NICE hypertension guideline 2011: evidence basedevolution
BMJ 13 January 2012د. حسين محمد جمعه
اختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2012
Diagnosis of hypertension
The 2011 guideline continued the risk based approach to diagnosis and starting treatment first proposed by the New Zealand guidelines in 1993 and included in subsequent British Hypertension Society and NICE guidelines since 1999. The method for diagnosing hypertension was refined after review of evidence from 19 studies considering the relative prognosticability of ambulatory or home blood pressure monitoring (ABPM and HBPM respectively) compared with clinic monitoring in determining outcome on the basis of baseline blood pressure.
These studies showed that “out of office measures” are better than clinic measurement at predicting
subsequent risk of cardiovascular events and many, including the PAMELA study, were included in Fagard and Cornelissen’s 2007 meta-analysis.8 This showed a hazard ratio for cardiovascular events of 1.12 (95% confidence interval 0.84 to 1.50) for “normal” ambulatory blood pressure with “raised”
clinic measurements (white coat hypertension) compared with “normal” ambulatory and clinic pressures (definitively normotension).
Included studies had follow-up periods of between 3.2 and 10.9 years (only one was less than five years)
suggesting no evidence of increased risk from white coat hypertension over and above normotension over a standard five yearly screening cycle. This provided the rationale for the recommendations on diagnosis, not a belief that hypertension is currently “over treated” as Brown and colleagues claim.
These data, combined with a new meta-analysis showing the relative test performance of clinic and home measurement, triggered the development of an economic model.
This showed that ambulatory monitoring was the dominant strategy for the
diagnosis of hypertension for both men and women in all age groups from 40-75 years and that this conclusion was robust to a wide variety of sensitivity analyses.
Exceptions to this were if normotensive people were assumed to benefit from blood pressure reduction or if the test performance of all three monitoring methods was considered equal. An absence of trials of the “polypill” approach as well as evidence from the systematic review of test performance in diagnosis meant that these analyses were not considered sufficient to overturn the main results.
If re-testing of all screened individuals took place annually rather than five yearly,
ambulatory monitoring was cost effective for people older than 60 but not younger. This scenario was deemed extreme as only people around the diagnostic threshold are likely to need frequent retesting, hence ambulatory monitoring retained its dominance. The guideline therefore recommended at least five yearly screening but annually for those close to the threshold.Ambulatory blood pressure thresholds
The thresholds for normal ambulatory blood pressure, unchangedfrom NICE guidelines since 2004, were not based on a single
study but were supported by the results of the prognostic
meta-analysis8 and are consistent with international
recommendations. Head and colleagues’ paper supports the
appropriateness of the ambulatory equivalents to clinic
measurements, particularly for the stage 1 and 2 thresholds.
Guideline targets are based on mean achieved blood pressure
as well as targets in trials, hence mean blood pressure
comparisons are relevant.
General considerations for choice of drugs
The general argument put forward by Sofat and colleagues that choice of antihypertensive drug should rest on cost, tolerability, and specific contraindications, ignores efficacy at preventing morbid and mortal cardiovascular events, which the guidelines
considered to be most important. Cost is now much less relevant as the main classes are available as generic drugs at broadly equivalent prices. The 2011 guidance aimed to recommend the most cost effective drugs, taking into account tolerability and most conceivable specific indications.
First line treatment
The other major area where changes were implemented in the 2011 guidelines was in treatment choice. Here again, the guideline development process was dictated by cost effectiveness. The 2006 model critiqued by Sofat and colleagues was updated to take account of the reduced costs of drugs as all are now available as generics.Importantly, the model showed that treating hypertension is cost saving versus no treatment.
As in 2006, calcium channel blockers emerged as the most cost effective option but now more so because of their availability as generic formulations. This was the principal driver for recommending calcium channel blockers as the preferred initial therapy for most people over the age of 55 years, the exception being people with evidence of heart failure or at higher risk of heart failure, for whom the sensitivity analysis suggested athiazide-like diuretic should be preferred as initial therapy.
That calcium channel blockers are also less likely to cause impaired glucose tolerance, electrolyte disturbances, and gout and have been reported to be particularly effective at reducing blood pressure variability (which has recently been suggested as an
independent predictor of risk, especially for stroke) further strengthened the rationale for this recommendation.
Another consideration was that the only trial to directly evaluate two drug combinations of treatments with a renin-angiotensin receptor system blocker, consistent with step two of the NICE
treatment algorithm, also showed that combination with acalcium channel blocker was better than with a thiazide diuretic for preventing cardiovascular outcomes.
The differentiation of drug choice for initial treatment according to age was maintained because sensitivity analyses showed that even small advantages in efficacy for drugs blocking the renin-angiotensin system over other classes made angiotensin
converting enzyme inhibitors and low cost angiotensin receptor blockers very cost effective in younger people. Diabetes, like hypertension, relies on an arbitrary cut-off for diagnosis, but why would somebody want to develop diabetes if it could be avoided?
The hazard ratio in the ASCOT trial for development
of diabetes was 0.70 (95% CI 0.63 to 0.78) for calcium channel blocker plus angiotensin converting enzyme inhibitor arm compared with β blocker plus thiazide. Sarwar and colleagues’outcome data show that diabetes is associated with increased hazard ratios for coronary heart disease of 2.00 (95% confidence interval 1.83 to 2.19) and 2.27 (1.95 to 2.65) for ischaemic stroke.
The NICE model assumed a doubling of cardiovascular
risk with diabetes in the base case, but at all levels of risk from diabetes calcium channel blockers remained the most cost effective option, even if the relative risk of cardiovascular events with diabetes was set to 1 (that is, no increase in risk of events).
Choice of diuretic
Another question in the scope related to the choice of diuretic.The United Kingdom is unique in the world in its almost
exclusive use of lower dose bendroflumethiazide (usually 2.5 mg once daily) to treat hypertension. The evidence review found no data evaluating and supporting effectiveness of this treatment
in preventing cardiovascular events. It was therefore difficult to continue to recommend it. More contemporary studies had used thiazide-like diuretics (chlortalidone and indapamide).
The evidence for these drugs at modern doses was substantial, including several large primary prevention trials such as SHEP,ALLHAT, and HYVET.18-20 Both are also available as low cost generic formulations and the decision, based on best available evidence, was that these should be the preferred diuretics.
Although chlortalidone is available in the UK only in higher doses (50 mg), it should not stretch the organisational capability of the NHS to respond because the recommended doses are widely available elsewhere.
β blockers
In the updated meta-analysis for this guideline, as in previously published independent meta-analyses, β blockers were the least cost effective treatment for hypertension and notably less effective than the recommended first line drugs.2 21 Law andcolleagues’ meta-analysis also found them to be significantly worse at preventing stroke than other drugs (relative risk 1.18 (1.03 to 1.36)). This may be a function of β blockers inferiority to calcium channel blockers or of less effective blood pressure reduction, but whatever the cause it is difficult to ignore when making recommendations for treating hypertension.
Fourth line treatment for resistant hypertension
The evidence for fourth line treatment options in hypertension is currently suboptimal. However, people with treatment resistant hypertension are a high risk group and the evidence,albeit primarily from six observational studies, suggested that low dose spironolactone can be very effective at further reducing blood pressure.
This strategy is common practice in specialist
hypertension clinics. Consequently, the 2011 guideline gave a“steer” towards the use of low dose spironolactone, while making clear in the research recommendations that more definitive evidence is needed. The ongoing PATHWAY studieswill hopefully provide clarity in this area.
Conclusions
Finally, although we welcome healthy academic debate about the finer detail of the guideline, the key to its success is buy-in from clinicians. To date this has been high, at least in part because of the continued support of the British Hypertension Society, which has made a series of videos covering key aspects of the guidance (www.bhsoc.org/stream/BHS_Annual_Scientific_Meeting_NICE_Hypertension_Guidelines.html).
These, along with the NICE implementation materials (http:// www.nice.org.uk/CG127), will help facilitate dissemination and implementation of this evidence based evolution of the NICE hypertension guidelines.
Differences in blood pressure between arms
May be diagnostically useful but needs further evaluation as a prognostic markerBMJ 20 March 2012
A difference in blood pressure readings between arms can be seen in congenital heart disease, aortic dissection, peripheral vascular disease, and unilateral neuromuscular abnormalities.
In the absence of these conditions, any discrepancy is small (mean difference: 5 mm Hg and 4 mm Hg for systolic and diastolic blood pressure, respectively).1 About 20% of patients in primary care or secondary care have a between arm blood pressure difference of 10 mm Hg or more and 4% have adifference of 20 mm Hg or more.
Although such a difference in blood pressure is thought to be a marker of atherosclerosis,
its clinical significance is not fully understood. In a linked research study (doi:10.1136/bmj.e1327), and in a recent meta-analysis,5 Clark and colleagues provide additional evidence on the diagnostic and prognostic relevance of this phenomenon.In their meta-analysis of cross sectional studies,5 Clark and colleagues reported that a between arm blood pressure difference of 15 mm Hg or more was associated with peripheral vascular disease (sensitivity 15% and specificity 96%) and with cerebrovascular disease (sensitivity 8% and specificity 93%),
but not with coronary artery disease. A difference of 10 mm Hg or more was associated only with peripheral vascular disease (sensitivity 32% and specificity 91%).5 The overall low sensitivity and high specificity suggest that the measurement of blood pressure in both arms is not a good screening test for asymptomatic peripheral vascular disease.
How should clinicians use measurements of bilateral arm blood pressure in their practice? As recommended by current hypertension guidelines, including the new update from the National Institute for Health and Clinical Excellence, bilateral blood pressure measurements should be done routinely to avoid delayed diagnosis or undertreatment of hypertension in those with a 10 mm Hg difference between arms (20% of primary and secondary care patients).
What about measurement technique? On the basis of available evidence and practicality, a sequential measurement, followed by confirmation with at least two simultaneous measurements using two automatic devices, seems to be a reasonable approach. The optimal number of repeated measurements and monitoring intervals are unknown.
If the difference is 10 mm Hg or more on repeated simultaneous measurements, the positive predictive value for peripheral vascular disease of the upper extremities is high and further diagnostic evaluation is warranted, especially in people with
risk factors for cardiovascular disease. Subsequent blood pressure monitoring should be performed in the arm with the higher readings..
In the absence of obstructive peripheral vascular
disease, there is insufficient evidence for evaluating prognosis or guiding treatment with antiplatelet drugs or statins on the basis of between arm differences in blood pressure. More workis needed to examine how well between arm difference in blood pressure compares with other markers of subclinical atherosclerosis, biomarkers, and clinical predictors as a predictor of mortality from cardiovascular disease.
Dual renin-angiotensin system blockade
Combining a renin inhibitor with an ACE inhibitor or ARB is riskyBMJ 1 February 2012
Some 30 years ago, captopril became the first angiotensin converting enzyme (ACE) inhibitor available for clinical use.
Since then, several other ACE inhibitors have been introduced, as well as drugs from the angiotensin II type 1 receptor blocker (ARB) family. More recently, an orally active renin inhibitor, aliskiren, has become available.
It is now possible to block renin itself, to inhibit the conversion of angiotensin I into angiotensin
II, and to block the main target receptors of angiotensin II in asingle patient. In the linked systematic review and meta-analysis of randomised controlled trials (doi:10.1136/bmj.e42), Harel and colleagues examined the safety of dual blockade of the renin-angiotensin system using the renin inhibitor aliskiren and
an ACE inhibitor or ARB.
Many investigators have evaluated whether delivering acombination of drugs that interfere with specific functions of the renin-angiotensin system would be more effective at reducing blood pressure or protecting target organs (or both)
than giving one drug only. Both dual and triple renin-angiotensin blockade have been investigated. Most trials did not consider the potential pathophysiological consequences of blocking the system at multiple levels.
A meta-analysis that compared combinations of an ACE inhibitor and ARB with single drug treatment—mainly in patients with diabetes or kidney
disease—showed that, among other positive findings, patients on dual treatment had a greater reduction in proteinuria than those taking a single drug.2 However, the authors cautioned that most of the included studies were small, varied in quality, and did not provide reliable data on adverse drug reactions. At thesame time concerns about the safety of combination treatmentwith an ACE inhibitor and an ARB, particularly in patients with left ventricular dysfunction, emerged from another meta-analysis.
Hypotension, worsening kidney function, and an increase in serum potassium were the most worrying adverse events. Two large randomised trials evaluating dual treatment
in high risk patients reported similar adverse findings.4 5 The
combination of an ACE inhibitor and an ARB seems to affect
potassium values more than it affects overall renal function,
which suggests that raised serum potassium is only marginally
related to a reduction in glomerular filtration rate, if at all. ACE
inhibitors and ARBs have opposite effects on angiotensin II
concentrations, and a reduction in glomerular filtration rate in
response to one of the agents may be partially offset by the other.
Both drugs, however, lower plasma aldosterone, and the combination of a fall in plasma angiotensin II together with adrenal angiotensin receptor blockade could possibly induce astate of relative hypoaldosteronism, leading to a rise in plasma potassium.
Less is known about safety when the new renin inhibitor aliskiren is combined with an ACE inhibitor or an ARB, which is why Harel and colleagues analysed the risk of acute kidney injury and hyperkalaemia when various blockers of the renin-angiotensin system are combined.
One message that emerged from their analysis of a large number of studies is that the risk of renal impairment associated with dual treatment with aliskiren and an ACE inhibitor or ARB is smaller than many clinicians might believe. However, their definition of acute kidney injury was fairly liberal, with a cut-off value that many nephrologists would consider too high, and it did not take into account baseline creatinine. Moreover, it is not certain that reported cases of renal impairment were truly related to kidney damage because a rise in creatinine can sometimes be explained entirely by intrarenal haemodynamic factors.
An important message from the current study is that use of acombination of aliskiren and an ACE inhibitor or ARB increases the risk of hyperkalaemia. This adverse effect could again be explained by suppression of endogenous aldosterone. The
patient’s clinical background is important, but the current study did not show a clear association between the risk of hyperkalaemia and the patient’s clinical state.
Although the authors tried to differentiate between low risk and high risk patients when estimating the risk of hyperkalaemia, they were not able to show a significant difference between the two groups
for this outcome. It is therefore not clear whether or not clinicians should exercise caution in prescribing dual treatment in the subgroup of patients with hypertension but no other risk factors for hyperkalaemia.
Another drawback is that ACE inhibitors and ARBs were considered together as a single class.
The authors understandably wished to simplify the analysis of data, but unfortunately this reduces the usefulness of the study’s findings from a pharmacological perspective. The two drug classes have divergent effects on angiotensin II concentrations and receptor occupancy, and their effects may also differ when combined with aliskiren. Finally, it is not clear whether the patients who were included in the various studies were representative of the general population of patients with hypertension.
Indeed, there was a preponderance of men and
younger people in the included trials. The risk estimates from the current meta-analysis may not accurately reflect risk in “real life”—the risk of developing severe hyperkalaemia may be greater still.It is noteworthy that the ALTITUDE trial, which was designed
to explore whether dual blockade with aliskiren combined withan ACE inhibitor or ARB would reduce morbidity and mortality
in a broad range of high risk patients with type 2 diabetes,6 was stopped prematurely in December 2011. The trial’s data safety monitoring board advised against continuing the study because “the active treatment group experienced an increased incidence of non-fatal stroke, renal complications, hyperkalaemia, and hypotension over 18 to 24 months of follow-up.” The committee concluded that patients were unlikely to benefit from aliskiren on top of standard antihypertensive treatment.
Given these concerns, clinicians should avoid using a
combination of renin-angiotensin system blockers in high risk patients and be extremely cautious when using combination treatment in low risk patients. Another lesson may be that sound pathophysiological data rather than logical reasoning shouldguide the design of large and costly trials. Future work should concentrate on how different renin-angiotensin system blockers interact and in which types of patients the combination can be used safely.
Controversies over hypertension guidelines
BMJ 25 January 2012The proper management of hypertension is arguably one of
modern medicine’s most effective preventive interventions. It’s
also one for which we have lots of clinical trial data, as well as
a good number of well done meta-analyses. Yet as this week’s
BMJ shows, controversy about how best to diagnose and treat
hypertension in adults is still alive and well.
The 2011 guidance from the UK’s National Institute for Health
and Clinical Excellence (NICE) has been met with a blast of
criticism. This week we present two different critiques: the first
suggesting that the guidelines are overcomplicated, the second
that they are insufficiently evidence based. In a third article, the
guidelines’ authors respond.
What did the 2011 update say? It was, in fact only a partial update, focusing on areas where the evidence was deemed to have moved on since 2006. But it made several key changes to previous guidance. These included advice to use ambulatory
and home blood pressure monitoring to confirm a raised clinic reading, and a different choice of drug class for first and second line treatment.
In place of the well established AB/CD
algorithm—angiotensin converting enzyme inhibitor or β blockers/calcium channel antagonist or diuretic—the updated guidance recommends ACD in people under 55: ACE inhibitor or angiotensin II receptor blocker, followed by a calcium channelblocker, followed by a thiazide-like diuretic. Patients over 55 are recommended to start on a calcium channel blocker.
Reecha Sofat and colleagues think this is overcomplicated(doi:10.1136/bmj.d8078). The most recent evidence suggests that the four drug classes are more similar than different in their
efficacy and safety, they say, and that their effects in combination are additive. This means that the initial choice of drug could rest on price, tolerability, and individual patients’ characteristics.
Morris Brown and colleagues take a different tack (doi:10.1136/ bmj.d8218). They say there are no outcome data from trials that justify the shift to ambulatory and home monitoring, and they
are surprised by NICE’s conclusion that ambulatory monitoring could cut the number of people starting on antihypertensive drugs by a quarter.
“The combination of a rise, compared to previous guidance, in the blood pressure threshold for treatment
and a longer interval before repeat monitoring is not plausible, evidence based, or safe,” they say. They are equally concerned about the relegation of diuretics from first to third line treatment, and the recommendation to use chlortalidone, for which no suitable 12.5 mg formulation is available in the UK. Good cheap drugs such as co-amilozide are overlooked, they say.
In reply, the guideline’s authors argue convincingly that the
recommendations are evidence based and that this latestguidance is an evolution that will continue as more evidence
accrues (doi:10.1136/bmj.e181). Both sets of critics say that, despite the many trials and meta-analyses already done, a great many questions remain. Brown and colleagues try to make the best of what they clearly see as a bad job in calling for the latest NICE guidelines to serve as a catalyst for more robust clinical trials. Sofat and colleagues call for an updated network
meta-analysis, taking into account the evidence from recent
large influential trials and meta-analyses.
CLINICAL IMPLICATIONS
Because of the risks for renal impairment, hypotension, and hyperkalemia, the FDA warns clinicians not to prescribe antihypertensive drugs containing aliskiren in combination with ACE inhibitors and ARBs for patients with diabetes. Clinicians should also avoid these drug combinations in patients with moderate to severe renal impairment.Valturna, which combines aliskiren and the ARB valsartan, will no longer be marketed by Novartis, but it will remain available until July 20 so that clinicians can help their patients make the transition to other drugs.
2012 Medscape
Other aliskiren-containing medications affected by the new FDA warning are Tekturna HCT, Tekamlo, and Amturnide. Labels on these drugs are being revised to reflect the new contraindication and warning.
The labeling changes are based on preliminary data from the ALTITUDE trial of aliskiren in combination with ACE inhibitors and ARBs. Because of an apparent lack of efficacy and higher risks for renal impairment, hypotension, and hyperkalemia, Novartis stopped the trial prematurely in December 2011. In this trial, aliskiren was associated with a "slight excess" in cardiovascular adverse events of unclear significance.
Oral Renin Inhibitors in Clinical Practice
Medscape 08/16/2012;The importance of the renin–angiotensin–aldosterone system (RAAS) in cardiovascular and renal diseases has long been
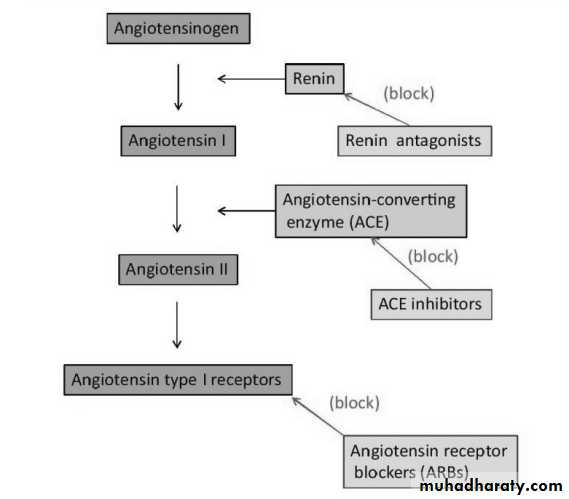
recognized. Secretion of renin is the first step in the RAAS cascade. Renin is secreted, in response to a variety of stimuli, from the juxtaglomerular cells in the kidneys.
Renin cleaves angiotensinogen to form angiotensin I (Ang I) which is then converted by angiotensin-converting enzyme (ACE) to the active angiotensin II (Ang II), the effector enzyme of the cascade.
Ang II interacts with type-1 angiotensin receptors (AT-1), inducing vasoconstriction and increasing blood pressure, promoting adrenal aldosterone secretion, renal sodium re-absorption and release of catecholamines from the adrenal medulla and
prejunctional nerve endings [Kim and Iwao, 2000].
This concept provides a rationale for the use of conventional therapies, such as ACE inhibitors (ACEIs), angiotensin receptor blockers (ARBs), β-blockers, aldosterone antagonists, and novel renin inhibitors in many cardiovascular conditions (Figure 1).
ACEIs and ARBs represent the backbone of current antihypertensive therapy.
The beneficial effects of these agents are attributed to the inhibition of deleterious AT1R stimulation and, therefore, prevention of Ang II-induced vasoconstriction, salt
and water retention, aldosterone and vasopressin release, stimulation of the sympathetic nervous system, inflammation, and
stimulation of cell growth [Unger, 2002]. However, clinical trials testing for ancillary-blood-pressure-independent benefits of
renin–angiotensin system inhibitors have yielded mixed results, in part because ACEIs and ARBs do not produce complete
renin–angiotensin system inhibition. By achieving more complete renin–angiotensin system inhibition, direct renin inhibitors
(DRIs) may afford greater protection from hypertensive complications [O'Brien et al. 2007].
The direct inhibition of renin is a logical target for pharmacologic suppression of the RAAS, because renin-mediated cleavage of angiotensinogen to form Ang I is a rate-limiting first step in the RAAS pathway. However, early attempts to develop DRIs
met with little success, and research subsequently focused on developing ACEIs and ARBs, with approval of ACEIs throughout the 1980s and ARBs beginning in the mid-1990s.
Direct Renin Inhibitors In the early 1980s, remarkable efforts were made to develop peptide analogues of angiotensinogen [Haber, 1983], which
through a number of chemical refinements led to the production of numerous compounds (e.g. enalkiren, ramikiren and zankiren) [Delabays et al. 1989; Kobrin et al. 1993; Menard et al. 1995]. However, none of these came to the point of being
used for treatment of patients, because of their low inhibiting activity, lack of oral bioavailability and short half-life [Staessen et al. 2006]
Reactive increases in plasma renin concentration (PRC) and plasma rennin activity (PRA) during ACEI treatment may result in increased Ang II production via non-ACE pathways. Moreover, higher levels of Ang I may overcome the ability of ACEIs to effectively suppress ACE activity. ACE escape also may relate to the relatively low binding affinity of ACEIs for ACE and the
relatively low levels of the dosing of ACEIs used in clinical practice to avoid drug-related adverse events.].
With ARBs, the reactive elevations in PRC and PRA lead to increases in Ang II levels [Schindler et al. 2007]. This may result in greater
competition and displacement of ARBs from AT1-R sites and reduced antihypertensive efficacy [Burnier and Brunner, 2000].
In contrast, DRIs bind directly to the catalytic site of renin, thereby inhibiting its ability to convert angiotensinogen to angiotensin I, the rate-limiting step in the formation of angiotensin II [Danser and Deinum, 2005; Wood et al. 2003].
Owing to this proximal renin–angiotensin system blockade, DRI therapy is accompanied by decreased Ang I and Ang II levels [Nussberger et al. 2002]. The resultant loss of feedback inhibition elicits a large reactive rise in renin secretion but PRA, the
enzymatic activity of renin, is markedly reduced by the DRI.[Nussberger et al. 2002] Thus, while PRA, Ang I and Ang II all increase reflexively with ARBs, they decrease markedly with a DRI [Nussberger et al. 2002
Aliskiren is the first DRI, suitable for oral administration, to reach the clinical arena [Van Tassell and Munger, 2007]. Aliskiren is characterized by high hydrophilicity which confers a good bioavailability; it is a potent human renin inhibitor [Wood et al.
2003]. Studies in normotensive subjects demonstrated that aliskiren suppressed PRA and plasma levels of Ang I and Ang II
[Nussberger et al. 2002]. The mean absolute bioavailability of aliskiren is 2.6% [Vaidyanathan et al. 2006], peak plasma
concentrations are reached 1–2 hours after dosing and steady state is reached after 5–8 days of once-daily administration
[Nussberger et al. 2002]. The main pathway of elimination for aliskiren is via biliary excretion as unmetabolized drug, less than 1% is excreted in urine [Nussberger et al. 2002].
Aliskiren is not metabolized by cytochrome P450 thus it shows no clinically relevant interaction with other commonly used drugs such as ramipril, valsartan, hydrochlorothiazide (HCTZ), amlodipine,
atenolol, lovastatin, warfarin, cimetidine, celecoxib. Age, gender, ethnicity, renal and hepatic impairment and diabetes do not
affect aliskiren pharmacokinetics [Ayalasomayajula et al. 2008; Dieterle et al. 2005; Vaidyanathan et al. 2006, 2007a, 2007b, 2007c, 2008].
One concern is the potential adverse effect of high circulating renin concentrations after aliskiren therapy. Aliskiren binds to the active site of renin, reducing its activity and Ang II production. Diminished Ang II levels stimulate renin secretion. The potential negative consequence of high renin concentration is that renin may bind to a renin receptor and trigger yet unknown events [Nguyen et al. 2002].
High prorenin levels are closely associated with the severity of diabetic complications. In this respect, in patients with
diabetes, increased prorenin levels have been shown to be associated with microalbuminuria and with the development of nephropathy [Chiarelli et al. 2001; Deinum et al. 1999].
Effect on Blood Pressure In the 8-week, placebo-controlled trials, monotherapy with aliskiren 150 mg/day or 300 mg/day reduced baseline systolic and diastolic blood pressure to a significantly greater extent than placebo in patients with stage 1 to stage 2 hypertension [Gradman et al. 2005; Kushiro et al. 2006; Oh et al. 2007; Oparil et al. 2007; Villamil et al. 2007]. The antihypertensive
efficacy of aliskiren monotherapy was also demonstrated in subgroups of patients, including diabetic and obese patients and
those with metabolic syndrome.[Weir et al. 2007]
Nussberger and coworkers [Nussberger et al. 2002] were the first to report the humoral effects of aliskiren in a comparative study with enalapril and placebo. In normal volunteers, they found a dose-dependent decrease in PRA, Ang I and Ang II in
response to increasing dosages of aliskiren from 40 to 640 mg/day. A reduction of 80% of all components of the RAAS from baseline was observed after 8 days of treatment with the maximal dose of aliskiren.
Plasma aldosterone was also decreased, whereas, not surprisingly in normotensive subjects, mean blood pressure was unmodified. Stanton and colleagues [Stanton et al. 2003] compared aliskiren 37.5, 75, 150 and 300 mg once daily with losartan 100 mg once a day in 226 patients with mildto-moderate essential hypertension showing after 4 weeks that aliskiren 75, 150 and 300 mg were similar to losartan 100 mg in reduction of systolic blood pressure.
In an 8-week placebo-controlled study, Gradman and colleagues [Gradman et al. 2005] randomized 652 hypertensive patients to aliskiren 150, 300, 600 or irbesartan 150 mg or placebo. Aliskiren 150 mg was as effective as irbesartan 150 mg, reducing systolic and diastolic blood pressure by 11.6 and 9.8 mmHg, respectively. A greater reduction in blood pressure was observed with aliskiren 300 and 600 mg. Strasser and colleagues [Strasser et al. 2007] compared aliskiren 150 mg/300 mg and lisinopril 20 mg/40 mg in 183 hypertensive patients showing a mean reduction in systolic and diastolic blood pressure of 20.0/18.5 mmHg and 22.3/20.1 mmHg with both drugs, respectively.
Aliskiren did not differ significantly from atenolol in lowering systolic blood pressure in a study in which patients received aliskiren 150 mg/day or atenolol 50 mg/day for 6 weeks followed by 6 weeks on double the initial dose of the agents [Dietz et
al. 2008]. However, reductions in diastolic blood pressure were significantly greater with atenolol than with aliskiren at both .
Several randomized double-blind or open-label multicentre trials have been conducted with aliskiren alone or in combination with HCTZ, valsartan, valsartan plus HCTZ, amlodipine, amlodipine plus HCTZ, ramipril, and atenolol.
Villamil and colleagues studied a total of 2776 patients with diastolic blood pressure 95–109 mmHg; patients were randomized to receive once-daily treatment with aliskiren (75, 150 or 300 mg), HCTZ (6.25, 12.5 or 25 mg), the combination of aliskiren and HCTZ, or placebo [Villamil et al. 2007].
The primary endpoint was the change in diastolic blood pressure from baseline to week 8. Aliskiren monotherapy was superior to placebo in reducing diastolic and systolic blood pressure. Combination treatment was superior to both monotherapies in reducing blood pressure; a reduction of 21.2/14.3 mmHg from baseline with
aliskiren/HCTZ 300/25 mg was observed.
The ACQUIRE (Aliskiren Alone or in Combination With Hydrochlorothiazide in Patients With Stage 2 Hypertension to Provide Quick Intensive Control of Blood Pressure) study [Black et al. 2010], performed in 688 patients with stage 2 hypertension,
demonstrated that the combination of aliskiren 150 mg/day with HCTZ 12.5 mg/day provides substantial reductions of blood pressure (30.0/12.6 mmHg), significantly greater than those achieved with aliskiren alone (20.3/8.2 mmHg).
Drummond and colleagues [Drummond et al. 2007] investigated the addition of aliskiren to amlodipine in 545 patients with mild-to-moderate hypertension that was inadequately controlled with amlodipine alone (diastolic blood pressure 90–100 mmHg). Patients were randomized to 6 weeks of double-blind treatment with amlodipine 5 mg plus aliskiren 150 mg,
amlodipine 5 mg, or amlodipine 10 mg. At the end of the study, systolic and diastolic blood pressure reductions with the combination of aliskiren 150 mg and amlodipine 5 mg were 11.0/8.5 mmHg, respectively: significantly greater than with
amlodipine 5 mg (5.0/4.8 mmHg), but similar to amlodipine 10 mg (9.6/8.0 mmHg).
In the Aliskiren and the Calcium Channel Blocker Amlodipine as an Initial Treatment Strategy for Hypertension Control (ACCELERATE) study [Brown et al. 2011], 1,254 hypertensive patients were randomly assigned to treatment with aliskiren or
amlodipine alone and in combination. After 16 weeks, patients taking the combination (aliskiren 300 mg/day and amlodipine 10 mg/day) had a 6.5/3.7 mmHg greater reduction in systolic and diastolic blood pressure than those treated with the monotherapies at equivalent doses.
Similar results were obtained in 489 hypertensive obese patients [Jordan et al. 2007] who were nonresponders to HCTZ 25 mg. Patients were randomly assigned to aliskiren 150 mg, irbesartan 150 mg, amlodipine 5 mg, or placebo plus HCTZ 25 mg for 4 weeks, followed by 8 weeks on double the initial doses of aliskiren, irbesartan, or amlodipine. After 8 weeks of doubleblind treatment, aliskiren/HCTZ lowered blood pressure by 15.8/11.9 mmHg, significantly more than placebo/HCTZ (8.6/7.9 mmHg) and similar to the reduction obtained with irbesartan/HCTZ and amlodipine/HCTZ (15.4/11.3 and 13.6/10.3 mmHg, respectively).
Pool and colleagues [Pool et al. 2007] investigated the blood-pressure-lowering effects of aliskiren, alone or in combination with valsartan. In a placebo-controlled, 8-week trial, 1123 patients with mild-to-moderate hypertension were randomized to
receive once-daily, double-blind oral treatment with placebo, aliskiren monotherapy (75, 150 or 300 mg), valsartan monotherapy (80, 160 or 320 mg), aliskiren and valsartan in combination, or valsartan/HCTZ (160/12.5 mg). Co-administration of aliskiren and valsartan produced a greater antihypertensive effect than either drug alone, comparable in magnitude to the
effect of valsartan/HCTZ, with a reduction in systolic and diastolic blood pressure of 18.9/13.5 mmHg.
In a double-blind study [Oparil et al. 2007], 1797 patients with hypertension were randomly assigned to receive once-daily aliskiren 150 mg (n = 437),
valsartan 160 mg (n = 455), a combination of aliskiren 150 mg and valsartan 160 mg (n = 446) or placebo (n = 459) for 4
weeks, followed by double dose for another 4 weeks. The primary endpoint was change in mean sitting diastolic blood
pressure from baseline to week 8. At week 8, the combination of aliskiren 300 mg and valsartan 320 mg lowered mean
diastolic blood pressure from baseline by 12.2 mmHg, significantly more than either monotherapy (aliskiren 300 mg, 9.0
mmHg decrease; valsartan 320 mg, 9.7 mmHg decrease) or with placebo (4.1 mmHg decrease).
Cardiovascular and Renal Protection .To examine whether aliskiren possesses cardiovascular protective actions, a vast program of clinical trials, Aliskiren Study in Post-MI Patients to Reduce Remodelling (ASPIRE-HIGHER), has been developed involving more than 35,000 patients in 14 randomized double-blind studies.
In the Aliskiren in Left Ventricular Hypertrophy (ALLAY) study, the investigators have shown that aliskiren was as effective as losartan in promoting left ventricular (LV) mass regression [Solomon et al. 2009].
They randomized 465 patients with hypertension, increased ventricular wall thickness, and body mass index >25 kg/m2 to receive aliskiren 300 mg, losartan 100 mg, or their combination, daily for 9 months. Patients were treated to standard blood pressure targets with add-on therapy, excluding other inhibitors of the RAAS and β-blockers.
The primary objective was to compare change in LV mass (assessed with magnetic resonance imaging) index from baseline to follow up in the combination and losartan arms; the secondary objective was to determine whether aliskiren was noninferior to losartan in reducing LV mass index from baseline to follow up.
Systolic and diastolic blood pressures were reduced similarly in all treatment groups, while LV mass index was reduced
significantly from baseline in all treatment groups. The reduction in LV mass index in the combination group was not
significantly different from that with losartan alone. Aliskiren was as effective as losartan in reducing LV mass index. Safety and tolerability were similar across all treatment groups.
In the Aliskiren Observation of Heart Failure Treatment (ALOFT) study patients with New York Heart Association class II to IV heart failure, current or past history of hypertension, and plasma brain natriuretic peptide (BNP) concentration >100 pg/ml who had been treated with an ACEI (or ARB) and β-blocker were randomized to 3 months of treatment with placebo (n = 146) or aliskiren 150 mg/day (n = 156) [Krum and Maggioni, 2010].
The primary efficacy outcome was the between-treatment difference in N-terminal pro-BNP (NT-proBNP). Plasma NT-proBNP rose by 762 ± 6123 pg/ml with placebo and fell by 244 ±
2025 pg/ml with aliskiren (p = 0.0106). BNP and urinary (but not plasma) aldosterone were also reduced by aliskiren.
Clinically important differences in blood pressure and biochemistry were not seen between aliskiren and placebo. The investigators concluded that addition of aliskiren to an ACEI (or ARB) and β-blocker had favourable neurohumoral effects in heart failure and appeared to be well tolerated.
The Aliskiren and Valsartan to Reduce pro-BNP via Renin–Angiotensin–aldosterone Blockade (AVANT-GARDE) trial is amultinational, double-blind trial, in which 1101 patients stabilized after acute coronary syndrome (ACS) without clinical evidence of heart failure or LV function ≤40% but with an increased level of natriuretic peptides (NT) have been randomized 3–
10 days after admission to aliskiren, valsartan, their combination, and placebo [Scirica et al. 2010].
The primary endpoint was the change in NT-proBNP from baseline to week 8. NT-proBNP declined significantly in each treatment arm, including
placebo, by week 8, although there were no differences in the reduction between treatment strategies (42% in placebo, 44% in aliskiren, 39% in valsartan and 36% in the combination arm). Several subgroups had higher baseline levels of NP and greater reductions over the study period; however, there were no differences among treatment groups in any subgroup.
It should be noted that there were more adverse events, including serious events and adverse events leading to early study drug discontinuation, in patients treated with active therapy. In conclusion, in this study performed in a high-risk population with
elevated levels of NPs but relatively preserved systolic function and no evidence of heart failure following ACS, there was no evidence for a benefit of early initiation of inhibition of RAAS with valsartan, aliskiren or their combination.
The results of this study are in contradiction with those of the ALOFT trial: an explanation could be the differences in
neurohumoral baseline features of the randomized patients of the two studies.
In the Aliskiren in the Evaluation of Proteinuria in Diabetes (AVOID) study, 599 hypertensive patients with type 2 diabetes and nephropathy received 6 months of aliskiren (150 mg daily titrated to 300 mg daily after 3 months) or placebo added to 100 mg losartan and optimal antihypertensive therapy [Parving et al. 2008]. By the end of the study period, treatment with aliskiren had reduced the mean urinary albumin-to-creatinine ratio by 20%, as compared with placebo (95% confidence interval [CI] 9–30; p < 0.001)
After adjustment for the change from baseline in systolic blood pressure, the reduction was 18% (95% CI 7–28; p = 0.002). A reduction of 50% or more in albuminuria was seen in 24.7% of the patients who received aliskiren, as compared with 12.5% of the patients who received placebo (p < 0.001). There was no difference in the overall incidence of adverse events between the aliskiren group and the placebo group (66.8% and 67.1%, respectively).
Hyperkalemia was reported in 5.0% of
the patients in the aliskiren group and in 5.7% of the patients in the placebo group. The hyperkalemia was transient. The authors concluded that aliskiren appears to have a renoprotective effect that is independent of its blood-pressure-lowering effect in patients with type 2 diabetes who are receiving the maximal recommended renoprotective treatment and optimal
antihypertensive therapy.
A post hoc analysis of the AVOID study assessed the efficacy and safety of aliskiren added to the maximal recommended dose of losartan according to baseline estimated glomerular filtration rate (eGFR; stage 1–3 chronic kidney disease [CKD]).
Exclusion criteria included eGFR <30 ml/min per 1.73 m2 and serum potassium >5.1 mmol/l [Persson et al. 2010].
Baseline characteristics were similar between treatment groups in all CKD stages. The antiproteinuric effects of aliskiren were consistent across CKD stages (19%, 22% and 18% reduction). In the stage 3 CKD group, baseline serum creatinine levels were equal, but renal dysfunction, prespecified as a postrandomization serum creatinine elevation >176.8 μmol/l (2.0 mg/dl)
occurred more frequently in the placebo group (29.2% versus 13.6%, p = 0.032).
Serum potassium elevations >5.5 mmol/l
(based on a single measurement) were more frequent with aliskiren (22.5% versus 13.6%) in stage 3 CKD. Adverse event rates were similar between treatments, irrespective of CKD stage.The authors concluded that aliskiren added to losartan reduced albuminuria and renal dysfunction and was well tolerated, except for hyperkalemia (stage 3), independent of baseline CKD stage in patients with type 2 diabetes, hypertension and nephropathy.
It is important also to mention that data deriving from experimental studies on animal models suggest that aliskiren can induce protection against atherosclerosis, attenuating insulin resistance and oxidative stress [Habibi et al. 2008; Imanishi et al. 2008]
Safety and Tolerability all studies Aliskiren was generally well tolerated at doses of 150 or 300 mg/day [Dugganet al. 2010] and resulted in an incidence of adverse events similar to placebo. Adverse events, including uncontrolled hypertension (2.2%), have generally been mild and have infrequently led to discontinuation of therapy [Vaidyanathan et al. 2007b]. The most common adverse events reported are headache (5.8%), nasopharyngitis (2.6%) and diarrhoea (1.4%) [Vaidyanathan et al. 2007c; Weir et al.
2007]. Aliskiren was also associated with a few cases of cough (1.1%), although, compared with ACEIs, the rate of cough
was approximately one-third to one-half that reported with ramipril or lisinopril.
In contrast, on 20 December 2011, Novartis has announced that an increase in adverse events and no apparent benefits among patients randomized to aliskiren (Rasilez®/Tekturna®, Novartis) in the ALTITUDE (Aliskiren Trial in Type 2 Diabetes
Using Cardio-Renal Endpoints) trial has prompted the data safety and monitoring board (DSMB) for the study to recommend its termination. ALTITUDE was studying aliskiren on top of ACEI or ARB therapy in patients with type 2 diabetes and renal
impairment compared with a placebo add-on.
In making its recommendation, the DSMB noted that the active-treatment group experienced an increased incidence of nonfatal stroke, renal complications, hyperkalemia and hypotension over 18–24 months of follow up. The committee concluded
that patients were unlikely to benefit from aliskiren on top of standard antihypertensive therapy. At the time in which this review has been written, Novartis is in ongoing discussions with health authorities worldwide about the implications of the findings from ALTITUDE for patients. As a precautionary measure Novartis is no longer recommending the use by physicians of aliskiren in combination with an ACEI or an ARB.
Conclusion
The direct inhibition of renin is a logical target for pharmacologic suppression of the RAAS, aliskiren is a new inhibitor of RAAS activity and actually is the only DRI suitable for oral administration. The studies performed show that as monotherapy or in combination with other drugs such as ACEIs, ARBs, diuretic and calcium channel blockers aliskiren significantly reduces blood pressure in patients with hypertension and recent observations suggest that it may have a renoprotective and cardioprotective effects.Aliskiren has a good tolerability profile, but the termination of the ALTITUDE trial for the increase of adverse events will probably limit its use in association with ARBs and ACEIs until new data will be collected.