د. حسين محمد جمعه
اختصاصي الامراض الباطنةالبورد العربي
كلية طب الموصل
2011
Diabetes: a driver for heart failure
• Heart 2011©BMJ Learning 2011
Diabetes and heart failure (HF) often co-exist, and since the number of people with either diabetes or HF is increasing worldwide, the number of diabetic HF patients is expected to grow exponentially. Patients with diabetes and HF have a poorer clinical outcome compared to diabetic patients without HF. Also, HF patients with diabetes have different characteristics compared to HF patients without diabetes. This observation suggests that diabetes might contribute to the onset and progression of HF. Several other findings support the concept that diabetes is a driver for HF.
Diabetes might increase the risk of developing HF through the increased risk of atherosclerotic disease. In addition, diabetes directly influences function and structure of the heart—for example, through altered insulin signalling and mitochondrial dysfunction, increased (deposition of) advanced glycation end products (AGEs), oxidative stress, and lipid accumulation.
Several treatment guidelines exist for diabetes and HF, but the specific treatment suggestions for diabetics with HF are sparse.
Moreover, some treatment options for diabetes are not recommended for those with concomitant HF. This article provides an overview of the current knowledge and understanding regarding the epidemiology, pathophysiology, and treatment of patients with HF and diabetes.
Epidemiology, clinical characteristics, and outcome of HF patients with diabetes
In the general population the prevalence of patients with both HF and diabetes is estimated at 0.5% in men and 0.4% in women. In patients with diabetes the prevalence of HF is between 9–22%, which is much higher compared to the general population.
In a hospital discharge database of the Kaiser Permanente Northwest Division the prevalence of HF was 11.8% in patients with diabetes compared to 4.5% in patients without diabetes (n=1566). The prevalence is highest in women aged 70 years and older. The prevalence of asymptomatic diastolic dysfunction in patients with diabetes is probably higher, and estimated to be between 52–60%. Not all patients with diastolic dysfunction have HF, but the prevalence of HF with preserved ejection fraction in patients with diabetes is probably more common but less well established (approximately 25%).
Prevalence of diabetes in patients with HF
In registries of patients with HF the prevalence of diabetes varies between 13–47%. In the Studies of Left Ventricular Dysfunction (SOLVD) registry with 6273 patients with systolic HF, defined as a left ventricular ejection fraction (LVEF) ≤45%, the prevalence of diabetes was 23%. The prevalence was higher (44%) in US patients hospitalised for acute HF in the Acute Decompensated Heart Failure National Registry (ADHERE). Interestingly, over a 10 year period, the percentage of patients with diabetes that were included in HF trials increased.In patients with HF and preserved ejection fraction, the prevalence of diabetes might be even higher. In four registries with HF patients and preserved ejection fraction, the prevalence of diabetes was 25% (n=10 072), 26% (n=368), 31% (n=1024), and 33% (n=312), respectively.w8–11 In clinical trials of HF such as the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM)-preserved, the prevalence of diabetes was 28% at baseline (n=3023).
Diabetes and the risk of development of HF
Patients with diabetes are at a higher risk of developing HF. In the Framingham Heart Study (n=5209, aged 45–74 years) the adjusted relative risk for developing HF in patients with diabetes was 1.82 for men and 3.75 for women. In the general population studied in the Cardiovascular Health Study (n=5888, aged 65–100 years), 33% of diabetic men and 45% of diabetic women developed HF during the 5.5 years of follow-up.Diabetes was a predictor for the development of HF independent of factors such as age, gender, coronary artery disease, and hypertension. Additionally, poor glucose control is associated with an increased risk of the development of HF. In a cohort study (n=48 858) with a follow-up of 2.2 years,
each 1% increase in glycosylated haemoglobin (HbA1c) was associated with an 8% increased risk of HF.
Outcome in patients with HF and diabetes compared with patients with HF without diabetes
Several studies have shown that HF patients with diabetes have a poorer outcome than HF patients without diabetes. In a cohort of patients with HF using the Framingham criteria, 5 year survival was 37% for patients with diabetes (n=128, mean age 75 years) compared to 46% for patients without diabetes (n=527, mean age 77 years).In the CHARM trial (n=7599), the risk of cardiovascular death and HF hospitalisation was higher in patients with diabetes with preserved ejection fraction compared to those with reduced ejection fraction (hazard ratio (HR) 2.0 vs 1.60, p<0.01). In hospitalised patients with and without preserved ejection fraction, acute hyperglycaemia and postprandial hyperglycaemia are associated with cardiac events and mortality.
How can diabetes cause HF?
Coronary artery disease is more often present and manifests earlier in life in patients with diabetes compared to patients without diabetes. Besides its higher prevalence, diabetics have more severe coronary artery disease, as illustrated by the higher incidence of three vessel disease. In a recent study in 839 patients with coronary artery disease it was shown that diabetes was a risk factor for HF independent of baseline functional assessment of ischaemia and myocardial infarction.
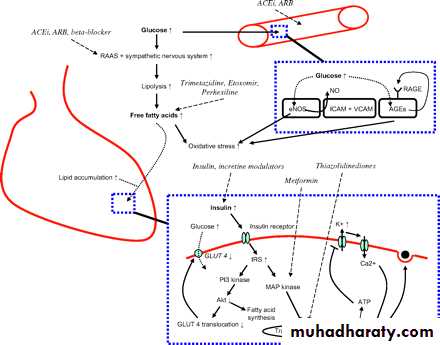
Therefore, the development of HF in patients with diabetes is only partly explained by the increased atherosclerotic risk and its cardiac and vascular complications. Diabetes also directly influences the structure and function of the heart—for example, through altered insulin signalling and mitochondrial dysfunction, increased AGEs, oxidative stress, and lipid accumulation.
In patients with diabetes, insulin mediated glucose uptake by the myocardium is decreased.
This so-called insulin resistance results from changes in insulin pathway signalling. Recent studies found that insulin stimulated Akt activity is diminished and the glucose transporter 4 is downregulated
(figure 1). Another effect of diminished glucose uptake, both of the heart and skeletal muscles, is hyperglycaemia.
Hyperglycaemia is associated with activation of the sympathetic nervous system and the renin–angiotensin–aldosterone system.
Activation of the sympathetic nervous system increases lipolysis resulting in raised plasma free fatty acid concentrations.
The myocardium uses the free fatty acids over glucose for energy production. To produce energy for its function, the heart needs more oxygen when using free fatty acids than when using glucose. Both higher oxygen need and higher concentrations of free fatty acids are associated with decreased contractility and diastolic dysfunction. Moreover, elevated concentrations of free fatty acids induce apoptosis of myocardial cells through oxidative stress, and are associated with accumulation of lipids in the myocardium.
Figure 1Schematic representation of the mode of action of drugs that interfere with diabetes mediated cardiac dysfunction. ACEi, angiotensin converting enzyme inhibitor; AGEs, advanced glycation end products; ARB; angiotensin receptor blocker; ICAM, intercellular adhesion molecule; RAAS, renin–angiotensin–aldosterone system; RAGE, receptor for advanced glycation end products; VCAM, vascular–cellular adhesion molecule.
Endothelial dysfunction and coronary microcirculation
Endothelial dysfunction appears to be closely associated with the development of microangiopathy in patients with diabetes. In a study of 94 patients with diabetes mellitus, the endothelial dysfunction marker factor VIII (Von Willebrand factor, vWF) was elevated and related to impaired outcome. Adhesion molecules (ICAMs and VCAMs) are elevated and vasoreactivity is altered in patients with diabetes compared to patients without diabetes.In diabetes higher tumour necrosis factor α (TNFα) values are associated vessel inflammation and oxidative stress, and thereby endothelial dysfunction. Endothelial dysfunction leads to thickened endothelial cytoplasm, with endothelial cell hypertrophy and cytoplasmic bridging resulting in complete obstruction of the capillary lumen.
The mismatch between capillary density and myocardium may lead to ischaemia and myocardial dysfunction.
Impaired calcium handling
Myofilament function is depressed in skinned fibres of diabetes patients as a result of decreased calcium sensitivity. This is suggested to result from an impaired sarcoplasmic reticulum function with reduced sarco(endo)plasmic reticulum calcium-ATPase (SERCA2q) pump activity, leading to disturbed intracellular calcium handling and impaired cardiac functionFibrosis and accumulation of advanced glycation end products
AGEs may be an important contributor to the development of HF among patients with diabetes. In 64 patients, it was found that those with HF and diabetes had increased myocardial AGE deposition and increased myocardial collagen fraction as indicated by biopsy. AGEs affect the physiological properties of proteins in tissues by creating crosslinks, and cause multiple vascular and tissue changes via the interaction with AGE receptors.Very recently, it was observed that the soluble form of the AGE receptor was directly associated with the extent of coronary disease, the severity of HF, and B-type natriuretic peptide (BNP) values in 103 HF patients. In a model of rats with diabetes compared to rats without diabetes, an increased cardiomyocyte diameter, myocardial AGE deposition, decreased myocardial contraction, delayed relaxation, and a decrease of collagen type I/type III absent myocardial distensibility was observed. In experiments AGEs also interacted with SERCA2q and may depress cardiac function through this pathway.
Increased neurohormones
Natriuretic peptides are good prognostic markers in HF patients with diabetes. HF patients with diabetes have higher N-terminal proBNP (NT-proBNP) values than patients without diabetes, while other neurohormones are found to be similar.Natriuretic peptides are related to diastolic dysfunction in patients with diabetes.
Which patients with diabetes develop HF?
A number of characteristics of patients with diabetes predict the development of HF. Established risk characteristics of patients are elevated body mass index or visceral fat and renal dysfunction. A higher HbA1c, the use of insulin, and a longer duration of diabetes are also strong predictors for the development of HF, illustrating the relation between glucose metabolism and the development of HF.Patients with diabetes had a stronger relative increase of left ventricular mass, a more pronounced increase of left ventricular end diastolic diameter, an increase in left atrial diameter, a decrease in ejection fraction, and a greater impairment of diastolic function during a follow-up period of 10 years. A recent study shows that patients with diabetes with echocardiographic signs of diastolic dysfunction (E/e' ratio >15) have a significant higher risk for HF compared to patients with diabetes without diastolic dysfunction (37% vs 17%).
Treatment of patients with HF and diabetes
Current recommendationsPrevention of HF
Although evidence has supported the hypothesis that diabetes contributes to the development of HF, a direct beneficial effect of glucose lowering in reducing the risk of HF has not been convincingly demonstrated. In the United Kingdom Prospective Diabetes Study (UKPDS) (n=4585) it was observed that for each decrease of 0.9% HbA1c the risk of an HF related event decreased with a non-significant 9%.
Treatment of coronary artery disease in diabetes
A recent meta-analysis of 10 randomised controlled trials with a total of 7812 patients demonstrated that the 5 year mortality in 1233 patients with diabetes undergoing coronary artery bypass grafting (CABG) was significantly lower compared to percutaneous coronary intervention (PCI) (12.3% vs 20.0%, respectively). However, both the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) and the Synergy between PCI with Taxus and Cardiac Surgery (SYNTAX) trials contradict these findings.
The BARI-2D demonstrated in 2368 type 2 diabetes patients that the absolute rate of major adverse cardiac events was comparable between patients treated with CABG (22.4%) or PCI (23%).
The SYNTAX trial, comparing drug eluting stents with CABG for multivessel disease in 1800 patients, observed equal rates of myocardial infarction and mortality with both treatment regimens. However, major adverse cardiac and cerebrovascular events were significantly more frequent in the PCI group, mainly due to higher rates of additional revascularisation. Data on the effect of coronary interventions on (prevention of) the onset of HF are lacking.
Treatment of patients with HF and diabetes
No randomised clinical trials have been performed to study specifically the effects of pharmacological intervention in patients with HF and diabetes.In current recommendations, only data from smaller studies and subgroup analyses are used.
Most guidelines indicate that angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) have equal benefit in patients with HF with and without diabetes to prevent morbidity and mortality.
The SOLVD intervention trial (n=2569) using enalapril and the Assessment of Treatment with Lisinopril and Survival (ATLAS) trial (n=3164) showed a similar effect in patients with and without diabetes.
The third Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico (GISSI 3) (n=18 131) and the Survival and Ventricular Enlargement (SAVE) trials (n=2231) both have shown a beneficial effect on morbidity and mortality of ACE inhibition in patients with diabetes after myocardial infarction. Similar results have been obtained with ARBs. In the Reduction of Endpoints in Non Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan (RENAAL) study, treatment with losartan resulted in a 32% risk reduction of first HF hospitalisation (n=1513) (p=0.005).
In the International Dietetics and Nutrition Terminology (IDNT), in patients with diabetes (n=1715) irbesartan reduced the incidence of HF compared to placebo (HR 0.72, p=0.048).w43 In the Losartan Intervention For Endpoint reduction in hypertension (LIFE) study (n=9193), losartan reduced the risk of first hospitalisation for HF when compared with atenolol (HR 0.59, p=0.019).w44 Therefore, ACE inhibitors and/or ARBs are indicated in all patients with diabetes and HF.
The current guidelines recommend the use of β-blockers in all patients with HF, including patients with diabetes. This is based on large randomised clinical trials, in which patients with diabetes were not excluded. In the majority of these trials, a subgroup analysis on patients with and without diabetes was not reported. However, both in the Metoprolol CR/XL Controlled Randomized Intervention Trial in Chronic HF (MERIT-HF) (n=3991) and the Study of Effects of Nebivolol Intervention on Outcomes and Rehospitalization in Seniors With Heart Failure (SENIORS) (n=2128) trials, the effect of β-blockers on their primary end points was not significant in patients with diabetes compared to patients without diabetes, with an HR of 1.04 (p=NS) versus an HR of 0.78 (p=0.006) in SENIORS.12
In addition, other smaller studies also indicate that patients with diabetes and HF may not accrue the same mortality benefit from β-blocker treatment as patients without diabetes.w45 The Carvedilol Or Metoprolol European Trial (COMET) (n=1511) suggested that the combined α- and β1-blocker carvedilol was more beneficial compared to metoprolol, a selective β1 receptor antagonist, in HF patients, with a similar reduction in mortality in those with diabetes.
It is assumed that stringent and meticulous metabolic control is beneficial in HF patients with diabetes. However, a meta-analysis showed that strict glucose control did not result in fewer congestive HF events. Moreover, in the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial (n=11 140), there was no effect of strict glucose control compared to lenient control on the onset of HF (3.9% vs 4.1%, p=NS). The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study (n=10 251) was stopped due to a higher event rate in the intensive glucose control group.
Combinations of medication were necessary to obtain glucose control, diminishing the potential beneficial effect of individual glucose lowering agents or specific combinations. New onset HF occurred more often in patients in the intensive treatment group compared to the lenient group (3.0% vs 2.4%, p=0.17), although the difference was small and not statistically significant. In the Diabetes mellitus And Diastolic Dysfunction (DADD) study (n=39), the effect of either insulin or oral hypoglycaemic agents did not result in a significant change in diastolic function despite improvement of HbA1c.
Current guidelines recommend HbA1c concentrations of <7.0%. How are these goals to be achieved in HF patients? Several hypoglycaemic agents can be used. In 2007, a systematic review of eight studies on different glucose lowering regimens in HF patients with diabetes showed that metformin was the only agent without a harmful effect. According to the HF guidelines metformin should be considered as a first line agent in overweight HF patients with type 2 diabetes without significant renal dysfunction.
Until recently metformin was contraindicated in HF, mainly because of concerns about lactic acidosis. In an attempt at a pilot study on metformin in HF patients, 27 out of 53 patients with contraindications already used metformin at inclusion, which resulted in the study being stopped. In a case–control study in patients with diabetes and HF (n=1633) metformin was associated with a reduction in all cause mortality (adjusted odds ratio (OR) 0.72, p=0.003).
In a Canadian study involving patients with a new diagnosis of HF, metformin monotherapy was associated with a reduced 1 year mortality when compared with sulfonylurea treatment.
One year mortality was also lower in patients taking metformin and sulfonylurea combination therapy compared with patients taking sulfonylurea monotherapy.
In a US study of patients admitted to hospital with HF, metformin use was associated with a lower 1 year mortality when compared to treatment with insulin or sulfonylurea (24.7% vs 36.0%, p<0.0001).w52 All cause readmission and HF hospitalisation were also less common in patients treated with metformin than in those not treated with an insulin sensitising drug. Therefore, although results from prospective randomised placebo controlled clinical trials are lacking, a large body of evidence suggests that metformin use is associated with a better clinical outcome in HF.
Sulfonylureas
A retrospective cohort study of over 16 000 patients with diabetes and HF found no relationship between sulfonylurea use and mortality.Compared with metformin, sulfonylureas do not seem to be beneficial.
Insulin
The main effect of insulin is to decrease blood glucose, but it may also increase myocardial blood flow, decrease heart rate, and cause a modest increase in cardiac output. Insulin treatment in patients with diabetes and HF is under debate. In a subgroup of the SAVE trial (n=496), those patients with diabetes and an ejection fraction <40% treated with insulin had a higher risk for cardiovascular mortality and HF hospitalisation.Similar findings were observed in CHARM, where the use of insulin was associated with mortality or hospital admission for HF.
Since these are observations from sub-analyses, further studies are needed to establish the specific role of insulin treatment beyond the role as a glucose lowering agent in patients with diabetes and HF.
Thiazolidinediones
Thiazolidinediones are peroxisome proliferator activated receptor γ (PPARγ) agonists, and increase insulin sensitivity. According to the guidelines, thiazolidinediones may be considered in patients with New York Heart Association (NYHA) functional class I–II HF with careful monitoring for fluid retention. Based on current evidence only pioglitazone, and not rosiglitazone, should be considered.
In a meta-analysis, rosiglitazone was the only oral hypoglycaemic agent associated with an increased risk of cardiovascular morbidity or mortality, albeit not statistically significant (OR 1.68). In the Prospective Pioglitazone Clinical Trial in Macrovascular Events (PROACTIVE) study, high risk patients with diabetes were randomised to pioglitazone (n=2536) or placebo (n=2633).
Patients treated with pioglitazone had a reduced risk of all cause mortality, non-fatal myocardial infarction, and stroke (HR 0.82, p=0.027), but the risk of hospitalisation for HF was increased. This might be explained by increased renal sodium reabsorption in pioglitazone treated patients. Therefore, pioglitazone should not be prescribed in patients with HF with NYHA functional class III–IV.
Incretine modulators
Drugs acting on the incretin system, such as glucagon-like peptide-1 (GLP-1) analogues and dipeptidyl peptidase-4 (DDP-4) inhibitors, act by insulin secretion through a glucose dependent mechanism. They have a positive effect on the β cell and are associated with weight loss. The GLP-1 analogues reduce HbA1c by approximately 1.5%.The effect on cardiovascular outcome and all cause mortality is under investigation. In a non-randomised study of 21 myocardial infarction patients, the infusion of GLP-1 during 72 h resulted in a significant increase in LVEF (29% to 39%, p<0.01). The infusion of GLP-1 during 5 weeks was also associated with an increase in LVEF (21% to 27%, p<0.01) in 12 HF patients compared to nine controls.
In a more recent randomised study in 20 patients with non-ischaemic HF and without diabetes, the infusion of GLP-1 during 48 h was not associated with an increase in LVEF compared to controls (28% to 30% vs 30% to 30%, P=NS). So, despite promising results in small studies, larger studies need to be performed before recommendations on the use of incretine modulators in diabetic HF patients can be made.
Potential agents
Aleglitazar is a combined PPARα/γ agonist that decreases HbA1c. However, oedema related to the use of PPARγ agonists might hamper its use in patients with HF. Other interesting novel glucose lowering compounds include trimetazidine, perhexiline, and etoxomir.Trimetazidine decreases free fatty acid oxidation without affecting the myocardial oxidative rate, which implies increased oxidation of glucose. In a study with 55 HF patients it was demonstrated that trimetazidine is associated with an increase in LVEF (36% to 43%, p=0.002). Similarly, in a recent placebo controlled study in 87 HF patients, it was observed that trimetazidine treatment during 3 months improved LVEF (33% to 42% vs 31% to 33%), especially in patients with diabetes.
Perhexiline is an antianginal drug that augments glucose metabolism by blocking muscle mitochondrial free fatty acid uptake, thereby increasing metabolic efficiency. In 56 patients optimally treated for non-ischaemic HF, perhexiline was related to an increase in LVEF (24% to 34%, p<0.001). Etoxomir is a carnitine palmitoyltransferase-1 inhibitor that shifts substrate oxidation in heart muscle from fatty acids to glucose, which might in turn improve contractile function. A randomised study with etoxomir in HF patients was stopped prematurely due to side effects (elevated liver enzymes).
Summary
The prevalence of patients with diabetes and HF is growing exponentially. Compared with patients with HF without diabetes, diabetic HF patients have a poorer prognosis and quality of life. Several lines of evidence suggest that diabetes is a driver for HF, although current knowledge is still limited. The difference in clinical characteristics, disease course, and outcome suggests that patients with HF and diabetes should be treated differently from those without diabetes.
All patients with HF should be treated with an ACE inhibitor and/or an ARB—diabetes augmenting the need for these treatments. Although the benefit of β-blockers might be less pronounced in HF patients with diabetes, all HF patients should still receive a β-blocker. Finally, metformin seems to be the preferred oral glucose lowering agent in patients with diabetes and HF. Randomised clinical trials of pharmacological treatments specifically in patients with HF and diabetes are lacking. Therefore, more knowledge about this growing group of patients with a poor outcome is strongly needed.
Diabetes and heart failure: key points
In the general population the prevalence of diabetes and heart failure is slightly higher in men than in women.Diabetes increases the risk for heart failure substantially.
Patients with both diabetes and heart failure have a worse prognosis, irrespective of left ventricular ejection fraction.
Heart failure in patients with diabetes is caused both by atherosclerosis and the effects of hyperglycaemia and insulin resistance on the myocardium.
For most patients with coronary artery disease and diabetes, coronary artery bypass surgery is the preferred treatment.
The evidence for treatment of patients with heart failure and diabetes with angiotensin converting enzyme inhibitors and angiotensin receptor blockers is strong.
The evidence for β-blockers is less established.
Metformin is the hypoglycaemic agent with the most favourable outcome compared to other agents.
Newer agents directed at influencing cardiac metabolism are sometimes promising, but evidence on clinical outcome is lacking.
The prevalence of diabetes among patients with heart failure with preserved ejection fraction in registries is approximately: 25–35%
The risk of developing heart failure in patients with diabetes is related to the percentage of HbA1c, ie, glucose regulation. With every 1% increase in HbA1c the risk of heart failure increases by: 8%
Neurohormones can differ in heart failure patients. For patients with diabetes compared to patients without diabetes: Only B-type natriuretic peptides are higher
Compared to patients without diabetes, diabetic patients with heart failure have: A relative increase of left ventricular mass, a more pronounced increase of left ventricular end diastolic diameter, an increase in left atrial diameter