د. حسين محمد جمعه
اختصاصي الامراض الباطنةالبورد العربي
كلية طب الموصل
2011
Thyroid eye disease
© 2011 BMJ Publishing Group LtdKey points
Thyroid eye disease affects 25% to 50% of patients with Graves' diseaseGraves' disease affects 1.0% to 2.7% of the UK population
Between 3% and 5% of patients with thyroid eye disease develop severe disease that threatens their sight.
Early recognition of thyroid eye disease and early referral to a specialist are crucial.
Cigarette smoking is the most important modifiable risk factor for the development of thyroid eye disease.
You should tell all patients to stop smoking. The mechanism by which smoking makes thyroid eye disease worse is unknown, although cigarette smoke extract can stimulate orbital fibroblasts to make fat and connective tissue and this could contribute to the adverse effects.
The current treatments for thyroid eye disease include:
• Steroids
• Radiotherapy to the orbit
• Surgery to decompress the orbit.
But these treatments do not work well enough. We need to develop new treatments for thyroid eye disease. Following available treatment67:
More than one half of patients have double vision
More than one third of patients are dissatisfied with the appearance of their eyes
More than one quarter have low visual acuity (20/25 vision or worse).
Clinical tips
Don't miss optic neuropathy. Ask the patient about:Blurred vision
Impaired perception of colour.
A patient with impaired perception of colour might complain that the intensity of colour perception (such as the greenness of the grass on a soccer pitch) is reduced in the affected eye or eyes. This may be more apparent if one eye is affected because the intensity of colour may be different between the two eyes.
Examine all patients for:
Reduced visual acuityA relative afferent pupillary defect (table 1)
Loss of visual fields.
It is important to refer urgently to ophthalmology a patient with thyroid eye disease with unexplained deterioration in vision, and/or awareness of any change in intensity or quality of colour vision in one or both eyes.
Table 1: Diagnosing a relative afferent pupillary defect - the swinging light test
You can perform the swinging test by shining a light into one eye and then the other and observing the results:
If the pupils are normal they should constrict each time you shine the light into them
If a relative afferent pupillary defect is present, the pupil will dilate when you shine light into it. This suggests a significant problem with the retina or optic nerve.
Background
Thyroid eye disease affects about 400 000 people in the United Kingdom. It can be an unpleasant and painful condition, and can change a person's facial appearance. Thyroid eye disease has a marked negative effect on quality of life, even many years after treatment. Occasionally it can threaten a person's sight, and unless you examine all patients for signs of optic neuropathy, a number of patients will lose their vision.Progress in the management of patients with thyroid eye disease has been limited; currently about one third of patients are disappointed with the outcome of treatment. Glucocorticoids, orbital radiotherapy, and orbital decompression are the mainstays of treatment.
Risk factors that you can change
SmokingThe main risk factor that you can change is smoking.
Thyroid eye disease is four times more common in smokers or ex-smokers than in patients who have never smoked. You should advise all smokers with Graves' disease to stop as this reduces their risk of developing eye disease. The outcome after surgery for thyroid eye disease is also worse in smokers.
Cigarette smoking also increases the risk of a worsening of ophthalmopathy after radioiodine therapy, so you should advise smokers to stop before they have this treatment.
Radioiodine
Treatment of hyperthyroidism with radioiodine may cause an increase in the inflammatory symptoms seen in thyroid eye disease, particularly if their eye disease is active.In a study of 443 patients with Graves' hyperthyroidism and slight or no thyroid eye disease, ophthalmopathy developed or worsened in 15% of patients who received radioiodine, compared with 3% of patients who received methimazole.18 The worsening was transient in 65% of these patients, but it persisted in 35%; these patients subsequently needed treatment for their eye disease. But ophthalmopathy did not occur in any of the patients who took prednisolone with radioiodine, so prednisolone may prevent worsening of ophthalmopathy after radioiodine therapy.
Sex
Females are much more likely to develop Graves' disease than males, so thyroid eye disease is more common in females. But once someone has Graves' disease, their sex has little effect on their risk.
Age
Men older than 60 years with Graves' disease may be at risk of more severe eye disease
Risk factors that you cannot change
Table 2: Features of Graves' disease
• Diffuse goitre
• Thyroid eye disease
• Pretibial myxoedema
• Thyroid acropachy
Learning bite
Antithyroglobulin and antimicrosomal antibodies are typically raised in patients with Graves' disease (but can be negative in 5% to 10%).Symptoms and signs of thyroid eye disease
Common symptoms include1:Pain
A feeling of pressure behind the eye
A gritty sensation in the eye
Double vision
Photophobia
The pain can be orbital, retro-orbital, or anterior (conjunctival or corneal). Pain is often an early feature and sometimes a presenting feature.
Common signs are:
Swelling and/or erythema of the conjunctiva (can be mistaken for conjunctivitis, and hence leads to delayed diagnosis)
Oedema and/or erythema of the eyelid
Retraction of the eyelid
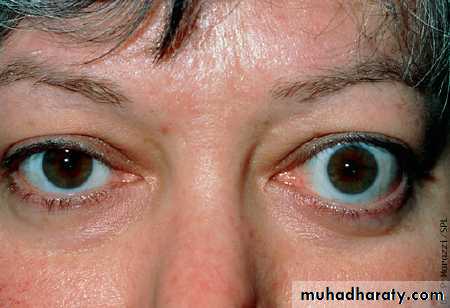
Exophthalmos
Divergent or convergent squint due to involvement of the extraocular muscles.
The extraocular muscles become involved in the acute inflammation, with invasion of macrophages and other inflammatory cells. This causes swelling and then fibrosis of the muscles. Either of these processes can stop the muscles working properly.
Scoring tools
You can measure the intensity of inflammation using the clinical activity score (table 3). You can also use this measurement to assess worsening or improvement of the disease and to help guide treatment. For example, if a patient's score worsens, the treatment may be increased (for example, by increasing the dose of steroids). As the score decreases, you can reduce the dose of steroids.Table 3: The clinical activity score
Score 1 for each item presentPain
1) Painful, oppressive feeling on or behind the globe during the last four weeks2) Pain on looking up or down or to the side during the last four weeksRedness
3) Redness of the eyelid(s)4) Diffuse redness of the conjunctiva, covering at least one quadrant
Swelling
5) Swelling of the eyelid(s)6) Chemosis (oedema of the bulbar conjunctiva)7) Swollen caruncle (the caruncle is the fleshy prominence at the inner angle of the eye)8) Increase by 2 mm or more in proptosis during a period of one to three months
Impaired function
9) Decrease in eye movements in any direction of five degrees or more during a period of one to three months10) Decrease in visual acuity (one or more lines on the Snellen chart using a pinhole) during a period of one to three months
You can classify the severity of eye changes with the NO SPECS system (table 4).
Table 4: NO SPECS classification of thyroid eye diseaseClass 0:
No signs or symptoms
Class 1:
Only signs (limited to upper lid retraction and stare, with or without lid lag)
Class 2:
Soft tissue involvement (oedema of conjunctivae and lids, conjunctival injection)
Class 3:
Proptosis
Class 4:
Extraocular muscle involvement (usually with double vision)
Class 5:
Corneal involvement, primarily due to lagophthalmos (lagophthalmos occurs when the eyelids are unable to close, usually because of forward protrusion of the eyeball)
Class 6:
Sight loss (due to optic nerve involvement)
Adapted from Mourits MP et al with permission from BMJ Publishing Group
When a patient has lid lag, the upper eyelid lags behind the cornea as the patient looks down. Lid retraction occurs when the muscles or tissues attached to the lids, or both, become shortened (due to fibrosis); the eyelids are pulled back and this causes increased exposure of the sclera.
You can measure exophthalmos objectively using an exophthalmometer. A Hertel exophthalmometer should be available in most endocrinology clinics. This is used by placing the plastic arms of the device against the lateral margin of the orbit, aligning the examiner's eye with a reference point on the angled mirror, and then reading from the mirror the point where the anterior surface of the globe is reflected.
Other scoring systems exist, including the EUGOGO recommendations aimed at generalists, specialists, and clinical researchers.
Optic neuropathy
It is important not to miss optic neuropathy. Ask the patient about:
• Blurred vision
• Impaired perception of colour.
You should remember that many patients are unaware of their loss of vision. This is why an examination by an ophthalmologist is essential, even if the patient says that they have no problems with their vision. In any case, visual acuity is not a good predictor of optic neuropathy because some patients will have normal acuity but a severely constricted visual field.
Examine all patients for:
Reduced visual acuityA relative afferent papillary defect
Visual field loss.
If you find any of these features or an unexplained deterioration in vision, and/or awareness of a change in the intensity or quality of colour vision in one or both eyes, you should refer the patient urgently to an ophthalmologist8; the shorter the duration of loss of vision, the better the chance of a good result after treatment.
Pitfalls in diagnosis
There are a number of pitfalls:Thyroid eye disease may affect only one eye
The patient may present with eye disease but no other features of Graves' disease
There may be optic neuropathy without obvious exophthalmos.
Optic neuropathy without exophthalmos can occur, and may be more common in older patients. This may occur in patients with more rigid connective tissue in the orbit that can withstand the increased pressure and so does not allow the eyeball to protrude forward.26
Thyroid eye disease typically affects patients with Graves' disease and hyperthyroidism, but it can occasionally affect those without clear features of Graves' disease, who may be euthyroid or hypothyroid.
If you are unsure of the diagnosis you should arrange the following tests:
Thyroid function tests (thyroid stimulating hormone (TSH), free T4 and T3)
Thyroid autoantibodies (antithyroid peroxidase, antithyroglobulin, and thyroid stimulating antibodies)
A radionuclide thyroid uptake scan (which shows increased uptake).
Patients who you suspect have thyroid eye disease should be referred to an ophthalmologist. Patients with unusual ocular features that may be due to thyroid eye disease should also be referred in order to establish the correct diagnosis. They may be able to help by finding features not typical of thyroid eye disease, such as a rapidly worsening clinical condition or the presence of lymphadenopathy.
A CT or MRI scan of the orbits can confirm involvement of the soft tissue and the extraocular muscles. But you need to interpret the radiological abnormalities in light of the clinical history. Occasionally, you may need to do an orbital biopsy to exclude other diseases, such as an orbital pseudotumour or a lymphoma.
What causes thyroid eye disease?
Target antigenWe don't know for sure why some patients with Graves' disease develop problems with their eyes, but it may be due to an abnormal immune response against a common autoantigen.
We don't know what this autoantigen is, but it is likely to be the TSH receptor.
Other possibilities include:
G2s, which has been identified as the terminal 141 amino acids of the winged-helix transcription factor FOXP1The flavoprotein (Fp) subunit of the mitochondrial enzyme succinate dehydrogenase
The 64 kDa protein
A non-tissue specific membrane protein called 1D
The calcium binding protein calsequestrin.
Levels of antibodies to the TSH receptor correlate directly with clinical features of thyroid eye disease, and TSH receptors are often present in orbital tissue. Even if the TSH receptor is not the autoantigen, it does appear to be involved in the disease process, as suggested by a report of thyroid eye disease becoming worse during administration of recombinant TSH.
Whatever the identity of the autoantigen, the processes involved in starting and continuing the inflammatory response are likely to be complex, with involvement of both antibody and cell-mediated responses.
Role of cellular immunity
In thyroid eye disease there is an inflammatory cellular infiltrate. This is more severe in early disease.Active thyroid eye disease coincides with:
Infiltration of monocytes into the periorbital region
Differentiation of macrophages.
There is also evidence that CD4+ gamma delta T cells may play an important role in pathogenesis. Researchers have found sensitised T lymphocytes that are specific for orbital tissue in the peripheral blood and orbit in patients with thyroid eye disease.
Role of antibodies
Levels of antibodies against the TSH receptor correlate with the clinical features of thyroid eye disease, but this may simply reflect the intensity of the autoimmune reaction. Other antibodies, such as those directed against muscles in the orbit, may also be pathogenic, but current evidence suggests that these are most likely to be secondary to cytotoxic T cell reactions.
There have been no proved episodes of thyroid eye disease in neonates born to mothers with active thyroid eye disease due to transfer of immunoglobulin G (IgG) across the placenta. This suggests that IgG antibodies are not sufficient on their own to cause active thyroid eye disease. But IgG from patients with thyroid eye disease can cause fibroblasts in their orbits to produce chemoattractants for T lymphocytes; these probably promote thyroid eye disease.
In addition, IgG from patients with Graves' disease can stimulate receptors for insulin like growth factor-I, so causing fibroblasts to make hyaluronan in the orbit.37 This suggests that receptors for insulin like growth factor-I and their activating antibodies may have a role in causing thyroid eye disease.37
Drug warning: thiazolidinediones
Peroxisome proliferator-activated receptor γ (PPAR-γ) agonists (such as the thiazolidinedione pioglitazone) stimulate orbital fat cells to grow in vitro. They also increase the expression of the orbital receptor for TSH in vitro.The thiazolidinedione pioglitazone has been found to promote eye protrusion in a subgroup of patients with type 2 diabetes. There has also been one report of a patient with thyroid eye disease that was inactive for more than two years. His symptoms got worse after he took pioglitazone. This suggests that you should not give PPAR-γ agonists to patients with thyroid eye disease
How do I treat it?
You should refer all patients with thyroid eye disease to a specialist with experience in its diagnosis and management. Ideally, patients should attend a combined clinic where they can see an endocrinologist and ophthalmologist. You should refer patients promptly because treatment is more likely to be effective while the ocular tissue is acutely inflamed.Most patients experience mild disease that needs only simple treatments, such as:
Artificial tearsAdvice to avoid dust
Advice to sleep propped up in bed.
You should refer all smokers for counselling to help them stop.36
Between 10% and 35% of patients need medical treatment for thyroid eye disease.41-43 An ophthalmologist should decide on the need for this treatment. The decision should be tailored to the individual patient and should take into account multiple factors, such as the:Activity of the disease
Threat to vision
Possible harms and benefits of treatment.
You should also involve the patient in the treatment decision.
Steroids
Observational studies suggest that steroids dampen down the acute inflammation in patients with thyroid eye disease (rates of response to steroids in patients with thyroid eye disease range from 33% to 66%). Reducing the extent of the acute inflammation reduces the severity of the symptoms experienced while the eye disease is active, such as eye pain. In addition, if you can limit the acute inflammation, you may be able to limit the severity of the symptoms that are due to post-inflammatory scarring (such as double vision).
It is not clear to what degree steroids improve symptoms; they may just shorten the time to recovery without improving the final outcome.
Steroids can cause serious side effects. You should use high doses initially, and then titrate the dose against the response, keeping it to the lowest effective dose for as short a time as possible (a specialist should determine this). You should not use steroids as long term treatment.
A typical starting regimen is prednisolone at a dose of 60 mg to 100 mg for seven to 14 days, followed by a dose reduction over several months.
Steroids cause systemic side effects, and they can also cause side effects that specifically affect the eyes. These include:
• Cataracts
• Glaucoma
• Thinning of the cornea and sclera
• Increased susceptibility to infections affecting the eye.
High dose intravenous steroids may be as effective and cause fewer side effects than high dose oral steroids. However, there have been a number of reports of severe and even fatal hepatitis occurring in patients receiving intravenous steroids for thyroid eye disease, although the risk of hepatic damage appears to be low if cumulative doses are kept below 8 g.
Many centres use oral steroids, only using high dose intravenous steroids in patients with severe ophthalmopathy. There has recently been more of a trend towards using intravenous steroids, as studies suggest that this treatment may be more effective and better tolerated than the oral route. Caution is still required, with low cumulative doses and careful monitoring of liver function recommended. You should consider starting patients taking steroids on treatments that may preserve bone density, such as bisphosphonates.
Radiotherapy to the orbits
The role of orbital radiotherapy is uncertain. Early studies that suggested it is effective were retrospective and uncontrolled. Recent placebo controlled trials have showed little or no improvement in symptoms or signs following radiotherapy.A study by Mourits et al in 2000 found that the only symptom which improved in the group receiving radiotherapy, as opposed to placebo, was up gaze (+4.9 degrees).52
All other variables remained unchanged (including down gaze, abduction, adduction, aperture of the eyelid, swelling of the eyelid, and proptosis). They concluded that in patients with moderately severe thyroid eye disease, you should use radiotherapy only to treat impairment of eye movements.
Studies by Gorman et al, which used each patient's other eye as an internal control, did not show any beneficial effect. This study attracted criticism because it included 19 patients (out of a total of 42) who had previously received corticosteroids. Also, the low dose of radiotherapy received by the other orbit may have had some positive influence on this internal control, thereby reducing any observed effect of treatment.
A study by Prummel et al looking at patients with mild thyroid eye disease found an increase in the motility of the eye (+6 degrees in downwards gaze) in patients receiving radiotherapy.54 Patients had less double vision and needed less immunosuppression. There was no effect on gaze in any other direction and no effect on exophthalmos, lid aperture, or clinical activity score.
In spite of this, radiotherapy does not prevent progression of the disease or improve the patient's quality of life.
Side effects of radiotherapy include:
Cataracts (12% of patients have cataracts after a median of 11 years)
Radiation retinopathy (usually, but not always, as a result of using wrong doses or wrong targeting)
Malignancy (calculated risk of 1.2% for malignancy and 0.7% risk of developing fatal cancer
There have been no reported cases of malignancy after orbital radiotherapy for thyroid eye disease. However, in the light of the calculated malignancy risk, you should probably only give this treatment to older patients.
You should not give radiotherapy to patients with diabetes who have pre-existing retinopathy.62
Ocular surgery
Surgery is an important part of the treatment of thyroid eye disease, particularly:When the disease is sight threatening and unresponsive to other treatments
For functional and cosmetic reasons once the disease has "burnt out."
Urgent surgery is the traditional treatment of choice for patients with optic neuropathy (although IV pulsed steroid treatment may be appropriate for some patients64).
You should refer all patients with thyroid eye disease to an ophthalmologist. If the ophthalmologist is unable to deal with the problem, they can refer the patient to a specialist oculoplastic surgeon.
Surgery attempts to:
Shrink swollen tissues or
Expand the volume of the orbit.
There are no prospective randomised controlled trials of surgery for thyroid eye disease. Many of the reports are case series. There is one comparative study (but patients were not randomised).
Untreated thyroid eye disease may improve spontaneously, so the conclusions we can draw from these uncontrolled studies are limited.
Surgical approach
Surgical outcomes are likely to be better at centres that have extensive experience in treating thyroid eye disease.Surgeons can use a number of techniques. Most involve making a hole in one or more walls of the orbit. Surgeons usually operate on the medial and inferior walls, although they sometimes do a balanced decompression of the medial and lateral walls. Another type of "decompression" removes fat without any removal of bone.
There are lots of other approaches, including transantral, lateral, transcaruncular, and endoscopic (transnasal). There is limited evidence that healing is faster and length of stay in hospital shorter with the endoscopic approach, and complications are fewer.
Effective decompression reduces proptosis and usually improves keratopathy and papilloedema. When optic neuropathy is present, the aim of decompression is to relieve the pressure on the nerve and so preserve vision.
One of the largest series to date, describing the outcome following transantral decompression in 428 patients, reported:
Average reduction in proptosis of 4.7 mm
Scotomas improving or resolving in 91% of eyes tested
Papilloedema resolving or improving in 94%
Exposure keratitis improving or resolving in 92%.
However, surgical complications are common and include:
Double vision (64%)Entropion of the lower eyelid (9%)
Numb lip (5%)
Sinusitis (4%)
Leaks of cerebrospinal fluid (4%)
One frontal lobe haematoma
Surgery on the medial and lateral walls may cause less double vision.
There are less data on the outcome following endoscopic decompression, but results from relatively small series are encouraging (for example, a mean decrease in proptosis of 3.3 mm, with 22% of patients developing double vision postoperatively). Because of the high rates of double vision following decompression, up to 70% of patients need further surgery to repair a squint.
Although decompression improves proptosis, there is often a need to do surgery on the eyelid at a later date. But if you do the decompression properly, you may be able to avoid surgery on the eyelid. The patients may need surgery to the eyelid to treat other problems, such as retraction of the upper eyelid and entropion.
Botulinum toxin
An injection can lower the upper eyelid in the short term in patients awaiting surgery. In one trial, botulinum toxin relieved symptoms and improved the appearance of the eye. However, the effect of treatment was difficult to predict and carried a risk of transient double vision.Recessions are the most frequently performed procedure for patients with double vision. A recession involves cutting the attachment of a muscle and reattaching it at a different point. For example, recession of the inferior rectus muscle involves cutting it and suturing it further back on the eyeball to allow the patient to look up (when previously the fibrotic and shortened muscle may have prevented this).
Patients who need both medial and inferior recessions should have the recessions done in separate sessions, starting with the horizontal muscle(s). But some surgeons say that they can do a recession of both sets of muscles in the same session.66
Thyroidectomy
Total thyroidectomy may have a positive influence on the course of thyroid eye disease. Retrospective, uncontrolled series support this view. Again, the conclusions that can be drawn from these reports are limited.For the treatment of hyperthyroidism, subtotal thyroidectomy and medical therapy with methimazole are both less likely than radioiodine to cause a flare in thyroid eye disease.
Current evidence suggests that subtotal or total thyroidectomy is unlikely to worsen thyroid eye disease. But you should not expect it to have a clinically significant beneficial effect on thyroid eye disease.
You should also advise patients on the other side effects of thyroidectomy, such as hypoparathyroidism and recurrent laryngeal nerve palsy. Recurrent laryngeal nerve palsy occurs in 1% to 2% of patients.
The future
Octreotide analogues showed early promise in small or unrandomised reports, but recent randomised controlled trials have not shown a clinical benefit.98 99Many cytokines are involved in thyroid eye disease. Interleukin 1 stimulates orbital fibroblasts to produce glycosaminoglycans in vitro; antagonists of interleukin 1 can block this effect. Agents that modulate the effects of certain cytokines, such as interleukin 1 or tumour necrosis factor α, may become a viable treatment.
There has been a pilot clinical trial of using a tumour necrosis factor α antagonist (etanercept) to treat thyroid eye disease. There appeared to be a reduction in severity of the eye disease during treatment and a worsening of severity after stopping treatment. But this study was uncontrolled, and the number of participants involved was too small to draw conclusions on effectiveness or safety.
Rituximab, an anti-CD20 antibody that causes a depletion of circulating B cells, has also been tried in thyroid eye disease, with case reports showing a beneficial effect on some aspects of the disease. Despite this success, adverse effects such as infusion reactions, and also development of ulcerative colitis and arthritis requiring steroids have been reported.
Randomised controlled trials are needed before conclusions can be drawn.
Other agents currently under investigation include:Triamcinolone, administered as a periocular injection
Antioxidants
Pentoxifylline.
These have all shown some promise in early studies, but these studies need to be repeated in larger controlled trials.
PPAR-γ agonists (such as the thiazolidinediones) may promote adipogenesis in thyroid eye disease, so it may be that antagonists of PPAR-γ may help thyroid eye disease. But any such agent may cause adverse metabolic effects that might limit its usefulness.
Learning bite
When patients with hyperthyroidism present with unusual symptoms, think about side effects of drugs. Carbimazole is a well known cause of neutropenia. Propylthiouracil can also cause neutropenia, but carbimazole is a more common cause of this side effect.Abnormal thyroid function tests despite a well patient - a guide to management
IntroductionLaboratory diagnosis of thyroid disorders has been around for decades. Initial laboratory assessment of suspected thyroid dysfunction usually includes measuring serum levels of: TSH, Free T4.
The combined results of these tests are then used to classify thyroid function into one of three major categories1:
Hyperthyroid (excess levels of free T4 and a depressed TSH level (<0.45 mIU/l))
Hypothyroid (deficient levels of free T4 and an elevated TSH level (>10.0 mIU/l))
Euthyroid (normal levels of free T4 and an TSH level between 0.45 mIU/l and 4.5 mIU/l).
Occasionally screening laboratory studies show an abnormal sTSH level but a normal free T3 and free T4 level. When this occurs the clinical condition is termed subclinical thyroid disease.
1. Does this laboratory diagnosis represent a disease?
Thirty years ago, before sTSH was measurable in the laboratory, a patient with a normal free T3 and free T4 level would have been considered euthyroid, even if they had signs or symptoms of thyroid disease (hyperthyroidism or hypothyroidism). So we need to expand the question: are we dealing with a clinical entity in its early stages, or is this a phenomenon of advancing technology (and no disease)?The diagnosis of subclinical thyroid disease raises three questions that need further study.
2. If subclinical thyroid disease is a real entity (ie the result of a physiological dysfunction) does it do harm?
If so, are definable conditions associated with the dysfunction that can adversely affect a person's health? Much ongoing research is trying to answer these questions by looking at6
Psychiatric and psychological disease (depression and mood disorders)
Neurological disease (cognitive impairment)Metabolic disease (osteoporosis and hyperlipidaemia)
Cardiovascular disease (atrial fibrillation, congestive heart failure, and hypertension) and premature mortality.
3. If subclinical thyroid disease does represent a real clinical entity and there are definable abnormalities associated with it that affect morbidity and mortality, does therapeutic intervention alter the course of the disease?
This question is the most difficult to answer.
How is subclinical thyroid disease defined?
Subclinical thyroid disease is divided into two entities based on the sTSH level:When the sTSH concentration is below normal reference range (<0.45 mIU/l), in association with normal free T4 levels, this constitutes subclinical hyperthyroidism
When the sTSH concentration is above the reference range (>4.5 mIU/l), in association with normal free T4 levels, this constitutes subclinical hypothyroidism.
Both diagnoses assume a well patient. An sTSH level <0.1 mIU/l or >10.0 mIU/l represents overt disease - hyperthyroidism or hypothyroidism, respectively.2
Remember that laboratory error can indicate subclinical thyroid disease. It is always prudent to repeat the sTSH test in two to 12 weeks before establishing the diagnosis and considering therapy.
Important - sick euthyroid syndrome is a different entity to subclinical thyroid disease
What is sick euthyroid syndrome?Serious illness has been shown to affect laboratory tests for thyroid function but there is no clear evidence that this reflects a disease state. Because there does not appear to be any direct adverse effect from these changes on the overall clinical condition of the patient, this situation has become known as the "sick euthyroid syndrome" or "sick euthyroidism."The generally accepted explanation for abnormal thyroid tests associated with sick euthyroid syndrome is impaired protein metabolism resulting from severe physiological stress.
In broad general terms sick euthyroid syndrome is of academic interest only and does not have direct bearing on the clinical course of the patient. However, several studies have looked at the predictive value of abnormal thyroid function tests when applied to survival outcomes in patients seriously ill with non-thyroid diseases. In these studies, mortality was predicted based on the level of circulating T4, independent of other thyroid parameters. If the serum T4 level was <3.1 g/dl, the predictive value for mortality had a sensitivity value of 75% and a specificity value of 80%.
Generally you should avoid doing thyroid function tests in patients who have coexisting acute illnesses such as pneumonia. This is because the thyroid function tests may be abnormal despite the thyroid gland working normally.
In non-thyroidal illness there is reduced conversion of T4 to T3 with a subsequent rise in serum T4 and fall in serum T3, often to the low-normal range.
What are the causes of subclinical hyperthyroidism?
It is important to ascertain the possible causes of subclinical hyperthyroidism, as this can affect management.The pituitary thyrotrophs, which produce TSH, respond to minor elevations of free T4 and free T3, even if these elevations are within the normal reference range, thus potentially decreasing, or even stopping, production and secretion of TSH.
Other common causes are:
Toxic multinodular goitre (Plummer's disease)Autonomous functioning nodule or a solitary toxic nodule
Excessive use of exogenous levothyroxine, whether iatrogenic or factitious.
Statistically, early Graves' disease is the most common cause for subclinical hyperthyroidism. If followed longitudinally some people will go on to develop overt disease with manifest thyrotoxicosis, while some people will remit spontaneously.
Toxic goitre and autonomously functioning nodule are second and third on the list of causes of subclinical hyperthyroidism. Over time these entities will cause signs and symptoms of thyrotoxicosis and are easily identified by radioactive iodine (I131) uptake and scan.
It is possible to find a depressed sTSH level and normal free T3 and free T4 levels in people with delayed pituitary recovery following treatment of thyrotoxicosis. Pregnancy, sick euthyroid syndrome, and medications (dopamine, glucocorticoids, and dobutamine) can also produce similar laboratory values. Although not common, another possible cause is central hypothyroidism secondary to pituitary failure. In these rare cases, following the patient over time will show a decline in circulating free T3 and free T4 levels and bring to light other deficiencies consistent with pituitary failure (hypogonadism and hypoadrenalism).
A careful history of current and past medications, menstrual pattern, and recent illness can generally exclude benign processes (for example pregnancy or medications). In these people all that is needed is a repeat test for sTSH, free T3, and free T4 level in one year's time.
Fast progression of subclinical hyperthyroidism to overt disease is rare.2 3 But the physiological stress resulting from thyrotoxicosis, especially in older people, is such that it is advisable to repeat the sTSH, free T3, and free T4 test within two weeks.20 If the patient is young and the repeat studies produce the same results, an examination at three and six months is sufficient.19 If the patient continues to be asymptomatic and laboratory studies are unchanged, annual visits are sufficient. Older people need closer follow up because of the increased incidence of atrial fibrillation associated with subclinical hyperthyroidism.10
Clinical implications of subclinical hyperthyroidism
Data showing adverse consequences from subclinical hyperthyroidism are less definitive than those associated with subclinical hypothyroidism. But there is good evidence that subclinical hyperthyroidism is associated with adverse eventsThe US Framingham study found an increased relative risk of 3:1 for the development of atrial fibrillation in patients with subclinical hyperthyroidism.10 Other studies have shown an increase in mortality from all causes3
Studies showing accelerated bone loss in women with hypothyroidism on replacement therapy with levothyroxine, whose laboratory findings are consistent with subclinical hyperthyroidism, are quoted as circumstantial evidence of similar outcomes in women with non-iatrogenic subclinical hyperthyroidism. There are no clinical data to support this supposition.7
Managing subclinical hyperthyroidism
The primary clinical entity that has been associated with subclinical hyperthyroidism is paroxysmal atrial fibrillation. This was identified in 1994, but the most appropriate therapeutic intervention to address this has not been clearly defined. Options might include beta blockers, antithyroid drugs, and thyroid ablation. There are no studies that show these interventions decrease the incidence of paroxysmal atrial fibrillation.Therapeutic intervention for subclinical hyperthyroidism will depend on the cause.
If Graves' disease is suspected as the cause of subclinical hyperthyroidism, a TSH receptor stimulator antibody level should be obtained. As long as the patient remains asymptomatic, intervention is not indicated. This is because Graves' disease can undergo spontaneous remission and, even when the disease is progressive, it could be years before symptoms occur. With early Graves' disease careful follow up is the therapeutic intervention of choice20Early toxic multinodular goitre (Plummer's disease) or toxic nodule can present as subclinical hyperthyroid disease. While close follow up is one course of action, this process is usually persistent, if not progressive, and has associated morbidity. Careful consideration of early intervention is advised. A radio isotope uptake scan can quickly help delineate this as the cause
The other two major causes of subclinical hyperthyroidism are overtreated hypothyroidism and factitious disease. Treatment of choice for both is reduction or discontinuation of levothyroxine, even when free T3 and free T4 levels are within normal ranges. This will result in the sTSH level returning to the normal range within four to eight weeks
Causes of subclinical hypothyroidism
Once you have excluded laboratory error the most common cause of subclinical hypothyroid disorder is early phase chronic autoimmune thyroiditis. In this disorder the serum levels of free T4 fall. Eventually the patient will develop the clinical symptoms of overt hypothyroidism such as fatigue, weight gain, feeling cold, amenorrhoea, dry skin, and thinning hair.Chronic autoimmune thyroiditis is found more often in women than men (5:1). It is most common in women older than 50. It progresses to overt disease at a rate of about 5% per year.3 Other entities that can present as subclinical hypothyroidism include:
Protracted recovery phase from acute thyroiditis
Insufficient replacement of levothyroxine in people with hypothyroidism.
Once you have confirmed the diagnosis with repeat sTSH tests, you should do follow up laboratory tests every three to 12 months. After one year if the values normalise or do not change an annual follow up is appropriate if the patient has no symptoms. Because chronic autoimmune thyroiditis is common in older women and a large number of patients with chronic autoimmune thyroiditis do progress to overt disease, additional studies including tests for antithyroid peroxidase antibodies may be indicated to identify this subgroup. You can identify patients in a protracted recovery phase of acute thyroiditis by repeat studies at the three to six month interval.
Clinical implications of subclinical hypothyroidism
Studies continue to be published supporting the premise that subclinical hypothyroidism is associated with a number of clinical conditions, not least progression to overt hypothyroidism4:There is evidence that subclinical hypothyroidism is associated with elevations in total cholesterol levels as well as low density lipoprotein cholesterol levels
Separate data have shown that women with subclinical hypothyroidism have a higher incidence of diastolic hypertension and hypertriglyceridaemia12
Mya et al showed the triad of increased prevalence of dyslipidaemia, coronary artery disease, and peripheral arterial disease in older people with subclinical hypothyroidism.
In 2005 Walsh et al published the results of a cross sectional study of 2108 archived serum samples from 1981 that showed a significantly higher prevalence of coronary heart disease in patients with subclinical hypothyroidism compared with euthyroid patients.
When looked at longitudinally the same study showed that the risk of coronary heart disease continued to be increased even after adjusting for standard cardiovascular risk factors. Patients with subclinical hyperthyroidism did not have this increased risk.9
A more recent study from the Netherlands (which has not yet come out in print) involving 11 554 individuals between 45 and 79 years old suggests thyroid status is not statistically associated with (significant) risk of future coronary heart disease or all cause mortality. This study was only directed toward risk and in its conclusions did not elucidate whether the individual had hyper- or hypothyroid dysfunction.
Another study published in 2005 showed an increased risk of congestive heart failure independent of other cardiovascular events and mortality in patients with an sTSH level ≥7.0 mIU/l11
Data from Japan indicate that subclinical hypothyroidism is associated with ischaemic heart disease and might affect mortality in men.
There have been few studies to determine the incidence of psychiatric disorders in people with subclinical thyroid disease:
One UK study showed people with subclinical hypothyroidism had an impaired perception of their health status when compared with test scores of health status perception of normative populations24
A more recent study, also from the UK, found no evidence to support a hypothesis that subclinical hyperthyroidism or hypothyroidism was associated with depression, anxiety, or cognitive dysfunction.6
Managing subclinical hypothyroidism
Guidelines by the British Thyroid Association state: "There is no evidence to support the benefit of routine early treatment with T4 in non-pregnant patients with a serum TSH between 4.5 and 10 mIU/l. Physicians may wish to consider the suitability of a therapeutic trial of T4 on an individual patient basis - for example patients with goitre or patients who plan to become pregnant."4 Treatment is generally not indicated in elderly patients and patients with underlying cardiac disease with aTSH of 4.5-10 mIU/l.Close follow up is required and treatment instituted should the patient become symptomatic. The American Association of Clinical Endocrinologists, however, disagrees - by recommending treatment for patients with an sTSH level >5.0 mIU/l who have positive antithyroid peroxidase antibodies.
Recent studies have shown the adjustment in levothyroxine dosing required to maintain a euthyroid state during pregnancy varies based on aetiology. Pregnant patients whose hypothyroidism is due to treated Graves' disease require the largest increase in dose, ranging between 27% to 51% depending on which trimester. Primary hypothyroidism (most likely Hashimoto's thyroiditis) requires the next largest increase, ranging between 13% and 26% depending on which trimester.
And lastly, patients whose hypothyroidism is due to treated thyroid cancer required the lowest adjustment, ranging between 9% and 26% depending on which trimester. The precise reason there is this much variability is as yet unknown.
Recommendations for screening patients
Guidelines published in July 2006 by the British Thyroid Association state: "Screening for thyroid dysfunction in a healthy adult population is not warranted." The guidelines go on to state that: "[Screening] in women at the menopause or if visiting a doctor in primary care with non-specific symptoms may be justified in view of the high prevalence of mild thyroid failure."While there are good scientific data to show an increased risk of paroxysmal atrial fibrillation in patients with subclinical hyperthyroidism, there are not sufficient data to show that treating this condition is beneficial in preventing paroxysmal atrial fibrillation and its potentially serious complications. except in the most unusual circumstances (patient with congestive heart failure New York Heart Association class 3 or class 4 or recurrent paroxysmal atrial fibrillation with embolic stroke
No authority recommends therapeutic intervention in all patients with subclinical thyroid disease based on laboratory testing alone.
Abnormal thyroid tests in critically ill patients have been shown to be predictive of mortality. When used alone, a free T4 level <3.1 µg/dl has a 75% sensitivity and 80% specificity for predicting death. When a free T4 level <3.1 µg/dl is combined with an 8 am serum cortisol level >30 µg/dl, sensitivity for predicting death rises to 100% and specificity to 100%. The predictive value for death from these two tests is better than the currently used APACHE II scoring system.
40 year old woman complains of gritty eyes. Her free T3 is elevated and her TSH is undetectable.
The most likely diagnosis is Graves' disease. Ophthalmopathy is not found in association with toxic multinodular goitre and the patient would be expected to have physical findings consistent with a goitre. It would be unusual (although not impossible) for the TSH to be undetectable with toxic multinodular goitre.
Although there are some data to support use of thyroxine replacement in subclinical hypothyroidism, no national or international groups advocate for this in an asymptomatic person.If she is trying to conceive, treatment with thyroxine is indicated to decrease the risk of fetal loss and stillbirth.
Thyroxine should be given to normalise serum thyroid stimulating hormone if the woman is thinking about becoming pregnant
علمني المطركيف اغسل همومي واحزانيوكيف اجدد حياتي كما تغسل قطرات المطراوراق الشجروتعيد لها الحياة