1
Cyanide poisoning
Prepared by:
ان يرفع اسم الجياشي عن السمنار هذا لتجاوز كبير على الامة العربيةو يعمل لاضعافها و توهينها
Collage of pharmacy
University of Al_Qadisiya
cyanide
It is a rapidly acting lethal agent that is limited in its military usefulness by its high LCt50 and high volatility.Physical characteristics:
cyanides are in liquid state in
munitions, but rapidly vaporize
upon detonation of the munitions.
The major threat is from the vapor .
Cyanide is hazardous by:
InhalationRapid onset: seconds to minutes
Ingestion
Delayed onset: 15 to 30 minutes
Skin contact
Delayed onset: 15 to 30 minutes
Death occurs in 6 to 8 minutes after inhalation of a high Concentration .
2 to 5 mg/kg of it is lethal .Plant source almond250 mg CN/100g plant tissue
Cassava104 mg CN/ 100 g plant tissue
Wild Cherries
140-370 mg CN/ 100 g plant materialMechanism of toxicity
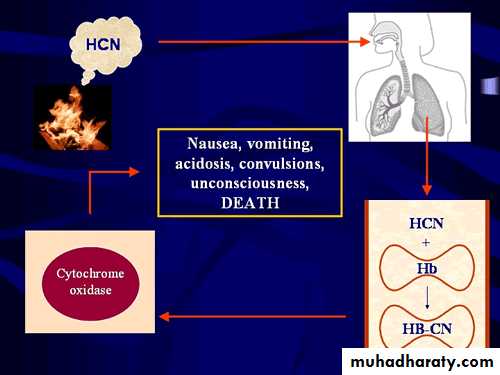
It produce cellular hypoxiaby binding to ferric iron
specially that present in
cytochrom oxidase system .
When it bind to this enzyme
complex electron transport is inhibited( ATP will not produced ) this is result in decrease cellular utilization of oxygen ( hypoxia ) .
Clinical manifestations
Common final pathway for cyanide intoxication is cellular hypoxiaMetabolic acidosis: nonspecific symptoms
CNS: dizziness, nausea, vomiting, drowsiness, tetany, trismus, hallucations
CV: arrhythmia, hypotension. Tachycardia and hypertension
Respiratory: dyspnea, initial hyperventilation followed by hypoventilation and pulmonary edema.Sign and symptom of its toxicity
Mild ToxicityNausea
Dizziness
Drowsiness
Moderate Toxicity
Loss of consciousness for a short period
Convulsion
Vomiting
Cyanosis
Severe Toxicity
Deep coma
Dilated non-reactive pupils
Deteriorating cardio-respiratory function
diagnosis
Case history
suspicion of exposureClinical presentation
metabolic acidosis, multisystem involvement
odor of bitter almonds
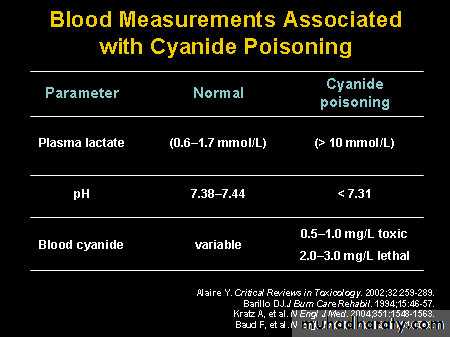
Laboratory diagnosis
blood cyanide levels can be drawn .
high anion gap metabolic acidosis
arterial and venous pO2 may be elevated .
Example on cyanide poisning
Cyanide used in its attack on the Kurdish village of Halabja in 1988. Five thousand people died and many were discovered sitting exactly where they were when the gas hit, their systems paralysed from within.On December 5, 2009, a fire in the night club Lame Horse (Khromaya Loshad) in the Russian city of Perm took the lives of 156 people. 111 people died on the spot and 45 later in hospitals
Properties that make cyanide more dangerous
1-in some case don’t have specific odour2- may don’t have color
3- large number of sorce in our life4-may found in many state
treatment
Treatment regimen depends on : severity of symptoms, route of exposure ,and what is availableTreatment options are:
• Sodium nitrite
• Sodium thiosulfate
• Amyl nitrite
• Activated charcoal
• Supplemental oxygen
• Hydroxocobalamin
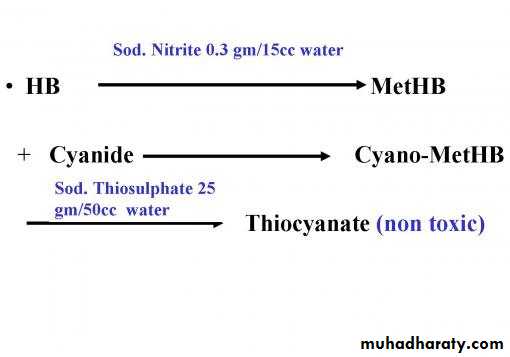
Commercial cyanide antidote kits contain Sodium nitrite & sodium thiosulfate
First step :
use Sodium nitrite : converts a portion of the hemoglobin into methemoglobin.
effectively pulling the cyanide off the cells and onto the methemoglobin. Once bound with the cyanide, the Methemoglobin becomes cyanomethemoglobin.
Second step : use sodium thiosulfate : which is administered IV. The sodium thiosulfate and cyano-methemoglobin become thiocyanate, releasing the hemoglobin, and the thiocyanate excreted by the kidneys .
• Amyl nitrite :
• -An inhaled drug, similar to sodium nitrite but with little systemic distribution: second line agent used when sodium nitrite is not available .• Activated charcoal :
• -For alert, asymptomatic patients
• following ingestion .
• Oxygen supplement :
• -100% for suspected exposure .
• : Hydroxocobalamin
• -Mechanism: direct binding agent,
• chelate the cyanide.( dose : 4 - 5 g IV )