Use of drug eluting stents in STsegment elevation myocardialinfarction
Heart 2010د. حسين محمد جمعه
اختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2011
Primary percutaneous coronary intervention (PCI)
is considered the optimal approach to themanagement of (STEMI) when the procedure is
performed expeditiously by an experienced team.
Drug eluting stents (DES) have been shown to
reduce the risks of both restenosis and target vessel
revascularisation (TVR) after elective PCI, as
compared with bare metal stents (BMS) in a broad
range of patients and lesions.
Reports from randomised trials and registries have suggested that DES may be associated with increased rates of late stent thrombosis.
Angioplasty with DES in this setting could therefore theoretically increase the rate of stent thrombosis, but data on this issue are conflicting.
To address these issues, several dedicated randomised controlled trials and registries have assessed the efficacy and safety of DES in the setting of primary PCI for AMI. Most of these studies were performed with sirolimus eluting stents (SES),Cypher (Cordis, Johnson and Johnson), and to a lesser extent with paclitaxel eluting stents (PES), (Taxus, Boston Scientific) and have yielded positive short and long term results in favour of SES and PES.
Yet, despite these positive findings, the use of
DES during PCI for AMI remains controversial andis still considered ‘off label’ in many countries.
The currently available data will be reviewed and put in perspective with clinical practice.
To date, 31 studies, 13 randomised trials, and 18
registries have directly compared SES or PES toBMS in the setting of AMI.
STRATEGY was the first trial to assess DES in
AMI. Patients with STEMI were randomly assigned
before obtaining the initial angiogram to single high
dose bolus tirofiban infusion followed by SES
implantation or abciximab and BMS implantation.
A total of 175 patients with STEMI were included.Three patients in the tirofiban SES group and five in the abciximabeBMS group did not undergo PCI.
Overall, 74 (85%) patients in the tirofiban SES and 77 (88%) in the abciximab BMS arm received the protocol mandated treatment combination. The primary end pointd composite of death, non-fatal myocardial infarction, stroke, or binary restenosis at 8 months was significantly lower in the tirofiban
plus SES group (19% vs 50%; hazard ratio (HR) 0.33,
95% confidence interval (CI) 0.18 to 0.60; p<0.001).
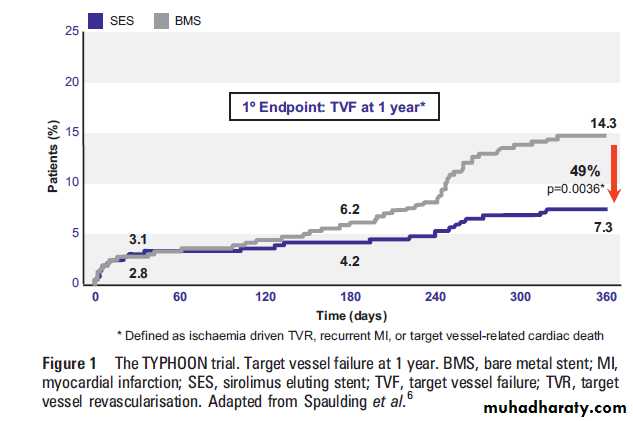
The TYPHOON trial was the first large randomised
controlled trial evaluating SES in 712 STEMI
patients. The primary end point of the study,
target vessel failure at 1 year (a composite of TVR,
recurrent infarction, or target vessel related cardiac
death), occurred in 7.3% of patients in the SES
group, and in 14.3% in the BMS group (p¼0.004)
(figure 1). This difference was driven by significant
differences in rates of TVR.
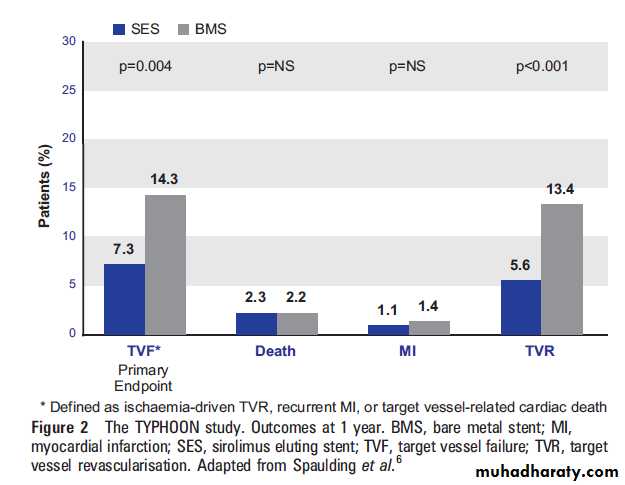
There was no difference between SES and BMS groups in death (2.2% in both, p¼1.0), re-infarction (1.1 vs 1.4%, p¼1.0) or stent thrombosis rates (3.4 vs 3.6%, p¼1.0) (figure 2). In the 210 patients of an angiographic substudy,SES were associated with significant reductions in in-stent late loss (0.1460.49 vs 0.8360.52 mm).
Target vessel failure was also reduced in patients
who did not undergo a systematic angiographic
control (6.8% vs 12.7%; p¼0.034), therefore indicating that the benefit of SES was not due to
revascularisation driven by the angiographic control.
The TYPHOON trial was performed in selected
patients. In contrast, the MULTISTRATEGY trialinclusion criteria were broad and close to daily
practice. Seven hundred and forty-four patients
were randomised to receive SES or BMS with
abciximab or tirofiban. High risk patients, such as
patients with cardiac failure, were included. Furthermore,no angiographic control was performed,therefore eliminating a bias induced by inappropriate TVR during the control angiograms.
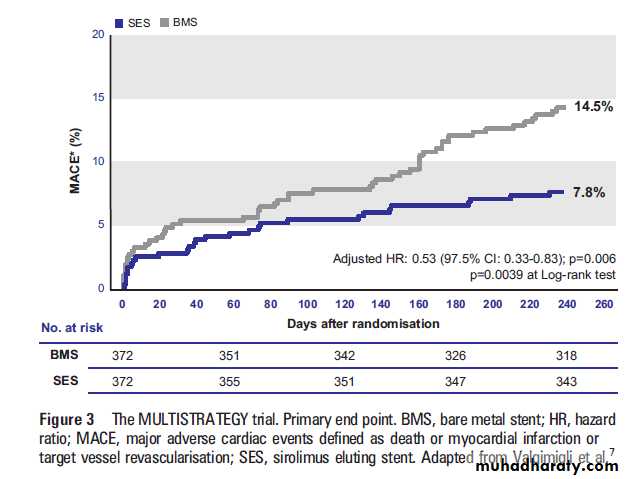
At 8 months a significant difference was noted in the
occurrence of the major cardiac events in favour of
the SES: 7.8% vs 14.5 % (adjusted HR 0.53, 95% CI
0.33 to 0.83; p¼0.006) (figure 3).
The SESAMI trial included 320 patients with
STEMI who were assigned to receive SES or BMS.
The primary end point, binary restenosis at 1 year,
was lower in the SES group than in the BMS group
(9.3% vs 21.3%, respectively; p¼0.032), as were the randomisation to bivalirudin or unfractionated
heparin and abciximab.
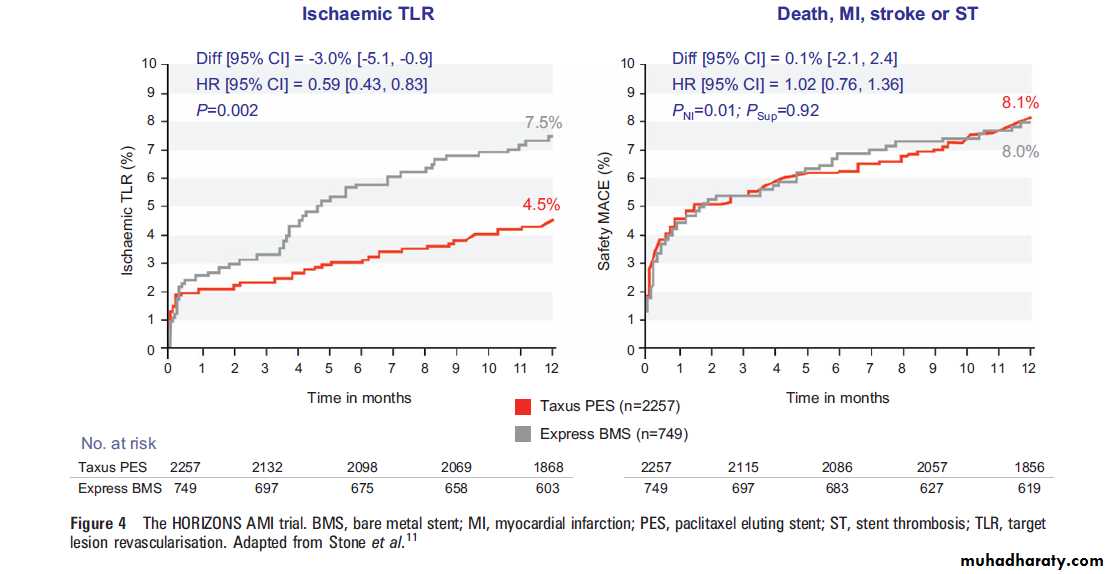
The composite end point included ischaemia driven target lesion revascularisation,all case mortality, reinfarction and stent thrombosis (definite or probable according to the ARC criteria). At 1 year, ischaemic target lesion revascularisation was reduced with PES (7.5% vs 4.5%; p¼0.02) and ischaemic events were similar between the two groups (figure 4).
Several registries have analysed the outcome of
patients receiving DES for AMI. The MASS-DACregistry12 included 4016 patients with STEMI and
non-STEMI (NSTEMI). The comparison of
matched patients treated with DES (72% SES) or
BMS shows a mortality reduction at 2 years
(3.1%, 95% CI 5.4 to 0.8%; p¼0.008) in
STEMI patients treated with DES. In contrast, the
GRACE registry reported an increased mortality in
patients treated with DES, between 6 months and
2 years after STEMI.
However, the overall mortality was significantly reduced among patients treated with DES over the 2 years (3.9% vs 5.3%;p¼0.04), and during hospital stay (2.0% vs 3.8%; p¼0.018). In addition, striking differences were noted in coronary risk factors between the two populations, therefore impacting long term outcomes,atherosclerosis progression, and acute ischaemic events. Finally, follow-up was suboptimal,
with data missing in over 30% of patients at 2 years.
A recent systematic review by Brar et al14
included 7352 patients from 13 randomised trialsand 26 521 patients from registry studies using
SES or PES. In randomised trials, DES significantly
reduced target vessel revascularisation (relative
risk (RR) 0.44, 95% CI 0.35 to 0.55), without
increasing death (RR 0.89, 95% CI 0.70 to 1.14),
MI (RR 0.82, 95% CI 0.64 to 1.05), or stent
thrombosis (RR 0.97, 95% CI 0.73 to 1.28).
These observations endured over 2 years. Among 18 registries (n¼ 26 521), DES significantly reduced target vessel revascularisation (RR 0.54, 95% CI 0.40 to 0.74) without an increase in myocardial infarction (RR 0.87, 95% CI 0.62 to 1.23). Death was significantly lower in the DES group within 1 year of the index PCI, but there were no differences
within 2 years (p¼ 0.45).
Thus, the use of DES appears safe and efficacious
in randomised trials and registries of patients withSTEMI. DES significantly reduce TVR compared
with BMS, without an increase in death, myocardial
infarction, or stent thrombosis within 2 years
of the index procedure. This clearly favours the
routine use of DES in AMI. However, several
questions remain on the rate of stent thrombosis,
long term safety, and the selection of compliant
patients to prolonged dual antiplatelet treatment in
an emergency setting.
STENT THROMBOSIS RATES IN AMI AND LONG
TERM SAFETY OF DES IN AMI
Thrombosis of DES has become an important topic
for interventional cardiologists and clinicians, despite
several analyses showing that the increase of stent
thrombosis with DES is modest .
Recently, the TRITON TIMI 38 study compared
prasugrel to clopidogrel in patients with acute
coronary syndromes.17 The choice of DES or BMS
was left to the discretion of the investigator. Of
interest, stent thrombosis rates increased according
to the severity of clinical presentation, with the
lowest rates in patients with unstable angina and
the highest in those with STEMI.
No difference was found in stent thrombus rates between DES and BMS in all subgroups of patients at 15 months.
Stent thrombosis after PCI for AMI is therefore high,
but does not seem to increase with the use of DES.
Pharmacological prevention of stent thrombosis in
this setting is of paramount importance. Of interest,
in the subgroup of STEMI patients included in the
TRITON TIMI 38 study, the rate of stent thrombosis
was halved in the prasugrel group (2.4% in the
clopidogrel group vs 1.2% in the prasugrel group; HR
0.49, 95% CI 0.28 to 0.84).
Although most trials on DES in AMI were performed recently, long term data are emerging.
In May 2009, results of the 5 year follow-up of
STRATEGY and 4 year follow-up of TYPHOON
were presented. Both studies yielded similar
results with a sustained effect of SES on the
reduction of TVR and no difference in safety end
points such as death or myocardial infarction. Of
interest, in the TYPHOON study, the majority of
stent thrombosis occurred early in the first month,
highlighting the importance of pharmacological
prevention.
PATIENT COMPLIANCE WITH DUAL ANTIPLATELET
THERAPY AFTER PRIMARY PCI FOR AMINon-compliance with dual antiplatelet therapy
during the first 6 months after implantation has been demonstrated as a predictive factor for DES
thrombosis. In the PREMIER registry, patients
treated with primary PCI for AMI who discontinued
clopidogrel after 30 days had a higher mortality
compared to patients on clopidogrel.
In the setting of an AMI, it is often difficult to assess a patient’s potential for compliance to medication and to enquire on a contraindication to prolonged dual antiplatelet therapy such as planned surgery. This could be a limitation to the implantation of a DES during primary PCI for AMI.
However, in most European countries, patients with AMI are triaged by pre-hospital or hospital emergency physicians who assess the patient’s past history and understanding of medication compliance. Furthermore,
several randomised studies and a recently published registry study clearly show that dual antiplatelet therapy with clopidogrel for at least a year reduces
major events in all AMI patients, including those
who receive a BMS. Therefore, long termcompliance
with dual antiplatelet therapy after AMI should be achieved by careful education in all AMI patients,
with or without BMS or DES implantation.
USE OF DES IN AMI IN CLINICAL PRACTICE
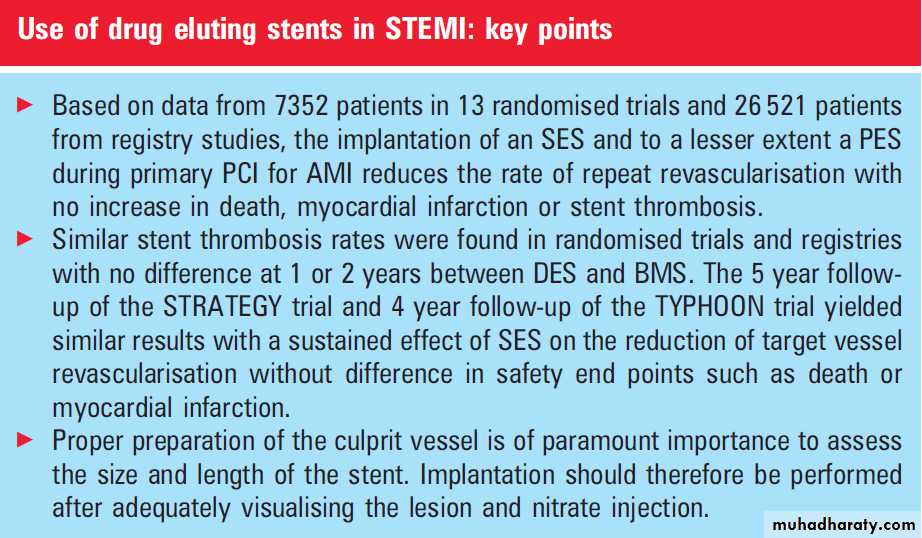
Based on data from 7352 patients in 13 randomisedtrials and 26 521 patients from registry studies, the
implantation of an SES and to a lesser extent PES
during primary PCI for AMI reduces the rate of
repeat revascularisation with no increase in death,
myocardial infarction or stent thrombosis. Cost
effectiveness studies in stable patients have shown
that the implantation of a DES is mostly beneficial
in patients with a high risk of restenosis such as
small vessels or long lesions.
In primary PCI for AMI, it seems reasonable to implant a DES in patients with high risk features for restenosis such as long lesions, small vessels, or diabetes. Proper preparation of the culprit vessel is of paramount importance to assess the size and length of the stent. Implantation should therefore be performed after visualising the lesion and nitrate injection.
DES implantation should be avoided in patients with permanent or temporary contraindications to dual antiplatelet therapy. Patient education on risk factor management and treatment compliance should start in the catheterisation laboratory, be continued during the
hospital stay, and pursued during follow-up.
CONCLUSION
Over the past 30 years, dramatic decreases in AMImortality have been achieved by increasing the
number of reperfused patients, reducing pre-hospital
and hospital delays, and obtaining adequate
coronary artery flow by primary angioplasty. DES
reduce the rate of repeat revascularisation and
therefore are an interesting asset to primary PCI
in selected patients.
علمني المطركيف اغسل همومي واحزانيوكيف اجدد حياتي كما تغسل قطرات المطراوراق الشجروتعيد لها الحياة