15-16 /د. بسام
كلية طب نينوى
1
LIPID BIOCHEMISTRY
Lipid Properties
1- Insoluble in water
(hydrophobic).
2- Soluble in nonpolar solvents such as ether & chloroform (lipophilic).
Importance of Lipid
1- Important dietary constituents.
2- Stored in adipose tissue where it serves as a thermal & mechanical insulator.
3- Acts as electrical insulators in nerve action.
Classification of Lipid
1. Simple lipids: Esters of fatty acids with various alcohols. eg, fat, oil & wax.
2. Complex lipids: Esters of fatty acids with various alcohols with additional
group, eg. Phospholipids, glycolipids, sulfolipids, aminolipids & lipoproteins.
3. Precursor & derived lipids: Include fatty acids, glycerol, steroids, fatty
aldehydes, ketone bodies, lipid-soluble vitamins & hormones.
F
F
a
a
t
t
t
t
y
y
A
A
c
c
i
i
d
d
s
s
Fatty acids are aliphatic carboxylic acids.
Presence of Fatty Acids
1- Present in the body mainly as esters, eg, triacylglycerol.
2- Present in the blood as unesterified form [free fatty acids or nonesterified
fatty acids (NEFA)] combined with albumin & in the cell they are attached to a
fatty acid-binding protein, so that in fact they are never
”free."
Classification of Fatty Acids
1- Saturated fatty acids: They have no double bonds.
2- Unsaturated Fatty Acids: They have one or more double bonds. They are
further subdivided into:
A- Monounsaturated (Monoenoic) fatty acids: Contain one double bond.
B- Polyunsaturated (Polyenoic) fatty acids: Contain two or more double bonds.
C- Eicosanoids: These compounds derived from eicosa (20-carbon) polyenoic
fatty acids. They include prostanoids, leukotrienes (LTs) & lipoxins (LXs).
Prostanoids
include
prostaglandins
(PGs),
prostacyclins
(PGIs)
&
thromboxanes (TXs).
Nomenclature of Fatty Acids
I-
Systematic Nomenclature:
1- Name according to number of carbon atoms that form the fatty acid with oic
being substituted for the final
e
.
15-16 /د. بسام
كلية طب نينوى
2
a- In saturated acids end in -anoic, eg, octanoic acid.
b- Unsaturated acids with single double bond (monoenoic acids) end in-enoic,
eg, octadecenoic acid, if two double bonds (dienoic acids) end in
-adienoic,
eg, octadecadienoic, if three double bonds (trienoic acids) end in
-atrienoic,
eg, octadecatrienoic & so on.
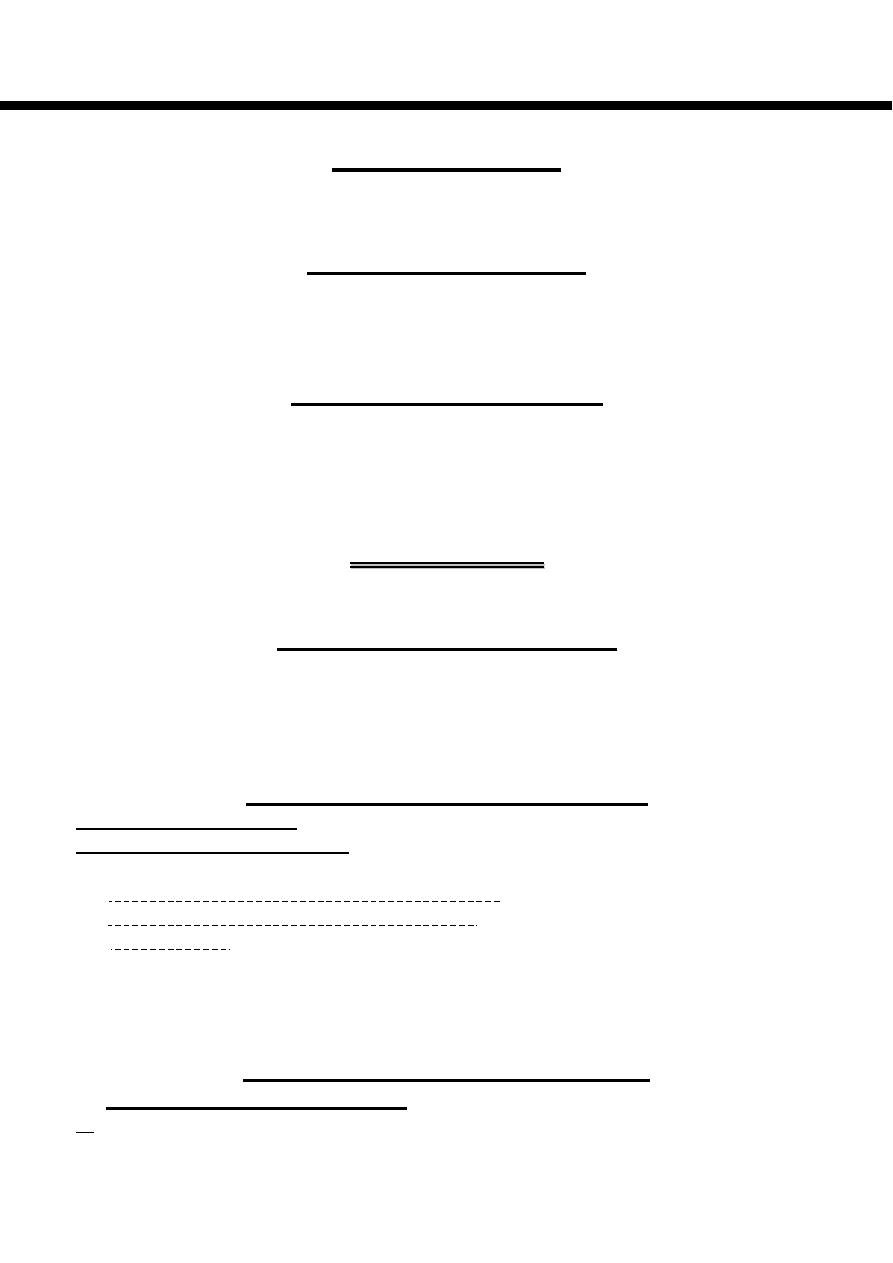
2- Carbon atoms are numbered from the carboxyl carbon (carbon No. 1). The
carbon atoms adjacent to the carboxyl carbon (Nos. 2, 3 & 4) are also known as
α, β & γ carbons respectively & the terminal methyl carbon is known as the ω or
n-carbon.
3- The number & position of the double bonds are indicated by:
a-
∆ using the number of preceding carbon atom in the double bond eg, ∆
9
indicates a double bond exist between carbons 9 & 10 of the fatty acid.
b-
ω using the number counting from the ω -carbon eg, ω
7
indicates a double
bond on the seventh carbon counting from the ω -carbon.
c- Write number of carbon atoms that form the fatty acid (eg, 16) : number of
double bonds (eg, 1) ; number of preceding carbon atom in the double bond
(eg, 9), therefore, it written 16:1;9
II-
Common Nomenclature:
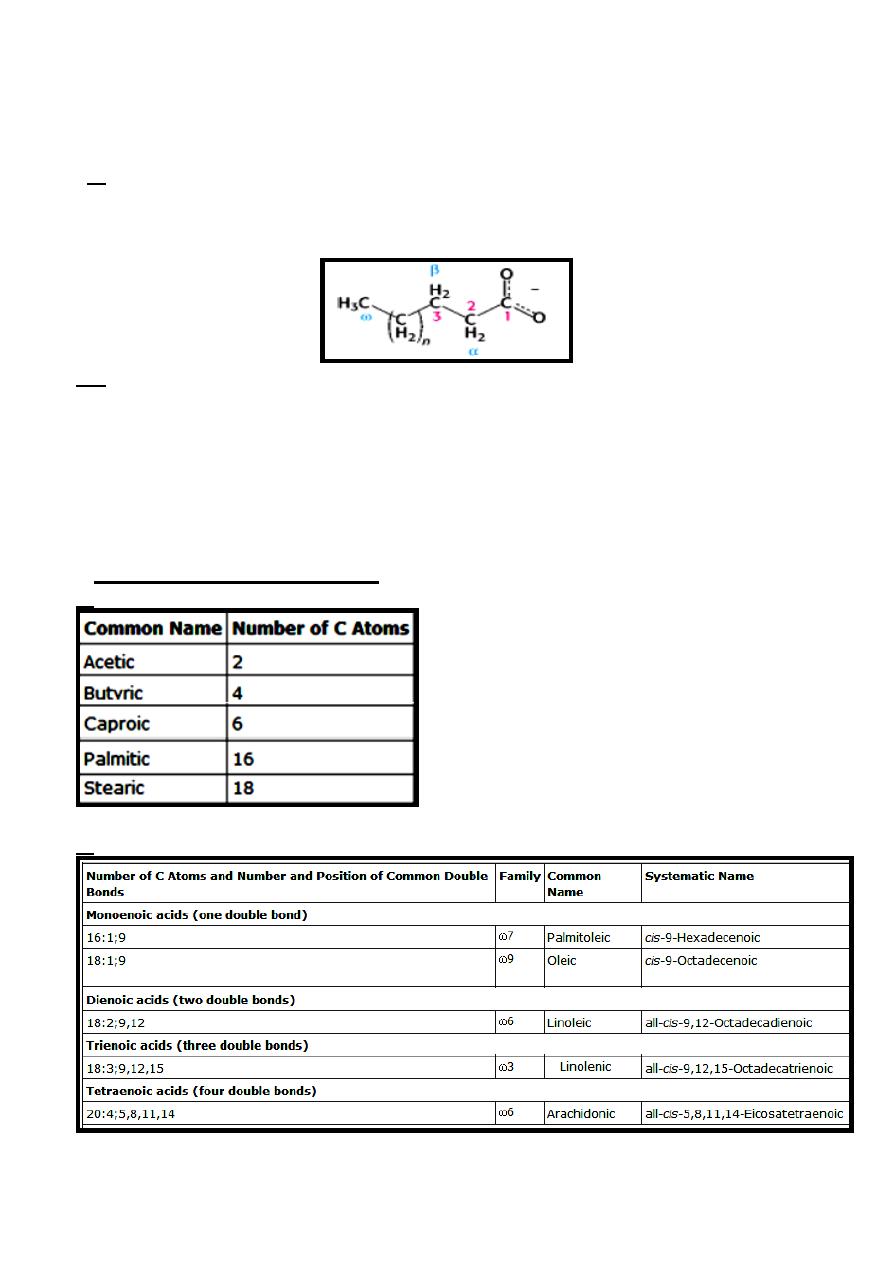
1- Common name for saturated fatty acids.
2- Common name for unsaturated fatty acids.
15-16 /د. بسام
كلية طب نينوى
3
Nutritionally Essential Fatty Acids(EFA)
Linoleic & linolenic fatty acids are known to be essential for the complete
nutrition in human, they are not synthesized in human (should be supplied in
diet) so they called nutritionally essential fatty acids (EFA).
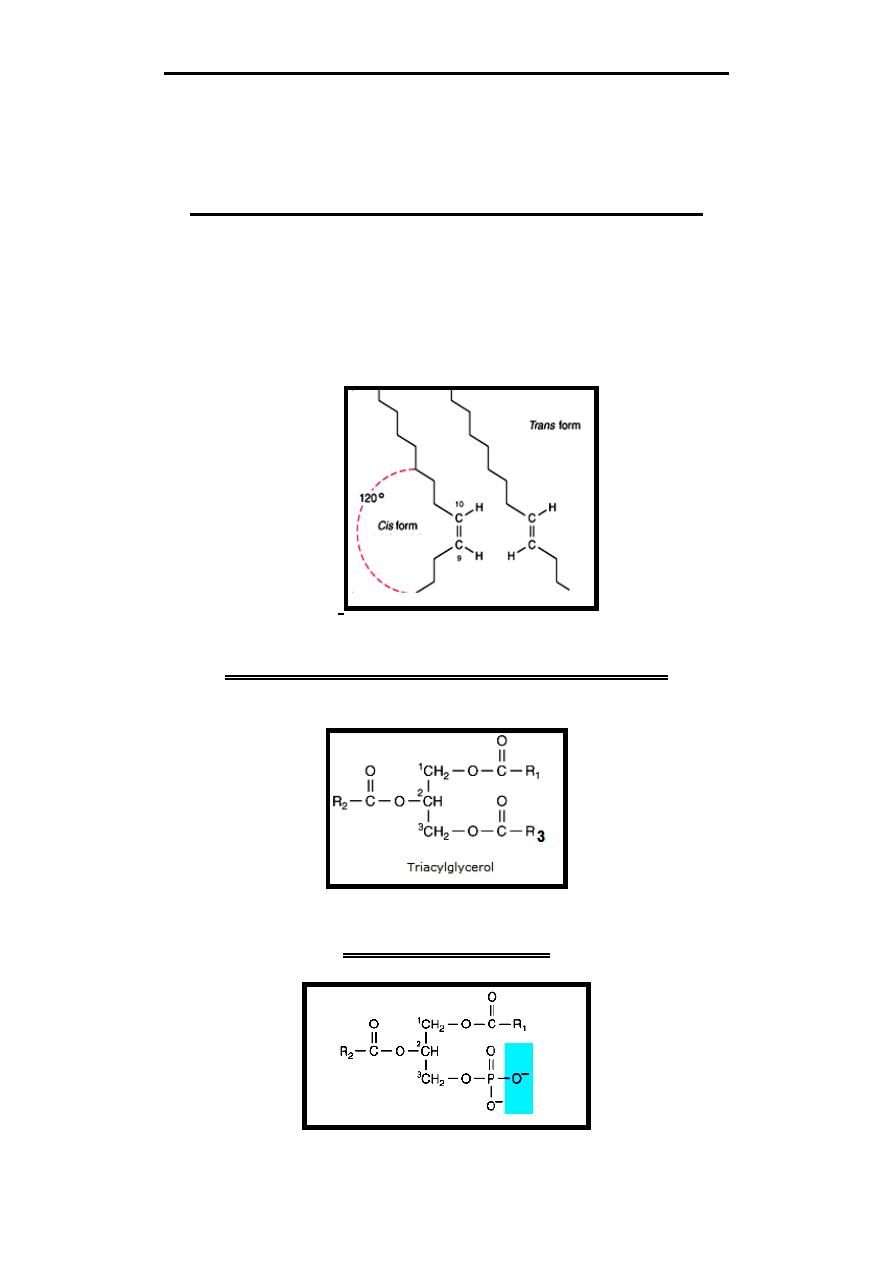
Geometric Isomerism of Fatty Acids
The carbon chains of saturated fatty acids form a zigzag pattern.
A type of geometric isomerism occurs in the unsaturated fatty acids,
depending on the orientation of atoms or groups around the axes of double
bonds. If the acyl chains are on the same side of the bond, it is cis-, if on
opposite sides, it is trans-, Double bonds in naturally occurring unsaturated
long-chain fatty acids are nearly all in the cis configuration, the molecules
being "bent" 120 degrees at the double bond.
Triacylglycerols (Triglycerides)
They are esters of glycerol with three fatty acids. It constitutes the majority of
lipids in the body & in food.
Phospholipids
They are esters of glycerol with fatty acid with additional phosphate group.
Phospholipids include mainly:
15-16 /د. بسام
كلية طب نينوى
4
1- Phosphatidylcholines (Lecithins.
2- Phosphatidylinositol.
3- Cardiolipin.
4- Lysophospholipids.
5- Plasmalogens.
6- Sphingomyelins.
Glycolipids
They are lipids with a carbohydrate attached
to it, widely distributed in every
body tissue particularly in nervous tissue.
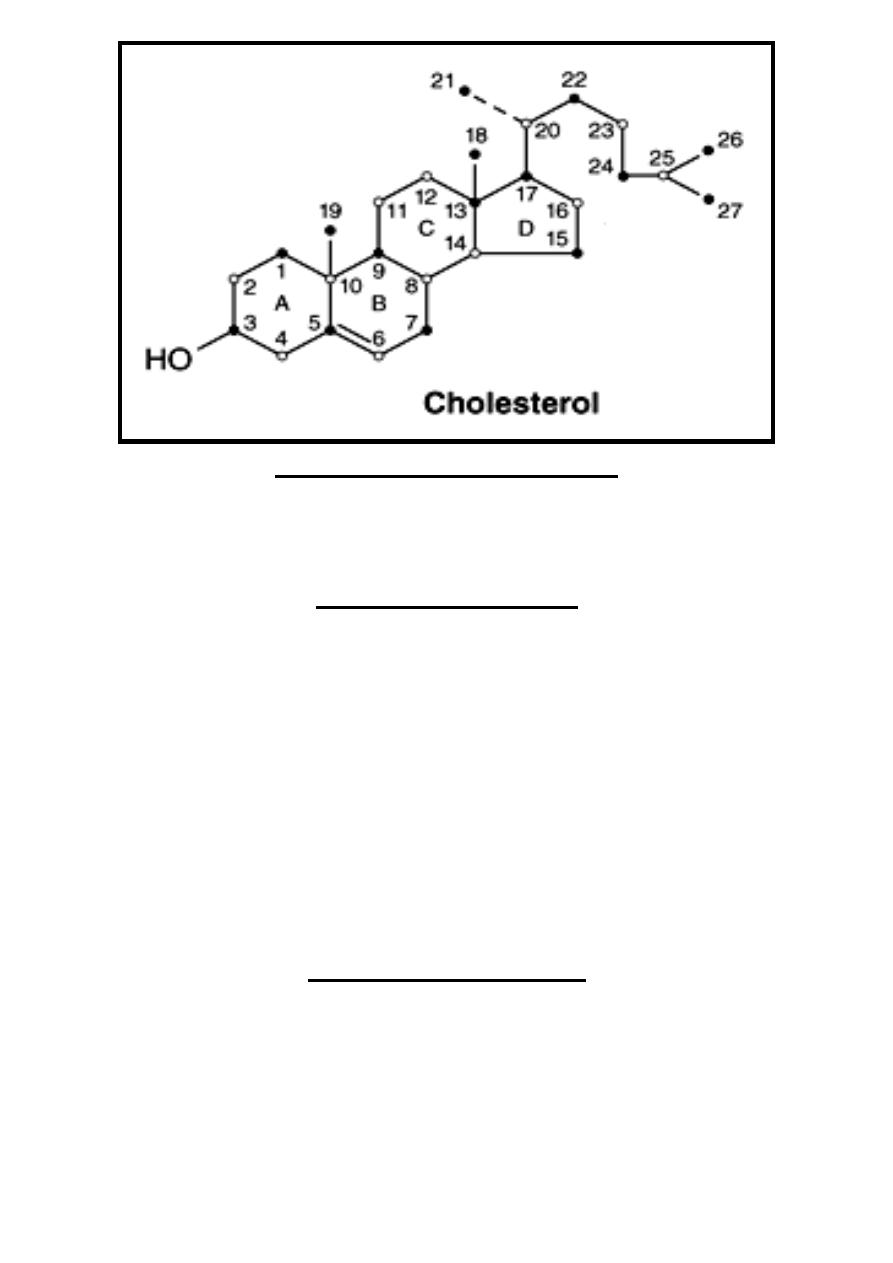
Steroids
Clinically cholesterol is the best-known steroid because of its association with
atherosclerosis & heart disease. Biochemically, it is also of significance
because it is the precursor of a large number of equally important steroids
including bile acids, adrenocortical hormones, sex hormones & vitamin D.
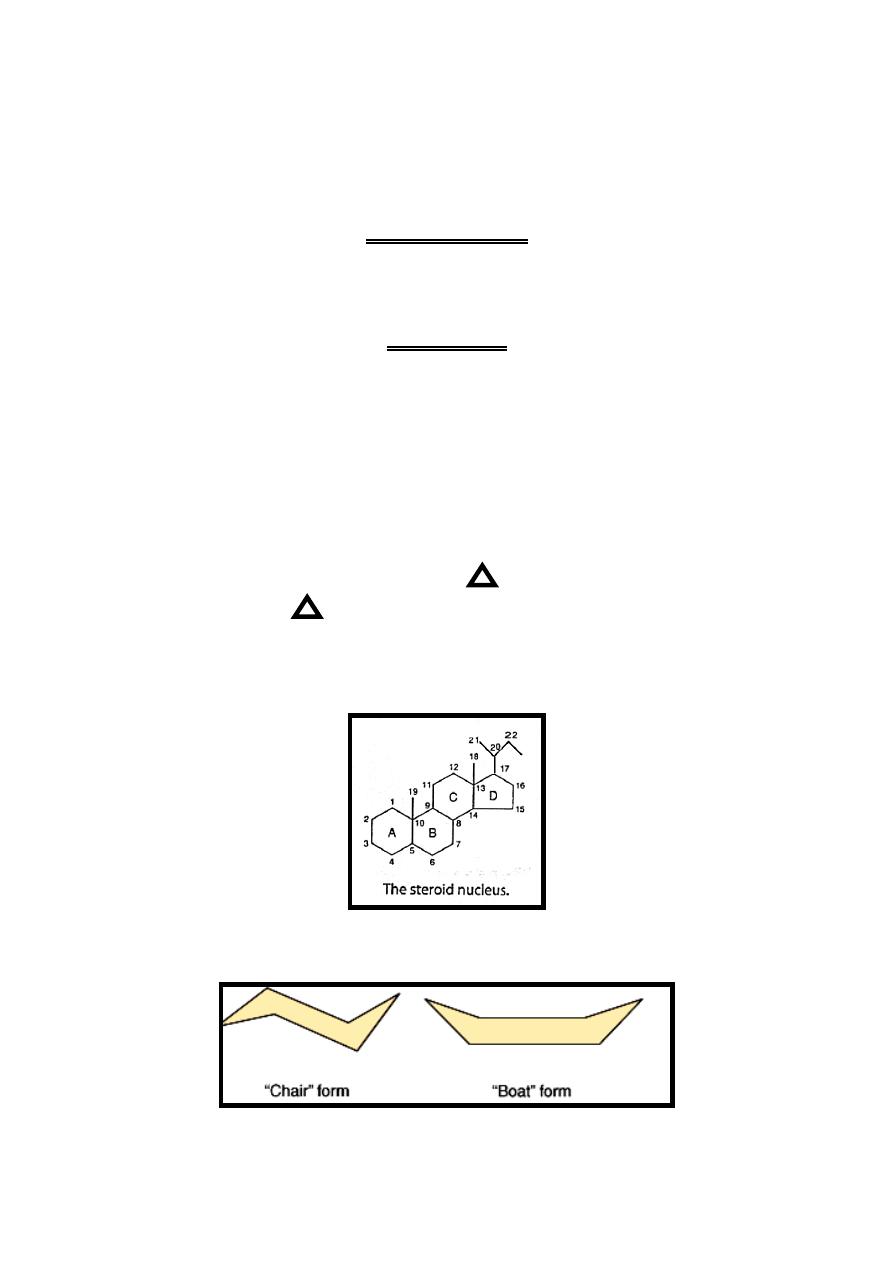
Steroids are characterized by having cyclopentanoperhydrophenanthrine
that have four rings (17-carbon atoms) with additional carbon atom at position
13(C18) & at position 10 (C19) & carbon atoms at position 17 (C 20,
21….).
If the side-
chain of steroid above (front) hormone plane it's called β cis while if
it's below (behind) hormone plane
it’s called α trans.
Double-bond in steroid represented by (Delta) with number of preceding
carbon atom (example 5 mean double bond between C5 & C6).
On drawing the structure of steroid, the carbon positions on the steroid
nucleus are numbered. All double bonds are shown as such. Methyl side chains
are shown as single bonds unattached at the farther (methyl) end.
β -bonds are
shown with bold solid lines
whereas α -bonds are indicated with broken lines.
Each ring of the steroid nucleus is capable of existing in the three-dimensional
conformation either of a "chair" or a "boat". In naturally occurring steroids,
virtually all the rings are in the "chair" form.
15-16 /د. بسام
كلية طب نينوى
5
Presence of Cholesterol
Cholesterol is widely distributed in all body cells. It present either as a free
cholesterol or more commonly as cholesterol ester in which the hydroxyl
group on position 3 is esterified with fatty acid.
Lipid Peroxidation
Peroxidation (auto-oxidation) of lipids when exposed to oxygen free radicals is
responsible not only for foods deterioration (rancidity) but also for damage to
tissues in vivo, where it may be a cause of cancer, inflammatory diseases,
atherosclerosis & aging. These deleterious effects are considered to be caused
by free radicals produced during lipid peroxidation.
Lipid peroxidation is a chain reaction involves three steps which are:
A- Initiation.
B- Propagation.
C- Termination.
The control & reduction of lipid peroxidation, is done by the use of antioxidants
The main sources of antioxidants are:
A- Food additives & supplements.
B- Naturally occurring: As vitamin E, urate & vitamin C.
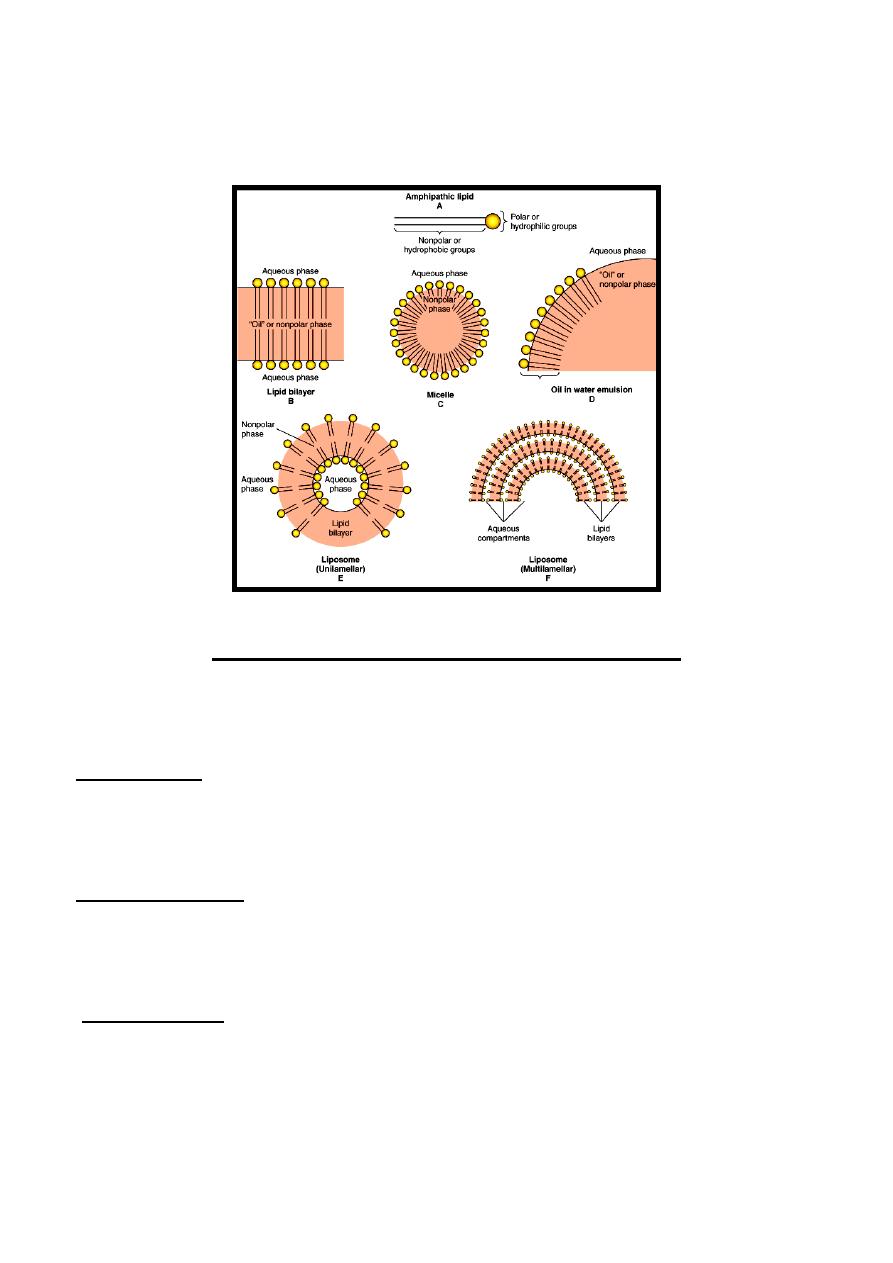
Amphipathic Lipids
In general, lipids are insoluble in water since they contain a predominance of
nonpolar groups. However, fatty acids, phospholipids, bile salts & to a lesser
extent, free cholesterol contain polar groups. Therefore, part of the molecule is
hydrophobic, or water-insoluble & part is hydrophilic, or water-soluble. Such
molecules are described as amphipathic. They become oriented at oil:water
interfaces with the polar group in the water phase & the nonpolar group in the
oil phase. Amphipathic lipids are present in the following:
15-16 /د. بسام
كلية طب نينوى
6
A- Biologic membranes.
B- Micelles: They are very small droplets present in an aqueous intestinal
medium to facilitate fat absorption.
C- Liposomes: It is an artificially-prepared vesicle that can be used for
administration of nutrients & pharmaceutical drugs.
D- Emulsions: They are much larger particles.
Digestion & Absorption of Lipids
The major lipids in the diet are triacylglycerols & to a lesser extent
phospholipids, cholesterol & fat-soluble vitamins (vitamins A, D, E & K). These
are hydrophobic molecules & have to be hydrolyzed & emulsified to very
small droplets (micelles) before they can be absorbed.
I- Hydrolysis
Hydrolysis of triacylglycerols into their constituents (fatty acids & glycerol) is
important for their absorption, this done by lipase enzyme which present in the
mouth (lingual lipase), stomach (gastric lipase) & pancreas (pancreatic
lipase).
II- Emulsification
Bile salts that formed in the liver & secreted in the bile, permit emulsification of
the products of lipid digestion together with phospholipids, cholesterol & fat-
soluble vitamins forming mixed micelles. Because the micelles are water
soluble, they allow their content of lipid to be absorbed by intestinal epithelium.
III- Absorption
Within the intestinal epithelium:
The products of lipid digestion within the intestinal epithelium (mainly
triacylglycerol, cholesterol ester, phospholipids & & fat-soluble vitamins), are
secreted as chylomicron into the lymphatics, entering the bloodstream via the
thoracic duct.
