Teaching Topics
د. حسين محمد جمعةاختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2011
Clinical Pearls
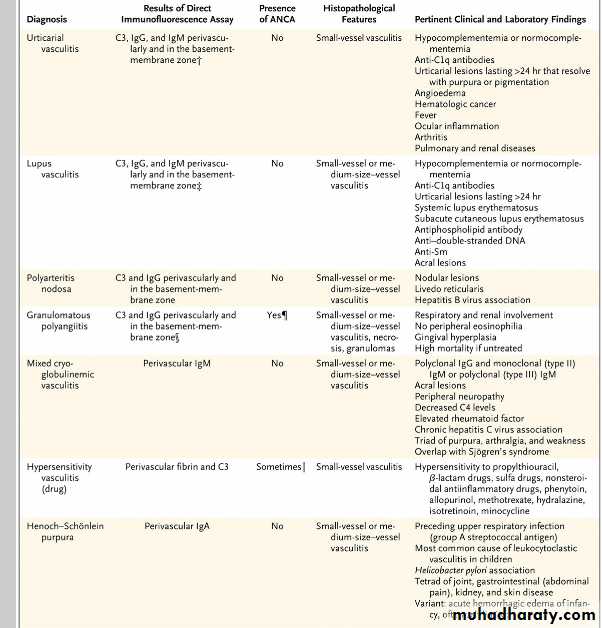
What rheumatologic disorders associated with vasculitis most frequently cause scleritis?Are rheumatoid arthritis and ANCA-associated vasculitides, particularly granulomatosis with polyangiitis. Less likely causes of scleritis are relapsing polychondritis, polyarteritis nodosa, systemic lupus erythematosus, Behçet’s disease, and hypocomplementemic urticarial vasculitis.
How can urticarial vasculitis be differentiated from simple urticaria?
Urticarial lesions include simple urticaria — pruritic, edematous, evanescent plaques that last less than 24 hours.In contrast, urticarial vasculitis tend to be painful or to burn rather than itch. They generally last longer than 24 hours and often heal with hyperpigmentation or purpura, particularly after repeated episodes of urticarials.
Q. How does Henoch–Schönlein purpura (HSP) typically present?
A. HSP is an immune-mediated vasculitis that causes arthritis, abdominal pain, renal disease, and palpable purpura. Purpura is the sine qua non of HSP. HSP accounts for 10% of all cutaneous vasculitides and more than 90% of all cases of vasculitis in children, but approximately 10% of cases of HSP occur in adults.
Q. What are the features of hypocomplementemic urticarial vasculitis?
A. Hypocomplementemic urticarial vasculitis is characterized by urticarial vasculitis, accompanied possibly by arthralgias, arthritis, abdominal pain, and angioedema, as well as fever.How should levels of parathyroid hormone (PTH) and vitamin D be interpreted in a pregnant woman with hypercalcemia?
PTH levels are typically in the low-to-midnormal range during pregnancy.
A normal PTH level in a pregnant woman with hypercalcemia is consistent with primary hyperparathyroidism.
Although an elevated level of 1,25-dihydroxyvitamin D is a recognized cause of hypercalcemia in nonpregnant patients with certain neoplastic or granulomatous disorders (e.g., lymphoma, sarcoidosis, or tuberculosis), in pregnant patients, the elevated level may simply reflect the physiological increase in 1,25-dihydroxyvitamin D during normal pregnancy.
What are the complications associated with hyperparathyroidism during pregnancy?
Fetal complications associated with maternal hyperparathyroidism include restriction of intrauterine growth, low birth weight, preterm delivery, stillbirth, miscarriage, and neonatal tetany. The maternal complications are similar to those seen in nonpregnant women and include nephrolithiasis, pancreatitis, bone disease, changes in mental status, and hypercalcemic crisis.Differential Diagnosis of Cutaneous Vasculitis.
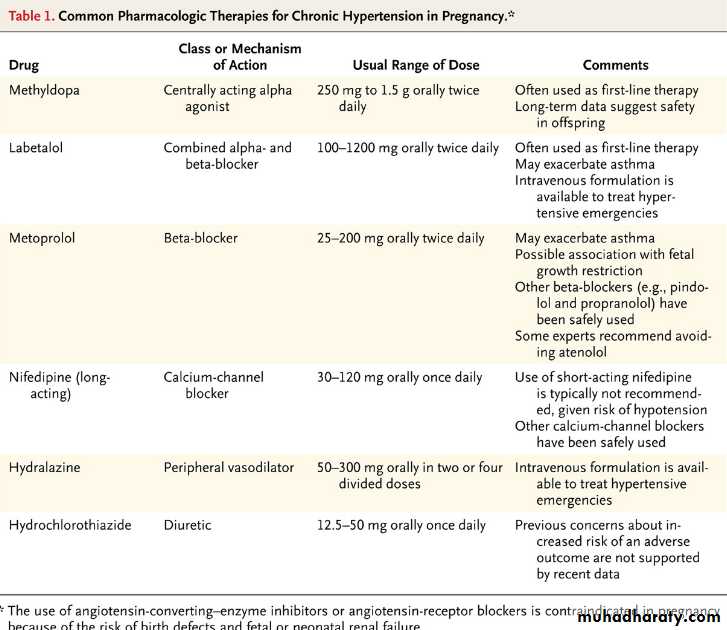
What are the risks associated with chronic hypertension in pregnancy?
Women with chronic hypertension have an increased frequency of preeclampsia (17 to 25% vs. 3 to 5% in the general population), as well as placental abruption, fetal growth restriction, preterm birth, and cesarean section. Preeclampsia is a leading cause of preterm birth and cesarean delivery in this population.
How does the blood pressure of women with chronic hypertension change during pregnancy?
Most women with chronic hypertension have a decrease in blood pressure during pregnancy, similar to that observed in normotensive women; blood pressure falls toward the end of the first trimester and rises toward prepregnancy values during the third trimester. As a result, antihypertensive medications can often be tapered during pregnancy.Q. What blood pressure targets are generally recommended during pregnancy?
A. Various professional guidelines provide disparate recommendations regarding indications for starting therapy (ranging from a blood pressure >159/89 mm Hg to >169/109 mm Hg) and for blood-pressure targets for women who are receiving therapy (ranging from <140/90 mm Hg to <160/110 mm Hg). For women whose antihypertensive therapy is continued, aggressive lowering of blood pressure should be avoided, though prospective controlled trials to support these recommendations are not available.Q. What medications are recommended to manage hypertension in pregnancy?
A. The antihypertensive agent with the largest quantity of data regarding fetal safety is methyldopa, which has been used during pregnancy since the 1960s. Labetalol, a combined alpha- and beta-receptor blocker, is often recommended as another first-line or second-line therapy for hypertension in pregnancy. Long-acting calcium-channel blockers also appear to be safe in pregnancy, although experience is more limited than with labetalol. Angiotensin-converting–enzyme inhibitors and angiotensin-receptor blockers are contraindicated in pregnancy.Of all breast cancers, only 1 to 6% are classified as inflammatory breast cancer. Approximately 20 to 40% of patients with inflammatory breast cancer have evidence of distant metastases at the time of presentation.
How can locally advanced breast cancer with secondary inflammatory changes be distinguished from inflammatory breast cancer?
Locally advanced breast cancer with secondary inflammatory changes typically results from a long history of neglected disease; a short clinical course is more consistent with inflammatory breast cancer. Relatively younger age also supports the diagnosis of inflammatory breast cancer; the age-specific rate of inflammatory breast cancer peaks and plateaus at 50 years, whereas the rate of locally advanced disease continues to increase after 50 years of age.
How should inflammatory breast cancer be optimally treated?
The optimal therapeutic approach for this disease is preoperative chemotherapy to render the tumor operable, followed by mastectomy and radiation.Q. What do skin biopsies from patients with inflammatory breast cancer typically reveal?
A. In cases of inflammatory breast cancer, skin-biopsy specimens characteristically reveal numerous tumor emboli occluding dermal lymphatics. Dermal lymphatic occlusion is believed to lead to increased vascular pressure and stasis, resulting in erythema, edema, and thickening of the skin. Although the changes mimic the appearance of an acute inflammatory process, inflammation does not actually contribute in any consequential way to the skin manifestations.
Q. What are the typical staining characteristics of inflammatory breast cancer?
A. Inflammatory breast carcinomas are more frequently negative for ER and PR than are noninflammatory breast carcinomas and have higher proliferation indexes. As compared with noninflammatory carcinomas, inflammatory carcinomas are more often positive for HER2.Multiple myeloma is a neoplastic plasma cell disorder characterized by clonal proliferation of malignant plasma cells in the bone marrow microenvironment, monoclonal protein in the blood or urine, and associated organ dysfunction.
It accounts for approximately 1% of neoplastic diseases and 13% of hematologic malignancies.
How is multiple myeloma diagnosed?
The diagnosis of myeloma is based on the presence of at least 10% clonal bone marrow plasma cells and serum or urinary monoclonal protein.What manifestations of disease are typically present at the time multiple myeloma is diagnosed?
Anemia, present in about 73% of patients at diagnosis, is generally related to myeloma marrow infiltration or renal dysfunction. Almost 80% of newly diagnosed patients develop bony lesions; 58% of patients reported bone pain. Renal impairment occurs in 20 to 40% of newly diagnosed patients, mainly due to direct tubular damage from excess protein load, dehydration, hypercalcemia, and the use of nephrotoxic medications. The risk of infection is increased with active disease, but decreases with response to therapy. Hypercalcemia is uncommon.
Q. How are high-risk disease and poor prognosis defined?
A. High-risk disease and poor prognosis are defined by the presence of one of the following: hypodiploidy, t(4;14), or deletion 17p13; high levels of serum β2-microglobulin or lactate dehydrogenase; and International Staging System stage III.Q. What treatment approach is recommended for patients with multiple myeloma?
A. Current data would support induction therapy with thalidomide, lenalidomide, or bortezomib plus hematopoietic stem-cell transplantation for patients aged less than 65 years without significant heart, lung, renal, or liver dysfunction.Reduced dose-intensity autologous transplantation should be considered for older patients or those with comorbidities.
Conventional therapy combined with thalidomide, lenalidomide, or bortezomib should be administered in patients older than 65 years of age. Less intense approaches that limit toxicity or prevent treatment interruption that would reduce the intended treatment effect should be considered in patients over 75 years of age or in younger patients with comorbidities.
How effective was omalizumab in reducing asthma exacerbations?
Adding omalizumab therapy to guideline-directed care for inner-city children, adolescents, and young adults with allergic asthma resulted in a significant and clinically meaningful decrease in asthma-related symptoms of 0.48 days per 2-week period, as compared with placebo (from 1.96 to 1.48 days), a reduction in the number of participants with at least one exacerbation (30.3% in the omalizumab group vs. 48.8% in the placebo group), fewer hospitalizations (1.5% vs. 6.3%), and a reduced need for inhaled glucocorticoids to maintain this improved level of asthma control.Did treatment with omalizumab reduce seasonal exacerbations?
In the control group, the average monthly rate of exacerbations in the fall and spring was almost twice the rate during the summer (9.0% and 8.1% vs. 4.6%), whereas the omalizumab group had only a small, nonsignificant, increase (4.3% and 4.2% vs. 3.3%).Q. What is the mechanism of action of omalizumab?
A. Omalizumab is a humanized monoclonal anti-IgE antibody.Q. How was omalizumab administered?
A. Each participant in the treatment arm received subcutaneous injections of omalizumab every 2 or 4 weeks for a total of 60 weeks (15 or 30 injections).
Approximately 10% of all cases of deep-vein thrombosis involve the upper extremities. Complications are less common with upper extremity deep-vein thrombosis than with lower extremity thrombosis, but include pulmonary embolism in 6% (versus 15 to 32% with lower extremity thrombosis), recurrence at 12 months in 2 to 5% (versus 10% with lower extremity thrombosis), and the post-thrombotic syndrome in 5% (versus up to 56% with lower extremity thrombosis).
What imaging technique is recommended to diagnose upper extremity deep-vein thrombosis?
Compression ultrasound, which relies on the finding that a thrombosed vein is incompressible, and is the clinical standard for diagnosing lower extremity thrombosis, is also the preferred imaging test for patients with suspected upper extremity deep-vein thrombosis.Data on the diagnostic accuracy of computed tomographic angiography or magnetic resonance angiography are limited, but either test may be useful for imaging the proximal arm veins if ultrasound is indeterminate. Ultrasound has virtually replaced conventional phlebography for diagnosing upper extremity deep-vein thrombosis; phlebography is occasionally performed in patients with indeterminate ultrasound results.
What type of initial anticoagulation is recommended for the management of patients with upper extremity deep-vein thrombosis?
Initial anticoagulation treatment thus usually involves low-molecular-weight heparin; unfractionated heparin is preferred in patients with severe renal dysfunction.
Q. How should catheter-associated thrombosis be managed?
A. In patients with catheter-associated thrombosis, routine catheter removal is not recommended. The decision whether to remove the catheter should consider the need for further intravenous medications, blood sampling, venous access difficulties, and patient preferences. Removal is generally warranted in cases of catheter malfunction or infection, contraindication to anticoagulation therapy, persistent symptoms or signs of upper extremity deep-vein thrombosis during initial anticoagulation therapy, or when the catheter is no longer needed.Q. What duration of treatment is recommended for long-term anticoagulation in patients with upper extremity deep-vein thrombosis?
A. Based on data from cohort studies demonstrating low recurrence rates with use of vitamin K antagonists for three to six months in patients with upper extremity deep-vein thrombosis, this duration of therapy is generally recommended, including in patients whose central venous catheter is removed. Vitamin K antagonists are generally used, but low-molecular-weight heparin is preferred in patients with cancer.
Among patients with decompensated heart failure, was there any advantage associated with continuous as compared to intermittent bolus treatment with furosemide?
Patients who were assigned to intravenous boluses of furosemide every 12 hours were more likely to require a dose increase at 48 hours than were those assigned to continuous intravenous infusion (21% vs. 11%, P=0.01).
Among patients with decompensated heart failure, was there any advantage associated with high-dose as compared to low-dose treatment with furosemide?
Patients assigned to the high-dose strategy were more likely to change to oral diuretics at 48 hours than were those assigned to the low-dose strategy (31% vs. 17%, P<0.001). Conversely, patients in the low-dose group were more likely to require a 50% increase in the dose at 48 hours than were those in the high-dose group (24% vs. 9%, P=0.003).
Q. Were any of the diuretic treatment strategies used in this study associated with a significantly greater increase in the serum creatinine?
A. There were no significant differences in patients’ change in renal function when diuretic therapy was administered by continuous infusion as compared with bolus infusion or at a high dose as compared with a low dose.
Q. Were any of the diuretic treatment strategies used in this study associated with a greater improvement in patients’ global assessment of symptoms?
A. There were no significant differences in patients’ global assessment of symptoms when diuretic therapy was administered by continuous infusion as compared with bolus infusion or at a high dose as compared with a low dose.
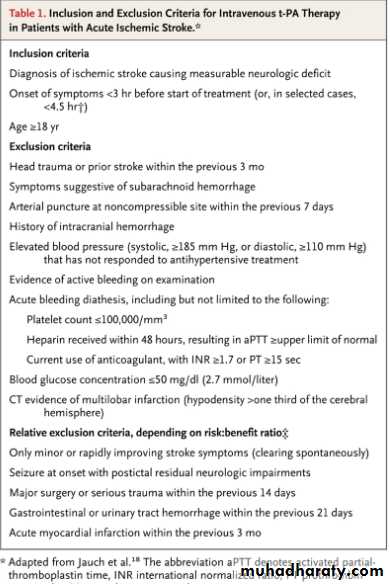
How soon after a stroke does rt-PA need to be given for its use to increase the probability of a favorable outcome?
Within 3 hours of onset of stroke, intravenous rt-PA increases the probability of favorable outcome. Some stroke centers now treat patients 3 to 4.5 hours from stroke onset. However, at present only treatment within 3 hours is approved by the FDA.
How should hypertension be managed when thrombolytic therapy for acute stroke is being considered?
Current guidelines recommend treatment of hypertension to achieve a systolic pressure ≤185 mm Hgsystolic and diastolic values ≤110 mm Hg prior to administering intravenous rt-PA. One or two doses of labetalol may be used to bring the blood pressure under these limits, but if blood pressure does not decrease to that level quickly, intravenous nicardipine, or, more rarely, sodium nitroprusside may be started to titrate blood pressure rapidly to this level.
Q. Current guidelines suggest that thrombolytic therapy be withheld from which patients with acute ischemic stroke?
A. If a focal area of low density (or “hypodensity”) is seen on computed tomography of the brain that involves more than 1/3 of the middle cerebral artery territory, most treatment protocols recommend withholding thrombolytic therapy, because this finding (which suggests irreversible injury) is predictive of subsequent hemorrhagic transformation of the infarct in some studies. Platelet count should be ≥100,000, prothrombin time <15 seconds (or INR <1.7) and glucose >50 mg/dl before rt-PA is administered.
Q. Which patients with acute stroke are at highest risk of developing a hemorrhage following treatment with rt-PA?
A. Symptomatic intracranial hemorrhage occurs in 1.7 to 8.0% of treated patients. In addition to age and NIH stroke scale score, other identified independent risk factors for symptomatic intracranial hemorrhage include CT hypodensity, elevated serum glucose and persistence of proximal arterial occlusion beyond 2 hours from rt-PA bolus.
How does coccidioidomycosis present?
Sixty percent of patients with primary infection are asymptomatic; the rest have fever, night sweats, cough, pleuritic chest pain, arthralgias, rash, headache, or some combination of these symptoms 1 to 3 weeks after exposure. Primary coccidioidomycosis is manifested as consolidation, nodules, or opacities on chest imaging, with concurrent hilar adenopathy in up to 40% of patients. Mediastinallymphadenopathy is less common. Dissemination occurs in about 1% of immunocompetent patients; the most common extrapulmonary sites are the meninges, skin, and skeletal system.How is coccidioidomycosis diagnosed?
The diagnosis can be established by serologic tests, histopathological identification of characteristic spherules, or growth of coccidioides species in culture. In patients with solitary pulmonary nodules, culture of respiratory secretions has a low yield and is not recommended as the sole means of ruling out the diagnosis.Q. What is the natural history of coccidioidomycosis?
A. Most patients with uncomplicated primary coccidioidomycosis do not require antifungal therapy; symptoms resolve spontaneously over a period of several weeks.Q. Which patients with coccidioidomycosis should be treated?
A. Antifungal therapy is indicated in patients with disseminated disease, concurrent immunosuppression, pregnant or postpartum status, or an age of more than 55 years, as well as in those with severe primary infection (as indicated by ongoing weight loss, persistent night sweats, infiltrates involving more than half of one lung or portions of both lungs, persistent lymphadenopathy, or a complement-fixation titer ≥1:16).
What endocrine tumors cause flushing?
Neuroendocrine tumors, including pheochromocytoma, vasoactive intestinal peptide–producing tumors, medullary thyroid carcinoma, and carcinoid tumors, may all secrete substances that cause flushing, hypotension or hypertension, diarrhea, and respiratory symptoms in various combinations.What are the characteristics of the carcinoid syndrome?
The carcinoid syndrome is characterized by cutaneous flushing, diarrhea, wheezing, and cardiac valvular lesions. Episodes are often precipitated by the ingestion of alcohol and chocolates. Facial telangiectasia and cyanosis and pellagra-like skin changes may be seen in chronic cases.Morning Report Questions
Q. How is mastocytosis diagnosed?A. Determination of the serum tryptase level is essential to establish the diagnosis of systemic mastocytosis and differentiate it from anaphylaxis.
Q. How is mastocytosis treated?
A. H1- and H2-histamine-receptor blockade (cetirizine, ranitidine); oral disodium cromoglycate, which blocks the release of mediators from mast cells; leukotriene-receptor blockade (montelukast); inhibition of PGD2 generation with acetylsalicylic acid; and a proton-pump inhibitor (omeprazole) are all used to treat systemic mastocytosis.
How should the symptom of abdominal distension be evaluated?
The isolated symptom of abdominal distention is frequently explained by functional gastrointestinal disorders such as the irritable bowel syndrome, but when it is accompanied by early satiety, fatigue, and dyspnea, more serious conditions such as ascites, subacute gastrointestinal hypomotility or obstruction, or an intraabdominal mass should be considered.What are the most common causes of ascites?
The most common cause of ascites in the United States is cirrhosis, followed distantly by a malignant condition, right heart failure, tuberculosis, pancreatic disease, and various rare infectious and hematologic diseases.Q. How can the causes of ascites be differentiated from each other using the serum–ascites albumin gradient (SAAG)?
A. Conditions such as peritoneal carcinomatosis and tuberculosis are associated with a low SAAG. An elevated SAAG (>1.1 g per deciliter) points to portal hypertension as the cause of ascites and suggests the need for further consideration of causes of portal hypertension, such as cirrhosis, heart failure, and the Budd–Chiari syndrome.
Q. How should a low level of B-type natriuretic peptide (BNP) be interpreted in a patient with an elevated venous pressure and ascites?
A. A low BNP level may occur in constrictive pericarditis, but would be unusual in a patient with biventricular or left-sided heart failure. Because the myocardium is encased in a constrictive pericardial sac, BNP values are typically normal or only slightly elevated because of a lack of cardiac stretch. A normal BNP level in a patient with ascites may lead clinicians to incorrectly dismiss the possibility of a cardiac cause.
How were the heparins administered in this study?
Local research pharmacists randomly assigned patients in this study to receive either subcutaneous dalteparin (at a dose of 5000 IU once daily) or unfractionated heparin (at a dose of 5000 IU twice daily).As compared to treatment with unfractionated heparin, did treatment with dalteparin significantly reduce the incidence of deep-vein thromboses?
No. The primary outcome of incident proximal deep-vein thrombosis developed in 96 of 1873 patients (5.1%) receiving dalteparin and in 109 of 1873 patients (5.8%) receiving unfractionated heparin (hazard ratio in the dalteparin group, 0.92; 95% confidence interval [CI], 0.68 to 1.23; P=0.57).
Q. As compared to treatment with unfractionated heparin, did treatment with dalteparin significantly reduce the incidence of pulmonary embolism?
A. Yes. Pulmonary embolism developed in significantly fewer patients receiving dalteparin (24 patients, 1.3%) than in those receiving unfractionated heparin (43 patients, 2.3%) (hazard ratio, 0.51; 95% CI, 0.30 to 0.88; P=0.01).
Q. As compared to treatment with unfractionated heparin, did treatment with dalteparin significantly reduce the incidence of heparin-induced thrombocytopenia?
A. The per-protocol analysis also had results similar to those in the main analysis, but the hazard ratio for the development of heparin-induced thrombocytopenia favoring dalteparin was significant (0.27; 95% CI, 0.08 to 0.98; P=0.046).
Q. What is the role of ultrasonography in diagnosing pneumothorax?
A. Ultrasonography for pneumothorax in trauma has shown to be much more sensitive and about equally specific compared to the supine chest x-ray typically used for the initial evaluation of trauma patients, with CT as a gold standard.Q. What is the role of ultrasonography for diagnosing other pulmonary conditions?
A. Ultrasonography has been shown to be more accurate than auscultation or chest radiography for pleural effusion, consolidation, and alveolar interstitial syndrome in the critical care setting.
What risks are associated with an increased BMI?
Obesity is associated with multiple chronic diseases, including type 2 diabetes, hypertension, coronary heart disease, stroke, and several cancers.
How does BMI differentially affect risk in Europe and Asia?
Studies have shown that for a given BMI, Asians generally have a higher percentage of body fat than do Europeans. Asian populations have also been shown to have an elevated risk of type 2 diabetes, hypertension, and hyperlipidemia at a relatively low level of BMI.Q. What BMI cutoff points for overweight and obesity have been suggested for Asian patients?
A. The suggested cutoff points for Asians are ≥23.0 for overweight and ≥25.0 for obesity.
Q. What did this study conclude?
A. Underweight was associated with a substantially increased risk of death in all Asian populations. The excess risk of death associated with a high BMI, however, was seen among East Asians but not among Indians and Bangladeshis.
What is the difference between high- and low-frequency ultrasound?
Ultrasound is defined as a frequency above what humans can hear, or greater than 20,000 Hz (20 kHz). Diagnostic ultrasound is in the millions of Hertz, or MHz range. Lower-frequency ultrasound will penetrate better, but with lower resolution. Higher-frequency ultrasound provides better images, but will not visualize deeper structures well. Therapeutic ultrasound, designed to create heat using mechanical sound waves, is typically lower in frequency than diagnostic ultrasound.For what conditions is screening ultrasonography recommended by the U.S. Preventive Services Task Force?
In 2005, the USPSTF gave a class B recommendation for one-time ultrasound screening for abdominal aortic aneurysm in males aged 65–75 who had ever smoked, leading to the incorporation of this screening into Medicare reimbursement. The USPSTF reports that ultrasonography has a sensitivity of 95% and a specificity of nearly 100% for this purpose when performed in “a setting with adequate quality assurance.” The USPSTF has specifically recommended against the use of ultrasound for routine screening for carotid stenosis, peripheral vascular disease, and ovarian cancer in the general population.
How does iron accumulation lead to organ dysfunction?
Non–transferrin-bound iron is a heterogeneous assortment of iron complexes that appear to be the major mediators of extrahepatic tissue damage in transfusional iron overload. Non–transferrin-bound plasma iron enters specific cells, particularly hepatocytes, cardiomyocytes, anterior pituitary cells, and pancreatic beta-cells. In these cells, iron accumulation leads to the generation of reactive oxygen species, resulting in cellular dysfunction, apoptosis, and necrosis.How are the available iron chelators administered?
Deferoxamine is administered subcutaneously or intravenously, usually with a portable pump, for 8 to 10 hours each day, 5 to 7 days per week. Subcutaneous administration is preferred except in patients with severe cardiac iron deposition, for whom continuous intravenous deferoxamine therapy is recommended. The synthetic chelator deferasirox is well absorbed from the gastrointestinal tract and thus can be administered orally. A third iron chelator, the synthetic oral agent deferiprone is not approved for use in the United States or Canada.Q. When should iron-chelating therapy be initiated?
A. Ideally, iron-chelating therapy should be initiated prophylactically, before clinically significant iron accumulation has occurred. Treatment should begin when patients have received 10 to 20 red-cell transfusions. Patients who have already undergone repeated transfusion without sufficient chelation can also be successfully treated, but they may require more intensive regimens.
Q. What is the clinical benefit of iron-chelating therapy?
A. Only one small, randomized trial has compared chelation plus deferoxamine with no therapy; this trial enrolled 20 children with β-thalassemia. After a mean of 5.8 years of treatment with intramuscular deferoxamine, the liver iron concentration was 25.9 mg per gram of liver tissue (dry weight) in the deferoxamine group and 42.2 mg per gram in the control group. At 14 years, one death had occurred in the deferoxamine group and six deaths had occurred in the control group.Gout is a type of inflammatory arthritis induced by the deposition of monosodium urate crystals in synovial fluid and other tissues.
It is associated with hyperuricemia, which is defined as a serum uric acid level of 6.8 mg per deciliter (404 μmol per liter) or higher, the limit of urate solubility at physiological temperature and pH.
What are the two clinical phases of gout?
Gout has two clinical phases.The first phase is characterized by intermittent acute attacks that spontaneously resolve, typically over 7 to 10 days, with asymptomatic periods between attacks.
The second phase is notable for inadequately treated hyperuricemia with a transition to chronic tophaceous gout, which is often characterized by polyarticular attacks, symptoms between attacks, and crystal deposition (tophi) in soft tissues or joints.
What are the risk factors for developing gout?
Factors that are associated with hyperuricemia and gout include the use of thiazide diuretics, cyclosporine, and low-dose aspirin, as well as insulin resistance, the metabolic syndrome, obesity, renal insufficiency, hypertension, congestive heart failure, and organ transplantation. The risk of incident gout is increased in persons with an increased intake of dietary purines (particularly meat and seafood), ethanol (particularly beer and spirits), soft drinks, and fructose and is decreased in those with an increased intake of coffee, dairy products, and vitamin C (lower urate).Q. How should acute gout be managed?
A. NSAIDs and colchicine (at a dose of 1.2 mg at the onset of a flare, followed by 0.6 mg 1 hour later) are first-line agents for acute attacks. When the use of NSAIDs or colchicine is poorly tolerated or contraindicated, glucocorticoids or ACTH may be used.Q. What urate lowering treatment is recommended?
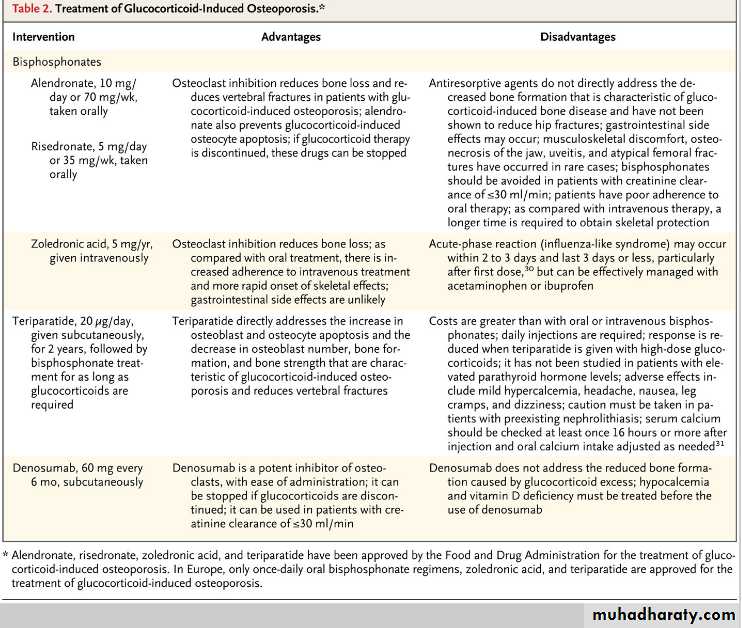
A. Urate-lowering therapy should not be initiated during acute attacks but rather started 2 to 4 weeks after flare resolution. The most commonly drug used to lower urate levels is allopurinol, which is effective in decreasing flares and tophi, particularly among patients with target urate levels. Febuxostat was approved for the treatment of hyperuricemia in gout in 2009. Because rapid lowering of urate levels is associated with gout flares, the general recommendation is to use colchicine at a dose of 0.6 mg once or twice daily for flare prophylaxis.At what dose of glucocorticoids is pharmacologic intervention to prevent bone loss recommended?
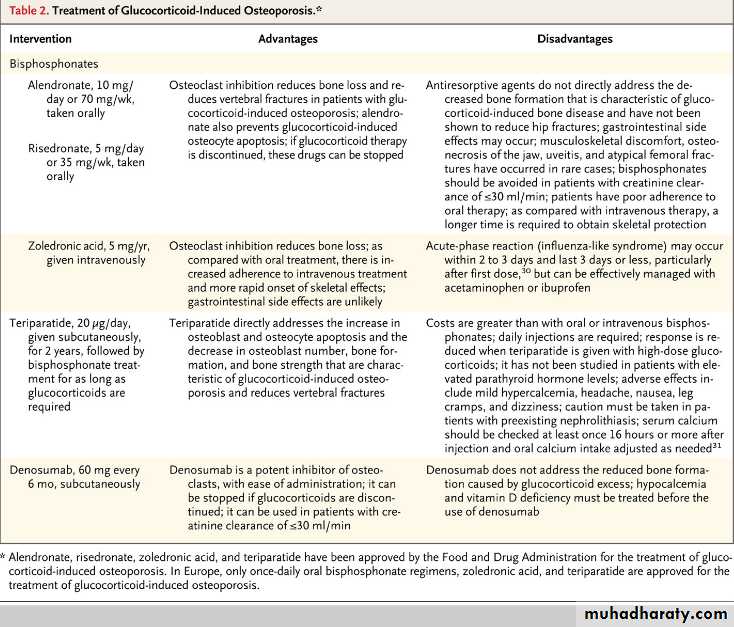
The American College of Rheumatology suggests that pharmacologic therapy is warranted when a patient is expected to be treated with at least 7.5 mg per day of prednisone (or equivalent) for at least 3 months. Patients at increased risk require treatment with any dose or duration.
What is the first-line treatment for the prevention of bone loss among glucocorticoid-treated patients?
Bisphosphonates are considered to be the first-line options for the treatment of glucocorticoid-induced osteoporosis; alendronate, risedronate, and zoledronic acid are approved by the Food and Drug Administration (FDA) for this indication. An alternative to bisphosphonates is teriparatide, recombinant human parathyroid hormone 1-34, which is approved by the FDA for the treatment of glucocorticoid-induced osteoporosis. Another potential treatment option is denosumab but it is not currently approved for the treatment of glucocorticoid-induced osteoporosis.
Q. How quickly does fracture risk increase after glucocorticoid treatment is initiated?
A. The loss of bone mineral density is biphasic; it occurs rapidly (6 to 12% loss) within the first year and more slowly (approximately 3% loss yearly) thereafter. However, the risk of fracture escalates by as much as 75% within the first 3 months after the initiation of therapy, typically before there is a substantial decline in bone mineral density, suggesting that there are adverse effects of glucocorticoids on bone that are not captured by bone densitometry.Q. What is the mechanism of glucocorticoid-induced bone disease?
A. Glucocorticoid excess directly reduces osteoclast production, but the lifespan of osteoclasts is prolonged, in contrast to the increase in osteoblast apoptosis. Therefore, with long-term therapy, the number of osteoclasts is usually maintained in the normal range, whereas the number of osteoblasts plummets and bone formation is substantially reduced.What are the classic manifestations of GCA?
The classic manifestations are headache, jaw claudication, polymyalgia rheumatica, and visual symptoms. However, 40% of patients present with less typical manifestations, such as breast or ovarian masses, peripheral neuropathy, the syndrome of inappropriate antidiuretic hormone secretion, or mesenteric ischemia.What laboratory findings are typical among patients with GCA?
In a large case series of patients with GCA, over 95% had an erythrocyte sedimentation rate above 50 mm/h, and over 40% had an erythrocyte sedimentation rate over 100mm/h. Elevation of C-reactive protein and hepatic enzymes, particularly alkaline phosphatase, a normocytic anemia, hypoalbuminemia, reactive thrombocytosis, and increased immunoglobulin levels are also commonly associated with GCA.Q. How is uncomplicated GCA generally managed?
A. Uncomplicated GCA can generally be controlled in the acute setting with 40 to 60 mg prednisone daily; this dose is typically tapered slowly over 9 to 12 months. For patients with visual loss, aortitis, or other widespread involvement, higher dose therapy is recommended (e.g., 1000 mg methylprednisolone intravenously each day for three days, followed by oral therapy of 1 mg/kg daily).Q. Is the use of glucocorticoid-sparing agents recommended for patients with GCA?
A. Randomized, placebo-controlled trials have evaluated the use of methotrexate, infliximab, and etanercept as glucocorticoid sparing agents; however, the data are conflicting and further investigation is needed.
Advanced-stage non–small-cell lung cancer (NSCLC) is currently considered an incurable disease for which standard chemotherapy provides marginal improvement in overall survival at the expense ofsubstantial morbidity and mortality. Even with the addition of newer agents, such as bevacizumab, to chemotherapy, the median overall survival of patients with metastatic NSCLC remains approximately 1 year.
Treatment of non–small-cell lung cancer with Erlotinib or Gefitinib
Which patients with non–small-cell lung cancer appear to benefit most substantially from treatment with erlotinib or gefitinib?
The available trial data suggest that EGFR tyrosine kinase inhibitors have efficacy that is similar to that of standard chemotherapy as second- or third-line treatment for patients with advanced NSCLC. Among patients receiving first-line therapy, tyrosine kinase inhibitors appear to be inferior to standard chemotherapy overall but superior for selected patients, especially for those with activating EGFRmutations.
For how long should treatment with erlotinib or gefitinib be continued?
Daily erlotinib or gefitinib therapy should be continued for as long as the patient’s performance status is adequate and there is no clinical or radiographic progression, since patients with stable disease have been shown to derive clinical benefit. Furthermore, data support the continuation of treatment even if a loss of response is documented, since tumor progression is accelerated to a greater degree if the agent is discontinued.Q. Concurrent treatment with which medications should be avoided in patients being treated with erlotinib or gefitinib?
A. The solubility of both erlotinib and gefitinib is pH dependent. Agents that alter gastric pH, such as H2-receptor antagonists and proton-pump inhibitors, can substantially reduce the plasma levels of the EGFR tyrosine kinase inhibitors, and their concomitant use should be avoided.
Q. What is the most common dose-limiting adverse effect that limits erlotinib or gefitinib dosing?
A. In phase 1 studies of both agents, diarrhea was the dose-limiting effect. Diarrhea occurs in up to 55% of patients who are treated with erlotinib, with severe diarrhea occurring in 6% of patients. The incidence of diarrhea in patients receiving gefitinib ranges from 27 to 35%. Unlike traditional cytotoxic agents, erlotinib and gefitinib do not typically cause myelosuppression, neuropathy, alopecia, or severe nausea.
What is the role of angiotensin-converting–enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) for the management of patients with type 2 diabetes?
ACE inhibitors and ARBs slow the worsening of the glomerular filtration rate and lower the rate of albumin excretion. ACE inhibition delays the onset of microalbuminuria in patients with hypertension, type 2 diabetes, normoalbuminuria, and normal renal function.
According to the results of this study, what were the benefits associated with the use of olmesartan as compared to placebo treatment?
Microalbuminuria developed in 8.2% of the patients in the olmesartan group (178 of 2160 patients who could be evaluated) and 9.8% in the placebo group (210 of 2139); the time to the onset of microalbuminuria was reduced by 23% with olmesartan (hazard ratio, 0.77; 95% confidence interval, 0.63 to 0.94; P=0.01).
Morning Report Questions
Q. What adverse events were associated with the use of olmesartan as compared to placebo treatment?A. The number of deaths from cardiovascular causes was higher in the olmesartan group than in the placebo group (15 vs. 3, P=0.01), owing primarily to more cases in the olmesartan group of fatalmyocardial infarctions (5 vs. 0) and sudden cardiac deaths (7 vs. 1).
Q. The increased death rate among patients treated with olmesartan was attributed to what patient group?
A. A greater number of fatal cardiovascular events in the olmesartan group was attributable in part to a higher rate of death from cardiovascular causes in the olmesartan group than in the placebo group among patients with preexisting coronary heart disease (11 of 564 patients [2.0%] vs. 1 of 540 [0.2%], P=0.02).
Recurrent miscarriage
• Recurrent miscarriage, defined as the loss of three or more consecutive pregnancies, occurs in approximately 1% of couples attempting to bear children.• Should women with recurrent miscarriage be investigated for the antiphospholipid syndrome or thrombophilias?
• Five to 15% of women with recurrent miscarriage have clinically important antiphospholipid antibody titers, as compared with 2% to 5% of unselected obstetrical patients. Because results may be transiently positive after infection, the antiphospholipid syndrome should be diagnosed only when two tests performed 12 or more weeks apart are positive. It also has become common to screen patients with recurrent miscarriage for thrombophilias, although it is uncertain whether this screening is warranted.
• What imaging is recommended in evaluating a woman with recurrent miscarriage?
• Uterine malformations, most commonly arcuate and septate uteruses, are detected in 10 to 25% of women with recurrent miscarriage as compared with 5% of women with sporadic miscarriage, and evaluation of the uterine cavity (primarily to look for a septum) is recommended by professional organizations in women with recurrent miscarriage. Sonohysterography and hysterosalpingography are noninvasive screening tests used to evaluate uterine cavity and shape; MRI, hysteroscopy, or both may be more informative but are more expensive and invasive, respectively.• Q. What is the role of parental karyotype testing for couples with recurrent miscarriage?
• A. In 3 to 6% of cases of recurrent miscarriage one partner (more often the woman) has a balanced chromosomal rearrangement. The most common abnormality is a translocation, either reciprocal or robertsonian. Parental karyotyping is expensive and is not always covered by third-party payers. Because available treatment (in vitro fertilization with preimplantation genetic diagnosis) has not been shown to improve the outcome, as compared with that of spontaneous conception, some couples may choose to forego parental karyotype testing.
Q. What is the role of performing a karyotype analysis of the conceptus?
A. Though controversial, some experts recommend karytoype analysis of the conceptus in couples with recurrent miscarriage to avoid unnecessary evaluation and treatment and because an aneuploid conceptus indicates a somewhat greater likelihood of success with a subsequent pregnancy.• Mild-to-moderate hypercalcemia (defined as a serum calcium level below 13.5 mg per deciliter [3.4 mmol per liter]) is commonly due to primary hyperparathyroidism and is typically diagnosed in the outpatient setting. Other possible causes are familial hypocalciuric hypercalcemia, granulomatous diseases, and in rare cases medications, including thiazide diuretics and, less frequently, calcium-containing antacids resulting in the milk alkali syndrome. Patients may be asymptomatic or have nonspecific symptoms, including nausea, vomiting, and constipation.
On the other hand, severe hypercalcemia (defined as a serum calcium level greater than or equal to 13.5 mg per deciliter) is often the result of a malignant condition. Symptoms are generally more pronounced than those in mild-to-moderate cases, with neuropsychiatric manifestations and other symptoms that develop over a period of weeks to months, including polyuria and polydipsia due to defects in the ability of the distal tubule to concentrate urine. Acute renal failure may result from a combination of volume contraction and hypercalcemia-induced renal vasoconstriction.
• What are the mechanisms of hypercalcemia of malignancy?
• Hypercalcemia of malignancy typically occurs through the following mechanisms:• a humoral effect mediated by PTHrP, osteolytic metastases, or ectopic1-alpha-hydroxylation of 25-hydroxyvitamin D resulting in increased calcitriol levels. In patients with myeloma, tumor-induced osteolysis causes hypercalcemia.
• How should severe hypercalcemia be managed?
• Intravenous fluid resuscitation is the mainstay of short-term treatment for hypercalcemia. Bisphosphonates are also warranted for severe hypercalcemia; although their potential nephrotoxicity in a patient with renal failure is a concern, no dose adjustment is recommended for initial treatment in a patient with hypercalcemia of malignancy if the serum creatinine level is less than 4.5 mg per deciliter (397.8 μmol per liter). Furosemide results in calciuresis, but its use should be limited to patients with signs of volume overload.• Q. How should calcitriol-mediated hypercalcemia be treated?
• A. In patients with calcitriol-mediated hypercalcemia, corticosteroids are the therapy of choice, reducing calcitriol production by macrophages and typically correcting the serum calcium within 3 to 5 days after the initial dose.• Q. What is the principal treatment for hypercalcemia of malignancy?
• A. For hypercalcemia of malignancy, bisphosphonates are the principal treatment — in particular, the potent agent zoledronic acid.
• Airway mucus traps inhaled toxins and transports them out of the lungs by means of ciliary beating and cough. Paradoxically, although a deficient mucous barrier leaves the lungs vulnerable to injury, excessive mucus or impaired clearance contributes to the pathogenesis of all the common airway diseases.
• What are the properties of healthy and diseased mucus?
• Healthy mucus contains 3% solids with the consistency of egg white. However, mucin hypersecretion or dysregulation of surface liquid volume may increase the concentration of solids up to 15%, resulting in viscous and elastic mucus that is not easily cleared. In addition, dehydrated mucus adheres more readily to the airway wall.• What is the effect of cigarette smoke on mucus?
• Cigarette smoke-induced mucus dysfunction is complex and incompletely delineated, but it involves adverse effects on the structure and function of cilia, activation of ErbB receptors, decreased function of CFTR, and proinflammatory effects that increase mucin production while decreasing mucus hydration and clearance. Cigarette smoke contains multiple toxins including particulate matter, oxidative chemicals, and organic compounds, among which acrolein is important because it potently induces mucin production.• Q. What is the effect of inhaled dornase alfa for patients with cystic fibrosis?
• A. Inhaled dornase alfa hydrolyzes DNA, improves lung function, and decreases the frequency of exacerbation in patients with cystic fibrosis, in whom airway mucus concentrations of DNA are very high (5–10 mg/ml). The concentration of DNA in other airway diseases, including non–cystic fibrosis bronchiectasis, COPD, and asthma, is 5 to 10 times lower; dornase alfa does not have beneficial effects in these diseases and may even be harmful.
Q. What is the effect of inhaled hypertonic saline on mucus clearance?
A. Treatment twice daily with aerosolized 7% hypertonic saline solution is associated with significant improvements in mucus clearance, modest improvements in airflow, and clinically meaningful reductions in rates of exacerbation. The mechanism of benefit is thought to be rehydration of the periciliary layer by drawing water from epithelial cells, but other mechanisms such as promotion of cough and direct effects on mucus elasticity and entanglement may also contribute.• How should inhaled short-acting β2-adrenergic agonists be administered to a patient with a severe asthma exacerbation?
• Most guidelines recommend the use of nebulizers for patients with severe exacerbations; metered-dose inhalers with holding chambers can be used for patients with mild-to-moderate exacerbations.
There is some evidence that continuous rather than intermittent administration of albuterol results in greater improvement in PEF and FEV1 and a greater reduction in the need for admission, particularly in patients with severe asthma. The recommended dose of nebulized albuterol is 2.5–5 mg every 20 min over the first hr; then 2.5–10 mg every 1–4 hours as needed or 10–15 mg/hr continuously.
• What corticosteroid regimen is recommended for patients with a severe asthma exacerbation?
• In most patients with exacerbations that necessitate treatment in the emergency department, systemic corticosteroids are warranted. Because comparisons of oral prednisone and intravenous corticosteroids have not shown differences in the rate of improvement of lung function or in the length of hospital stay.
the oral route is preferred for patients with normal mental status and without conditions expected to interfere with gastrointestinal absorption.
The most recent National Asthma Education and Prevention Program Expert Panel Report 3 recommends the use of 40 to 80 mg per day in one dose or two divided doses.
• Q. What criteria can be used to determine suitability for admission to the hospital?
• A. After treatment in the emergency department for 1 to 3 hours, patients who have an incomplete or poor response, defined as an FEV1 or PEF of less than 70% of the personal best or predicted value, should be evaluated for admission to the hospital. Patients who have an FEV1 of less than 40%, continuing moderate-to-severe symptoms, drowsiness, confusion, or a partial pressure of arterial carbon dioxide of 42 mm Hg or greater should be admitted.• Q. What features can be used to determine readiness for discharge?
• A. According to the authors, patients may be discharged if the FEV1 or PEF after treatment is 70% or more of the personal best or predicted value and the lung function and improvement of symptoms are sustained for at least 60 minutes. After discharge, patients should continue to use inhaled short-acting β2-adrenergic agonists as needed and should be prescribed oral corticosteroids for 3 to 10 days.• The World Health Organization (WHO) estimates that worldwide there are 66,000 deaths annually from skin cancer, with approximately 80% due to melanoma. In the United States alone, an estimated 8600 persons died from melanoma in 2009.
Metastatic Melanoma
• What is the median survival of patients with melanoma who have distant metastases?
• The median survival of patients with melanoma who have distant metastases is less than 1 year. There has been no therapy that has been shown in a phase 3, randomized, controlled trial to improve overall survival in patients with metastatic melanoma.• What effect did treatment with ipilimumab have on overall survival according to the results of this study?
• The median overall survival was 10.0 months among patients receiving ipilimumab plus gp100, 10.1 months with ipilimumab alone, and 6.4 months among patients receiving gp100 alone.
• Q. What is the mechanism of action of ipilimumab?
• A. CTLA-4 is an immune checkpoint molecule that down-regulates pathways of T-cell activation. Ipilimumab is a fully human monoclonal antibody (IgG1) that blocks CTLA-4 to promote antitumor immunity.Q. What adverse effects are most common with ipilimumab?
A. Grade 3 or 4 immune-related adverse events occurred in 10 to 15% of patients treated with ipilimumab. Diarrhea and fatigue were the most frequently reported adverse events, occurring in more than 30% of patients treated with ipilimumab. Pyrexia, headache and pruritus were reported by less than a quarter of treated patients.When is oxygen therapy indicated in patients with severe COPD?If the partial pressure of arterial oxygen is at or below 55 mm Hg, or if the arterial oxygen saturation is at or below 88%, home use of oxygen should be prescribed for at least 18 hours daily, including sleep time, with flow rates that maintain the oxygen saturation above 90%. There was an absolute reduction in death from any cause of about 20% in both multi-year trials of oxygen therapy.
What bronchodilator treatment should be offered to patients with severe COPD?Many patients with severe COPD obtain symptomatic relief from the use of inhaled bronchodilators. Short-acting β2-adrenergic agonists (e.g., albuterol) and ipratropium bromide, a short-acting anticholinergic agent, are used singly and in combination. Long-acting bronchodilators are now commonly used, but a short-acting bronchodilator should be provided for rescue therapy. Both classes of drugs also reduce the risk of exacerbation by 15 to 20% (relative risk reduction), and this may be their most important clinical benefit.
• Q:
• What course of corticosteroid treatment is appropriate for severely symptomatic patients seen in an outpatient setting?• A:
• Severely symptomatic patients seen in an outpatient setting are likely to benefit from systemic corticosteroids, although data from trials of outpatient corticosteroid therapy for severe symptoms are lacking. In most instances, 40 mg of prednisone taken once daily for 10 to 14 days should suffice.
• Q:
• What is the role of pulmonary rehabilitation for patients with severe COPD?• A:
• Randomized, controlled trials of pulmonary rehabilitation consist mostly of small, single-center studies, generally involving patients with severe disease according to spirometric criteria (FEV1:FVC <0.70; FEV1, 30 to 49% of predicted value). A systematic review concluded that pulmonary rehabilitation significantly improved both functional exercise capacity (assessed by measuring the distance walked in 6 minutes) and respiratory quality of life.
How do frontal-lobe disorders present?Apathy, perseveration, decreased verbal fluency, and gait disorder suggest localization to the frontal lobes. Other clinical signs of frontal-lobe dysfunction are palmar grasp reflexes and urinary or bowel incontinence.
How does Wernicke's aphasia present?Wernicke's aphasia results from lesions — often strokes — in the left temporal lobe. Patients have fluent but nonsensical speech with paraphasic errors, neologisms, and impaired comprehension of spoken and written instructions.
This is commonly mistaken for a confusional state, particularly because of the absence of localizing signs such as hemiplegia. The speech of the confused patient may be rambling and
• Q:
• How does progressive multifocal leukoencephalopathy (PML) present?• A:
• A steadily increasing frontal-lobe dysfunction over the course of several weeks is consistent with this diagnosis. CSF examination to detect JC virus helps to establish a diagnosis of PML.
• Q:
• What cerebrovascular disease affects patients with the antiphospholipid syndrome?
• A:
• Antiphospholipid antibodies, which this patient had for many years, may lead to venous thrombosis, which can affect the superior sagittal sinus and cause bilateral frontal-lobe infarcts.
What are the most common causes of oral ulcers?The most common causes are minor trauma, infection with herpes simplex virus, and aphthous ulcers (canker sores). Patients with HSV infection or aphthous ulcers generally have a history of ulcers. These two types of ulcers are associated with physical and emotional stresses, such as upper respiratory tract infection, malnutrition, and fatigue; they heal spontaneously, usually within 3 weeks. Oral ulcers due to mechanical or thermal trauma, an ill-fitting denture, a sharp-edged tooth or filling, or hot sticky foods should heal within 3 weeks after removal of the cause of irritation.
What is the most common location for an oral squamous-cell carcinoma?The most common sites for oral squamous-cell carcinoma are the lateral tongue (40% of cases), the floor of the mouth (30%), and the soft palate. Black race, use of alcohol, smoking, and infection with human papillomavirus increase the risk of oral squamous-cell carcinoma.
• Q:
• How does primary syphilis present?• A:
• The chancre of primary syphilis typically develops 1 to 3 weeks after exposure. When the solitary chancre of primary syphilis occurs in the oral cavity, it is typically on the upper lip in men and on the tongue in women, because of the anatomy of heterosexual oral sexual activity. However, the chancre may occur anywhere on the body, including any part of the oral cavity. In men who have sex with men, the chancre may be located on the tongue rather than the lip. The chancres are often multiple, and although they are typically painless when located on the penis or vulva, they can be painful when located on the tongue, fingers, and anus.
• Q:
• What are some of the typical characteristics of secondary syphilis?
• A:
• Painful, shallow oral mucosal ulcers occur in up to 30% of cases of secondary syphilis. The oval or crescentic ulcers (mucous patches) may coalesce and form serpiginous lesions, sometimes termed snail-track ulcers. The oral ulcers of secondary syphilis are often associated with symmetric maculopapular skin lesions on the extremities. Sore throat, malaise, fever, and weight loss are common. Regional lymphadenopathy may occur in either primary or secondary syphilis.
How should acute variceal hemorrhage be treated in patients with compensated cirrhosis?Patients who have Child class A or B disease or who have an hepatic venous pressure gradient (HVPG) of less than 20 mm Hg have a low or intermediate risk and should receive standard therapy — specifically, the combination of a vasoconstrictor (terlipressin, somatostatin, or analogues, administered from the time of admission and maintained for 2 to 5 days) and endoscopic therapy (preferably endoscopic variceal ligation, performed at diagnostic endoscopy <12 hours after admission), together with short-term prophylactic antibiotics (either norfloxacin or ceftriaxone). Placement of a transjugular intrahepatic portosystemic shunt is currently considered a salvage therapy for the 10 to 20% of patients in whom standard medical therapy fails.
• What treatments should be used to prevent recurrent variceal hemorrhage?Given the high recurrence rate, patients who survive an acute variceal hemorrhage should receive therapy to prevent recurrence before they are discharged from the hospital. Combination pharmacologic therapy (nonselective beta-blockers such as propranolol or nadolol plus nitrates) or combination endoscopic variceal ligation plus drug therapy are warranted because of the high risk of recurrence, even though the side effects will be greater than those with single-agent therapy (recommended for primary prophylaxis).
• Q:
• Should patients with cirrhosis but without varices be treated with non-selective beta-blockers for primary prophylaxis?• A:
• Patients without gastroesophageal varices or with gastroesophageal varices that have never bled are at relatively low risk for bleeding and death; therefore, therapies for these patients should be the least invasive. In patients without varices, treatment with nonselective beta-blockers is not recommended because they do not prevent the development of varices and are associated with side effects.
• Q:
• In patients with cirrhosis without varices, which one of the following measurements is the best method to stratify risk?
• A:
• In patients without varices and in those with variceal hemorrhage, measurement of portal pressure with the use of the HVPG is the best method to stratify risk. Portal hypertension is present when the HVPG is greater than 5 mm Hg, but it is considered clinically significant when the HVPG is greater than 10 mm Hg, because in patients without varices, this pressure is the strongest predictor of the development of varices, clinical decompensation, and hepatocellular carcinoma. The HVPG is obtained by means of catheterization of a hepatic vein with a balloon catheter through a jugular or femoral vein.
• Q:
• How does diabetes insipidus present?• A:
• The development of hypernatremia during a period of fluid restriction suggests diabetes insipidus. Polyuria is typical at the time of presentation.
• Q:
• How does hypothalamic injury present?
• A:
• The neurons that produce vasopressin are located in the hypothalamus and project axons to nerve terminals in the posterior pituitary gland; as a result, diabetes insipidus is seen more commonly with hypothalamic conditions than with pituitary disease. In addition to hormonal deficiencies, hypothalamic involvement is often manifested as impairment of thirst, which is regulated by osmostats in the hypothalamus, and as imbalances in energy homeostasis due to loss of normal appetite and energy regulation. Loss of circadian rhythms results in altered sleep–wake cycles. Dysregulation of body temperature, memory loss, and personality changes are also common.
What is the pattern of cortisol in a patient with secondary adrenal insufficiency?A stimulated cortisol level above 18 μg per deciliter 60 minutes after the administration of synthetic corticotropin indicates that the adrenal glands are capable of responding normally to corticotropin.
A normal stimulated response with a low basal cortisol level can indicate secondary adrenal insufficiency of recent onset
How does hepatic encephalopathy present?Hepatic encephalopathy is a neuropsychiatric syndrome that is manifested as progressive deterioration in mental status, with psychomotor dysfunction, impaired memory, increased reaction time, sensory abnormalities, poor concentration, disorientation, and in severe forms coma.
• How is hepatic encephalopathy diagnosed?The clinical diagnosis of overt hepatic encephalopathy is based on two concurrent types of symptoms: impaired mental status, as defined by the Conn score (West Haven criteria, on a scale from 0 to 4, with higher scores indicating more severe impairment), and impaired neuromotor function. The Conn score is recommended by the Working Party on Hepatic Encephalopathy for the assessment of overt hepatic encephalopathy in clinical trials. Signs of neuromotor impairment include hyperreflexia, rigidity, myoclonus, and asterixis (a coarse, myoclonic, “flapping” muscle tremor), which is measured with the use of an asterixis severity scale.
• Q:
• What is the mechanism of action of rifaximin?• A:
• Rifaximin is a minimally absorbed antimicrobial agent that is concentrated in the gastrointestinal tract, has broad-spectrum in vitro activity against gram-positive and gram-negative aerobic and anaerobic enteric bacteria, and has a low risk of inducing bacterial resistance.
• Q:
• What is the efficacy of rifaximin as compared to lactulose alone?
• A:
• In the study by Bass and colleagues, breakthrough episodes of hepatic encephalopathy were reported in 31 of 140 patients in the rifaximin group (22.1%) and 73 of 159 patients in the placebo group (45.9%). The hazard ratio for the risk of a breakthrough episode in the rifaximin group, as compared with the placebo group, was 0.42 (95% confidence interval [CI], 0.28 to 0.64; P<0.001), reflecting a reduction in the risk of a breakthrough episode by 58% with rifaximin as compared with placebo during the 6-month study period.
What is the typical presentation of prolactinomas in women?Clinical symptoms and signs of hyperprolactinemia in women include oligoamenorrhea, infertility, and galactorrhea. In women with hyperprolactinemia who continue to have menses, luteal-phase abnormalities can lead to infertility. Estrogen deficiency in amenorrheic women with untreated prolactinomas causes low bone mass, which is associated with an increased risk of fracture.
What is the typical presentation of prolactinomas in men?In men, hyperprolactinemia may lead to hypogonadism, decreased libido, erectile dysfunction, infertility, gynecomastia, and, in rare instances, galactorrhea. Decreased bone mass and anemia result from testosterone deficiency. In contrast with women, who usually present with microadenomas, most men present with macroadenomas, often with headache, visual symptoms, or both, in addition to hypogonadism.
• Q:
• Other than prolactinoma, what are other causes of hyperprolactinemia?• A:
• Secretion of prolactin is under tonic inhibitory control by hypothalamic dopamine; levels of prolactin can be increased in the presence of other tumor types, inflammatory disorders such as lymphocytic hypophysitis, cysts (e.g., Rathke's cysts, which disrupt dopamine transport down the pituitary stalk), or medications that interfere with normal secretion of hypothalamic dopamine. These medications include antidepressants and antipsychotic agents (risperidone, in particular), other dopaminergic blockers (e.g., metoclopramide and sulpiride), some antihypertensive agents, opiates, and H2-receptor blockers.
• Q:
• What treatment rapidly normalizes prolactin levels, restores reproductive function, reverses galactorrhea, and decreases tumor size in most patients with a prolactinoma?
• A:
• Dopamine agonists are the primary therapy for both microadenomas that require treatment and macroprolactinomas. They rapidly normalize prolactin levels, restore reproductive function, reverse galactorrhea, and decrease tumor size in most patients. Dopamine agonists include bromocriptine and cabergoline (both ergot derivatives) and quinagolide (not approved for use in the United States).
• Q:
• How should H. pylori be eradicated?• A:
• Various drug regimens are used to treat H. pylori infection. Most include two antibiotics plus a proton-pump inhibitor or a bismuth preparation (or both). The most commonly used initial treatment is triple therapy consisting of a proton-pump inhibitor plus clarithromycin and amoxicillin, each given twice per day for 7 to 14 days. Metronidazole is used in place of amoxicillin in patients with a penicillin allergy. The duration of triple therapy that is typically recommended is 10 to 14 days in the United States and 7 days in Europe.
• Q:
• What treatment is indicated if initial therapy fails?
• A:
• If initial therapy did not include a bismuth salt, bismuth-based quadruple therapy is commonly used as second-line therapy, with eradication rates in case series ranging from 57 to 95%. Triple therapies have also been tested as second-line therapies in patients in whom initial therapy failed. A proton-pump inhibitor used in combination with metronidazole and either amoxicillin or tetracycline is recommended in patients previously treated with a proton-pump inhibitor, amoxicillin, and clarithromycin.
• Endometriosis, a major contributor to pelvic pain and subfertility, is characterized by endometrial-like tissue outside the uterus, primarily on the pelvic peritoneum, ovaries, and rectovaginal septum, and rarely on the diaphragm, pleura, and pericardium. Endometriosis affects 6 to 10% of women of reproductive age, 50 to 60% of women and teens with pelvic pain, and up to 50% of women with infertility.
What is the first-line treatment for women with endometriosis and dysmenorrhea?Combined oral contraceptives can be used cyclically or continuously for endometriosis-related pain and are commonly combined with NSAIDs, although there is a 20-25% failure rate. This approach is first-line therapy in patients without contraindications to combined oral contraceptive use.
• How should GnRH agonist treatment be used to treat endometriosis?Since GnRH agonist therapy causes significant hypoestrogenic side effects, including bone loss (up to 13% over 6 months, partly reversible on cessation of therapy), estrogen plus progestagen “add-back” therapies are recommended.
• The “estrogen threshold hypothesis” suggests that maintaining E2 levels between 30 to 45 pg/ml will maintain bone mineral density without stimulating disease.
• Q:
• What are the risk factors for endometriosis?• A:
• Risk factors for endometriosis include obstruction of menstrual outflow (e.g., mullerian anomalies), in utero DES exposure, prolonged exposure to endogenous estrogen (e.g., because of early menarche, late menopause, or obesity), short cycles, low birth weight, and exposure to endocrine-disrupting chemicals. Prolonged lactation and multiple pregnancies are protective.
• Q:
• What are the most effective treatments for women with endometriosis and infertility?
• A:
• Gonadotropins and intrauterine insemination (IUI), as well as in vitro fertilization, are efficacious treatments for infertile women with endometriosis. In a large randomized trial comparing 4 treatment strategies in 932 couples with stage I/II endometriosis or unexplained infertility, cumulative pregnancy rates within 4 treatment cycles were: intracervical insemination (10%); IUI (18%); gonadotropin/intracervical insemination (19%); gonadotropin/IUI (33%).
• What is the auscultatory presentation of mitral-valve prolapse?A late-systolic murmur is more common than a mid-systolic click. When there is chordal rupture with the development of a flail leaflet, the murmur becomes holosystolic. The radiation of the murmur follows the direction of the regurgitant jet. With a flail posterior leaflet, the murmur radiates anteriorly and may mimic aortic stenosis, while the murmur associated with a flail anterior leaflet radiates to the back. Severe regurgitation may be associated with a displaced apical impulse, an S3, and an accentuated P2 due to pulmonary venous hypertension.
• Q:
• When is surgical repair for mitral regurgitation indicated?• A:
• Surgery is recommended for management of chronic severe MR in symptomatic patients and asymptomatic patients with evidence of LV dysfunction, defined as a left ventricular ejection fraction of 30 to 60% and a left ventricular end-systolic dimension 40 mm or greater; the guidelines note that patients with severely reduced ejection fractions may not benefit from surgery. Other reasonable indications for surgery in the asymptomatic patient include elevation of pulmonary artery pressure and new onset of atrial fibrillation and the asymptomatic patient with normal LV function in whom repair is likely.
• Q:
• How do outcomes following mitral-valve repair and replacement differ?
• A:
• The overall reoperation rate is similar for mitral-valve repair and replacement. In a recent meta-analysis of 29 observational studies, the early (30-day) operative mortality, as well as longer term mortality, were significantly lower following repair than replacement; however, the analyses were not adjusted for age or other co-morbidities. The risk of endocarditis is lower in patients who undergo repair (approximately 1.5% at 15 years) than replacement (0.3% to 1.2%/yr, similar for mechanical and bioprosthetic valves). In an observational study comparing different surgeries, 10 year stroke rates were 10% for patients with mitral-valve repair, 12% for those with bioprosthetic mitral-valve replacement, and 23% for those with mechanical valve replacement, with only the latter having rates higher than expected for the population.
who undergo repair (approximately 1.5% at 15 years) than replacement (0.3% to 1.2%/yr, similar for mechanical and bioprosthetic valves). In an observational study comparing different surgeries, 10 year stroke rates were 10% for patients with mitral-valve repair, 12% for those with bioprosthetic mitral-valve replacement, and 23% for those with mechanical valve replacement, with only the latter having rates higher than expected for the population.
Is testosterone replacement effective?In an analysis of all the men who underwent at least one outcome assessment after randomization in this study, men who were assigned to testosterone, as compared with those assigned to placebo, had significantly greater increases in leg-press strength, chest-press strength, and stair-climbing power while carrying a load. Changes in gait speed without a load and stair-climbing power without a load did not differ significantly between the groups.
What adverse events were more common among testosterone-treated as compared with placebo-treated patients?During the course of the study, the testosterone group had higher rates of cardiac, respiratory, and dermatologic events than did the placebo group.
A total of 23 subjects in the testosterone group, as compared with 5 in the placebo group, had cardiovascular-related adverse events. The relative risk of a cardiovascular-related adverse event remained constant throughout the 6-month treatment period
• Q:
• How did metabolic parameters change in the intervention group?
• A:
• In the testosterone group, as compared with the placebo group, there was a significant increase in hemoglobin and hematocrit levels and a significant decrease in high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels.
• Q:
• Is there a mechanistic link between testosterone administration and cardiovascular events?
• A:
• Testosterone causes salt and water retention, particularly in older men, and this could contribute to edema, hypertension, and congestive heart failure, although there are some trials in which testosterone has been administered in men with congestive heart failure. Testosterone and associated increases in estradiol may promote inflammation, coagulation, and platelet aggregation. The use of anabolic steroids has been associated with left ventricular hypertrophy and systolic and diastolic dysfunction.
• Q:
• Which of the phosphate-binding agents appears to have the highest incidence of gastrointestinal adverse effects?• A:
• Sevelamer hydrochloride and sevelamer carbonate appear to have a higher incidence of gastrointestinal adverse effects (nausea, vomiting, abdominal pain, bloating, diarrhea, and constipation) than the other listed phosphate binders. The approximate mean incidence of gastrointestinal adverse effects for calcium salts, lanthanum, magnesium salts, and sevelamer are listed at 21.5%, 7.5%, 19.5%, and 37.5% respectively.
• Q:
• What adverse effects are associated with the use of lanthanum for the management of hyperphosphatemia?
• A:
• Lanthanum is associated with peripheral edema (approximately 23.4% of patients), myalgia (approximately 20.8% of patients), gastrointestinal effects (approximately 7.5% of patients), muscular cramping (approximately 7.1% of patients), and hypercalcemia (approximately 5.7% of patients).
What is the efficacy of the current standard treatment for patients infected with genotype 1 of the hepatitis C virus?Currently available treatment for HCV infection requires peginterferon alfa injections combined with ribavirin orally for 24 or 48 weeks (based on HCV genotype). Overall, 40% to 50% of genotype 1, treatment-naïve patients achieve the short- and longer-term benefits of a sustained viral response.
• What treatment protocol had the greatest sustained virologic response?In patients who failed initial peginterferon/ribavirin treatment, telaprevir in combination with peginterferon and ribavirin was more effective than retreatment with peginterferon and ribavirin alone.
• Q:
• What is the mechanism of action of telaprevir?• A:
• Telaprevir, an orally bioavailable inhibitor of the NS3/4A HCV protease, is one of several investigational agents that specifically targets HCV with the goal of improving the chance of a sustained viral response.
• Q:
• What adverse effects are most typically associated with telaprevir?
• A:
• Rash and pruritus were more common in the groups that received telaprevir, as described in prior studies of treatment-naïve patients. The rashes in patients in the telaprevir treatment groups were generally described as maculopapular, and were grade 3 in up to 5% of subjects. A decrease in hemoglobin levels was more common in patients receiving telaprevir-based regimens than in controls, and was reversible on discontinuation of telaprevir.
When is the D-dimer assay useful in the diagnosis of acute pulmonary embolism?In patients with hemodynamic stability, the diagnosis of pulmonary embolism should follow a sequential diagnostic workup that integrates clinical probability assessment, D-dimer testing, and (if necessary) multidetector computed tomography or ventilation–perfusion scanning.
The use of the D-dimer assay is of limited value in patients with a high clinical probability of pulmonary embolism. The specificity of an increased D-dimer level is reduced in patients with cancer, pregnant women, and hospitalized and elderly patients. Most hospitalized patients should not undergo D-dimer testing when pulmonary embolism is suspected.
• What duration of anticoagulation is recommended after acute pulmonary embolism?The duration of long-term anticoagulation should be based on the risk of recurrence after stopping vitamin K antagonists, the risk of bleeding while receiving treatment, and the patient's preference. In patients with pulmonary embolism secondary to a temporary (reversible) risk factor, therapy with vitamin K antagonists should be given for 3 months. Patients with unprovoked pulmonary embolism, those with cancer, and those with recurrent unprovoked pulmonary embolism are candidates for indefinite anticoagulation with periodic reassessment of the risk–benefit ratio.
• Q:
• How should hemodynamically unstable patients with acute pulmonary embolism be managed?• A:
• Hemodynamically unstable patients are candidates for more aggressive treatment, such as pharmacologic or mechanical thrombolysis. Percutaneous mechanical thrombectomy (thrombus fragmentation and aspiration) and surgical embolectomy should be restricted to high-risk patients with an absolute contraindication to thrombolytic treatment or those in whom thrombolytic treatment has not improved hemodynamic status or as an alternative to surgical embolectomy if immediate access to cardiopulmonary bypass is unavailable.
• Q:
• What risk factors should be considered when evaluating a patient with acute pulmonary embolism?
• A:
• Shock and sustained hypotension identify patients at high risk for an adverse outcome. Right ventricular hypokinesis and dilatation have been shown to be independent predictors of 30-day mortality in hemodynamically stable patients. Patients with elevated levels of B-type natriuretic peptide (BNP) and pro-BNP were shown to be at increased risk for an adverse in-hospital outcome, as compared with patients with normal levels. Among hemodynamically stable patients, the association between an increased troponin level and right ventricular dysfunction on echocardiography identifies a group of patients at particularly high risk for an adverse outcome.
• Q. How should abnormalities of thyroid function be interpreted and managed in women with nausea and vomiting in pregnancy?
• A. As hCG cross-reacts with TSH and stimulates the thyroid gland, high levels of hCG that occur in women with nausea and vomiting in pregnancy can be associated with mild hyperthyroidism. A pelvic ultrasound should be performed to detect multiple gestation or hydatidiform mole in pregnant patients who develop a suppressed TSH. This type of hCG-induced hyperthyroidism usually resolves spontaneously, and treatment with propylthiouracil does not help the nausea and vomiting.
Q. What medication is recommended as first-line treatment for nausea and vomiting in pregnancy?
A. Oral vitamin B6 and doxylamine are available over the counter in the United States. The combination has been well studied in over 6000 patients and controls, with no evidence of teratogenicity, and, in randomized trials, a 70% reduction in nausea and vomiting. It is recommended by the American College of Obstetricians and Gynecologists as first-line therapy for nausea and vomiting in pregnancy.
• When do nausea and vomiting in pregnancy most typically resolve?
• The onset of the nausea is within four weeks of the last menstrual period in most patients. The problem typically peaks at approximately 9 weeks of gestation.• Sixty percent of cases resolve by the end of the first trimester, and 91% by 20 weeks gestation.
• What are some protective factors against nausea and vomiting in pregnancy?
• Nausea and vomiting are less common in older women, multiparous women, and smokers, an observation that has been attributed to their smaller placental volumes .
• Q:
• How should hospital-acquired pneumonia be diagnosed?• A:
• The diagnosis of ventilator-associated pneumonia remains challenging, with no easily obtained reference standard. Apart from clinical criteria, microbiologic assessment is important to help guide therapy. For patients suspected of having ventilator-associated pneumonia, a fluid sample from the lower respiratory tract should be obtained by means of endotracheal aspiration, bronchoalveolar lavage, or a protected specimen brush (depending on the resources available) for microscopy and culture before antibiotics are administered. Although each sampling method has its limitations, the most important point is to obtain the sample in a timely manner.
• Q:
• What patients with bloodstream infection should receive empirical antibiotic coverage?
• A:
• Delays in the administration of appropriate antibiotic therapy are associated with excess mortality among patients with hospital-acquired bloodstream infection. Empirical antibiotic coverage for gram-negative bacteria should be considered for patients who are immunosuppressed, those in the ICU, those with a femoral catheter, those with gram-negative bacterial infection at another anatomical site (particularly the lung, genitourinary tract, or abdomen), and those with other risk factors for resistant organisms.
• Q. What outcome in this study was unexpectedly poor among kidney-allograft recipients infected with HIV?
• A. The main finding of concern in this study, as well as in our pilot study, was the unexpectedly higher rejection rates (by a factor of 2 to 3) in the HIV-infected kidney recipients, as compared with recipients who did not have HIV infection. About half these episodes were glucocorticoid-resistant, which is characteristic of aggressive rejection.
Q. What immunosuppressive regime may be preferred for patients with HIV who are kidney transplant recipients?
A. Since rejection rates were significantly increased with cyclosporine-based maintenance therapy, tacrolimus may be the calcineurin inhibitor of choice. Nonetheless, for patients coinfected with HCV, cyclosporine may be optimal on the basis of its in vitro efficacy against this virus. Antithymocyte globulin induction therapy should be restricted to patients at very high immunologic risk for rejection.
• Q. When should platelets be administered to patients with TTP?
• A. Platelet transfusion in patients with TTP may be associated with acute deterioration or even death. For this reason, platelet transfusion is relatively contraindicated in patients with thrombotic thrombocytopenic purpura and should be limited to the treatment of life-threatening bleeding.Q. What is the treatment of choice for patients with TTP?
A. In patients with idiopathic TTP, microvascular hemostasis is disrupted; plasma exchange is a life-saving procedure for these patients, because it corrects the perturbation by replacing the ADAMTS 13 enzyme and removing, over the course of several procedures, the autoantibody inhibitor that is often detected during the acute illness. Patients with idiopathic TTP used to be treated with plasma infusion or plasma exchange, but a randomized, controlled trial conducted approximately 20 years ago showed the superiority of plasma exchange.• What types of tics are most typical of Tourette’s syndrome?
• Motor tics include simple tics such as twitching, eye blinking, facial grimacing, or head jerking; slow twisting movements (dystonic tics); isometric contractions (tonic tics) such as tensing of the abdominal muscles; and more complicated, purposeful-looking movements (complex motor tics) such as touching or tapping.Vocal tics (also called phonic tics) include inarticulate noises such as throat clearing, sniffing, or coughing (simple vocal tics) and words or partial words (complex vocal tics). The most notorious tic of Tourette’s syndrome, obscene or insulting utterances (coprolalia), occurs in less than 50% of cases in reported series.
• What problems frequently coexist in patients with Tourette’s syndrome?
• Tourette’s syndrome is now viewed as a neuropsychiatric spectrum disorder in which tics are commonly associated with obsessive–compulsive symptoms that do not always meet the full diagnostic criteria for obsessive–compulsive disorder (OCD) and with disturbances of attention that do not always meet the full criteria for attention deficit–hyperactivity disorder (ADHD
• Q. What behavioral treatments have been effective for patients with Tourette’s syndrome?
• A. Clinical trials have shown that a form of cognitive behavioral therapy termed habit-reversal treatment is efficacious in suppressing tics. This form of therapy involves training patients to monitor their tics and premonitory sensations and to respond to them with a voluntary behavior that is physically incompatible with the tics.
Q. What pharmacologic therapy is effective for patients with disabling tics?
A. When tics are disabling, tic-suppressing therapy is indicated. The only medications for Tourette’s syndrome that have been approved by the Food and Drug Administration (FDA) are the classic neuroleptic antipsychotic agents haloperidol and pimozide, which block D2 dopamine receptors. Data from controlled clinical trials provide support for their efficacy. Randomized, controlled trials have also provided support for the efficacy of a newer atypical antipsychotic agent, risperidone, in suppressing tics, with a magnitude of benefit that is similar to that of the classic neuroleptics.• Q. When should platelets be administered to patients with TTP?
• A. Platelet transfusion in patients with TTP may be associated with acute deterioration or even death. For this reason, platelet transfusion is relatively contraindicated in patients with thrombotic thrombocytopenic purpura and should be limited to the treatment of life-threatening bleeding.Q. What is the treatment of choice for patients with TTP?
A. In patients with idiopathic TTP, microvascular hemostasis is disrupted; plasma exchange is a life-saving procedure for these patients, because it corrects the perturbation by replacing the ADAMTS 13 enzyme and removing, over the course of several procedures, the autoantibody inhibitor that is often detected during the acute illness. Patients with idiopathic TTP used to be treated with plasma infusion or plasma exchange, but a randomized, controlled trial conducted approximately 20 years ago showed the superiority of plasma exchange.• Retinal-vein occlusion is a common cause of vision loss in older persons, and the second most common retinal vascular disease after diabetic retinopathy. In branch retinal-vein occlusion, the occlusion is at an arteriovenous intersection; in central retinal-vein occlusion, the occlusion is at or proximal to the lamina cribosa of the optic nerve, where the central retinal vein exits the eye.
• How does branch retinal-vein occlusion present?
• Patients with retinal-vein occlusion typically present with sudden, unilateral, painless loss of vision. The degree of vision loss depends on the extent of retinal involvement and on macular-perfusion status. Some patients with branch retinal-vein occlusion report only a peripheral visual-field defect.• What are the major risk factors for branch retinal-vein occlusion?
• The strongest risk factor for branch retinal-vein occlusion is hypertension, but associations have been reported for diabetes mellitus, dyslipidemia, cigarette smoking, and renal disease.• Q. What is the recommended management of a patient with branch retinal-vein occlusion?
• A. The authors suggest that first-line treatment of branch retinal-vein occlusion is grid laser photocoagulation, since longer-term data from clinical trials have shown improvement in visual acuity, lower rates of adverse effects, and lower costs with this treatment than with anti-VEGF therapy. Grid, or focal, laser photocoagulation is used for the treatment of macular edema resulting from branch retinal-vein occlusion. Treatment with a dexamethasone implant is another option, but evidence is lacking to demonstrate an improvement in visual acuity beyond 3 months.
Q. What is the recommended management of a patient with a central retinal-vein occlusion?
A. Whereas macular edema resulting from branch retinal-vein occlusion is susceptible to treatment with grid laser photocoagulation, that resulting from central retinal-vein occlusion is not. Ranibizumab and bevacizumab are widely used in the treatment of central retinal-vein occlusion, as well as in the treatment of branch retinal-vein occlusion, and have been demonstrated to improve vision. The study of intravitreal injection of dexamethasone through an implant showed that the intervention improved vision in patients with central retinal-vein occlusion.• What is the approximate recurrence risk for patients with calcium stones?
• After passage of a first stone, the risk of recurrence is 40% at 5 years and 75% at 20 years. Among patients with recurrent calcium stones who have served as control subjects in randomized, controlled trials of interventions, new stones formed in 43 to 80% of subjects within 3 years.• What is the most common metabolic abnormality in patients with recurrent calcium stones?
• Hypercalciuria, the most common metabolic abnormality found in patients with recurrent calcium stones, is most often familial and idiopathic and is strongly influenced by diet.
• Q. How should symptomatic stones be managed?
• A. Stones that have formed in kidneys do not require removal or fragmentation unless they cause obstruction, infection, serious bleeding, or persistent pain. Ureteral stones of less than 10 mm in diameter may be followed with conservative treatment in the absence of fever, infection, or renal failure, if pain is controlled.Opioid analgesics and nonsteroidal antiinflammatory agents are both effective for pain control in acute colic. Therapy with drugs that block α1-adrenergic receptors or calcium-channel blockers may facilitate passage of ureteral stones.
In general, stones larger than 10 mm in diameter will not pass, and those smaller than 5 mm will.
• Q. What recommendations can be made to reduce the risk of recurrent stone formation?
• A. Patients should be advised to increase fluid intake to at least 2 liters daily and reduce sodium intake to 2300 mg or less and protein intake to 0.8 to 1 g per kilogram of body weight per day, since these dietary interventions have reduced stone recurrence in randomized trials. Calcium intake should not be reduced below the recommended intake for sex and age. Thiazide-type diuretics decrease urine calcium excretion, and in randomized, controlled trials, these medications significantly reduced recurrence rates of calcium stones by more than 50% during a 3-year period, as compared with placebo.• Q:
• How does salt restriction lead to a lower blood pressure in patients with essential hypertension?
• A:
• In essential hypertension sodium excretion is impaired. It is hypothesized that in most cases essential hypertension is a genetic disorder involving many individual genes, each of which influences the body's handling of sodium to varying degrees and becomes expressed in the context of an unhealthful dietary environment, particularly one characterized by excessive intake of salt. Reductions in dietary salt lessen the amount of sodium the kidney has to excrete to restore normal blood volume. Aortic and carotid artery compliance is significantly improved in older patients with hypertension when sodium intake is reduced. Reduction in sodium intake also improves arterial vasodilation.
• Q:
• What is the recommended dietary approach to managing hypertension?
• A:
• The authors recommend the American Heart Association's guidelines for cardiovascular health and the dietary management of hypertension. These guidelines endorse foods and approaches to diet similar to those included in the DASH diet and cite intake of 65 mmol, or 1.5 g, of sodium per day as optimal. In addition, a target BMI of less than 25 is recommended. Finally, the guidelines recommend no more than two alcoholic drinks per day for men and one for women and people of lighter weight.
• Q:
• Where in the brain does expressive aphasia localize?• A:
• An aphasia that is more expressive than receptive in nature (such as a patient who is mute, with partially preserved comprehension) suggests that the lesion is located in the inferior frontal lobe, although partial temporal-lobe involvement is also possible.
• Q:
• What are the emergency treatment options for acute stroke?
• A:
• Intravenous recombinant tissue plasminogen activator (rt-PA) is currently approved by the Food and Drug Administration for the treatment of ischemic stroke within 3 hours after onset; however, a recent study has shown that rt-PA therapy can be effective when administered up to 4 1/2 hours after the onset of symptoms. After that time, stroke patients are still eligible for several short-term endovascular interventions, including mechanical embolectomy and intraarterial thrombolysis, although the effects of such procedures on morbidity have not been well established.
• Which infections are particularly increased among patients treated with TNF inhibitors?
• TNF inhibitors are associated with an increased risk of infection, particularly tuberculosis, atypical mycobacterial disease, histoplasmosis, and listeriosis .
• What are the features of drug-induced lupus, which is described in patients treated with TNF inhibitors?
• The serologic findings supported drug-induced lupus, which is rare but well described in patients treated with TNF inhibitors and may include fever, rash, and elevated levels of antinuclear antibodies and antibodies against double-stranded DNA. Drug-induced lupus is usually characterized by inflammatory symptoms such as fever, rash, pleurisy, and arthritis, combined with serologic abnormalities.
• Q. How do TNF inhibitors lead to an increased infection risk?
• A. The biologic activity of TNF includes macrophage activation and chemotaxis; inhibition of these activities might be expected to decrease the host’s ability to handle intracellular infections, such as those due to fungi, mycobacteria, and L. monocytogenes, thus increasing the risk of infection.Q. What is the risk of infection among patients treated with TNF inhibitors?
A. A recent meta-analysis of randomized, controlled trials of infliximab and adalimumab in patients with rheumatoid arthritis showed an increased risk of serious infection with the use of these drugs (relative risk, 2.0; 95% confidence interval, 1.3 to 2.1). Of the 126 infections observed in the treated patients, 12 were granulomatous. Furthermore, in one large post-marketing study, 19 intracellular infections occurred in more than 11,000 patients with rheumatoid arthritis, and all occurred in patients treated with TNF inhibitors• What is the optimal management of calcium and phosphate levels in patients receiving hemodialysis?
• According to the authors, it is appropriate to aim for a serum calcium level of 8.4 to 9.5 mg per deciliter and a serum phosphate level of 3.5 to 5.5 mg per deciliter. The serum levels of intact PTH should be monitored, although the optimal target PTH level has not been well defined, but it should be maintained at least twice the upper limit of the normal range to minimize risk of low bone turnover. Rising PTH should be suppressed with vitamin D analogues, calcimimetics, or both.
• What is the optimal management of anemia in patients receiving hemodialysis?
• The authors recommend a target hemoglobin level of 10 to 12 g per deciliter (although current recommendations may change on the basis of results from clinical trials involving patients with chronic kidney disease). High-dose erythropoietin should be avoided; patients with erythropoietin resistance should be evaluated for inflammation and iron deficiency. Iron levels should be monitored and iron deficiency should be treated; the long-term safety and efficacy of iron administration in patients with high ferritin levels has not been well established.• Q. How should the presence of cardiovascular risk factors be managed in patients receiving hemodialysis?
• A. The authors suggest that an LDL cholesterol level of <100 mg/dl is appropriate, but acknowledge that statins are without proven benefit. The optimal targets and management strategies for hypertension have not been well defined. For patients with diabetes mellitus, clinicians must balance benefits of tighter glycemic control with increased risk for hypoglycemia; HbA1c targets have not been well defined.
Q. What is the death rate in the first two years after starting maintenance hemodialysis?
A. The steady improvement in procedure-focused and process-related measures of quality has led to a noticeable improvement in survival over the past two decades. Nevertheless, the death rate among U.S. patients undergoing dialysis continues to exceed 20% per year during the first 2 years after maintenance dialysis is begun.• What is the classic triad associated with tubulointerstitial nephritis?
• The classic triad of fever, peripheral eosinophilia, and rash is present in only a fraction (approximately 10%) of patients with allergic tubulointerstitial nephritis, although at least one of these features is present in the majority of cases.• What urinary findings are typical in patients with tubulointerstitial nephritis?
• Giemsa staining of a cytospin preparation of urinary sediment shows lymphocytes, plasma cells, and eosinophils in cases of acute tubulointerstitial nephritis. The presence of eosinophils in urine can be helpful in suggesting the diagnosis and can be detected with the use of Wright, Giemsa, or Hansel staining; however, the positive and negative predictive values of this finding are low. White-cell casts or red-cell casts would point to a diagnosis of acute tubulointerstitial nephritis or glomerulonephritis.• Q. What are the most typical causes of tubulointerstitial nephritis?
• A. The causes of tubulointerstitial nephritis include drug-induced allergic reactions, infection, autoimmune inflammation (e.g., systemic lupus erythematosus and Sjögren’s syndrome), and the syndrome of tubulointerstitial nephritis and uveitis. Medications account for about 70% of cases.Q. How should allergic interstitial nephritis be managed?
A. The initial approach to the treatment of allergic interstitial nephritis is to remove the offending agent. Conclusive data are lacking to support the use of immunosuppressants as adjunctive therapy. The findings in retrospective case series have been inconsistent.Autosomal dominant polycystic kidney disease (ADPKD) is the most frequent hereditary kidney disease and the cause of end-stage renal disease in 7 to 10% of all patients undergoing dialysis. As yet, no treatment is available to delay disease progression
• What is the natural history of ADPKD?
• The disease is characterized by the growth of numerous kidney cysts, which leads to progressive destruction of the adjacent renal parenchyma and massive enlargement of the kidneys. Renal function is often preserved until the age of 40 years because functioning nephrons undergo compensatory hypertrophy. Subsequently, the glomerular filtration rate decreases, and end-stage renal disease ensues in many patients by the fifth decade.• What is one cell signaling system that is aberrantly activated in patients with ADPKD and how can it be inhibited?
• The mammalian target of rapamycin (mTOR) is aberrantly activated in cystic epithelia in patients with ADPKD. Sirolimus inhibits mTOR, and in an orthologous mouse model of ADPKD and other rodent models of polycystic kidney disease, sirolimus significantly improved renal cystic disease.
• Q. As compared to standard treatment, what was the effect of sirolimus treatment on kidney size?
• A. According to the results of this study, the median increase in kidney volume over the 18-month period was 99 cm3 (interquartile range, 43 to 173) in the sirolimus group and 97 cm3 (interquartile range, 37 to 181) in the control group. At 18 months, the median total kidney volume in the sirolimus group was 102% of that in the control group (95% confidence interval [CI], 99 to 105; P=0.26).
Q. As compared to standard treatment, what was the effect of sirolimus on the glomerular filtration rate?
A. The glomerular filtration rate did not differ significantly between the sirolimus and standard care groups; however, the urinary albumin excretion rate was higher in the sirolimus group.
• Approximately 1 to 2% of the population in developed countries has heart failure, with the prevalence rising to 10% or more among persons 70 years of age or older. At least half the patients with heart failure have a low ejection fraction (40% or less), and coronary artery disease is the cause of approximately two thirds of the cases of systolic heart failure.
Which patients with heart failure should be treated with angiotensin-converting–enzyme (ACE) inhibitors?ACE inhibitors are the first-line therapy for patients with systolic heart failure; therapy should be initiated promptly after diagnosis and continued indefinitely. ACE inhibitors increase the ejection fraction modestly and reduce ventricular size, symptoms, hospitalization, and overall mortality. ACE inhibitors also reduce the risk of myocardial infarction.
When should spironolactone be used in patients with heart failure?In a large, placebo-controlled, randomized trial in which patients received spironolactone in addition to a diuretic, digoxin, and an ACE inhibitor, a reduction in symptoms and in hospital admissions, and a 30% reduction in mortality, were seen among patients with severe systolic heart failure (NYHA class III or IV). Therefore, the addition of an aldosterone antagonist should be considered for any patient who remains in NYHA class III or IV, despite treatment with a diuretic, an ACE inhibitor (or angiotensin-receptor blocker [ARB]), and a beta-blocker.
• Q:
• Is measurement of natriuretic peptides useful when heart failure is suspected?• A:
• Measurement of the plasma concentration of natriuretic peptides is recommended, since natriuretic peptides are secreted into the circulation increased amounts by the failing heart, and a normal concentration virtually rules out a diagnosis of heart failure (although this observation may not hold true in the case of obese persons).
• Q:
• Which patients with heart failure benefit from an implantable cardioverter–defibrillator?
• A:
• An implantable cardioverter–defibrillator is indicated for secondary prevention, in the case of any patient who survives an unprovoked episode of ventricular fibrillation or sustained ventricular tachycardia and for primary prevention, in the case of patients in NYHA functional class II or III who have an ejection fraction that is persistently 35% or less despite optimal medical therapy and who are expected to survive for at least 1 year with a reasonable quality of life and functional status.
• Q:
• How is Chagas' disease diagnosed?• A:
• In the acute phase, the diagnosis of Chagas' disease is made by visualization of parasites on thin and thick blood smears stained with Giemsa, but the parasites become difficult to detect by 3 months after infection. During the chronic phase, immunodiagnosis is most commonly used. Two tests are routinely performed: an enzyme-linked immunosorbent assay (ELISA) and an indirect immunofluorescence test for antibodies.
• Q:
• How is Chagas' disease treated?
• A:
• There are currently only two agents with antitrypanosomal activity: benznidazole and nifurtimox. Antiparasitic treatment is recommended in acute or congenital infection, in children with chronic infection, and in immunosuppressed patients. Among adults 19 to 50 years of age, antiparasitic therapy should generally be offered to women of childbearing age and to adults with the indeterminate form of the disease or mild-to-moderate cardiomyopathy. Treatment is considered optional in patients over 50 years of age and is generally not offered to patients who have severe cardiomyopathy or megaesophagus with swallowing difficulty.
• Cushing's syndrome results from sustained hypercortisolemia. The most common cause is administration of exogenous glucocorticoids. Secretion of corticotropin from the pituitary (Cushing's disease) accounts for approximately 70% of endogenous cases; adrenal tumors and the ectopic production of corticotropin each account for approximately 15% of cases.
How does ectopic corticotropin syndrome present?In patients with cancer, Cushing's syndrome generally develops quickly and is associated with extremely high levels of corticotropin and severe hypercortisolemia. Metabolic abnormalities tend to predominate in the clinical presentation. Hypokalemia is present in approximately 70% of patients with Cushing's syndrome and is related to the degree of hypercortisolemia.
The physical phenotype of Cushing's disease (i.e., facial fullness, a dorsocervical fat pad, and striae) may be absent in patients with ectopic corticotropin production, since these signs take weeks or months to develop.
Pigmentation appears to be common in patients with small-cell lung cancer, and neuropsychiatric abnormalities have a particular association with neuroendocrine tumors.
What are the typical tumors associated with the ectopic corticotropin syndrome?Small-cell lung cancers, bronchial carcinoids, thymic tumors, islet-cell tumors of the pancreas, medullary thyroid carcinomas, and pheochromocytomas have all been associated with ectopic corticotropin secretion. The source of ectopic corticotropin remains unidentified in approximately 10% of patients with the syndrome, but that percentage has declined with improvements in imaging.
• Q:
• How should possible Cushing's syndrome be evaluated?• A:
• The diagnosis of Cushing's syndrome requires the confirmation of hypercortisolism, generally with the measurement of 24-hour urinary cortisol excretion, measurement of midnight salivary cortisol levels, or both. Autonomous production of cortisol can be demonstrated with the use of a dexamethasone (1-mg) suppression test. Once hypercortisolism is established, a corticotrophin level of more than 20 pg per milliliter (4.4 pmol per liter) suggests corticotrophin dependency; a level below 5 pg per milliliter (1 pmol per liter) suggests an adrenal source. When corticotrophin dependency is established, magnetic resonance imaging can identify pituitary tumors approximately 60% of the time.
• Q:
• How can hypercortisolemia be managed medically?
• A:
• Medical management of hypercortisolemia is often necessary in preparation for surgery or for palliation. The principal treatments are metyrapone and ketoconazole. Other drugs include aminoglutethimide, mitotane, mifepristone, and somatostatin analogues.
• Approximately 5% of thyroid nodules in adults are malignant, whereas approximately 20% of thyroid nodules in pediatric patients are cancers (reported range, 2 to 40%).
What is the most common cause of thyroid cancer in children?
Papillary thyroid cancers are the most common type of thyroid cancer in children, followed by follicular and medullary thyroid cancers. Anaplastic cancers, primary thyroid lymphomas, and metastatic cancer are typically seen in older adults and rarely in children.What are the operative risks during thyroidectomy?
Permanent injury to one recurrent laryngeal nerve occurs in 1 to 2% of patients who undergo thyroid surgery with an experienced surgeon but can occur in up to 7% of patients whose surgeons are inexperienced.Bilateral nerve injury is very rare. Permanent hypoparathyroidism, which occurs when none of the four parathyroid glands retain function after thyroid surgery, is seen in 2% of patients; temporary hypoparathyroidism occurs in up to 20% of patients.
Q. How should levothyroxine therapy be managed following thyroidectomy for a thyroid cancer?
A. Slightly supraphysiological doses of thyroid hormone are administered to suppress serum thyrotropin levels, since thyrotropin is a growth factor for persistent disease. The suppression of thyrotropin levels is associated with better outcomes in young patients.
Q. What is the most sensitive imaging modality to detect recurrence of thyroid cancer?
A. Ultrasonography is the most sensitive imaging technique for detecting the recurrence of thyroid cancer in the anterior neck; it is also the primary imaging technique for long-term follow-up.
Nanomedicine
The field of nanomedicine aims to apply nanotechnology to the diagnosis and treatment of diseases at the molecular level.What do nanomaterials consist of?
Nanomaterials generally consist of metal atoms, nonmetal atoms, or a mixture of metal and nonmetal atoms, commonly referred to as metallic, organic, or semiconducting particles, respectively. The surface of nanomaterials is usually coated with polymers or biorecognition molecules for improved biocompatibility and selective targeting of biologic molecules.What physical feature is common to all nanomaterials?
A common feature of all nanomaterials is their large ratio of surface area to volume, which may be orders of magnitude greater than that of macroscopic materials.
Cutting a 1-cm cube into 1021 cubes that are each 1 nm will result in the same volume and mass, but the surface area will be increased by a factor of 10 million. Thus, the advantage of using nanomaterials as carriers is that their surface can be coated with many molecules.
Q. Why are nanoparticles particularly appropriate for use as radiocontrast agents?
A. Nanoparticles have a larger, localized magnetic field as compared with that of larger particles. This larger magnetic field can increase the contrast on imaging (MRI), since more protons interact in a larger field.Q. Why are nanomaterials particularly suitable for use in chemotherapy?
A. Nanomaterials infused into the bloodstream can accumulate in tumors owing to the enhanced permeability and retention effect when the vasculature of immature tumors has pores smaller than 200 nm, permitting extravasation of nanoparticles from blood into tumor tissue. With nanomaterials, the high ratio of surface area to volume permits high surface loading of the therapeutic agent; in the case of organic nanomaterials, their hollow or porous core allows encapsulation of hundreds of drug molecules into a single carrier particle.What clinical features are consistent with a diagnosis of Cushing’s syndrome?
Classical features include weight gain, facial rounding, hyperglycemia, ecchymoses, wide striae, edema, muscle weakness, hypokalemia, thrombotic events, and multiple infections. Osteopenia and osteoporosis are often seen given prolonged glucocorticoid exposure.Once a patient is suspected of having excess cortisol levels, laboratory testing is advisable for the detection of pathologic hypercortisolism and to distinguish it from other conditions. Laboratory tests for Cushing’s syndrome are conducted in cases of excessive cortisol secretion (which leads to increased excretion in the urine, as measured by 24-hour urine free cortisol), blunted normal circadian rhythm of cortisol secretion (which leads to high nocturnal cortisol in the blood or saliva), and diminished sensitivity to negative feedback regulation of cortisol secretion by glucocorticoids (which leads to lack of suppression of early-morning serum cortisol levels after the oral administration of dexamethasone, known as the dexamethasone suppression test).
What laboratory finding is pathognomonic of Cushing’s syndrome?
A measured urine free cortisol level that is at least four times as high as the upper end of the normal range is pathognomonic of Cushing’s syndrome.Cortisol excess of a lesser magnitude may be present in other conditions, such as pregnancy, severe obesity, or poorly controlled diabetes mellitus, which need to be distinguished from Cushing’s syndrome.
What medical therapy may be offered to patients with symptoms associated with Cushing’s syndrome?
Inhibitors of steroidogenesis, including ketoconazole and metyrapone can be used to treat symptoms associated with Cushing’s syndrome.
Ketoconazole inhibits the first step in cortisol synthesis as well as inhibiting the conversion of 11-deoxycortisol to cortisol.
Metyrapone works by blocking the conversion of 11-deoxycortisol to cortisol.
What is Nelson’s syndrome?
Nelson’s syndrome is estimated to affect 8 to 38% of patients with Cushing’s disease after bilateral adrenalectomy. The first reports of Nelson’s syndrome described the onset of cutaneous manifestations, such as the development of diffuse skin pigmentation, which may be more prominent in scars or in skin areas subject to friction and may affect the buccal mucosa.
These manifestations occur as a result of rising plasma corticotropin levels, despite adequate glucocorticoid replacement, which stimulates melanocortin 1 receptors in the skin.
The onset of Nelson’s syndrome occurs from a few months to 24 years after adrenalectomy. Patients may present with large, locally aggressive corticotroph tumors, leading to mass effect (headache, visual-field defects, ophthalmoplegia, or hypopituitarism). The precise pathogenesis of Nelson’s syndrome has not been elucidated, but presumably, after bilateral adrenalectomy, the absence of the negative feedback by glucocorticoids may lead to stimulation of an undetected small pituitary tumor and uncontrolled corticotropin secretion.