Medical Disorders and Drug Use during Pregnancy and breast feeding
د. حسين محمد جمعهاختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2012
At the end of this talk, you will be able to:
• Confidently prescribe needed medications in pregnancy• Order diagnostic imaging safely for your pregnant patients
• Act on evidence-based recommendations for management of common medical problems in pregnancy
Goals
There are four important clinical principles
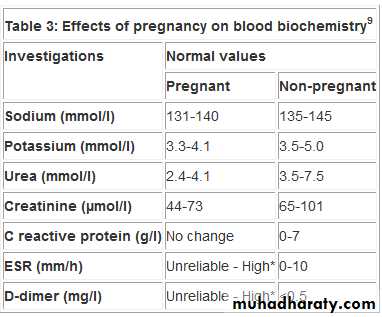
Firstly, medical disorders are affected by pregnancy,when important physiological changes occur in almost every system in the body. Haemodynamic changes may lead to an additional burden on the cardiovascularsystem, which may predispose to the occurrence of
heart failure in women with cardiac disease or hypertension.
Secondly, medical disorders may affect the pregnancy. Diabetes may
lead to foetal macrosomia while chronic hypertension or renal disease can result in foetal growth retardation.Thirdly, physiological changes during pregnancy
make the diagnosis of a medical disorder more difficult.Sometimes abnormal symptoms due to medical disorders may be attributed to the pregnancy, leading to a delay in diagnosis, while physiological symptoms and signs may lead to overdiagnosis of some medical disorders.Finally, the treatment of medical disorders during pregnancy may be different from their treatment in the non-pregnant state. In a pregnant woman,there are two patients—the mother and the foetus.
The physician and the obstetrician have to balance the risks and benefits to both the mother and foetus when deciding on treatment.
In the management of more common medical disorders such as diabetes
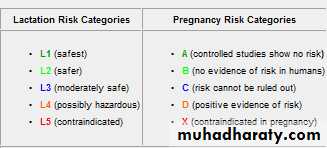
or cardiac disease, a combined clinic with the participation of both physicians and obstetricians is ideal.Category A
Adequate and well-controlled studies have failed to demonstrate a risk to the fetus in the first trimester of pregnancy (and there is no evidence of risk in later trimesters).Category B
Animal reproduction studies have failed to demonstrate a risk to the fetus and there are no adequate and well-controlled studies in pregnant women.
Category C
Animal reproduction studies have shown an adverse effect on the fetus and there are no adequate and well-controlled studies in humans, but potential benefits may warrant use despite potential risks.
Category D
There is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience or studies in humans, but potential benefits may warrant use of the drug in pregnant women despite potential risks.Category X
Studies in animals or humans have demonstrated fetal abnormalities and/or there is positive evidence of human fetal risk based on adverse reaction data from investigational or marketing experience, and the risks involved in use of the drug in pregnant women clearly outweigh potential benefits.Normal changes in blood pressure during pregnancy
First trimester there is a fall in BP caused by vasodilation(prostacyclin and nitric oxide),primarily affects the diastolic pressure, and a drop of 10 mm Hg is usual by 13-20 weeks' gestation.BP continues to fall until 22-24 weeks. After this, there is a gradual increase until term, when BP returns to the level it was before pregnancy.Immediately after delivery BP usually falls and then increases over the next five days
ECG changes in pregnancy
ECG readings are also altered in pregnant women because the heart is pushed up and rotated by the gravid uterus. Normal ECG findings in a pregnant woman can include:• Atrial and ventricular ectopics
• Small Q waves and inverted T waves in lead III
• ST depression and T wave inversion in inferior and lateral leads
• Left shift in QRS axis.
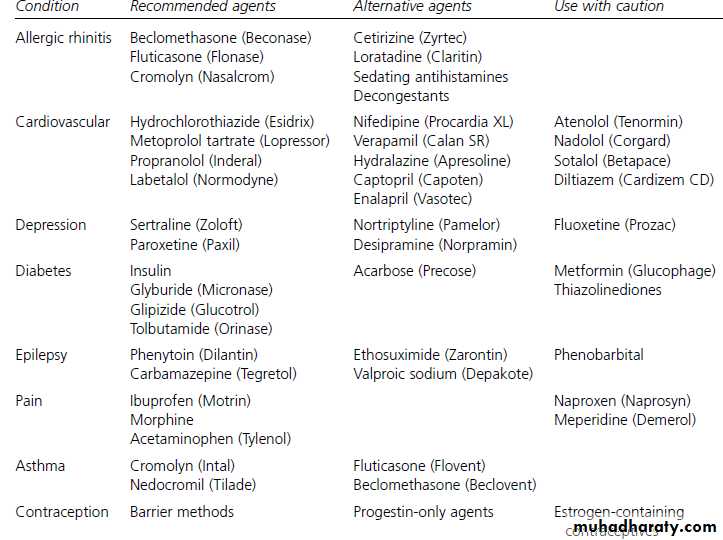
Minimizing Potential Risk to Nursing Infants from Maternal Medications
General considerationsAvoid drug therapy when possible.
Use topical therapy when possible.
Medications that are safe for use directly in an infant of the nursing infant’s age are generally safe for the breast-feeding mother.
Medications that are safe in pregnancy are not always safe in breast-feeding mothers.
Use reliable references for obtaining information on medications .
Medication selection
Choose medications with the shortest half-life and highest protein-binding ability.Choose medications that are well-studied in infants.
Choose medications with the poorest oral absorption.
Choose medications with the lowest lipid solubility.
Medication dosing
Administer single daily-dose medications just before the longest sleep interval for the infant, usually after the bed-time feeding.
Breast-feed infant immediately before medication dose when multiple daily doses are needed.
Pregnancy and Diabetes
Gestational diabetes
Increased insulin resistance during pregnancy due to the insulin antagonistic effects of human placental lactogen and cortisol,1-2% of pregnant women develop gestational diabetesRisk factors include
Obesity
Family history
Previous baby >4.5kg
Gestational Diabetes
All pregnant women should be screened for gestational diabetes unless they are in a low-risk group.Women at low risk for gestational diabetes are those <25 years of age; those with a body mass index < 25 kg/m2, no maternal history of macrosomia or gestational diabetes, and no diabetes in a first-degree relative.
40% of women with a history of gestational diabetes develop diabetes at some point in the future.
Most women with gestational diabetes can control blood glucose levels with dietary changes, while 10-15% may require insulin injections.
About 1 to 3% of pregnant women develop diabetes during pregnancy. Of all the women who have diabetes during pregnancy, 90% have gestational diabetes. Gestational diabetes is between 24-28 weeks. Unrecognized and untreated, gestational diabetes can increase the risk of health problems for pregnant women and their fetus and the risk of death for the fetus.
Because gestational diabetes typically occurs late in the second trimester when the baby’s body is already formed, it does not usually increase the risk of birth defects, but is associated with a chance for
delivering a large baby. If gestational diabetes is not well controlled, there is an increased chance for the baby to have hypoglycemia and breathing problems at birth. increased risk of preeclampsia
In rare cases where gestational diabetes is present in the first trimester, there may be a small increased risk for birth defects similar to that seen with other forms of diabetes.
Gestational Diabetes: Treatment
Dietary intervention followed by insulin injections if diet alone does not adequately control blood sugar .[fasting glucose < 5.6 mmol/L (<100 mg/dL) and 2-h post-prandial <7.0 mmol/L (<126 mg/dL)] is associated with a decreased risk of birth trauma for the fetus.Risks of Diabetes During Pregnancy: If diabetes is poorly controlled early in the pregnancy, the risk of an early miscarriage birth defects, larger Babies, cesarean , immature lungs , preeclampsia.Newborns with low sugar, low calcium, and high bilirubin levels in the blood.
Preconception counseling and treatment are important for the diabetic patient contemplating pregnancy and can reduce the risk of congenital malformations and improve pregnancy outcome.
Patients should take a prenatal vitamin containing at least 1.0 mg of folic acid daily for at least 3 months prior to conception to minimize the risk of neural tube defects in the fetus.
Pregnancy management of diabetes.
Dietary therapyThe goal is to avoid single large meals and a large percentage of simple carbohydrates.
6 feedings per day is preferred, with 3 major meals and 3 snacks to limit the amount of energy intake presented to the bloodstream at any interval. Examples include foods with complex carbohydrates, (whole grain breads and legumes). Carbohydrates no more than 50% of the diet, protein and fats equally accounting for the remainder.
Glucose monitoring
The availability of capillary glucose test strips should now be considered the standard of care for pregnancy monitoring.A typical schedule involves capillary glucose checks upon awakening in the morning, 1 hour after breakfast, before and after lunch, before dinner, and at bedtime.. Superb glycemic control requires attention to both preprandial and postprandial glucose levels.
Insulin therapy
The goal of insulin therapy during pregnancy is to achieve glucose profiles similar to those of nondiabetic pregnant women Insulins lispro, aspart, regular and NPH are well-studied in pregnancy and regarded as safe and efficacious. Insulin glargine is less well-studied, and given its long pharmacologic effect, may exacerbate periods of maternal hypoglycemia.As pregnancy progresses, the increasing fetal demand for glucose the progressive lowering of fasting and between-meal blood sugar levels increases the risk of symptomatic hypoglycemia. Thus, the regimens must be continuously modified as the patient progresses from the first to the third trimester and insulin resistance rises.
Insulin pump
In a select group of patients, use of an insulin pump may improve glycemic control while enhancing patient convenience. These devices can be programmed to infuse varying basal and bolus levels of insulin, which change smoothly even while the patient sleeps or is otherwise preoccupied.The effectiveness of continuous subcutaneous insulin infusion in pregnancy is well established.Oral hypoglycemic agents - Glyburide
Glyburide is minimally transported across the human placenta. This is probably largely due to the high plasma protein binding coupled with a short half-life.Glyburide should not be used in the first trimester because its effects, if any, on the embryo are unknown.
Several prospective and retrospective studies involving more than 775 pregnancies have concluded glyburide is equally as safe and efficacious as insulin. Glyburide has been shown to be safe in breastfeeding, with no transfer into human milk. More data on the safety and efficacy of glyburide for the management of gestational diabetes are needed before it supplants insulin as the treatment agent of choice.
If any of the sulfonylureas are used, it is important to monitor the nursing infant for signs of hypoglycemia, such as increased fussiness or somnolence.
The alpha-glucosidase inhibitors, are unlikely to be excreted into breast milk in clinically significant amounts.
Because of the potential for serious side effects (e.g., lactic acidosis, hepatotoxicity) in adults, it may be advisable to avoid the use of metformin and thiazolidinediones
until more information is available on their use in breast-feeding.
In pregnancy, the fetoplacental unit induces major metabolic changes, the purpose of which is to shunt glucose and amino acids to the fetus while the mother uses ketones and triglycerides to fuel her metabolic needs. These metabolic changes are accompanied by maternal insulin resistance, caused in part by placental production of steroids, a growth hormone variant, and placental lactogen. Although pregnancy has been referred to as a state of "accelerated starvation," it is better characterized as "accelerated ketosis.
" In pregnancy, after an overnight fast, plasma glucose is lower by 0.8–1.1 mmol/L (15–20 mg/dL) than in the nonpregnant state. This is due to the use of glucose by the fetus. In early pregnancy, fasting may result in circulating glucose concentrations in the range of 2.2 mmol/L (40 mg/dL) and may be associated with symptoms of hypoglycemia. In contrast to the decrease in maternal glucose concentration, plasma hydroxybutyrate and acetoacetate levels rise to two to four times normal after a fast.
Folate supplementation reduces the incidence of fetal neural tube defects, which occur with greater frequency in fetuses of diabetic. Optimizing glucose during periods of organogenesis reduces other congenital anomalies including sacral agenesis, caudal dysplasia, renal agenesis, and ventricular septal defect. Control should be more aggressively than in the nonpregnant state. Fasting blood glucose levels should be maintained at <5.8 mmol/L (<105 mg/dL).
Statins are contraindicated during pregnancy and breastfeeding, Because statins interfere with the synthesis of cholesterol, fetal harm or death may result if they are given during pregnancy. Options during pregnancy include intensifying diet therapy and/or starting a nonsystemic agent like a bile acid sequestrant (prevents absorption of bile acids in the digestive system), e.g., cholestyramine, colestipol, or colesevelam, which are rated pregnancy category B. The HMG-CoA reductase inhibitors (US Food and Drug Administration pregnancy category X).
Treatment of Hyperlipidemia during pregnancy
ACE inhibitors: renal dysgenesis
Tetracycline: abnormalities of bone and teethFluoroquinolones: abnl cartilage development
Systemic retinoids: CNS, craniofacial, CV defects
Warfarin: skeletal and CNS defects
Valproic acid: neural tube defects
NSAIDS: bleeding, premature closure of the ductus arteriosis
Live vaccines (MMR, oral polio, varicella, yellow fever): may cross placenta
Drugs to avoid in pregnancy
• FDA and AAP Approved Medications and Drugs
• Here is a list of Safe Drugs for Breastfeeding approved by the American Academy of Pediatrics.
•
most antihypertensives
most thyroid medicationsPenicillin
famotidine
omeprazole quinine
Metoclopramide
Azithromycin
sertraline
All vaccines
acetaminophen
Cephalosporin CodeineMetronidazole
Heparin
ibuprofen
propranolol
antiepileptics (although Primidone, should be given with caution)insulin Morphine
most antihistamines
ANTIBIOTICS
Penicillins and cephalosporins, which are excreted in milk in trace amounts, are compatible with breast-feeding. A remote possibility exists that the child will experience an allergic reaction to the antibiotic or develop diarrhea caused by changes in gut flora. Trimethoprimsulfamethoxazole is compatible with breast-feeding, but its use should be avoided when nursing infants are younger than two months because of its potential for causing increased bilirubin levels.
Tetracycline
Excreted in small amounts in breast milk, but the calcium in breast milk limits its absorption. Although tetracycline is compatible with breast-feeding, other antibiotics are preferred, especially for long-term use.Doxycycline
Should be avoided because of higher absorption by infants and toxicity in children (e.g.,dental staining, decreased bone growth).Quinolones
Have not been well studied in breast milk and are not rated by the AAP.They should be used in the breast-feeding mother
only when other, better-studied options cannot
be used and after the risks and benefits have been assessed.
Metronidazole is rated by the AAP as a drug whose effect on infants is unknown, Nevertheless, the amount transferred to the infant through breast milk is much lower than the therapeutic dosage for infants, and no adverse effects have been reported from exposure through breast milk.Following a 2-g dose, cessation of breastfeeding for 12 to 24 hours is recommended by the AAP.
Fluconazole (Diflucan) is commonly prescribed for yeast infections of the nipple in breast-feeding mothers. It is present in breast milk, but the nursing infant can only ingest
5 % of the usual pediatric dosage,topical antifungal agents, such as clotrimazole or miconazole produce very low maternal serum concentrations,and their use should pose little risk to the nursing infant
ANTIDEPRESSANTS
Tricyclic antidepressants have been shown to have little to no effect on the breastfeeding infant, although the AAP finds most tricyclic agents to be of possible concern.Taking a single daily dose at bedtime will limit the infant’s exposure.The selective serotonin reuptake inhibitors (SSRIs) are generally the first choice. Sertraline (Zoloft) is likely to be the safest choice among them because it has been studied extensively and because drug levels found in nursing infants are usually minimal.Fluoxetine
Fluoxetine’s long half-life and potential for accumulation in breast milk has prompted some recommendations to avoid its use in women who are breast-feeding young infants. Colic and fussiness have been attributed to elevated serum concentrations of fluoxetine and its metabolite in nursing infants.
ANALGESICS
Of the (NSAIDs), ibuprofen is the preferred choice because it has poor transfer into milk and has been well-studied in children.Long half-life NSAIDs such as naproxen ,sulindac and piroxicam can accumulate in the infant with prolonged use. Morphine,codeine and hydrocodone are considered compatible.
Meperidine is not the preferred analgesic for use in breast-feeding women .
Acetaminophen Use During Pregnancy: Better Than Safe?
Surprisingly, pregnant women who used acetaminophen for fever were less likely to have infants with certain birth defects.Journal Watch Women's Health January 28, 2010
CONTRACEPTIVE AGENTS
Are not harmful to infants but, because estrogen diminishes the maternal milk supply, should be avoided especially during the first two months of breast-feeding. Progestin-only contraceptives are preferable, although these also may decrease milk supply. When appropriate, the use of an intrauterine contraceptive device is ideal.ANESTHETIC AGENTS
limited information is available , use of propofol, thiopental sodium (Pentothal) and enflurane , result in negligible amounts of drug exposure to the nursing infant. In general, the healthy term infant can safely nurse as soon after surgery as the mother is awake and alert.Thyroid Disease
In pregnancy, the estrogen-induced increase in thyroxine-binding globulin causes an increase in circulating levels of total T3 and total T4. The normal range of circulating levels of free T4, free T3, and thyroid-stimulating hormone (TSH) remain unaltered by pregnancy.Graves' Disease
Abnormal antibodies stimulate the thyroid gland. These antibodies can cross the placenta and stimulate the thyroid gland in the fetus. As a result, the fetus may have a rapid heart rate and may not grow as much as expected. The fetus's thyroid gland may enlarge, forming a goiter. Very rarely, a goiter is so large that it interferes with delivery through the vagina.
Graves' disease is treated with the lowest possible dose of propylthiouracil .
The drug may slow the activity of the thyroid of the fetus. It may also cause a goiter. Methimazole may be used instead of propylthiouracil,has been associated with fetal aplasia cutis.Treating hyperthyroidism during pregnancy
The specialist will see the patient each month during their pregnancy.Radio-iodine is absolutely contraindicated in pregnancy because it is concentrated by the fetal thyroid, causing hypothyroidism.
The mainstay of treatment is drug therapy using the minimum dose that maintains normal thyroid function.
Propylthiouracil is the drug of choice, because it is believed to be less teratogenic than carbimazole.
Untreated hyperthyroidism is associated with:
• Miscarriage
• Premature labour
• Low birth weight
• Eclampsia.
Clinical hypothyroidism in pregnancy
Thyroxine is safe to use in pregnancy and while breast feeding.Subclinical hypothyroidism in pregnancy
Women with subclinical hypothyroidism who are trying to get pregnant should be treated with thyroxine. Untreated hypothyroidism in pregnancy is associated with an increased risk of fetal loss, stillbirth, and premature labour
The aim is to normalise women's serum thyroid stimulating hormone before pregnancy, even in mild thyroid failure
The daily dose of thyroxine may need to increase by 25 to 50 µg during pregnancy
This dose increase must happen within the first 12 weeks of the pregnancy. Do not delay until the antenatal booking consultation
Hematologic Disorders
Pregnancy has been described as a state of physiologic anemia. Part of the reduction in hemoglobin concentration is dilutional, but iron and folate deficiencies are the major causes of correctable anemia during pregnancy.In populations at high risk for hemoglobinopathies hemoglobin electrophoresis should be performed as part of the prenatal screen. Hemoglobinopathies can be associated with increased maternal and fetal morbidity and mortality.
Thrombocytopenia occurs commonly during pregnancy. The majority of cases are benign gestational thrombocytopenias, but the differential diagnosis should include immune thrombocytopenia and preeclampsia. Maternal thrombocytopenia may also be caused by catastrophic obstetric events such as retention of a dead fetus, sepsis, abruptio placenta, and amniotic fluid embolism.
Chronic renal disease
Complications depend on the degree of renal compromise .Mother has increased risk of pre-eclampsia which can be difficult to diagnose in the background or proteinurea and hypertensionAlso associated with IUGR and preterm labour
Renal function and BP must be monitored as must fetal growth and development
Women on dialysis have impaired fertility and conception is not common.
When it does occur it has a very high rate of miscarriage and termination is often offeredWhen pregnancy does occur it is associated with pre-eclampsia, hypertension and volume overload
Gastrointestinal and Liver Disease
Up to 90% of pregnant women experience nausea and vomiting during the first trimester of pregnancy. Occasionally, hyperemesis gravidarum requires hospitalization to prevent dehydration, and sometimes parenteral nutrition is required.Crohn's disease may be associated with exacerbations in the second and third trimesters. Ulcerative colitis is associated with disease exacerbations in the first trimester and during the early postpartum period. management is identical to the management in the nonpregnant state .
Exacerbation of gall bladder disease is commonly observed during pregnancy. In part this may be due to pregnancy-induced alteration in the metabolism of bile and fatty acids.
Intrahepatic cholestasis of pregnancy is generally a third-trimester event. Favorable results with ursodiol have been reported.
Acute fatty liver is a rare complication of pregnancy. Frequently confused with the HELLP syndrome ,diagnosis by imaging studies and laboratory evaluation, markedly increased levels of bilirubin and ammonia and by hypoglycemia. Management of acute fatty liver of pregnancy is supportive.
Pregnancy and Infections
InfectionsBacterial Infections
The most common infections involve the urinary tract .Many pregnant women have asymptomatic bacteriuria, most likely due to stasis caused by progestational effects on ureteral and bladder smooth muscle and later in pregnancy due to compression effects of the enlarging uterus, if asymptomatic bacteriuria is left untreated, pyelonephritis may occur.
Indeed, ~75% of cases of pregnancy-associated pyelonephritis are the result of untreated asymptomatic bacteriuria. All pregnant women should be screened with a urine culture for asymptomatic bacteriuria at the first prenatal visit. Subsequent screening with nitrite/leukocyte esterase strips is indicated for high-risk women, such as those with sickle cell trait or a history of urinary tract infections. All women with positive screens should be treated.
Untreated tuberculosis (TB) represents a greater hazard to a pregnant woman and her fetus than does its treatment. Infants born to women with untreated TB may be of lower birth weight than those born to women without TB and, rarely, the infant may be born with TB. Although the drugs used in the initial treatment regimen cross the placenta, they do not appear to have harmful effects on the fetus.
Tuberculosis and Pregnancy
Active TB should be treated, even in women in the first stage of pregnancy. Isoniazid, rifampin, and ethambutol may be used. In the United States, pyrazinamide is reserved for women with suspected MDR-TB. Elsewhere in the world, pyrazinamide is commonly used in pregnant women with TB.
How Does Maternal TB Affect Foetus and
Neonate?A foetus can get TB infection either by haematogenous spread through umbilical vein to foetal liver or by ingestion or aspiration of infected amniotic fluid. True congenital TB is believed to be rare. The risk to neonate of getting TB infection shortly after the birth is greater. A neonate having congenital TB may present with respiratory distress, fever, poor feeding,lethargy, irritability, abdominal distention, lymphadenopathy and hepato-splenomegaly.
A failure to obtain favourable response with broad spectrum antibiotics along with negative results for other congenital infections should lead to the suspicion of
congenital TB. An abnormal chest radiograph is found in all such cases, half of whom have a miliary pattern. The overall mortality for congenital TB is 38% in the untreated and 22% in the treated.
Care of neonates
Neonates born to mothers having infectious TB should be given chemoprophylaxis with INH for 3 months or till the mother becomes noninfectious.BCG vaccination may be postponed or done with INH-resistant BCG vaccine. After 3 months, if mother has a negative sputum smear and the neonate (with a normal chest XR) has anegative Mantoux test, then INH chemoprophylaxis may be discontinued. In case, the Mantoux test is positive, a thorough search should be made for locating the presence of pulmonary or extrapulmonary focus and administration of ATT may be decided accordingly.Patients with congenital tuberculosis
Neonates should receive isoniazid (10 to 15 mg per kilogram of body weight per day), rifampin (10 to 20 mg per kilogram per day), pyrazinamide (15 to 30 mg per kilogram per day), and either streptomycin (20 to 30 mg per kilogram per day) or ethambutol (15 to 25 mg per kilogram per day) for the first 2 months, followed by isoniazid and rifampin for 4 to 10 months, depending on the severity of the disease. Because of the potential for optic neuritis, streptomycin is generally preferred over ethambutol in infants. In communities where rates of resistance to isoniazid are 4 percent or less, initial regimens of three drugs may be acceptable.Effects on Breast feeding
Use of Isoniazid, Rifampicin, Ethambutol,Pyrazinamide, Streptomycin, Kanamycin and
Cycloserine has been considered safe for breast
feeding. The effect of these drugs gets minimized, if the mother breast feeds before taking the drugs and substitutes the
next feed with formula preparation.
breast-feeding of neonates is recommended
regardless of the mother’s TB status.
Streptomycin, kanamycin, amikacin, capreomycin and fluoroquinolones listed as contraindicated for use by pregnant.
Breastfeeding
should not be discouraged for women being treated with the first-line antituberculosis drugs because the concentrations of these drugs in breast milk are too small to produce toxicity in the nursing newborn.
Pyridoxine supplementation (25 mg/day) is recommended for all women taking INH who are either pregnant or breastfeeding.
Viral Infections
Cytomegalovirus Infection
As many as 50–90% of women of childbearing age have antibodies to CMV, but only rarely does CMV reactivation result in neonatal infection. More commonly, primary CMV infection during pregnancy creates a risk of congenital CMV. No currently accepted treatment of CMV
Rubella
First-trimester rubella carries a high risk of fetal anomalies, though the risk decreases significantly later in pregnancy. Congenital rubella may be diagnosed by percutaneous umbilical blood sampling with the detection of IgM antibodies in fetal blood.All pregnant women should be screened for their immune status to rubella. Indeed, all women of childbearing age, regardless of pregnancy status, should have their immune status for rubella verified and be immunized if necessary.
Herpesvirus
Associated with spontaneous abortion, prematurity, and congenital and neonatal herpes. The risk can be reduced by prescribing acyclovir for the last 4 weeks of pregnancy.Herpesvirus infection in the newborn can be devastatingfrom CNS involvement.
It is recommended that pregnant women with active genital herpes lesions at the time of presentation in labor be delivered by cesarean.
HIV Infection
The predominant cause of HIV infection in children is transmission of the virus from the mother to the newborn during the perinatal period. Exposures, which increase the risk of mother-to-child transmission, include vaginal delivery, preterm delivery, trauma to the fetal skin, and maternal bleeding.Breast-feeding may also transmit HIV to the newborn and is therefore contraindicated in most developed countries for HIV-infected mothers.
Zidovudine (ZDV) administered during pregnancy and labor and to the newborn reduces the risk of vertical transmission by 70%. Cesarean section is associated with additional risk reduction compared to vaginal delivery.
Hepatitis during pregnancy
Fortunately, transmission of most forms of hepatitis via breastfeeding is rare.
Some experts have advised giving the infant immune globulin if the mother has the onset of symptoms in the period from two weeks before to one week after delivery. Even without immune globulin, severe disease has not been reported in infants. Careful hand washing should still be emphasized to the mother.
Hepatitis A, even during the acute infectious period, is not a contraindication to breastfeeding.
Hepatitis B Virus (HBV)
Transmitted by sexual contact, perinatally, and rarely congenitally (mother-to-child during pregnancy). A major route of transmission from an infected mother to her baby is via contact with blood at the time of birth.Infants born to known Hepatitis B positive should receive hepatitis B immune globulin and the first dose of hepatitis B vaccine within 12 hours of birth. The second dose of vaccine should be given at aged 1–2 months, and the third dose at aged 6 months.
Mothers with Hepatitis B should be encouraged to breastfeed.
The infant should be tested after completion of the vaccine series, at aged 9–18 months to determine if the vaccine worked and the infant is not infected with HBV through exposure to the mother's blood during the birth process.
All mothers who breastfeed should take good care of their nipples to avoid cracking and bleeding.
HCV ,the risk of perinatal transmission is of approximately 4 %
Several recent studies demonstrate no increased risk of transmission caused by breastfeeding. HCV-positive mother's nipples and/or surrounding areola are cracked and bleeding, she should stop nursing temporarily.Urinary tract infections Asymptomatic bacteriuria is very common in pregnant women because of the altered dynamics of the urinary tract. If untreated, this frequently progresses to acute cystitis (1-2%) and/or pyelonephritis. The symptoms of acute cystitis are the same as in non-pregnant women:Frequency .Urgency Cloudy, smelly urine .Dysuria
Those of acute pyelonephritis are:
Pyrexia Rigors .Flank pain. Nausea & vomiting Headache .Frequency & dysuria
Management
Urine should be tested by dipstick. Confirmation is with urine microscopy and culture.Treat with trimethoprim, a cephalosporin or nitrofurantoin (except where otherwise contraindicated). In recurrent UTI, consider prophylactic nitrofurantoin but stop before delivery.
Consequences
premature labour and low birth weight.
A chest x ray exposes a fetus to a negligible amount of radiation at any gestation, so if a chest x ray is clinically indicated, you should arrange one for a pregnant patient without delay.
Which Drug for the Pregnant Woman with Epilepsy?
More than 90 percent of pregnant women who have epilepsy deliver healthy babies. For most pregnant women who have epilepsy, seizures remain the same.How does epilepsy affect pregnancy?
Higher risk of pregnancy-related complications, including:Severe morning sickness
Anemia
Vaginal bleeding
placental abruption
preeclampsia
Premature birth
uncontrolled seizures may deprive the baby of oxygen. Seizures can also increase the risk of miscarriage or stillbirth.
What about medication?
Birth defects — including cleft palate, neural tube defects, skeletal abnormalities, and congenital heart and urinary tract defects — are a major concern with seizure medications.
For babies whose mothers take seizure medication, the risk of birth defects is 4 to 8%
Carbamazepineappears comparatively safe with respect to malformations .
More recent research consistently indicate that the risk of major congenital malformations is two to four times as high with the use of valproate as with the use of other antiepileptic drugs such as carbamazepine and lamotrigine.
A trial of lamotrigine could be considered, since malformation rates associated with its use are similar to those associated with the use of carbamazepine.
However, lamotrigine is difficult to use in pregnancy because of the risk of breakthrough seizures.
Cognitive development of a child may be affected by exposure throughout pregnancy.
Children exposed to valproate in utero had significantly lower IQs
Babies born to mothers who have epilepsy also have a slightly higher risk of developing seizures as they get older.
The safety of topiramate and levetiracetam, other newer-generation antiepileptic drugs with efficacy in generalized epilepsies, has not been sufficiently assessed for use during pregnancy.
A low dose of valproate remains an option if seizures cannot be controlled by other drugs. Doses below 800 mg per day may not be associated with fetal risks that are any greater than the risks associated with the use of other antiepileptic drugs
Because of the risks associated with a loss of seizure control during pregnancy, changes from valproate to another antiepileptic drug should be made and evaluated before conception.
By the time a woman realizes that she is pregnant, switching drugs is unlikely to reduce the risk of birth defects.
Because safe practice involves changing antiepileptic drugs only over a prolonged period (i.e., typically over a period of months, with the use of polytherapy during the transition period), a switch from valproate once pregnancy has been established is also unlikely to eliminate the risk of cognitive impairment in the child.
Folic acid helps prevent neural tube defects, serious abnormalities of the brain and spinal cord. Because some seizure drugs affect the way the body uses folic acid, a high-dose folic acid supplement is recommended.
Ideally starting three months before conception.
Babies who had mom's milk for more than 9 months had fewer seizures than babies who had breast milk for a shorter time, report the authors in the Journal of Pediatrics.
Breast-feeding is encouraged for most women who have epilepsy, even those who take seizure medication.
Four antiepileptic drugs were studied including carbamazepine, lamotrigine, phenytoin and valproate.
This preliminary analysis failed to demonstrate any negative effects of breastfeeding during antiepileptic drug therapy on IQ of children who have been exposed to the medication by breast milk.
Breast feeding is not contraindicated. However, if the drugs are sedating, monitor the baby for sedation
Enzyme inducing anti-epileptic drugs can cause vitamin K deficiency in children born to women with epilepsy. Vitamin K (10 mg per day) can be prescribed in the last month of pregnancy. Alternatively, the baby should get 1 mg of vitamin K parenterally as soon as possible after labour.
Nausea and Vomiting in Pregnancy
About 50% of women have nausea and vomiting in early pregnancy, and an additional 25% have nausea alone. The popular term“morning sickness” is a misnomer, as it often persists throughout the day. In about 35% of those who have this condition, nausea and vomiting are clinically significant.When do nausea and vomiting in pregnancy most typically resolve?
The onset is within four weeks of the LMP in most patients.Typically peaks at approximately 9 weeks.
Sixty percent of cases resolve by the end of the first trimester, and
91% by 20 weeks gestation. Adequate oral hydration and avoidance of dietary triggers are
often sufficient, but a proportion of women with severe andprotracted nausea and vomiting will need antiemetic drugs.
Preventable rare maternal complications of hyperemesis gravidarum include
peripheral neuropathies due to vitamin B6 and B12 deficiencies.
Wernicke’s encephalopathy due to thiamine (vitamin B1) deficiency. Characterized by the triad of ophthalmoplegia, ataxia, and confusion, this condition may occur after at least 3 weeks of persistent vomiting.
If patients are treated with intravenous dextrose without thiamine, metabolism
of the dextrose rapidly consumes the available B1, triggering acute encephalopathy.Incidence of hyperemesis gravidarum is 0.3 to 1.0%; this condition is characterized by persistent vomiting, weight loss of more than 5%,ketonuria, hypokalemia, and dehydration . Although the cause of nausea and vomiting in pregnancy is unclear, the observation that pregnancies with a complete hydatidiform mole (no fetus) are associated
with clinically significant nausea and vomiting indicates that the stimulus is produced by the placenta, not the fetus.
It is theorized that hCG may stimulate estrogen production from the ovary; estrogen is known to increase nausea and vomiting.
Women with twins or hydatidiform moles, who have higher hCG levels than do other pregnant women, are at higher risk for these symptoms. Another theory is that vitamin B deficiency may contribute, since the use of vitamin B reduces the incidence of nausea and vomiting.
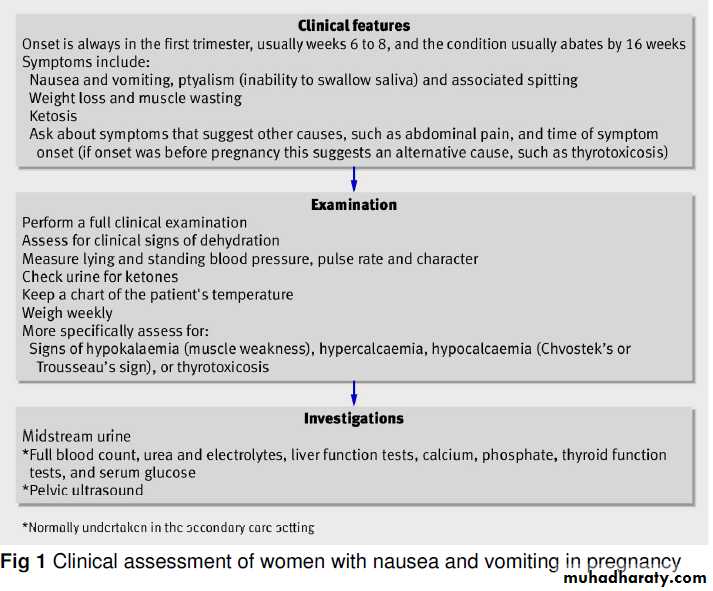
Evaluation
Hyperemesis gravidarum must be distinguished from other conditions that may cause persistent vomiting in pregnancy, including gastrointestinal conditions (e.g., appendicitis, hepatitis, pancreatitis, or biliary tract disease), pyelonephritis,and metabolic disorders such as diabetic ketoacidosis, porphyria, or Addison’s disease.An onset of nausea and vomiting more than 8 weeks after the last menstrual period is rare in pregnancy.
The presence of fever, abdominal pain, or headache is atypical in women with hyperemesis and suggests another cause.
Laboratory testing should generally include measurement of levels of urinary ketones, blood urea nitrogen, creatinine, alanine aminotransferase, aspartate aminotransferase,electrolytes, amylase, and thyrotropin (as well as free thyroxine [T4] if thyrotropin is suppressed).
Because hCG cross-reacts with thyrotropin and stimulates the thyroid gland, thyrotropin is typically suppressed in these patients. This apparent hyperthyroidism usually resolves spontaneously.
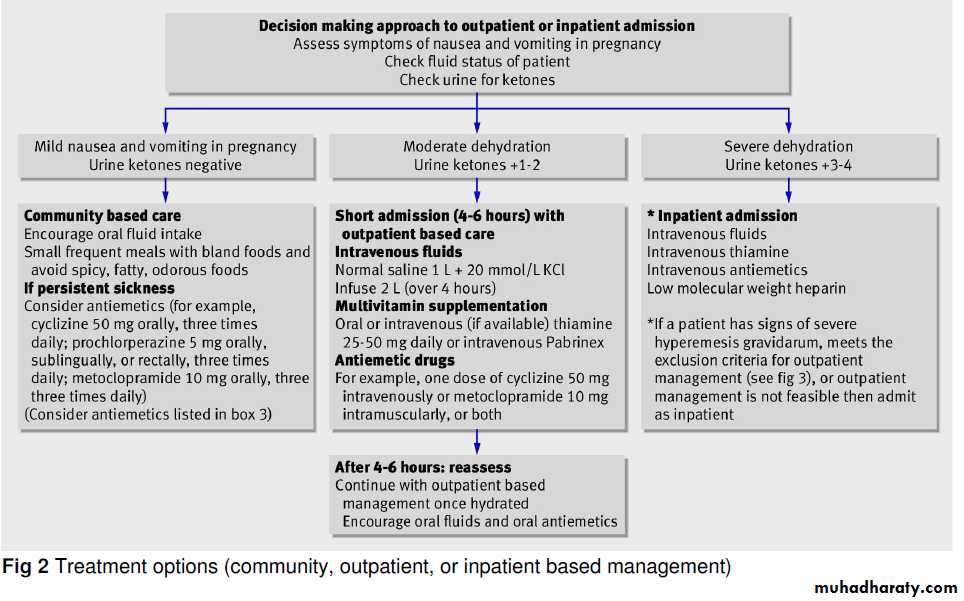
Management
Women should be advised to avoid exposure to odors, foods, or supplements that appear to trigger nausea; common triggers include fatty or spicy foods and iron tablets. Clinical experience suggests that eating small amounts of food several times a day and drinking fluids between meals may be helpful, as may bland, dry, and high-protein foods.Dietary advice (frequent small meals) may be helpful.
protein-predominant meals reducenausea more than meals containing equal amounts of calories from carbohydrates and fat. Women who have persistent nausea and vomiting and high concentrations of ketones require intravenous hydration with multivitamins, including thiamine, with measurement s of urinary ketones and electrolytes.Antiemetic agents should be prescribed.
Pharmacologic Therapies
Approximately 10% of women with nausea andvomiting in pregnancy require medication. Pharmacologic therapies include vitamin B6, antihistamines,prokinetic agents, and other medications.
Oral vitamin B6 and antihistamine doxylamine are available over the counter in the United States,with no evidence of teratogenicity, and, a 70% reduction in nausea and vomiting,
recommended by the American College of Obstetricians and Gynecologists as first-line therapy
Phenothiazine or metoclopramide is usually prescribed if antihistamines fail. Prochlorperazine is also available as a buccal tablet ,which is usually associated with less drowsiness and sedation than oral tablets.Metoclopramide is a prokinetic agent,a dopamine antagonist. associated in rare cases with tardive dyskinesia. The risk of the development of this complication increases with the duration of treatment and the total cumulative dose; treatment for more than 12 weeks should be avoided.
Intravenous metoclopramide and intravenous promethazine had similar efficacy in the treatment of hyperemesis,
but metoclopramide caused
less drowsiness and dizziness.
The 5-hydroxytryptamine3-receptor antagonists,such as ondansetron (Zofran), are increasingly used for hyperemesis in pregnancy, but information is limited to inform their use in pregnant women.
Methylprednisolone is an option in refractory
cases. In a randomized trial involving 40 women,methylprednisolone was superior to promethazine
for treating nausea and vomiting in pregnancy. a meta-analysis of four studies, use of glucocorticoids
before 10 weeks of gestation was associated
with a risk of cleft lip with or without cleft
palate that was increased by a factor of 3 to 4;
higher doses were associated with greater risks.
Thus, it is recommended that glucocorticoids be
used only after 10 weeks of gestation.
Randomized, double-blind trials have provided support for a benefit of ginger in the management of nausea and vomiting in pregnancy.
Management of Refractory Cases
Patients with nausea and vomiting that are not controlled with outpatient regimens require intravenous hydration and nutritional supplementation.Enteral tube feeding may be effective, although some patients continue to have persistent emesis.
Total parenteral nutrition is associated with a substantial risk of line sepsis (25%), steatohepatitis may also occur with the use of lipid emulsion during pregnancy. Given these risks, total parenteral nutrition should be reserved for patients with clinically significant weight loss (>5% of body weight) who have had no response to antiemetic regimens and whose condition cannot be managed with enteral feedings.
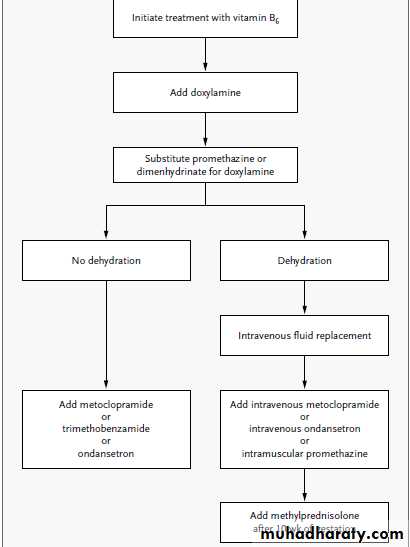
Given data from randomized trials suggesting that vitamin B6 and doxylamine are beneficial, B6 10 to 25 mg every 8 hours, and doxylamine, 25 mg at bedtime and 12.5 mg each in the morning and afternoon. If this regimen is not effective, a phenothiazine,metoclopramide, or ondansetron can be tried in succession. Methylprednisolone should be reserved for refractory cases after 10 weeks of gestation.
Alternative remedies such as ginger and acupuncture may be tried at any time.
Pregnant women with dehydration should receive intravenous fluid replacement with multivitamins,especially thiamine. If, after 12 hours of intravenous therapy, the vomiting continues, hospitalization may be required. Enteral or parenteral nutrition should be reserved for patients in whom weight loss continues despite pharmacologic therapies.
NEJM 2010
Management of hypertension during pregnancy
Blood pressure should be measured in the sitting position, cuff at the level of the heart. Inferior vena caval compression by the gravid uterus while the patient is supine leading to an underestimation of the blood pressure., left lateral position similarly may yield falsely low values if the blood pressure is measured in the higher arm, unless the cuff maintained at the level of the heart.Hypertension is the most common medical problem seen in pregnancy 15% of pregnancies
First, the priority is in making the correct diagnosis, with the emphasis on distinguishing preexisting (chronic) from pregnancy induced (gestational hypertension and the syndrome of preeclampsia).Second, much of the obstetric literature distinguishes blood pressure (BP) levels as either mild (140 to 159/90 to 109 mm Hg) or severe (160/110 mm Hg), rather than as stages .
Update on the Use of Antihypertensive Drugs in Pregnancy
Third, in contrast to hypertension guidelines in adults, which emphasize the importance of systolic BP, much of the obstetric literature focuses on diastolic rather than systolic BP, in part because of the lack of clinical trials to support one approach versus another.
Even women whose blood pressure was normal throughout pregnancy may experience transient hypertension in the early period post partum. This perhaps reflects a degree of vasomotor instability.
The main types of hypertensive disease in pregnancy
• pre-existing hypertension• gestational hypertension
• pre-eclampsia
The presence of mild pre-existing hypertension approximately
doubles the risk of pre-eclampsia and increases the risk of abruption of the placenta and restriction of growth in the fetus.
Gestational hypertension
hypertension occurring in the second half of pregnancy without significant proteinuria or other features of pre-eclampsia in a woman with previously normal blood pressure. It complicates 6-7% of pregnancies.The risk of pre-eclampsia is 15-26%.
Blood pressure usually returns to normal by six weeks after birth.
NEJM September 5, 2011
In the absence of definitive recommendationswith respect to optimal blood-pressure
targets during pregnancy, we aim to adjust
medications to maintain blood pressure between
130/80 mm Hg and 150/100 mm Hg.
NEJM September 5, 2011
Pre-eclampsia and eclampsia
usually occurs after 20 weeks' gestation and is a multisystem disorder.It was classically defined as a triad of:
Hypertension
Oedema
Proteinuria.
But a more modern definition of pre-eclampsia concentrates on a rise in blood pressure during pregnancy together with >0.3 g proteinuria in 24 hours. Oedema is no longer included because it is not specific.
In most normal pregnancies, the woman has some lower extremity edema by the third trimester. In contrast,
a sudden worsening in dependent edema, edema in nondependent areas (such as the face and hands),
or rapid weight gain suggest a pathologic process and warrant further evaluation for preeclampsia.
The onset of significant proteinuria, in the absence of renal disease, is one of the best indicators of pre-eclampsia. headache, visual disturbance, and abdominal pain are well recognised signs of severe pre-eclampsia .
Preeclampsia-eclampsia is a syndrome that manifests clinically as new-onset hypertension in later pregnancy (any time after 20 weeks, but usually closer to term), with associated proteinuria: 1 on dipstick and, officially, 300 mg per 24-hour urine collection.
2008 American Heart Association.
occurs in 5% to 8% of all pregnancies and is thought to be a consequence of abnormalities in the maternal vessels supplying the placenta,leading to poor placental perfusion and release of factors,causing widespread endothelial dysfunction with multiorgan system clinical features, such as hypertension, proteinuria,and cerebral (edema, occipital headaches, or seizures) and hepatic dysfunction (extension to hemolysis elevation of liver enzymes, low platelets).
Preeclampsia-eclampsia
The HELLP syndrome(hemolysis, elevated liver enzymes, low platelets) is a special subgroup of severe preeclampsia and is a major cause of morbidity and mortality in this disease. The presence of platelet dysfunction and coagulation disorders further increases the risk of stroke.
Preeclampsia
Although the exact pathophysiologic mechanism is not clearly understood, preeclampsia is primarily a disorder of placental dysfunction leading to a syndrome of endothelial dysfunction with associated vasospasm.In most cases, pathology demonstrates evidence of placental insufficiency with associated abnormalities such as diffuse placental thrombosis, an inflammatory placental decidual vasculopathy, and/or abnormal trophoblastic invasion of the endometrium. This supports abnormal placental development or placental damage from diffuse microthrombosis as being central to the development of this disorder.
Evidence also indicates that an altered maternal immune response to fetal/placental tissue may contribute to the development of preeclampsia. Risk factors for the development of preeclampsia include nulliparity, diabetes mellitus, a history of renal disease or chronic hypertension, a prior history of preeclampsia, extremes of maternal age (>35 or <15 years), obesity, factor V Leiden mutation, antiphospholipid antibody syndrome, and multiple gestation.
Deterrence/Prevention
Multiple interventions to prevent preeclampsia have been investigated. Pharmacologic treatment and normalization of chronic hypertension does not reduce the risk of developing superimposed preeclampsia. Other therapies that have been tried include low-dose acetylsalicylic acid (ASA), supplemental calcium, salt restriction, supplemental magnesium, and fish oil therapy.
While several large trials of ASA in high-risk populations showed minimal benefit in reducing the frequency of preeclampsia, a meta-analysis reported an approximate
15% reduction in preeclampsia among pregnant women taking low-dose ASA. This therapy appears very safe and might be considered in high-risk women. None of the other therapies have demonstrated any significant preventive benefit.
Magnesium sulfate is the treatment of choice for the prevention and treatment of eclamptic seizures. Two large randomized clinical trials have demonstrated the superiority of magnesium sulfate over phenytoin and diazepam, and a recent large randomized clinical trial has demonstrated the efficacy of magnesium sulfate in reducing the risk of seizure and possibly reducing the risk of maternal death.
Given the difficulty of predicting eclamptic seizures on the basis of disease severity, it is recommended that once the decision to proceed with delivery is made, all patients carrying a diagnosis of preeclampsia be treated with magnesium sulfate.
When to treat hypertension during pregnancy
Significant hypertension must be treated to reduce the incidence of maternal intracranial haemorrhage.The level at which you should start antihypertensive treatment for non-severe hypertension is controversial. Most doctors start antihypertensives when:
Systolic blood pressure is >140-160 mm Hg or
Diastolic pressure is >90-110 mm Hg.
The blood pressure you should
aim to achieve is also controversial, but many practitioners would treat to keep the mean arterial pressure <125 mm Hg . for example, a blood pressureof < 150/100 mm Hg.
Lowering the blood pressure too much may lead to placental hypoperfusion because placental blood flow is not autoregulated. This will affect the fetus.
Unfortunately there is no evidence that treating chronic or gestational hypertension protects against the development of pre-eclampsia.
Drug treatment
All antihypertensive drugs cross the placenta and reach the fetal circulation. Most of the antihypertensive agents in routine use are not teratogenic, although ACE inhibitors and angiotensin receptor blockers are, and should be avoided.A teratogenic substance is one that interferes with the formation of major body structures during the first trimester, during the period of organogenesis
A fetotoxic drug interferes with the subsequent growth and development of the fetus
Mild to moderate hypertension
Treating mild to moderate pre-existing HP benefits the mother, but there is no clear evidence of an enhanced outcome for the baby.Some women with mild treated pre-existing hypertension are able to stop their medication in the first half of pregnancy because of the physiological fall in blood pressure. But should be monitored and treatment restarted as soon as necessary.
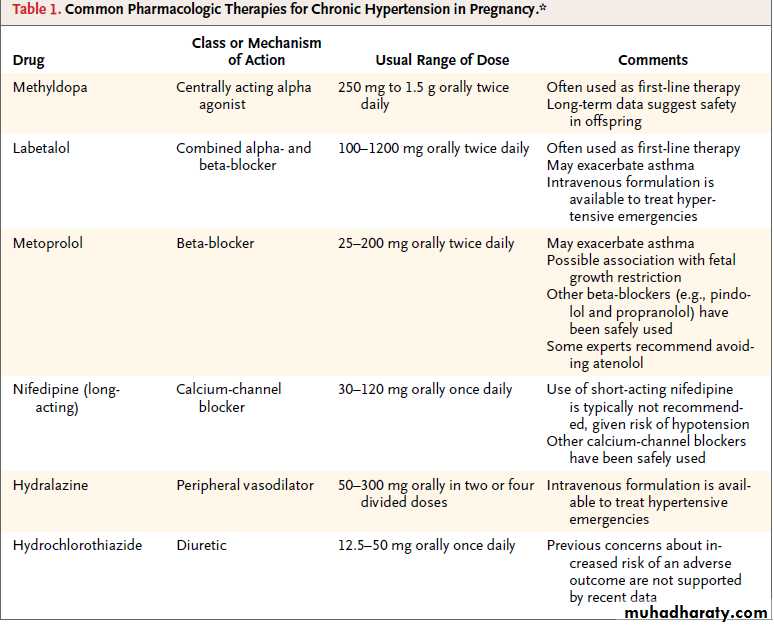
First line drugs
MethyldopaCentrally acting agent and first choice.
You should warn women that it can cause sedation; this can limit the dose used. The drug may cause liver transaminases to rise (in up to 5% of women) or a positive Coomb's test (although haemolytic anaemia is uncommon). You should not give methyldopa to women with a prior history of depression, because of the increased risk of postnatal depression.
Second line drugs
You should use these drugs when monotherapy with methyldopa is insufficient or when women cannot tolerate methyldopa.Nifedipine
It is safe at any stage of gestation. You should avoid sublingual nifedipine(placental hypoperfusion).
Giving concomitant magnesium sulphate can exacerbate abrupt hypotension. Amlodipine has been used in pregnancy, but there are little safety data.
Oral labetalol
This is used by some as a first line agent. alpha and beta blocker and appears safe in pregnancy. Since beta blockers have been associated with growth restriction when used from the first trimester, our preference is to avoid labetalol until later in pregnancy.
Oral hydralazine
Safe throughout pregnancy, although there have been reports of lupus-like syndromes in the mother and neonate.more frequently used as an infusion for treating acute severe hypertension.
Third line drugs
Alpha and beta adrenergic blockersBB associated with an increased risk of fetal growth restriction. Atenolol in particular has often been singled out but labetalol seems safe.
you should avoid BB in the first half of pregnancy because of concerns about growth restriction.
Prazosin is safe and effective in pregnancy. Doxazosin appears to be safe, although data are limited.
Thiazide diuretics
These drugs do not appear to be teratogenic. Although they reduce the expansion in plasma volume associated with normal pregnancy, this has not been proved to impair fetal growth.They are usually avoided in the treatment of hypertension in pregnancy, their use is reserved for cardiac disease and pulmonary oedema.
Hydrochlorothiazide may be continued during pregnancy;the use of low doses (12.5 to 25 mg daily) may minimize untoward metabolic effects, such as impaired glucose tolerance and hypokalemia. Triamterene and amiloride are not teratogenic based on small numbers of case reports. Spironolactone is not recommended because of its antiandrogenic effects during fetal development.
However,mild volume contraction with diuretic therapy may lead to hyperuricemia and in so doing invalidate serum uric acid levels as a laboratory marker in the diagnosis of superimposed preeclampsia.
Different units have their preferences for intravenous hydralazine or labetalol, which are equally effective, but the latter has fewer side effects. Oral nifedipine may also be used.
You should give hydralazine only after giving intravenous fluid: this reduces the reflex tachycardia, and abrupt hypotension, precipitated by vasodilation.
Severe hypertension
Good control of hypertension in severe pre-eclampsia does not halt the progression of the disease, only delivery can do this, but it can reduce the incidence of complications such as cerebral haemorrhage. In patients with severe hypertension you may gain only hours or days.
Women with gestational hypertension or pre-eclampsia are usually able to stop all antihypertensives within six weeks after birth. Women with pre-existing hypertension can resume the drugs they were taking before they became pregnant.
But women who want to breast feed should avoid diuretics because they can increase thirst.
Proteinuria in pre-eclamptic women will usually resolve by three months post partum if there is no underlying renal abnormality.
Breast feeding
You can give most antihypertensive drugs used in routine practice to women who are breast feeding.Antihypertensive treatment post partum and during breast feeding
Blood pressure typically rises after delivery over the first five days. May be normotensive immediately after the birth, but then become hypertensive again in the first postnatal week.You should avoid methyldopa post partum because of the risk of postnatal depression. Our first line agent is atenolol, plus nifedipine or an ACE inhibitor if another agent is required.
Neonatal exposure to methyldopa via nursing is likely low, and it is generally considered safe .Atenolol and metoprolol are concentrated in breast milk,possibly to levels that could affect the infant; by ontrast,exposure to labetalol and propranolol seems low. Although milk concentrations of diuretics are low and considered safe,these agents can decrease milk production significantly.
2008 American Heart Association.
There are reports of calcium channel blocker transfer into breast milk, apparently without adverse effects. Sufficient data exist for the safety of 2 ACE-Is, captopril and enalapril.
2008 American Heart Association.
Diuretics and beta blockers, commonly preferred antihypertensives, are safe for use in lactating women, with some precautions. In general, it is preferable to avoid high dosages of any one medication by either changing medications or adding an additional agent.
Low dosages of thiazide diuretics (e.g.,25 mg per day or less are excreted in small amounts into the breast milk but do not suppress lactation and, consequently, are compatible with nursing.
Beta blockers vary widely in the amount excreted into breast milk. Propranolol , metoprolol and labetalol are excreted in small quantities and are compatible with breastfeeding even in compromised infants.Atenolol ,nadolol and sotalol are excreted in higher amounts, which can lead to hypotension,bradycardia and tachypnea in the infant.
Nonsteroidal anti-inflammatory drugs
Oral contraceptivesCorticosteroids
Cyclosporine
Erythropoietin
Cocaine
Alcohol abuse
Licorice (excessive amounts)
Drugs that increase BP
Cardiomyopathy during pregnancy
Peripartum cardiomyopathy is one of the most common causes of maternal death in the United Kingdom and United States; the estimated mortality rate is 9% to 50%.
Patients have a 20% to 50% risk of recurrence in subsequent pregnancies, and if they did not recover full cardiac function after the first episode, patients have a risk of death of up to 20% in a future pregnancy. It is therefore imperative that pre-pregnancy counselling occurs prior to consideration of a future pregnancy
Women may develop cardiomyopathy during pregnancy, or the physiological changes of pregnancy may uncover a previously asymptomatic condition. Signs include:
• Increasing exertional dyspnoea
• Orthopnoea
• Tachycardia
• Raised jugular venous pressure
• Third or fourth heart sound.
Peripartum cardiomyopathy
Is a condition specific to pregnancy defined as
cardiac failure, which develops:
Between the last month of pregnancy and five months post partum In the absence of an identifiable cause or recognisable heart disease prior to the last month of pregnancy With left ventricular systolic dysfunction
left ventricular systolic dysfunction, shown by both:
• Left ventricular ejection fraction <45%• A left ventricular end diastolic dimension of greater than 2.7 cm/m2 of body surface area.
Peripartum cardiomyopathy has an incidence of about 1 in 10 000 pregnancies.
Factors which place women at increased risk include:
• Multiple pregnancy• Hypertension
• Multiparity
• Increased maternal age
• African ethnic origin.
Peripartum cardiomyopathy
uncommon disorder of pregnancy associated with myocarditis, and its etiology remains unknown. Treatment is directed toward symptomatic relief and improvement of cardiac function. Many patients recover completely; others are left with a progressive dilated cardiomyopathy. Recurrence in a subsequent pregnancy has been reported, and women should be counseled to avoid pregnancy after a diagnosis of peripartum cardiomyopathy.Management of cardiomyopathy
You should manage in a similar way to non-pregnant patients. However, you need to give special consideration to the safety of cardiac drugs in pregnancy and for the breastfeeding mother. Specifically, angiotensin converting enzyme (ACE) inhibitors should be avoided, but patients may remain on beta blockers and some types of diuretic. You should consider prescribing thromboprophylaxis, especially if there is a history of arrhythmia or transient ischaemic attack.Heart valve disease
during pregnancy
Heart valve disease
The physiological changes of pregnancy include systemic vasodilation and a fall in peripheral vascular resistance. So regurgitation at a valve is generally better tolerated than stenosis, provided that there is no associated left ventricular dysfunction.Valve stenosis is generally less well tolerated in pregnancy as it limits the physiological increase in cardiac output.
Mitral Regurgitation and Aortic Regurgitation
These are generally well tolerated during pregnancy. The pregnancy-induced decrease in systemic vascular resistance reduces the risk of cardiac failure with these conditions.Patients who were previously asymptomatic may experience symptoms from
Mitral stenosis and develop pulmonary oedema. This particularly occurs during the third trimester or immediately after delivery.Aortic stenosis may develop syncope, exertional dyspnoea, or chest pain.
Medical treatment for aortic stenosis includes:
Giving beta blockers to treat symptoms like chest pain, syncope, and dyspnoeaAvoiding vasodilators like nifedipine.
Medical treatment for mitral stenosis includes:
Use of diuretics and beta blockers to control symptoms like pulmonary oedema or chest pain.Considering using thromboprophylaxis in patients with an enlarged left atrium. Balloon valvulotomy can be carried out during pregnancy.
Congenital Heart Disease
Increases the risk of congenital cardiac disease in the newborn. Atrial or ventricular septal defect is usually well tolerated during pregnancy in the absence of pulmonary hypertension, provided that the woman's prepregnancy cardiac status is favorable. Use of air filters on IV sets during labor and delivery in patients with intracardiac shunts is generally recommended.
Supraventricular tachycardia is a common cardiac complication of pregnancy. Treatment is the same as in the nonpregnant patient, and fetal tolerance of medications such as adenosine and calcium channel blockers is acceptable. When necessary, electrocardioversion may be performed and is generally well tolerated by mother and fetus.
Pulmonary Hypertension
Maternal mortality in the setting of severe pulmonary hypertension is high, and primary pulmonary hypertension is a contraindication to pregnancy. Termination of pregnancy may be advisable in these circumstances to preserve the life of the mother. In the Eisenmenger syndrome, i.e., the combination of pulmonary hypertension with right-to-left shunting due to congenital abnormalities maternal and fetal death occur frequently.Deep Venous Thrombosis and Pulmonary Embolism
A hypercoagulable state is characteristic of pregnancy. Pulmonary embolism is one of the most common causes of maternal death in the United States. In pregnant women, DVT occurs much more commonly in the left leg than in the right leg, due to the compression of the left iliac vein by the iliac artery and the uterus.Approximately 25% of women with DVT during pregnancy carry the factor V Leiden allele. The presence of the factor V Leiden mutation also increases the risk for severe preeclampsia.
Deep Venous Thrombosis: Treatment
Aggressive diagnosis and management of DVT and suspected pulmonary embolism optimize the outcome for mother and fetus. In general, all diagnostic and therapeutic modalities afforded the nonpregnant patient should be utilized in pregnancy. Anticoagulant therapy with low-molecular-weight heparin (LMWH) or unfractionated heparin is indicated.LMWH may be associated with an increased risk of epidural hematoma in women receiving an epidural anesthetic in labor. One approach to this problem is to switch from LMWH to unfractionated heparin before the anticipated delivery date.
Warfarin therapy is contraindicated in the first trimester due to its association with fetal chondrodysplasia punctata. In the second and third trimesters, warfarin may cause fetal optic atrophy and mental retardation. When DVT occurs in the postpartum period, LMWH therapy for 7–10 days may be followed by warfarin therapy for 3–6 months. Warfarin is not contraindicated in breast-feeding women.
radiation exposure
The dose of absorbed radiation is measured in milligrays (mGy) (1 mGy = 0.1 rads).
A typical person is exposed to about1 mGy a year of natural “background radiation.”
The mean values of radiation exposure to a fetus from imaging procedures in common use have been estimated as:
Chest x ray: <0.01 mGy
Perfusion scan: <0.08 mGy
Ventilation scan: <0.1 mGy
CTPA: <0.13 mGy
Abdominal x ray: 1.4 mGy
Intravenous urogram: 1.7 mGy
Pelvis CT: 25 mGy.
Exposure to ionising radiation in the first two weeks of conception tends to have an “all or nothing” effect, leading to either miscarriage or no discernable effects. Exposure later in pregnancy tends to have more graduated effects depending on the dose and the gestation at exposure.
A radiological procedure causing fetal exposure of less than 10 mGy is considered safe at any gestation.
Asthma
Asthma is a chronic inflammatory disorder of the airways characterised by:• Pulmonary symptoms
• Reversible airway obstruction
• Evidence of bronchial hyper-reactivity.
Asthma seen in 8% of pregnant. Although uncontrolled asthma may increase the risk of adverse outcomes well-controlled asthma generally have good pregnancy outcomes.
Asthma may improve, worsen, or remain unchanged; the mechanisms remain undefined.
The diagnosis of asthma is usually straightforward .
The most common alternative diagnosis isdyspnea of pregnancy, which is not associated with cough, wheezing, chest tightness, or airway obstruction.
Other potential diagnoses include cough due to reflux or postnasal drip, bronchitis, laryngeal dysfunction, hyperventilation, pulmonary edema, and pulmonary embolism.
Spirometry is the preferred method for pulmonary function testing.
However, peak expiratory flow measurement with a peak flow meter is also adequate.patients should be monitored with testing of peak expiratory flow rate and forced expiratory volume in 1 second as well as by following their symptoms during pregnancy.
• The demonstration of a reduced FEV1 or ratio of FEV1 to forced vital capacity with a 12% or greater improvement in FEV1 after the administration of inhaled albuterol confirms a diagnosis of asthma in pregnancy.
Schatz M and Dombrowski M. N Engl J Med 2009;360:1862-1869
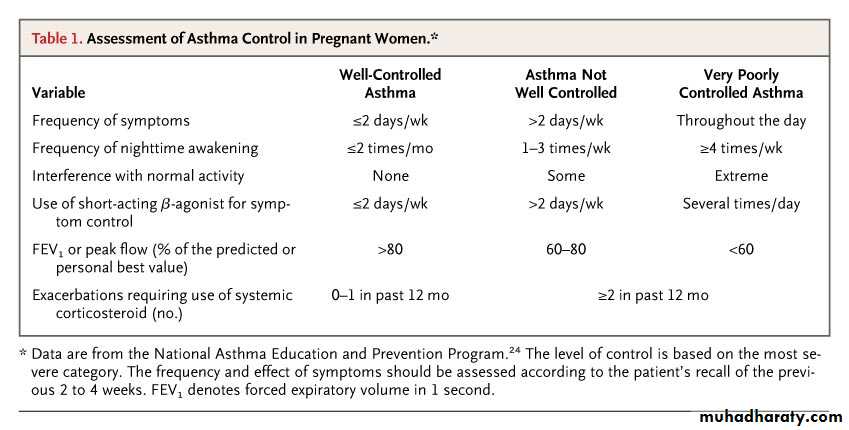
Assessment of Asthma Control in Pregnant WomenSchatz M and Dombrowski M. N Engl J Med 2009;360:1862-1869
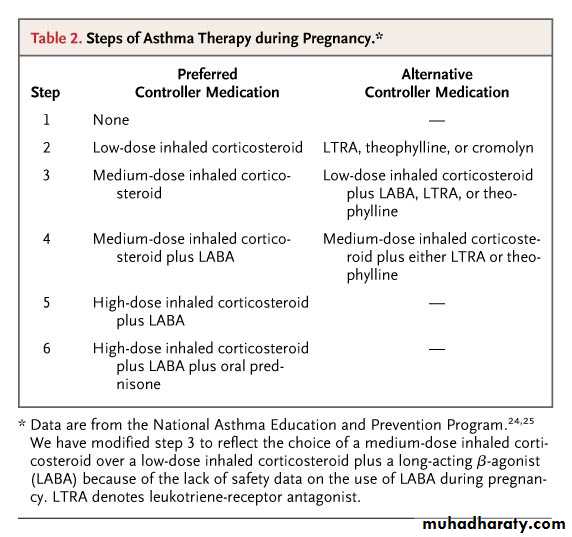
Steps of Asthma Therapy during PregnancySchatz M and Dombrowski M. N Engl J Med 2009;360:1862-1869
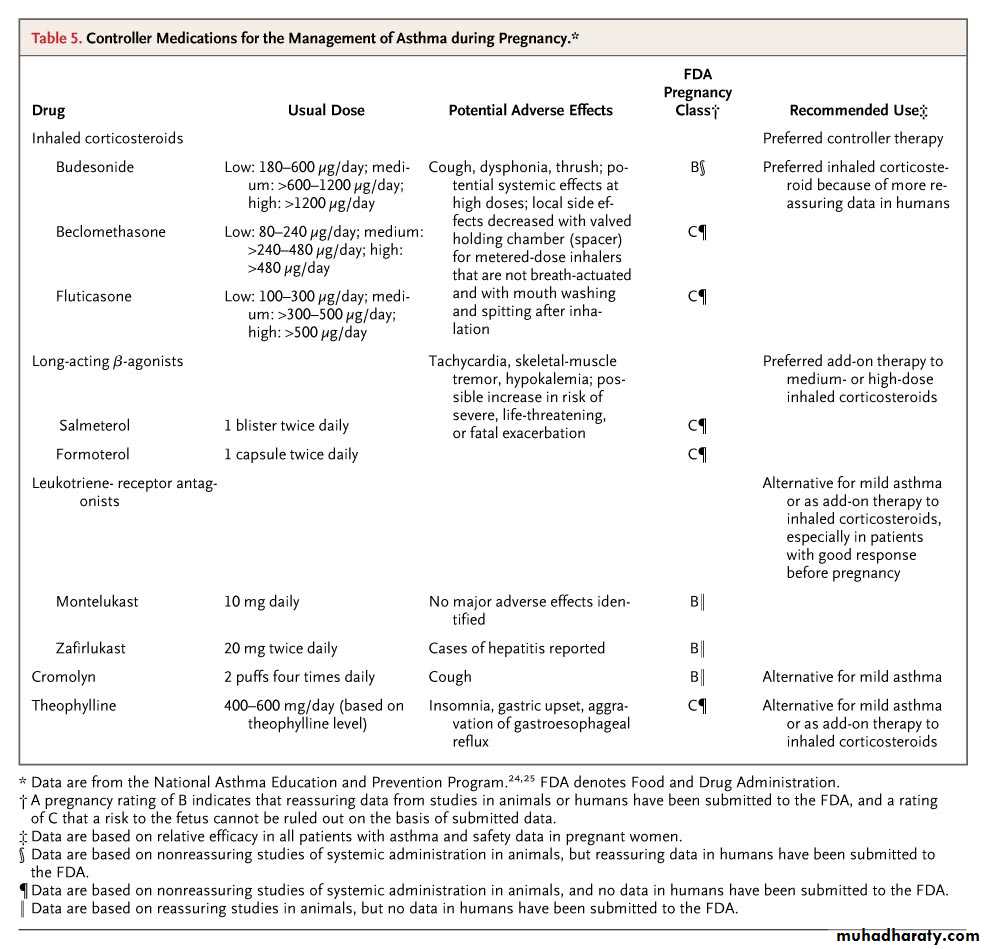
Controller Medications for the Management of Asthma during Pregnancy• The main goal of asthma treatment is to maintain sufficient oxygenation of the fetus by preventing hypoxic episodes in the mother
• long-term controller medications that prevent the manifestations of asthma (inhaled corticosteroids, long-acting β-agonists, leukotriene modifiers, cromolyn, and theophylline) .
• rescue therapy that provides quick relief of symptoms (short-acting inhaled β-agonists).
Medications
All patients should be educated
In studies, inhaled corticosteroids were the most effective controller medications in terms of reducing symptoms and exacerbations and improving pulmonary function.
Long-acting β-agonists have been shown to be more effective than leukotriene-receptor antagonists or theophylline as add-on therapy to inhaled corticosteroids.
Many studies have shown no increased perinatal risks (including preeclampsia, preterm birth, low birth weight, and congenital malformations) associated with the use of inhaled β-agonists or inhaled corticosteroids,
The use of oral corticosteroids among pregnant women with asthma has been associated with increased risks of preeclampsia and prematurity among their offspring, as compared with the use of other asthma medications
Reassuring data on the use of cromolyn and theophylline in pregnant women have been published.
Data on the use of leukotriene-receptor antagonists during pregnancy are more limited; Data are lacking regarding the safety of long-acting β-agonists, although the inhalational route and the generally reassuring data on short-acting β-agonists suggest that these agents are probably safe.
A possible association between long-acting β-agonists and an increased risk of severe and even fatal asthma exacerbations has been observed in patients who were not pregnant. Although the data are sparse, expert panels suggest that the benefits of the use of long-acting β-agonists appear to outweigh the risks as long as they are used concurrently with inhaled corticosteroids.
should be managed with inhaled β-agonists, inhaled anticholinergic drugs, and systemic corticosteroids. Maintenance of an arterial oxygen saturation of at least 95% measured by means of pulse oximetry, is recommended to ensure sufficient oxygenation in both the mother and the fetus.
Asthma Exacerbations
If oxygen saturation remains below a level of at least 95% (as measured by means of pulse oximetry) while the patient is breathing ambient air, if FEV1 or peak expiratory flow remains below 70% of the predicted value, or if there is evidence of fetal compromise, the patient should be hospitalized, with careful medical and obstetrical surveillance.
Assessment of the fetus during an acute asthma episode depends on the stage of the pregnancy, but continuous electronic fetal monitoring, a biophysical profile, or both should be considered if the fetus has reached the stage of viability.
In general, data are lacking on the optimal obstetrical care of patients with asthma, Adequate hydration and analgesia should be maintained during labor and delivery; analgesia should not compromise the patient's respiratory status, and insufficient pain control could trigger bronchospasm.
Obstetrical Care
asthma medications should be continued during labor and delivery. It is commonly recommended that women who are currently taking systemic corticosteroids or who have received several short courses of systemic corticosteroids during pregnancy receive intravenous corticosteroids (e.g., hydrocortisone at a dose of 100 mg every 8 hours) during labor and for 24 hours after delivery in order to prevent adrenal crisis.
Cesarean delivery is rarely required. In general, only small amounts of asthma medications enter breast milk. During breast-feeding, use of prednisone, theophylline, antihistamines, inhaled corticosteroids, beta2-agonists, and cromolyn is therefore not contraindicated.
During pregnancy, budesonide is the preferred inhaled corticosteroid.
For pregnant women with asthma, recommended rescue therapy is inhaled albuterol (Salbutamol).• Symptoms (no more than 2 days per week)
• Use your rescue inhaler(no more than 2 days/week)• Awakenings with asthma symptoms (no more than 2 times per month)
• Interferes with daily activities (not at all)
• Peak flow readings (normal between asthma attacks)
• Use of oral steroids (no more than once a year)
Mild intermittent asthma
• Symptoms more than 2 days per week, but not daily
• Use rescue inhaler more than 2 days per week, but not daily• Wake up 3 to 4 nights per month
• Minor interference with daily activities
• Have a FEV1 greater than 80% of predicted or normal lung function most of the time
Mild persistent asthma
• Symptoms daily.
• Rescue inhaler daily.
• Wake up from asthma more than one night per week, but not every night.
• Moderately interferes with daily activities.
• FEV1 greater than 60% but less than 80% of predicted.
Moderate persistent asthma
• Have symptoms throughout the day
• Wake up from your asthma nightly• Use your rescue inhaler multiple times per day
• Have extreme interference with your daily activities
• Have a FEV1 less than 60% of predicted
Severe Persistent Asthma
For mild intermittent asthma
albuterol should be given as needed.For mild persistent asthma
low-dose inhaled corticosteroid, with alternative treatments being cromolyn, a leukotriene receptor antagonist, or theophylline to a target serum level of 5 to 12 µg/ mL.Recommendations for step therapy medical management of asthma during pregnancy are as follows:
For moderate persistent asthma
low-dose inhaled corticosteroid and salmeterol or medium-dose inhaled corticosteroid or medium-dose inhaled corticosteroid and salmeterol if needed. An alternative regimen is a low-dose or medium-dose (if needed) inhaled corticosteroid with either a leukotriene receptor antagonist or theophylline to a target serum level of 5 to 12 µg/ mL
For severe persistent asthma
high-dose inhaled corticosteroid and salmeterol, plus oral corticosteroid if needed. An alternative regimen is a high-dose inhaled corticosteroid and theophylline to a target serum level of 5 to 12 µg/ mL, plus an oral corticosteroid if needed.Advising on travel during pregnancy
Travel during pregnancy may carry additional risksThe second trimester of pregnancy is considered the safest in which to travel.
Air travel may carry risk of miscarriage, preterm birth, and thromboembolism.
Adequacy of obstetric and neonatal care facilities at destinations is varied…..
Women should obtain adequate insurance and check with their airline for restrictions on travel
Communicable diseases acquired abroad may increase risks of perinatal morbidity.
BMJ 28 April 2011
Paracetamol is one of the most common overdoses in pregnancy and is capable of crossing the placenta. Cytochrome 2E1 does not become active until 14 weeks' gestation and, therefore, the fetus is unable to metabolise paracetamol to NAPQI during this period. Immediate treatment of paracetamol ingestion should be the same as for a non-pregnant female. Neither paracetamol overdose nor acetylcysteine administration appears to have a significant detrimental effect on the developing fetus.