د. حسين محمد جمعه
اختصاصي الامراض الباطنةالبورد العربي
كلية طب الموصل
2012
Statins: established indications andcontroversial subgroups
Heart 2008Statins are, to date, the most powerful cholesterol
lowering drugs. In brief, they inhibit 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase,
which is a key enzyme in the initial chain of
the steroid synthesis of cholesterol. As a response
to the decreased synthesis of cholesterol in the
liver, the low density lipoprotein (LDL) receptors
are stimulated and the result is an increased
clearance of LDL cholesterol (LDL-C) accompanied
by its decreasing blood concentrations.
Akira Endo and Masao Kuroda from Japan are acknowledged pioneers of the research into inhibitors of HMGCoA reductase and their work dates back as early as 1971. Despite more than 35 years of research, statins still remain a Pandora’s box for physicians, both in regard to their exact mode of action as well as clinical efficacy.
Bearing in mind the skyrocketing social and economic burden of cardiovascular disease worldwide, one can easily understand why statins are now more appealing than ever, but also feared because of the high total
prescription costs accompanying the broadening
indication area.
This paper presents the unanimously accepted
evidence with regard to statins, and then discusses
more controversial topics such as categories of
patients in whom statin use still remains unclear
STATINS ARE EFFICIENT IN WIDE GROUPS OF
PATIENTSStatins are first choice medication for reducing
LDL-C values, and clinical trials have demonstrated
beyond doubt that lowering LDL-C with statins
considerably diminishes the risk for cardiovascular
disease in a wide range of patients. The first large
clinical study to show truly significant beneficial
effects of statins was the Scandinavian Simvastatin
Survival Study (4S) published in 1994.
total of 4444 high risk patients with documented coronary heart disease (CHD) and high baseline cholesterol were randomised to double blind treatment with simvastatin or placebo and followed for a median of 5.4 years. Simvastatin produced mean changes of total cholesterol (TC), LDL-C and high density lipoprotein cholesterol (HDL-C) of
-25%, -35% and +8%, respectively, and notable adverse events were rare (there was one case of rhabdomyolysis).
Enzymatic creatine kinase, aspartate aminotransferase
and alanine aminotransferase increases above the upper normal limit occurred in a similar manner in both simvastatin and placebo groups.Statin treatment reduced the overall risk of death by 30%, the risk of coronary death by 42%, and the
risk of major coronary events by 34%. Other benefits of treatment included a 37% reduction in the risk of undergoing myocardial revascularisation procedures.
One year later, the West Of Scotland Coronary
Prevention Study Group (WOSCOPS)2 results werepublished, this time from patients with high baseline cholesterol and risk profile but without overt CHD. A total of 6595 men <65 years old were randomised to receive pravastatin or placebo, and were followed for an average period of 4.9 years.
Pravastatin lowered TC by 20% and LDL-C by 26%, and increased HDL-C by 5%.
Pravastatin reduced the overall risk of death by22%, the risk of definite non-fatal myocardial
infarction by 31%, the risk of death from definite
CHD by 28%, and the risk of death from all
cardiovascular causes by 32%.
Another step further was made in 1998 when two studies, Cholesterol and Recurrent Events (CARE) and Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID), demonstrated the beneficial effect of statins in patients with CHD but with average cholesterol concentrations.
Similar relative risk reductions with regard to major CHD events were observed with again amild side effect profile.
Around the same time results from two angiographic
trials were published: the Regression
Growth Evaluation Statin Study (REGRESS)5 and
the Multicentre Anti-Atheroma Study (MAAS).
They showed a slowing down of the coronary
atheroma progression but, on average, no complete
cessation or net regression, thereby providing an
indication that a complete abolishment of clinical
events was not to be expected.
Thus, in patients with established CHD, almost
regardless of their lipoprotein values, as well as inpatients at clearly increased CHD risk but without
yet overt CHD, medium dose statin treatment is of
proven value with an overall favourable safety
profile, and is judged to be cost effective.
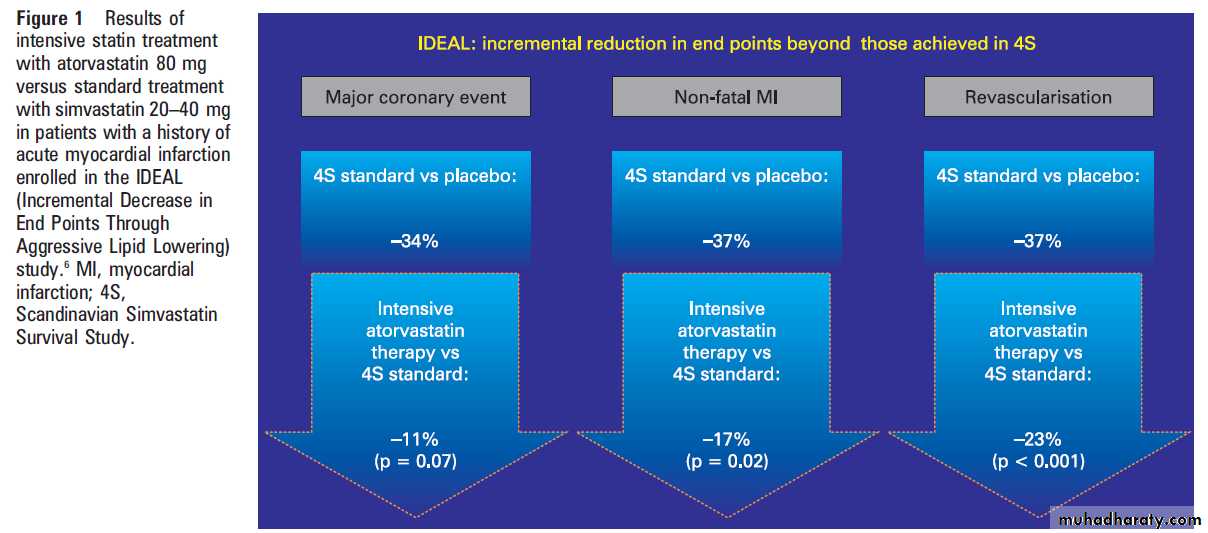
How low to go with cholesterol lowering: IDEAL
study starts where 4S ended In the 4S study,1 patients had a baseline LDL-Cconcentration of 4.9 mmol/l (188 mg/dl) and at
the end of the study achieved an average 35%
reduction up to the LDL-C value of 3.2 mmol/l
(125 mg/dl).
The Incremental Decrease in End Points Through Aggressive Lipid Lowering(IDEAL) study was a randomised trial which
enrolled 8888 patients with a history of acute
myocardial infarction.
The average baseline LDL-C was 3.1 mmol/l (122 mg/dl). Patients were randomly assigned to receive either high dose atorvastatin (40–80 mg daily) or usual dose simvastatin (20–40 mg daily) and were followed up for
amedian of 4.8 years.
The changes obtained in the lipid profile at the end of the follow-up were: LDLC was lowered to 2 mmol/l (80 mg/dl) in the intensive atorvastatin group and to 2.5 mmol/l (100 mg/dl) in the standard simvastatin group; TC was lowered to 4 mmol/l (154 mg/dl) in the intensive atorvastatin arm and to 4.6 mmol/l
(177 mg/dl) in the standard simvastatin group.
What extra benefits did this intensive cholesterol
bring? The authors reported a reduction in theprimary outcome of major coronary events that just
failed to reach statistical significance (p=0.07), and
there was no difference in cardiovascular or all cause
mortality during the approximately 5 years of
follow-up.
The intensive atorvastatin treatment reduced the risk of non-fatal acute myocardial infarction by an additional 17%, the risk of major cardiovascular events by an additional 13%, and the risk of revascularisation by an additional 23%
compared to the simvastatin group (fig 1). Patients
in the atorvastatin group had higher rates of drug
discontinuation due to non-serious adverse events
and transaminase elevation. Serious myopathy and
rhabdomyolysis were rare in both groups.
Should we go even lower?
As already indicated from angiographic studies, the progression of atherosclerotic disease may be
slowed with mean doses of statins, but can we
do better? A quite interesting vision towards this
question emerges from the publication of O’Keefe
et al.
They support the idea that major modifications in the diet and lifestyle of humans appeared too recent on the evolutionary time scale for the human genome to adjust. Thus, although an LDLC value between 1.3–1.8 mmol/l (50–70 mg/dl) seems excessively low by modern standards, the authors suggest that this is precisely the normal range for individuals with the diet and lifestyle for which we were genetically programmed.
To support this concept further, O’Keefe et al7 provide
two types of evidence. First, they show that, evento date, hunter–gatherer populations living in their
indigenous lifestyle have TC values of 2.6–3.9 mmol/l (100–150 mg/dl) and LDL-C values of 1.3–1.9 mmol/l (50–75 mg/dl) and have no evidence of atherosclerosis, even at advanced age.
The LDL-C values of healthy neonates are in the 0.8–
1.8 mmol/l (30–70 mg/dl) range and healthy wildadult primates have LDL-C values of 1.0–2.0 mmol/l (40–80 mg/dl). People with heterozygous
hypobetalipoproteinaemia have TC values as
low as 2.0 mmol/l (80 mg/dl) and LDL-C values as
low as 0.8 mmol/l (30 mg/dl) and this status is
associated with longevity, probably due to the
absence of atherosclerosis.
Secondly, authors analysed data from studies
correlating LDL-C treatment and the risk of CHD.
Based on linear regression analysis they show that
atherosclerosis progression as measured by mean
luminal diameter of the coronary artery may reach
approximately 0 mm/year at an LDL-C value of
1.8 mmol/l (70 mg/dl).
The LDL-C value at which the cardiovascular event rate is predicted to approach 0 is 1.47 mmol/l (57 mg/dl) for primary prevention and 0.8 mmol/l (30 mg/dl) for secondary prevention. Of course, this is an interesting
theoretical exercise but does not provide definite
clinical proof.
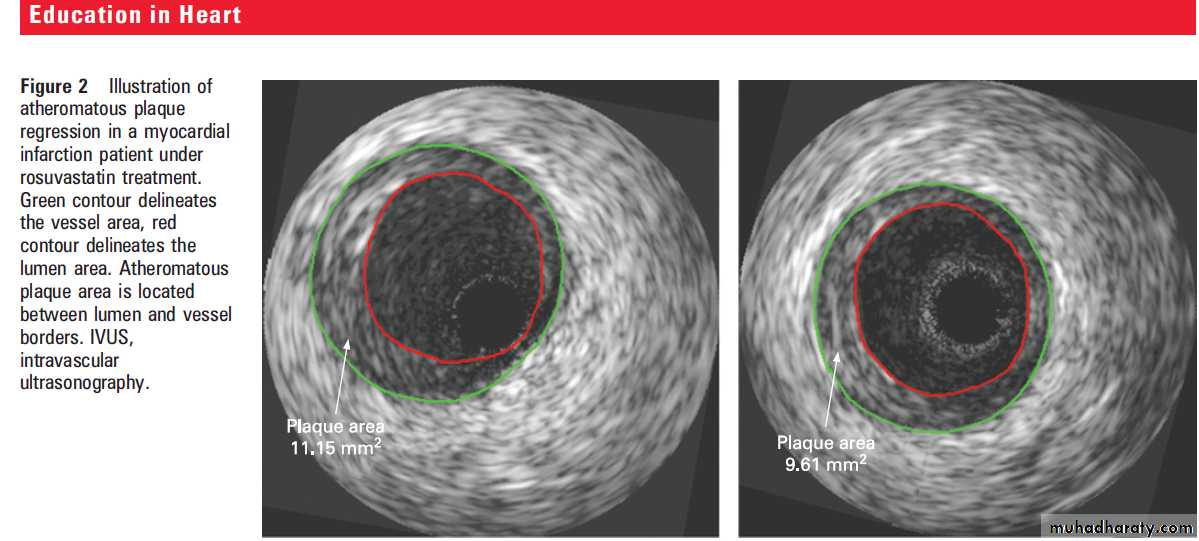
Is plaque regression also possible?
On average, statin treatment diminishes the progressionof coronary atherosclerosis, but regression has sometimes been observed on an individual basis
with serial coronary angiographies. In 2004, using
intravascular ultrasonography (IVUS) analyses (as
illustrated in fig 2), Nissen et al published the results
from the Reversal of Atherosclerosis with Aggressive
Lipid Lowering (REVERSAL) study.
This was arandomised trial in which 502 patients received either 40 mg of pravastatin or an intensive lipid lowering regimen of 80 mg of atorvastatin.
Coronary arteries were assessed by serial IVUS at
baseline and after 18 months of treatment. Initial
LDL-C concentrations (3.9 mmol/l or 150 mg/dl in
both groups) were reduced during the treatment by
27% in the moderate regimen group (pravastatin)
and by 47% in the intensive regimen group.
Compared to baseline, progression of coronary
atherosclerosis occurred in the pravastatin group(2.7%) and did not occur in the atorvastatin group
(20.4%). The percentage change in atheroma
volume (PAV) showed a significantly lower progression
rate in the atorvastatin group. Similar differences
between groups were reported for change in
total atheroma volume (TAV) and change in
atheroma volume in the most severely diseased
10 mm vessel sub-segment.
Two years later, Nissen et al published the
results of a prospective trial, A Study to Evaluatethe Effect of Rosuvastatin on Intravascular
Ultrasound-Derived Coronary Atheroma Burden
(ASTEROID), where 349 patients received 40 mg
of rosuvastatin daily and were evaluated by IVUS
at baseline and after 24 months.
The mean baseline LDL-C of 3.4 mmol/l (130 mg/dl) decreased by 53% while the mean HDL-C value increased by 15% from 1.1 mmol/l (43 mg/dl) at baseline. The
mean change of PAV compared with baseline was
1% for the entire vessel. The total atheroma
volume showed a 6.8% median reduction and the
mean change in atheroma volume in the most
diseased 10 mm subsegment was -6.1 mm3.
Although these two studies do not report impressive values per se with regard to regression of atheroma, one has to keep in mind that they represent valuable evidence that progression may be stopped or even regression of atherosclerotic disease may be achieved, at least in some patients, with the current drugs in high dosages.
Does the baseline cholesterol matter?
The Pravastatin or Atorvastatin Evaluation andInfection Therapy-Thrombolysis in Myocardial
Infarction 22 (PROVE IT-TIMI 22) trial enrolled
4162 patients hospitalised for an acute coronary
syndrome within the preceding 10 days.
Participants were randomly assigned to receive
40 mg of pravastatin daily (standard treatment) or
80 mg of atorvastatin daily (intensive treatment).
Is there evidence to treat all CHD patients
aggressively with statins?
The primary end point was a composite of death
from any cause, myocardial infarction, documented
unstable angina requiring rehospitalisation,and stroke. At the end of the mean 24 month follow-up, a 16% (95% confidence interval (CI) 5% to 26%) reduction in the hazard ratio in favour of atorvastatin (p=0.005) was reported for the primary end point, demonstrating the benefit of early aggressive statin treatment after acute coronary syndromes.
A recent report by Giraldez et al is an important substudy of this PROVE-IT TIMI 22 trial. For this substudy only statin naïve patients were selected. The substudy shows convincingly and in great detail that the additional benefit of intensive treatment with statins compared with moderate treatment declines with
decreasing baseline LDL-C values and that a benefit
is no longer seen in patients starting with baseline
LDL-C of 1.7 mmol/l (66 mg/dl).
In summary, high dose (intensive) statin treatment
with simvastatin 80 mg, atorvastatin 80 mgor possibly rosuvastatin 20–40 mg daily (no existing
clinical event trials are yet available in this respect for high dose rosuvastatin) is advisable in
patients with a history of acute myocardial
infarction/post-acute coronary syndrome, and
brings extra beneficial effects when compared to
usual dose statin treatment.
This is proven now for at least some years after the index event.
It is uncertain if this high dose statin treatment should be maintained over a person’s lifetime. The above presented findings strongly suggest that intensive statin treatment may be unwarranted with regard to reduction of CHD events in all patients and may induce more unnecessary side effects in patients with low baseline LDL-C.Does only LDL-C matter?
In addition to LDL-C, the importance of other lipid
fractions in the development of atherosclerosis is
being increasingly recognised. Epidemiological studies
have proven an inverse relationship between
HDL-C values and the risk for CHD. While LDL-C
remains the primary target for prevention of CHD,
the LDL-C/HDL-C, TC/HDL-C and apolipoprotein
B/A-I ratios significantly improve cardiovascular
risk prediction.
For example, analysis of data from over 2000 patients in the Helsinki Heart Study showed that the LDL-C/HDL-C ratio was the best single predictor of cardiac events. In another study of more than 175 000 individuals followed up to 5.5 years, apolipoprotein B concentration was ahighly significant predictor of fatal acute myocardial infarction and the strongest univariate predictor was the apolipoprotein B/A-I ratio.
If HDLC should be a target besides LDL-C, is there any evidence for a clinically meaningful differential
effect of statins on HDL-C?
In the Rosuvastatin and Atorvastatin in different
Dosages And Reverse cholesterol (RADAR) study,
effects of increasing dosages of rosuvastatin and
atorvastatin on the lipid profile were compared in
patients with cardiovascular disease and low
(<1.0 mmol/l or 40 mg/dl) HDL-C.
A total of 461 patients entered a 6 week dietary run-in period before randomisation to rosuvastatin 10 mg or atorvastatin 20 mg for 6 weeks. Doses were
increased after 6 weeks to rosuvastatin 20 mg or
atorvastatin 40 mg, and after 12 weeks to rosuvastatin
40 mg or atorvastatin 80 mg. Rosuvastatin was more effective than atorvastatin in reducing LDL-C and TC, and improving the LDL-C/HDL-C,TC/HDL-C and apolipoprotein B/A-I ratios at all three time points.
Although rosuvastatin increased HDL-C numerically more than atorvastatin at 12 and 18 weeks of treatment, this difference did not reach significance between the groups. Interestingly, the HDL-C response to atorvastatin
diminished with increasing doses, albeit nonsignificantly. Paraoxonase-1 (PON-1) is an HDL associated enzyme involved in the protective mechanisms of HDL.
A substudy13 from RADAR aimed to compare the effect of treatment with rosuvastatin and atorvastatin on serum PON-1 activity.
Rosuvastatin treatment resulted in a significant
increment of serum PON-1 activity with increasing
dose, while this was not observed with atorvastatin.
These findings suggest that, although they seem
to be similar, differential effects in the lipid profilemay appear between statins and with different
dosages. These subtle changes may translate into
important clinical outcomes as was suggested by
alipidomics substudy14 of the RADAR trial, and
therefore attention should not be limited to the
classic lipid values.
Direct comparisons between (high dose) statins evaluating atherosclerosis and clinical events seems warranted in order to settle this issue.
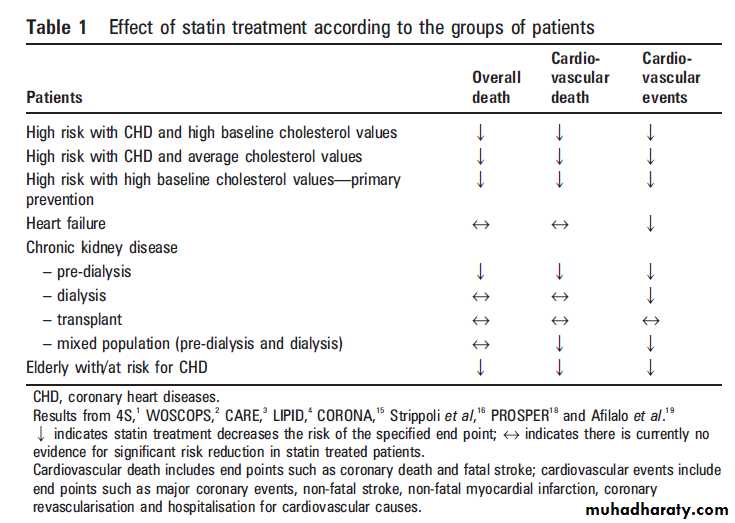
THE CONTROVERSIAL GROUPS OF PATIENTS
Where as statin treatment has, as described above,
proven its efficacy in a broad range of patient
groups, data have to be interpreted cautiously
when it comes to using statins in certain groups of
patients, of which the most clinically relevant
currently seem to be:
(1) patients with (ischaemic)heart failure,
(2) patients with chronic kidneydisease; and
(3) the elderly at risk (table 1).
Statins in (ischaemic) heart failure
Patients with systolic heart failure have been generally excluded from statin trials. Besides the beneficial effects of statins on clinical events in non-heart failure patients, statins are also reported to stabilise vulnerable plaque, reduce inflammation,restore autonomic function and reduce the risk for ventricular fibrillation and subsequent sudden death.These effects of statins might also be beneficial in patients with heart failure, but may be balanced by a worsening prognosis due to impaired neutralisation of inflammatory cytokines and endotoxins that enter the circulation via the intestines due to reduced concentrations of LDL-C.
Moreover, statins may decrease the production of
co-enzyme Q10, increase the oxidative stress and
affect the mitochondrial function worsening heart failure. Therefore, the effects of statins in heart
failure require a separate prospective investigation.
The CORONA study is a randomised trial
where a total of 5011 patients aged >60 years withNew York Heart Association (NYHA) functional
class II to IV ischaemic systolic heart failure
received 10 mg of rosuvastatin or placebo. During
a median follow-up of 32.8 months, patients in the
rosuvastatin group had a 45% reduction in LDL-C
and a 37% reduction in C-reactive protein (CRP).
The risk of death from cardiovascular disease, nonfatal
myocardial infarction or non-fatal stroke was
non-significantly reduced by 8% in the rosuvastatin
group. There were no significant differences
between the two groups in term of death from all
causes or death from cardiovascular causes.
There were, however, significantly fewer hospitalisations
for cardiovascular causes in the rosuvastatin groupthan in the placebo group. Since patients with
heart failure are at higher risk of arrhythmic death,
as was also observed in this study, for which statin
treatment apparently does not help, it would be
interesting to investigate whether prophylactic
implantable cardioverter-defibrillator (ICD)
implantation together with statin treatment could
result in a significant reduction in risk of death
from all (cardiovascular) causes.
Statins in chronic kidney disease (pre-dialysis,
dialysis, and transplant population)Strippoli et al16 recently published a meta-analysis
of 50 trials and 30 144 patients that analysed the
benefits and side effects of statins in patients with
chronic kidney disease (pre-dialysis, dialysis, and
transplant populations).
Compared with placebo, statins significantly
reduced TC and LDL-C concentrations by approximately 1.1 mmol/l (43 mg/dl). In terms of all cause mortality, statins significantly reduced the risk by
19% in pre-dialysis patients but had no significant
risk reduction in dialysis and transplant patients.
In terms of cardiovascular mortality, statins
significantly reduced the risk by 20% in pre-dialysis
patients and a trend towards risk reduction was
seen in dialysis patients (17%) and transplant
patients (32%), albeit borderline non-significant.
For cardiovascular events, statins offered asignificant risk reduction of 25% in pre-dialysis
patients and 14% in dialysis patients, and a trend
for significant risk reduction of 30% in transplant
patients. Overall, statin treatment significantly
reduced the risk of cardiovascular events by 22%.
This meta-analysis also showed that the side effect
of statins was similar to that of placebo and statins
did not produce an excess of withdrawal of the
patients with chronic kidney disease when compared
to placebo. Cardiac disease is the major cause
of death in dialysis patients.
The single largest cause of death is linked to ventricular arrhythmias and is followed by sudden cardiac death.
An ongoing study, Prevention of sudden cardiac death: Rationale and design of the Implantable Cardioverter Defibrillators in Dialysis patients (ICD2) Trial, is investigating the benefit of prophylactic ICD therapy in dialysis patients. As in the case of (ischaemic) heart failure patients, it is of particular interest to investigate whether the association of statin treatment with prophylactic ICD implantation can add a significant cardiovascular risk reduction in this group of patients.
Statins in the elderly at risk
In 2002, Shepherd et al published promisingresults from the PROspective Study of
Pravastatin in the Elderly at Risk (PROSPER) with
regard to the beneficial effects of statins in the
elderly with, or at high risk of developing,
cardiovascular disease and stroke. A total of 5804
participants aged 70–82 years were randomly
assigned to pravastatin 40 mg daily or placebo.
The mean follow-up was 3.2 years and the primary
end point was a composite of coronary death, nonfatalmyocardial infarction, and fatal or non-fatal
stroke. Pravastatin lowered LDL-C by 34% and
reduced the risk of the primary end point by 15%
(95% CI 0.74 to 0.97; p=0.014). The authors
reported a higher new cancer diagnosis rate in the
pravastatin group compared to the placebo group
(hazard ratio 1.25, 95% CI 1.04 to 1.51; p=0.02).
A recently published meta-analysis gathered all
the available data to determine whether statins
reduce all cause mortality in elderly patients at
enhanced risk with CHD. Nine trials with a total
of 19 569 patients aged 65–82 years were included.
Over 5 years, statins reduced the all cause mortality
by 22%, CHD mortality by 30%, non-fatal
myocardial infarction by 26%, need for revascularisation by 30%, and stroke by 25%.
Older patients attained a greater reduction of all cause mortality than younger patients: the relative risk reduction was 50% in patients .80 years, 44% in patients aged 65–79, and 30% in patients ,65 years. Thus, elderly patients at enhanced CHD risk should not
be excluded from statin treatment, provided that
they have a reasonable life expectancy.
SAFETY CONSIDERATIONS IN STATIN TREATMENT
As already discussed for the individual studies,statin treatment in general is not associated with
significant adverse effects when compared to
placebo. A meta-analysis of data from 90 056
participants in 14 randomised trials of statins
showed no evidence that lowering LDL-C by
1 mmol/l during 5 years of statin treatment may
increase the risks of any specific non-vascular cause
of death or of any specific type of cancer.
Rhabdomyolysis occurred in 9 out of 39 884 statin
patients versus 6 out of 39 817 controls; the 5 year
excess risk with statin was 0.01% (p=0.4).
The side effects associated with intensive versus
moderate statin treatment were analysed in a
recent meta-analysis of six randomised control
trials. Intensive statin treatment was associated
with an increase in hepatic transaminases >3 times normal (odds ratio (OR) 3.7, 95% CI 2.1 to 6.6) and a trend for creatine kinase >10 times normal and rhabdomyolysis (OR 1.9, 95% CI 0.5 to 7.6).
Therefore, intensive statin treatment may be optimally prescribed in carefully selected patients at high risk for cardiovascular events where standard regimens appear safe in all categories of patients who have an indication for statin treatment. Needless to say that, in individual cases, unwanted (serious) side effects indeed may prompt immediate discontinuation or change in the type of statins.
CONCLUSIONS
Statin treatment offers beneficial effects in termsof primary and secondary prevention of cardiovascular disease and subsequent death.
High dose treatment is now proven to be beneficial in patients with acute myocardial infarction/postacute coronary syndrome for at least some years after the index event.
It is still unclear if high dose treatment should be recommended during the lifetime of these patients.
The protective role of statins applies to numerous categories of population, including the elderly at risk. Yet more controversial effects are mainly related to chronic kidney disease and heart failure patients. They might benefit more from statin treatment if it is combined with ICD therapy in properly selected subgroups; however, this remains to be proven.
Newly developed techniques such as intravascular
ultrasonography have revealed that newly developed statins are able to control and sometimes even to reverse coronary atherosclerotic plaques.In conclusion, because they are efficient, generally
safe and have few contraindications, statins are
expected to become even more increasingly used in
the prevention and treatment of cardiovascular disease.