Effects of glucagon-like peptide-1 receptor agonistson weight loss: systematic review and meta-analysesof randomised controlled trials
BMJ10 January 2012
د. حسين محمد جمعه
اختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2012
Results 25 trials were included in the analysis. GLP-1R agonist groups
achieved a greater weight loss than control groups (weighted mean difference −2.9 kg, 95% confidence interval –3.6 to –2.2; trials, 6411participants). We found evidence of intertrial heterogeneity, but no evidence of bias or small study effects in regression analyses. The results
were confirmed in sequential analyses.
We recorded weight loss in the GLP-1R agonist groups for patients without diabetes (–3.2 kg, –4.3 to –2.1; three trials) as well as patients with diabetes (–2.8 kg, –3.4 to –2.3;18 trials). In the overall analysis, GLP-1R agonists had beneficial effects
on systolic and diastolic blood pressure, plasma concentrations of cholesterol, and glycaemic control, but did not have a significant effect on plasma concentrations of liver enzymes. GLP-1R agonists were associated with nausea, diarrhoea, and vomiting, but not with hypoglycaemia.
In the United States, more than two thirds of the population is overweight (body mass index 25-29.9) or obese (body mass index ≥30). This proportion is smaller in Europe, but continues to increase. The World Health Organization estimates that 1.5 billion adults worldwide are overweight and 500 million are obese. Almost three million adults die each year as a result of being overweight or obese. An estimated 44% of the burden for diabetes has been attributed to these weight problems, as well as 23% and 7-41% of the burdens for ischaemic heart disease and specific cancers, respectively.
Weight loss is not easily accomplished or maintained.
Meta-analyses of clinical trials on non-pharmacological strategies for weight reduction have reported 1-6 kg losses that have been difficult to maintain. Meta-analyses of sibutramine and orlistat trials report average weight reductions of 3 kg to 5 kg, but some of the included trials had attrition rates of up to 50% that were possibly due to adverse events, suggesting that the interventions could be less effective in clinical practice.Meta-analyses have found that bariatric surgery reduces long term mortality in obese patients,9 10 but the safety risks and the costs of this intervention limit the use for large patient populations. The risk of developing diabetes escalates with the degree of excess body weight, increasing threefold with a body mass index of 25.0 to 29.9, and 20-fold with an index of 35 and higher compared with a healthy index of 18.5-24.9.
The difficultie encountered in the management of type 2 diabetes are indicated by the low proportion (<50%) of patients treated to therapeutic goals. Problems in treatment management might be related to shortcomings with the currently available drugs, including bodyweight increase (thiazolidinediones, sulphonylureas, and insulin), hypoglycaemia (sulphonylureas, repaglinides, and insulin), and gastrointestinal side effects (metformin and alpha
glucosidase inhibitors).
Glucagon-like peptide-1 (GLP-1) is a gut hormone that is secreted from the intestine in response to meal ingestion. GLP-1 based therapy was recently introduced as a new treatment for patients with type 2 diabetes mellitus.
Treatment with GLP-1 enhances the endogenous secretion of insulin induced by meal ingestion and inhibits glucagon secretion, thereby improving
glucose homoeostasis. Notably, it also suppresses food intake and appetite. Trials of patients with type 2 diabetes suggest that agonists of GLP-1 receptor (GLP-1R) have beneficial effects
on metabolic regulation and could lead to weight loss. We did a systematic review and meta-analysis to provide an up to date overview of the beneficial and harmful effects of GLP-1R agonists in patients who are overweight or obese.
Discussion
The present meta-analyses include data from randomised controlled trials assessing clinically relevant doses of GLP-1R agonists given for at least 20 weeks. The results indicate that treatment with GLP-1R agonists help reduce body weight in patients who are overweight or obese. Three of the included trials assessed the effect of GLP-1R agonists on patients without type 2 diabetes and assessed patients with type 2 diabetes.GLP-1R agonists also had beneficial effects on systolic and diastolic blood pressure. In subgroup and sensitivity analyses, the liver enzyme activity was lower in the GLP-1R agonist groups than in the control groups. However, the overall effect of GLP-1R agonists on liver enzymes was not clear. For patients
with type 2 diabetes, GLP-1R agonists improved glycaemic control (as assessed by HbA1c percentage and fasting plasma glucose) and increased the proportion of patients who achieved the target haemoglobin.
GLP-1R agonists were associated with several adverse events.
Gastrointestinal side effects (such as nausea, vomiting, and diarrhoea) were common, but did not seem to affect the number of losses to follow-up. These findings concur with recent evidence showing that the overall patient satisfaction with GLP-1R agonist treatment is relatively high.
In patients with type 2 diabetes, improved glycaemic control is often associated with increased body weight. We found that participants without diabetes achieved greater reductions in body weight than those with diabetes. Our results suggest that
treatment with GLP-1R agonists are an effective intervention for participants who are overweight, irrespective of whether they have diabetes. GLP-1R agonists could be especially relevant for patients with hypertension, raised cholesterol, or other conditions in the metabolic syndrome. However, additional
trials investigating the effect of comorbidities (such as
cardiovascular diseases) are still warranted.
In developed countries, obesity increases mortality and
morbidity as well as the frequency of type 2 diabetes,hypertension, and non-alcoholic fatty liver disease. Obesity, particularly with an excess of visceral or ectopic fat, is an independent risk factor for heart disease and several studies suggest that obesity has detrimental effects on complications to infectious diseases, quality of life and depression, and
cancer related mortality.
These diseases have an important impact on the individuals affected, and the management of
complications to obesity puts an increasing strain on the healthcare systems. A recent German study found that the cost associated with obesity is apparent even from childhood. Since nearly 50 million children under the age of 5 years are overweight, we are facing a huge medical, as well as financial,challenge worldwide. Accordingly, identification of effective interventions for weight reductions is crucial. The costs with interventions that lead to lasting weight reduction should be considered in relation to those with the treatment of complications to obesity.
In 2005, the US Food and Drug Administration approved the first long acting stable GLP-1R agonist. Two products are currently available on the market: exenatide (Byetta; Eli Lilly) and liraglutide (Victoza; Novo Nordisk). Both drugs are injectable medications that can be used in combination with oral antidiabetic drugs such as metformin, thiazolidinediones, or sulphonylurea compounds.
The treatments are approved for patients with type 2 diabetes who have not achieved adequate
glycaemic control after treatment with conventional antidiabetic interventions. Exenatide treatment is initiated at a 5 μg dose given twice daily for at least one month to improve tolerability.
The dose can subsequently be increased to a maximum of 10 μg twice daily to improve glycaemic control.48
In October 2011, exenatide as a once weekly injection
(Bydureon; Eli Lilly) reached the European market for the treatment of type 2 diabetes, with similar indications as for exenatide twice daily and liraglutide. The starting dose of exenatide once weekly is 2 mg/week with no need for titration.
The starting dose of liraglutide is 0.6 mg/day; after one week, the daily dose is increased to 1.2 mg, based on the clinical response and side effects, and can be increased further to 1.8 mg/day the following week, depending on efficacy and adverse events. The trials included in the present review followed these
intervention regimens, although one trial also assessed liraglutide doses of 2.4-3.0 mg/day in obese individuals without diabetes. These doses seemed to further increase not only the achievedbodyweight loss, but also the number of gastrointestinal adverse
events. None of the remaining trials assessed equally high doses.
Patients with obesity and type 2 diabetes have an increased risk of developing non-alcoholic fatty liver disease. This disease could have a benign course, although it progresses to non-alcoholic steatohepatitis in about 20-30% of patients. Although histological markers remain the best method for assessing the severity of fatty liver disease, liver enzymes are associated with the disease activity in the early stages of non-alcoholic steatohepatitis.
We found no clear effect of GLP-1R agonists on liver enzyme activity. However, since we did not analyse histological changes, additional trials are needed to investigate the effects of GLP-1R agonists in participants with overweight and non-alcoholic fatty liver disease.
Strengths and limitations of the findings
Unlike previous reviews, we were able to gather data for outcomes that were not described in the published reports. Outcome measures are less likely to be described in published reports if the result is not significant.Therefore, the retrieval of unpublished outcome data could reduce the risk of reporting bias (that is, selective reporting of outcomes with a positive result).
The present review also differed from previous reviews by the inclusion of patients without type 2 diabetes mellitus. Although the non-specific
inclusion criteria increased intertrial heterogeneity, they also improved the external validity of our findings. Furthermore, to achieve and maintain clinically relevant weight loss, we only included trials that followed participants for a sufficient period
(20 weeks or more).
Inadequate randomisation and attrition bias could lead to overestimated benefits of an intervention. None of the included trials had deficiencies in the reported randomisation methods and all accounted for the intention to treat population in their results and analyses. These aspects support the internal validity of our findings. Since we only included trials that used clinically relevant doses given for clinically relevant treatment periods,
the results can be extrapolated to clinical practice.
Accordingly, the present review can help evidence based practitioners determine the size of the treatment benefit.
All the included trials received industry funding. Previous evidence and clinical examples have described that financial interests could lead to bias in intervention comparisons. On the other hand, funding could be associated with adequate control of bias.
Furthermore, in a large cohort of randomised trials, industry funding was associated with more positive author conclusions, but not with the quantitative results of the trials.Since we did not identify trials without funding, we were unable to make subgroup analyses to explore the potential effect of competing interests. We did, however, undertake rigorous assessments of the quality of included trials. None of our analyses showed any evidence of selection, assessment, attrition,
or outcome reporting bias. These analyses support the validity of our results.
Conclusions and clinical implications
Participants who are overweight are likely to develop type 2 diabetes, hypertension, hypercholesterolaemia, liver disease, and eventually cardiovascular disease. Accordingly, these participants have a considerably elevated risk of morbidity and mortality. Traditional treatments for obesity are, unfortunately, often short lived and consequently not especially effective.Current treatments for patients with type 2 diabetes are associated with shortcomings (weight gain, hypoglycaemia, and other side effects) that limit the number of patients reaching acceptable therapeutic goals. The present meta-analysis provides
convincing evidence that GLP-1R agonists, when given to obese patients with or without diabetes, results in clinically relevant beneficial effects on body weight.
Additional beneficial effects on blood pressure and total cholesterol might also be achieved.
The intervention should be considered in patients with diabetes who are obese or overweight. Further studies are needed to elucidate the effects of GLP-1R agonists in the treatment of obese patients without diabetes.
What is already known on this topic
Improved glycaemic control is associated with increased body weight Agonists to the glucagon-like peptide-1 receptor (GLP-1R) enhance glucose homoeostasis and suppress food intake and appetite.What this study adds
Treatment with clinically relevant doses of GLP-1R agonists for at least 20 weeks leads to weight loss in obese or overweight patients with or without type 2 diabetes mellitus in spite of an improved metabolic regulation .The effect of GLP-1R agonists could be more pronounced in patients without diabetes
GLP-1R agonists also reduce systolic and diastolic blood pressure and total cholesterol.
Several questions require further clarification in future studies, including delineation of the mechanisms that underlie weight reduction, which seem to be mainly related to a centrally mediated reduction in food intake; examination of the efficacy and safety of longer acting once weekly and once monthly preparations; and elucidation of the clinical relevance of apossible β cell mass preserving effect seen in animal models.
BMJ 10 January 2012
However, the most important unanswered question relates to the safety of GLP-1 agonists. Animal studies have raised concerns of an increased risk of pancreatitis, pancreatic metaplasia, and thyroid C cell tumours. The clinical relevance in humans is unknown and may take decades to assess fully, although data from post-marketing surveillance studies and
meta-analyses of the (admittedly) highly selected patient populations enrolled in randomised controlled trials have been reassuring. Nevertheless, continued and close surveillance of these new agents using all available data sources is warranted.
Diabetes mellitus is the seventh leading cause of death in the United States. In addition, it is a leading cause of morbidity and leads to microvascular (retinopathy, nephropathy, and neuropathy) and macrovascular (coronary artery, cerebrovascular, and peripheral vascular disease) complications. Type 2 diabetes mellitus is the most common form of the disease (affecting 90% to 95% of persons with diabetes)T2DM is the leading cause of blindness, nontraumatic lower-limb amputation, and chronic kidney disease in the United States
Glycemic Management of Type 2 Diabetes Mellitus
NEJM Teaching Topics | April 5, 2012
It is a major cause of cardiovascular disease (CVD), leading to early mortality. According to the Centers for Disease Control and Prevention, the number of persons with T2DM in the United States will more than triple by 2050 from the current estimate of 26 million. The increasing incidence of T2DM is largely attributable to changes in lifestyle (diet and activity levels) and obesity.
• Clinical Pearls
• What are the American Diabetes Association criteria for diagnosis of Type 2 diabetes mellitus?• The diagnosis of T2DM, as outlined by the American Diabetes Association (ADA), is based on a HbA1c level ≥6.5%, or fasting plasma glucose level ≥126 mg/dl (7.0 mmol/l), or a 2-hour plasma glucose ≥200 mg/dl (11.1 mmol/l) during an oral glucose tolerance test. The diagnosis can also be established by classic symptoms of hyperglycemia and a random plasma glucose ≥200 mg/dl. Test results need confirmation using the above criteria, unless the diagnosis is obvious based on symptoms.
What are the goals of glycemic control in patients with type 2 diabetes?
Long-term follow-up of patients with newly-diagnosed T2DM enrolled in the U.K. Prospective Diabetes Study showed a reduced risk of CVD events 10 years after the end of the trial among those initially randomized to intensive glycemic management, as compared with conventional therapy (average HbA1c of 7.0% versus 7.9%).Results of three trials in older patients with established T2DM and a history of or risk factors for CVD showed no reduction in total mortality or CVD-related mortality (and increased mortality in the ACCORD study) from intensive lowering of glucose to near-normal levels using multiple agents, compared to standard glycemic control.
A first step in glycemic management is setting an appropriate glycemic target in each individual patient. Current guidelines specify HbA1c targets of <7.0% or <6.5%. However, the appropriateness of these goals vary with different clinical characteristics and psychosocial factors, including the patient’s capacity for self-management and home support systems. In general, in patients with recently recognized T2DM and few (or no) complications (especially younger patients), a near-normal glycemic target aimed at prevention of complications over the many years of life can be suggested. In contrast, in older individuals with CVD (or multiple CVD risk factors) higher targets are often appropriate.
Q. What are appropriate lifestyle modifications for patients with Type 2 DM?
A. Weight loss and exercise are important nonpharmacologic approaches to improve glycemic control. A balanced diet rich in fiber, whole grains, and legumes, containing <7% saturated fat and reduced trans fats, and limited in calories and high glycemic index foods, is recommended by the ADA. Exercise has an additive effect to caloric restriction on glycemic control. Patients should be encouraged to perform at least 150 minutes per week of moderate-intensity aerobic exercise.Q. What are the guidelines for medical treatment in Type 2 DM?
A. Of the potential strategies for glycemic control, lifestyle modification and metformin are preferred and are cost-effective. By stimulating AMP-activated protein kinase, metformin reduces hepatic glucose production. It causes no weight gain or slight weight loss, and rarely causes hypoglycemia. Patients with high chronic baseline HbA1c (~9.0%) are unlikely to achieve adequate glycemic control with metformin alone, and in patients with significant hyperglycemia (>300 to 400 mg/dl; HbA1c >10–12%), initial insulin therapy should be considered.If metformin monotherapy cannot be used, other oral agents such as sulfonylureas (insulin secretagogues), dipeptidyl peptidase IV inhibitors (e.g., sitagliptin, inhibits the degradation of GLP-1 and results in modest elevations of circulating GLP-1 levels; does not cause weight gain), pioglitazone (activates PPAR-γ, which enhances insulin sensitivity in peripheral tissue and reduces hepatic glucose production) or a GLP-1 receptor agonist (e.g., exenetide or liraglutide, injectable agents that are structurally similar to endogenous GLP-1) can be initiated.
Over time, additional medications become necessary for glycemic control. A logical strategy is to consider agents with complementary modes of action. Strong evidence is lacking to support any one particular second agent over another. Perhaps due to reluctance of patients and providers, insulin is generally added much later than medically indicated.
Clinical Context
Type 2 diabetes is most often managed with oral medications in the United States, according to a study of data from the National Health and Nutrition Survey by Dodd and colleagues. Their research, which was published in the July 2009 issue of Current Medical Research and Opinion, found that the most common oral medications used to treat diabetes changed from sulfonylureas in 1999 to 2000 to thiazolidinediones in 2003 to 2004.Best Second-Line Therapy for Diabetes Treatment Identified
Medscape Education © 2012 Medscape, LLC
Slightly more than half of patients achieved a glycated hemoglobin A1c (A1C) level of less than 7.0%.
Metformin is now accepted as first-line therapy for type 2 diabetes, but many patients require additional medications to achieve glycemic control. What is the best choice of the many options available? The current study by Liu and colleagues uses a network meta-analysis to try to reach a conclusion as to which drug balances treatment efficacy and safety.
Study Synopsis and Perspective
The top 3 drugs for the reduction of A1C levels are biphasic insulin, glucagon-like peptide 1 (GLP-1) analogues, and basal insulin. Although most oral antidiabetic drugs had a similar effect on A1C, GLP-1 analogues had the additional advantages of weight reduction in the absence of an increased risk for hypoglycemia.Sung-Chen Liu, MD, from the Mackay Memorial Hospital, Division of Endocrinology and Metabolism, Department of Internal Medicine, Taipei, Taiwan, and colleagues published their network meta-analysis of different treatments for diabetes online April 9 in Diabetes, Obesity and Metabolism. The meta-analysis examined the use of antidiabetic agents for the treatment of type 2 diabetes that was inadequately controlled by metformin.
The authors searched PubMed and the Cochrane Central Register of Controlled Trials for randomized controlled trials written in English through December 2011. The search identified 819 articles, 70 of which were reviewed as full-text articles. The authors included 39 randomized controlled trials involving 17,860 patients.
Previous reports indicated that A1C reductions ranged from −0.64% for α-glucosidase inhibitors to −0.97% for GLP-1 analogues. The results of this study are consistent with those of previous network meta-analyses. GLP-1 analogues resulted in greater decrease in A1C levels compared with sulfonylureas (−0.20%; 95% confidence interval [CI], −0.34% to −0.04%), glinides (−0.31%; 95% CI, −0.61% to −0.02%), thiazolidinediones (−0.20%; 95% CI, −0.38% to −0.00%), α-glucosidase inhibitors (−0.36%; 95% CI, −0.64% to −0.07%), and dipeptidyl peptidase IV inhibitors (DPP-4 inhibitors; −0.32%; 95% CI, −0.47 to −0.17%), and resulted in A1C levels comparable to basal insulin and biphasic insulin.
The decrease in A1C levels was greater for sulfonylureas compared with DPP-4 inhibitors (−0.12%; 95% CI, −0.23% to −0.03%), and for biphasic insulin compared with glinides (−0.36%; 95% CI, −0.82% to −0.11%).
The authors found that sulfonylureas, glinides, basal insulin, and biphasic insulin treatments were associated with an increased risk for hypoglycemia compared with placebo.
علمني المطركيف اغسل همومي واحزانيوكيف اجدد حياتي كما تغسل قطرات المطراوراق الشجروتعيد لها الحياة
Patients receiving sulfonylureas, glinides, thiazolidinediones, basal insulin, and biphasic insulin gained weight, and patients receiving α-glucosidase inhibitors and GLP-1 analogues lost weight. The authors acknowledge that their analysis was limited by the fact that the duration of treatment was only 12 to 52 weeks.
The authors have disclosed no relevant financial relationships. Diabetes Obes Metab. Published online April 9, 2012.
Study Highlights
Researchers searched major medical databases for randomized controlled trials of all major medications used to treat type 2 diabetes. All studies were written in English, were conducted among adults 18 years or older, were between 12 and 52 weeks in duration, and were published before December 2011.The following outcomes were used to compare different treatments of diabetes (other than metformin):
Reductions in A1C levels
Changes in body weight
Risk for hypoglycemia
Medscape Education © 2012 Medscape, LLC
Of 819 articles identified in the original search, 39 were included in the meta-analysis. These studies enrolled a total of 17,860 adults collectively. The mean age of study participants varied between 51 and 62 years, and the mean duration of diabetes was 4.6 to 9.5 years. The mean baseline A1C values were 7.3% to 9.9%.
The average reduction in A1C levels compared with placebo was as follows:
Biphasic insulin: −1.07% (95% CI, −1.46 to −0.69%)GLP-1 analogues: −1.02% (95% CI, −1.17 to −0.86%)
Basal insulin: −0.88% (95% CI, −1.21 to −0.56%)
Sulfonylureas: −0.82% (95% CI, −0.95 to −0.70%)
Glinides: −0.71% (95% CI, −1.01 to −0.43%)
DPP-4 inhibitors: −0.69% (95% CI, −0.79 to −0.61)
α-glucosidase inhibitors: −0.66% (95% CI, −0.90 to −0.42%)
GLP-1 analogues were significantly more effective in reducing A1C levels vs all active treatments except insulin.
There was limited change to the overall result in subgroup analyses based on baseline A1C or body mass index values.
The risk for hypoglycemia was significantly elevated with the sulfonylureas, insulins, and glinides, with odds ratios from 4.77 to 17.78 vs placebo. The other agents did not significantly increase the risk for hypoglycemia vs placebo.
Treatment with sulfonylureas, glinides, thiazolidinediones, basal insulin, and biphasic insulin was associated with significant increases in body weight vs placebo. The average weight gain values associated with these medications were 2.17 kg, 1.40 kg, 2.46 kg, 1.38 kg, and 3.41 kg, respectively.
In contrast, GLP-1 analogues were associated with a mean weight loss of 1.66 kg, and α-glucosidase inhibitors reduced weight by a mean of 1.01 kg vs placebo.
Given the stronger reduction in A1C levels along with a low risk for hypoglycemia and superior body weight outcomes, the authors recommend GLP-1 analogues as the best treatment of type 2 diabetes after metformin.
Clinical Implications
A previous study by Dodd and colleagues found that most type 2 diabetes was managed with oral medications, and slightly more than half of patients had achieved good glycemic control. There was a shift in the most popular oral medications from sulfonylureas to thiazolidinediones during the study period from 1999 to 2004.GLP-1 analogues are recommended as second-line treatment of type 2 diabetes in the current study by Liu and colleagues based on their efficacy in reducing both A1C levels and body weight while presenting a low risk for hypoglycemia.
Diabetic Retinopathy
nejm.org march 29, 2012
Until recently, the treatment for diabetic retinopathy relied almost exclusively on managing the metabolic dysregulation of diabetes mellitus
until the severity of vascular lesions warranted laser surgery. Intensive metabolic control remains a highly effective means of controlling retinopathy and
other diabetes-related complications in many patients.
Recent research has identified the central role of vascular endothelial growth factor (VEGF) in the vascular lesions observed in diabetic retinopathy, and new agents that block VEGF action provide an effective treatment for this debilitating disease in patients for whom metabolic control alone is insufficient. The fact that treatment of vascular complications in the retina preserves visual acuity in patients with diabetic retinopathy highlights the interconnectedness of the neural retina with the retinal vasculature and the functional neurovascular unit in the retina.
In this article, we highlight the principles underlying metabolic control and anti-VEGF therapies in the treatment of diabetic retinopathy. We also explore the molecular interactions of neuronal, glial, and vascular cells in the retina as the basis
of the neurovascular unit and examine the effect of diabetes on the function of the neurovascular unit in order to highlight new therapeutic approaches that are needed to address the large increase in the worldwide prevalence of diabetes.
DIABETIC RETINOPATHY IN THE PAST AND PRESENT
The features of diabetic retinopathy, as detected by ophthalmoscopy, were describedmin the 19th century. They begin with microaneurysms and progress into exudative changes (leakage of lipoproteins [hard exudates] and blood [blot hemorrhages]) that lead to macular edema (Fig. 1), ischemic changes (infarcts of the nerve-fiber layer [cotton-wool spots]), collateralization (intraretinal microvascular abnormalities) and dilatation of venules (venous beading), and proliferative changes (abnormal vessels on the optic disk and retina, proliferation of fibroblasts, and vitreous hemorrhage).Persons with mild-to-moderate nonproliferative retinopathy have impaired contrast sensitivity and visual fields that cause difficulty with driving, reading, and managing diabetes and other activities of daily living. Visual acuity, as determined with the use of Snellen charts, declines when the central macula is affected by edema, ischemia,
epiretinal membranes, or retinal detachment.
Fifty years ago, proliferative diabetic retinopathy was treated by means of pituitary ablation, but the high frequency of complications related to hypopituitarism,
including death, prompted the development of panretinal photocoagulation. In 1968,the Airlie House Symposium led to a standard classification system for diabetic retinopathy and laid the groundwork for the Diabetic Retinopathy Study.
These clinical trials showed the dramatic effects of retinal photocoagulation,
which significantly reduced the severe visual loss
due to proliferative diabetic retinopathy and macular
edema, and led to guidelines and screening
programs for the timely detection and treatment
of diabetic retinopathy.
The incidence and the risk of progression of
diabetic retinopathy have both declined over thepast 30 years, from up to 90% to less than 50%.
The population-based Wisconsin Epidemiologic
Study of Diabetic Retinopathy showed that, from
1980 to 2007, the estimated annual incidence of
proliferative diabetic retinopathy decreased by 77%
and vision impairment decreased by 57% among
persons with type 1 diabetes.
Persons with recently diagnosed type 1 or type 2 diabetes have amuch lower risk of proliferative diabetic retinopathy, macular edema, and visual impairment (Fig. 2A), as compared with patients from earlier periods. The marked reduction in the prevalence and incidence of retinopathy and vision impairment over the past few decades reflects improved management of glycemia, blood pressure, and lipid levels.
These improvements have resulted from the introduction of new devices for self-monitoring of blood-glucose levels and the administration of insulin, new medications (e.g., statins and hypoglycemic agents), surgical interventions (including vitrectomy), an increased awareness of the need for intensive control of glycemia and blood pressure, and the implementation of educational and screening programs (Fig. 2B). The benefits of intensive control are offset, however, by a 33% increase in the frequency of hypoglycemia and a 100% increase in the prevalence of overweight or obesity among adults with diabetes.
The percentage of persons with type 2 diabetes
who meet the target levels for glycated hemoglobin,
blood pressure, or serum total cholesterol, as
recommended by the American Diabetes Association,
increased by 30 to 50% from 2000 to
2006.13 However, only 7% of patients meet all
three targets,14 and non-Hispanic blacks and Mexican Americans meet them less commonly than
whites.
THE DIABETES EPIDEMIC
The number of persons with diabetes worldwideis predicted to grow to 429 million by 2030, owing
to the rising frequency of obesity, increasing life
span, and improved detection of the disease.
In India, an estimated 32 million persons had diabetes
in 2000, and roughly 79 million will be affected
by 2030. If the prevalence of complications
remains unchanged, approximately 0.7 million Indians
will have proliferative diabetic retinopathy and 1.8 million will have clinically significant macular edema.
Improved delivery of health care reduces the incidence of vision impairment in whites
in developed countries (e.g., Denmark, Sweden,and the United States), but it remains uncertain
whether the lifestyle changes that are associated
with urbanization in India and other developing
countries will result in uncontrolled glycemia,
blood pressure, and lipid levels and a higher frequency of severe diabetic retinopathy in persons
with type 2 diabetes.
These data portend a huge population of persons at high risk for diabetes induced visual impairment for whom current approaches to treatment are inadequate. Little information exists on the risk of retinopathy and other diabetes-related complications in developing countries, so continued epidemiologic surveillance is needed to determine trends, properly allocate resources, and develop cost-effective preventive interventions.
Epidemiologic studies have shown the effects
of hyperglycemia, hypertension, and dyslipidemia— and, to a lesser extent, a high body-mass index,
a low level of physical activity, and insulin resistance
— on the incidence and progression of
diabetic retinopathy and clinically significant
macular edema. The Diabetes Control and Complications Trial (DCCT; NCT00360815) showed
that intensive metabolic control reduces the incidence
and progression of diabetic retinopathy.
Although the glycated-hemoglobin level is the
strongest risk factor for predicting the developmentand progression of diabetic retinopathy,
glycated hemoglobin accounted for only 11% of
the risk of retinopathy in the DCCT.19 Similarly,
the values for glycated hemoglobin, blood pressure,
and total serum cholesterol together accounted
for only 9 to 10% of the risk of retinopathy
in the Wisconsin Epidemiologic Study of Diabetic Retinopathy.
Therefore, the prevention and treatment of diabetic complications should include other modifiable factors. Data from several studies suggest roles for other factors, including sleep apnea, nonalcoholic fatty
liver disease, and serum prolactin, adiponectin,
and homocysteine levels, as well as genetic
factors, including mutations in the erythropoietin
gene promoter. However, the relative
contributions of these factors to the risk of retinopathy in populations remain uncertain.
Despite advances in diabetes care, complications
persist for various reasons. Proliferative diabeticretinopathy and other complications develop
after 30 years in up to 20% of persons with diabetes who have been treated with intensive metabolic control, and ideal metabolic control is difficult to achieve because of the increased risk of
hypoglycemia and the nonphysiologic route of insulin administration.
Only 17% of persons in the DCCT who were followed in the Epidemiology of Diabetes Interventions and Complications study (NCT00360893) had glycated-hemoglobin levels less than 7% at their last visit. In developing countries, the resources needed to implement good diabetes control are generally unavailable.
Therefore, greater emphasis must be placed on
preventing complications, which will require both
a better understanding of the mechanisms by
which diabetes affects the retina and an improved
means of detecting retinopathy.
CLINICAL TRIALS OF RETINOPATHY
THER APIESLarge, randomized trials have shown the benefits
of systemic and ocular therapies for the
prevention or treatment of diabetic retinopathy
(Table 1) and have revealed that metabolic control,
the renin–angiotensin system, peroxisome
proliferator–activated receptor α (PPAR-α), and
VEGF contribute to human pathophysiology.
Notably, renin–angiotensin system inhibitors reduce
the incidence and risk of progression of
diabetic retinopathy in persons with type 1 diabetes
and are now standard therapy.12,29,32,33
The PPAR-α agonist, fenofibrate, reduces the risk
of progression by up to 40% among patients with
nonproliferative retinopathy, as shown in the
Fenofibrate Intervention and Event Lowering in
Diabetes (FIELD; Current Controlled Trials
number, ISRCTN64783481)37 and the Action to
Control Cardiovascular Risk in Diabetes (ACCORD;
NCT00000620) studies.
Whether the mechanism of action underlying this preventive effect of fenofibrate is related to its lipid-lowering action remains unclear. The ACCORD study did not show an effect of intensive blood-pressure control on retinopathy progression but did show the benefit of intensive glycemic control in preventing the progression of retinopathy.
Eye-specific treatments are beneficial in patients
whose vision is threatened by macular edema.
Use of the VEGF-neutralizing antibodies bevacizumab
and ranibizumab improves visual acuity by an average of one to two lines on a Snellen chart, with an improvement of three or more lines in 25 to 30% of patients, and loss of visual acuity decreased by one third. These improvements, which are seen over a period of 2 years after approximately 10 intraocular injections, are significantly better than the results of laser treatment alone.The VEGF aptamer, pegaptanib, improves visual acuity by approximately one line.
Sustained intravitreal delivery of fluocinoloneyields a similar likelihood of gaining three or more
lines of acuity but with a 60% increase in the risk
of glaucoma and a 33% increase in the need for
cataract surgery.
The same implant technology delivering a lower dose of fluocinolone did not increase the risk of cataract or glaucoma. Glucocorticoids
such as fluocinolone reduce retinal
inflammation and may restore the integrity of the
blood–retina barrier by increasing tight-junction
protein expression. These initial treatments
for diabetic retinopathy reflect the gains in our
understanding of how diabetes impairs vision and
set the stage for further advances in the management of this disorder.
THE NEUROVASCULAR UNIT
New insights into retinal physiology suggest thatthe retinal dysfunction associated with diabetes
may be viewed as a change in the retinal neurovascular unit. The neurovascular unit refers to the physical and biochemical relationship among neurons,glia, and specialized vasculature and the close interdependency of these tissues in the central nervous system (Fig. 3).
This intimate association of glia with neurons allows for energy homeostasis and neurotransmitter regulation. Furthermore, glial-cell, pericyte, and neural interactions promote formation of the blood–brain and blood–retina barriers, which control the flux of fluids and bloodborne metabolites into the neural parenchyma.
Neurodegenerative conditions such as stroke, Alzheimer’s disease, amyotrophic lateral sclerosis,
and Parkinson’s disease alter the neurovascular
unit, with changes in neural function and neurotransmitter metabolism and loss of the blood–
brain barrier. If the neurovascular unit is similarly
involved in diabetes, then new therapeutic
approaches addressing both vascular dysfunction
and neural degeneration may be required.
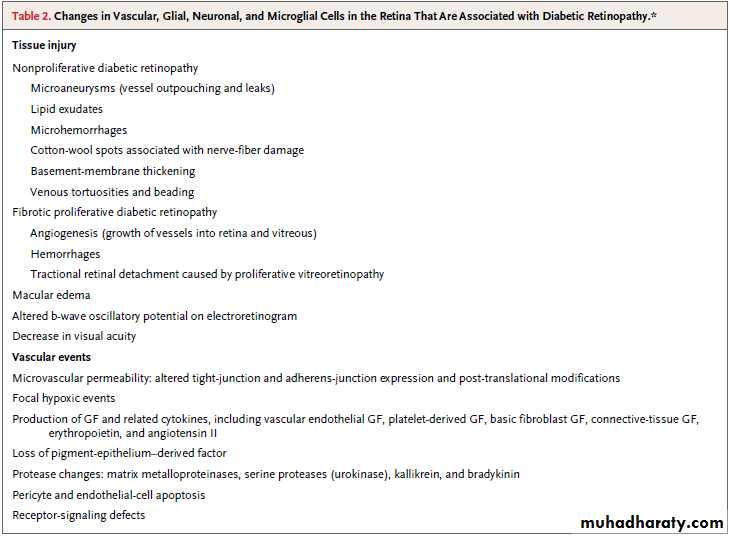
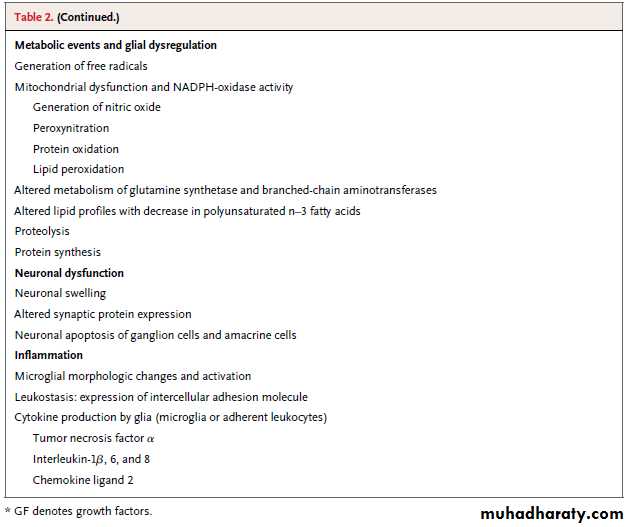
Table 2lists alterations in the neurovascular unit in diabetic retinopathy.
The retinal architecture confers unique characteristics
to the neurovascular unit. The inner retina has capillary beds in the ganglion-cell and inner nuclear layers.
The neurovascular unit includes astrocytes and Müller cells, and amacrine and ganglion neurons reside in close proximity to microvascular segments that deliver oxygen andnutrients. The close coupling of neurovascular
units is shown by the autoregulation of retinal
vascular blood flow by local metabolite levels (the
lactate level and the partial pressure of oxygen and
of carbon dioxide) and glial cells.
The outer retina consists of photoreceptor neurons and Müllercells, which are metabolically coupled to support the generation of electrochemical impulses in response to stimulation with light, with nutrients
and oxygen diffusing from choroidal vessels
through the pigmented epithelial-cell layer.
VASCULAR LEAKAGE AND ANGIOGENESIS
Diabetic retinopathy involves occlusion and leakageof retinal vessels, leading to macular edema in
the nonproliferative phase and angiogenesis
and to tufts of highly permeable vessels in the
proliferative phase. Macular edema (present in
25% of persons with diabetes) remains the clinical
feature most closely associated with vision
loss, with thickening of the central fovea evident
on optical coherence tomography and fluorescein
leakage visible on angiographic testing.
The duration of central foveal thickening and the degree of fluorescein leakage are major factors in
accounting for reduced visual acuity. The efficacy
of treatment with the anti-VEGF agents ranibizumab
and bevacizumab indicates that VEGF contributes to the pathogenesis of diabetic macular edema and reflects successful translational research.
Conditional deletion of the VEGF gene from
Müller cells reveals the importance of glial cellsfor VEGF production in oxygen-induced retinopathy
models of angiogenesis, and this finding underscores
the consequences of altered glial–vascular
communication.
The mechanism ofVEGF-induced vascular permeability involves activation of classical protein kinase C isoforms, particularly protein kinase C beta. Recently,
the tight-junction protein occludin was identified
as a target of protein kinase C beta, leading to
ubiquitin-mediated endocytosis of tight-junction
components and increased vascular permeability
and providing a molecular mechanism for
the regulation of the properties of the blood–retina
barrier in response to VEGF.
Studies in animals and initial clinical reports suggest that inhibiting protein kinase C beta with ruboxistaurin reduces diabetic macular edema. In a combined analysis of data from two clinical trials of oral ruboxistaurin, the proportion of patients with sustained moderate visual loss was smaller in the group of patients who received ruboxistaurin than in the placebo group (6.1% vs. 10.2%)60,61; however, the Food and Drug Administration asked to see additional confirmatory phase 3 clinical-trial results
before approving the drug for clinical use.
Other potential targets of VEGF-receptor signaling
include inhibition of the soluble tyrosine
kinase Src to regulate vascular permeability.
Using small-molecule inhibitors of Src in animals with
src-gene deletion, Scheppke and colleagues62 foundthat the requirement for Src activation in VEGF
induced retinal vascular permeability. Although
no data from clinical trials are available yet, topical
application to the cornea of a dual Src and
VEGF receptor inhibitor prevented VEGF-induced
vascular permeability in animals.
Signaling pathways also contribute to vascular
permeability in diabetic retinopathy. Massspectrometry analysis of vitreous fluid in patients
with proliferative retinopathy allowed Gao and
colleagues63 to identify the plasma kallikrein system
that leads to bradykinin-receptor activation.
Kallikrein inhibitors prevent retinal vascular permeability in diabetic rodents, and kallikrein injection acts synergistically with diabetes to increase
retinal vascular leakage.
Other extracellular proteases, such as urokinase plasminogen activator and matrix metalloproteases 2 and 9, may also contribute to the degradation of tight-junction protein and to retinal vascular permeability.
The blood–retina barrier requires proper pericyte
function, and loss of pericytes may contribute
to vascular permeability (Fig. 4). Pericyte dropout
is a feature of diabetic retinopathy, and genetic
ablation of platelet-derived growth factor
(PDGF) β causes pericyte loss and a phenotype
that resembles diabetic retinopathy, with increased vascular damage and angiogenesis.
Geraldes et al.68 recently found that hyperglycemia induces
expression of protein kinase C delta, which upregulatesSrc-homology domain–containing tyrosine phosphatase. This tyrosine phosphatase inhibits PDGF signaling through the Akt survival pathway, contributing to pericyte-cell death and
vascular derangement.
These findings underscore the cell-to-cell communication necessary for proper retinal function and maintenance of the blood–
retina barrier.
Retinal angiogenesis (neovascularization) usually
arises on the optic disk and at the junctionof nonperfused retinal vessels and perfused vessels
that are leaking, with growth into the posterior
surface of the vitreous. Untreated neovascularization leads to vitreous contraction, vitreous hemorrhage, and tractional retinal detachment.
Don't drink your calories
Health tipAn increased ratio of proangiogenic factors (VEGF
and erythropoietin) to antiangiogenic factors (pigment- epithelium–derived factor) promotes neovascularization, and the ratio is decreased afterlaser treatment. However, VEGF inhibition and
regression of active neovascularization are associated with increased expression of connectivetissue growth factor in the vitreous, which contributes to vitreoretinal fibrosis.
NEURONAL DYSFUNCTION
In addition to vascular abnormalities, the neurosensoryretina is altered in diabetes. The neurosensory
retina generates vision but is transparent
and largely undetectable by standard clinical examination, so its role in diabetic retinopathy has
been difficult to determine in humans. However,
most retinal neurons and glial cells are altered
concomitantly with the development of microvascular
lesions and are progressively impaired with
worsening retinopathy.
These alterations include biochemical defects, such as impaired control of glutamate metabolism (the major neurotransmitter), as well as loss of synaptic activity and dendrites, apoptosis of neurons primarily in the
ganglion-cell and inner nuclear layers, and activation
of microglial cells that may protect the inner
retina from injury and contribute to the inflammatory
response.
In experimental models of diabetes, insulinreceptor
signaling is impaired in the retina as itis in peripheral tissues, and the actions of
brain-derived neurotrophic factor are also reduced.
Thus, just as the loss of PDGF signaling
contributes to pericyte loss, the loss of neu-rotrophic signals that support cell survival and
cell–cell interactions at synapses probably contributes
to the pathological features of retinopathy.
Furthermore, changes in retinal blood flow and
vasoreactivity in response to oxygen may indicateimpaired autoregulation and impaired control of
vascular integrity by the neural retina. Diabetic
retinopathy includes reduced electrical activity
and alterations of nerve fibers.
Together with reduced corneal-nerve sensation and impaired autonomic innervation of the pupil, altered function of the retinal sensory nerve indicates that
diabetes causes denervation of multiple sensory
inputs to the eye. Thus, although the retinal neuronal
structure differs from the peripheral sensory
system, diabetic retinopathy resembles diabetic
peripheral sensory neuropathy.
INFLAMMATION IN DIABETIC RETINOPATHY
The concept of the neurovascular unit extends tothe presence of activated microglia in diabetic retinopathy.
Systemic inflammation is an intrinsic response to overfeeding, obesity, and diabetes,
and diabetes increases the release of retinal inflammatory mediators (interleukin-1β, tumor necrosis
factor α [TNF-α], intercellular adhesion molecule
[ICAM] 1, and angiotensin II) and activation
of microglial cells in early retinopathy.
Leukostasis occurs in diabetic mice and rats, and deletion of the genes for the adhesion protein ICAM or its leukocyte binding partner, CD18, ameliorates leukostasis and permeability.34 Vascular permeability, leukostasis, CD18 and ICAM expression, and nuclear factor κB activation are normalized by treatment with high-dose aspirin, a cyclooxygenase- 2 inhibitor, meloxicam, or a soluble TNF-α receptor–Fc hybrid, such as etanercept. These findings suggest that TNF-α and cyclooxygenase-2 contribute to diabetic retinopathy, perhaps by preventing endothelial-cell damage from adhering leukocytes.
Furthermore, a newly discovered inhibitor of atypical protein kinase C prevents TNF-α–induced retinal vascular permeability and VEGF-induced permeability (according to an unpublished study), providing a broad target for potential control of edema. Interleukin-1β and TNF-α levels increase in the vitreous of patients with proliferative diabetic retinopathy. Progressive
retinal injury may impair the blood–retina barrier
and lead to macrophage migration into the neurosensory retina or increased adherence to the vasculature, as well as accumulation of inflammatory
and angiogenic mediators in the vitreous cavity.
Collectively, the data suggest that inflammation
contributes to the development and progression
of retinopathy. Antiinflammatory treatment
with intravitreal glucocorticoids and anti-VEGF
therapy reduce the overall severity of retinopathy
and macular edema and restore the blood–retina
barrier. Further investigation is needed to develop therapies that control inflammation in diabetic retinopathy.
FUTURE CHALLENGES
AND OPPORTUNITIESThe large worldwide increase in diabetes provides
an imperative to prevent retinopathy and other
complications before the advanced stages of disease.
Improved outcomes of treatments for cancer
have resulted from advances in clinical-trial end
points that reflect the pathophysiology of the disease,such as molecular biomarkers of tumor activity and positron-emission–tomographic scanning.
Likewise, new end points reflecting the
pathophysiological features and full phenotype ofdiabetic retinopathy are needed for sensitive, quantitative, and predictive assessment of the severity
of retinopathy. Vascular lesions change slowly, and
photographic staging alone cannot facilitate shortterm (<1 year) proof-of-concept trials to evaluate pathophysiological mechanisms and therapies.
Standard measures are now being supplement-
ed with sensitive indexes of retinal function and
structure to determine the nature of early retinopathy.
Flavoprotein spectrophotometry reveals
defects in mitochondrial metabolism, reduced
electroretinographic responses suggest reduced
cellular signal transmission and predict subsequent
microvascular lesions and responses to
improved metabolic control, and subtle defects
in visual function are detected by contrast sensitivity
and visual-field defects.
Optical coherence tomography detects thinning of the neuronal and synaptic layers of mild retinopathy.
Metabolic and blood-pressure control have reduced
the incidence of diabetic retinopathy and
vision impairment and remain the foundation for
controlling retinopathy and other complication of
diabetes.
However, these approaches do not ameliorate visual impairment and may have adverse effects.
Furthermore, economic barriers often prevent
the implementation of these approachesamong patients who are poor and underinsured.
Research into the molecular causes of diabetic
retinopathy reveals changes affecting all cells
within the retina, including those in the microvasculature, glia, neurons, and microglia. These
changes in the retina, which can be viewed as a
disruption of the neurovascular unit, contribute
to the pathophysiology of diabetic retinopathy.
Intraocular administration of VEGF inhibitors and
glucocorticoids has launched an era of biologically
based pharmacologic treatment that complements
surgical approaches for advanced stages
of retinopathy. Further advances require an
understanding of how the metabolic changes in
diabetes disrupt the neurovascular unit, as well as
focused efforts to develop clinical-trial end points
and biomarkers.
The expected increase in diabetic
retinopathy due to the increasing incidence oftype 2 diabetes requires the elimination of socio
economic barriers so that research advances can
be translated into effective, accessible care for all
persons with diabetes.
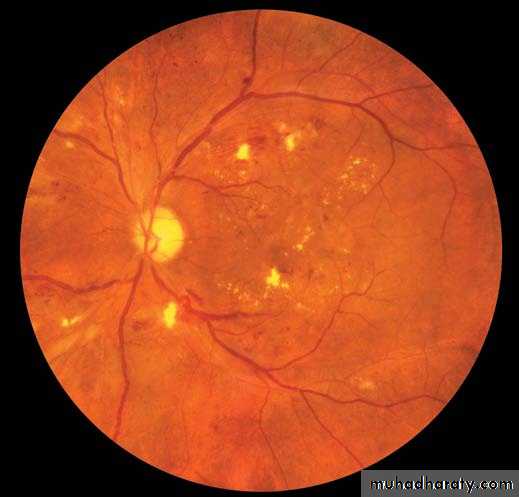
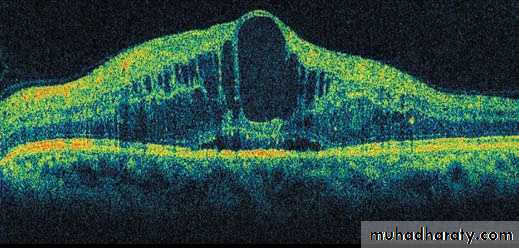
Figure 1. Clinical Features of Diabetic Retinopathy.
A fundus photograph (Panel A) shows the left eye of a 57-year-old man with20/200 visual acuity, signs of hypertension, and proliferative diabetic retinopathy
with macular edema (the region of macular edema is indicated by the
bracket). Notable features include arteriolar narrowing (AN), nerve-fiber
hemorrhage (NFH), hard exudates (HE), cotton-wool spots (CWS), venous
beading (VB), and preretinal hemorrhage (PRH). Optical coherence tomography
(Panel B) with a horizontal scan through the central fovea (corresponding
to the horizontal line in Panel A) reveals marked thickening and edema
of the macula with cysts (C) and subretinal fluid (SRF). (Images courtesy of
Richard Hackel, M.A., C.R.A.)
Everolimus-Eluting Stents Perform Best in Diabetics
August 13, 2012 (New York, New York)— All drug-eluting coronary stents (DES) are more effective than their bare-metal counterparts in diabetic patients, without any compromise in safety, but it is likely that the everolimus-eluting stent is the most effective DES in this population, a meta-analysis shows [1].Dr Sripal Bangalore (New York University, NY) and colleagues analyzed 42 trials comparing four major types of DES--sirolimus, paclitaxel, everolimus, and zotarolimus--with each other and with bare-metal stents for the treatment of de novo coronary lesions in patients with diabetes.
The results in this diabetic subgroup, published online August 10, 2012 in BMJ, largely mirror that of this group's overall meta-analysis of 76 randomized controlled trials with 117 762 patient-years of follow-up, published in Circulation.
Overall, the analysis covers a total of 22 844 patient-years of follow-up. All of the studies selected for this meta-analysis had at least 50 diabetic patients.
Although the analysis shows that all four of the DES evaluated reduced target vessel revascularization compared with bare-metal stents (37% vs 69%) without any offsetting safety concerns, the DES did not all perform equally as well. Based on all of the comparisons, the authors calculate there is an 87% probability that everolimus-eluting stents are the most efficacious compared with all others and a 62% chance that everolimus-eluting stents are the least likely to develop any stent thrombosis.
Bangalore et al note that a 2007 pooled patient-level meta-analysis of sirolimus-eluting-stent trials suggested an increased risk of mortality with sirolimus-eluting stents compared with bare-metal stents in the subgroup with diabetes. But that study included only 428 patients with diabetes, and the study by Bangalore et al found no such safety concerns in a sample 25 times as large.
This analysis found that Endeavor (Medtronic) zotarolimus-eluting stents were associated with a higher rate of repeat revascularization than either sirolimus-eluting stents or everolimus-eluting stents and a higher rate of MI than sirolimus-eluting stents, paclitaxel-eluting stents, or everolimus-eluting stents. However, the authors point out that the data on Resolute-eluting stents are still limited, and the analysis could include only two published trials of these stents, TWENTE and RESOLUTE All-Comers.
"The credibility interval around the estimates for zotarolimus-eluting stents were wide, suggesting less precision and confidence in the estimates, probably because of limited published data on the use of zotarolimus-eluting stents in patients with diabetes," Bangalore et al explain. "This analysis on the zotarolimus-eluting Resolute stent should therefore be viewed as highly exploratory."
Bangalore is on the advisory boards of Boehringer Ingelheim and Daiichi Sankyo. Disclosures for the coauthors are listed in the paper.
New Guidelines Stress Hyperglycemia Management in Diabetic Patients
Clinical ContextLong-term glycemic control is one of the most important treatment goals for type 2 diabetes, and the authors of the current position statement provide a broad overview of research into different levels of glycemic control among patients with type 2 diabetes.
Tighter glycemic control has been most associated with a lower risk for microvascular complications, such as retinopathy and nephropathy.
However, very tight glycemic control appears to be less beneficial regarding the risk for cardiovascular disease, with some research suggesting that tight control actually increases the risk for cardiovascular mortality.
Regardless of the specific glycemic control target, patient-centered care is an important strategy to help patients achieve their treatment goal. The current position statement from the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) emphasizes the role of patient-centered care for patients with type 2 diabetes
Study Synopsis and Perspective
The ADA and the EASD have issued a joint position statement emphasizing patient-specific treatment of hyperglycemia in persons with type 2 diabetes. The new guidelines are reported concurrently in the April 19 online edition of Diabetes Care and in Diabetologia."All guidelines are in a state of evolution based on new information, and the overall standard of care is updated every January," Vivian Fonseca, MD, ADA president of medicine and science, told Medscape Medical News in a telephone interview.
"The last guidelines specific to management of hyperglycemia were published about 4-5 years ago, and more recent developments have been incorporated into the new guidelines."
The impetus underlying the new guidelines was the growing complexity and controversy surrounding contemporary glycemic management in persons with type 2 diabetes.
Factors complicating management include the increasing number and variety of available pharmacotherapy, issues regarding potential adverse effects, and new uncertainties concerning the effects of intensive glycemic control on macrovascular complications.
Dr. Fonseca, who is also professor of medicine and endocrinology at Tulane University in New Orleans, Louisiana, explained that there has been a small change in what the optimal blood glucose goal should be.
On the basis of findings from ACCORD (Action to Control Cardiovascular Risk in Diabetes study) and other studies, the ADA has set the hemoglobin A1c (HbA1c) goal at 7% in general, but with some individualization.
"For patients with advanced cardiovascular disease, reduced life expectancy, and multiple medical problems, for example, the goal may be higher," Dr. Fonseca said. "For patients who are newly diagnosed and very motivated, the goal may be lower."
Another recent change underlying the new guidelines is the recognition that many people with diabetes will need multiple agents. For example, the dipeptidyl peptidase-4 (DPP4) inhibitors have become available since the last hyperglycemia guideline was published.
Key Points
Key recommendations in the new ADA/EASD statement include the following:Glycemic targets and treatments to lower glucose must be individualized according to specific patient characteristics.
The mainstay of any type 2 diabetes treatment program is still diet, exercise, and education.
Metformin is the preferred first-line drug, in the absence of contraindications. Data are limited regarding use of agents other than metformin. A reasonable approach is combination therapy with 1 to 2 additional oral or injectable agents, with the goal of minimizing side effects to the extent possible.To maintain glycemic control, many patients will ultimately need insulin monotherapy or in combination with other medications.Whenever possible, the patient should participate in all treatment decisions, focusing on their preferences, needs, and values.
A major treatment goal must be comprehensive cardiovascular risk reduction.
Others Weigh in on the New Guidelines
In an accompanying editorial, Diabetes Care editor William Cefalu, MD, notes that some experts prefer an algorithm-based management plan offering consistent treatment guidelines, whereas others favor flexible treatment options based on specific pathophysiology."The most attractive aspect of the new position statement is that more than any other previously reported guidelines to date, it clearly emphasizes that 'one size clearly does not fit all'," Dr. Cefalu writes.
EASD president Andrew J.M. Boulton also commended the new guidelines for their patient-specific approach.
"The new guidelines were prepared using the best available evidence," Dr. Boulton said in a news release. "Diabetes is a condition which affects people in a multitude of ways: the new guidelines take a more holistic approach, focusing on treating the patient as an individual and understanding that treatments need to be 'made to measure,' an approach that will likely improve not only patient care, but also quality of life."
Study Highlights
Current guidelines suggest that the goal HbA1c level should be less than 7%. This goal can be achieved with fasting glucose levels of less than 130 mg/dL and postprandial glucose levels of less than 180 mg/dL.More strict HbA1c targets may be considered among patients with long life expectancy and no history of cardiovascular disease.
Although all patients with diabetes should receive instruction on a healthy lifestyle, this advice should be personalized to account for a patient's personal and cultural preferences.
Advice on exercise should target a minimum of 150 minutes per week of activity that might include aerobic, resistance, and flexibility training.
Metformin is the mainstay of oral therapy for type 2 diabetes because it effectively lowers serum glucose levels without raising body weight or the risk for hypoglycemia. It should be initiated at a low dose to reduce the incidence of gastrointestinal adverse effects.
Patients with poor glycemic control at baseline, as characterized by an HbA1c level of 9% or more, may receive combination therapy at the outset of treatment.
Insulin therapy should be considered as the initial therapy for patients with plasma glucose levels of more than 300 mg/dL or HbA1c levels greater than 10%.
Adding a second agent is associated with an average additional reduction of HbA1c levels of 1%.
The choice of a second agent should be based not only on improving HbA1c values but also on improving the treatment's adverse effect profile and cost.
Patients with an HbA1c value exceeding 8.5% with 2-drug therapy should receive insulin as opposed to a third antidiabetic agent.
Insulin is usually started at a low dose of 0.1 to 0.2 unit/kg/day. It is reasonable to have patients slowly titrate up their insulin dose once or twice weekly if glycemic targets are not met.
The addition of prandial to basal insulin is usually necessary when the dose of insulin climbs to 0.5 to 1 unit/kg/day.
Insulin secretagogues such as sulfonylureas may be continued during treatment with basal insulin only but should be stopped when prandial insulin is prescribed.
HbA1c targets up to 8% may be acceptable among older and frail patients with diabetes. Older adults are at higher risk for severe complications related to hypoglycemia, and this complication should be assiduously avoided among these patients.
There are no sex-based differences in response to treatment with antidiabetic drugs. Although Latinos tend to have greater insulin resistance and persons of East Asian descent are more likely to have beta-cell dysfunction, these broad traits should not necessarily influence treatment decisions for the individual.
Metformin may be used among patients with mild heart failure, and current practice standards regarding the use of metformin in the setting of chronic kidney disease are too restrictive. Metformin can be used until the estimated glomerular filtration rate decreases to 30 mL/minute or less.
Some evidence exists that pioglitazone may be particularly advantageous for patients with mild steatohepatitis.
Clinical Implications
Strict glycemic control appears to reduce the risk for microvascular complications of diabetes, such as retinopathy and nephropathy, to a greater extent than reducing the risk for cardiovascular disease.The current position statement from the ADA/EASD endorses metformin as the initial therapy for most patients with type 2 diabetes. Metformin should be initiated at a low dose to avoid adverse effects and can be continued even in cases of moderate chronic kidney disease. Patients with an HbA1c value exceeding 8.5% with 2-drug therapy should receive insulin as opposed to a third antidiabetic agent.
Statins Associated With Significant Increase in Diabetes Risk
January 9, 2012 (Boston, Massachusetts) — Statin use in postmenopausal women is associated with a significantly increased risk of diabetes mellitus, research shows [1]. New data from the Women's Health Initiative (WHI) hints that the risk of diabetes is higher than suggested by previous studies, with investigators reporting a 48% increased risk of diabetes among the women taking the lipid-lowering medications."With this study, what we're seeing is that the risk of diabetes is particularly high in elderly women, and this risk is much larger than was observed in another previous meta-analysis," senior investigator Dr Yunsheng Ma (University of Massachusetts Medical School, Boston) told heartwire . "For doctors treating patients, we would like them to really look at the risk-benefit analysis, especially in different age groups, such as older women."
Annie Culver (Mayo Clinic, Rochester, MN), a pharmacist and lead investigator of the study, published online January 9, 2012 in the Archives of Internal Medicine, said that "close monitoring and an individualized risk-versus-benefit assessment is really a good thing, as well as an emphasis on continued lifestyle changes." Culver added that as the population ages, and because these patients have a higher vulnerability to diabetes anyway, monitoring for diabetes in statin-treated patients becomes more important.
"I think the risk [of diabetes] is definitely there for statins," Culver told heartwire , "and I think physicians are probably aware of this risk. I think we now need more information and more research about precisely how this risk translates to different people and different populations."
Previously Published Data on Statins and Diabetes Risk
Recently published data reported by heartwire highlighted the potential risk of diabetes with statin therapy. In June, Dr Kausik Ray (St George's University of London, UK) and colleagues published a meta-analysis of PROVE-IT, A to Z, TNT, IDEAL, and SEARCH--five trials testing high-dose statin therapy--and found a significant increase in risk of diabetes with higher doses of the lipid-lowering drugs. A meta-analysis published in the Lancet in 2010 by Dr Naveed Sattar (University of Glasgow, UK) also showed that statin therapy was associated with a 9% increased risk of diabetes.
In the present study, Culver, Ma, and colleagues analyzed data from the WHI, an analysis that included 153 840 postmenopausal women aged 50–79 years old. Information about statin use was obtained at enrollment and year three; the current analysis includes data up until 2005. At baseline, 7.0% of women were taking statins, with 30% of women taking simvastatin, 27% taking lovastatin, 22% taking pravastatin, 12.5% taking fluvastatin, and 8% taking atorvastatin. During the study period, 10 242 incident cases of diabetes were reported.
In an unadjusted risk model, statin use at baseline was associated with a 71% (95% CI 1.61–1.83) increased risk of diabetes. After adjusting for potential confounding variables, the risk of diabetes associated with statin therapy declined to 48% (95% CI 1.38–1.59). The association was observed for all types of statins.
"The association between diabetes risk and statin therapy was not observed with any one type of statin, and it seems to be a class effect," said Ma.
Subgroup Risk
A significantly increased risk of diabetes was observed in white, Hispanic, and Asian women (an increased risk of 49%, 57%, and 78%, respectively). Among African Americans, who made up 8.3% of the population studied, there was a nonsignificant 18% increased diabetes risk associated with statin use at baseline.Statin use and diabetes risk was also observed in women across a range of body mass indices (BMIs <25.0, 25.0–29.9, and >30.0 kg/m2). Women with the lowest BMI (<25.0 kg/m2), appeared to be at higher risk of diabetes compared with obese women, a finding the investigators speculate is related to phenotype or hormonal differences between the women.
In an editorial [2], Dr Kirsten Johansen (University of California, San Francisco), Editor of the Archives, noted that the increased risk of diabetes in women without CVD has "important implications for the balance of risk and benefit of statins in the setting of primary prevention in which previous meta-analyses show no benefit on all-cause mortality."
Ma agreed, noting to heartwire that statins are used with increasing frequency, including in primary prevention, and--based on the JUPITER trial--in patients with normal LDL cholesterol, but elevated C-reactive protein (>2.0 mg/L). In the present study, baseline statin therapy was associated with a significant 46% and 48% increased risk of diabetes in women with CVD and without CVD, respectively.
Just 7% of women in the WHI study were taking statins in the analysis, but today that number would be significantly higher, making the potential risk of diabetes at the population level much more widespread. Ma said that physicians need to evaluate the risk of diabetes as well as the potential benefits of statin therapy in elderly female patients, and start statins after lifestyle interventions have been attempted.
Pain and Other Symptoms Common in Patients With Diabetes
August 13, 2012 — In a large study of adults with type 2 diabetes, pain and nonpain symptoms were common among all patients, not just among those near the end of life, according to a new study.Rebecca L. Sudore, MD, from the San Francisco VA Medical Center and the Division of Geriatrics, University of California, San Francisco, and colleagues published their findings online August 2 in the Journal of General Internal Medicine.
According to the researchers, the prevalence of pain and nonpain symptoms across the age spectrum and at varying points before death or survival status in patients with type 2 diabetes has not been explored.
"To maximize quality of life, in addition to risk factor control, diabetes care management and quality improvement efforts should consider broadening efforts to include symptom palliation and palliative services for patients with type 2 diabetes across the disease course," Dr. Sudore and colleagues write.
The current study examined a sample of 13,171 adults with type 2 diabetes, aged 30 to 75 years, from Kaiser Permanente, Northern California. Patients had answered a baseline symptom survey in 2005 to 2006. The researchers sought to determine whether symptom burden differed by survival status and by age.
Survival status from baseline was categorized into alive for less than or equal to 6 months, more than 6 to 24 months, or more than 24 months.
Mean age of the patients was 60 years. Just under half (48%) were women, and 57% were white.
Of the participants, 41.8% reported having acute pain and 39.7% reported chronic pain. In addition, 24.6% had fatigue, 23.7% had neuropathy, 23.5% had depression, 24.2% had insomnia, and 15.6% had physical/emotional disability.
The proportion of patients experiencing symptoms was high regardless of which survival status category patients were in, but symptoms were most common in those with the shortest survival (P < .001).
The researchers also found that adults who were at least 60 years of age and who were alive for more than 2 years reported more physical symptoms such as acute pain and dyspnea, although the differences in pain by age were likely related to comorbid conditions. In contrast, participants younger than 60 years reported more psychosocial symptoms, such as depressed mood, fatigue, and insomnia.
"Our study suggests that patients with diabetes would also benefit from palliative care services over the disease course and in conjunction with aggressive disease management," they conclude.