• Panorama 8
• In Medicineد. حسين محمد جمعه
اختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2012
• ABO Blood Type Is a Risk Factor for Coronary Heart Disease
Heartwire © 2012 Medscape, LLCAugust 14, 2012 (Boston, Massachusetts) — Data from two prospective cohort studies have identified the ABO blood group as a risk factor for the development of coronary heart disease [1]. Individuals with blood groups A, B, or AB were 5% to 23% more likely to develop coronary heart disease compared with subjects with O blood type, and the associations were not altered by multivariate adjustment of other risk or dietary factors.
The analysis, led by Dr Meian He (Harvard School of Public Health, Boston, MA), included 62 073 women from the Nurses' Health Study (NHS) and 27 428 men from the Health Professionals Follow-up Study (HPFS) and is published in the September 2012 issue of Arteriosclerosis, Thrombosis, and Vascular Biology.
In the NHS and HPFS, the incident rates of coronary heart disease per 100 000 person-years were 125, 128, 142, and 161 for women with type O, A, B, and AB, respectively, and 373, 382, 387, and 524 for men with type O, A, B, and AB, respectively. Compared with individuals with O blood type, individuals with blood group A, B, or AB had a respective 5%, 11%, and 23% increased risk of developing coronary heart disease in an age-adjusted model.
These associations were not significantly altered in the multivariable-adjusted risk model.
The researchers also performed an analysis examining the risk of coronary heart disease in patients with non-O blood type. Compared with individuals with O blood type, those with A, B, and AB had a 9% increased risk of developing coronary heart disease, and this risk was unaltered after adjustment for other risk factors. Similarly, a combined analysis of NHS and HPFS with four other prospective studies, an analysis that included 114 648 subjects, found there was a 6% increased risk of coronary heart disease for those with non-O blood type compared with individuals with O blood.
In total, just over 6% of the coronary heart disease cases were attributable to the A, B, or AB blood types, according to the researchers.
In terms of possible underlying mechanisms for the increased risk, He et al note that in non-O individuals, plasma levels of factor VIII-von Willebrand factor (vWF) are approximately 25% higher than in individuals with type O blood type. Elevated levels of factor VIII-vWF have been previously identified as a risk factor for coronary heart disease.
"The vWF has an important role in hemostasis and thrombosis by mediating platelet adhesion to the vascular wall, especially under high shear stress conditions," explain He and colleagues.
"Along with fibrinogen, vWF also participates in platelet aggregation and plays a role in the development of atherosclerosis." In addition, the A blood group has been shown to have higher levels of total and LDL cholesterol.
In a study reported online June 22nd in Circulation, Dr. Dawn and colleagues systematically reviewed the effects of adult bone marrow cell transplantation on clinical and surrogate endpoints in patients with ischemic heart disease.
Bone marrow cell transplantation was associated with a 3.96% improvement in left ventricular ejection fraction (p<0.00001), a 4.03% reduction in infarct size (p<0.00001), an 8.9 mL reduction in left ventricular end systolic volume (p<0.00001), and a 5.23 mL reduction in left ventricular end diastolic volume (p<0.0001), compared with standard treatment.
The improvement in left ventricular ejection fraction persisted for at least 24 months, whereas the other improvements persisted for at least 12 months.
Circulation 2012.
Injection of at least 40 million bone marrow cells resulted in significant improvement in all four of these primary outcome measures, while injection of fewer than 40 million bone marrow cells did not show improvement in any outcome.
Additionally, compared with patients who received standard therapy, bone marrow cell-treated patients had a 61% decrease in all-cause mortality (p<0.00001), a 59% decrease in cardiac mortality (p=0.005), a 75% decrease in recurrent myocardial infarction (p=0.001), and a 66% decrease in stent thrombosis (p=0.04).
There were trends toward a reduction in the incidence of heart failure and cerebrovascular events among bone marrow cell-treated patients.
"Patients with acute myocardial infarction...and patients with chronic ischemic heart disease are both likely to benefit from bone marrow cell therapy," Dr. Dawn said. "Moreover, these benefits are noted independent of the location of myocardial infarction (anterior wall vs. other walls). However, patients with worse heart function (lower ejection fraction) at baseline seem to derive greater benefits from bone marrow cell therapy."
Circulation 2012.
April 25, 2012 (Bern, Switzerland and Gateshead, United Kingdom)— Mild degrees of hyperthyroidism or hypothyroidism both appear to be associated with an increased risk of cardiovascular events [1,2]. And treatment of hypothyroidism with levothyroxine may reduce this risk, at least in younger patients.
These are the conclusions of two new studies published online April 23, 2012 in the Archives of Internal Medicine.
On the mechanisms involved in the link between thyroid disease and heart disease, Rodondi said these were clearer for hypothyroidism, which is associated with traditional cardiovascular risk factors such as increases in weight, cholesterol, and blood pressure. "The mechanisms connecting hyperthyroidism to heart disease are more complex. It could be related to a faster heart rate, as hyperthyroidism does seem to be associated with arrhythmias."
Warfarin and Aspirin in Patients
with Heart Failure and Sinus RhythmN Engl J Med May 3, 2012.
Among patients with reduced LVEF who were in sinus rhythm, there was no significant overall difference in the primary outcome between treatment with warfarin
and treatment with aspirin. A reduced risk of ischemic stroke with warfarin was offset by an increased risk of major hemorrhage. The choice between warfarin and aspirin should be individualized. (Funded by the National Institute of Neurological Disorders and Stroke; WARCEF ClinicalTrials.gov number, NCT00041938.)
The WARCEF trial was designed to determine whether warfarin or aspirin is a better treatment for patients with a reduced LVEF who are in sinus rhythm. Previous studies either were retrospective or lacked the power to adequately address this issue. As a result, there has been insufficient evidence to support any strong treatment recommendations regarding the use of warfarin or aspirin in these patients.
Discussion
Our trial had a double-blind design with sham INRs, similar to that used in the Warfarin–Aspirin Recurrent Stroke Study (WARSS, NCT00027066), and used centralized INR processing centers to ensure that the INR data would be of high quality.
Our results show no significant overall difference
between warfarin and aspirin therapies inpreventing the primary outcome. Although there
may have been a small benefit with warfarin
among patients followed for 4 or more years, it was of borderline statistical significance and uncertain clinical significance. There was a consistent
and significant benefit of warfarin as compared
with aspirin with respect to the prevention of ischemic
stroke throughout the follow-up period.
This benefit was suggested in the WATCH trial and has now been confirmed in the WARCEF trial, which included more patients and a longer followup period.
However, the benefit was offset by the increase in the incidence of major bleeding. The relative reduction in the risk of ischemic stroke with warfarin among the patients in our study,
who had heart failure, is similar to that observed
among patients with atrial fibrillation.21 However,
the absolute risk of ischemic stroke among patients
with a low LVEF who are in sinus rhythm is significantly lower than that among patients with atrial fibrillation.
With respect to the main secondary outcome,which included myocardial infarction and hospitalization
for heart failure, in addition to theprimary outcome, there was no significant differencebetween the warfarin group and the aspirin group. There was a trend toward an increased rate of hospitalization for heart failure in the warfarin group, a finding that is in direct contrast to the results of the WASH and WATCH trials, which suggested an increased rate of hospitalization for heart failure among patients receiving aspirin.
There has been speculation that aspirin may interfere with prostaglandin synthesis, leading to a reduced effectiveness of ACE inhibition. In our trial, however, no increase in the rate of hospitalization for heart failure was seen in the aspirin group as compared with the warfarin group, even though a large proportion of patients in the aspirin group were treated with an ACE
inhibitor.
In the warfarin group, the INR was in the therapeutic range of 2.0 to 3.5 for 63% of the total treatment time. We set the INR target above that used in trials involving patients with atrial fibrillation, because among trials involving patients who had had a myocardial infarction, those with higher INR targets and values showed the superiority of warfarin over aspirin, whereas those with lower INR targets and values did
not. In our study, patients received either warfarin
or aspirin and did not take both medications.
The side-effect profile in the case of both warfarin and aspirin was generally acceptable,
and there was a low rate of intracerebral hemorrhage.The rate of major hemorrhage was significantly
increased with warfarin therapy but was lower than that seen in the warfarin group in recent trials involving patients with atrial fibrillation and similar to that seen in the WARSS and WATCH trials.
The limitations of our study include the smallerthan-
anticipated number of patients enrolled, and,
given the variable length of follow-up, the relatively
small numbers of patients who were still
being followed in years 5 and 6. The time in the
therapeutic range among patients in the warfarin
group was relatively low at 63%.
In addition, in both groups, there was a substantial portion of follow-up time during which the patients did not receive the assigned study treatment. However, this duration was similar in the two treatment groups, thus minimizing any bias. Since newer antithrombotic agents, as compared with warfarin, are easier to administer and may be associated with better long-term adherence to therapy, they may increase
the time in the therapeutic range and reduce the
time during which patients do not receive the assigned therapy.26-28 If so, they may prove to be more effective than warfarin or aspirin.
In summary, this trial showed no significant overall difference between warfarin and aspirin with respect to the primary outcome of death,ischemic stroke, or intracerebral hemorrhage.
However, among patients followed for 4 or more years, there may have been a small benefit, of uncertain clinical significance, with warfarin. Warfarin was associated with a reduction in the risk of ischemic stroke throughout the follow-up period. Given the finding that warfarin did not provide an overall benefit and was associated with an increased risk of bleeding, there is no compelling reason to use warfarin rather than aspirin in patients with a reduced LVEF who are in sinus rhythm.
Effects in Breastfed Infants
Forty nursing mothers used nitroglycerin ointment (dosage not specified) topically for the treatment of postpartum anal fissures for durations ranging from 1 use to 12 months of intermittent use. All but 9 of the women reported side effects from therapy, primarily headache, but also dizziness or lightheadedness. None of the mothers reported any side effects in their breastfed infants.Nitroglycerin
• Therapeutic actions• Relaxes vascular smooth muscle with a resultant decrease in venous return and decrease in arterial BP, which reduces left ventricular workload and decreases myocardial oxygen consumption.
•
Indications
· Sublingual, translingual preparations: Acute angina
· Oral SR, sublingual, topical, transdermal, translingual, transmucosal preparations: Prophylaxis of angina
· IV: Angina unresponsive to recommended doses of organic nitrates or beta-blockers
· IV: Perioperative hypertension
· IV: CHF associated with acute MI
· IV: To produce controlled hypertension during surgery
· Unlabeled uses: Reduction of cardiac workload in acute MI and in CHF (sublingual, topical); adjunctive treatment of Raynaud's disease (topical)
• Contraindications and cautions
• · Contraindicated with allergy to nitrates, severe anemia, early MI, head trauma, cerebral hemorrhage, hypertrophic cardiomyopathy, pregnancy, lactation.• · Use cautiously with hepatic or renal disease, hypotension or hypovolemia, increased intracranial pressure, constrictive pericarditis, pericardial tamponade, low ventricular filling pressure or low PCWP.
•
• Available forms
• Injection—0.5, 5 mg/mL; injection solution—25, 50, 100, 200 mg; sublingual tablets—0.3, 0.4, 0.6 mg; translingual spray—0.4 mg/spray; transmucosal tablets—1, 2, 3 mg; transmucosal SR tablets—1, 2, 2.5, 3, 5 mg; oral SR capsules—2.5, 6.5, 9 mg; transdermal—0.1, 0.2, 0.3, 0.4, 0.6, 0.8 mg/hr; topical ointment—2%
Dosages
ADULTSIV
Initial dose, 5 mcg/min delivered through an infusion pump. Increase by 5-mcg/min-increments every 3–5 min as needed. If no response at 20 mcg/min, increase increments to 10–20 mcg/min. Once a partial BP response is obtained, reduce dose and lengthen dosage intervals; continually monitor response and titrate carefully.
Sublingual
· Acute attack: Dissolve 1 tablet under tongue or in buccal pouch at first sign of anginal attack; repeat every 5 min until relief is obtained. Do not take more than 3 tablets/15 min. If pain continues or increases, patient should call physician or go to hospital.
· Prophylaxis: Use 5–10 min before activities that might precipitate an attack.
Sustained-release (oral)
Initial dose, 2.5–9 mg q 12 hr. Increase to q 8 hr as needed and tolerated. Doses as high as 26 mg given qid have been used.
Topical
Initial dose, one-half inch q 8 hr. Increase by one-half inch to achieve desired results. Usual dose is 1–2 inches q 8 hr; up to 4–5 inches q 4 hr have been used. 1 inch = 15 mg nitroglycerin.
Transdermal
Apply one patch each day. Adjust to higher doses by using patches that deliver more drug or by applying more than one patch. Apply patch to arm; remove hs.
Translingual
Spray preparation delivers 0.4 mg/metered dose. At onset of attack, spray one to two metered doses into oral mucosa; no more than three doses/15 min should be used. If pain persists, seek medical attention. May be used prophylactically 5–10 min prior to activity that might precipitate an attack.Transmucosal
1 mg q 3–5 hr during waking hours. Place tablet between lip and gum above incisors, or between cheek and gum.
• Route
• Onset• Duration
• IV
• 1–2 min
• 3–5 min
• Sublingual
• 1–3 min
• 30–60 min
• Translingual spray
• 2 min
• 30–60 min
• Transmucosal tablet
• 1–2 min
• 3–5 min
• Oral, SR
• 20–45 min
• 8–12 hr
• Topical ointment
• 30–60 min
• 4–8 hr
• Transdermal
• 30–60 min
• 24 hr
• Pharmacokinetics
• Metabolism: Hepatic; T1/2: 1–4 min
• Distribution: Crosses placenta; enters breast milk
• Excretion: Urine
•
• IV facts
• Preparations: Dilute in 5% dextrose injection or 0.9% sodium chloride injection. Do not mix with other drugs; check the manufacturer's instructions carefully because products vary considerably in concentration and volume per vial. Use only with glass IV bottles and the administration sets provided. Protect from light and extremes of temperature.
• Infusion: Do not give by IV push; regulate rate based on patient response.
• Incompatibilities: Do not mix in solution with other drugs.
Adverse effects
· CNS: Headache, apprehension, restlessness, weakness, vertigo, dizziness, faintness· CV: Tachycardia, retrosternal discomfort, palpitations, hypotension, syncope, collapse, orthostatic hypotension, angina
· Dermatologic: Rash, exfoliative dermatitis, cutaneous vasodilation with flushing, pallor, perspiration, cold sweat, contact dermatitis—transdermal preparations, topical allergic reactions—topical nitroglycerin ointment
· GI: Nausea, vomiting, incontinence of urine and feces, abdominal pain
· Local: Local burning sensation at the point of dissolution (sublingual)
· Other: Ethanol intoxication with high-dose IV use (alcohol in diluent)
•
• Interactions• Drug-drug
• · Increased risk of hypertension and decreased antianginal effect with ergot alkaloids
• · Decreased pharmacologic effects of heparin
• · Risk of severe hypotension and adverse CV events with sildenafil, tadalafil, vardenafil; avoid this combination
• Drug-lab test
• · False report of decreased serum cholesterol if done by the Zlatkis-Zak color reaction
Nursing considerations
CLINICAL ALERT!
Name confusion has occurred between NitroBid (nitrogylcerin) and Nicotral (nicotine); between nitroglycerin and nitroprusside; use caution.
Assessment
· History: Allergy to nitrates, severe anemia, early MI, head trauma, cerebral hemorrhage, hypertrophic cardiomyopathy, hepatic or renal disease, hypotension or hypovolemia, increased intracranial pressure, constrictive pericarditis, pericardial tamponade, low ventricular filling pressure or low PCWP, pregnancy, lactation
· Physical: Skin color, temperature, lesions; orientation, reflexes, affect; P, BP, orthostatic BP, baseline ECG, peripheral perfusion; R, adventitious sounds; liver evaluation, normal output; liver and renal function tests (IV); CBC, Hgb
• Interventions
• · Give sublingual preparations under the tongue or in the buccal pouch. Encourage patient not to swallow. Ask patient if the tablet "fizzles" or burns. Always check the expiration date on the bottle; store at room temperature, protected from light. Discard unused drug 6 mo after bottle is opened (conventional tablets); stabilized tablets (Nitrostat) are less subject to loss of potency.• · Give SR preparations with water; warn the patient not to chew the tablets or capsules; do not crush these preparations.
• · Administer topical ointment by applying the ointment over a 6 × 6 inch area in a thin, uniform layer using the applicator. Cover area with plastic wrap held in place by adhesive tape. Rotate sites of application to decrease the chance of inflammation and sensitization; close tube tightly when finished.
• · Administer transdermal systems to skin site free of hair and not subject to much movement. Shave areas that have a lot of hair. Do not apply to distal extremities. Change sites slightly to decrease the chance of local irritation and sensitization. Remove transdermal system before attempting defibrillation or cardioversion.
• · Administer transmucosal tablets by placing them between the lip and gum above the incisors or between the cheek and gum. Encourage patient not to swallow and not to chew the tablet.
• · Administer the translingual spray directly onto the oral mucosa; preparation is not to be inhaled.
• · WARNING: Arrange to withdraw drug gradually. 4–6 wk is the recommended withdrawal period for the transdermal preparations.
•
• Teaching points
• · Place sublingual tablets under your tongue or in your cheek; do not chew or swallow the tablet; the tablet should burn or "fizzle" under the tongue. Take the nitroglycerin before chest pain begins, when you anticipate that your activities or situation may precipitate an attack. Do not buy large quantities; this drug does not store well. Keep the drug in a dark, dry place, in a dark-colored glass bottle with a tight lid; do not combine with other drugs.• · Do not chew or crush the timed-release preparations; take on an empty stomach.
• · Spread a thin layer of topical ointment on the skin using the applicator. Do not rub or massage the area. Cover with plastic wrap held in place with adhesive tape. Wash your hands after application. Keep the tube tightly closed. Rotate the sites frequently to prevent local irritation.
• · To use transdermal systems, you may need to shave an area for application. Apply to a slightly different area each day. Remove the old system before you apply a new one. Use care if changing brands; each system has a different concentration.
• · Place transmucosal tablets between the lip and gum or between the gum and cheek. Do not chew; try not to swallow.
• · Spray translingual spray directly onto oral mucous membranes; do not inhale. Use 5–10 min before activities that you anticipate will precipitate an attack.
• · You may experience these side effects: Dizziness, light-headedness (may be transient; change positions slowly); headache (lie down in a cool environment and rest; OTC preparations may not help); flushing of the neck or face (transient).
• · Report blurred vision, persistent or severe headache, rash, more frequent or more severe angina attacks, fainting.
•
Adverse effects in Italic are most common; those in Bold are life-threatening.
As compared with the general population, patients with heart failure have an increased risk of stroke and of
systemic thromboembolic events, which are believed
to arise from within the heart as a result of
left ventricular stasis, endocardial dysfunction, and
a systemic hypercoagulable state.2 Heart failure
is a risk factor for atrial fibrillation, which, even if
asymptomatic, further increases the risk of stroke.3
Warfarin in Heart Failure
nejm.org may 17, 2012
Consequently, one would expect that patients with
heart failure could benefit from oral anticoagulanttherapy. However, until now, the hypothesis
that patients with heart failure benefit from longterm
anticoagulant therapy has been tested only
in modest-sized randomized, controlled trials-
In the Warfarin versus Aspirin in Reduced Cardiac
Ejection Fraction (WARCEF) trial reported byHomma et al.,7 2305 patients (mean age, 61 years
— relatively young for a population with heart
failure) who had severe left ventricular dysfunction
(mean left ventricular ejection fraction of 25%) were randomly assigned to receive warfarin (with a target international normalized ratio [INR] of 2.0 to 3.5) or aspirin (at a dose of 325 mg per day), with treatment continued for a mean of 3.5 years.
The benefit of warfarin in reducing the rate of ischemic stroke was offset by an increase in the rate of major bleeding (1.78 events per 100 patient-years with warfarin vs. 0.87 events per 100 patient-years with aspirin, P<0.001; number needed to harm, 110), although there was no excess of intracerebral bleeding events (0.12 events per 100 patient-years with warfarin and 0.05 events per 100 patient-years with aspirin, P = 0.35). Patients with atrial fibrillation place a higher value
on prevention of stroke than on reduction of the risk of bleeding events,9 but the modest absolute reduction in the rate of stroke (number needed to treat, 156) and the lack of mortality benefit in the WARCEF trial provide little support for the use of warfarin in preference to aspirin in patients with heart failure.
The WARCEF trial provides clear evidence that anticoagulant therapy prevents stroke, probably embolic stroke, in patients with heart failure who
have severe systolic dysfunction, but the rates of
stroke are too low to justify the routine clinical
use of warfarin in most patients with heart failure,
in light of the increase in the risk of bleeding.
Warfarin in Heart Failure
In conclusion, the results of the WARCEF trial
are consistent with those of three previous smallerrandomized, controlled trials in showing that
warfarin anticoagulant therapy, as compared with
aspirin, is not associated with a reduction in
mortality among patients with heart failure. The
WARCEF trial provides clear evidence that anticoagulant therapy prevents stroke, probably embolic stroke, in patients with heart failure who
have severe systolic dysfunction, but the rates of
stroke are too low to justify the routine clinical
use of warfarin in most patients with heart failure,
in light of the increase in the risk of bleeding.
From 2002 to 2010 in England, the age standardised total mortality rate fell by about half, whereas the age standardised event and case fatality rates both declined by about one third.
BMJ 25 January 2012
In men, the acute myocardial infarction event, case fatality, and total mortality rates declined at an average annual rate of, respectively, 4.8% (95% confidence interval
3.0% to 6.5%), 3.6% (3.4% to 3.7%), and 8.6% (5.4% to 11.6%). In women, the corresponding figures were 4.5% (1.7% to 7.1%), 4.2% (4.0% to 4.3%), and 9.1% (4.5% to 13.6%). Overall, the relative contributions of the reductions in event and case fatality rates to the decline in acute myocardial infarction mortality rate were, respectively,
57% and 43% in men and 52% and 48% in women; however, the relative contributions differed by age, sex, and geographical region.
From 2002 to 2010, the age standardised case fatality for acute myocardial infarction decreased in men from 42.0% (95% confidence interval 41.6% to 42.4%) to 32.1% (31.7% to 32.6%) and in women from 42.2% (41.7% to 42.7%) to 29.9% (29.4% to 30.4%) (table 2). Comparing 2002 with 2010, case fatality rates fell by 24% in men and by 29% in women. A declining case fatality rate was seen in all age groups and for both sexes.
The rates of decline were broadly similar between age groups in men and in women, with only slightly higher rates in middle aged people and slightly lower rates in younger and older people.
Just over half of the decline in deaths from acute
myocardial infarction during the 2000s in England can be attributed to a decline in event rate and just less than half to improved survival at 30 days. Both prevention of acute myocardial infarction and acute medical treatment have contributed to the decline in deaths from acute myocardial infarction over the past decade.BMJ 25 January 2012
Both increased fatness and reduced fitness were associated with increased risks for hypertension, hypercholesterolemia, and metabolic syndrome, even when accounting for other factors. However, improvements in fitness attenuated the effects of increased body-mass index and percent body fat, and vice versa. The ideal, then, is to motivate our patients to both maintain a normal body weight and improve their fitness level, not just one or the other.
Journal Watch General Medicine March 20, 2012
Frying modifies the nutritional content of foods and the frying medium. For example, frying leads to an increase in trans fats and a decrease in unsaturated fats in foods. Frying also increases the energy density of food and makes food more palatable,
which may lead to the consumption of larger amounts. Indeed,consumption of fried foods has been found to increase the likelihood of cardiovascular risk factors, including arterial hypertension, low concentrations of high density lipoprotein-cholesterol, and adiposity.
Future studies should therefore characterise fried foods in more detail by including information on the type of oil used for frying, the type of frying procedure performed (deep fried or pan fried),
the time and temperature used for frying, and the degree to which oils are reused.
In conclusion, evidence for antiplatelet agents in persons
with CKD and various cardiovascular diseases is of
low quality. Glycoprotein IIb/IIIa inhibitors or clopidogrel
given in addition to standard care have little or no effect on
death, myocardial infarction, or coronary revascularization
and may increase major bleeding in persons with CKD and
acute coronary syndromes or those having high-risk coronary
artery intervention. Antiplatelet agents reduce myocardial
infarction in persons with CKD but have uncertain
effects on stroke and mortality and may increase bleeding.
Bleeding hazards and lack of clear efficacy in reducing cardiovascular morbidity and mortality need to be acknowledged
when patients with CKD are being counseled about
acute or long-term antiplatelet therapy.
Ann Intern Med. 2012
Results
Venous thromboembolism recurred in 28 of the 205 patients who received aspirin and in 43 of the 197 patients who received placebo (6.6% vs. 11.2% per year; hazardratio, 0.58; 95% confidence interval [CI], 0.36 to 0.93) (median study period, 24.6 months). During a median treatment period of 23.9 months, 23 patients taking aspirin and 39 taking placebo had a recurrence (5.9% vs. 11.0% per year; hazard
ratio, 0.55; 95% CI, 0.33 to 0.92). One patient in each treatment group had a major bleeding episode. Adverse events were similar in the two groups.
NEJM may 24, 2012
Conclusions
Aspirin reduced the risk of recurrence when given to patients with unprovoked venous thromboembolism who had discontinued anticoagulant treatment, with
no apparent increase in the risk of major bleeding.
On the basis of the available evidence, patients
with unprovoked venous thromboembolism whoare at low-to-moderate risk for bleeding are expected to derive the greatest overall benefit from
extending anticoagulant therapy.
nejm.org may 24, 2012
The findings of the Aspirin for the Prevention of Recurrent Venous Thromboembolism (the Warfarin and Aspirin[WARFASA]) study are compelling and may signal an important step in the evolution of care; however, confirmatory studies will be required to establish a role in daily clinical practice for the use of aspirin among patients who are at high risk for bleeding due to anticoagulant therapy or for whom ongoing investigations identify and subsequently validate a clinical or biomarker-based profile associated with a low risk of recurring venous thromboembolism.
The ongoing Aspirin to Prevent Recurrent Venous Thromboembolism (ASPIRE) study (Australian New Zealand Clinical Trials Registry number, ACTRN012605000004662) has recruited
822 patients with a first unprovoked event,
as documented by means of objective testing, who
have received warfarin anticoagulant therapy for
3 to 6 months (but not longer than 12 months).
Patients have been randomly assigned to either
aspirin (100 mg daily) or placebo for a medianof 3 years and are being followed for a first occurrence of symptomatic and objectively confirmed
deep-vein thrombosis or nonfatal or fatal pulmonary
embolism with the use of an intention to-treat approach. Results of this study are expected
during 2012. A prospectively planned
combined analysis of the ASPIRE and WARFASA
trials (ACTRN12611000684921) may provide more
reliable evidence of the effect of aspirin in patients
with first unprovoked venous thromboembolism.
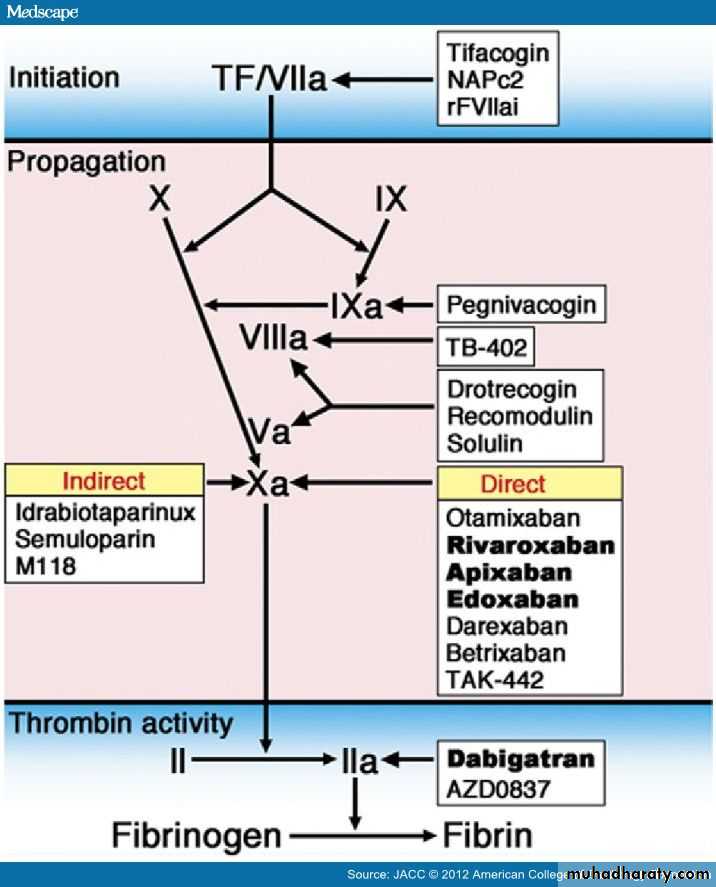
• Figure 1. Targets of Novel Anticoagulants for Long-Term UseBesides the indirect thrombin inhibitors (unfractionated heparin [UFH] and low molecular weight heparin [LMWH]), direct thrombin inhibitors bind directly to thrombin and prevent fibrin formation as well as thrombin-mediated activation of factor (F) V, FVIII, FXI, and FXIII. They also prevent thrombin-mediated activation of platelets, inflammation, antifibrinolysis, and the anticoagulant protein C/protein S/thrombomodulin pathway.
Parenteral direct thrombin inhibitors include hirudin, bivalirudin, and argatroban. Oral direct thrombin inhibitors are prodrugs that generate an active compound able to bind directly to the catalytic site of thrombin: examples include ximelagatran (withdrawn from development), AZD0837, now under evaluation, and dabigatran etexilate.
Drugs that target coagulation proteases that drive the propagation phase include agents that block FIXa (such as the DNA aptamer pegnivacogin), FVIIIa (TB-402), or jointly FVa/FVIIIa, cofactors that are critical for the generation of thrombin (drotrecogin, which is a recombinant form of human activated protein C and recomodulin and solulin, both of which are recombinant soluble derivatives of human thrombomodulin).
Blockers of the propagation phase also include FXa inhibitors. At variance from the parenteral indirect FXa inhibitors, such as UFH, LMWH, and pentasaccharide derivatives (fondaparinux, idrabiotaparinux), which exert their effects equally on thrombin and FXa (UFH), prevalently on FXa (LMWH), or exclusively on FXa (fondaparinux, biotaparinux), all by potentiating the natural inhibitor antithrombin (AT) (AT III), a number of oral direct (i.e., non–AT-mediated) FXa inhibitors are in clinical trials.
To target the initiation of coagulation, inhibitors toward the tissue factor/FVIIa complex have been developed, such as recombinant TFPI (tifacogin), recombinant nematode anticoagulant protein (NAP)C2, active site–inhibited recombinant (r) FVIIa inhibitors (rFVIIaI) and monoclonal antibodies against TF.
Figure illustration by Craig Skaggs. The direct thrombin inhibitors (DTI) (gatrans) bind to thrombin and block its capacity to convert fibrinogen to fibrin; to amplify its own generation through activation of FV, FVIII, and FIX; and to serve as a potent platelet agonist.[3] In contrast to indirect thrombin inhibitors, such as heparin, DTIs not only inhibit free thrombin, but also inhibit thrombin bound to fibrin.[4] Currently, only 1 DTI (dabigatran etexilate) has completed phase III clinical evaluation for stroke prevention in atrial fibrillation. only 1 DTI (dabigatran etexilate) has completed phase III clinical evaluation for stroke prevention in atrial fibrillation.
Drugs that target coagulation proteases that drive the propagation phase also decrease thrombin generation and include agents that block FIXa (such as the DNA aptamer pegnivacogin), FVIIIa (TB-402), or, jointly, FVa/FVIIIa, cofactors that are critical for the generation of thrombin (drotrecogin, which is a recombinant form of human activated protein C; recomodulin and solulin, both recombinant soluble derivatives of human thrombomodulin)[4] .
None of these new drugs have yet reached phase III development with cardiological indications. The largest family of new anticoagulants for long-term use is the FXa inhibitors. Parenteral synthetic pentasaccharides mediate indirect, antithrombin-dependent inhibition of FXa. The prototype of such drugs, fondaparinux, has been in clinical use for the treatment of acute coronary syndromes for a number of years. Idrabiotaparinux, a hypermethylated derivative of fondaparinux that possesses a biotin moiety to enable reversal, has been evaluated for the treatment of venous thromboembolism and as an alternative to warfarin for stroke prevention in patients with atrial fibrillation.
The atrial fibrillation trial was stopped early, and the future of this agent is uncertain. Consequently, idrabiotaparinux is not further reviewed here. A large series of compounds is now being developed to target FXa directly (direct FXa inhibitors, xabans), most of which are orally active. Only 3 such compounds (apixaban, rivaroxaban, edoxaban) have, however, completed or are now undergoing phase III clinical development for stroke prevention in atrial fibrillation. Rivaroxaban and apixaban have also undergone phase III clinical trials for the prevention of recurrent ischemia in acute coronary syndromes.
Summary points
Primary hyperparathyroidism (PHPT) is the most common cause of hypercalcaemia in the ambulatory setting; malignancy and other
secondary causes must be excluded
Primary hyperparathyroidism is diagnosed when intact parathyroid hormone is raised or mid to high normal in the setting of raised total
or ionised calcium after exclusion of conditions that mimic PHPT.
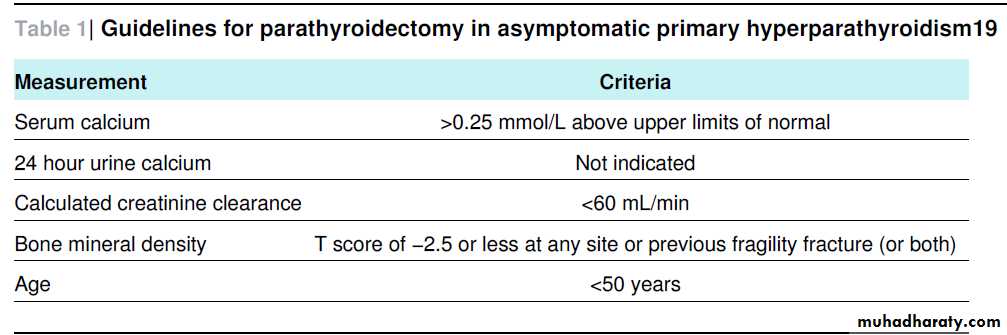
Medical surveillance comprises annual measurement of serum calcium and creatinine, plus measurement of bone mineral density (at three sites) every one to two years
Medical management options for select patients and those who do not meet parathyroidectomy guidelines include bisphosphonates and oestrogen replacement (both provide skeletal protection) and the calcimimetic cinacalcet, which can reduce serum calcium and parathyroid hormone values
Sestamibi imaging is used for localisation before surgery and is not a diagnostic tool—a negative scan does not exclude the diagnosis of PHPT.
Secondary hyperparathyroidism is commonly caused by vitamin D inadequacy or chronic kidney disease.
Differential diagnosis of hypercalcaemia
Parathyroid hormone (PTH) mediatedPrimary hyperparathyroidism
Familial hypocalciuric hypercalcaemia
Tertiary hyperparathyroidism
Ectopic PTH production by a tumour
PTH independent
Cancer: secretion of PTH related peptide, increased calcitriol, bone metastases
Granulomatous diseases
Vitamin D intoxication
Drugs: thiazides, lithium, vitamin A
Milk alkali syndrome
Adrenal insufficiency
Hyperthyroidism
Immobilisation
Vitamin A toxicity
Chronic renal failure
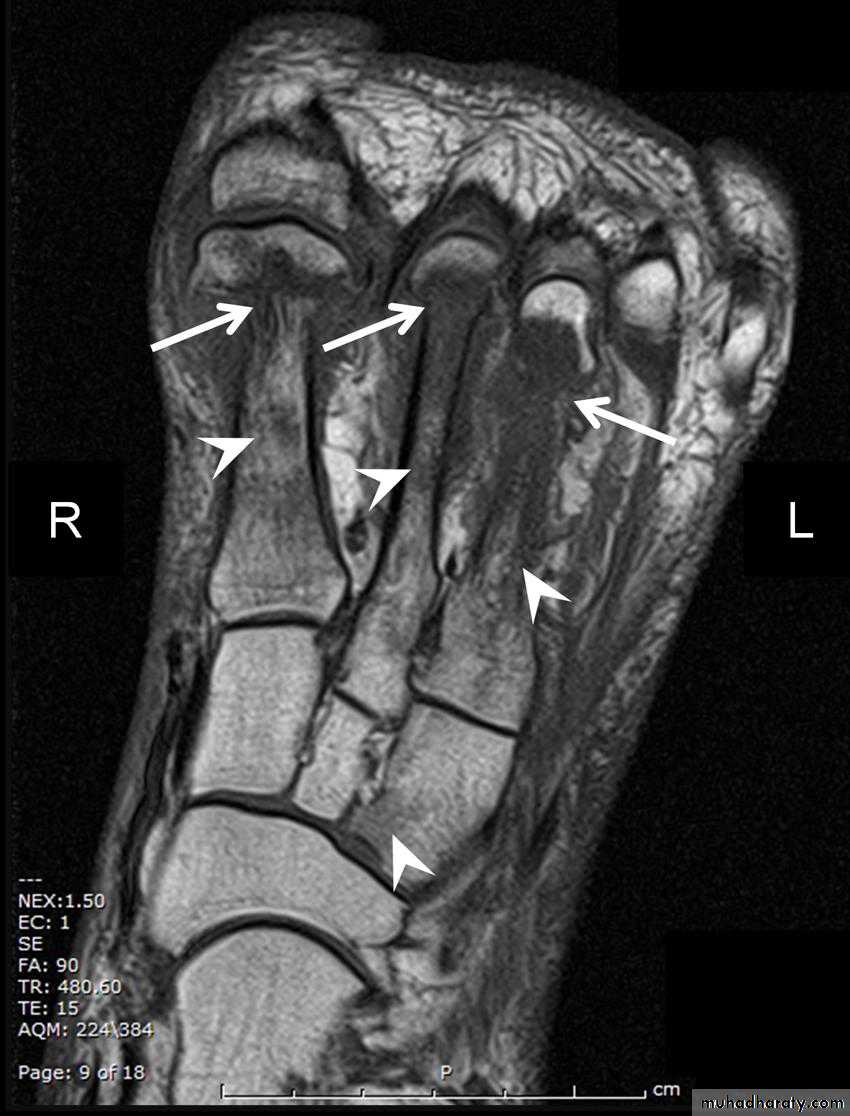
Axial T1 weighted MRI showing pathological fractures through the necks of the first three metatarsals (arrows) with
oedema in metatarsal shafts and lateral cuneiform bones (arrow heads). These features, in a patient with diabetes and no
history of trauma, suggest acute Charcot foot.
Minimizing Unnecessary Surgery for Thyroid Nodules
NEJM June 25, 2012Thyroid follicles remodel continuously, responding
to stimuli such as thyrotropin, growth factors,cytokines, and iodine. Nodules develop when
these growth signals drive hyperplasia or when a
follicular cell acquires a genetic mutation that confers
autonomous growth. Thyroid nodules are
commonly seen in clinical practice. With the use
of ultrasonography, nodules can be detected in at
least 25 to 50% of adults and are more common
in women and with increasing age.
When a patient presents with a thyroid nodule,
the primary concern is whether it is benign
or malignant. Findings on fine-needle aspiration,
ideally performed with ultrasonographic guidance,
are the mainstay of clinical decision making.
1 When the diagnosis is in doubt, most physicians
and patients opt for hemithyroidectomy or
total thyroidectomy, hedging against the risk of a
potential cancer and allowing a thorough pathological
examination.
The quest for better diagnostic tests for patients with thyroid nodules has been a long but successful journey. In addition to fine-needle aspiration,valuable tests have included radionuclide
scanning with iodine-123 or technetium-99m, ultrasonography,
and positron-emission tomography.
In recent years, genetic abnormalities associated with thyroid cancer have offered promise as a more definitive means of distinguishing benign and malignant lesions. Unfortunately, most thyroid nodules do not contain one of these highrisk mutations, necessitating lobectomy or thyroidectomy in nodules with mutation-negative cytologic findings.
Alexander et al.3 now provide evidence in the Journal that a new gene-expression classifier test can be used to identify low-risk thyroid nodules among cytologically indeterminate fine-needle aspirates. This test was based on an empirical assessment of more than 247,000 mRNA transcripts associated with pathologically proven benign
or malignant thyroid lesions. The primary finding is that the gene-expression classifier test has a high negative predictive value for cytologically indeterminate nodules (95% for an atypical or follicular lesion of undetermined significance, 94% for a follicular neoplasm or lesion
suggestive of follicular neoplasm, and 85%
for a lesion suggestive of cancer).
It bears emphasizing that this test does not have sufficient
specificity to inform decision making for samples
with clear-cut cytologic results (i.e., benign
or malignant).
Can this new gene-expression test reduce unnecessary surgery? The answer is seemingly “yes,”
but with important caveats. Approximately 75,000 operations are performed for cytologically indeterminate nodules in the United States each year.
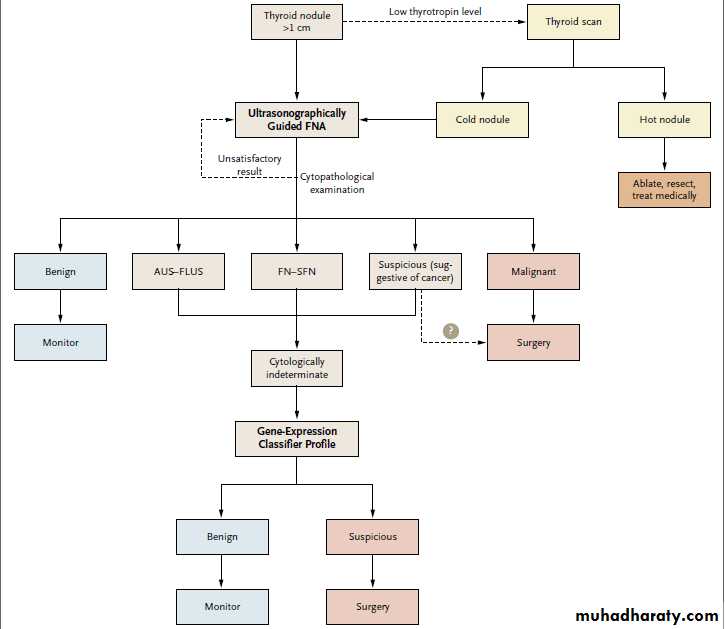
If results of the gene-expression classifier test were used to inform clinical decision making (Fig. 1), it might be possible to reduce surgery for nodules with indeterminate cytology by at least
one third, or about 25,000 operations per year.
Even with the added cost of the test, this approach
could result in substantial cost savings.However, the risk of this approach is that 5 to 10%
of nodules classified as benign (false negatives)
are likely to be malignant, particularly those
that are cytologically indeterminate but suggestive
of cancer. Because this group is at high risk
for cancer, it might be reasonable to repeat the
fine-needle aspiration biopsy or perform a diagnostic hemithyroidectomy even when the geneexpression classifier indicates a benign profile.
Algorithm for Evaluating Thyroid Nodules.
The gene-expression classifier profile allows cytologically indeterminate fine-needle aspirates to be divided into either benign or suspicious
groups, thereby informing the need for and extent of surgery. AUS–FLUS denotes atypical or follicular lesion of undetermined significance,
FNA fine-needle aspiration, and FN–SFN follicular neoplasm or lesion suggestive of follicular neoplasm.
For patients being monitored, it will be important to have a low threshold to repeat fine-needle aspiration if ultrasonographic findings indicate rapid growth or characteristics suggestive of cancer.
Over time, we can anticipate that additional molecular tests will further refine diagnostic accuracy. For example, when known mutations are present (e.g., BRAF V600E, RET/PTC, and PAX8–PPARγ [peroxisome proliferator–activated
receptor gamma 1]), the risk of malignancy is close to 100%.2 In this era of focusing on highquality outcomes at lower cost, this new geneexpression classifier test is a welcome addition to the tools available for informed decision making about the management of thyroid nodules.