د. حسين محمد جمعه
اختصاصي الامراض الباطنةالبورد العربي
كلية طب الموصل
2012
Management of acute upper gastrointestinal bleeding:summary of NICE guidance
BMJ 13 June 2012Acute upper gastrointestinal bleeding is the commonest medical emergency managed by gastroenterologists in the United Kingdom. The most frequently identified source of bleeding is peptic ulcer disease, but other important causes exist, particularly oesophageal or gastric varices, which are classically associated
with more severe bleeding. A large audit in the UK in 2007 indicated that the rate of mortality from acute upper gastrointestinal bleeding (about 7%) has not changed much over the past 50 years, and thatservice provision varies considerably across the UK.
Recommendations
NICE recommendations are based on systematic reviews of the best available evidence and explicit consideration of cost effectiveness. When minimal evidence is available, recommendations are based on the Guideline Development Group’s experience and opinion of what constitutes good practice.Evidence levels for the recommendations are given in italic in square brackets.
Risk assessment
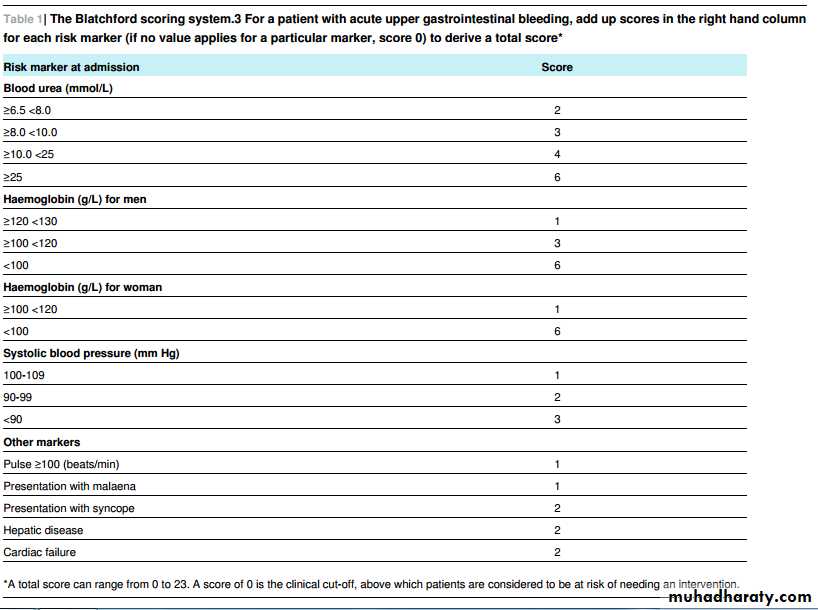
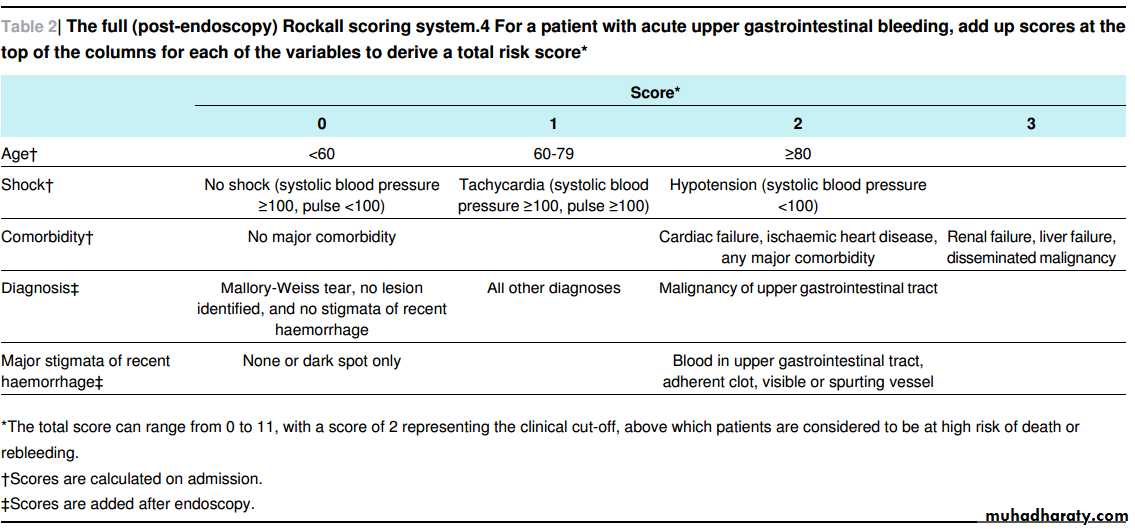
At presentation with acute upper gastrointestinal bleeding, assess for risk of serious adverse events or need for intervention. To do this use the following formal risk assessment scoring systems for all patients with acute gastrointestinal bleeding: the Blatchford scoring system at first assessment and the full Rockall scoring system after endoscopy (tables 1⇓ and 2⇓).
[Based on low to very low quality evidence from prospective and retrospective case reviews]
Resuscitation and initial management
Patients with massive bleedingTransfuse with blood, platelets, and clotting factors in line with local protocols for managing massive bleeding.
[Based on the experience and opinion of the GDG]
Blood products
Base decisions on blood transfusion on the full clinicalpicture, recognising that overtransfusion may be as
damaging as undertransfusion. [Based on very low quality evidence from a randomised control trial and observational studies and on the experience and opinion of the Guideline Development Group (GDG)]
Do not offer platelet transfusion to patients who are not actively bleeding and who are haemodynamically stable.
[Based on the experience and opinion of the GDG]
Offer platelet transfusion to patients who are actively
bleeding and have a platelet count of <50×109/L. [Based on the experience and opinion of the GDG]
Offer fresh frozen plasma to patients who have (a) afibrinogen concentration <1 g/L or (b) a prothrombin time (international normalised ratio) or activated partial thromboplastin time that is more than 1.5 times the normal
level.
[Based on the experience and opinion of the GDG]
Do not use recombinant factor VIIa except when all other methods have failed.
[Based on high to low quality evidence from randomised controlled studies].
Patients who are taking warfarin
Offer prothrombin complex concentrate to patients who are taking warfarin and are actively bleeding.
[Based onthe experience and opinion of the GDG]
Treat patients who are taking warfarin and whose upper gastrointestinal bleeding has stopped in line with local warfarin protocols.
[Based on the experience and opinion of the GDG]
Timing of endoscopy
Offer endoscopy to unstable patients with severe acuteupper gastrointestinal bleeding immediately after
resuscitation.
[Based on the experience and opinion of the GDG]
Offer endoscopy within 24 hours of admission to all otherpatients with upper gastrointestinal bleeding.
Units seeing more than 330 cases a year should offer daily endoscopy lists; units seeing fewer than this should arrange their service according to local circumstances. [Based onvery low quality evidence from randomised controlled
trials, one cost effectiveness study with minor limitations
and partial applicability, an original economic model with
potentially seriouslimitations and direct applicability, and
on the experience and opinion of the GDG]
Management of non-variceal bleeding
Proton pump inhibitorsDo not offer acid suppression drugs(proton pump inhibitors or H2 receptor antagonists) before endoscopy to patients with suspected non-variceal upper gastrointestinal bleeding.
[Based on moderate to low quality evidence from
randomised controlled studies and economic evidence with
direct to partial applicability and minor to potentially
serious limitations]
Offer proton pump inhibitors to patients with non-variceal upper gastrointestinal bleeding and stigmata of recent haemorrhage shown at endoscopy. [Based on moderate to very low quality evidence from randomised controlled studies and economic evidence with direct applicability and minor serious limitations]
Endoscopic treatment
Do not use adrenaline as monotherapy for the endoscopic treatment of non-variceal upper gastrointestinal bleeding.
[Based on low to very low quality evidence from randomised controlled studies]
For the endoscopic treatment of non-variceal upper
gastrointestinal bleeding, use one of the following
[Based on low to very low evidence from randomised controlled
studies and the experience and opinion of the GDG]:
-A mechanical method—for example, clips with or without adrenaline
-Thermal coagulation with adrenaline
-Fibrin or thrombin with adrenaline.
Unstable patients who rebleed after endoscopic
Treatment:Offer interventional radiology; if this is not promptly available, refer urgently for surgery. [Based on very low quality evidence from observational studies and the
experience and opinion of the GDG]
Management of variceal upper gastrointestinal
bleedingTerlipressin :Offer terlipressin, a vasopressin analogue, to patients with
suspected variceal bleeding at presentation. Stop treatment after definitive haemostasis has been achieved or after five days, unless there is another indication for its use, such as renal failure.
[Based on very low to moderate quality evidence from randomised controlled studies and on economic evidence of direct applicability and minor limitations]
At the time of publication (June 2012),
terlipressin was indicated for the treatment of bleeding from oesophageal varices, with a maximum duration of treatment of 72 hours. Prescribers should consult the relevant summary of product characteristics and obtain and
document informed consent for off-label use.
Antibiotics
Offer prophylactic antibiotic treatment at presentation to patients with suspected or confirmed variceal bleeding.[Based on low to very low quality evidence from randomised controlled studies and on economic evidence of partial applicability and potentially serious limitations]
Oesophageal varices
Use band ligation in patients with bleeding fromoesophageal varices.
[Based on moderate to very low quality evidence from randomised controlled trials and on economic evidence of partial applicability and potentially serious limitations]
Consider using transjugular intrahepatic portosystemic shunts if oesophageal variceal bleeding is not controlled by band ligation.
[Based on the experience and opinion of the GDG]
Gastric varices
Offer endoscopic injection of N-butyl-2-cyanoacrylate topatients with bleeding from gastric varices.
[Based onmoderate to very low quality evidence from randomised controlled trials and on economic evidence of partial applicability and potentially serious limitations]
Offer transjugular intrahepatic portosystemic shunts if bleeding from gastric varices is not controlled by endoscopic injection of N-butyl-2-cyanoacrylate.
[Based on the experience and opinion of the GDG]
Primary prophylaxis in acutely ill patients
Offer acid suppression treatment (H2 receptor antagonists or proton pump inhibitors) for primary prevention of upper gastrointestinal bleeding in acutely ill patients admitted to critical care. If possible, use the oral form of the drug.
[Based on low to very low quality evidence from randomised controlled studies and on economic evidence of partial applicability and potentially serious limitations]
Review the ongoing need for acid suppression drugs for primary prevention of upper gastrointestinal bleeding in acutely ill patients when they recover or are discharged
from critical care.
[Based on the experience and opinion of the GDG]
Control of bleeding and prevention of
re-bleeding in patients taking NSAIDS, aspirin,or clopidogrel
A substantial proportion of acute peptic ulcer bleeds occur in patients taking non-steroidal anti-inflammatory drugs(NSAIDs), aspirin, or clopidogrel. In patients with upper gastrointestinal bleeding who are taking these drugs:
Continue low dose aspirin for secondary prevention of vascular events once haemostasis has been achieved.
[Based on high to moderate quality evidence from one randomised controlled study]
Stop other non-steroidal anti-inflammatory drugs(including cyclo-oxygenase-2 inhibitors) during the acute phase of bleeding.
[Based on the experience and opinion of the GDG]
Discuss the risks and benefits of continuing clopidogrel
(or any other thienopyridine antiplatelet agents) with the appropriate specialist (for example, a cardiologist or astroke specialist) and with the patient.
[Based on the experience and opinion of the GDG]
Overcoming barriers
Some recommendations in this guideline may pose difficulties forsome services, in particular doing endoscopy within 24 hours for any patient presenting with acute gastrointestinal bleeding
(sooner if the patient is unstable). The health economic model devised to inform this recommendation showed that, for units treating a large number of acute upper gastrointestinal bleeds annually, it was highly likely that providing daily endoscopy lists would reduce length of the hospital stay and that this reduction would offset the cost of additional staffing for weekend lists.
The cost effectiveness of weekend lists in smaller
units is less certain, and this strategy of providing endoscopy could not be firmly recommended in smaller units on health economic grounds. The GDG therefore stipulated that all patients should receive endoscopy within 24 hours without specifying how smaller unitsshould achieve this; the possibilityof network arrangements may offer one potential mechanism for smaller providers.