndings are generally nonspecifi c. In a retrospective review of over 70 patients
with pleuropulmonary paragonimiasis, chest radiographs demonstrated pleural
lesions in over half of the cases reviewed, with less than 20% showing evidence
of pleural eff usion. Nonspecifi c fi ndings of the parenchymal lung disease on
radiographs include air space consolidation, cystic changes and peripheral linear
densities. Th e radiographically opaque linear densities have been attributed to the
parasite’s migration tracks through the tissues.
Computed tomography (CT) has been used to aid in the diagnosis of pulmo-
nary and extrapulmonary paragonimiasis. CT is more sensitive in demonstrating
small pleural eff usions and better depicts the mass lesions seen on radiographs.
Masses usually consist of one or more peripheral, irregularly shaped densities which
calcify with time. Characteristically, the mass lesions may display either a low at-
tenuation signal at their center or show evidence of an air-fl uid level, associated with
ring-like enhancement at the edges. In setting of pulmonary paragonimiasis, these
lesions have been described as “worm cysts.” Recently, Kuroki et al evaluated eight
patients with high-resolution CT and were better able to demonstrate bronchial
102
Medical Parasitology
15
thickening and ground-glass opacities in association with the usual CT fi ndings
of pulmonary paragonimiasis.
Radiologic imaging studies serve as an important adjunct to the diagnosis of
paragonimiasis, but defi nitive diagnostic evaluation today requires microbiologic
and, increasingly, serologic methods. It may take up to 3 weeks for fl ukes to mature
and produce eggs visible for microscopic diagnosis. To augment microscopic ex-
amination in cases where infection may be early or those samples diffi cult to access
(extrapulmonary disease), immunologic tests have been developed.
Th e diagnosis of patients with early pulmonary disease or those with extra-
pulmonary disease has been improved with the development of serologic testing.
While a simple intradermal test has been developed, the cutaneous reaction cannot
accurately distinguish between active or past infection despite good sensitivity.
Enzyme linked immunosorbent assays (ELISA) with greater specifi city and sensi-
tivity for infection have been developed, including an ELISA using the parasite’s
excretory-secretory antigens. PCR assays are under development and are not yet
commercially available; however these assays hold the promise of being able to
diff erentiate among the various Paragonimus species in addition to diagnosing
infection.
Treatment and Prevention
Th e current treatment of choice for paragonimiasis is praziquantel. It is given
orally at 25 mg/kg, three times a day for two days for those patients with pulmonary
disease. Longer treatment courses may be necessary for extrapulmonary infection,
particularly lesions involving the brain. Surgical resection, combined with medical
therapy, may be necessary in severe plueropulmonary disease.
Praziquantel is associated with headache and drowsiness, but is generally well
tolerated. It is highly eff ective against all species of Paragonimus, with a cure rate
exceeding 95%. Bithionol, which was used in the past, required up to 25 days of
therapy, but has been largely replaced by praziquantel due to compliance and toxic-
ity issues. Approved only for veterinary use, triclabendazole as a single 10 mg/kg
dose is also eff ective and is used outside the US in the treatment of pulmonary
disease.
Th e in vitro eff ect of praziquantel on Paragonimus has been examined by elec-
tron microscopy. Parasites under treatment exhibit severe vacuolization in a variety
of organs, including the tegument and reproductive system. Th e worms are both
immobilized and unable to produce eggs aft er praziquantel therapy. Th e partially
disintegrated or intact, immobilized adults or the eggs may persist in tissues for
weeks aft er treatment. Resolution by radiologic imaging may be delayed despite
clinical response. Th e dead and dying parasites and/or eggs create a persistent
antigenic challenge, decreasing the utility of following posttreatment serologies.
In endemic areas the main risk for human acquisition of disease is food
preparation technique. Prevention can best be achieved by avoiding undercooked
freshwater crabs and crayfi sh and in improving the monitoring of imported of
pickled or frozen raw products imported from endemic countries. Transmission
to humans can be minimized by targeting treatment of municipal water supplies
in endemic areas. By reducing fecal contamination, transmission in both animal
and human hosts can be reduced.

103
Paragonimiasis
15
Suggested Reading
1. Blair D, Xu ZB, Agatsuma T. Paragonimiasis and the genus Paragonimus. Adv
Parasitol 1999; 42:113-222.
2. Bunnag D, Cross JH, Bunnag T. Lung Fluke Infections: Paragonimiasis, In:
Strickland, GT ed. Hunter’s Tropical Medicine and Emerging Infectious Diseases,
8th Edition. Philadelphia: WB Saunders, 2000:847-51.
3. Cho SY, Hong ST, Rho YH et al. Application of micro-ELISA in serodiagnosis
of Human paragonimiasis. Kisaengchunghak Chapchi 1981; 19:151-6.
4. Harinasuta T, Pungpak S, Keystone JS. Trematode infections. Opisthorchiasis,
clonorchiasis, fascioliasis and paragonimiasis. Infect Dis Clin North Am 1993;
7:699.
5. Im JG, Whang HY, Kim WS et al. Pleuropulmonary paragonimiasis: radiologic
fi ndings in 71 patients. Am J Roentgenol 1992; 159:39-43.
6. Keiser J, Utzinger J. Chemotherapy for major food-borne trematodes: a review.
Expert Opin Pharmacother 2004; 5:1711-26.
7. Kuroki M, Hatabu H, Nakata H et al. High-resolution computed tomography
fi ndings of P. westermani. J Th orac Imaging 2005; 20:210-3.
8. Lee SH, Park HJ, Hong SJ et al. In vitro eff ect of praziquantel on Paragonimus
westermani by light and scanning electron microscopic observation.
Kisaengchunghak Chapchi 1987; 25:24-36.
9. Li AH, Na BK, Kong Y et al. Molecular cloning and characterization of copper/
zinc-superoxide dismutase of Paragonimus westermani. J Parasitol 2005; 91:293-9.
10. Maleewong W. Recent advances in diagnosis of paragonimiasis. Southeast Asian
J Trop Med Public Health 1997; 28:134-8.
11. Min DY, Lee YA, Ryu JS et al. Caspase-3-mediated apoptosis of human eosino-
phils by the tissue-invading helminth Paragonimus westermani. Int Arch Allergy
Immunol 2004; 133:357-64.
12. Moyou-Somo R, Kefi e-Arrey C, Dreyfuss G et al. An epidemiological study of
pleuropulmonary paragonimiasis among pupils in the peri-urban zone of Kumba
town, Meme Division, Cameroon. BMC Public Health 2003; 3:40.
13. Narain K, Devi KR, Mahanta J. Development of enzyme-linked immunosorbent assay
for serodiagnosis of human paragonimiasis: Indian J Med Res 2005; 121:739-46.
14. Narain K, Rekha Devi K, Mahanta J. A rodent model for pulmonary paragoni-
miasis. Parasitol Res 2003; 91:517-9.
15. Park H, Kim SI, Hong KM et al. Characterization and classifi cation of fi ve cysteine
proteinases expressed by Paragonimus westermani adult worm. Exp Parasitol 2002;
102:143-9.
16. Pezzella AT, Yu HS, Kim JE. Surgical aspects of pulmonary paragonimiasis.
Cardiovasc Dis 1981; 8:187-94.
17. Shin MH, Lee SY. Proteolytic activity of cysteine protease in excretory-secretory
product of Paragonimus westermani newly excysted metacercariae pivotally regulates
IL-8 production of human eosinophils. Parasite Immunol 2000; 22:529-33.
18. Shin MH, Seoh JY, Park HY et al. Excretory-secretory products secreted by Paragonimus
westermani delay the spontaneous cell death of human eosinophils through autocrine
production of GM-CSF. Int Arch Allergy Immunol 2003; 132:48-57.
19. Singh TS, Mutum SS, Razaque MA. Pulmonary paragonimiasis: clinical features,
diagnosis and treatment of 39 cases in Manipur. Trans R Soc Trop Med Hyg 1986;
80:967-71.
20. Spitalny KC, Senft AW, Meglio FD et al. Treatment of pulmonary paragonimiasis with
a new broad-spectrum anthelminthic, praziquantel. J Pediatr 1982; 101:144-6.
21. Th e Trematodes. In: Markell EK, John DT, Krotoski WA, eds. Medical Parisitology,
8th Edition. Philadelphia: WB Saunders, 1999:225-31.
22. Velez ID, Ortega JE, Velasquez LE. Paragonimiasis: a view from Columbia. Clin
Chest Med 2002; 23:421-31, ix-x.

C
HAPTER
16
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2008 Landes Bioscience.
Intestinal Trematode Infections
Sharon H. Wu, Peter J. Hotez and Thaddeus K. Graczyk
Introduction and General Epidemiology
Th e intestinal trematodes are estimated to account for almost 1.3 million
of the 40-50 million food-borne trematode infections world-wide, particularly
within endemic foci in Southeast Asia and the Western Pacifi c region where they
are signifi cant causes of pediatric malnutrition. Rural poverty and inadequate
sanitation represent major risk factors for this food and waterborne infection, in
addition to unique cultural traditions of raw food consumption, agricultural use
of “night soil” (the use of human feces as fertilizer), promiscuous defecation and
an absence of health education. Transmission occurs focally in endemic areas,
which are characterized by remote, rural and semi-urban communities that prac-
tice small-scale farming. Infections rarely cause mortality; however, they infl ict
considerable morbidity, particularly seen in school-aged children with chronic
infections resulting in malnutrition and stunted growth.
Human intestinal trematodiases are caused by some 70 diff erent species span-
ning 13 families within the Digenea subclass, parasitising all major vertebrate
groups as defi nitive hosts, gastropods and other mollusk groups as fi rst intermediate
hosts and humans as second intermediate hosts. Th is discussion focuses on major
intestinal fl ukes belonging to the families Fasciolidae (e.g., Fascioloposis buski),
Echinostomatidae (Echinostoma spp.) and Heterophyidae (Heterophyes heterophyes
and Metagonimus yokogawai), which are responsible for the bulk of intestinal
trematodiases in humans.
Fasciolidae
Etiology and Defi nitive Hosts
Fasciolopsis buski is the only species within the Fasciolidae family that regularly
infects humans and it is one of the largest, typically ranging from 8-10 cm in length
and 1-3 cm wide. Pigs are the most important reservoir though they carry only
3 to 10 adult fl ukes which produce fewer eggs/adult than in humans, whereas
up to 800 fl ukes/child has been reported with a mean egg production of 16,000
eggs/fl uke per day.
Geographical Distribution and Epidemiology
Fasciolopsis buski is endemic in the Far Eastern and Southeast Asian countries
of mainland China (including Hong Kong), Taiwan, Th ailand, Vietnam, Lao
DR, Cambodia, Philippines, Singapore, Indonesia and Malaysia, as well as in
India (Assam, Maharashtra, Tamil Nadu and Uttar Pradesh) and Bangladesh.
105
Intestinal Trematode Infections
16
Fasciolopsiasis is predominantly a neglected tropical disease of school-aged children
in rural and remote areas with freshwater habitats and bodies of stagnant water. In
such areas, endemic foci range in prevalence from 57% of children in China reported
from as many as 10 diff erent provinces, 25% in Taiwan, 50% in Bangladesh, 60% in
India and 10% in Th ailand. Two studies have reported a slightly greater predilec-
tion in females than males in Th ailand, the opposite trend reported in Taiwan. Th e
infection is underreported and the exact global prevalence is unknown. However, an
estimated 200,000 and 10,000 infections occur in China and Th ailand, respectively.
Endemic areas are characterized by standing or slow-moving freshwater habitats of
food plants such as the water caltrop, water chestnut, watercress, water bamboo,
water lily, gankola, or morning glory, feeding both livestock and humans. Infection
occurs through ingestion of metacercarial cysts on raw or undercooked water plants,
drinking contaminated water and the handling or processing of water plants through
the use of teeth to shuck the outer layers.
Life Cycle and Transmission
Indiscriminate defecation and inadequate sanitation play a large role in continu-
ing the life cycle and transmission of this intestinal fl uke. Humans shed parasite
eggs through the feces and upon contact with water, eggs hatch aft er a period of
3-7 weeks, releasing miracidium. Th ese then swim to fi nd their fi rst intermediate
hosts: snails of the genera Segmentina, Hippeutis and Gyraulus, among others.
Miracidia attach and penetrate the snails’ mantle, tentacles or feet, with sporocyst
development occurring within 2 days. Mother radiae develop rapidly inside the
sporocyst, emerging within 9-10 days aft er miracidial penetration. Th ey then mi-
grate to the ovotestis of the snail, where daughter radiae develop and mature, each
harboring up to 45 cercariae. Th ese then emerge from the daughter radiae (average
patency period 21 days postinfection) and the snails begin to shed cercariae in ir-
regular patterns, all snails dying aft er their fi rst heavy shedding of 50 cercariae or
more. Fasciolopsis buski parasitism of the snail causes physiological and histological
damage to the ovotestis resulting in castration and death. Cercariae released into
water then encyst on fresh water plants or on the water surface, surviving from
64-72 days. Upon ingestion, typically while peeling the plants using one’s teeth,
the encysted metacercariae exyst in the duodenum and attach to the intestinal wall
where, aft er about 3 months, they develop into adult worms. Although pigs are a
potential source of eggs in the environment, the overall contribution of zoonotic
transmission to humans is unknown.
Human Disease
Th e site of infection occurs mainly in the duodenum and jejunum, though
heavy infections can aff ect the entire intestinal tract including the stomach.
Clinical symptoms are related to parasite load, with light infections generally
being asymptomatic or associated with mild bouts of diarrhea, abdominal pain,
anemia, anorexia, eosinophilia, headache and dizziness. Moderate to heavy
infections can result in a severe enteritis with extensive intestinal and duodenal
erosions, ulcerations, hemorrhaging and abscesses. Fasciolopsis enteritis can result
in severe epigastric and abdominal pain, acute ileus, bowel obstruction, nausea,
fever, vomiting and marked eosinophilia and leukocytosis. Ascites, general edema

106
Medical Parasitology
16
and facial edema can result, possibly from elevated immunologic response to
absorption of toxic and allergic worm metabolites. In very heavy infections, intes-
tinal obstruction can result in death. Fasciolopsiasis has been shown to decrease
intestinal absorption of vitamin B12, though having no eff ect on carbohydrate,
fat and protein absorption. Feces are characterized by a yellow-brown or green
color with pieces of undigested food.
Diagnosis and Treatment
Diagnosis of fasciolopsiasis is done by fecal examination to identify the charac-
teristic large, ellipsoidal, operculate eggs, or expelled adult worms. In some cases,
patients vomit adult worms, which are subsequently identifi ed. Th e treatment of
choice for fasciolopsiasis is praziquantel, which induces rapid vacuolisation and
disintegration of the tegument of the parasite. For children and adults the recom-
mended dose is 75 mg/kg/d in 3 doses given over a period of one day. However,
some investigators recommend a single praziquantel dose of 15 mg/kg for children
at bedtime, that being the lowest dose with no side eff ects, and 25 mg/kg for adults.
In the past, niclosamide has been recommended as an alternative treatment.
Economic Impact
Fasciolopsiasis is considered to be a major cause of malnutrition in children
in endemic areas of underdeveloped countries. During this particularly crucial
time of growth and development, improving children’s health could have positive
economic impact through increasing school attendance, learning and healthy
behaviors leading to less-parasitized adults. Measures of disease mortality and
morbidity specifi c for F. buski are not available yet, but like other food-borne
zoonotic trematodiases, fasciolopsiasis is aggravated by socio-economic factors,
particularly practices of free-food markets which lack regulatory inspection and
sanitation. It has also important to consider the eff ect of polyparasitism on child
growth. In China, soil-transmitted helminthiases and foodborne trematodiases
are prevalent among children in schistosome-endemic areas.
Control and Prevention
Th e main goal of current control strategies for other helminthiases (e.g., schis-
tosomiasis, soil-transmitted helminthiases) is to prevent disease through large-scale
treatment programs rather than reducing or eradicating transmission. Control
of intestinal trematode infections requires a slightly diff erent approach. Since
morbidity is directly associated with intensity of infection, prevalence is not the
best indicator of disease morbidity within a population. A reduction in prevalence
may not refl ect a reduction of infection intensity aft er anthelminthic treatment
alone, but rather the eff ects of several interventions acting in concert. A decreas-
ing prevalence pattern of intestinal trematodiases observed currently in Southeast
Asian countries proves to be the result of industrialization of animal production,
food processing, health education, environmental alteration and changes in solid
waste management. WHO and FAO have developed a conceptual framework
for integrated food-borne trematode control comprised of (1) diagnosis through
routine stool examination, community participation and self-reliance; (2) treat-
ment with anthelminthics to reduce morbidity and human host reservoirs; and
107
Intestinal Trematode Infections
16
(3) prevention though health education and reduction of risk behavior to break
the cycle of transmission.
Prevention of food-borne trematode infections involves economic develop-
ment, industrialization, agricultural guidelines, changes in eating behavior,
changes in defecation behavior and sanitation along with sustained praziquantel
treatment of existing infections and reinfections. Th e simplest method of pre-
vention is health education to thoroughly cook raw vegetables before ingestion
and the containment of fecal matter separate from food vegetation. Because
the disease is most prevalent in school-aged children, aggressive school-based
education programs have been implemented to target those who may not be as
entrenched in their eating habits and hygienic customs.
Echinostomatidae
Etiology and Defi nitive Hosts
Human echinostomiasis is caused by up to 20 diff erent species across eight
genera. It diff ers from other food-borne trematodiases in that its range of defi ni-
tive and intermediate hosts is quite broad, making parasite transmission especially
diffi cult to interrupt. Th e reservoir for echinostomes include domesticated and
wild waterfowl, other birds, mammals and any aquatic organisms that maintain
the intermediate hosts. Intermediate hosts include snails, bivalves, crustaceans,
fi sh, amphibians, rats, dogs, humans and a variety of other mammals. Th e adult
fl ukes are typically 3-10 mm in length, 1-3 mm wide with a large ventral sucker
and numerous collar spines.
Geographical Distribution and Epidemiology
Echinostomiasis is endemic in China, Taiwan, India, Korea, Malaysia, the
Philippines and Indonesia, with some foci occurring in Th ailand, Hungary, Italy,
Romania and Egypt. Prevalence of infection in certain endemic foci is estimated
to be 5% in China, 65% in Taiwan, 22% in Korea, 44% in the Philippines and
50% in northern Th ailand, with 150,000 people infected in China and 56,000
in Korea. Th ree species of echinostomes have been reported in Korea: E. hortense
with over 100 proven cases and 50,000 estimated cases, E. cinetorchis with four
proven cases and 1,000 estimated cases and E. japonicus with four proven cases and
5,000 estimated cases. Infection is associated with consumption of the intermediate
host, usually raw or inadequately cooked fi sh, snails, shrimp, frogs, tadpoles, clams
and mussels. Endemic foci are characterized by fresh or brackish water habitats
containing intermediate hosts that are consumed raw or undercooked and socio-
economic factors that bring populations into contact with aquatic environments
for food sources.
Life Cycle and Transmission
Humans ingest metacercariae in raw or undercooked intermediate hosts which
then exyst in the intestinal tract, maturing into adults. Th e adults lay eggs which
hatch in the environment, releasing miracidia which then infect an intermediate
host, usually a snail or fi sh. Cercariae develop and are released, encysting in the
environment for the next host. Humans are accidental intermediate hosts and
echinostomes demonstrate a sylvatic cycle as well as a human cycle.

108
Medical Parasitology
16
Human Disease
Clinical symptoms associated with light to moderate infections include anemia,
headache, dizziness, stomachache, gastric pain and loose stools while heavy infec-
tions can cause eosinophilia, abdominal pain, profuse watery diarrhea, anemia,
edema and anorexia. Th e worms cause pathological changes in the intestinal lumen.
Erosions, destruction of villi, loss of mucosal integrity and catarrhal infl ammation
have all been reported.
Diagnosis and Treatment
Diagnosis of echinostomiasis is done by identifying eggs upon fecal examina-
tion. Praziquantel is probably the drug of choice for the treatment of human
echinostomiasis, although it has been reported that infections can also be treated
with mebendazole, albendazole, or niclosamide.
Economic Impact
Echinostomiasis is an underreported infection in endemic areas, which tend to be
remote places where the burden of infection is carried by the rural poor and women
of child-bearing age. Like fasciolopsiasis, echinostomiasis is a disease of poverty and
poor sanitation. Education, industrialization, changes in ecology and agricultural
practices have reduced prevalence of some trematodiases in endemic areas. In Lindu
Valley, Indonesia, predation of cercaria-infected Corbicula clams by Tilapia mos-
sambica fi sh drastically reduced echinostomiasis infection in the region.
Control and Prevention
Th e control of echinostomiasis, like that of fasciolopsiasis, involves interrupting
the life cycle through diagnosis, treatment and prevention of reinfection. Within
its endemic boundaries, however, echinostomiasis is diffi cult to control or eradi-
cate due to the operation of three independent transmission cycles, i.e., human
(anthroponotic), zoonotic (domesticated livestock) and sylvatic (wildlife). Th us,
any successful control program involving anthelminthic treatment would have to be
sustained to cure re-infections in humans as well as animal populations. Treatment
with broad-spectrum anthelminthics would also cure concurrent helminthiases in
polyparasitized individuals. Prevention of echinostomiasis follows a similar strategy
for fasciolopsiasis, relying on health education targeted to school-aged children to
change people’s eating habits. Industrialization and economic development would
have the greatest impact on reducing transmission since vector and intermediate
host control is not possible.
Heterophyidae
Etiology and Defi nitive Hosts
While there are many genera of heterophyids containing species that cause
human trematodiasis, this discussion focuses on Heterophyes heterophyes and
Metagonimus yokogawai, the two most prevalent species infecting humans.
Defi nitive host range for H. heterophyes and M. yokogawai include various
fi sh-eating mammals such as dogs, cats, foxes, jackals, birds and humans; fi rst in-
termediate hosts are littorine snails; and second intermediate hosts are shore-fi sh
and brackish water fi shes such as cunners, gudgeon, charr, perch, shad, mullet
109
Intestinal Trematode Infections
16
and goby. Th ese worms are small (<0.5 mm in length), egg-shaped and possess
a unique morphological feature of a gonotyl or genital sucker.
Geographical Distribution and Epidemiology
H. heterophyes have been reported in Egypt, Sudan, Iran, Turkey, Tunisia, China,
Taiwan, the Philippines, Indonesia, Africa and India. In one Egyptian village, the
highest prevalence of infection, 37%, was found among individuals aged 15-45
years, greater for females than males, followed by 28% prevalence in children
under 5 years of age. Over 10,000 people are estimated to be infected in Egypt. H.
heterophyes in China occurs in approximately 230,000 infected people.
M. yokogawai is mainly distributed in China, Japan, Korea, Taiwan, Indonesia,
Russia, Israel, the Balkans and Spain. An estimated 500,000 people are infected
in South Korea, 150,000 in Japan and 12,000 in Russia. Epidemiologic studies
have compared prevalence of food-borne trematode infections in villages close to
water bodies and found a relative risk of 5.01 to 7.44 in Republic of Korea for M.
yokogawai infection associated with proximity to fresh water. Twenty-fi ve percent
of food fi shes like perch and mullet studied from Jinju Bay, Korea, were infected
with heterophyid metacercaria.
Life Cycle and Transmission
Transmission of heterophyids occurs through consumption of raw, undercooked
or improperly processed metacercaria-infected fi shes.
Human Disease
H. heterophyes and M. yokogawai invade intestinal mucosa of the small intestine,
causing infl ammation, ulceration and superfi cial necrosis. Heavy infections are asso-
ciated with diarrhea, mucus-rich feces, pain, dyspepsia, anorexia, nausea and vomit-
ing. Worm eggs may also enter the lymphatic vessels via the crypts of Lieberkühn,
enter the circulation and may occlude cardiac vessels leading to heart damage and
fi brosis. Rare cases of egg emboli in the spinal cord and brain have been reported.
Diagnosis and Treatment
Diagnosis is done by fecal examination and treatment is praziquantel admin-
istered in three doses of 25 mg/kg in a single day (75 mg/kg/d in 3 doses × 1 d)
or as a single dose.
Control and Prevention
Heterophyiases and metagonimiases overlap geographically with other
food-borne trematodiases, particularly the liver fl ukes that cause clonorchiases and
opisthorchiases. Control and prevention are similar to those strategies employed for
fasciolopsiasis and echinostomiasis. In addition, the major soil-transmitted helmint-
hiases and schistosomiases are coendemic and while the number of these infections
has decreased due to large-scale deworming programs in many areas, overall the
number of cases of food-borne trematode infections have not decreased signifi cantly.
Th is could be due to a number of factors, such as the rapidly growing aquaculture
industry in Asian countries playing a key role in transmission. Prevention would
then require strict regulation of this expanding sector of food production along with
praziquantel-based treatment programs.

110
Medical Parasitology
16
Suggested Reading
1. Control of foodborne trematode infections. Report of a WHO Study Group. World
Health Organ Tech Rep Ser 1995; 849:1-157.
2. Abdussalam M, Kaferstein FK, Mott KE. Food safety measures for the control of
foodborne trematode infections. Food Control 1995; 6:71-79.
3. Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Di 2005;
11:1507-14.
4. Mas-Coma S, Valero B. Fascioliasis and other plant-borne trematode zoonoses. Int
J Parasitol 2005; 35:1255-78.
5. Manning GS, Ratanarat C. Fasciolopsis buski (Lankester, 1957) in Th ailand. Amer
J Trop Med Hyg 1970; 19:613-9.
6. Sadun EH, Maiphoom C. Studies on the epidemiology of the human intestinal
fl uke, Fasciolopsis buski (Lankester) in Central Th ailand. Am J Trop Med Hyg 1953;
2:1070-84.
7. Joint WHO/FAO workshop on food-borne trematode infections in Asia. WHO,
Regional Offi ce for the Western Pacifi c 2002.
8. Graczyk TK, Alam K, Gilman RH et al. Development of Fasciolopsis buski
(Trematoda: Fasciolidae) in Hippeutis umbilicalis and Segmentina trochoideus
(Gastropoda: Pulmonata). Parasitol Res 2000; 86:324-32.
9. Lo CT. Life history of the snail, Segmentina hemisphaerula (Benson) and its experi-
mental infection with Fasciolopsis buski (Lankester). J Parasitol 1967; 53:735-8.
10. Jaroonvesama N, Charoenlarp K, Areekul S. Intestinal absorption studies in
Fasciolopsis buski infection. Southeast Asian J Trop Med Public Health 1986;
17:582-5.
11. Le TH, Nguyen VD, Phan BU et al. Case report: unusual presentation of Fasciolopsis
buski in a Viet Namese child. Trans R Soc Trop Med Hyg 2004; 98:193-4.
12. Gupta A, Xess A, Sharma HP et al. Fasciolopsis buski (giant intestinal fl uke)—a case
report. Indian J Pathol Microbiol 1999; 42:359-60.
13. Keiser J, Utzinger J. Chemotherapy for major food-borne trematodes: a review. Exp
Opin Pharmacother 2004; 5:1711-26.
14. Zhou H, Ohtsuka R, He Y et al. Impact of parasitic infections and dietary intake on
child growth in the Schistosomiasis-endemic Dongting Lake region, China. Am J
Trop Med Hyg 2005; 72:534-9.
15. Graczyk TK, Fried B. Echinostomiasis: a common but forgotten food-borne disease.
Am J Trop Med Hyg 1998; 58:501-4.
16. Chai JY, Lee SH. Intestinal trematodes of humans in Korea: Metagonimus,
Heterophyids and Echinostomes. Kisaengchunghak Chapchi 1990; 28:103-22.
17. Kim DG, Kim TS, Cho SH et al. Heterphyid metacercarial infections in brackish
water fi shes from Jinju-man (Bay), Kyongsangnam-do, Korea. Korean J Parasitol
2006; 44:7-13.
18. Abou-Basha LM, Abdel-Fattah M, Orecchia P et al. Epidemiological study of hetero-
phyiasis among humans in an area of Egypt. Eastern Med Health J 2000; 6:932-8.
19. Goldsmid JM. Ecological and cultural aspects of human trematodiasis (excluding
schistosomiasis) in Africa. Cent Afr J Med 1975; 21:49-52.
20. Olson PD, Cribb TH, Tkach VV et al. Phylogeny and classifi cation of the Digenea
(Platyhelminthes: Trematoda). Int J Parasitol 2003; 33:733-55.
21. Bhatti HS, Malla N, Mahajan RC et al. Fasciolopsiasis—a re-emerging infection in
Azamgarh (Uttar Pradesh). Indian J Pathol Microbiol 2000; 43:73-6.
22. Th e Medical Letter on Drugs and Th erapeutics. Drugs for Parasitic Infections 2004:4,
www.medicalletter.org
23. Cross JH. Changing patterns of some trematode infections in Asia.
Arzneimittelforschung 1984; 34:1224-6.
24. Brooker S, Whawell S, Kabatereine NB et al. Evaluating the epidemiological impact
of national control programmes for helminths. Trends Parasitol 2004; 20:537-45.

C
HAPTER
17
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Schistosomiasis: Schistosoma japonicum
Edsel Maurice T. Salvana and Charles H. King
Background
Schistosoma japonicum, also known as the oriental blood fl uke, is one of the
causative agents of chronic intestinal schistosomiasis in humans. Th is condition,
which frequently progresses to liver fi brosis, portal hypertension and splenomegaly,
is endemic to southeastern and eastern Asia, particularly parts of China, Sulawesi,
Indonesia, Th ailand, Laos, Cambodia and the Philippines. At one time, it was
endemic in southern Japan, where Katsurada fi rst described the adult form of the
S. japonicum parasite. However, transmission of this infection was completely
eliminated in Japan as of 1977.
In the late 1950s, an estimated 11.6 million people in China were infected with
S. japonicum. A national control program was launched, with emphasis on inter-
mediate host snail control through environmental management, and this decreased
the numbers of infected individuals dramatically. Recently between 2000 and 2003,
there has been an upsurge of cases in China, which suggests a reemergence of the
disease. More than 1.3 million people in the world are currently infected with S.
japonicum; the ∼843,000 in China and over 500,000 in the Philippines make up
the greater part of present S. japonicum cases.
S. japonicum is a trematode parasite or fl uke that requires a snail of the genus
Oncomelania as an intermediate host. Compared to S. mansoni and S. hae-
matobium, S. japonicum is associated with more severe disease manifestations.
Ecologically, it is perhaps the most diffi cult schistosome species to control, in part
due to its higher egg production and its ability to infect a wider range of defi nitive
mammalian hosts, including common domestic animals such as dogs, cats, pigs
and cows, as well as feral animals such as mice, rabbits and monkeys. Nevertheless,
nations such as Japan, China and the Philippines have made signifi cant progress
in S. japonicum control, mostly linked to economic development and focused ef-
forts at improved sanitation. Geographically distinct strains of S. japonicum have
diff erent preferred hosts and appear to have varying degrees of pathogenicity.
Moreover, diff erent geographic strains elicit distinct immune responses, which are
not necessarily cross-protective. Th is phenomenon means that a universal vaccine
against S. japonicum will be diffi cult to develop.
Several S. japonicum-like fl ukes have been identifi ed in Th ailand and Malaysia
that are of uncertain clinical signifi cance for humans. In addition, in 1978, a new
species of Schistosoma was recognized within the Mekong River basin in Laos to
Cambodia. Th is new species, S. mekongi, utilizes the snail Neotricula aperta as its
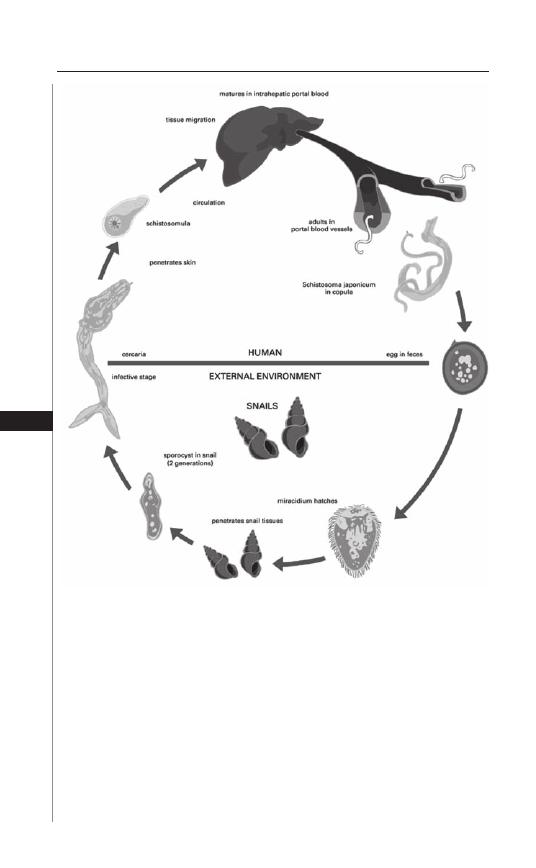
112
Medical Parasitology
17
intermediate host. In human infection, its clinical manifestations and pathogenesis
are similar to those of S. japonicum.
Th e S. japonicum life cycle (Fig. 17.1) includes both parasitic and free-living
stages. Th e infective stage for humans is the cercaria (pl. cercariae), which is
free-living and free-swimming, but short-lived (24-72 h). Th e S. japonicum cercariae
gain entry to the host through penetration of skin immersed in water. Th e cercariae
then transform into larval schistosomula, which penetrate the circulation through
subcutaneous vessels and reach the pulmonary circulation. In the lungs, the schisto-
somules elongate, break into the pulmonary veins and then travel through the heart
to the systemic capillary bed. If the schistosomulum reaches the splanchnic vessels,
Figure 17.1. Life cycle of Schistosoma japonicum. Modifi ed with permission
from: Belizario VY, de Leon WU, eds. Philippine Textbook of Parasitology,
2nd Ed. Manila: University of the Philippines, 2004:196.

113
Schistosomiasis: Schistosoma japonicum
17
it moves across the capillary bed to the portal circulation (otherwise, it returns to
the heart to circulate again). From the mesenteric capillaries, the schistosomulum
travels to the liver, where it goes into the intrahepatic branches of the portal vein
and matures into an adult schistosome. Adult S. japonicum are dioecious, i.e., either
male or female and these migrate back to the mesenteric vessels to pair, mate and
begin oviposition in the wall of the bowel. Eggs leave the human body in feces
and when exposed to fresh water, these hatch to release miracidia, which in turn
infect intermediate host snails, which complete the parasite life cycle by releasing
cercariae 4-12 weeks aft er infection.
Areas with high rates of S. japonicum infection have habitats that are rich in
water and provide a favorable breeding ground for snails, as well as opportunities
for the cercariae to infect defi nitive hosts. Rural areas with rice fi elds and seasonal
fl ooding are ideal for the parasite to thrive. Control of infection has been attempted
in the past through eradication of the intermediate snail hosts, and this was done
successfully in Japan. Lately, the focus has been on mass treatment of aff ected hu-
man populations, especially in areas with high disease prevalence.
Disease Signs and Symptoms
Acute Schistosomiasis
Th e penetration of cercariae into the skin causes localized infl ammation and
pruritus known as cercarial dermatitis or “swimmer’s itch.” Th is is not unique to
S. japonicum and characterizes the trematode cercariae across the parasite genus
and family, including those bird schistosomes such as Trichobilharzia that cause
zoonotic swimmer’s itch. S. mansoni and S. haematobium are more likely to cause
dermatitis than S. japonicum.
More commonly, successful cercarial entry to the body and development of
larvae in the bloodstream brings about a self-limiting febrile illness known as
“snail fever” or Katayama fever. Th is can be accompanied by arthralgias, myalgias
and abdominal pain, as well as fi ndings of lymphadenopathy, hepatosplenomegaly
and eosinophilia.
As the maturing worms migrate through the circulatory system, they may rarely
end up in aberrant areas such as the brain or spinal cord, where direct occlusion of
blood vessels may cause transient ischemic attacks, strokes, paraparesis, seizures,
or hydrocephalus.
Repeated infections can stimulate partial immunity in the host and, due to a
compensatory immunomodulation of antiparasite immune responses, the derma-
titis and systemic manifestations of acute infection may actually be decreased when
infected individuals are exposed to subsequent cercarial penetration. A proportion
of immune patients may spontaneously clear their infections without treatment,
but the remainder go on to develop chronic schistosomiasis with variable degrees
of morbidity.
Chronic Schistosomiasis
Schistosoma japonicum causes chronic pathology when the adult worms fi nd
their way into the portal circulation. A mated pair of worms produces 300 to
3,000 eggs per day, which are released into the capillaries and portal veins. In
114
Medical Parasitology
17
order to be expelled through the intestinal wall, an egg must be inserted into a
terminal vein within the bowel wall and then be ulcerated (via immune-mediated
infl ammation) through the mucosa into the bowel lumen. Bleeding and polyp
formation in the bowel wall are thus common complications of egg transfer
from the venule to the bowel lumen. Th e process of local infl ammation can lead
to protein loss, iron loss, anemia of chronic disease, diarrhea and in some cases,
intestinal obstruction.
More than half of the worm eggs become permanently trapped in host tissues,
where they each evoke an immune-mediated infl ammatory granuloma. Th e signifi -
cant numbers of eggs ultimately deposited in tissues cause widespread fi brosis. Th is
is especially apparent in the liver, where a classic pattern of periportal “pipestem”
fi brosis is manifested, which leads to portal hypertension and its sequelae of varix
formation, ascites and splenomegaly.
As portal hypertension increases, eggs are shunted into the pulmonary circula-
tion, where local fi brosis causes pulmonary hypertension, which may lead to cor
pulmonale. Eggs reaching the central nervous system lead to local granuloma
formation, which can cause seizures, hydrocephalus and space occupying lesions.
Pediatric Infection
Infection with S. japonicum has detrimental eff ects on the growth of children in
endemic areas. A recent study in China noted that height, weight and mid-upper
arm circumference were all signifi cantly reduced in S. japonicum-infected children
compared to controls and this eff ect was most severe among girls. Comparable
studies in the Philippines have shown S. japonicum-infected children to have an
increased prevalence of nutritional morbidity, as well as poorer performance on
standardized tests of learning. Because of their chronic, long-term persistence over
many years, these ‘subtle’ morbidities constitute a signifi cant lifetime burden of
disease for endemic populations.
Diagnosis
Acute Infection
Acute infection with schistosomiasis is diffi cult to diagnose defi nitively. Clinical
symptoms are nonspecifi c. A history of exposure of skin to water in endemic areas
followed by a suitable clinical syndrome should increase the index of suspicion
for acute schistosomiasis. Antischistosome serologic tests may be performed at
this juncture, although a positive result does not distinguish a recent infection
from a remote one. However, among returning travelers who have no history of
prior exposure, a negative serology is quite helpful in ruling out the possibility of
schistosome infection.
Chronic Infection
Direct stool examination using the Kato-Katz technique is the method of choice
for determining the presence of infection and the associated egg density in aff ected
humans. S. japonicum eggs have a distinct appearance, which is ovoidal with a small
knob near one of the poles (Fig. 17.2). Th e typical egg measures around 100
μ
m
on the long axis and 60
μ
m on the short axis.

115
Schistosomiasis: Schistosoma japonicum
17
Concentration techniques are helpful for processing large stool volumes but are
not completely sensitive for light infections. Common concentration techniques
include formaldehyde-ether concentration technique, merthiolate-formaldehyde
concentration technique and merthiolate-iodine-formaldehyde concentration
technique.
In consideration of cases of very light infection, a rectal biopsy can be useful if
repeated stool examinations remain negative in the face of a high clinical suspicion
of infection. An estimation of the viability of any recovered eggs is appropriate, as
large numbers of eggs can concentrate in the rectal mucosa and remain there even
if active infection has ceased. Liver biopsy can also demonstrate worm eggs in the
parenchyma, but this procedure is more invasive, risky and requires specialized
equipment.
Immunologic diagnosis is available using both S. japonicum egg and adult worm
extracts, although the World Health Organization recommends that testing be
performed with crude egg antigens for greater specifi city. Currently available tests
include the circumoval precipitin test (COPT), indirect hemagglutination test
and ELISA against soluble schistosome antigens.
Th e COPT tests patient’s serum for reactivity with S. japonicum eggs. Upon
serum exposure, a precipitate forms on the egg surface that can be visualized
through the microscope. Like the ELISA for anti-S. japonicum antibodies, the
COPT does not distinguish an active from a remote infection.
Antigen detection through ELISA is emerging as the most specifi c test for
detecting active infection. Monoclonal antibodies against specifi c S. japonicum
antigens are used to detect circulating antigens, including gut-associated antigens
such as circulating cathodic antigen (CAA). Th e CAA assay is particularly useful
Figure 17.2. Schistosoma japonicum egg.
116
Medical Parasitology
17
because the antigen can be detected in urine, which should facilitate its use in
large-scale fi eld studies.
Liver ultrasound and computed tomography can identify a distinctive pattern
of periportal fi brosis and a “network” pattern in S. japonicum. Th is can be useful
in distinguishing liver disease secondary to the parasite from other etiologies.
However, more imaging studies are required to validate these fi ndings.
Treatment
Praziquantel remains the mainstay of therapy for active S. japonicum disease.
While individuals with acute infection can initially be observed and treated symp-
tomatically, evidence of chronic infection, as manifested by recovery of eggs from
stool or tissue requires anthelminthic therapy to prevent signifi cant morbidity, as
well as to prevent further transmission.
For S. japonicum treatment, praziquantel should be given at an oral dose of 60
mg/kg in two to three divided doses over the period of one day. Th is regimen results
in an 80-90 % cure rate. Common side-eff ects are mild and include abdominal dis-
comfort, low-grade fevers, nausea, dizziness and diaphoresis. Th ere is evidence that
treatment of chronic schistosome infection, if given early in the course of the disease,
can reverse infection-associated morbidity such as hepatosplenomegaly. Among
patients with extensive fi brosis and obstruction, however, treatment may have little
eff ect, with only minimal regression of clinical and ultrasound fi ndings.
Alternative drug regimens include niridazole and antimony-based therapy, but
these drugs are not FDA-approved and are associated with much lower cure rates
and with signifi cant toxicity. Artemisinin derivatives such as artemether have been
shown to have antiparasitic activity, particularly against juvenile forms of the worm,
but they are less active against the mature, adult forms of the parasite. Combination
treatment with artemether and praziquantel has been more highly eff ective in
reducing worm burdens in laboratory animals and is believed to have considerable
potential for interrupting transmission in high-transmission communities.
Recently, calcium-channel blockers have been shown to aff ect the eggshell
formation in S. mansoni and these agents may hold some promise in reducing
or interrupting S. japonicum transmission. Several unique schistosomal proteins
involved in eggshell synthesis have been identifi ed and are currently being studied
as targets for treatment.
Prevention and Prophylaxis
Th e mainstay of epidemiologic control involves interruption of transmission. In
areas of high prevalence, mass chemotherapy is the main control strategy. Although
mass treatment may reduce transmission, it does not assure signifi cant interruption of
transmission. Environmental control of the Oncomelania host snail is a strategy that
has been used successfully in Japan and parts of China. Elimination of snails involves
elimination of breeding sites as well as the use of chemical molluscicides to kill snails
at transmission sites. Improved sanitation (to prevent S. japonicum eggs in feces from
reaching fresh water snail habitat) is likewise an important control measure.
No eff ective chemoprophylaxis currently exists for S. japonicum. Some fi eld
studies involving artemether for prophylaxis have been promising, but results
from large-scale intervention trials are still pending. Th ere have been studies

117
Schistosomiasis: Schistosoma japonicum
17
involving chemical repellants to prevent penetration of cercariae but these have
been largely unsatisfactory. Th erefore, travelers have no protective options and
should be strongly cautioned against participating in wading or swimming activi-
ties in any unchlorinated fresh water found in S. japonicum-endemic areas.
Vaccine Research
An eff ective vaccine against the diff erent geographic strains of S. japonicum will
be a major breakthrough in the control of schistosomiasis in Asia. Th is is because
current chemotherapy does little to interrupt transmission and does nothing to
prevent reinfection. A number of vaccines based on novel target antigens are in
development. A transmission-blocking veterinary vaccine that targets infection
in water buff aloes has completed fi eld trails and may have a major role in inte-
grated schistosomiasis control programs in the future. Recent elucidation of the
S. japonicum genome is expected to further boost research into fi nding targets for
drug treatment and vaccine development.
Suggested Reading
1. Blas B, Rosales M, Lipayon I et al. Th e schistosomiasis problem in the Philippines:
a review. Parasitol Int 2004; 53:127-34.
2. Bonn D. Schistosomiasis: a new target for calcium channel blockers. Lancet Infect
Dis 2004; 4:190.
3. Coutinho HM, McGarvey ST, Acosta LP et al. Nutritional status and serum cytokine
profi les in children, adolescents and young adults with Schistosoma japonicum-as-
sociated hepatic fi brosis, in Leyte, Philippines. J Infect Dis 2005; 192:528-36.
4. Ezeamama AE, Friedman JF, Acosta LP et al. Helminth infection and cognitive
impairment among Filipino children. Am J Trop Med Hyg 2005; 72:540-8.
5. Garcia EG, Belizario VY Jr. Trematode infections: blood fl ukes. In: Belizario VY,
de Leon WU, eds. Philippine Textbook of Medical Parasitology, 2nd Edition.
Manila: Th e Publications Program University of the Philippines, 2004:195-211.
6. Hirayama K. Immunogenetic analysis of postschistosomal liver fi brosis. Parasitol
Int 2004; 53:193-6.
7. King CH. Acute and chronic schistosomiasis. Hosp Pract 1991; 26:117-30.
8. Li Y, Herter U, Ruppel A. Acute, chronic and late-stage infections with Schistosoma
japonicum: reactivity of patient sera in indirect immunofl uorescence tests. Ann Trop
Med Parasitol 2004; 98:49-57.
9. McManus DP. Prospects for development of a transmission blocking vaccine against
Schistosoma japonicum. Parasite Immunol 2005; 27:297-308.
10. Ohmae H, Sy OS, Chigusa Y et al. Imaging diagnosis of schistosomiasis japonica—
the use in Japan and application for fi eld study in the present endemic area. Parasitol
Int 2003; 52:385-93.
11. Olveda RM. Disease in schistosomiasis japonica. In: Mahmoud AAF, ed.
Schistosomiasis. Tropical Medicine: Science and Practice. London: Imperial
College Press, 2001:361-89.
12. Ross AGP, Bartley PB, Sleigh AC et al. Current Concepts: Schistosomiasis. N Engl
J Med 2002; 346:1212-20.
13. Vennervald BJ, Dunne DW. Morbidity in schistosomiasis: an update. Curr Opin
Infect Dis 2004; 17:439-47.
14. Zhou H, Ohtsuka R, He Y et al. Impact of parasitic infections and dietary intake
on child growth in the schistosomiasis-endemic Dongting Lake Region, China.
Am J Trop Med Hyg 2005; 72:534-9.
15. Zhou XN, Wang LY, Chen MG et al. Th e public health signifi cance and control
of schistosomiasis in China—then and now. Acta Trop 2005; 96:97-105.

C
HAPTER
18
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Schistosomiasis: Schistosoma mansoni
Wafa Alnassir and Charles H. King
Background
Archeological evidence in Africa and China indicates that the parasitic trema-
tode infection schistosomiasis has been part of human life for at least four millen-
nia. In 1852, Dr. Th eodore Bilharz, a German physician working in Egypt, fi rst
described the presence of adult worms in postmortem examinations of aff ected
patients and since then, the disease is oft en referred to generically as ‘Bilharziasis’.
It was not until a half-century later (in 1907) that Dr. Patrick Manson defi ned the
existence of diff erent Schistosoma species and later in 1908, Sambon established
the distinct species we now call Schistosoma mansoni.
Epidemiology
Internationally, Schistosoma mansoni is the most prevalent of the schistosome
species that aff ect the intestines and liver. An estimated 62 million persons are
infected worldwide. S. mansoni is known to occur in 52 countries, including
sub-Saharan Africa (where around 85% of the global burden is concentrated),
North African and Eastern Mediterranean countries, South American countries
(Brazil, Venezuela, Surinam) as well as several Caribbean countries (Saint Lucia,
Montserrat, Martnique, Guadeloupe, Dominican Republic and Puerto Rico)
(Fig. 18.1).
Life Cycle
Among all species of human schistosome parasites (S. mansoni, S. haemato-
bium, S. japonicum, S. intercalatum, S. mekongi), the schistosome life cycle is very
similar, with the exception that diff erent species diff er in the fi nal location where
the adult worms prefer to reside within the human body. Adults of S. mansoni and
S. japonicum favor the intestines, whereas S. haematobium prefer the urinary tract.
Th e adult form of S. mansoni most frequently lives in the mesenteric veins of the
small and large intestines and its eggs typically pass out of the body in the feces.
However, in heavy infection S. mansoni will fi nd space in other pelvic abdominal
organs and S. mansoni eggs can be passed in the urine as well.
Th e life cycle of the schistosome worms involves an adult dioecious (i.e., either
male or female) sexual stage within the defi nitive human host and an asexual re-
productive stage within an intermediate host snail. When parasite eggs (ova) reach
fresh water, they hatch and free-swimming miracidial forms are released. Th ese pen-
etrate the bodies of suitable aquatic snails (for S. mansoni, these are Biomphalaria
species snails (Fig. 18.3)) and for the next 3-5 weeks they multiply asexually to
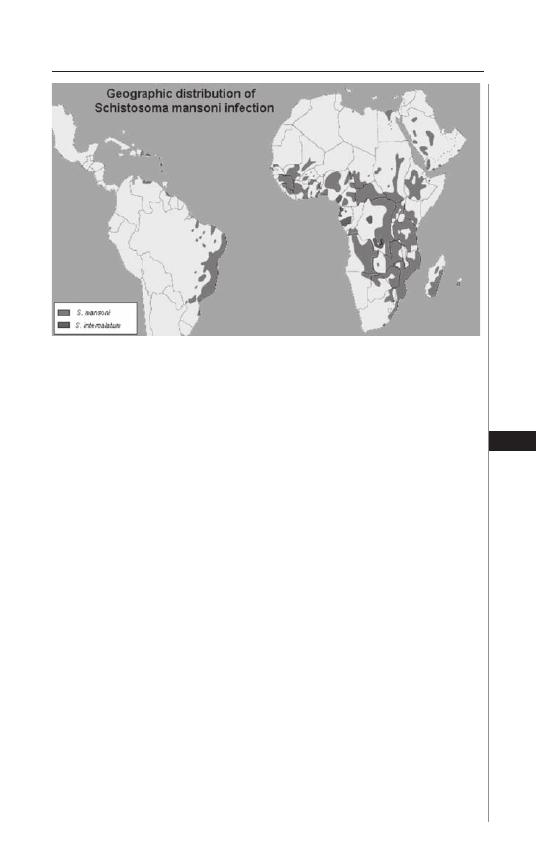
119
Schistosomiasis: Schistosoma mansoni
18
form hundreds of fork-tailed cercariae. Th e cercariae leave the snail and swim to a
human or nonhuman animal, where they penetrate the skin (Fig. 18.2).
Inside the human body, the larval forms, or schistosomula, are transported
through blood or lymphatics to the right side of the heart, then to the lungs, where
they mature for a period of 7-10 days. Th ey then break into the pulmonary veins
and travel through the systemic circulation to reach the portal circulation and the
liver, where they develop into adult worms (Fig. 18.3). Ultimately, the mature adult
worms migrate from the liver through the portal veins to the mesentery and the
wall of the bowel. Th ese adult worms do little damage to the host. Th ey feed on
circulating erythrocytes and plasma glucose but usually don’t cause symptoms.
Th e time between the fi rst skin penetration by a cercaria to the fi rst ova produc-
tion by an adult worm is around 4-6 weeks. Th e female S. mansoni adult worm
mates with the male worm and lays around 100-300 ovoid, laterally spined eggs
each day (Fig. 18.3). Th is morphological appearance of the eggs allows very clear
diagnostic identifi cation of the infecting species. Th e female worm lives approxi-
mately 3-8 years and, when mated, lays eggs throughout her adult life. About half
of all eggs reach the lumen of the bowel by a process of infl ammatory ulceration
through the bowel wall. Th e remainder of schistosome eggs remains trapped
within the human host’s body and result in acute and chronic granulomatous
infl ammation (Fig. 18.3).
Th e host immune response to schistosome eggs is the hallmark of disease due to
schistosomiasis, in that immunological reaction and granuloma formation in the
tissues is responsible for the morbidity and mortality of chronic schistosomiasis.
In terms of disease risk, no clear-cut racial predilection exists, although diff erent
populations have been found to have varying risk for fi brotic liver disease. Men oft en
have a higher incidence of infection and disease than women, most likely because of
their increased exposure to infested water via bathing, swimming and agricultural
Figure 18.1. Geographic distribution of Schistosoma mansoni infection.
Adapted with permission from the Atlas of Medical Parasitology, Carlo
Denegri Foundation.
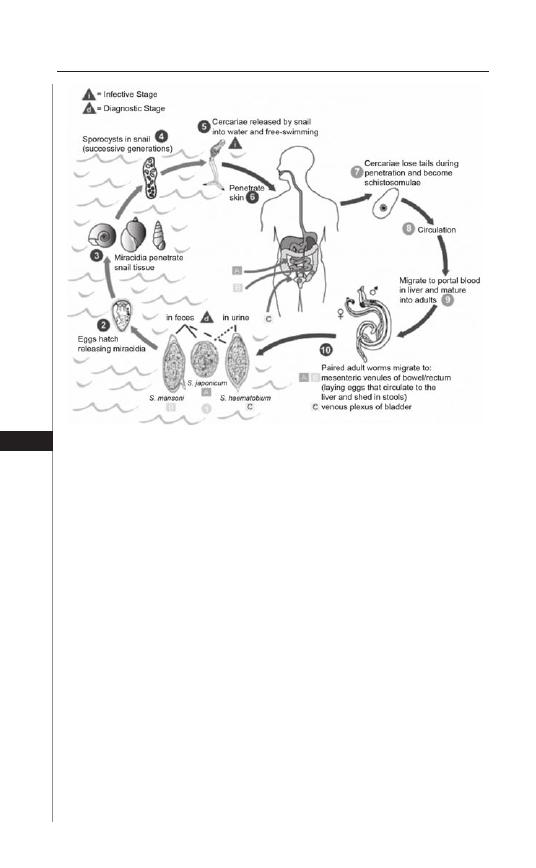
120
Medical Parasitology
18
activities. In terms of age-related risk, schistosome exposure can start shortly aft er
birth, but tends to increase during late childhood, with maximum exposure occurring
among people aged 10-14 years. Th ere is typically a lower incidence of infection and
disease among adults, probably due to a combination of acquired partial immunity
and an age-related decrease in exposure to infected water.
Typical Risk Factors
People who live or travel in areas where schistosomiasis is endemic and who
are exposed to or swim in standing or running freshwater wherever the appropri-
ate type of Biomphalaria snails are present, are at risk for infection. Transmission
is intermittent, but under the right circumstances only a brief (<2 min) exposure
can result in infection.
Disease Signs and Symptoms
Acute Manifestations
Aft er cercarial penetration, there can be localized pruritus lasting for few hours
(‘swimmers itch’) followed by urticarial rash or papuloerythematous exanthem.
Most people will be initially asymptomatic aft er this fi rst stage of infection.
However, for some infected individuals (especially in non-immune individuals),
symptoms of ‘snail fever’ or Katayama syndrome may develop aft er an incubation
Figure 18.2. Life cycle of schistosomiasis. Reproduced from: Centers for
Disease Control and Prevention (CDC) (http://www.dpd.cdc.gov/dpdx).
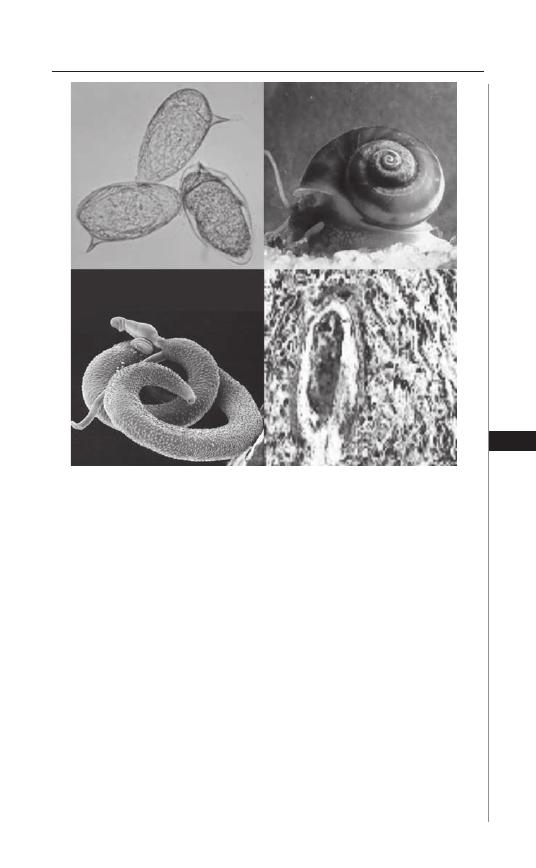
121
Schistosomiasis: Schistosoma mansoni
18
period of 1-2 months. Th is syndrome is associated with abrupt onset of fever, oft en
accompanied by headache, shivering, anorexia, myalgia and right upper quadrant
pain and less commonly with nausea, vomiting, diarrhea, cough and mild broncho-
spasm. Some may also develop hypersensitivity reaction to initial egg deposition,
in the form of urticaria, generalized pruritus, facial edema, erythematus plaques
or purpuric lesions.
Signs of chronic systemic infl ammatory illness may persist and weight loss
is the rule. Clinical signs include hepatosplenomegaly, which may disappear in
few months. Laboratory fi ndings include leukocytosis up to 50,000 per
μ
L, with
pronounced eosinophilia (typically, 20-30% up to 70%), increased serum gamma
globulins, an increase in the sedimentation rate and possibly interstitial infi ltrates
on the chest X-ray.
Figure 18.3. Upper left) S. mansoni eggs (reproduced from: Centers for
Disease Control and Prevention (CDC) (http://www.dpd.cdc.gov/dpdx)).
Upper right) Biomphalaria snail (reproduced from: WHO/TDR). Lower left)
Female S. mansoni worm mated and resident in the groove of an adult male,
(reproduced with permission from Davies Laboratory Uniformed Services
University, Bethesda, MD). Lower right) S. mansoni pathology in liver tissue
(reproduced with permission from the Atlas of Medical Parasitology, Carlo
Denegri Foundation).
122
Medical Parasitology
18
Chronic Manifestations
Intestinal schistosomiasis: Due to chronic egg-mediated infl ammation of the
bowel wall, patients with intestinal schistosomiasis may present with fatigue,
vague abdominal pain, diarrhea alternating with constipation and sometimes
dysentery-like illness with bloody bowel movements. Eggs in the gut wall induce
infl ammation, hyperplasia, ulceration, microabscess formation and polyposis.
Detection of occult blood in the stools is common. Intestinal obstruction second-
ary to marked polyposis is a rare complication.
Hepatosplenic schistosomiasis: Th is complication occurs in 4-8% of patients
with chronic infection and is associated with pronounced host infl ammatory
response and/or sustained heavy infection. With development of portal hyperten-
sion secondary to egg-mediated periportal fi brosis (Symmers’ pipe-stem fi brosis),
secondary phenomena such as ascites, pedal edema, hepatosplenomegaly and
variceal shunting will develop. Th e liver is usually hard with a nodular surface and
frequently a prominent left lobe. Hematemesis secondary to esophageal varices is
frequent and may prove fatal.
Other Manifestations and Complications
1. Cardiopulmonary schistosomiasis: As presinusoidal portal hypertension
develops (see above) it fosters the development of portosystemtic collateral
vessels that allow schistosome eggs to embolize into the pulmonary circulation,
where the eggs can set up an infl ammatory reaction with granuloma forma-
tion, which may block the small pulmonary arterioles. Intractable pulmonary
hypertension and cor pulmonale may result.
2. CNS schistosomiasis may develop due to aberrant worm migration or egg
deposition. A presentation with focal or generalized seizures can occur
with S. mansoni, but transverse myelitis is the most common neurological
manifestation of infection by this species. Myeloradiculopathy may also
occur.
3. In high-risk populations, anemia, growth retardation in children and malnu-
trition are associated with S. mansoni infection, probably as a consequence of
the chronic infl ammation that is associated with infection.
4. Proteinuria can be found in 20-25% of S. mansoni cases with hepatosplenic
disease. In addition, a distinct form of glomerulopathy is associated with
chronic S. mansoni infection.
Interaction with Other Infections
Human Immunodefi ciency Virus
HIV infection may exert a deleterious eff ect on the natural course of schisto-
somiasis in diff erent ways: time until reinfection with schistosomiasis is shorter
in HIV-positive than in HIV-negative individuals. On the other hand, treatment
and response to praziquantel, a drug requiring a functioning immune system to be
eff ective, is not impaired in patients with HIV co-infection. Evidence also suggests
that Schistosoma infection may render the host more susceptible to HIV infection
by interfering with specifi c immune responses.

123
Schistosomiasis: Schistosoma mansoni
18
Salmonella
Prolonged or refractory septicemic salmonellosis is described in S. mansoni -en-
demic areas. Treatment of schistosomiasis is oft en required to cure the Salmonella
infection.
HBV Infection
Signifi cant interactions between schistosomiasis and hepatitis B have been
reported. Th ose people with coinfection are thought to have more severe disease
and a worse prognosis.
Malaria
Malaria and schistosomiasis are co-endemic in many areas and studies have shown
that co-infection with malaria may increase the level of morbidity in hepatosplenic
schistosomiasis and alter the host immune response towards Schistosoma antigens.
Diagnosis
Routine Laboratory Testing
Peripheral eosinophilia is usually present in acute schistosomiasis, but in the
chronic form, the peripheral eosinophilic counts may be minimal, even though
pronounced tissue eosinophilia persists.
Th e detection of S. mansoni eggs in standardized fecal smear preparations
(Kato-Katz method) is the diagnostic method of choice. Th e extent of egg produc-
tion fl uctuates over time, and as many as three separate stool specimens may be
required for diagnosis in some patients. Th e use of formalin-based techniques for
sedimentation and concentration may increase the diagnostic yield.
Other Laboratory Testing
Egg viability testing may help to assess the presence or absence of active
infection, particularly aft er treatment. Testing involves mixing stool samples with
room temperature distilled water and observing the excreted S. mansoni eggs for
hatching of live miracidia.
Stool occult blood can be noted in intestinal schistosomiasis, although it is an
inconstant fi nding and may be due to other disease processes.
Liver function testing is usually within normal limits until the end-stage of
the disease. If LFTs are abnormal, then one should consider co-infection, e.g.,
viral hepatitis.
Anti-S. mansoni serology—Antibody testing is a useful epidemiological tool,
but cannot diff erentiate active from past infection and, unlike egg counts, it does
not allow quantifi cation of infectious burden. Serology may be used to help in the
diagnosis of patients from nonendemic areas, because negative antibody titers are
expected and negative testing may help to exclude diagnosis.
Other investigational blood testing involves detection of circulating parasite
antigens such as CAA and CCA (circulating anodic and cathodic proteoglycan
antigens); these are thought to indicate active infection and also to quantify the
intensity of infection. Studies are underway to evaluate the sensitivity and specifi c-
ity of these tests under fi eld conditions.
124
Medical Parasitology
18
Imaging
Ultrasound of the liver and spleen is an early and accurate diagnostic method
for detecting S. mansoni-associated periportal fi brosis and hepatosplenomegaly
and also serves to grade or stage the hepatosplenic disease. Portable ultrasound
machines are becoming relatively inexpensive and scanning may serve as an eff ective
diagnostic method for S. mansoni-related disease in endemic areas.
Invasive procedures—Rectal biopsies and mucosal biopsies of the bowel are
eff ective in visualizing eggs and helpful when the stool sample testing is negative,
typically in the case of light infection. It is recommended to obtain multiple biopsy
samples and to crush them between two slides to increase volume of sampling and to
increase the likelihood of fi nding the eggs in the tissues. In advanced disease, upper
gastrointestinal endoscopy may be used to assess and treat esophageal varices.
Treatment
Acute Schistosomiasis
For acute schistosomiasis mansoni, the treatment is usually supportive and
focused on controlling symptoms. In the case of severe and persistent symptoms,
specifi c antiparasitic treatment combined with anti-infl ammatory therapy may
play a role. Prednisone 1 mg/kg a day before antiparasite treatment, for 1 wk,
starting one day before the antiparasitic treatment, followed by 0.5 mg for the
second week and 0.25 mg for the third week, may increase the chances of cure
and improve symptoms.
Chronic Schistosomiasis
Th e aim of chemotherapy in chronic schistosomiasis is two-fold. Th e fi rst goal
is to cure the disease, or at least minimize its associated morbidity. Th e second goal
is to limit transmission of the parasite within the endemic area. Praziquantel and
oxamniquine are commonly used for S. mansoni treatment, but because praziquan-
tel is active against all Schistosoma species, it is, overall, the preferred treatment for
schistosomiasis. Clinical studies have shown that artemether, which is an eff ective
antimalarial treatment, is also active against immature forms of all three major
schistosome parasites. Trials that involve the combination of artemether and prazi-
quantel treatment indicate that combination therapy might have a more benefi cial
eff ect than monotherapy in terms of cure rate and morbidity reduction.
Praziquantel (Biltricide and generics), is a pyrazinoisoquinolone derivative. It
is currently the mainstay of treatment of schistosomiasis mansoni, as it is the most
eff ective and most readily available agent. It plays a critical part of community-based
schistosomiasis control programs. Treatment is usually well tolerated, although side
eff ects can include dizziness, drowsiness, headache, nausea, vomiting, abdominal
pain, pruritus, hives and diarrhea. Such side eff ects are not long lasting and usually
resolve in less than 2 hours. Praziquantel may provoke signifi cant infl ammatory
symptoms when given to patients with neurocysticercosis, so that the drug should
be used with caution in areas where S. mansoni and T. solium are both endemic.
Praziquantel’s mechanism of action is complex. It is thought to damage the
worm’s outer tegument membrane (the natural covering of the worm body) and
expose the worm to the body’s immune response, which ultimately results in worm

125
Schistosomiasis: Schistosoma mansoni
18
death. In treating S. mansoni infection, the praziquantel-mediated cure rate is
equal to or greater than 85-90%. Among those persons not cured, the worm load
and associated egg burden are markedly decreased. Th e recommended dose for
praziquantel treatment is a single treatment of 40 mg/kg as a single dose or divided
into two doses over 1 day. When possible, rescreening for persistent infection 6-8
weeks aft er initial therapy, followed by repeat dosing as needed, will provide optimal
cure rates and morbidity control.
Oxamniquine (Vansil), is a tetrahydroquinoline antischistosomal agent that
is solely eff ective against S. mansoni. In animal models, the drug is seen to cause
paralysis of the worm with a consequent shift of worms into the liver. Th is appears
to be associated with damage the tegumental surface of male schistosome worms, so
that the host immune system is able to kill them. It also stops female worms from
producing eggs, with a subsequently marked reduction in host morbidity. Dosing
is 15 mg/kg once (in S. America) or twice daily for 2 days (in Africa). Overall cure
rate is 60-100%. Th is drug is not available in the United States.
Artemisinin derivatives, artemether and artesunate, are sesquiterpene lactones
derived from the active components of the plant Artemisia annua, and these drugs
are best known for their antimalarial properties. Th e antischistosomal activities of
artemisinin derivatives were discovered in the 1980s, with initial interest focusing
on its eff ects on S. japonicum. Animal studies have since suggested that these drugs
are also eff ective against S. mansoni and clinical testing of artmether for prevention
of schistosomiasis mansoni suggests that the artemesinins are safe and eff ective
drugs for treatment of early stages of infection.
Combination chemotherapy with praziquantel and oxamniquine has been
tested—initial studies suggest some synergy, but direct comparison against mono-
therapy has not been done and most of the populations studied have had concurrent
infections with S. haematobium, which is not susceptible to oxamniquine. Further
studies are warranted.
Initial laboratory and nonrandomized studies of combined praziquantel and
artemesinin derivatives suggested greater eff ectiveness for the combination therapy.
A recent randomized, double blind, placebo-controlled trial in Gabon indicated
that the overall cure rate with combination therapy was not substantially higher
compared to praziquantel alone, but in terms of the egg count reduction, the
praziquantel-artemsinin combination had greater benefi cial eff ect.
Emergence of drug resistance or tolerance is a threat to all chemotherapy-based
control programs. Risk of schistosome resistance to praziquantel has been recently
reviewed by Cioli who concluded that resistance is likely to exist, but it is currently
of low-level and its clinical importance is, so far, minimal. Th e conclusion from
recent studies has been that there is no conclusive evidence of emerging resistance
of S. mansoni to praziquantel, and the observational studies that previously sug-
gested drug failure may be explained by the specifi c transmission charcteristics.
Unfortunately, there is no reliable testing available to determine praziquantel
resistance in vitro.
Resistance to oxamniquine is undisputedly documented in vitro and in vivo,
but its impact has been limited and it is not yet considered to be a problem for
control program operations.
126
Medical Parasitology
18
Advanced S. Mansoni-Associated Disease
For patients with late, severe complications of S. mansoni infection such
as GI bleeding, intestinal obstruction, cardiopulmonary syndromes, or CNS
disease, in-patient treatment and supportive measures (beyond antiparasite
therapy) are needed. Endoscopy and variceal sclerosis or surgical treatment
(portosystemic shunting) are indicated for severe bleeding secondary to portal
hypertension.
Prognosis aft er Treatment
Response to treatment is evaluated by assessing the amount of decrease in egg
excretion. In the fi rst two weeks aft er treatment, excreted egg counts may not de-
crease because eggs that were laid before the treatment can require up to 2 weeks
to be shed. Even with eff ective therapy, some viable eggs can be excreted for up to
6-8 weeks aft er treatment. Newer tests that measure circulating parasite antigens
(CCA, CAA), when measured 5-10 days aft er treatment, may better assess acute
antiworm therapeutic response. Persistent circulating antigen, or the persistent
excretion of eggs at 6-8 weeks, indicates residual infection. Such patients should
be retreated as needed to eff ect a cure.
Signifi cant Points about Th erapy
• Early intestinal, hepatic and portal vein disease usually improves with
treatment.
• In the absence of other forms of liver disease, hepatosplenic schistosomiasis
can have a relatively good prognosis because hepatocyte function is preserved.
Late disease (in which variceal bleeding occurs) may be irreversible and result
in fatality due to severe hemorrhage and its acute complications. In commu-
nity treatment, approximately 40% of hepatosplenic disease is reversed with
therapy.
• S. mansoni-associated cor pulmonale is a form of advanced schistosomiasis and
usually does not improve signifi cantly with antiparasitic treatment. Adjunctive
treatment with selective vasodilators (sildenafi l) might prove benefi cial.
• Spinal cord schistosomiasis carries a guarded prognosis. Pharmacologic or
surgical decompression as appropriate, combined with specifi c antiparasite
praziquantel treatment, should be administered as soon as possible.
Prevention and Prophylaxis
Over the last two-to-three decades, large-scale national and regional schis-
tosomiasis control programs have been implemented in a number of areas with
varying levels of success. Th e most important examples of successful schistosomiasis
control have been reported from Brazil, Venezuela, Cambodia, China, Egypt and
the Philippines. Given the strong link between environmental factors, poverty and
schistosomiasis transmission, it is imperative to take full advantage of the renewed
contemporary international emphasis on the provision of of clean water and
sanitation, as this represents a fundamental basis for schistosomiasis transmission
control. A combined transmission reduction strategy would mutually reinforce
chemotherapy-based morbidity control and provide the optimal preventive strategy
for resource-poor developing areas.

127
Schistosomiasis: Schistosoma mansoni
18
Programs for controlling Schistosomiasis mansoni within an endemic area should
consider (and optimally include) all of the following:
• Population-based chemotherapy.
• Provision of a safe water supply and washing/swimming facilities to reduce
exposure.
• Health education that promotes improved sanitation, including means for
reduction in local water contamination by schistosome egg-containing urine
or stool.
• Control of intermediate host snails (Biomphalaria spp.).
• Instruction to travelers and immigrants to avoid contact with fresh water in
endemic areas.
• As local transmission falls, anticipate increased incidence of acute schistoso-
miasis in the setting of any recent freshwater contact. Make early treatment for
S. mansoni available if diagnostic test results are positive or clinical suspicion
is high.
• Consider clinical trials (involving human volunteers) towards development
of an eff ective vaccine against Schistosomiasis mansoni.
Suggested Reading
1. Case records of the Massachusetts General Hospital. Weekly clinicopathological
exercises. Case 21-2001. A 31-year-old man with an apparent seizure and a mass
in the right parietal lobe. N Engl J Med 2001; 345:126-31.
2. Carod Artal FJ, Vargas AP, Horan TA et al. Schistosoma mansoni myelopathy:
clinical and pathologic fi ndings. Neurology 2004; 63:388-91.
3. Cioli D. Praziquantel: is there real resistance and are there alternatives? Curr Opin
Infect Dis 2000; 13:659-63.
4. Corachan M. Schistosomiasis and international travel. Clin Infect Dis 2002;
35:446-50.
5. Gellido CL, Onesti S, Llena J et al. Spinal schistosomiasis. Neurology 2000;
54:527.
6. Ferrari ML, Coelho PM, Antunes CM et al. Effi cacy of oxamniquine and prazi-
quantel in the treatment of Schistosoma mansoni infection: a controlled trial. Bull
World Health Organ 2003; 81:190-6.
Figure 18.4. Left panel) Spray mollusciciding for snail control. Right
panel) Water contact activities associated with risk for S. mansoni infection.
Reproduced with permission from the Atlas of Medical Parasitology, Carlo
Denegri Foundation.
128
Medical Parasitology
18
7. King CH. Acute and chronic schistosomiasis. Hosp Pract 1991; 26:117-30.
8. Mahmoud AA, ed. Schistosomiasis. London: Imperial College Press, 2001.
9. Richter J, Hatz C, Haussinger D. Ultrasound in tropical and parasitic diseases.
Lancet 2003; 362:900-2.
10. Ross AG, Bartley PB, Sleigh AC et al. Schistosomiasis. N Engl J Med 2002;
346:1212-20.
11. Utzinger J, Keiser J, Shuhua X et al. Combination chemotherapy of schistosomiasis
in laboratory studies and clinical trials. Antimicrob Agents Chemother 2003;
47:1487-95.
12. Utzinger J, N’Goran EK, N’Dri A et al. Oral artemether for prevention of
Schistosoma mansoni infection: randomised controlled trial. Lancet 2000;
355:1320-5.
13. van Lieshout L, Polderman AM, Deelder AM. Immunodiagnosis of by determina-
tion of the circulating antigens CAA and CCA, in particular in individuals with
recent or light infections. Acta Trop 2000; 77:69-80.
14. Vennervald BJ, Dunne DW. Morbidity in schistosomiasis: an update. Curr Opin
Infect Dis 2004; 17:439-47.
15. Wynn TA, Th ompson RW, Cheever AW et al. Immunopathogenesis of schistoso-
miasis. Immunol Rev 2004; 201:156-67.

C
HAPTER
19
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Schistosomiasis: Schistosoma haematobium
Vijay Khiani and Charles H. King
Background
Schistosoma haematobium is a parasitic trematode that infects over 111 mil-
lion people, mostly in Africa and the Middle East. It is the most common cause
of urinary schistosomiasis, which is caused by antiparasite infl ammation in the
wall of the human host’s bladder, ureters, or kidneys. Because the syndrome is
unfamiliar to most physicians in the United States, they may oft en overlook
the diagnosis of S. haematobium infection and its associated disease when pre-
sented with ‘occult’ cases of hematuria, hematospermia, pelvic infl ammation, or
infertility. While the typical returning traveler or immigrant is more likely to
be infected with hepatitis, malaria, or intestinal helminths, it should be noted
that schistosome infection is not uncommon aft er either short- or long-term
travel to endemic areas.
S. haematobium infection is prevalent in areas that have a reservoir of human
infection, the presence of an intermediate Bulinus species snail host and the poor
socioeconomic conditions or poor sanitation that allow urinary contamination
of local freshwater. Th ese factors are all essential components of the parasitic
trematode life cycle, which requires transmission from a defi nitive human host
(in which adult worms undergo sexual reproduction) to an intermediate snail host
(in which asexual multiplication of larvae occurs) and then the reverse process of
snail-to-human transmission.
The mode of transmission of S. haematobium to humans begins with
exposure to fresh water that contains infected snail intermediate hosts (Fig.
19.1). Of note, the transmission of schistosomiasis is NOT person-to-person.
Infected snails release free-swimming cercariae, which seek and penetrate human
skin. The cercariae are typically 2 mm in length and have acetabular and head
glands to aid in attachment and penetration of the skin. Upon penetrating the
epidermal layer, the cercariae reach the dermis and transform into immature
larval forms called schistosomula. Over a period of two months, these schis-
tosomula migrate first to the lungs (through venous circulation) and then to
the left heart and into the systemic circulation. The schistosomula proceed
to the liver, where they mature into either male or female adult worms. The
S. haematobium worms, now 1 to 2 cm in length, migrate to the veins of the
bladder (or, less frequently, the bowel), where the worms lie together in pairs
and the female worms lay their eggs in the wall of the nearby urinary collect-
ing system. The mature female worms deposit several hundred eggs each day.
Approximately 50% of these eggs will become inadvertently trapped in host
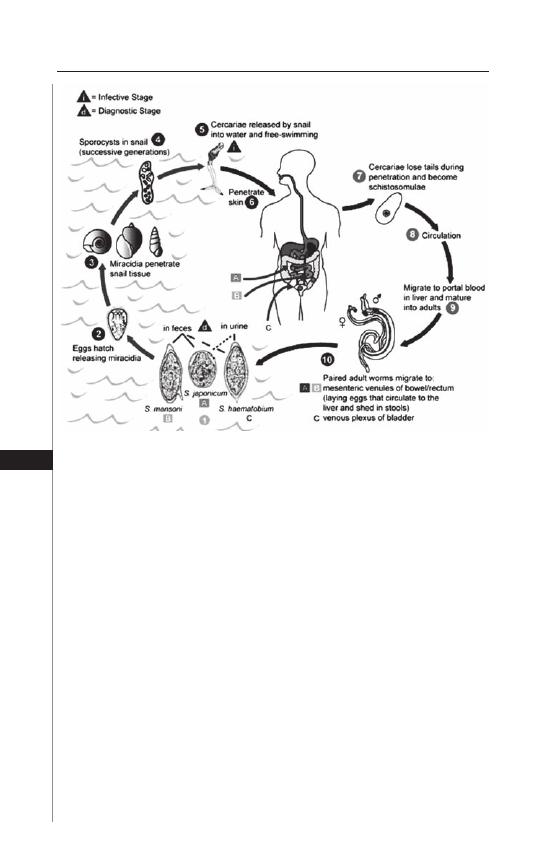
130
Medical Parasitology
19
tissues, while the other half manage (via local inflammation and ulceration) to
penetrate through to the lumen of the ureter or bladder. From there, the eggs
pass in the urine into the environment to reach bodies of fresh water. These
eggs hatch and release motile ciliated forms called miracidia into the water,
which seek out and infect the intermediate Bulinus species snail hosts by direct
penetration. In the snail, the miracidia develop into sporocysts, which complete
the life-cycle by a process of asexual reproduction that yields multiple infectious
cercariae after a period of 1-2 months.
Most infected humans (50 to 75%) carry light infection with relatively low
worm burdens, while a minority (1 to 5%) carry very heavy infection (defi ned as
excreting > 400 eggs/10 mL urine), which results in the production of thousands
of eggs daily. As a consequence, heavily infected hosts are most prone to the pro-
gressive disease sequelae of S. haematobium, including severe hematuria, fi brosis,
ureteral stricture and calcifi cation of the urinary tract. However, any level of infec-
tion can cause disease, as a function of the relative intensity of the aff ected host’s
anti-egg immune response. In some endemic areas, there is a defi nite link between
S. haematobium and squamous cell carcinoma of the bladder and S. haematobium
has been formally listed as a carcinogen by the WHO. Genetic variation of the
parasite and host are also likely factors in determining the pathogenesis and clinical
outcomes of infection and disease.
Figure 19.1. Transmission and life cycle of human schistosomes. Reproduced
from: Centers for Disease Control and Prevention (CDC), Atlanta, GA (http://
www.dpd.cdc.gov/dpdx).
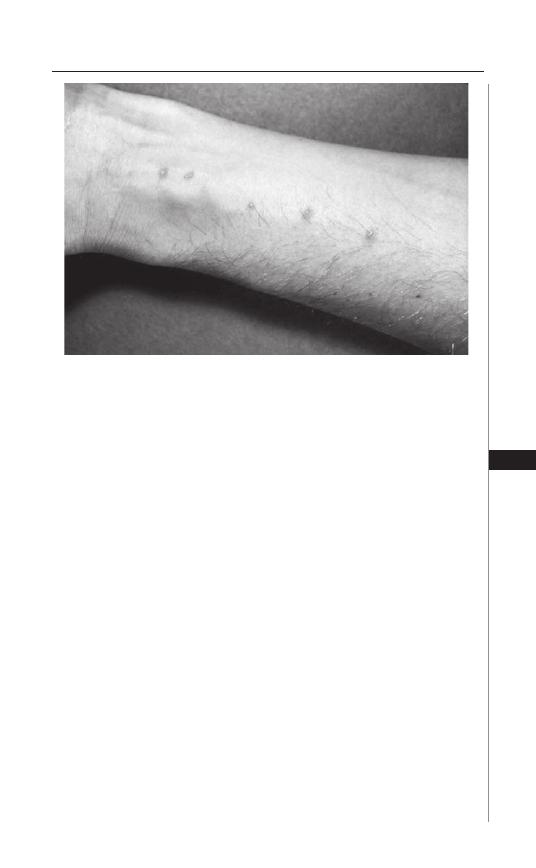
131
Schistosomiasis: Schistosoma haematobium
19
Disease Signs and Symptoms
Immediate Manifestations
Th e earliest form of infection-associated disease for patients aff ected by S. hae-
matobium is cercarial dermatitis (Fig. 19.2), which is, in essence, a maculopapular,
blistering eruption in the area of skin exposed to parasite penetration. Th is presenta-
tion, which can be due either to an immediate or delayed hypersensitivity, usually
begins 1-2 days aft er exposure, lasts for a few days and is self-limited.
Acute Schistosomiasis
Acute schistosomiasis, also known as Katayama fever or snail fever, is most
oft en seen in travelers who visit endemic areas for the fi rst time, contract a
schistosome infection and then present with signs of fever, lymphadenopathy,
hepatosplenomegaly and blood eosinophilia some 4-6 weeks later. Th is process is
usually triggered during the early egg deposition period of the parasite’s life cycle
and tends to aff ect the adolescent or young adult patient most frequently.
Th is acute process is related to an initial allergic hypersensitivity to the parasite,
as well as subsequent formation of soluble immune complexes that can cause a
serum-sickness type of illness. Acute schistosomiasis is usually a self-limited process,
but can become quite severe and debilitating. In some cases, because the process
of infl ammation is antigen driven, acute schistosomiasis may even worsen with
antiparasitic therapy. In such cases, it is recommended to start with symptomatic
therapy to eff ect control of the process of infl ammation, a step which usually
requires the use of corticosteroids. Th is is then followed by specifi c antiparasitic
therapy (praziquantel) to eliminate the causative infecting worms.
Figure 19.2. Cercarial dermatitis cause by exposure to schistosome-infested
water. Reproduced from: Centers for Disease Control and Prevention (CDC)
(http://phil.cdc.gov/phil/details.asp).
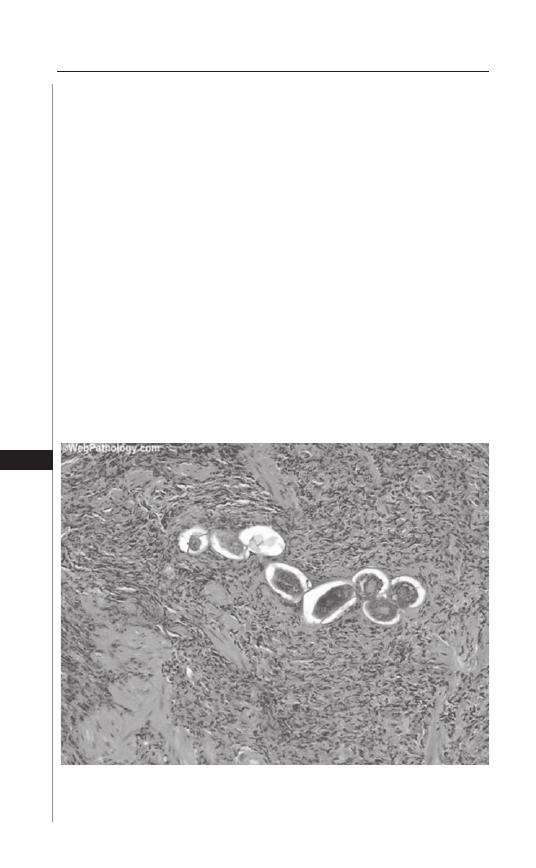
132
Medical Parasitology
19
Chronic Schistosomiasis
Chronic schistosomiasis in humans is a consequence of prolonged (multi-year)
and repeated infection with schistosome parasites. In endemic areas, chronic
schistosomiasis is oft en established by age fi ve, but as a consequence of repeated
exposure, the intensity of worm burden increases over time, with a peak, maximum
burden of infection experienced at ∼13 years of age. Th e chronic form of disease
develops as many S. haematobium eggs remain trapped in the host tissues and
become surrounded by delayed-type hypersensitivity granulomatous infl amma-
tion (Fig. 19.3). Th is infl ammation is formed by host lymphocytes, eosinophils
and macrophages and ultimately kills and destroys the parasite eggs. However,
the resolution of the infl ammation is associated with collagen deposition and scar
formation. Gradual accumulation of this scar tissue within bladder and ureters
can lead to deformity, contractile incoordination and obstruction, resulting in
hydroureter, hydronephrosis and ascending bacterial infection due to urinary
refl ux. Infl ammation can result in local ulceration and signifi cant blood loss in
the urine and/or feces. Additionally, the chronic presence of infl ammation may
be associated with dyserythropoesis (anemia of chronic disease) and signs of iron
and/or protein-calorie malnutrition.
Th e severity of the manifestations associated with chronic schistosomiasis
is related both to the extent of exposure to the infection and to the intensity
of host immune response. For those with less intense exposure, the early signs
and symptoms include dysuria, proteinuria and bladder polyps. Later signs and
Figure 19.3. S. haematobium eggs surrounded by host infl ammation in bladder
wall. Reproduced from: http://webpathology.com/image.cfm?case=51&n=6,
with kind permission of Dharam M. Ramnani, MD.
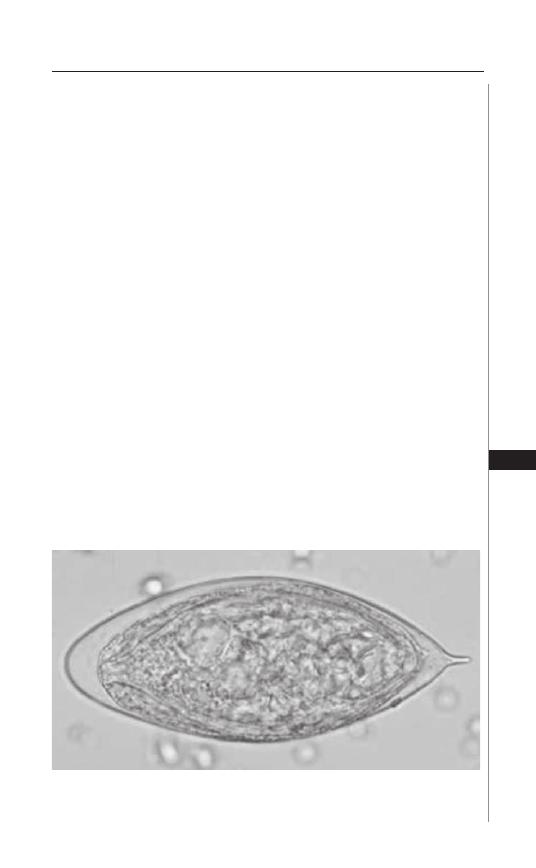
133
Schistosomiasis: Schistosoma haematobium
19
symptoms include hydronephrosis, hydroureter, bladder calcifi cation, increased
risk of urinary tract infections and ultimately squamous cell carcinoma of the
bladder. For hosts with eggs in the bladder or lower ureters, over 50% of the
patients have symptoms of dysuria, frequency and terminal hematuria. Cor
pulmonale can result via direct passage of eggs through the ureteral veins to
the systemic veins, resulting in egg transport to the pulmonary circulation and
trapping in the lung tissues. Central nervous system disorders can result from
eggs passing into the brain, spinal cord, meninges, or ventricles, or from aberrant
migration of worms into CNS tissues. Th e resulting infl ammation can lead to
seizures, spinal cord compression, or hydrocephalus.
Diagnosis
Th e most important aspect of making the diagnosis of S. haematobium is to
appreciate risk of exposure. Possible urinary schistosomiasis can be established by
means of a thorough history and physical examination. Specifi cally, the history
should focus on specifi cs of travel history, including the geographic areas visited
and known or possible exposures to freshwater bodies while abroad. Upon comple-
tion of the history and examination, one may begin to pursue tests and studies to
arrive at the fi nal diagnosis.
Th e majority of cases of S. haematobium are diagnosed by the fi nding of
parasite eggs in urine or feces. Concentration methods are helpful in increasing
the sensitivity of urine testing. Sedimentation or membrane fi ltration techniques
are oft en used when analyzing the urine for S. haematobium. Cystoscopy is rarely
required, but may be helpful in recovering parasite eggs in cases of light infection
with signifi cant pathology. S. haematobium eggs are spindle shaped, usually around
140-150 by 60 micrometers (Fig. 19.4). Th e eggs have a terminal spine, which is
similar to the appearance of another, less common human parasite, S. intercalatum.
Th e two species can be diff erentiated by the Ziehl-Nielsen test, which is negative
for eggs of S. haematobium.
Figure 19.4. S. haematobium egg in urine sediment. Note characteristic
terminal spine at right. Reproduced from: Centers for Disease Control and
Prevention (CDC), Atlanta, GA (http://www.dpd.cdc.gov/dpdx).

134
Medical Parasitology
19
Parasite eggs may persist in human tissues for a number of years, even fol-
lowing eff ective therapy. Because of this, egg viability testing (by vital staining or
miracidial hatching) may be necessary to establish the presence of active infection.
Serologic testing for S. haematobium is sensitive, but not very specifi c due to the
fact that antiparasite antibodies persist aft er infection resolves. In consequence, the
specifi city is too low to be used as a diagnostic tool for patients in endemic areas.
Radiologic testing remains yet another option that can provide useful diagnostic
information. For example, an ultrasound may demonstrate evidence of obstructive
uropathy with fi ndings of bladder wall thickening and characteristic granulomas,
calcifi cation, hydroureter and hydronephrosis.
Treatment
For patients with S. haematobium infection who present with cercarial derma-
titis, this initial presentation is usually self-limited and responds to local topical
care. Treatment of acute schistosomiasis, as described earlier, focuses on control
of infl ammation fi rst, followed by specifi c antiparasite therapy. It is important to
note that immature schistosome parasites are relatively resistant to the eff ects of
the standard antischistosomal drug, praziquantel. To fully eradicate infection, it
may be necessary to provide retreatment of patients with acute schistosomiasis at
a point 2-3 months aft er last exposure.
For chronic schistosomiasis haematobia, the treatment of choice is praziqu-
antel, 40 mg/kg, as a single oral dose or in two divided doses. Praziquantel is
formulated as scored, breakable 600 mg pills, so that dose can be appropriately
adjusted to body weight (see Table 19.1).
A single treatment is 80-90% eff ective with limited side eff ects. Praziquantel side
eff ects include sedation, malaise, headache, dizziness, abdominal discomfort and
nausea. Th e minority of patients who fail to clear their S. haematobium infection
aft er praziquantel therapy nevertheless typically have a >95% decrease in their egg
burden, which indicates signifi cant reduction of infection. If egg excretion persists
for more than one month, an additional round of praziquantel therapy (40 mg/kg,
PO, once) should be given. Th ere are some patients that have an acute exacerbation
of symptoms during praziquantel treatment and these individuals may need a short
course of anti-infl ammatory medications such as antihistamines or corticosteroids.
Metrifonate is an alternative treatment of choice for patients with S. haematobium
infection, but may not be commercially available.
Table 19.1. Praziquantel doses to treat schistosomiasis
No. of Praziquantel Tablets
Body Weight (kg)
Height
(600 mg tablet)
10.0-14.9 (94-110
cm) 1
15.0-22.4 (110-125
cm) 1½
22.5-29.9 (125-138
cm) 2
30.0-37.4 (138-150
cm) 2½
37.5-44.9 (150-160
cm) 3
45.0-59.9 (160-178
cm) 4
60.0-75.0 (>178
cm) 5

135
Schistosomiasis: Schistosoma haematobium
19
Prevention and Control
Disease due to S. haematobium infection remains the most prevalent form
of urinary schistosomiasis in sub-Saharan Africa. Th e WHO has established the
goal to treat 75% of school-age children with infection and at risk of morbidity by
2010. Morbidity is particularly associated with cases with S. haematobium infection
who develop immunopathological sequelae, such as hepatosplenomegaly, bladder
deformity and hydronephrosis. Endoscopic/surgical interventions may be needed
for hematuria, urinary obstruction, bladder cancer and CNS disease. For persons
living in high transmission areas, regular treatment may prevent S. haematobium
infection from developing into serious disease.
Even though an estimated 150,000 individuals die from schistosomiasis infec-
tion on a yearly basis, there is still no form of prophylaxis for patients with risk of
exposure. However, there are many important ways to improve upon current risk for
transmission. It is vital to avoid fresh immersion in any freshwater in contaminated
areas. Because transmission is highly focal and sporadic, one should avoid cutane-
ous exposure to all ponds, lakes, rivers and streams in endemic areas. Bath water
should be heated for at least 5 minutes at 150 degrees F, or let stand in a cistern or
container (known to be completely free of snails) for 3 days.
For residents of endemic areas, sanitation, socioeconomic development and
health education are key components.
Suggested Reading
1. Brouwer KC, Ndhlovu PD, Wagatsuma Y et al. Urinary tract pathology attributed
to Schistosoma haematobium: does parasite genetics play a role? Am J Trop Med
Hyg 2003; 68:456-62.
2. Centers for Disease Control. Schistosomiasis among river raft ers—Ethiopia.
MMWR 1983; 32:585-6.
3. Gonzalez E. Schistosomiasis, cercarial dermatitis and marine dermatitis. Dermatol
Clin 1989; 7:291-300.
4. King CH, Mahmoud AA. Drugs fi ve years later: praziquantel. Ann Intern Med
1989; 110:290-6.
5. King CH. Disease in Schistosomiasis Haematobia. In: Mahmoud AAF, ed.
Schistosomiasis. London: Imperial College Press, 2001:265-96.
6. King CH. Ultrasound monitoring of structural urinary tract disease in S. haematobium
infection. Memorias do Instituto Oswaldo Cruz 2002; 97:149-52.
7. King CL. Initiation and regulation of disease in schistosomiasis. In: Mahmoud
AAF, ed. Schistosomiasis. London: Imperial College Press, 2001:213-64.
8. Smith JH, Christie JD. Th e pathobiology of Schistosoma haematobium infection
in humans. Hum Pathol 1986; 17:333-45.
9. Peters PAS, Kazura JW. Update on diagnostic methods for schistosomiasis. In:
Mahmoud AAF, ed. Balliere’s Clinical Tropical Medicine and Communicable
Diseases, Schistosomiasis, Vol. 2. London: Bailliere Tindall, 1987:419-33.
9. van der Werf MJ, de Vlas SJ, Brooker S et al. Q uantifi cation of clinical morbidity associ-
ated with schistosome infection in sub-Saharan Africa. Acta Trop 2003; 86:125-39.
10. Visser LG, Polderman AM, Stuiver PC. Outbreak of schistosomiasis among
travelers returning from Mali, West Africa. Clin Infect Dis 1995; 20:280-5.
11. World Health Organization. IARC Monographs on the Evaluation of
Carcinogenic Risks to Humans. Schistosomes, Liver Flukes and Helicobacter
pylori. Vol. 61 Geneva: World Health Organization, 1994.
12. World Health Organization. Prevention and control of schistosomiasis and
soil-transmitted helminthiasis: Report of a WHO expert committee. Technical
Report Series 912 2002. Report No. 912.


S
ECTION
III
Cestodes

C
HAPTER
20
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Taeniasis and Cyticercosis
Hannah Cummings, Luis I. Terrazas and Abhay R. Satoskar
Background
Taeniasis and cysticercosis are diseases resulting from infection with parasitic
tapeworms belonging to Taenia species. Approximately 45 species of Taenia have
been identifi ed; however, the two most commonly responsible for human infec-
tion are the pork tapeworm Taenia solium and the beef tapeworm Taenia saginata.
Parasitic tapeworm infections occur worldwide, causing sickness, malnutrition and
oft en resulting in the death of their host. Infection with adult tapeworms of either
T. solium or T. saginata cause taeniasis in humans. Th e metacestode, or larval stage,
of Taenia solium causes the tissue infection, cysticercosis. Clinical manifestations
associated with the tapeworm infection can vary greatly and may range from mild
forms where patients exhibit little to no symptoms, to severe life-threatening forms
which are oft en fatal.
Geographic Distribution and Transmission
Taenia infections are estimated to aff ect 100 million people worldwide, with
major endemic areas located primarily in the developing countries of South
America, Africa, India, China and Southeast Asia. Th e ingestion of cysticerci
from raw or undercooked meat facilitates the transmission of T. solium from
pigs to humans and is presumably responsible for the high prevalence of hu-
man cysticerosis in these regions. It is estimated that anywhere between 5-40%
of individuals carrying the adult tapeworm will develop cysticercosis. Taenia
infections are less common in North America; however neurocysticercosis has
been recognized as an important health problem in California. Although this
disease is mainly seen in migrant workers from Latin American, it has also been
reported in US residents who have not traveled to endemic countries.
Life-Cycle
Th e complete life-cycle of Taenia solium involves two hosts: the pig and
the human, whereas that of Taenia saginata involves the cow and the human
(Fig. 20.1). Humans act as the defi nitive host and harbor the adult tapeworm in
the small intestine. Infection is acquired either through the accidental ingestion
of embryonated eggs passed in the feces of an individual infected with the adult
tapeworm, or through the consumption of raw or poorly cooked meat containing
cysticerci. Th e cysticerca develops into an adult worm in the gut; these worms
can survive up to 25 years. Depending on the species of Taenia, an adult worm
can reach lengths between 2-25 meters and may produce as many as 300,000 eggs

139
Taeniasis and Cyticercosis
20
per day. Th e morphology of the adult worm consists of a scolex and a strombila.
Th e scolex acts as the organ of attachment and consists of four suckers equipped
with hooklets. Th e strombila consists of several segments (proglottids) with the
gravid or egg-carrying proglottids located toward the posterior end of the worm
(Fig. 20.2). Individual proglottids may contain as many as 40,000 eggs in T. solium
or as many as 100,000 eggs in T. saginata.
Both the proglottids and the eggs are released with the feces of infected
individuals and serve as a source of infection for pigs and cattle, which act as
intermediate hosts for these parasites. Following the ingestion of eggs, mature
larvae (onchospheres) are released in the gut. Th ese onchospheres enter the blood
stream by penetrating the small intestine and migrate to skeletal and cardiac
muscles where they develop into cysticerci. Cysticerci may survive in the host
tissues for several years causing cysticercosis (Fig. 20.3). Th e consumption of
raw or undercooked meat containing cysticerci facilitates the spread of infec-
tion from pigs to humans. In humans, cysticerci transform into adult tapeworms
which persist in the small intestines for years causing taeniasis. Th e time between
initial infection and the development of the adult worm occurs over a period of
approximately 2 months. In some instances, an infected individual harboring
the adult worm can become auto-infected through the accidental ingestion of
eggs released in the feces.
Figure 20.1. Life cycles of the beef tapeworm, Taenia saginata and the pork
tapeworm, T. solium. Reproduced from: Nappi AJ, Vass E, eds. Parasites of
Medical Importance. Austin: Landes Bioscience, 2002:61.
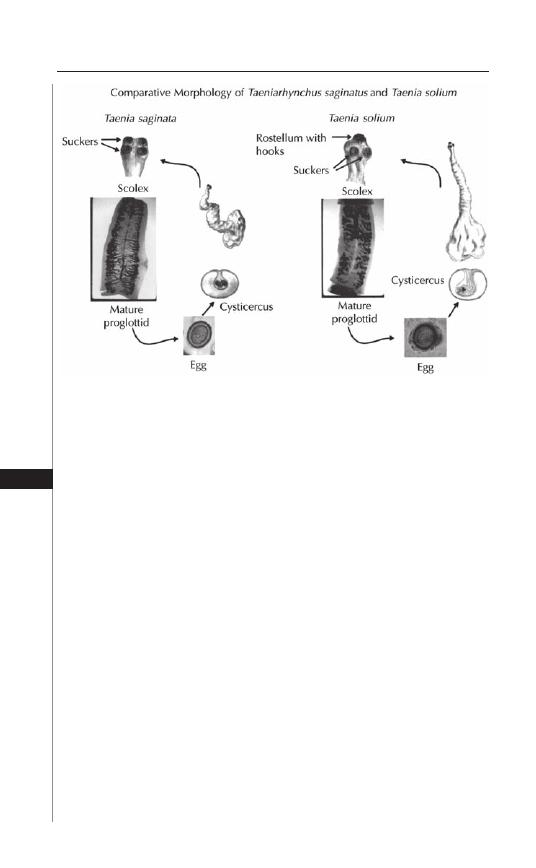
140
Medical Parasitology
20
Immunobiology
Infection with the adult tapeworm occurs in the small intestine of the human
host and has been shown to induce a Th 2-type immune response characterized
by high levels of IL-4 and IL-10 expression and an increase in immunoglobulin
production, primarily IgG. Antibodies produced in response to parasite antigens
appear to be somewhat eff ective in the destruction of the early larval form, but
off er little to no protection against cysticerci present within the tissues.
Viable cysticerci produce little to no infl ammation within the surrounding
tissues and their ability to suppress the host infl ammatory response undoubt-
edly plays a major role in their ability to survive within the host for extended
periods of time. In contrast, the death or destruction of cysticerci within host
tissues has been shown to induce a strong Th 1-type cell-mediated infl ammatory
response, characterized by high levels of interferon-gamma and the formation
of granulomas containing lymphocytes, eosinophils, granulocytes and plasma
cells. Experimental data using a mouse model suggest that the development of
a Th 1 cell-mediated infl ammatory response controls parasite growth, whereas a
Th 2-type response increases levels of susceptibility to chronic infection.
Th ese parasites have developed numerous methods for evading the host immune
response. Although the ingested oncospheres which are capable of penetrating the
intestinal mucosa are susceptible to destruction by host compliment and antibody
responses, the time required to generate these antibodies allows the oncosphere
to transform into the highly resistant metacestode form. Th e metacestode form,
resistant to complement-mediated destruction, produces a variety of molecules
eff ective in evading the host immune response. Th e serine-threonine protease
Figure 20.2. Morphology of Taenia saginata and T. solium. Reproduced
from: Nappi AJ, Vass E, eds. Parasites of Medical Importance. Austin: Landes
Bioscience, 2002:62.
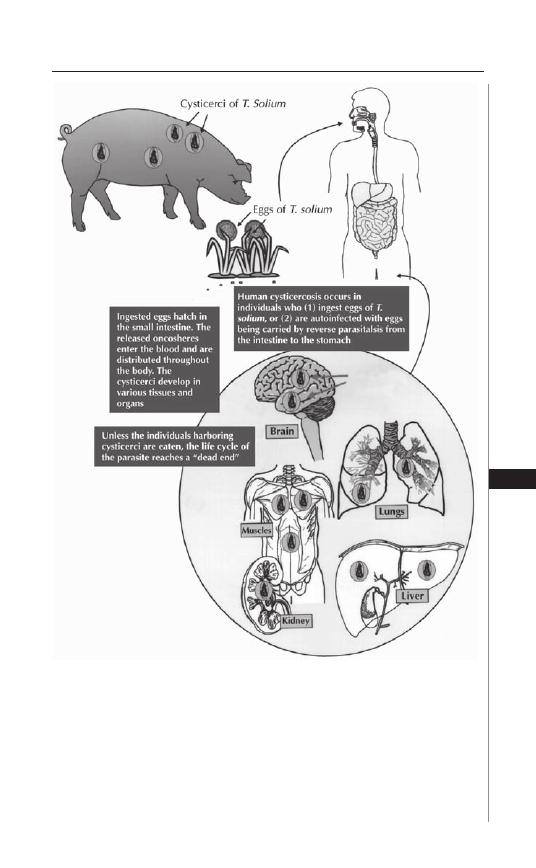
141
Taeniasis and Cyticercosis
20
inhibitor, Taeniastatin, inhibits complement activation, blocks cytokine produc-
tion and interferes with neutrophil function. Paramyosin renders parasite killing
by the host complement cascade ineff ective, primarily through inhibiting the
activity of C1q. Activated complement is directed away from the parasite by the
production of sulfated polysaccharides. Antibodies produced by the host bind the
metacestode form through Fc receptors and are degraded, possibly functioning as
Figure 20.3. Development of cysticercosis in humans. Reproduced from:
Nappi AJ, Vass E, eds. Parasites of Medical Importance. Austin: Landes
Bioscience, 2002:63.

142
Medical Parasitology
20
a source of amino acids for the parasite. Glutathione S-transferase and other small
molecules produced by the cyst form are involved in the detoxifi cation of toxic
oxygen intermediates and the suppression of host infl ammation.
Signs and Symptoms
Taeniasis
Taeniasis is an infection with the adult tapeworm which usually remains con-
fi ned to the small intestine. Most oft en, such infection results in minor gastroin-
testinal irritation and is frequently accompanied by nausea, diarrhea, constipation,
hunger pains, chronic indigestion and passage of proglottids in the feces. Although
these symptoms are usually milder when the infection is caused by T. solium, the
risk of developing cysticercosis remains high.
Cysticercosis
Cysticercosis refers to the tissue infection caused by the metacestode, or
larval stage, of Taenia solium and is acquired by the accidental ingestion of eggs.
Th e clinical manifestations associated with cysticercosis are a direct result of
the infl ammatory response induced to control parasite growth and may occur
months to years aft er initial infection. Manifestations of disease are dependent
upon a variety of factors including the site of infection as well as the number of
cysticerci present within the tissues, which most oft en localize to sites within the
eyes, skeletal muscles and brain. Cysticercosis is the most common intra-orbital
parasitic infection and is observed in 13-46% of infected individuals. Infection
may involve the sub-retinal space (intra-ocular) or the extraocular muscles, eyelid
and/or lachrymal glands (extra-ocular) surrounding the eye(s). Patients suff ering
from ocular infection frequently experience pain in the eyes accompanied by blur-
riness and partial or complete loss of vision. In extreme cases, infection may cause
complete detachment of the retina.
Patients infected with cysticerci in the skeletal muscles and/or subcutaneous
tissues are usually asymptomatic. In most cases, multiple cysts are present within
the tissues, although solitary cysts may also be detected. Cysts range from 10-15
mm in length and arrange themselves in the same orientation as the muscle fi bers.
Leakage of fl uid into the tissues, or death of the parasite, can trigger a strong
infl ammatory response, resulting in sterile abscess formation accompanied by
localized pain and swelling.
Neurocysticercosis
Neurocysticercosis is the most common parasitic infection of the human
central nervous system and is observed in 60-90% of infected patients. Cysts
localized within the brain may range anywhere from 4-20 mm in length, but most
commonly average between 8-10 mm. As with cysts localized in skeletal muscles
and subcutaneous tissues, the destruction of parasites induces an infl ammatory
response, granulomas and fi brosis which may result in a subacute encephalitis.
Seizures are the most common symptom reported in patients with neu-
rocysticercosis and occur in 70-90% of infected patients. Other commonly
associated clinical manifestations include headache, dizziness, involuntary
muscle movement, intercranial hypertension and dementia. Not all patients

143
Taeniasis and Cyticercosis
20
with neurocysticercosis are symptomatic; a certain percentage of patients with
neurocysticercosis never develop any symptoms and these infections are oft en
self-resolving.
Diagnosis
Diagnosis is oft en diffi cult due to the nonspecifi c nature of symptoms associated
with cysticercosis. Th erefore, proper diagnosis of the diseases is most oft en based
on a combination of clinical, serological and epidemiological data.
MRI and CAT scans are considered to be the most sensitive methods of detec-
tion of neurocysticercosis and are useful in establishing diagnosis. However, the
high costs associated with these radiologic methods greatly restrict the availability
and/or accessibility of these tests in most underdeveloped countries where the
disease is endemic.
Serological methods of detection most often include the ELISA (en-
zyme-linked immunoassays) and the EITB (enzyme-linked immunoelectrotrans-
fer blot) and involve the detection of antibodies against cysticerci. EITB is
highly sensitive and is considered to be the best immunological diagnostic test
available. However, EITB is not eff ective in the detection of antibodies when only
one cyst is present. Th e ELISA, while not as sensitive, is technically simpler and
is therefore used extensively in clinical settings. It should be noted, however, that
detection of anticysticercal antibodies may simply indicate previous exposure or
infection and is not an exclusive indication of a current, active infection within
the host. Other methods of detection include compliment fi xation and indirect
haemagglutination assays.
Treatment
Praziquantel and albendazole are the two anticysticercal drugs used to treat pa-
tients diagnosed with cysticercosis in the brain and skeletal muscles. Treatment with
praziqauntel (50-100 mg/kg/d × 30 d) and albendazole (400 mg bid for 8-30 d)
has been shown to completely eliminate cysts in 80% of treated patients, with an
additional 10% of patients experiencing a signifi cant reduction in the number of
cysts present. Some investigators recommend 100 mg/kg/d in three divided doses
×
1 day and then 50 mg/kg/d in 3 doses for 29 days of praziquantel. Neither drug
is toxic; however, a percentage of patients undergoing therapy experience adverse
side eff ects such as headache, nausea, vomiting, dizziness and increased pressure
on the brain. Th ese eff ects are most likely a result of the host immune response
resulting from the massive destruction of parasites and therefore, treatment with
either praziquantel or albendazole is oft en administered concomitantly with corti-
costeroids in order to prevent excessive infl ammation. Dexamethasone is the steroid
most oft en administered in conjunction with either praziquantel or albendazole.
Prednisone may be used as a replacement in patients when long-term therapy is
required. Antiepileptic drugs may be necessary adjuncts for treatment of seizures
in patients being treated for neurocysticercosis.
Surgical removal of cysts from infected tissues is possible and, prior to the de-
velopment of anticysticercal drugs, was the primary means of treatment. However,
the invasiveness and high risk of complications associated with surgery makes this
method less favorable to treatment with chemotherapeutic agents.

144
Medical Parasitology
20
Prevention and Prophylaxis
Th e most eff ective means of preventing infection is to ensure that meats are cooked
thoroughly prior to consumption. Good hygiene and sanitation are highly eff ective
in decreasing the risk of infection associated with fecal-oral transmission.
Th e costs associated with chemotherapy and other medical resources, as well as
losses in production, are enormous and eff orts to prevent and/or eliminate disease
have been a primary concern for public health systems in endemic countries for
a long time. More recently, an increase in the number of imported cysticercoses
in developed countries has made the eradication of the diseases a primary health
concern worldwide.
Improvements in sanitation and public health care are essential for preventing the
further spread of disease. Altering the infrastructure to keep pigs from roaming freely
and contacting human feces will help reduce human-to-pig transmission. Eff ective
measures to control and regulate meat inspection at slaughterhouses has been ex-
tremely eff ective in Europe and North America; however, programs to ensure proper
compensation for the loss of infected livestock must be developed in order to discour-
age the underground traffi cking of livestock by local farmers in endemic regions.
Vaccines aimed at preventing infection in pigs may play a role in eff orts to con-
trol the spread of disease. Due to their typically short-life span (approximately one
year), pigs do not require long-term immunity; therefore, vaccines which provide
only short term resistance may be suffi cient to prevent the spread of infection to
humans. Additionally, the vaccination, rather than the confi scation, of pigs is oft en
a more favorable alternative to local farmers.
To date, the most eff ective vaccines have involved the expression of recombi-
nant oncosphere antigens TSOL18 and TSOL45 in E. coli. TSOL18 appears to
be more eff ective, inducing greater than 99% protection in the fi ve vaccine trials
undertaken thus far. Current eff orts are focused on developing the methods neces-
sary to make the vaccine widely available and successful on a practical scale. Th e use
of recombinant vaccines in pigs, combined with anticysticercal chemotherapy in
humans, seems to be the most eff ective approach in the battle against cysticercosis
and appears to have potential to control and/or eradicate the disease.
Concluding Remarks
Cysticercosis and taeniasis resulting from tapeworm infections currently
aff ect millions of people worldwide and continue to exert increasing pressure
on public health care systems in endemic countries and non-endemic coun-
tries alike. Th e high prevalence of the diseases in endemic countries as well as
increasing incidences of these diseases in non-endemic regions has grabbed
the attention of health offi cials worldwide. Further research to elucidate the
mechanisms of the host immune response to parasitic infection, including the
mechanisms by which parasites are able to evade destruction by the host, will
likely facilitate the development of eff ective vaccines to control the further
spread of disease. Successful programs to eradicate the diseases will require
the combined eff orts of scientists and physicians as well as the development of
social and economic programs geared towards improving public education and
the quality of life in many impoverished, underdeveloped countries in which
Taenia infections are endemic.

145
Taeniasis and Cyticercosis
20
Suggested Reading
1. Carpio A. Neurocysticercosis: an update. Th e Lancet Infectious Diseases 2002;
2:751-62.
2. Hoberg EP. Phylogeny of Taenia: species defi nitions and origins of human parasites.
Parasitol Int 2006; 50::S23-30.
3. Singh G, Prabhakar S. Taenia solium Cysticercosis: From Basic to Clinical Science.
New York: CABI Publishing, 2002.
4. Becker H. Out of Africa: Th e origins of the tapeworms. Agricultural Research
2001; 49:16-8.
5. Sciutto E, Fragoso G, Fleury A et al. Taenia solium disease in humans and pigs: an
ancient parasitosis disease rooted in developing countries and emerging as a major
health problem of global dimensions. Microbes and Infection 2000; 2:1875-90.
5. Wandra T, Ito A, Yamasaki H et al. Taenia solium Cysticercosis, Irian Jaya,
Indonesia. Emerg Infect Dis 2003; 9:884-5.
6. White AC Jr, Robinson P, Kuhn RE. Taenia solium cysticercosis: host-parasite
interactions and the immune response. Chem Immunol 1997; 66:209-30.
7. Rahalkar MD, Shetty DD, Kelkar AB et al. Th e Many Faces of Cysticercosis. Clin
Radiol 2000; 55:668-74.
8. Sloan L, Schneider S, Rosenblatt J. Evaluation of Enzyme-Linked Immunoassay
for Serological Diagnosis of Cysticercosis. J Clin Microbiol 1995; 33:3124-8.
9. Garcia H, Evans C, Nash TE et al. Current Consensus Guidelines for Treatment
of Neurocysticercosis. Clin Microbiol Rev 2002; 15:747-56.
10. Garg RK. Drug treatment of neurocysticercosis. Natl Med J India 1997;
10:173-77.
11. Th e Medical Letter (Drugs for Parasitic Infections) 2004; 46:e1-e12.

C
HAPTER
21
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Hydatid Disease
Hannah Cummings, Miriam Rodriguez-Sosa
and Abhay R. Satoskar
Background
Hydatid disease, also called hydatidosis or echinococcosis, is a cyst-forming
disease resulting from an infection with the metacestode, or larval form, of parasitic
dog tapeworms from the genus Echinococcus. To date, fi ve species of Echinococcus
have been characterized. Th e vast majority of human diseases are from Echinococcus
granulosus and Echinococcus multioccularis which cause cystic echinococcosis and
alveolar echinococcosis, respectively. Millions of people worldwide are aff ected
by human hydatid disease and as a result, the diagnosis, treatment and prevention
of the disease has become a serious concern for public health care systems around
the world.
Geographic Distribution
Echinococcus infections are estimated to aff ect between 2-3 million people
worldwide with endemics located primarily in regions of North and South
America, Europe, Africa and Asia associated with the widespread raising of sheep
and other livestock.
Life Cycle
Hydatid disease is caused by infection with the larval form of E. granulosus (and/
or E. multiocularis) and results in the formation of cysts within various host tissues.
Th e complete life cycle of Echinococcus granulosus requires two hosts (Fig. 21.1).
Domestic dogs act as the primary defi nitive host of the mature adult worms and a
single infected dog may harbor millions of adult worms within its intestines. Other
canines such as wild dogs, wolves, coyotes, foxes and jackals may also act as a defi ni-
tive host harboring the adult tapeworms. Intermediate hosts become infected with
the larval form of the parasite and include a wide range of herbivorous animals,
primarily sheep, cattle, pigs, goats and horses. Th e life cycle is completed by the
ingestion of one or more cysts and its contents by the canine host through the
consumption of infected viscera of sheep and and/or other livestock. Protoscoleces
released in the small intestine attach to the intestinal wall through the action of
four suckers and a row of hooks and within two months mature into adult worms
capable of producing infective eggs.
Humans may become infected though the ingestion of food and/or water
contaminated with infective eggs released in the feces of dogs harboring the adult
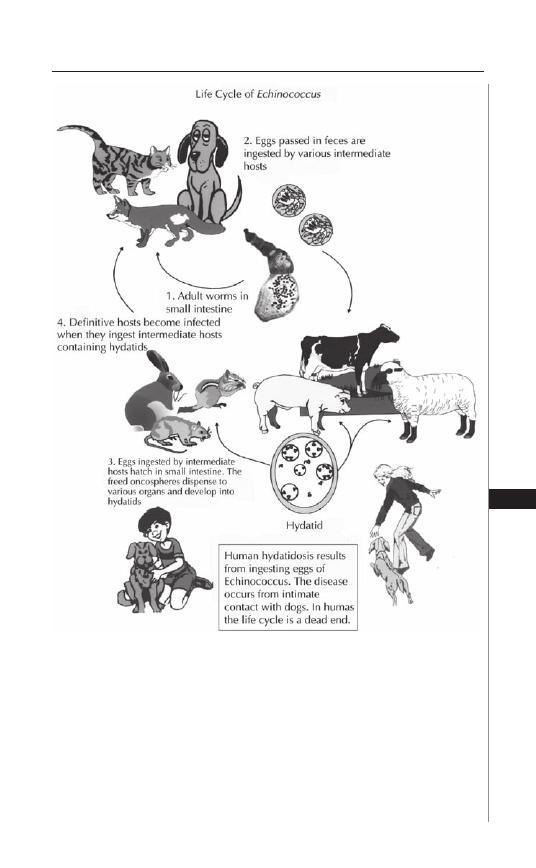
147
Hydatid Disease
21
tapeworm(s). Once ingested, the eggs release oncospheres capable of actively
penetrating the intestinal mucosa. Th ese oncospheres gain access to the blood
stream via the hepatic portal vein and migrate to various internal organs where
they develop into cysts. Hydatid cysts most oft en localize within the liver and the
lungs; however, cysts may also form in the bones, brain, skeletal muscles, kidney
and spleen. Th e clinical manifestations of hydatid disease vary depending on a
variety of factors including the location, size and number of cysts present within
the infected tissues.
Figure 21.1. Life cycle of Echinococcus. Reproduced from: Nappi AJ, Vass E,
eds. Parasites of Medical Importance. Austin: Landes Bioscience, 2002:65.

148
Medical Parasitology
21
Similar to E. granulosus, the complete life cycle of E. multiocularis also requires
two hosts. Th e primary defi nitive host for E. multiocularis is the fox, although the
parasite may also infect wild and domesticated dogs and occasionally cats. Rodents
such as fi eld mice, voles and ground squirrels act as natural intermediate hosts and
acquire infection by ingesting infective eggs released into the environment.
Immunobiology
Th e development of an immune response to infection with the larval form of the
parasite is generally divided into two broad phases: the preencystment phase and
the postencystment phase. Both cellular and humoral immunity are induced during
each phase; however, neither response is suffi cient to eliminate the parasite.
Early stages of a primary infection with E. granulosus are characterized by the
substantial activation of a cell-mediated type immune reaction against the parasite.
Th e release of oncospheres promotes an increase in leukocytosis, primarily by eo-
sinophils, lymphocytes and macrophages. Host complement pathways contribute
to the host infl ammatory response and are activated by both living organisms as
well as by material derived from dead parasites. Intense, dense granulomas form
around the cyst and are responsible for much of the tissue destruction and subse-
quent clinical pathology associated with the disease.
Parasite-specifi c antibodies can be detected in the sera of patients shortly aft er
infection and include IgG, IgA and IgM. Studies suggest that early oncospheres
may be killed through antibody-dependent cell-mediated cytotoxicity reactions
involving neutrophils. A certain percentage of patients develop an immediate-type
hypersensitivity reaction to larval antigens, characterized by the nonspecifi c de-
granulation of basophils and increased levels of circulating IgE. Anaphylaxis-type
reactions may occur and are oft en induced by the rupture of a cyst or the leakage
of hydatid cyst fl uid within the tissues.
Th e postencystment phase of infection is marked by an increase in the levels of
IgG, IgM and IgE. Th e infi ltration of eosinophils, neutrophils, macrophages and
fi brocytes initiated early in infection persists throughout the later phases of cyst
development; however, the presence of mature cysts within the tissues does not
result in an intense infl ammatory response.
Cytokine profi les of infected patients suggest the development of both a Th 1-
and Th 2-type immune response to infection. Live parasites have been shown to
actively induce Th 1 cytokines, suggesting that the development of a Th 2-type
response is involved in host susceptibility to infection. In addition, Th 2 cyto-
kines are the predominant cytokines detected in sera from patients with active or
transitional cysts. In contrast, patients with inactive cysts or undergoing eff ective
chemotherapy exhibit a strong Th 1-type response. Th is Th 1 response dominates
the Th 2 response and suggests that a predominant Th 1 response induced late in
infection may be responsible for the successful resolution of infection.
Signs and Symptoms
Echinococcus granulosus and Echinococcus multiocularis are the two species
most oft en identifi ed in human hydatid disease. Cystic echinococcosis, caused
by E. granulosus, is the most common and accounts for approximately 95% of all
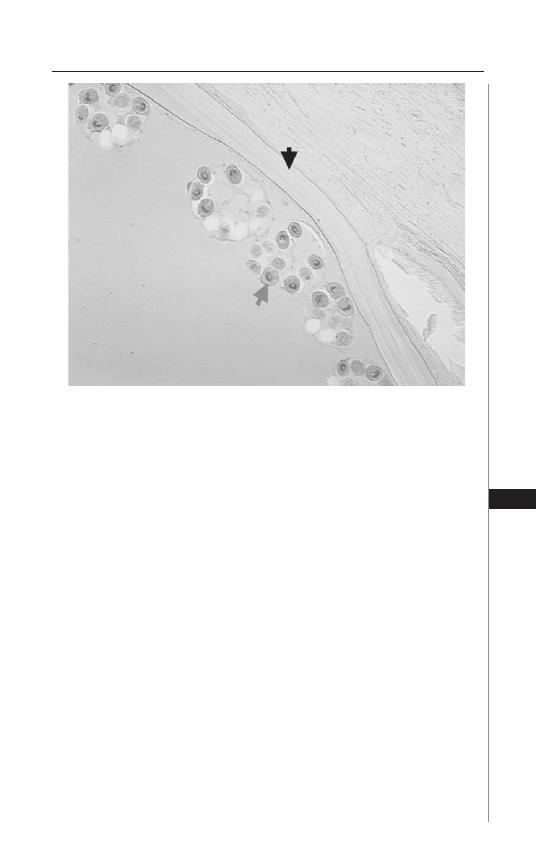
149
Hydatid Disease
21
global cases. Cystic echinococcosis may aff ect people of all ages, but hydatid cysts
are most oft en present in patients between 15-35 years of age.
Infection with E. granulosus results in the rapid growth of large, uniocular cysts
fi lled with fl uid (Fig. 21.2). Most cysts develop within the tissues of the liver and lung,
with 55-75% of cysts found in the liver and 10-30% of cysts found in the lungs. Cysts
may survive in the liver for several years and oft en do not cause any symptoms in the
infected host. Symptoms arise when the cysts become large enough to be palpable
and/or cause visual abdominal swelling and pressure. Patients frequently experience
abdominal pain in the right upper quadrant, oft en accompanied by nausea and vomit-
ing. Th e rupture or leakage of cysts within the tissue can result in anaphylactic shock
and facilitate the spread of secondary cysts through the release and dissemination of
germinal elements. Biliary tract disease and portal hypertension may complicate liver
involvement and postobstructive infection due to erosion of cysts into the biliary tract
may further complicate echinococcal infection. Pulmonary cystic echinococcosis is
acquired early during childhood, but the clinical manifestations associated with the
disease do not typically appear until the third or fourth decade of life.
Cysts residing within the lung tissue oft en remain silent producing little to
no symptoms. Problems arise when cysts grow large enough to obstruct or erode
a bronchus, oft en causing the rupture of cysts and the dissemination of cystic
fl uids. Patients infected with pulmonary cysts frequently experience chronic dry
cough, chest pain and hemoptysis oft en accompanied by headache, sweating,
fever and malaise.
Figure 21.2. Photomicrograph of a hydatid cyst from the liver. Note the
hyaline membrane (black arrow) and the protoscolex in the brood capsules
(gray arrow).

150
Medical Parasitology
21
Alveolar echinococcosis aff ects between 0.3-0.5 million people and is usu-
ally caused by Echinococcus multiocularis. It is characterized by the formation of
multiocular hydatid cysts which contain little to no fl uid. Th ese cysts lack both
the hyaline membrane and the brood capsules which facilitate the widespread
metastasis of larvae into the surrounding tissues. Th ese larvae invade adjacent tis-
sues and proliferate indefi nitely causing extensive and progressive tissue necrosis
and eventual death in 70% of infected patients.
Hydatid disease can aff ect a wide range of organs including the bones, central
nervous system, heart, spleen, kidneys, muscles and eyes. Patients diagnosed with
the disease should be screened for the presence of multiple cysts in various tissues.
Diagnosis
Proper diagnosis and treatment of hydatid disease is diffi cult. Individuals oft en
remain asymptomatic for several years aft er initial infection, allowing time for the
growth of large, debilitating cysts. Various imaging techniques are used to visually
detect cysts present within host tissues. CT scans and MRIs are used extensively
in clinical settings and are useful in the detection of developing, dying or dead
cysts. Typical features include thick cyst walls, detached germinal membranes,
internal septae and/or the presence of daughter cysts. X-ray, ultrasound and scin-
tillography may also be useful in the detection of hydatid cysts and in diagnosis
of the disease.
Numerous serological assays are currently available and are useful in the detec-
tion and diagnosis of hydatid disease. Common detection methods include indirect
hemagglutination assays (IHA), indirect immunofl uorescence, counter-current
immunoelectrophoresis (CIEP), enzyme-linked immunoassays (ELISA) and
enzyme-linked immunotransfer blots (EITB). Most serological assays involve the
detection of specifi c serum antibodies, primarily the detection of IgG to hydatid
cyst fl uid-derived or recombinant antigen B subunits. Although high levels of sen-
sitivity have been achieved (92.2%), complications may arise due to cross-reactivity
between hydatid disease and cysticercosis.
Detection of mitochondrial DNA using molecular techniques like PCR is
extremely useful and is oft en used to analyze genotypic variations between species
and/or strains.
Treatment
Surgery remains the treatment of choice for the removal of cysts. Patients
diagnosed with multiple cysts often require numerous staged operations.
Complete excision of the cysts is diffi cult: surgical removal may cause the rup-
ture or leakage of cysts/cystic fl uid resulting in the release and dissemination
of infective protoscoleces.
Albendazole is frequently used to treat patients with hydatid disease. Patients
typically receive 10 mg/kg/d or 400 mg orally twice per day for 1-6 months.
Although neither regimen has been proven to be eff ective in resolving the disease
alone, the use of drug therapy in conjunction with surgical treatment has shown
to greatly reduce the risk of development of new cysts and is currently the therapy
of choice.

151
Hydatid Disease
21
PAIR, or percutaneous aspiration, followed by injection of 95% ethanol or
another scolicidal agent and then reaspiration, may sometimes be used as an alter-
native to therapy, especially for the treatment of inoperable cysts.
Prevention and Prophylaxis
Th e most eff ective means to control hydatid disease in humans and eliminate
the consequences of Echinococcus infections in livestock is through the broad- range
education of people living in endemic regions. Education to prevent the feeding
of infected viscera to dogs is essential for controlling the spread of infection from
livestock to dogs. Most human infections are due to close contact with infected
dogs. Deliberate actions aimed at reducing the rate of dog infection in endemic
regions will undoubtedly reduce the number of human infections. In addition, the
reduction and removal of stray and unwanted dogs, as well as the regular treatment
of dogs with anthelminthic drugs, will facilitate the widespread eff orts geared
towards controlling disease transmission.
Th e development of vaccines designed to prevent infection of either or both
the defi nitive and intermediate host(s) off ers the greatest possibility of success in
the control and eradication of hydatid disease in both the livestock and human
populations. EG95 is a 16.5 kDa recombinant GST fusion protein derived from
E. granulosus oncospheres and functions as a highly eff ective vaccine for grazing
livestock. EG95, which induces immunity through complement-fi xing antibodies,
has been shown to induce high levels of protection (96-98%) against the develop-
ment of hydatid cysts.
Concluding Remarks
Human hydatid disease aff ects millions of people and has attracted the attention
of health professionals around the world. Th e treatment of echinococcus infections
within the domestic animal population would likely result in a reduction in the
number of human cases of hydatid disease and, therefore, has become the focus of
many studies aimed at the development of eff ective vaccines to control the spread
of disease. Although vaccines are an invaluable tool for the control and eradication
of disease, increasing public education and awareness of the eff ects of infection and
the mode of transmission will be essential for control within remote areas where
the disease is endemic.
Suggested Reading
1. Th ompson RCA. Th e Biology of Echinococcus and Hydatid Disease. London:
George Allen & Unwin Ltd, 1986:85.
2. Zhang W, Li J, McManus DP. Concepts in immunology and diagnosis of hydatid
disease. Clin Microbiol Rev 2003; 16:18-36.
3. Craig PS, McManus DP, Lightlowlers MW et al. Prevention and control of cystic
echinococcosis. Lancet Infect Dis 2007; 7:385-94.
4. Sturton SD. Geographic distribution of hydatid disease. Chest 1968; 54:78.
5. Ceran S, Sunam GS, Gormus N et al. Cost-eff ective and time-saving surgical treat-
ment of pulmonary hydatid cysts with multiple localization. Surg Today 2002;
32:573-6.
6. Jenkins DJ, Power K. Human hydatidosis in New South Wales and the Australian
Capital Territory, 1987-1992. Med J Aust 1996; 164:14-7.

152
Medical Parasitology
21
7. Goldsmith RS. 35 Infectious diseases: protozoal and helminthic. Current Medical
Diagnosis and Treatment 2007; 46:
8. Dickson DD, Gwadz RW, Hotez PJ. Parasitic Diseases, 3rd Edition. New York:
Springer-Verlag, 1995:93-8.
9. Parija SC. Text Book of Medical Parasitology: Protozoology and Helminthology.
Chennai: All India Publishers & Distributors, 2001: 214-9.
10. Arora DR, Arora B. Medical Parasitology. New Delhi: CBS Publishers and
Distributors, 2002:120-3.
11. Moro P, Schantz PM. Cystic echinococcosis in the Americas. Parasitol Internat
2005; 55:S181-6.
12. Magambo J, Njoroge E, Zeyhle E. Epidemiology and control of echinococcosis in
sub-Sahara Africa. Parasitol Internat 2006; 55:S193-5.
13. Torgerson PR, Oguljahan B, Muminov AE et al. Present situation of cystic echi-
nococcosis in Central Asia. Parasitol Internat 2006; 55:S207-12.
14. Shaikenov BS, Vaganov TF, Torgerson PR. Cystic Echinococcosis in Kazakhstan:
An emerging disease since independence from the Soviet Union. Parasitol Today
1999; 15:173-4.
15. Romig T, Dinkel A. Mackenstedt. Th e present situation of echinococcosis in
Europe. Parasitol Internat 2006; 55:S187-91.
16. Baz A, Ettlin GM, Dematteis S. Complexity and function of cytokine responses
in experimental infection by Echinococcus granulosus. Immunobiology 2006;
211:3-9.
17. Warren KS. Immunology and Molecular Biology of Parasitic Infections, 3rd
Edition. Chelsea: Blackwell Scientifi c Publications, 1993:438-48.
18. Ferreira M, Irigoin F, Breijo M et al. How echinococcus granulosus deals with
compliment. Parasitol Today 2000; 16:168-72.
19. Zhang W, You H, Zhang Z et al. Further studies on an intermediate host murine
model showing that a primary Echinococcus granulosus infection is protective
against subsequent oncospheral challenge. Parasitol Internat 2001; 50:279-83.
20. Rosenzvit M, Camicia F, Kamenetzky L et al. Identifi cation and intra-specifi c
variability analysis of secreted and membrane-bound proteins from Echinococcus
granulosus. Parasitol Internat 2006; 55:S63-7.
21. Kizaki T, Kobayashi S, Ogasawara K et al. Immune Suppression Induced by
Protoscoleces of Echinococcus multiocularis in Mice: Evidence for the Presence
of CD8
+
dull Suppressor Cells in Spleens of Mice Intraperitoneally Infected with
E. multiocularis. J Immunol 1991; 147:1659-66.
22. Markel EK, John DT, Krotoski WA. Markell and Voge’s Medical Parasitology, 8th
Edition. Philadelphia: W.B. Saunders Company, 1999:254-60.
23. Tor M, Atasalihi, Altuntas N et al. Review of cases with cystic hydatid lung dis-
ease in a tertiary referral hospital in an endemic region: a 10 Years’ experience.
Respiration 2000; 67:539-42.
24. Elton C, Lewis M, Jourdan MH. Unusual site of hydatid disease. Lancet 2000;
355:2132.
25. Bahloul K, Ghorbel M, Boudouara MZ et al. Primary vertebral echinococcosis:
four case reports and review of literature. Br J Neurosurg 2006; 20:320-3.
26. Todorov T, Mechkov G, Vutova K et al. Benzimidazoles in the treatment of
abdominal hydatid disease: a comparative evaluation. Parasitol Internat 1998;
47:105-31.
27. Heath D, Yang W, Tiaoying L et al. Control of hydatidosis. Parasitol Internat 2006;
55:S247-52.
28. Parija SC. A review of some simple immunoassays in the serodiagnosis of cystic
hydatid disease. Acta Tropica 1998; 70:17-24.

S
ECTION
IV
Protozoans

C
HAPTER
22
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
American Trypanosomiasis
(Chagas Disease)
Bradford S. McGwire and David M. Engman
Introduction
American trypanosomiasis is a vector-borne infection caused by the protozoan
parasite Trypanosoma cruzi. Also called Chagas disease, named aft er the Brazilian
physician Carlos Chagas who described the infection in 1909, it is found only
on the American continent. Th e parasite alternately infects triatomine insects
(reduviid, assassin or “kissing” bugs) and a wide range of vertebrate hosts in a
complex lifecycle. Human infection results in a myriad clinical syndromes result-
ing from localized and disseminated infection arising from the initial deposition
of infective parasites during feeding of the blood sucking triatomine. Chagas
disease is an important public health concern, being widespread in Central and
South America and chronic infection is the leading cause of heart failure in these
regions. Transmission via transfusion of blood products and organ transplantation
is a matter of concern, even in North America. Th is review will cover the lifecycle
and epidemiology, pathogenesis, clinical diagnosis, management and prevention
of T. cruzi infection.
Epidemiology of T. cruzi Infection
Th e triatomine insects that transmit T. cruzi are present throughout the
Americas, spanning vast regions from the central United States throughout
Central and South America, extending to the south-central portions of Chile and
Argentina. T. cruzi infection is primarily a zoonosis and humans are only incidental
hosts; thus, natural transmission occurs primarily in rural areas where insects are
abundant. Th e incidence of human infection is increasing in these regions due to
deforestation for farming, which has caused the insects to migrate to the rudi-
mentary human dwellings made of mud and thatch, wood or stone. Despite the
presence of T. cruzi-infected insects in the United States, the low incidence of acute
Chagas disease in this country is thought to be due to the relatively high quality
of housing. Th e World Health Organization currently estimates that 13 million
people are infected with T. cruzi, with 200,000 new infections occurring annually
in 15 countries. In addition to insect-borne disease, T. cruzi can also be transmitted
congenitally or by blood transfusion or organ transplantation. Transmission of T.
cruzi infection by blood transfusion is increasing in the US due to the increasing
infl ux of infected immigrants who donate blood. Th us, there is a pressing need to
implement widespread screening of blood products for the presence of T. cruzi.
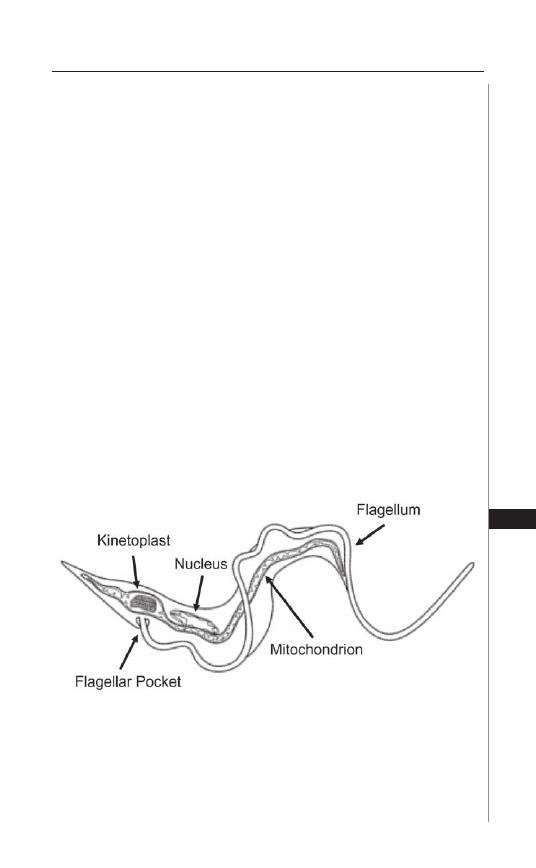
155
American Trypanosomiasis (Chagas Disease)
22
Figure 22.1. Cellular features of a Trypanosoma cruzi trypomastigote.
Trypanosoma cruzi is a protozoan parasite, possessing the organelles of
all eukaryotes including a membrane bound nucleus and mitochondrion. A
single membrane-bound fl agellum emerges from the trypanosome’s fl agellar
pocket and runs the length of the cell, attached to the cell body membrane
via a desmosome-like adhesive junction. At its origin, the fl agellum is physi-
cally connected to the mitochondrial DNA, which resides in a specialized
region of the mitochondrion termed the kinetoplast.
T. cruzi Life Cycle and Transmission
Trypanosoma cruzi is a eukaryote possessing a membrane bound nucleus
and mitochondrion. Th e mitochondrial DNA is a complex structure which
resides in a specialized region (kinetoplast) adjacent to the base of the fl agel-
lum (Fig. 22.1). T. cruzi has four distinct life cycle stages (Fig. 22.2). Within the
midgut of the reduviid bug, parasites replicate as fl agellated epimastigotes (epi).
As epis replicate and increase in number they migrate to the hindgut of the bug
where they diff erentiate into infective metacyclic trypomastigotes (meta). Metas
are discharged in the feces of the bug as they take a blood meal. Infection results
from the contamination of the insect bite or open wounds, mucous membranes
or conjunctiva with parasite laden bug feces. Once in the vertebrate host, the
meta, which is unable to replicate, must invade host cell within which it can
diff erentiate into the replicating amastigote (ama). During invasion the meta is
initially present within a membrane bound vacuole, but it escapes this vacuole
and diff erentiates into the afl agellated ama, which divides in the cytoplasm. Aft er
a number of rounds of replication, the amas fi ll the cytoplasm and diff erentiate
into motile trypomastigotes (tryp), which lyse the infected cell and escape to
infect adjacent cells or disseminate throughout the body via the bloodstream
and lymphatics. Tryps, like metas, cannot replicate and must invade host cells
and diff erentiate into amas to survive. Alternatively, they may be taken up by a
triatomine insect during a blood meal and diff erentiate into epis in the insect
midgut, thereby completing the life cycle. Within the vertebrate host, parasites
can infect any nucleated cell, but have a predilection for muscle, particularly of
the heart and gastrointestinal tract. Th is tissue tropism ultimately leads to the
two predominant clinical forms of chronic T. cruzi infection: cardiomyopathy
and megacolon/megaesophagus.
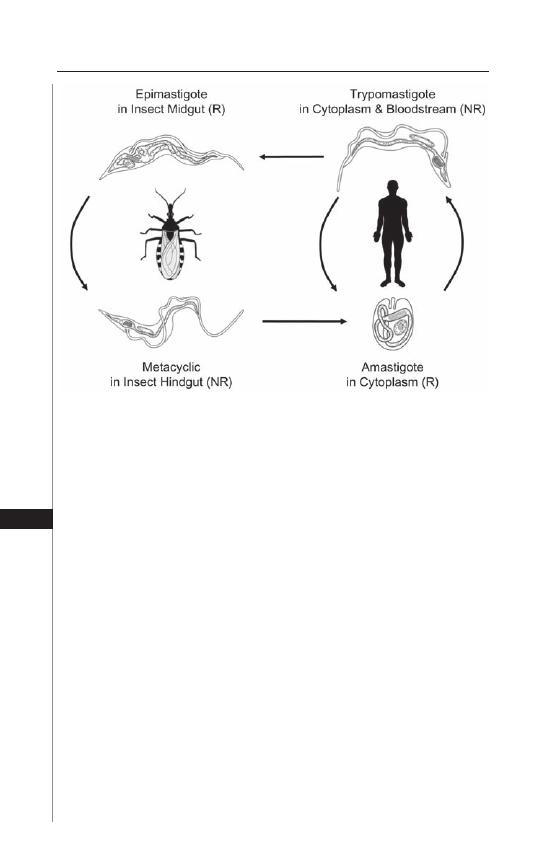
156
Medical Parasitology
22
Pathogenesis of Chagas Disease
Acute T. cruzi infection results from the contamination of wounds or
mucous membranes with insect feces containing expelled infective parasites.
Locally deposited parasites bind to and invade host tissue and transform into
and replicate as intracellular amastigotes. Infection leads to the formation of
parasite “pseudocysts,” so named because the amastigote nests are intracellular.
Th is stimulates a localized infl ammatory response mediated predominantly by
lymphocytes and macrophages. Lymphatic drainage of the infected area into
regional lymph nodes results in activation and proliferation of cells, resulting
in regional lymphadenopathy. As the process continues, the amas transform
into trypomastigotes, escape host cells and disseminate throughout the body.
Infection and lysis of liver cells results in transient increases in serum liver
enzyme levels. In chronic infection, tissue parasites are diffi cult to detect but
signifi cant interstitial fi brosis occurs, damaging the aff ected tissue. Th e molecular
Figure 22.2. Life cycle of Trypanosoma cruzi. T. cruzi possesses four basic
life cycle stages. In the insect, noninfectious epimastigotes replicate (R) in the
midgut and differentiate into infectious but nonreplicating (NR) metacyclics
as they migrate to hindgut. The fecal material of the insect, which contains
metacyclics is deposited on the skin during a bloodmeal and infection occurs
when this material contaminates the insect bite or a mucous membrane, which
the trypomastigotes can penetrate. Within the human host, metacyclics invade
host cells and differentiate into amastigotes, which replicate, burst out of the
cell and either invade other cells or are taken up by another insect. Within
the insect gut, the trypomastigotes differentiate into replicating epimastigotes,
thus completing the cycle.

157
American Trypanosomiasis (Chagas Disease)
22
pathogenesis of Chagas disease is not completely understood, but likely results
from (i) parasite-mediated tissue destruction, (ii) infl ammation and fi brosis
resulting from immune responses generated to parasites and residual parasite
antigen, (iii) parasite-induced microvascular spasm and ischemic damage and/
or (iv) autoimmune responses triggered by release of self-antigen during parasite
lysis of host cells. Because there are many outcomes of chronic T. cruzi infec-
tion (see below), it is likely that each of these mechanisms occurs in isolation
or in combination in a given individual, depending on the specifi c pathogenic
potential of the strain of parasite (tissue tropism, replication rate, etc.) and the
immunogenetic susceptibility of the infected individual.
Clinical Syndromes of Chagas Disease
Acute infection by T. cruzi is marked by the development of localized swelling
and erythema at the site of the insect bite, which is termed a chagoma. Th is is a
result of the local replication of parasites and the infl ux of fl uid and infl amma-
tory cells into the infected area. Infection through the conjunctiva can result in
periorbital swelling, termed Romaña’s sign (Fig. 22.3D). As parasites disseminate
patients experience nonspecifi c symptoms such as fever, malaise and anorexia.
Parasite infestation of peripheral tissues can give rise to hepatosplenomegaly and,
in some cases, meningeal signs. Initial infection of heart tissue can lead to acute
myocarditis and cardiac sudden death due to parasitization of the cardiac conduc-
tion system. Th e signs and symptoms of acute T. cruzi infection can last from days
to weeks but are oft en unrecognized due to their nonspecifi c nature. Th e disease
then proceeds to a quiescent phase lasting months to years and oft en decades,
prior to the onset of chronic disease. It should be noted that the majority of T.
cruzi-infected individuals do not develop any parasite-related disease and simply
harbor low levels of parasites for life. Less than one-third of infected people develop
chronic Chagas disease. Th e two hallmarks, usually mutually exclusive, disorders
that occur in chronically infected patients are cardiomyopathy and megaorgan
syndromes (Fig. 22.3E and G. respectively).
Cardiac involvement is heralded by the development of fi brosis within the
heart muscle (Fig. 22.3F) and conduction system which leads to arrhythmias
and heart failure, that latter being predominantly right-sided. Loss of ventricular
muscle leads to wall thinning which can be associated with the development of
apical aneurysms and subsequent formation of thrombi, which may have seri-
ous thromboembolic consequences (Fig. 22.3E). In the gastrointestinal tract,
chronic infection leads to parasympathetic denervation, resulting in massive
dilatation of the esophagus and/or colon. Esophageal involvement results in
achalasia, associated odynophagia, dysphagia and esophageal dysmotility, oft en
resulting in aspiration pneumonia. Colonic involvement results in abdominal
pain, constipation, obstruction with perforation and secondary intrabdominal
infection. Immunosuppression of patients with chronic Chagas disease, regard-
less of the mechanism (HIV infection, usage of immunosuppressive drugs in
organ transplantation) can lead to recrudescence of parasite replication, massive
parasitosis and death. Clinical disease in this setting is oft en fulminant with more
extensive involvement of the central nervous system.
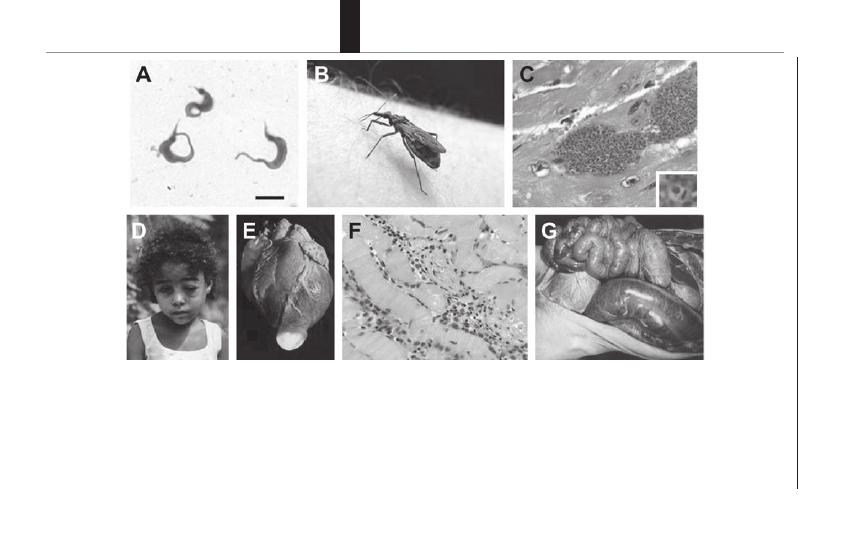
158
Medical Parasitology
22
Figure 22.3. Various aspects of Trypanosoma cruzi biology and Chagas disease. A) T. cruzi trypomastigotes stained with Giemsa
(bar = 5
μ
m). Note the prominent darkly-stained kinetoplast DNA. B) Reduviid bug. C) Nests of amastigotes in heart tissue, often termed
“pseudocysts” since they are intracellular collections of parasites. The inset shows an amastigote with clearly visible nucleus (round
structure) and kinetoplast (bar-like structure). D) Romaña’s sign. E) Apical aneurysm (illuminated by light bulb) can occur after chronic
fi brosis and weakening of the apical wall of left ventricle. F) Histopathology of Chagas heart disease: myofi brillar swelling and degen-
eration, mononuclear cell infi ltration, fi brosis and edema in the absence of parasites are typical. G) Megacolon: a serious sequela of
infection that is poorly understood. Photographs are courtesy of Cheryl Olson (A,C,F), Dr. Chris Beard (B), Dr. Michael Miles (D), Prof.
F. Köberle (E,F,G).

159
American Trypanosomiasis (Chagas Disease)
22
Diagnosis of T. cruzi Infection
Th e diagnosis of T. cruzi infection initially requires a high degree of clinical
suspicion. History of potential exposure to T. cruzi is important to document.
Patients with a history of travel to or having had blood transfusion within endemic
areas are at increased risk of T. cruzi infection. Th e presence of, or recent history
of a chagoma or Romaña’s sign are indicators of recent infection. Th e mainstay
of diagnosis is detection of trypomastigotes in the blood or the presence of T.
cruzi-specifi c antibodies in serum to indicate acute or chronic infection, respec-
tively. Direct detection of parasites in blood is easier in immunocompromised
patients in whom the immunologic control of parasites is not as effi cient. Heavy
parasite burdens in the tissues of such patients can permit diagnosis via direct
examination of tissue (lymph nodes, or bone marrow) or fl uids (cerebrospinal or
pericardial fl uid). In addition these specimens can be cultured in vitro in liquid
medium or by growth within uninfected insect vectors (xenodiagnosis). During
chronic infection parasites are frequently not detectable in the blood, and the
presence of T. cruzi IgG, using commercial immunoassays, ELISA, complement
fi xation, or hemagglutination based tests, establishes the diagnosis. Direct detec-
tion of parasites using PCR based testing has been demonstrated but is not yet
available for routine laboratory diagnosis. Potential blood donors throughout
the Americas are asked questions related to risk factors of T. cruzi infection, but
transfusion-associated disease remains a serious problem. As a result, the blood
in much of South and Central America is screened for T. cruzi-specifi c antibod-
ies, and many feel that the United States blood supply will be screened beginning
within a few years.
Treatment of T. cruzi Infection
Benznidazole, an imidazole (trade name Rochagan, produced by Roche in
Brazil) and Nifurtimox, a nitrofuran (trade name Lampit, produced by Bayer
in Germany), are the two agents approved for treatment of Chagas disease and
are available in the United States through contact with the Centers for Disease
Control in Atlanta, Georgia. Th ese agents have similar effi cacy but have many
adverse eff ects. Benznidazole is given orally for 1-3 months at a dose of 5-7
mg/kg/d in two divided doses. Th e side eff ects of this medication include rash and
peripheral neuropathy but can also include bone marrow suppression. In adults,
Nifurtimox is given for 120 days at a dose of 8-10 mg/kg/d in four divided doses.
In children the drug is given for 90 days in four divided doses but the amount
is based on age: 11-16 years (12.5-15 mg/kg/d); and under 11 years (15-20
mg/kg/d). Gastrointestinal maladies (nausea, vomiting, abdominal pain) are the
predominant side eff ects of this medication but up to 30% of patients can also
experience central nervous system eff ects such as polyneuritis, confusion or focal
or generalized seizures. Skin rash can also develop in some patients. Individuals
with glucose-6-phosphate dehydrogenase defi ciency can experience drug-induced
hemolytic anemia. Treatment is undertaken in cases of acute or congenital infection
natural infection or in cases of accidental laboratory inoculation. Recent systematic
reviews of clinical trials of trypanocidal therapy in patients with chronic T. cruzi
infection suggest that treatment of asymptomatic immunocompetent patients may
result in a reduction of progression to chronic disease (development of megaorgan

160
Medical Parasitology
22
syndromes, cardiomyopathy and arrhythmia). In contrast, there is no convincing
data that support the use of trypanocidal therapy in patients who have already
manifested end-organ damage as a result of chronic T. cruzi infection. It is clear
that randomized controlled trials are necessary to truly understand the clinical
benefi t of trypanocidal therapy in chronic Chagas disease. Th e management of T.
cruzi induced cardiac failure, achalasia and megacolon are approached in the same
way that these end-organ problems are approached due to other causes.
Prevention of T. cruzi Infection
Limiting exposure to T. cruzi infected insects and blood is the mainstay of
the prevention of Chagas disease. Persons living in or traveling to areas endemic
for T. cruzi should avoid residing in substandard housing frequented by reduviid
bugs. Th e use of bed nets and insect repellent are also recommended for this pur-
pose. Barrier protection for those working with T. cruzi in the laboratory setting,
such as protective clothing, gloves and eyewear is a must. Since the incidence of
transfusion- and transplantation-associated T. cruzi infection is increasing in the
Americas, serologic screening of donated blood seems advisable. Such is the practice
in endemic countries within South America. As the number of potentially-infected
immigrants to the United States increases, this will likely increase the number
of transfusion-associated T. cruzi infections despite the presence of blood bank
questionnaires.
Suggested Reading
1. Engman DM, Leon JS. Pathogenesis of Chagas heart disease: role of autoimmunity.
Acta Trop 2002; 81:123-32.
2. Kirchhoff LV. Trypanosoma Species (American Trypanosomiasis, Chagas
Disease): Biology of Trypanosomes. In: Mandell GL, Douglas RG, Bennett
JE, eds. Principles and Practice of Infectious Diseases, 6th Edition. New York:
Churchill Livingstone, 2005:
3. Mascola L, Kubak B, Radhakrishna S et al. Chagas disease aft er organ trans-
plantation—Los Angeles, California. MMWR Morb Mortal Wkly Rep 2006;
55:789-800.
4. Villar JC, Marin-Neto JA, Ebrahim S et al. Trypanocidal drugs for chronic
asymptomatic Trypanosoma cruzi infection. Cochrane Database Syst Rev 2002;
CD003463.
5. Tyler KM, Miles MA. American Trypanosomiasis. Norwell: Kluwer Academic
Publishers, 2003.

African Trypanosomiasis
Guy Caljon, Patrick De Baetselier and Stefan Magez
Abstract
African trypanosomiasis is a vector-born disease that severely aff ects a broad
range of vertebrate hosts, including humans, on the sub-Saharan African continent.
Th e infection is caused by fl agellated unicellular parasites (Trypanosoma sp.) and is
lethal without treatment. Disease manifestations are pleotropic and are dependent
on the host and infection-stage. Currently available diagnostic tests are adapted
for fi eld usage but have a low specifi city, while an accurate diff erential diagnosis
of human pathogenic Trypanosoma subspecies and correct determination of the
infection stage is essential for appropriate treatment. For treatment of human
African trypanosomiasis (HAT), four drugs with signifi cant side-eff ects are cur-
rently available, with only one of them being registered in the last 50 years. Th is
chapter will introduce the disease, its diagnosis, treatment and prospects for new
therapeutic approaches.
Introduction
African trypanosomes are extracellular protozoan parasites that cause lethal
infections in humans and livestock in large parts of sub-Saharan Africa. Th e re-
sponsible fl agellated parasite (Trypanosoma sp.) is approximately twice the size of
erythrocytes (15-30
μ
m, Fig. 23.1A) and relies on tsetse fl ies for its transmission
(Fig. 23.1B). Th ese arthropods are obligate bloodsucking insects (genus Glossina),
that get infected through feeding on a parasitized host and accommodate the
trypanosome during their entire lifespan. Engorged trypanosomes colonize the
midgut, proliferate and undergo diff erentiation while directionally migrating
towards the insect salivary glands. Th e vertebrate-infective metacyclic form of the
parasite resides in the salivary glands or mouthparts of the fl y, using the bloodfeed-
ing behaviour for its transmission to a new host. Upon transmission to the verte-
brate host, trypanosomes will transform into actively proliferating (long slender)
forms to allow a systemic colonization of the host. Eventually, trypanosomes in
the bloodstream become quiescent (short stumpy) and pre-adapt to uptake and
subsequent survival in the tsetse fl y. During the complex life cycle (Fig. 23.1C)
of the parasite in the insect and vertebrate host, trypanosomes undergo several
metabolic changes for the acquisition of free-energy from diff erent available sources
and modify mechanisms for the uptake of host nutrients, such as iron complexed
with transferrin. In the fl y, trypanosomes utilize amino acids (e.g., proline) as
primary energy sources while trypanosomes in the vertebrate hosts metabolize
glucose via glycolysis in a unique organelle, the glycosome. Only two subspecies,
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
C
HAPTER
23
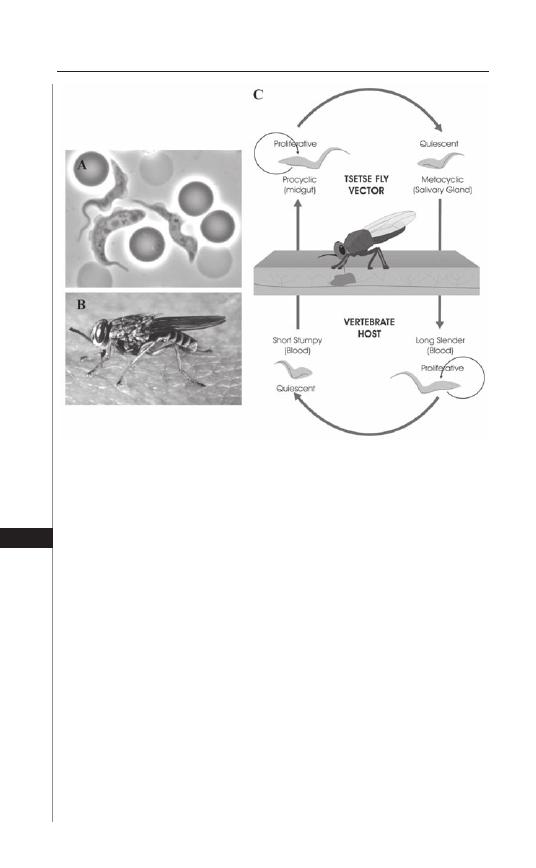
Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense, have the ad-
ditional feature of resistance to normal human serum (NHS) that is trypanolytic
for strictly livestock-threatening trypanosomes. Although both subspecies are
pathogenic to human, they diff er signifi cantly in virulence and geographical oc-
currence (Fig. 23.2). T. b. gambiense causes chronic infections in West and Central
Africa which can persist up to 10 years while T. b. rhodesiense is more prevalent
in Eastern Africa and mostly results in acute human infections that can be lethal
within a few months. Th e diseases caused by both subspecies are categorized
under human African trypanosomiasis, better known as sleeping sickness and are
responsible for an estimated 50,000 deaths a year. In contrast, T. congolense, T. vivax
and T. brucei brucei are trypanosomes species that cause the majority of livestock
infections with an estimated loss in agriculture of more than 1 billion $ per annum.
Although sleeping sickness was largely controlled by the early 1960s, the disrup-
tion of health infrastructures and population displacement, as well as the lack of
human and fi nancial resources for disease control, led to a current epidemic scale
of the disease in specifi c regions of Africa. Moreover, the development of protec-
Figure 23.1. The trypanosome’s lifecycle: A) a microscopic image (obtained
with permission from Dr. David Pérez-Morga) of the causative agent of HAT,
the trypanosome; B) a photograph of the vector of the disease, the tsetse fl y
(Glossina morsitans) (obtained with permission from Jan Van Den Abbeele);
C) the lifecycle in the mammalian and insect host, indicating a proliferative
stage for host colonization and a quiescent form, pre-adapted to survival in
a new host (obtained with permission from Guy Caljon).
162
Medical Parasitology
23
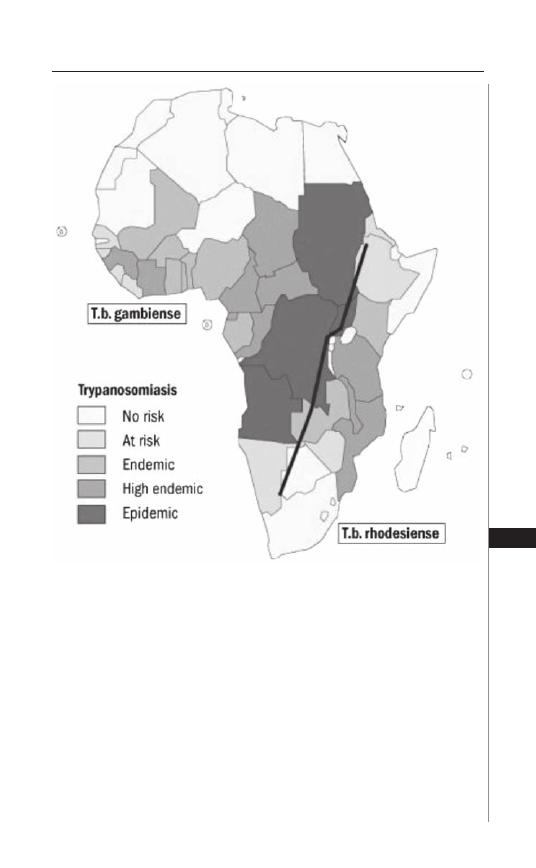
tive vaccines has been unsuccessful until now, mainly due to the ability of African
trypanosomes to escape adaptive immune responses by antigenic variation of the
most abundant surface glycoprotein VSG (variant-specifi c surface glycoprotein).
Th ese VSG molecules form a densely packed coat of up to 10
7
identical copies per
cell, making invariant epitopes as potential immune targets inaccessible to conven-
tional antibodies. Th ere are about 1,000 genes present in the trypanosome genome
that encode for these VSGs, but, due to a strictly controlled gene expression, only
one gene at a time is translated to construct the actual outer protein coat. As the
vertebrate host mounts an effi cient antibody response against the VSG leading to
partial parasite clearance of the major variant antigenic type (VAT), a minor part
Figure 23.2. Distribution of HAT in sub-Sahara Africa: predominant occur-
rence of T. b. gambiense in Central and West Africa and T. b. rhodesiense
in East Africa. The colour-scale indicates the incidence of HAT in the different
countries. This fi gure was reproduced with permission from the World Health
Organization (http://www.who.int/en/).
163
African Trypanosomiasis
23
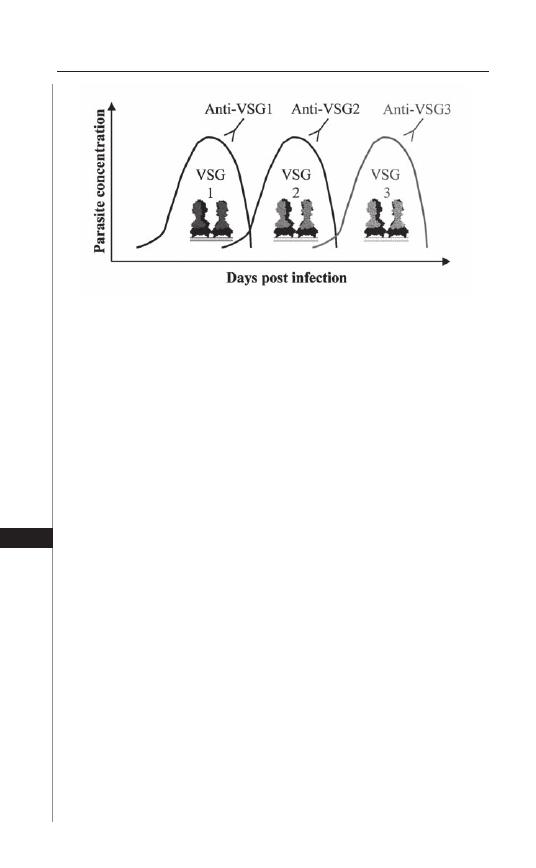
of the parasite population will initiate transcription from another VSG gene or
undergo genetic rearrangements, resulting in the expression of a new VSG type.
As such, a new wave of trypanosomes will emerge, expressing a VSG that is not
recognized by the raised anti-VSG immune response (Fig. 23.3). Together with
the modulation of functions of antigen presenting cells (e.g., macrophages) and
T-lymphocytes, the trypanosome is able to avoid elimination by the immune system
and to maintain a well controlled growth in a broad range of hosts.
Pathology
African trypanosomiasis is lethal unless the parasite is completely eliminated
from the body of the infected individual by drug treatment. However, death
in infected hosts is rarely due to an uncontrolled parasite expansion. In human
infections, mortality results from neurological complications after penetra-
tion of the parasite into the central nervous system, while cattle succumb from
infection-induced severe anemia or complications associated with secondary
opportunistic infections.
Human African trypanosomiasis (HAT) is characterized by two disease stages.
During the fi rst (haemolymphatic) stage of the infection, parasites will proliferate in
the blood and the lymphatic circulation. Symptoms at this stage are pleotropic and
nonspecifi c and include fever, lymphadenopathies, splenomegaly and endrocrine
disorders. Systemic infl ammation fi nally leads to increased blood-brain barrier
(BBB) permeability allowing parasites to penetrate the central nervous system
and cerebrospinal fl uid, ushering in the second (encephalitic) stage of HAT. Th e
symptoms of this stage include sensory, motoric and psychic disturbances, neu-
roendocrine abnormalities and disturbed circardian rhythms, eventually resulting
in coma and death. Th e disturbed day-night cycles in the late stage of infection are
characteristic for “sleeping sickness”.
Figure 23.3. Antigenic variation of VSG: illustration of the escape of the
trypanosome from specifi c host antibody responses. Each parasitemia wave
represents a population that expresses another VSG-molecule or variant
antigenic type (VAT) that escaped the host antiparasite immunity. Obtained
with permission from Guy Caljon.
164
Medical Parasitology
23

Experimental models for trypanosomiasis indicated that the transition from the
fi rst to second stage of HAT is dependent on infl ammatory cytokine secretion by
macrophages and microglial cells, which in turn can activate matrix metalloprotei-
nases that selectively cleave basement membrane components from BBB endothelial
cells. Th is could facilitate the migration of leukocytes and trypanosomes across the
BBB. Tumor necrosis factor (TNF) seems to be especially involved in all stages of
the infl ammatory pathology of trypanosomiasis. In this context, TNF was fi rst
identifi ed as cachexin, the causative agent of the tremendous weight loss (cachexia)
observed during cattle trypanosomiasis. Circulating serum TNF concentrations and
disease severity are correlated for HAT, cattle trypanosomiasis and experimental
mouse infections. Interestingly, TNF was also demonstrated to exert an eff ector
function in parasitemia control, attributed to a direct trypanolytic eff ect of this
cytokine. A TNF
–/–
mouse model confi rmed the involvement of TNF in both
parasite control and immune pathology, as knockout mice show signifi cantly less
signs of morbidity as compared to wildtype mice although having 20-fold higher
parasite concentrations in the blood (10
9
/ml versus 5 × 10
7
/ml).
Another aspect of trypanosomiasis-associated pathology, causing extensive
morbidity during animal trypanosomiasis, is anemia. Th e mechanism underly-
ing trypanosomiasis-elicited anemia was proposed to rely on (i) the release of
trypanosome components lytic to red blood cells (RBCs), (ii) antibody-mediated
lysis and/or phagocytosis of opsonized erythrocytes and (iii) suppression of
RBC replenishment by erythropoiesis. Detailed analysis of anemia has recently
uncovered a major role of T-cells, found to be a major source of IFNγ during
infection, and responsible for excessive macrophage activation and TNF produc-
tion. As such, activated macrophages and TNF are proposed to play key roles in
both the induction of pathology and the control of parasitemia.
Beside the pathology occurring during infection, treatment with a trypanocidal
drug during second stage HAT can prove fatal due to several complications includ-
ing cardiomyopathy, hepatitis and especially posttreatment reactive encephalopathy
(PTRE). To study PTRE, several mouse models were generated mainly relying on
sub-curative drug treatment. Th is treatment clears parasites from the circulation,
but not from the central nervous system, eventually leading to encephalitic shock.
Immunological analysis revealed that severe PTRE was associated with astrocyte
activation and increased IL (interleukin)-1
α
, -4, -6, MIP-1 (macrophage infl am-
matory protein-1) and TNF mRNA levels in the brain. Th is indicates that infl am-
matory responses in the central nervous system are associated with the occurrence
of encephalopathy aft er treatment. A profound continued analysis of PTRE is
mandatory in order to develop appropriate treatment protocols that will reduce
fatalities during HAT treatment.
Diagnosis
Th e main bottlenecks in HAT diagnosis are the identifi cation of the infecting
trypanosome subspecies as well as the determination of the disease stage. Correct
diff erential diagnosis of T. b. gambiense and T. b. rhodesiense infections will deter-
mine the applied treatment, as T. b. rhodesiense is acute and refractory to two of the
four trypanocidal drugs. Th e disease stage will determine whether BBB-crossing
drugs that are generally more toxic, should be administered. To date, diagnosis of
165
African Trypanosomiasis
23

fi rst stage HAT mainly relies on microscopic detection of trypanosomes in blood
smears and lymph node aspirates. Second stage HAT diagnosis is based on para-
site detection or lymphocyte counting in the cerebrospinal fl uid (CSF) taken by
lumbar puncture. So far, molecular and serological tools cannot substitute for the
classical parasitological procedures. For fi eld conditions, the card agglutination
test for trypanosomiasis (CATT) is the preferred fi rst-line serological detection
method for T. b. gambiense, but must be followed by parasitological confi rmation
and stage determination. Th e assay relies on the detection of anti-VSG antibodies
in an agglutination reaction. However, since this diagnostic test is based on the
recognition of one variable antigen type (VAT), LiTat 1.3, antigenic variation
of the parasite population in a given foci can result in the disappearance of this
VAT and false negative CATT assay results. In addition, a variable percentage of
CATT seropositive individuals shows no clinical signs of infection and cannot be
confi rmed by parasitological detection. In order to complement the CATT assay,
a T. b. gambiense specifi c polymerase chain reaction (PCR) was recently developed,
based on the presence of a T. b. gambiense-specifi c gene, i.e., tgsGP. Th is PCR
was proven to be unaff ected by antigenic variation of the VSG and able to detect
infections in individuals that scored negative in the antibody-based CATT test.
In addition, false positive CATT results could be excluded by this PCR-based
technique. Finally, since trypanosome-specifi c antibodies remain in the circula-
tion aft er curative HAT treatment, the serological CATT assay cannot be used to
detect relapses or re-infection. Here, the further development of a TgsGP reversed
transcription PCR (RT-PCR) for the detection of mRNA from living parasites
could provide a discriminative diagnosis in previously infected individuals.
For T. b. rhodesiense, infections can only be diagnosed by microscopic analysis
as no serological fi eld test is available. Based on advances in molecular parasitology,
a new T. b. rhodesiense diagnostic PCR method has been developed based on the
restricted presence of the serum resistance antigen (SRA) gene. Th e SRA-based
PCR could be appropriate for diagnosis and to delineate the distribution pattern
of T. b. rhodesiense in livestock, an issue that will become crucial for correct HAT
management.
In addition to PCR diagnostics, rapid and low cost diagnostic approaches
are developed and validated with a focus on increased sensitivity and specifi city
(overview Fig. 23.4). As an alternative for PCR, a new DNA amplifi cation method
under isothermal conditions, the loop-mediated isothermal amplifi cation (LAMP)
for the detection of African trypanosomes has been developed for easier fi eld use.
As alternative serological tests, the immunofl uorescent antibody test (IFAT) and
enzyme-linked immunosorbent assay (ELISA) methods have been proposed.
However, due to the simplicity and rapidity of the CATT, it remains the most
effi cient fi eld serological test. As an alternative for the CATT, the LATEX/T. b.
gambiense has been developed. Th is test is, similarly to the CATT test, based on
an agglutination reaction, using latex particles coated with three purifi ed vari-
able surface antigens, LiTat 1.3, 1.5 and 1.6. In recent fi eld studies conducted in
several West and Central African countries, LATEX/T. b. gambiense showed a
higher specifi city (96 to 99%) but a lower or similar sensitivity (71 to 100%) as
compared to the CATT. Further evaluation of this test is ongoing before it can be
recommended for routine fi eld use.
166
Medical Parasitology
23

Another approach is to improve the microscopic detection of trypanosomes
by including a parasite concentration step. Th ree techniques have been proposed
for this purpose: (i) the microhematocrit centrifugation technique (mHCT), (ii)
the quantitative buff y coat (QBC) and (iii) the mini-anion-exchange centrifuga-
tion technique (mAECT). Th e mHCT and QBC techniques are based on the
concentration of parasites in the white blood cell fraction of total blood by high
speed centrifugation in capillary tubes. Both techniques enhance the detection
limit signifi cantly (<100-500 trypanosomes/ml), but remain quite labor inten-
sive. Th e mAECT allows the separation of trypanosomes from blood cells, based
on the diff erences in surface electrical charge. Validation of a newly produced
mAECT version for fi eld usage is ongoing. Recently, spotting and methanol-fi xing
of blood samples aft er erythrocyte lysis on microscopy slides followed by specifi c
trypanosome-detection by fl uorescence in situ hybridization (FISH) with peptide
nucleic acid (PNA) probes was proposed as alternative approach with improved
detection limits (5 trypanosomes/ml). PNA probes are pseudopeptides that are
resistant to nucleases and proteases and hybridize specifi cally to a complementary
nucleic acid target (DNA or RNA). Using Trypanozoon 18S ribosomal DNA
sequences that are not aff ected by the mechanisms of antigenic variation, specifi c
probes were generated for batch hybridization assays. Together, detection limits
of microscopic diagnosis can be improved by several techniques but make the
procedure more labor intensive and require mobile teams to be equipped with
additional apparatus for fi eld diagnosis.
Treatment
In T. b. gambiense infections, the human reservoir is the primary source for new
HAT cases as the disease is chronic and might take years to result in fatal outcome.
Treatment relies on suramine, pentamidine, melarsoprol and efl ornitine. Suramine
is a polysulphonated symmetrical naphthalene derivative fi rst used to treat HAT
in 1922. Th e drug is administered through slow intravenous injection, typically
Figure 23.4. Overview of the major diagnostic tests based on microscopy,
serology and molecular techniques for fi eld diagnosis of T. b. rhodesiense and
T. b. gambiense infections. Obtained with permission from Guy Caljon.
167
African Trypanosomiasis
23

100-200 mg (test dose), then 1 g IV on days 1, 3, 7, 14 and 21. Severe side eff ects
have oft en been reported, including anaphylactic shock, severe cutaneous reac-
tions, neurotoxic signs and renal failure. Th e exact trypanocidal mode of action of
suramine remains to be elucidated. However, as suramine does not cross the BBB,
its application is limited to the treatment of the haemolymphatic stage HAT.
Compared to suramine, pentamidine is better tolerated and has been in use
since 1940. It is an aromatic diamidine that exerts a direct trypanocidal activity
but also does not cross the BBB. Th e typical administration protocol is a regime of
seven intramuscular doses of 4 mg/kg per injection given daily or every alternate
day. Hypotension and hypoglycemia are the most common side eff ects. As a polyca-
tion, pentamidine interacts with polyamines and circular DNA molecules in the
mitochondrion upon uptake into the parasite by a specifi c receptor/transporter
(P2 amino-purine transporter). As such, specifi c point-mutations or loss of expres-
sion of this transporter can render parasites resistant to pentamidine treatment.
Interestingly, loss of this transporter function also renders trypanosomes resistant
to melarsoprol, the main drug for treatment of second stage HAT.
Th e melaminophenyl arsenical melarsoprol is a trivalent organo-arsenical com-
pound that was fi rst used in HAT treatment in 1949. Th e drug is water-insoluble
and is dissolved in 3.6% propylene glycol. Generally, melarsoprol treatment is
preceded by one or two injections with suramine or pentamidine to clear the
parasites from the bloodstream. Th e most common treatment protocol consists
of 3 to 4 series of intravenous injections separated by rest periods of at least 1 week
(8-10 days), as melarsoprol is a highly toxic drug that penetrates the central nervous
system. Each series consists of one intravenous injection of 2-3.6 mg/kg/d on 3
consecutive days. Although this arsenical derivate very effi ciently lyses trypano-
somes, posttreatment reactive encephalopathy occurs as an adverse drug reaction
in up to 12% of the cases. A potential target of melarsoprol is trypanothione
(N
1
,N
8
-bis-glutathionylspermidine), a low molecular weight thiol comprising
two glutathione molecules conjugated with spermidine. In trypanosomatids, try-
panothione fulfi lls most of the roles carried out by glutathione as the major redox
reactive metabolite in mammalian cells. As arsenic is documented to interact very
stably with thiols, complexation with trypanothione could account for a complete
disturbance of the redox balance and a rapid trypanotoxic eff ect.
Th e fourth drug used to treat T. b. gambiense is efl ornithine (DFMO or
dl-alpha-difl uoromethylornithine). Th is is the only new molecule registered for
HAT treatment in the last 50 years and was fi rst used in 1981. DFMO is diffi cult to
administer as it requires to be given at 400 mg/kg/d in 4 doses for 14 days. While it
is better tolerated than melarsoprol for the treatment of second stage HAT, it still
can cause pancytopenia, diarrhea, convulsions and hallucinations. DFMO is an
analogue of ornithine, which acts as a specifi c inhibitor of ornithine decarboxylase
(ODC) resulting in a suppression of the trypanothione and polyamine biosynthe-
sis. Specifi city for parasite killing results from a several orders of magnitude faster
turnover of ODC in mammalian cells as compared to trypanosomes.
In contrast to T. b. gambiense, T. b. rhodesiense is a zoonotic parasite that mainly
infects livestock and wild animals. Infections in humans are acute and require a
fast and accurate diagnosis to initiate an appropriate treatment. Treatment relies
168
Medical Parasitology
23

only on suramine (fi rst stage) and melarsoprol (second stage) as T. b. rhodesiense
is refractory to pentamidine and DFMO.
In the context of novel chemotherapies against HAT, ongoing preclinical
and clinical trials are focusing on combinational treatment strategies in order
to increase cure rates by lower dosages and milder treatment schedules. An
example of such approach is the combination of efl ornitine with nifurtimox
(5-nitrofuran), an orally administered drug that is used for the treatment of
Chagas disease and sometimes for the encephalitic stage of T. b. gambiense HAT
if efl ornitine or melarsoprol are ineff ective. Combination of efl ornitine (DFMO)
and suramine is also in trial for treatment of second stage T. b. rhodesiense
infection. Other compounds are being tested for targeting several biochemi-
cal pathways in the trypanosome including, e.g., the polyamine biosynthesis,
trypanothione reductase and glycolytic enzymes. In that context, DB289, an
aromatic diamidine (pentamidine analog) and prodrug of the active metabolite
diphenyl furan diamidine (DB75), is currently in Phase III clinical trials as a new
orally administered candidate drug to treat 1st stage HAT.
A novel immunotherapeutic approach in the preclinical phase is based on the
generation of a 15 kD VSG-recognizing molecule derived from nonconventional
heavy chain camel antibodies. In contrast to most other mammals, camelids have
a separate class of single chain antibodies that enable the engineering of small
antigen-specifi c moieties (nanobodies) through a relatively simple procedure of
cloning and affi nity panning. Th e generated nanobody was shown to be a promising
tool for targeting eff ector molecules to the trypanosome membrane as it is able to
penetrate into the VSG coat and bind to conserved trypanosome surface epitopes
that are inaccessible to lager conventional antibodies. In the further development
of immunotoxins for trypanosomiasis therapy, trypanosome-specifi c nanobodies
might be coupled to conventional drugs or new trypanocidal molecules. Recently,
a toxin was generated from apolipoprotein L-1 (ApoL-1), a naturally occurring
trypanolytic component in normal human serum and coupled to a VSG recogniz-
ing nanobody for in vivo use.
Conclusion
African trypanosomiasis is a devastating disease that is making a fast comeback
in sub-Saharan Africa. Limited local resources for trypanosomiasis prevention
and control have made this disease a major humanitarian and economic disaster
aff ecting more than 10 million Km
2
of the African continent. Currently, no
serological fi eld test for T. b. rhodesiense is available, while diff erential diagnosis
of the two human-pathogenic subspecies relies on relatively sophisticated molec-
ular-based (PCR) tests. Moreover, available trypanocidal drugs have considerable
levels of toxicity and are generally used for a specifi c disease stage. Combined
eff orts for new drug design approaches will be needed to combat this disease
in the future. Beside toxicity, the rise of drug-resistance in trypanosomes is an
important issue to be taken into account, urging that mechanisms of resistance
need to be elucidated. One of those mechanisms is dependent on the reduced
uptake of drugs through the P2 amino-purine transporter leading to resistance
of trypanosomes to pentamidine, melaminophenyl arsenicals and potentially all
169
African Trypanosomiasis
23

new drug variants derived hereof. While preclinical and clinical studies on new
chemotherapeutic and immunotherapeutic approaches seem to off er alternative
approaches, further research is needed to evaluate pharmacological properties and
applicability in the fi eld. Other lines of research that might lead to the discovery
of new low-toxicity antitrypanosomal agents will probably emerge through
unraveling unique biochemical pathways utilized by the parasite. For instance,
the specifi c compartmentalization of the glycolysis in glycosomes or unique
metabolic features of the trypanosome could yield new antiparasite drug-targets.
In this context, the full sequencing of the trypanosome genome will probably
also contribute to the identifi cation of new potential drug targets.
Suggested Reading
1. Vanhamme L, Lecordier L, Pays E. Control and function of the bloodstream variant
surface glycoprotein expression sites in Trypanosoma brucei. Int J Parasitol 2001;
31:523-31.
2. Magez S, Radwanska M, Beschin A et al. Tumor necrosis factor alpha is a key
mediator in the regulation of experimental Trypanosoma brucei infections. Infect
Immun 1999; 67:3128-32.
3. De Baetselier P, Namangala B, Noel W et al. Alternative versus classical macrophage
activation during experimental African trypanosomosis. Int J Parasitol 2001;
31:575-87.
4. Pays E, Vanhollebeke B, Vanhamme L et al. Th e trypanolytic factor of human
serum. Nat Rev Microbiol 2006; 4:477-86.
5. Chappuis F, Loutan L, Simarro P et al. Options for fi eld diagnosis of human African
trypanosomiasis. Clin Microbiol Rev 2005; 18:133-46.
6. Fairlamb AH. Chemotherapy of human African trypanosomiasis: current and
future prospects. Trends Parasitol 2003; 19:488-94.
170
Medical Parasitology
23

C
HAPTER
24
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Visceral Leishmaniasis (Kala-Azar)
Ambar Haleem and Mary E. Wilson
Abstract
Amongst the many clinical forms taken by leishmaniasis, visceral leishmani-
asis is the form that most oft en leads to a fatal outcome. Ninety percent of cases
world-wide occur in three regions: northeast India/Bangladesh/Nepal, the Sudan
and northeast Brazil. Th e disease is most oft en caused by L. donovani or L. infan-
tum in the Old World, or by L. chagasi in the New World. Th e clinical presenta-
tion diff ers somewhat in diff erent geographic regions, with humans serving as a
main reservoir of infection in India due to the high incidence of PKDL, but dogs
serving as a major reservoir in Brazil and around the Mediterranean. Treatment
of visceral leishmaniasis is complicated by a need to administer standard therapy
parenterally, toxicity of therapeutic agents and emerging parasite resistance to
standard medications.
Introduction
Leishmaniasis is a vector-borne disease caused by obligate, intracellular protozoa
belonging to the genus Leishmania; 21 out of the 30 mammalian-infecting species
of Leishmania cause disease in humans.
5,13,14
Th e etiologic parasite was discovered
in 1903, when Leishman and Donovan separately described the protozoan now
called Leishmania donovani in splenic tissue from patients in India. Th ey correctly
identifi ed this as the causative agent of the life threatening disease visceral leish-
maniasis.
5
Th e insect vector is a female phlebotomine sand fl y which acquires the
parasite while feeding on an infected mammalian host. A total of about 30 sand
fl y species have been identifi ed as vectors transmitting the diff erent Leishmania
species, although not all sand fl ies are capable of hosting all Leishmania species.
5
Th e disease leishmaniasis refers to several clinical syndromes. Th e most common
are visceral (VL), cutaneous (CL) and mucocutaneous (MCL) leishmaniasis,
which result from pathological changes in the reticuloendothelial organs, dermis
and naso-oropharynx, respectively. Th e following chapter will include a discussion
of the epidemiology, pathogenesis, clinical features and diagnostic and therapeutic
approaches employed in visceral leishmaniasis.
Leishmaniasis is caused by a large number of Leishmania species that lead to
characteristic clinical syndromes, with some overlap between species. Th e distri-
bution of the diff erent Leishmania infections is very regional and treatment is
challenging. With the spread of AIDS, visceral leishmaniasis (VL) has become
recognized as an opportunistic co-infection in HIV-infected people particularly
in the Iberian Peninsula. Th e high morbidity and mortality associated with visceral

172
Medical Parasitology
24
leishmaniasis, lack of available and aff ordable diagnostic and therapeutic modali-
ties and increasing drug resistance in the developing world continue to pose major
challenges in eradication of this infection.
Epidemiology
Leishmania donovani and Leishmania infantum in the Old World (particu-
larly India, Nepal, Bangladesh and Sudan) and Leishmania chagasi in the New
World (Latin America) are responsible for most of the cases of visceral leishmani-
asis worldwide. Some of the Leishmania spp. that are commonly associated with
CL (L. amazonensis in Latin America and L. tropica in Middle East and Africa)
are on occasion, also isolated from patients with visceral disease.
5,13,14
Molecular
techniques have revealed that most likely L. chagasi and L. infantum are the same
organism causing disease in diverse geographic locations.
7
Leishmaniasis has been reported from 88 countries around the world.
Approximately 90% of the estimated 500,000 new annual cases of visceral disease
occur in rural areas of India, Nepal, Bangladesh, Sudan and Brazil. (http://www.
cdc.gov/ncidod/dpd/parasites/leishmania/). Disease is transmitted primarily
through the bite of a sand fl y, although rarely disease is transmitted by the con-gen-
ital route, blood transfusions, accidental needle stick injuries in the laboratory or
by sharing of leishmania-contaminated needles by intravenous drug users.
13
Visceral leishmaniasis encompasses a broad range of clinical manifestations.
Infection can assume an asymptomatic/subclinical and self-resolving form, or
follow an aggressive, systemic course of illness (classic kala-azar or black fever).
Th e disseminated form of infection is fatal if untreated and has resulted in mass
epidemics in India and Sudan.
Most leishmania infections are zoonotic, with dogs or rodents as reservoir hosts.
Only two species can maintain an anthroponotic cycle (human reservoir).
14
Th ese
two species are L. donovani, responsible for VL in the Indian subcontinent (par-
ticularly Bihar and Assam states) and East Africa, as well as L. tropica that causes
CL in the Old World. Particularly in East Africa, people aff ected by post-kala-azar
dermal leishmaniasis (PKDL) may serve as a reservoir for visceral disease.
13
Pathogenesis
Leishmania spp. parasites exist in two stages, the promastigote and the amas-
tigote. Th e promastigote is a15-20
μ
m × 1.5-3.5
μ
m fl agellated form found in the
gut of sand fl ies. Th e amastigote is a nonfl agellated, intracellular form measuring
2-4
μ
m in diameter that replicates in macrophage phagosomes. Amastigotes are
the only form present in mammalian hosts.
Aft er inoculation into skin by a sand fl y, promastigotes are phagocytosed by
dermal macrophages, where they convert to amastigotes and multiply within acidic
parasitophorous vacuoles. Additional mononuclear phagocytes are attracted to the
site of the initial lesion and become infected (Fig. 24.1). Amastigotes then dissemi-
nate through regional lymphatics and the vascular system to infect mononuclear
phagocytes throughout the reticuloendothelial system (Fig. 24.1). Progressive
recruitment of amastigote-infected mononuclear phagocytes and infl ammatory
cells within organs results in distortion of the native tissue architecture and oft en,
massive hepatosplenic enlargement. Parasitized reticuloendothelial cells can be
found in bone marrow, lymph nodes, skin and other organs.
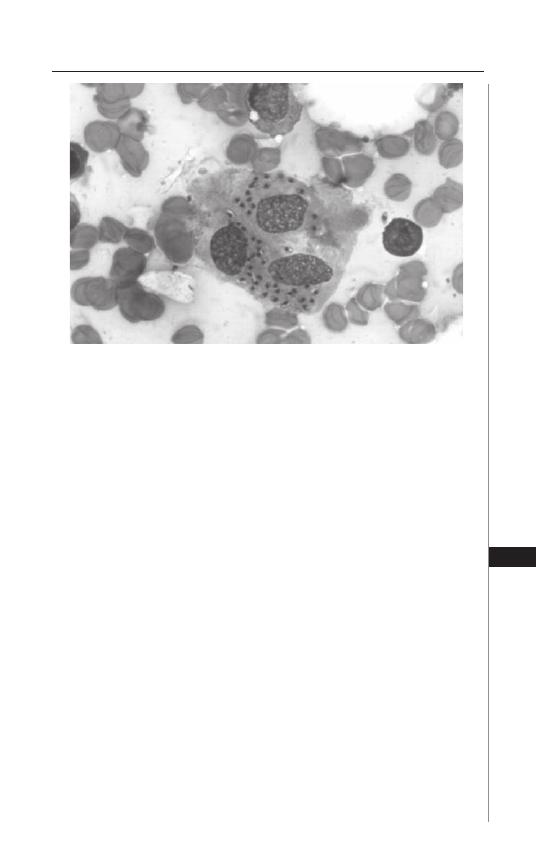
173
Visceral Leishmaniasis (Kala-Azar)
24
Why the infection follows a self-resolving course in certain human hosts and
progresses to overwhelming, life-threatening disease in others remains an area of
intense research. Mice self-cure infection and thus are a better model of asymptom-
atic infection than disease. Although murine models cannot completely explain the
unique milieu present during human infection, murine models have illuminated the
cytokines and chemokines that play key roles in determining whether the parasite
replicates within quiescent macrophages or is killed by activated macrophages.
Some mouse strains are inherently susceptible or resistant to Leishmania spp.
infections. Similarly, genetically determined human immune responses infl uence
the manifestations of leishmania infection in the human host.
5,12,20
Early in infection of genetically resistant mouse strains, expansion of leishma-
nia-specifi c CD4
+
T-cells of the Th 1 type that secrete interferon gamma (IFN-
γ
)
and interleukin 2 (IL-2) confers resistance to disease progression.
20
In contrast,
expansion of Th 2-type CD4
+
cells producing IL-4, IL-10 and IL-13 leads to
progression of infection caused by L. major or other species inducing CL in mice.
Transforming growth factor
β
(TGF-
β
) in the absence of a Th 2 response promotes
progressive murine infection due to the visceralizing Leishmania species. IL-2
enables diff erentiation of Th 1 cells and production of IFN-
γ
, which then activates
murine macrophages to kill amastigotes largely through nitric-oxide dependent
mechanisms.
20
Similar to mice, humans who have either had self-limiting infection with L.
donovani or L. infantum/L. chagasi, or who have been successfully treated for
symptomatic VL, develop protective Type 1 immunity against the same parasite.
Leishmania-specifi c Type 1 responses are lacking in human hosts during progressive
VL, although there is not oft en a clear expansion of Type 2 or TGF-
β
response during
progressive infection.
20
Nonetheless, antileishmanial antibodies from polyconal B-cell
Figure 24.1. Bone marrow aspirate from a patient suffering from VL showing
amastigotes in the macrophages. Photo kindly provided by Selma Jeronimo,
MD, PhD, Universidade Federal do Rio Grande do Norte, Natal RN Brazil.

174
Medical Parasitology
24
activation are produced in high titer during progressive VL, but are not protective,
similar to the murine Th 2 response. Humans can develop reactivation of disease in
the setting of immune suppression such as occurs during HIV-1 co-infection.
Clinical Manifestations of Kala Azar
Infection with the Leishmania species causing visceral leishmaniasis can mani-
fest as a progressive fatal disease or as an asymptomatic form. Th e incubation period
typically varies from 3 to 8 months, but can be weeks or years. Typical, symptomatic
VL is associated with heavily infected mononuclear phagocytes throughout the
reticuloendothelial system and suppressed cellular immune responses. VL can be
fatal if left untreated.
Th e onset of disease is insidious in most cases and marked by the progressive
development of fever, weakness, anorexia, weight loss and abdominal enlargement
from hepatosplenomegaly. Fever, accompanied by chills is usually intermittent or
remittent with twice-daily temperature spikes. During the less common acute cases,
fever can be of abrupt onset and have a periodicity similar to that of malaria.
Progressive and massive hepatosplenomegaly is characteristic of VL. Infected
individuals in the Sudan oft en also develop lymphadenopathy (Fig. 24.2) and in
India patients with VL commonly develop hyperpigmentation of extremities,
face and abdomen. Hemorrhage can occur from various sites. Severe cachexia is
a prominent feature of VL, driven in part by high levels of TNF-
α
. Death from
VL occurs either from the primary, multisystem disease causing malnutrition and
bone marrow suppression and/or from secondary bacterial infections such as
tuberculosis, dysentery, pneumonia and measles.
13
Important laboratory fi ndings in advanced visceral disease include profound
pancytopenia, eosinopenia, hypoalbuminemia and hypergammaglobulinemia
(mainly IgG). Th e erythrocyte sedimentation rate is usually elevated. Kidneys may
show evidence of immune complex deposition, but renal failure is rare.
Several infectious and hematologic diseases can mimic visceral leishmaniasis.
Th ese include malaria, schistosomiasis, miliary tuberculosis, African trypanoso-
miasis, typhoid fever, brucellosis, histoplasmosis, bacterial endocarditis, lymphoma
and leukemia.
Coinfection with HIV-1
Reactivation (or newly acquired) visceral leishmaniasis is a recognized oppor-
tunistic infection in T-cell impaired/defi cient persons. Examples include individu-
als with HIV-1 infection, neoplasm, or receiving steroids, cancer chemotherapy
or antirejection agents in organ transplantation. Th e leishmania parasite may be
a cofactor in the pathogenesis of HIV infection. A major surface molecule, the
lipophosphoglycan of L. donovani, induces transcription of HIV in CD4
+
cells.
4
Most of the data on HIV co-infected persons with VL is derived from three
countries in southern Europe, in particular from Spanish patient cohorts. Based on
these studies, it appears that most HIV-infected patients manifest VL late in the
course of HIV infection (CD4 cell count <200 cells/mm
3
in 90% of patients). Th e
clinical presentation can be atypical.
1,5,6,13
Splenomegaly may be absent, whereas the
gastrointestinal tract and oro-mucosal surfaces are commonly involved. Visceral
leishmaniasis usually follows a chronic and relapsing course in HIV-positive
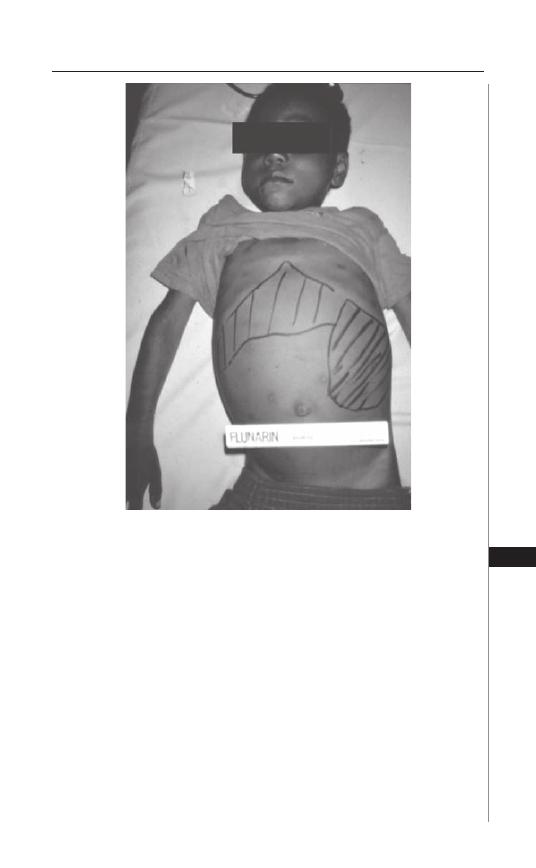
175
Visceral Leishmaniasis (Kala-Azar)
24
patients. Initial responses to traditional VL therapy are lower in these hosts and
adverse drug reactions are frequent. 50-70% of HIV-infected patients relapse within
12 months aft er discontinuing treatment.
10,13
Post-Kala-Azar Dermal Leishmaniasis
PKDL is a syndrome encountered aft er completion or premature cessation of
treatment for visceral leishmaniasis due to L. donovani. PKDL occurs in 5-10% of
persons with VL in India and approximately 50% of those in Sudan.
13
PKDL may
also occur in some HIV-coinfected people. Th e clinical presentation of PKDL in
India and Sudan is similar, although the onset and duration of skin lesions diff ers
between these two patient populations. In India, skin lesions typically appear 1
to 2 years aft er therapy and can persist for as long as 20 years, whereas the timing
of appearance and persistence of lesions is much shorter in Sudan. PKDL lesions
presumably serve as a source of leishmania infection for sand fl ies and the long
duration of PKDL in Indian patients helps explain the fact that humans serve as
the major reservoir of disease in this country.
Figure 24.2. Hepatosplenomegaly in patients suffering from VL. Photo kindly
provided by Dr. John David, Harvard School of Public Health, Boston.

176
Medical Parasitology
24
PKDL is generally asymptomatic other than widespread skin lesions on the face,
trunk, extremities, oral mucosa or genitalia. Th ese can vary from hyperpigmented
macules to overt nodules. Lesions may resemble the lesions of leprosy clinically
and pathologically.
Diagnosis
Th e clinical features of visceral leishmaniasis are highly suggestive of, but not
specifi c for this disease. Particularly in developing countries the diff erential is wide,
including leukemia and a variety of tropical infections such as malaria, schistoso-
miasis and tuberculosis amongst others. Moreover, people from non-endemic areas
or those with HIV co-infection can manifest atypical manifestations of VL. Hence,
diagnosis must be confi rmed by demonstration of the parasite in tissues.
5
Tissue Diagnosis
Reliable diagnostic methods for leishmaniasis primarily involve invasive
procedures with visualization of amastigotes in Wright-Giemsa stained smears
of tissues, or by culture of promastigotes from human samples.
5,14
Splenic, liver
or bone marrow biopsy, lymph node aspirates (particularly in Sudan) or buff y
coat of peripheral blood can be utilized to look for the parasite microscopically.
Splenic aspiration, although incurring a risk of hemorrhage, is the most sensitive
means (95%) for diagnosing leishmaniasis.
5,14
Bone marrow biopsy demonstrates
amastigotes in approximately two-thirds of patients. Liver biopsy is less sensitive
than either splenic or bone marrow specimens.
13
Syndromes such as VL or PKDL are characterized by many parasites in tissues,
whereas lesions of mucosal leishmaniasis characteristically exhibit an exuberant
infl ammatory infi ltrate with few parasites present. Logically, the abundance of
parasites in tissues of the reticuloendothelial system during visceral leishmaniasis
enables relatively easy demonstration of parasites from tissue smears as compared
to diagnosis in cutaneous and mucosal syndromes of leishmaniasis. Furthermore,
in HIV co-infected individuals parasites may be isolated and cultured from
a multitude of sites, oft en atypical. Th ese include bronchoalveolar lavage and
pleural fl uid, biopsies of the gastrointestinal tract or peripheral blood smears. Th e
Giemsa-stained peripheral blood smear has a sensitivity of about 50% and parasite
culture of a buff y coat preparation, about 70% for diagnosis of VL in HIV-positive
patients.
5,13
In post-kala-azar dermal leishmaniasis syndrome, diagnosis is primarily
clinical although amastigotes can be readily visualized in dermal macrophages in
80% of Sudanese patients.
In order to make an accurate diagnosis of leishmaniasis, amastigotes should be
visualized by light microscopy under oil immersion. Identifying features are the
parasite size (2-4
μ
m in diameter), shape (round to oval) and morphologic charac-
teristics (nucleus and kinetoplast).
5
Th e kinetoplast is a rod-shaped mitochondrial
structure that contains the extranuclear mitochondrial DNA.
14
In vitro culture of promastigotes from tissue aspirates should be performed
in concert with microscopic demonstration of amastigotes. However, it can take
several weeks to achieve a detectable concentration of parasites in culture. Th e
standard method for Leishmania species identifi cation is by isoenzyme analysis
of cultured promastigotes. Various molecular methods are promising tools but

177
Visceral Leishmaniasis (Kala-Azar)
24
require further assessment for fi eld application. Th ese can employ polymerase chain
reaction (PCR) to detect leishmanial DNA and RNA targets.
14
Serology
Serological assays are sensitive tools for the diagnosis of VL in immuncompetent
persons. Leishmania parasite-specifi c antibody responses peak during active VL and
decline aft er treatment or spontaneous resolution of infection, although a period of
seropositivity aft er recovery can confound the interpretation of a positive response.
Antibodies do not correlate with protective immunity and do not remain posi-
tive for life and as such they cannot be used for studies of prevalence or presumed
immunity to reinfection. Th ey are, however, extremely useful for the diagnosis of
VL. Factors that preclude widespread use of serological and immunodiagnostic
methods are test costs, availability of a full-equipped laboratory, adaptability to
fi eld/rural conditions in developing countries and confl icting results in immu-
nocompromised patients. Antibodies produced during several other infections
(leprosy, cutaneous leishmaniasis, Chagas disease) can yield false-positive results
on tests for VL, whereas patients with AIDS may have false-negative results owing
to absence of an antileishmanial humoral response.
Recent advances that have increased the feasibility of serodiagnosis include the
discovery that humoral responses to recombinant K39 antigen of L. chagasi/L. infan-
tum are highly correlated with acute disease and the development of an rK39 dipstick
test for use in the fi eld. Nonetheless, it is sometimes diffi cult to distinguish between
remote and recent infection. Th ere is no one diagnostic method that is perfect.
5,13,14
Commonly employed antigen-based tests are the enzyme linked immunosor-
bent assay (ELISA) using whole parasite lysate, the rK39 ELISA and the direct
agglutination test (DAT). Indirect fl uorescent antibody detection test (IFAT) is
the only one that has been used on a limited scale.
14
Th e DAT entails agglutination
of Coomassie blue-stained promastigotes in serum dilutions and is simple and reli-
able for fi eld use. Th e ELISA assay is highly sensitive (80-100%) but the specifi city
varies with the antigen used, from 80-94% with whole parasite lysate to or 100%
with rK39. Studies have found that dipstick tests using a recombinant kinesin-like
antigen, rK39 from L. infantum/L. chagasi have 100% sensitivity and specifi city
for diagnosis of VL and that the test has good predictive value in detecting VL in
immunocompromised patients.
5,13,14
Antigen-impregnated nitrocellulose paper strips with rK39 antigen are being
used successfully on the fi eld for VL diagnosis. Th e DAT assay has been found to
be 91-100% sensitive and 72-100% specifi c in various studies.
Skin Testing
It should be mentioned that the delayed-type hypersensitivity (DTH) leish-
mania antigen skin test (Montenegro test) is generally negative during active VL
and may be negative during post-kala-azar dermal leishmaniasis. Th e test becomes
positive in the majority of people in whom infection spontaneously resolves or who
have undergone successful therapy. However, the lag between clinical recovery and
DTH development can be months or longer. DTH testing is very useful in the
diagnosis of cutaneous leishmaniasis. It is utilized primarily as an epidemiological
tool and has little role in establishment of a diagnosis of acute VL.
13

178
Medical Parasitology
24
Treatment
Th e decision to treat leishmaniasis depends on the clinical syndrome, the infect-
ing Leishmania spp., the immunologic status of the host and drug availability and
cost. Th e goal of antileishmanial therapy is to prevent death from visceral disease
and limit morbidity from cutaneous and mucocutaneous syndromes.
Th erapeutic strategies for treatment of VL have evolved since 1912 when the
fi rst drugs against Leishmania species, the organic pentavalent antimonials were
developed. Two commonly used pentavalent antimony preparations are stiboglu-
conate sodium and meglumine antimoniate. However, the recent emergence of
parasite strains resistant to pentavalent antimony (Sb) preparations has limited the
utility of this mainstay of thereapy, primarily in the Indian subcontinent which
harbors the bulk of VL cases globally. In the early 1990s, failure rates of Sb reached
65% in the endemic Indian state of Bihar. In spite of treatment advances in VL, all
currently approved drugs are parenteral, toxic and must be administered for long
durations. Th e high cost of alternative medications has been a major drawback to
the use and development of newer agents in developing countries. Furthermore,
for HIV co-infected patients where relapse of visceral disease is common aft er
therapy there are few, good treatment options.
Outside of India, pentavalent antimonials are still the mainstay of therapy for
visceral disease in both children and adults. Th e recommended dosage is 20 mg/
kg/d for 28-30 days with usual cure rates of ∼90%.
10
Inadequate response to the
initial course of therapy or infection relapse may require a second course of treat-
ment with the same agent. Common side eff ects are mainly gastrointestinal and
include abdominal pain, nausea, vomiting and anorexia. Chemical hepatitis, chemi-
cal pancreatitis, arthralgias and myalgias can also occur. Th ese drugs should be used
cautiously in the elderly and in persons with heart disease, since dose-dependent
arrhythmias and sudden death have been reported. Th is is a particular risk with
doses >20 mg of Sb/kg/d. Renal failure can rarely occur.
Over the past 10 years practitioners in Sb-resistant areas of India have turned to
amphotericin B for fi rst-line therapy. Th e target of amphotericin B is ergosterol-like
sterols, the major membrane sterols of Leishmania spp., leading to parasite kill-
ing through the creation of membrane pores. At a dose of 0.75-1 mg/kg for 15
infusions on alternate days, the drug cures ∼95-97% of patients.
8,10,19
Occasional
relapses (∼1%) with amphotericin B can be successfully treated with the same
drug.
14
Limitations to the use of amphotercin B in developing countries include
the need for parenteral administration, cost and serious adverse eff ects such as
nephrotoxicity.
Lipid formulations of amphotericin B are eff ective and less toxic than ampho-
tericin B deoxycholate. In these preparations, various liposomes or other lipid com-
ponents have been used to replace the usual carrier deoxycholate. Liposomes target
the drug toward macrophages of the reticuloendothelial system and thus are ideal
for treatment of VL in which the parasite resides in these same cells. Renal toxicity
is a less frequent complication compared to conventional amphotercin B because
lipid drug formulations are specifi cally targeted toward macrophage-rich organs
and not to the kidney. Th is also allows for higher daily doses and shorter courses
of amphotercin therapy to be delivered.
2,15
Lipid formulations of amphotericin B

179
Visceral Leishmaniasis (Kala-Azar)
24
include liposomal amphotericin B (L-Amb: Ambisome), amphotericin B colloidal
dispersion (ABCD: Amphocil) and amphotericin B lipid complex (ABL: Abelecet).
Th e US Food and Drug Administration licensed liposomal amphotericin B in 1999
for treatment of VL and has recommended treating immunocompetent persons
with 3 mg/kg daily on days 1-5,14 and 21 (total 21 mg/kg) and immunosuppressed
patients with 4 mg/kg daily on days 1-5, 10, 17, 24, 31 and 38 (total 40 mg/kg).
3
An
alternative recommendation for immunocompetent persons is treatment on days 1-5
and day 10 with 3-4 mg/kg daily in Europe and Brazil, 3 mg/kg daily in Africa and
2-3 mg/kg daily in India.
3
Although the reason is not known, the continent-specifi c
therapeutic requirements could refl ect diff erent causative Leishmania species and/
or diff erences in the susceptible populations. Ambisome seems best-suited phar-
macologically for ultra-short-course of therapy. Indeed, a trial in India showed the
treatment duration can be compressed to a single day in India in adults and children
(one infusion of 5 or 7.5 mg/kg)
17
and to two days in children in Greece (two
infusions of 10 mg/kg each)
18
with some loss of cure rate but much improved cost
and compliance. Relapses can occur aft er treatment with the lipid formulations in
persons with AIDS and repeat courses may be necessary. However, the high cost,
even with lower total doses and short courses of therapy, oft en proves prohibitive
for use outside the developed world.
Another approved agent for treatment of visceral leishmaniasis is parenteral
pentamidine. Promising investigational treatment agents include parenteral paro-
momycin and the only oral drug, Miltefosine, which has proven eff ective for
Indian VL but is under investigation in other countries. Pentamidine has consider-
able toxicity and is not eff ective for Indian VL. Paromomycin is an inexpensive
aminoglycoside that has appeared promising in limited studies. Miltefosine is an
exciting oral option for therapy for VL in naive and Sb-refractory patients across
the world. It was registered for use in India for both children and adults in 2002
10
at a dose of 50-100 mg (∼2.5 mg/kg) for 4 weeks.
16
Th e drug is still undergoing
trials for therapy of VL in other regions of the world. Major limitations to the use
of miltefosine include its potential for teratogenicity and its long median half-life
of 154 hrs, which could encourage emergence of drug resistance.
14
Transient,
minor side eff ects are anorexia, diarrhea and nausea.
Th e imidazoles, ketoconazole and itraconazole, have been used successfully
in some cases of cutaneous leishmaniasis, but primary failures occur in visceral
leishmaniasis and they are not recommended for general use. Combination drug
therapy can be an attractive approach for enabling cure of VL. At present, the only
potential combination therapy is miltefosine and paromomycin but adequate
clinical trials are lacking in this area.
Persons co-infected with HIV-1 pose a major challenge to eff ective therapy
because of frequent relapses and reduced treatment responses. Most HIV-positive
patients are given one of the standard regimens for VL. Concomitant HAART
therapy and resulting increase in CD4 count >200 cells/mm
3
would be expected
to enhance the effi cacy of antileishmanial therapy and reduce relapse rates, but no
randomized controlled trials have been conducted to investigate this hypothesis.
Moreover, there is no general consensus about long-term maintenance therapy for
VL in HIV-coinfected patients.
9

180
Medical Parasitology
24
Antileishmanial treatment is indicated in Indian PKDL, and treatment
duration oft en spans 4 months. Most PKDL lesions of the Sudanese form
self-resolve. When therapy is necessary, stibogluconate sodium 20 mg/kg/d for
2 months is given.
Most patients with visceral leishmaniasis become afebrile during the fi rst week
of treatment.
5
Splenomegaly and biochemical abnormalities oft en take weeks to
resolve. Freedom from clinical relapse for at least 6 months is usually indicative of
cure.
5
Even aft er symptomatic cure, parasites can remain in tissues indefi nitely and
do not necessarily represent an indication for retreatment. However, if symptoms
relapse or do not resolve, a re-evaluation of the diagnosis is warranted followed
sometimes by retreatment. Relapses usually occur within 6 months of therapy and
are more common in persons with AIDS than in immunocompetent persons.
References
1. Albrecht H, Sobottka I, Emminger C et al. Visceral leishmaniasis emerging as an
important opportunistic infection in HIV-infected persons living in areas nonen-
demic for Leishmania donovani. Arch Pathol Lab Med 1996; 120:189-98.
2. Berman JD. Human leishmaniasis:clinical, diagnostic and chemotherapeutic
developments in the last 10 years. Clin Infect Dis 1997; 24:684-703.
3. Berman JD. US Food and Drug Administration approval of AmBisome (liposomal am-
photericin B) for treatment of visceral leishmaniasis. Clin Infect Dis 1999; 28:49-51.
4. Bernier R, Barbeau B, Tremblay MJ et al. Th e lipophosphoglycan of Leishmania
donovani up-regulates HIV-1 transcription in T-cells through the nuclear
factor-kappaB elements. J Immunol 1998; 160:2881-8.
5. Herwaldt BL. Leishmaniasis. Lancet 1999; 354:1191-9.
6. Lopez-Velez R. Th e impact of highly active antiretroviral therapy (HAART) on
visceral leishmaniasis in Spanish patients who are co-infected with HIV. Ann Trop
Med Parasitol 2003; 97:143-7.
7. Maurício IL, Stothard JR, Miles MA. Th e strange case of Leishmania chagasi.
Parasitol Today 2000; 16:188-9.
8. Mishra M, Biswas UK, Jha DN et al. Amphotericin versus pentamidine in antimony-
unresponsive kala-azar. Lancet 1992; 340:1256-7.
9. Murray HW. Kala-azar—progress against a neglected disease. N Engl J Med 2002;
347:1793-4.
10. Murray HW. Progress in the treatment of a neglected infectious disease:visceral
leishmaniasis. Expert Rev Anti Infect Th er 2004; 2:279-92.
11. Murray HW. Treatment of visceral leishmaniasis in 2004. Am J Trop Med Hyg
2004; 71:787-94.
12. Reed SG, Scott P. T-cell and cytokine responses in leishmaniasis. Curr Opin
Immunol 1993; 5:524-31.
13. Selma MB, Jeronimo AD, Richard QS et al. Leishmania species: Visceral
(Kala-Azar), cutaneous and mucocutaneous leishmaniasis. In: Mandell GL,
Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. London:
Churchill Livingstone, 2004.
14. Singh RK, Pandey HP, Sundar S. Visceral leishmaniasis (kala-azar): challenges
ahead. Indian J Med Res 2006; 123:331-44.
15. Sundar S, Goyal AK, More DK et al. Treatment of antimony-unresponsive Indian
visceral leishmaniasis with ultra-short courses of amphotericin-B-lipid complex.
Ann Trop Med Parasitol 1998; 92:755-64.
16. Sundar S, Jha TK, Th akur CP et al. Oral miltefosine for Indian visceral leishmani-
asis. N Engl J Med 2002; 347:1739-46.

181
Visceral Leishmaniasis (Kala-Azar)
24
17. Sundar S, Jha TK, Th akur CP et al. Single-dose liposomal amphotericin B in the
treatment of visceral leishmaniasis in India: a multicenter study. Clin Infect Dis
2003; 37:800-4.
18. Syriopoulou V, Daikos GL, Th eodoridou M et al. Two doses of a lipid formulation
of amphotericin B for the treatment of Mediterranean visceral leishmaniasis. Clin
Infect Dis 2003; 36:560-6.
19. Th akur CP, Kumar M, Pandey AK. Comparison of regimes of treatment of
antimony-resistant kala-azar patients: a randomized study. Am J Trop Med Hyg
1991; 45:435-41.
20. Wilson ME, Jeronimo SM, Pearson RD. Immunopathogenesis of infection with
the visceralizing Leishmania species. Microb Pathog 2005; 38:147-60.

Cutaneous Leishmaniasis
Claudio M. Lezama-Davila, John R. David
and Abhay R. Satoskar
Background
Th e leishmaniases comprise several diseases that are caused by Leishmania
species, intracellular protozoan parasites which lead to a wide spectrum of clinical
manifestations. Over 12 million people currently are infected with Leishmania and
approximately 2 million are infected annually, making it a major health problem
in parts of Asia, Africa, the Middle East, Southern Europe and Latin America.
Th e clinical manifestations depend on the parasite species. Visceral leishmaniasis
(VL or kala-azar) is caused by Leishmania donovani or Leishmania infantum (the
latter called chagasi in Latin America). Cutaneous leishmaniasis (CL) is caused by
Leishmania mexicana and Leishmania brasiliensis complexes in the New World and
by Leishmania major, Leishmania tropica and L. aethiopica complexes in the Old
World. Leishmania parasites are transmitted by about 50 diff erent species of new
(Lutzomia) and old (Phlebotomus) world sand fl ies. Most cases of human CL are
due to a zoonotic mode of transmission (animal to man), but L. tropica infections
are due to an anthroponotic mode of transmission (human to human).
Life Cycle
Th e female sand fl ies inoculate infective Leishmania metacyclic promastig-
otes during blood meals taken for egg production. Macrophages migrate to the
inoculation site and phagocytose parasites which then transform into amastigotes.
Amastigotes multiply in infected cells and skin and depending in part on the
Leishmania species, migrate to other organs. Th is is the basis of the diverse clinical
manifestations of leishmaniasis. Th en, more sand fl ies become infected when they
ingest infected macrophages full of amastigotes while taking blood meals. In the
sand fl y’s midgut, amastigotes diff erentiate into promastigotes, which multiply and
migrate to the proboscis transforming into the infective metacyclic stage. For life
cycle details, see Figure 25.1.
Geographical Distribution
Th e disease is found in 88 tropical and subtropical countries around the world.
Approximately 350 million people live in these areas. Each year, an estimated
1-1.5 million children and adults develop symptomatic cutaneous leishmaniasis.
Th e settings in which cutaneous leishmaniasis is found range from rain forest and
other vegetative sites in Mexico, Central and South America to desert-like areas
in West Asia and Africa.
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
C
HAPTER
25
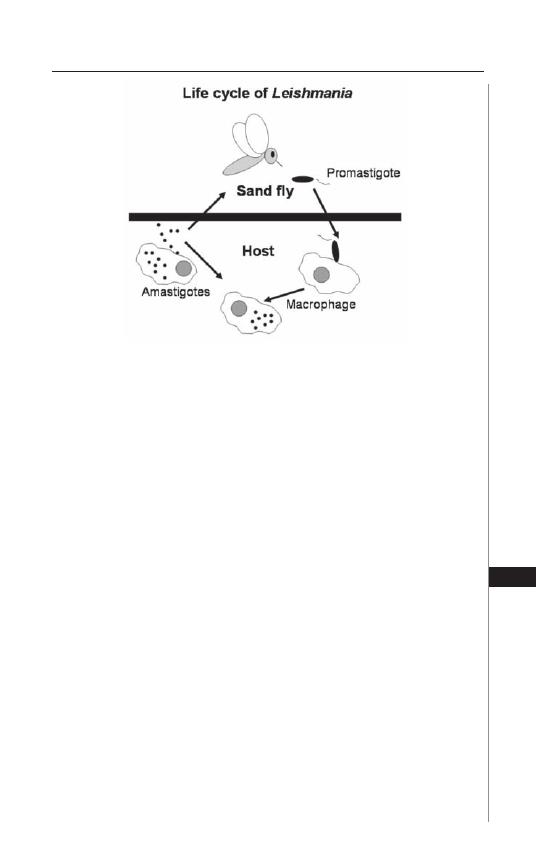
New World cutaneous leishmaniasis in the Americas spreads from Northern
Argentina to Southern Texas, although most aff ected places are in Mexico, Central
America (Belize, Guatemala, Nicaragua, Honduras, El Salvador and Panama) and
South America (Guyana, Brazil, Colombia, Venezuela, Bolivia, Peru, Ecuador and
Argentina. Th e United States of America presents some endemic foci in Texas.
Old World cutaneous leishmaniasis in Africa spreads more abundantly in East and
North Africa, with some cases elsewhere. In Asia and the Middle East the aff ected
countries include Afghanistan, Pakistan, India, Iran, Iraq, Saudi Arabia, Palestine
and Israel. More than 90% of the world’s cases of cutaneous leishmaniasis occur in
Afghanistan, Algeria, Brazil, Iran, Iraq, Peru, Saudi Arabia and Syria.
Immunobiology
Macrophages and other monocytic lineage cells are the primary target cells
for Leishmania, but they are also the principle eff ector cells that kill intracellular
parasites via nitric oxide (NO)-dependent and-independent mechanisms when
activated by the cytokines IFN-
γ
, MIF and TNF-
α
. Activated macrophages also
function as antigen presenting cells (APCs) and secrete cytokines such as IL-12,
IL-18 and TNF-
α
, all of which regulate innate and acquired immunity during CL.
Th e dendritic cells (DCs) also play a critical role in induction of acquired immunity
against CL. Th e disease protective role of DCs has been attributed to their ability
to present parasite antigens to CD4
+
T-cells, as well as produce cytokines such as
IL-12, which is required for NK cell activation and subsequent diff erentiation of
CD4
+
T-cells into a Th 1 subset. Th ese lymphocytes produce IFN-
γ
, which not
only activates macrophages to kill Leishmania but also facilitates Th 1 diff erentia-
tion of CD4
+
T-cells by signaling via the STAT1-mediated pathway. In contrast,
CD4
+
Th 2 cells, which produce IL-4, IL-10 and IL-13, are believed to play a role
in disease exacerbation in CL. Genetic factors also infl uence outcomes of CL. It
is important to note that particular alleles at the TNF-
α
and TNF-
β
genetic loci
183
Cutaneous Leishmaniasis
25
Figure 25.1. Life cycle of Leishmania.
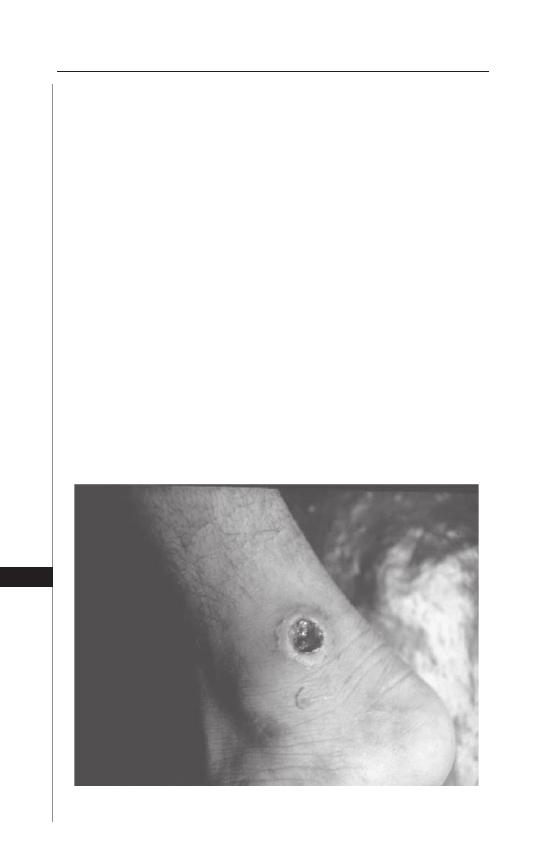
and overproduction of TNF-
α
are associated with a higher risk of CL in studies
with Venezuelan, Brazilian and Mexican patients. However, the overall group of
genes associated with resistance or susceptibility of diff erent forms of cutaneous
leishmaniasis remains to be defi ned.
Signs and Symptoms
Skin-lesions appear within weeks or months aft er the sand fl y bite. Lesions
are normally painless clean ulcers, but can be nodular or plaque-like. Lesions may
self heal, but may take months or even years to resolve without treatment. Th ey
frequently can become secondarily infected. Some lesions may also grow and leave
disfi guring scars. Th ey rarely spread to the mouth or nose unless they are caused
by L. brasiliensis or L. aethiopica.
Localized Cutaneous Leishmaniasis (LCL)
Th is disease is caused by L. tropica, L. major and L. aethiopica in the Old World
and by L. (V) brasiliensis, L. (V) guyanensis, L. (V) panamensis, L. (V) peruviana,
L. (L) mexicana, L. (L) amazonensis and L. (L) garhani in the New World. Th e
incubation period of the disease varies between 1 week to 3 months, depending on
the size of the inoculum and parasite species, but it may be shorter in travelers from
non-endemic countries who are infected in endemic regions. Th e infection caused
by most of these Leishmania species commonly manifests as painless ulcerative
skin lesion(s) at the site of parasite inoculation, which resolve aft er antimonial
treatment in most patients or self-heal (Fig. 25.2). However, L. tropica infection
can produce disfi guring scars on the face in some patients and L. aethiopica and
L. amazonensis can disseminate to other skin sites.
Figure 25.2. A typical ulcerating skin lesion in a patient suffering from local-
ized cutaneous leishmaniasis (LCL). Courtesy Dr. J.R. David.
184
Medical Parasitology
25

Lesions that resolve within a year are considered acute cutaneous leishmaniasis
patients. On the other hand, patients with persistent lesions for more than a year
(1-2 years) are considered chronic CL patients. Th ese patients present a higher
morbidity since they develop chronic, large lesions which may be diffi cult to
diagnose because they contain few or undetectable parasites.
Mucocutaneous Leishmaniasis (MCL)
Th is disease is caused most commonly by L. (V) brasiliensis, but occasionally
by L. (V) panamensis and L. (V) guayanensis. It begins as a single lesion that dis-
seminates and produces new metastases aff ecting the mucosa, mouth, palate and
nose (Fig. 25.3). Th e extensive mucocutaneous damage associated with MCL is
mediated by an exaggerated host immune response against the parasite. MCL
can be highly disfi guring if not promptly treated and is diffi cult to treat in some
patients. Severe MCL can diminish the ability to eat and can be fatal.
Disseminated Cutaneous Leishmaniasis (DCL)
Th is form of the disease is normally produced by L. aethiopica and L. amazonensis,
although some cases of disseminated cutaneous leishmaniasis have been reported
where the causative agent is L. major or L. mexicana and is characterized by an ini-
tial lesion that disseminates throughout the skin (Fig. 25.4). Th is disease is usually
associated with the failure of the host to mount an antigen-specifi c T-cell-response
against the parasite. Lesions in patients with DCL present as nodules and ery-
thematous plaques with variable degrees of verrucous changes, scaling and scarring.
Microscopically, these lesions show abundance of parasites with minimal infl amma-
tion. DCL has been described in patients co-infected by L. braziliensis and HIV.
Leishmaniasis Recidiva Cutis (also known as Lupoid
Leishmaniasis and Leishmaniasis recidivans)
Th is clinical form of the disease is rare and peculiar form provoked by L. tropica
in the Old World and L. (V) brasiliensis in the New World. Th e disease is associ-
ated with the development of new lesions within the scar of a healed acute lesion,
mimicking lupus vulgaris. Lesions appear as scaly erythematous papules that may
evolve before the classic ulcer has healed or develop aft erwards.
Post-Kala-Azar Dermal Leishmaniasis
Th is is a manifestation that happens in 5 and 20% of patients recovering from
visceral leishmaniasis in Africa and India, respectively. It is less prevalent in the New
World. Th e eruption is papular and lasts for months in African patients, whereas
in India the lesions usually start as erythematous and hypopigmented macules
that enlarge into patches. Later these asymptomatic patches become nonulcerative
erythematous nodules that are particularly disfi guring.
Cutaneous Leishmaniasis and Immunodefi ciency Virus (HIV)
Co-Infection
With the increased transmission of human immunodefi ciency virus infection
(HIV) from urban and peri-urban locations to rural areas where leishmaniasis is en-
demic, incidence of CL as a co-infection in patients infected with HIV is increasing.
Most cases (90%) of coinfections have been described in Southern Europe (Spain,
185
Cutaneous Leishmaniasis
25
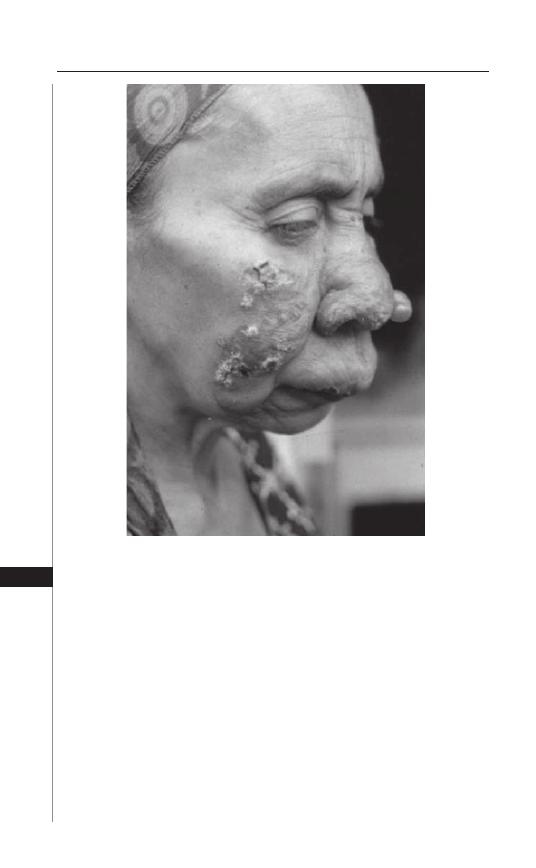
France and Italy) and it is suspected that the mode of transmission of parasites is by
shared contaminated needles among intravenous drug abusers. It has been described
in Brazil in patients co-infected with L. braziliensis and HIV. Clinically, cutaneous
lesions in these patients manifest as papulonodular, ulcerative, diff use, kaposi-like
and psoriasis-like forms, with spread to the mucosa in some patients.
Diagnosis and Treatment
Microscopic examination of Giemsa-stained imprint smears, hematoxylin
eosin-stained punch biopsy specimens, or needle aspirations from suspected le-
sions are the most commonly used techniques for detecting amastigote forms of
the parasites. Additionally, parasites can also be isolated from the ulcer by cultur-
ing a lesion biopsy or aspirate in tissue culture media (e.g., Schneider’s medium,
RPMI-1640, M199 medium) supplemented with 20% fetal bovine serum and
penicillin/streptomycin or by inoculation into laboratory animals such as hamsters.
Th is method not only allows parasitologic confi rmation of the diagnosis, but also
Figure 25.3. Clinical manifestations in a patient suffering from mucocutaneous
leishmaniasis caused by L. brasiliensis. Courtesy Dr. P. Marsden.
186
Medical Parasitology
25
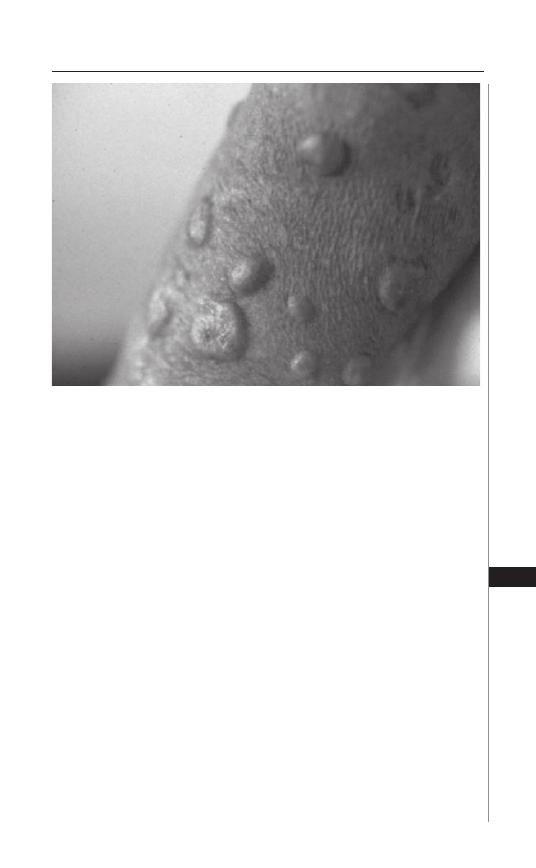
provides suffi cient number of promastigotes for further investigations (e.g., identi-
fi cation of Leishmania species by isoenzyme analysis). Other diagnostic techniques
exist that allow parasite detection and/or species identifi cation using immunologic
(monoclonal antibodies and immunoassays) and molecular (PCR) approaches.
Such techniques, however, are expensive and may not be readily available in general
diagnostic laboratories in some developing countries or in the fi eld.
Pentavalent antimony (Sb
v
) drugs such as sodium stibogluconate (Pentostam™)
or meglumine antimoniate (Glucantime™) have been used for more than 50 years to
treat cutaneous leishmaniasis. Both these drugs are required to be administered daily
via intravenous or intramuscular route for 20 days and sometimes several (20 mg/
kg/d) courses are needed. Th ese drugs, however, are not approved by the Food and
Drug Administration, although they are approved and produced in Great Britain
(Pentostam™) and France, (Glucantime™). Th ey must be administered under an
Investigational New Drug (IND) protocol. Pentostam™ is available under an IND
protocol from the CDC Drug Service to physicians. Paramomycin can also be used
topically for the treatment of CL caused by Leishmania species that have low potential
to spread to mucosa and Imiquimod in combination with Glucantime™ has been used
to treat CL patients in the New World that are refractory to Glucantime™ alone.
Drugs of choice for treating MCL are Pentostam™, Glucantime™ or Amphotericin
B. Both Pentostam™ and Glucantime™ can be administered (20 mg/kg IV or IM) daily
for 28 days whereas as Amphotericin B (0.5-1 mg/kg) is administered IV daily or
every second day for up to 8 wks. New formulations of Amphotericin B encapsulated
into liposomes are being investigated. Oral Miltefosine (2.5 mg/kg/d oral) × 28 d was
Figure 25.4. Disseminated cutaneous leishmaniasis (DCL) caused by L.
amazonensis. This infection clinically manifests as multiple skin lesions.
Courtesy of Dr. J.R. David.
187
Cutaneous Leishmaniasis
25

also found to be eff ective in treatment of L. panamensis, but not L. brazilliensis or L.
mexicana. Physicians may consult the CDC to obtain information on how to treat
leishmaniasis. Walter Reed Army Medical Center (WRAMC) in Washington and
Brooke Army Medical Centre Medical at Fort Sam Houston, Texas off er treatment
to military benefi ciaries, including reservists no longer on active duty. An alterna-
tive treatment for CL is radiowave-induced heat by a small portable battery-driven
instrument. Th e lesions are anesthetized and a small applicator producing heat to
50˚C is applied for 30 seconds across the lesions. One treatment lasting less than 5
minutes is usually suffi cient. Cryotherapy using liquid nitrogen has also been used
but this requires numerous applications. For L. major infections it is suggested that
fl uconazole may decrease the time of healing and, once the parasite species has been
determined as the causative agent, a course of 6 weeks treatment is advised only for
the clinical form associated to this particular species. Also under investigation are
extracts from certain plants that have been used successfully for the treatment of
CL in Mexico.
Prevention and Prophylaxis
Th e best prevention for leishmaniasis is to avoid sand fl ies bites. Some useful
measures are as follows:
a. Stay in air-conditioned rooms from dusk to dawn or at least in a properly
inspected closed tents free of sand fl ies.
b. Wear long sleeved shirts, long pants and socks when going outside. Tuck under-
shirts into pants and pants into socks. Insect repellent should be used liberally
on the face, under the ends of the sleeves and pant legs. Clothing should be
treated with permethrine in aerosol spray and retreated every six washes.
c. People not having access to air-conditioned rooms or screened tents should
use thin mess nets with at least 18 holes per inch and cover and tuck it into the
mattress. Th e bed and the mattress should be treated with permethrin, since
sometimes sand fl ies can fl y through the mesh.
d. Domestic pets such as dogs and rodents should be avoided near the sleeping
area.
Presently, there are no commercial vaccines that protect humans against
any type of leishmaniasis. Several experimental vaccines, however, are currently
under investigation. One of them under study in human populations contains
a mixture of parasite antigens and is known as Leish 111f. Another strategy is
the use of immunotherapy-based vaccines in humans that include BCG (Bacille
Calmette-Guerin) and a mixture of killed parasites. Th is preparation is useful to
improve chemotherapy, thus reducing eff ective doses of antimonial treatments.
Concluding Remarks
Although each of the Leishmania species may have its own manifestations
and areas of endemicity, none of the clinical forms of this disease are unique to
a particular species because of considerable clinical diversity and overlap. Th us,
the clinical picture is dependent on determinants associated with parasite and
host interactions, such as parasites-virulence, genetic susceptibility and immune
responses. New chemotherapeutic contributions, such as development of liposomal
amphotericin B and miltefosine to treat resistant visceral leishmaniasis and the
188
Medical Parasitology
25

development of new vaccines for human application are needed to establish better
control of this disease in endemic areas around the world.
Suggested Reading
1. Murray WH, Berman JD, Davis CR et al. Advances in leishmaniasis. Lancet 2005;
366:1561-77.
2. Mott KE, Nuttalli I, Desjeux P et al. New geographical approaches to control of
some parasitic zoonoses. Bull World Health Organ 1995; 73:247-57.
3. Alexander J, Bryson K. T helper (h)1/Th 2 and Leishmania: paradox rather than
paradigm. Immunol Lett 2005; 99:17-23.
4. Berman JD. Human leishmaniasis: clinical, diagnostic and chemotherapeutic
developments in the last 10 years. Clin Infect Dis 1997; 24:684-703.
5. Malla N, Nahajan RC. Pathophysiology of visceral leishmaniasis—some recent
concepts. Indian J Med Res 2006; 123:267-74.
6. Schwartz E, Hatz C, Blum J. New World cutaneous leishmaniasis in travellers.
Lancet Infect Dis 2006; 6:342-9.
7. Zijlstra EE, Musa AM, Khalil EAG et al. Post-kala-azar dermal leishmaniasis.
Lancet Infect Dis 2003; 3:87-98.
8. Singh S. New developments in diagnosis of leishmaniasis. Indian J Med Res 2006;
123:311-30.
9. Roscoe M. Leishmaniasis: early diagnosis is key. JAAPA 2005; 18:47-50,53-4.
10. Vega-Lopez F. Diagnosis of cutaneous leishmaniasis. Curr Opin Infect Dis 2003;
16:97-101.
11. Blum J, Desjeux P, Schwartz E et al. Treatment of cutaneous leishmaniasis among
travellers. J Antimicrob Chemother 2004; 53:158-66.
12. Croft SL, Coombs GH. Leishmaniasis—current chemotherapy and recent
advances in the search for novel drugs. Trends Parasitol 2003; 19:502-8.
13. Magill AJ. Cutaneous leishmaniasis in the returning traveler. Infect Dis Clin North
Am 2005; 19:241-66.
14. Davies CR , Kaye P, Croft SL et al. Leishmaniasis: new approaches to disease
control. BMJ 2003; 326:377-82.
15. Bogdan C, Guessner A, Solbach W et al. Invasion, control and persistence of
Leishmania parasites. Curr Opin Immunol 1996; 8:517-25.
16. Convit J. Leishmaniasis: Immunological and clinical aspects and vaccines in
Venezuela. Clin Dermatol 1996; 14:479-87.
17. Khamesipour A, Rafati S, Davoudi N et al. Leishmaniasis vaccine candidates for
development: a global overview. Indian J Med Res 2006; 123:423-38.
18. Reithinger R, Mohsen M, Wahid M et al. Effi cacy of thermotherapy to treat
cutaneous leishmaniasis caused by Leishmania tropica in Kabul, Afghanistan: a
randomized, controlled trial. Clin Infect Dis 2005; 40:1148-55.
19. Lobo IMF. Soares MBP, Correia TM et al. Heat therapy for cutaneous
leishmaniasis elicits a systemic cytokine response similar to that of antimonial
(Glucantime) therapy.Trans Roy Soc Trop Med Hyg 2006; 100:642-9.
20. Drugs for parasitic infections. Th e Medical Letter 2004; 46:e5.
189
Cutaneous Leishmaniasis
25

C
HAPTER
26
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Toxoplasmosis
Sandhya Vasan and Moriya Tsuji
Defi nition
Toxoplasmosis is an infection caused by the protozoan obligate intracellular
parasite Toxoplasma gondii.
Overview and Incidence
Th e incidence of toxoplasmosis varies greatly by country and by age, but may
aff ect up to one-third of the global human population. Th e majority of immu-
nocompetent adults, pregnant women and children infected with Toxoplasma
gondii experience no or mild symptoms during acute infection. Infants of women
who seroconvert during pregnancy may develop congenital toxoplasmosis. Th is
incidence ranges from one to ten per 10,000 live births. Immunocompromised
individuals are at risk for reactivation of latent infection, including potentially
fatal encephalitis.
Causes and Risk Factors
Th e house cat and other members of the family Felidae serve as defi nitive hosts
in which the sexual stages of the parasite develop. Th e life cycle of Toxoplasma gondii
begins when a cat ingests toxoplasma-infected tissue from an intermediate host,
usually a rodent. Tissue cysts within the muscle fi bers or brain are digested in the
cat’s digestive tract. Th e parasite then undergoes sexual development, multiplies in
the intestine of the cat and is eventually shed in cat feces, mainly into litter boxes
and garden soil. A human may become infected in one of the following ways:
1. By accidentally ingesting oocysts passed in cat feces through contaminated
soil or handling of cat litter.
2. By ingesting tissue cysts within raw or undercooked meat (lamb, pork and
beef ), drinking unpasteurized milk, contaminated water, or unwashed fruits
or vegetables.
3. By direct transmission of tachyzoites from mother to fetus through the pla-
centa (congenital infection) or, rarely, by blood transfusion or solid organ
transplantation from a positive donor to a previously uninfected host.
Parasite Life Cycle (Fig. 26.1)
Oocysts: Infective stages transmitted via cat feces aft er sexual development
in cat.
Tachyzoites: Infective stages that infect macrophages and are carried through-
out the human body via macrophages, causing pathology.
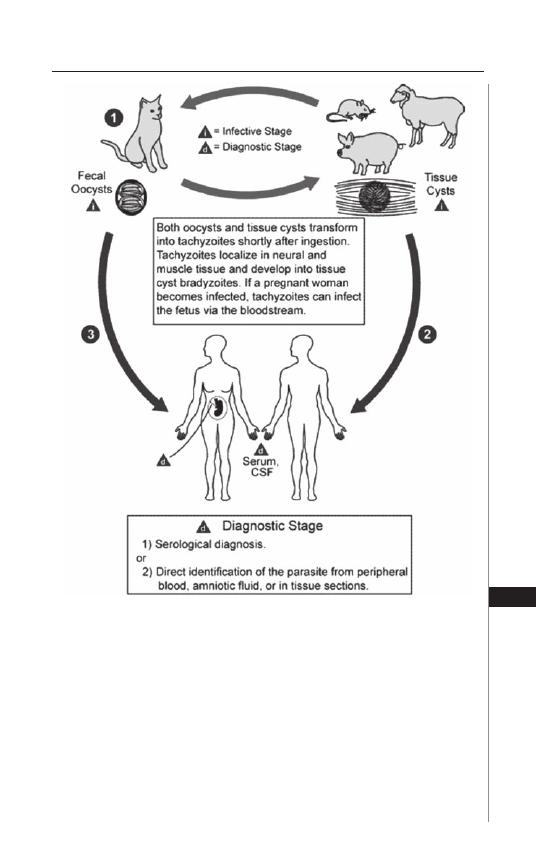
191
Toxoplasmosis
26
Tissue cysts (pseudocysts): Large cyst-like forms that become quiescent in
response to host adaptive immune responses.
Bradyzoites: Slowly developing forms within tissue cyst.
Pathology
T. gondii invades numerous organs, infecting a broad spectrum of cell types.
Tachyzoites infect macrophages and are disseminated through the blood to many
organs, where they invade, asexually multiply and cause cellular disruption, lead-
ing to cell death. Th e resulting necrosis attracts infl ammatory host cells, such as
lymphocytes and monocytes. It is this infl ammatory response that causes the major
pathology in infected individuals.
Figure 26.1. Toxoplasmosis Life Cycle. Image reproduced with permission of the
Centers for Disease Control and Prevention (CDC) (http://www.cdc.gov).

192
Medical Parasitology
26
As host resistance develops, usually around 3 weeks post infection, tissue cysts
may form in many organs, primarily in brain and muscle. Th ese quiescent cysts
enable Toxoplasma gondii to evade the adaptive host immune. As tissue cysts pe-
riodically rupture, the released bradyzoites are killed by the host immune system.
If immune surveillance becomes compromised, such as due to chemotherapy or
AIDS, these bradyzoites develop into tachyzoites, causing active toxoplasmosis.
Clinical Manifestations
Over 80-90% of primary infections produce no symptoms. Th e incubation
period for symptoms is 1 to 2 weeks. Mild symptoms of primary infection include
localized, painless cervical or occipital lymphadenopathy, usually persisting 4-6
weeks, or nonspecifi c symptoms including myalgia, headache, rash or sore throat
that persist for one month or longer. Recently, newer more virulent strains causing
severe symptoms in immunocompetent individuals have been reported.
Congenital toxoplasmosis is caused by infection with Toxoplasma gondii in a
pregnant woman. Infants born to women who were infected before conception
do not develop disease due to protection by maternal antibodies. In contrast, new
infections with detectable maternal parasitemia are associated with up to a 50%
transmission rate to the fetus. Th e likelihood of transmission and severity of disease
in the fetus are inversely proportional. Mothers who develop acute toxoplasmosis
in the fi rst trimester have a much lower fetal transmission rate than in the third
trimester, but fetuses exposed early are at much higher risk for severe symptoms
or death and spontaneous abortion.
Up to 85% of newborns with congenital toxoplasmosis show no initial symp-
toms. Infants may show signs of central nervous system disorders such as hydro-
cephalus, microcephaly, or mental retardation. Hepatomegaly, splenomegaly, rash,
fever, jaundice, anemia may also be present.
Th e most common pathology is chorioretinitis, which may result in strabismus
or blindness. Th e age of onset ranges from 1-2 months to several years. A character-
istic residual pigmented scar is left on the retina aft er resolution of infection.
Severe toxoplasmosis occurs most oft en in immunocompromised adults that
develop either acute infection or reactivation from quiescent tissue cysts. In these
cases, the disease may aff ect the brain, lung, heart, eyes, or liver. Brain lesions are
associated with fever, headache, confusion, seizures and abnormal neurological fi nd-
ings. Systemic manifestations include myocarditis, pneumonitis and chorioretinitis,
although immunocompetent individuals may also experience ocular damage from
toxoplasmosis. Th e hallmark fi nding of chorioretinitis is white focal retinal lesions
accompanied by vitreous infl ammaion.
Diagnosis
Diagnosis relies on either indirect serological tests or direct detection of the
organism. Serologic tests, indicating recent or past infection, are most eff ective in
immunocompetent adults who are able to mount a humoral response to the parasite.
Th ese include ELISA, IFA, complement fi xation and in the past, the Sabin-Feldman
dye test to detect IgG antibodies. IgG antibodies develop 1-2 weeks post infection
and then persist and therefore will not distinguish between recent and past infection.
Rising titers on serial examination may be indicative of active or ongoing infection.
Th e presence of a high IgM titer in the absence of a signifi cant IgG titers indicates

193
Toxoplasmosis
26
early stages of primary infection. A negative IgM titer is helpful for ruling out recent
infection. However, due to considerable variability in tests and a high false positive
rate, a positive IgM test should be verifi ed in a reference laboratory (e.g., the US
Centers for Disease Control or Toxoplasmosis Serology Lab, Palo Alto Medical
Foundation).
Direct diagnosis can be made by PCR on several bodily fl uids and tissues,
including blood, bronchioalveolar lavage, vitreous fl uid, amniotic fl uid aft er 18
weeks gestation and brain biopsies. It is particularly useful in cases of advanced
immunosuppression, where antibody titers may be less reliable. Diagnosis is oc-
casionally made from identifi cation of trophozoites in infected tissue. Histologic
examination of lymph nodes may reveal a characteristic histiocytic hyperplasia.
Additional indirect diagnostic tests include cranial MRI or CT scan for cysts in
brain tissue and ocular slit lamp examination for characteristic retinal lesions.
In infants, it is important to distinguish between maternal antibodies found in a
non-infected infant versus a high titer of antibodies being produced by an infected
infant. In this regard, the simultaneous measurement of maternal and infant IgG
antibodies specifi c for the parasites is critical. An infant: maternal IgG ratio of four
or higher is indicative of new infection. Th is test should be repeated in the infant at
4 months of age. In addition, the presence of high titers of specifi c IgM antibodies
in the infant’s serum is diagnostic. Current procedures allow diagnosis of an ac-
tive infection in the fetus in utero by means of PCR of amniotic fl uid obtained by
amniocentesis. Th e majority of infants will appear normal on prenatal ultrasound
although fi ndings may include intracranial calcifi cations, ventricular dilatation,
hepatic enlargement, increased placental thickness and ascites.
Treatment
Th e immunocompetent, nonpregnant individual over 5 years of age with acute
toxoplasmosis is treated with specifi c medications only if signs and symptoms are
severe or persistent, or in the case of active chorioretinitis. Immunocompromised
patients must be treated, oft en for 4 to 6 weeks aft er cessation of symptoms.
Treatment of new infections in pregnant women is controversial because of the
toxicity of the medications, but treatment is still advocated. Congenitally-infected
newborns are treated aggressively.
Medications to treat the infection include: pyrimethamine (25-100 mg/d × 3-4
wks) plus either trisulfapyrimidines or sulfadiazine (1-1.5 gm qid 3-4 wks). Pregnant
women are usually treated with spiramycin. Th e length of treatment varies widely
between countries and may be followed by a course of pyrimethadine/sulfadiazine.
Additional medications include sulfonamide drugs, folinic acid, clindamycin and
trimethoprim-sulfamethoxazole. In immunocompetent adults and newborns with
chorioretinitis, corticosteroids may help suppress the acute vitreous infl ammatory
response. Treatment in AIDS patients is continued as long as the individual is
considered immunocompromised, usually until the CD4
+
T-cell count is above
200 cells/
μ
L, to prevent reactivation of the disease.
Prognosis
Toxoplasmosis in immunocompetent adults carries a good prognosis without long
term sequellae. In contrast, infection in immunocompromised adults can be fatal if
not treated early, oft en due to neurological complications. Congenital toxoplasmosis

194
Medical Parasitology
26
can result in permanent disability in infants due to ocular and neurological involve-
ment, including blindness, learning disorders and mental retardation.
Prevention
General
•
Protect children’s play areas from cat and dog feces. Cover sandboxes when
not in use to avoid cat defecation.
•
Wash hands thoroughly aft er contact with soil that may be contaminated with
animal feces.
•
Control fl ies and cockroaches as much as possible. Th ey can spread contami-
nated soil or cat feces onto food.
•
Avoid rubbing eyes or face when preparing food, especially raw meat or poultry.
Aft er food preparation, wash hands thoroughly with soap and water and clean
the counter.
•
Avoid ingesting raw or undercooked meat or poultry, raw eggs and unpasteur-
ized milk. Fruits and vegetables should be peeled or thoroughly washed.
During Pregnancy
•
Pregnant women should have their serologic testing for toxoplasma antibod-
ies that may be repeated several times during the pregnancy depending upon
initial results.
•
Avoid exposure to cat feces by having other family members change the cat
litter box. If the litter box must be changed, wear rubber gloves to avoid contact
with the litter. Wash hands thoroughly with soap and water aft erwards.
•
Use work gloves when gardening and wash hands aft erwards.
HIV-Infected Individuals
Patients with HIV disease should have toxoplasma antibody titers checked. If the
results are positive and if the CD4
+
T-cell count is less than 200 cells/
μ
L, patients
should be given prophylactic antibiotics, such as trimethoprim-sulfamethoxazole,
in conjunction with antiretroviral therapy until the CD4
+
T-cell count has risen.
Selected Internet Resources
http://www.cdc.gov/ncidod/dpd/parasites/toxoplasmosis/default.htm—
CDC Division of Parasitic Diseases.
www.cfsph.iastate.edu/Factsheets/pdfs/toxoplasmosis.pdf—Center for Food
Security and Public Health, Institute for International Cooperation in Animal
Biologics, Iowa State university, 2005.
Suggested Reading
1. Lopez A, Dietz VJ, Wilson M et al. Preventing congenital toxoplasmosis. MMWR
2000; 49:57-75.
2. Olliaro P. Congenital toxoplasmosis. Clin Evid 2004; 12:1058-61.
3. Kravetz JD, Federman DG. Toxoplasmosis in pregnancy. Am J Med 2005;
118:212-6.
4. Montoya JG, Liesenfi eld O. Toxoplasmosis. Lancet 2004; 363:1965-1976.
5. Bossi P, Bricaire F. Severe acute disseminated toxoplasmosis. Lancet 2004;
364:579.

C
HAPTER
27
Giardiasis
Photini Sinnis
Introduction
Giardia intestinalis, also called Giardia lamblia and Giardia duodenalis, is one
of the most common intestinal parasites in the world, occurring in both industrial-
ized and developing countries with an estimated 2.8 million new cases annually.
First observed by Anton Van Leuwenhoek in 1681 in a sample of his own diarrheal
stool, and later described in greater detail by Vilem Lamble, Giardia was initially
thought to be a commensal and has only been recognized as a pathogen since the
mid 1900s. In this chapter, salient features of the parasite and the disease it causes
are described.
Life Cycle and Structure
Th is one-celled fl agellated protozoan has a simple life cycle consisting of two
stages: trophozoite and cyst (Fig. 27.1). Cysts are the transmission stage and are
excreted in the feces of infected individuals into the environment where they can
survive for weeks. When ingested, exposure to the low pH of the stomach and
pancreatic enzymes induces excystation, with two trophozoites developing from
each cyst. Trophozoites attach to epithelial cells of the upper intestine, primarily
the jejeunum but also the duodenum, where they grow and divide. Attachment
is required to prevent being swept away by peristalsis and is mediated by the
ventral disk of the trophozoite as well as adhesins on the parasite surface. As the
intestinal epithelial cell surface is renewed, trophozoites move and reattach to
other epithelial cells. In some cases, the detached trophozoite is carried down the
intestinal tract where exposure to bile salts, which occurs when the trophozoite
is no longer protected by the mucous layer of the epithelium, and cholesterol
starvation induce encystation.
Th e structure of trophozoite and cyst are shown in Figure 27.2. Trophozoites
have two nuclei and each nucleus contains a prominent karyosome, giving the
parasite its characteristic face-like appearance. In addition it has four pairs of fl a-
gella, an axostyle (a microtubule-containing organelle to which the fl agella attach),
a ventral disk and two median bodies, organelles whose function is not known.
Cysts, which are slightly smaller than trophozoites, have a carbohydrate-rich cell
wall which likely protects them from the environment and two to four nuclei.
Giardia is an aerotolerant anaerobe that metabolizes glucose and scavenges cho-
lesterol, phospholipids, purines, pyrimidines and amino acids. Giardia does not
contain classical mitochondria but does make ATP in double-membraned organ-
elles called mitosomes, which may represent degenerate mitochondria. Th e lack of
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
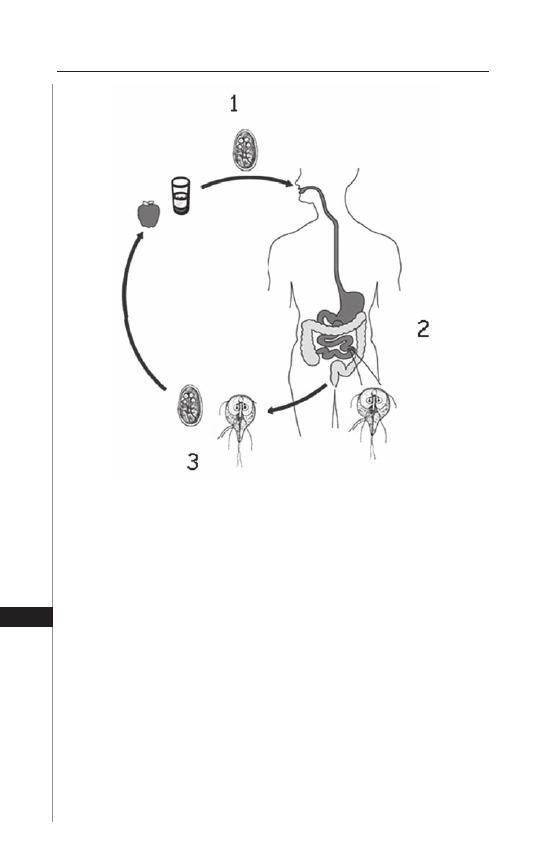
196
Medical Parasitology
27
mitochondria originally led people to classify these parasites as early eukaryotes;
however, the recent sequencing of the Giardia genome has identifi ed genes that
can be traced to the common prokaryotic ancestor of the mitochondria raising
the possibility that mitosomes have evolved from mitochondria as an adaptation
to the microaerophilic environment of the gut and that Giardia is an ancestor of
an aerobic mitochondria-containing fl agellate.
Epidemiology
Giardia transmission occurs by the fecal-oral route, either directly, via person
to person contact or indirectly, via contamination of surface water or food Th e
salient features of Giardia cysts that infl uence disease transmission include their
stability in the environment, their immediate infectivity upon leaving the host and
the small number of cysts required to cause infection.
Figure 27.1. Lifecycle of Giardia intestinalis. 1) Ingestion of cysts either di-
rectly or via fecally-contaminated water or food. 2) Excystation of trophozoites
in small intestine where they grow and multiply. 3) Encystation as the tropho-
zoite leaves the small intestine and excretion of cysts into the environment.
Some trophozoites may be found in feces but are not adapted for survival in
the environment. Adapted from: Centers for Disease Control and Prevention
(CDC) (http://www.dpd.cdc.gov/dpdx/images/ParasiteImages).
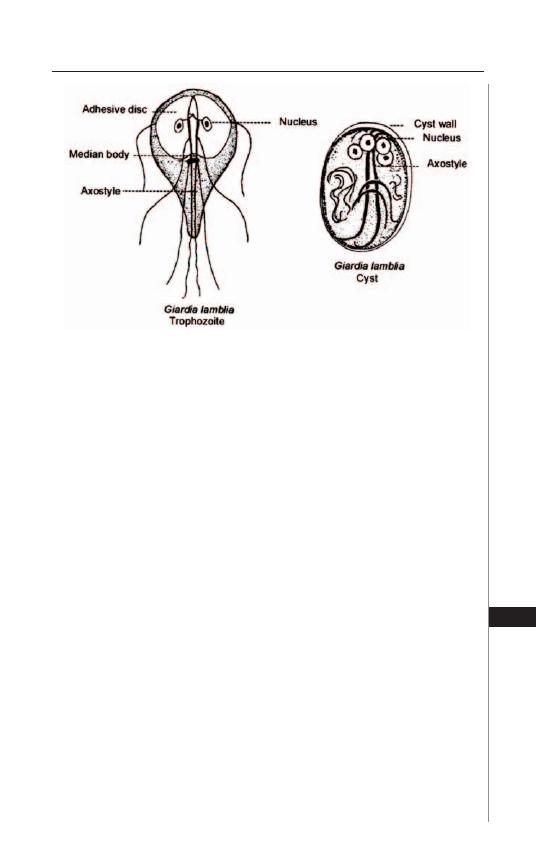
197
Giardiasis
27
Th e stability of cysts in the environment means that fecal contamination of
both food and surface water can lead to disease transmission. Common source
outbreaks of giardiasis are primarily the result of contaminated water, although
foodborne outbreaks have been reported. In the United States, Giardia is the
most common cause of waterborne diarrheal disease. Risk factors for Giardia
infection via waterborne transmission include drinking tap water, swallowing
water while swimming, contact with recreational fresh water and eating lettuce,
the last factor likely refl ecting the use of contaminated water or fertilizer in farm-
ing. Giardia cysts can also be transmitted directly from person to person and this
commonly occurs in daycare centers, custodial institutions and among men who
have sex with men. In developing countries inadequate means of disposing of
human waste leads to very high rates of infection, likely via both contamination
of drinking water and direct person to person contact.
Th ere are fi ve species of Giardia, only one of which, G. intestinalis, infects
humans; the others infect a variety of domestic and wild animals. G. intestinalis,
can be further subdivided into six genetically distinct groups or Assemblages,
only two of which, Assemblages A and B, are associated with human infection.
Further molecular typing may fi nd that these genotypes actually represent distinct
species. Th e two human-infecting genotypes have a broad host-range that includes
aquatic mammals such as seals, domestic animals and beavers. Although it has
been suggested that these animals serve as reservoirs for human infection, recent
studies using molecular tools that allow for precise genotyping of isolates have sug-
gested that, on the contrary, other animals are indicator species for environmental
contamination with human waste. More studies that incorporate genotyping of
Giardia isolates are required before we fully understand the zoonotic potential of
the Giardia parasites that infect humans.
Figure 27.2. Structure of trophozoite (10 - 20 microns by 5 - 15 microns)
is shown on the left and the slightly smaller cyst (7 - 10 microns by 8 - 12
microns) is on the right. Reproduced from: Nappi AJ, Vass E, eds. Parasites
of Medical Importance. Austin: Landes Bioscience, 2002:16.

198
Medical Parasitology
27
Clinical Presentation
In the early 1920s, Rendtorff gave human volunteers Giardia cysts in gela-
tin capsules and found that although infection can occur aft er ingestion of as
few as 10 cysts, it more reliably occurs upon ingestion of 100 or more cysts.
More recent studies demonstrate that ingestion of cysts results in one of three
outcomes: no infection, asymptomatic infection with excretion of cysts, or
symptomatic infection. Analysis of infection rates during outbreaks indicates
that 35 to 70% of exposed people remain uninfected, without symptoms or any
trace of infection, possibly in part due to a low innoculum, 5 to 15% of people
remain completely asymptomatic but pass cysts in their feces and 15 to 60% of
people become symptomatic. Th e majority of symptomatic people have an acute
self-limited diarrhea, lasting 7 to 15 days while a small proportion of symptomatic
patients develop chronic diarrhea accompanied by signs of malabsorption and
signifi cant weight loss.
Symptoms usually begin 7 to 14 days aft er cyst ingestion but can begin as late
as 4 weeks later. Th e onset is usually acute with diarrhea accompanied by diff use
abdominal cramping and discomfort, bloating, fl atulence and fatigue. Nausea can
be present but vomiting is rare. Fever, when present, is low-grade and seen early
in the course of infection. Initially stools are usually profuse and watery but later
in the course of the disease can become greasy and malodorous. Stools usually do
not contain blood or white blood cells, the peripheral white blood cell count is
not elevated and there is no increase in the absolute eosinophil count. Weight loss
is common and it is not unusual for patients to present with a greater than 10 lb
weight loss. Very rarely, extraintestinal symptoms such as urticaria and polyarthritis
are seen and there are a few case reports in which Giardia infection extended into
the biliary tract. Two distinguishing features of Giardia infection are its duration,
usually in the range of 2 to 4 weeks, and the associated weight loss. Although the
majority of symptomatic Giardia infections are self-limiting, symptoms can none-
theless be quite severe and occasionally lead to hospitalization, usually secondary to
volume depletion. In an outbreak in New Hampshire, it was estimated that about
13% of symptomatic individuals required hospitalization.
A small percentage of symptomatic individuals will have chronic infection,
lasting months or longer. Th ese patients can have chronic diarrhea or a waxing
and waning course characterized by intermittent symptoms of diff use abdominal
discomfort, malaise and diarrhea. Chronic giardiasis is frequently accompanied
by weight loss, which can be signifi cant, and malabsorption. Malabsorption
of fats, vitamins A and B12, disacharrides, especially lactose, and protein are
observed, with malabsorption of fats and lactose being most common. Lactose
malabsorption is likely due to damage to the brush border which aff ects the
disaccharidase-containing microvilli. All of these absorption defi cits resolve with
treatment of the infection. However, it can take several weeks for full restoration
of the disaccharidase activity of the brush border so that lactose intolerance can
persist even aft er the infection is eradicated.
More severe and prolonged symptoms are observed in patients with immu-
nodefi ciencies, especially those aff ecting the humoral arm of the immune system.
Adults with common variable immunodefi ciency (T and B cell defi cits resulting
in impaired production of most immunoglobulins), selective IgA defi ciency and

199
Giardiasis
27
X-linked agammaglobulinemia have an increased incidence of symptomatic disease
with a prolonged course. HIV patients do not have more severe disease, with the
exception of a subset of AIDS patients with low CD4 counts who have severe
symptoms and are diffi cult to cure. Exactly how this group of AIDs patients diff er
from most is not clear.
One group of patients worth particular mention is children living in poor
communities where baseline nutritional status is borderline and infection with
intestinal helminths and protozoans is common. Many studies in poor communi-
ties in Central and South America, the Middle East and other places have found
that Giardia infection in children is strongly associated with growth stunting. In
children harboring several intestinal parasites, Giardia infection is most robustly
associated with decreased growth. In one study, it was found that more than one
episode per year of giardiasis was associated with a decrease in cognitive function.
In contrast to these fi ndings, growth of children in a daycare setting in Israel, was
not aff ected by Giardia infection, despite the fact that prevalence of infection was
high (20 to 40%). It is likely that underlying conditions, such as protein-energy
malnutrition, aff ect the ability of the child to mount an eff ective immune response,
resulting in more severe, chronic infection and leading to malabsorption of essential
vitamins and fats. Th is situation is compounded by the fact that malabsorption in a
child with borderline nutritional status will have more far-ranging and deleterious
eff ects than it would in otherwise healthy children.
It is not yet known why there is such a large range of outcomes aft er ingestion
of Giardia cysts. Infectious dose does not seem to play a role in outcome other than
the fact that very low infectious doses may not result in infection at all. Likely both
host and parasite factors are involved. Th e immune status of the host is clearly im-
portant with nonimmune and immunocompromised hosts more likely to become
ill or severely ill. In addition, some studies suggest that diff erent strains of Giardia
diff er in their capacity to cause disease. Th ese types of studies, however, are still
in their infancy and there is not yet consensus as to which strains or Assemblages
of Giardia are more virulent. Undoubtedly as studies are performed with more
markers, the role of strain variation in virulence will become clear.
Immunity
Th ere is immunity to Giardia infection but it is not complete and reinfection is
common. Th e existence of immunity is supported by several clinical observations:
First, in developing countries where the prevalence of Giardia infection is high,
the incidence of giardiasis is higher in younger age groups, suggesting immunity
develops with exposure. Second, infection is typically more severe and of longer
duration in people with immunodefi ciencies, especially those with defi ciencies in
the humoral immune response.
In mouse models, using either G. lamblia or the rodent parasite G. muris, it has
been shown that mice clear the infection and become resistant to reinfection, indicat-
ing that there is an immune response to the parasite in mice. Immunodefi cient mice
such as nude mice or thymectomized mice cannot clear the parasite and have chronic
persistant infections. More detailed studies have found that depletion of B-cells
leads to chronic giardiasis, indicating a role for antibodies in parasite clearance. IgA
knockout mice cannot clear the infection although they control the infection better

200
Medical Parasitology
27
than B-cell knockout mice, suggesting that IgA antibodies are critical for parasite
clearance but that other isotypes play a role in controlling infection or can partly
compensate for the defi ciency in IgA. Other experiments using rodent models found
that the cytokine IL-6 is important in the acute phase of infection. IL-6 knockout
mice had normal IgA production and could control G. lamblia infection but did so
more slowly. Overall, the work from rodent models suggests that there is an early
B-cell independent response to the parasite followed by an antibody-dependent
phase, with secretory IgA being one of the most important components of the im-
mune response. In humans its been suggested that failure to mount an IgA response
is correlated with chronic giardiasis.
Work with rodent models also suggests that in addition to being important
in clearing the infection, the host immune response may also lead to some of the
pathology observed during infection. For example, one study found that adop-
tive transfer of either CD8
+
T-cells or whole unfractionated lymphocytes from
Giardia-infected mice to naïve recipients resulted in diff use shortening of microvilli
and loss of brush border surface area in the recipients. In another study, Giardia
infection in immunocompetent mice resulted in diff use loss of the brush border
microvillus surface whereas T-cell defi cient mice infected with the same load of
parasites had no change in microvillus surface area or structure.
Protection against both infection and disease is associated with breast feeding
and it has been shown that both immune and nonimmune mechanisms are involved
in this protection. Specifi c antibodies are transferred in milk from mothers previ-
ously infected with Giardia and are associated with a decreased risk of acquiring
the infection and of developing severe giardiasis. Human breast milk also protects
against Giardia infection because it contains a specifi c lipase that releases free fatty
acids from milk triglycerides upon stimulation by bile salts (bile salt stimulated
lipase), that kill Giardia trophozoites.
Pathogenesis
Th e mechanisms by which Giardia causes diarrhea and malabsorption have not
been elucidated. Th ere is no evidence that Giardia produces an enterotoxin or that
it invades the intestinal epithelial cells. Electron microscopy shows that the ventral
disk embeds the parasite into the epithelial microvillus layer and “footprints” of
formerly adherent trophozoites are visible on the epithelial cell surface. However,
even in a heavy infection, the surface area covered and possibly damaged by the
adherent trophozoites cannot account for the symptoms. In humans, biopsy of
the infected gut shows little abnormality. In a European study in which over 500
biopsy specimens from Giardia-infected patients were observed, slightly over
96% had normal looking mucosa and 3.7% had mild villous shortening with a
small amount of neutrophil and lymphocyte infi ltration. Th e lack of histologic
abnormalities in the majority of symptomatic patients has also been observed in
other, smaller studies. In one study in which patients with villous shortening and
infl ammatory infi ltration were followed with serial biopsies, these abnormalities
all resolved aft er the infection was eradicated. In the murine models of giardiasis,
similar fi ndings of villous atrophy and infl ammatory infi ltration of villous epithe-
lium can be observed. However as with humans, the fi ndings are subtle and the
infl ammatory changes mild.
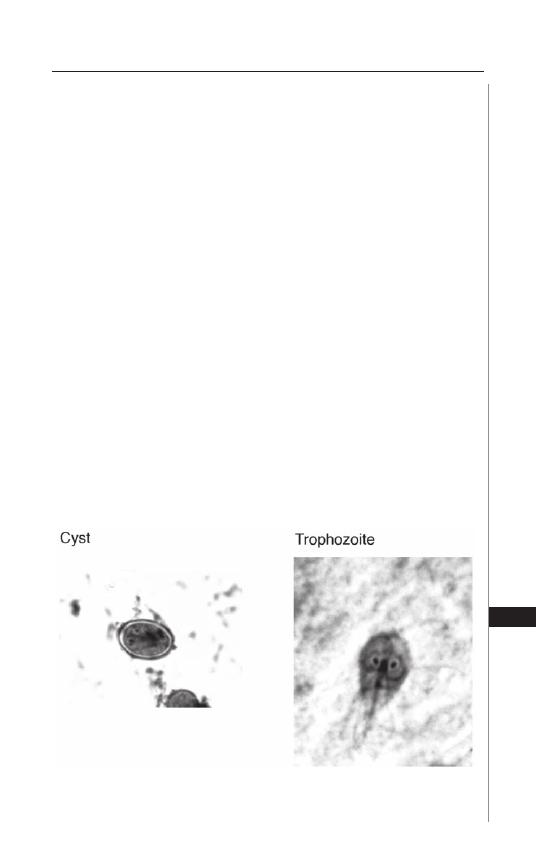
201
Giardiasis
27
In conclusion, the cause of diarrhea and malabsorption in Giardia infection is
likely to be multifactorial, involving the host immune response to the pathogen
as well as, yet to be identifi ed, cytopathic substances that the parasite may secrete.
Additionally, it has been suggested that Giardia may cause pathology by alteration
of the bile content or endogenous fl ora of the small intestine which in turn could
aff ect the absorptive function of gut. Th ese hypotheses must now be formally tested
before a more complete picture emerges.
Diagnosis
Th e traditional method of diagnosis is examination of stool for trophozoites or
cysts (stool O&P). Both fresh and fi xed stool specimens are usually examined. Cysts
are normally found but motile trophozoites can be observed in a fresh specimen
of loose stool (Fig. 27.3). Because the parasites are normally found in the small
intestine and are shed intermittently, the sensitivity of one stool specimen is low,
in the range of 50 - 70%. However, examination of three specimens, from three
diff erent days, increases the sensitivity to 85 - 90%; specifi city is close to 100%.
Th is assay remains the most widely used method to diagnose Giardia infection
and is the gold standard to which other newer assays are usually compared. It is
important to note that there can be a delay between the onset of symptoms and the
excretion of cysts so that a negative stool sample in someone in whom giardiasis
is suspected warrants reanalysis at a later time.
Recently, new assays have been developed based on detection of Giardia anti-
gens. Th e direct fl uorescent antibody test (DFA), uses a Giardia-specifi c antibody
conjugated to a fl uorophore to stain stool specimens. Because the parasites are
labeled, much larger regions of the slide can be scanned more quickly and the likeli-
hood of detecting the parasite is increased. On a single stool specimen the sensitivity
is between 96 - 100%. Other antigen-detection tests detect soluble Giardia-specifi c
proteins in the stool. Th ere are two diff erent types of soluble-antigen-detection
Figure 27.3. Light microscopy of G. intestinalis trophozoites and cyst in stool
sample. Reproduced with permission from Apple Trees Productions, LLC, New
York and generously supplied by Dr. Dickson Despommier.

202
Medical Parasitology
27
tests, the enzyme immunoassays (EIA) and immunochromatographic dipsticks
(ICT) which work on the same overall principal, i.e., immobilization of antibod-
ies specifi c for Giardia proteins on a plate (EIA) or a piece of chromatography
paper (ICT) that then capture Giardia proteins in the stool. ICTs are relatively
new and have a lower sensitivity than EIAs (80% versus 94 to 98%). Importantly,
these can be positive even aft er a person stops shedding intact organisms and can
give false-positive results in someone who has been treated and cured of Giardia
infection. Specifi city for all of the antigen-based detection assays is 90 to 100%.
Although these tests are faster and do not require a highly trained technician, in
contrast to the stool O&P which does, stool for O&P can be more economical
when screening for more than one intestinal parasite. However antigen tests can
be very useful during outbreaks or in institutional settings when screening large
numbers of people.
Both stool O&P and the antigen detection tests give false-negative results
when there are very few organisms in the stool. In these cases, the physician may
decide to directly sample the contents of the duodenum using either the string test
(Entero-Test, HDC Corporation, San Jose, CA) or by endoscopy accompanied
by duodenal fl uid sampling and biopsy. Th e mucus adherent to the string and the
duodenal aspirate fl uid are examined for organisms, primarily trophozoites, directly
and aft er staining. In cases where biopsy is performed, it is processed, stained and
then examined by a pathologist for trophozoites. Th ese more invasive tests are not
more sensitive for detecting parasites but can be used when the more widely-used
diagnostic methods fail.
Assays which detect Giardia-specifi c nucleic acid, using polymerase chain
reaction (PCR), are being developed but are currently experimental and not in
clinical use.
Treatment
Treatment of giardiasis is summarized in Table 27.1. Th e nitroimidazole, met-
ronidazole is the most commonly used drug to treat Giardia infection although it
has never been FDA approved for this use. In adults, 250 mg tid for 5-7 days has
been shown to be eff ective in 80 to 95% of cases. Shorter courses of higher doses
lead to better compliance but are less eff ective and have a worse side eff ect profi le.
Metronidazole is mutagenic to bacteria and carcinogenic in laboratory rodents; for
this reason most physicians prefer not to use it in children although no mutagenic
eff ects have been observed in humans.
Tinidazole is another nitroimidazole that until recently was not available in the
United States but was commonly used in Europe and in parts of the world where
the prevalence of Giardia infection is very high. It has a longer half-life than met-
ronidazole and is 80 to 100% eff ective as a single 2 gm dose. It is likely that given
its recent availability in the United States and FDA approval for use in giardiasis,
combined with its effi cacy as a single dose, tinidazole may replace metronidazole
as the most frequently prescribed drug for the treatment of giardiasis.
Nitazoxanide, discovered in the 1980s by Jean Francois Rossignol at the Pasteur
Institute, is a new drug in the anti-Giardia arsenal and was recently approved by the
FDA to treat giardiasis in children aged 1-11 and in adults. It is a thiazolide that
interferes with pyruvate-ferredoxin oxidoreductase-dependent electron transport

203
Giardiasis
27
which is required for anaerobic respiration. It is not mutagenic and so is more
appropriate for use in children. Effi cacy is over 85% and it is well-tolerated with a
good side eff ect profi le. Nitazoxanide's effi cacy extends well beyond the treatment
of giardiasis, and it has been found to be eff ective in treating Cryptosporidium,
Entamoeba, Cyclospora, Isospora as well as many intestinal helminthes.
Albendazole, a benzimidazole commonly used to treat intestinal helminth in-
fections, is also eff ective against Giardia. Its cure rates are similar to metronidazole
when 400 mg/d is given for 5 to 7 days. Interestingly, the other benzimidazole,
mebendazole, is not as eff ective in the treatment of Giardia infection and so is
generally not used for this purpose.
Pregnant women with Giardia infection can be diffi cult to manage because
none of the aforementioned drugs has been shown to be safe for the fetus. Th e ni-
troimidazoles (metronidazole and tinidazole) rapidly enter the fetal circulation aft er
absorption from the mother's GI tract. Although large retrospective studies have not
demonstrated any adverse eff ects on the fetus in the 2nd and 3rd trimester, in one
large study of pregnant women, a small increase in fetal malformations was associated
with fi rst trimester exposure to metronidazole. Albendazole, and the other benzimi-
dazole, mebendazole, are teratogenic in animals at high doses and are also generally
not used in pregnant women. Th e use of these drugs in mass treatment campaigns to
eradicate fi lariasis or treat intestinal helminths has led to their inadvertent use in early
pregnancy, and no increased risk of fetal malformations has been observed. However,
given that the doses used were much lower than that needed to eradicate Giardia and
that the numbers of pregnant women in these studies was small, it is not advisable
Table 27.1. Treatment of Giardia infection in adults and children
Dosing
Drug
Adult Dose
in Children*
Comments
Metronidazole 250 mg tid x 5 mg/kg tid x
5 -10 days
5 -10 days
Tinidazole
2 gm single
50 mg/kg
Only recently available
dose
single dose
in the United States
Nitazoxanide
500 mg bid x 1-3 year-old: 100
A recent addition to the
3 days
mg bid x 3 days
anti-Giardia arsenal
4-11 yrs: 200 mg
bid x 3 days
Albendazole
400 mg qd x
15 mg/kg/d x
5d
5-7 d
Paromomycin
500 mg tid x
10 mg/kg tid x
Used primarily in
5-10 days
5-10 days
pregnant women
Quinacrine
100 mg tid x 2 mg/kg tid x
Used primarily in
5 - 7 days
5 - 10 days
refractory cases in
combination
with
a
nitroimidazole
*Tinidazole, Nitazoxanide and Albendazole available in liquid form.

204
Medical Parasitology
27
to use these drugs to treat giardiasis in pregnant women. Lastly, there are no safety
data available regarding the use of the recently discovered nitazoxanide in pregnant
women. So how to treat the pregnant woman with giardiasis? If her symptoms are
not severe and pose no threat to herself or the fetus, she can be given supportive
treatment to maintain her fl uid and nutritional status during the fi rst trimester and
if possible, the entire pregnancy. If treatment becomes necessary, paromomycin, an
aminoglycoside that is not absorbed in signifi cant quantities from the GI tract is
usually tried fi rst. It is not as eff ective as metronidazole, with effi cacies ranging from
55 to 88% and is usually administered for 10 days. If necessary, metronidazole can
be given during the 2nd and 3rd trimesters using the dosing schedule outlined in
Table 27.1 rather than a high-dose short-course regimen.
Although most people respond to treatment with one of the aforementioned
drugs, there is a small subset of patients who do not. In a series of six patients with
refractory infections, the majority of which were immunodefi cient, the combina-
tion of a nitroimidazole, either metronidazole or tinidazole, and quinacrine (100
mg tid), administered for 2 to 3 weeks, resolved the infection. Quinacrine is an old
antimalarial drug that is no longer used to treat malaria, is no longer produced in the
United States, and has a high incidence of side eff ects. However, in combination with
a nitroimidazole, it appears to be eff ective in eradicating Giardia in refractory infec-
tions. It can be ordered from an independent compounding pharmacy, Panorama
Pharmacy, Panorama City, CA (http://www.panoramapharmacy.com/).
Since a large proportion of infected people are asymptomatic, the question
arises as to whether they should be treated. It is generally accepted that if they
pose a risk to others and are unlikely to rapidly become re-infected, they should be
treated. Th is would mean that asymptomatic children in daycare centers who are
excreting cysts should be treated, as should asymptomatic carriers in institutional
settings. In developing countries the likelihood that an infected, asymptomatic
child will become re-infected aft er treatment is high and for this reason, treatment
is not always recommended. However, in studies demonstrating that Giardia in-
fection in preschool children with borderline nutritional status is associated with
reduced growth, it was found that eradicating the infection allows for catch-up
growth. For this reason, Giardia infection should be treated in children in areas
where there is a high prevalence of giardiasis and malnutrition.
Prevention
Providing safe drinking water is critical if Giardia transmission is to be con-
trolled. Th e traditional methods employed to insure water safety are protection
of the watershed, fl occulation and sedimentation (using compounds such as alum
to bridge contaminating organisms into clumps that can then be removed by
sedimentation), chemical disinfection usually with chlorine, and fi ltration. As the
population increases and expands into areas once considered remote and pristine,
our watersheds are becoming more diffi cult to protect from human and animal
waste. A 1991 study found that 81% of the raw surface water entering 66 diff erent
water purifi cation plants was contaminated with Giardia cysts. Short of assuring a
pristine watershed, fi ltration is the most important component of the standard water
purifi cation process in the prevention of waterborne outbreaks of Giardia. Numbers
of cysts can be decreased by fl occulation and sedimentation but not to acceptable

205
Giardiasis
27
levels. Although Giardia cysts are sensitive to chlorine, their inactivation requires
high levels of chlorine and long exposures that are not routinely implemented in
water treatment plants. Proper fi ltration of surface water, or ground water under the
direct infl uence of surface water, is required and can decrease cyst number by 2 to 3
orders of magnitude. However, fi ltration to remove all cysts on a large scale is not
feasible so that drinking water continues to pose some risk. In New York City, the
Department of Environmental Protection began, in 1992, to monitor the level of
Giardia and Cryptosporidium contamination in the city's source water. Th ese data
can be viewed at: http://www.ci.nyc.ny.us/html/dep/html/pathogen.html.
Backpackers and hikers drinking untreated water and travelers to areas where
Giardia is prevalent and safe drinking water is not available can perform one of the
following to insure the safety of their water: Boil for 1 minute or at high altitude
for 3 minutes; fi lter through a 2 micron pore size fi lter; treat with iodine (12.5
ml/liter for 30 minutes) or halazone (5 tablets/liter for 30 minutes).
Conclusion
Giardia is an important cause of diarrhea worldwide. Most symptomatic
patients recover with treatment although disease is more prolonged and can be
diffi cult to treat in certain groups of immunocompromised patients. Th e great-
est impact of giardiasis is likely on children of marginal nutritional status where
the malabsorption associated with infection can cause growth stunting. As our
watersheds become increasingly contaminated with human waste, it is likely that
the incidence of the disease will increase. Th e recent availability of tinidazole in
the United States combined with the discovery of nitazoxanide should make the
treatment of giardiasis simpler and more straightforward.
Suggested Reading
1. Appelbee AJ, Th ompson ARC, Olson ME. Giardia and Cryptosporidium in mam-
malian wildlife - current status and future needs. Trends Parasitol 2005; 21:370-5.
2. Gardner TB, Hill DR. Treatment of Giardiasis. Clin Microbiol Rev 2001;
14:114-28.
3. Nash TE, Ohl CA, Th omas E et al. Treatment of patients with refractory Giardiasis.
Clin Infect Dis 2001; 33:22-8.
4. Fox LM, Saravolatz LD. Nitazoxanide: A new Th iazolide antiparasitic agent. Clin
Infect Dis 2005; 40:1173-80.
5. Farthing MJG, Mata L, Urrutia JJ, Kronmal RA. Natural history of Giardia infection
of infants and children in rural Guatemala and its impact on physical growth. Am J
Clin Nutr 1986; 43:395-405.
6. Berkman DS, Lescano AG, Gilman RG et al. Eff ects of stunting, diarrhoeal disease
and parasitic infection during infancy on cognition in late childhood: A follow-up
study. Lancet 2002; 359:564-71.
7. Lopez CE, Dykes AC, Juranek DD et al. Waterborne giardiasis: A communitywide
outbreak of disease and a high rate of asymptomatic infection. Am J Epidemiol 1980;
112:495-507.
8. Adam RD. Biology of Giardia lamblia. Clin Microbiol Rev 2001; 14:447-475.
9. Petri WA. Treatment of Giardiasis. Curr Treat Options Gastroenterol 2005;
8:13-7.
10. Hill DR. Giardia lamblia. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and
Practics of Infectious Diseases, 6th Edition. Philadelphia: Churchill Livingstone,
2005:3198-203.

C
HAPTER
28
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Amebiasis
Daniel J. Eichinger
Introduction
Th e causative agent of intestinal amebiasis is the single-celled protozoan parasite
Entamoeba histolytica. Th is parasite is endemic in most tropical and subtropical
areas of the world, where it causes millions of cases of dysentery and liver abscess
each year. Infected persons display a wide range of disease severity, refl ecting the
contributions of the patient’s immune and nutritional status, the infective dose
and the pathogenic potential of the infecting organism. It is only one of several
Entamoeba organisms that can reside in the human GI tract, which in the past
has made diagnosis diffi cult. It is uniquely adapted to the conditions found in
the lumen of the large intestine and as a result is sensitive to drugs that target
anaerobic organisms.
Life Cycle and Structure
Th ere are only two stages to the life cycle of E. histolytica, the infectious cyst
stage and the multiplying, disease-causing trophozoite stage. In the majority of
cases infection results from the ingestion of fecally-contaminated water or food
that contains E. histolytica cysts. Much less oft en the cyst or the trophozoite forms
can be transmitted as a result of oral or oral/anal sexual practices. Cysts are 8-10
μ
m in diameter and when they are released from infected persons they are stable
for days to weeks in low-temperature aqueous conditions. Re-ingested cysts are
resistant to the low pH of the stomach and appear to be triggered to exit the cyst
capsule (excyst) by components of bile and bicarbonate that are encountered in
the small intestine. Th ese metacystic amoebae are carried to the colon where the
10-30
μ
m diameter trophozoite form adheres to the mucus layer overlying the
colonic epithelium. Th is adherence is mediated by lectins found on the surface of
the trophozoite that have specifi city for ligands expressing appropriately spaced
terminal N-acetylgalactosamine and galactose sugar residues, as are found on the
colonic form of mucin. Th e trophozoite stage of the parasite feeds on host ingesta,
components of mucus and the resident bacteria and multiplies by asexual binary
fi ssion. In response to conditions and stimuli that are not yet completely defi ned,
trophozoites stop multiplying and revert to the cyst form (encyst). Th ese cysts are
released in the tens of millions per gram of feces to allow for completion of the life
cycle upon infection of another person. Most cysts are released from asymptomatic
carriers of the parasite, suggesting that conditions within the intestine that are
conducive to cyst formation are not signifi cantly abnormal. Recent reports have
also revealed an ability of products (short-chain fatty acids) normally produced

207
Amebiasis
28
by the enteric bacteria to regulate the encystment process. Mixtures of cysts and
trophozoites are more commonly released by symptomatic (dysenteric) persons, but
the trophozoite form is generally not considered infectious due to its sensitivity to
hypoosmotic conditions outside the body and the low pH of the stomach.
Th e trophozoite form contains a single nucleus and many internal vesicles
(Fig. 28.1). Th e nucleus has a thin continuous rim of heterochromatin and a cen-
trally located nucleolus (karyosome). In fresh wet mounts the trophozoite displays
ameboid motion with rapid extension of ectoplasmic pseudopods. Th e cyst form
contains a refractile chitin-containing cyst wall, four nuclei and, in many cases,
crystalline, bullet-shaped aggregations of ribosomes called chromatoid bodies.
Th ese features of the trophozoite nucleus and the cyst distinguish E. histolytica
from other species of nonpathogenic amoebae that can also reside in the human
colon, such as E. coli, E. nana or Iodamoeba butschlii.
Epidemiology
Most parasite transmission follows the oral-fecal route in which cysts are in-
gested. More rarely cysts are transmitted directly between persons. Th e worldwide
distribution of E. histolytica therefore primarily refl ects the incomplete separation
of human feces from water and food sources typical of densely populated areas of
developing countries although infrequent outbreaks in developed countries have
occurred. Th e previous worldwide estimates of up to 500 million Entamoeba-
infected persons now need to be qualifi ed with the recent understanding that
approximately 90% of those infections are with the morphologically indistinguish-
able but genetically distinct organism E. dispar, which does not cause disease. An
estimated 40-100,000 persons die each year from infection with E. histolytica,
however, and many more are either asymptomatically infected or present with
varying degrees of dysentery and extraintestinal disease. Experimental establish-
ment of infection with E. coli, a nonpathogenic parasite of humans, was shown
Figure 28.1. Schematic depictions of the morphology of the E. histolytica
trophozoite and cyst, as compared to other amoebae found in the human
intestine. Reproduced from Nappi AJ, Vass E, eds. Parasites of Medical
Importance. Austin: Landes Bioscience, 2002:20.

208
Medical Parasitology
28
to be possible with as few as 1-10 cysts, with a prepatent period (time following
ingestion to the detection of cysts in stool) ranging from 6 to 23 days, an average
of 10 days.
In closely examined areas where E. histolytica is endemic, most infected adults
are asymptomatic. Depending on the age of the person, the time of asymptomatic
infection can be considerable, with a half-life of parasite persistence of about 13
months in adults in one area of Vietnam where the 10% of adults are infected. Th e
time of parasite persistence in children appears to be shorter, which may refl ect the
higher incidence of diarrhea episodes from multiple unrelated causes in that age
group that serves to physically remove the parasite. Th e long (13 month) half life
of asymptomatic parasite infection in adults suggests that up to 5% of individu-
als can retain the parasite for up to 5 years. Asymptomatic persons can therefore
develop intestinal or extraintestinal disease (liver abscess) months to years aft er
visiting areas where parasite transmission is known or suspected to occur. Most
cases of diagnosed E. histolytica infection in the US occur in immigrants, par-
ticularly those from Mexico, central and South America and Asia. Seropositivity,
refl ecting the tissue-invasive capacity of E. histolytica, is approximately 8% in the
entire population of Mexico.
In contrast to disease restricted to the lumen of the intestine, which occurs
nearly equally in males and females, for unknown reasons extraintestinal liver
abscess shows a 3-20 fold higher incidence in males vs females and in individuals
that abuse alcohol.
Clinical Presentation
Presentation of amebiasis can take several forms, depending on the severity of
the disease within the intestine and the involvement of extraintestinal sites.
Intestinal Disease
Th e greatest numbers of infected individuals have parasites restricted to the
lumen of the intestine and are asymptomatic. 90-96% of these would normally
clear the parasite spontaneously, but the potential for disease development in
the remaining 4-10% requires that even asymptomatic individuals be treated if
E. histolytica is defi nitively identifi ed. Th ose persons presenting with colitis typi-
cally have a history of several weeks of gradually increasing abdominal cramping,
tenderness, weight loss and a range of bowel function alterations, ranging from
frequent mucoid stools to watery and bloody diarrhea, oft en with periods of
dysentery alternating with constipation. As these symptoms are also typical of
those elicited by a variety of bacterial pathogens, diff erential diagnosis should
include other infectious as well as noninfectious causes of colitis. Stools are nearly
always heme-positive due to the invasive nature of the parasite, and rectal release
of blood without diarrhea is not uncommon in children. Fever is present in less
than half of intestinal amebiasis patients. However, fulminant necrotizing colitis,
developing in less than 1% of patients, has a high mortality rate (40%) and pres-
ents with fever, bloody mucoid diarrhea, leukocytosis and peritoneal tenderness.
Intestinal perforation occurs in the majority of patients with fulminant colitis,
and both it and toxic megacolon, associated with corticosteroid use, require
surgical intervention. Another infrequent intestinal manifestation, ameboma,

209
Amebiasis
28
mimics colonic carcinoma in its radiologic presentation as an annular deposition
of granulomatous tissue that locally narrows the lumen of the colon.
Liver Abscess
Th e most common site of extraintestinal infection (up to 9% of amebiasis cases)
is the liver, resulting from hematogenous spread (via portal circulation primarily
to the right lobe) of trophozoites that have eroded through the colonic mucosa.
Within the liver trophozoites multiply while degrading liver tissue in a spherically
expanding abscess that becomes fi lled with necrotic liver cells. Hepatic amebiasis
can present months to years aft er an individual has traveled to or resided in an
endemic area, during which time the patient may be completely asymptomatic and
negative for stool parasites. Only 20% of patients with liver abscess have a prior
history of clinical dysentery. Not all liver abscesses will progress to a symptomatic
stage and some can self-resolve subclinically. Once they do become apparent, how-
ever, liver abscess symptoms develop relatively rapidly, over a course of 10 days to
several weeks and can include right upper quadrant pain, fever, point tenderness
of the liver, anorexia and weight loss. Abscesses located just below the diaphragm
can lead to pleural pain or referred right shoulder pain. Liver alkaline phosphatase
levels are normal and alanine aminotransferase levels are elevated in acute liver
abscess, which may, however, reverse over time. Males are ten times more likely to
present with liver abscess than females and middle-age and young adults more than
children. Careful history elicitation is important because of the possible length of
time ensuing between presentation with liver abscess and past residence or visitation
in an endemic geographic region. Serum antibodies to amoeba antigens are nearly
always found in such patients, but if residence in an endemic area was prolonged,
such antibodies may be the result of prior infection.
Pathogenesis
Th e species name, histolytica, refers to the impressive ability of this organism
to degrade a variety of host tissues. Th e parasite expresses a large number of factors,
including lytic peptides (amebapores), cysteine proteinases and phospholipases
that are presumably designed to aid in the ingestion and digestion of bacteria and
other food materials. Th ese products are considered virulence factors as they can
also lyse colonic epithelial, liver and immune cells that come into contact with the
trophozoite via its galNAc-specifi c lectin. Entamoeba trophozoites are actively
phagocytic and the presence of ingested red blood cells is diagnostic of E. histolytica
organisms that are found in suitably stained biopsy specimens (Fig. 28.2). As a result
of the variability of these activities, the range of pathological fi ndings in the colitic
colon includes increased height of the mucosa, isolated ulcerations of the mucosal
layer, invasion and erosion of the submucosa (Fig. 28.3), areas of necrosis with loss
of large patches of mucosa and complete perforation of the colon. Invasion of the
mucosal layer is oft en stopped by underlying muscularis layers, forcing the parasite
to erode tissues laterally in a manner that generates ulcers with a fl ask-shaped cross
sectional appearance.
Liver abscesses are well circumscribed with an outer wall of connective tissue
surrounded by normal liver cells, an underlying layer of trophozoites and dying
hepatocytes and a central area fi lled with dead hepatocytes and immune cells. A
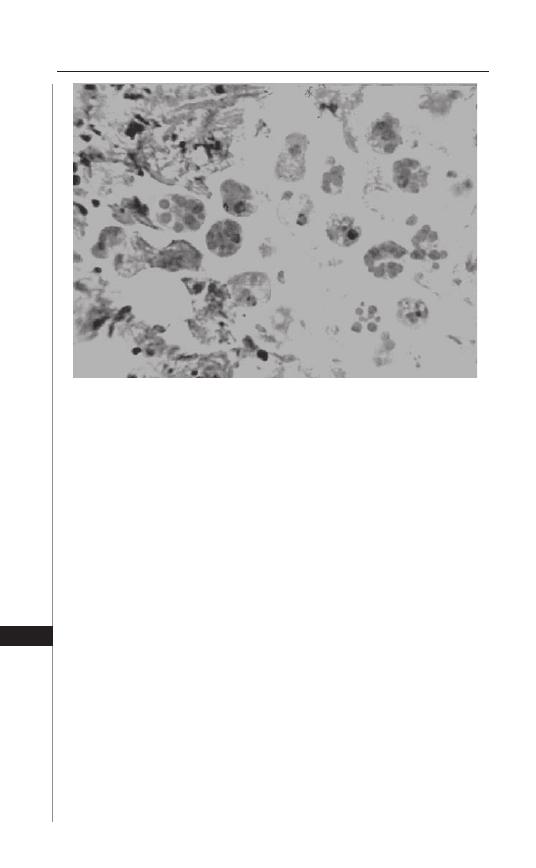
210
Medical Parasitology
28
complication of liver abscess that results from suffi cient expansion of the abscess
to make contact the liver capsule is the rupture of the abscess into surrounding
anatomic spaces, which occurs in up to 20% of abscess patients. Rupture of liver
abscess through the diaphragm can yield pleuropulmonary amebiasis that presents
with cough, chest pain and respiratory distress. Signifi cant leakage of liver abscess
material into the lung can yield cough producing brown sputum. Erosions of liver
abscesses into the peritoneal and pericardial spaces are less frequent but can be of
greater clinical signifi cance even though the liver abscess contents are sterile. Much
less common but with highest mortality is the dissemination of tropohozoites from
liver abscesses via general circulation to the brain.
Diagnosis
Blood cell parameters of amebiasis patients are not grossly abnormal, but
elevated total white cell counts are found in >75%. Unlike infection with invasive
helminth parasites, there is typically no eosinophilia with amebiasis. Chemistry
changes are usually limited to increased alkaline phosphatase levels in the majority
of liver abscess patients.
Microscopic identifi cation of trophozoites or cysts in stool samples or biopsy
specimens is the most defi nitive method of diagnosis. However, cyst passage is
known to be inconsistent in asymptomatic carriers and to require the examina-
tion of multiple samples. Visualization of the organism is therefore being sup-
planted by ELISA assays that detect stool antigen rather than whole cells and,
importantly, are capable of distinguishing between pathogenic E. histolytica and
nonpathogenic E. dispar. With high sensitivity (80%), specifi city (99%) and
Figure 28.2. Biopsy specimen containing E. histolytica trophozoites with
ingested red blood cells. Original image courtesy of Dr. William Petri,
University of Virginia.

211
Amebiasis
28
commercial availability, these tests will make diagnosis of intestinal amebiasis
more reliable. Invasive disease presenting as colitis or liver abscess can addition-
ally be diagnosed serologically, as antibodies are present in >90% of such patients
and detectable by ELISA and various agglutination and electrophoresis methods.
Antibody titers increase with length of infection, so patients presenting with
acute suspected disease may be negative initially but positive within 2 weeks.
Asymptomatic patients can also develop positive antibody titers, allowing for
determination of the infecting organism when stool samples are positive for
cysts and consideration of treatment.
Amebic liver abscesses are readily detected radiographically with ultrasound,
CT scan or MRI methods, which, when combined with serology, can distinguish
the amebic abscess from other space-occupying lesions such as hepatoma, pyogenic
or hydatid abscesses. (Fig. 28.4). Nearly all amebic liver abscesses completely resolve,
but a small number will leave residual radiographic lesions that do not require
further treatment. Amebomas can be visualized by barium contrast radiography,
taking into account the risk of perforation if colitis is also present.
Treatment
Since most asymptomatic persons diagnosed with amebiasis are infected with
E. dispar and do not require treatment, it is important to establish that E. his-
tolytica is present to justify treatment. In contrast, asymptomatic infections with
E. histolytica do require treatment because of the possibility of eventual disease
development. Paromomycin (Humatin) and diloxanide furoanate (Furamide) are
the two drugs recommended for treatment of these patients. Recent analysis of
Figure 28.3. Isolated amoeba-induced lesions, as well as areas of sloughed
mucosa resulting from fusion of adjacent lesions, are evident in this patho-
logical colon specimen.
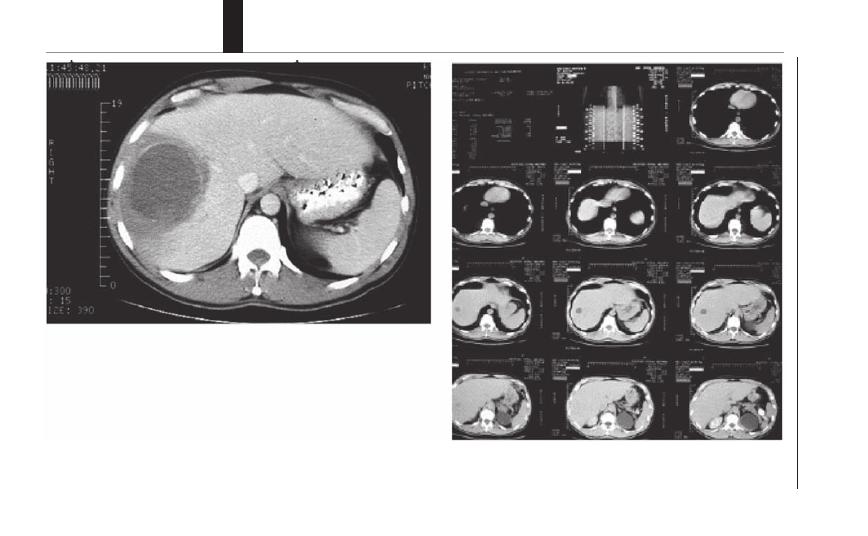
212
Medical Parasitology
28
Figure 28.4. CT scan images of a patient with a right lobe amoebic liver abscess, upon admission (left), and six weeks following per-
cutaneous drainage and a course of metronidazole (right).

213
Amebiasis
28
the relative eff ectiveness of these two drugs against E. histolytica as compared to
E. dispar showed that paromomycin (25-35 mg/kg/d in three doses for 7 days) is
85% eff ective against E. histolytica and an equal course of diloxanide furoate is 51%
eff ective. Successful treatment is documented with follow-up stool (microscopic or
ELISA) examination 2-4 weeks later. Treatment failure rates can be as high as 15%.
An alternative drug is iodoquinol (Yodoxin), which, however, requires a longer
treatment course (20 days) and has iodine-related toxicities.
As compared with luminal asymptomatic infection, the drug of choice for sus-
pected or defi ned invasive (colitis) and extraintestinal (hepatic abscess) disease is
metronidazole (Flagyl). Paroral metronidazole is completely absorbed and recent
studies have shown that the previously recommended 750 mg tid 7-10 day course
for adults can be reduced to one daily 2.4 g dose for 3 days with equal effi cacy.
Because it is so readily absorbed, treatment with metronidazole must be combined
(either concurrently or sequentially) with a lumenally-retained agent to eliminate
potential parasites within the intestine whether or not they are detected by stool
examination. Metronidazole appears not to have adverse aff ects on fetal develop-
ment during the fi rst trimester. Percutaneous drainage of liver abscess is warranted
only if medical therapy appears not to be working, abscess rupture appears to be
imminent, or left lobe abscesses threaten to rupture into the pericardium.
Suggested Reading
1. Petri WA, Singh U. Diagnosis and management of amebiasis. Clin Infect Dis 1999;
29:1117-25.
2. Stanley SL. Amoebiasis. Lancet 2003; 361:1025-34.
3. Haque R, Huston CD, Hughes M et al. Amebiasis. New Engl J Med 2003;
348:1565-73.
4. Blessman J, Tannich E. Treatment of asymptomatic intestinal Entamoeba histo-
lytica infection. New Engl J Med 2002; 347:1384.
5. Blessman J, Ali IKM, Nu PAT et al. Longitudinal study of intestinal Entamoeba
histolytica infections in asymptomatic adult carriers. J Clin Microbiol 2003;
41:4745-50.

C
HAPTER
29
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Cryptosporidiosis
Gerasimos J. Zaharatos
Introduction
Although the fi rst human cases of Cryptosporidium were described in 1976,
the contribution of this protozoan parasite to gastrointestinal disease was not
fully appreciated until the 1980s when scores of cases were described among
patients with acquired immunodefi ciency syndrome (AIDS). Th e disease gained
greater notoriety aft er a massive outbreak of waterborne cryptosporidial infection
in Milwaukee, Wisconsin in 1993. Watery diarrhea and malabsorption are the
usual sequelae of symptomatic infection. In addition to Cryptosporidium causing
chronic diarrhea and extraintestinal disease in immunocompromised individuals,
the parasite is an important source of self-limited diarrhea in immunocompetent
individuals and a major contributor to persistent diarrhea in children through-
out the developing world. Routine fecal specimen testing, for enteric pathogens
including ova and parasites, will fail to detect this organism and thus self-limited
cases may remain undiagnosed. Limited therapeutic options for persistent and
chronic disease present an additional challenge to clinicians and treatment of any
underlying immunodefi ciency is paramount.
Th e Parasite
Th e genus Cryptosporidium consists of at least 10 species. Th is group of or-
ganisms resides within the subphylum Apicomplexa, along with other protozoan
parasites such as Plasmodium species. It is most closely related to coccidian para-
sites including other intestinal pathogens such as Cyclospora and Isospora species.
Cryptosporidium species have been detected in the gastrointestinal tract of a
number of mammalian and vertebrate species. Its presence in ruminants has been
most widely described. C. parvum, a species commonly found in bovine hosts, was
formerly the species most oft en associated with human disease. However, genotypic
and phenotypic diff erences among isolates eventually led to the recognition of two
separate species, C. hominis (formerly “human” genotype or C. parvum genotype 1)
and C. parvum (“bovine” genotype or C. parvum genotype 2). Humans are most
commonly infected by C. hominis or C. parvum and on occasion by species nor-
mally present in other animal hosts. It appears that C. hominis only infects humans.
Mixed infections have been rarely described in immunocompromised patients. As
the genetic diversity of various host-adapted species is better appreciated, it is likely
that the present nomenclature will evolve and the relative importance of zoonotic
transmission will undergo further re-evaluation.

215
Cryptosporidiosis
29
Lifecycle, Pathogenesis and the Host Response
Th e lifecycle of Cryptosporidium can be completed entirely within a single
host (Fig. 29.1A). Subsequent to oocyst ingestion and activation in the upper GI
tract, the organisms excyst to release sporozoites. Th ese sporozoites bind intestinal
epithelial cells and via induction of actin polymerization, provoke their own en-
gulfment to eventually reside in a parasitophorous vacuole within the microvillus
layer. In this sequestered environment, the parasites undergo asexual reproduction
(termed merogony) and ultimately produce merozoites that are released intralumi-
nally. Th ese forms in turn bind and are again engulfed by epithelial cells and thus
perpetuate the cycle. Alternatively the engulfed merozoites may undergo sexual
diff erentiation and ultimately the fertilization of macrogamonts by microgametes
will yield new oocysts. Th ese new oocysts may either be shed into the environment
or excyst within the same host.
Th e organism can be found throughout the gastrointestinal tract; however it ap-
pears to have an affi nity for epithelial cells in the jejunum, ileum and proximal colon.
Cholangiocytes are also susceptible to infection, and apoptosis of these epithelial
cells likely contributes to biliary tract disease. Th e respiratory tract also appears to
be a site of infection in immunocompromised individuals. Epithelial cell death,
by both apoptotic and necrotic mechanisms, has been noted in involved regions.
Th ere is evidence that infected epithelial cells can induce apoptosis in neighboring
uninfected cells. Epithelial cell infection usually culminates in dysregulation of cell
signaling pathways including upregulation of proinfl ammatory cascades as well as
cyclooxygenase-2, prostaglandins and neuropeptide production. Th ese perturba-
tions result in epithelial barrier dysfunction, augmented intestinal permeability,
dysregulation of electrolyte absorption and secretion and fl uid malabsorption.
Accordingly, symptomatic infection usually manifests as watery diarrhea.
Epidemiology
Cryptosporidium has a wide geographic distribution, though infection is
more prevalent in regions of the world with poor sanitary conditions. Infection
is more common during warm rainy months. Th e reported prevalence of infec-
tion varies widely and is infl uenced by geographic region, age, immune status,
local outbreaks and the range of sensitivities and specifi cities off ered by diff erent
diagnostic modalities. In general, exposure rates based on seroprevalence stud-
ies suggest that in North America at least 30% of adults have been previously
exposed to Cryptosporidium species. However, seroprevalence rates are as high as
90% in the developing world. In moist environments, Cryptosporidium oocysts
may remain infectious for 6 months. Th e infectious dose can be as low as 10
oocysts, though considerable variability exists among isolates and a much higher
infectious dose is oft en required in previously exposed seropositive individuals.
Oocysts have been detected in apparently pure surface water sources, though
protected spring water sources are less likely to be contaminated. Untreated or
raw waste water is substantially contaminated with oocysts. Moreover munici-
pal wastewater treatment centers, runoff from animal agriculture and various
wildlife populations all contribute to a remarkable release of oocysts into the
aquatic environment. Oocysts are highly resistant to chlorination and can by-
pass certain fi ltration methods. Accordingly, sources of treated potable water

216
Medical Parasitology
29
can contain signifi cant numbers of oocysts. Oocysts can survive for a period
of time in seawater, and indeed shellfi sh in coastal areas have been found to be
contaminated with infectious oocysts. Most cases among immunocompetent
hosts have been associated with waterborne outbreaks and have involved either
contaminated drinking water or recreational water sources such as swimming
pools and lakes. Disease is also well-described in returning travelers, persons
with animal contact (e.g., farmers) and amongst daycare personnel working with
young children. Direct person-to-person transmission via the fecal-oral route
is also common in a number of settings including during sexual activity. Health
care workers should be cognizant of the potential for nosocomial transmission.
Foodborne transmission, though relatively infrequent, has been reported from a
number of sources including inadequately pasteurized beverages and raw fruits
and vegetables.
Clinical Manifestations and the Host Response
Nonbloody diarrhea is the most common clinical presentation of cryptosporidi-
osis; however clinical fi ndings may vary widely and are dependent on the aff ected
host population being considered. Th e severity and duration of diarrhea may be
quite variable. Th e incubation period is usually 7 to 10 days, though it can range
from several days to weeks.
Th e Developed World: Immunocompetent Hosts
Among immunocompetent adults, the most common presentation is watery,
occasionally mucoid, diarrhea. Th e severity and frequency of diarrhea can be
variable ranging from small volume intermittent stools to continuous and volu-
minous unformed or watery stools. Diarrhea is usually self-limited and persists
for up to 14 days, though it can persist in normal hosts for a more prolonged
period. Diarrhea may be accompanied by abdominal cramping, fever, malaise,
nausea and occasionally vomiting. Concurrent respiratory symptoms have also
been reported. Despite an initial resolution of symptoms, a considerable pro-
portion of infected individuals eventually have recurrent disease within days
to weeks. Some individuals with C. hominis infection report development of
extraintestinal symptoms late in their course including recurrent headaches,
fatigue, dizziness, ocular pain and arthralgias. Accumulating evidence suggests
that mild or asymptomatic infection may also be common. Previously exposed
seropositive individuals appear to be more resistant to reinfection and when
reinfected have milder forms of disease.
Figure 29.1, viewed on following page. A) Life cycle of Cryptosporidium
species. From the CDC Public Health Image Library (PHIL #3386). Image
credit: Alexander J da Silva and Melanie Moser (CDC). B) Modifi ed Acid Fast
Stain. Examination of fecal specimens may reveal round pink or red oocysts
of 4-6 microns in diameter on a blue or blue-green background (represented
here as black oocysts on a grey background). Sporozoites may be visual-
ized inside individual oocysts. The assessment of three separate specimens
and use of an oocyst concentration technique prior to staining may increase
diagnostic yield. From the CDC Public Health Image Library (PHIL# 7829).
Image credit: Melanie Moser (CDC/ DPDx).
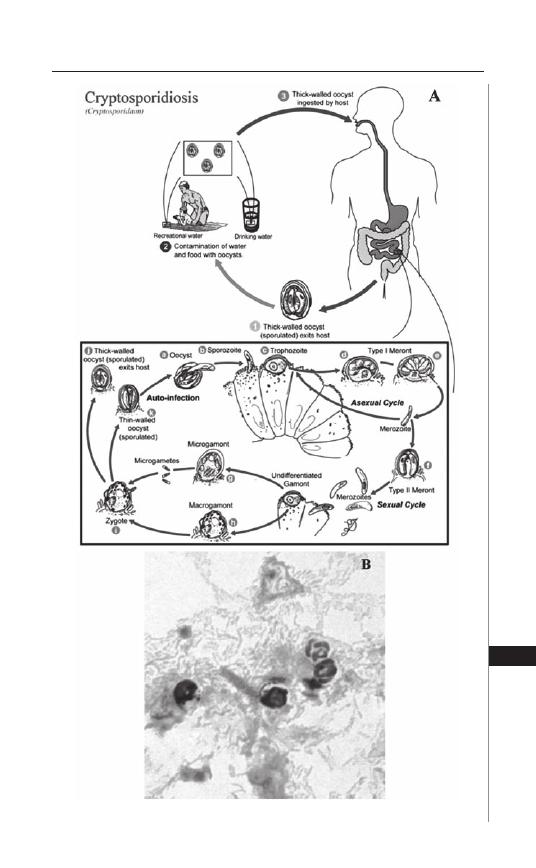
217
Cryptosporidiosis
29
Figure 29.1. Please see fi gure legend on previous page.

218
Medical Parasitology
29
Th e Developing World: Childhood Diarrhea and Malnutrition
In the developing world, diarrhea amongst children is the most common clinical
presentation of cryptosporidiosis. Little distinguishes Cryptosporidium-associated
diarrhea from other infectious causes of childhood diarrhea except for its propen-
sity to cause persistent diarrhea beyond 2 weeks duration. Persistent diarrhea is
associated with greater morbidity and mortality. Relapsing diarrhea, signifi cant
weight loss and growth rate reduction are common sequelae of infection in this
population. Th e relationship between cryptosporidiosis and malnutrition is com-
plex. It is unclear whether malnourished children have an increased susceptibility
to infection and predilection for more severe and persistent disease.
Th e Immunocompromised Host: AIDS and Other
Immunodefi ciencies
In the patient with HIV, the course of cryptosporidiosis oft en correlates with
the immune status of the individual. Patients with CD4 counts above 200 cells/
μ
L
are likely to have a clinical course similar to immunocompetent hosts. Patients with
AIDS and progressively declining CD4 counts are more likely to present with foul
smelling bulky stools in the context of chronic diarrhea and weight loss. Severely im-
munocompromised individuals with CD4 counts less than 50 cells/microL develop
a more fulminant cholera-like disease with watery and voluminous diarrhea. Biliary
and respiratory tract disease is more likely to manifest in severely immunocompro-
mised persons with CD4 counts less than 50 cells/
μ
L. Biliary tract involvement may
result in biliary strictures, papillary stenosis, pancreatitis, acalculous cholecystitis, or
sclerosing cholangitis. Th ese may manifest with right upper quadrant pain, nausea,
vomiting and low grade fever. Although oocysts have been detected in respiratory
secretions of immunocompromised patients, a causal link between Cryptosporidium
and pulmonary disease is usually diffi cult to establish given the occurrence of
coexistent opportunistic pathogens in this population. More severe or persistent
cryptosporidiosis has also been described in other immunocompromised settings,
including organ transplantation, immunosuppressive therapy, chemotherapy, primary
immunodefi ciencies, hematologic malignancies, cytokine defi ciencies and a variety
of other conditions associated with cell mediated immune dysfunction. CD4 T-cells
and the induction of certain cytokines, particularly interferon-gamma, play a critical
role in controlling infection. Th e role of other cytokines in both pathogenesis and
eff ective immunity or parasitologic clearance continues to be investigated. Th e role of
antibodies in immune protection remains contentious and immunoglobulin defi cits
or B-cell dysfunction do not preclude contemporaneous defi cits in T-cell or den-
dritic cell function. Moreover, patients with AIDS can mount apparently adequate
humoral responses yet remain susceptible to severe or persistent cryptosporidiosis.
Interestingly, fulminant disease has been noted in patients with severe combined
immunodefi ciency syndrome, immunoglobulin defi ciencies and subset of hyper IgM
syndromes. Th e latter syndromes are a heterogeneous and rare group of disorders
which manifest high levels of IgM but markedly reduced levels of other Ig isotypes.
Th e X-linked form is associated with inadequate levels of CD40L expression, defec-
tive immunoglobulin class switching and ineff ective generation of antigen specifi c
responses. Despite immunoglobulin replacement therapy, these patients remain
susceptible to certain opportunistic infections including cryptosporidiosis.

219
Cryptosporidiosis
29
Diagnosis
Routine stool examination for ova and parasites will generally fail to detect
the organism and the clinician should specify that Cryptosporidium species is in
the diff erential diagnosis. Th e sample should be preserved in 10% buff ered for-
malin, though some laboratories use a concentration method with formalin-ethyl
acetate fi xation that may augment the sensitivity of detection. Examination
of unfi xed specimens should be discouraged to reduce the risk to laboratory
workers. A number of diagnostic modalities with varying sensitivities and
specifi cities are now available. Traditionally, the diagnosis of cryptosporidiosis
has been made on the basis of microscopic identifi cation of round oocysts of
4-6
μ
m in diameter (Fig. 29.1B). Standard staining techniques include modifi ed
acid fast staining in which oocysts appear pink or red on a blue or blue-green
background, allowing clear diff erentiation from morphologically similar yeasts.
Sporozoites may be seen in individual oocysts and their visualization may
assist in the diagnosis. White blood cells are usually not seen. At least three
separate specimens should be examined to improve the diagnostic yield. Th e
yield may be enhanced if the stool specimen is unformed and if the laboratory
uses a concentration technique prior to staining. Fluorescent auramine stains
are potentially useful but provide little specifi city by themselves. Malachite
green, Giemsa and hematoxylin and eosin staining techniques have been used
to detect the organism with varying success and are inferior to modifi ed acid
fast staining. Immunofl uorescence assays are now commonly used and may be
a log more sensitive than acid-fast stains.
Antigen-detection assays in the form of ELISA or immunochromatographic
kits are now more widely used. A number of commercially available kits now have
excellent sensitivity and specifi city relative to the modifi ed acid fast technique.
Th e clinician should nevertheless review the diagnostic reliability of such kits be-
fore excluding the diagnosis. PCR remains investigational, is more labor intensive
and is not widely used though signifi cantly more sensitive than any microscopic
technique. Validated in-house assays can be more sensitive than antigen-detection
assays and may have the capability to distinguish between diff erent species.
Although serological testing has been instrumental in determining the prevalence
of cryptosporidiosis, it is of limited usefulness given the persistence of antibody
titers aft er remote infection in otherwise healthy individuals.
In the immunocompromised patient with biliary disease or pancreatitis, the
clinician should maintain a high index of suspicion for a cryptosporidiosis. One
should not exclude the diagnosis based on negative stool specimen testing; as
such investigations may be negative in this setting. Without frank obstruction,
hyperbilirubinemia may be unimpressive or absent though elevated alkaline
phosphatase and glutamyl transpeptidase, as well as elevated amylase and lipase
in the setting of pancreatitis, may point to the diagnosis of biliary tract infection.
Ultrasonographic abnormalities may include a thickened gallbladder wall as well
as dilated and irregular intrahepatic and extrahepatic bile ducts. A suspicion of
Cryptosporidium-related cholangiopathy should prompt an endoscopic assessment
of biliary involvement with tissue biopsy for histology as well as examination of
bile for oocysts.

220
Medical Parasitology
29
Management
Supportive care of the patient with cryptosporidiosis includes fluid and
electrolyte replacement, adequate nutritional intake and a lactose-free diet. Fluid
absorption may be improved by supplementation with glutamine. Antimotility
agents like loperamide are a mainstay of symptomatic treatment, though opiates
and octreotide have been used as adjuncts in more severe disease. In the setting of
obstruction secondary to papillary stenosis, endoscopic sphincterotomy with or
without stent placement may off er a symptomatic benefi t.
Th e effi cacy of antiparasitic therapy has historically been quite disappointing,
particularly amongst immunocompromised or malnourished patients. Th ree an-
tiparasitic agents are presently considered as having some in vivo activity against
Cryptosporidium: paromomycin, azithromycin and nitazoxanide. Amongst im-
munocompetent individuals, nitazoxamide (500 mg po BID for 3 days) has more
recently been shown to speed the resolution of diarrhea and oocyst shedding.
Nitazoxanide is also available as a suspension for pediatric use and is approved
for use in the United States for children older than 12 months of age at a range of
age-adjusted doses. Th e clinician should be cognizant of the signifi cant likelihood
of relapsing disease, even in the immunocompetent host.
In patients with immune dysfunction, eff orts to improve immune function
are paramount. In persons with AIDS, diarrhea may improve dramatically and
resolve on eff ective antiretroviral therapy as a result of immune reconstitution.
Interestingly, some investigators have reported that protease inhibitors have some
antiparasitic activity. Nevertheless, treatment of the patient with advanced AIDS
may be challenging in that antiretroviral medications may be poorly absorbed in the
setting of active cryptosporidiosis. For this reason, some authorities suggest initially
controlling disease with antiparasitic drugs prior to instituting a new antiretroviral
regimen. Antiparasitic therapy can speed resolution of diarrhea and oocyst shed-
ding, however a transient response to therapy is to be expected in patients with
advanced AIDS if immune reconstitution does not eventually occur.
Paromomycin has been shown to improve diarrhea to some extent in patients
with AIDS and in combination with azithromycin has been eff ective in dimin-
ishing symptoms and oocyst shedding in some patients. However, this regimen
does not usually result in resolution of disease in patients with AIDS. Numerous
isolated case reports have suggested a response to various combinations of a num-
ber of alternative agents. Such regimens have contributed transciently, if at all, to
improvement of symptoms and should be considered as adjuncts of limited eff ec-
tiveness. Th ese agents include spiramycin, clarithromycin, atovaquone, letrazuril
and hyperimmune bovine colostrum. Interestingly, rifaximin has been shown to
improve diarrhea to some extent in patients with HIV. A recent small case series
has also described its success in treating patients with advanced AIDS, but such
reports remain preliminary.
Promising data has been garnered from compassionate use programs for
nitazoxamide in patients with AIDS, suggesting that most recipients have a
sustained clinical response to this agent. However, short courses of nitazoxanide
appear ineff ective in children with AIDS not receiving ongoing eff ective antire-
troviral therapy. Judging the eff ectiveness of the drug in HIV-infected and other

221
Cryptosporidiosis
29
immunocompromised hosts will require further formal investigation. Higher
doses and/or a more extended duration of this therapy (500 to 1000 mg po BID
for 14 days in adults) should be considered for such populations, and immune
reconstitution remains fundamental to any sustained response. Th erapy may
need to be prolonged until suffi cient immune reconstitution has been achieved.
In hosts with intractable immunodefi ciency, parasitologic cure is very unlikely
irrespective of the regimen chosen and persistent fulminant diarrhea portends
a dismal prognosis.
Suggested Reading
1. Bushen OY, Lima AA, Guerrant RL. Cryptosporidiosis. In: Guerrant RL,
Walker DH, Weller PF: Tropical infectious diseases. Principle, pathogens and
practice. Philadelphia, PA: Elsevier Churchill Livingstone 2006; 1003-14.
2. Huang DB, White AC. An updated review on Cryptosporidium and Giardia.
Gastroenterol Clin N Am 2006; 35: 291-314.
3. White AC. Cryptosporidiosis (Cryptosporidium hominis, Cryptosporidium
parvum and other species). In: Mandell, Bennett & Dolin: Principles and Practice
of Infectious Diseases. 6th Edition. Philadelphia: Elsevier Churchill Livingstone,
2005:3215-28.
4. Xiao L, Fayer R, Ryan U et al. Cryptosporidium taxonomy: recent advances and
implications for public health. Clin Microbiol Rev 2004; 17:72-97.
5. Hunter PR, Nichols G. Epidemiology and clinical features of Cryptosporidium in-
fection in immunocompromised patients. Clin Microbiol Rev 2002; 15:145-54.
6. Centers for disease control and prevention and the national center for infec-
tious diseases, division of parasitic diseases. Parasitic disease information:
Cryptosporidium Infection/Cryptosporidiosis. http://www.cdc.gov/ncidod/
dpd/parasites/cryptosporidiosis/default.htm

C
HAPTER
30
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Trichomoniasis
Raymond M. Johnson
Introduction
Trichomonas vaginalis is a fl agellated single cell eukaryote with a relatively
simple lifecycle. T. vaginalis exists only in the trophozoite form and divides by
simple binary fi ssion in its human host. Th ere are no T. vaginalis cysts, portions
of the parasite lifecycle that occur outside its human host, or known animal or
environmental reservoirs. A closely related relative Tritrichomonas foetus causes
commercially important reproductive tract and fetal infections in cattle.
T. vaginalis carries the distinction of being the only truly sexually transmitted
parasitic infection in humans.
1
It is very successful as a pathogen causing roughly
the same number of STDs as Chlamydia trachomatis, the most prevalent sexually
transmitted bacterial pathogen. In the U.S. there are an estimated 3-5 million new
cases of ‘trich’ every year with an infected pool of approximately 20 million indi-
viduals. Worldwide the prevalence of T. vaginalis varies from 2% to greater than
50% depending on region, country, gender and demographics of the population
specifi cally evaluated.
T. vaginalis is highly adapted to the human urogenital tract and is never found in
stool specimens. Th e unique adaptation of T. vaginalis to the urogenital tract allows
it to be easily identifi ed in urogenital tract clinical specimens without concern about
other parasite species. T. vaginalis thrives in the microaerophilic environment of
the vaginal mucosa. To live in the low oxygen tension it utilizes an organelle called
a hydrogenosome to generate ATP. T. vaginalis lack mitochondria that generate
ATP for oxygen-dependent eukaryotes. Instead the hydrogenosome generates
ATP utilizing a pathway similar to mitochondria except that the fi nal electron
acceptor is hydrogen rather than oxygen, generating hydrogen gas as a byproduct
of metabolism. Th e hydrogenosome is also the Achille’s heel of T. vaginalis as it
metabolizes the 5-nitroimidazole antibiotics metronidazole and tinidazole into
toxic anion radicals that kill the parasite.
Clinical Disease
Trichomonas vaginalis typically comes to medical attention through one of
four basic scenarios:
1. Women seeking evaluation for a vaginal discharge.
2. Women incidentally found to be infected with T. vaginalis during prenatal
care visits or examination of routine Pap smears.
3. Male partners of women diagnosed with T. vaginalis via (1) or (2).
4. Men with nongonococcal urethritis (NGU) that does not respond to usual
NGU therapy.

223
Trichomoniasis
30
T. vaginalis is only found in the lower urinary and reproductive tracts of men
and women. In women it causes a superfi cial infection of the vagina and urethra,
occasionally ascending further to cause cystitis (bladder infections). In males
the infection is largely confi ned to the urethra, but can ascend into the prostate
and epididymis. T. vaginalis is not known to disseminate from the urogenital
tract to cause deep seated infections in other parts of the body. Neonates acquir-
ing Trichomonas vaginalis during transit through the birth canal rarely develop
pneumonia and a single case of a trauma related T. vaginalis perinephric abscess
has been reported.
Consistent with its tropism, T. vaginalis causes lower reproductive tract symp-
toms in women including vaginal discharge, vulvar irritation and dysparunia.
Colonization of the urinary tract is associated with dysuria, urinary frequency,
post voiding discomfort and lower abdominal pain. Th e vaginal discharge caused
by T. vaginalis tends to be copious and can be ‘frothy’. While women seeking
medical attention for the above conditions are subjectively and objectively ill,
many women carry T. vaginalis asymptomatically for protracted periods of time.
40-50% of women infected with T. vaginalis do not report a vaginal discharge.
2
A recent study in adolescent women over approximately 2 years had an incident
rate of infection of 23%, with roughly a third of the infected adolescent women
remaining asymptomatic.
3
Th e majority of infected men are asymptomatic, however T. vaginalis can
cause a symptomatic nongonococcal urethritis (NGU).
4
Th e urethral discharge
associated with the infection tends to be minimal compared with other NGU
etiologies. Because diagnostic testing for T. vaginalis in men is suboptimal, it is
common practice in many STD clinics to empirically treat for T. vaginalis in men
that have failed standard nongonococcal urethritis therapy and observe for resolu-
tion of symptoms. T. vaginalis rarely ascends into the prostate or epididymis to
cause symptomatic infections.
Th e natural history of T. vaginalis infections is poorly understood. It is estimated
that only 20% of women and 40% of men spontaneously clear their infections. Th e
long duration of infection combined with a high rate of asymptomatic carriage
likely account for the success of T. vaginalis as a sexually transmitted parasite.
Th e relative insensitivity of currently utilized laboratory tests for diagnosing T.
vaginalis allows it to escape detection and likely further facilitates its prevalence
throughout the world.
Diagnosis
Th e approach to diagnosing T. vaginalis infection diff ers between men and
women. A vaginal discharge may be caused by multiple etiologies and concurrent
infections with two or more pathogens are common. Th e physical exam should
include inspection of the cervix for presence of mucopurulent cervicitis associated
with chlamydia and gonorrhea infections and the so-called ‘strawberry cervix’, a
rare physical fi nding associated with T. vaginalis infection. Th e exam should also
include evaluation for cervical motion tenderness, the physical exam fi nding that
correlates with pelvic infl ammatory disease.
Th e nature of the vaginal discharge itself is not particularly informative,
though experienced STD clinic practitioners develop some ability to diff erentiate
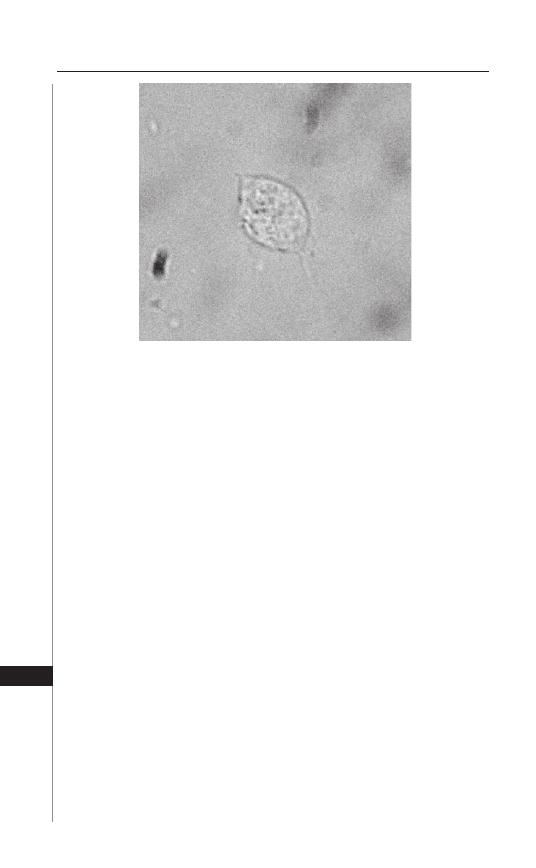
224
Medical Parasitology
30
trichomonas infections from bacterial vaginosis and other infectious etiologies. An
abundant frothy vaginal discharge is highly suspicious for T. vaginalis infection, but
is not specifi c enough to establish a diagnosis and defer further diagnostic evalu-
ation. Vaginal fl uid specimens collected with swabs should be tested for pH and
normal saline and KOH slides prepared. Typical cases of vaginal T. vaginalis infec-
tions have pH values greater than 4.5, but a normal vaginal pH does not rule out a T.
vaginalis infection. Th is fi nding also does not distinguish T. vaginalis from bacterial
vaginosis that is also associated with elevated pH. A positive KOH ‘whiff ’ test for
amines (fi shy smell) implies bacterial vaginosis but does not exclude T. vaginalis
co-infection. Th e normal saline wet prep slide on microscopy may show clue cells
(vaginal epithelial cells coated with bacteria) consistent with bacterial vaginosis, or
motile trichomonads diagnostic for T. vaginalis infection, or both. Visualization
of motile trichomonads on microscopic examination is somewhat dramatic and
is diagnostic of a ‘trich’ infection (Fig. 30.1). Unfortunately examination of the
normal saline slide is only about 60% sensitive for making the diagnosis of a T.
vaginalis infection. However, a positive T. vaginalis wet prep is all that is needed
to make the diagnosis in the majority of patients and therefore is an important
part of the initial medical evaluation in women with vaginal discharges.
A sizeable minority of women with T. vaginalis infections will have a negative
saline wet prep. Th ere is no consensus among STD care providers on how the sub-
sequent evaluation should proceed. For patients diagnosed with bacterial vaginosis
(BV), systemic metronidazole given to treat BV would eradicate a coincident T.
vaginalis infection while topical metronidazole gel therapy is not eff ective ‘trich’
therapy. Also, missing the diagnosis of a co-incident T. vaginalis infection means
that the patient’s male partner will go untreated and the patient will likely be
reinfected immediately aft er coming off antibiotic therapy.
Figure 30.1. Trichomonas vaginalis visualized on normal saline wet prep
microscopy. Image courtesy of Barbara Van Der Pol, Indiana University
School of Medicine, Indianapolis, IN.

225
Trichomoniasis
30
In medical settings where the test is available, the InPouch™ Trichomonas
vaginalis culture system (Biomed) is probably the best and most aff ordable
second screening test. It does however have several drawbacks. Samples must be
inoculated into the culture medium within 30 minutes as the T. vaginalis must
be viable for the culture to give a positive result. Also, because it is a culture
system, there is an incubation period of 1 to 5 days before the test turns positive.
In addition a microbiology technician must be available to evaluate the culture
system microscopically for identifi cation of motile T. vaginalis forms to fi nalize
the test results. Th e culture technique is currently considered the gold standard
for making a T. vaginalis diagnosis and is the most sensitive of any diagnostic
test currently available for diagnosing T. vaginalis in women. PCR tests for T.
vaginalis in vaginal fl uid specimens are not commercially/clinically available
and most of those in development do not appear to be more sensitive than
Trichomonas vaginalis culture. Because a vaginal discharge represents more than
a simple inconvenience for most women, a more rapid point of care diagnostic
test would be desirable.
Th ere are two nonculture clinical tests available for diagnosing T. vaginalis
vaginal infections. A DNA probe test (Affi rm™ VPII, Becton Dickinson) is capable
of identifying Trichomonas vaginalis, Gardnerella vaginalis and Candida species
in vaginal fl uid specimens. Th e DNA probe test has a sensitivity of roughly 90%
compared with culture. It has the advantage of potentially identifying polymicro-
bial infections and has more forgiving specimen handling requirements. However,
it requires specialized equipment specifi c to the assay and has potential delays in
diagnosis depending on individual laboratory protocols for processing specimens
and reporting results. A simple point of care test based on Trichomonas vaginalis
antigen (OSOM® Trichomonas Rapid Test, Genzyme Diagnositics) is commer-
cially available and FDA approved in the US. Th e antigen-based test is about 80%
sensitive compared with culture. Once vaginal swabs have been obtained, sample
processing and test completion take about 10 minutes. In some clinical settings
where culture is unavailable or immediate results are desired, examination of a
saline wet mount combined with a point of care antigen test for negative wet
preps may be a practical approach for diagnosing T. vaginalis.
In men, microscopic examination of urethral swabs or urine sediment are
insensitive tests for diagnosing ‘trich’ infections and are not routinely performed.
Culture is the test of choice for making a defi nitive diagnosis with a sensitivity of
roughly 60-70%. To get maximum sensitivity both urethral swab and urine sedi-
ment cultures should be obtained. Semen may be the most sensitive clinical sample
for culturing Trichomonas vaginalis, though it is not routinely used. In men, the
development of urine sediment PCR assay for T. vaginalis holds the promise of
being a more sensitive and convenient test. Currently urine sediment PCR test-
ing is only available on an experimental basis or in clinical labs with ‘home brew’
PCR assays. Because of the diffi culty inherent in diagnosing T. vaginalis in men
with currently available tests, many men are treated empirically for T. vaginalis
because a female partner has been diagnosed with T. vaginalis or because they have
an NGU that did not respond to standard therapy. While there are no defi nite
statistics available, asymptomatic infected men likely represent a major reservoir
for ongoing transmission of T. vaginalis infections.

226
Medical Parasitology
30
Treatment
Trichomonas vaginalis is susceptible to the 5-nitroimidazole antibiotics metro-
nidazole and tinidazole. Th e standard therapies for both men and women are:
1. Metronidazole 2 g orally in a single dose, or
2. Metronidazole 500 mg orally twice a day for 7 days, or
3. Tinidazole 2 g orally in a single dose
4. A single 2 g dose of metronidazole is recommended for pregnant women.
Th ere are no major advantages of tinidazole over metronidazole, with the
latter being less costly than the former. Tinidazole, commonly used outside the
U.S. and recently approved in the U.S., has a longer half-life and may be better
tolerated. Patients and their partners are generally treated with a single 2 g dose
of metronidazole or tinidazole. Patients should abstain from sexual activity
until asymptomatic or, more specifi cally, for at least one week aft er completing
antibiotic therapy. Th e seven day course of metronidazole therapy is generally
reserved for initial treatment failures. Multiple treatment failures refl ect either
untreated ongoing sexual partners or 5-nitroimidazole resistance. 5-nitroimida-
zole resistance is estimated to be 2-5%.
5
Suspicious cases should be referred to
an infectious diseases specialist or gynecologist with expertise and in the U.S.
arrangements should be made to send cultures to the Centers for Disease Control
and Prevention (CDC; Consultation is available via tel: 770-488-4115 or website:
http://www.cdc.gov/std/). Th erapy with metronidazole or tinidazole is generally
well tolerated, but patients should be warned to avoid drinking alcohol for at
least 48 hours aft er their last dose of either metronidazole or tinidazole to avoid
severe ‘hangovers’ that result from antibiotic inhibition of alcohol metabolism.
In addition, patients should be warned about a metallic taste in the back of their
mouths especially those on a 7 day course of therapy. For a very small minority of
patients nausea/vomiting is a signifi cant side eff ect of the medications.
Th e possibility the Trichomonas vaginalis infections contribute to the spread
of HIV combined with its high prevalence has altered “trich’s” status from that
of a mere ‘nuisance’ to that of an important sexually transmitted microbial
pathogen.
6
References
1. Schwebke JR, Burgess D. Trichomoniasis. Clin Microbiol Rev 2004; 17:794-803,
table of contents.
2. Fouts AC, Kraus SJ. Trichomonas vaginalis: reevaluation of its clinical presentation
and laboratory diagnosis. J Infect Dis 1980; 141:137-43.
3. Van Der Pol B, Williams JA, Orr DP et al. Prevalence, incidence, natural history
and response to treatment of Trichomonas vaginalis infection among adolescent
women. J Infect Dis 2005; 192:2039-44.
4. Krieger JN. Trichomoniasis in men: old issues and new data. Sex Transm Dis 1995;
22:83-96.
5. Cudmore SL, Delgaty KL, Hayward-McClelland SF et al. Treatment of infections
caused by metronidazole-resistant Trichomonas vaginalis. Clin Microbiol Rev
2004; 17:783-93, table of contents.
6. Cohen MS, Hoff man IF, Royce RA et al. Reduction of concentration of HIV-1 in
semen aft er treatment of urethritis: implications for prevention of sexual transmis-
sion of HIV-1. AIDSCAP Malawi Research Group. Lancet 1997; 349:1868-73.

C
HAPTER
31
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Pneumocystis Pneumonia
Allen B. Clarkson, Jr. and Salim Merali
Introduction
Pneumocystis is an opportunistic fungal pathogen causing Pneumocystis pneu-
monia (PcP) in mammals with compromised immunity. It was most oft en classifi ed
as a protozoan until, beginning in the late 1980s, genetic and other data showed
conclusively that Pneumocystis is a fungus, albeit an unusual one. Because it appears
similar in all mammals and produces similar pneumonias, all Pneumocystis was once
classifi ed as the single species, P. carinii. When genetic analyses in recent years showed
wide divergence amongst Pneumocystis infecting diff erent animals, the need for
diff erentiation became clear. Initially the species P. carinii was divided into several
form specialis, but that clumsy nomenclature has been superseded by a widely, but
not yet universally, accepted classifi cation with multiple species within the genus
Pneumocystis with P. jiroveci for the species infecting humans, P. carinii for one of
two species specifi c for rats and other names for Pneumocystis species infecting other
mammals. Identifi cation of genetically distinct strains within P. jiroveci has proven
helpful for epidemiological studies. For practical reasons, the established acronym
PCP (or PcP) remains in use, but now refers to Pneumocystis pneumonia rather than
P. carinii pneumonia. Figure 31.1 shows Pneumocystis in the lung at the electron
microscope level and Figure 31.2 at the light microscope level.
Populations at Risk
Pneumocystis was described in animal lungs in 1912, but was not known to be
a human pathogen until 1951 when it was associated with interstitial plasma-cell
pneumonia in malnourished institutionalized children. Subsequently, PCP was
recognized as important complication for children being treated for acute lympho-
cytic leukemia and the introduction of cotrimoxazole to treat PCP comorbidity in
1975 greatly improved outcomes. PCP remains a threat for patients given drugs that
suppress immunity. Without prophylaxis, the rate of PCP aft er organ transplant
ranges from 5-25% and for cancer treatment from 1-25%. Drugs used for rheumatic
disease can also increase susceptibility. However, advanced HIV disease accounts
for the greatest number of cases. Th e rate of PCP associated with HIV infection
dropped when prophylaxis was widely adopted in the early 1990s and dropped
further in the mid 1990s with the introduction of HAART to suppress viral load.
But for untreated or nonresponsive advanced HIV disease, the rate remains above
50%. Although the risk of HIV-associated PCP can be nearly eliminated by good
compliance with a correctly chosen and tolerated chemoprophylactic regimen,
PCP remains the most common opportunistic infection associated with AIDS.
228
Medical Parasitology
31
Recent anecdotal information suggests the overall rate has risen with the shift in
HIV infection patterns to populations with a lower probability of seeking and less
access to timely medical care. PCP was thought to be rare in the huge population
of HIV-infected people in sub-Saharan Africa, but recent studies show a high
prevalence there as well—especially in children.
Life Cycle and Transmission
Because Pneumocystis cannot yet be cultured well and genetic manipulation is
not yet possible, it is diffi cult to study and much remains unknown. Two distinct life
cycle forms are known: cyst and trophozoite. Th e mature cyst has a thick cell wall
and eight intracystic bodies. Good evidence indicates that intracystic bodies are the
product of meiosis, but details are lacking. Upon cyst rupture, intracystic bodies are
released and become trophozoites which either grow and divide by binary fi ssion or
develop into precysts then cysts. Mature cysts have a characteristic indented-sphere
Figure 31.1. Transmission electron micrograph showing Pneumocystis
trophozoites attached to Type 1 pneumocytes: These organisms reduce gas
exchange, although the degree of involvement seen here is light. As the
disease progresses and alveoli of lobules become completely fi lled with
organisms, gas exchange becomes totally blocked. However, progression
is slow with areas of high involvement becoming consolidated and fi brous
tissue replacing portions of lung parenchyma. *) Pneumocystis organisms; 1)
capillary lumen; 2) erythrocyte; 3) alveolar air space; 4) area of consolida-
tion with cross-sectioned collagen fi bers seen as small dots.

229
Pneumocystis Pneumonia
31
shape and a narrow size range, but trophozoites are highly variable in both size and
shape. Transmission from animal to animal by air has been directly demonstrated and
air sampling fi lters placed in hospital rooms of patients with PCP trap particulates
containing more copies of Pneumocystis-specifi c nucleic acid sequences than fi lters
from control areas clinical. Th e thick presumably environmentally resistant cyst wall
suggests a role in transmission, but animal studies show both forms can transmit
infection when instilled directly into lungs. Speculation exists regarding a cryptic
life cycle stage outside mammalian hosts, but evidence is lacking.
A long-standing question has been whether PCP develops upon immunosup-
pression due to activation of nearly universal colonization or as the result of a new
inoculation. If the former is true, patients with PCP present no risk to other im-
munosuppressed persons. However, accumulating data support the second hypoth-
esis. Th ese data include reports of clusters of PCP cases, analyses showing related
groups of patients to have the same genetic strain and examinations of Pneumocystis
taken from patients during multiple episodes of PCP that showed diff erent genetic
strains to be involved in each episode. Th e risk of those with active PCP present
to other immunosuppressed persons is unclear, but several recent reports suggest
it is signifi cant.
Diagnosis
Presenting symptoms of PCP can include general fatigue, dry cough, night
sweats, low grade fever, tachypnea and history of progressive dyspnea most no-
ticeable on exertion. Auscultation is characteristically uninformative, but fi ne
Figure 31.2. Lung sections silver-stained and counterstained with eosin: The
dark bodies are cysts, but the far more numerous trophozoites are not selec-
tively stained. The left panel shows a region with moderate pathology; septa
are not yet markedly thickened, although gas exchange will have already
been compromised by trophozoites attached to Type 1 pneumocytes. The
right panel shows a region with greater involvement; septa are thickened and
some regions show consolidation. As the disease progresses, gas exchange
becomes more compromised eventually to the point of asphyxiation.

230
Medical Parasitology
31
dry rales may be noted. Presentation can include pneumothorax, but this is rare.
For those with advanced HIV disease, symptoms may progress slowly so the
patient isn’t aware of the developing pneumonia and attributes breathlessness
upon exertion as general fatigue. Clinical data associated with PCP include chest
X-ray showing a bilateral fi ne smooth “ground glass” opacity, positive gallium
scan, hypoxia indicated by arterial oxygen saturation <85%, abnormal diff using
capacity and elevated serum lactate dehydrogenase. Although all these symptoms
and data can all result from other causes and thus are not diagnostic, the presence
of any requires PCP be considered, especially for persons with impaired immune
function. Furthermore, since PCP may be the fi rst indication of developing HIV
disease, particularly for those unaware of their infection, it should be considered
even in the absence of known immunosuppression. A presumptive diagnosis of
PCP is sometimes given based on knowledge of immunosuppression in combi-
nation with a set of symptoms and clinical data; response to specifi c therapy is
considered confi rmation. However, the particular set of symptoms and laboratory
data varies among physicians and hospitals and there is no accepted standard.
Although presumptive diagnoses by experienced clinicians can be timely, accurate,
cost eff ective and benefi cial to patients, most authorities recommend the eff ort
to obtain a defi nitive diagnosis by demonstrating presence of Pneumocystis using
histologic, cytologic, or nucleic acid-based methods. For some years following
discovery of HIV infection, diagnosis based on open lung biopsy was common,
but this has been replaced by less invasive procedures. Bronchial alveolar lavage
(BAL) is the current gold standard and is sometimes combined with transbron-
chial biopsy, depending on hospital policy. Figure 31.3 shows sediment from BAL
fl uid stained to reveal cysts. Examination of sputum induced by inhalation of an
aerosol of hypertonic saline is also very eff ective. In some hospitals, diagnoses
have been made using oral washings. Success of these progressively less invasive
diagnostic procedures has depended upon progressive improvements in both
detection methods and laboratory experience. Because P. jiroveci cysts have a
thick fungal-type, glycan-containing cell wall, they are revealed by fungal cell
wall stains such as methenamime silver, cresyl echt violet and toluidine blue as
well as by selective uptake of fl uorescent dyes such as calcofl uor white. Th ese are
not specifi c for Pneumocystis so diff erentiation from other fungi depends on the
characteristic size (∼5
μ
M) and shape of cysts (individual indented spheres with
a smooth surface). As a consequence, identifi cation of individual cysts in sections
is not as easy as in preparations such as smears that preserve the shape of the intact
organism. However, sections reveal alveoli fi lled with “foamy exudate” characteris-
tic of PCP and can be useful for diagnosis. Although trophozoite numbers exceed
cysts by an order of magnitude, they can be less useful for diagnosis. Th ey are not
stained by fungal cell wall reagents. Th ey do take up hematological stains such as
Wrights and Giemsa, but host material and other pathogens are also stained by
these. Trophozoites are highly variable in both size (0.5-8
μ
m) and shape (from
round to almost stellate and from a smooth to a highly irregular surface) so that
reliable identifi cation requires training, skill and experience. Because intracystic
bodies within cysts stain similarly to trophozoites, they are sometimes seen as a
cluster of eight intracystic bodies. IFA kits licensed for PCP diagnosis are com-
mercially available; these label both trophozoites and cysts selectively and strongly.
231
Pneumocystis Pneumonia
31
When used with BAL samples, they oft en reveal distinctive fl uorescent clusters
of trophozoites and cysts enmeshed in exudate that is also labeled. Although IFA
provides high sensitivity and specifi city, this diagnostic tool may not be available
due to drawbacks of reagent cost, fl uorescence microscope requirement and the
time, skill and equipment needed for processing the specimens. PCR-based nucleic
acid detection off ers exquisite sensitivity and specifi city, but suff ers from lack of
FDA-approved kits as well as the general problems of PCR-based diagnoses: costs
of special reagents, special equipment, technical training, inclusion of extensive
positive and negative controls and need for rigid procedures to prevent false
positives due to contamination. Furthermore, the extreme sensitivity of PCR
presents a problem in that colonization with small numbers of P. jiroveci can be
detected even if they are not causing disease. A well-designed and fully developed
quantitative-PCR protocol with a clear “initial target copy number” threshold
could resolve many of these issues and encouraging clinical studies have been
done; however, even if fully developed, general disadvantages of PCR diagnosis
will remain. Reported success rates for the various diagnostic methods vary from
study to study, partially due to the experience and expertise of physicians and
laboratories, but trends are clear. Open lung or transbronchial biopsy specimens
stained for cysts provide diagnostic sensitivity and specifi city of 98+ and 100%,
respectively, but both procedures are invasive and present risks to the patient.
Sensitivities and specifi cities for BAL fl uid samples stained for cysts are reported
as high as 95 and 100%, respectively; IFA can improve sensitivity to 98% while
retaining selectivity. Reports of effi cacy using induced sputum samples have a much
broader spread with sensitivity ranging from 30 to 90%; specifi city remains high.
Figure 31.3. BAL sample silver-stained and counterstained with Fast Green: Due
to their shape and dark gray staining, cysts are readily recognizable. When
present in adequate numbers, the diagnosis can be made with confi dence.

232
Medical Parasitology
31
Finally, because those persons vulnerable to PCP can and oft en do have multiple
respiratory infections, the presence of other pathogens and their possible contri-
bution to disease must be considered. Th is is true even if all symptoms and data
are consistent with PCP, P. jiroveci is positively identifi ed in patient specimens
and the disease responds to treatment.
Course of Disease and Pathology
PCP in infants from age 1 to 7 months of age, particularly from 2 to 4 months,
develops more rapidly and becomes more severe than in older children or adults.
Onset in infants can be insidious with the fi rst signs being tachypnea and slight
perioral cyanosis; coughing and fever may not be signifi cant. Th e disease may
progress over several weeks with increased tachypnea, dyspnea with sternal
contraction and cyanosis. However, progression can be very rapid with death
preceding diagnosis. Th ere is no information on the eff ectiveness of maternally
transferred immunity, but animal studies showing antibody protection suggests
a role for maternal antibodies. Since antibodies specifi c for Pneumocystis com-
monly develop in early childhood without any history of PCP, the severity of PCP
in immunosuppressed infants may be due to a combination of fading maternal
protection and primary exposure. Interestingly, recent information indicates
that even in otherwise healthy infants primary infections may not be completely
silent. When nasopharyngeal samples from 105 infants (2 or 3 months old with
respiratory infection symptoms) were tested for the presence of P. jiroveci, 48%
were positive; the frequency was markedly lower for younger or older infants with
similar symptoms. Examination of lungs from sudden infant death syndrome
victims revealed a high proportion infected with P. jiroveci. However, the question
remains open as to whether PCP contributed to death, whether the presence P.
jiroveci refl ects another underlying pathological condition, or whether this fi nd-
ing is irrelevant because P. jiroveci colonization is common in infants of that age
and causes no signifi cant pathology without immunosuppression. Progression
of symptoms in older children and adults is slower, oft en over several months.
Progression in HIV-infected children and adults is still slower than in those
immunosuppressed from other causes. Th e best evidence suggests that, despite
immunosuppression, disease is more the result of residual immune function
than any direct damage by P. jiroveci. Th is may explain why, despite numbers of
organisms being greater for PCP associated with HIV, symptoms develop more
slowly and the probability of successful treatment is better than other causes
of immunosuppression. Regardless the cause of immunosuppression, disease
progression always involves increasing numbers of alveoli becoming fi lled with
organisms and exudate which leads to impairment of air exchange and ultimately
asphyxiation. While mechanical ventilation can be helpful in maintaining blood
gases, that requirement is associated with poor prognosis. Postmortem examina-
tion reveals heavy, fi rm lungs that retain shape aft er removal from the thoracic
cavity. H&E stained sections from biopsy and postmortem specimen show al-
veoli fi lled with an eosinophilic foamy or honeycomb exudate. When treatment
is successful, there may be residual fi brosis and pulmonary defi cit. While rare,
extrapulmonary pneumocystosis does occur and infections have been reported
at sites such brain, spleen, middle ear and peritoneum.

233
Pneumocystis Pneumonia
31
Treatment
Th e antifolate combination of trimethoprim (15 mg/kg/d) and sulfamethox-
azole (75 mg/kg/d in 3-4 doses) (cotrimoxazole, TMP-SMZ) for 14-21 d is widely
considered fi rst line treatment for PCP. Advantages include effi cacy equal or better
than for all other treatments, favorable cost and availability and generally good tol-
erance. Drawbacks include gathering genetic evidence suggesting development of
resistance as well as more frequent and more severe adverse drug reactions (ADRs)
in patients immunosuppressed due to HIV infection (40-60% or higher depend-
ing on the study vs 8-10% for patients without HIV). However, clinical resistance
has not been demonstrated to date and ADRs are not always of such severity to
necessitate a change in therapy. Second line drugs include clindamycin with pri-
maquine, other antifolate combinations such as trimethoprim or pyrimethamine
combined with dapsone, pentamidine either by aerosol or i.v. and atovaquone as a
single agent or combined with azithromycin. All treatments for PCP have associated
ADRs and some interact negatively with drugs used to suppress HIV replication.
While ADRs are frequently mild, they may be severe and changes in therapy are
more oft en due to ADRs than lack of response. For TMP-SMZ and dapsone, the
ADR spectra are similar, yet it is not uncommon for a patient to be intolerant of
one and do well with the other. Th e most frequent ADRs for these drugs are fever,
rash, nausea and vomiting, elevation of plasma liver enzymes and leukopenia. For
TMP-SMZ the most severe ADR is debilitating Stevens-Johnson syndrome-like
skin reaction that sometimes leads to extensive skin loss. TMP-SMZ and dapsone
can also cause hemolytic anemia, especially for patients with G-6-PD defi ciency.
Occasionally other severe systemic reactions involve liver, kidney, bone marrow
and heart. Clindamycin plus primaquine can cause rash, liver enzyme elevation,
leukopenia, or anemia. Pentamidine can result in nephrotoxicity, hypoglycemia,
pancreatitis and arrhythmias. Pentamidine delivery by aerosol has a lower ADR
rate, but such delivery may not provide adequate dosage to upper portions of
the lung and cannot treat rare extrapulmonary infection sites. Atovaquone when
used as a single agent can cause rash, nausea, diarrhea or headache, but the rate of
ADRs is lower than for other drugs; effi cacy, however, is lower as well. Combining
azithromycin with atovaquone improves effi cacy, but the rate of ADRs also rises.
Atovaquone can slow metabolism of zidovudine thus may signifi cantly increase
ADRs for that drug. Documentation of diff erences in effi cacies or ADR rates for
approved anti-Pneumocystis regimens is made diffi cult by the wide variation in clini-
cal responses with any one drug, diff erences in study populations and diff erences
in study designs. Furthermore, actual diff erences may be small. Should a change in
treatment be necessary due to poor response or ADRs, any of the approved drugs
can be substituted. However, one large study did fi nd clindamycin/primaquine and
atovaquone to be the most successful salvage therapies, regardless of the primary
treatment. Trimetrexate with leucovorin to reduce toxicity has been used success-
fully for salvage, but suff ers from a higher rate of relapse.
Th e evidence for spreading resistance to TMP-SMZ is emergence of mutations
in P. jiroveci similar to those of other microbes that are known to confer resistance
to the sulfamethoxazole component of TMP-SMZ. Th ese mutations are found with
higher frequency in populations where TMP-SMZ has been used extensively to treat
and/or prevent PCP as well as in individual patients who have used TMP-SMZ for

234
Medical Parasitology
31
prophylaxis. Th e same relationship exists for emergence of mutations associated with
resistance to atovaquone. However, direct evidence of clinical resistance is lacking
and obtaining such evidence is problematic because response to treatment is vari-
able and in vitro testing is not possible because the organism cannot be cultured
from patient samples.
Response to treatment is oft en slow with improvement sometimes seen only aft er
a week or more; lung function improvement oft en precedes changes detectable by
radiology. Slow response can be problematic if there is any doubt of the diagnosis
and, even if the diagnosis is confi rmed cytologically, the possibility of one or more
comorbidities arises. Several approaches to a response-to-treatment assay have been
suggested and promising data have been collected. Th ese include a plasma assay
for glycan shed from cyst walls, a sputum assay using PCR to detect changes in P.
jiroveci DNA or RNA content and a plasma assay for changes in host metabolic
intermediates infl uenced by P. jiroveci. None, however, are available for clinical use.
Controlled trials needed to determine optimal duration of therapy are lacking, but
clinical experience has led to treatment usually lasting 14 to 21 days, even if response
is relatively rapid. Adjuvant corticosteroid treatment for severe PCP was evaluated
by controlled trials and the consensus recommendation of an expert panel in 1990
was they be used for treatment of severe PCP (arterial O
2
partial pressure <70 mm
Hg or alveolar-arterial gradient >35 mm Hg, both on room air). Meta-analysis of
data from randomized trials published through 2004 confi rmed and quantifi ed this
benefi t. Th e advantage of steroid treatment can be demonstrated by radiology and
lung performance studies showing that anti-Pneumocystis drugs used alone cause an
initial increase in lung opacity and a decline in lung function parameters. Animal
model data show treatment exacerbates infl ammation leading to a reduction in
gas exchange effi ciency. For moderate PCP the case is not as strong and there is no
indication for steroids in treatment of mild disease.
Treatment Outcomes
Factors associated with poor outcomes include age, poor oxygenation on ad-
mission, elevated serum LDH, low hemoglobin, low serum albumin, presence of
pulmonary co-pathogens, neutrophils in BAL fl uid, delay in needed ICU admission
aft er start of anti-PCP treatment, high APACHE II score and pneumothorax either
at presentation or aft er initiation of mechanical ventilation. PCP is a serious disease
but it is oft en now described as a “treatable disease”, refl ecting the perception that
outcomes are generally better now than early in the HIV epidemic. Any improve-
ments must be attributed to clinical skills derived from experience since, other
than the introduction of adjuvant steroid treatment, the list of approved drugs has
not changed for over a decade and effi cacies from oldest to newest drugs are very
similar. A sobering view is provided by two recent papers, one based on data from a
major UK and the other from a major US medical center. Both studies included all
patient records with laboratory-confi rmed PCP diagnoses. Th e UK data covered the
period from 1985 -2006 and included 494 patients; the US data from 1990 -2001
and 488 patients. Both reported reductions in PCP mortality aft er the introduc-
tion of HAART in 1996. UK data showed a 16.9% mortality rate for the period
1990 -1996 and 9.7% for the period 1996 -2006; however, the 1985 -1989 rate was
10.1% so real improvement is certain. US data showed a 47% mortality rate for the

235
Pneumocystis Pneumonia
31
period 1990 -1995 and 37% for the period 1996 -2001; the higher US mortality
likely refl ects the “inner city” patient population of the study. Since these data are
from major centers where there is considerable experience and expertise in treating
PCP, broader mortality rates may be even higher.
Prophylaxis
Th e overall incidence of PCP associated with HIV infection dropped with wide
acceptance of prophylaxis guidelines published in 1989 and dropped further upon
introduction of HAART in the mid-1990s. Currently, discontinuation of prophylaxis
is now recommended when HAART allows restoration of CD4 cells to >200 cell
μ
L
−
1
, but risk of PCP is not a threshold phenomenon: an increase in CD4 count
from 100 to 200 cells
μ
L
−
1
is associated with a 10x reduction in risk of PCP, but there
is a further 10X reduction in risk with an increase from 200 to 500 CD4 cells
μ
L
−
1
.
Drugs used for prophylaxis include all the drugs used for treatment. TMP-SMZ is the
mainstay and the advantages/disadvantages are the same as for treatment; however,
due to time factors, desensitization using a slowly increasing dosage plays a bigger
role in prophylaxis. Aerosolized pentamidine has advantages of a low ADR rate and
good protection but has drawbacks: upper lung regions are poorly treated and this can
allow intense local involvement without symptoms so pneumothorax may be a result.
Extrapulmonary sites are also not protected, but infection beyond the lungs is rare.
Diffi culties and costs associated with aerosol administration are also disadvantages.
Dapsone used as a single prophylactic agent has effi cacy and ADR profi les similar
to aerosolized pentamidine, but without the upper lung and extrapulmonary site
limitations. However, one of the few studies of prophylaxis failure reported the vast
majority of probable failures to be associated with dapsone.
Suggested Reading
Biology of Pneumocystis
1. Wakefi eld AE. Pneumocystis carinii. British Medical Bulletin 2002; 61:175-88.
2. Stringer JR, Beard CB, Miller RF et al. A new name (Pneumocystis jiroveci) for
pneumocystis from humans. Emerg Infect Dis 2002; 8:891-6.
3. Huang L, Morris A, Limper AH et al. ATS pneumocystis workshop participants.
An offi cial ATS workshop summary: recent advances and future directions in
pneumocystis pneumonia (PCP). Proc Am Th orac Soc 2006; 3:655-64.
Course of Disease and Prognosis
1. Miller RF, Allen E, Copas A et al. Improved survival for HIV infected severe
Pneumocystis jirovecii pneumonia independent of highly active antiretroviral.
Th orax 2006; 61:716-72.
2. Festic E, Gajic O, Limper AL et al. Acute respiratory failure due to pneumocystis
pneumonia in patients without human immunodefi ciency virus infection: outcome
and associated features. Chest 2005; 128:573-9.
3. Fujii T, Nakamura T, Iwamoto A. Pneumocystis pneumonia in patients with HIV
infection: clinical manifestations, laboratory fi ndings and radiological features. J
Infect Chemother 2007; 13:1-7.
4. Walzer, PD, Evans HE, Copas AJ et al. Early predictors of mortality from
Pneumocystis jirovecii pneumonia in HIV-infected patients: 1985-2006. Clin
Infect Dis 2008; 46(4):625-33.
5. Tellez I, Barragán M, Franco-Paredes C et al. Pneumocystis jiroveci pneumonia
in patients with AIDS in the inner city: a persistent and deadly opportunistic
infection. Am J Med Sci 2008; 335(3):192-7.

236
Medical Parasitology
31
Adjuvant Steroids for Treating Severe PCP and Insight into Benefi cial Eff ect
1. Briel M, Boscacci R, Furrer H et al. Adjunctive corticosteroids for Pneumocystis
jiroveci pneumonia in patients with HIV infection: a meta-analysis of randomised
controlled trials. BMC Infect Dis 2005; 5:101-8.
2. Gigliotti F, Wright TW. Immunopathogenesis of Pneumocystis carinii pneumonia.
Expert Rev Mol Med 2005; 7:1-16.
Prophylaxis
1. Rodriguez M, Fishman JA. Prevention of infection due to Pneumocystis spp. in
human immunodefi ciency virus-negative immunocompromised patients. Clin
Microbiol Rev 2004; 17:770-82.
2. Podlekareva D, Mocroft A, Dragsted UB et al. EuroSIDA study group. Factors
associated with the development of opportunistic infections in HIV-1-infected adults
with high CD4
+
cell counts: a EuroSIDA study. J Infect Dis 2006; 194:633-41.
3. Green H, Hay P, Dunn DT et al. STOPIT Investigators. A prospective multicentre
study of discontinuing prophylaxis for opportunistic infections aft er eff ective
antiretroviral therapy. HIV Med 2004; 5:278-83.
Current Recommendations for Treatment and Prophylaxis
1. Morbidity and Mortality Weekly Report (MMWR) http://www.cdc.gov/mmwr/
2. Th e Medical Letter on Drugs and Th erapeutics http://www.medicalletter.org/
(fee-based access).

C
HAPTER
32
Malaria
Moriya Tsuji and Kevin C. Kain
Introduction
Malaria parasites are members of the Apicomplexa, characterized by the presence
of a unique organelle called an apical complex. Th ere are four malaria species that
infect and cause disease in humans, Plasmodium falciparum, P. vivax, P. malariae and
P. ovale, although cases of human infection with Plasmodium knowlesi, a monkey ma-
laria, have very recently been reported in Malaysia. Each Plasmodium sp. is associated
with a specifi c cyclic fever, caused by the synchronous release of parasites from the
erythrocytes in which they had been developing and multiplying. Th e diff erent spe-
cies may be distinguished by their morphological characteristics on Giemsa-stained
thin blood smears. Th ey are also distinct in their clinical course, including incubation
period, pathophysiology and associated morbidity and mortality.
Life Cycle
Malaria infection may be acquired congenitally from mother to baby across the
placenta, from platelet or blood transfusions and from the use of shared needles;
however it is most frequently initiated with the bite of an infected, female Anopheles
mosquito, which injects the sporozoite stage of the parasite with its bite. A typi-
cal life cycle of Plasmodium is shown in Figure 32.1. Usually 20-30 sporozoites
are transmitted to the host by a single mosquito bite and some of the sporozoites
rapidly reach the liver of the host via the blood circulation and thereby invade
hepatocytes. Once inside the hepatocyte, the parasite undergoes asexual division
and develops into liver schizonts within the infected hepatocytes over a period of
approximately 1-2 weeks, depending on the species of Plasmodium. Because the
initial stage of development within the liver occurs outside the bloodstream, this
hepatic stage is generally referred to as the exo-erythrocytic stage. At the end of
the hepatic stage of development, a single sporozoite can develop into a schizont
that contains thousands of daughter parasites that fi ll the hepatocyte. Infected
hepatocytes burst and release numerous merozoites into the bloodstream. P.
falciparum can complete this liver stage within 7 days and each of its sporozoites
produces about 40,000 daughter parasites. For P. vivax, these values are 6-8 days
and 10,000 merozoites; for P. malariae, 12-16 days and 2000 merozoites; and for
P. ovale, 9 days and 15,000 merozoites.
Th e next stage of development, called the erythrocytic or blood stage, is initiated
when exo-erythrocytic merozoites from the liver invade red blood cells (RBCs).
Merozoites of P. falciparum can infect RBCs of all ages, whereas those of P. vivax
and P. ovale infect reticulocytes and those of P. malariae invade only older RBCs.
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
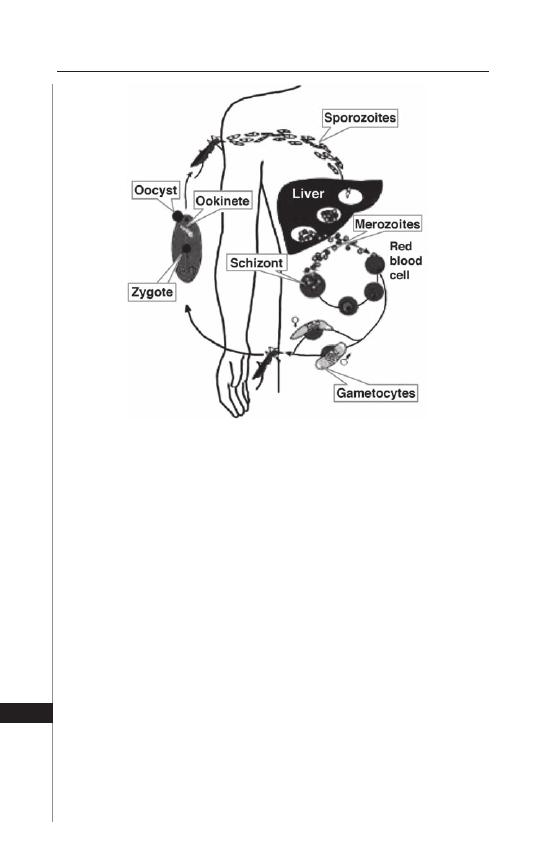
238
Medical Parasitology
32
Shortly aft er merozoites are released from hepatocytes, they invade RBCs and
over a period of 2 or 3 days, develop asexually. Th e stages of asexual development
include the ring (early trophozoite), trophozoite and schizont stages. Th e diagnosis
of malaria can be made upon the identifi cation of the parasites within erythrocytes
on Giemsa-stained blood smears (see Table 32.1). Th e distinct appearance of
these stages of development on Giemsa-stained thin blood smears, allows one to
determine the specifi c species infecting the host.
Th e ring stage derives its name from its signet ring-like appearance, with
a blue-stained nucleus and a pink-stained ring of cytoplasm. Th e trophozoite
is the feeding stage of the parasite and contains a single nucleus with pigment
granules, called hemozoin (a product of hemoglobin digestion), located within
the cytoplasm of the parasite. Th e schizont stage is initiated by the division of the
trophozoite nucleus. Further nuclear division leads to enlargement of the parasite.
Each individual nucleus then becomes surrounded by parasite cytoplasm to form
a merozoite.
At maturation, the schizont bursts and releases merozoites into the blood
circulation. Most of the released merozoites re-invade a new erythrocyte, thereby
repeating their asexual life cycle (blood stage cycle). In some instances, however,
invasion of an erythrocyte by a merozoite initiates sexual development instead of
asexual development. Th us, merozoites may develop into male gametocytes (mi-
crogametocytes) or female gametocytes (macrogametocytes). Th ese gametocytes
Figure 32.1. Life cycle of malaria parasite. Modifi ed from an original, kindly
provided by Drs. Chris Janse and Andy Waters at the Leiden University
Medical Centre, The Netherlands.

239
Malaria
32
Table 32.1. Differential features of infected RBCs among various plasmodial species
P. falciparum
P. vivax
P. malariae
P. ovale
Characteristics of infected
red blood cell (RBC)
RBC not enlarged
Schüffner’s dots in cytoplasm
of RBC; RBC enlarged
RBC not enlarged
Schüffner’s dots in
cytoplasm of RBC;
Compact trophozoite;
RBC enlarged
Ring forms
Smaller rings; Multiple rings
per cell; Double nuclei;
Appliqué forms (Fig. 32.2)
Large rings
Medium sized rings
Large rings
Trophozoites
Seldom seen in peripheral
blood
Parasite “active”; amoeboid
shape with pseudopodia
“Band” forms
Compact
Mature schizonts
Seldom seen in peripheral
blood
Schüffner’s dots in cytoplasm
of RBC; RBC enlarged
“Rosette” forms
Schüffner’s dots; fewer
merozoites per cell;
RBC elongated
Gametocytes
“Crescent” shaped
Round; within enlarged RBC
Round; within
non-enlarged RBC
Round; within enlarged
RBC

240
Medical Parasitology
32
can develop further only when they are taken up by an appropriate species of
Anopheles mosquito during a blood meal. Th ey subsequently mate within the gut
of the mosquito, the defi nitive host. Th e parasites eventually become sporozoites,
which reach the salivary gland of the mosquito. With the next bite, the infected
mosquito releases sporozoites into the host, thereby completing the life cycle.
Pathology
Malaria is often classified as uncomplicated or complicated/severe.
Uncomplicated malaria can be caused by all four species and is characterized by
periodic fever and chills, mild anemia and splenomegaly. Uncomplicated malaria
is rarely fatal unless it is left untreated and it progresses to severe disease. Severe
or complicated malaria is almost exclusively caused by P. falciparum infections
(although occasionally by P. vivax and other species) and is associated with higher
parasite burdens and vital organ dysfunction including CNS (coma, seizures etc.)
and pulmonary compromise (pulmonary edema, ARDS, respiratory distress etc.),
acute renal failure, severe anemia and metabolic acidosis. Anemia arises in part
from the destruction of erythrocytes when merozoites burst out of the infected
RBC and RBC production is further compromised by bone marrow suppression or
dyserythropoeisis. In falciparum malaria, anemia can be dramatic and life threaten-
ing. Th e rise in temperature is also correlated with the rupture of schizonts with
release of pyrogens together with merozoites from the bursting infected RBCs. Th e
pathogenesis of general malaise, myalgia and headache is ill-defi ned. Th e classic
periodicity of the fever (P. vivax/ovale = every 2nd day; P. malariae = every 3rd
day), based on synchronous infections, is oft en not observed particularly early in
the course of infection. In the early phase of infection, the growth of the parasites
is not synchronous, RBC rupture is more random and consequently fever can be
erratic. In addition, some infections may be due to two or more broods of parasites,
with the periodicity of one being independent of that of the others. Th is is more
oft en seen in the case of severe falciparum malaria.
Most malaria deaths are associated with P. falciparum infections. RBCs infected
with the maturing forms of this parasite express parasite proteins called PfEMP-1
associated with morphological structures (“knobs”) that permit them to stick to
endothelial cells lining the blood vessels and result in sequestration of these infected
RBCs within the vascular bed of vital organs. When this occurs in the brain, the
resulting cerebral malaria may lead to coma and death. Renal, pulmonary and GI
complications may also be seen. Congenital malaria and infection of the placenta
may result in stillbirth, low birth weight infants, or perinatal mortality.
Aft er the initiation of blood stage infection by the parasite, the repeated in-
fection of erythrocytes by merozoites results in exponential growth. As a result,
the parasitized RBCs accumulate in the capillaries and sinusoids of blood vessels,
causing general congestion in the peripheral blood circulation. Th e congestion
causes organomegaly, notably splenomegaly and possibly hepatomegaly and
contributes to anemia, leukopenia and thrombocytopenia. In vivax malaria, these
processes occur rather acutely and the aff ected organs, particularly the spleen,
become susceptible to rupture following trauma. In severe falciparum malaria, the
kidneys may show punctate hemorrhages and tubular necrosis. Severe hemolysis
and damage in the renal tubules results in hemoglobinuria or in its most severe

241
Malaria
32
form “blackwater fever”. In fact, the latter condition has been oft en associated with
massive intravascular hemolysis in the context of prior treatment with quinine
or treatment with primaquine in those with glucose-6 phosphate dehydrogenase
defi ciency (G6PD). Chronic P. malariae infection can be associated with nephrotic
syndrome, a condition in which the kidney shows histological hypertrophy, caused
by the deposition of immune complexes.
Epidemiology
Malaria is the most important parasitic disease in the world accounting for
over 500 million clinical infections and 1 million deaths every year. Ecologic
change, economic and political instability, combined with escalating malaria drug
resistance, has led to a worldwide resurgence of this parasitic disease. However,
malaria is not just a problem in the developing world. Th e combination of
burgeoning international travel and increasing drug resistance has resulted in
a growing number of travelers at risk of contracting malaria. It is estimated
that as many as 30,000 travelers from industrialized countries contract malaria
each year. However, this incidence is likely to be an underestimate because of
the prevalence of underreporting. Th e majority of P. falciparum cases imported
into North America and Europe are acquired in Africa (85%) and travel to the
African continent is currently on the rise.
P. ovale infection can be distinguished from P. vivax infection in part by its
epidemiology, i.e., the distribution of P. ovale is limited to tropical Africa and
to discrete areas of the Western Pacifi c. Most West Africans are negative for the
Duff y blood-type, which is shown to be associated with receptor sites for P. vivax
merozoites on the RBC. Th erefore, many West Africans are not susceptible to
infection with P. vivax. Falciparum malaria is generally confi ned to tropical and
subtropical regions, particularly in sub-Saharan Africa, the Amazon region of
South America, rural forested areas of Southeast Asia and urban and rural areas of
the Indian subcontinent. Individuals with sickle-cell trait (AS) are more resistant
to severe falciparum malaria than normal homozygotes (AA). Th e SS individuals
are also protected, but their sickle-cell disease leads to an early death. P. malariae
has a wide, but spotty distribution throughout the world.
Clinical Manifestations
Aft er being bitten by a malaria-infected Anopheles mosquito, the fi rst symptoms
appear aft er an incubation period ranging from 7 to 30 days. A shorter incubation
period is most frequently observed with P. falciparum, whereas the incubation
period for P. malariae can be quite lengthy. Typical symptoms include fever, chills,
sweats, rigors, headache, nausea and vomiting, body aches and general malaise.
Th ese symptoms may be seen in all types of malaria and the malaria paroxysm is
typically accompanied by sudden shaking chills. Th is may last 10 to 15 minutes or
longer. During this stage, the patient complains of feeling extremely cold, despite
a steady elevation of body temperature. Chills may be followed by severe frontal
headache and myalgia (muscular pain) in the limbs and back. Th is stage lasts 2-6
hours in P. vivax and P. ovale infections, 6 hours or more in P. malariae infection
and considerably longer in falciparum malaria. Finally, the patient starts to sweat
profusely for several hours and usually begins to feel better until the onset of the

242
Medical Parasitology
32
next paroxysm. Fever occurs on alternate days with P. vivax and P. ovale and every
3 days with P. malariae. With falciparum malaria, fever may be asynchronous,
recurring every 36 to 48 hours. P. falciparum is a deadly parasite, causing death
as quickly as 36 hours from the onset of symptoms in non-immune individuals.
P. vivax is a relatively benign parasite that elicits alternate day fever without caus-
ing mortality. P. ovale also produces alternate day fever and is clinically similar to
vivax malaria.
Relapsing and recrudescent disease must also be diff erentiated. Relapsing
disease implies the reappearance of parasitemia in sporozoite-induced infec-
tion, following adequate antiblood stage therapy. In the case of P. vivax and P.
ovale, the development of exo-erythocytic forms allows the parasite to remain
dormant within the hepatocyte. Th ese dormant parasites are called hypnozoites.
Accordingly, despite eradication of parasites from the peripheral circulation with
conventional antimalarial drugs, a fresh wave of exo-erythrocytic merozoites can
emerge from the hepatocytes and reinitiate the infection. Th e hypnozoites can
remain quiescent in the liver for more than fi ve years. In order to achieve radical
cure, therefore, it is necessary to destroy not only the blood circulating parasites,
but also the hypnozoites. P. falciparum and P. malariae do not develop hypnozoites
and do not cause relapsing disease. Recrudescence is the recurrence of symptoms
of malaria aft er a subclinical or asymptomatic level of parasitemia for a certain
period of time. Th is recrudescence likely occurs in cases where the blood stages
of malaria are maintained at very low levels aft er inadequate drug treatment. Such
parasites may become drug resistant. All malaria species can cause recrudescence.
In the case of P. falciparum, the parasites can recrudesce aft er one or two days,
whereas P. malariae can do so for up to 30 years.
Diagnosis
For over a century, malaria diagnosis has relied on the microscopic detection of
Plasmodium sp. on Giemsa-stained blood smears as no other reliable and relatively
rapid method for the detection of infection and quantifi cation of parasite burden
has been available. A detailed study of malaria parasites can be accomplished with
Giemsa stained thin blood smears (Fig. 32.2), whereas thick smears are more sensi-
tive, because greater volume of blood can be examined. As shown in Table 32.1,
there are a number of diff erential features of infected RBCs among the various
species. It should be noted that a diagnosis oft en cannot be made on the basis of a
single slide. In practice, several slides must be read systematically before a negative
report may be given. It is noteworthy that P. ovale, the least common of the malaria
species, resembles P. vivax in morphology and in biology.
Although microscopic detection of parasites has been the reference standard,
its reliability highly depends on the technical expertise of the microscopist. Th e
ability to maintain the required level of expertise in malaria diagnostics may
be problematic especially in peripheral medical centers in countries where the
disease is not endemic. Consequently recent eff orts have focused on developing
sensitive and specifi c nonmicroscopic malaria-diagnostic devices including those
based on PCR or the detection of malaria antigen in whole blood. Many fi rst
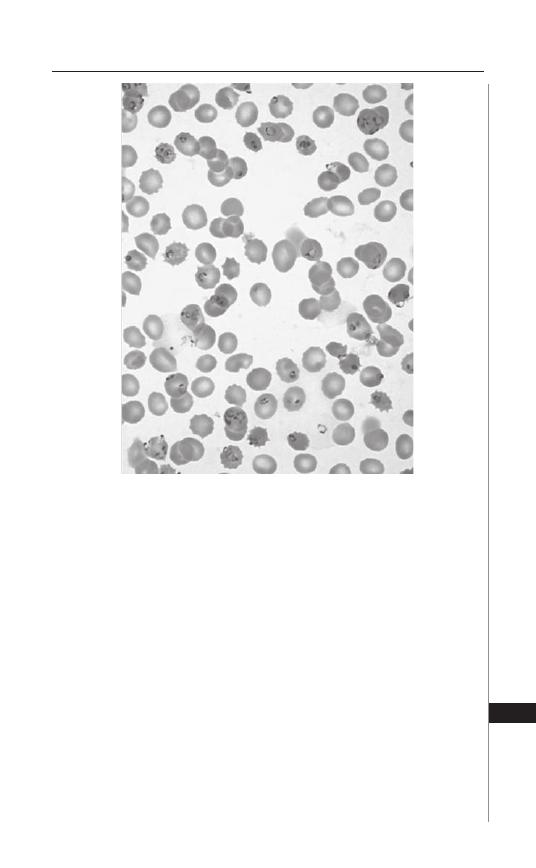
243
Malaria
32
generation rapid diagnostic products relied on the detection of the histidine-rich
protein II (HRP II) antigen of P. falciparum and therefore could not detect other
Plasmodium species. Th e next generation of rapid diagnostic devices based on
antigen capture with immunochromatographic (ICT) strip technology, utilize
monoclonal antibodies to HRP II for the detection of P. falciparum as well as
aldolase, a pan- Plasmodium antigen, thus facilitating identifi cation of nonfalci-
parum infections. One such assay has now been licensed and is available for sale
in the United States. Within the last decade, PCR-based diagnostic methods
for malaria have been developed and surpass microscopic methods with respect
to sensitivity and specifi city. Currently reported amplifi cation-based methods
for malaria diagnosis, particularly nested PCR-based methods, are sensitive
and specifi c but are also labour intensive with turnaround times that are gener-
ally too long for routine clinical application. Moreover these are open systems
that require considerable pre- and post-sample handling and therefore special
eff orts need to be employed in order to prevent false positive assays. Real-time
quantitative PCR technology has recently been developed and overcomes these
limitations, off ering a simple, time-eff ective and quantitative diagnostic option,
if an appropriate laboratory setting is readily available.
Figure 32.2. Thin blood smear showing P. falciparum-infected erythrocytes.

244
Medical Parasitology
32
Table 32.2. Summary of CDC guidelines for treatment of malaria in the
United States
Clinical Diagnosis/
Plasmodial Species
Region Infection
Acquired
Recommended Drug
Uncomplicated
malaria/P. falciparum or
Species unidentifi ed (If
subsequently diagnosed
as P. vivax or P. ovale,
then treatment with
primaquine is required
provide G6PD levels are
normal)
Chloroquine-sensitive
(Central America west of
Panama Canal; Haiti; the
Dominican Republic; and
most of the Middle East)
Chloroquine phosphate
Chloroquine-resistant
or unknown resistance
(All malarious regions
except those specifi ed
as chloroquine-sensitive
listed above)
A. Quinine sulfate plus
Doxycycline, Tetracycline
or Clindamycin
B. Atovaquone-proguanil
C. Mefl oquine
Uncomplicated
malaria/P. malariae
All regions
Chloroquine phosphate
Uncomplicated
malaria/P. vivax or
P. ovale
All regions
Chloroquine phosphate
followed by Primaquine
phosphate
Uncomplicated
malaria/P. vivax
Chloroquine-resistant
(Papua New Guinea
and Indonesia)
A. Quinine sulfate
plus Doxycycline or
Tetracycline, followed by
Primaquine phosphate
B. Mefl oquine followed by
Primaquine phosphate
Uncomplicated malaria:
alternatives for pregnant
women
Chloroquine-sensitive
Chloroquine phosphate
Chloroquine-resistant
P. falciparum
(regions except
Chloroquine-sensitive
regions listed above)
Quinine sulfate plus
Clindamycin
Chloroquine-resistant
P. vivax (Papua New
Guinea and Indonesia)
Quinine sulfate
Severe falciparum
malaria
All regions
A. Parenteral quinidine or
quinine plus Doxycycline,
Tetracycline or Clindamycin
B. Artesunate* followed by
Atovaquone, Doxycycline
or Mefl oquine
*Investigational new drug available from CDC.

245
Malaria
32
Treatment
Table 32.2 summarizes the CDC guidelines for treatment of malaria in the
United States.
Key tips to stepwise approaches for malaria:
i. Fever in a returned traveler or immigrant from a malaria endemic area is con-
sidered malaria until proven otherwise.
ii. Malaria is a medical emergency and needs STAT diagnosis and treatment.
iii. Treat all falciparum malaria as drug-resistant unless you know without ques-
tion that the infection was acquired in an area of chloroquine sensitivity.
iv. Decide if the case is falciparum or not and what the parasitemia is (percentage
of RBCs infected with parasites on a blood smear).
v. Is it complicated malaria (i.e., any evidence of organ dysfunction etc.) or not.
vi. If complicated then you need to use parenteral quinine/quinidine including
a loading dose (parenteral drug should also be used in any case for any species
in which the patient can not tolerate oral therapy); alternatively, parenteral
Artesunate is available through the CDC as an investigational new drug.
vii. If falciparum and the parasitemia is high (>10%) consider exchange transfu-
sion and seek expert consultation.
Prevention and Control
Th ere are four principles—adapted from the WHO’s ABCD of malaria protec-
tion—of which all travelers to malarious areas should be informed:
A. Be Aware of the risk, the symptoms and understand that malaria is a serious
infection.
B. Avoid mosquito Bites.
C. Take Chemoprophylaxis when appropriate.
D. Seek immediate Diagnosis and treatment if they develop fever during or
aft er travel.
Protection against malaria can be summarized into the following four
principles:
Assessing Individual Risk
Estimating a traveler’s risk is based on a detailed travel itinerary and the specifi c
risk behaviors of the traveler. Th e risk of acquiring malaria varies according to the
geographic area visited (e.g., Southeast Asia versus Africa), the travel destination
within diff erent geographic areas (urban versus rural area), type of accommoda-
tions (well-screened or air conditioned versus camping), duration of stay (1 week
business travel versus 3-month overland trek), season of travel (low versus high
malaria transmission season) and elevation of destination (malaria transmission is
rare above 2000 meters). In addition to the location, travelers can infl uence their
own risk by how well they comply with preventive measures such as treated bed
nets and chemoprophylactic drugs and the effi cacy of these measures. Additional
information can be obtained from good sources of updated malaria information
and country-specifi c risk are available on line from the WHO and Centers for
Disease Control and Prevention (CDC).

246
Medical Parasitology
32
Preventing Mosquito Bites (Personal Protection Measures)
All travelers to malaria-endemic areas need to be instructed in how best to avoid
bites from Anopheles mosquitoes that transmit malaria. Any measure that reduces
exposure to the evening and night-time feeding female Anopheles mosquito will
reduce the risk of acquiring malaria. Insecticide-impregnated bed nets (permethrin
or similarly treated) are safe for children and pregnant women and are an eff ective
prevention strategy that is underused by travelers. Insect repellents that contain
DEET (N,N-diethyl-meta-toluamide) can also help you to avoid contracting ma-
laria. Insect repellents should be applied sparingly, only to exposed skin or clothing,
and from dusk to dawn—the time malaria mosquitoes bite the most. Th e use of
repellents should be minimized in pregnant and nursing women.
Use of Chemoprophylactic Drugs where Appropriate
Th e use of antimalarial drugs and their potential adverse eff ects must be weighed
against the risk of acquiring malaria (as described previously). Th e following ques-
tions should be addressed before prescribing any antimalarial:
a. Will the traveler be exposed to malaria?
b. Will the traveler be in a drug-resistant P. falciparum zone?
c. Will the traveler have prompt access to medical care (including blood smears
prepared with sterile equipment and then properly interpreted) if symptoms
of malaria were to occur?
d. Are there any contraindications to the use of a particular antimalarial drug?
If the traveler will be in chloroquine-sensitive areas, the drug of choice is
chloroquine phosphate at 5 mg base/kg/wk beginning 1-2 weeks before travel
until 4 weeks aft er leaving the malarious area. For the adult traveler heading to
chloroquine-resistant areas, the primary choice of the prophylaxis is atovaquone/
proquantil at 1 adult tab/d beginning 1-2 days before travel until 1 week aft er
leaving, or mefl oquine at 250 mg once/wk beginning 1-2 weeks before travel until
4 weeks aft er leaving. It is important to note that a number of travelers to low-risk
areas, such as urban areas and tourist resorts of Southeast Asia, continue to be
inappropriately prescribed antimalarial drugs that result in unnecessary adverse
events but off er little protection. Improved traveler adherence with antimalarial
drugs is more likely when travel medicine practitioners make a concerted eff ort
to identify and carefully counsel the high-risk traveler and avoid unnecessary
drugs in the low-risk individual.
Seeking Early Diagnosis and Treatment if Fever Develops
during or aft er Travel
Travelers should be informed that although personal protection measures and
antimalarials can markedly decrease the risk of contracting malaria, these interven-
tions do not guarantee complete protection. Symptoms resulting from malaria may
occur as early as 1 week aft er fi rst exposure and as late as several years aft er leaving
a malaria zone whether or not chemoprophylaxis has been used. Most travelers
who acquire falciparum malaria will develop symptoms within 2 months of expo-
sure. Falciparum malaria can be eff ectively treated early in its course, but delays in
therapy may result in a serious and even fatal outcome. Th e most important factors
that determine outcome are early diagnosis and appropriate therapy. Travelers and

247
Malaria
32
health care providers alike must consider and urgently rule out malaria in any febrile
illness that occurs during or aft er travel to a malaria-endemic area.
In addition to travelers from endemic areas, an important source of malaria
infection includes immigrants from endemic areas. Also, it is noteworthy that a
person who has never travelled to endemic areas could get malaria by the bites of
malaria-infected mosquitoes carried in airplanes from endemic areas. Th is is called
“Airport (Baggage) malaria.”
Both adequate mosquito control and an eff ective malaria vaccine remain
unavailable, though promising work is proceeding towards development of these
pivotal preventive strategies.
Suggested Reading
1. Centers for Disease Control and Prevention: Health information for international
travel, Washington DC, 2005-2006, Government Printing Offi ce.
2. Katz, Despommier, Gwadz. Parasitic Diseases, Second Edition. Springer-Verlag
1988.
3. Fiona E, Lovegrove, Kevin C. Kain. Malaria prevention. In: Jong E, ed. Th e Travel
and Tropical Medicine Manual. 4th Edition. Philadelphia: WB Saunders, 2007:
Chapter 5.
4. Neva FA, Brown HW. Basic Clinical Parasitology, 6th Edition. Norwalk: Appleton
& Lange, 1994.
5. World Health Organization: International travel and health 2006. Geneva
Switzerland, 2006.
6. Nappi AJ, Vass E, eds. Parasites of Medical Importance. Austin: Landes Bioscience,
2002:29.


S
ECTION
V
Arthropods

C
HAPTER
33
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Clinically Relevant Arthropods
Sam R. Telford III
Introduction
Arthropods are animals with segmented bodies covered by a chitin exoskel-
eton. Familiar arthropods include fl ies, fl eas, bees, spiders, crabs and shrimp.
Medically important arthropods are typically thought of as vectors of infectious
agents or as infestations causing direct injury. Arthropods may also actively
or passively defend themselves against crushing or swatting by biting, sting-
ing, piercing, or secreting noxious chemicals; or by means of urticarial hairs.
Arthropods may also be medically important due to a patient’s fear of insects,
delusional parasitosis, or allergy due to dust mites. Th e diversity of ways in which
arthropods may aff ect human health refl ects the great diversity of these animals.
More than 80% of all known animal species are arthropods. Given their presence
in all environments, pathology may also sometimes be erroneously attributed
to their presence.
Arthropods may transmit infectious agents including viruses, bacteria, pro-
tozoa and helminths. Such agents may require an arthropod for perpetuation
(biological transmission) or may simply contaminate an arthropod (mechanical
transmission). Leishmania parasites have a complex developmental cycle within
certain phlebotomine sandfl ies and could not move from animal to animal
without the sandfl y. Housefl ies may have salmonella contaminating their outer
surfaces or mouthparts, which may be transferred to animals by the act of landing
and crawling; but, salmonella does not need the housefl y because it is an enteric
commensal of many kinds of animals. Few agents can survive long enough to
be transmitted by mechanical means: HIV, for example, has never been epide-
miologically linked with mosquitoes or other arthropods even though it might
be taken up during the act of bloodfeeding, probably because the virus is labile
outside of host tissues and enough infected lymphocytes may never contaminate
the small surface area of mosquito mouthparts.
Th ere are fi ve major groups of vectors, the diptera (fl ies and mosquitoes);
hemiptera (kissing bugs); siphonaptera (fl eas); anoplura (lice); and acarines (ticks
and mites). In addition, several other groups of arthropods may be medically
important. Th e general life history strategies and clinical signifi cance for each
group are briefl y reviewed; specifi c vector-pathogen relationships are discussed
in detail in chapters focusing on the respective agents.

251
Diseases Transmitted by Arthropods
33
Hexapoda (Insects)
Diptera
Th e dipteran vectors are winged insects that include mosquitoes, sandfl ies,
blackfl ies, gnats, horse/deerfl ies and tsetse fl ies. Th ese range in size from minute
(ceratopogonid midges less than 2 mm in length) to large (horsefl ies more than
2 cm in length). Unlike other winged insects, dipterans have only one pair of
wings. Th ose that take bloodmeals as adult females may serve as vectors or pests.
Bloodmeals are used as nutrient to produce eggs; once those eggs are laid, another
bloodmeal may be taken and more eggs produced. Th us, unless a mosquito (as an
example of a dipteran) inherits infection (transovarial or vertical transmission),
the fi rst bloodmeal infects it and the second allows the agent to be transmitted;
under favorable environmental circumstances, a mosquito may survive for several
weeks and take more than two bloodmeals. Eggs, larvae and pupae would never
be associated with illness; the only medical relevance is that these stages are oft en
targeted when trying to control infestations.
Host-seeking cues for the bloodfeeding diptera include body heat, carbon
dioxide (exhaled by the host), mechanical vibrations and lactic acid or other skin
associated compounds. Repellants such as DEET (diethyltoluamide) work by
disrupting the airborne gradients of chemical cues that allow diptera to home in
to their hosts. Once a host has been identifi ed, feeding is initiated and completed
within minutes. Sandfl ies, blackfl ies and mosquitoes have a diverse salivary ar-
mamentarium of pharmacologically active substances that promote fi nding and
removing blood. People may develop immediate and delayed-type hypersensitivity
to dipteran bites, manifesting as erythema or welts accompanied by itch. Itch may
be severe enough to cause scratching and secondary infection. Th e transient nature
of infestation by bloodfeeding diptera means that few specimens are submitted
to clinical laboratories. Other than analyses related to confi rming the diagnosis
of a vector borne infection, dipterans would come to the attention of physicians
mainly for issues related to hypersensitivity, or for myiasis.
The muscoid diptera include the muscids (houseflies, stable flies); the
Calliphoridae (blowfl ies); and the Sarcophagidae (the fl esh fl ies). Egg deposi-
tion and larval development occurs in characteristic materials, viz., fecal mate-
rial for housefl ies, decaying plant material for stable fl ies, live fl esh for blowfl ies
and carrion for the fl esh fl ies. Housefl ies have received much attention for their
potential health burden because of their association with poor hygiene. A large
fl eshy structure at the apex of the proboscis provides a surface for contamination,
as does the hairy body and legs of the fl y. In addition, fl ies may regurgitate while
feeding and the vomitus may contain organisms that were acquired in a previous
landing. Housefl ies commonly feed on human excrement and virtually every
possible enteric pathogen (those causing amebiasis, cholera, typhoid, hepatitis
A, poliomyelitis; even roundworms and Helicobacter pylori) has been detected
within or upon them. With few exceptions, such fi ndings are epidemiologically
irrelevant inasmuch as all of the agents perpetuate in their absence. It is likely that
individual cases of enteric disease may derive from fl y contamination of food, but
whether the risk of such an event merits worry by patients or their health care
providers remains unclear.

252
Medical Parasitology
33
Myiasis is the infestation of human or animal tissue by fl y larvae, deposited as
eggs or fi rst stage larvae; the larvae develop by feeding on the surrounding tissue,
emerge as third stage larvae and pupate in the environment. A variety of clinical
presentations are evident depending on the site where the larvae are present. Botfl ies
cause furuncular lesions or migratory integumomyiasis (a serpiginous track may
be produced in the skin). Wound myiasis comprises shallow or pocketlike initial
lesions that become more deeply invasive. Maggots may invade the nose and ac-
companying structures, causing nasal or oral myiasis. Maggots may get into the ears,
producing aural myiasis. Ophthalmomyiasis is due to external or internal infesta-
tion. Enteric, vaginal, or vesicomyiasis is due to invasion of the gut or genitalia. In
all presentations, pathology may be due to tissue trauma or local destruction, but
is more oft en associated with secondary bacterial infection. On the other hand,
many maggots do not promote bacterial infection, but rather secrete bacteriolytic
compounds and have been used as a surgical intervention to debride wounds. Th us,
the development of secondary bacterial infection in myiasis may suggest the death
of the maggot. In all cases, treatment is by removal of the maggots, laboriously pick-
ing them out using forceps. Th e maggots causing furuncular myiasis or migratory
integumomyiasis will have their posterior end visible within the lesion, exposing the
spiracular plates that cover their tracheolar breathing apparatus. Although much
lore exists on how to best remove such maggots, including “luring” the maggots
with bacon or pork fat, the simplest method is to cover the lesion with vaseline,
thereby preventing the maggot from breathing. It will eventually move out enough
in an attempt to get air so that it may be grasped with forceps.
Hemiptera
Th e only hemiptera that serve as vectors are the kissing or reduviid bugs, minute
to large insects with compound eyes, antennae, sucking mouthparts, two pairs of
wings (one delicate pair hidden under an outer pair of more robust ones) and a
segmented abdomen. All are easily seen without magnifi cation, adult bedbugs being
slightly less than 1 cm in length and adult triatomines ranging in size from 1 cm
to more than 5 cm. Reduviid bugs are cryptic, living within cracks of mud walls or
other narrow, confi ned spaces. Th ey serve as vectors of trypanosomes (Trypanosoma
cruzi, the agent of Chagas disease) in Latin America. Transmission of T. cruzi to
humans requires contamination of the site of the bite or mucosa by trypanosomes
that are excreted in the bug feces. As with mosquitoes, salivary products from
reduviids contain a variety of pharmacologically active compounds. Unlike those
of mosquitoes, repeated reduviid feedings may cause a dangerous anaphylactoid
reaction in residents of houses where the bugs are common. Th e resulting itching
and irritation promote the entry of bug feces directly into the bite site or indirectly
by contamination of mucosa. Although it is possible that a true reduviid bug may
be presented by a patient for identifi cation in clinical settings outside of Latin
America, it is more likely that such specimens are related heteropterans such as
assassin bugs, which are insect predators, or the plant-feeding stink bugs, chinch
bugs, harlequin bugs, or squash bugs.
Bedbugs (Cimex lectularius, C. hemipterus) are hemipterans, but are not known
to serve as vectors for any pathogen. Th ese bugs with short broad heads, oval bodies
and 4-jointed antennae undergo incomplete metamorphosis, with four nymphal

253
Diseases Transmitted by Arthropods
33
stages, each taking a bloodmeal in order to develop. Th ey are cryptic and require
hiding places such as cracks in walls, mattress foundations, or rattan furniture. At
night, bedbugs will emerge and infest sleeping people, taking 10-20 minutes for
engorgement. Feeding is oft en interrupted by the movements of people during
their sleep and so multiple bites may result from a single bedbug. Erythematous,
itching bites, oft en several in number, are typically observed as a result; treatment
is symptomatic. Bedbug infestations are currently increasing in prevalence, in all
socioeconomic levels. Elimination of these pests can be diffi cult, particularly in
multi-family dwellings. Mattresses and box springs must be decontaminated or
destroyed; baseboards of walls must be sprayed.
Blattaria
Roaches are dorsoventrally fl attened, smooth-bodied, winged insects that
resemble the hemiptera but are closely related to termites and mole crickets, with
long antennae, biting-type mouthparts and abdominal projections. Th e outer pair
of wings is thick and leathery and the inner pair membranous. Roaches may fl y, but
usually scuttle about on long spiny legs. Roaches can live for months without food,
but water seems critical. Th ey are omnivorous, feeding on the fi nest of foods to the
vilest of waste, usually at night. Secretions deposited by scent glands (including trail
and aggregation pheromones) give rise to a characteristic disagreeable odor that
confi rms an infestation even when live roaches cannot be found. Common roaches
range in size from the small German cockroach (Blatella germanica) about half an
inch in length, to the American cockroach, nearly two inches. As with fl ies, the
presence of roaches suggests poor environmental hygiene, but only rare instances
of enteric disease might be associated with them. Dense infestations may produce
large amounts of antigenic material (feces, molted cuticle, or parts from carcasses),
which may be implicated in allergic or asthmatic reactions. Control of roach in-
festations can be diffi cult. Boric acid, deposited along walls, behind moldings and
around other sites where they may hide, is eff ective in killing adults and nymphs
by abrading cuticle between abdominal segments, rendering the roach prone to
dessication. Removing standing water (wiping up and getting rid of clutter around
sinks) can also reduce infestations by preventing access to water.
Siphonaptera
Th e fl eas are bilaterally compressed, heavily chitinized insects with greatly modi-
fi ed hind legs for their characteristic jumping mode of locomotion; they lack wings.
Fleas are generally small, no larger than 5 mm in length. Th e female fl ea requires
blood for egg production. Most fl eas are what are known as “nest parasites”, par-
ticularly of rodents. Individual fl eas may live as long as a year but usually only for a
couple of months, laying eggs daily. Only a few species are of medical importance.
Th ese include the “human” fl ea (Pulex irritans); the dog and cat fl eas (Ctenocephalides
canis and C. felis); the main plague vector (Xenopsylla cheopis) and the sticktight fl ea
(Echidnophaga gallinacea). Of these, C. felis are the most notorious pests, feeding
voraciously and rapidly developing dense infestations. Chronic infestation of homes
are largely due to the presence of a cat or dog (despite its name, this fl ea will feed on
either animal), their bedding, wall-to-wall carpeting and relatively great humidity
within the home. Cold climates rarely have self sustaining infestations because winter

254
Medical Parasitology
33
heating tends to dry out the carpets and molding and other places where the larval
fl eas tend to be hidden. Although bites sustained over several weeks will usually
induce a typical delayed type hypersensitivity reaction, with an intensely itching
red spot developing at the site of the bite (usually around the ankles), note that not
all members of a household will react in the same manner.
An unusual fl ea, the chigoe or jigger (Tunga penetrans) will attach to a host and
maintain a feeding site. Originally found in Latin America, the chigoe has been
carried across to sub-Saharan Africa by humans and may be found anywhere there.
Th is fl ea will oft en penetrate under toenails, or burrow into skin between toes, or
in the soles of feet, an infestation known as tungiasis. When the fl ea dies, the lesion
becomes secondarily infected, causing great irritation and pain. Tourists will oft en
become infested by walking barefoot in shady spots around beaches.
Phthiraptera
Infestation by lice is called pediculosis. Th e lice are wingless, fl at (dorsoven-
trally), elongate, small (0.4-10 mm) insects that are generally characterized by strong
host specifi city. Lice undergo incomplete (hemimetabolous) development, with
nymphal forms resembling adult lice and are oft en found concurrently with the
adults. Th us, size may appear to greatly vary within a single collection of specimens
from one host. All lice are delicate and very sensitive to temperature and humid-
ity requirements; all die within days without the host. Th e lice of greatest clinical
importance are the head louse (Pediculus humanus capitis); body louse (Pediculus
humanus corporis); and the pubic louse (Phthiris pubis). All of these sucking lice
have prominent claws attached to each of their legs, morphologically adapted for
grasping the hairs of their host. Th ey feed at least daily and deposit 1-10 eggs from
each bloodmeal, gluing one egg at a time onto the shaft s of hairs (or in the case of the
body louse, onto threads within clothing). Th e eggs, or nits, are almost cylindrical
in shape and have an anterior operculum. Lice are transferred between hosts by
close physical contact, or by sharing clothing in the case of body lice.
Body or pubic louse infestation may result in intense irritation for several days,
with each bite generating a red papule. Chronically infested individuals may become
desensitized, or may develop a nonspecifi c febrile illness with lymphadenopathy,
edema and arthropathy (although such signs and symptoms should prompt a search
for the agent of trench fever). Although body louse or pubic louse infestation may
be considered evidence of poor hygiene or poor judgement, infestation by head lice
should not be a stigma, occurring in the best of families. Nor should head louse
infestation be considered to be a public health menace, or even a clinical problem.
Very few infestations are dense enough to cause signs or symptoms and head lice are
not vectors. Head or pubic louse infestations may be easily treated by shaving the
head or pubes; body lice, by changing clothing. Several permethrin-based shampoos
have great effi cacy, although resistance has been reported.
Hymenoptera
Wasps, bees and ants all belong to the order Hymenoptera, which are minute to
medium-sized insects with compound eyes, mandibles and two pairs of transparent
wings (although in the ants, wings may be seen on males or females only at certain
times of the year). Th ese insects have a complex social behavior, with males, females

255
Diseases Transmitted by Arthropods
33
and worker castes. Workers have a stinging organ, which is used for defending the
colony or capturing prey. Most stings by hymenoptera cause localized reactions,
sometimes with extreme pain and resulting in a transient induration with hyperemia.
By virtue of their living in large colonies, bees and ants may swarm an intruder and
dozens if not hundreds of stings may be sustained. Airway obstruction may result
should multiple stings be received on the face or neck. Bees diff er from wasps and ants
in that their stinging apparatus is forcibly torn out during the act of stinging, thereby
ensuring the death of individual bee as a result. Wasps and ants may sting multiple
times; some fi re ants may hang on by their mandibles and repeatedly insert their pos-
teriorly located stinger. Bee venom has been well characterized and consists of a large
amount of a polypeptide (melittin), phospholipase A2, histamine, hyaluronidase,
mast cell discharging peptide and apamin. Histamine appears to be the cause of the
acute pain of bee stings. Th e most important clinical manifestation of bee or wasp
stings is anaphylaxis. Chest tightness, nausea, vertigo, cyanosis and urticaria may be
seen even in individuals who apparently had never previously been exposed. Dozens
of people die each year in the US due to bee sting anaphylaxis. Ants, on the other
hand, rarely pose a risk for anaphylaxis but produce a reaction that may persist for a
longer duration. An induration or wheal may be observed immediately aft er the sting
and a papule may develop that itches or remains irritated for several days.
Lepidoptera
Caterpillars with urticating hairs are associated with rashes caused by contact
with hairs (erucism, or erucic rash). A common shade tree pest, the brown tailed
moth (Nygmia phaeorrhoea), in Europe and northeastern North America, liberates
tiny barbed hairs when the caterpillar molts. Th ese hairs are blown about by the
wind and when skin is exposed, a severe dermatitis results; ingestion or inhalation
can also cause signifi cant irritation of the mucosa or bronchospasm. Contact with
the eye may induce conjunctivitis. Dermatitis produced by urticating hairs typically
comprises itchy, erythematous patches associated with small vesicles and edema.
Th ese lesions are only where the hairs have free access to the skin or where contact
is made. Use of a masking tape-type lint roller can be very eff ective in removing
urticating hairs, which may or may not be visible.
Beetles
Some beetles may induce vesication: direct contact with live or crushed beetles
may expose a person to cantharidin. Bombardier beetles will spray a boiling hot
jet of benzoquinone as a defense, causing burns and blistering. Blistering is also
produced by crushing the staphylinid beetle Paederus fusca of Southeast Asia,
which contain a toxic alkaloid, pederin. More commonly, carpet beetles (der-
mestids) which feed on wool rugs and other animal fur products are associated
with a papulovesicular eruption. In particular, larval dermestids and their hairs
or shed skins (exuviae) cause a contact dermatitis.
Arachnida
Th e acarines are a subclass within the Class Arachnida, which also contains the
spiders and scorpions. Acarina comprise the mites and ticks, tiny to small arthro-
pods with eight legs as nymphs and adults (as opposed to six for insects) and with
fused main body segments as opposed to three discrete ones for insects.

256
Medical Parasitology
33
Rickettsialpox and scrub typhus are currently the only known infections trans-
mitted by mites. Mites are, however, important for their pest potential, causing
itch, dermatitis and allergy. Th e most common of the ectoparasite-caused direct
injury is scabies, caused by infestation with the human scabies mite (Sarcoptes
scabiei). Infestation may occur anywhere in the world. Canine sarcoptic mange is
commonly associated with scabies infestations in the owners of the dogs. In either
animal or human scabies, transmission is by direct personal contact and infestations
oft en cluster among groups of people, particularly families. Th ere is little evidence
that environments become contaminated; fomites have not been identifi ed. Th e
female scabies mite burrows beneath the stratum corneum, leaving behind eggs and
highly antigenic feces within a track like trail. Nocturnal itching begins within a
month of the fi rst infestation, but may begin within a day in previously exposed
individuals. Erythematous papules and vesicles fi rst appear on the webs of fi ngers
and spread to the arms, trunk and buttocks. Interestingly, in individuals who are
immunocompromised, hundreds of female mites may be found, itching is minimal,
but a hyperkeratosis is prominent. Such “crusted” or Norwegian scabies are highly
infectious to other people.
Scabies infestations can be easily diagnosed by scraping a newly developed
papule (not one that has been scratched) with a scalpel coated with mineral oil.
Th e scrapings in oil may be transferred to a slide and examined at X100 or X400
brightfi eld for 300-400
μ
m mites or the smaller black fecal granules. Scabies may
be treated by 5% permethrin cream or 1% permethrin rinse (Nix, same as is used
for head lice). Th e method of choice until recently was topical lindane (Kwell),
but FDA now suggests the use of permethrin fi rst and lindane only if that fails.
Lindane can be neurotoxic to children and small adults.
Demodex folliculorum, the follicle mite, infests virtually everybody. Th is elongate
500
μ
m long mite may be found within follicles on the face, the ear and breast
and are oft en brought to the attention of a physician because a tweezed eyelash
may have a few mites at the base of the hair shaft . Although they appear to largely
be nonpathogenic, the same species causes demodectic mange in dogs and cattle.
Pityriasis folliculorum, with small pustules appearing on the forehead, has been
attributed to them.
Dust mite allergies (one of many causes of asthma, rhinitis and atopic dermati-
tis) are due to inhalation of feces excreted by Dermatophagoides farinae or related
pyroglyphid mites, which are human commensals that feed on fl akes of skin shed
from a person. Th e mites themselves do not infest a person, but remain in the en-
vironment (usually within bedding or carpets) to feed and develop. About a half
gram of skin may be shed from a person each day; one female mite lays one egg a
day, for about 2 months; thus, large accumulations of mites may readily develop.
Th e 300-400
μ
m long mites may be presented by patients as suspects for other
nonspecifi c lesions or sets of signs and symptoms because they may be found in
virtually all houses and can be detected if dust is allowed to settle on standing
water; the mites fl oat and will move and can be seen at X20. Humidity less than
60% will greatly reduce dust mite infestations, as will periodic vacuuming and
washing of bedding and carpets.
Ticks are prolifi c vectors, with more recognized transmitted agents than any
arthropod other than mosquitoes. Hard ticks are so named because of the hardened

257
Diseases Transmitted by Arthropods
33
dorsal shield or scutum. In female hard ticks, the scutum is on the anterior third of
the body with the remainder consisting of pleated, leathery cuticle that allows for
tremendous expansion during bloodfeeding. In male hard ticks, which may or may
not feed at all, the scutum extends the length of the body. In contrast, soft ticks
have no scutum; their entire body is leathery. Th e “head”, or capitulum, consists
of the holdfast (hypostome), chelicerae (which are homologs of insect mandibles)
and the palps, which cover the mouthparts (hypostome and chelicerae). Chelicerae
act as cutting organs, the two sides sliding past each other, with the cutting teeth
at the end gaining a purchase into a host’s skin. Th e hypostome is thereby inserted
and allows anchoring of the entire tick due to recurved, backward facing teeth or
denticles. Many hard ticks also secrete a cement around the hypostome. Oft en,
when removing an attached tick from a host, a large piece of skin comes with the
hypostome, mostly cement and surrounding epidermis. Th us, by virtue of the ce-
ment and denticles, tick hypostomes rarely emerge intact when a tick is removed.
“Leaving the head in” is not critical and most times the remnant will be walled off as
a foreign body, or will work itself out, perhaps by the act of scratching. Treatment,
therefore, should simply be disinfection of the site of the bite and certainly not
excavation of the epidermis looking for the “head”. Soft ticks are transient feeders
and will only rarely be found attached.
Hard ticks require several days to complete their bloodmeal; the number of
days depends on the species and stage of the tick. (In contrast, soft ticks are more
like mosquitoes in their feeding, spending tens of minutes to no more than a few
hours feeding, usually as their host is sleeping.) North American deer ticks (Ixodes
dammini) will feed 3 days as a larva, 4 days as a nymph and 7 days as the female.
During the fi rst 70% of the feeding process, very little blood or lymph appears to
be present within ticks, which remain dorsoventrally fl at. Hemoglobin is excreted
from the anus, lipids are retained and water from the blood is recycled back into
the host as saliva. In the last day, usually in the last 3 or 4 hours of the bloodmeal,
the tick takes what has been termed “the big sip”, removing a large volume of whole
blood, then detaching and dropping from the host. Because they must remain
attached for days, hard ticks have evolved means of temporarily disabling a host’s
local infl ammatory response, which might inhibit its feeding. Hard tick saliva is
an extremely complex mixture of anticoagulant, anti-infl ammatory and antihe-
mostatic agents that act mainly at the site of the feeding lesion. Hosts that have
never been exposed to ticks will not realize that a tick is attached. Indeed, most
cases of Lyme disease or spotted fever never knew that they had been “bitten”. Th e
most dangerous tick is not necessarily the one that a patient fi nds, removes and
aborts the transmission process, but the one that he or she never knew was there
and which was able to complete its feeding. Because most tick-borne pathogens
require at least 24 hours to attain infectivity, prompt removal of an attached tick
will usually abort infection.
An unusual toxicosis due to tick bite is tick paralysis. Th e presence of certain
feeding ticks (Australian Ixodes holocyclus, Western American Dermacentor ander-
soni) induces an acute ascending paralysis. Children are the usual victims of tick
paralysis, with ticks attached at the nape of the neck. Th e illness is characterized by
fatigue, irritability, distal paresthesias, leg weakness with reduced tendon refl exes,
ataxia and lethargy. Unless the tick is removed, quadriplegia and respiratory failure

258
Medical Parasitology
33
may result; the case fatality rate without treatment can be 10%. Removal of the
tick induces a miraculous recovery within 48 hours.
Scorpions
Scorpions are arachnids with a characteristic crablike appearance. Th e seg-
mented body ends in a segmented tail, which terminates in a prominent stinging
apparatus. Th e four pairs of legs includes well defi ned pincers on the fi rst pair of
legs. Scorpions may range in size from 2-10 cm. Scorpions are problems mainly
in the warmer climates. Th e bulbous end of the tail contains muscles that force
venom through the stinger. Humans are stung by walking barefoot at night; by
not shaking their shoes out in the morning in an endemic area; lift ing rocks or
logs; or in bedding that is on the fl oor. Th e stings cause local pain (probably due
to the great biogenic amine content of the venom), edema, discoloration and
hypesthesia. Systemic signs can include shock, salivation, confusion or anxiety,
nausea, tachycardia and tetany. Venom characteristics diff er depending on the ge-
nus of scorpion: some stimulate parasympathetic nerves and can lead to secondary
stimulation of catecholamines resulting in sympathetic stimulation, which in turn
may contribute to respiratory failure. Others aff ect the central nervous system, are
hemolytic, or cause local necrosis. Treatment is usually symptomatic.
Spiders
Even though all spiders have stout chelicerae and can bite and all spiders have
a venom with which to immobilize their prey, most are too small to be noticed by
humans even if they were to be bitten. Th e clinical manifestations and complica-
tions of envenomenation may diff er between the four main kinds of medically
important spiders and the syndromes caused by each have been given names that
refl ect the identity of the spider. With spider bites in general there is local pain
and erythema at the site of the bite and this may be accompanied by fever, chills,
nausea and joint pains. In loxoscelism (recluse spiders), the site of the bite will
ulcerate and become necrotic. Skin may slough and there may be destruction of
the adjacent tissues. Hemolysis, thrombocytopenia and renal failure may ensue.
Necrotic dermal lesions are oft en classifi ed as loxoscelism even if the appropriate
spiders are not known to be present in the area. In addition, severe reactions to
tick bite, or even the erythema migrans of Lyme disease may be confused with
recluse spider bites. With latrodectism (black widow spiders), phoneutrism and
funnel-web neurotoxicity, the venoms have a strong neurotoxic action. Muscle
rigidity and cramping (similar to acute abdomen) is seen with latrodectism;
complications include EKG abnormalities and hypotension. With phoneutrism,
visual disturbances, vertigo and prostration may occur; complications include
hypotension and respiratory paralysis. Funnel web spiders induce autonomic
nervous system excitation, with muscular twitching, salivation and lachrymation,
nausea and vomiting and diarrhea; fatal respiratory arrest may result from apnea
or laryngospasm. Funnel web spider bites require prompt fi rst aid and the same
recommendations could be used for latrodectism or phoneutrism. A compres-
sion bandage should be applied over the site of the bite and the aff ected limb
immobilized by splinting with a compression bandage if bitten on an extremity
(standard procedures for snakebite). Th is may help prevent the venom from

259
Diseases Transmitted by Arthropods
33
moving from the local lymphatics. Th e patient should seek medical attention as
soon as possible for antivenom treatment. Otherwise, treatment is symptomatic
with analgesics and antipyretics.
Myriapods
Th e centipedes and millipedes are elongate, vermiform arthropods with dozens
of segments, each of which bears a pair of legs. “Centipedes” would suggest having
100 segments (and pairs of legs) or fewer; “millipedes”, more than 100 and up to
1000. Th e diversity of both millipedes and centipedes is greatest in the tropics and
virtually all those of medical importance are found in warm climates. Millipedes
may squirt a noxious, corrosive fl uid from pores on their segments. Such fl uid may
contain benzoquinone, aldehydes and hydrocyanic acid and cause an immediate
burning sensation followed by erythema and edema, even progressing to blistering.
Most millipedes also have a repugnant smell; both the corrosive fl uid and smell
tend to protect them from predation. People become exposed when they step on or
sleep on millipedes, or provoke them (children playing with them are oft en victims).
Treatment consists of washing the aff ected site as soon as possible to dilute and
remove the corrosive fl uids and symptomatic for the skin lesions and pain.
Centipedes have powerful biting mandibles and small fang-like structures
situated between them and derived from the fi rst pair of legs that may inject a
venom. Centipede bites occur when people step on or sleep on them, or play with
them. Envenomation is manifested by local pain and swelling, with proximal
lymphadenopathy. Headache, nausea and anxiety are common. Skin lesions may
ulcerate and become necrotic.
Suggested Reading
1. Centers for Disease Control and Prevention. Pictorial keys to arthropods, reptiles,
birds and mammals of public health signifi cance. [online] http://www.cdc.gov/
nceh/ehs/Pictorial_Keys.htm
2. Eldridge BF, Edman JD. Medical Entomology: A Textbook on Public Health
and Veterinary Problems Caused by Arthropods. New York: Kluwer Academic
Publishers, 2004.
3. Harwood RF, James MT. Entomology in Human and Animal Health, 7th Edition.
New York: Macmillan, 1979.
4. Herms WB. Medical Entomology, 3rd Edition. New York: Macmillan, 1939.
5. Horsfall WR. Mosquitoes: Th eir Bionomics and Relation to Disease. London:
Constable, 1955.
6. Kettle DS. Medical and Veterinary Entomology. London: Croom Helm, 1984.
7. Marquardt WC, Black WC IV, Freier JE et al. Biology of Disease Vectors, 2nd
Edition. Burlington: Elsevier Academic Press, 2005.
8. Peters W. A Colour Atlas of Arthropods in Clinical Medicine. London: Wolfe
Publishing Ltd, 1992.
9. Smith KGV. Insects and Other Arthropods of Medical Importance. London:
British Museum of Natural History, 1973.
10. Sonenshine DE. Biology of Ticks, vol 1. New York: Oxford Press, 1991.
11. Sonenshine DE. Biology of Ticks, vol 2. New York: Oxford Press, 1994.


A
PPENDIX
Medical Parasitology, edited by Abhay R. Satoskar, Gary L. Simon, Peter J. Hotez
and Moriya Tsuji. ©2009 Landes Bioscience.
Infection/Drug
Adult Dosage
Pediatric Dosage
Acanthamoeba keratitis—see footnote 1
Amebiasis (Entamoeba histolytica)
Asymptomatic
Iodoquinol
D,2
650 mg PO tid x 20 d
30-40 mg/kg/d (max 2 g) PO in 3
doses x 20 d
Paromomycin
3
25-35 mg/kg/d PO
25-35 mg/kg/d PO in 3 doses x 7 d
in 3 doses x 7 d
Diloxanide furoate
4*
500 mg PO tid x 10 d
20 mg/kg/d PO in 3 doses x 10 d
Mild to moderate intestinal disease
Metronidazole
D,5
500-750 mg PO tid
35-50 mg/kg/d PO in 3 doses
x 7-10 d
x 7-10 d
Tinidazole
6
2 g once PO daily x 3 d ≥3 yrs: 50 mg/kg/d (max 2 g) PO
in 1 dose x 3 d
either followed by
Iodoquinol
2
650 mg PO tid x 20 d
30-40 mg/kg/d (max 2 g) PO in 3
doses x 20 d
Paromomycin
3
25-35 mg/kg/d PO in 3 25-35 mg/kg/ d PO in 3 doses x 7 d
doses x 7 d
Severe intestinal and extraintestinal disease
Metronidazole
D
750 mg PO tid x 7-10 d 35-50 mg/kg/d PO in 3 doses
x 7-10 d
Tinidazole
6
2 g once PO daily x 5 d ≥3 yrs: 50 mg/kg/d (max 2 g) PO
in 1 dose x 3 d
either followed by
Iodoquinol
2
650 mg PO tid x 20 d
30-40 mg/kg/d (max 2 g) PO in 3
doses x 20 d
Paromomycin
3
25-35 mg/kg/d PO
25-35 mg/kg/d PO in 3 doses x 7 d
in 3 doses x 7 d
Amebic meningoencephalitis—primary and granulomatous
Naegleria
Amphotericin B
D,7,8
1.5 mg/kg/d IV in 2
1.5 mg/kg/d IV in 2 doses x 3 d,
doses x 3 d, then
then 1 mg/kg/d x 6 d plus
1 mg/kg/d x 6 d plus 1.5 mg/d intrathecally x 2 d,
1.5 mg/d intrathecally then 1 mg/d every other
x 2 d, then 1 mg/d
day x 8 d
every other day x 8 d
Drugs for Parasitic Infections
Appendix information from: The Medical Letter, “Drugs for Parasitic Infections”, vol. 5,
Supplement 2007, last modifi ed August 2008; with permission.

262
Medical Parasitology
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
Acanthamoeba—see footnote 9
Balamuthis mandrillaris—see footnote 10
Sappinia diploidea—see footnote 11
Ancylostoma caninum (Eosinophilic enterocolitis)
Albendazole
D,7,12
400 mg PO once
400 mg PO once
Mebendazole
100 mg PO bid x 3 d
100 mg PO bid x 3 d
Pyrantel
11 mg/kg (max 1 g)
11 mg/kg (max 1 g) PO x 3 d
pamoate
7,13*
PO x 3 d
Endoscopic removal
Ancylostoma duodenale—see Hookworm
Angiostrongyliasis (Angiostongylus cantonensis, Angiostrongylus
costaricensis)
14
Anisakiasis (Anisakis spp.)—see footnote 15
Surgical or endoscopic removal
Ascariasis (Ascaris lumbricoides, roundworm)
Albendazole
D,5,7,12
400 mg PO once
400 mg PO once
Mebendazole
100 mg bid PO x 3 d
100 mg PO bid x 3 d or 500 mg
or 500 mg once
once
Ivermectin
7,16
150-200 mcg/kg PO once 150-200 mcg/kg PO once
Babesiosis (Babesia microti)
Clindamycin
D,7,17,18
1.2 g bid IV or 600 mg
20-40 mg/kg/d PO in 3 doses
tid PO x 7-10 d
x 7-10 d
plus quinine
7,19
650 mg PO tid x 7-10 d 30 mg/kg/d PO in 3 doses x 7-10 d
or Atovaquone
7,20
750 mg PO bid x 7-10 d 20 mg/kg/d PO in 2 doses x 7-10 d
plus azithromycin
7
600 mg PO daily x 7-10 d 12 mg/kg/d PO x 7-10 d
Balamuthia mandrillaris—see Amebic meningoencephalitis, primary
Balantidiasis (Balantidium coli)
Tetracycline
D,7,21
500 mg PO qid x 10 d
40 mg/kg/d (max 2 g) PO in
4 doses x 10 d
Metronidazole
A,7
750 mg PO tid x 5 d
35-50 mg/kg/d PO in 3 doses x 5 d
Iodoquinol
A,2,7
650 mg PO tid x 20 d
30-40 mg/kg/d (max 2 g) PO in
3 doses x 20 d
Baylisascariasis (Baylisascaris procyonis)—see footnote 22
Blastocystis hominis infection—see footnote 23
Capillariasis (Capillaria philippinensis)
Mebendazole
D,7
200 mg PO bid x 20 d
200 mg PO bid x 20 d
Albendazole
A,7,12
400 mg PO daily x 10 d 400 mg PO daily x 10 d
Chagas’ disease—see Trypanosomiasis
Clonorchis sinensis—see Fluke infection
Cryptosporidiosis (Cryptosporidium)
Non-HIV infected
Nitazoxanide
D,5
500 mg PO bid x 3 d
1-3 yrs: 100 mg PO bid x 3 d
4-11 yrs: 200 mg PO bid x 3 d
>12 yrs: 500 mg PO q 12 h x 3 d
HIV infected—see footnote 24

263
Appendix
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
Cutaneous larva migrans (creeping eruption, dog and cat hookworm)
Albendazole
D,7,12,25
400 mg PO daily x 3 d
400 mg PO daily x 3 d
Ivermectin
7,16
200 mcg/kg PO daily
200 mcg/kg PO daily x 1-2 d
x 1-2 d
Cyclosporiasis (Cyclospora cayetanensis)
Trimethoprim/sulfa- TMP 160 mg/SMX
TMP 5 mg/kg/SMX
methoxazole
D,7,26
800 mg (1 DS tab) PO
25 mg/kg/d PO in 2 doses
bid x 7-10 d
x 7-10d
Cysticercosis—see Tapeworm infection
Dientamoeba fragilis infection
27
Iodoquinol
D,2,7
650 mg PO tid x 20 d
30-40 mg/kg/d (max 2 g) PO in 3
doses x 20 d
Paromomycin
3,7
25-35 mg/kg/d PO in 3 25-35 mg/kg/d PO in 3 doses
doses x 7 d
x 7 d
Tetracycline
7,21
500 mg PO qid x 10 d
40 mg/kg/d (max 2 g) PO in 4
doses x 10 d
Metronidazole
7
500-750 mg PO tid x 10 d 35-50 mg/kg/d PO in 3 doses x 10 d
Diphyllobothrium latum—see Tapeworm infection
Dracunculus medinensis (guinea worm) infection—see footnote 28
Echinococcus—see Tapeworm infection
Entamoeba histolytica—see Amebiasis
Enterobius vermicularis (pinworm) infection
Mebendazole
D,29
100 mg PO once; repeat 100 mg PO once; repeat in 2 wks
in 2 wks
Pyrantel pamoate
13*
11 mg/kg base PO once 11 mg/kg base PO once (max 1 g);
(max 1 g); repeat
repeat in 2 wks
in 2 wks
Albendazole
7,12
400 mg PO once; repeat 400 mg PO once; repeat in 2wks
in 2wks
Fasciola hepatica—see Fluke infection
Filariasis
30
Wuchereria bancrofti, Brugia malayi, Brugia timori
Diethylcarbama- 6
mg/kg/d PO in 3
6 mg/kg/d PO in 3 doses x 12 d
32,33
zine
D,31
*
doses x 12 d
32,33
Loa loa
Diethylcarbama-
6 mg/kg/d PO in 3
6 mg/kg/d PO in 3 doses x 12 d
32,33
zine
34
*
doses x 12 d
32,33
Mansonella ozzardi—see footnote 35
Mansonella perstans
Albendazole
D,7,12
400 mg PO bid x 10 d
400 mg PO bid x 10 d
Mebendazole
7
100 mg PO bid x 30 d
100 mg PO bid x 30 d
Mansonella streptocerca
Diethylcarbama- 6
mg/kg/d PO x 12 d
33
6 mg/kg/d PO x 12 d
33
zine
D,36
*
Ivermectin
7,16
150 mcg/kg PO once
150 mcg/kg PO once

264
Medical Parasitology
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
Filariasis (continued)
30
Tropical Pulmonary Eosinophilia (TPE)
37
Diethylcarbama- 6
mg/kg/d in 3 doses
6 mg/kg/d in 3 doses x 12-21 d
33
zine
D
*
x 12-21 d
33
Onchocerca volvulus (River blindness)
Ivermectin
D,16,38
150 mcg/kg PO once,
150 mcg/kg PO once, repeated
repeated every 6-12
every 6-12 mos until
mos until asymptomatic asymptomatic
Fluke—hermaphroditic, infection
Clonorchis sinensis (Chinese liver fl uke)
Praziquantel
D,39
75 mg/kg/d PO in 3
75 mg/kg/d PO in 3 doses x 2 d
doses x 2 d
Albendazole
7,12
10 mg/kg/d PO x 7 d
10 mg/kg/d PO x 7 d
Fasciola hepatica (sheep liver fl uke)
Triclabendazole
D,40
* 10 mg/kg PO once
10 mg/kg PO once or twice
41
or twice
41
Bithionol
A,
*
30-50 mg/kg on alternate 30-50 mg/kg on alternate days
days x 10-15 doses
x 10-15 doses
Nitazoxanide
A,5,7
500 mg PO bid x 7 d
1-3 yrs: 100 mg PO q 12 h x 7 d
4-11 yrs: 200 mg PO q 12 h x 7 d
>12 yrs: 500 mg PO q 12 h x 7 d
Fasciolopsis buski, Heterophyes heterophyes, Metagonimus yokogawai (intestinal fl ukes)
Praziquantel
D,7,39
75 mg/kg/d PO in 3
75 mg/kg/d PO in 3 doses x 1 d
doses x 1 d
Metorchis conjunctus (North American liver fl uke)
Praziquantel
D,7,39
75 mg/kg/d PO in 3
75 mg/kg/d PO in 3 doses x 1 d
doses x 1 d
Nanophyetus salmincola
Praziquantel
D,7,39
60 mg/kg/d PO in 3
60 mg/kg/d PO in 3 doses x 1 d
doses x 1 d
Opisthorchis viverrini (Southeast Asian liver fl uke)
Praziquantel
D,39
75 mg/kg/d PO in 3
75 mg/kg/d PO in 3 doses x 2 d
doses x 2 d
Paragonimus westermani (lung fl uke)
Praziquantel
D,7,39
75 mg/kg/d PO in 3
75 mg/kg/d PO in 3 doses x 2 d
doses x 2 d
Bithionol
A,42
*
30-50 mg/kg on alternate 30-50 mg/kg on alternate days
days x 10-15 doses
x 10-15 doses
Giardiasis (Giardia duodenalis)
Metronidazole
7
250 mg PO tid x 5-7 d
15 mg/kg/d PO in 3 doses x 5-7 d
Tinidazole
6
2 g PO once
50 mg/kg PO once (max 2 g)
Nitazoxanide
5
500 mg PO bid x 3 d
1-3 yrs: 100 mg PO q 12 h x 3 d
4-11yrs: 200 mg PO q12h x 3d
>12yrs: 500 mg PO q12h x 3d
Paromomycin
A,3,7,
25-35 mg/kg/d PO in
25-35 mg/kg/d PO in 3 doses
43,44
3 doses x 5-10 d
x 5-10 d
Furazolidone
A,43
*
100 mg PO qid x 7-10 d 6 mg/kg/d PO in 4 doses x 7-10 d
Quinacrine
A,4,43,45*
100 mg PO tid x 5 d
2 mg/kg/d PO in 3 doses x 5 d
(max 300 mg/d)

265
Appendix
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
Gnathostomiasis (Gnathostoma spinigerum)
46
Albendazole
T,7,12
400 mg PO bid x 21 d
400 mg PO bid x 21 d
Ivermectin
7,16
200 mcg/kg/d PO x 2 d 200 mcg/kg/d PO x 2 d
either
± Surgical removal
Gongylonemiasis (Gongylonema sp.)
47
Surgical removal
T
Albendazole
7,12
400 mg/d PO x 3 d
400 mg/d PO x 3 d
Hookworm infection (Ancylostoma duodenale, Necator americanus)
Albendazole
D,7,12
400 mg PO once
400 mg PO once
Mebendazole
100 mg PO bid x 3 d
100 mg PO bid x 3 d or 500 mg
or 500 mg once
once
Pyrantel
11 mg/kg (max 1 g)
11 mg/kg (max 1 g) PO x 3 d
pamoate
7,13
*
PO x 3 d
Hydatid cyst—see Tapeworm infection
Hymenolepis nana—see Tapeworm infection
Isosporiasis (Isospora belli)
Trimethoprimsulfa-
TMP 160 mg/SMX 800 TMP 5 mg/kg/d/SMX 25 mg/kg/d
methoxazole
D,7,48
mg (1 DS tab) PO bid PO in 2 doses x 10 d
x 10 d
Leishmania
Visceral
49,50
Liposomal
3 mg/kg/d IV d 1-5,
3 mg/kg/d IV d 1-5, 14 and 21
52
amphotericin B
D,51
14 and 21
52
Sodium
20 mg Sb/kg/d IV or
20 mg Sb/kg/d IV or IM x 28 d
stibogluconate*
IM x 28 d
Miltefosine
53
*
2.5 mg/kg/d PO (max
2.5 mg/kg/d PO (max 150 mg/d)
150 mg/d) x 28 d
x 28 d
Meglumine
20 mg Sb/kg/d IV or
20 mg Sb/kg/d IV or IM x 28 d
antimonate
A
*
IM x 28 d
Amphotericin B
A,7
1 mg/kg IV daily x 15-20 1 mg/kg IV daily x 15-20 d or every
d or every second
second day for up to 8 wks
day for up to 8 wks
Paromomycin
7,13,54
* 15 mg/kg/d IM x 21 d
15 mg/kd/d IM x 21 d
Cutaneous
49,55
Sodium
20 mg Sb/kg/d IV or
20 mg Sb/kg/d IV or IM x 20 d
stibogluconate
D
* IM x 20 d
Meglumine
20 mg Sb/kg/d IV or
20 mg Sb/kg/d IV or IM x 20 d
antimonate*
IM x 20 d
Miltefosine
53
*
2.5 mg/kg/d PO (max 2.5 mg/kg/d PO (max 150 mg/d)
150 mg/d) x 28 d
x 28 d
Paromomycin
A,7,
Topically 2 x/d x
Topically 2 x/d x 10-20 d
13,54,56*
10-20 d
Pentamidine
7,56
2-3 mg/kg IV or IM
2-3 mg/kg IV or IM daily or every
daily or every second
second day x 4-7 doses
57
day x 4-7 doses
57

266
Medical Parasitology
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
Leishmania (continued)
Mucosal
49,58
Sodium
20 mg Sb/kg/d IV or
20 mg Sb/kg/d IV or IM x 28 d
stibogluconate
D,
* IM x 28 d
Meglumine
20 mg Sb/kg/d IV or
20 mg Sb/kg/d IV or IM x 28 d
antimonate*
IM x 28 d
Amphotericin B
7
0.5-1 mg/kg IV daily
0.5-1 mg/kg IV daily or every
or every second day
second day for up to 8 wks
for up to 8 wks
Miltefosine
53
*
2.5 mg/kg/d PO (max 2.5 mg/kg/d PO (max 150 mg/d)
150 mg/d) x 28d
x 28d
Lice infestation (Pediculus humanus, P. capitis, Phthirus pubis)
0.5% Malathion
D,60
Topically
Topically
1% Permethrin
61
Topically
Topically
Pyrethrins with
Topically
Topically
piperonyl
butoxide
A,61
Ivermectin
A,7,16,62
200 mcg/kg PO
>15 kg: 200 mcg/kg PO
Loa loa—see Filariasis
Malaria, treatment of (Plasmodium falciparum,
63
P. vivax,
64
P. ovale, and P. malariae
65
)
Oral:
66
P. falciparum or unidentifi ed species acquired in areas of chloroquine-resistant
P. falciparum
63
Atovaquone/
2 adult tabs bid
69
or 4
<5 kg: not indicated
proguanil
D,68
adult tabs once/d x 3 d 5-8 kg: 2 peds tabs once/d x 3 d
9-10 kg: 3 peds tabs once/d x 3 d
11-20 kg: 1 adult tab once/d x 3 d
21-30 kg: 2 adult tabs once/d x 3 d
31-40 kg: 3 adult tabs once/d x 3 d
>40 kg: 4 adult tabs once/d x 3 d
Quinine sulfate
650 mg q 8 h x 3 or 7 d
70
30 mg/kg/d in 3 doses x 3 or 7 d
70
plus
doxycycline
7,21,71
100 mg bid x 7 d
4 mg/kg/d in 2 doses x 7 d
or plus
tetracycline
7,21
250 mg qid x 7 d
6.25 mg/kg/d in 4 doses x 7 d
or plus
clindamycin
7,21,72
20 mg/kg/d in 3
20 mg/kg/d in 3 doses x 7 d
doses x 7d
73
Mefl oquine
A,67,74,75
750 mg followed 12 hrs 15 mg/kg followed 12 hrs later by
later by 500 mg
10 mg/kg
Artemether/lume-
6 doses over 3d (4
6 doses over 3d at same intervals
fantrine
A,67,76,77
*
tabs/dose at 0, 8, 24, as adults;
36, 48 and 60 hrs)
<15 kg: 1 tab/dose
15-25 kg: 2 tabs/dose
25-35 kg: 3 tabs/dose
>35 kg: 4 tabs/dose
Artesunate
A,76
*
4 mg/kg/d x 3 d
4 mg/kg/d x 3 d
plus see footnote 78

267
Appendix
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
Malaria, treatment of (continued)
P. vivax acquired in areas of chloroquine-resistant P. vivax
64
Mefl oquine
D,67,74
750 mg PO followed
15 mg/kg PO followed 12 hrs later
12 hrs later by 500 mg by 10 mg/kg
Atovaquone/
2 adult tabs bid
69
or 4
<5 kg: not indicated
proguanil
68
adult tabs once/d x 3 d 5-8 kg: 2 peds tabs once/d x 3 d
9-10 kg: 3 peds tabs once/d x 3 d
11-20 kg: 1 adult tab once/d x 3 d
21-30 kg: 2 adult tabs once/d x 3 d
31-40 kg: 3 adult tabs once/d x 3 d
>40 kg: 4 adult tabs once/d x 3 d
either followed by
primaquine
30 mg base/d PO
0.6 mg/kg/d PO x 14 d
phosphate
79
x 14 d
Chloroquine
25 mg base/kg PO in
25 mg base/kg PO in 3 doses
phosphate
A,67,80
3 doses over 48 hrs
81
over 48 hrs
81
Quinine sulfate
A
650 mg PO q 8 h
30 mg/kg/d PO in 3 doses
x 3-7 d
70
x 3-7 d
70
plus
doxycycline
7,21,71
100 mg PO bid x 7 d
4 mg/kg/d PO in 2 doses x 7 d
either followed by
primaquine
30 mg base/d PO
0.6 mg/kg/d PO x 14 d
phosphate
79
x 14 d
All Plasmodium species except chloroquine-resistant P. falciparum
63
and chloroquine-
resistant P. vivax
64
Chloroquine
1 g (600 mg base) PO, 10 mg base/kg (max 600 mg base)
phosphate
D,67,80
then 500 mg (300 mg PO, then 5 mg base/kg 6 hrs later,
base) 6 hrs later, then then 5 mg base/kg at 24
500 mg (300 mg base) and 48 hrs
81
at 24 and 48 hrs
81
Parenteral:
66
All Plasmodium species (Chloroquine-sensitive and resistant)
Quinidine
10 mg/kg IV loading
10 mg/kg lV loading dose
gluconate
D,67,82,83
dose (max 600 mg) in
(max 600 mg) in normal saline
normal saline over 1-2 hrs, over 1-2 hrs, followed by continuous
followed by continuous infusion of 0.02 mg/kg/min
infusion of 0.02
until PO therapy can be started
mg/kg/min until PO
therapy can be started
Quinine dihydro-
20 mg/kg IV loading
20 mg/kg IV loading dose in 5%
chloride
83
*
dose in 5% dextrose over
4 hrs, followed by
dextrose over 4 hrs, followed by
10 mg/kg over 2-4 hrs 10 mg/kg over 2-4 hrs q 8 h (max
q 8 h (max 1800 mg/d) 1800 mg/d) until PO therapy can
until PO therapy can
be started
be started
Artesunate
76
*
2.4 mg/kg/dose IV
2.4 mg/kg/dose IV x 3d at 0, 12,
x 3d at 0, 12, 24,
24, 48 and 72 hrs
48 and 72 hrs
plus see footnote 78

268
Medical Parasitology
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
Malaria, prevention of
84
All Plasmodium species in chloroquine-sensitive areas
63-65
Chloroquine phos- 500 mg (300 mg base) 5 mg/kg base PO once/wk, up to
phate
D,67,80,85,86
PO once/wk
87
adult dose of 300 mg base
87
All Plasmodium species in chloroquine-resistant areas
63-65
Atovaquone/
1 adult tab/d
88
5-8 kg: 1/2 peds tab/d
68,88
proguanil
D,67,68
9-10 kg: 3/4 peds tab/d
68,88
11-20 kg: 1 peds tab/d
68,88
21-30 kg: 2 peds tabs/d
68,88
31-40 kg: 3 peds tabs/d
68,88
>40 kg: 1 adult tab/d
68,88
Doxycycline
7,21,71
100 mg PO daily
89
2 mg/kg/d PO, up to 100 mg/d
89
Mefl oquine
74,75,90
250 mg PO once/wk
91
5-10 kg: 1/8 tab once/wk
91
11-20 kg: 1/4 tab once/wk
91
21-30 kg: 1/2 tab once/wk
91
31-45 kg: 3/4 tab once/wk
91
>45 kg: 1 tab once/wk
91
Primaquine
30 mg base PO daily
93
0.6 mg/kg base PO daily
93
phosphate
A,7,79
Malaria, prevention of relapses: P. vivax and P. ovale
67
Primaquine
30 mg base/d PO
0.6 mg base/kg/d PO x 14 d
phosphate
D,79
x 14 d
Malaria, self-presumptive treatment
94
Atovaquone/
4 adult tabs once/d
<5 kg: not indicated
proguanil
D,7,68
x 3 d
69
5-8 kg: 2 peds tabs once/d x 3 d
9-10 kg: 3 peds tabs once/d x 3 d
11-20 kg: 1 adult tab once/d x 3 d
21-30 kg: 2 adult tabs once/d x 3d
31-40 kg: 3 adult tabs once/d x 3 d
>40 kg: 4 adult tabs once/d x 3 d
69
Quinine sulfate
650 mg PO q 8 h
30 mg/kg/d PO in 3 doses
x 3 or 7 d
70
x 3 or 7 d
70
plus
doxycycline
7,21,71
100 mg PO bid x 7 d
4 mg/kg/d PO in 2 doses x 7 d
Artesunate
76
*
4 mg/kg/d PO x 3 d
4 mg/kg/d PO x 3 d
plus see footnote 78
Microsporidiosis
Ocular (Encephalitozoon hellem, E. cuniculi, Vittaforma corneae [Nosema corneum])
Albendazole
D,7,12
400 mg PO bid
plus fumagillin
95
*
Intestinal (E. bieneusi, E. [Septata] intestinalis)
E. bieneusi
Fumagillin
D,96
*
20 mg PO tid x 14 d
E. intestinalis
Albendazole
D,7,12
400 mg PO bid x 21 d
Disseminated (E. hellem, E. cuniculi, E. intestinalis, Pleistophora sp., Trachipleistophora
sp. and Brachiola vesicularum)
Albendazole
D,7,12,97
* 400 mg PO bid

269
Appendix
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
Mites —see Scabies
Moniliformis infection
Pyrantel
11 mg/kg PO once,
11 mg/kg PO once, repeat twice,
pamoate
D,7,13
*
repeat twice, 2wks apart 2wks apart
Naegleria species—see Amebic meningoencephalitis, primary
Necator americanus, see Hookworm infection
Oesophagostomum bifurcum—see footnote 98
Onchocerca volvulus—see Filariasis
Opisthorchis viverrini—see Fluke infection
Paragonimus westermani—see Fluke infection
Pediculus capitis, humanus, Phthirus pubis—see Lice
Pinworm—see Enterobius
Pneumocystis jiroveci (formerly carinii) pneumonia (PCP)
99
Trimethoprim/
TMP 15 mg/SMX
TMP 15 mg/SMX 75 mg/kg/d,
sulfamethoxazole
D
75 mg/kg/d, PO or IV PO or IV in 3 or 4 doses x 21 d
in 3 or 4 doses x 21 d
Primaquine
A,7,79
30 mg base PO daily
0.3 mg/kg base PO daily x 21 d
x 21 d
plus clindamycin
7,18
600 mg IV q 6 h x 21 d, 15-25 mg/kg IV q 6 h x 21 d, or
or 300-450 mg PO
10 mg/kg PO q 6 h x 21 d
q 6 h x 21 d
Trimethoprim
A,7
5 mg/kg PO tid x 21 d
5 mg/kg PO tid x 21 d
plus dapsone
7
100 mg daily x 21 d
2 mg/kg/d PO x 21 d
Pentamidine
A
3-4 mg/kg IV daily
3-4 mg/kg IV daily x 21 d
x 21 d
Atovaquone
A
750 mg PO bid x 21 d
1-3 mos: 30 mg/kg/d PO x 21 d
4-24 mos: 45 mg/kg/d PO x 21 d
>24 mos: 30 mg/d PO x 21 d
Primary and secondary prophylaxis
100
Trimethoprim/
1 tab (single or double
TMP 150 mg/SMX 750 mg/m
2
/d
sulfamethoxazole
D
strength) daily or 1 DS PO in 2 doses 3 d/wk
tab PO 3 d/wk
Dapsone
A,7
50 mg PO bid or
2 mg/kg/d (max 100 mg) PO or
100 mg PO daily
4 mg/kg (max 200 mg) PO each wk
Dapsone
A,7
50 mg PO daily or
200 mg PO each wk
plus
pyrimethamine
101
50 mg PO or 75 mg
PO each wk
Pentamidine
A
300 mg aerosol inhaled ≥5 yrs: 300 mg inhaled monthly via
monthly via Respirgard Respirgard II nebulizer
II nebulizer
Atovaquone
A,7,20
1500 mg PO daily
1-3 mos: 30 mg/kg/d PO
4-24 mos: 45 mg/kg/d PO
>24 mos: 30 mg/kg/d PO

270
Medical Parasitology
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
River Blindness—see Filariasis
Roundworm—see Ascariasis
Sappinia diploidea—see Amebic meningoencephalitis, primary
Scabies (Sarcoptes scabiei)
5% Permethrin
D
Topically
once
102
Topically
once
102
Ivermectin
A,7,16,103,104
200 mcg/kg PO once
102
200 mcg/kg PO once
102
10% Crotamiton
A
Topically once/d x 2
Topically once/d PO x 2
Schistosomiasis (Bilharziasis)
S. haematobium
Praziquantel
D,39
40 mg/kg/d PO in
40 mg/kg/d PO in 2 doses x 1 d
2 doses x 1 d
S. japonicum
Praziquantel
D,39
60 mg/kg/d PO in
60 mg/kg/d PO in 3 doses x 1 d
3 doses x 1 d
S. mansoni
Praziquantel
D,39
40 mg/kg/d PO in
40 mg/kg/d PO in 2 doses x 1 d
2 doses x 1 d
Oxamniquine
A,105
* 15 mg/kg PO once
106
20 mg/kg/d PO in 2 doses x 1 d
106
S. mekongi
Praziquantel
D,39
60 mg/kg/d PO in
60 mg/kg/d PO in 3 doses x 1 d
3 doses x 1 d
Sleeping sickness, see Trypanosomiasis
Strongyloidiasis (Strongyloides stercoralis)
Ivermectin
D,16,107
200 mcg/kg/d PO x 2 d 200 mcg/kg/d PO x 2 d
Albendazole
A,7,12
400 mg PO bid x 7 d
400 mg PO bid x 7 d
Tapeworm infection
Adult (intestinal stage):
Diphyllobothrium latum (fi sh), Taenia saginata (beef), Taenia solium (pork), Dipylidium
caninum (dog)
Praziquantel
D,7,39
5-10 mg/kg PO once
5-10 mg/kg PO once
Niclosamide
A,108
*
2 g PO once
50 mg/kg PO once
Hymenolepis nana (dwarf tapeworm)
Praziquantel
D,7,39
25 mg/kg PO once
25 mg/kg PO once
Nitazoxanide
A,5,7
500 mg PO once/d
1-3 yrs: 100 mg PO bid x 3 d
109
or bid x 3 d
109
4-11 yrs: 200 mg PO bid x 3 d
109
Larval (tissue stage):
Echinococcus granulosus (hydatid cyst)
Albendazole
D,12,110
400 mg PO bid
15 mg/kg/d (max 800 mg)
x 1-6 mos
x 1-6 mos
Echinococcus multilocularis—see footnote 111
T
Taenia solium (Cysticercosis)
Treatment of choice—see footnote 112
Albendazole
A,12
400 mg PO bid x 8-30 d; 15 mg/kg/d (max 800 mg) PO in
can be repeated as
2 doses x 8-30 d; can be
necessary
repeated as necessary
Praziquantel
A,7,39
100 mg/kg/d PO in 3
100 mg/kg/d PO in 3 doses x 1 d
doses x 1 d then 50 mg/ then 50 mg/kg/d in 3 doses
kg/d in 3 doses x 29 d x 29 days

271
Appendix
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
Toxocariasis—see Visceral larva migrans
Toxoplasmosis (Toxoplasma gondii)
Pyrimetha-
25-100 mg/d PO
2 mg/kg/d PO x 2d, then 1 mg/
mine
D,113,114
x 3-4 wks
kg/d (max 25 mg/d) x 4 wks
115
plus
sulfadiazine
116
1-1.5 g PO qid x 3-4 wks 100-200 mg/kg/d PO x 3-4 wks
Trichinellosis (Trichinella spiralis)
Steroids for severe
symptoms
D
plus
albendazole
7,12
400 mg PO bid x 8-14 d 400 mg PO bid x 8-14 d
Mebendazole
A,7
200-400 mg PO tid
200-400 mg PO tid x 3 d, then
x 3 d, then 400-500
400-500 mg tid x 10 d
mg tid x 10 d
Trichomoniasis (Trichomonas vaginalis)
Metronidazole
D,117
2 g PO once or 500
15 mg/kg/d PO in 3 doses x 7 d
mg bid x 7 d
Tinidazole
6
2 g PO once
50 mg/kg once (max 2 g)
Trichostrongylus infection
Pyrantel
11 mg/kg base PO
11 mg/kg PO once (max 1 g)
pamoate
D,7,13
*
once (max 1 g)
Mebendazole
A,7
100 mg PO bid x 3 d
100 mg PO bid x 3 d
Albendazole
A,7,12
400 mg PO once
400 mg PO once
Trichuriasis (Trichuris trichiura, whipworm)
Mebendazole
D
100 mg PO bid x 3 d
100 mg PO bid x 3 d or 500 mg
or 500 mg once
once
Albendazole
A,7,12
400 mg PO x 3 d
400 mg PO x 3 d
Ivermectin
A,7,16
200 mcg/kg PO daily
200 mcg/kg/d PO x 3 d
x 3 d
Trypanosomiasis
T. cruzi (American trypanosomiasis, Chagas’ disease)
Nifurtimox
D
*
8-10 mg/kg/d PO in
1-10 yrs: 15-20 mg/kg/d PO in
3-4 doses x 90-120 d
4 doses x 90-120 d
11-16 yrs: 12.5-15 mg/kg/d in
4 doses x 90-120 d
Benznidazole
119
*
5-7 mg/kg/d PO in 2
≤12 yrs: 10 mg/kg/d PO in 2 doses
doses x 30-90 d
x 30-90 d
>12 yrs: 5-7 mg/kg/d in 2 doses
x 30-90 d
T. brucei gambiense (West African trypanosomiasis, sleeping sickness)
Hemolymphatic stage:
Pentamidine
D,7,120
4 mg/kg/d IM x 7 d
4 mg/kg/d IM x 7 d
Suramin
A
*
100-200 mg (test dose) 20 mg/kg on d 1,3,7,14 and 21
IV, then 1 g IV on days
1,3,7,14 and 21

272
Medical Parasitology
Appendix
Infection/Drug
Adult Dosage
Pediatric Dosage
Trypanosomiasis (continued)
T. brucei gambiense (continued)
Late disease with CNS involvement:
Efl ornithine
D,121
*
400 mg/kg/d IV in 4
400 mg/kg/d IV in 4 doses x 14 d
doses x 14d
Melarsoprol
122
2.2 mg/kg/d IV x 10 d 2.2 mg/kg/d IV x 10 d
T. b. rhodesiense (East African trypanosomiasis, sleeping sickness)
Hemolymphatic stage:
Suramin
D
*
100-200 mg (test dose)
20 mg/kg on d 1,3,7,14 and 21
IV, then 1 g IV on days
1,3,7,14 and 21
Late disease with CNS involvement
Melarsoprol
D,122
2-3.6 mg/kg/d IV x 3 d; 2-3.6 mg/kg/d x 3 d; after 7 d
after 7 d 3.6 mg/kg/d 3.6 mg/kg/d x 3 d; repeat again
x 3 d; repeat again
after 7d
after 7d
Visceral larva migrans
123
(Toxocariasis)
Albendazole
D,7,12
400 mg PO bid x 5 d
400 mg PO bid x 5 d
Mebendazole
7
100-200 mg PO bid
100-200 mg PO bid x 5 d
x 5 d
Whipworm—see Trichuriasis
Wuchereria bancrofti—see Filariasis
D Drug of choice
A Alternative drug choice
T Treatment of choice
* Availability problems. See following table.
1. Topical 0.02% chlorhexidine and polyhexamethylene biguanide (PHMB, 0.02%), either
alone or in combination, have been used successfully in a large number of patients.
Treatment with either chlorhexidine or PHMB is often combined with propamidine isethionate
(Brolene) or hexamidine (Desmodine). None of these drugs is commercially available or
approved for use in the US, but they can be obtained from compounding pharmacies (see
footnote 2). Leiter’s Park Avenue Pharmacy, San Jose, CA (800-292-6773; www.leiterrx.
com) is a compounding pharmacy that specializes in ophthalmic drugs. Propamidine is
available over the counter in the UK and Australia. Hexamidine is available in France.
The combination of chlorhexidine, natamycin (pimaricin) and debridement also has been
successful (K Kitagawa et al, Jpn J Ophthalmol 2003; 47:616). Debridement is most useful
during the stage of corneal epithelial infection. Most cysts are resistant to neomycin; its
use is no longer recommended. Azole antifungal drugs (ketoconazole, itraconazole) have
been used as oral or topical adjuncts (FL Shuster and GS Visvesvara, Drug Resist Update
2004; 7:41). Use of corticosteroids is controversial (K Hammersmith, Curr Opinions
Ophthal 2006; 17:327; ST Awwad et al, Eye Contact Lens 2007; 33:1).
2. Iodoquinol should be taken after meals.
3. Paromomycin should be taken with a meal.
4. Not available commercially. It may be obtained through compounding pharmacies
such as Panorama Compounding Pharmacy, 6744 Balboa Blvd, Van Nuys, CA 91406
(800-247-9767) or Medical Center Pharmacy, New Haven, CT (203-688-6816). Other
compounding pharmacies may be found through the National Association of Compounding
Pharmacies (800-687-7850) or the Professional Compounding Centers of America
(800-331-2498, www.pccarx.com).
5. Nitazoxanide may be effective against a variety of protozoan and helminth infections (DA
Bobak, Curr Infect Dis Rep 2006; 8:91; E Diaz et al, Am J Trop Med Hyg2003; 68:384).

273
Appendix
Appendix
It was effective against mild to moderate amebiasis, 500 mg bid x 3 d, in a recent study
(JF Rossignol et al, Trans R Soc Trop Med Hyg 2007 Oct; 101:1025 E pub 2007 July 20).
It is FDA-approved only for treatment of diarrhea caused by Giardia or Cryptosporidium
(Med Lett Drugs Ther 2003; 45:29). Nitazoxanide is available in 500-mg tablets and an
oral suspension; it should be taken with food.
6. A nitroimidazole similar to metronidazole, tinidazole appears to be as effective as
metronidazole and better tolerated (Med Lett Drugs Ther 2004; 46:70). It should be taken
with food to minimize GI adverse effects. For children and patients unable to take tablets,
a pharmacist can crush the tablets and mix them with cherry syrup (Humco, and others).
The syrup suspension is good for 7 days at room temperature and must be shaken before
use (HB Fung and TL Doan et al, Clin Ther 2005; 27:1859). Ornidazole, a similar drug,
is also used outside the US.
7. Not FDA-approved for this indication.
8.
Although
a
Naegleria fowleri infection was treated successfully in a 9-year-old girl with
combination of amphotericin B and miconazole both intravenous and intrathecal, plus oral
rifampin (JS Seidel et al NEJM 1982;306:346). Amphotericin B and miconazole appear
to have a synergistic effect, but Medical Letter consultants believe the rifampin probably
had no additional effect (GS Visvesvara et al, FEMS Immunol Med Microbiol 2007;
50:1). Parenteral miconazole is no longer available in the US. Azithromycin has been
used successfully in combination therapy to treat Balmuthia infection, but was changed to
clarithromycin because of toxicity concerns and for better penetration into the cerebrospinal
fl uid. In vitro, azithromycin is more active than clarithromycin against Naegleria, so may
be a better choice combined with amphotericin B for treatment of Naegleria (TR Deetz
et al, Clin Infect Dis 2003; 37:1304; FL Schuster and GS Visvesvara, Drug Resistance
Updates 2004; 7:41). Combinations of amphotericin B, ornidazole and rifampin (R Jain
et al, Neurol Indian 2002; 50:470) and amphotericin B fl uconazole and rifampin have
also been used (J Vargas-Zepeda et al, Arch Med Research 2005;36:83). Case reports
of other successful therapy have been published (FL Schuster and GS Visvesvara, Int J
Parasitol 2004; 34:1001).
9. Several patients with granulomatous amebic encephalitis (GAE) have been successfully
treated with combinations of pentamidine, sulfadiazine, fl ucytosine, and either fl uconazole
or itraconazole (GS Visvesvara et al, FEMS Immunol Med Microbiol 2007; 50:1, epub
Apr 11). GAE in an AIDS patient was treated successfully with sulfadiazine, pyrimethamine
and fl uconazole combined with surgical resection of the CNS lesion (M Seijo Martinez et
al, J Clin Microbiol 2000; 38:3892). Chronic Acanthamoeba meningitis was successfully
treated in 2 children with a combination of oral trimethoprim/sulfamethoxazole, rifampin
and ketoconazole (T Singhal et al, Pediatr Infect Dis J 2001; 20:623). Disseminated
cutaneous infection in an immunocompromised patient was treated successfully with
IV pentamidine, topical chlorhexidine and 2% ketoconazole cream, followed by oral
itraconazole (CA Slater et al, N Engl J Med 1994; 331:85) and with voriconazole and
amphotericin B lipid complex (R Walia et al, Transplant Infect Dis 2007; 9:51). Other
reports of successful therapy have been described (FL Schuster and GS Visvesvara, Drug
Resistance Updates 2004; 7:41). Susceptibility testing of Acanthamoeba isolates has
shown differences in drug sensitivity between species and even among strains of a single
species; antimicrobial susceptibility testing is advisable (FL Schuster and GS Visvesvara,
Int J Parasitiol 2004; 34:1001).
10. B. mandrillaris is a free-living ameba that causes subacute to fatal granulomatous amebic
encephalitis (GAE) and cutaneous disease. Two cases of Balamuthia encephalitis have been
successfully treated with fl ucytosine, pentamidine, fl uconazole and sulfadiazine plus either
azithromycin or clarithromycin (phenothiazines were also used) combined with surgical
resection of the CNS lesion (TR Deetz et al, Clin Infect Dis 2003; 37:1304). Another case
was successfully treated following open biopsy with pentamidine, fl uconazole, sulfadiazine
and clarithromycin (S Jung et al, Arch Pathol Lab Med 2004; 128:466).
11. A free-living ameba once thought not to be pathogenic to humans. S. diploidea has been
successfully treated with azithromycin, pentamidine, itraconazole and fl ucytosine combined
with surgical resection of the CNS lesion (BB Gelman et al, J Neuropathol Exp Neurol
2003; 62:990).

274
Medical Parasitology
Appendix
12. Albendazole must be taken with food; a fatty meal increases oral bioavailability.
13. Pyrantel pamoate suspension can be mixed with milk or fruit juice.
14. A. cantonensis causes predominantly neurotropic disease. A. costaricensis causes
gastrointestinal disease. Most patients infected with either species have a self-limited
course and recover completely. Analgesics, corticosteroids and careful removal of CSF at
frequent intervals can relieve symptoms from increased intracranial pressure (V Lo Re III
and SJ Gluckman, Am J Med 2003; 114:217). Treatment of A. cantonensis is controversial
and varies across endemic areas. No anthelminthic drug is proven to be effective and
some patients have worsened with therapy (TJ Slom et al, N Engl J Med 2002; 346:668).
Mebendazole and a corticosteroid, however, appear to shorten the course of infection (H-C
Tsai et al, Am J Med 2001; 111:109; V Chotmongkol et al, Am J Trop Med Hyg 2006;
74:1122). Albendazole has also relieved symptoms of angiostrongyliasis (XG Chen et
al, Emerg Infect Dis 2005; 11:1645).
15. A Repiso Ortega et al, Gastroenterol Hepatol 2003; 26:341. Successful treatment of
Anisakiasis with albendazole 400 mg PO bid x 3-5d has been reported, but the diagnosis
was presumptive (DA Moore et al, Lancet 2002; 360:54; E Pacios et al, Clin Infect Dis
2005; 41:1825).
16. Safety of ivermectin in young children (<15 kg) and pregnant women remains to be
established. Ivermectin should be taken on an empty stomach with water.
17. Exchange transfusion has been used in severely ill patients and those with high (>10%)
parasitemia (VI Powell and K Grima, Transfus Med Rev 2002; 16:239). In patients who
were not severely ill, combination therapy with atovaquone and azithromycin was as
effective as clindamycin and quinine and may have been better tolerated (PJ Krause
et al, N Engl J Med 2000; 343:1454). Longer treatment courses may be needed in
immunosuppressed patients and those with asplenia. Patients are commonly co-infected
with Lyme disease (Med Lett Drugs Ther 2007; 49:49; AC Steere et al, Clin Infect Dis
2003; 36:1078).
18. Oral clindamycin should be taken with a full glass of water to minimize esophageal
ulceration.
19. Quinine should be taken with or after a meal to decrease gastrointestinal adverse
effects.
20. Atovaquone is available in an oral suspension that should be taken with a meal to increase
absorption.
21. Use of tetracyclines is contraindicated in pregnancy and in children <8 years old.
Tetracycline should be taken 1 hour before or 2 hours after meals and/or dairy
products.
22. No drug has been demonstrated to be effective. Albendazole 25 mg/kg/d PO x 20 d
started as soon as possible (up to 3 d after possible infection) might prevent clinical
disease and is recommended for children with known exposure (ingestion of raccoon
stool or contaminated soil) (WJ Murray and KR Kazacos, Clin Infect Dis 2004; 39:1484).
Mebendazole, levamisole or ivermectin could be tried if albendazole is not available.
Steroid therapy may be helpful, especially in eye and CNS infections (PJ Gavin et al,
Clin Microbiol Rev 2005; 18:703). Ocular baylisascariasis has been treated successfully
using laser photocoagulation therapy to destroy the intraretinal larvae (CA Garcia et al,
Eye 2004; 18:624).
23. Clinical signifi cance of these organisms is controversial; metronidazole 750 mg PO tid x
10 d, iodoquinol 650 mg PO tid x 20 d or trimethoprim/sulfamethoxazole 1 DS tab PO
bid x 7d have been reported to be effective (DJ Stenzel and PFL Borenam, Clin Microbiol
Rev 1996; 9:563; UZ Ok et al, Am J Gastroenterol 1999; 94:3245). Metronidazole
resistance may be common in some areas (K Haresh et al, Trop Med Int Health 1999;
4:274). Nitazoxanide has been effective in clearing organism and improving symptoms (E
Diaz et al, Am J Trop Med Hyg 2003; 68:384; JF Rossignol, Clin Gastroenterol Hepatol
2005; 18:703).
24. No drug has proven effi cacy against cryptosporidiosis in advanced AIDS (I Abubakar
et al, Cochrane Database Syst Rev 2007; 1:CD004932). Treatment with HAART is the
mainstay of therapy. Nitazoxanide (JF Rossignol, Aliment Pharmacol Ther 2006; 24:807),
paromomycin (P Maggi et al, Clin Infect Dis 2000; 33:1609), or a combination of
paromomycin and azithromycin (NH Smith et al, J Infect Dis 1998; 178:900) may be

275
Appendix
Appendix
tried to decrease diarrhea and recalcitrant malabsorption of antimicrobial drugs, which
can occur with chronic cryptosporidiosis.
25. G Albanese et al, Int J Dermatol 2001; 40:67; D Malvy et al, J Travel Med 2006;
13:244.
26. HIV-infected patients may need higher dosage and long-term maintenance. Successful use
of nitazoxanide (see also footnote 5) has been reported in one patient with sulfa allergy
(SM Zimmer et al, Clin Infect Dis 2007; 44:466).
27. A Norberg et al, Clin Microbiol Infect 2003; 9:65; O Vandenberg et al, Int J Infect Dis
2006; 10:255.
28. No drug is curative against Dracunculus. A program for monitoring local sources of
drinking water to eliminate transmission has dramatically decreased the number of cases
worldwide (M Barry, N Engl J Med 2007; 356:2561). The treatment of choice is slow
extraction of worm combined with wound care and pain management (C Greenaway,
CMAJ 2004; 170:495).
29. Since family members are usually infected, treatment of the entire household is
recommended.
30. Antihistamines or corticosteroids may be required to decrease allergic reactions to
components of disintegrating microfi lariae that result from treatment, especially in
infection caused by Loa loa. Endosymbiotic Wolbachia bacteria may have a role in fi larial
development and host response, and may represent a potential target for therapy. Addition
of doxycycline 100 or 200 mg/d PO x 6-8 wks in lymphatic fi lariasis and onchocerciasis
has resulted in substantial loss of Wolbachia and decrease in both micro- and macrofi lariae
(MJ Taylor et al, Lancet 2005; 365:2116; AY Debrah et al, Plos Pathog 2006; e92:0829);
but use of tetracyclines is contraindicated in pregnancy and in children <8 yrs old.
31. Most symptoms are caused by adult worm. A single-dose combination of albendazole (400
mg PO) with either ivermectin (200 mcg/kg PO) or diethylcarbamazine (6 mg/kg PO) is
effective for reduction or suppression of W. bancrofti microfi laria, but the albendazole/
ivermectin combination does not kill all the adult worms (D Addiss et al, Cochrane Database
Syst Rev 2004; CD003753).
32. For patients with microfi laria in the blood, Medical Letter consultants start with a lower
dosage and scale up: d1: 50 mg; d2: 50 mg tid; d3: 100 mg tid; d4-14: 6 mg/kg in 3
doses (for Loa loa d4-14: 9 mg/kg in 3 doses). Multi-dose regimens have been shown
to provide more rapid reduction in microfi laria than single-dose diethylcarbamazine, but
microfi laria levels are similar 6-12 months after treatment (LD Andrade et al, Trans R Soc
Trop Med Hyg 1995; 89:319; PE Simonsen et al, Am J Trop Med Hyg 1995; 53:267).
A single dose of 6 mg/kg is used in endemic areas for mass treatment (J Figueredo-Silva
et al, Trans R Soc Trop Med Hyg 1996; 90:192; J Noroes et al, Trans R Soc Trop Med
Hyg 1997; 91:78).
33. Diethylcarbamazine should not be used for treatment of Onchocerca volvulus due to the
risk of increased ocular side effects including blindness associated with rapid killing of
the worms. It should be used cautiously in geographic regions where O. volvulus coexists
with other fi lariae. Diethylcarbamazine is contraindicated during pregnancy. See also
footnote 38.
34. In heavy infections with Loa loa, rapid killing of microfi lariae can provoke encephalopathy.
Apheresis has been reported to be effective in lowering microfi larial counts in patients
heavily infected with Loa loa (EA Ottesen, Infect Dis Clin North Am 1993; 7:619).
Albendazole may be useful for treatment of loiasis when diethylcarbamazine is ineffective
or cannot be used, but repeated courses may be necessary (AD Klion et al, Clin Infect Dis
1999; 29:680; TE Tabi et al, Am J Trop Med Hyg 2004; 71:211). Ivermectin has also been
used to reduce microfi laremia, but albendazole is preferred because of its slower onset of
action and lower risk of precipitating encephalopathy (AD Klion et al, J Infect Dis 1993;
168:202; M Kombila et al, Am J Trop Med Hyg 1998; 58:458). Diethylcarbamazine,
300 mg PO once/wk, has been recommended for prevention of loiasis (TB Nutman et
al, N Engl J Med 1988; 319:752).
35. Diethylcarbamazine has no effect. A single dose of ivermectin 200 mcg/kg PO reduces
microfi laria densities and provides both short- and long-term reductions in M. ozzardi
microfi laremia (AA Gonzalez et al, W Indian Med J 1999; 48:231).

276
Medical Parasitology
Appendix
36. Diethylcarbamazine is potentially curative due to activity against both adult worms and
microfi lariae. Ivermectin is active only against microfi lariae.
37. AK Boggild et al, Clin Infect Dis 2004; 39:1123. Relapses occur and can be treated with
a repeated course of diethylcarbamazine.
38. Diethylcarbamazine should not be used for treatment of this disease because rapid killing
of the worms can lead to blindness. Periodic treatment with ivermectin (every 3-12 months),
150 mcg/kg PO, can prevent blindness due to ocular onchocerciasis (DN Udall, Clin
Infect Dis 2007; 44:53). Skin reactions after ivermectin treatment are often reported in
persons with high microfi larial skin densities. Ivermectin has been inadvertently given to
pregnant women during mass treatment programs; the rates of congenital abnormalities
were similar in treated and untreated women. Because of the high risk of blindness from
onchocerciasis, the use of ivermectin after the fi rst trimester is considered acceptable
according to the WHO. Doxycycline (100 mg/day PO for 6 weeks), followed by a single
150 mcg/kg PO dose of ivermectin, resulted in up to 19 months of amicrofi laridermia and
100% elimination of Wolbachia species (A Hoerauf et al, Lancet 2001; 357:1415).
39. Praziquantel should be taken with liquids during a meal.
40. Unlike infections with other fl ukes, Fasciola hepatica infections may not respond to
praziquantel. Triclabendazole (Egaten - Novartis) appears to be safe and effective, but
data are limited (DY Aksoy et al, Clin Microbiol Infect 2005; 11:859). It is available from
Victoria Pharmacy, Zurich, Switzerland (www.pharmaworld.com; 41-1-211-24-32) and
should be given with food for better absorption. Nitazoxanide also appears to have effi cacy
in treating fascioliasis in adults and in children (L Favennec et al, Aliment Pharmacol Ther
2003; 17:265; JF Rossignol et al, Trans R Soc Trop Med Hyg 1998; 92:103; SM Kabil
et al, Curr Ther Res 2000; 61:339).
41. J Keiser et al, Expert Opin Investig Drugs 2005; 14:1513.
42. Triclabendazole may be effective in a dosage of 5 mg/kg PO once/d x 3 d or 10 mg/
kg PO bid x 1 d (M Calvopiña et al, Trans R Soc Trop Med Hyg 1998; 92:566). See
footnote 40 for availability.
43. Another alternative is albendazole 400 mg/d PO x 5 d in adults and 10 mg/kg/d PO
x 5 d in children (K Yereli et al, Clin Microbiol Infect 2004; 10:527; O Karabay et al,
World J Gastroenterol 2004; 10:1215). Combination treatment with standard doses of
metronidazole and quinacrine x 3 wks has been effective for a small number of refractory
infections (TE Nash et al, Clin Infect Dis 2001; 33:22). In one study, nitazoxanide was
used successfully in high doses to treat a case of Giardia resistant to metronidazole and
albendazole (P Abboud et al, Clin Infect Dis 2001; 32:1792).
44. Poorly absorbed; may be useful for treatment of giardiasis in pregnancy.
45. Quinacrine should be taken with liquids after a meal.
46. P Nontasut et al, Southeast Asian J Trop Med Pub Health 2005; 36:650; M de Gorgolas
et al, J Travel Med 2003; 10:358. All patients should be treated with medication whether
surgery is attempted or not.
47. ME Wilson et al, Clin Infect Dis 2001; 32:1378; G Molavi et al, J Helminth 2006;
80:425.
48. Usually a self-limited illness in immunocompetent patients. Immunosuppressed patients may
need higher doses, longer duration (TMP/SMX qid x 10 d, followed by bid x 3 wks) and
long-term maintenance. In sulfonamide-sensitive patients, pyrimethamine 50-75 mg daily
in divided doses (plus leucovorin 10-25 mg/d) has been effective.
49. To maximize effectiveness and minimize toxicity, the choice of drug, dosage, and duration
of therapy should be individualized based on the region of disease acquisition, a likely
infecting species, and host factors such as immune status (BL Herwaldt, Lancet 1999;
354:1191). Some of the listed drugs and regimens are effective only against certain
Leishmania species/strains and only in certain areas of the world (J Arevalo et al, Clin
Infect Dis 2007; 195:1846). Medical Letter consultants recommend consultation with
physicians experienced in management of this disease.
50. Visceral infection is most commonly due to the Old World species L. donovani (kala-azar)
and L. infantum and the New World species L. chagasi.
51. Liposomal amphotericin B (AmBisome) is the only lipid formulation of amphotericin B
FDA-approved for treatment of visceral leishmania, largely based on clinical trials in

277
Appendix
Appendix
patients infected with L. infantum (A Meyerhoff, Clin Infect Dis 1999; 28:42). Two other
amphotericin B lipid formulations, amphotericin B lipid complex (Abelcet) and amphotericin
B cholesteryl sulfate (Amphotec) have been used, but are considered investigational for
this condition and may not be as effective (C Bern et al, Clin Infect Dis 2006; 43:917).
52. The FDA-approved dosage regimen for immunocompromised patients (e.g., HIV infected) is
4 mg/kg/d IV on days 1-5, 10, 17, 24, 31 and 38. The relapse rate is high; maintenance
therapy (secondary prevention) may be indicated, but there is no consensus as to dosage
or duration.
53. Effective for both antimony-sensitive and -resistant L. donovani (Indian); miltefosine
(Impavido) is manufactured in 10- or 50-mg capsules by Zentaris (Frankfurt, Germany
at info@zentaris.com) and is available through consultation with the CDC. The drug is
contraindicated in pregnancy; a negative pregnancy test before drug initiation and effective
contraception during and for 2 months after treatment is recommended (H Murray et al,
Lancet 2005; 366:1561). In a placebo-controlled trial in patients ≥12 years old, oral
miltefosine 2.5 mg/kg/d x 28 d was also effective for treatment of cutaneous leishmaniasis
due to L.(V.) panamensis in Colombia, but not L.(V.) braziliensis or L. mexicana in Guatemala
(J Soto et al, Clin Infect Dis 2004; 38:1266). “Motion sickness,” nausea, headache and
increased creatinine are the most frequent adverse effects (J Soto and P Soto, Expert Rev
Anti Infect Ther 2006; 4:177).
54. Paromomycin IM has been effective against leishmania in India; it has not yet been tested
in South America or the Mediterranean and there is insuffi cient data to support its use
in pregnancy (S Sundar et al, N Engl J Med 2007; 356:2371). Topical paromomycin
should be used only in geographic regions where cutaneous leishmaniasis species
have low potential for mucosal spread. A formulation of 15% paromomycin/12%
methylbenzethonium chloride (Leshcutan) in soft white paraffi n for topical use has been
reported to be partially effective against cutaneous leishmaniasis due to L. major in Israel
and L. mexicana and L. (V.) braziliensis in Guatemala, where mucosal spread is very
rare (BA Arana et al, Am J Trop Med Hyg 2001; 65:466). The methylbenzethonium is
irritating to the skin; lesions may worsen before they improve.
55. Cutaneous infection is most commonly due to the Old World species L. major and L. tropica
and the New World species L. mexicana, L. (Viannia) braziliensis, and others.
56. Although azole drugs (fl uconazole, ketoconazole, itraconazole) have been used to treat
cutaneous disease, they are not reliably effective and have no effi cacy against mucosal
disease (AJ Magill, Infect Dis Clin North Am 2005; 19:241). For treatment of L. major
cutaneous lesions, a study in Saudi Arabia found that oral fl uconazole, 200 mg once/d
x 6 wks appeared to speed healing (AA Alrajhi et al, N Engl J Med 2002; 346:891).
Thermotherapy may be an option for cutaneous L. tropica infection (R Reithinger et al, Clin
Infect Dis 2005; 40:1148). A device that generates focused and controlled heating of
the skin has been approved by the FDA for this indication (ThermoMed—ThermoSurgery
Technologies Inc., Phoenix, AZ, 602-264-7300; www.thermosurgery.com).
57. At this dosage pentamidine has been effective in Colombia predominantly against L. (V.)
panamensis (J Soto-Mancipe et al, Clin Infect Dis 1993; 16:417; J Soto et al, Am J Trop
Med Hyg 1994; 50:107). Activity against other species is not well established.
58. Mucosal infection is most commonly due to the New World species L. (V.) braziliensis, L.
(V.) panamensis, or L. (V.) guyanensis.
59. Pediculocides should not be used for infestations of the eyelashes. Such infestations are
treated with petrolatum ointment applied 2-4 x/d x 8-10 d. Oral TMP/SMX has also been
used (TL Meinking and D Taplin, Curr Probl Dermatol 1996; 24:157). For pubic lice,
treat with 5% permethrin or ivermectin as for scabies. TMP/SMX has also been effective
when used together with permethrin for head lice (RB Hipolito et al, Pediatrics 2001;
107:E30).
60. Malathion is both ovicidal and pediculocidal; 2 applications at least 7 days apart are
generally necessary to kill all lice and nits.
61. Permethrin and pyrethrin are pediculocidal; retreatment in 7-10 d is needed to eradicate
the infestation. Some lice are resistant to pyrethrins and permethrin (TL Meinking et al,
Arch Dermatol 2002; 138:220).

278
Medical Parasitology
Appendix
62. Ivermectin is pediculocidal, but more than one dose is generally necessary to eradicate
the infestation (KN Jones and JC English 3rd, Clin Infect Dis 2003; 36:1355). The number
of doses and interval between doses has not been established, but in one study of body
lice, 3 doses administered at 7-day intervals were effective (C Fouault et al, J Infect Dis
2006; 193:474).
63. Chloroquine-resistant P. falciparum occurs in all malarious areas except Central America
(including Panama north and west of the Canal Zone), Mexico, Haiti, the Dominican
Republic, Paraguay, northern Argentina, North and South Korea, Georgia, Armenia, most
of rural China and some countries in the Middle East (chloroquine resistance has been
reported in Yemen, Oman, Saudi Arabia and Iran). For treatment of multiple-drug-resistant P.
falciparum in Southeast Asia, especially Thailand, where mefl oquine resistance is frequent,
atovaquone/proguanil, quinine plus either doxycycline or clindamycin, or artemether/
lumefantrine may be used.
64. P. vivax with decreased susceptibility to chloroquine is a signifi cant problem in Papua-New
Guinea and Indonesia. There are also a few reports of resistance from Myanmar, India,
the Solomon Islands, Vanuatu, Guyana, Brazil, Colombia and Peru (JK Baird et al, Curr
Infect Dis Rep 2007; 9:39).
65. Chloroquine-resistant P. malariae has been reported from Sumatra (JD Maguire et al,
Lancet 2002; 360:58).
66. Uncomplicated or mild malaria may be treated with oral drugs. Severe malaria (e.g.
impaired consciousness, parasitemia >5%, shock, etc.) should be treated with parenteral
drugs (KS Griffi n et al, JAMA 2007; 297:2264).
67. Primaquine is given for prevention of relapse after infection with P. vivax or P. ovale. Some
experts also prescribe primaquine phosphate 30 mg base/d (0.6 mg base/kg/d for
children) for 14 d after departure from areas where these species are endemic (Presumptive
Anti-Relapse Therapy [PART], “terminal prophylaxis”). Since this is not always effective as
prophylaxis (E Schwartz et al, N Engl J Med 2003; 349:1510), others prefer to rely on
surveillance to detect cases when they occur, particularly when exposure was limited or
doubtful. See also footnote 79.
68. Atovaquone/proguanil is available as a fi xed-dose combination tablet: adult tablets
(Malarone; 250 mg atovaquone/100 mg proguanil) and pediatric tablets (Malarone
Pediatric; 62.5 mg atovaquone/25 mg proguanil). To enhance absorption and reduce
nausea and vomiting, it should be taken with food or a milky drink. Safety in pregnancy
is unknown; outcomes were normal in 24 women treated with the combination in the
2nd and 3rd trimester (R McGready et al, Eur J Clin Pharmacol 2003; 59:545). The
drug should not be given to patients with severe renal impairment (creatinine clearance
<30mL/min). There have been isolated case reports of resistance in P. falciparum in
Africa, but Medical Letter consultants do not believe there is a high risk for acquisition of
Malarone-resistant disease (E Schwartz et al, Clin Infect Dis 2003; 37:450; A Farnert et
al, BMJ 2003; 326:628; S Kuhn et al, Am J Trop Med Hyg 2005; 72:407; CT Happi et
al, Malaria Journal 2006; 5:82).
69. Although approved for once-daily dosing, Medical Letter consultants usually divide the
dose in two to decrease nausea and vomiting.
70. Available in the US in a 324-mg capsule; 2 capsules suffi ce for adult dosage. In Southeast
Asia, relative resistance to quinine has increased and treatment should be continued for 7 d.
Quinine should be taken with or after meals to decrease gastrointestinal adverse effects.
71. Doxycycline should be taken with adequate water to avoid esophageal irritation. It can
be taken with food to minimize gastrointestinal adverse effects.
72. For use in pregnancy and in children <8 yrs.
73. B Lell and PG Kremsner, Antimicrob Agents Chemother 2002; 46:2315; M Ramharter et
al, Clin Infect Dis 2005; 40:1777.
74. At this dosage, adverse effects include nausea, vomiting, diarrhea and dizziness. Disturbed
sense of balance, toxic psychosis and seizures can also occur. Mefl oquine should not
be used for treatment of malaria in pregnancy unless there is no other treatment option
because of increased risk for stillbirth (F Nosten et al, Clin Infect Dis 1999; 28:808).
It should be avoided for treatment of malaria in persons with active depression or with
a history of psychosis or seizures and should be used with caution in persons with any
psychiatric illness. Mefl oquine can be given to patients taking
β
-blockers if they do not have

279
Appendix
Appendix
an underlying arrhythmia; it should not be used in patients with conduction abnormalities.
Mefl oquine should not be given together with quinine or quinidine, and caution is required
in using quinine or quinidine to treat patients with malaria who have taken mefl oquine
for prophylaxis. Mefl oquine should not be taken on an empty stomach; it should be taken
with at least 8 oz of water.
75. P. falciparum with resistance to mefl oquine is a signifi cant problem in the malarious areas
of Thailand and in areas of Myanmar and Cambodia that border on Thailand. It has also
been reported on the borders between Myanmar and China, Laos and Myanmar, and in
Southern Vietnam. In the US, a 250-mg tablet of mefl oquine contains 228 mg mefl oquine
base. Outside the US, each 275-mg tablet contains 250 mg base.
76. The artemisinin-derivatives, artemether and artesunate, are both frequently used globally
in combination regimens to treat malaria. Both are available in oral, parenteral and rectal
formulations, but manufacturing standards are not consistent (HA Karunajeewa et al, JAMA
2007; 297:2381; EA Ashley and NJ White, Curr Opin Infect Dis 2005; 18:531). In the US,
only the IV formulation of artesunate is available; it can be obtained through the CDC under
an IND for patients with severe disease who do not have timely access, cannot tolerate, or
fail to respond to IV quinidine (www.cdc.gov/malaria/features/artesunate_now_available.
htm). To avoid development of resistance, monotherapy should be avoided (PE Duffy and
CH Sibley, Lancet 2005; 366:1908). In animal studies artemisinins have been embryotoxic
and caused a low incidence of teratogenicity; no adverse pregnancy outcome has been
observed in limited studies in humans (S Dellicour et al, Malaria Journal 2007; 6:15).
77. Artemether/lumefantrine is available as a fi xed-dose combination tablet (Coartem in
countries with endemic malaria, Riamet in Europe and countries without endemic malaria);
each tablet contains 20 mg artemether and 120 mg lumefantrine (M van Vugt et al, Am J
Trop Med Hyg 1999; 60:936). It is contraindicated during the fi rst trimester of pregnancy;
safety during the second and third trimester is not known. The tablets should be taken with
food. Artemether/lumefantrine should not be used in patients with cardiac arrhythmias,
bradycardia, severe cardiac disease or QT prolongation. Concomitant use of drugs that
prolong the QT interval or are metabolized by CYP2D6 is contraindicated.
78. Adults treated with artesunate should also receive oral treatment doses of either
atovaquone/proguanil, doxycycline, clindamycin or mefl oquine; children should take
either atovaquone/proguanil, clindamycin or mefl oquine (F Nosten et al, Lancet 2000;
356:297; M van Vugt, Clin Infect Dis 2002; 35:1498; F Smithuis et al, Trans R Soc Trop
Med Hyg 2004; 98:182). If artesunate is given IV, oral medication should be started
when the patient is able to tolerate it (SEAQUAMAT group, Lancet 2005; 366:717).
79. Primaquine phosphate can cause hemolytic anemia, especially in patients whose red
cells are defi cient in G-6-PD. This defi ciency is most common in African, Asian and
Mediterranean peoples. Patients should be screened for G-6-PD defi ciency before treatment.
Primaquine should not be used during pregnancy. It should be taken with food to minimize
nausea and abdominal pain. Primaquine-tolerant P. vivax can be found globally. Relapses
of primaquine-resistant strains may be retreated with 30 mg (base) x 28 d.
80. Chloroquine should be taken with food to decrease gastrointestinal adverse effects. If
chloroquine phosphate is not available, hydroxychloroquine sulfate is as effective; 400
mg of hydroxychloroquine sulfate is equivalent to 500 mg of chloroquine phosphate.
81. Chloroquine combined with primaquine was effective in 85% of patients with P. vivax
resistant to chloroquine and could be a reasonable choice in areas where other alternatives
are not available (JK Baird et al, J Infect Dis 1995; 171:1678).
82. Exchange transfusion is controversial, but has been helpful for some patients with
high-density (>10%) parasitemia, altered mental status, pulmonary edema or renal
complications (VI Powell and K Grima, Transfus Med Rev 2002; 16:239; MS Riddle et
al, Clin Infect Dis 2002; 34:1192).
83. Continuous EKG, blood pressure and glucose monitoring are recommended, especially
in pregnant women and young children. For problems with quinidine availability, call
the manufacturer (Eli Lilly, 800-821-0538) or the CDC Malaria Hotline (770-488-7788).
Quinidine may have greater antimalarial activity than quinine. The loading dose should
be decreased or omitted in patients who have received quinine or mefl oquine. If more
than 48 hours of parenteral treatment is required, the quinine or quinidine dose should
be reduced by 30-50%.

280
Medical Parasitology
Appendix
84. No drug guarantees protection against malaria. Travelers should be advised to seek medical
attention if fever develops after they return. Insect repellents, insecticide- impregnated bed
nets and proper clothing are important adjuncts for malaria prophylaxis (Med Lett Drugs
Ther 2005; 47:100). Malaria in pregnancy is particularly serious for both mother and
fetus; prophylaxis is indicated if exposure cannot be avoided.
85. Alternatives for patients who are unable to take chloroquine include atovaquone/proguanil,
mefl oquine, doxycycline or primaquine dosed as for chloroquine-resistant areas.
86. Has been used extensively and safely for prophylaxis in pregnancy.
87. Beginning 1-2 wks before travel and continuing weekly for the duration of stay and for 4
wks after leaving.
88. Beginning 1-2 d before travel and continuing for the duration of stay and for 1 wk after
leaving. In one study of malaria prophylaxis, atovaquone/proguanil was better tolerated
than mefl oquine in nonimmune travelers (D Overbosch et al, Clin Infect Dis 2001;
33:1015). The protective effi cacy of Malarone against P. vivax is variable ranging from
84% in Indonesian New Guinea (J Ling et al, Clin Infect Dis 2002; 35:825) to 100%
in Colombia (J Soto et al, Am J Trop Med Hyg 2006; 75:430). Some Medical Letter
consultants prefer alternate drugs if traveling to areas where P. vivax predominates.
89. Beginning 1-2 d before travel and continuing for the duration of stay and for 4 wks after
leaving. Use of tetracyclines is contraindicated in pregnancy and in children <8 years old.
Doxycycline can cause gastrointestinal disturbances, vaginal moniliasis and photosensitivity
reactions.
90. Mefl oquine has not been approved for use during pregnancy. However, it has been
reported to be safe for prophylactic use during the second and third trimester of pregnancy
and possibly during early pregnancy as well (CDC Health Information for International
Travel, 2008, page 228; BL Smoak et al, J Infect Dis 1997; 176:831). For pediatric
doses <½ tablet, it is advisable to have a pharmacist crush the tablet, estimate doses by
weighing, and package them in gelatin capsules.There is no data for use in children <5
kg, but based on dosages in other weight groups, a dose of 5 mg/kg can be used. Not
recommended for use in travelers with active depression or with a history of psychosis or
seizures and should be used with caution in persons with psychiatric illness. Mefl oquine
can be given to patients taking
β
-blockers if they do not have an underlying arrhythmia;
it should not be used in patients with conduction abnormalities.
91. Beginning 1-2 wks before travel and continuing weekly for the duration of stay and for 4 wks
after leaving. Most adverse events occur within 3 doses. Some Medical Letter consultants
favor starting mefl oquine 3 weeks prior to travel and monitoring the patient for adverse
events, this allows time to change to an alternative regimen if mefl oquine is not tolerated.
92. The combination of weekly chloroquine (300 mg base) and daily proguanil (200 mg)
is recommended by the World Health Organization (www.WHO.int) for use in selected
areas; this combination is no longer recommended by the CDC. Proguanil (Paludrine—
AstraZeneca, United Kingdom) is not available alone in the US but is widely available
in Canada and Europe. Prophylaxis is recommended during exposure and for 4 weeks
afterwards. Proguanil has been used in pregnancy without evidence of toxicity (PA
Phillips-Howard and D Wood, Drug Saf 1996; 14:131).
93. Studies have shown that daily primaquine beginning 1d before departure and continued
until 3-7 d after leaving the malarious area provides effective prophylaxis against
chloroquine-resistant P. falciparum (JK Baird et al, Clin Infect Dis 2003; 37:1659). Some
studies have shown less effi cacy against P. vivax. Nausea and abdominal pain can be
diminished by taking with food.
94. A traveler can be given a course of medication for presumptive self-treatment of febrile
illness. The drug given for self-treatment should be different from that used for prophylaxis.
This approach should be used only in very rare circumstances when a traveler would not
be able to get medical care promptly.
95. CM Chan et al, Ophthalmology 2003; 110:1420. Ocular lesions due to E. hellem in
HIV-infected patients have responded to fumagillin eyedrops prepared from Fumidil-B
(bicyclohexyl ammonium fumagillin) used to control a microsporidial disease of honey bees (MJ
Garvey et al, Ann Pharmacother 1995; 29:872), available from Leiter’s Park Avenue Pharmacy
(see footnote 1). For lesions due toV. corneae, topical therapy is generally not effective and
keratoplasty may be required (RM Davis et al, Ophthalmology 1990; 97:953).

281
Appendix
Appendix
96. Oral fumagillin (Flisint—Sanofi -Aventis, France) has been effective in treating E. bieneusi
(J-M Molina et al, N Engl J Med 2002; 346:1963), but has been associated with
thrombocytopenia and neutropenia. Highly active antiretroviral therapy (HAART) may
lead to microbiologic and clinical response in HIV-infected patients with microsporidial
diarrhea. Octreotide (Sandostatin) has provided symptomatic relief in some patients with
large-volume diarrhea.
97. J-M Molina et al, J Infect Dis 1995; 171:245. There is no established treatment for
Pleistophora. For disseminated disease due toTrachipleistophora or Brachiola, itraconazole
400 mg PO once/d plus albendazole may also be tried (CM Coyle et al, N Engl J Med
2004; 351:42).
98. Albendazole or pyrantel pamoate may be effective (JB Ziem et al, Ann Trop Med Parasitol
2004; 98:385).
99. Pneumocystis has been reclassifi ed as a fungus. In severe disease with room air PO
2
≤
70 mmHg or Aa gradient ≥ 35 mmHg, prednisone should also be used (S Gagnon et al,
N Engl J Med 1990; 323:1444; E Caumes et al, Clin Infect Dis 1994; 18:319).
100. Primary/secondary prophylaxis in patients with HIV can be discontinued after CD4 count
increases to >200 x 10
6
/L for >3 mos.
101. Plus leucovorin 25 mg with each dose of pyrimethamine. Pyrimethamine should be taken
with food to minimize gastrointestinal adverse effects.
102. Treatment may need to be repeated in 10-14 days. A second ivermectin dose taken 2
weeks later increases the cure rate to 95%, which is equivalent to that of 5% permethrin
(V Usha et al, J Am Acad Dermatol 2000; 42:236; O Chosidow, N Engl J Med 2006;
354:1718; J Heukelbach and H Feldmeier, Lancet 2006; 367:1767).
103. Lindane (γ-benzene hexachloride) should be reserved for treatment of patients who fail to
respond to other drugs. The FDA has recommended it not be used for immunocompromised
patients, young children, the elderly, pregnant and breast-feeding women, and patients
weighing <50 kg.
104. Ivermectin, either alone or in combination with a topical scabicide, is the drug of choice
for crusted scabies in immunocompromised patients (P del Giudice, Curr Opin Infect Dis
2004; 15:123).
105. Oxamniquine, which is not available in the US, is generally not as effective as praziquantel.
It has been useful, however, in some areas in which praziquantel is less effective (ML Ferrari
et al, Bull World Health Organ 2003; 81:190; A Harder, Parasitol Res 2002; 88:395).
Oxamniquine is contraindicated in pregnancy. It should be taken after food.
106. In East Africa, the dose should be increased to 30 mg/kg, and in Egypt and South Africa
to 30 mg/kg/d x 2 d. Some experts recommend 40-60 mg/kg over 2-3 d in all of Africa
(KC Shekhar, Drugs 1991; 42:379).
107. In immunocompromised patients or disseminated disease, it may be necessary to prolong
or repeat therapy, or to use other agents. Veterinary parenteral and enema formulations
of ivermectin have been used in severely ill patients with hyperinfection who were unable
to take or reliably absorb oral medications (J Orem et al, Clin Infect Dis 2003; 37:152;
PE Tarr Am J Trop Med Hyg 2003; 68:453; FM Marty et al, Clin Infect Dis 2005; 41:e5).
In disseminated strongyloidiasis, combination therapy with albendazole and ivermectin
has been suggested (S Lim et al, CMAJ 2004; 171:479).
108. Niclosamide must be chewed thoroughly before swallowing and washed down with
water.
109. JO Juan et al, Trans R Soc Trop Med Hyg 2002; 96:193; JC Chero et al, Trans R Soc
Trop Med Hyg 2007; 101:203; E Diaz et al, Am J Trop Med Hyg 2003; 68:384.
110. Patients may benefi t from surgical resection or percutaneous drainage of cysts. Praziquantel
is useful preoperatively or in case of spillage of cyst contents during surgery. Percutaneous
aspiration-injection-reaspiration (PAIR) with ultrasound guidance plus albendazole therapy
has been effective for management of hepatic hydatid cyst disease (RA Smego, Jr. et al,
Clin Infect Dis 2003; 37:1073; S Nepalia et al, J Assoc Physicians India 2006; 54:458;
E Zerem and R Jusufovic Surg Endosc 2006; 20:1543).
111. Surgical excision is the only reliable means of cure. Reports have suggested that in
nonresectable cases use of albendazole (400 mg bid) can stabilize and sometimes cure
infection (P Craig, Curr Opin Infect Dis 2003; 16:437; O Lidove et al, Am J Med 2005;
118:195).

282
Medical Parasitology
Appendix
112. Initial therapy for patients with infl amed parenchymal cysticercosis should focus on
symptomatic treatment with anti-seizure medication (LS Yancey et al, Curr Infect Dis
Rep 2005; 7:39; AH del Brutto et al, Ann Intern Med 2006; 145:43). Patients with
live parenchymal cysts who have seizures should be treated with albendazole together
with steroids (dexamethasone 6 mg/d or prednisone 40-60 mg/d) and an anti-seizure
medication (HH Garcia et al, N Engl J Med 2004; 350:249). Patients with subarachnoid
cysts or giant cysts in the fi ssures should be treated for at least 30 d (JV Proaño et al, N
Engl J Med 2001; 345:879). Surgical intervention (especially neuroendoscopic removal)
or CSF diversion followed by albendazole and steroids is indicated for obstructive
hydocephalus. Arachnoiditis, vasculitis or cerebral edema is treated with prednisone
60 mg/d or dexamethasone 4-6 mg/d together with albendazole or praziquantel (AC
White, Jr., Annu Rev Med 2000; 51:187). Any cysticercocidal drug may cause irreparable
damage when used to treat ocular or spinal cysts, even when corticosteroids are used.
An ophthalmic exam should always precede treatment to rule out intraocular cysts.
113. To treat CNS toxoplasmosis in HIV-infected patients, some clinicians have used
pyrimethamine 50-100 mg/d (after a loading dose of 200 mg) with sulfadiazine and, when
sulfonamide sensitivity developed, have given clindamycin 1.8-2.4 g/d in divided doses
instead of the sulfonamide. Treatment is usually given for at least 4-6 weeks. Atovaquone
(1500 mg PO bid) plus pyrimethamine (200 mg loading dose, followed by 75 mg/d PO)
for 6 weeks appears to be an effective alternative in sulfa-intolerant patients (K Chirgwin
et al, Clin Infect Dis 2002; 34:1243). Atovaquone must be taken with a meal to enhance
absorption. Treatment is followed by chronic suppression with lower dosage regimens of
the same drugs. For primary prophylaxis in HIV patients with <100 x 106/L CD4 cells,
either trimethoprim-sulfamethoxazole, pyrimethamine with dapsone, or atovaquone with or
without pyrimethamine can be used. Primary or secondary prophylaxis may be discontinued
when the CD4 count increases to >200 x 106/L for >3mos (MMWR Morb Mortal Wkly
Rep 2004; 53 [RR15]:1). In ocular toxoplasmosis with macular involvement, corticosteroids
are recommended in addition to antiparasitic therapy for an anti-infl ammatory effect.
In one randomized single-blind study, trimethoprim/sulfamethoxazole was reported to
be as effective as pyrimethamine/sulfadiazine for treatment of ocular toxoplasmosis (M
Soheilian et al, Ophthalmology 2005; 112:1876). Women who develop toxoplasmosis
during the fi rst trimester of pregnancy should be treated with spiramycin (3-4 g/d). After
the fi rst trimester, if there is no documented transmission to the fetus, spiramycin can be
continued until term. If transmission has occurred in utero, therapy with pyrimethamine and
sulfadiazine should be started (JG Montoya and O Liesenfeld, Lancet 2004; 363:1965).
Pyrimethamine is a potential teratogen and should be used only after the fi rst trimester.
114. Plus leucovorin 10-25 mg with each dose of pyrimethamine. Pyrimethamine should be
taken with food to minimize gastrointestinal adverse effects.
115. Congenitally infected newborns should be treated with pyrimethamine every 2 or 3
days and a sulfonamide daily for about one year (JS Remington and G Desmonts in JS
Remington and JO Klein, eds, Infectious Disease of the Fetus and Newborn Infant, 6th
ed, Philadelphia: Saunders, 2006:1038).
116. Sulfadiazine should be taken on an empty stomach with adequate water.
117. Sexual partners should be treated simultaneously with same dosage. Metronidazole-resistant
strains have been reported and can be treated with higher doses of metronidazole (2-4
g/d x 7-14 d) or with tinidazole (MMWR Morb Mortal Wkly Rep 2006; 55 [RR11]:1).
118. MP Barrett et al, Lancet 2003; 362:1469. Treatment of chronic or indeterminate Chagas’
disease with benznidazole has been associated with reduced progression and increased
negative seroconversion (R Viotti et al, Ann Intern Med 2006; 144:724).
119. Benznidazole should be taken with meals to minimize gastrointestinal adverse effects. It
is contraindicated during pregnancy.
120. Pentamidine and suramin have equal effi cacy, but pentamidine is better tolerated.
121. Efl ornithine is highly effective in T.b. gambiense, but not in T.b. rhodesiense infections. In
one study of treatment of CNS disease due to T.b. gambiense, there were fewer serious
complications with efl ornithine than with melarsoprol (F Chappuis et al, Clin Infect Dis
2005; 41:748). Efl ornithine is available in limited supply only from the WHO. It is
contraindicated during pregnancy.

283
Appendix
Appendix
122. E Schmid et al, J Infect Dis 2005; 191:1922. Corticosteroids have been used to prevent
arsenical encephalopathy (J Pepin et al, Trans R Soc Trop Med Hyg 1995; 89:92). Up
to 20% of patients with T.b.gambiense fail to respond to melarsoprol (MP Barrett, Lancet
1999; 353:1113). In one study, a combination of low-dose melarsoprol (1.2 mg/kg/d
IV) and nifurtimox (7.5 mg/kg PO bid) x 10d was more effective than standard-dose
melarsoprol alone (S Bisser et al, J Infect Dis 2007; 195:322).
123. Optimum duration of therapy is not known; some Medical Letter consultants
would treat x 20 d. For severe symptoms or eye involvement, corticosteroids can
be used in addition (D Despommier, Clin Microbiol Rev 2003; 16:265).

284
Medical Parasitology
Appendix
Safety of Antiparasitic Drugs
in Pregnancy
Drug
Toxicity in Pregnancy
Recommendations
Albendazole (Albenza)
Teratogenic and embryotoxic
Caution*
in animals
Amphotericin B
None known
Caution*
(Fungizone, and others)
Amphotericin B liposomal None known
Caution*
(AmBisome)
Artemether/lumefantrine
Embryocidal and teratogenic
Caution*
(Coartem, Riamet)
1
in animals
Artesunate
1
Embryocidal and teratogenic
Caution*
in animals
Atovaquone (Mepron)
Maternal and fetal toxicity
Caution*
in animals
Atovaquone/proguanil
Maternal and fetal toxicity
Caution*
(Malarone)
2
in animals
Azithromycin (Zithromax, None known
Probably safe
and others)
Benznidazole (Rochagan) Unknown
Contraindicated
Chloroquine (Aralen,
None known with doses
Probably safe in low
and others)
recommended for malaria
doses
prophylaxis
Clarithromycin (Biaxin,
Teratogenic in animals
Contraindicated
and others)
Clindamycin (Cleocin,
None known
Caution*
and others)
Crotamiton (Eurax) Unknown
Caution*
Dapsone
None known; carcinogenic
Caution*, especially
in rats and mice; hemolytic
at term
reactions in neonates
Diethylcarbamazine
Not known; abortifacient
Contraindicated
(DEC; Hetrazan)
in one study in rabbits
Diloxanide (Furamide)
Safety not established
Caution*
Doxycycline (Vibramycin, Tooth discoloration and dysplasia, Contraindicated
and others)
inhibition of bone growth in fetus;
hepatic toxicity and azotemia
with IV use in pregnant patients
with decreased renal function
or with overdosage
Efl ornithine (Ornidyl)
Embryocidal in animals
Contraindicated
Fluconazole (Difl ucan,
Teratogenic
Contraindicated for
and others)
high dose; caution*
for single dose
Flucytosine (Ancoban)
Teratogenic in rats
Contraindicated
Furazolidone (Furoxone)
None known; carcinogenic in
Caution*;
rodents; hemolysis with
contraindicated
G-6-PD defi ciency in newborn
at term

285
Appendix
Appendix
Drug
Toxicity in Pregnancy
Recommendations
Hydroxychloroquine
None known with doses
Probably safe
(Plaquenil)
recommended for malaria
in low doses
prophylaxis
Itraconazole (Sporanox,
Teratogenic and embryotoxic
Caution*
and others)
in rats
Iodoquinol (Yodoxin,
Unknown
Caution*
and others)
Ivermectin (Stromectol)
Teratogenic in animals
Contraindicated
Ketoconazole (Nizoral,
Teratogenic and embryotoxic
Contraindicated;
and others)
in rats
topical probably safe
Lindane
Absorbed from the skin; potential Contraindicated
CNS toxicity in fetus
Malathion, topical (Ovide) None known
Probably safe
Mebendazole (Vermox)
Teratogenic and embryotoxic in rats Caution*
Mefl oquine (Lariam)
3
Teratogenic in animals
Caution*
Meglumine (Glucantine) Not
known
Caution*
Metronidazole (Flagyl,
None known—carcinogenic
Caution*
and others)
in rats and mice
Miconazole (Monistat i.v.) None known
Caution*
Miltefosine (Impavido)
Teratogenic in rats and induces
Contraindicated;
abortions in animals
effective contra-
ception must be
used for 2 months
after the last dose
Niclosamide (Niclocide)
Not absorbed; no known toxicity Probably safe
in fetus
Nitazoxanide (Alinia) None
known
Caution*
Oxamniquine (Vansil)
Embryocidal in animals
Contraindicated
Paromomycin (Humatin)
Poorly absorbed; toxicity in fetus Oral capsules
unknown
probably safe
Pentamidine (Pentam 300, Safety not established
Caution*
NebuPent, and others)
Permethrin (Nix, and
Poorly absorbed; no known
Probably safe
others)
toxicity in fetus
Praziquantel (Biltricide)
Not known
Probably safe
Primaquine
Hemolysis in G-6-PD defi ciency
Contraindicated
Pyrantel pamoate
Absorbed in small amounts;
Probably safe
(Antiminth, and others)
no known toxicity in fetus
Pyrethrins and piperonyl
Poorly absorbed; no known
Probably safe
butoxide (RID, and others) toxicity in fetus
Pyrimethamine
Teratogenic in animals
Caution*; contra-
(Daraprim)
4
indicated during
1st trimester
Quinacrine (Atabrine)
Safety not established
Caution*
Quinidine
Large doses can cause abortion
Probably safe
Quinine (Qualaquin)
Large doses can cause abortion; Caution*
auditory nerve hypoplasia,
deafness in fetus; visual
changes, limb anomalies,
visceral defects also reported
286
Medical Parasitology
Appendix
Drug
Toxicity in Pregnancy
Recommendations
Sodium stibogluconate
Not known
Caution*
(Pentostam)
Sulfonamides
Teratogenic in some animal
Caution*; contra-
studies; hemolysis in newborn
indicated at term
with G-6-PD defi ciency;
increased risk of kernicterus
in newborn
Suramin sodium
Teratogenic in mice
Caution*
(Germanin)
Tetracycline (Sumycin,
Tooth discoloration and dysplasia, Contraindicated
and others)
inhibition of bone growth in
fetus; hepatic toxicity and
azotemia with IV use in pregnant
patients with decreased renal
function or with overdosage
Tinidazole (Tindamax)
Increased fetal mortality in rats
Caution*
Trimethoprim (Proloprim, Folate antagonism; teratogenic
Caution*
and others)
in rats
Trimethoprim-sulfa-
Same as sulfonamides and
Caution*; contra-
methoxazole (Bactrim,
trimethoprim
indicated at term
and others)
* Use only for strong clinical indication in absence of suitable alternative.
1. See also footnote 76 in previous table.
2. See also footnote 68 in previous table.
3. See also footnotes 74 and 90 in previous table.
4. See also footnote 113 in previous table.

287
Appendix
Appendix
Manufacturers of Drugs Used to Treat
Parasitic Infections
albendazole—Albenza
(GlaxoSmithKline)
Albenza (GlaxoSmithKline)—albendazole
Alinia
(Romark)—nitazoxanide
AmBisome (Gilead)—amphotericin B, liposomal
amphotericin
B—Fungizone (Apothecon), others
amphotericin B, liposomal—AmBisome (Gilead)
Ancobon (Valeant)—fl ucytosine
§ Antiminth (Pfi zer)—pyrantel pamoate
• Aralen (Sanofi )—chloroquine HCl and chloroquine phosphate
§ artemether—Artenam (Arenco, Belgium)
§ artemether/lumefantrine—Coartem, Riamet (Novartis)
§ Artenam (Arenco, Belgium)—artemether
§ artesunate—(Guilin No. 1 Factory, People’s Republic of China)
atovaquone—Mepron (GlaxoSmithKline)
atovaquone/proguanil—Malarone (GlaxoSmithKline)
azithromycin—Zithromax (Pfi zer), others
• Bactrim (Roche)—TMP/Sulfa
§ benznidazole—Rochagan (Brazil)
• Biaxin (Abbott)—clarithromycin
§ Biltricide (Bayer)—praziquantel
† bithionol—Bitin (Tanabe, Japan)
† Bitin (Tanabe, Japan)—bithionol
§ Brolene (Aventis, Canada)—propamidine isethionate chloroquine HCl
and chloroquine phosphate—Aralen (Sanofi ), others
clarithromycin—Biaxin (Abbott), others
• Cleocin (Pfi zer)—clindamycin
clindamycin—Cleocin (Pfi zer), others
Coartem (Novartis)—artemether/lumefantrine
crotamiton—Eurax (Westwood-Squibb)
dapsone—(Jacobus)
§ Daraprim (GlaxoSmithKline)—pyrimethamine USP
† diethylcarbamazine citrate (DEC)—Hetrazan
• Difl ucan (Pfi zer)—fl uconazole
§ diloxanide
furoate—Furamide (Boots, United Kingdom)
doxycycline—Vibramycin (Pfi zer), others
efl ornithine (Difl uoromethylornithine, DFMO)—Ornidyl (Aventis)
§ Egaten (Novartis)—triclabendazole
Elimite (Allergan)—permethrin
Ergamisol (Janssen)—levamisole
Eurax (Westwood-Squibb)—crotamiton
• Flagyl (Pfi zer)—metronidazole
§ Flisint (Sanofi -Aventis, France)—fumagillin
fl uconazole—Difl ucan (Pfi zer), others
fl ucytosine—Ancobon (Valeant)
§ fumagillin—Flisint (Sanofi -Aventis, France)
• Fungizone (Apothecon)—amphotericin
§ Furamide (Boots, United Kingdom)—diloxanide furoate

288
Medical Parasitology
Appendix
§ furazolidone—Furozone (Roberts)
§ Furozone (Roberts)—furazolidone
† Germanin (Bayer, Germany)—suramin sodium
§ Glucantime (Aventis, France)—meglumine antimonate
† Hetrazan—diethylcarbamazine citrate (DEC)
Humatin (Monarch)—paromomycin
§ Impavido (Zentaris, Germany)—miltefosine
iodoquinol—Yodoxin (Glenwood), others
itraconazole—Sporanox (Janssen-Ortho), others
ivermectin—Stromectol (Merck)
ketoconazole—Nizoral (Janssen), others
† Lampit (Bayer, Germany)—nifurtimox
L ariam (Roche)—mefl oquine
§ Leshcutan (Teva, Israel)—topical paromomycin
levamisole—Ergamisol (Janssen)
lumefantrine/artemether—Coartem, Riamet (Novartis)
Malarone (GlaxoSmithKline)—atovaquone/proguanil
malathion—Ovide (Medicis)
mebendazole—Vermox (McNeil), others
mefl oquine—Lariam (Roche)
§ meglumine
antimonate—Glucantime (Aventis, France)
† melarsoprol—Mel-B
† Mel-B—melarsoprol
Mepron (GlaxoSmithKline)—atovaquone
metronidazole—Flagyl (Pfi zer), others
§ miconazole—Monistat i.v.
§ miltefosine—Impavido (Zentaris, Germany)
§ Monistat i.v.—miconazole
NebuPent (Fujisawa)—pentamidine isethionate
Neutrexin (US Bioscience)—trimetrexate
§ niclosamide—Yomesan (Bayer, Germany)
† nifurtimox—Lampit (Bayer, Germany)
nitazoxanide—Alinia (Romark)
• Nizoral (Janssen)—ketoconazole
Nix (GlaxoSmithKline)—permethrin
§ ornidazole—Tiberal (Roche, France)
Ornidyl (Aventis)—efl ornithine (Difl uoromethylornithine, DFMO)
Ovide (Medicis)—malathion
§ oxamniquine—Vansil (Pfi zer)
§ Paludrine (AstraZeneca, United Kingdom)—proguanil
paromomycin—Humatin (Monarch); Leshcutan (Teva, Israel; (topical formulation not
available in US)
Pentam 300 (Fujisawa)—pentamidine isethionate
pentamidine
isethionate—Pentam 300 (Fujisawa), NebuPent (Fujisawa)
† Pentostam (GlaxoSmithKline, United Kingdom)—sodium stibogluconate
permethrin—Nix (GlaxoSmithKline), Elimite (Allergan)
§ praziquantel—Biltricide (Bayer)
primaquine phosphate USP
§ proguanil—Paludrine (AstraZeneca, United Kingdom)
proguanil/atovaquone—Malarone (GlaxoSmithKline)
§ propamidine
isethionate—Brolene (Aventis, Canada)
§ pyrantel
pamoate—Antiminth (Pfi zer)
289
Appendix
Appendix
pyrethrins and piperonyl butoxide—RID (Pfi zer), others
§ pyrimethamine
USP—Daraprim (GlaxoSmithKline)
Qualaquin—quinine sulfate (Mutual Pharmaceutical Co/AR Scientifi c)
* quinidine gluconate (Eli Lilly)
§ quinine
dihydrochloride
quinine
sulfate—Qualaquin (Mutual Pharmaceutical Co/AR Scientifi c)
Riamet (Novartis)—artemether/lumefantrine
• RID (Pfi zer)—pyrethrins and piperonyl butoxide
• Rifadin (Aventis)—rifampin
rifampin—Rifadin (Aventis), others
§ Rochagan (Brazil)—benznidazole
* Rovamycine (Aventis)—spiramycin
† sodium stibogluconate—Pentostam (GlaxoSmithKline, United Kingdom)
* spiramycin—Rovamycine (Aventis)
• Sporanox (Janssen-Ortho)—itraconazole
Stromectol (Merck)—ivermectin
sulfadiazine—(Eon)
† suramin
sodium—Germanin (Bayer, Germany)
§ Tiberal (Roche, France)—ornidazole
Tindamax (Mission)—tinidazole
tinidazole—Tindamax (Mission)
TMP/Sulfa—Bactrim (Roche), others
§ triclabendazole—Egaten (Novartis)
trimetrexate—Neutrexin (US Bioscience)
§ Vansil (Pfi zer)—oxamniquine
• Vermox (McNeil)—mebendazole
• Vibramycin (Pfi zer)—doxycycline
• Yodoxin (Glenwood)—iodoquinol
§ Yomesan (Bayer, Germany)—niclosamide
• Zithromax (Pfi zer)—azithromycin
* Available in the US only from the manufacturer.
§ Not available in the US; may be available through a compounding pharmacy (see footnote
4 in previous Drugs for Parasitic Infections table).
† Available from the CDC Drug Service, Centers for Disease Control and Prevention, Atlanta,
Georgia 30333; 404-639-3670 (evenings, weekends, or holidays: 770-488-7100).
• Also available generically.


Index
I
NDEX
A
Acute schistosomiasis 113, 114, 123,
124, 127, 131, 134
Affi rm™ VPII 225
Afr ican trypanosomiasis 161, 162,
164, 169, 174, 271, 272
AIDS 35, 171, 177, 179, 180, 192,
193, 199, 214, 218, 220, 227,
273, 274
Albendazole 5, 6, 11, 12, 18, 19, 29,
37, 43, 56, 65, 69, 74, 82, 83, 91,
96, 108, 143, 150, 203, 274-276,
281, 282, 284, 287
Amebiasis 206, 208-211, 251, 261,
263, 273
Ameboma 208, 211
Amphotericin B 178, 179, 187, 188,
273, 276, 277, 284, 287
Ancyclostoma braziliense 63, 64
Ancylostoma 21, 23, 60, 63, 64, 262,
265
Anemia 10, 12, 21, 25-27, 73, 93,
105, 108, 114, 122, 132, 159,
164, 165, 192, 233, 240, 279
Angioedema 54, 55
Animal hookworm 63, 64
Arachnida 255
Ascaris lumbricoides 14-16, 18, 19,
24, 71, 262
Atovaquone 220, 233, 234, 244,
246, 266-268, 274, 278-280,
282, 284, 287, 288
Autoinfection 2, 6, 34, 35, 37, 38
Azithromycin 220, 233, 273, 274,
284, 287, 289
B
Baylisascariasis 67, 68, 262, 274
Baylisascariasis procyonis 67-69
Beef tapeworm 138, 139
Benzimidazole 11, 12, 29, 30, 43, 74,
95, 203
Biliary duct 93
Biliary tract 86, 88, 89, 91, 93, 95,
149, 198, 215, 218, 219
Blood smear 99, 166, 176, 237, 238,
242, 243, 245, 246
Bradyzoite 191, 192
C
Calabar swelling 53, 54, 56
Calcifi cation 60, 130, 133, 134, 193
Canine 146, 256
Card agglutination test for
trypanosomiasis (CATT) 166
Cardiomyopathy 41, 155, 157, 160,
165
Cercariae 89, 93, 105, 107, 112, 113,
117, 119, 129, 130
Cerebral malaria 240
Chagas disease 154, 156-160, 169,
177, 252, 262, 271, 282
Chloroquine resistant 244, 246,
266-268, 278, 280
Cholangiocarcinoma 89, 91
Chorioretinitis 49, 50, 192, 193
Chronic schistosomiasis 113, 119,
124, 132, 134
Chrysops spp 53
Clindamycin 193, 233, 244, 274,
278, 279, 282, 284, 287
Clonorchiasis 86-91, 109
Clonorchis 86-88, 262, 264
Colitis 4, 5, 10, 11, 208, 211, 213
Congenital toxoplasmosis 190, 192,
193
Copepod 58, 59, 61
Corticosteroid 35, 43, 56, 69, 74,
131, 134, 143, 193, 208, 234,
272, 274, 275, 282, 283

292
Medical Parasitology
Index
Creeping eruption 63, 64, 263
Cryptosporidium 203, 205,
214-216, 218-220, 262, 273
Cutaneous larva migrans (CLM) 21,
26-29, 63-65, 263
Cutaneous leishmaniasis 177, 179,
182-185, 187, 277
Cyclic fever 237
Cyclopiscide 61
Cyst 41, 101, 105, 142, 143,
146-151, 190-193, 195-199,
201, 204-208, 210, 211, 222,
228-231, 234, 265, 270, 272,
281, 282
Cysticerca 138
D
Dapsone 233, 235, 269, 282, 284,
287
DFMO 168, 169, 287, 288
Diagnosis 4-6, 11, 17, 18, 27, 37,
43, 49, 55, 60, 65, 68, 73, 74,
80, 82, 90, 93-96, 98, 101, 102,
106, 108, 109, 114, 115, 123,
129, 133, 143, 146, 150, 154,
159, 161, 165-169, 176, 177,
180, 186, 192, 193, 201, 206,
208, 210, 211, 219, 223-225,
229-232, 234, 238, 242-246,
251, 274
Diarrhea 10, 11, 26, 27, 29, 35, 36,
42, 89, 95, 105, 108, 109, 114,
121, 122, 124, 142, 168, 179,
198, 200, 201, 205, 208, 214,
215, 216, 218, 220, 221, 233,
258, 273, 275, 278, 281
Diethylcarbamazine (DEC) 49, 50,
55, 56, 74, 82, 83, 275, 276, 284,
287, 288
Diff use unilateral subacute
neuroretinitis (DUSN) 68
Disseminated cutaneous leishmaniasis
(DCL) 185, 187
Diurnal rhythm 53
Dracunculiasis 58, 60, 61
Dracunculus medinensis 58, 263
Duff y 241, 279
Dust mite 250, 256
Dysentery 10, 122, 174, 206-209
E
Echinococcosis 146, 148-150
Echinococcus granulosus 146, 148,
149, 151, 270
Echinostome 107
Efl ornithine 168, 282, 284, 287, 288
Elephantiasis 79-81
Encephalitis 142, 190, 273
Endomyocardial fi brosis 55
Entamoeba 203, 206, 207, 209, 261,
263
Enterobiasis 2-4, 6
Eosinophilia 4, 10, 16, 18, 26-28,
31, 35, 36-38, 43, 54-56, 60, 65,
68, 72, 73, 75, 80, 89, 90, 93, 99,
101, 105, 108, 113, 121, 123,
131, 210, 264
Eosinophilic meningoencephalitis 68,
73
Eradication program 61
Erythrocytic (blood) stage 237, 238,
240, 242
Exo-erythrocytic (hepatic) stage 237
Eye worm 53
F
Fasciola hepatica 92-95, 263, 264,
276
Fascioliasis 92, 93, 95, 96, 276
Fasciolopsis buski 104-106, 264
Fecal-oral 87, 91, 144, 196, 216
Food-borne trematode control 106
Fungal infection 79, 227

Index
293
Index
G
Gastrointestinal disease 214, 274
Giardia duodenalis 195, 264
Giardia intestinalis 195-197, 201
Giardia lamblia 195, 199, 200
Giardiasis 195, 197-205, 264, 276
Global program for elimination of
lymphatic fi lariasis (GPELF) 83
Glossina 161, 162
Guinea worm 58, 60, 61, 263
H
Hanging groin 48
Harada-Mori 28
Helminth 2, 8, 12, 16, 19, 21, 24, 35,
37, 58, 60, 70, 82, 83, 129, 199,
203, 210, 250, 272, 276
Hematuria 55, 78, 80, 129, 130, 133,
135
Hepatic abscess 17, 213
Heterophyes heterophyes 104, 108,
109, 264
Hexapoda 251
Histolytica 206-211, 213, 261, 263
HIV 35, 122, 157, 171, 174-176,
178, 179, 185, 186, 194,
199, 218, 220, 226-228, 230,
232-235, 250, 262, 275, 277,
280-282
Hookworm 8-11, 21-30, 37, 63, 64,
262, 263, 265, 269
Human African trypanosomiasis
(HAT) 161-169
Hydatid cyst 147-151, 265, 270, 281
Hydatidosis 146
Hydrocele 55, 79-82
Hyperinfection 34-38, 281
I
Immunocompromised 159, 177,
190, 192, 193, 199, 205, 214,
215, 218-221, 256, 273, 277, 281
Immunodefi ciency 122, 185, 198,
199, 214, 218, 221
Immunosuppression 157, 193, 229,
230, 232
InPouch™ 225
Intestinal fl uke 104, 105, 264
Iron defi ciency 27
Ivermectin 6, 29, 37, 38, 49-51, 55,
56, 61, 65, 69, 82, 83, 274-278,
281, 285, 288, 289
K
Kato-Katz 114, 123
L
Liposomal amphotericin 179, 188,
276
Liver fl uke 90, 92, 109, 264
Loa loa 50, 51, 53-56, 263, 266, 275
Loiasis 53, 55, 56, 275
Lung fl uke 98, 264
Lymphatic 21, 34, 41, 45, 76-83,
109, 119, 155, 156, 164, 172,
259, 275
Lymphedema 47, 79, 82
M
Macrofi lariae 45, 49-51, 275
Malaria 21, 25, 123, 129, 174,
176, 204, 237, 238, 240-247,
266-268, 278-280, 284, 285
Malnutrition 10, 12, 21, 25, 27, 29,
34, 36, 104, 106, 122, 132, 138,
174, 199, 204, 218
Mango fl y 53
Mazzotti reaction 49, 50
Mebendazole 5, 6, 11, 18, 19, 29, 43,
61, 69, 74, 108, 203, 262, 265,
274, 285, 288, 289
Megacolon 155, 158, 160, 208
Megaesophagus 155
Melarsoprol 167-169, 282, 283, 288

294
Medical Parasitology
Index
Meningoencephalitis 55, 68, 73,
261, 262, 269, 270
Metacercariae 89, 91, 93, 98, 99,
105, 107
Metagonimus yokogawai 104, 108,
109, 264
Metronidazole 61, 96, 202-204, 212,
213, 222, 224, 226, 273, 274,
276, 282, 285, 287, 288
Microfi laria 45, 47-51, 53-56, 76-78,
80, 82, 275, 276
Miltefosine 179, 187, 188, 277, 285,
288
Mosquito 76, 77, 82, 237, 240, 241,
245-247, 250-252, 256, 257
Mucocutaneous leishmaniasis 185,
186
Muscle 39, 41-43, 89, 98, 99, 139,
142, 143, 147, 150, 155, 157,
190, 192, 258
Myocarditis 42, 44, 73, 157, 192
Myositis 64
N
Necator 21, 22, 64, 265, 269
Neglected tropical disease 105
Nematode 2, 6, 8, 11, 14, 21, 31, 39,
45-48, 53, 63, 64, 67, 68, 76, 77
Neural larva migrans (NLM) 68, 69
Neurocysticercosis 124, 138, 142,
143
Neurotrichinellosis 44
Nongonococcal urethritis (NGU)
222, 223, 225
Nylon fi lter 61
O
Obstructive jaundice 17, 91
Ocular larva migrans (OLM) 68, 69,
70, 72-74
Onchocerca volvulus 45-47, 50, 264,
269, 275
Onchocercoma 45, 48, 49
Onchocercosis 45, 48-51
Oocyst 190, 215, 216, 218-220
Opisthorchiasis 86-90, 109
Opisthorchis 86, 87, 90, 264, 269
Opportunistic infection 164, 174,
218, 227
Oriental blood fl uke 111
OSOM® 225
P
Paragonimiasis 98-102
Parasite 2, 3, 6, 9, 14-17, 21-23, 26,
27, 32, 35, 40-43, 46, 47, 49, 50,
54, 55, 58, 60, 65, 67, 68, 70, 71,
74, 76, 77, 78, 80, 82, 83, 87-89,
94, 95, 98-102, 105-107, 111,
113, 116, 118, 123, 124, 126,
130-134, 139-144, 146-148,
154-159, 161, 163-168, 170-174,
176-178, 180, 182-188, 190,
192-197, 199-202, 206-210,
213-215, 219, 222, 223,
237-240, 242, 245, 250, 253
PCP treatment 234
Pentamidine 167-169, 179, 233,
235, 273, 277, 282, 285, 288
Pentavalent antimony 178, 187
Periorbital swelling 157
Phlebotomine 171, 250
Pinworm 2, 4, 6, 263, 269
Plasmodium falciparum 237,
239-244, 246, 266, 278-280
Plasmodium malariae 237, 239-242,
244, 278
Plasmodium ovale 237, 239, 241,
242, 244, 266, 278
Plasmodium vivax 237, 239-242,
244, 266-268, 278-280
Pneumocystis carinii 227
Pneumocystis jiroveci 227, 230-234,
269
Pneumocystis pneumonia (PCP)
227-230, 232-235, 269

Index
295
Index
Polymerase chain reaction (PCR)
43, 49, 50, 56, 82, 91, 102, 150,
159, 166, 169, 177, 187, 193,
202, 219, 225, 231, 234, 242,
243
Poor sanitary conditions 8, 12, 215
Pork 39, 44, 138, 139, 190, 252, 270
Pork tapeworm 138, 139
Post-kala-azar dermal leishmaniasis
(PKDL) 171, 172, 175-177,
180, 185
Praziquantel 91, 96, 102, 106-109,
116, 122, 124-126, 131, 134,
143, 276, 281, 282, 285, 287,
288
Primaquine 233, 241, 244, 267, 268,
278-280, 285, 288
Prophylaxis 6, 12, 51, 56, 65, 69, 91,
96, 116, 126, 135, 144, 151, 188,
227, 234, 235, 246, 278-282,
284, 285
Proteinuria 41, 55, 79, 80, 122, 132
Protozoa 11, 171, 250
Protozoan 154, 155, 161, 171, 182,
190, 195, 199, 206, 214, 227,
272
Punctate keratitis 49, 50
Pyrimethamine 193, 233, 273, 276,
281, 282, 285, 287, 289
R
Raccoon 67-69, 274
Recrudescence 49, 51, 52, 157, 242
Rectal prolapse 10
Reduviid bug 155, 158, 160, 252
Relapse 65, 166, 175, 178-180, 233,
268, 276-279
Reservoir 8, 23, 53, 58, 78, 92, 98,
104, 106, 107, 129, 167, 171,
172, 175, 197, 222, 225
River blindness 45, 264, 270
Roundworm 14, 63, 70, 251, 262,
270
Rural areas 6, 8, 21, 31, 70, 76, 92,
104, 105, 108, 113, 154, 172,
177, 185, 241, 245, 278
S
Sabin-Feldman dye test 192
Sand fl y 171, 172, 175, 182, 184,
188, 250, 251
Schistosoma haematobium 111, 113,
118, 125, 129-135, 270
Schistosoma japonicum 111-118, 125,
270
Schistosoma mansoni 111, 113, 116,
118, 119, 121-127, 270
Schistosomiasis 106, 109, 111, 113,
114, 117-120, 122-127, 129,
131-135, 174, 176, 270
Scotch tape 4, 5
Serpinginous track 64
Sheep liver fl uke 92, 264
Sickle cell 241
Simulium 45, 48
Skin infection 63, 101
Skin ulcer 60
Sleeping sickness 162, 164, 270-272
Soil-transmitted infection 8, 12, 14,
21, 106, 109
Sowda 46, 47
Sporozoite 215, 216, 219, 237, 240,
242
Strongyloidiasis 31, 34-37, 270, 281
Subcutaneous nodule 45, 64, 101
Sulfadiazine 193, 273, 282, 289
Suramine 167-169
Syncytial tunnel 8
T
Tabanid fl y 53, 138, 139, 144
Tachyzoite 190-192
Taenia saginata 138-140, 270
Taeniasis 138, 142
Taenia solium 138, 142, 270

296
Medical Parasitology
Index
Temephos 61
Tetanus 58, 60
Tick 250, 255-258
Tinidazole 202-205, 222, 226, 273,
282, 286, 289
Tissue cyst 190-192
Toxocariasis 67, 70, 72-75, 271, 272
Toxocariasis canis 70, 73
Toxocariasis cati 70
Toxoplasma gondii 190-192, 271
Treatment 5, 6, 10-12, 18, 19, 28-30,
34, 37, 43, 50, 51, 55, 56, 60, 61,
65, 69, 74, 75, 82, 91, 95, 96,
102, 106-109, 113, 116, 117,
122-127, 134, 135, 143, 146,
150, 151, 159, 161, 164-169,
171, 175, 177-180, 184,
186-188, 193, 198, 202-205,
211, 213-215, 220, 226, 227,
232-235, 241, 242, 244-246,
252, 253, 257-259, 266, 267,
270, 272-282
Trematode 92, 95, 98, 101, 104, 106,
107, 109, 111, 113, 118, 129
Trichinellosis 39, 42-44, 271
Trichomonas vaginalis 222-226, 271
Trichuriasis 8, 10-12, 271, 272
Triclabendazole 95, 102, 276, 287,
289
Trimethoprim-sulfamethoxazole
193, 194, 282
Trimetrexate 233, 288, 289
Trisulfapyrimidine 193
Trophozoite 193, 195-197, 200-202,
206, 207, 209, 210, 222,
228-231, 238, 239
Trypanosoma 154-156, 158, 161,
162, 252
Trypanosoma brucei gambiense 162,
271, 272
Trypanosoma brucei rhodesiense 162
Trypanosomiasis 154, 161, 162,
164-166, 169, 174, 262, 270-272
Tsetse fl y 161, 162, 251
U
Uveitis 49, 50, 73
V
Vaginal discharge 222-225
Visceral larva migrans (VLM) 67,
68, 70, 72-74, 271
Visceral leishmaniasis 171, 172,
174-176, 179, 180, 182, 185, 188
W
Waterborne 93, 104, 197, 204, 214,
216
Watercress 92, 93, 96, 105
Water sanitation 61
Whipworm 8, 271, 272
Wild game 39, 44
Wolbachia endosymbiont 51
Worm extraction 17, 55, 61, 275
Satoskar
Simon
Hotez
Tsuji
Medical P
ar
asitolog
y
ad
e
m
e
c
u
m
V
V
m
a d e m e c u
Medical
Parasitology
Abhay R. Satoskar, Gary L. Simon,
Peter J. Hotez and Moriya Tsuji
LANDES
B I O S C I E N C E
LANDES
B I O S C I E N C E
m
V
a d e m e c u
Table of contents
Section I. Nematodes
Section II. Trematodes
Section III. Cestodes
Section IV. Protozoans
Section V. Arthropods
The Vademecum series includes subjects generally not covered in other handbook
series, especially many technology-driven topics that reflect the increasing
infl uence of technology in clinical medicine.
The name chosen for this comprehensive medical handbook series is Vademecum,
a Latin word that roughly means “to carry along”. In the Middle Ages, traveling
clerics carried pocket-sized books, excerpts of the carefully transcribed canons,
known as Vademecum. In the 19th century a medical publisher in Germany, Samuel
Karger, called a series of portable medical books Vademecum.
The Landes Bioscience Vademecum books are intended to be used both in the
training of physicians and the care of patients, by medical students, medical house
staff and practicing physicians. We hope you will fi nd them a valuable resource.
All titles available at
www.landesbioscience.com
LANDES
B I O S C I E N C E
ISBN 978-1-57059-695-7
9 7 8 1 5 7 0 5 9 6 9 5 7
