د. حسين محمد جمعه
اختصاصي الامراض الباطنةالبورد العربي
كلية طب الموصل
2011
Diabetes mellitus type 2: an update on management
• Heart 2011©BMJ Learning 2011
How should I treat it?
Principles of managementPatients should receive ongoing structured care to evaluate microvascular and cardiovascular risk
All patients should be entered into practice disease registers to allow patient call and recall
All clinical information that relates to diabetes should be collected in electronic templates with locally or nationally agreed READ code datasets. This will help you achieve care standards and the quality targets in the general medical services contract.
Targets should be tailored to the individual patient, according to what it is possible to achieve. Overambitious targets can be counterproductive and risk severe hypoglycaemia
The impact of other cardiovascular risk factors should be taken into consideration when agreeing targets.
Table 1. Targets for controlling diabetes and cardiovascular risk factors in people with diabetes.
Risk factor
OptimalBorderline
PoorValues are mmol/l unless otherwise specified
Plasma glucose
Fasting4.4-6.1
6.2-7.8>7.8
Postprandial
4.4-8.0
8.1-10.0>10.0
Total cholesterol
<4.0
4.0-5.5
>6.5
High density lipoprotein cholesterol
>1.1
0.9-1.1<0.9
Fasting triglycerides
<1.7
1.7-2.2>2.2
Body mass index (kg/m2)
Men
20-25
26-27>27
Women
19-24
25-26
>26
Blood pressure (mm Hg)
<130/80*
130/80-140/80>140/80
Smoking
Non-smoker
Pipe/cigarettes*Stricter targets are needed in younger people and in people with early nephropathy who have a good life expectancy.
• Treatment targets
• Table 1 sets out recommended targets for controlling glucose, blood pressure, and lipids.Evidence for treatment targets
Blood glucoseThe ADVANCE trial (2008), assessed the benefits of improved glucose control on the development of microvascular and macrovascular complications in type 2 diabetes. Over a 5.5 year period intensive blood glucose control (HbA1c 6.5% v 7.3%) resulted in:
A 14% reduction in microvascular complications
A non-significant reduction in cardiovascular morbidity and mortality.
In contrast, for a population of patients with type 2 diabetes at high risk for CVD and an HbA1C of ≥7.5%, a therapeutic strategy that targets HbA1C <6% v 7.0-7.9% increased mortality over 3.5 years in the ACCORD study.5 This outcome is probably consequent to cardiovascular complications of severe hypoglycaemia and highlights the importance of individual treatment targets for patients with type 2 diabetes.
A 2011 Cochrane Review of 20 trials assessed the benefit of intensive glucose lowering treatment on all cause mortality, cardiovascular death, and microvascular events in patients with type 2 diabetes. This demonstrated:
No significant difference between intensive and conventional glycaemic control for all cause mortality (RR 1.01, 95% CI 0.90 to 1.13) or cardiovascular mortality (RR 1.06, 95% CI 0.90 to 1.26)
Targeting intensive glycaemic control (HbA1c 6.5% v 7.3%) reduced the risk of amputation (RR 0.64, 95% CI 0.43 to 0.95; P = 0.03), the risk of retinopathy (RR 0.79, 95% CI 0.68 to 0.92; P = 0.002; 10,986 participants, 8 trials) and nephropathy (RR 0.78, 95% CI 0.61 to 0.99; P = 0.04).
Three other comprehensive meta-analyses of intensive glucose lowering also conclude:
There is no benefit of intensive glucose lowering treatment on all cause mortality or death from cardiovascular causes in adults with type 2 diabetes. And compared with standard treatment, the risk of severe hypoglycaemia is more than twice as high in the intensive treatment group (RR 2.33, 95% CI 1.62 to 3.36).7Picture 1: Example of a glucometer
HypertensionHypertension is:
Defined by the British Hypertension Society as blood pressure ≥140/90 mm Hg
1.5-3.0 times more prevalent in patients with type 2 diabetes than in controls.
The UKPDS's hypertension in diabetes study compared "tight" blood pressure control (144/82 mm Hg) with "less tight" control (154/87 mm Hg).10 Tight blood pressure control reduced the incidence of myocardial infarction (14% in the tight control group v 21% in the less tight group) and stroke (5% in the tight control group v 8.7% in the less tight group).
The trial found that tight blood pressure control in patients with hypertension and type 2 diabetes achieved a clinically important reduction in the risk of deaths related to diabetes, complications related to diabetes, progression of diabetic retinopathy, and deterioration in visual acuity.
The Hypertension Optimal Treatment (HOT) trial compared an intensive treatment group, whose target diastolic was 80 mm Hg, with a conventional treatment group, whose target blood pressure was 90 mm Hg.
Intensive treatment reduced the incidence of all cardiovascular events (4.4% in the intensive treatment group v 9% in the conventional treatment group).
Lipids
The Heart Protection Study looked at the role of simvastatin 40 mg in the primary prevention of cardiovascular disease.12 It included 3985 patients aged 40-80 with type 1 or type 2 diabetes with no previous history of cardiovascular disease and total cholesterol >3.5 mmol/l.
In total, 9.1% of patients who received simvastatin experienced a coronary event, stroke, or revascularisation procedure compared with 13.5% of those who received placebo.
In June 2004, results from the Collaborative Atorvastatin Diabetes Study (CARDS) were presented at the annual meeting of the American Diabetes Association. This primary prevention study investigated the benefit of treatment with atorvastatin 10 mg in patients with:
Type 2 diabetes
A low-density lipoprotein cholesterol ≤4.14 mmol/l
One other cardiovascular risk factor (hypertension, smoking, retinopathy, microalbuminuria).
Atorvastatin reduced the incidence of major cardiovascular events by 37% compared with placebo (this is a relative risk reduction).
The Joint British Societies (JBS) Guideline recommends that all patients who have had type 2 diabetes for more than 10 years and all patients with type 2 diabetes who are older than 40 years, have a sufficient level of cardiovascular risk to benefit from statin therapy, unless they have specific contraindications to its use.
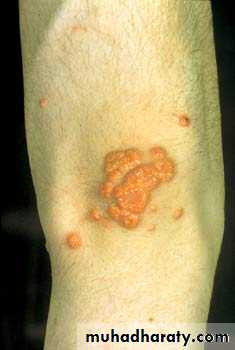
Picture 2: Xanthomata on the knee from severe hyperlipidaemia. This typically occurs in patients with familial hypercholesterolaemia, but it may occur in patients with diabetes
• Aspirin
• Current JBS guidelines recommended low dose aspirin (75 mg) is prescribed for patients who have had type 2 diabetes for more than 10 years and all patients with type 2 diabetes who are older than 50 years.• However, a meta-analysis of the primary prevention of cardiovascular events in people with diabetes in 2009 demonstrates any CV benefit is offset by an increased risk of cerebral and gastrointestinal bleeding.
The Medicines and Healthcare products Regulatory Authority (MHRA) Drug safety update (Volume 3, Issue 3, October 2009) recommends if aspirin is used in primary prevention, the balance of benefits and risks should be considered for each individual, particularly the presence of risk factors for vascular disease and the risk of gastrointestinal bleeding.
• Diabetes mellitus type 2: an update on management
• Non-drug measuresThe most important lifestyle advice for patients is to stop smoking and to stick to an appropriate diet.
Education
Tailor patients' educational needs to their beliefs and culture
Listen and respond to preconceived ideas and anxieties
Explain the aims of treatment and the relationship between blood glucose and diet and exercise
Explain the consequences of poor glucose control for the development of complications
Explain the importance of screening for complications
Explain the importance of a healthy lifestyle, especially physical activity and stopping smoking
Explain the role and importance of self monitoring (for example, monitoring glucose concentrations at home)
Explain the importance of regular foot care
Dietary advice
Eat regular meals planned around starchy food such as bread, potatoes, rice, and pastaReduce consumption of fried and fatty foods
Use skimmed or semi skimmed milk, rather than full fat milk
Eat at least five portions of fruit, vegetable, or pulses a day
Avoid eating chocolate and sweets
Reduce consumption of salt
Drink alcohol only in moderation
Other lifestyle advice
Other lifestyle advice includes:
Reduce weight to a realistic target - body mass index of 25 (80-90% of patients with type 2 diabetes are overweight).
Encourage 30 minutes of moderate physical exercise or activity on at least five days of the week.
Diabetes mellitus type 2: an update on management
Drug managementThe principles of managing type 2 diabetes are:
All patients should be encourage to modify lifestyle (good diet, lose weight, exercise, and stop smoking)
Metformin is first line drug therapy for most patients.
Recommendations from both the European Association for the Study of Diabetes and the American Diabetes Association state that metformin should be prescribed alongside lifestyle modification from the time of diagnosis of type 2 diabetes. It is no longer advised that patients are given a "trial of lifestyle change" without metformin therapy.
Add a sulphonylurea to metformin if glucose control is inadequate.
The following drugs can also be used under specific circumstances
ThiazolidinedionesDPP-4 inhibitors
GLP-1 analogues
Postprandial glucose regulators
Acarbose
Insulin.
Conventional drug treatment
Metformin
Metformin reduces output of glucose from the liver. It also enhances uptake and use of glucose by muscle cells.
Benefits
Metformin reduces the risk of microvascular and macrovascular complications.
Side effects
Metformin can cause anorexia, nausea, and diarrhoea. Rarely, it can cause lactic acidosis (the extremely rare cases of lactic acidosis with metformin are normally seen in association with renal impairment).
Metformin is contraindicated in patients with:
• Renal impairment (where the eGFR is <30 ml/min)• Liver failure
• Alcoholism
• Diabetic ketoacidosis
• Major surgery
• Sepsis.
For patients undergoing radiological intravascular contrast investigations metformin should be suspended prior to the test and restarted after 48 hours, provided renal function has returned to baseline.
Evidence
Metformin reduces the risk of microvascular and macrovascular complications. A substudy of the United Kingdom Prospective Diabetes Study looked at the use of metformin as intensive therapy in overweight people with type 2 diabetes. These patients had fewer hypoglycaemic episodes and less weight gain on metformin than on other drug therapies.16Patients likely to benefit
Patients should be offered metformin as first line treatment from the point of diagnosis of type 2 diabetes. Overweight patients especially are likely to benefit.Metformin can be used as monotherapy or in combination with sulphonylureas.
Dose
Patients should start with 500 mg metformin daily. The dose should be increased gradually to 1 g twice daily or the maximum tolerated dose over the course of 2-3 months.
Sulphonylureas
Sulphonylureas stimulate insulin release from the pancreas.
Benefits
Sulphonylureas control blood sugar and reduce the incidence of long term microvascular complications.
Side effects
Weight gain with sulphonylureas is variable and may be up to 4 kg. Hypoglycaemia can be a problem, especially in:
Older people
People with poor hepatic or renal function
People with erratic meal schedules.
Alcohol can also contribute to hypoglycaemia. Long acting sulphonylureas can cause delayed hypoglycaemia, which may be prolonged.
Evidence
Sulphonylureas cause a mean decrease in HbA1c of about 1-2% compared with placebo.Sulphonylurea treatment reduces the incidence of long term microvascular complications.
Patients likely to benefit
Sulphonylureas should be used with metformin when glucose control deteriorates. They should be considered as an option for first line treatment if metformin is not tolerated or is contraindicated, or if the patient is not overweight.Sulphonylureas can also be used with acarbose, thiazolidinediones, GLP-1 analogues, DPP-4 inhibitors, or insulin.Dose
All sulphonylureas should be started at the lowest dose and the dose should be titrated upwards according to response.
DA Approves BYDUREON™ -- The First and Only Once-Weekly Treatment for Type 2 Diabetes
Provides Glycemic Control in a Once-Weekly DoseSAN DIEGO and DUBLIN, Jan. 27, 2012 /PRNewswire/ -- Amylin Pharmaceuticals, Inc. (Nasdaq: AMLN) andAlkermes plc (Nasdaq: ALKS) today announced that the U.S. Food and Drug Administration (FDA) has approved BYDUREON™ (exenatide extended-release for injectable suspension) – the first once-weekly treatment for type 2 diabetes. BYDUREON is a glucagon-like peptide-1 (GLP-1) receptor agonist indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes in multiple clinical settings. BYDUREON will be available in pharmacies nationwide in February.
With BYDUREON, U.S. physicians and patients can now choose a therapy that offers continuous blood sugar control in just one dose per week," said John Buse, M.D., Ph.D., professor of medicine, director of the Diabetes Care Center and chief of the Division of Endocrinology at the University of North Carolina School of Medicine in Chapel Hill. "New treatment options are essential for the millions of adults with type 2 diabetes who continue to struggle to achieve optimal blood sugar control."
The approval of BYDUREON (pronounced by-DUR-ee-on) was based on safety and efficacy data from the DURATION clinical trial program, in which treatment with BYDUREON resulted in improvements in glycemic control with just one dose per week. The approval was also based on clinical experience with BYETTA®(exenatide) injection, a twice-daily form of exenatide that has been available in the U.S. since June 2005 and is used in nearly 80 countries worldwide. BYDUREON uses Alkermes' proprietary technology for long-acting medications to provide a controlled release of exenatide.
"As the first and only once-weekly diabetes treatment, BYDUREON represents an important milestone in Amylin's promise to bring to market innovative therapies to help improve the lives of people with type 2 diabetes," said Daniel M. Bradbury, president and chief executive officer, Amylin Pharmaceuticals. "BYDUREON builds upon the proven benefits of BYETTA, offering significant improvements in glycemic control in a single weekly dose."
In the DURATION-5 head-to-head clinical study, after 24 weeks of treatment, patients taking once-weekly BYDUREON experienced a statistically superior reduction in A1C of 1.6 percentage points from baseline, compared to a reduction of 0.9 percentage points for patients taking BYETTA. A1C is a measure of average blood sugar over three months. Both treatment groups achieved statistically significant weight loss by the end of the study, with an average loss of 5.1 pounds for patients taking BYDUREON and 3.0 pounds for patients taking BYETTA (weight loss was a secondary endpoint).
The most frequently reported adverse event in both groups was nausea, reported less frequently by BYDUREON users (14 percent) than by BYETTA users (35 percent). Other common treatment-emergent adverse events in the BYDUREON group included diarrhea, upper respiratory tract infection and injection site nodules. There were no major hypoglycemic events.
BYDUREON has been approved with a Risk Evaluation and Mitigation Strategy (REMS) to ensure that the benefits of BYDUREON outweigh the risk of acute pancreatitis and the potential risk of medullary thyroid carcinoma. As part of the REMS, Amylin has established a communication plan for healthcare professionals to help minimize these risks. In addition, Amylin will fulfill a number of post-marketing requirements to further assess the impact of BYDUREON on medullary thyroid cancer and cardiovascular disease.
BYDUREON is provided in a straightforward single-dose tray so that patients can self-administer the once-weekly subcutaneous injection. In the DURATION clinical studies, the delivery system was well accepted by patients and physicians.
About BYDUREON™ (exenatide extended-release for injectable suspension)BYDUREON, previously known as exenatide once weekly, is the first and only once-weekly medicine to be approved by the FDA for the treatment of type 2 diabetes. BYDUREON works with the body to help make its own insulin when needed, providing continuous glycemic control with just one dose per week. Each dose of BYDUREON is made up of biodegradable microspheres that provide a controlled release of exenatide throughout the week.
BYDUREON is an injectable prescription medicine that may improve blood sugar (glucose) in adults with type 2 diabetes mellitus, and should be used along with diet and exercise.
BYDUREON is not recommended as the first medication to treat diabetes.
BYDUREON is a long-acting form of the medication in BYETTA® (exenatide) injection so both drugs should not be used together. BYDUREON is not insulin and should not be taken instead of insulin. BYDUREON is not for people with type 1 diabetes or people with diabetic ketoacidosis. BYDUREON is not recommended for use in children.
It is not known if BYDUREON is safe and effective in people with a history of pancreatitis or severe kidney problems.
Important Safety Information for BYDUREON™ (exenatide extended-release for injectable suspension)
In animal studies,
BYDUREON caused rats to develop tumors of the thyroid gland. Some tumors were cancers. It is not known if BYDUREON causes thyroid tumors or a type of thyroid cancer called medullary thyroid cancer (MTC) in people. BYDUREON should not be used if there is a personal or family history of MTC or Multiple Endocrine Neoplasia syndrome type 2.
Based on post-marketing data, exenatide has been associated with acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis. Patients should be observed for signs and symptoms of pancreatitis after initiation of BYDUREON.
The risk of getting low blood sugar is higher if BYDUREON is taken with another medicine that can cause low blood sugar, such as a sulfonylurea. The dose of sulfonylurea may need to be lowered while BYDUREON is used.
BYDUREON should not be used in people who have or had severe kidney problems and may cause or worsen problems with kidney function, including kidney failure.
Patients should talk with their healthcare provider if they have severe problems with their stomach, such as delayed emptying of the stomach (gastroparesis) or problems with digesting food.
Antibodies may develop with use of BYDUREON, which may lead to worsening or failure to achieve adequate glycemic control. Severe allergic reactions can happen with BYDUREON. There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with BYDUREON or any other antidiabetic drug.
The most common side effects with BYDUREON include nausea, diarrhea, headache, vomiting, constipation, itching at injection site, a small bump (nodule) at the injection site, and indigestion.
Nausea most commonly happens when first starting BYDUREON, but may become less over time.
About BYETTA® (exenatide) injectionBYETTA was the first glucagon-like peptide-1 (GLP-1) receptor agonist to be approved by the FDA for the treatment of type 2 diabetes. BYETTA exhibits many of the same effects as the human incretin hormone GLP-1. GLP-1 improves blood sugar after food intake through multiple effects that work in concert on the stomach, liver, pancreas and brain.
BYETTA is an injectable prescription medicine that may improve blood sugar (glucose) control in adults with type 2 diabetes mellitus, when used with a diet and exercise program. It can also be used with metformin, a sulfonylurea, a thiazolidinedione or Lantus® (insulin glargine), which is a long-acting insulin.
BYETTA is not insulin and should not be taken instead of insulin. BYETTA should not be taken with short- and/or rapid-acting insulin. BYETTA is not for people with type 1 diabetes or people with diabetic ketoacidosis. BYETTA has not been studied in patients with a history of pancreatitis. Other antidiabetic therapies should be considered for these patients.
BYETTA provides sustained A1C control with potential weight loss (BYETTA is not a weight-loss product). BYETTA was approved in the U.S. in April 2005 and in Europe in November 2006 and has been used by more than 1.8 million patients since its introduction.
Important Safety Information for BYETTA® (exenatide) injection
Based on post-marketing data, BYETTA has been associated with acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis. Patients should be observed for signs and symptoms of pancreatitis after initiation or dose escalation of BYETTA.
the risk of getting low blood sugar is higher if BYETTA is taken with another medicine that can cause low blood sugar, such as a sulfonylurea or insulin. The dose of sulfonylurea or insulin may need to be lowered while BYETTA is used. BYETTA should not be used in people who have severe kidney problems and may cause or worsen problems with kidney function, including kidney failure. Patients should talk with their healthcare provider if they have severe problems with their stomach, such as delayed emptying of the stomach (gastroparesis) or problems with digesting food.
Antibodies may develop with use of BYETTA. Patients who develop high titers to exenatide could have worsening or failure to achieve adequate glycemic control. Severe allergic reactions can happen with BYETTA. There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with BYETTA or any other antidiabetic drug.
The most common side effects with BYETTA include nausea, vomiting, diarrhea, feeling jittery, dizziness, headache, acid stomach, constipation and weakness. Nausea most commonly happens when first starting BYETTA, but may become less over time.
These are not all the side effects from use of BYETTA. A healthcare provider should be consulted about any side effect that is bothersome or does not go away.
Diabetes affects nearly 26 million people in the U.S. and an estimated 347 million adults worldwide.[i],[ii]Approximately 90-95 percent of those affected have type 2 diabetes. In the U.S., diabetes costs more than$174 billion per year in direct and indirect medical expenses.[i
According to the Centers for Disease Control and Prevention's National Health and Nutrition Examination Survey, approximately 60 percent of people with diabetes do not achieve their target blood sugar levels with their current treatment regimen.[iv] In addition, 85 percent of type 2 diabetes patients are overweight and 55 percent are considered obese.[v] Data indicate that weight loss (even a modest amount) supports patients in their efforts to achieve and sustain glycemic control.[vi]
Bydureon is a longer-lasting version of Amylin’s existing drug Byetta, which is injected twice a day. Alkermes, which supplied the technology that slowly releases Bydureon inside the body, is entitled to royalties on sales of the drug.
Bydureon, Byetta and Victoza are drugs called GLP-1 receptor agonists, which mimic the effect of glucagonlike peptide- 1, a hormone that increases insulin production when blood sugar is high.
The main ingredient in both Bydureon and Byetta is exenatide, a hormone derived from the saliva of the Gila monster, a poisonous lizard found in the Southwestern United States and Mexico.
Despite the less frequent injections, Bydureon will not automatically be preferred over Victoza.
That is in part because there is some data from one of Amylin’s own trials suggesting Bydureon is slightly less effective in controlling blood sugar than Victoza. Also, it is more cumbersome for a patient to prepare Bydureon for each injection and the needle used is larger than for Victoza.
Amylin said the wholesale price of Bydureon would be $323.44 for four doses, or about $4,200 a year. That is between the roughly $3,400 for the low dose of Victoza and $5,000 for the high dose, said Mark Schoenebaum, an analyst at the ISI Group.
The F.D.A. is requiring Amylin to conduct a clinical trial, which has begun, to assess whether Bydureon increases the risk of heart attacks and other cardiovascular problems. The same trial will also look at whether the drug increases the risk for thyroid cancer, pancreatitis and other health problems.
Amylin, founded in 1987, has never been profitable from drug sales and it still faces hurdles. It must pay Lilly $1.2 billion over time from sales of Bydureon.
Robyn Karnauskas, an analyst at Deutsche Bank, said Amylin might be a takeover candidate for a big company.