Liver
د. حسين محمد جمعهاختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2011
Chronic Hepatitis C Infection
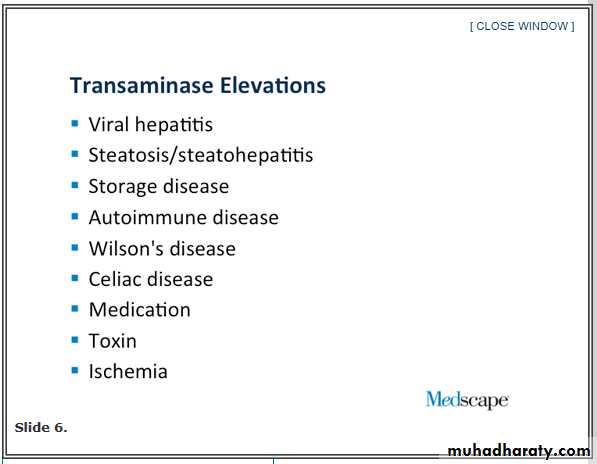
Infection with HCV affects an estimated 180 million people globally.It is a leading cause of chronic hepatitis, cirrhosis, and liver cancer and a primary indication for liver transplantation in the Western world.
NEJM june 23, 2011
There are at least six major HCV genotypes
Genotype 1 accounts for the majority of infections in North America, South America, and Europe. The predominant riskfactor for HCV acquisition is injection-drug use; among U.S. adults 20 to 59 years of age with any history of illicit injection-drug use, the prevalence of HCV infection is greater than 45% Other risk factors include blood transfusion before 1992, high
lifetime number of sexual partners, and iatrogenic transmission, including through dialysis; in large series, 15 to 30% of patients report no risk factors.
15 to 30% of patients in whom chronic infection develops have progression to cirrhosis over the ensuing three decades.
Patients with HCV-related cirrhosis warrant surveillance for complications, including hepatocellular carcinoma, which develops in 1 to 3% of such patients per year. For patients with clinically significant hepatic fibrosis (Metavir stage ≥2 or Ishak stage ≥3) ,there is widespread agreement that antiviral therapy is indicated because of the high risk of cirrhosis.
Prospective data indicate that the stage of fibrosis predicts clinical outcomes; the cumulative 6-year incidence of liver transplantation or liver-related death ranges from 4% for an Ishak fibrosis score of 2 to 28% for an Ishak score of 6. Because of the extended interval between infection and the
emergence of complications, the HCV-related disease burden is projected to increase severalfold over the next 20 years.
Diagnosis and Clinical Staging
Liver biopsy remains the standard for assessmentof hepatic fibrosis and is helpful for prognostication
and decision making. The histologic pattern
of HCV infection consists of lymphocyte infiltration
of the parenchyma, lymphoid follicles in portal
areas, and reactive bile-duct changes. However,
liver biopsy is costly and invasive, and it carries a
risk of complications (e.g., 1 to 5% of patients who
undergo the procedure require hospitalization).
Additional limitations of biopsy include sampling
error and interobserver variability.
Several methods have been used to quantify
hepatic fibrosis, including the simple aspartateaminotransferase:platelet ratio index (APRI) and
commercially available assays of some or most
of the following biomarkers:
α2-macroglobulin,
α2-globulin,
γ-globulin,
apolipoprotein A-I,
γ-glutamyltransferase,
total bilirubin, and hyaluronic acid.
Management
Interferon-Based Antiviral Therapy
Substantial progress has been made in the treatment
of HCV infection. The goals of therapy are
to prevent complications and death from HCV infection;regardless of the stage of fibrosis, symptomatic extrahepatic HCV (e.g.,cryoglobulinemia)
is an indication for therapy.
Over the past decade,on the basis of considerable data from randomized trials,
pegylated interferon (peginterferon)
plus ribavirin became the standard of care
for all HCV genotypes.
The two licensed peginterferons (Pegasys,
Roche, and PegIntron, Merck) have been shown inhead-to-head comparison to be equivalent in efficacy and to have similar safety profiles. Among patients with genotype 1 who are treated with peginterferon at the standard weight-based dose of ribavirin (1000 or 1200 mg per day) for 48 weeks, 40 to 50% have a sustained virologic response(defined as an undetectable HCV RNA level
24 weeks after the cessation of antiviral therapy).
A shorter course of treatment and a lower ribavirin dose are associated with lower rates of sustainedm virologic response (and higher relapse rates) among genotype 1–infected patients.
In contrast,
patients with genotype 2 or 3, who accountfor approximately one quarter of HCV-infected
patients in the United States, have rates of sustained virologic response in the range of 70 to
80% after taking peginterferon and ribavirin at
a reduced dose (800 mg per day) for 24 weeks.
A sustained virologic response is associated with permanent cure in the vast majority of patients.
Regardless of the infecting genotype, the likelihood of a sustained virologic response is lower among patients
with a high pretreatment HCV RNA level (with a
high level defined as >600,000 IU per milliliter in
some studies and >800,000 IU per milliliter in
others) and higher among patients with better
adherence to antiviral therapy (receiving 380% of
total interferon and ribavirin doses for 380% of
the expected duration of therapy).
Adherence can be problematic because of the plethora of side effects, including fevers,
influenza-like symptoms,headache,
cytopenias,
fatigue, anorexia,
depression,and anxiety.
On-treatment viral kinetics have emerged as
important predictors of the likelihood of responseand are used to guide the duration of therapy.
An early virologic response is defined as a decrease in the HCV RNA level of at least 2 log10
IU per milliliter or the complete absence of serum
HCV RNA at week 12 of therapy.
The lack of such a response in a patient has a very high negative predictive value for a sustained virologic response.
Among patients with previously untreated genotype 1 infection, more than 97% of those who do not have an early virologic response to treatment will not have a sustained response.
A rapid virologic response, defined as an undetectable HCV RNA level (<50 IU per milliliter) at week 4 of treatment, has been shown to predict a sustained virologic response, as well as to identify those patients for whom the duration of therapy can be shortened without compromising the virologic response.
A rapid virologic response, defined as an undetectable HCV RNA level (<50 IU per milliliter) at week 4 of treatment,
Has been shown to predict
a sustained virologic response, (i.e. the infection was no longer detected in the blood 24 weeks after stopping treatment)as well as to identify those patients
for whom the duration of therapy can be shortened without compromising the virologic response.A recent metaanalysis of seven randomized trials has shown that genotype 1–infected patients with a low baseline HCV RNA level (<400,000 IU per milliliter) who have a rapid virologic response may discontinue therapy at 24 weeks rather than continue for the standard 48 weeks.32 A reduction of the treatment duration has the added benefits of decreased costs and side effects.
Race is another important predictor of response
to antiviral therapy. Black patients havesignificantly lower rates of sustained virologic
response than white patients (28% vs. 52%).
Although the reasons for this difference have
been uncertain, recent data from genomewide
association studies have indicated that singlenucleotide polymorphisms (SNPs) on chromosome 19 in or near the interleukin-28B gene (IL28B,encoding interferon lambda-3) are highly predictive of successful antiviral treatment.
In an analysis that was adjusted for other predictors, the chance of cure was more than doubled with homozygosity for the C allele at the rs12979860SNP, as compared with the TT genotype (78% for the CC genotype, 38% for the TC genotype, and 26% for the TT genotype). The C allele is much more frequent in white and Asian populations than in black populations.
The nonstructural 3 (NS3) serine protease inhibitors
are the furthest along in development. Inaddition to ablating replication, protease inhibition
blocks the ability of the NS3/4A serine protease
to cleave the HCV polyprotein and interferon-β
promoter stimulator 1, thus restoring innate immune
signaling within hepatocytes (Fig. 3B).15
Two protease inhibitors, telaprevir and boceprevir,
were recently approved by the Food and Drug
Administration (FDA).
Two protease inhibitors,
telaprevir andboceprevir
were recently approved by the
(FDA).
In the Protease Inhibition for Viral Evaluation
1 trial ,which involved genotype 1–infected patients who had not previously received treatment, the rates of sustained virologic response were 61% and 69%, respectively, among
those who received a 12-week course of telaprevir,
an orally bioavailable inhibitor of NS3/4A,38 in
combination with peginterferon–ribavirin, which
was continued for an additional 12 weeks (total
duration of antiviral therapy, 24 weeks; T12PR24
As compared with standard therapy with peginterferon–ribavirin, the addition of telaprevir resulted in a shorter median time to achieve an undetectable HCV RNA level (<30 days, vs.113 days). Major side effects of telaprevir included rash, pruritus, anemia, and gastrointestinal
symptoms. The observation that viral relapse
(detectable HCV RNA level during the 24-week
posttreatment period in patients with an end-oftreatment response) occurred in 48% of patients
who did not receive ribavirin (T12P12 in Fig. 4)
underscores the critical role of this agent in preventing
relapse and the emergence of telaprevir
resistance.
The ADVANCE (A New Direction in HCV Care:
A Study of Treatment-Naive Hepatitis C Patientswith Telaprevir) trial (NCT00627926), a phase III
randomized trial reported in this issue of the
Journal, incorporated on-treatment response to
tailor the duration of additional peginterferon–
ribavirin. Telaprevir and peginterferon–ribavirin
were administered for the first 12 weeks or
for 8 weeks, followed by 4 weeks of placebo.
Extended rapid virologic response was defined as
an undetectable HCV RNA level (<25 IU per milliliter) at week 4 and week 12 of therapy; patients who did not have an extended rapid virologic response received 36 additional weeks of
peginterferon–ribavirin, for a total of 48 weeks.
More than half of the telaprevir-treated patients
had an extended rapid virologic response, and
24 weeks of total therapy was associated with
a rate of sustained virologic response that was
higher than 80% among these patients.
As in all the other telaprevir studies, virologic failure was more common in patients with genotype 1a than in those with genotype 1b. The REALIZE (Retreatment of Patients with Telaprevir-based Regimen to Optimize Outcomes) study,
Showed that the addition of telaprevir to peginterferon– ribavirin significantly increased the rate of sustained virologic response among patients who had previously received treatment, particularly in prior relapsers (patients with an undetectable
HCV RNA level at the end of a prior course of
peginterferon–ribavirin therapy but with a detectable
HCV RNA level thereafter).
The Serine Protease Inhibitor Therapy 1 trial
and the SPRINT-2 trialhave shown the efficacy of boceprevir
in combination with peginterferon alfa-2b
and ribavirin in genotype 1–infected patients who had not previously received treatment .
Principal side effects of boceprevir included anemia
(necessitating treatment with erythropoietin
analogues in many patients) and dysgeusia, which
appeared to be more common than previously
reported with telaprevir; rash was reported less
frequently than in the telaprevir trials.
Mathematical modeling has projected that if
the rate of response to antiviral therapy increasesto 80%, which appears to be likely in the foreseeable future, treatment of half of HCV-infected persons would reduce cases of cirrhosis by
15%, cases of hepatocellular carcinoma by 30%,
and deaths due to liver disease by 34% after just
10 years.
Areas of Uncertainty
Transient elastography (FibroScan, Echosens) isa novel noninvasive technique that measures liver stiffness by assessing the velocity of a shear wave created by a transitory vibration. Thresholds for a high likelihood of clinically significant fibrosis
(Metavir score ≥2) have been defined.
The technique has an increased failure rate among obese patients, and it has not been approved by the FDA.
Whether modifications of existing technologies
(e.g., computed tomography and magnetic resonance imaging) will provide sensitive differentiation of levels of hepatic fibrosis requires further study.
Although peginterferon–ribavirin is likely to
remain the backbone of antiviral therapy for the foreseeable future, options for treating HCV are
expected to expand rapidly in upcoming years.
The optimal combination of agents (including
nucleoside and nonnucleoside polymerase inhibitors, inhibitors of NS4B and NS5A proteases,
modulators of the immune response, and medications that interfere with lipid metabolism,
which is essential for the assembly and maturation
of HCV particles) and duration of therapy will
need to be defined, in order to maximize rates
of sustained virologic response while minimizing
the risk that resistance will develop.
A recent pilot study of a combination of directly acting antiviral agents suggests the possibility of treating HCV infection with an interferon-free, oral approach. Further study is needed in subgroups of patients with lower response rates, including black patients, patients without a response to prior treatment, liver-transplant recipients, and those who have coinfection with HIV, a high baseline viral load, advanced fibrosis, or insulin resistance.
Guidelines
The American Association for the Study of LiverDiseases and the American Gastroenterological
Association have published guidelines for the
assessment and management of chronic HCV infection, but these guidelines were issued before
the publication of data from randomized trials of
directly acting antiviral agents. Newer European
guidelines take these data into account; the recommendations provided below are generally consistent with these guidelines.
Conclusions and
RecommendationsHCV genotype 1 with a high viral load,should be vaccinated for hepatitis A because of an increased risk of liver failure among patients with chronic hepatitis C infection; hepatitis B vaccination is also indicated in those without evidence of prior exposure. Possible contraindications to treatment
(e.g., depression) should be determined, and the
patient should be informed about potential side effects of antiviral therapy.
Although some clinicians would administer treatment without performing a liver biopsy, I would recommend a biopsy to assess the degree of fibrosis. For a patient with
clinically significant fibrosis (Metavir score ≥2), triple antiviral therapy with peginterferon–ribavirin
and an NS3/4A protease inhibitor, either telapreviror boceprevir, should be recommended.
On the basis of data from recent randomized
trials, a reasonable initial regimen would be telaprevirwith peginterferon–ribavirin for 12 weeks.
If tests for HCV RNA were negative at weeks
4 through 12 (indicating an extended rapid virologic
response), only 12 additional weeks of peginterferon–
ribavirin would be recommended,
whereas if an extended rapid virologic response
were not achieved, peginterferon–ribavirin would
be continued for an additional 36 weeks.
If boceprevir were used, according to new FDA guidelines, a 4-week lead-in phase of peginterferon–ribavirin would be followed by peginterferon–ribavirin and boceprevir for 24 weeks (a totalof 28 weeks) if tests for HCV RNA were negative at weeks 8 through 24 of treatment.
If the tests were positive between weeks 8 and 24 but negative at week 24, peginterferon–ribavirin and boceprevir would be continued for an additional 8 weeks, followed by an additional 12 weeks of peginterferon–ribavirin (a total of 48 weeks).
Alternatively, if the patient has milder fibrosis and is reluctant to receive treatment, it would be reasonable to wait and reevaluate as new therapeutic agents become available.
NEJM june 23, 2011
FDA approves Incivek for hepatitis C
The U.S. Food and Drug Administration approved Incivek (telaprevir) (May 23, 2011) to treat certain adults with chronic hepatitis C infection. Incivek is used for patients who have either not received interferon-based drug therapy for their infection or who have not responded adequately to prior therapies. Incivek is approved for use with interferon therapy made up of peginterferon alfa and ribavirin.
The current standard of care for patients with chronic hepatitis C infection is peginterferon alfa and ribavirin taken for 48 weeks. Less than 50 percent of patients respond to this therapy.
The safety and effectiveness of Incivek was evaluated in three phase 3 clinical trials with about 2,250 adult patients who were previously untreated, or who had received prior therapy. In all studies patients also received the drug with standard of care. In previously untreated patients, 79 percent of those receiving Incivek experienced a sustained virologic response (i.e. the infection was no longer detected in the blood 24 weeks after stopping treatment) compared to standard treatment alone.
The sustained virologic response for patients treated with Incivek across all studies, and across all patient groups, was between 20 and 45 percent higher than current standard of care.
The studies indicate that treatment with Incivek can be shortened from 48 weeks to 24 weeks in most patients. Sixty percent of previously untreated patients achieved an early response and received only 24 weeks of treatment (compared to the standard of care of 48 weeks).
The sustained virologic response for these patients was 90 percent.
When a person achieves a sustained virologic response after completing treatment, this suggests that the hepatitis C infection has been cured. Sustained virologic response can result in decreased cirrhosis and complications of liver disease, decreased rates of liver cancer, and decreased mortality.People can get HCV in a number of ways, including: exposure to blood that is infected with the virus; being born to a mother with HCV; sharing a needle; having sex with an infected person; sharing personal items such as a razor or toothbrush with someone who is infected with the virus, or from unsterilized tattoo or piercing tools.
Incivek is a pill taken three times a day with food. Incivek should be taken for the first 12 weeks in combination with peginterferon alfa and ribavirin. Most people with a good early response to the Incivek combination regimen can be treated for 24 weeks rather than the recommended 48 weeks of treatment with the standard of care. Incivek is part of a class of drugs referred to as protease inhibitors, which work by binding to the virus and preventing it from multiplying.
The most commonly reported side effects in patients receiving Incivek in combination with peginterferon alfa and ribavirin include rash, low red blood cell count (anemia), nausea, fatigue, headache, diarrhea, itching (pruritus), and anal or rectal irritation and pain. Rash can be serious and can require stopping Incivek or all three drugs in the treatment regimen.
On May 13, FDA approved Victrelis (boceprevir), another new treatment for chronic hepatitis C, marketed by Merck of Whitehouse Station, N.J.
Incivek (telaprevir)
Victrelis (boceprevir)
protease inhibitors
• What are the predominant risk factors for hepatitis C virus infection?
• The predominant risk factor for HCV acquisition is injection-drug use; among U.S. adults 20 to 59 years of age with any history of illicit injection-drug use, the prevalence of HCV infection is greater than 45%. Other risk factors include blood transfusion before 1992, high lifetime number of sexual partners, and iatrogenic transmission, including through dialysis; in large series, 15 to 30% of patients report no risk factors.Approximately what percentage of patients with chronic hepatitis C virus infection have progression to cirrhosis?
Although the natural history of HCV infection is highly variable, an estimated 15 to 30% of patients in whom chronic infection develops have progression to cirrhosis over the ensuing three decades.
A number of factors, including a longer duration of infection, an older age at the time of exposure, male sex, coinfection with other viruses such as HIV, and daily alcohol consumption, but not viral level or genotype, have been consistently associated with an increased risk of fibrosis.
Triple Therapy for Genotype 1 Hepatitis C Infection
A new regimen that included boceprevir yielded high rates of sustained virologic response.Triple therapy with peginterferon, ribavirin, and a specifically targeted antiviral agent such as a protease inhibitor will soon be available to improve sustained virologic response (SVR) in patients infected with hepatitis C virus (HCV) genotype 1. In this industry-funded, open-label, multicenter, phase II trial, researchers assessed the efficacy of 800 mg of boceprevir (thrice daily), an NS3 protease inhibitor, in combination with peginterferon alfa-2b (1.5 µg/kg) and ribavirin (800 to 1400 mg/day).
Published in Journal Watch Gastroenterology August 20, 2010
In all, 520 treatment-naive patients were randomly assigned to one of five regimens:
Peginterferon plus ribavirin for 48 weeks (control group)Peginterferon plus ribavirin for 4 weeks and then both drugs plus boceprevir for 24 weeks
Peginterferon plus ribavirin for 4 weeks and then both drugs plus boceprevir for 44 weeks
Peginterferon, ribavirin, and boceprevir for 28 weeks
Peginterferon, ribavirin, and boceprevir for 48 weeks
Subsequently, 75 patients were randomized to receive triple therapy in which the ribavirin was administered at either the standard dose (800–1400 mg/day) or a lower dose (400–1000 mg/day).
In an intent-to-treat analysis, rates of the primary endpoint — SVR at 24 weeks — were significantly higher in the boceprevir groups (range, 54% with 28-week triple therapy to 75% with 44-week triple therapy preceded by a 4-week lead-in) than in the control group (38%); comparisons with the control group ranged from P=0.013 to P<0.0001.
Among the boceprevir groups, the rate of viral breakthrough (with mutations) was nonsignificantly lower in those who received a two-drug lead-in than in those who did not (4% and 9%; P=0.057). Low-dose ribavirin recipients had a high rate of viral breakthrough with resistance. Compared with the control group, the boceprevir groups had higher rates of anemia (55% vs. 34%) and dysgeusia (27% vs. 9%).
Comment: In this phase II study involving treatment-naive patients with HCV genotype 1 infection, triple therapy with peginterferon, ribavirin, and boceprevir for 44 weeks — preceded by a 4-week lead-in without the boceprevir — achieved an SVR rate of 75%. If a nearly complete phase II trial verifies this striking result, clinicians will soon be able to choose between this and a triple-therapy option involving telaprevir (JW Gastroenterol Apr 29 2009). Both the telaprevir and boceprevir regimens are likely to yield high SVR rates, but boceprevir will be given for 48 weeks in most cases, and telaprevir for 24 weeks. Both drugs will add to the adverse effects: skin rash with telaprevir, anemia with boceprevir.
Published in Journal Watch Gastroenterology August 20, 2010
HCV genotype and virologic response to treatment determine the duration of treatment. Patients with genotypes 1 and 4 are treated for 48 weeks, and those with genotypes 2 and 3 are treated for 24 weeks. Multidrug regimens may be developed in the future, using new agents in combination with current therapies.
Am Fam Physician. 2010
"The quantitative HCV RNA level is used to assess response to therapy and as a guide to discontinue treatment," the review authors write.
"A negative viral load test after four weeks of therapy is predictive of sustained virologic response.
In contrast, failure to achieve a 100-fold reduction in viral load by week 12 of therapy has a strong negative predictive value for sustained virologic response and suggests that treatment is likely ineffective and should be stopped."
Absolute contraindications to treatment of HCV infection include
active alcohol or substance abuse,active autoimmune hepatitis or other condition known to be exacerbated by interferon and ribavirin, known
hypersensitivity to medications used to treat HCV infection, and
pregnancy or lack of compliance with adequate contraception.
For ribavirin only, renal failure is an absolute contraindication.
Other absolute contraindications to treatment of HCV infection are severe concurrent cardiopulmonary disease; uncontrolled major depressive illness, psychosis, or bipolar disorder; and untreated hyperthyroidism.
Relative contraindications to treatment of HCV infection include laboratory values suggesting decompensated cirrhosis, and baseline hematologic and biochemical indices.
Treatment of patients with chronic HCV infection should include counselling them to abstain from alcohol use. Although no vaccine currently exists to prevent HCV infection, persons infected with HCV should be vaccinated against hepatitis A and B. Persons with chronic HCV infection and cirrhosis should periodically undergo ultrasound imaging as surveillance for hepatocellular carcinoma, according to recommendations from the American Association for the Study of Liver Diseases.
Am Fam Physician. 2010
Nucleic Acid Testing to Detect HBV
Infection in Blood DonorsTriplex nucleic acid testing detected potentially infectious HBV, along with HIV and
HCV, during the window period before seroconversion.
HBV vaccination appeared to be protective, with a breakthrough subclinical infection occurring with non-A2 HBV subgenotypes and causing clinically inconsequential outcomes.
nejm.236 org january 20, 2011
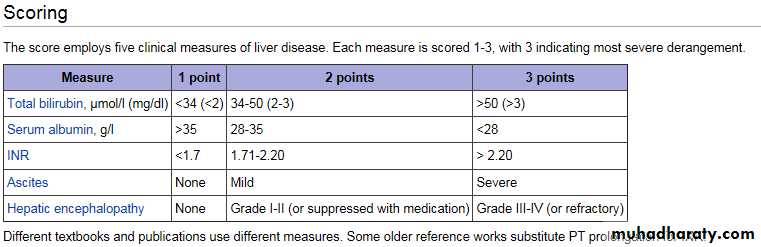
Acute liver failure (ALF) is an uncommon condition in which the rapid deterioration of liver function results in coagulopathy and alteration in the mental status of a previously healthy individual. Acute liver failure often affects young people and carries a very high mortality. The term acute liver failure is used to describe the development of coagulopathy, usually an international normalized ratio (INR) of greater than 1.5, and any degree of mental alteration (encephalopathy) in a patient without preexisting cirrhosis and with an illness of less than 26 weeks' duration.
Acute liver failure is a broad term and encompasses both fulminant hepatic failure (FHF) and subfulminant hepatic failure (or late-onset hepatic failure). Fulminant hepatic failure is generally used to describe the development of encephalopathy within 8 weeks of the onset of symptoms in a patient with a previously healthy liver. Subfulminant hepatic failure is reserved for patients with liver disease for up to 26 weeks before the development of hepatic encephalopathy.
The risk of mortality increases with the development of any of the complications, which include cerebral edema, renal failure, adult respiratory distress syndrome (ARDS), coagulopathy, and infection.
Grade
Level of ConsciousnessPersonality and Intellect
Neurologic Signs
Electroencephalogram (EEG) Abnormalities
0
Normal
Normal
None
None
Subclinical
Normal
Normal
Abnormalities only on psychometric testing
None
1
Day/night sleep reversal, restlessness
Forgetfulness, mild confusion, agitation, irritability
Tremor, apraxia, incoordination, impaired handwriting
Triphasic waves (5 Hz)
2
Lethargy, slowed responses
Disorientation to time, loss of inhibition, inappropriate behavior
Asterixis, dysarthria, ataxia, hypoactive reflexes
Triphasic waves (5 Hz)
3
Somnolence, confusion
Disorientation to place, aggressive behavior
Asterixis, muscular rigidity, Babinski signs, hyperactive reflexes
Triphasic waves (5 Hz)
4
Coma
None
Decerebration
Delta/slow wave activity
Grading of Hepatic Encephalopathy
Causes
Numerous causes of fulminant hepatic failure exist, but drug-related hepatotoxicity due to acetaminophen and idiosyncratic drug reactions is the most common cause of acute liver failure in the United States.
For nearly 15% of patients, the cause remains indeterminate.
Hepatitis A and B are the typical viruses that cause viral hepatitis and may lead to hepatic failure. Hepatitis C rarely causes acute liver failure. HDV (co-infection or superinfection with HBV) can lead to fulminant hepatic failure. HEV (often observed in pregnant women) in endemic areas is an important cause of fulminant hepatic failure.
Other atypical viruses can cause viral hepatitis and fulminant hepatic failure.
Cytomegalovirus
Hemorrhagic fever viruses
Herpes simplex virus
Paramyxovirus
Epstein-Barr virus
The incidence of acute fatty liver of pregnancy, frequently culminating in fulminant hepatic failure, has been estimated to be 0.008% (typically in the third trimester; preeclampsia develops in approximately 50% of these patients). However, the most common cause of acute jaundice in pregnancy is acute viral hepatitis, and most of these patients do not develop fulminant hepatic failure.
The one major exception to this is the pregnant patient who develops HEV infection and in whom an exposure history is usually remarkable for travel and/or residence in the Middle East, India and the subcontinent, Mexico, or other endemic areas. In these patients, progression to fulminant hepatic failure is unfortunately common and often fatal. In the United States, it is relatively uncommon but must be considered in the appropriate setting.
The HELLP syndrome occurs in 0.1-0.6% of pregnancies and is usually associated with preeclampsia.
Incidence of fulminant hepatic failure following other liver diseases is less well established.
Many drugs (both prescription and illicit) are implicated in the development of FHF. The list provided is incomplete, and only the more common agents are identified. Consult an appropriate pharmacy reference text if concerns exist regarding a specific medication. Idiosyncratic drug reactions may occur with virtually any medication. Fortunately, these appear to lead to fulminant hepatic failure only rarely, although they are the most common form of drug reaction to lead to fulminant hepatic failure (with the exception of acetaminophen poisoning).
Drug toxicity – Acetaminophen
Intentional or accidental overdose. In the US Acute Liver Failure (ALF) study, unintentional acetaminophen use accounted for 48% of cases, whereas 44% of cases were due to intentional use; in 8% of cases, the intention was unknown.Dose-related toxicity
May have greatly increased susceptibility to hepatotoxicity with depleted glutathione stores in the setting of chronic alcohol use (consider increased susceptibility due to chronic alcohol use)
Prescription medications (idiosyncratic hypersensitivity reactions)
Antibiotics (ampicillin-clavulanate, ciprofloxacin, doxycycline, erythromycin, isoniazid, nitrofurantoin, tetracycline)
Antivirals (fialuridine)
Antidepressants (amitriptyline, nortriptyline)
Antidiabetics (troglitazone)
Antiepileptics (phenytoin, valproate)
Anesthetic agents (halothane)
Lipid-lowering medications (atorvastatin, lovastatin, simvastatin)
Immunosuppressive agents (cyclophosphamide, methotrexate)
(NSAIDs)
Salicylates (Reye syndrome)
Oral hypoglycemic agents (troglitazone)
Others (disulfiram, flutamide, gold, propylthiouracil)
Illicit drugs
Ecstasy (3,4-methylenedioxymethamphetamine [MDMA])Cocaine (may be the result of hepatic ischemia)
Herbal or alternative medicines
Ginseng
Pennyroyal oil
Teucrium polium
Chaparral or germander tea
Kawakawa
The following toxins are associated with dose-related toxicity:
Amanita phalloides mushroom toxin[14 ]
Bacillus cereus toxin
Cyanobacteria toxin
Organic solvents (eg, carbon tetrachloride)
Yellow phosphorus
The following are vascular causes of hepatic failure:
Ischemic hepatitis (consider especially if in the setting of severe hypotension or recent hepatic tumor chemoembolization)
Hepatic vein thrombosis (Budd-Chiari syndrome)
Hepatic veno-occlusive disease
Portal vein thrombosis
Hepatic arterial thrombosis (consider posttransplant)
The following metabolic diseases can cause hepatic failure:
Acute fatty liver of pregnancyAlpha1 antitrypsin deficiency
Fructose intolerance
Galactosemia
Lecithin-cholesterol acyltransferase deficiency
Reye syndrome
Tyrosinemia
Wilson disease
Autoimmune disease (autoimmune hepatitis) can cause hepatic failure.
Malignancy can cause of hepatic failure.Primary liver tumor (usually hepatocellular carcinoma, rarely cholangiocarcinoma)Secondary tumor (extensive hepatic metastases or infiltration from adenocarcinoma, such as breast, lung, melanoma primaries [common]; lymphoma; leukemia)The following are miscellaneous causes of hepatic failure:
Adult-onset Still disease
Heat stroke
Primary graft nonfunction (in liver transplant recipients)
Differential Diagnoses
Other Problems to Be Considered
Acute fatty liver of pregnancyAdult-onset Still diseaseA phalloides mushroom poisoningB cereus toxinFructose intoleranceGalactosemiaHELLP syndrome of pregnancyHemorrhagic viruses (Ebola virus, Lassa virus, Marburg virus)Idiopathic drug reaction (hypersensitivity)Neonatal iron storage diseaseParamyxovirusPrimary graft nonfunction (in liver transplant recipients)TyrosinemiaYellow phosphorus poisoningAcetaminophen poisoning
Complete blood cell (CBC) count: Results may indicate thrombocytopenia.
PT and/or international normalized ratio (INR)Hepatic enzymes
Serum bilirubin
Serum ammonia
Serum glucose: levels may be very low and pose a serious hazard.
Serum lactate
Serum creatinine: levels may be elevated, signifying the development of hepatorenal syndrome or some other cause of acute renal failure.
Blood cultures
Serum-free copper
Serum phosphate
Viral serologies
Autoimmune markers: Antinuclear antibody (ANA), anti-smooth muscle antibody (ASMA), and immunoglobulin levels are important markers for a diagnosis of autoimmune hepatitis.
Acetaminophen level
Drug screen: Consider a drug screen in a person who is an IV drug abuser.
Hepatic enzymes Levels of the transaminases (aspartate aminotransferase [AST]/serum glutamic-oxaloacetic transaminase [SGOT]
and alanine aminotransferase [ALT]/serum glutamic-pyruvic transaminase [SGPT]) are often elevated dramatically as a result of severe hepatocellular necrosis.
In instances of acetaminophen toxicity (especially alcohol-enhanced), the AST level may be well over 10,000 U/L.
The alkaline phosphatase (ALP) level may be normal or elevated.
Serum glucose: levels may be very low and pose a serious hazard.
This results from impairments in glycogen production and gluconeogenesis.
Serum lactate .Arterial blood lactate levels either at 4 hours (>3.5 mmol/L) or at 12 hours (>3.0 mmol/L) are early predictors of outcome in acetaminophen-induced acute liver failure. levels are often elevated as a result of both impaired tissue perfusion (increases production) and decreased clearance by the liver.An increased anion gap metabolic acidosis is associated with this condition (although it may be accompanied by a respiratory alkalosis as a result of hyperventilation).
Arterial blood gases
(ABGs): These may reveal hypoxemia, which is a significant concern as a result of adult respiratory distress syndrome (ARDS) or other causes (eg, pneumonia).Serum creatinine:
levels may be elevated, signifying the development of hepatorenal syndrome or some other cause of acute renal failure.
Serum ammonia
This level may be elevated dramatically in patients with fulminant hepatic failure. Arterial blood is the best way to measure ammonia.The arterial serum ammonia level is most accurate, but venous ammonia levels are generally acceptable.
It does not exclude the possibility of another cause for mental status changes (notably increased ICP and seizures).
Treatment
Medical CareThe most important step is to identify the cause of liver failure.
Prognosis of acute liver failure is dependent on etiology. A few etiologies of acute liver failure demand immediate and specific treatment. It is also critical to identify those patients who will be candidates for liver transplantation.
The most important aspect of treatment in patients with acute liver failure is to provide good intensive care support. Patients with grade II encephalopathy should be transferred to the intensive care unit (ICU) for monitoring. As the patient develops progressive encephalopathy, protection of the airway is important.
Most patients with acute liver failure tend to develop some degree of circulatory dysfunction. Careful attention should be paid to fluid management, hemodynamics, metabolic parameters, and surveillance of infection. Maintenance of nutrition and prompt recognition of gastrointestinal bleeding are crucial. Coagulation parameters, CBC count, and metabolic panel should be checked frequently. Serum aminotransferases and bilirubin are generally measured daily to follow the course of infection. Intensive care management includes recognition and management of complications.
Airway protection
As the patients with fulminant hepatic failure drift deeper into coma, their ability to protect their airway from aspiration decreases.
Patients who are in stage III coma should have a nasogastric tube (NGT) for stomach decompression. When patients progress to stage III coma, intubation should be performed.
Short-acting benzodiazepines in low doses (eg, midazolam 2-3 mg) may be used before intubation or propofol (50 mcg/kg/min) may be initiated before intubation and continued as an infusion. Propofol is also known to decrease the cerebral blood flow and ICH. It may be advisable to use endotracheal lidocaine before endotracheal suctioning.
Encephalopathy and cerebral edema
Patients with grade I encephalopathy may sometimes be safely managed on a medicine ward. Frequent mental status checks should be performed with transfer to an ICU warranted with progression to grade II encephalopathy.Head imaging with CT scanning is used to exclude other causes of decline in mental status, such as intracranial hemorrhage.
Sedation should be avoided if possible; unmanageable agitation may be treated with short-acting benzodiazepines in low doses.
Patients should be positioned with the head elevated at 30°.
Efforts should be made to avoid patient stimulation. Maneuvers that cause straining or, in particular, Valsalva-like movements may increase ICP.
There is increasing evidence that ammonia may play a pathogenic role in the development of cerebral edema. Reducing elevated ammonia levels with enteral administration of lactulose might help prevent or treat cerebral edema.
ICP monitoring helps in the early recognition of cerebral edema.
The clinical signs of elevated ICP, including hypertension, bradycardia, and irregular respirations (Cushing triad), are not uniformly present; these and other neurologic changes, such as pupillary dilatation or signs of decerebration, evident only late in the course.
CT scanning of the brain does not reliably demonstrate evidence of edema, especially at early stages. A primary purpose of ICP monitoring is to detect elevations in ICP and reductions in cerebral perfusion pressure (CPP; calculated as mean arterial pressure [MAP] minus ICP) so that interventions can be made to prevent herniation while preserving brain perfusion.
The ultimate goal of such measures is to maintain neurologic integrity and prolong survival while awaiting receipt of a donor organ or recovery of sufficient functioning hepatocyte mass. Additionally, refractory ICH and/or decreased CPP is considered a contraindication to liver transplantation in many centers.
Cardiovascular monitoring
Homodynamic derangements consistent with multiple organ failure occur in acute liver failure. Hypotension (systolic, <80 mm Hg) may be present in 15% of patients. Most patients will require fluid resuscitation on admission. Intravascular volume deficits may be present on admission due to decreased oral intake or gastrointestinal blood loss. Hemodynamic derangement resembles that of sepsis or cirrhosis with hepatorenal syndrome (low SVR with normal or increased cardiac output). An arterial line should be placed for continuous blood pressure monitoring.A Swan–Ganz catheter should be placed and fluid replacement with colloid albumin should be guided by the filling pressure. If needed, dopamine or norepinephrine can be used to correct hypotension.
Management of renal failure: Hemodialysis may significantly lower the mean arterial pressure such that cerebral perfusion pressure is compromised. Continuous veno-venous hemofiltration is preferred.
Management of coagulopathy
In the absence of bleeding, it is not necessary to correct clotting abnormalities with fresh frozen plasma (FFP); the exception is when an invasive procedure is planned or in the presence of profound coagulopathy (INR >7). (PT and PTT become prolonged when plasma coagulation components are diluted to less than 30%, and abnormal bleeding occurs when they are less than 17%. One unit of FFP increases the coagulation factor by 5%; 2 units increase it by 10%.) FFP of 15 mL/kg of body weight or 4 units correct deficiency. If the fibrinogen level is very low (<80 mg/dL), consider cryoprecipitation.Recombinant factor VIIa may be used in patients whose condition is nonresponsive to FFP. It is used in a dose of 4 µg/kg IV push over 2-5 minutes. PT is normalized in 20 minutes and remains normalized for 3-4 hours.
Platelet transfusions are not used until the count is less than 10,000/µL or if an invasive procedure is being done and the platelet count is less than 50,000/µL. Six to 8 random donor platelets (1 random donor unit platelet/10 kg) will increase the platelet count to greater than 50,000/µL. The platelet count should be checked after 1 hour and 24 hours. Transfused platelets survive 3-5 days.
Managing poisonings (eg, acetaminophen, mushroom) requires specific treatment distinct from other, more general issues related to fulminant hepatic failure.Treat acetaminophen (paracetamol, APAP) overdose with N-acetylcysteine (NAC). Researchers theorize that this antidote works by a number of protective mechanisms. Early after overdose, NAC prevents the formation and accumulation of N-acetyl-p-benzoquinone imine (NAPQI), a free radical that binds to intracellular proteins, nonspecifically resulting in toxicity.
NAC increases glutathione stores, combines directly with NAPQI as a glutathione substitute, and enhances sulfate conjugation. NAC also functions as an anti-inflammatory and antioxidant and has positive inotropic and vasodilating effects, which improve microcirculatory blood flow and oxygen delivery to tissues. These latter effects decrease morbidity and mortality once hepatotoxicity is well established.
The protective effect of NAC is greatest when administered within 8 hours of ingestion; however, when indicated, administer regardless of the time since the overdose. Therapy with NAC has been shown to decrease mortality in late-presenting patients with fulminant hepatic failure (in the absence of acetaminophen in the serum).
A phalloides mushroom intoxication is much more common in Europe as well as in California. Treat with IV penicillin G, even though its mode of action is unclear. Silibinin, a water-soluble derivative of silymarin, may be administered orally, and oral charcoal may be helpful by binding the mushroom toxin.
Surgical Care
Liver transplantation is the definitive treatment in liver failure, but a detailed discussion is beyond the scope of this article. Although, 2 recent studies regarding liver transplantation are mentioned below, preoperative management is emphasized in this section. Lerut et al evaluated the effect of tacrolimus monotherapy in 156 adults receiving a primary liver graft, randomizing them to receive tacrolimus-placebo and tacrolimus-low-dose, short-term (64 days), steroid immunosuppression. There were no exclusion criteria at randomization, and all patients had a 12-month follow-up (range, 12-84).[20 ]The investigators found that the patients in the tacrolimus-steroid group had higher 3- and 12-month survival rates, as well as higher 12-month graft survival rates, relative to those in the tacrolimus-placebo group. Not only were fewer patients in the tacrolimus-steroid group administered rejection treatment at 3 and 12 months, but fewer individuals in this group and the group of 145 patients transplanted without artificial organ support demonstrated corticosteroid-resistant rejection at 3 and 12 months.[20
By 1 year, 82% (64/78) of those in the tacrolimus steroid group were on tacrolimus monotherapy compared with 78.2% (61/78) of those in the tacrolimus-placebo group (P = 0.54). However, when considering the 74 tacrolimus-steroid and 67 tacrolimus-placebo survivors, rates of monotherapy were lower in the tacrolimus-steroid group versus the tacrolimus-placebo group (P = 0.39).[20 ]
Lerut et al concluded that tacrolimus monotherapy can be achieved safely without compromising graft nor patient survival in a primary, even unselected, adult liver transplant population and that such a strategy may lead to further large-scale minimization studies in liver transplantation.[20 ]The investigators attributed the higher incidence of early corticosteroid-resistant rejection in the tacrolimus-placebo group to the significantly higher number of patients transplanted while being on artificial organ support and recommended that the monodrug immunosuppressive strategy would require adaptation in this setting.[20 ]
In a retrospective study, Taketomi et al evaluated donor safety in adult-to-adult living donor liver transplantation by establishing a selection criterion for donors in which the left lobe was the first choice of graft.[21 ]Two hundred and six consecutive donors were divided into 2 groups according to the graft type (left [n = 137] vs right lobe [n =69]). Mean intraoperative blood loss was significantly increased in the left lobe donors compared with right lobe donors; however, mean peak postoperative total bilirubin levels and duration of hospital stay after surgery were significantly less for those in the left lobe group (P <0.05).[21 ]
No donor died or suffered a life-threatening complication during the study period. The investigators noted that logistic regression analysis revealed that only graft type (left vs right lobe) was significantly related to the occurrence of biliary complications (odds ratio 0.11; P = 0.0012).[21 ]However, there were no significant differences regarding the cumulative overall graft survival rates between the recipients with left lobe grafts and those with right lobe grafts.
In selected patients for whom no allograft is immediately available, consider support with a bioartificial liver. This is a short-term measure that only leads to survival if the liver spontaneously recovers or is replaced.
In the future, hepatocyte transplantation, which has shown dramatic results in animal models of acute liver failure, may provide long-term support, but it remains investigational.
Artificial liver support systems
Artificial liver support systems can be divided into 2 major categories: biologic (bioartificial) and nonbiologic.The bioartificial liver is composed of a dialysis cartridge with mammalian or porcine hepatocytes filling the extracapillary spaces. These devices have undergone controlled trials. One multicenter trial reported improved short-term survival for a subgroup of patients with acute liver failure who were treated with a porcine hepatocyte-based artificial liver.[25 ]
Nonbiologic extracorporeal liver support systems, such as hemodialysis, hemofiltration, charcoal hemoperfusion, plasmapheresis, and exchange transfusions, have been used; however, no controlled study has shown long-term benefit.
These modalities permit temporary liver support until a suitable donor liver is found. Although extracorporeal hemoperfusion of charcoal and other inert substances provide some measure of excretory function, no synthetic capacity is provided.
Among the liver support systems currently available, albumin dialysis using the molecular adsorbent recirculating system (MARS) is the one that has been most extensively investigated. In this device, blood is dialyzed across an albumin-impregnated membrane against 20% albumin. Charcoal and anion exchange resins columns in the circuit cleanse and regenerate the albumin dialysate.
Clinical studies have shown that it improves hyperbilirubinemia and encephalopathy.
Two other systems based on the removal of albumin bound toxins, the Prometheus, using the principle of fractionated plasma separation and adsorption (FPSA), and the single pass albumin dialysis (SPAD), are also undergoing clinical studies for acute liver failure.Currently available liver support systems are not routinely recommended outside of clinical trials.
Diet
Patients with acute liver failure are, by necessity, nothing by mouth (NPO). They may require large amounts of IV glucose to avoid hypoglycemia.
When enteral feeding via a feeding tube is not feasible (eg, as in a patient with paralytic ileus), institute total parenteral nutrition (TPN). (See also Nutritional Requirements of Adults Before Transplantation and Nutritional Requirements of Children Prior to Transplantation).
Restricting protein (amino acids) to 0.6 g/kg body weight per day was previously routine in the setting of hepatic encephalopathy, but this may not be necessary.
Activity
Bedrest is recommended.Medication
Multiple medications may be necessary in patients with acute liver failure because of the wide variety of complications that may develop from fulminant hepatic failure. Decreased hepatic metabolism and the potential for hepatotoxicity become central issues. Antidotes that effectively bind or eliminate A phalloides toxin and toxic metabolites of acetaminophen are essential.
Acetaminophen ingestion of more than 10 g may be hepatotoxic due to formation of a highly reactive toxic intermediate metabolite, which is ordinarily metabolized further in the presence of glutathione to N -acetyl-p-aminophenol-mercaptopurine. Administering NAC permits restitution of intrahepatic glutathione. NAC is most effective when administered within 12-20 hours following acetaminophen overdose. Never administer aminoglycosides and NSAIDs, because the potential for nephrotoxicity is exaggerated greatly in this setting.
Complications
Hepatic encephalopathyManage hepatic encephalopathy in the conventional way, by providing lactulose and avoiding sedatives. In the late stages of encephalopathy, avoid providing lactulose by mouth or nasogastric tube without previous intubation, considering the risk of aspiration.Hepatic encephalopathy is not truly a complication because it is required for the diagnosis of fulminant hepatic failure, but evolution to higher stages of hepatic encephalopathy may result in patients losing their abilities to maintain their airways.
Cerebral edema
The occurrence of cerebral edema and ICH in patients with acute liver failure is related to the severity of encephalopathy. Cerebral edema is seldom observed in patients with grades I-II encephalopathy. The risk of edema increases to 25-35% with progression to grade III and to 65-75% (or more) in patients reaching grade IV coma.Patients in the advanced stages of encephalopathy require close follow-up care. Monitoring and management of hemodynamic and renal parameters, as well as glucose, electrolytes, and acid/base status, become critical. Frequent neurologic evaluation for signs of elevated ICP should be conducted.
ICP monitoring
ICP monitoring helps in the early recognition of cerebral edema. The clinical signs of elevated ICP, including hypertension, bradycardia, and irregular respirations (Cushing triad), are not uniformly present; these and other neurologic changes, such as papillary dilatation or signs of decerebration, are typically evident only late in the course.
CT scanning of the brain does not reliably demonstrate evidence of edema, especially in the early stages. A primary purpose of ICP monitoring is to detect elevations in ICP and reductions in CPP, so that interventions can be made to prevent herniation while preserving brain perfusion.
The ultimate goal of such measures is to maintain neurologic integrity and to prolong survival while awaiting receipt of a donor organ or recovery of sufficient functioning hepatocyte mass.
Additionally, refractory ICH and/or decreased CPP is considered a contraindication to liver transplantation in many centers.
In patients with grade III or IV encephalopathy, consider placement of ICP monitors.
Correct coagulopathy and bleeding tendencies with the use of FFP and platelet infusion.If an ICP monitor is placed, ICP should be maintained below 20-25 mm Hg, if possible, with CPP maintained above 50-60 mm Hg. Support of systemic blood pressure may be required to maintain adequate CPP.
ICH is managed initially by the use of mannitol. Osmotic diuresis with IV mannitol is effective in the short term in decreasing cerebral edema. Administration of IV mannitol (in a bolus dose of 0.5-1 g/kg or 50-100 g) is recommended to treat ICH in acute liver failure. The dose may be repeated once or twice, as needed, provided serum osmolality has not exceeded 320 mOsm/L. Volume overload is a risk with mannitol use in patients with renal impairment and may necessitate the use of dialysis to remove excess fluid.
Other therapies used to decrease ICH but not routinely recommended may be considered in refractory ICH.
A controlled trial of administration of 30% hypertonic saline, 5-20 mL/h, to maintain serum sodium levels of 145-155 mmol/L in patients with acute liver failure and severe encephalopathy suggested that induction and maintenance of hypernatremia may be used to prevent the rise in ICP values.
Barbiturate agents (thiopental or pentobarbital) may also be considered when severe ICH does not respond to other measures; administration has been shown to effectively decrease ICP. Significant systemic hypotension frequently limits their use and may necessitate additional measures to maintain adequate mean arterial pressure.Thiopental 5-10 mg/kg loading dose followed by 3-5 mg/kg IV infusion.Pentobarbital 3-5 mg/kg IV loading dose followed by 1-3 mg/kg/h infusion.
Seizures, which may be seen as a manifestation of the process that leads to hepatic coma and ICH, should be controlled with phenytoin. The use of any sedative is discouraged in light of its effects on the evaluation of mental status. Only minimal doses of benzodiazepines should be used given their delayed clearance by the failing liver. Seizure activity may acutely elevate and may also cause cerebral hypoxia and, thus, contribute to cerebral edema.
Hemorrhage
This develops as a result of the profoundly impaired coagulation that manifests in these patients.Correct coagulopathy, as earlier outlined.
The transfusion requirements for coagulation products (FFP, platelets) may be enormous. Multiple transfusions with packed red blood cells may be needed.
Gastrointestinal bleeding may develop from esophageal, gastric, or ectopic varices as a result of portal hypertension. Portal hypertensive gastropathy and stress gastritis may also develop.
Any minor trauma may result in extensive percutaneous bleeding or internal hemorrhage.
Consider retroperitoneal hemorrhage if large transfusion requirements are not matched by an obvious blood loss.
Infection prophylaxis and treatment
Periodic surveillance cultures should be performed to detect bacterial and fungal infections.
Empiric broad-spectrum antibiotics and antifungals should be given in the following circumstances:
Progressive encephalopathy (All patients listed for transplantation start antibiotics.)
Signs of systemic inflammatory response syndrome (SIRS) (temperature, >38ºC or <36ºC; white blood cell [WBC] count, >12,000/μL or <4000/μL; pulse rate, >90 bpm)
Persistent hypotension
Zosyn and fluconazole should be the initial choice. In hospital-acquired IV catheter infections, consider vancomycin.
Renal electrolyte and acid-base imbalances
Acute renal failure is a frequent complication in patients with acute liver failure and may be due to dehydration, hepatorenal syndrome, or acute tubular necrosis.Maintain adequate blood pressure, avoid nephrotoxic medications and NSAIDs, and promptly treat infections.
When dialysis is needed, continuous (ie, continuous venovenous hemodialysis [CVVHD]) rather than intermittent renal replacement therapy is preferred.
Metabolic concerns
Alkalosis and acidosis occur; identify and treat the underlying cause.Base deficits can be corrected by THAM solution (tromethamine injection), which prevents a rise in carbon dioxide, osmolality, and serum sodium.
Severe hypoglycemia occurs in approximately 40% of patients with fulminant hepatic failure. Although hypoglycemia occurs more frequently in children, it needs to be monitored in adult patients as well.
Blood sugars should be maintained in the range of 60-200 mg/dL with the infusion. Use 10% dextrose solution and glucose monitoring.
Phosphate, magnesium, and potassium levels are low and require frequent supplementation.
Prognosis
Prognosis is highly dependent on the inciting cause of fulminant hepatic failure. Prognostic indices have been developed to identify patients who require liver transplantation. The development of complications is the other factor that largely determines survival.Viral hepatitis
Approximately 50-60% of patients with fulminant hepatic failure due to HAV infection survive.
These patients account for a substantial proportion (10-20%) of the pediatric liver transplants in some countries, despite the relatively mild infection observed in many children infected with HAV.
The outcome for patients with fulminant hepatic failure as the result of other causes of viral hepatitis is much less favorable.
Acetaminophen toxicity
Fulminant hepatic failure due to acetaminophen toxicity generally has a relatively favorable outcome, and prognostic variables permit reasonable accuracy in determining the need for OLT.Patients presenting with deep coma (hepatic encephalopathy grades 3-4) have increased mortality when compared with those with milder encephalopathy.
An arterial pH of less than 7.3 and either a PT greater than 100 seconds or serum creatinine greater than 300 mcg/mL (3.4 mg/dL) are independent predictors of a poor prognosis.
Non–acetaminophen-induced fulminant hepatic failure
A PT greater than 100 seconds and any 3 of the following 5 criteria are independent predictors[10 ]:(1) age younger than 10 years or older than 40 years; (2) fulminant hepatic failure due to non-A, non-B, non-C hepatitis; halothane hepatitis; or idiosyncratic drug reactions;
(3) jaundice present longer than 1 week before onset of encephalopathy;
(4) PT greater than 50 seconds; or
(5) serum bilirubin greater than 300 mmol/L (17.5 mg/dL).Once these patients are identified, arrange appropriate preparations for OLT.
The above criteria, developed at King's College Hospital in London, have been validated in other centers; however, significant variability occurs in the patient populations encountered at any center, and this heterogeneity may preclude widespread applicability.
Other prognosticating tests have been proposed. Reduced levels of Gc-globulin (a molecule that binds actin) have been reported in fulminant hepatic failure, and a persistently increasing PT portends death. These and other parameters have not been widely validated yet.
Wilson disease: Wilson disease presenting as fulminant hepatic failure is almost uniformly fatal without OLT.
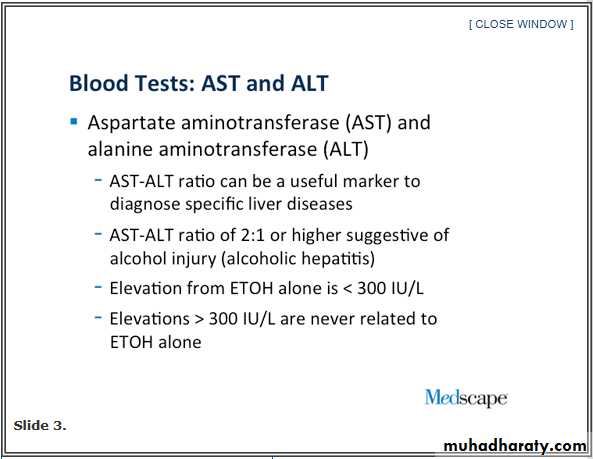
We are always taught that there is a disproportion in patients that have alcohol [injury]. The AST goes up, and the ALT lags behind. There is a 2:1 ratio that we are all taught and is somewhat strongly suggestive of alcohol injury. Why is that? It is because of where the enzymes live in the cell. For alcohol injury, it is a subcellular enzyme. It does not necessarily require the whole cell to be killed to have this released; but, the ALT is a cytosolic component to the enzyme, so you really have to kill the whole cell to get the ALT released.
The injury from alcohol does not necessarily have to kill the whole cell, so the AST goes up, and the ALT lags behind, in particular, unless the whole cell is killed. That can happen with alcohol, too.
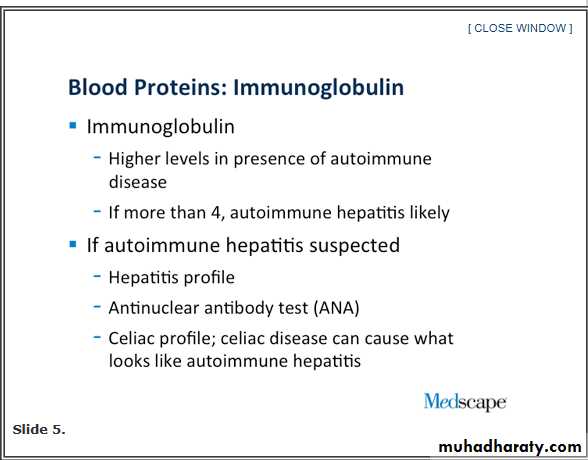
I always look at the immunoglobulin fraction. This is helpful because it may pinpoint you very quickly to a patient with autoimmune hepatitis. The immunoglobulin fraction is something you can easily calculate; it is simple subtraction. You start with the total protein and then subtract the albumin. It should be less than 4. If it is less than 4, autoimmune hepatitis is less likely, if it is over 4, you are starting to think very quickly about autoimmune hepatitis.
We are seeing an incredible number of people with celiac disease, and their only manifestation, as it was in this case, potentially, was that they just had their blood drawn and their liver tests are up. Celiac disease can cause what looks like an autoimmune-type hepatitis pattern. It is something that we are seeing more and more. It has responded nicely to control of celiac disease. I would consider a celiac profile, maybe not on the first pass of this patient, but, if there is persistent elevation, I would consider it on the second pass.
you should consider Wilson's disease. It is something that we used to tell people, you never screen them over 40 .We do see that Wilson's disease will typically present earlier in life with liver disease. In patients younger than 25-30 years, Wilson's disease may present with hemolysis and liver test abnormalities. Wilson's disease can cause fulminant hepatic failure. Older patients, that is, older than 35 years or so, may present more with neurologic sequelae. We have seen Wilson's disease reported now in patients in their seventh decade of life. A ceruloplasmin is an easy one to get, and I never fault the residents for ordering a ceruloplasmin even on patient like this.
By the Numbers: Liver Disease and Patient Age
What else do I do as far as looking at these people? I think about different ages.
If a young patient comes in (the patient in their 20s, or sub 20s), you think about things like Wilson's disease and autoimmune hepatitis. That would be something that you really could not afford to miss.
When [patients] get into their 20s and 30s, you start to think about things like hemochromatosis, Wilson's disease continues, and also viral hepatitis.
For patients in the 30s, 40s, and early 50s, you start to worry about things like primary biliary cirrhosis, especially in women. The tip here is that these patients will typically have pruritus when they come in. It is very common to see pruritus, even though they are not even quite jaundice.
Interestingly, intrahepatic cholestasis, meaning not obstructive biliary disease, is very likely to cause pruritus early as opposed to a patient who comes in and is profoundly jaundice from a malignancy or something.
Those patients get pruritus (itching) later in the stages of their disease, as opposed to [patients with] primary biliary cirrhosis who may have [pruritus] very early in their presentation.
So for patients in the 30s, 40s, and 50s, I am also thinking about autoimmune hepatitis. Alpha-1 antitrypsin deficiency is one we had not mentioned yet, but it is simple enough to look for. It typically does not present as liver disease until in the fourth, maybe fourth to fifth, decade, so it is later in presentation. A simple test here is a serum protein electrophoresis, looking at the Alpha-1 level, in particular. If you see any abnormalities, then go straight to an Alpha-1 level, it is a lot more expensive to order that test up front, so I do not typically do that. I order a serum protein electrophoresis.
For patients in the 50s, 60s, and 70s, you start to always think about bad things, such as infiltrative diseases and metastatic diseases. There is second peak for autoimmune hepatitis in the 50s and 60s. Think again about primary biliary cirrhosis. Think about other things, particularly drug-induced abnormalities, as these patients are exposed to more drugs as they age.
One thing that we start to see in the older patient population too is, as they may have a component of congestive failure, we may see some elevation there; this more or less creates an ischemic pattern of injury, and, in that patient, I may also look at hepatic portal venous flow and do hepatic duplex, looking at their right heart influence on the hepatic flow.
An Interferon-Free Regimen for Chronic Hepatitis C Virus Infection
Combining a protease inhibitor and a polymerase inhibitor led to declines in viral load, even among patients with previous treatment failure.In 2011, protease inhibitors will become available for the treatment of chronic hepatitis C virus (HCV) infection. Previous studies have shown that adding one of these agents to peginterferon plus ribavirin improves the rate of sustained virologic response (SVR) but also increases the likelihood of adverse effects and withdrawal (JW Gastroenterol Apr 7 2010 and JW Gastroenterol Aug 20 2010).
Now, in the industry-funded INFORM-1 trial, researchers have evaluated the safety, tolerability, and antiviral activity of an interferon-free regimen consisting of two new oral anti-HCV agents: danoprevir (an NS3/4A protease inhibitor) and RG7128 (a nucleoside polymerase inhibitor).
Patients with HCV genotype 1 infection (some treatment naive, some with previous treatment failure) were randomized to receive placebo or a combination of danoprevir (100 mg or 200 mg every 8 hours or 600 mg or 900 mg twice daily) plus RG7128 (500 mg or 1000 mg twice daily) for 13 days. A total of 73 patients received the combination treatment, and 14 received placebo. The mean baseline viral load was 6.4 log10 IU/mL.
By the end of treatment, the median change in viral load for the various dosing groups ranged from –3.7 to –5.2 log10 IU/mL, with 18 patients achieving undetectable viral loads.
At the highest dosing combination studied (1000 mg of RG7128 twice daily and 900 mg of danoprevir twice daily), the median decline in viral load was similar between treatment-naive patients and those with previous treatment failure (–5.2 and –4.9 log10 IU/mL, respectively). Overall, the regimen was well tolerated, with no safety issues or resistance concerns identified.
Comment: This proof-of-concept study demonstrates the short-term benefits of combining a polymerase inhibitor and a protease inhibitor for the treatment of HCV. Significant declines in viral load were seen at 14 days, even among patients with previous treatment failure, and some patients achieved undetectable viral loads. Longer studies are needed to determine whether interferon-free regimens such as this one will achieve SVR and whether resistance will be an issue. In the meantime, this study gives hope that HCV might eventually be treatable with a well-tolerated, oral, interferon-free regimen.
Published in Journal Watch Gastroenterology November 19, 2010
In cirrhotic patients, esophageal variceal bleeds are common, with a mortality rate for first bleed reaching 50%. Endoscopic injection sclerotherapy is a well-established method in the management of acute bleeding from esophageal varices; however, it is not recommended for prophylaxis of the first episode of variceal hemorrhage. Currently, endoscopic variceal ligation has replaced endoscopic injection sclerotherapy as the endoscopic treatment of choice of bleeding esophageal varices.
Beta-Blockers Alone May Be Preferred to Prevent First Variceal Bleeding
MedscapeCME 06/18/2010;
In addition, nonselective beta-blockers have been well documented in reducing the risk for variceal bleed.
Thus, both nadolol and ligation have proven to be effective in the prophylaxis of first variceal bleeding. It has been suggested that combining the 2 approaches may enhance their effectiveness; however, the results of this combination are still unknown. This aim of this study was to evaluate the effects and safety of combining nadolol with ligation in the primary prophylaxis of variceal bleeding.
Cirrhotic patients with high-risk esophageal varices but without a bleeding history were considered for enrollment.
140 eligible patients were randomly assigned to receive band ligation plus nadolol (combined group, 70 patients) or nadolol alone (nadolol group, 70 patients).
The severity of liver disease of each patient was assessed at the time of presentation according to Pugh's modification of Child's classification.
Patients received regular ligation treatment at an interval of 4 weeks until all varices were obliterated or were too small to be ligated.
Nadolol was administered at a dose to reduce 25% of the pulse rate in both the combined group and the nadolol group.
Both groups were comparable in baseline data.
In the combined group, 50 patients (71%) achieved variceal obliteration.
The mean dose of nadolol was 52 ± 16 mg in the combined group and 56 ± 19 mg in the nadolol group.
During a median follow-up of 26 months, 18 patients (26%) in the combined group and 13 patients (18%) in the nadolol group experienced upper gastrointestinal tract bleeding (P = .42).
Esophageal variceal bleeding occurred in 10 patients (14%) in the combined group and 9 patients (13%) in the nadolol group (P = .60).
No relationship existed between esophageal variceal bleeding and Child-Pugh class or the model for end-stage liver disease (MELD) score in both the treatment groups.
Multivariate analysis revealed that only bilirubin (odds ratio [OR], 1.28; 95% confidence interval [CI], 1.08 - 1.52; P < .005) was the factor predictive of first rebleeding.
Adverse events were noted in 48 patients (68%) in the combined group and 28 patients (40%) in the nadolol group (P = .06).
16 patients in each group died. The most common cause of death was hepatic failure, followed by sepsis.
The cause of death ascribed to variceal bleeding occurred in 1 patient in the combined group and 2 patients in the nadolol group.
Multivariate analysis revealed that ascites and encephalopathy were predictive factors of mortality.
Additionally, patients with a higher baseline MELD score at enrollment in the nadolol group had a higher mortality rate.
Clinical Implications
Both nadolol and ligation have been proven to be effective in the prophylaxis of first variceal bleeding.This study demonstrated that the addition of ligation to nadolol may increase adverse events and did not enhance the effectiveness in the prophylaxis of first variceal bleeding.
• Drugs that can cause jaundice:
• Aldomet• Aminosalicylate sodium
• Chemotherapy drugs:
• The administration of medicines that kill cancer cells.
• Erythromycin
• Flucytosine
• Oral contraceptives
• Oral diabetes medicines (e.g. tolbutamide, chlorpropamide)
• Phenothiazines:
• Chlorpromazine (Thorazine)
• Fluphenazine (Prolixin)
• Trifluoperazine (Stelazine)
• Perphenazine (Trilafon)
• Thioridazine (Mellaril)
• Prochlorperazine (Compazine)
Propylthiouracil
Rifampin
Steroids:
Prednisone
Medrol
Sulfa drugs:
Bactrim
Septra
Testosterone
Tiopronin
Treatment for hepatitis B
BMJ 5 January 2010
Hepatitis B virus is estimated to have infected 350 million individuals globally, accounting for over
500 000 deaths each year. An effective and widely available vaccine provides protection from infection, but treatment is rarely curative. Recent developments in antiviral treatment have brought the opportunity for greatly improved management of those chronically infected with hepatitis B virus, and for patients infected both with HIV and hepatitis B virus there is now the potential to treat both viruses with a simplified combination of drugs.
The screening test for hepatitis B is the presence in blood of hepatitis B surface antigen.
Chronic hepatitis B infection is defined as persistence of hepatitis B surface antigen for
more than six months.
In patients without evidence of hepatitis B surface antigen where it is important to know whether there has been previous infection—for example, in those about to receive chemotherapy—previous exposure can be assessed by presence of hepatitis B core antibody.
• Investigations under specialist careInvestigations related to hepatitis B
• Surface antigen (HBsAg), hepatitis Be antigen (HBeAg), anti-HBe, anti-HBs, anti-HBcore• Quantitative hepatitis B virus DNA
• Hepatitis B virus genotype (for those considered for interferon)
• Delta virus serology
• General
• Full blood count
• Bilirubin
• Liver enzymes
• Clotting
Ferritin
Lipid profile
Autoantibody screen
Caeruloplasmin
Tests for hepatitis C virus and HIV
Screening for liver cancer
Ultrasonography
fetoprotein
Staging of disease
Fibroelastography
Liver biopsy
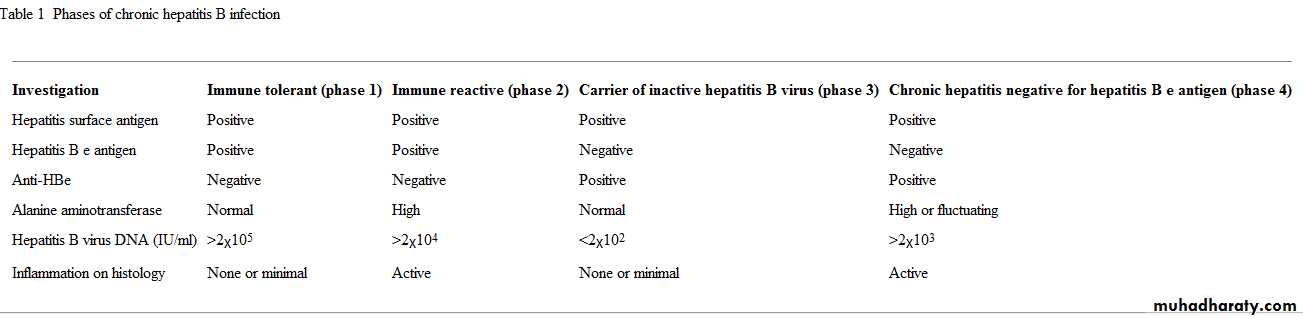
• The distinction between hepatitis B viral activity and liver disease related to hepatitis B virus is important and sometimes the source of confusion. Individuals infected in childhood (which is most of those infected worldwide) often experience a period of immunotolerance during which the virus is highly replicative but minimal liver damage occurs.The infection will eventually progress to a hepatitic phase associated with inflammation and liver fibrosis.
Spontaneous resolution of the hepatitic phase may occur with loss of hepatitis B e antigen (HBeAg) and appearance of anti-HBe. This results in an "asymptomatic" phase with normal liver functions tests and low viral load (<2x103 IU/ml). Many adult patients with chronic hepatitis B virus infection in European settings will present in this phase of disease. In the past they may have been falsely reassured and discharged. It is now clear that viral reactivation will occur in 15-20% of this group of patients, causing HBeAg negative hepatitis
The role of liver biopsy
Liver biopsy continues to play an important role in determining who should start treatment. The information it can provide on the aetiology and severity of liver disease cannot easily be substituted by non-invasive tests. Different histological grading systems are used in different centres. The most commonly used are the Ishak and Metavir scores, but all systems provide some measure of inflammation (current activity) and fibrosis (morechronic scarring).Although in the past physicians and patients have expressed concerns about the safety of liver biopsy, developments in technology, particularly ultrasonography, mean that complications from the procedure are now rare when the procedure is carried out regularly.15 Newer, non-invasive imaging methods, particularly the FibroScan technique,16 are gaining favour in routine practice, though validation data are still needed in different settings.
The hope has been that non-invasive biomarkers of liver fibrosis, including proprietary tests such as ELF (enhanced liver fibrosis), FibroTest, and others,17 can substitute for biopsy, which at best is unpleasant. However, the imperfect performance of non-invasive markers in clinical practice means they have yet to be widely adopted and further improvements are a priority for research.
What is the goal of treatment?
The goal of treatment for individuals with chronic hepatitis B virus infection is to prevent morbidity and mortality related to the disease. This can be achieved with a finite course of interferon therapy or long term viral suppression with nucleoside/nucleotide analogues.7 There are recommended surrogate end points for treatment.13 Loss of hepatitis B surface antigen may be achieved, and this can be regarded as remission of the infection.In patients positive for HBeAg, seroconversion to become negative for HBeAg or positive for anti-HBe is associated with sustained suppression of hepatitis B virus DNA after treatment withdrawal and can be considered an end point of therapy. However, cohort studies have identified the importance of high viral load in determining disease progression. The availability of newer treatments means that increasing emphasis is placed on the need to suppress viral load to below the limit of detectable DNA (usually below 10-15 IU/ml, depending on the assay), with long term therapy.
Who should be treated and with what?
A systematic review of numerous clinical trials of interferon therapy in chronic hepatitis B infection found that for a subgroup of patients a finite course of treatment with pegylated interferon could be given without virological resistance and with a lasting response. Several factors have been associated with a good response to interferon . However, even with stringent patient selection, response rates are in the region of 30% at one year for patients positive for HBeAg and 15% for patients negative for HBeAg. Associated side effects mean that pegylated interferon is not often used in clinical practice.Characteristics associated with a good response to interferon among patients with chronic hepatitis B virus infectionAlanine aminotransferase more than twice the upper limit of normal
Hepatitis B virus DNA <106 IU/ml
Age <50 years
Hepatitis B virus genotype A or B
Female
Non-vertical transmission
• The development of newer, potent, oral antivirals with a favourable toxicity profile has made long term suppression of hepatitis B virus replication feasible, with sustained viral suppression response rates that are cumulatively better than interferon treatment.
Long term lamivudine monotherapy has been shown to improve clinical outcomes, particularly in those with late stage disease, and to prevent progression to cirrhosis or liver cancer.
Adefovir, sometimes used in combination with lamivudine, has also shown some benefit, but its use is limited by its slow action, lack of potency, and potential for renal toxicity.
The best choice of antiviral is contentious. Although lamivudine is cheap and well tolerated, the rate at which viral resistance emerges is unacceptably high. Newer agents that can provide improved viral suppression with lower rates of emerging resistance and less toxicity have recently been licensed and reviewed by the National Institute for Health and Clinical Excellence (NICE): entecavir25 and tenofovir26 were both recommended as options for the treatment of individuals with chronic hepatitis B (both HBeAg positive and HBeAg negative), but telbivudine was not.27 Although most specialists would now use tenofovir or entecavir as first line treatment, some still prefer combination treatments in those at high risk of resistance.
• Side effects of treatment
• Of drugs active against hepatitis B virus, the greatest clinical experience is with lamivudine, used widely in the treatment of patients with hepatitis B virus or HIV, or with both. The side effect profile of lamivudine is good, with very few patients experiencing problems; its use is decreasing, however, primarily because of concerns about the emergence of resistant virus.28 Tenofovir is being used increasingly in HIV patients, and in these patients renal toxicity and the metabolic consequences of urinary phosphate loss have been• observed . Experience is more limited in those infected with hepatitis B virus, and renal toxicity has not been described.
What are the risks of poor adherence to treatment?
Two major risks come from poor adherence to treatment. Firstly, stopping suppressive therapy for hepatitis B virus can lead to a flare-up of liver disease related to the virus. This can be fatal, although more commonly in patients with advanced cirrhosis.30 Secondly, there is a risk of developing resistance. This can lead to a flare-up of disease and to the development of accumulation of resistance mutations, some of which may compromise alternative treatment options.Special situations
Acute hepatitisThe differentiation between acute hepatitis B, a flare-up of chronic hepatitis B, and hepatitis resulting from other viruses can sometimes be difficult as IgM against the core antigen can be positive in all settings. Of patients with acute hepatitis B virus, 95-99% will develop antibody to hepatitis B surface antigen and recover spontaneously. Some evidence exists that antivirals can be beneficial in the acute setting when there is derangement of coagulation (international normalised ratio >1.5), but no randomised trials have been conducted to help understand this better.• Coinfection of hepatitis B virus and HIVHepatitis B virus and HIV have an important relation, not only because of their overlapping transmission risks but also because of the clinically important interactions of the two diseases.. For HIV patients, who are often also infected with hepatitis B virus, both viruses can be treated with a simplified combination of drugs (usually tenofovir and emtricitabine). However, patients with hepatitis B virus who are starting antiviral therapy must be offered HIV testing as monotherapy or even dual therapy can rapidly lead to the emergence of HIV resistance mutation, thereby limiting the treatment options.
PregnancyThe universal prevention of transmission of hepatitis B virus from mother to child during the perinatal period remains an achievable goal. Testing of mothers for hepatitis B surface antigen allows those with a positive result to get hepatitis B immune globulin and early vaccination for their children, a strategy that has been proved to reduce transmission by 95-99%.
The advent of low toxicity antiviral treatment might help in this regard too, with emerging evidence that addition of antivirals in the third trimester, particularly for those with high viral loads, might further reduce infant infection.
Risk of reactivation of hepatitis with immunosuppressionIndividuals positive for hepatitis B surface antigen are at risk of a flare-up in disease if given immunosuppressants or cytotoxic chemotherapy.33 In addition to routine screening for hepatitis B surface antigen, it is now recommended that exposure to hepatitis B virus should also be assessed using anti-HBcore antibody as even patients who are negative for the surface antigen are at risk of reactivation and should be given prophylactic treatment. Guidelines recommend that patients positive for hepatitis B surface antigen should receive antiviral therapy for the duration of the immunosuppressive period and for 12 months thereafter.
Guidelines recommend that patients positive for hepatitis B surface antigen should receive antiviral therapy for the duration of the immunosuppressive period and for 12 months thereafter.
Investigation
Immune tolerant (phase 1)
Immune reactive (phase 2)
Carrier of inactive hepatitis B virus (phase 3)
Chronic hepatitis negative for hepatitis B e antigen (phase 4)
Hepatitis surface antigen
Positive
Positive
Positive
Positive
Hepatitis B e antigen
Positive
Positive
Negative
Negative
Anti-HBe
Negative
Negative
Positive
Positive
Alanine aminotransferase
Normal
High
Normal
High or fluctuating
Hepatitis B virus DNA (IU/ml)
>2x105
>2x104
<2x102
>2x103
Inflammation on histology
None or minimal
Active
None or minimal
Active
Phases of chronic hepatitis B infection
A global perspective and future developmentsThe coming years will bring important changes in our approach to hepatitis B virus. With a growing range of treatments available, the rationale for active case finding and screening will become stronger and needs to be informed by better evidence. How new treatments are best deployed will need to be explored through trials of different drug combinations and treatment strategies. With the existing tools for treatment of hepatitis B virus, the time is right for a global initiative to deliver the treatment to those most in need.34 This initiative will be debated at the World Health Assembly in early 2010, and it has to be hoped that advances in treatment that have been achieved in recent years can be implemented beyond wealthy countries.
Questions for future researchWho should be screened for hepatitis B virus?
Which patients will not get complications and could avoid treatment?How do we best monitor patients before and after starting treatment?
What is the most efficient way to eradicate hepatitis B virus infection?
How do we best use the new antivirals available for treatment?
How do we get treatments for hepatitis B virus to resource-poor settings?
Tips for non-specialistsConsider hepatitis B in anyone born in a country with high prevalence
A positive hepatitis B surface antigen result requires either investigation or referral
A flare-up of hepatitis requires detailed assessment to establish the cause
All patients with hepatitis B require HIV testing (and vice versa)
Myth of γ glutamyltransferase
Cobbold and colleagues state that γ-glutamyltransferase should be measured in all patients with raised serum alanine aminotransferase because if raised it would indicate alcohol related liver disease.Because of its ubiquitous distribution within the liver, γ glutamyltransferase is raised in all types of liver disease. In the absence of serious liver disease, the myth that a raised γ glutamyltransferase is sensitive and specific for alcohol excess persists. In these circumstances, for example, γ glutamyltransferase is raised in only 52% of alcoholic patients, but it is also raised in 50% of patients with non-alcoholic fatty liver disease.
Guidelines recommend that it is measured only to identify the likely origin of an isolated raised alkaline phosphatase, because if raised it indicates a hepatic rather than bony origin.
We therefore suggest that measuring γ glutamyltransferase when investigating raised serum transaminases is unnecessary and potentially misleading.
• Body fat and increased risk of cirrhosis
• Overweight (defined for European populations as a body mass index (BMI) of between 25 and <30) and obesity (defined as BMI > 30) have become a considerable threat to public health worldwide. Established consequences of obesity include cardiovascular disease, diabetes, musculoskeletal problems, gallstones, and various cancers. In the linked studies, Liu and colleagues assess the link between BMI, alcohol intake, and liver disease.BMJ 11 March 2010
Liu and colleagues found that the relative risk of liver cirrhosis increased by 28% for every 5 unit increase in BMI above 22.5 in each stratum of alcohol consumption. Women were recruited between 1996 and 2001 at a mean age of 56 years from NHS breast screening centres in the United Kingdom, and they self reported height and weight (with measured data available for a subgroup).
A key question is what proportion of cirrhosis can be attributed to modifiable risk factors? In tackling this question, the investigators concluded that during the study period 42% of all hospital admissions or deaths from liver cirrhosis could be attributed to alcohol consumption and 17% were caused by excess body weight.
Interestingly, the combination of obesity and an alcohol consumption of 150 g (about 18 units) or more each week was associated with a marked increased risk of cirrhosis (about fivefold) compared with that seen in obese women who drank less than 70 g of alcohol a week. The important question of whether alcohol and excess body fat have a simple additive adverse effect on the liver, or whether these two factors exert a synergistic effect to produce liver disease, is dealt with in the second of the two linked papers. Hart and colleagues analysed data from the Midspan prospective cohort studies of 9559 men in Scotland and investigated the relation between baseline BMI and self reported alcohol consumption at baseline on liver related morbidity and mortality after a median of 29 years (maximum 42 years).
Both BMI and alcohol consumption were strongly and independently associated with mortality from liver disease, as shown by Liu and colleagues. Hart and colleagues also show that being overweight or obese and drinking 15 units of alcohol or more each week has a synergistic effect, which amplifies the insult to the liver and greatly increases the risk of liver related morbidity and mortality.
The association between increased BMI and cirrhosis can be explained by the biochemical and physiological consequences of obesity on the liver. Twenty years ago researchers began to appreciate the importance of insulin resistance in relation to cardiometabolic risk in what was first termed "syndrome X," but is now known as the metabolic syndrome—a cluster of risk factors associated with central obesity.
The presence of "ectopic" fat is thought to represent a key component of the metabolic syndrome with the accumulation of ectopic fat in the liver resulting in non-alcoholic fatty liver disease. This disease represents a spectrum of associated liver conditions, from simple steatosis to end stage cirrhosis and hepatocellular carcinoma. In as many as 40% of people with non-alcoholic fatty liver disease, the condition progresses over time to non-alcoholic steatohepatitis, with the development of additional hepatic inflammation. Non-alcoholic steatohepatitis may progress to advanced fibrosis and cirrhosis and carries an increased risk of hepatocellular carcinoma.
Progressive fibrotic scarring results in a loss of hepatic parenchymal cells and thereby loss of fat laden liver cells, removing the clue that non-alcoholic fatty liver disease originally caused the liver fibrosis and cirrhosis.
BMI is a good proxy measure of central obesity and visceral body fat in epidemiological studies. Increased BMI in both the Million Women Study and the Midspan studies probably reflects the presence of central obesity and non-alcoholic fatty liver disease at the time of recruitment to the study.
What does this association mean for clinicians? Establishing a diagnosis of non-alcoholic fatty liver disease is difficult. Simple liver function tests, such as the measurement of serum alanine transaminase (ALT), have poor sensitivity and specificity as diagnostic tests. In addition, although liver ultrasound can detect liver fat, it cannot detect inflammation or early fibrosis. Non-alcoholic steatohepatitis can be identified only by liver biopsy—no other validated tests are available.
Simpler, inexpensive, less invasive tests are needed to identify people with hepatic steatosis who are at risk of inflammation and progressive liver disease.
Risk factors for progression of non-alcoholic fatty liver disease include increasing age, smoking, and obesity. The role of moderate alcohol consumption is unclear because by definition non-alcoholic fatty liver disease should be diagnosed only in people who drink less than 10 g of alcohol a day. It is hoped that simple algorithms based on these risk factors and blood tests can be developed to identify people with non-alcoholic steatohepatitis so that liver biopsy will no longer be necessary.10
The causes of non-alcoholic steatohepatitis are complex and many unanswered questions remain, not least what causes progression of liver disease from simple steatosis to non-alcoholic steatohepatitis. Future research must focus on developing an approach to diagnosing and treating non-alcoholic fatty liver disease. In the meantime, the old adage of "prevention is better than cure" remains pertinent to dealing with the problem of non-alcoholic fatty liver disease.
the old adage of "prevention is better than cure" remains pertinent to dealing with the problem of non-alcoholic fatty liver disease.
The increasing prevalence of obesity and alcohol consumption over time, together with the increasing prevalence of hepatitis C, are contributing to the increasing incidence and prevalence of liver disease. Data from the Scottish health survey for 2003 (SHS 2003) show that the prevalence of obesity in women over 50 years was 32% compared with 18% for participants in the Million Women Study (between 1996 and 2001).
Among men in SHS 2003, the prevalence of obesity was 24%, compared with 6% for Midspan participants (between 1965 and 1973). Even more worrying, the proportion of people who are obese and consume excess amounts of alcohol has increased over time. The proportion of men who were both obese and reported drinking more than 15 units of alcohol a week was 9.4% in SHS 2003, compared with 1.7% in Midspan.
Reducing alcohol consumption and obesity are, at present, our only weapons against non-viral liver disease. The progression of non-alcoholic fatty liver disease to end stage liver disease can now be added to the list of the undesirable consequences of modern lifestyles.
BMJ 2010;340
• Raised serum alanine aminotransferase values usually indicate hepatocellular damage. Aminotransferase values of less than five times the upper limit of normal are often considered mild, while those more than five times the upper limit of normal are severe, representing more extensive hepatocellular injury.
BMJ 30 July 2010
Mildly raised values do not exclude severe chronic liver disease; indeed evidence exists that substantial liver damage may be present even with relatively mild biochemical derangements.1 Therefore we recommend that even mild derangements of aminotransferase values that persist on retesting over a three month period should be investigated further.
If clinical features suggest a more pressing need for investigation (such as jaundice or raised bilirubin, deranged clotting, or hepatic decompensation) investigation should be expedited.
Non-alcoholic fatty liver diseaseNon-alcoholic fatty liver disease is best considered as the hepatic manifestation of the metabolic syndrome. The prevalence of non-alcoholic fatty liver disease is estimated to be 20-30% in Western populations. Of the patients with the disease who have raised aminotransferase levels, 43-55% have histological steatohepatitis, and it is these patients who are at greatest risk of progressing to cirrhosis.
With such a high proportion of the adult population having non-alcoholic fatty liver disease, the challenge facing clinicians both in primary and secondary care is to identify and target treatment at those at greatest risk of cirrhosis without the routine need for liver biopsy. Optimum management remains an area of active research but is probably best delivered as a partnership between general practice and hepatology services.
Associated cardiovascular risk factors (obesity, hypertension, dyslipidaemia, insulin resistance, and diabetes mellitus) should be sought and tackled as these may represent a greater risk to the patient than the liver disease. Currently, specialist hepatology referral for additional investigation and risk stratification using non-invasive biochemical or imaging modalities (and in selected cases liver biopsy) should be considered on a case by case basis.
In patients in whom the results of investigations are consistent with non-alcoholic fatty liver disease and aminotransferase values return persistently to within normal ranges on adoption of sustained lifestyle changes, onward referral may not be required.
The prevalence of non-alcoholic steatohepatitis with advanced fibrosis is higher among patients with a raised alanine aminotransferase value and insulin resistance.
The "NAFLD (non-alcoholic fatty liver disease) fibrosis score" based on readily available anthropometric and biochemical indices suggested an indeterminate risk for advanced fibrosis, and so a liver biopsy was performed that confirmed the presence of steatohepatitis with moderate fibrosis, indicating the patient to be of higher risk of future liver related morbidity.
• Long-Term Outcomes in Carriers of Inactive Hepatitis B Virus
• Such carriers are at elevated risk for hepatocellular carcinoma and cirrhosis.• Previous data from a prospective cohort study in Taiwan demonstrated an increased risk for hepatocellular carcinoma (HCC) and cirrhosis among hepatitis B virus (HBV)-infected patients with viral loads 10,000 copies/mL. Now, a report from the same study describes these risks among 1932 carriers of inactive HBV (i.e., nonreplicative HBV infection), compared with 18,137 controls.
By definition, the carriers of inactive HBV met the following criteria at study entry: an HBV DNA level <10,000 copies/mL, a normal serum alanine aminotransferase (ALT) level (<45 U/L), hepatitis B e antigen negativity, and hepatitis B surface antigen (HBsAg) positivity. The controls were all HBsAg negative. Mean follow-up time was 13 years. New HCC cases were identified through a national cancer registry, and liver-related deaths were ascertained through death certificates.
The annual incidence rate of HCC was low overall but significantly higher among carriers than controls (0.6% vs. 0.2%; adjusted hazard ratio, 4.6; 95% confidence interval, 2.5–8.3). A similar pattern was seen for liver-related death (0.04% vs. 0.02%; AHR, 2.1; 95% CI, 1.1–4.1). Among carriers, alcohol use and older age were independent risk factors for incident HCC.
Comment: Traditionally, HBV treatment is considered only for patients with baseline HBV DNA levels 10,000 copies/mL. However, these data suggest that patients with normal ALT levels and HBV DNA levels below this threshold also have an elevated risk for HCC and cirrhosis. One important limitation of this study is that it included only baseline HBV DNA levels, not levels during follow-up; thus, patients might not have remained in the inactive carrier state for the duration of the study. Regardless, these results suggest that, at a minimum, carriers of inactive HBV should be followed long term.
Published in Journal Watch Gastroenterology June 18, 2010
Lanreotide for Polycystic Liver Disease
Compared with placebo, lanreotide significantly reduced liver volume in patients with polycystic liver.Current therapy for patients with problematic liver cysts is radiographic-guided drainage or surgical resection. However, these techniques are invasive and less feasible for those with multiple cysts. Somatostatin analogues have been shown to reduce cyst volume in polycystic disease in humans and rodent models, likely by activating signaling cascades through inhibitory G protein, which suppresses cyclic adenosine monophosphate.
To assess the efficacy of a long-acting somatostatin analogue in this setting, investigators conducted an industry-supported, multicenter, randomized, double-blind, placebo-controlled trial involving 54 polycystic liver patients who were divided evenly to receive either lanreotide (120 mg) or placebo every 28 days for 24 weeks. A sample size of 19 patients per group was estimated to achieve a study power of 80%.
The mean liver volume decreased by 2.9% (from 4606 mL to 4471 mL) in the lanreotide group, compared with an increase of 1.6% (4689 mL to 4896 mL) in the placebo group (P<0.01). Lanreotide was well tolerated; the main adverse effect was diarrhea.
Comment: This well-designed study demonstrated that, compared with placebo, lanreotide significantly reduced liver volume in patients with polycystic liver and that lanreotide administration was relatively convenient and safe. However, these findings should be viewed as a "proof of principle." Before this therapy can be used in routine practice, further studies will have to demonstrate clinically relevant results, such as improved symptomatic outcomes, durability of effect, and cost-effectiveness.
Published in Journal Watch Gastroenterology January 8, 2010
Jaundice of pregnancy There are some additional causes of jaundice that are unique to pregnancy.Cholestasis of pregnancy. is an uncommon condition that occurs in pregnant women during the third trimester. The cholestasis is often accompanied by itching but infrequently causes jaundice. The itching can be severe, but there is treatment (ursodeoxycholic acid or ursodiol). Pregnant women with cholestasis usually do well although they may be at greater risk for developing gallstones. More importantly, there appears to be an increased risk to the fetus for developmental abnormalities.
Cholestasis of pregnancy is more common in certain groups, particularly in Scandinavia and Chile, and tends to occur with each additional pregnancy. There also is an association between cholestasis of pregnancy and cholestasis caused by oral estrogens, and it has been hypothesized that it is the increased estrogens during pregnancy that are responsible for the cholestasis of pregnancy.
Pre-eclampsia. is a disease that occurs during the second half of pregnancy and involves several systems within the body, including the liver. It may result in high blood pressure, fluid retention, and damage to the kidneys as well as anemia and reduced numbers of platelets due to destruction of red blood cells and platelets. It often causes problems for the fetus. Although the bilirubin level in the blood is elevated in pre-eclampsia, it usually is mildly elevated, and jaundice is uncommon. Treatment of pre-eclampsia usually involves delivery of the fetus as soon as possible if the fetus is mature.
Acute fatty liver of pregnancy. Acute fatty liver of pregnancy (AFLP) is a very serious complication of pregnancy of unclear cause that often is associated with pre-eclampsia. It occurs late in pregnancy and results in failure of the liver. It can almost always be reversed by immediate delivery of the fetus. There is an increased risk of infant death. Jaundice is common, but not always present in AFLP. Treatment usually involves delivery of the fetus as soon as possible.
• Interleukin-28B and Clearance of Hepatitis C Virus in HIV-Coinfected Patients
• Sequences near the IL-28B gene predict response to HCV therapy in HCV/HIV-coinfected patients.• Recent studies involving patients with hepatitis C virus (HCV) monoinfection have demonstrated that certain sequences near the interleukin (IL)-28B gene — known as the rs12979860 genotype — are associated with both spontaneous and treatment-induced HCV clearance. Now, investigators have examined whether these same sequences predict HCV treatment response in HIV-coinfected patients.
The study involved 164 patients in Spain, most of whom had well-controlled HIV infection: The median CD4 count was 468 cells/mm3, and 73% of patients had undetectable HIV RNA levels on antiretroviral therapy.
The rate of sustained virologic response (SVR) to peginterferon/ribavirin therapy was 55% overall but was significantly higher in patients with the rs12979860 CC genotype than in patients with the CT or TT genotype .The CC genotype remained a strong independent predictor of HCV response even after adjustment for other prognostic factors.
Among patients with HCV genotype 1 or 4, the SVR rate was 100% among those who had the CC genotype, along with an HCV RNA level <600,000 IU/mL and no signs of advanced liver fibrosis — versus only 12% among patients without any of these protective factors.
Comment: These findings suggest that IL-28B genotyping is useful for predicting response to peginterferon/ribavirin in patients coinfected with HIV and the difficult-to-treat HCV genotypes 1 and 4. (SVR rates in patients with HCV genotype 3 are excellent regardless of IL-28B genotype). Whether this test will also forecast the activity of HCV protease-inhibitor therapy remains to be seen. Understanding the mechanism by which IL-28B (also known as IFN- -3) affects HCV clearance may result in improved therapies in the future.
Published in Journal Watch HIV/AIDS Clinical Care May 17, 2010
Hepatocellular Carcinoma and Hepatitis C Virus–Related Cirrhosis
Among cirrhotic HCV patients, those who achieved sustained virologic response from interferon therapy had lower risk for developing HCC than nonresponders.The efficacy of interferon (IFN) for reducing risk for hepatocellular carcinoma (HCC) in patients with hepatitis C virus (HCV) infection is controversial. To address this issue, investigators conducted a meta-analysis of 32 studies that evaluated the use of IFN in this setting. The primary outcome in each study was occurrence of HCC at the last follow-up evaluation.
In 20 studies that compared cirrhotic HCV patients treated with IFN (with or without ribavirin) versus untreated patients, pooled data showed that treated patients had lower risk for HCC (risk ratio, 0.43; 95% confidence interval, 0.33–0.56). However, the data were heterogeneous; metaregression analysis revealed that studies with follow-up duration of >5 years contributed to the heterogeneity.
In 14 studies that reported HCC development based on sustained virologic response (SVR) to IFN therapy, patients who achieved SVR had significantly lower risk for HCC than nonresponders (RR, 0.35; 95% CI, 0.26–0.46). Analysis of five studies showed that among patients who received IFN plus ribavirin, those who achieved SVR had the lowest risk for HCC development versus nonresponders (RR, 0.25; 95% CI, 0.14–0.46).
In four studies that analyzed the effect of maintenance IFN in nonresponders to initial therapy, patients who received maintenance IFN achieved no benefit in terms of HCC risk reduction.
Comment: This excellent systematic review and meta-analysis suggests that cirrhotic HCV patients who achieve SVR from IFN therapy are at lower risk for developing HCC than nonresponders. The evidence is less convincing that receiving treatment of any kind for reducing HCC risk is better than receiving no treatment at all. Also, maintenance therapy with IFN in nonresponders does not offer any benefit for HCC risk reduction. This study provides additional rationale for treating HCV cirrhotic patients with IFN-based therapy.
Published in Journal Watch Gastroenterology April 2, 2010
Hepatitis C Virus Transmission at an Endoscopy Clinic
Eight cases were identified; contamination and reuse of open propofol vials was the likely source.Hepatitis C is the most common bloodborne infection in the U.S. Although nosocomial transmission of hepatitis C virus (HCV) is considered rare, the number of cases associated with nonhospital medical settings is increasing. Now, researchers describe an outbreak of HCV infection at an endoscopy clinic.
During a 5-week period in 2007, three patients developed acute hepatitis after undergoing endoscopy at a single clinic in Las Vegas. Among the 123 additional patients who underwent endoscopy at that clinic on the same dates as these individuals, 6 were known to be HCV infected and were considered potential source patients; the remaining 117 were advised to undergo screening for antibodies to HCV. This testing identified an additional five patients who met the case definition for clinic-acquired HCV infection.
Genetic analysis of the HCV from the eight patients with clinic-acquired infections and from the six patients known to have been infected before their procedures allowed the identification of the source patient for each endoscopy date. Among HCV-susceptible individuals who underwent endoscopy after the source patients, HCV infection developed in 1 of 49 (2%) whose procedures occurred on the first date and in 7 of 38 (18%) whose procedures occurred on the second date. During the investigation, an anesthetist was observed placing a new needle on a syringe, then refilling the syringe from a propofol vial that was intended for single use but had been used for other patients.
Comment: This outbreak of HCV infection was likely related to contamination of open propofol vials through refilling of syringes that had become contaminated with the source patients' blood. This practice was routine in the clinic, so it is surprising that more infections did not occur.
Published in Journal Watch Infectious Diseases July 21, 2010
Hepatitis C Virus Survival in Syringes
Under certain conditions, HCV can survive longer than 2 months.Researchers have estimated that the incidence of hepatitis C virus (HCV) infection among injection-drug users ranges from 16% to 42% per year, and that, for each exposure to a contaminated syringe, the probability of HCV acquisition is 5- to 20-fold higher than the probability of HIV acquisition. Believing that prolonged virus survival in contaminated syringes might be playing a role, investigators used a microculture assay to evaluate the viability of HCV at various temperatures.
Syringes were loaded with blood that had been spiked with a genetically modified laboratory HCV clone; after storage, the contents were cultured in human hepatoma cells. HCV survival was examined for two syringe types: the low void volume insulin syringe with an attached 27-gauge needle and the high void volume tuberculin syringe with a detachable 26-gauge needle (average residual volume after complete depression of the plunger, 2 and 32 µL, respectively).
Viable virus was recovered for up to 7 days from low void volume syringes stored at 4°C, but not beyond day 1 from those stored at 22° or 37°. HCV persisted for much longer in the high void volume syringes: After 63 days of storage, viable virus was recovered from 13% of syringes stored at 4°C, 20% of those stored at 20°, and 6% of those stored at 37°.
Comment: Although survival of the laboratory clone used in this research might differ from survival of clinical isolates, the study shows that viable HCV may persist for months in high void volume syringes. As noted by editorialists, this finding should inform development of more-effective HCV prevention methods and public health interventions.
Published in Journal Watch Infectious Diseases September 8, 2010
• Functional Renal Failure in Patients with Cirrhosis and Ascites
• Nearly half of patients developed FRF, which was associated with poor prognosis.• Hepatorenal syndrome is known to be associated with high morbidity and mortality in patients with cirrhosis, especially those with ascites. To characterize less well-understood forms of functional renal failure (FRF) in this clinical setting, investigators conducted a nested cohort study that was part of a long-term analysis of the natural history of ascites in patients with cirrhosis.
The researchers enrolled 263 patients (mean age, 61; 65% men) who experienced their first episodes of ascites-related decompensation during a 4-year period at two sites in Spain; all participants were followed for 6 months. Cirrhosis was related to hepatitis C virus infection in 52% of patients and to alcohol use in 48%. Renal failure was defined as a serum creatinine level 1.5 mg/dL or, in cases of renal insufficiency, a creatinine-level increase of 50% from baseline.
FRF was defined as prerenal failure, renal failure induced by infection that did not lead to HRS, or HRS based on preestablished criteria. Complete clinical and laboratory data were collected prospectively.
During follow-up (mean, 41 months), 49% of the cohort developed some form of FRF: an absolute 27% due to prerenal causes, 14% due to infectious causes, and only 8% due to HRS. The 1-year probability of experiencing a first episode of any type of FRF was 23.6%. Patients who developed FRF during follow-up had a 1-year probability of survival of 47%, compared with 91% for patients who did not develop FRF. Independent predictors of FRF were older age, higher baseline creatinine level, and Child-Pugh score.
Comment: Functional renal failure was common and associated with a substantially reduced survival rate in this study, which had a retrospective basic design but used clinical and laboratory data that were collected prospectively. Notably, most of the incident cases of FRF were due to prerenal or infectious causes, although those cases together represented 41% of all the patients with cirrhosis. Clinicians should proactively diagnose and then address these causes — for example, by aggressively treating or preventing volume depletion and using diuretics judiciously.
Published in Journal Watch Gastroenterology September 24, 2010
Gastroesophageal varices are present in 50% of patients with cirrhosis, and variceal hemorrhage develops in up to one third of these patients. The risk of variceal hemorrhage is increased in patients who have large varices and advanced stages of liver disease, as assessed on the basis of the Child–Pugh class. Several studies published between 1942 and 1981 showed poor outcomes after variceal hemorrhage, with mortality rates of 40% at 6 weeks and 70% at 1 year.
Early TIPS to Improve Survival in Acute Variceal Bleeding
NEJM June 24, 2010
Over the past five decades, a number of randomized trials have shown an improvement in the efficacy of endoscopic, pharmacologic, surgical, and radiologic techniques for arresting hemorrhage, but most of these studies were not powered to determine whether these therapies resulted in a survival benefit.
Subsequently, retrospective single-center and multicenter studies have shown a decrease in in-hospital mortality associated with variceal hemorrhage over the past two decades. The decrease in mortality was largely due to the prevention of rebleeding with the use of earlier, more effective endoscopic therapy in combination with vasoactive medications and to the prevention of sepsis through the use of antibiotic prophylaxis.
Despite this improvement, however, the mortality at 30 days among patients in Child–Pugh class C is still 32%, and 75% of the patients who require transjugular intrahepatic portosystemic shunt (TIPS) as rescue therapy to control index bleeding are in Child–Pugh class C. In addition, a Child–Pugh score above 9 has been identified as an independent risk factor for death, with a hazard ratio of 1.45 for each 1-point increase in the score.
Current practice guidelines for treating patients with acute variceal bleeding recommend fluid resuscitation, antibiotic prophylaxis, and vasoactive drugs such as glypressin or somatostatin analogues, followed by early endoscopy and either ligation or sclerosis of the varices. Despite these measures, failure to control index bleeding occurs in 10 to 20% of patients. An elevated hepatic venous pressure gradient (>20 mm Hg) measured within 24 hours after the start of bleeding is the best predictor of treatment failure.
The use of TIPS to control variceal bleeding has largely been reserved for patients who require rescue therapy because hemostasis has not been achieved, either during the index bleeding or during the secondary-prophylaxis period.
TIPS is extremely effective in controlling bleeding, with a reported rate of immediate hemostasis of 93% and with rebleeding in only 12% of patients.
Nevertheless, mortality at 6 weeks among patients treated with rescue TIPS for uncontrolled index bleeding and rebleeding is very high (35%), reflecting the severity of their underlying liver disease as well as additional organ dysfunction that may have occurred owing to hypotension, infection, and aspiration.
In this issue of the Journal, García-Pagán and colleagues report the results of a randomized, multicenter study that compared early TIPS with optimal medical therapy (endoscopic therapy plus vasoactive drugs) in patients at high risk for rebleeding who were either in Child–Pugh class B with active bleeding at endoscopy or in Child–Pugh class C.
After the acute bleeding, the medical-therapy group received endoscopic therapy until obliteration of the varices, followed by surveillance, beta-blockade (in 80% of patients), and nitrates (in 39% of patients). Thirty-one of the 32 patients randomly assigned to the early-TIPS group underwent shunting within 72 hours after endoscopy, and the portal-pressure gradient was reduced to less than 12 mm Hg in all but 2 of these 31 patients.
This study shows the benefit of early TIPS in patients with Child–Pugh class B or C disease who are at high risk for uncontrolled bleeding with standard therapy. Patients who were randomly assigned to receive TIPS had a significantly better chance of remaining free of bleeding than did those who received the standard care (97% vs. 50%), possibly owing to a greater reduction in portal pressure with TIPS than could be achieved with pharmacologic therapy.
The rate of survival at 6 weeks was 97% in the TIPS group as compared with 67% in the medical-therapy group, as a result of reductions in rebleeding, sepsis, and liver failure. However, the 86% 1-year survival rate in the TIPS group is somewhat surprising for patients with Child–Pugh class B or C disease who have variceal bleeding.
Does early TIPS alter the natural history of cirrhosis, or were these findings attributable to abstinence from alcohol in the large proportion of study patients with a diagnosis of alcohol-related cirrhosis (66%), half of whom were actively drinking at the time of presentation? Given that abstinence has been associated with improvement in liver function, it would be interesting to know whether any of these patients had improvement in the Model for End-Stage Liver Disease (MELD) score or the Child–Pugh class.
An alternative theory is that the placement of TIPS early in the illness results in a large reduction in portal pressure and preservation of liver function, with a reduction in the risk of liver decompensation that has not typically been associated with the use of rescue TIPS.
Use of the newer stents, which are covered with extended polytetrafluoroethylene (e-PTFE), probably has an important bearing on the outcome of this study. For reasons that are unclear, e-PTFE–covered stents, as compared with bare-metal stents, are associated with better long-term survival among patients undergoing TIPS.13 This finding may be related to a reduced rate of TIPS dysfunction and thus fewer complications of portal hypertension with the newer stents, but there are no clearly established reasons for this phenomenon.14
In conclusion, the study by García-Pagán and colleagues should stimulate a reevaluation of how we approach variceal bleeding in patients with Child–Pugh class B or C disease. Instead of taking a wait-and-see approach, physicians should consider the early use of TIPS with an e-PFTE–covered stent as first-line therapy rather than as rescue treatment if rebleeding occurs in high-risk patients with Child–Pugh B or C disease. Additional clinical trials of adequate size should be performed to confirm these findings and to examine the effect of a rapid reduction in portal pressure on disease progression in patients with cirrhosis of other causes.
The Child-Pugh score (sometimes the Child-Turcotte-Pugh score) is used to assess the prognosis of chronic liver disease, mainly cirrhosis. Although it was originally used to predict mortality during surgery, it is now used to determine the prognosis, as well as the required strength of treatment and the necessity of liver transplantation.
Bilirubin:
<2 mg/dl (34 uM/l) 2-3 mg/dl (34-50 uM/l)>3 mg/dl (50 uM/l)Albumin:
>3.5 g/dl 3.5-2.8 <2.8
PT prolongation (INR):
<4 seconds (<1.7)4-6 seconds (1.7-2.3)>6 seconds (>2.3)
Ascites:
Absent Mild-Moderate Severe/Refractory
Encephalopathy:
Absent Mild (I-II) Severe (III-IV)
• Child-Pugh Score Calculator
• Child-Pugh Score: Interpretation:Class A: 5-6Class B: 7-9Class C: 10-15
Indications
Evaluating prognosis in Cirrhosis
Criteria
A-Total Serum Bilirubin
Bilirubin <2 mg/dl: 1 point
Bilirubin 2-3 mg/dl: 2 points
Bilirubin >3 mg/dl: 3 points
B.Serum Albumin
Albumin >3.5 g/dl: 1 point
Albumin 2.8 to 3.5 g/dl: 2 point
Albumin <2.8 g/dl: 3 point
C.INR
INR <1.70: 1 point
INR 1.71 to 2.20: 2 point
INR >2.20: 3 point
D.Ascites
No Ascites: 1 point
Ascites controlled medically: 2 point
Ascites poorly controlled: 3 point
E.Encephalopathy
No Encephalopathy: 1 point
Encephalopathy controlled medically: 2 point
Encephalopathy poorly controlled: 3 point
Child-Pugh Score
Interpretation
A.Child Class A: 5 to 6 points
Life expectancy: 15 to 20 years
Abdominal surgery peri-operative mortality: 10%
B.Child Class B: 7 to 9 points
Indicated for liver transplantation evaluation
Abdominal surgery peri-operative mortality: 30%
C.Child Class C: 10 to 15 points
Life expectancy: 1 to 3 years
Abdominal surgery peri-operative mortality: 82%
Other scoring systems
Although the Child-Turcotte scoring system was the first of its kind in stratifying the seriousness of end-stage liver disease, it is by no means the only one. The Model for End-Stage Liver Disease (MELD) is used increasingly to assess patients for liver transplantation, although both scores seem to be more or less equivalent.
Rifaximin Treatment in Hepatic Encephalopathy
ABSTRACTBackground Hepatic encephalopathy is a chronically debilitating complication of hepatic cirrhosis. The efficacy of rifaximin, a minimally absorbed antibiotic, is well documented in the treatment of acute hepatic encephalopathy, but its efficacy for prevention of the disease has not been established.
NEJM March 25, 2010
Methods In this randomized, double-blind, placebo-controlled trial, we randomly assigned 299 patients who were in remission from recurrent hepatic encephalopathy resulting from chronic liver disease to receive either rifaximin, at a dose of 550 mg twice daily (140 patients), or placebo (159 patients) for 6 months. The primary efficacy end point was the time to the first breakthrough episode of hepatic encephalopathy. The key secondary end point was the time to the first hospitalization involving hepatic encephalopathy.Results
Rifaximin significantly reduced the risk of an episode of hepatic encephalopathy, as compared with placebo, over a 6-month period (hazard ratio with rifaximin, 0.42; 95% confidence interval [CI], 0.28 to 0.64; P<0.001).A breakthrough episode of hepatic encephalopathy occurred in 22.1% of patients in the rifaximin group, as compared with 45.9% of patients in the placebo group.
A total of 13.6% of the patients in the rifaximin group had a hospitalization involving hepatic encephalopathy, as compared with 22.6% of patients in the placebo group, for a hazard ratio of 0.50 (95% CI, 0.29 to 0.87; P=0.01). More than 90% of patients received concomitant lactulose therapy. The incidence of adverse events reported during the study was similar in the two groups, as was the incidence of serious adverse events.
Conclusions Over a 6-month period, treatment with rifaximin maintained remission from hepatic encephalopathy more effectively than did placebo.
Rifaximin treatment also significantly reduced the risk of hospitalization involving hepatic encephalopathy.
• Discussion
• The prevention of episodes of hepatic encephalopathy is an important goal in the treatment of patients with liver disease, especially since symptoms of overt encephalopathy are debilitating and decrease the ability for self-care, leading to improper nutrition and nonadherence to a therapeutic regimen, which in turn leads to severe symptoms, frequent hospitalizations, and a poor quality of life.
Our study showed that the use of rifaximin reduced the risk of a breakthrough episode of hepatic encephalopathy during a 6-month period among patients in remission who had a recent history of recurrent overt hepatic encephalopathy ( 2 episodes within the previous 6 months) before enrollment. The reduced risk was seen across subgroups, further showing the consistency of the results, which expand previously reported findings of the efficacy of rifaximin in the treatment of overt hepatic encephalopathy.
The current study differs from previous randomized studies in that it examined the protective effect of rifaximin against breakthrough episodes of hepatic encephalopathy rather than its effect in the treatment of acute, overt symptoms; the study also involved a larger group of patients and a longer study period. In previous randomized studies, rifaximin was administered for 21 days or less or intermittently, for 14 or 15 days per month for 3 or 6 months.
Our study shows the superiority of rifaximin therapy over treatment with lactulose alone. More than 90% of patients received concomitant lactulose during the study period, and a significant treatment effect was noted within 28 days after randomization. In contrast, a recent single-center, open-label study of 120 patients showed that although lactulose therapy was more effective than no active treatment in the prevention of overt hepatic encephalopathy, the treatment effects favoring lactulose were apparent only after approximately 4 months.
In the current, prospective study, rifaximin therapy reduced the risk of hospitalization involving hepatic encephalopathy, reflecting the clinical significance of our efficacy findings. Also, the reduced risk of hospitalization supports the results of retrospective chart reviews, which have shown that rifaximin, as compared with lactulose, is associated with a significantly lower frequency and duration of hospitalization and lower hospital costs.
The incidences of adverse events in general and adverse events consisting of infection in particular were similar in the rifaximin group and the placebo group. The safety profile of rifaximin appears to be superior to that of systemic antibiotics, particularly for patients with liver disease. The occurrence of nephrotoxicity and ototoxicity with the use of aminoglycosides (e.g., neomycin and paromomycin) and of nausea and peripheral neuropathy with prolonged use of metronidazole restricts their use in patients with hepatic encephalopathy.
The risk of bacterial resistance appears to be lower with rifaximin than with systemic antibiotics. Plasma levels of rifaximin are negligible; therefore, bacteria outside the gastrointestinal tract are not exposed to appreciable selective pressure. In addition, whereas resistance to other antimicrobial agents is plasma-mediated, resistance to rifaximin is mediated through reversible genomic change.
For chromosomally mediated mutation and selection to result in clinically relevant resistance, the mutation cannot be lethal and cannot significantly decrease virulence; otherwise, the resistant trait will not be transmitted. Both in vitro and in vivo studies of the effects of rifaximin on commensal flora suggest that rifaximin-resistant organisms have low viability.
In summary, this study shows a robust protective effect of rifaximin against episodes of hepatic encephalopathy. Rifaximin also reduces the risk of hospitalization involving hepatic encephalopathy.
Transjugular Intrahepatic Portosystemic Shunts for Refractory Hepatohydrothorax
Symptomatic relief was achieved by most patients, but overall survival was poor.Hepatohydrothorax occurs in 5% to 12% of patients with cirrhosis and is less common than other complications, such as abdominal ascites and variceal bleeding. First-line therapy typically entails sodium restriction and diuretics.
However, in refractory cases, management is more difficult. Repeated thoracentesis and chest-tube placement, although effective, are associated with substantial risks, including pneumothorax and infection. Transjugular intrahepatic portosystemic shunts (TIPS) is another treatment for refractory hepatohydrothorax, but most studies of this procedure involved relatively short follow-up periods and yielded conflicting results.
To examine long-term outcomes of TIPS in this setting, investigators retrospectively reviewed 73 consecutive patients who underwent TIPS for refractory hepatohydrothorax between 1992 and 2008. Uncovered stents were used until 2000; covered stents then became available and were used thereafter. Clinical response (complete resolution of hepatohydrothorax symptoms with or without diuretics) was assessed within 1 month after TIPS and at 6 months.
Mean duration of follow-up was 759 days. Results were as follows:
The rate of clinical response within 1 month and at 6 months was 79% and 75%, respectively.Short-term survival rates in clinical responders at 30, 60, and 90 days were 81%, 78%, and 72%, respectively.
At 30 days, 42.8% of deaths were due to liver failure, 28.5% to acute respiratory failure, 14.3% to renal failure, and 14.3% to sepsis.
Long-term survival rates at 1, 3, and 5 years were 48%, 26%, and 15%, respectively.
Independent factors associated with overall survival were pre-TIPS model for end-stage liver disease (MELD) scores <15 (hazard ratio, 1.9; P=0.039) and TIPS clinical response (P=0.003; HR, 2.5).Hepatic encephalopathy occurred in 15.1% of patients.
Comment: These results show that most patients who underwent TIPS placement for refractory hepatohydrothorax achieved symptomatic relief. However, overall survival was poor, highlighting that these patients have advanced disease and should be referred early for liver-transplant consideration. Also, care should be taken in patient selection for TIPS, given that nearly 50% of deaths at 30 days were due to the precipitation of liver failure.
Published in Journal Watch Gastroenterology May 7, 2010
Hepatitis G virus (HGV), also known as GB virus-C (GBC), is a benign virus that infects humans, but has not been proven to cause disease. Although the virus lives in the blood, and is genetically similar to hepatitis C, there is no indication that it results in liver damage like other types of hepatitis.
It was first discovered in 1995, and is believed to infect between two and five percent of people worldwide.
Hepatitis G is known to cause persistent infection in 15 to 30 percent of adults for as long as nine years. Many times infected persons will not be aware that they carry the virus, because symptoms are non-existent. It is commonly found in co-infections with other viruses, such as hepatitis C and human immunodeficiency virus (HIV).
In fact, more than one third of people infected with HIV are also infected with hepatitis G.
The hepatitis G virus is transmitted through blood. Sharing personal care items such as razors and toothbrushes infected with the virus can spread the disease, as well as sexual intercourse, from mother to child at birth, intravenous drug use, or other blood to blood contact.
The virus cannot be contracted through saliva, semen, or any other bodily fluids other than blood.
Some people are at higher risk for contracting hepatitis G than others. Hemodialysis patients, users of injected drugs, and health care workers exposed to blood on a regular basis, are at the highest risk for becoming infected with the virus. Anyone who receives a tattoo, acupuncture, or a body piercing is at medium risk, if the tools being used are not properly sterilized.
Because hepatitis G was so recently discovered, there is no cure or recommended treatment. There is research being performed on the virus currently, but little is known aside from the fact that it doesn’t seem to cause liver damage. Individual response to the virus will vary, and so will treatment options. However, getting enough rest, eating a balanced diet, and avoiding alcohol and other liver irritants are all recommended for sufferers of hepatitis G.
Hepatitis F is the name that had been given to a form of viral hepatitis that seemed to be unexplained by the viruses that cause hepatitis A-G.
It has been debated whether this form of hepatitis is caused by a separate virus or by a variant of one of the other hepatitis viruses.
Ursodeoxycholic Acid for Nonalcoholic Steatohepatitis
High-dose UDCA was ineffective, as was a low-dose regimen in a prior study.No specific medical therapy exists for patients with nonalcoholic steatohepatitis (NASH). One therapy that previously failed in a large, randomized, controlled trial was low-dose ursodeoxycholic acid (UDCA; 13–15 mg/kg/day).
Now, to evaluate the efficacy of high-dose UDCA (23–28 mg/kg/day) in this setting, investigators conducted a multicenter, randomized, placebo-controlled, double-blind study involving 185 patients with biopsy-proven NASH.
Patients were randomized to receive either high-dose UDCA or placebo in three divided daily doses for 18 months. The primary endpoint was histological improvement based on nonalcoholic fatty liver disease activity scores (NAS) and modified Brunt scores. End-of-treatment biopsies were available in 139 patients.
Histology (NAS or Brunt scores) and adverse events were similar in both groups, and no changes in fibrosis scores or body weight were seen in either group. Only lobular inflammation was significantly improved in the UDCA group versus the placebo group (P=0.005 for NAS; P=0.011 for Brunt score).
Comment: This well-designed, adequately powered, and well-executed study demonstrated that higher-dose UDCA was ineffective in treating patients with NASH. These results, combined with findings of prior studies showing a lack of efficacy of low-dose UDCA, conclusively demonstrate that UDCA should not be used as monotherapy for NASH patients. Furthermore, a recent study of high-dose UDCA in primary sclerosing cholangitis raised safety concerns about this agent (JW Gastroenterol Oct 23 2009).
Published in Journal Watch Gastroenterology October 1, 2010