TUBERCULOSIS
د. حسين محمد جمعةاختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2011
Tuberculosis can affect virtually any organ system in the body and can be devastating if left untreated. The increasing prevalence of tuberculosis in both immunocompetent and immunocompromised individuals in recent years makes this disease a topic of universal concern. Because tuberculosis demonstrates a variety of clinical and radiologic findings and has a known propensity for dissemination from its primary site, it can mimic numerous other disease entities.
Basic facts about TB
TB is a bacterial disease caused byMycobacterium tuberculosis (and occasionally by Mycobacterium bovis And Mycobacterium africanum ).
These organisms are also known as tubercle bacilli (because they cause lesions called tubercles) or as acid-fast bacilli (AFB).When sputum containing bacilli is stained with certain dyes and examined under the microscope, the bacilli look red.This is because they are acid-fast (they have kept the dye even after being washed with acid and alcohol).Tubercle bacilli can remain dormant in tissues and persist for many years.
Sources of infection
The most important source of infection is the patient with pulmonary TB (PTB),and who is coughing. This person is usually sputum smear-positive.Coughing produces tiny infectious droplet nuclei (infectious particles of respiratory secretions usually less than 5 µm in diameter and containing tubercle bacilli). Asingle cough can produce 3000 droplet nuclei.Droplet nuclei can also be spread into the air by talking, sneezing, spitting and singing, and can remain suspended in the air for long periods.Direct sunlight kills tubercle bacilli in 5 minutes, but they can survive in the dark for long periods.Transmission therefore generally occurs indoors.
Droplet nuclei are so small that they avoid the defences of the bronchi and penetrate into the terminal alveoli of the lungs, where multiplication and infection begin.
Two factors determine an individual's risk of exposure:
the concentration of droplet nuclei in contaminated air and
the length of time he or she breathes that air.
Routes by which TB is not transmitted
TB is not transmitted throughfood and water or by
sexual intercourse,
blood transfusion,
or mosquitoes.
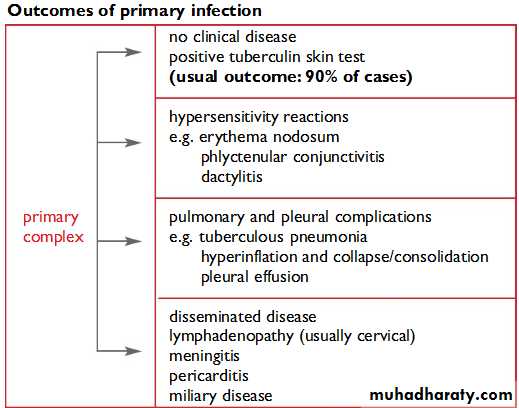
Once infected , a person can stay infected for many years, probably for life.The vast majority (90%) of people without HIV infection who are infected with M. Tuberculosis do not develop TB. In these, asymptomatic but infected individuals, the only evidence of infection may be a positive tuberculin skin test.
Infected persons can develop TB at any time.The disease can affect most tissues and organs, but especially the lungs.The chance of developing disease is greatest shortly after infection and steadily lessens as time goes by. Infected infants and young children are at greater risk of developing disease than older people because they have an immature immune system.TB is also more likely to spread from the lungs to other parts of the body in this age group.
Children who develop disease usually do so within two years following exposure and infection. Most do not develop disease in childhood but may do so later in life.Various physical or emotional stresses may trigger progression of infection to disease.The most important trigger is weakening of immune resistance,especially by HIV infection.
Natural history of untreated TB
Without treatment, by the end of 5 years50% of PTB patients will be dead,
25% will be healthy (self-cured by a strong immune defence) and
25% will remain ill with chronic infectious TB
Pathogenesis of TB
Primary infection
Primary infection occurs in people who have not had any previous exposure to tubercle bacilli. Droplet nuclei, which are inhaled into the lungs, are so small that they avoid the mucociliary defences of the bronchi and lodge in the terminal alveoli of the lungs. Infection begins with multiplication of tubercle bacilli in the lungs.The resulting lesion is the Ghon focus. Lymphatics drain the bacilli to the hilar lymph nodes.The Ghon focus and related hilar lymphadenopathy form the primary complex. Bacilli may spread in the blood from the primary complex throughout the body.
The immune response (delayed hypersensitivity and cellular immunity) develops about 4–6 weeks after the primary infection.The size of the infecting dose of bacilli and the strength of the immune response determine what happens next. In most cases, the immune response stops the multiplication of bacilli. However, a few dormant bacilli may persist.A positive tuberculin skin test would be the only evidence of infection. In a few cases the immune response is not strong enough to prevent multiplication of bacilli, and disease occurs within a few months.
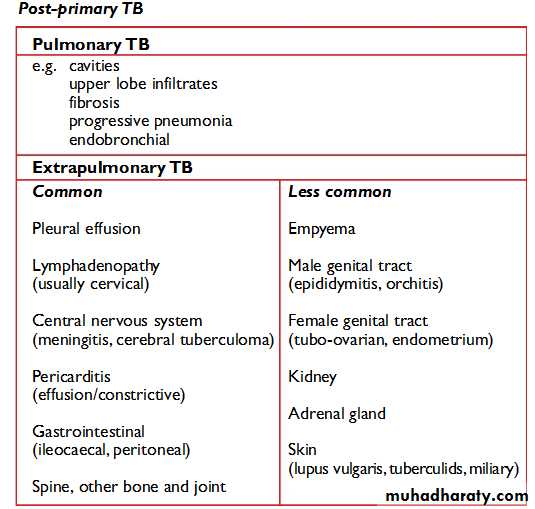
Post-primary TB
occurs after a latent period of months or years following primary infection. It may occur either by reactivation of the dormant tubercle bacilli acquired from a primary infection or by reinfection. Reactivation means that dormant bacilli persisting in tissues for months or years after primary infection start to multiply.This may bein response to a trigger,such as weakening of the immune system by HIV infection. Reinfection means a repeat infection in a person who has previously had a primary infection.The immune response of the patient results in a pathological lesion that is characteristically localized,often with extensive tissue destruction and cavitation.Post-primary TB usually affects the lungs but can involve any part of the body.
The characteristic features of post-primary PTB are the following:• extensive lung destruction with cavitation;
• positive sputum smear;
• upper lobe involvement;
• usually no intrathoracic lymphadenopathy. Patients with these lesions are the main transmitters of infection in the commmunity.
Post-primary infection with pulmonary disease usually occurs in adults and leads to microscopy-positive sputum smears.
Mycobacteria are "acid- and alcohol-fast bacilli" (AAFB),often shortened to
"acid-fast bacilli" (AFB). The waxy coat of mycobacteria retains an aniline dye (e.g. carbol fuchsin) even after decolorization with acid and alcohol.Conditions that may suppress the tuberculin skin test
• HIV infection• Malnutrition
• severe bacterial infections, including TB itself
• viral infections, e.g. measles, chickenpox, glandular fever
• Cancer
• immunosuppressive drugs, e.g. steroids
• incorrect injection of PPD
Tuberculosis treatment refers to the medical treatment of the infectious disease tuberculosis (TB).
Active tuberculosis will kill about two of every three people affected if left untreated.
Treated tuberculosis has a mortality rate of less than 5%. Directly observed therapy (DOT), in which patients are observed to ingest each dose of antituberculosis medications, to maximize the likelihood of completion of therapy.
The standard "short" course treatment for TB is isoniazid, rifampicin ,pyrazinamide, and ethambutol for two months, then isoniazid and rifampicin alone for a further four months. The patient is considered cured at six months
(although there is still a relapse rate of 2 to 3%).
For latent tuberculosis, the standard treatment is six to nine months of isoniazid alone.
If the organism is known to be fully sensitive, then treatment is with isoniazid, rifampicin, and pyrazinamide for two months, followed by isoniazid and rifampicin for four months. Ethambutol need not be used.
• Drug regimens are similarly abbreviated in
• a standardised manner. The drugs are listed using their single letter abbreviations (in the order given above, which is roughly the order of introduction into clinical practice).• A prefix denotes the number of months the treatment should be given for; a subscript denotes intermittent dosing (so 3 means three times a week) and no subscript means daily dosing.
Most regimens have an initial high-intensity phase, followed by a continuation phase (also called a consolidation phase or eradication phase): the high-intensity phase is given first, then the continuation phase, the two phases divided by a slash.
So,
2HREZ/4HR3
means isoniazid, rifampicin, ethambutol, pyrazinamide daily for two months, followed by four months of isoniazid and rifampicin given three times a week.
Second line
There are six classes of second-line drugs (SLDs). A drug may be classed as second-line instead of first-line for one of three possible reasons: it may be less effective than the first-line drugs (e.g., p-aminosalicylic acid); or, it may have toxic side-effects (e.g., cycloserine); or it may be unavailable in many developing countries (e.g., fluoroquinolones):aminoglycosides: e.g., amikacin ,kanamycin (KM);
polypeptides: e.g., capreomycin, viomycin, enviomycin;
Fluoroquinolones: e.g., ciprofloxacin (CIP), levofloxacin, moxifloxacin (MXF);thioamides: e.g. ethionamide, prothionamide,cycloserine (the only antibiotic in its class);p-aminosalicylic acid (PAS or P).
Third line
Other drugs that may be useful, but are not on the WHO list of SLDs:Rifabutin ,macrolides: e.g., clarithromycin (CLR);
linezolid (LZD);thioacetazone (T);thioridazine;arginine;
vitamin D;R207910. These drugs may be considered "third-line drugs" and are listed here either because they are not very effective (e.g., clarithromycin) or because their efficacy has not been proven (e.g., linezolid, R207910). Rifabutin is effective, but is not included on the WHO list because for most developing countries, it is impractically expensive.
Rationale and evidence for the standard regimen
Tuberculosis has been treated with combination therapy for over fifty years. Drugs are not used singly (except in latent TB or chemoprophylaxis), and regimens that use only single drugs result in the rapid development of resistance and treatment failure.The rationale for using multiple drugs to treat TB are based on simple probability. The frequency of spontaneous mutations that confer resistance to an individual drug are well known: 1 in 107 for EMB, 1 in 108 for STM and INH, and 1 in 1010 for RMP.
A patient with extensive pulmonary TB has approximately 1012 bacteria in his body, and therefore will probably be harboring approximately 105 EMB-resistant bacteria, 104 STM-resistant bacteria, 104 INH-resistant bacteria and 10² RMP-resistant bacteria. Resistance mutations appear spontaneously and independently, so the chances of him harbouring a bacterium that is spontaneously resistant to both INH and RMP is 1 in 108 x 1 in 1010 = 1 in 1018, and the chances of him harbouring a bacterium that is spontaneously resistant to all four drugs is 1 in 1033. This is, of course, an oversimplification, but it is a useful way of explaining combination therapy.
There are other theoretical reasons for supporting combination therapy. The different drugs in the regimen have different modes of action. INH are bacteriocidal against replicating bacteria. EMB is bacteriostatic at low doses, but is used in TB treatment at higher, bactericidal doses. RMP is bacteriocidal and has a sterilizing effect. PZA is only weakly bactericidal, but is very effective against bacteria located in acidic environments, inside macrophages, or in areas of acute inflammation.
All TB regimens in use were 18 months or longer until the appearance of rifampicin. In 1953, the standard UK regimen was 3SPH/15PH or 3SPH/15SH2. Between 1965 and 1970, EMB replaced PAS.
RMP began to be used to treat TB in 1968 and the BTS study in the 1970s showed that 2HRE/7HR was efficacious. In 1984, a BTS study showed that 2HRZ/4HR was efficacious, with a relapse rate of less than 3% after two years. In 1995, with the recognition that INH resistance was increasing, the BTS recommended adding EMB or STM to the regimen: 2HREZ/4HR or 2SHRZ/4HR, which are the regimens currently recommended.
The WHO also recommend
a six-month continuation phase of HR• if the patient is still culture positive after 2 months of treatment (approximately 15% of patients with fully sensitive TB) and
• for those patients who have extensive bilateral cavitation at the start of treatment.
Monitoring, DOTS, and DOTS-Plus
DOTS stands for "Directly Observed Treatment, Short-course" and is a major plank in the WHO Global Plan to Stop TB. The DOTS strategy focuses on five main points of action. These include government commitment to control TB, diagnosis based on sputum-smear microscopy tests done on patients who actively report TB symptoms, direct observation short-course chemotherapy treatments, a definite supply of drugs, and standardized reporting and recording of cases and treatment outcomes.The WHO advises that all TB patients should have at least the first two months of their therapy observed (and preferably the whole of it observed): this means an independent observer watching patients swallow their anti-TB therapy. The independent observer is often not a healthcare worker and may be a shopkeeper or a tribal elder or similar senior person within that society. DOTS is used with intermittent dosing (thrice weekly or 2HREZ/4HR3). Twice weekly dosing is effective but not recommended by the WHO, because there is no margin for error (accidentally omitting one dose per week results in once weekly dosing, which is ineffective).
Treatment with properly implemented DOTS has a success rate exceeding 95% and prevents the emergence of further multi-drug resistant strains of tuberculosis.Administering DOTS, decreases the possibilities of tuberculosis from recurring, resulting in a reduction in unsuccessful treatments. This is in part due to the fact that areas without the DOTS strategy generally provide lower standards of care. Areas with DOTS administration help lower the number of patients seeking help from other facilities where they are treated with unknown treatments resulting in unknown outcomes.
However if the DOTS program is not implemented or done so incorrectly positive results will be unlikely. In order for the program to work efficiently and accurately health providers must be fully engaged, links must be built between public and private practitioners, health services must be available to all, and global support is provided to countries trying to reach their TB prevention, and treatment aims. Some researchers suggest that, because the DOTS framework has been so successful in the treatment of tuberculosis in sub-Saharan Africa, DOTS should be expanded to non-communicable diseases such as diabetes mellitus, hypertension, and epilepsy.
The WHO extended the DOTS programme in 1998 to include the treatment of MDR-TB (called "DOTS-Plus"). Implementation of DOTS-Plus requires the capacity to perform drug-susceptibility testing (not routinely available even in developed countries) and the availability of second-line agents, in addition to all the requirements for DOTS. DOTS-Plus is therefore much more resource-expensive than DOTS, and requires much greater commitment from countries wishing to implement it. Resource limitations mean that the implementation of DOTS-Plus may lead inadvertently to the diversion of resources from existing DOTS programmes and a consequent decrease in the overall standard of care.
Monthly surveillance until cultures convert to negative is recommended for DOTS-Plus, but not for DOTS.
If cultures are positive or symptoms do not resolve after three months of treatment, it is necessary to re-evaluate the patient for drug-resistant disease or nonadherence to drug regimen. If cultures do not convert to negative despite three months of therapy, some physicians may consider admitting the patient to hospital so as to closely monitor therapy.
Extra-pulmonary tuberculosis
Tuberculosis not affecting the lungs is called extra-pulmonary tuberculosis. Disease of the central nervous system is specifically excluded from this classification.The UK and WHO recommendation is 2HREZ/4HR; the US recommendation is 2HREZ/7HR. There is good evidence from randomised-controlled trials to say that in tuberculous lymphadenitis and in TB of the spine,the six-month regimen is equivalent to the nine-month regimen; the US recommendation is therefore not supported by the evidence.
Up to 25% of patients with TB of the lymph nodes (TB lymphadenitis) will get worse on treatment before they get better and this usually happens in the first few months of treatment. A few weeks after starting treatment, lymph nodes often start to enlarge, and previously solid lymph nodes may become fluctuant. This should not be interpreted as failure of therapy and is a common reason for patients (and their physicians) to panic unnecessarily. With patience, two to three months into treatment the lymph nodes start to shrink again and re-aspiration or re-biopsy of the lymph nodes is unnecessary.
if repeat microbiological studies are ordered, they will show the continued presence of viable bacteria with the same sensitivity pattern, which further adds to the confusion: physicians inexperienced in the treatment of TB will then often add second-line drugs in the belief that the treatment is not working. In these situations, all that is required is re-assurance. Steroids may be useful in resolving the swelling, especially if it is painful, but they are unnecessary. Additional antibiotics are unnecessary and the treatment regimen does not need to be lengthened.
Tuberculosis of the central nervous system
Tuberculosis may affect the central nervous system (meninges, brain or spinal cord) in which case it is called TB meningitis, TB cerebritis, and TB myelitis respectively; the standard treatment is 12 months of drugs (2HREZ/10HR) and steroid are mandatory.Diagnosis is difficult as CSF culture is positive in less than half of cases, and therefore a large proportion of cases are treated on the basis of clinical suspicion alone. PCR of CSF does not significantly improve the microbiology yield; culture remains the most sensitive method and a minimum of 5 ml (preferably 20 ml) of CSF should be sent for analysis.
TB cerebritis (or TB of the brain) may require brain biopsy in order to make the diagnosis, because the CSF is commonly normal: this is not always available and even when it is, some clinicians would debate whether it is justified putting a patient through such an invasive and potentially dangerous procedure when a trial of anti-TB therapy may yield the same answer; probably the only justification for brain biopsy is when drug-resistant TB is suspected.
It is possible that shorter durations of therapy (e.g., six months) may be sufficient to treat TB meningitis, but no clinical trial has addressed this issue. The CSF of patients with treated TB meningitis is commonly abnormal even at 12 months,the rate of resolution of the abnormality bears no correlation with clinical progress or outcome,and is not an indication for extending or repeating treatment; repeated sampling of CSF by lumbar puncture to monitor treatment progress should therefore not be done.
Although TB meningitis and TB cerebritis are classified together, the experience of many clinicians is that their progression and response to treatment is not the same. TB meningitis usually responds well to treatment, but TB cerebritis may require prolonged treatment (up to two years) and the steroid course needed is often also prolonged (up to six months). Unlike TB meningitis, TB cerebritis often required repeated CT or MRI imaging of the brain to monitor progress.
CNS TB may be secondary to blood-borne spread: therefore some experts advocate the routine sampling of CSF in patients with miliary TB.The anti-TB drugs that are most useful for the treatment of CNS TB are:INH (CSF penetration 100%),RMP (10–20%),EMB (25–50% inflamed meninges only),PZA (100%)
STM (20% inflamed meninges only),LZD (20%)
Cycloserine (80–100%),Ethionamide (100%)
PAS (10–50%) (inflamed meninges only)
The use of steroids is routine in TB meningitis (see section below). There is evidence from one poorly designed trial that aspirin may be beneficial,but further work is required before this can be recommended routinely.
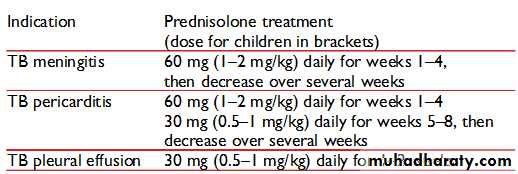
Steroids
The usefulness of corticosteroids (e.g., prednisolone or dexamethasone) in the treatment of TB is proven for TB meningitis and TB pericarditis. The dose for TB meningitis is dexamethasone 8 to 12 mg daily tapered off over six weeks .The dose for pericarditis is prednisolone 60 mg daily tapered off over four to eight weeks.Steroids may be of temporary benefit in pleurisy, extremely advanced TB, and TB in children:
Pleurisy: prednisolone 20 to 40 mg daily tapered off over 4 to 8 weeks
Extremely advanced TB: 40 to 60 mg daily tapered off over 4 to 8 weeks
TB in children: 2 to 5 mg/kg/day for one week, 1 mg/kg/day the next week, then tapered off over 5 weeks
Steroids may be of benefit in peritonitis, miliary disease, tubercular osteomyelitis, TB osteomyelitis, laryngeal TB, lymphadenitis and genitourinary disease, but the evidence is scant and the routine use of steroids cannot be recommended. Steroid treatment in these patients should be considered on a case by case basis by the attending physician.
Thalidomide may be of benefit in TB meningitis and has been used in cases where patients have failed to respond to steroid treatment.
Non-compliance
Patients who take their TB treatment in an irregular and unreliable way are at greatly increased risk of treatment failure, relapse and the development of drug-resistant TB strains.There are variety of reasons why patients fail to take their medication. The symptoms of TB commonly resolve within a few weeks of starting TB treatment and many patients then lose motivation to continue taking their medication. Regular follow-up is important to check on compliance and to identify any problems patients are having with their medication.
Patients need to be told of the importance of taking their tablets regularly, and the importance of completing treatment, because of the risk of relapse or drug-resistance developing otherwise.
One of the main complaints is the bulkiness of the tablets. The main offender is PZA (the tablets being the size of horse tablets). PZA syrup may be offered as a substitute, or if the size of the tablets is truly an issue and liquid preparations are not available, then PZA can be omitted altogether. If PZA is omitted, the patient should be warned that this results in a significant increase in the duration of treatment (details of regimens omitting PZA are given below).
The other complaint is that the medicines must be taken on an empty stomach to facilitate absorption. This can be difficult for patients to follow (for example, shift workers who take their meals at irregular times) and may mean the patient waking up an hour earlier than usual everyday just to take medication. The rules are actually less stringent than many physicians and pharmacists realise: the issue is that the absorption of RMP is reduced if taken with fat, but is unaffected by carbohydrate, protein,[24] or antacids.[25]
So the patient can in fact have his or her medication with food as long as the meal does not contain fat or oils (e.g., a cup of black coffee or toast with jam and no butter). Taking the medicines with food also helps ease the nausea that many patients feel when taking the medicines on an empty stomach.
The effect of food on the absorption of INH is not clear: two studies have shown reduced absorption with food but one study showed no difference.There is a small effect of food on the absorption of PZA and of EMB that is probably not clinically important.
It is possible to test urine for isoniazid and rifampicin levels in order to check for compliance. The interpretation of urine analysis is based on the fact that isoniazid has a longer half-life than rifampicin:
urine positive for isoniazid and rifampicin patient probably fully compliant
urine positive for isoniazid only patient has taken his medication in the last few days preceding the clinic appointment, but had not yet taken a dose that day.
urine positive for rifampicin only patient has omitted to take his medication the preceding few days, but did take it just before coming to clinic.
urine negative for both isoniazid and rifampicin patient has not taken either medicine for a number of days
In countries where doctors are unable to compel patients to take their treatment (e.g., the UK), some say that urine testing only results in unhelpful confrontations with patients and does not help increase compliance. In countries where legal measures can be taken to force patients to take their medication (e.g., the US), then urine testing can be a useful adjunct in assuring compliance.
RMP colours the urine and all bodily secretions (tears, sweat, etc.) an orange-pink colour and this can be a useful proxy if urine testing is not available (although this colour fades approximately six to eight hours after each dose).
Adverse effects
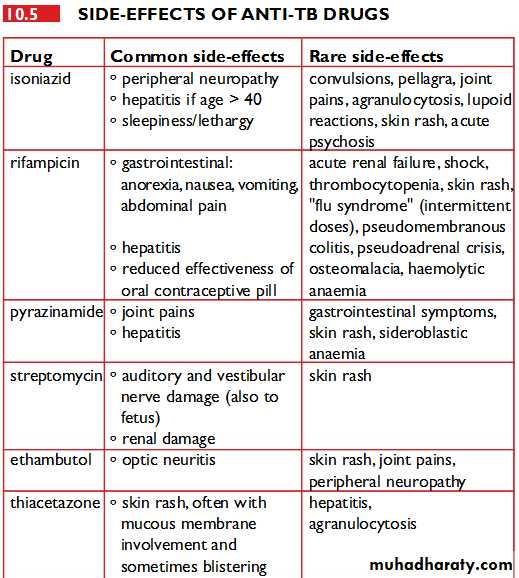
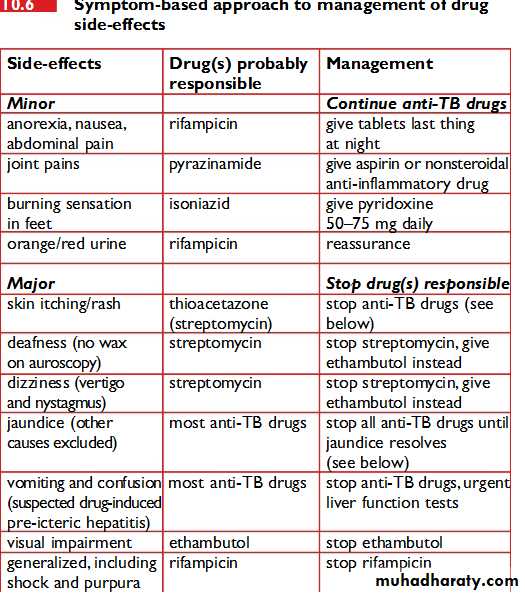
For information on adverse effects of individual anti-TB drugs, please refer to the individual articles for each drug.The relative incidence of major adverse effects has been carefully described:
INH 0.49 per hundred patient months
RMP 0.43
EMB 0.07
PZA 1.48
All drugs 2.47
It can be extremely difficult identifying which drug is responsible for which side effect, but the relative frequency of each is known. The offending drugs are given in decreasing order of frequency:
Thrombocytopenia: RMP
Neuropathy: INH
Vertigo: STM
Hepatitis: PZA, RMP, INH
Rash: PZA, RMP, EMB
Thrombocytopenia is only caused by RMP and no test dosing need be done.
The most frequent cause of neuropathy is INH. The peripheral neuropathy of INH is always a pure sensory neuropathy. Once a peripheral neuropathy has occurred, INH must be stopped and pyridoxine should be given at a dose of 50 mg thrice daily. Simply adding high dose pyridoxine to the regimen once neuropathy has occurred will not stop the neuropathy from progressing. Patients at risk of peripheral neuropathy from other causes (diabetes mellitus, alcoholism, renal failure, malnutrition, pregnancy, etc.) should all be given pyridoxine 10 mg daily at the start of treatment.Rashes are most frequently due to PZA, but can occur with any of the TB drugs. Test dosing using the same regimen as detailed below for hepatitis may be necessary to determine which drug is responsible.
Itching RMP commonly causes itching without a rash in the first two weeks of treatment: treatment should not be stopped and the patient should be advised that the itch usually resolves on its own. Short courses of sedative antihistamines such as chlorpheniramine may be useful in alleviating the itch.
Fever during treatment can be due to a number of causes. It can occur as a natural effect of tuberculosis (in which case it should resolve within three weeks of starting treatment). Fever can be a result of drug resistance (but in that case the organism must be resistant to two or more of the drugs). Fever may be due to a superadded infection or additional diagnosis (patients with TB are not exempt from getting influenza and other illnesses during the course of treatment). In a few patients, the fever is due to drug allergy. The clinician must also consider the possibility that the diagnosis of TB is wrong.
If the patient has been on treatment for more than two weeks and if the fever had initially settled and then come back, it is reasonable to stop all TB medication for 72 hours. If the fever persists despite stopping all TB medication, then the fever is not due to the drugs. If the fever disappears off treatment, then the drugs need to be tested individually to determine the cause. The same scheme as is used for test dosing for drug-induced hepatitis (described below) may be used. The drug most frequently implicated as causing a drug fever is RMP
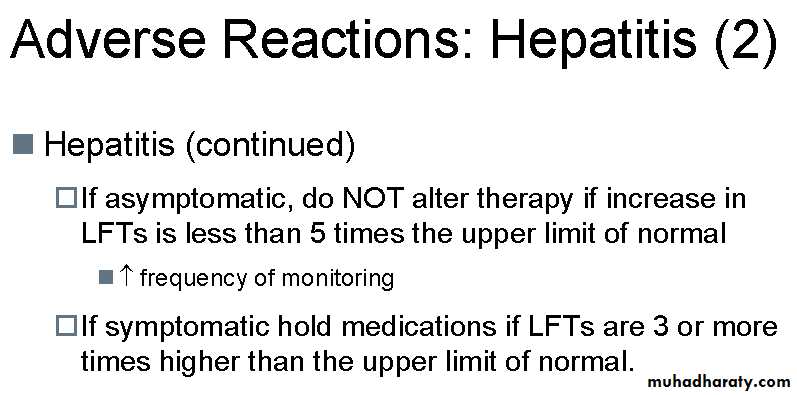
Drug-induced hepatitis
The single biggest problem with TB treatment is drug-induced hepatitis, which has a mortality rate of around 5%. Three drugs can induce hepatitis: PZA, INH and RMP (in decreasing order of frequency). It is not possible to distinguish between these three causes based purely on signs and symptoms. Test dosing must be carried out to determine which drug is responsible.Liver function tests (LFTs) should be checked at the start of treatment, but, if normal, need not be checked again; the patient need only be warned of the symptoms of hepatitis. Some clinicians insist on regular monitoring of LFT's while on treatment, and in this instance, tests need only be done two weeks after starting treatment and then every two months thereafter, unless any problems are detected.
Elevations in bilirubin must be expected with RMP treatment (RMP blocks bilirubin excretion) and usually resolve after 10 days (liver enzyme production increases to compensate). Isolated elevations in bilirubin can be safely ignored.
Elevations in liver transaminases (ALT and AST) are common in the first three weeks of treatment. If the patient is asymptomatic and the elevation is not excessive then no action need be taken; some experts suggest a cut-off of four times the upper limit of normal, but there is no evidence to support this particular number over and above any other number.
Some experts consider that treatment should only be stopped if jaundice becomes clinically evident.
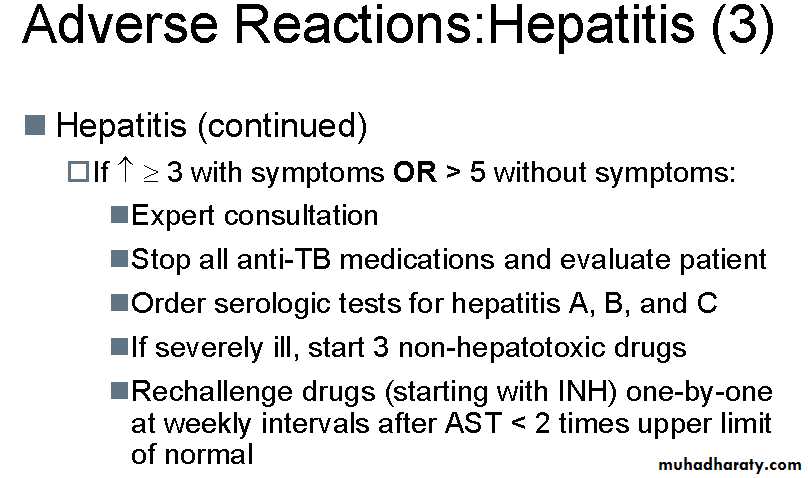
If clinically significant hepatitis occurs while on TB treatment, then all the drugs should be stopped until the liver transaminases return to normal.
If the patient is so ill that TB treatment cannot be stopped, then STM and EMB should be given until the liver transaminases return to normal (these two drugs are not associated with hepatitis).
Fulminant hepatitis can occur in the course of TB treatment, but is fortunately rare; emergency liver transplantation may be necessary and deaths do occur.
Test dosing for drug-induced hepatitis
Drugs should be re-introduced individually. This cannot be done in an outpatient setting, and must be done under close observation. A nurse must be present to take patient's pulse and blood pressure at 15 minute intervals for a minimum of four hours after each test dose is given (most problems will occur within six hours of test dosing, (if they are going to occur). Patients can become very suddenly unwell and access to intensive care facilities must be available. The drugs should be given in this order:Day 1: INH at 1/3 or 1/4 dose
Day 2: INH at 1/2 dose
Day 3: INH at full dose
Day 4: RMP at 1/3 or 1/4 dose
Day 5: RMP at 1/2 dose
Day 6: RMP at full dose
Day 7: EMB at 1/3 or 1/4 dose
Day 8: EMB at 1/2 dose
Day 9: EMB at full dose
No more than one test dose per day should be given, and all other drugs should be stopped while test dosing is being done. So on day 4, for example, the patient only receives RMP and no other drugs are given. If the patient completes the nine days of test dosing, then it is reasonable to assume that PZA has caused the hepatitis and no PZA test dosing need be done. The reason for using the order for testing drugs is because the two most important drugs for treating TB are INH and RMP, so these are tested first: PZA is the most likely drug to cause hepatitis and is also the drug that can be most easily omitted.
EMB is useful when the sensitivity pattern of the TB organism are not known and can be omitted if the organism is known to be sensitive to INH. Regimens omitting each of the standard drugs are listed below.
The order in which the drugs are tested can be varied according to the following considerations:
The most useful drugs (INH and RMP) should be tested first, because the absence of these drugs from a treatment regimen severely impairs its efficacy.
The drugs most likely to be causing the reaction should be tested as late as possible (and possibly need not be tested at all). This avoids rechallenging patients with a drug to which they have already had a (possibly) dangerous adverse reaction.
A similar scheme may be used for other adverse effects (such as fever and rash), using similar principles.
Deviations from the standard regimen
There is evidence supporting some deviations from the standard regimen when treating pulmonary TB. Sputum culture positive patients who are smear negative at the start of treatment do well with only 4 months of treatment ,and sputum culture negative patients do well on only 3 months of treatment .It is unwise to treat patients for only three or four months, but all TB physicians will have patients who stop their treatment early (for whatever reason), and it can be re-assuring to know that sometimes retreatment is unnecessary. Elderly patients who are already taking a large number of tablets may be offered 9HR, omitting PZA which is the bulkiest part of the regimen.It may not always be necessary to treat with four drugs from the beginning. An example might be a close contact of a patient known to have a fully sensitive strain of tuberculosis: in this case, it is acceptable to use 2HRZ/4HR (omitting EMB and STM) in the expectation that their strain will be INH susceptible also. Indeed, this was previously the recommended standard regimen in many countries until the early 1990s, when isoniazid-resistance rates increased.
TB involving the brain or spinal cord (meningitis, encephalitis, etc.) is currently treated with 2HREZ/10HR (12 months of treatment in total), but there is no evidence to say that this is superior to 2HREZ/4HR, it is merely that no-one has been brave enough to do the clinic trial that answers the question if the short course is equivalent.
Regimens omitting isoniazid
Isoniazid resistance in the UK accounts for approximately 6 to 7% of isolates at time of writing (25 Feb 2006). Worldwide, it is the most common type of resistance encountered, hence the current recommendation of using HREZ at the beginning of treatment until sensitivities are known. It is useful to know of current reported outbreaks (like the current outbreak of INH-resistant TB in London).If a patient is discovered to be infected with an isoniazid-resistant strain of TB having completed 2 months of HREZ, then he should be changed to RE for a further 10 months, and the same thing if the patient is intolerant to isoniazid (although 2REZ/7RE may be acceptable if the patient is well supervised). The US recommendation is 6RZE with the option of adding a quinolone such as moxifloxacin. The level of evidence for all these regimens is poor, and there is little to recommend one over the other.
Regimens omitting rifampicin
It is rare for TB strains to be resistant to rifampicin without also being resistant to isoniazid,but rifampicin intolerance is not uncommon (hepatitis or thrombocytopaenia being the most common reasons for stopping rifampicin). Of the first-line drugs, rifampicin is also the most expensive, and in the poorest countries, regimens omitting rifampicin are therefore often used. Rifampicin is the most potent sterilising drug available for the treatment of tuberculosis and all treatment regimens that omit rifampicin are significantly longer than the standard regimen.The UK recommendation is 18HE or 12HEZ. The US recommendation is 9 to 12HEZ, with option of adding a quinolone (for example, MXF).
Regimens omitting pyrazinamide
PZA is a common cause of rash, hepatitis and of painful arthralgia in the HREZ regimen, and can be safely stopped in those patients who are intolerant to it. Isolated PZA resistance is uncommon in M. tuberculosis, but M. bovis is innately resistant to PZA. PZA is not crucial to the treatment of fully sensitive TB, and its main value is in shortening the total treatment duration from nine months to six.There is good evidence from UK trials that a regimen of 9HR is adequate for M. tuberculosis; this is also the first-line regimen used to treat M. bovis.
Regimens omitting ethambutol
EMB intolerance or resistance is rare. If a patient is truly intolerant or is infected with TB that is resistant to EMB, then 2HRZ/4HR is a perfectly acceptable regimen. EMB has no part to play in the treatment of TB that is sensitive to both INH and RMP, and the only reason for including it in the initial regimen is because of increasing rates of INH resistance. If INH resistance rates are known to be low, or if the infecting TB strain is known to be INH-sensitive, then there is no need to use EMB anyway.
Liver disease
People with alcoholic liver disease are at an increased risk of tuberculosis. The incidence of tuberculous peritonitis is particularly high in patients with cirrhosis of the liver.No dosing change needs to be made in the dosing of patients with known liver disease, unless the liver disease is thought to have been caused by TB treatment. Some authorities recommend avoiding PZA in patients with known liver disease, because of the five first-line drugs, PZA has the highest risk of producing drug-induced hepatitis.
Patients with pre-existing liver disease should have their liver function tests monitored regularly throughout TB treatment.
Pregnancy
Pregnancy itself is not a risk factor for TB.Rifampicin makes hormonal contraception less effective, so additional precautions need to be taken for birth control during tuberculosis treatment.
Untreated TB in pregnancy is associated with an increased risk of miscarriage and major fetal abnormality, and treatment of pregnant women. The US guidelines recommend omitting PZA when treating TB in pregnancy; the UK and WHO guidelines make no such recommendation, and PZA is commonly used in pregnancy. There is extensive experience with the treatment of pregnant women with TB and no toxic effect of PZA in pregnancy has ever been found. Pyridoxine supplementation (25 mg/day) is recommended for all women taking INH who are either pregnant or breastfeeding.
High doses of RMP (much higher than used in humans) causes neural tube defects in animals, but no such effect has ever been found in humans. There may be an increased risk of hepatitis in pregnancy and during the puerperium. It is prudent to advise all women of child-bearing age to avoid getting pregnant until TB treatment is completed.
Aminoglycosides (STM, capreomycin, amikacin) should be used with caution in pregnancy, because they may cause deafness in the unborn child. The attending physician must weigh the benefits of treating the mother against the potential harm to the baby, and good outcomes have been reported in children whose mothers were treated with aminoglycosides.Experience in Peru shows that treatment for MDR-TB is not a reason to recommend termination of pregnancy, and that good outcomes are possible.
Kidney disease
Patients with renal failure have a 10 to 30-fold increase in risk of getting TB. Patients with kidney disease who are being given immunosuppressive drugs or are being considered for transplant should be considered for treatment of latent tuberculosis if appropriate.Aminoglycosides (STM, capreomycin and amikacin) should be avoided in patients with mild to severe kidney problems because of the increased risk of damage to the kidneys. If the use of aminoglycosides cannot be avoided (e.g., in treating drug-resistant TB) then serum levels must be closely monitored and the patient warned to report any side-effects (deafness in particular). If patient have end-stage renal failure and have no useful remaining kidney function, then aminoglycosides can be used, but only if drug levels can be easily measured (often only amikacin levels can be measured).
In mild renal impairment, no change needs to be made in dosing any of the other drugs routinely used in the treatment of TB. In severe renal insufficiency (GFR<30), the EMB dose should be halved (or avoided altogether). The PZA dose is 20 mg/kg/day (UK recommendation) or three-quarters the normal dose (US recommendation), but not much published evidence is available to support this.
When using 2HRZ/4HR in patients on dialysis, the drugs should be given daily during the initial high-intensity phase. In the continuation phase, the drugs should be given at the end of each haemodialysis session and no dose should be taken on non-dialysis days.
HIV
In patients with HIV, treatment for the HIV should be delayed until TB treatment is completed, if possible.
The current UK guidance (provided by the British HIV Association) is
CD4 count over 200—delay treatment until the six months of TB treatment are complete.
CD4 count 100 to 200—delay treatment until the initial two-month intensive phase of therapy is complete
CD4 count less than 100—the situation is unclear and patients should be enrolled in clinical trials examining this question. There is evidence that if these patients are managed by a specialist in both TB and HIV then outcomes are not compromised for either disease.[40]
If HIV treatment has to be started while a patient is still on TB treatment, then the advice of a specialist HIV pharmacist should be sought. In general, there is no significant interactions with the NRTI's. Nevirapine should not be used with rifampicin. Efavirenz may be used, but dose used depends on the patient's weight (600 mg daily if weight less than 50 kg; 800 mg daily if weight greater than 50 kg). Efavirenz levels should be checked early after starting treatment (unfortunately, this is not a service routinely offered in the US, but is readily available in the UK). The protease inhibitors should be avoided if at all possible: patients on rifamycins and potease inhibitors have an increased risk of treatment failure or relapse.[41]
The WHO warns against using thioacetazone in patients with HIV, because of the 23% risk of potentially fatal exfoliative dermatitis
Epilepsy
INH may be associated with an increased risk of seizures. Pyridoxine 10 mg daily should be given to all epileptics taking INH. There is no evidence that INH causes seizures in patients who are not epileptic.TB treatment involves numerous drug interactions with anti-epileptic drugs and serum drug levels should be closely monitored. There are serious interactions between rifampicin and carbamazepine, rifampicin and phenytoin, and rifampicin and sodium valproate. The advice of a pharmacist should always be sought.
Drug-resistant tuberculosis (MDR- and XDR-TB)
DefinitionsMulti-drug resistant tuberculosis (MDR-TB) is defined as TB that is resistant at least to INH and RMP. Isolates that are multiply resistant to any other combination of anti-TB drugs but not to INH and RMP are not classed as MDR."Extensively drug-resistant tuberculosis" (XDR-TB) is defined as MDR-TB that is resistant to quinolones and also to any one of kanamycin, capreomycin, or amikacin.The old case definition of XDR-TB is MDR-TB that is also resistant to three or more of the six classes of second-line drugs.This definition should no longer be used, but is included here because many older publications refer to it.
The principles of treatment for MDR-TB and for XDR-TB are the same. The main difference is that XDR-TB is associated with a much higher mortality rate than MDR-TB, because of a reduced number of effective treatment options.The epidemiology of XDR-TB is currently not well studied, but it is believed that XDR-TB does not transmit easily in healthy populations, but is capable of causing epidemics in populations which are already stricken by HIV and therefore more susceptible to TB infection.
Treatment of MDR-TB
The treatment and prognosis of MDR-TB are much more akin to that for cancer than to that for infection. It has a mortality rate of up to 80%, which depends on a number of factors, includingHow many drugs the organism is resistant to (the fewer the better),
How many drugs the patient is given (patients treated with five or more drugs do better),
Whether an injectable drug is given or not (it should be given for the first three months at least),
The expertise and experience of the physician responsible,
How co-operative the patient is with treatment (treatment is arduous and long, and requires persistence and determination on the part of the patient),
Whether the patient is HIV positive or not (HIV co-infection is associated with an increased mortality).
Treatment courses are a minimum of 18 months and may last years; it may require surgery, though death rates remain high despite optimal treatment. That said, good outcomes are still possible. Treatment courses that are at least 18 months long and which have a directly observed component can increase cure rates to 69%.
The treatment of MDR-TB must be undertaken by a physician experienced in the treatment of MDR-TB. Mortality and morbidity in patients treated in non-specialist centres is significantly superior to those patients treated in specialist centres.
In addition to the obvious risks (i.e., known exposure to a patient with MDR-TB), risk factors for MDR-TB include male sex, HIV infection, previous incarceration, failed TB treatment, failure to respond to standard TB treatment, and relapse following standard TB treatment.
Treatment of MDR-TB must be done on the basis of sensitivity testing: it is impossible to treat such patients without this information. If treating a patient with suspected MDR-TB, the patient should be started on SHREZ+MXF+cycloserine pending the result of laboratory sensitivity testing.
A gene probe for rpoB is available in some countries and this serves as a useful marker for MDR-TB, because isolated RMP resistance is rare (except when patients have a history of being treated with rifampicin alone). If the results of a gene probe (rpoB) are known to be positive, then it is reasonable to omit RMP and to use SHEZ+MXF+cycloserine. The reason for maintaining the patient on INH despite the suspicion of MDR-TB is that INH is so potent in treating TB that it is foolish to omit it until there is microbiological proof that it is ineffective.
There are also probes available for isoniazid-resistance (katG and mabA-inhA, but these are less widely available.
When sensitivities are known and the isolate is confirmed as resistant to both INH and RMP, five drugs should be chosen in the following order (based on known sensitivities):
an aminoglycoside (e.g., amikacin, kanamycin) or polypeptide antibiotic (e.g., capreomycin) PZA EMB,a fluoroquinolones: moxifloxacin is preferred (ciprofloxacin should no longer be used[62]);rifabutin
Cycloserine,a thioamide: prothionamide or ethionamide,PAS,a macrolide: e.g., clarithromycin,linezolid,high-dose INH (if low-level resistance),interferon-γ[63],thioridazine
meropenem and clavulanic acid
Drugs are placed nearer the top of the list because they are more effective and less toxic; drugs are placed nearer the bottom of the list because they are less effective or more toxic, or more difficult to obtain.
Resistance to one drug within a class generally means resistance to all drugs within that class, but a notable exception is rifabutin: rifampicin-resistance does not always mean rifabutin-resistance and the laboratory should be asked to test for it. It is only possible to use one drug within each drug class. If it is difficult finding five drugs to treat then the clinician can request that high level INH-resistance be looked for. If the strain has only low level INH-resistance (resistance at 1.0 mg/l INH, but sensitive at 0.2 mg/l INH), then high dose INH can be used as part of the regimen. When counting drugs, PZA and interferon count as zero; that is to say, when adding PZA to a four drug regimen, you must still choose another drug to make five.
It is not possible to use more than one injectable (STM, capreomycin or amikacin), because the toxic effect of these drugs is additive: if possible, the aminoglycoside should be given daily for a minimum of three months (and perhaps thrice weekly thereafter). Ciprofloxacin should not be used in the treatment of tuberculosis if other fluoroquinolones are available.
There is no intermittent regimen validated for use in MDR-TB, but clinical experience is that giving injectable drugs for five days a week (because there is no-one available to give the drug at weekends) does not seem to result in inferior results.
Directly observed therapy certainly helps to improve outcomes in MDR-TB and should be considered an integral part of the treatment of MDR-TB.
Response to treatment must be obtained by repeated sputum cultures (monthly if possible). Treatment for MDR-TB must be given for a minimum of 18 months and cannot be stopped until the patient has been culture-negative for a minimum of nine months. It is not unusual for patients with MDR-TB to be on treatment for two years or more.
Patients with MDR-TB should be isolated in negative-pressure rooms, if possible. Patients with MDR-TB should not be accommodated on the same ward as immunosuppressed patients (HIV infected patients, or patients on immunosuppressive drugs). Careful monitoring of compliance with treatment is crucial to the management of MDR-TB (and some physicians insist on hospitalisation if only for this reason). Some physicians will insist that these patients are isolated until their sputum is smear negative, or even culture negative (which may take many months, or even years). Keeping these patients in hospital for weeks (or months) on end may be a practical or physical impossibility and the final decision depends on the clinical judgement of the physician treating that patient. The attending physician should make full use of therapeutic drug monitoring (particularly of the aminoglycosides) both to monitor compliance and to avoid toxic effects.
Some supplements may be useful as adjuncts in the treatment of tuberculosis, but the for the purposes of counting drugs for MDR-TB, they count as zero (if you already have four drugs in the regimen, it may be beneficial to add arginine or vitamin D or both, but you still need another drug to make five).
arginine (peanuts are a good source)
Vitamin D
The drugs listed below have been used in desperation and it is uncertain whether they are effective at all. They are used when it is not possible to find five drugs from the list above.
imipenem
co-amoxiclav
clofazimine
prochlorperazine
metronidazole[
• The follow drugs are experimental compounds that are not commercially available, but which may be obtained from the manufacturer as part of a clinical trial or on a compassionate basis. Their efficacy and safety are unknown:
• There is increasing evidence for the role of surgery (lobectomy or pneumonectomy) in the treatment of MDR-TB, although whether this is should be performed early or late is not yet clearly defined.
Patients who fail treatment
Patients who respond to treatment and appear to be cured after completing a course of TB treatment are not classed as treatment failures, but as relapses and are discussed in a separate section below.Patients are said to have failed treatment if they
fail to respond to treatment (cough and sputum production persisting throughout the whole of treatment), or
only experience a transient response to treatment (the patient gets better at first, but then get worse again, all the while on treatment).
Patients who fail treatment must be distinguished from patients who relapse. A patient is said to relapse if he gets better while on treatment and only gets worse again after stopping treatment; patients who relapse are discussed in a separate section below.
It is very uncommon for patients not to respond to TB treatment at all (even transiently), because this implies resistance at base-line to all of the drugs in the regimen. Patients who fail to get any response at all while on treatment should first of all be questioned very closely about whether or not they have been taking their medicines, and perhaps even be admitted to hospital to be observed taking their treatment. Blood or urine samples may be taken to check for malabsorption of TB drugs. If it can be shown that they are fully compliant with their medication, then the probability that they have another diagnosis (perhaps in addition to the diagnosis of TB) is very high. These patients should have their diagnosis carefully reviewed and specimens obtained for TB culture and sensitivity testing.
Patients who get better and then get worse again should likewise be question very closely about adherence to treatment. If adherence is confirmed then they should be investigated for resistant TB (including MDR-TB), even if a specimen has already been obtained for microbiology before commencing treatment.
Prescription or dispensing errors will account for a proportion of patients who fail to respond to treatment. Immune defects are a rare cause of non-response. In a tiny proportion of patients, treatment failure is a reflection of extreme biological variation and no cause is found.
In a proportion of patients, all medical and surgical options for treatment will be exhausted and when that point arrives, the patient and his family should be informed that the patient will most likely die of tuberculosis. Care should then be focussed on relief of respiratory symptoms, nutritional requirements and psychological support to enable a dignified death.
Patients who relapse
A patient is said to relapse if he improves while on treatment, but becomes ill again after stopping treatment. Patients who experience only a transient improvement while on treatment, or who never respond to treatment are said to have failed treatment and are discussed above.There is a small relapse rate associated with all treatment regimens, even if the treatment has been taken religiously with 100% compliance (the standard regimen 2HREZ/4HR has a relapse rate of 2 to 3%). The majority of relapses occur within 6 months of finishing treatment. Patients who are more likely to relapse are those who took their medication in an unreliable and irregular fashion.
The probability of resistance is higher in those patients who relapse and every effort must be made to obtain a specimen that can be cultured for sensitivities. That said, most patients who relapse do so with a fully sensitive strain and it is possible that these patients have not relapsed, but have instead been re-infected; these patients can be re-treated with the same regimen as before (no drugs need to be added to the regimen and the duration need not be any longer).
The WHO recommends a regimen of 2SHREZ/6HRE when microbiology is not available (the majority of countries where TB is highly endemic). This regimen was designed to provide optimal treatment for fully sensitive TB (the most common finding in patients who have relapsed) as well as to cover the possibility of INH-resistant TB (the most common form of resistance found).
Because of the life-long risk of relapse, all patients should be warned of the symptoms of TB relapse upon finishing treatment and given strict instructions to return to their doctor if symptoms recur.
Trial of TB treatment
In areas where TB is highly endemic, it is not unusual to encounter patient with a fever, but in whom no source of infection is found. The physician may then, after extensive investigation has excluded all other diseases, resort to a trial of TB treatment.The regimen used is HEZ for a minimum of three weeks; RMP and STM are omitted from the regimen because they are broad spectrum antibiotics, whereas the other three first-line drugs treat only mycobacterial infection. Resolution of the fever after three weeks of treatment is good evidence for occult TB and the patient should then be changed to conventional TB treatment (2HREZ/4HR). If the fever does not resolve after three weeks of treatment then it is reasonable to conclude that the patient has another cause for his fever.• Surgical treatment
• Surgery has played an important part in the management of tuberculosis since the 1940s.• Historical surgical management
• The first successful treatments for tuberculosis were all surgical. They were based on the observation that healed tuberculous cavities were all closed. Surgical management was therefore directed at closing open cavities in order to encourage healing. These procedures were all used in the pre-antibiotic era. There exists a myth that surgeons believed that the purpose was to deprive the organism of oxygen: it was however well known that the organism survives anaerobic conditions. Although these procedures may be considered barbaric by today's standards, it must be remembered that these treatments represented a potential cure for a disease that at the time had a mortality at least as bad as lung cancer today.
Recurrent or persistent pneumothorax
The simplest and earliest procedure was to introduce air into the pleural space so as to collapse the affected lung and therefore the open cavity. There was always spontaneous resolution of the pneumothorax and the procedure had to be repeated every few weeks.
Phrenic nerve crush
The phrenic nerve (which supplies the diaphragm) was cut or crushed so as to permanently paralyse the diaphragm on that side. The paralysed diaphragm would then rise up and the lung on that side would collapse, thus closing the cavity.
Thoracoplasty
When the cavity was located in the apex of the lung, thoracoplasty could be performed. Six to eight ribs were broken and pushed into the thoracic cavity to collapse the lung beneath. This was a disfiguring operation, but it avoided the need for repeated procedures.Plombage
Plombage reduced the need for a disfiguring operation. It involved inserting porcelain balls into the thoracic cavity to collapse the lung underneath.
Surgical resection of infected lung was not possible in the 1940s and 1950s, because the science of anaesthesia at the time was not sufficiently advanced to permit surgery on the lungs of an anaesthetised patient.
Modern surgical management
In modern times, the surgical treatment of tuberculosis is confined to the management of multi-drug resistant TB. A patient with MDR-TB who remains culture positive after many months of treatment may be referred for lobectomy or pneumonectomy with the aim of cutting out the infected tissue. The optimal timing for surgery has not been defined, and surgery still confers significant morbidity The centre with the largest experience in the US is the National Jewish Medical and Research Center in Denver, Colorado.From 1983 to 2000, they performed 180 operations in 172 patients; of these, 98 were lobectomies, and 82 were pneumonectomies. They report a 3.3% operative mortality, with an additional 6.8% dying following the operation; 12% experienced significant morbidity (particularly extreme breathlessness).Of 91 patients who were culture positive before surgery, only 4 were culture positive after surgery.
Some complications of treated tuberculosis like recurrent hemoptysis, destroyed or bronchiectaic lungs and empyema (a collection of pus in the pleural cavity) are also amenable to surgical therapy.
In extrapulmonary TB, surgery is often needed to make a diagnosis (rather than to effect a cure): surgical excision of lymph nodes, drainage of abscesses, tissue biopsy, etc. are all examples of this. Samples taken for TB culture should be sent to the laboratory in a sterile pot with no additive (not even water or saline) and must arrive in the laboratory as soon as possible. Where facilities for liquid culture are available, specimens from sterile sites may be inoculated directly following the procedure: this may improve the yield.
In spinal TB, surgery is indicated for spinal instability (when there is extensive bony destriction) or when the spinal cord is threatened. Therapeutic drainage of tuberculous abscesses or collections is not routinely indicated and will resolve with adequate treatment. In TB meningitis, hydrocephalus is a potential complication and may necessitate the insertion of a ventricular shunt or drain.
Nutrition
It is well known that malnutrition is a strong risk factor for becoming unwell with TB, that TB is itself a risk factor for malnutrition,and that malnourished patients with TB (BMI less than 18.5) are at an increased risk of death even with appropriate antibiotic therapy.Knowledge about the association between malnutrition and TB is prevalent in some cultures, and may reduce diagnostic delay and improve adherence to treatment.
Vitamin D and tuberculosis epidemiology
Vitamin D deficiency is a risk factor for tuberculosis,[97] and vitamin D deficiency appears to impair the body's ability to fight tuberculosis,[98] but there is no clinical evidence to show that treating vitamin D deficiency prevents tuberculosis,[99] although the available evidence is that it ought to. Reduced levels of vitamin D may explain the increased susceptibility of African-Americans to tuberculosis,and may also explain why phototherapy is effective for lupus vulgaris (tuberculosis of the skin) (a finding which won Niels Finsen the Nobel Prize in 1903), because skin exposed to sunlight naturally produces more vitamin D.Concerns that tuberculosis treatment itself decreases vitamin D levels appear not to be an issue in clinical practice.
Genetic differences in the vitamin D receptor in West African,[107]Gujarati[108] and Chinese[109] populations have been noted to affect susceptibility to tuberculosis, but there is no data available in any population that shows vitamin D supplementation (that is, giving extra vitamin D to people with normal vitamin D levels) has any effect on susceptibility to TB.
Vitamin D and tuberculosis treatment
Giving vitamin D to TB patients who are vitamin D deficient is beneficial in a proportion of patients. In a subset of patients with the tt genotype of the TaqI vitamin D receptor and who are vitamin D deficient, vitamin D supplementation appears to hasten sputum culture conversion.Giving vitamin D supplements to TB patients who have normal vitamin D levels does not provide any benefit from the point of view of TB.It was noted as early as the mid-19th century that cod liver oil (which is rich in vitamin D) improved patients with tuberculosis,and the mechanism for this is probably an enhancement of immune responses to tuberculosis.
The addition of vitamin D appears to enhance the ability of monocytes and macrophages to kill M. tuberculosis in vitroas well as ameliorating potentially harmful effects of the human immune system.
Latent tuberculosis
The treatment of latent tuberculosis infection (LTBI) is essential to controlling and eliminating TB by reducing the risk that TB infection will progress to disease.The terms "preventive therapy" and "chemoprophylaxis" have been used for decades and are preferred in the UK because it involves giving medication to people who have no active disease and are currently well, the reason for treatment is primarily to prevent people from becoming unwell. The term "latent tuberculosis treatment" is preferred in the US because the medication does not actually prevent infection: it prevents an existing silent infection from becoming active.
The feeling in the US is that the term "treatment of LTBI" promotes wider implementation by convincing people that they are receiving treatment for disease. There are no convincing reasons to prefer one term over the other.
It is essential that assessment to rule out active TB is carried out before treatment for LTBI is started. To give LTBI treatment to someone with active TB is a serious error: the TB will not be adequately treated and there is a risk of developing drug-resistant strains of TB.
There are several treatment regimens available:
9H—Isoniazid for 9 months is the gold standard and is 93% effective.
6H—Isoniazid for 6 months might be adopted by a local TB program based on cost-effectiveness and patient compliance. This is the regimen currently recommended in the UK for routine use. The US guidance exclude this regimen from use in children or persons with radiographic evidence of prior tuberculosis (old fibrotic lesions). (69% effective)
6 to 9H2—A twice-weekly regimen for the above two treatment regimens is an alternative if administered under Directly observed therapy (DOT).
4R—Rifampicin for 4 months is an alternative for those who are unable to take isoniazid or who have had known exposure to isoniazid-resistant TB.
3HR—Isoniazid and rifampicin may be given for 3 months.
2RZ—The 2-month regimen of rifampicin and pyrazinamide is no longer recommended for treatment of LTBI because of the greatly increased risk of drug-induced hepatitis and death.
3RPT/INH - 3-month (12-dose) regimen of weekly rifapentine and isoniazid.
Current research
There is some evidence from animal[125] and clinical studies[126] that suggests that moxifloxacin-containing regimens as short as four months may be as effective as six months of conventional therapy.[127]Bayer is currently running a Phase II clinical trial in collaboration with the TB Alliance to evaluate shorter treatment regimens for TB;[128] encouragingly, Bayer have also promised that if the trials are successful, Bayer will make moxifloxacin affordable and accessible in countries that need it.The following drugs are experimental compounds that are not commercially available, but which may be available from the manufacturer as part of a clinical trial or on a compassionate basis. Their efficacy and safety are unknown:
PA-824[78] (manufactured by PathoGenesis Corporation, Seattle, Washington)R207910[79] (under development by Johnson & Johnson)
A Ukrainian herbal product has been the subject of several small, open label clinical trials, with promising results in TB patients[129][130] and in patients with TB/HIV coinfection.[131] Open label trials with Dzherelo/Immunoxel have also been positive in Multi-drug-resistant tuberculosis patients[132] and Extensively drug-resistant tuberculosis patients.[133] Stirling Products Ltd of Australia has announced further work in drug-resistant TB and in TB/HIV in trials in Nigeria.[134]
V-5 Immunitor (known as "V5") is an oral hepatitis B and hepatitis C[135] treatment vaccine administered as simple tablets. In patients co-infected with hepatitis C and tuberculosis, TB sputum clearance was unexpectedly noted within only one month. Further blinded studies at multiple trial centres suggest that V5 is equally effective against multiple drug resistant tuberculosis (MDR-TB).[
Various pharmaceutical tuberculosis treatments their actions
MANAGEMENT OF HEPATITIS
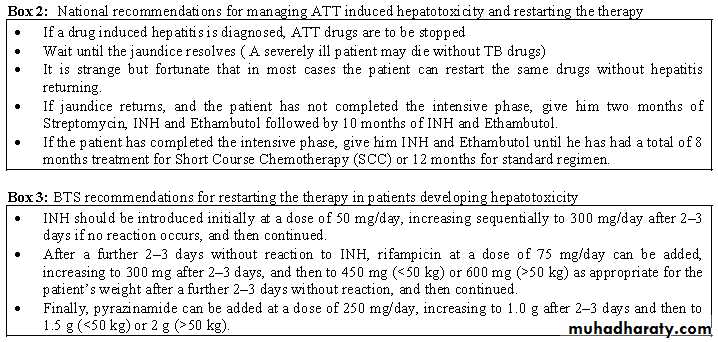
Most anti-TB drugs can damage the liver.Isoniazid and pyrazinamide are most commonly responsible. Ethambutol is rarely responsible.When apatient develops hepatitis during anti-TB treatment, the cause may be the anti-TB treatment or something else. It is often difficult to find out.Try to rule out other possible causes before deciding that the hepatitisis drug-induced. Hepatitis presents with anorexia, jaundice and often liver enlargement.If you diagnose drug-induced hepatitis,stop the anti-TB drugs.Wait until the jaundice or hepatic symptoms have resolved and the liver enzymes have returned to baseline.
If liver enzymes cannot be measured, then itis advisable to wait two weeks after the jaundice has disappeared beforere commencing anti-TB treatment.
It is strange, but fortunate,that in most cases the patient can restart the same anti-TB drugs without hepatitis returning.This can be done either gradually or all at once (if the hepatitis was mild). If the hepatitis has been life-threatening,it is probably safer to use the standard regimen of streptomycin, isoniazid and ethambutol.
A severely ill TB patient may die without anti-TB drugs.In this case,treat the patient with 2 of the least hepatotoxic drugs, streptomycin and ethambutol. When the hepatitis resolves, restart usual anti-TB treatment. In the face of extensive TB, the fluoroquinolones, especial lyofloxacin, can be considered in conjunction with streptomycin and ethambutol as an interim non-hepatotoxic regimen.
USE OF ANTI-TB DRUGS IN SPECIAL SITUATIONS
Pregnancy* Streptomycin during pregnancy can cause permanent deafness in the baby.*
Do not give streptomycin in pregnancy .
Use ethambutol instead.* Isoniazid, rifampicin, pyrazinamide and ethambutol are safe to use.*
Second-line drugs such as fluoroquinolones,
ethionamide andprotionamide are teratogenic, and should not be used.
Breastfeeding women
* All anti-TB drugs are compatible with breastfeeding.
Renal failure
Rifampicin, isoniazid and pyrazinamide are safe and can be given in normal dosages. Patients with severe renal failure should receive pyridoxine with isoniazid to prevent peripheral neuropathy.* Ethionamide and protionamide are also safe.* The excretion of streptomycin is renal.The excretion of ethambutol and thioacetazone is partly renal.*
Avoid streptomycin and ethambutol if there are alternatives.Otherwise give in reduced doses at less frequent intervals.*Do not give thioacetazone.The margin between the therapeuticand toxic dose is too narrow.* The safest regimen to give to patients in renal failure is 2HRZ/4HR
Liver disease
* Most anti-TB drugs can cause liver damage and therefore care is needed.*Do not give pyrazinamide because this is the most hepatotoxic anti-TB drug.
* Isoniazid and rifampicin plus one or two non-hepatotoxic drugs, such as streptomycin and ethambutol, can be used for a total treatment duration of eight months.* If the patient has severe liver damage, an alternative regimen is streptomycin plus isoniazid plus ethambutol in the initial phase followed by isoniazid and ethambutol in the continuation phase with atotal duration of 12 months.* Recommended regimens are 2SRHE/6HE or 2SHE/10HE.
What are the indications for treatment with steroids?
• TB meningitis (decreased consciousness, neurological defects, or spinal block).*• TB pericarditis (with effusion or constriction).*
• TB pleural effusion (when large with severe symptoms).*
• Hypoadrenalism (TB of adrenal glands).*
• TB laryngitis (with life-threatening airway obstruction).*
• Severe hypersensitivity reactions to anti-TB drugs.*
• Renal tract TB (to prevent ureteric scarring).*
• Massive lymph node enlargement with pressure effects.
What are the recommended treatment doses of prednisolone?
Rifampicin is a potent inducer of hepatic enzymes that metabolize steroids. The effective dose of prednisolone is therefore half the prescribed treatment dose given to the patient.The table below shows suggested treatment doses of prednisolone.Frequently Asked Questions about Tuberculosis by
DEPARTMENT OF RESPIRATORY MEDICINE, QUEEN ELIZABETH HOSPITAL in Malaysia.January 16, 2010
What does the result sputum direct smear AFB >50/L or 2/3L mean?>50/L means more than 50 AFBs are seen on one line on the slide under the microscope.2/3L means only 2 AFBs are seen on 3 lines on the slide. The letter L does not stand for litre. Obviously >50/L is more infectious than 2/3L and you can use this result to monitor your patient progress while on TB treatment.
When is it mandatory to admit a TB patient to the ward? Admit your TB patient if he develops complications such as massive haemoptysis or pneumothorax, if you suspect your patient will be non-compliant to treatment, poor family support or no proper home. TB involving the vital organs such as the brain, heart, adrenal glands, kidneys, spine etc also need admission.
What category of TB patient is infectious? Only pulmonary tuberculosis (TB of lung parenchyma) is infectious in the following order:-Smear positive case – very infectiousSmear negative but culture positive – less infectiousSmear negative and culture negative – not infectiousAll extra pulmonary TB are non-infectiousWhen does a smear positive TB patient become non-infectious? As a general rule, if the AFB germs are sensitive to the first line TB drugs, the patient will be non-infectious after one month of treatment.
What is the duration of treatment for TB lymphadenitis? At least 9 months. What is the duration of treatment for other extra pulmonary TB? Should be longer than 9 months since it is difficult to assess response to treatment in extra pulmonary TB unlike pulmonary tuberculosis where you can use chest X-ray. The duration is not fixed and you have to use good clinical judgment to decide the duration of treatment. The longer the duration of treatment, the less chance of relapse.
When do you give Isoniazide prophylaxis and what is the duration of prophylaxis? Isoniazide prophylaxis is a controversial issue but Isoniazide prophylaxis is given in only 2 conditions
• children under 5 years old who are contacts of smear positive cases with positive Mantoux AND
• HIV positive patients with positive Mantoux. The duration of prophylaxis is between 9 months to 1 year. IT IS IMPORTANT TO EXCLUDE ACTIVE TUBERCULOSIS BEFORE YOU DECIDE GIVING PROPHYLAXIS.
My patient develops mild pruritus or mild skin rash after initiation of TB treatment, what should I do? If it is just pruritus or very mild skin rash, reassure the patient and give symptomatic treatment; it is better to continue the TB drugs and advise the patient to come back if it gets worse. You can also give symptomatic treatment for mild gastro-intestinal upset.
My patient develops severe skin rash with anti-TB drugs; what should I do? Stop the TB drugs and review the patient regularly till the rashes subside. Thereafter, you start drug challenge (one drug at a time). The first drug you give is Isoniazide; please give full dose (based on patient's body weight) on the first day then followed by full dose of Rifampicin, Pyrazinamide, Ethambutol and or Streptomycin if necessary. Do not give low dose on day 1 and then increase gradually on day 2 and so on. The idea is to identify the offending drug. After you have identified the offending drug, please consult a respiratory physician for further advice.
My patient has raised liver enzymes; can I give anti-TB drugs? give anti-TB drugs if the liver enzymes are only twice elevated but if more than three times the normal limit, you have to wait till they normalize. If the patient develops hepatitis, you have to stop the treatment and wait till the hepatitis resolves. You may do re-challenge to identify the offending drug causing hepatitis. Giving lower dosage is NOT the way to prevent hepatitis as this can lead to resistance. In some cases where patients are very ill and smear positive with severe hepatitis, I do not do re-challenge as it is time consuming and I will advise you the alternate regimen to use.
My patient has TB but she is pregnant, are the TB drugs safe in pregnancy? Just give EHRZ. Streptomycin is contraindicated in pregnancy.My patient has TB but has renal failure; what regimen do I give? Just use HRZ regimen and prolong the duration of treatment. Streptomycin and Ethambutol are contraindicated in renal failure.
What is the re-treatment regimen for relapsed TB cases (cured of TB in the past and now AFB smear is positive again)?WHO recommends 2 months of SEHRZ, 1 month of EHRZ and 5 months of EHR ALL DAILY. Please send sputum for AFB culture and sensitivity in all relapsed PTB cases to ensure no resistant TB. Please review the AFB culture results.
What are the ‘10 principles’ of TB management? The ‘10 principles’ are: NEVER ADD A SINGLE DRUG TO A FAILING REGIMEN (repeat this 10 times and it becomes the ‘10 principles’). A single drug in a failing regimen is as good as monotherapy and this will lead to resistance to that drug.
How effective is BCG vaccination in preventing TB? BCG vaccination has been proven to be effective in reducing the risk of miliary TB and TB meningitis in children. It is not effective in preventing TB in adults. We are waiting for better TB vaccines in future.
Is steroid useful in tuberculous pleural effusion? No. I would not advocate steroid in tuberculous pleural effusion. A Spanish study and a report from South Africa showed no benefit of steroid in tuberculous pleural effusion. It is more important to achieve early complete drainage of the pleural effusion to prevent pleural fibrosis.
Latent tuberculosis is where a patient is infected with Mycobacterium tuberculosis, but does not have active tuberculosis disease. Patients with latent tuberculosis are not infectious, and it is not possible to get TB from someone with latent tuberculosis.
The main risk is that approximately 10% (5% in the first two years after infection and 0.1% per year thereafter but higher risk if immunosuppressed) of these patients will go on to develop active tuberculosis at a later stage of their life.
This is particularly true if there is development of a disease affecting the immune system (such as AIDS) or a disease whose treatment affects the immune system (such as chemotherapy in cancer or systemic steroids in asthma or Enbrel, Humira or Orencia in rheumatoid arthritis) or in cirucumstances resulting in malnutrition (such as illness or injury affecting the digestive system, or prolonged period of not eating, or disturbance in availability such as famine, residence in refugee camp or concentration camp, or civil war. We also recall that just "old age" affects the immune system also.The identification and treatment of people with latent TB is an important part of controlling this disease.
Tests for latent tuberculosis
There are currently two major classes of tests used to identify patients with latent tuberculosis: tuberculin skin tests and γ-interferon tests. The tuberculin skin tests in use include (but are not limited to)Mantoux test
Heaf test
Tine test (often misspelled as tyne)
There are currently three γ-interferon (interferon gamma release assay - IGRA) tests available.
• T-SPOT.TB
• QuantiFERON-TB Gold
• QuantiFERON-TB Gold In-Tube
Tuberculin skin testing
The tuberculin skin test has its origins in the late 19th century. Crudely speaking, tuberculin (also called purified protein derivative or PPD) is a standardised killed extract of cultured TB, injected into the skin to measure the reaction that person's immune response to TB. There are three methods of testing, the Mantoux test, the Heaf test and the Tine test. The Heaf test was preferred in the UK because it requires less training to administer and because there is less interobserver variation in its interpretation. The Heaf test was stopped in 2005 because the manufacturer did not find it financially sustainable to continue manufacturing the test.• Mantoux test
• The Mantoux test is now standardised by the WHO. 0.1 ml of tuberculin (100 units/ml) is given by intradermal injection into the volar surface of the forearm (subcutaneous injection results in false negative results). A waterproof ink mark is drawn around the injection site so as to avoid difficulty finding it later if the level of reaction is small. The test is read two to seven days afterwards. The area of induration (NOT erythema) is measured transversely across the forearm (left to right, not up and down) and recorded to the nearest millimetre.• Heaf test
• first described in 1951 (Heaf 1951, pp. 151–3). The test uses a Heaf gun with disposable single-use heads; each head has six needles arranged in a circle. There are standard heads and pediatric heads: the standard head is used on all patients aged 2 years and older; the pediatric head is for infants under the age of 2. For the standard head, the needles protrude 2 mm when the gun is actuated; for the pediatric heads, the needles protrude 1 mm. Skin is cleaned with alcohol, then tuberculin (100,000 units/ml) is evenly smeared on the skin (about 0.1 ml); the gun is then applied to the skin and fired. The excess solution is then wiped off and a waterproof ink mark is drawn around the injection site. The test is read 2 to 7 days later.It is not easy to translate between Heaf and Mantoux, but
Heaf grade 0 & 1 ~ Mantoux less than 5 mm;Heaf grade 2 ~ Mantoux 5–14 mm;
Heaf grade 3 & 4 ~ Mantoux 15 or greater
The tuberculin used for Heaf tests is 1000 times more concentrated than that used for Mantoux tests. In countries where both tests are used, use of the correct concentration avoids false positive and false negative results.
Tuberculin conversion
Tuberculin conversion is said to occur if a patient who has previously had a negative tuberculin skin test develops a positive tuberculin skin test at a later date. This is strong evidence for significant exposure to TB. The UK recommendation is that the two tests be done at least six weeks apart; the U.S. recommendation is that the two tests can be done as little as one week apart.Boosting
The phenomenon of boosting occurs when people who have had remote infection with Mycobacterium tuberculosis and/or previous exposure to Bacille Calmette Guerin of Mycobacterium bovis (BCG), an attenuated strain of Mycobacterium bovis that is used in some countries as a vaccine against tuberculosis are given repeated tuberculin skin tests. In these people, the first test revives or primes the immune response so that on repeat testing, the response is much stronger and the patient then looks as if he now has a positive reaction. The second tuberculin skin test result is the correct one.
The UK and U.S. guidelines approach the phenomenon of boosting differently. Under U.S. guidelines, which say to ignore previous immunisation with BCG, a person showing the phenomenon of boosting will be falsely described as a tuberculin converter. On the other hand, UK guidelines advise two tuberculin skin tests one week apart if boosting is suspected, taking the result of the second test as being the true result.
Boosting can occur up to two years after the first Mantoux test.
Interpretation of tuberculin skin tests
According to the U.S. guidelines, latent tuberculosis is diagnosed on a Mantoux test if there is more than 10 (recent immigrant, under age of 4 or have other risk factors for the disease) or 15 mm (no risk factors) of induration. In high risk groups, such as those who are HIV positive, the cut-off is 5 mm on induration. The U.S. guidelines recommend that a history of previous BCG vaccination should be ignored. For details of tuberculin skin test interpretation, please refer to the CDC guidelines (reference given below).The UK guidelines are formulated according to the Heaf test: In patients who have had BCG previously, latent TB is diagnosed if the Heaf test is grade 3 or 4 and have no signs or symptoms of active TB; if the Heaf test is grade 0 or 1, then the test is repeated and. In patients who have not had BCG previously, latent TB is the Heaf test if grade 2, 3 or 4, and have no signs or symptoms of active TB. Repeat Heaf testing is not done in patients who have had BCG (because of the phenomenon of boosting). For details of tuberculin skin test interpretation, please refer to the BTS guidelines (references given below).
Given that the US recommendation is that prior BCG vaccination be ignored in the interpretation of tuberculin skin tests, false positives are possible as a result of:
(1) people who have previously had BCG (even many years ago) may still have a false positive Mantoux test, and
(2) serial testing with tuberculin skin tests boosts the immunological response in those people who have previously had BCG, so these people will falsely appear to be tuberculin converters. This may lead to treating more people than necessary, with the possible risk of those patients suffering adverse drug reactions. However, as Bacille Calmette-Guérin vaccine is not 100% effective, and is less protective in adults than pediatric patients, not treating these patients could lead to a possible infection.
The current US policy seems to a reflect a desire to err on the side of safety (of the general public).
The U.S. guidelines also allow for tuberculin skin testing in immunosuppressed patients (those with HIV, or who are on immunosuppressive drugs), whereas the UK guidelines recommend that tuberculin skin tests should not be used for such patients because it is unreliable.
• γ-interferon testing
• The role of gamma interferon tests is under going constant review and various guidelines have been published with the option for revision as new data comes to hand.CDC:MMWR Health Protection Agency:UK• There are currently (26 March 2009) three commercially available interferon-γ release assays (IGRAs):
• QuantiFERON-TB Gold,
• QuantiFERON-TB Gold In-Tube and
• T-SPOT.TB.
• These tests are not affected by prior BCG vaccination, and look for the body's response to specific TB antigens not present in other forms of mycobacteria and BCG (ESAT-6). Whilst these tests are new they are now becoming available globally.
CDC recommends that QFT-G may be used in all circumstances in which the TST is currently used, including contact investigations, evaluation of recent immigrants, and sequential-testing surveillance programs for infection control (e.g., those for health-care workers).
HPA Interim Guidance:
The HPA recommends the use of IGRA testing in health care workers, if available, in view of the importance of detecting latently infected staff who may go on to develop active disease and come into contact with immunocompromised patients and the logistical simplicity of IGRA testing.
Treatment
The treatment of latent tuberculosis infection (LTBI) is essential to controlling and eliminating TB by reducing the risk that TB infection will progress to disease. The gold standard of treatment is nine months of isoniazid, and this regimen is still widely used in the U.S. (but not elsewhere).Terminology
There is no agreement regarding terminology: the terms preventive therapy and chemoprophylaxis have been used for decades, and are preferred in the UK because it involves giving medication to people who have no disease and are currently well: the reason for giving medication is primarily to prevent people from becoming unwell. In the U.S., physicians talk about latent tuberculosis treatment because the medication does not actually prevent infection: the person is already infected and the medication prevents existing silent infection from becoming active disease. There are no convincing reasons to prefer one term over the other.Treatment regimens
It is essential that assessment to rule out active TB be carried out before treatment for LTBI is started. To give treatment for latent tuberculosis to someone with active tuberculosis is a serious error: the tuberculosis will not be adequately treated and there is a serious risk of developing drug-resistant strains of TB.There are several treatment regimens currently in use:
9H — Isoniazid for 9 months is the gold standard (93% effective).6H — Isoniazid for 6 months might be adopted by a local TB program based on cost-effectiveness and patient compliance. This is the regimen currently recommended in the UK for routine use. The U.S. guidance excludes this regimen from use in children or persons with radiographic evidence of prior tuberculosis (old fibrotic lesions) (69% effective).
6 to 9H2 — An intermittent twice-weekly regimen for the above 2 treatment regimens is an alternative if administered under Directly observed therapy (DOT).
4R — Rifampin for 4-months is an alternative for those who are unable to take isoniazid or who have had known exposure to isoniazid-resistant TB.
3HR — Isoniazid and rifampin may be given daily for three months.
2RZ — The two month regimen of rifampin and pyrazinamide is no longer recommended for treatment of LTBI because of the greatly increased risk of drug-induced hepatitis and death.[3]
3RPT/INH - three-month (12-dose) regimen of weekly rifapentine and isoniazid.[
Multi-drug-resistant TB
There is no evidence for any regimen used for persons with known exposure to MDR-TB and no consensus on optimal treatment. A regimen consisting of ethambutol and PAS has been used before. It would appear sensible to choose a combination of antibiotics based on the known sensitivities of the organism. The CDC have recommended a combination of pyrazinamide and ethambutol, with either pyrazinamide or fluoroquinolone. Immunocompetent contacts should be treated for 6 months; immunocompromised contacts should be treated for 12 months.
Research
Although a tuberculosis cure was developed more than 50 years ago, TB continues to kill from 2 to 3 million people every year. According to the World Health Organization (WHO) approximately 36 million people will die of tuberculosis by 2020 if it is not controlled. They add that eight million people develop active tuberculosis every year. Approximately 98 percent of those victims are from developing countries.[9] Furthermore, WHO estimates that approximately one-third of the world's population are carriers of latent tuberculosis.Therefore, the scientific community continues its research efforts to learn more from this disease in order to develop more options for possible treatments.
The factors that trigger latent tuberculosis infection to progress into active disease have been unknown. However, Professor David Russell and his group at Cornell University in New York, USA, recently demonstrated that the bacteria responsible for causing TB are able to hijack fat metabolism in the host to drive the progression of the disease.
The research made clear that the Mycrobacterium tuberculosis is able to stimulate the immune cells they infect resulting in the accumulation of fat droplets. These droplets turn into what they called “foamy” cells and such transformation can trigger a reawakening of the TB infection from its latent state. Professor Russell believes that more knowledge about the bacterium's life cycle and its host interactions will open possibilities to develop new treatments.[10]
Another group of researchers at Sydney's Centenary Institute made an important discovery that could lead to the development of a new drug for TB. The team, led by Dr Nick West, investigated a protein that is essential for TB to survive and later they reported some success in developing a drug that would inhibit this protein. If the group is successful in finding a way to treat latent tuberculosis, millions of people around the world could be saved.[11]
Furthermore, researchers from the Genome Institute of Singapore together with collaborators in The Netherlands, Indonesia, United Kingdom, and the Russian Federation, identified a new gene that would provide susceptibility to pulmonary tuberculosis. The gene is named Toll-like receptor 8 (TLR8) and it recognizes some factors from viruses such as HIV. This gene is believed to also have a role in human susceptibility to Mycobacterium tuberculosis infections. This discovery could open new options for therapeutic interventions.
Elevations in bilirubin must be expected with RMP treatment (RMP blocks bilirubin excretion) and usually resolve after 10 days (liver enzyme production increases to compensate). Isolated elevations in bilirubin can be safely ignored.
Elevations in liver transaminases (ALT and AST) are common in the first three weeks of treatment. If the patient is asymptomatic and the elevation is not excessive then no action need be taken; some experts suggest a cut-off of four times the upper limit of normal, but there is no evidence to support this particular number over and above any other number.
Some experts consider that treatment should only be stopped if jaundice becomes clinically evident.
If clinically significant hepatitis occurs while on TB treatment, then all the drugs should be stopped until the liver transaminases return to normal.
If the patient is so ill that TB treatment cannot be stopped, then STM and EMB should be given until the liver transaminases return to normal (these two drugs are not associated with hepatitis).
Thrombocytopenia is only caused by RMP and no test dosing need be done. Regimens omitting RMP are discussed below. Please refer to the entry on rifampicin for further details.
The most frequent cause of neuropathy is INH. The peripheral neuropathy of INH is always a pure sensory neuropathy and finding a motor component to the peripheral neuropathy should always prompt a search for an alternative cause. Once a peripheral neuropathy has occurred, INH must be stopped and pyridoxine should be given at a dose of 50 mg thrice daily. Simply adding high dose pyridoxine to the regimen once neuropathy has occurred will not stop the neuropathy from progressing.
Patients at risk of peripheral neuropathy from other causes (diabetes mellitus, alcoholism, renal failure, malnutrition, pregnancy, etc.) should all be given pyridoxine 10 mg daily at the start of treatment. Please refer to the entry on isoniazid for details on other neurological side effects of INH.
Rashes are most frequently due to PZA, but can occur with any of the TB drugs. Test dosing using the same regimen as detailed below for hepatitis may be necessary to determine which drug is responsible.
Itching RMP commonly causes itching without a rash in the first two weeks of treatment: treatment should not be stopped and the patient should be advised that the itch usually resolves on its own. Short courses of sedative antihistamines such as chlorpheniramine may be useful in alleviating the itch.
Fever during treatment can be due to a number of causes. It can occur as a natural effect of tuberculosis (in which case it should resolve within three weeks of starting treatment). Fever can be a result of drug resistance (but in that case the organism must be resistant to two or more of the drugs). Fever may be due to a superadded infection or additional diagnosis (patients with TB are not exempt from getting influenza and other illnesses during the course of treatment). In a few patients, the fever is due to drug allergy.
The clinician must also consider the possibility that the diagnosis of TB is wrong. If the patient has been on treatment for more than two weeks and if the fever had initially settled and then come back, it is reasonable to stop all TB medication for 72 hours. If the fever persists despite stopping all TB medication, then the fever is not due to the drugs. If the fever disappears off treatment, then the drugs need to be tested individually to determine the cause. The same scheme as is used for test dosing for drug-induced hepatitis may be used. The drug most frequently implicated as causing a drug fever is RMP.
What new regimen is effective for the treatment of latent tuberculosis?
This study showed that for the treatment of latent M. tuberculosis, directly observed, once-weekly therapy with rifapentine plus isoniazid for 3 months was as effective as self-administered daily isoniazid for 9 months, with the rate of tuberculosis in the combination-therapy group approximately half that in the isoniazid-only group.Teaching Topics NEJM | December 8, 2011
Q. Which patients are at highest risk of conversion from latent M. tuberculosis infection to active disease?
A. Persons at high risk of conversion from latent tuberculosis to active disease include close contacts of a patient with culture-confirmed tuberculosis, a recent conversion to a positive tuberculin skin test (PPD), immunocompromised patients with a positive PPD, as well as patients with a positive PPD who have fibrotic changes on chest radiography consistent with previously untreated tuberculosis.
Teaching Topics NEJM | December 8, 2011
Q. What adverse effects were more common in the combination-therapy group (rifixamin plus isoniazid) than in the isoniazid-only group?
A. Among other adverse events attributed to study drug, the proportion of participants with possible hypersensitivity was higher in the combination-therapy group. The proportion of participants who permanently discontinued a study drug because of possible hypersensitivity was 2.9% in the combination-therapy group and 0.4% in the isoniazid-only group (P<0.001).
Teaching Topics NEJM | December 8, 2011
Three Months of Rifapentine and Isoniazid for Latent Tuberculosis Infection
NEJM december 8, 2011
Background
Treatment of latent Mycobacterium tuberculosis infection is an essential component oftuberculosis control and elimination. The current standard regimen of isoniazid for
9 months is efficacious but is limited by toxicity and low rates of treatment completion.
Methods
We conducted an open-label, randomized noninferiority trial comparing 3 months of directly observed once-weekly therapy with rifapentine (900 mg) plus isoniazid(900 mg) (combination-therapy group) with 9 months of self-administered daily isoniazid (300 mg) (isoniazid-only group) in subjects at high risk for tuberculosis.
Subjects were enrolled from the United States, Canada, Brazil, and Spain and followed for 33 months. The primary end point was confirmed tuberculosis, and the noninferiority margin was 0.75%.
Results
In the modified intention-to-treat analysis, tuberculosis developed in 7 of 3986 subjects in the combination-therapy group (cumulative rate, 0.19%) and in 15 of3745 subjects in the isoniazid-only group (cumulative rate, 0.43%), for a difference of 0.24 percentage points. Rates of treatment completion were 82.1% in the combination-therapy group and 69.0% in the isoniazid-only group (P<0.001). Rates of permanent drug discontinuation owing to an adverse event were 4.9% in the combination-therapy group and 3.7% in the isoniazid-only group (P = 0.009). Rates of investigator-assessed drug-related hepatotoxicity were 0.4% and 2.7%, respectively (P<0.001).
Conclusions
The use of rifapentine plus isoniazid for 3 months was as effective as 9 months of isoniazid alone in preventing tuberculosis and had a higher treatment-completion rate.Long-term safety monitoring will be important. (Funded by the Centers for Disease
Control and Prevention; PREVENT TB ClinicalTrials.gov number, NCT00023452.)
New drug combination for TB is tested in unique trial
BMJ 25 July 2012
Researchers have found that a new combination of three drugs is as effective as the standard treatment for tuberculosis.
The novel combination, called PaMZ, is made up of PA-824, abrand new drug candidate for treatment of tuberculosis;moxifloxacin, an established antibiotic not yet approved for use in first line treatment of the disease; and pyrazinamide, which is often used in combination with established tuberculosis
treatments.
Results from a small study involving just 80 patients found that, after two weeks of treatment, the mean early bactericidal activity (EBA, as measured by the daily rate of change of log10 colony
forming units in sputum) of PaMZ (n=13; EBA 0·233 (SD 0·128)) was significantly higher than that of bedaquiline (n=14; 0·061 (0·068)), bedaquiline with pyrazinamide (n=15; 0·131(0·102)), and bedaquiline with PA-824 (n=14; 0·114 (0·05)) but not PA-824 with pyrazinamide (n=14; 0·154 (0·04)) and was similar to that of the standard treatment of rifampicin, isoniazid, and pyrazinamide (n=10; 0·14 (0·094)).
The trial is the first in which more than one unapproved drugs have been tested together rather than separately and could speed up the approval
process for new drugs. Andreas Diacon, principal investigator of the study, said, “With this new approach, we can now test drugs in combination right
from the start, and we can possibly have a new drug regimen in five years. From a doctor’s perspective that’s amazing progress. Before, I was anticipating it could take decades for anew TB drug regimen, but now it looks like it will take years.”
Researchers have already begun recruiting patients into a larger trial being carried out at eight sites in South Africa, Tanzania,and Brazil to test the drugs over two months.
Mel Spigelman, chief executive and president of the non-profit TB Alliance, which funded the study, said, “A new regimen like this holds tremendous potential for those with multiple drug resistant TB.
We could be reducing their treatment by two years or even longer. The regimen also promises to be 90% cheaper than the current regimens. In the end, that means we could soon have a dramatically shorter, simpler, cheaper, and more effective
treatment at the same time.”
In an accompanying commentary Giovanni Migliori, of the WHO Collaborating Centre for Tuberculosis and Lung Diseases, and Giovanni Sotgiu, of the University of Sassari, Italy, say that the small number of patients in the study does not allow any
conclusions to be drawn about the safety of the new combination.
They also warn that studies that measure early
bactericidal activity have “instrinsic unpredictability” because of patients’ clinical features and the method of sputum sampling. Migliori and Sotgiu say that the need for new drugs to treat tuberculosis is urgent and that “mistakes that have been made with the most effective drugs we have to treat tuberculosis
(rifampicin and fluoroquinolones) should be kept in mind.”
They conclude, “The international community has the chance to prevent the misuse of new drugs and regimens. To protect the investment in these drugs, the rational use of antibiotics within strengthened health systems is necessary to avoid the real risk of losing these new agents in a time shorter than that
needed to develop them.
Confronting Multidrug-Resistant Tuberculosis
nejm.org june 7, 2012Microbial resistance to antituberculosis drugs has existed since the dawn of the antibiotic era.
Multidrug-resistant (MDR) tuberculosis, defined
by resistance to isoniazid and rifampin, requires
treatment for up to 2 years with expensive secondline
drugs that have poor side-effect profiles; success
rates rarely exceed 65 to 75%.
Extensively drug-resistant (XDR) tuberculosis, defined by additional resistance to two second-line drug classes,is more difficult to treat and may be incurable.
Fewer than 3% of all patients with diagnosed
tuberculosis worldwide have drug-susceptibility
testing performed, and only about 10% of the
estimated half-million new cases of MDR tuberculosis annually are treated appropriately. Patients with ineffectively treated MDR tuberculosis acquire further resistance and may continue the spread of drug-resistant organisms.
Two articles in this issue of the Journal address
some of the enormous challenges that must be
confronted if MDR and XDR tuberculosis are to
be controlled. Zhao and colleagues from the Chinese Center for Disease Control and Prevention
provide worrisome data on the extent of the MDR
and XDR tuberculosis problem in China, a country
with 1 million incident cases of tuberculosis
annually.
The authors systematically conducted drug-susceptibility testing of nearly 4000 patients with tuberculosis to measure the prevalence of drug resistance, and the results are sobering.
Among patients with newly diagnosed tuberculosis,
34.2% had some form of drug resistance,
5.7% had MDR tuberculosis, and 0.5% had XDR
tuberculosis. Among previously treated patients,
54.5% had some form of resistance, 25.6% had
MDR tuberculosis, and 2.1% had XDR tuberculosis
the prevalence of MDR tuberculosis was higher among previously treated patients, the majority of the estimated 110,000 cases of MDR tuberculosis in China annually are due to transmission of resistant strains to patients who had never before been treated for tuberculosis, rather than mismanagement of a previous episode.
How did resistance become so prevalent in
China? First, poor management of the disease
created the initial drug-resistant cases, and persons
with these cases of drug-resistant disease
transmitted the infection to others.
Improper treatment of these resistant cases selected more extensive resistance, and the onward cycle repeated.
Currently, 1 in 10 patients with tuberculosis
in China has MDR tuberculosis and 1 in 120 has
XDR tuberculosis. These data demand a radical
change in the global approach to MDR tuberculosis.
Despite abundant evidence that drug-resistant
tuberculosis strains are transmitted to others,3clinicians and public health authorities often focus
diagnostic efforts on “high-risk” patients,
such as patients who have not had a response to
therapy or who have had a relapse. Testing only
patients with a history of prior treatment might
seem to be more efficient, but that approach misses
most resistant cases of tuberculosis.
Global policies aspire to testing all patients, but most
programs fail to do so.4 In addition, antiquateddiagnostic tests must be replaced. The most widely
used test for diagnosing tuberculosis, the sputum
smear, misses 50 to 70% of cases and provides
no information about drug resistance.
Rapid molecular assays for detecting tuberculosis and drug resistance are now available. The use of a molecular assay for all patients in a South African
program doubled the yield of MDR diagnoses,
accounted for by increased detection in previously
untreated patients. Investment in the laboratory
infrastructure and human resources needed
to ensure broad access to these new technologies
is urgently needed.
Effective treatment of MDR tuberculosis is another
unmet need. Gler and colleagues report resultsfrom a phase 2 trial of delamanid, a novel
nitro-dihydro-imidazooxazole drug active against
Mycobacterium tuberculosis strains that are resistant
to conventional agents.8 Patients with MDR tuberculosis
received one of two doses of delamanid
or a matching placebo, together with a background
regimen of second-line drugs.
Sputum culture conversion at 8 weeks increased from
30% in the control group to 42 to 45% in thedelamanid groups. No dose–response effect was
observed, and the margin of benefit was significant
but modest. Nonetheless, increased sputumculture
conversion rates of this magnitude are
historically associated with a reduction in the
duration of treatment needed to cure tuberculosis.
Delamanid had an acceptable adverse-event
profile, but cardiotoxicity is a potential concern.
A study of another novel agent, bedaquiline (formerly TMC207),9 showed that the response rates at 8 weeks among patients receiving bedaquiline were similar to those among patients receiving delamanid in the study by Gler et al. (48% and 42 to 45%, respectively), but the response rates in the placebo group were substantially lower in the bedaquiline trial than in the delamanid trial (9% vs. 30%).
Differences in the study populations
and in the definitions of the end points couldaccount for this, but the inclusion of more potent
fluoroquinolones in the background regimen in
the delamanid trial is a better explanation.
Regimens containing two or more agents that
are known to be effective offer the greatest hope for “turning back the clock” on MDR tuberculosis.Both delamanid and bedaquiline enhance the
activity of second-line regimens, but how should
we use these drugs going forward? Unfortunately,
neither study provides an answer.
The drug development process requires companies to show the independent effects of their candidate agents;
however, to treat tuberculosis, clinicians need to
know what combination regimens to use, in what
configuration, and for what duration. Delamanid
and bedaquiline may receive regulatory approval
soon, yet we don’t know whether they can be
used together safely and effectively. Despite the
obvious need, most sponsors are (understandably)
reluctant to combine novel agents, citing proprietary,
safety, and regulatory concerns.
It is important to accelerate research to identify the best regimens of new and existing drugs and guide clinicians in the most effective application of these drugs.
Regulatory agencies should consider this
imperative in their guidance to prospective sponsorsand in their review of applications for the
registration of new agents.
MDR and XDR tuberculosis are now widespread
throughout the world, with the increase
driven largely by transmission.
Efforts to control drug-resistant tuberculosis can no longer focus solely on high-risk patients but must be incorporated into basic tuberculosis-control programs.
Creating the capacity to make an accurate diagnosis
of MDR tuberculosis and to treat the patients
with this disease appropriately is a monumental
task but one that cannot be avoided if
tuberculosis is to be contained.