Shock-Wave Lithotripsy for Renal Calculi
nejm. org july 5, 2012د. حسين محمد جمعه
اختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2012
A 42-year-old man without a history of kidney stones had intermittent left flank pain for several weeks before being seen by his primary care doctor. Urinalysis revealed microhematuria. Computed tomography (CT) of the abdomen and pelvis without contrast enhancement identified a calcification 12 mm in diameter in the left renal pelvis, associated with mild hydronephrosis and a normal-caliber ureter. The attenuation coefficient of the stone was 790 Hounsfield units, and the skin-to-stone distance was 8.5 cm. He was referred to a urologist, who reviewed the CT scan and recommended treatment with extracorporeal shock-wave lithotripsy.
The Clinical Problem
Nephrolithiasis is a common condition, with a lifetime prevalence of approximately13% in men and 7% in women in the United States.1 Data from the National
Health and Nutrition Examination Survey (NHANES) II (1976 to 1980) and NHANES
III (1988 to 1994) showed an increase in prevalence of 37% between these two time
periods — an increase that encompassed all age groups and both sexes.
Although many kidney stones are asymptomatic and are detected only incidentally on imaging that is performed for unrelated reasons, some stones cause pain, urinary tract obstruction, infection, or loss of renal function. Patients with symptomatic
stones often require visits to the emergency department or doctor’s office, admission to the hospital, and surgical intervention. Between 1.0% and 1.7% of emergency department visits (1 to 2 million visits annually) are accounted for by a primary diagnosis of renal colic or renal calculus.
The economic burden of urolithiasis is immense. One analysis used retrospective claims data from a privately insured, employed population in 2000 to estimate
direct and indirect costs associated with nephrolithiasis.4 Taking into account the cost of evaluation, hospitalization, and treatment of nephrolithiasis in this population,total health care expenditures reached nearly $4.5 billion annually, and this figure
increased to $5.3 billion when the indirect costs of lost workdays were included.
Pathophysiology and Effect of Therapy
Approximately 80% of upper urinary tract stones are calcium-based (composed of calcium oxalate, calcium phosphate, or brushite), with the remaining 20% composed of uric acid, struvite, cystine, or, rarely, other components. The pathogenesisof stone formation is complex and involves not only urinary supersaturation with stone-forming salts but also processes that are localized to microenvironments
within the renal papilla.
The underlying pathophysiological mechanisms involved in the formation of uric acid, struvite, and cystine stones are predictable (e.g., acidic urine in the case of uric acid stones and recurrent urinary tract infections in the case of struvite stones). In contrast,
calcium stone formation is multifactorial, and avariety of pathophysiological abnormalities may contribute to the risk of stone formation, including
hypercalciuria, hyperoxaluria, hyperuricosuria,
hypocitraturia, and low urine pH. Dietary factors have also been implicated in the formation of stones.
Extracorporeal shock-wave lithotripsy is anoninvasive therapeutic intervention for renal and ureteral calculi that uses an external source to deliver pulses of energy into a fluid chamber, generating a shock wave. The shock wave is transmitted unimpeded through the fluid and then
through the patient’s soft tissues (which have approximately the same density as fluid) until it
encounters an abrupt change in acoustic density
from body tissue to stone.
By focusing the shock waves on a single focal point, the lithotripter concentrates energy at the site in which the stone is located. Fragmentation of the stone by shock waves occurs as a consequence of both direct mechanical stress from the incident shock wave and indirect forces as a result of collapse of cavitation bubbles generated by the trailing negative-pressure wave.
Several types of lithotripters are in clinical use, and they differ from one another primarily
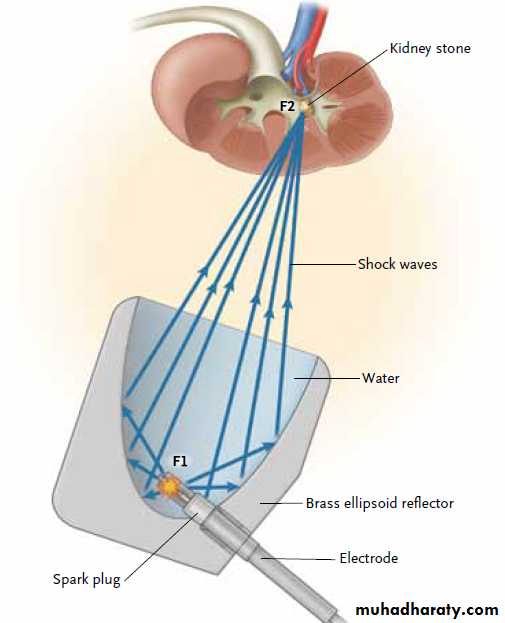
in the way they generate the shock waves. Electrohydraulic (spark-gap) lithotripters rely on an
underwater discharge of a high-voltage spark that
rapidly vaporizes the surrounding water, generating
spherically expanding shock waves (Fig. 1).
Placement of the electrode within an ellipsoid
reflector allows the shock waves to be focusedon the second focal point of the ellipse. Electromagnetic
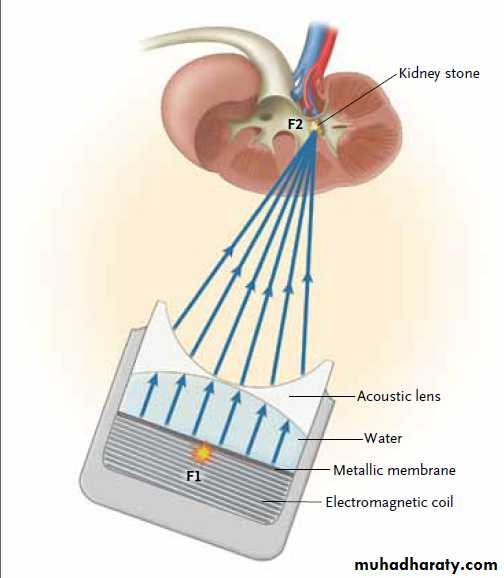
lithotripters are composed of an electromagnetic
coil and a closely approximated metallic
plate (or membrane) inside a water-filled
shock tube (Fig. 2). When an electrical impulse
is applied to the coil, the thin membrane responds
with a repulsive force, thereby generating
shock waves that are directed toward the
focal point by means of an acoustic lens.
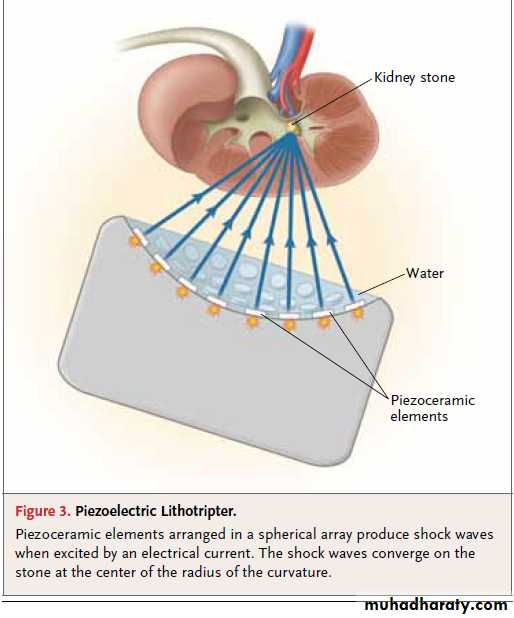
Piezoelectric lithotripters produce shock waves with
the use of a spherical dish containing an arrayof small ceramic elements. In response to a highvoltage pulse, these elements rapidly expand,
generating shock waves (Fig. 3). The spherical
shape of the dish allows the shock waves to
converge at the center of the radius of curvature
where the stone is positioned.
Clinical Evidence
There have been few randomized, controlled trials
comparing various strategies for the treatment of
renal calculi. Open surgery, once a standard approach
to the treatment of stones, has all but disappeared
from clinical practice in the United States, and no randomized trials have compared shock-wave lithotripsy with open surgery. Two other approaches in current clinical use are percutaneous nephrolithotomy and ureteroscopy with fragmentation or retrieval of the stone, both of which have been compared with shock-wave lithotripsy in randomized, clinical trials.
Figure 1. Electrohydraulic Lithotripter.
Shock waves (blue arrows) are generated by the ignition of a spark plug locatedin the center of a water-filled ellipsoid reflector (F1). The spherical
shock waves are reflected off the ellipsoid and focused on the kidney stone
at the second focal point (F2).
A 2009 Cochrane review identified only three
published randomized trials that comparedshock-wave lithotripsy with other minimally invasive
methods of treatment. In one of these
trials, 128 patients with symptomatic lower-pole
renal calculi 3 cm or less in diameter were randomly
assigned to either shock-wave lithotripsy (68 patients) or percutaneous nephrolithotomy (60 patients).
Among the 107 patients with follow-up data available at 3 months, the overall stone-free rate was 37% with shock-wave lithotripsy and 95% with percutaneous nephrolithotomy,and the disparity between these rates increased with the size of the stone. Among patients with stones less than 10 mm in diameter, 63% of those who underwent lithotripsy, as compared with 100% of those who underwent
percutaneous nephrolithotomy, were free of stones
at 3 months.
Among patients with stones greater than 1 cm in diameter, stone-free rates were unacceptably low with shock-wave lithotripsy.
Another randomized trial that was limited to patients with symptomatic lower-pole stones 1 cm or less in diameter compared shock-wave lithotripsy (32 patients) with ureteroscopy (35 patients).10 The stone-free rates were lower with shock-wave lithotripsy than with ureteroscopy (35% vs. 50%), but the difference was not significant.
Although the mean duration of the procedure was 24 minutes shorter with shockwave lithotripsy than with ureteroscopy, the rates of intraoperative and postoperative
complications
and the hospital lengths of stay were similar in the two treatment groups.A third randomized trial assigned patients with
renal calculi 3 to 30 mm in diameter to either
shock-wave lithotripsy (30 patients) or percutaneous
nephrolithotomy (25 patients). Among 40 patients who completed 4 weeks of follow-up, 76% of those who underwent shock-wave lithotripsy,as compared with 100% of the patients who underwent percutaneous nephrolithotomy, were free of stones or were left with residual fragments that were less than 4 mm in diameter.
The definition of a successful outcome in
this trial was less rigorous than in the two previouslydescribed trials, both of which required
that there be no detectable residual fragments.
Clinical Use
Renal calculi that do not cause symptoms or obstructionand are not associated with infection may
be managed with observation alone, although the
initiation of a medical prophylactic program, consisting
of serum and 24-hour urine testing to direct dietary measures and medical therapy aimed at the prevention of stone formation and stone growth is advisable.
Renal calculi associated with pain, obstruction, infection, or continued growth should be treated to prevent sepsis or loss of renal function.
The primary therapeutic options for surgical management of renal calculi are shockwave
lithotripsy, ureteroscopy, and percutaneous
nephrolithotomy. Among these, shock-wave lithotripsy
has consistently been the most commonly
used approach, representing 49 to 54% of procedures to treat renal stones.
Choosing the appropriate treatment method
for renal calculi is complex: not only success
rates (as determined by the stone-free rate), but
also rates of retreatment, rates of complications,
and patient and physician preference must be
taken into account.
Figure 2. Electromagnetic Lithotripter.
Shock waves are produced by the movement of a metallic membrane drivenby an electromagnetic coil (F1) and are focused by means of an acoustic
lens on the kidney stone located at the second focal point (F2).
The outcomes of shock-wave lithotripsy depend on a variety of factors, including the size, location, and composition of the stone and the anatomy of the collecting system.
Shock-wave lithotripsy is generally most effective
for stones that are less than 1.5 to 2.0 cm in diameter. Although this method is generally not recommended for patients with branched (or staghorn) calculi, limited success has been achieved with shock-wave treatment of smallvolume staghorn calculi (less than 500 mm2) in
nondilated collecting systems.
The outcome in patients with lower-pole renal calculi is generally poorer than the outcome in those with
stones in other locations in the kidney, probably
owing to impaired clearance of fragments from the dependent lower-pole calyces. Therefore, treatment of lower-pole stones with shockwave lithotripsy should be limited to stones that are less than 10 mm in diameter. Larger stones should generally be treated with ureteroscopy or percutaneous nephrolithotomy.
The ease with which shock waves fragment a
stone varies according to the composition of thestone.15 Cystine and brushite stones are the most
resistant to shock waves, followed in descending
order of resistance by calcium oxalate monohydrate,
struvite, calcium oxalate dihydrate, and uric
acid stones.16 Although the composition of the
stone may not be known before treatment, this
factor should be considered in the decision-making
process in the case of patients who have had
previous stones analyzed.
CT imaging can provide additional information
to aid in the selection of patients for shockwavelithotripsy. Less dense stones (i.e., those with
an attenuation coefficient below 900 Hounsfield
units) are more likely than denser stones to be
successfully managed with shock-wave lithotripsy.
Skin-to-stone distance also correlates with the success of shock-wave lithotripsy; shorter distances (less than 10 cm) are associated with agreater likelihood of success.
Contraindications to shock-wave lithotripsy
include active urinary tract infection, uncorrected
bleeding diathesis or coagulopathy, distal obstruction, and pregnancy. Obesity and orthopedic
or spinal deformities may preclude shock-wave
lithotripsy because the patient may not be able
to be properly positioned or adequately imaged.
For patients who are selected for shock-wave
lithotripsy, preoperative preparation includes thediscontinuation of aspirin-containing medications,
anticoagulants, platelet inhibitors, and nonsteroidal antiinflammatory agents for 7 to 10 days before the procedure. Documentation of a negative urine culture is essential to prevent postprocedural urinary tract infection or sepsis.
In women of childbearing age, a pregnancy test
is administered if ionizing radiation is to be used during the procedure, as is frequently the case in the United States but less often in Europe.
Initial imaging studies should confirm that the stone is radiopaque and that there is no distal obstruction.
If proper positioning of the patient or adequate
visualization of the stone is questionableowing to extremes in body habitus (e.g., obesity
or severe scoliosis) or renal anomalies (e.g.,
horseshoe or pelvic kidneys), a dry-run procedure
can be performed some time in advance of
the scheduled procedure to be sure that the
stone can be localized.
Depending on the size, radiopacity, and location of the stone, patients may be advised to drink clear liquids, take alaxative, or both the day before the procedure so that visualization of the stone can be enhanced
by a reduction in overlying stool and bowel gas.
Patients are instructed to abstain from food and
drink the night before the procedure in accordance
with standard guidelines for anesthesia.
Figure 3. Piezoelectric Lithotripter.
Piezoceramic elements arranged in a spherical array produce shock waveswhen excited by an electrical current. The shock waves converge on the
stone at the center of the radius of the curvature.
Shock-wave lithotripsy is an outpatient procedure
that can be performed in a hospital, anambulatory surgery center, or a mobile lithotripter.
The anesthesia that is used varies according
to needs of the patient, the preferences of
the physician and anesthesiologist, the type of
lithotripter, and the power settings used.
The type of anesthesia ranges from topical anesthetics
(e.g., lidocaine–prilocaine cream) to conscious
sedation or general anesthesia. Outcomes with some newer-generation lithotripters have been shown to be superior when general anesthesia is used because patient movement is reduced and more consistent and sustained targeting of the stone can be achieved. The pain associated with lithotripsy treatment varies inversely with the diameter of the lithotripter aperture and the area of the skin where the shock waves enter; however, lithotripsy always requires the administration of some form of anesthesia or analgesia.
In most centers, a confirmatory plain abdominal
radiograph is obtained on the day of theprocedure to ensure visualization of the stone
before anesthesia is administered. The use of
periprocedural antibiotics is not uniform, but
the American Urological Association (AUA) Best
Practice Policy20 recommends administration of
an antibiotic (fluoroquinolone or trimethoprim–
sulfamethoxazole) before the procedure and for
up to 24 hours postoperatively.
Positioning of the patient is dictated by the
location of the stone. The supine position isused for renal and most ureteral calculi; however,
for stones located in the middle ureter
(which overlies the bony pelvis) and in patients
with horseshoe, transplanted, or pelvic kidneys,
the patient should be treated in the prone position
to accommodate the more anterior location
of the ureter or kidney and to prevent attenuation
of the shock waves by the bony pelvis.
Once positioned, the patient is “coupled” to the lithotripter, in most cases by means of a water cushion coated with an acoustic gel that enables the shock waves to be delivered to the patient without a change in acoustic density or loss of energy.
With the treating physician, technician, or
both seated at a console remote from the lithotripter, the stone is targeted with the use of fluoroscopy or ultrasonography, and the table is moved until the stone lies within the focal point of the lithotripter, indicated by cross-hairs on the monitor.
The number, energy, and pattern of delivery
of the shock waves vary according to the preferenceof the treating physician and are often
customized to the size of the stone and its composition (if the latter is known), as well as the appearance of the stone as treatment progresses.
A common initial treatment plan calls for a
treatment rate of 60 to 120 shock waves per
minute, for a total of approximately 1000 to
2000 shocks administered. Periodic monitoring
with fluoroscopy or ultrasonography during treatment
allows the operator to assess the fragmentation
of the stone and determine the need to reposition
the patient to maintain the stone within the focal zone.
Renal calculi often appear to spread out or even disappear as they break because the fragments become dispersed within the collecting system, whereas ureteral stones typically show no change in radiographic appearance because the fragments are confined by the ureter. The maximum number of shock waves allowable is
set by the Food and Drug Administration for each
approved lithotripter, but it is advisable to deliver
only as many shock waves as necessary to pulverize
the stone in order to limit potential injury to the kidney.
Multiple stones may be treated in one session provided that the total number of shocks does not exceed the maximum number allowable.
Treatment of each stone requires repositioning of
the patient.
At the time of discharge, patients are given a
prescription for narcotic analgesics and a strainer
to collect stone fragments during voiding for
later analysis. A number of agents have been
shown to enhance the clearance of stone fragments
after shock-wave lithotripsy and are increasingly
prescribed after the procedure.
In several trials, the use of either potassium citrate or tamsulosin with or without methylprednisolone.
has resulted in superior stone-free rates, as compared
with placebo or controls.
After the procedure, patients are instructed to watch for signs of infection or obstruction as stone fragments are passed. A few days of mild hematuria and flank soreness can be expected,
but persistent, heavy, gross hematuria or severe pain should prompt a reevaluation and an assessment for bleeding complications.
Patients should be reevaluated 2 to 4 weeks after the procedure by means of a urinalysis and imaging (typically abdominal radiography and renal ultrasonography) to assess the effectiveness of stone fragmentation and to verify the absence of obstruction.
For patients with stones that failed to fragment or were incompletely treated, repeat lithotripsy may be considered 2 weeks or more after the initial treatment, or an alternative treatment may be recommended.
In general, passage of fragments may continue for up to 3 months; therefore, stone-free status is not assessed until the 3-month followup examination has been performed.
The cost of lithotripsy is driven largely by the
high capital cost of the equipment. In one comparative
study from 2005 that was based on data
from an international cost survey, the estimated
cost of shock-wave lithotripsy ranged from $380
in Germany (in U.S. dollars) to $6,697 in the
United States.
Adverse Effects
Adverse effects associated with shock-wave lithotripsy can be related to obstructive problems associated with stone passage, direct tissue effects, or both. Obstruction associated with passage of fragments often occurs transiently and resolves spontaneously. However, occasionally a column of stone fragments (Steinstrasse [German for “stone street”]) will accumulate in the ureter inassociation with an initial obstructing fragment.
This phenomenon has been reported to occur in
6 to 20% of patients after shock-wave lithotripsy,with an incidence that varies inversely with the
size of the stone.25-27 In most cases, this type of
ureteral obstruction resolves spontaneously. Placement of a nephrostomy tube promotes the passage of these fragments in recalcitrant cases, but
occasionally lithotripsy of an obstructing lead fragment, ureteroscopy, or percutaneous nephrolithotomy is necessary to clear the stones.
Clinically significant subcapsular hematomas
develop after lithotripsy in less than 1% of cases.However, magnetic resonance imaging or CT
can detect hematomas in up to 20% of patients
undergoing shock-wave lithotripsy, depending
on the type of lithotripter used.28,29 Risk factors
for hematoma include older age,30 uncontrolled
hypertension, diabetes, coronary artery disease,
and obesity. The mechanism of hematoma
formation has been ascribed to shock-wave–induced
rupture of blood vessels.32 Most hematomas
resolve spontaneously with bed rest and only rarely require arterial embolization to stop ongoing bleeding.
Vascular injury associated with shock-wave
lithotripsy may also lead to long-term effects.
Studies in animals have shown that renal vasoconstriction,
which is manifested as a decrease
in the glomerular filtration rate and in renal
plasma flow, occurs as an immediate response
to lithotripsy and, if prolonged, may result in
inflammation, ischemia, and renal fibrosis.
Clinically, it has been suggested that shock-wave
lithotripsy may result in increased rates of both
hypertension and diabetes, but other studies
have failed to validate these potential risks.
Areas of Uncertainty
Many questions remain regarding the appropriateuse and long-term safety of shock-wave lithotripsy,
and the ideal lithotripter remains elusive.
Relatively few studies have compared the different
types of devices directly, and those that have
done so have not reached consistent conclusions.
In addition, the anatomical and radiographic
features associated with outcomes after
lithotripsy and the exact cutoff points distinguishing
success from failure have not been firmly established;
however, it is clear that the size and
location of stones do have an influence on the
likelihood that a patient will receive a benefit
from lithotripsy. The role of shock-wave lithotripsy
in the treatment of renal calculi is still evolving,
and well-designed, prospective clinical series and
trials are needed to directly compare lithotripsy
with ureteroscopy and percutaneous nephrolithotomy
in patients with moderate-sized (less than
2 cm in diameter), non–lower-pole renal calculi.
Guidelines
Validated guidelines for the treatment of renalcalculi are limited. A guidelines panel directed
by the AUA has made specific recommendations
with respect to staghorn calculi.14 According to
these recommendations, shock-wave lithotripsy
should be considered as primary therapy only in
the case of small-volume staghorn calculi with
minimal dilation of the collecting system — a
rare clinical scenario for which stone-free rates
were found to be acceptable with lithotripsy.
Unfortunately, no formal guidelines have been developed by the AUA for management of the more
common, nonstaghorn renal calculi.
In 2011, the European Association of Urology
Urolithiasis Guideline Panel released compre-
hensive guidelines,45 recommending shock-wave
lithotripsy as first-line therapy for non–lower-pole
renal calculi less than 2 cm in diameter and for
lower-pole renal calculi less than 1 cm in diameter.
For larger stones in either location, percutaneous
nephrolithotomy is the recommendedprimary treatment. For lower-pole stones between 1 and 2 cm in diameter without unfavorable anatomical factors or shock-wave–resistant stone composition, lithotripsy can be considered as an option for primary management.
Recommendations
The patient described in the vignette has a stonewith a size (less than 2 cm in diameter) and location
(non–lower pole) that is considered to be favorable
for treatment with shock-wave lithotripsy.
Findings on CT imaging, including the attenuation
coefficient (less than 1000 Hounsfield units) and
the skin-to-stone distance (less than 10 cm), also
predict a favorable outcome.
Although the stone composition cannot be reliably predicted by means of current CT findings, and the patient did not previously have a stone with a known composition, the favorable findings on CT suggest that the stone will probably fragment well and that the risk of obstructive complications will be low.