Hussien Mohammed Jumaah
CABMLecturer in internal medicine
Mosul College of Medicine
Thursday, 24 march, 2016
CHRONIC KIDNEY DISEASE
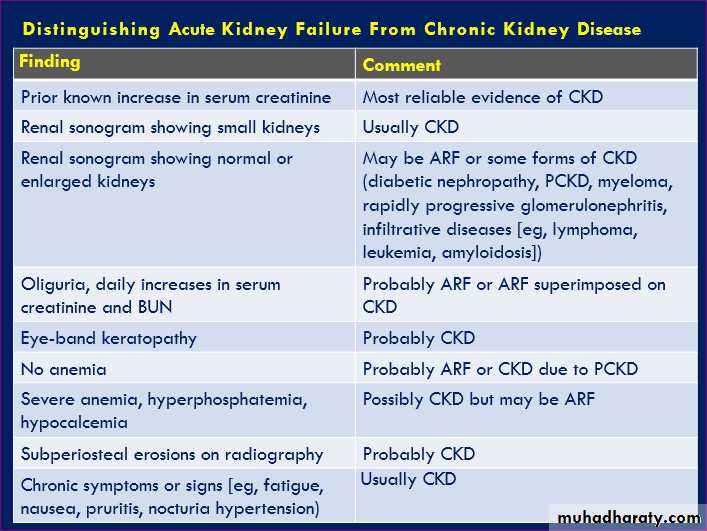
Chronic kidney disease (CKD), previously termed
chronic renal failure, irreversible deterioration in renal function ,develop over a period of years .Initially, manifest only as a biochemical abnormality,
eventually, loss of the excretory, metabolic and endocrine functions of the kidney leads to the symptoms and signs of renal failure, collectively referred to as uraemia.
When death is likely without renal replacement therapy (RRT) (CKD stage 5), it is called end-stage renal disease or failure (ESRD or ESRF).
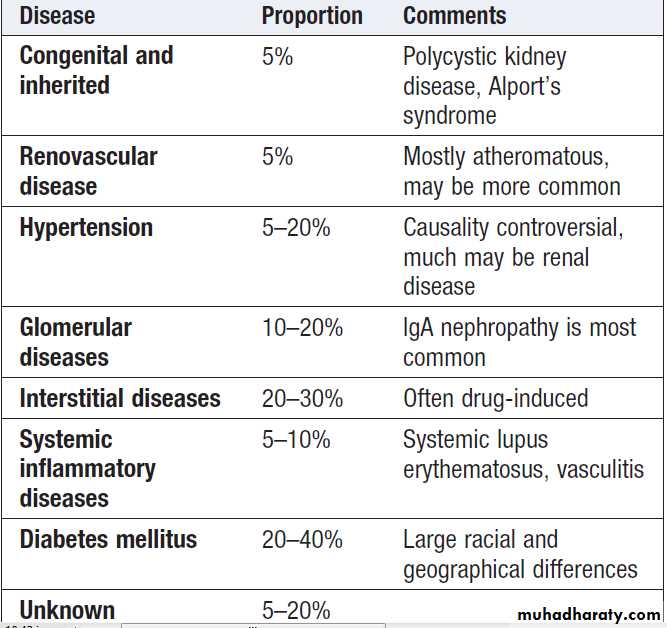
Common causes of ESRD
The severity CKD is described by stages,first three also depend whether there is
evidence of kidney damage,
((markers of damage, abnormalities in blood , urine test e.g. proteinuria or imaging studies)):
0) Normal kidney function – GFR above 90mL/min/1.73m2 and no proteinuria
1) CKD1 – GFR above 90mL/min/1.73m2 with evidence of kidney damage
2) CKD2 (Mild) – GFR 60 to 89 mL/min/1.73 m2 with evidence of kidney damage
3) CKD3 (Moderate) – GFR of 30 to 59 mL/min/1.73m2
4) CKD4 (Severe) – GFR of 15 to 29 mL/min/1.73m2
5) CKD5 Kidney failure (RRT , dialysis or kidney transplant needed) – GFR < 15 mL/min/1.73m2
Clinical features of CKD
The typical presentation is a raised urea and creatinine found during routine tests, hypertension, proteinuria or anaemia.1. General symptoms
Most are asymptomatic until (stage 4 or 5) , symptoms and signs affect almost all systems.
Nocturia(loss of concentrating ability).Tiredness , breathlessness, related to renal anemia and metabolic acidosis (Kussmaul’s respiration), pruritus, anorexia, nausea , vomiting , hiccups, weight loss, muscular twitching, fits, drowsiness and coma.
Clinical features of CKD (continued)
2. Electrolyte abnormalitiesHyperkalaemia and metabolic acidosis .
Fluid retention is common in advanced CKD sometimes leading to pulmonary oedema.
Conversely, some patients with tubulo-interstitial disease can develop ‘salt-wasting’ disease and may require a high sodium and water intake, to prevent fluid depletion and worsening of renal function.
Acidosis increase tissue catabolism and decreased protein synthesis, exacerbate bone disease and the rate of decline in renal function.
Clinical features of CKD (continued)
3. Immune dysfunctionCellular and humoral immunity is impaired , increased susceptibility to infections, the second most common cause of death in dialysis patients, after CVD. Many infections are associated with access devices and pneumonia.
4. Haematological
Increased bleeding tendency in advanced CKD, ecchymoses and mucosal bleeds. Platelet function is impaired. Adequate dialysis partially corrects the bleeding tendency, they are at increased risk from all anticoagulants, required during dialysis. Anaemia is common. Haemoglobin can be as low as 50–70 g/L in CKD stage 5, although it is often less severe or absent in patients with polycystic kidney disease.
Clinical features of CKD (continued)
5. Endocrine functionIn both genders, there is loss of libido related, in part, to hypogonadism due to hyperprolactinaemia .The half-life of insulin is prolonged , there is also insulin resistance. Because of this, insulin requirements are unpredictable in diabetic with advanced CKD.
6. Neurological and muscle function
Myopathy occur due to poor nutrition, vitamin D deficiency hyperparathyroidism, and electrolyte abnormalities. Muscle cramps are common. The ‘restless leg syndrome’, in which the patient’s legs are jumpy during the night. Sensory and motor neuropathy can arise, presenting as paraesthesia and foot drop, respectively, often improve on dialysis.
Clinical features of CKD (continued)
7. Cardiovascular diseaseThe risk of CVD is substantially increased in patients with CKD stage 3 or worse and those with microalbuminuria or proteinuria. Left ventricular hypertrophy, secondary to hypertension, may account for the increased risk of sudden death (dysrhythmias). Pericarditis complicate untreated or inadequately treated ESRD ,cause pericardial tamponade or constrictive pericarditis.
Hyperphosphataemia contribute to the increased risk of CVD and itching.
Medial vascular calcification is common ((the raised serum phosphate complex with calcium, causing ectopic calcification in blood vessels and other tissues)).
Clinical features of CKD (continued)
8. Metabolic bone diseaseDisturbances of calcium and phosphate metabolism are
almost universal in advanced CKD, there is impaired conversion of 25-hydroxyvitamin D to its active metabolite, 1,25(OH)2D, due in part to tubular cell damage.
The reduced 1,25(OH)2D levels impair intestinal absorption of calcium, causing hypocalcaemia, which leads to parathyroid hypertrophy and secondary hyperparathyroidism, increased PTH and metabolic bone disease may occur, including osteitis fibrosa cystica, osteomalacia and osteoporosis.
In some , tertiary HP supervenes, due to autonomous production of PTH, this presents with hypercalcaemia.
Causes of anaemia in CKD
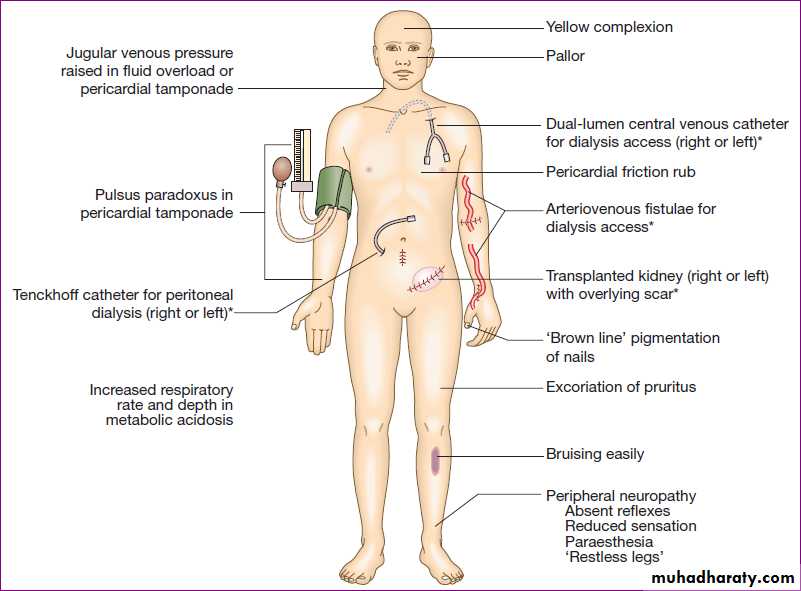
Physical signs in advanced chronic kidney disease. (*Features of renal replacement therapy)
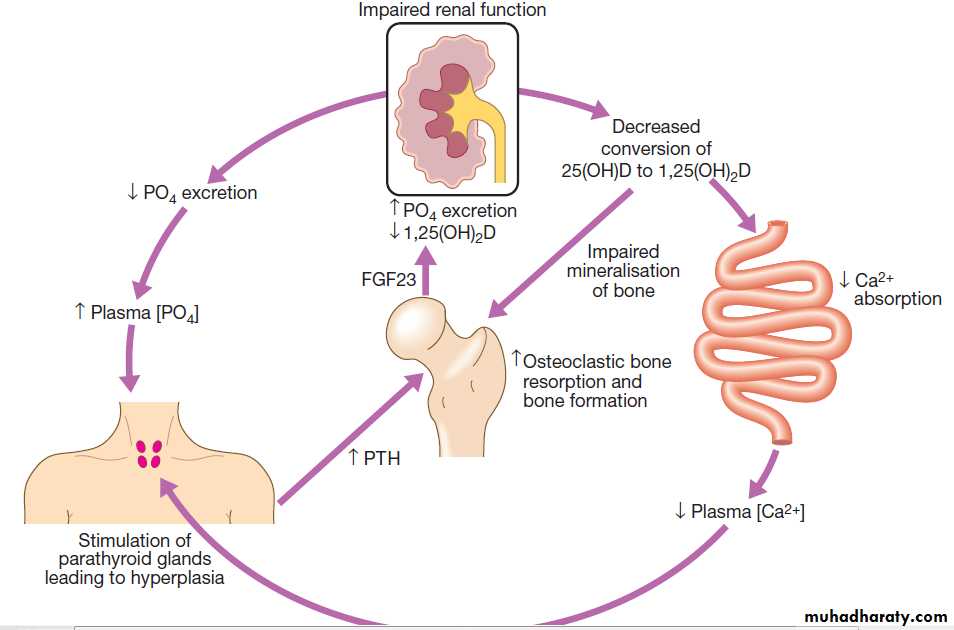
Pathogenesis of renal osteodystrophy. Low 1,25(OH)2D levels cause calcium malabsorption and this, combined with high phosphate levels, causes hypocalcaemia, which increases PTH production by the parathyroid glands. The raised level of PTH increases osteoclastic bone resorption and bone formation. Although production of FGF23 from osteocytes also increases, promoting phosphate excretion, this is insufficient to prevent hyperphosphataemia in advanced CKD.
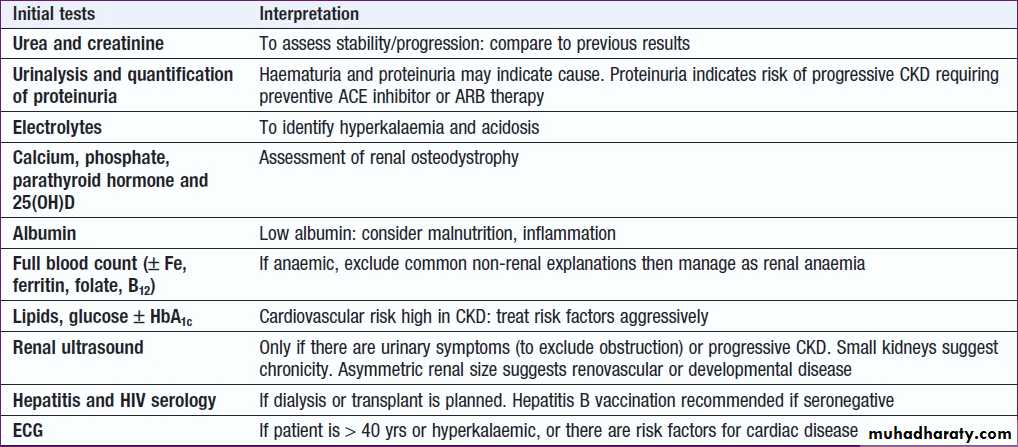
CKD investigations : to
a. Identify the underlying causeb. Identify reversible factors that may worsen renal function, such as
hypertension, urinary tract obstruction, nephrotoxic drugs, and salt
and water depletion
c. Screen for complications, as anaemia and renal osteodystrophy
d. Screen for CV risk factors.
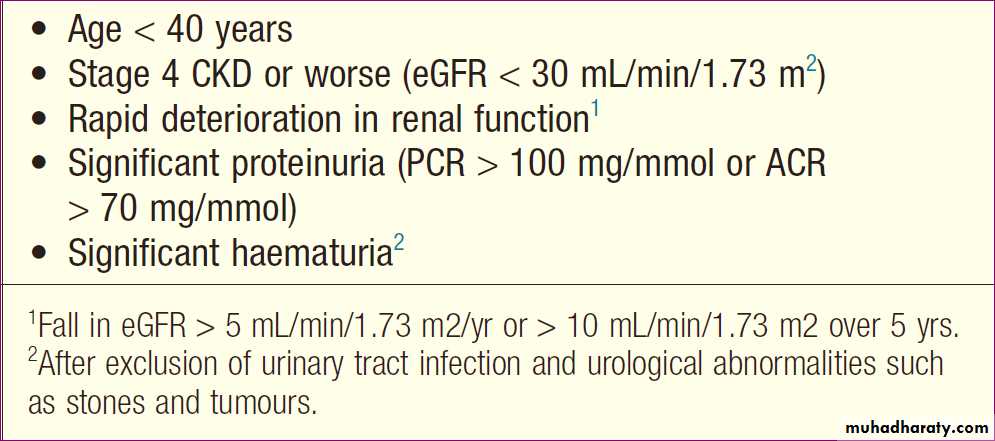
Criteria for referral of CKD patients to a nephrologist
Referral to a nephrologist is appropriate for patientswith potentially treatable underlying disease and those
who are likely to progress to ESRD.
Management of CKD
The aims are to prevent or slow further renal damage; to limit the adverse physiological effects; to treat risk factors for CVD; and to prepare for RRT.1. Antihypertensive therapy
Slows the rate of renal declines, lowering the risk of hypertensive heart failure, stroke , peripheral vascular disease, and proteinuria. Various targets have been suggested, 130/80 mmHg for uncomplicated ,and 125/75 mmHg for CKD complicated by significant proteinuria of more than 1 g/day .
2. Reduction of proteinuria .There is strong evidence that reducing proteinuria reduces the risk of progression. ACEI and ARBs reduce proteinuria and retard the progression of CKD .
Management of CKD (continued)
These effects are partly due to the reduction in blood pressure .In addition, ACE inhibitors have been shown to reduce the risk of cardiovascular events and all-cause mortality in CKD. ACEI and ARBs may be accompanied by an immediate reduction in GFR when treatment is initiated, due to a reduction in glomerular perfusion pressure.Treatment can be continued so long as the reduction in GFR is < 20% and is not progressive.
Accordingly ACE inhibitors and/or ARBs should be prescribed to all diabetic nephropathy and those with proteinuria, irrespective of whether or not hypertension is present, providing that hyperkalaemia does not occur.
Management of CKD (continued)
3. Lipid-lowering therapyHypercholesterolaemia is almost universal, and increased triglyceride also common. Lipid lowering has been shown to reduce vascular events in non-dialysis CKD patients.
There is evidence that control of dyslipidaemia with statins may slow the rate of progression of renal disease.
4. Dietary and lifestyle interventions
Restricting dietary protein can reduce progression of CKD. All patients with stage 4 and 5 CKD should be given dietetic advice aimed at preventing excessive consumption of protein, ensuring adequate calorific intake and limiting potassium and phosphate intake.
Severe protein restriction is not recommended.
Management of CKD (continued)
5. All patients should be advised to stop smoking. Exercise and weight loss reduce proteinuria and CV risk.6. Maintaining fluid and electrolyte balance
Dietary sodium intake limited to 100 mmol/d, but often loop diuretics may also be required to treat fluid overload.
If hyperkalaemia occurs, reduce or stop potassium-sparing diuretics, ACE inhibitors and ARBs. Correction of acidosis may be helpful, and limiting potassium intake to about 70 mmol/day. Potassium-binding resins, such as calcium resonium, may be useful in the short term. The plasma bicarbonate should be maintained above 22 mmol/L by giving sodium bicarbonate supplements . If the increased sodium intake induces hypertension or oedema, calcium carbonate may be used as an alternative, since this has the advantage of also binding dietary phosphate.
Management of CKD (continued)
7. Treatment of anaemiaAnaemia is common with a GFR below 30 mL/min/1.73 m2. Recombinant human erythropoietin is effective in correcting the anaemia of CKD and improving the associated morbidity. Correcting haemoglobin to normal levels may carry some extra risk, including hypertension and thrombosis (including thrombosis of the AVF).The target haemoglobin is usually between 100 and 120 g/L .
Erythropoietin is less effective in the presence of iron deficiency, active inflammation or malignancy, and aluminium overload, which may occur in dialysis.
Management of CKD (continued)
8. Renal bone diseaseTreatment with active vitamin D metabolites (1-α(OH) D or 1,25(OH)2D) in patients who are found to have hypocalcaemia or serum PTH levels more than twice the normal, but care to avoid hypercalcaemia.
Hyperphosphataemia should be treated by dietary restriction of foods with high phosphate content (milk, cheese, eggs and protein-rich foods) and by the use of phosphate-binding drugs, including calcium carbonate, aluminium hydroxide, and phosphate binders such as sevelamer. Parathyroidectomy may be required for the treatment of tertiary hyperparathyroidism. An alternative is calcimimetic agents, such as cinacalcet, which bind to the calcium-sensing receptor and reduce PTH secretion. They have a place if parathyroidectomy is unsuccessful or not possible.
Management of CKD (continued)
9. Renal replacement therapy (RRT) may be required on atemporary basis in patients with AKI or on a permanent
basis for those with CKD.
The aim of RRT is to replace the excretory functions of the kidney, and to maintain normal electrolyte concentrations and fluid balance.
Following initiation of dialysis in the UK, the survival
is 84% at 1 year and 50% after 5 years.
Comorbid conditions, such as diabetes mellitus and generalised vascular disease, also have a strong influence on mortality.
Various options are available, including haemodialysis, haemofiltration, haemodiafiltration, peritoneal dialysis and renal transplantation.
Management of CKD (continued)
Conservative treatmentIn older patients with multiple comorbidities, conservative treatment of stage 5 , aimed at limiting the adverse effects of ESRD without commencing RRT, is increasingly viewed as a positive choice .
Current evidence suggests that survival of these patients can be similar or only slightly shorter than that of patients who undergo RRT, but they avoid the hospitalisation and interventions associated with dialysis. Patients are offered full medical, psychological and social support to optimise and sustain their existing renal function and to treat complications, such as anaemia, for as long as possible. Many enjoy a good quality of life for several years.
It is also appropriate to discontinue dialysis, with the consent of the patient, and to offer conservative therapy when quality of life on dialysis is inadequate.
Preparing for renal replacement therapy
Patients who have progressive CKD are prepared well in advance for RRT, should be started at least 12 months before the predicted start date, providing the patient with psychological and social support, assessing home circumstances and discussing the various choices of treatment ,the overall aim is to commence RRT by the time symptoms have started to appear but before serious complications have occurred.
For those that decide to go ahead with RRT, further choices between haemo and peritoneal dialysis between hospital and home treatment, and R. transplantation.
At the present time, the average eGFR at the time of initiating RRT in the UK is about 8 mL/min/1.73 m2 but there is wide variation.
Management of CKD (continued)
Haemodialysis (HD)Haemodialysis is the most common form of RRT in
ESRD and is also used in AKI.
Haemodialysis involves gaining access to the circulation, either through an arteriovenous fistula, a central venous catheter or an arteriovenous shunt, (a Scribner shunt). The patient’s blood is pumped through a haemodialyser, which allows bidirectional diffusion of solutes between blood and the dialysate across a semipermeable membrane down a concentration gradient .
Haemodialysis in AKI
Offers the best rate of small solute clearance, but should be started gradually because of the risk of confusion and convulsions due to cerebral oedema (dialysis disequilibrium). Typically, 1–2 hours of dialysis is prescribed initially but, subsequently, stable patients can be treated by 4–5 hours of HD on alternate days.For short dialyses and in patients with abnormal clotting, it may be possible to avoid anticoagulation altogether.
In AKI, dialysis is performed through a large-bore, dual-lumen catheter inserted into the femoral or internal jugular vein. Subclavian lines are avoided where possible, as thromboses or stenoses here will compromise the ability to form a functioning fistula in the arm if the patient fails to recover renal function and needs chronic dialysis.
Haemodialysis in CKD
Vascular access for is gained by formation of an AV fistula, usually in the forearm, up to a year before dialysis is contemplated. After 4–6 weeks, increased pressure transmitted from the artery to the vein causes distension and thickening of the vessel wall (arterialisation).Large-bore needles can then be inserted into the vein to provide access for haemodialysis.All patients must be screened for hepatitis B, C and HIV, and vaccinated against hepatitis B if they are not immune. All dialysis units should have segregation facilities for hepatitis B-positive patients, given its easy transmissibility. Patients with hepatitis C and HIV are less infectious and can be treated satisfactorily using machine segregation and standard infection control measures.
Haemodialysis is usually carried out for 3–5 hours
three times weekly, either at home or in an outpatientdialysis unit.The intensity and frequency of dialysis adjusted to achieve a reduction in urea of over 65%. Most patients notice an improvement in symptoms during the first 6 weeks of treatment.
Plasma urea and creatinine are lowered by each treatment but do not return to normal.
Haemofiltration
principally used in the treatment of AKI.
Haemodiafiltration
This combines haemodialysis with approximately 20–30 litres of ultrafiltration over a 3–5-hour treatment. It is sometimes used in the treatment of AKI but is increasingly favoured in the treatment of CKD. It is more expensive .
Indications for dialysis -CKD
Uremic PericarditisUremic Encephalopathy or Neuropathy
Pulmonary edema (unresponsive to diuretics)
Severe Hypertension
Severe hyperkalemia
Intractable acidosis
Severe Bleeding diathesis
Persistent gastrointestinal symptoms
Medical Complications during hemodialysis
Common: hypotension , chest Pain , muscle cramps, nausea ,vomiting, headache, itching, fever and chills .Serious complications
• Disequilibrium syndrome, Intracranial bleeding , seizures .
• Arrhythmia , cardiac tamponade .
• Hemolysis, air embolism .
Peritoneal dialysis
Principally used in the treatment of CKD.It requires the insertion of a permanent Silastic catheter into the peritoneal cavity Two types are in common use.
1. In continuous ambulatory PD(CAPD), about 2 litres of sterile, isotonic dialysis fluid are introduced and left in place for approximately 4–6 hours.
The fluid is then drained and fresh dialysis fluid introduced, in a continuous four-times-daily cycle. Metabolic waste products diffuse from peritoneal capillaries into the dialysis fluid down a concentration gradient.The fluid is then drained and fresh dialysis fluid introduced, in a continuous four-times-daily cycle. The patient is mobile and able to undertake normal daily activities.
Peritoneal dialysis (continued)
2. Automated PD (APD) is similar to CAPD but uses a mechanical device to perform the fluid exchanges during the night, leaving the patient free, or with only a single exchange to perform, during the day.is particularly useful in children, as a first treatment in adults with residual renal function, and as a treatment for elderly patients with CV instability.
The long-term use of peritoneal dialysis may be limited by episodes of bacterial peritonitis and damage to the peritoneal membrane
• Specific indications for peritoneal dialysis
• Patients with cardiovascular or hemodynamic instability• Vascular access failure or can not be created .
• High risk of anticoagulation .
• older age group (over 65) and small children .
• Severe hemodialysis-related symptoms or disequilibrium .
Contraindication for Peritoneal dialysis
• Peritoneal fibrosis , Pleuroperitoneal leak .
• Recent Abdominal and thoracic surgery .
• Extensive Abdominal adhesions .
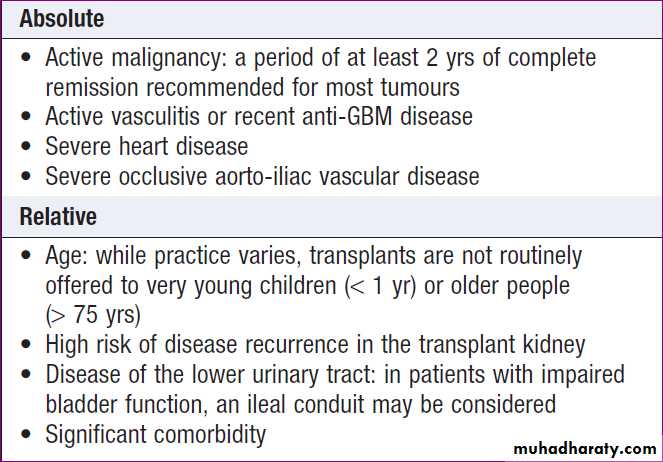
Renal transplantation
Offers the best chance of long-term survival in ESRD, and is the most cost-effective treatment.Transplantation can restore normal kidney function and correct all the metabolic abnormalities .
but requires long-term immunosuppression with its
attendant risks . All ESRD patients should be considered for transplantation, unless there are contraindications .Compatibility of ABO blood group between donor and recipient is usually required and the degree of matching for major histocompatibility (MHC) antigens, particularly HLA DR, influences the incidence of rejection.
Renal transplantation (continued)
Immediately prior to transplantation, tests should be performed for antibodies against HLA antigens and for antibodies that can bind to lymphocytes of the donor .Positive tests predict early rejection.
Although some ABO- and HLA-incompatible transplants are now possible, this involves appropriate preparation with pre-transplant plasma exchange and/or immunosuppression, so that recipient antibodies to the donor’s tissue are reduced to acceptably low levels.
During the transplant, the kidney is placed in the pelvis; the donor vessels are usually anastomosed to the recipient’s internal iliac artery and vein, and the donor ureter anastomosed to the bladder .
The failed kidneys are left in place.
Renal transplantation (continued)
Once the graft begins to function, near-normal biochemistry is usually achieved within days to weeks. All patients require regular life-long follow-up to monitor renal function and immunosuppression to prevent rejection but needs to be more intensive in the early period, when the risk is highest.A common regimen is triple therapy with prednisolone; ciclosporin or tacrolimus; and azathioprine or mycophenolate mofetil. Sirolimus (rapamycin) is an alternative. Antibodies to deplete or modulate specific lymphocyte populations are increasingly used; targeting the lymphocyte interleukin (IL)-2 receptor is particularly effective for preventing rejection.
Renal transplantation (continued)
Acute rejection is usually treated, in the first instance, by short courses of high-dose corticosteroids, such as methylprednisolone 500 mg IV on 3 consecutive days. Other therapies, such as antilymphocyte antibodies, intravenous immunoglobulin and plasma exchange, can be used for episodes of acute rejection that do not respond to high dose corticosteroids.Complications of immunosuppression include
Infections and malignancy .
Approximately 50% of white patients develop skin malignancy by 15 years after transplantation.
The prognosis after kidney transplantation
is good. Recent UK statistics for transplants from cadaver donors indicate96% patient survival and 93% graft survival at 1 year, and 88% patient survival and 84% graft survival at 5 years.
Even better figures are obtained with
living donor transplantation
(91% graft survival at 5 years).
Advances in immunosuppression have greatly improved results from using genetically unrelated donors