UROLOGY
د. حسين محمد جمعةاختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2011
In the United States, ~13% of men and 7% of women will develop a kidney stone during their lifetime. Ureteral calculi almost always originate in the kidneys, although they may continue to grow once they lodge in the ureter. calculi consist of aggregates of crystals containing small amounts of proteins and glycoprotein, probably as a consequence of dietary and environmental factors, but genetic factors may also contribute.
Nephrolithiasis
In Europe, 80% of renal stones contain crystals of calcium (most commonly as oxalate, but also as phosphate).
About 15% contain magnesium ammonium phosphate (struvite; these are often associated with infection),
and small numbers of pure cystine or uric acid stones are found. Rarely, drugs may form stones (e.g. indinavir, ephedrine).
Calcium phosphate, calcium carbonate, and magnesium phosphate stones develop in alkaline urine.
Uric acid, cystine, and calcium oxalate stones precipitate in acidic urine; in this situation, the urine should be kept alkaline or less acidic than normal. Drugs such as streptomycin, neomycin, and kanamycin are effective in treating urinary tract infections if the urine is alkaline.
During treatment with sulfa drugs, alkaline urine helps prevent formation of sulfonamide crystals.
Normal urine is sterile. E. coli causes roughly 90% of urinary infections, and this bacterium does NOT contain the enzyme urease.
Therefore in over 90% of urinary infections, you will NOT see elevation of urine pH.
• urine contains proteins,
• glycosaminoglycans• pyrophosphate and
• citrate
• which help to keep otherwise insoluble salts in solution.
• Low urine volumes: high ambient temperatures, low fluid intake
• Diet: high protein intake, high sodium, low calcium
• High sodium excretion
• High oxalate excretion
• High urate excretion
• Low citrate excretion
Acquired causes
Hypercalcaemia of any cause.Chemical analysis of stones is helpful. Since most stones pass spontaneously through the urinary tract, urine should be sieved for a few days after an episode of colic in order to collect the calculus for analysis.
• Around 90% of stones less than 4 mm in diameter will pass spontaneously, but only 10% of stones of more than 6 mm will pass and these may require active intervention.
Acute loin pain radiating to the groin ('renal colic'), together with haematuria, is typical of ureteric obstruction most commonly due to calculi, although a sloughed renal papilla, tumour or blood clot may be responsible.
Pathophysiology
Urinary tract stone disease is likely caused by two basic phenomena.The first phenomenon is supersaturation of the urine by stone-forming constituents, including calcium, oxalate, and uric acid. Crystals or foreign bodies can act as nidi, upon which ions from the supersaturated urine form microscopic crystalline structures.
The second etiology, which is most likely responsible for calcium oxalate stones, is deposition of stone material on a renal papillary calcium phosphate nidus, typically a Randall plaque.
Calcium phosphate precipitates in the basement membrane of the thin loops of Henle, erodes into the interstitium, and then accumulates in the subepithelial space of the renal papilla. The subepithelial deposits, which have long been known as Randall plaques, eventually erode through the papillary urothelium. Stone matrix, calcium phosphate, and calcium oxalate gradually deposit on the substrate to create a urinary calculus. Randall plaques are always composed of calcium phosphate.
Magnesium and especially citrate are important inhibitors of stone formation in the urinary tract. Decreased levels of these in the urine predispose to stone formation.
A low fluid intake, with a subsequent low volume of urine production, produces high concentrations of stone-forming solutes in the urine. This is an important, if not the most important, environmental factor in kidney stone formation.
The exact nature of the tubular damage or dysfunction that leads to stone formation has not been characterized.
Animal proteins cause calcium to be leached from the bones and excreted in the urine where it can form stones. Diets rich in animal proteins also increase uric acid excretion.
subjects on a diet eliminating animal protein had less than half the calcium loss that they had on their baseline diet.
The Harvard study mentioned earlier found that even a modest increase in animal protein, from less than 50 grams to 77 grams per day, was associated with a 33 percent increased risk of stones in men. The same is true for women.
Animal Protein
The association between animal proteins and stones probably relates both to the amount of protein they contain and to their content of the sulfur-containing amino acids. In particular, the sulfur in cystine and methionine is converted to sulfate, which tends to acidify the blood.As a part of the process of neutralizing this acid, bone is dissolved, and bone calcium ends up in the urine.
Meats and eggs contain two to five times more of these sulfur-containing amino acids than are found in grains and beans.
High-Potassium Foods A study of 46,000 men conducted by Harvard University researchers found that a high potassium intake can cut the risk of kidney stones in half. Potassium helps the kidneys retain calcium, rather than sending it out into the urine. Potassium supplements are not generally necessary. Rather, a diet including regular servings of fruits, vegetables, and beans supplies plenty of potassium.
Sodium. Sodium increases the passage of calcium through the kidney and increases the risk of stones. When people cut their salt (sodium chloride) intake in half, they reduce their daily need for calcium by about 160 milligrams.
Dairy products and meats contain more salt than plant products, and table salt, frozen meals, and canned and snack foods are the highest-sodium food products.
Sugar. Sugar accelerates calcium losses through the kidney.
Climate. Kidney stones are also more common in warm climates, presumably because perspiration leads to dehydration and a more concentrated urine, and because sunlight increases the production of vitamin D in the skin which, in turn, increases calcium absorption from the digestive tract.Surprisingly, oxalate-rich foods, such as chocolate, nuts, tea, and spinach, are not associated with a higher risk of renal stones, nor is vitamin C, even though it can be converted to oxalate. A large study of men taking vitamin C supplements found that they had no more kidney stones than men who do not take them.
Plants of any kind—grains, vegetables, legumes, and fruits,contain almost no sodium at all unless it is added during canning or other processing.
Patients with urinary calculi may report pain, infection, or hematuria. Small nonobstructing stones in the kidneys only occasionally cause symptoms. If present, symptoms are usually moderate and easily controlled.
The passage of stones into the ureter with subsequent acute obstruction, proximal urinary tract dilation, and spasm is associated with classic renal colic.
Renal colic is characterized by undulating cramps and severe pain and is often associated with nausea and vomiting.
As the stone travels through the ureter, the pain moves from the flank to the lower abdomen, down to the groin, and eventually to the scrotal or labial areas.
Associated irritative bladder symptoms are common when the stone is located in the distal or intramural ureter.
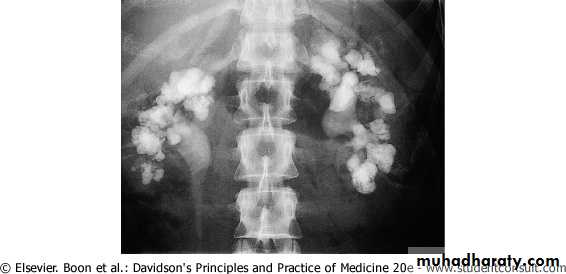
Patients with large renal stones known as Staghorn are often relatively asymptomatic.
Staghorn refers to the presence of a branched kidney stone occupying the renal pelvis and at least one calyceal system.
Such calculi usually manifest as infection and hematuria rather than as acute pain.
Asymptomatic bilateral obstruction, which is uncommon, manifests as symptoms of renal failure.
Staghorn calculi are most frequently composed of
mixtures of magnesium ammonium phosphate (struvite) and/or calcium carbonate apatite.cystine or uric acid,either in pure form or mixed with other components, can also grow in a "staghorn“.
but calcium oxalate or phosphate stones only rarely grow in this configuration.
Struvite/calcium carbonate apatite stones also are referred to as "infection stones" because of
their strong association with urinary tract infection caused by specific organisms that produce the
enzyme urease that promotes the generation of ammonia and hydroxide from urea
Sex
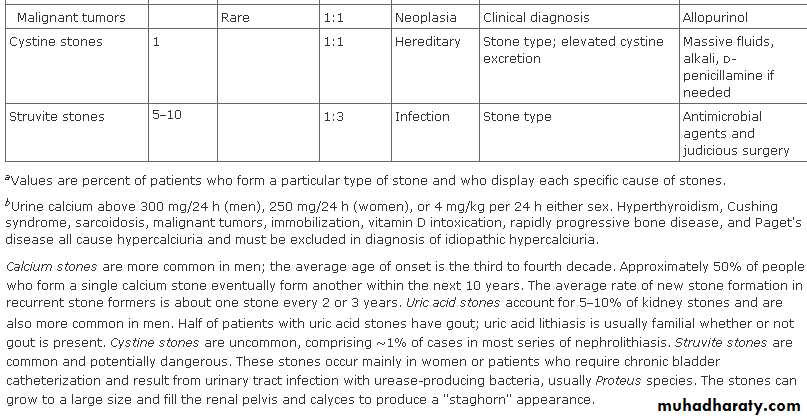
In general, urolithiasis is more common in males (male-to-female ratio of 3:1).
Stones due to infection (struvite calculi) are more common in women than in men.
Age
Most urinary calculi develop in persons aged 20-49 years.
An initial stone attack after age 50 years is relatively uncommon.
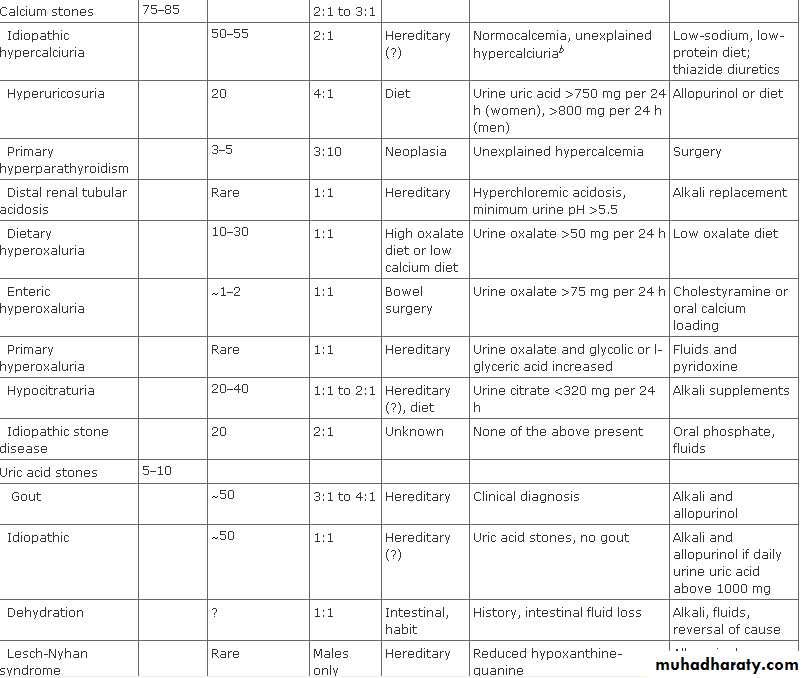
Causes
Hypercalciuria is the most common metabolic abnormality. Some cases of hypercalciuria are related to increased intestinal absorption of calcium ,some are related to excess resorption of calcium from bone (ie, hyperparathyroidism), and some are related to an inability of the renal tubules to properly reclaim calcium in the glomerular filtrate (renal-leak hypercalciuria).Magnesium and especially citrate are important inhibitors of stone formation in the urinary tract. Decreased levels of these in the urine predispose to stone formation.
A low fluid intake
The most common findings on 24-hour urine studies include hypercalciuria, hyperoxaluria, hyperuricosuria, hypocitraturia, and low urinary volume.
Types of Stones
Calcium salts, uric acid, cystine, and struvite (MgNH4PO4) are the basic constituents of most kidney stones in the western hemisphere . Calcium oxalate and calcium phosphate stones make up 75–85% of the total and may be admixed in the same stone. Calcium phosphate in stones is usually hydroxyapatite [Ca5(PO4)3OH] or less commonly, brushite (calcium monohydrogen phosphate). (CaHPO4H2O).• Classification of kidney stones by composition
• Calcium oxalate, phosphate, or both (70-80%)• Uric acid (5-10%)
• Cystine (1%)
• Struvite (magnesium ammonium phosphate) (5-15%)
• Other (such as xanthine, guaifenesin) (1%)
Presence of systemic illness
Primary hyperparathyroidismRenal tubular acidosis
Cystinuria
Gout
Diabetes mellitus
Inflammatory bowel disease
Renal insufficiency
Sarcoidosis
Medullary sponge kidney
Important factors to identify in the patient's history
Anatomical features
Presence of horseshoe kidney
Previous urinary diversion
Obstruction of the ureteropelvic junction
Solitary kidney
Previous renal or ureteral surgery
Previous kidney disease
History of urinary tract infection or pyelonephritis, or both
Family history of urolithiasis
Detailed history of previous stone events
Drugs that affect stone disease
Carbonic anhydrase inhibitors (topirimate)Ephedrine
Guaifenesin
Calcium with vitamin D
Triamterene
Indinavir or sulfadiazine
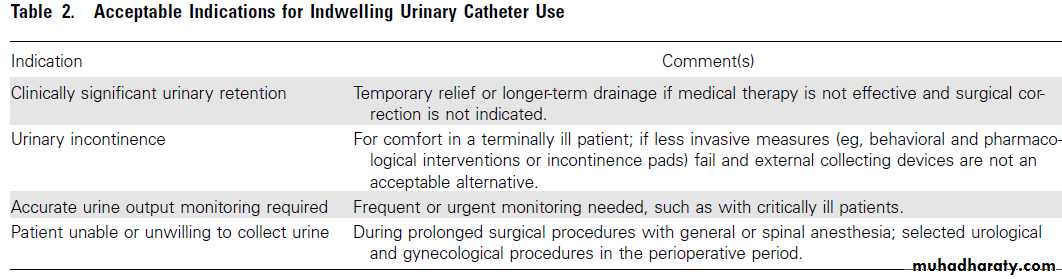
How are kidney stones treated ? depends on the size and type of stone, the underlying cause, the presence of urinary infection, and whether the condition recurs. Stones 4 mm and smaller pass without intervention in 90% of cases; those 5 – 7 mm do so in 50% of cases, and those larger than 7 mm rarely pass. Calcium: Plenty in diet (because calcium forms an insoluble salt with dietary oxalate, lowering oxalate absorption and excretion) Avoid supplements away from meals (increase calcium excretion without reducing oxalate excretion)Oxalate:Avoid foods that are rich in oxalate (e.g. rhubarb)
• Indications for urgent intervention
• Presence of infection with urinary tract obstruction• Urosepsis
• Intractable pain or vomiting, or both
• Impending acute renal failure
• Obstruction in a solitary or transplanted kidney
• Bilateral obstructing stones
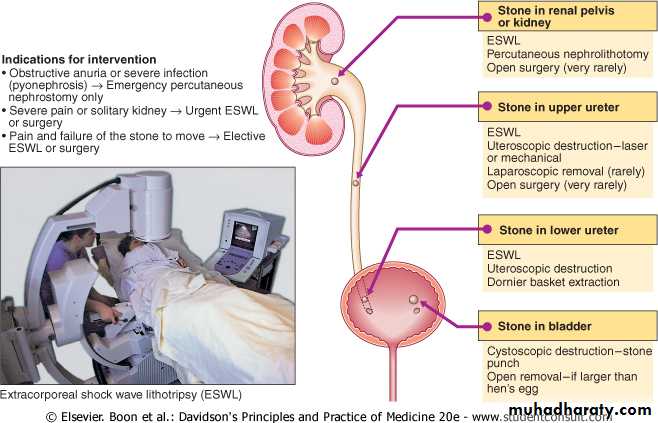
• 1-Lithotripsy.
• effective for stones in the kidney or upper ureter. It uses an instrument, machine, or probe to break the stone into tiny particles that can pass naturally. Lithotripsy is not appropriate for patients with very large stones or other medical conditions.• 2-Ureteroscopy.
• This is a fiberoptic long, thin telescope (ureteroscope) inserted through the urethra and passed through the bladder to the stone used to remove or break up (fragment) stones with a laser. A small tube (or stent) may be left in the ureter for a few days after treatment to promote healing and prevent blockage from swelling or spasm. This is performed in an outpatient setting.
A retrospective study showed that ureteroscopy
Is useful• when lithotripsy fails;
• when complex or lower pole renal calculi are present; or
• when patient factors such as pregnancy, coagulopathy, or morbid obesity preclude lithotripsy.
disadvantage of ureteroscopy is that a ureteral stent, which causes considerable discomfort in some patients, is often necessary to prevent obstruction from ureteral oedema or stone fragments.
3-Percutaneous Nephrostolithotomy (PCNL)
accomplished by the surgeon threading various catheters over the guidewires into the kidney and manipulates surgical instruments through the catheters to fragment and remove kidney stones. This procedure usually requires hospitalization, and most patients resume normal activity within 2 weeks.
• 4-Laparoscopic Surgery
Three small 3 to 5 mm incisions are made and the patient's abdomen is distended with gas. The stone is extracted through an incision in the ureter or kidney, which is then repaired in a minimally invasive setting. Most patients require overnight hospitalization.
Although shock wave lithotripsy is the most common treatment for urolithiasis, it can have side effects. In human and animal models it can cause acute renal injury. Computed tomography and magnetic resonance imaging have demonstrated renal injury in 63-85% of patients treated with shock wave lithotripsy. A recent retrospective case-control study with 19 year follow-up noted an association between shock wave lithotripsy and the development of hypertension and diabetes mellitus. In the lithotripsy group, diabetes developed in 16.8% of patients versus 6.6% of controls. The chronic effects of shock wave lithotripsy are an area of ongoing research.
Prevention of renal stone depends on the type of stone produced, underlying urinary chemical risk factors, and the patient’s willingness to undergo a long-term prevention plan. Drink a minimum of half of body weight in ounces of water daily. Proper hydration helps prevent the urine from becoming concentrated with crystals, which can lead to stone formation. It also reduces the risk for urinary tract infections, which may lessen the risk for struvite stones. Urine color may indicate the level of concentration: dark or bright yellow urine indicates highly concentrated urine; pale or colorless urine indicates dilute urine. Lemonade with real lemon juice is a good source of citrate and may be recommended as an alternative to water.
Limiting meat, salt, and foods high in oxalate (eg, green leafy vegetables, chocolate, nuts) in the diet may also be recommended. Medication may be prescribed and treatment for an underlying condition that causes renal stone disease may be necessary.
• drinking lots of water every day. Ideally, at least eight glasses of distilled water every day.
• Calcium from dairy products is good in dissolving kidney stones.
• Do not eat salty foods.
• Abstain from sodas as much as possible because they contain phosphorus, the buildup of which leads to kidney stones.
• Sugar also promotes higher risks of kidney stone.
• It is important not to lounge after eating. You should go out for a short walk after eating to prevent kidney stone formation because exercise helps digest the foods that we eat. The longer the food stays in our intestines the more prone we are to having kidney stones.
• Eat lots of foods high in fiber like fruits, vegetables, salads, grains and oat meal.
• Hypercalciuria is a common clinical pediatric problem that in some children is associated with renal stones. Most renal stones (80%) are formed by calcium oxalate, calcium phosphate phases,Calcium phosphate in stones is usually hydroxyapatite [Ca5(PO4)3OH] orless commonly brushite (CaHPO4H2O calcium monohydrogen phosphate). Hypercalciuria can be either primary (accounts for the vast majority of children with calcium stones) or secondary. Uric acid stones are the most common cause of radiolucent kidney stones in children.
High amounts of calcium in the urine (hypercalciuria) can cause development of kidney stones in children. Treatment for these children includes plenty of fluids, a low-salt diet and medications such as potassium citrate. A major advantage of potassium citrate, as compared to hydrochlorothiazide, is its lack of side effects. One problem the researchers and others have observed is that some children continue to form kidney stones despite correction of hypercalciuria with potassium citrate. One possible explanation is that in some individuals potassium citrate therapy results in an excessive elevation of urine pH, a situation that may predispose to calcium phosphate stone formation.
• Treatment for children with calcium stones involves non-pharmacological and pharmacological interventions. Non-pharmacological interventions include high fluid intake, low sodium, and potassium enhanced diet, with RDA calcium and protein. Historically, the specific treatment for hypercalciuric stone formers has included thiazides, which reduce calciuria, lower the urinary saturation of calcium oxalate and phosphate, and restore normal intestinal calcium absorption. However thiazides induce hypokalemia and hypocitraturia, and the latter attenuates the beneficial effects of the drug on stone formation.
Currently, the drug of choice replacing thiazides in treating idiopathic hypercalciuria is potassium citrate. Potassium citrate is readily absorbed from the gastrointestinal tract, and after being excreted in the urine, it inhibits the crystallization of stone forming calcium salts by binding the calcium ion, thus decreasing its urinary saturation and inhibiting the nucleation and crystal growth of calcium oxalate; therefore,
potassium citrate is an effective stone inhibitor agent. A major advantage of potassium citrate is its lack of side effects.
One of the problems seen in clinical practice is that some children with primary hypercalciuria, even after the calciuria is treated successfully with potassium citrate, continue to develop stones. It has been suggested that an elevation in urine pH, seen in some patients treated with potassium citrate, may result in an alkaline urinary milieu which promotes calcium phosphate stone formation. The researchers will try to identify whether the beneficial effects of potassium citrate supplementation on lowering urine calcium and increasing citrate might be offset by too high urine pH (>8) which could promote the formation of calcium
Pediatric StonesClassification
• Metabolic Stones• Renal Tubular Disorders
• Cystinuria
• Hypercalcemia/Hypercalciuria
• Hypercalciuria
• Uric Acid Lithiasis
• Enzyme Defects Xanthinuria Hyperoxaluria
• Infection Stones
• Anatomically Related Stones
• Idiopathic (Endemic Stones)
Renal Tubular Disorders
The two most common types of renal tubular disorders are renal tubular acidosis Type I (RTA) and cystinuria. Type I RTA is a disorder of hydrogen ion (H+) excretion, resulting from a tubular defect within the nephron which prevents a patient from generating a normal pH gradient between the blood and tubular urine pH. As a result, hyperchloremic acidosis is produced with excessive urinary losses of calcium, sodium, potassium, and phosphate.Nephrocalcinosis and calcium phosphate stone formation can occur as a result of hypercalciuria and low urinary citrate excretion.
The diagnosis results from the patient's inability to form an acidic urine (pH less than 5.5).
Treatment
Type I RTA is replacement of bicarbonate, sodium, and potassium with either sodium bicarbonate and potassium supplements or (Polycitra K) which contains sodium citrate, potassium citrate and citric acid.
The goal of treatment is to treat the hypocitraturia.
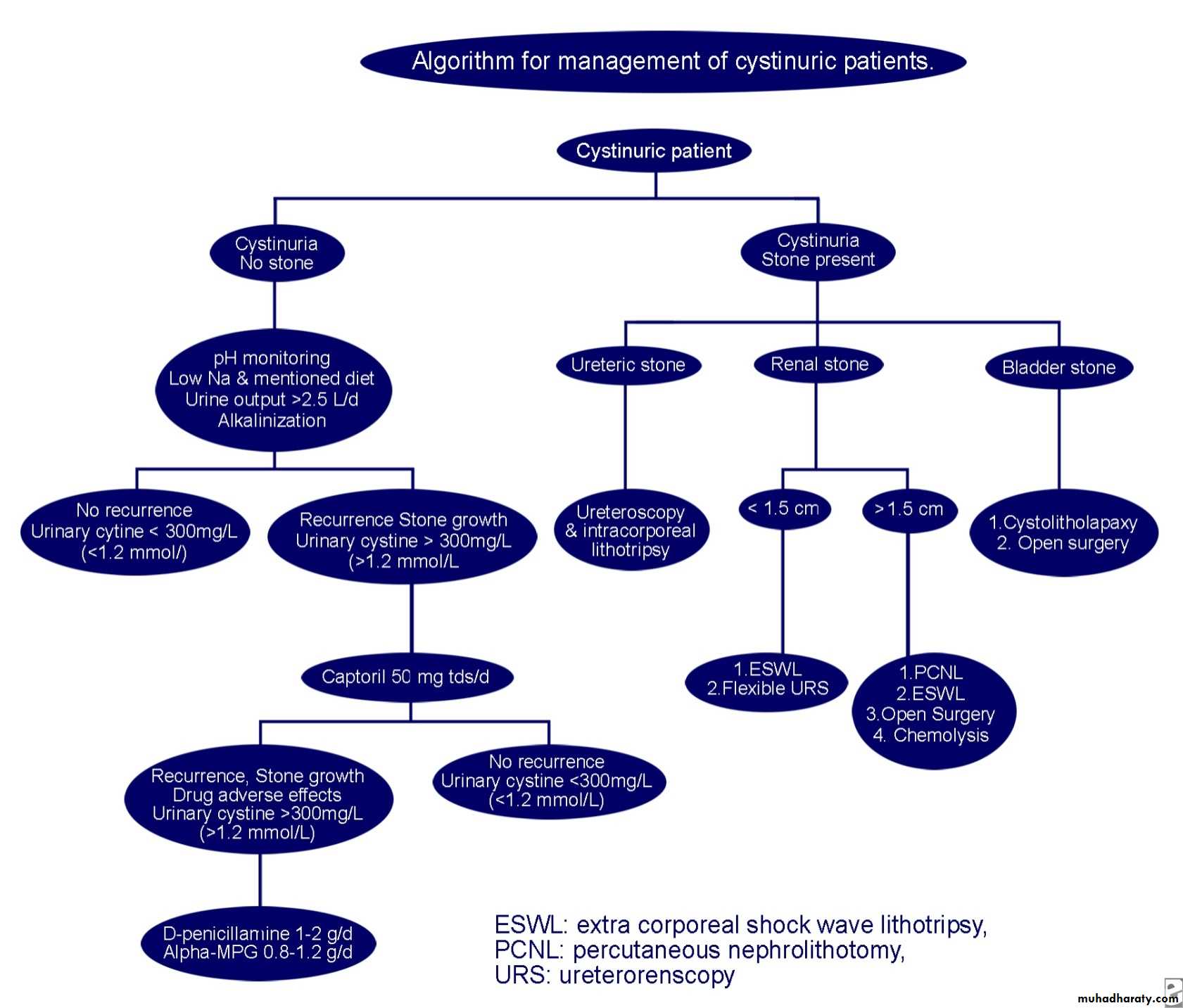
Cystinuriaautosomal recessively inherited disorder of tubular amino acid transport. This results in excessive urinary excretion of the basic amino acids, including cystine, ornithine, lysine, and arginine (C-O-L-A) and the formation of renal calculi.
Normal individuals excrete less than 60 mg of cystine per 1.73 m2 of body surface area per day (1.73m2/per day). Patients who have this disorder have the excretion of cystine greater than 400 mg per 1.73m2/per day.
Cyanide nitroprusside test remains a widely used screening technique.
The treatment of cystinuria involves urinary alkalization and hydration. Medical therapy includes the use of D-Penicillamine, Thiola, or Captopril when stone formation reoccurs.
Potassium citrate is the first-line alkalinizing drug. The typical adult dose is 60-80 mEq/d divided into 3-4 doses (15-20 mL/d), titrating the dose as needed to maintain a urine pH level within the target range of 7-7.5.Paradoxically, a urine pH level of more than 7.5 can cause a predisposition to the formation of calcium phosphate calculi, so urine must be monitored with dipsticks to maintain a pH level of 7-7.5 for stone prevention.
Captopril
In 1987, Sloand and Izzo reported the effectiveness of captopril in the treatment of patients with cystinuria.29Captopril is a thiol first-generation ACE inhibitor and has been shown to form a thiol-cysteine mixed disulfide. This complex is 200 times more soluble than cystine.
Newer thiol compounds, such as thiophosphate and meso-2-3-dimercaptosuccinic acid, have been used both in vitro and in a few clinical trials.
Captopril at doses of 75-100 mg was used in 2 patients, and cystine excretion decreased 70% and 93%. However, as reported by Sloand and Izzo, various follow-up studies have reported conflicting results.29
Captopril can be used to treat patients whose conditions fail to respond to standard treatment and to treat patients with cystinuria who are hypertensive.
Extracorporeal shockwave lithotripsy (ESWL)
ESWL is especially effective for cystine stones smaller than 1.5 cm in diameter, although overall stone-free rates are lower compared with rates for stones of other composition.Because of their hardness and homogenous amino acid composition, most cystine stones require 2-3 times the usual number of shocks to adequately fragment the stone. Multiple treatments are often necessary to achieve acceptable stone-free rates.
When considering candidates for ESWL, some authors suggest an upper limit of 1.5 cm for upper ureteral or renal cystine calculi. As reported by Kachel et al in 1991, these authors prefer to limit ESWL to renal calculi smaller than 1 cm in diameter.
ESWL is appropriate in the treatment of ureteral cystine calculi. Stones not visualized after fluoroscopy can still be opacified by either retrograde or intravenous contrast administration to allow for lithotripsy.
Patients taking thiol derivatives may have cystine calculi that are more fragile because the cystine is replaced by apatite in approximately 30% of cases. These calculi may be easier to treat with ESWL.
Hypercalcemia/Hypercalciuria
Primary HyperparathyroidismHypervitaminosis DSarcoidosisMilk/Alkali SyndromeNeoplasiaCushing's SyndromeHyperthyroidismImmobilizationImmobilization as a cause for stone disease occurs primarily in the pediatric population with head injuries, orthopedic injuries and severe burns. As a result of immobilization, there is increased bone reabsorption resulting in both serum and urine calcium levels to rise. The management of these patients should include adequate hydration and early ambulation, or calcitonin therapy if ambulation is not possible.
In the United States, Hypercalciuria appears to be a leading metabolic cause of stone disease accounting for 27-42% of pediatric patients with stone disease.
The etiology of hypercalciuria relates to
1) Absorptive Hypercalciuria, which is the hyperabsorption of intestinal calcium.2) Renal Hypercalciuria, which is the defective renal tubular absorption of calcium. Primary hyperabsorption of calcium from the gut increases serum levels of calcium, causing an increase of calcium in the urine.
Treatment has been directed toward decreasing intestinal calcium absorption with neutral phosphate supplements and a low calcium diet.
HypercalciuriaRenal hypercalciuria is characterized by the primary leakage of calcium from the kidney. The resulting mild hypocalcemia leads to an increase in calcium absorption in the gut. This condition is diagnosed by the presence of hypercalciuria in the fasting state. Thiazide diuretics and a low calcium diet are effective in reducing calcium excretion in patients with renal idiopathic hypercalciuria.
The current recommendation for determining the etiology of hypercalciuria is to obtain a 24-hour urine calcium excretion following one week of dietary calcium and sodium restriction.
If urinary calcium remains elevated during calcium restriction, the patient is considered to have renal leak hypercalciuria.
The correction of hypercalciuria by dietary restriction is consistent with the diagnosis of absorptive hypercalciuria.
If a child has absorptive hypercalciuria, dietary calcium restriction to 400-600 milligrams per day is recommended. If these recommendations do not result in normalization of urine calcium excretion, hydrochlorothiazide (2mg/kg per day) is added. For a child with renal leak hypercalciuria that persists despite hydration and sodium restriction, hydrochlorothiazide (2mg/kg per day) is added.
A unique form of hypercalciuria related to stone disease has been recognized in neonates receiving furosemide therapy. It has become more prevalent with increasing numbers of neonates in intensive care settings who require furosemide for their lung disease.
Furosemide results in hypercalciuria and calcium oxalate and calcium phosphate stone formation. The treatment of this disorder is medical and involves replacing furosemide with hydrochlorothiazide. In the majority of neonates managed medically, subsequent x-ray studies demonstrate a decrease or disappearance of renal calcifications.
Uric Acid LithiasisIn the pediatric population, uric acid stones are seen primarily in children with
1) myeloproliferative or
2) intestinal tract disease.
Myeloproliferative diseases such as
1) leukemia and
2) lymphoma, typically lead to uric acid stone formation following courses of chemotherapy which result in increased purine turnover and excessive uric acid production.
Patients with intestinal tract diseases such as
1) regional enteritis and2) ileostomy can experience excessive fluid and bicarbonate losses resulting in low volumes of acidic urine. This predisposes to uric acid crystal formation, which occurs at a pH of 5.7.
Treatment of uric acid stone formation is medical. Alkalinization of the urine to pH equal to 6.5 and maintenance of a high urine volume results in stone dissolution in the majority of cases. Allopurinol may also be used in dissolving uric acid stones. works by inhibition of the enzyme xanthine oxidase, thereby decreasing the concentration of urinary uric acid
Enzyme DefectsXanthinuriaXanthinuria is a rare, autosomal recessive disorder of purine metabolism caused by a deficiency of the enzyme xanthine oxidase, resulting in increased urinary xanthine and xanthine calculi, as well as hypouricemia and hypouricosuria. The most common cause of xanthinuria is the use of the medication allopurinol.
Hyperoxaluriauncommon autosomal recessive disorder of glyoxylic acid metabolism that results in excessive synthesis and excretion of oxalate. This disease results in urolithiasis or nephrocalcinosis before the age of 5. The diagnosis is confirmed by 24-hour urine excretion of oxalate in excess of the normal 40 milligrams per 24 hours.
Infection StonesAlthough infection stones are relatively uncommon in the United States, infection is the leading cause of pediatric stone disease in the United Kingdom, accounting for 2/3 of all cases. These stones are composed of magnesium ammonium phosphate, also referred to as struvite stones. The bacterium, Proteus mirabilis, is the most common organism in these types of stones.
Infection stones present earlier than other stone types. The male to female ratio is 2:1. The vast majority of infection stones were located in the upper urinary tracts (85%) and 15% were bilateral. Vesicoureteric reflux was diagnosed in 12% of children with infection stones.
The treatment of infection calculi include complete stone clearance and prevention of further infections. Nearly all patients with recurrent stones had reinfections. Therefore, it is important to correct any underlying cause for infection and keep these children on long term antibiotic prophylaxis.
Anatomically-Related StonesCongenital anomalies of the urinary tract have been recognized as contributing factors of pediatric stone formation. 10-44% of children with stones are associated with congenital abnormalities of the urinary tract. Of the various congenital anomalies, ureteral pelvic junction obstruction was the most common factor seen in 54-65% of patients. Approximately 15% of anatomic stones were located in the lower urinary tract and were most commonly seen in children with bladder reconstruction or neuropathic bladder.
Treatment involves simultaneous correction of the obstructing abnormality at the time of surgical stone removal. In this group of patients, there is a high stone recurrence rate of approximately 27%.
Idiopathic (endemic) StonesIdiopathic stone formers comprise approximately 25% of pediatric stones. The syndrome of idiopathic calcium oxalate stone formation includes a group of heterogenous abnormalities for which the underlying causes are incompletely, or not well defined. This is a diagnosis of exclusion, made after other primary metabolic causes of stone formation have been eliminated. Seventy to 80 per cent of the patients who form stones in industrialized countries have this syndrome.
In children, this percentage may not be this high, yet it remains the most common metabolic cause of stone formation within the urinary tract. Most often, it becomes symptomatic after the onset of puberty, and when patients with this syndrome begin to form stones before the age of 20, they usually demonstrate recurrent stone formation requiring specific treatment adjustments, including medication, to prevent further stone formation. This syndrome occurs commonly in families, and when it does, there is an autosomal dominant pattern of inheritance.
Imaging Studies
Plain abdominal radiographyPlain abdominal radiography (also known as a flat plate or kidney, ureter, and bladder [KUB] radiography) is useful for assessing total stone burden, as well as the size, shape, and location of urinary calculi in some patients. It is also helpful in determining the progress of the stone without the need for more expensive tests with greater radiation exposures.
Calcium-containing stones (approximately 85% of all upper urinary tract calculi) are radiopaque, but pure uric acid, indinavir-induced, and cystine calculi are relatively radiolucent on plain radiography.
When used with other imaging studies, such as a renal ultrasonography or, particularly, CT scanning, the plain film helps provide a better understanding of the size, shape, location, orientation, and composition of urinary stones revealed with these other imaging studies. This may also be helpful in planning surgical therapy and in tracking progress of the stone over time.
Renal ultrasonography
frequently adequate to determine the presence of a renal stone. The study is mainly used alone in pregnancy or in combination with plain abdominal radiography to determine hydronephrosis or ureteral dilation associated with an abnormal radiographic density believed to be a urinary tract calculus.A stone easily identified with renal ultrasonography but not visible on the plain radiograph may be a uric acid or cystine stone, which is potentially dissolvable with urinary alkalinization therapy.
Ureteral calculi, especially in the distal ureter, and stones smaller than 5 mm are not easily observed with ultrasonography.
A helical CT scan without contrast material is currently believed to be the best initial radiographic examination for acute renal colic. If positive, KUB radiography is recommended to assist in follow-up and planning.
IVU is very labor intensive and is no longer the standard for the initial evaluation of a patient with a kidney stone. It involves intravenous injection of potentially allergic and mildly nephrotoxic contrast material.
IVU is helpful in identifying the specific problematic stone among numerous pelvic calcifications and in establishing that the other kidney is functional. These determinations are particularly helpful if the degree of hydronephrosis is mild and the non-contrast CT scan findings are not definitive. The so-called delayed nephrogram on the IVU is one of the hallmark signs of acute urinary tract obstruction. CT scanning with delayed contrast series and thin slices has reduced the need for IVU in the evaluation of problematic ureteral stones.
CT scanning is the most sensitive clinical imaging modality for calcifications. Even calculi that are radiolucent on a plain radiograph (except for indinavir-induced stones) are clear and distinct on a CT scan. Contrast is not used in the initial screening study because it makes the entire urinary collecting system appear white on the study, thus masking the stones.
CT scanning with contrast, obtained after the noncontrast study, is useful in treatment planning and in distinguishing problematic radio-opacities.
Adding plain radiography to noncontrast CT scanning increases the value of the study by allowing visualization of the size, shape, and relative position of the stone.
A lucent stone that is not visible on the KUB radiograph that is clearly visible on the CT scan may indicate a uric acid calculus. This suggests a different diagnosis and therapy (allopurinol and/or urinary alkalinization) than for a calcium stone. For these reasons, many institutions routinely perform KUB radiography whenever renal colic noncontrast CT scanning is performed.
Advantages of a CT scanning include the following:
It can reveal other pathologyabdominal aneurysms
appendicitis
cholecystis.
It can be performed quickly.
It avoids the use of intravenous contrast materials.
Disadvantages of CT scanning include the following:
It cannot be used to assess individual renal function.
It can fail to reveal some unusual radiolucent stones, such as those caused by indinavir, which are invisible on the CT scan. Because of this possibility, IVUs with contrast should be used for patients taking indinavir.
It is relatively expensive.
It exposes the patient to a relatively high radiation dose.
Precise identification of small distal stones is occasionally difficult.
It is not suitable for tracking the progress of the stone over time, supporting the recommendation for KUB radiography along with the CT scan.
Plain renal tomography
Although largely replaced by helical CT scanning without contrast, plain renal tomography is often helpful in finding small stones in the kidneys, especially in patients who are large or obese whose bowel contents complicate observation of any renal calcifications.Tomography does not require extensive preparation and can be performed quickly. In addition, the cost and radiation dosage to the patient are less than with CT scanning.
Plain renal tomography is most useful when monitoring a difficult-to-observe stone after therapy or for clarification of stones not clearly detected or identified with other studies.
Plain renal tomography is also useful for determining the number of stones present in the kidneys before a stone-prevention program is instituted. This information is used to better differentiate stones formed before therapy began from those formed later.
Laboratory Studies
UrinalysisApproximately 85% of patients with urinarycalculi exhibit gross or microscopic hematuria.
Complete blood cell count
In the context of nephrolithiasis, an elevated white blood cell count suggests renal or systemic infection.
A depressed red blood cell count suggests a chronic disease state or severe ongoing hematuria.
Serum electrolytes, creatinine, calcium, uric acid, parathyroid hormone (PTH), and phosphorus studies
Twenty-four–hour urine collection for levels of pH, calcium, oxalate, uric acid, sodium, phosphorus, citrate, magnesium, creatinine, and total volume . The following are objective indications for a metabolic evaluation with a 24-hour urinalysis:
Residual calculi after surgical treatment
Initial presentation with multiple calculi
Initial presentation before age 30 years
Renal failure
Solitary kidney (including renal transplant)
Family history of calculi
More than one stone in the past year
Bilateral calculi
Patient preference: An important consideration in determining whether to perform a 24-hour urine study is the patient's interest. If a patient is strongly motivated to follow a protracted stone-prevention treatment plan (involving diet, supplements, medications, or a combination), obtain the study. If a patient is unlikely or unwilling to follow a long-term treatment plan, a metabolic evaluation is probably unwarranted. Patients have to understand that stone disease is a chronic disease. If they do not commit to helping themselves in behavior modification, dietary changes, or medical compliance, they are prone to more frequent calculi formation.
Hypercalciuria can be subdivided into absorptive, resorptive, and renal-leak categories based on the results of blood tests and 24-hour urinalysis on both regular and calcium-restricted diets.
treatment of absorptive hypercalciuria may include modest dietary calcium restriction, thiazide, oral calcium binders, or phosphate supplementation.
Resorptive hypercalciuria is primary hyperparathyroidism and requires parathyroidectomy, when possible. If parathyroid surgery is not possible, phosphate supplementation is usually recommended.
Renal-leak hypercalciuria, which is less common than absorptive hypercalciuria, is usually associated with secondary hyperparathyroidism and is best managed with thiazide diuretics.
Indiscriminate dietary calcium restriction is not advantageous and in fact may increase formation of calculi owing to a secondary increase in oxalate absorption. The reduced dietary calcium reduces the oxalate-binding sites in the gastrointestinal tract, increasing the free dietary oxalate and leading to increased oxalate absorption. The final product of this is a net increase in stone production.
Hyperoxaluria may be primary (a rare genetic disease), enteric (due to malabsorption and associated with chronic diarrhea or short-bowel syndrome), or idiopathic. Oxalate restriction and vitamin B-6 supplementation are somewhat helpful in patients with idiopathic hyperoxaluria.
Enteric hyperoxaluria is the type that is most amenable to treatment; dietary calcium supplementation often produces dramatic results.
Calcium citrate is the recommended supplement because citrate tends to further reduce stone formation. Calcium carbonate supplementation is less expensive but does not provide citrate's added benefit. Calcium therapy works as an oxalate binder, reducing oxalate absorption from the intestinal tract. Calcium should be administered with meals, especially those that contain high-oxalate foods. The supplement should not contain added vitamin D because this increases calcium absorption, leaving less calcium in the intestinal tract to bind to oxalate. The optimal 24-hour urine oxalate level is 20 mg/d or less.
Hyperuricosuria predisposes to the formation of calcium-containing calculi because sodium urate can produce malabsorption of macromolecular inhibitors or can serve as a nidus for the heterogeneous growth of calcium oxalate crystals. Gouty diathesis, a condition of increased stone production associated with high serum uric acid levels, is also possible. Therapy involves potassium citrate supplementation, allopurinol, or both. In general, patients with pure uric acid stones and hyperuricemia are treated with allopurinol, and those with hyperuricosuric calcium stones are treated with citrate supplementation. The optimal 24-hour urine uric acid level is 600 mg/d or less.
Sodium and phosphorus
Excess sodium excretion can contribute to hypercalciuria by a phenomenon known as solute drag. Elevated urinary sodium levels are almost always associated with dietary indiscretions. Decreasing the oral sodium intake can decrease calcium excretion, thereby decreasing calcium saturation.An elevated phosphorus level is useful as a marker for a subtype of absorptive hypercalciuria known as renal phosphate leak (absorptive hypercalciuria type III). Renal phosphate leak is identified by high urinary phosphate levels, low serum phosphate levels, high serum 1,25 vitamin D-3 (calcitriol) levels, and hypercalciuria. This type of hypercalciuria is uncommon and does not respond well to standard therapies.
Citrate and magnesium
Magnesium and, especially, citrate are important chemical inhibitors of stone formation. Hypocitraturia is one of the most common metabolic defects that predispose to stone formation, and some authorities have recommended citrate therapy as primary or adjunctive therapy to almost all patients who have formed recurrent calcium-containing stones.A pH level of 6.5 is usually considered optimal. A pH level over 7.0 should be discouraged, as it prompts calcium phosphate precipitation.
lemon juice provide an excellent source of citrate, or, alternatively, large quantities of lemonade can be ingested, which, of course, has the added benefit of providing increased fluid intake.
Potassium citrate is the preferred type of pharmacologic citrate supplement, although a potassium/magnesium preparation is under investigation.
Magnesium is a more recently recognized inhibitor of stone formation, and the clinical role of magnesium replacement therapy is less well defined than that of citrate.
PH: Some stones, such as those composed of uric acid or cystine, are pH-dependent, meaning that they can form only in acidic conditions.
Calcium phosphate and struvite only form when the urine pH is alkaline. Although the other parameters in the 24-hour urine usually identify patients at risk of forming these stones, pH studies can be important in monitoring these patients, in optimizing therapy with citrate supplementation, and in identifying occult stone disease in some patients.
If a patient older than 40 years has formed a single stone that passed spontaneously or was easily treated, follow-up care for recurrent stones may be unnecessary. This patient is at a reasonably low risk for recurrence if adequate fluid intake is maintained.
Indications for comprehensive metabolic evaluation
• Family history of urolithiasis
• bilateral stone disease
• inflammatory bowel disease, chronic diarrhoea, or malabsorption
• History of bariatric surgery
• Concurrent medical conditions associated with urolithiasis (primary hyperparathyroidism, gout, renal tubular acidosis)
• Presence of nephrocalcinosis
• Presence of osteoporosis or pathological skeletal fractures
• Stones are formed from cystine, uric acid, or calcium phosphate
• The patient is a child
Components of a comprehensive metabolic evaluation
Analysis of stone compositionTwo 24 hour urine collections for:
a-Volume, pH, calcium, oxalate, citrate, uric acid, phosphate, sodium, potassium, magnesium, ammonium, chloride, sulfate, and creatinine
b-Cystine screen
serum calcium, bicarbonate, creatinine, chloride, potassium, magnesium, phosphate, and uric acid
Measurement of blood urea nitrogen
In cystinuric patients, evaluation as above and 24 hour measurement of cystine
In hypercalcaemic patients, intact parathyroid hormone and 1,25 dihydroxyvitamin D
Complications
• Abscess formation
• Serious infection of the kidney that diminishes renal function
• Urinary fistula formation
• Ureteral scarring and stenosis
• Ureteral perforation
• Extravasation
• Urosepsis
• Renal loss due to long-standing obstruction
The usually quoted recurrence rate for urinary calculi is 50% within 5 years and 70% or higher within 10 years.
Metabolic evaluation and treatment are indicated for patients at greater risk for recurrence, including those who present with multiple stones, who have a personal or family history of previous stone formation, who present with stones at a younger age, or who have residual stones after treatment.
Prognosis
Increased fluids and dietary moderation can cut the stone recurrence rate by 60%. The most morbid and potentially dangerous aspect of stone disease is the combination of urinary tract obstruction and upper urinary tract infection. Pyelonephritis, pyonephrosis, and urosepsis can ensue. Early recognition and immediate surgical drainage are necessary in these situations.• After diagnosing renal (ureteral) colic, determine the presence or absence of obstruction or infection.
• Obstruction in the absence of infection can be initially managed with analgesics and with other medical measures to facilitate passage of the stone.
• Infection in the absence of obstruction can be initially managed with antimicrobial therapy. In either case, promptly refer the patient to a urologist.
• If neither obstruction nor infection is present, analgesics and other medical measures to facilitate passage of the stone can be initiated with the expectation that the stone will likely pass from the upper urinary tract if its diameter is smaller than 5-6 mm (larger stones are more likely to require surgical measures).
• If both obstruction and infection are present, emergent decompression of the upper urinary collecting system is required (see Surgical Care). Immediately consult with a urologist for patients whose pain fails to respond to ED management.
General guidelines for emergency management
Treatment
The cornerstone of ureteral colic management is analgesia, which can be achieved most expediently with parenteral narcotics or nonsteroidal anti-inflammatory drugs (NSAIDs).
Morphine sulfate is the narcotic analgesic drug of choice for parenteral use.
Ketorolac tromethamine is the only NSAID approved for parenteral use in the United States, and it is often effective when used for renal colic.
Antiemetic agents such as metoclopramide HCl and prochlorperazine may also be added as needed.
If oral intake is tolerated, the combination of
oral narcotics (eg, codeine, oxycodone, hydrocodone, usually in a combination form with acetaminophen), NSAIDs, and antiemetics, as needed, is a potent outpatient management approach for renal colic.
Treatment approach has recently been improved with the application of active medical expulsive therapy (MET). NSAIDs have ureteral-relaxing effects and, as such, can be considered a form of MET . MET should be considered in any patient with a reasonable probability of stone passage. Stones smaller than 3 mm are already associated with an 85% chance of spontaneous passage, and, as such, MET is probably most useful for stones 3-10 mm in size. Overall, MET is associated with a 65% greater likelihood of stone passage.
Although corticosteroids are effective, concerns about their side effects limited the acceptance of MET.
The calcium channel blocker nifedipine relaxes ureteral smooth muscle and enhances stone passage.
The alpha-blockers, such as terazosin, and the alpha-1 selective blockers, such as tamsulosin, also relax musculature of the ureter and lower urinary tract, markedly facilitating passage of ureteral stones.
MET with calcium channel blockers and alpha-blockers also appear to improve the results of extracorporeal shock-wave lithotripsy.
Analgesic therapy combined with MET dramatically improves the passage of stones, addresses pain, and reduces the need for surgical treatment. A typical regimen for this aggressive management is 1-2 oral narcotic/acetaminophen tablets every 4 hours as needed for pain, 600-800 mg ibuprofen every 8 hours, and MET with 30 mg nifedipine extended-release tablet once daily, 0.4 mg tamsulosin once daily, or 4 mg of terazosin once daily. Limit MET to a 10- to 14-day course, as most stones that pass during this regimen do so in that time frame. If outpatient treatment fails, promptly consult a urologist.
Urinary calculi composed predominantly of calcium cannot be dissolved with current medical therapy; however, medical therapy is important in the long-term chemoprophylaxis of further calculus growth or formation.
Prophylactic therapy might include limitation of dietary components, addition of stone-formation inhibitors or intestinal calcium binders, and, most importantly, augmentation of fluid intake.
Besides advising patients to avoid excessive salt and protein intake and to increase fluid intake, base medical therapy for the chronic chemoprophylaxis of urinary calculi on the results of a 24-hour urinalysis for chemical constituents.
Uric acid and cystine calculi
can be dissolved with medical therapy. Patients with uric acid stones who do not require urgent surgical intervention for reasons of pain, obstruction, or infection can often have their stones dissolved with alkalization of the urine.
Sodium bicarbonate can be used as the alkalizing agent, but potassium citrate is usually preferred because of the availability of slow-release tablets and the avoidance of a high sodium load.
The dosage of the alkalizing agent should be adjusted to maintain the urinary pH between 6.5 and 7.0. Urinary pH of more than 7.5 should be avoided because of the potential deposition of calcium phosphate around the uric acid calculus, which would make it undissolvable. Both uric acid and cystine calculi form in acidic environments.
Even very large uric acid calculi can be dissolved Roughly 1 cm per month dissolution can be achieved.
Practical ability to alkalinize the urine significantly limits the ability to dissolve cystine calculi. Chemoprophylaxis of uric acid and cystine calculi consists primarily of long-term alkalinization of urine.
If hyperuricosuria or hyperuricemia is documented in patients with pure uric acid stones (present in only a relative minority), allopurinol (300 mg qd) is recommended because it reduces uric acid excretion.
Pharmaceuticals that can bind free cystine in the urine (eg, D-penicillamine, 2-alpha-mercaptopropionyl-glycine) help reduce stone formation in cystinuria. Therapy should also include long-term urinary alkalinization and aggressive fluid intake. Captopril has been shown to be effective in some trials, although, again, strong data are lacking. Routine use should be avoided but can be added in patients who have difficulty in dissolving and preventing cystine stones.
Surgical CareEndoscopic surgery is often required for stones, but open surgery is now almost never needed except for large bladder stones. All stones are potentially infected and surgery should be covered with appropriate antibiotics.
The primary indications for surgical treatment include pain, infection, and obstruction.
General contraindications to definitive stone manipulation include the following:
• Active, untreated urinary tract infection
• Uncorrected bleeding diathesis
• Pregnancy (a relative contraindication)
For an obstructed and infected collecting system secondary to stone disease, virtually no contraindications exist for emergency surgical relief either by ureteral stent placement (a small tube placed endoscopically into the entire length of the ureter from the kidney to the bladder) or by percutaneous nephrostomy (a small tube placed through the skin of the flank directly into the kidney).
The vast majority of symptomatic urinary tract calculi are now treated with noninvasive or minimally invasive techniques, while open surgical excision of a stone from the urinary tract is now limited to isolated atypical cases.
In general, stones that are 4 mm in diameter or smaller will probably pass spontaneously, and stones that are larger than 8 mm are unlikely to pass without surgical intervention. With MET, stones 5-8 mm in size often pass, especially if located in the distal ureter.
The 2005 American Urological Association staghorn calculus guidelines recommend
percutaneous nephrostolithotomy as the cornerstone of management.
In the ureteral stone guidelines produced by a joint effort of the American Urological Association and the European Association of Urology, ESWL and ureteroscopy are both recognized as first-line treatments for ureteral stones.
Extracorporeal shockwave lithotripsy ESWL
Most urinary tract calculi that require treatment are currently managed with this ESWL, which is the least invasive of the surgical methods of stone removal. ESWL is limited somewhat by the size and location of the calculus.A stone larger than 1.5 cm in diameter or one located in the lower section of the kidney is treated less successfully. Fragmentation still occurs, but the large volume of fragments or their location in a dependent section of the kidney precludes complete passage. In addition, results may not be optimal in large patients, especially if the skin-to-stone distance exceeds 10 cm.
Ureteroscopy
Ureteroscopic manipulation of a stone is the next most commonly applied modality. A small endoscope, which may be rigid, semirigid, or flexible, is passed into the bladder and up the ureter to directly visualize the stone.The typical patient has acute symptoms caused by a distal ureteral stone, usually measuring 5-8 mm. This calculus can be rapidly addressed with miniaturized instruments. A stone can be either directly extracted using a basket or grasper or broken into small pieces using various lithotrites (eg, laser, ultrasonic, lectrohydraulic, ballistic).
Often, a ureteral stent must be placed following this procedure in order to prevent obstruction from ureteral spasm and edema. A ureteral stent is often uncomfortable; consequently, many urologists eschew stent placement following ureteroscopy in selected patients.
allows fragmentation and removal of large calculi from the kidney and ureter and is often used for the many ESWL failures. A needle, and then a wire, over which is passed a hollow sheath, are inserted directly in the kidney through the skin of the flank.
Percutaneous access to the kidney typically involves a sheath with a 1-cm lumen. Relatively large endoscopes with powerful and effective lithotrites can be used to rapidly fragment and remove large stone volumes.
Percutaneous nephrostolithotomy
Because of their increased morbidity compared with ESWL and ureteroscopy, percutaneous procedures are generally reserved for large and/or complex renal stones and failures from the other two modalities.In some cases, a combination of ESWL and a percutaneous technique is necessary to completely remove all stone material from a kidney. This technique, called sandwich therapy, is reserved for staghorn or other complicated stone cases.
Diet
Increase in fluid intake and, therefore, an increase in urine output is likely the single most important aspect of stone prophylaxis.
The other dietary guidelines are to avoid excessive salt and protein intake. Moderation of calcium and oxalate intake is also reasonable, Excessive dietary calcium restriction can increase the risk of calcium oxalate stone formation .
Dietary calcium should not be restricted beyond normal unless specifically indicated based on 24-hour urinalysis findings. Urinary calcium levels are normal in many patients with calcium stones. Reducing dietary calcium in these patients may actually worsen their stone disease, because more oxalate is absorbed from the gastrointestinal tract in the absence of sufficient intestinal calcium to bind with it. This results in a net increase in oxalate absorption and hyperoxaluria, which tends to increase new kidney stone formation in patients with calcium oxalate calculi. An empiric restriction of dietary calcium may also adversely affect bone mineralization and may have osteoporosis implications, especially in women. This practice should be condemned unless indicated based on a metabolic evaluation.
As a rule, dietary calcium should be restricted to 600-800 mg/d in patients with diet-responsive hypercalciuria who form calcium stones. This is roughly equivalent to a single high-calcium or dairy meal per day.
High-protein diets are associated with low-grade, chronic metabolic acidosis, which can increase urinary nitrogen and calcium excretion, stimulate muscle breakdown and negatively influence bone remodeling,
High-protein diet was associated with significant increases in net acid excretion, urinary calcium and urinary nitrogen, as well as an increase in serum levels of insulin-like growth factor (IGF). Supplementation with KHCO3 reduced by almost half the rise in urinary nitrogen excretion that accompanied increased protein intake, KHCO3 was also associated with increased levels of IGF-1, which the authors speculate may be the mediator of KHCO3-induced nitrogen sparing and calcium absorption.
Bilateral staghorn calculi. The intravenous pyelogram demonstrates that, while some dye is being excreted by the right kidney, there is little function on the left.
Surgical options for urinary stones.
Calcium, cystine, and struvite stones are all radiopaque, whereas pure uric acid, indinavir-induced are relatively radiolucent on plain radiography.
Staghorn Calculi: struvite , calcium carbonate apatite.
cystine or uric acid,either in pure form or mixed with other components, can grow in a "staghorn“.
(but calcium oxalate or phosphate stones only rarely grow in this configuration. )
They gradually fill the renal pelvis and may extend outward through the infundibula to the calyces themselves. Very large staghorn stones can have surprisingly few symptoms and may lead to the eventual loss of kidney function.
Struvite stones are caused by bacterial infection that hydrolyzes urea to ammonium and raises urine pH to neutral or alkaline values.
Urea-splitting organisms (urease-producing bacteria) include Proteus, Pseudomonas, Klebsiella, Staphylococcus, and Mycoplasma.
The solubility of calcium oxalate is not influenced by changes in urine pH.
• Inhibitors of Crystal Formation• Urine contains potent inhibitors of nucleation, growth, and aggregation for calcium salts. Inorganic pyrophosphate is a potent inhibitor that appears to affect formation of calcium phosphate more than calcium oxalate crystals. Citrate inhibits crystal growth and nucleation, although most of the stone inhibitory activity of citrate is due to lowering urine supersaturation via complexation of calcium. Other urine components such as glycoproteins inhibit calcium oxalate crystallization.
• Increasing urine volume to 2.5 L per day resulted in a 50% reduction of stone recurrence compared to the control group. high fluid intake so that the urine specific gravity remains at ≤ 1.005 throughout the day and night.
• Hypocitraturia
• Urine citrate prevents calcium stone formation by creating a soluble complex with calcium, effectively reducing free urine calcium. Hypocitraturia is found in 20–40% of stone formers, either as a single disorder or in combination with other metabolic abnormalities. It can be secondary to systemic disorders, such as RTA, chronic diarrheal illness, or hypokalemia, or it may be a primary disorder, in which case it is called idiopathic hypocitraturia.• Treatment
• Treatment is with alkali, which increases urine citrate excretion; generally bicarbonate or citrate salts are used. Potassium salts are preferred as sodium loading increases urinary excretion of calcium, reducing the effectiveness of treatment.
About 20% of calcium oxalate stone formers are hyperuricosuric, primarily because of an excessive intake of purine from meat, fish, and poultry. The mechanism of stone formation is probably due to salting out calcium oxalate by urate.
A low-purine diet is desirable but difficult for many patients to achieve. The alternative is allopurinol, which has been shown to be effective in a randomized, controlled trial.
A dose of 100 mg bid is usually sufficient.
Idiopathic Hypercalciuria
Is the most common metabolic abnormality found in patients with nephrolithiasis . It is familial and is likely a polygenic trait. diagnosed by the presence of hypercalciuria without hypercalcemia and the absence of other systemic disorders known to affect mineral metabolism. In the past, the separation of "absorptive" and "renal" forms of hypercalciuria was used to guide treatment. However, these may not be distinct entities. Vitamin D overactivity, either through high calcitriol levels or excess vitamin D receptor, is a likely explanation for the hypercalciuria in many of these patients.5-year prospective trial compared the efficacy of a low-calcium diet to a low-protein, low-sodium, normal-calcium diet in preventing stone recurrence in male calcium stone formers. The group on the low-calcium diet had a significantly greater rate of stone relapse. Low-calcium diets are of unknown efficacy in preventing stone formation and carry a long-term risk of bone disease in the stone-forming population. Low-sodium and low-protein diets are a superior option in stone formers. If diet therapy is not sufficient to prevent stones, then thiazide diuretics may be used. Thiazide diuretics lower urine calcium and are effective in preventing the formation of stones.
Three 3-year randomized trials have shown a 50% decrease in stone formation in the thiazide-treated groups as compared to the placebo-treated controls. The drug effect requires slight contraction of the extracellular fluid volume, and high dietary NaCl intake reduces its therapeutic effect.
Thiazide-induced hypokalemia should be aggressively treated since hypokalemia will reduce urine citrate, an important inhibitor of calcium crystallization.
Nephrocalcinosis:
Calcium stones grow on the papillae. Most break loose and cause colic, but they may remain in place so that multiple papillary calcifications are found by x-ray, a condition termed nephrocalcinosis. Papillary nephrocalcinosis is common in hereditary distal renal tubular acidosis (RTA) and in other types of severe hypercalciuria.In medullary sponge kidney disease , calcification may occur in dilated distal collecting ducts.
struvite crystals in urine, rectangular prisms said to resemble coffin lids .
cystinuria reveals typical hexagonal, platelike cystine crystals. Cystinuria can also be detected using the urine sodium nitroprusside test.Uric Acid Lithiasis: Treatment
The two goals of treatment are to raise urine pH and to lower excessive urine uric acid excretion to <1 g/d. Supplemental alkali, 1–3 mmol/kg of body weight per day, should be given in three or four divided doses, one of which should be given at bedtime. The goal of treatment should be a urine pH between 6.0 and 6.5 in a 24-h urine collection. Increasing urine pH above 6.5 will not provide additional benefit in preventing uric acid crystallization but does increase the risk of calcium phosphate stone formation.The form of the alkali may be important. Potassium citrate may reduce the risk of calcium salts crystallizing when urine pH is increased, whereas sodium citrate or sodium bicarbonate may increase the risk. A low-purine diet should be instituted in uric acid stone formers with hyperuricosuria. Patients who continue to form uric acid stones despite treatment with fluids, alkali, and a low-purine diet should have allopurinol added to their regimen.
Normal urine is sterile. UTI can therefore be diagnosed if a single viable gram negative bacterium inhabits the urinary tract (kidney, ureters, bladder). In reality, the bacteria causing UTI multiply in log phase growth in normal urine, and most people with urinary tract infection have 104-106 bacteria/ml. The acute number will depend on the urine flow rate, characteristics of the urine, the duration of infection, etc. The problem in diagnosis is that of contamination arising from voided specimens passing through the non-sterile distal urethra. For this reason, clinicians use the criteria of 105 bacteria/ml of “clean catch” urine to diagnose UTI.
At this level (105 bacteria/ml), < 1% of the represent contaminants. At counts of 1000-10,000/ml, there is a 50/50 chance the result represents contamination. Such a count may represent true infection, but to be sure a second culture showing the same organism might be more convincing.
The second criteria for diagnosing UTI is the presence of pyuria
(> 5 WBC/HPF) on the urinalysis.
Suprapublic aspirates done under sterile conditions are the “gold standard” for diagnosing UTI, and would reveal the true populations of infected and non-infected women. Clean catch collections or samples collected by “sterile” bladder catheterization would approximate this distribution but due to many contaminated cultures there would be many more false positive cultures. Random voided cultures are essentially useless – a positive culture is much more likely to represent contamination than true infection. In practice, use the clean catch or bladder catheterization technique.
February 6, 2009 — In patients with non-advanced chronic kidney disease, elevated urinary and serum levels of neutrophil gelatinase-associated lipocalin (NGAL) are a strong and independent predictor of disease progression, researchers from Italy have found.
NGAL is a small 25-kD protein massively released from renal tubular cells after various injuring stimuli, Dr. Michele Buemi and colleagues from University of Messina explain in their report published online ahead of print in the February issue of the Clinical Journal of the American Society of Nephrology. patients with higher baseline NGAL levels showed a considerably increased risk of worsening renal function within 1 year compared with those with lower baseline NGAL levels.
Independent predictors of progression of CKD in patients with nonterminal CKD are eGFR, fibrinogen, urinary NGAL levels, and serum NGAL levels.
Urinary NGAL levels and serum NGAL levels are independent predictors of progression of CKD, and an increase of 10 ng/mL predicts a 2% to 3% risk for progression.
Which of the following is least likely to be an independent predictor of progression in patients with nonterminal CKD?
• Serum NGAL levels
• Urinary NGAL levels
• eGFR
• Proteinuria
Most often, the urine pH is not altered at all by the infection.the normal urine pH is very variable, but usually in the range of 6.0 -- almost always less than 7.
Only bacteria that contain the enzyme "urease" will cause an obvious elevation in urine pH. This enzyme will split urea into ammonia plus CO2. The ammonia then combines with water, forming ammonium ions plus hydroxyl ions, thus raising the pH. So infections with "urease positive" bacteria can raise the pH of the urine to something higher than 7 -- as high as 8.0.
Having said this, you need to know that E. coli causes roughly 90% of urinary infections, and this bacterium does NOT contain the enzyme urease. Therefore in over 90% of urinary infections, you will NOT see elevation of urine pH.
The urine pH in almost all infections is normal, therefor.Classically, if you DO see a high urine pH, you are supposed to look for proteus infection, which can result in the formation of struvite stones.
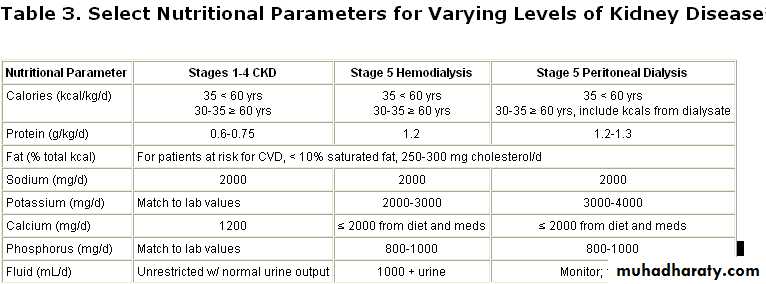
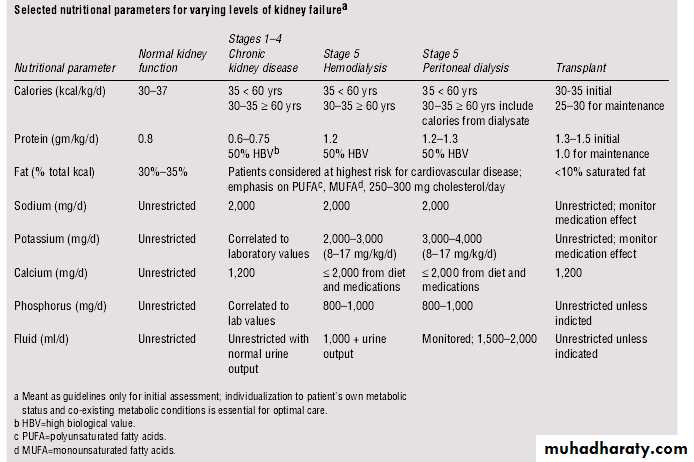
Nutrition and Renal Disease
According to K/DOQI guidelinesNational Kidney Foundation's Kidney Dialysis Outcome Quality Initiative (K/DOQI)
in CKD patients stage 1-4, sodium is restricted to 2000 mg/d, calcium is restricted to 1200 mg/d, and potassium and phosphorus intakes should be correlated with laboratory values. Fluid intake can be unrestricted assuming normal urine output. Careful monitoring of laboratory values is necessary.
In stage 5, potassium, phosphorus, and fluid, as well as sodium and calcium, are restricted, depending upon the type of dialysis the patient is undergoing.
Patients on dialysis (stage 5) are known to lose certain water-soluble vitamins. However, patients in renal failure have decreased excretion of vitamin A, and vitamin A toxicity has been reported in some cases. Therefore, patients on dialysis should receive a multivitamin supplement that avoids excessive vitamin A.
The severity of chronic kidney disease (CKD) is described by 6 stages, the most severe three are defined by the MDRD Modification of Diet in Renal Disease (MDRD) formula -e(estimated)GFR value, and first three also depend whether there is other evidence of kidney disease (e.g. proteinuria):
0) Normal kidney function – GFR above 90mL/min/1.73m2 and no proteinuria
1) CKD1 – GFR above 90mL/min/1.73m2 with evidence of kidney damage
2) CKD2 (Mild) – GFR of 60 to 89 mL/min/1.73m2 with evidence of kidney damage
3) CKD3 (Moderate) – GFR of 30 to 59 mL/min/1.73m2
4) CKD4 (Severe) – GFR of 15 to 29 mL/min/1.73m2
5) CKD5 Kidney failure (dialysis or kidney transplant needed) – GFR less than 15 mL/min/1.73m2
Current K/DOQI guidelines suggest a protein intake of 0.6-0.75 grams of protein per kilogram of body weight per day (g/kg/d) for patients in stages 1-4 of CKD.
In stage 5, when patients are receiving dialysis, increased protein intake is suggested (approx. 1.2 g/kg/d).
most (but not all) literature suggests that decreasing protein intake in CKD stages 1-4 can delay progression into stage 5.
Also, the type of protein consumed by a patient needs to be considered. This is because greater than 50% of CKD stage 5 patients ultimately die of cardiovascular events. While in stage 5, proteins of high biologic value are recommended, which typically means proteins from animals, milk, and eggs. It is well known that there are higher-fat and higher-cholesterol choices associated with this group of foods which could predispose patients already at risk for cardiovascular disease. Careful dietary counseling can steer patients in the direction of healthier protein choices of high biologic value. An example of lower-fat and -cholesterol protein items to substitute is given below. ( Table 1 ).
Finally, the amount of calories consumed in addition to the protein reduction is critical.
Enough calories need to be consumed by CKD patients in stages 1-4 to spare protein from being used as a fuel. This prevents loss of lean muscle mass and protein-calorie malnutrition. Because of the cardiovascular risk discussed above, these extra calories should not come from foods that increase risk for cardiovascular disease.
35 kcals per kilogram of body weight per day for patients younger than 60 years for all 5 stages. An example of foods to add to the diet to increase calorie intake is given below .
Decreased Intake
AnorexiaGastroparesis
Intraperitoneal instillation of dialsate in CAPD
Uremia
Increased Leptin
• Diet Restrictions
• Loss of nutrients in dialysate• Concurrent illness and hospitalizations
• Increased inflammatory and catabolic cytokines
• Chronic blood loss
• Acidosis
• Accumulation of toxins such as aluminum
• Endocrine disorders
• Insulin resistance
• Hyperglucogonemia
Factors contributing to malnutrition in renal failure
Diet for Nephrotic Syndrome
A well-planned diet can replace lost protein and ensure efficient utilization of ingested proteins through provision of adequate calories. Dietary changes can also help control hypertension, edema, and hyperlipidemia, and slow the progression of renal disease.Protein: High-protein diets are not recommended as they may encourage damage to the nephrons, leading to a progression of renal insufficiency. Since albumin losses in nephrotic patients are due to increased catabolism, rather than a reduction in protein synthesis, low-protein diets, which decrease catabolism, may be more beneficial.
The optimal amount of dietary protein necessary to prevent protein catabolism and progression of renal disease has not been established. A common recommendation is 0.6 grams of protein per kilogram of ideal body weight, adjusted depending on the glomerular filtration rate and nutritional status, plus gram-for-gram replacement of urinary protein losses.
Sodium and Fluid: A limit on sodium of 1-3 grams per day is usually recommended to control edema and hypertension. Diuretics may also be used. A fluid restriction is not warranted unless renal failure occurs.
Lipids: A diet low in saturated fat and cholesterol, combined with loss of excess weight, is recommended to reduce the risk of cardiovascular disease. Many clinicians recommend limiting cholesterol to less than 300 milligrams per day and fat intake to 30 percent of calories. low-fat vegetarian diets are much more effective for lipid control. Cholesterol-lowering drugs can be used adjunctively if needed.
Energy: Calorie intake should be adequate to achieve and maintain ideal body weight and maintain protein stores. Foods rich in complex carbohydrates should provide the majority of calories.
Supplements: Patients with nephrotic syndrome are often low in B vitamins and zinc, and can benefit from supplements. In addition, since a significant portion of serum calcium is protein-bound, it tends to be low when serum proteins are reduced. No modification is routinely needed for potassium, but potassium losses due to secondary hyperaldosteronism may require replacement.
Nonsurgical Treatments for Urinary incontinence in women
Urinary incontinence in women is a common problem that adversely affects quality of life. In general, urinary incontinence affects about 19% of women age 19 to 44 years, 25% of those age 45 to 64 years, and 30% of those age 65 years and older. Moderate levels of evidence suggest thatpelvic floor muscle and bladder training resolved urinary incontinence in women. Anticholinergic drugs resolved urinary incontinence, with similar effects from oxybutynin or tolterodine. Duloxetine improved but did not resolve urinary incontinence. The effects of electrostimulation, medical devices(urethral insert device , A disposable intravaginal device) , injectable bulking agents(intraurethral collagen injection) , and local estrogen therapy were inconsistent.
Oral hormone administration resulted in higher rates of urinary incontinence in most studies . In contrast, transdermal or vaginal administration of estrogen resulted in inconsistent improvement in urinary incontinence . The highest rates of continence were reported after transdermal administration of an estrogen patch (100%) and estrogen gel (90%) among postmenopausal women with self-reported urinary symptoms . Topical estrogen in suppositories or creams combined with physiotherapy and electrical stimulation resolved urinary incontinence in 22% of women age 50 to 74 years with regular mild incontinence (>2 leakage episodes per month) compared with 0% in the control group, which did not receive hormone treatment.
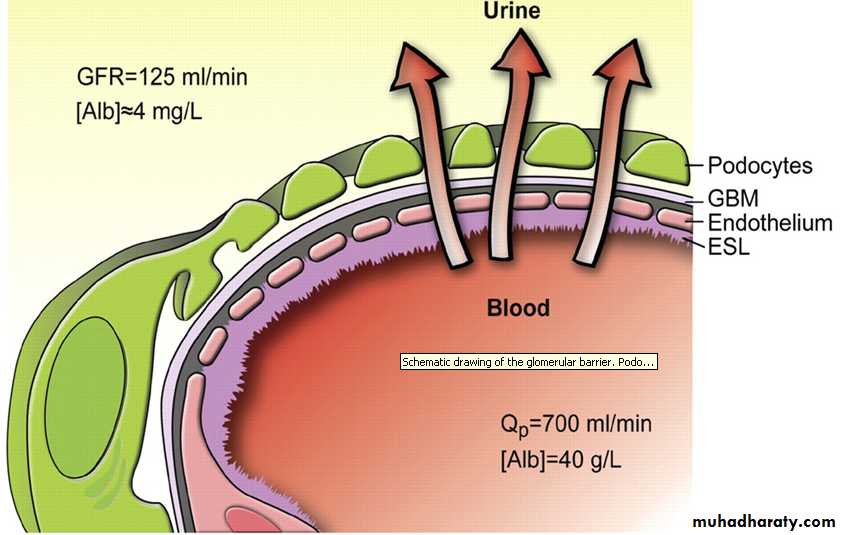
Creatinine clearance approximation of GFR
In clinical practice, however, creatinine clearance is used to measure GFR. Creatinine is produced naturally by the body (creatinine is a metabolite of creatine, which is found in muscle). It is freely filtered by the glomerulus, but also actively secreted by the renal tubules in very small amounts such that creatinine clearance overestimates actual GFR by 10-20%. This margin of error is acceptable considering the ease with which creatinine clearance is measured. Unlike precise GFR measurements involving constant infusions of inulin, creatinine is already at a steady-state concentration in the blood and so measuring creatinine clearance is much less cumbersome.Indications and contraindications of dialysis
Indications for Dialysis -In Chronic Renal FailureIn patients with chronic renal failure factors to be considered before initiating dialysis should include comorbid conditions and patient preference. Timing of therapy is dictated by serum chemistries and symptoms.
A) Absolute indications
Uremic PericarditisUremic Encephalopathy or Neuropathy
Pulmonary edema (unresponsive to diuretics)
Severe Hypertension
Severe hyperkalemia
Intractable acidosis
Severe Bleeding diathesis
Persistent gastrointestinal symptoms
S.Creatinine more than 12 mg/dl, BUN more than 100 mg/dl
B) Relative indications
Mild encephalopathy or neuropathy
Severe edema (unresponsive to diuretics)
Progressive gastrointestinal symptoms
Recurrent GI “itis”: stomatitis, gastritis, dudenitis, pancreatitis
Ascitis without hepatic disease
Anemia refractory to Erythropoietin
Mild Bleeding diathesis
Pruritus
Infectious complications
Depression
C) Early indications
Decrease ideal body weightDecrease in muscle mass
Decrease in s.albumin to less than 4 g/l
GFR less than 15 ml/min
S. Creatinine >10 mg/dl and bun >100 mg /dl
Decrease in s.transferrin
Low total cholesterol
Growth retardation in children
• D) Specific indications for peritoneal dialysis
• Patients with cardiovascular or hemodynamic instability
• Hemodialysis patients with vascular access failure or can not be created (e.g. diabetic patients(
• High risk of anticoagulation
• Patients in the older age group (over 65) and small children
• Severe hemodialysis-related symptoms or disequilibrium
• Social reason
• Indications for Dialysis other than chronic renal failure
• Acute renal failure• Poisons and Drug intoxication
• Hypercalcaemia
• Hyperuricemia
• Hypothermia
• Metabolic alkalosis.
Dialyzable drugs and Poisons
a.Barbiturates : Phenobarbital -Pentobarbital -b.Alcohol's : Methanol -Ethanol -Ethylene glycol -
c.Analgesics : Acetylsalicylic acid -Methylsalicylate
d.Metals : Calcium -Potassium -Sodium -Lithium
e.Endogenous toxins : Uric acid Uremic toxins -Hyperosmolar state
f.Halides : Bromide
e.Miscellaneous : Theophylline -Mannitol -Radiocontrast -Thiocynate -
Boric acid –Aniline
NB Dialysis for poisoning should be considered only when supportive measures are ineffective or there is impending irreversible organ toxicity.
Hemoperfusion is required in some cases
Factors Determinants for Dialysis
Factors determining the mode of chronic dialysis should include medical and non medical factors which have an impact on the treatment modality. Physicians have the responsibility to discuss the therapeutic options and offer their advice and recommendations about the choices. In general renal transplantation should be recommended as the preferred mode of renal replacement therapy in whom surgery and immunosupression is safe and feasible.• Medical Factors
• Age• Comorbid medical illnesses
• Patient survival
• Patient rehabilitation
• Quality of life
• Non Medical Factors
• Government-imposed Economic limitations
• Physician and Patient bias
• Resource availability
• Social ,Religious ,Cultural mores
• Availability of transplantation
• Family support
• Cost , race , sex , reimbursement
Contraindications of Dialysis therapy
Principally there is no absolute contraindication to dialysis therapy. Advanced age in and of itself is not a contraindication to dialysis therapy. Many elderly are physiologically equivalent to young patients.
• Relative contraindications to dialysis therapy
• Advanced malignancy (except multiple myeloma(• Alzheimer’s disease
• Multi-infarct dementia
• Hepatorenal syndrome
• Advanced liver cirrhosis with encephalopathy
• Hypotension unresponsive to pressors
• Terminal illness
• Organic brain syndrome
• Absolute
• Peritoneal fibrosis• Pleuroperitoneal leak
• Relative Major
• Chronic Ostomies
• Severe hypercatabolic state
• Fresh aortic prosthesis
• Recent Abdominal surgery
• Recent Thoracic surgery
• Extensive Abdominal adhesions
• Quadriplegia
• Blindness
• Physical handicaps
• Mental Retardation
• Polycystic Kidney disease
• Diverticulosis
• Obesity
• Peripheral vascular disease
• Hyperlipidemia
• Social
Complications during Hemodialysis
a. Medical Complications
Common Complications during Hemodialysis
1- Hypotension (20-30% of dialyses)
2- Muscle Cramps (5-20% of dialyses)
3- Nausea and Vomiting (5-15%of dialyses)
4- Headache (5% of dialyses)
5- Chest Pain (2-5% of dialyses)
6- Back Pain (2-5%of dialyses)
7- Itching (5% of dialyses)
8- Fever and chills (<1%of dialyses)
Serious complications during hemodialysis
1-Disequilibrium syndrome2-First use syndrome
3-Arrhythmia
4-Cardiac tamponade
5-Intracranial bleeding
6-Seizures
7-Hemolysis
8-Air embolism
b. Mechanical Complications
1.Rupture dialyzer
2.Clotted dialyzer
3. Air embolism
4.High conductivity
5.Low conductivity
6.Low water pressure
7.High venous pressure
8.Abnormal arterial pressure
9.Electrical failure
10.Needle-site bleeding
11.Hemolysis
Dialysis disequilibrium syndrome
DDS, is the occurrence of neurologic signs and symptoms, attributed to cerebral edema,during or following shortly after intermittent hemodialysis. Classically, DDS arises in individuals starting hemodialysis due to chronic renal failure and is associated, in particular, with "aggressive" (high solute removal) dialysis. However, it may also arise in fast onset, i.e. acute renal failure in certain conditions. Clinical signs of cerebral edema, such as focal neurological deficits, papilledema and decreased level of consciousness.The liver produces urea in the urea cycle as a waste product of the digestion of protein
7 to 21 mg of urea nitrogen per 100 mlAn elevated BUN in the setting of a relatively normal creatinine may reflect
• A physiological response to a relative decrease of blood flow to the kidney (as seen in heart failure or dehydration) without indicating any true injury to the kidney.
• Excessive formation of urea seen in bleeding in the upper gastrointestinal tract. The nitrogenous compounds from the blood are resorbed as they pass through the rest of the GI tract and then broken down to urea by the liver
• Enhanced metabolism of proteins will also increase urea production, as may be seen with high protein diets, steroid use, burns, or fevers .
When the ratio of
BUN to creatinine is greater than 20, the patient is suspected of having pre-renal azotemia. This means that the pathologic process is unlikely to be due to intrinsic kidney damage.• A low BUN usually has little significance, but its causes include
• liver problems• malnutrition
• excessive alcohol consumption.
• Overhydration from intravenous fluids.
• pregnancy
Urea itself is not toxic. This was demonstrated by Johnson et al. by adding large amounts of urea to the dialysate of hemodialysis patients for several months and finding no ill effects. However, BUN is a marker for other nitrogenous waste. Thus, when renal failure leads to a buildup of urea and other nitrogenous wastes (uremia), an individual may suffer neurological disturbances such as altered cognitive function (encephalopathy), impaired taste (dysgeusia) or loss of appetite (anorexia). The individual may also suffer from nausea and vomiting, or bleeding from dysfunctional platelets. Prolonged periods of severe uremia may result in the skin taking on a grey discolouration or even forming frank urea crystals ("uremic frost") on the skin.
Because multiple variables can interfere with the interpretation of a BUN value, GFR and creatinine clearance are more accurate markers of kidney function. Age, sex, and weight will alter the "normal" range for each individual, including race. In renal failure or chronic kidney disease (CKD), BUN will only be elevated outside "normal" when more than 60% of kidney cells are no longer functioning.
mg /dL of BUN to mmol/L of urea, divide by 2.8
Urea = BUN × 2.14 ... (mg/dL)
• Albumin is the main protein in the blood. Proteins are the building blocks for all body parts, including muscles, bones, hair, and nails. Proteins in the blood also perform a number of important functions. They protect the body from infection, help blood clot, and keep the right amount of fluid circulating throughout the body.
Microalbuminuria is defined as an albumin excretion rate (AER) above normal but below that detectable by certain dipstick methods (e.g. Albustix). There are different ways of expressing microalbuminuria, which has in the past caused confusion. In atimed overnight urine collection, microalbuminuria represents an AER of 20–200 μg/min. A positive microalbuminuria screen is defined as an
AER of 20–200 μg/min
or an albumin:creatinine ratio > 2 mg/mmol
in at least two of three consecutive collections.
Using microalbuminuria as a screening test in elderly patients will be subject to error due to urinary tract infection, prostatic disease and heart failure, all of which may cause raised AER unrelated to diabetic nephropathy. For this reason, it is our practice not to screen beyond the age of 70 years.
Microalbuminuria is not in itself diagnostic of diabetic
nephropathy or glomerular damage since other renal disease will increase AER, as will strenuous exercise, urinary or systemic infection,or contamination during menstruation. It is lowest when the subject is lying down and the preferred urine specimen is the one collected immediately on rising. Microalbuminuria is also found in patients with hypertension and cardiovascular disease who do not have diabetes. Microalbuminuria can occur within one year of type 1 diabetes and therefore early screening seems logical. However, persistent elevation of AER is more common after five years.It has been known since 1982 that microalbuminuria is predictive of the development of overt diabetic kidney disease and subsequent progressive renal failure. In type 2 diabetes, it predicts not only nephropathy but also premature death from cardiovascular or cerebrovascular disease. In the presence of microalbuminuria, there is evidence of significant endothelial dysfunction. Markers such as transcapillary albumin escape rate, circulating levels of Von Willebrand factor, plasminogen activator inhibitor (PAI-1), thrombomodulin, homocysteine and fibrinogen can be found to be abnormal .
The prevalence of microalbuminuria
type 1 diabetes has been quoted at 4% but increases after 15 years duration of the illness to more than 20%.type 2 diabetes, the prevalence is between 20–36%
Microalbuminuria is diagnosed either from
24-hour urine collection(20 to 200 µg/min)
(30 to 300 mg/L or day) or
albumin:creatinine ratio > 2 mg/mmol on at least two occasions. ≥2.8 mg/ mmol (female)
An albumin level above these values is called "macroalbuminuria", or sometimes just albuminuria
ACR, defined as
normal (ACR 30 mg/g)mild (ACR30-300 mg/g)
heavy (ACR 300 mg/g).
Microalbuminuria A value > 30 mg/g suggests microalbuminuria. The reliability of the test is best when a midmorning specimen is used, vigorous exercise is avoided before the test, and unusual creatinine production (in cachectic or very muscular patients) is not present. Microalbuminuria can occur in all of the following:
• Diabetes mellitus
• Hypertension
• Renal allograft dysfunction
• Preeclampsia
• UTI
Microalbuminuria is highly predictive of subsequent nephropathy in type 1 but not type 2 diabetes. Microalbuminuria is a risk factor for cardiovascular disorders and early cardiovascular mortality independent of diabetes or hypertension.
• The following five types of proteinuria are distinguished by milligrams (mg) of protein measured during a 24-hour urine collection
• Microalbuminuria (30–150 mg /24-hour urine)
• Mild (150–500 mg(
• Moderate (500–1000 mg(
• Heavy (1000–3000 mg(
• Nephrotic range (more than 3500 mg(
In fact, patients with heavy proteinuria but without overtly abnormal eGFR appeared to have worse clinical outcomes than those with moderately reduced eGFR but without
proteinuria. For example, the age-adjusted rates of all-cause mortality and kidney failure appear to vary up to 4- and 50-fold (depending on the severity of proteinuria)
within a given stage as defined by the current scheme.
Relation Between Kidney Function,
Proteinuria, and Adverse Outcomes
Similarly, a patient with an eGFR of 80 mL/min/1.73 m2 and 3 proteinuria on dipstick reading (or ACR of 400 mg/g) would be assigned to stage 1 CKD under the current system—even though his or her age-adjusted risks of death and the need for renal replacement therapy would be approximately 2 and 10 times higher, respectively, than an otherwise similar patient with an eGFR of 50 mL/min/1.73 m2 but no evidence of proteinuria (stage 3 disease).
In conclusion, we found that the risks
of death, myocardial infarction, and progressionto kidney failure at a given level of eGFR were independently increased in individuals with higher levels of proteinuria.
These findings suggest that future revisions of the classification system for CKD should incorporate information from proteinuria.
JAMA. 2010
Examples of proteinuria that are not so important are
The upper limit of normal urinary protein excretion is considered to be 150 mg/day, which can be measured in a 24-h urine collection or estimated by random urine protein/creatinine ratio (values < 0.3 are abnormal); for albumin it is about 30 mg/day.
Albumin excretion between 30 and 300 mg/day (20 to 200 µg/min) is considered microalbuminuria, and higher levels are considered macroalbuminuria.
Mechanisms of proteinuria may be categorized as
Glomerular
Tubular
Overflow
Functional
Tubular proteinuria results from renal tubulointerstitial disorders that impair reabsorption of protein by the proximal tubule, causing proteinuria (mostly from smaller proteins such as immunoglobulin light chains rather than albumin).
Overflow proteinuria occurs when excessive amounts of small plasma proteins (eg, immunoglobulin light chains produced in multiple myeloma) exceed the reabsorptive capacity of the proximal tubules.
Functional proteinuria occurs when increased renal blood flow (eg, due to exercise, fever, high-output heart failure) delivers increased amounts of protein to the nephron, resulting in increased protein in the urine (usually < 1 g/day). Functional proteinuria reverses when renal blood flow returns to normal.
The commonly used dip-and-read test strip (dipstick) mainly detects albumin among the various proteins in urine. This test is sensitive to albumin concentrations as low as 15 mg/dL. However, it is not sufficiently sensitive for detecting albumin in the range of microalbuminuria ie, albumin excretion of 30-300 mg/d in an adult.The threshold for transition from microalbuminuria to dipstick-detectable albuminuria (300 mg albumin excreted per day in an adult) corresponds to a concentration of 15 mg/dL if the daily urine volume is 2000 mL. This level gives a trace result with the dipstick.Specialized test strips are now available to specifically detect microalbuminuria as a marker for early kidney involvement due to hyperfiltration injury, as is observed in diabetes, hypertension, or other kidney diseases with reduced nephron mass.
Dilute urine may clinically mask significant proteinuria. Concentrated urine may suggest clinically significant proteinuria when the actual degree of proteinuria is not severe. If highly dilute or concentrated urine is positive for proteinuria, a more reliable estimate than this is made by using the protein-to-creatinine ratio .
A false-positive result may occur if the urine is highly alkaline (pH >8) or if a skin-disinfecting agent, such as chlorhexidine or benzalkonium chloride, contaminates the specimen. The dipstick result may be difficult to read if the urine is abnormally colored because of nitrofurantoin, riboflavin, or azo-containing sulfonamide antimicrobials.
Proteinuria is often diagnosed by a simple dipstick test although it is possible for the test to give a false negative even with nephrotic range proteinuria if the urine is dilute. False negatives may also occur if the protein in the urine is composed mainly globulin or bence jones protein .
Protein/Creatinine Ratio (PCR). The 2005 UK Chronic Kidney Disease guidelines states that PCR is a better test than 24 hour urinary protein measurement.
Proteinuria is defined as a Protein:creatinine ratio >45 mg/mmol (which is equivalent to Albumin:creatinine ratio of >30 mg/mmol) with very high levels of nephrotic syndrome being for PCR > 100 mg/mmol.
The dipstick test for protein provides a crude semiquantitative estimation of protein concentration, with results as follows
Trace = 5-20 mg/dL
1+ = 30 mg/dL
2+ = 100 mg/dL
3+ = 300 mg/dL
4+ = Greater than 2000 mg/dL
Nephrotic range proteinuria will be apparent by 3+ or 4+ readings on the dipstick, or by semiquantitative testing by sulfosalicylic acid. A 3+ reading is 300 mg/dL of urinary protein or more, which is 3 g/L or more and thus in the nephrotic range. The chemistry of the dipsticks is such that albumin is the major protein that is tested.
Waxy casts mark proteinuric renal disease. By use of a polarizing microscope, one can see oval fat bodies and also fatty casts. These point to the nephrotic syndrome. They occur because of glomerular filtration of lipoproteins.
more than 2 red blood cells (RBCs) per high power field is indicative of microhematuria. Glomerular disease may allow RBCs to traverse the damaged glomerular basement membrane, and the RBCs in the sediment may then be deformed, or dysmorphic. This points to glomerular disease with inflammation and destruction of the normal structures, ie, a nephritis (and thus a nephritic picture). This could occur in, for example, nephrotic syndromes associated with IgA nephropathy or proliferative glomerulonephritis.
More than 2 granular casts in the entire sediment is a biomarker for renal parenchymal disease. Variable-caliber granular casts point to reduced renal function.
Orthostatic proteinuria is the most common cause of a positive result for proteinuria in pediatric patients (often tall, physically active adolescents with a slender body habitus). In this circumstance, the detection of isolated proteinuria (in the absence of hematuria) in an asymptomatic individual based on a random specimen collected during the day must be confirmed by repeating the test on a specimen collected immediately upon the patient's awakening in the morning. For this purpose, the child should not be allowed to be active after arising from recumbency until the moment of urine collection. Moreover, to ensure that no residual urine originating from the previous day is in the bladder, the child must completely empty his or her bladder before going to bed the night before the collection. Strictly speaking, orthostatic proteinuria is not a kidney disease; it is a benign condition without clinical significance.
A healthy child with orthostatic proteinuria may have a considerable quantity of protein in a 24-hour urine collection, perhaps up to 1000 mg, and may be falsely identified as having kidney disease. As mentioned above, only a first-voided morning specimen should be tested.
Although orthostatic proteinuria does not generally persist beyond the third decade of life, testing for proteinuria on an annual basis is prudent, especially because both pathologic and physiologic proteinuria (ie, the small amount of protein normally present in urine) also has an orthostatic component. If the first-voided morning specimen has a 1+ or greater reaction for protein, further studies are indicated.
• Bence-Jones proteins
• Waldenstrom's macroglobulinemia• Chronic lymphocytic leukemia
• Amyloidosis
• Malignancies (e.g., lymphoma, other cancers)
• Multiple myeloma
Distinguishing nephrotic-range proteinuria from nonnephrotic (or subnephrotic) proteinuria is clinically useful. In adults, nephrotic-range proteinuria refers to excretion of more than 3-3.5 g of protein per 24 hours or a protein-to-creatinine ratio that exceeds PCR 2.5-3 in a random specimen. In adults, the albumin-to-creatinine ratio corresponds to the 24-hour albumin excretion in a roughly linear manner. For example, a ratio of 3 is predictive of an excretion of about 3 g of protein in 24 hours.
Proteinuria may originate from a unilateral diseased kidney in the presence of an apparently normal contralateral kidney, as with
reflux nephropathy or with a
hypoplastic or dysplastic kidney. However, if heavy proteinuria is found, evaluation of the presumably normal contralateral kidney should be considered.
In children and young adults the ankles may be less affected and the abdomen and face more affected. Most ankle swelling is caused by other diseases ;
nephrotic syndrome is a rare cause of ankle swelling. Urine tests and blood samples are required to prove that nephrotic syndrome is the cause. The protein leak can sometimes make the urine frothy. Some people feel tired.
Myoglobinuria
HemoglobinuriaPorphyrinuria
Porphobilinuria
Food-induced urine coloring (some foods, eg, beets, rhubarb, sometimes food coloring, may make urine appear red)
Drug-induced urine coloring ,phenazopyridine but sometimes cascara, diphenylhydantoin, methyldopa phenacetin, phenindione, phenolphthalein, phenothiazines, and senna .
Red urine
Foamy urine and swelling (edema) are two signs of proteinuria
foamy urine may also be caused by bilirubin in the urineretrograde ejaculation,
pneumaturia (air bubbles in the urine) due to a fistula,
or drugs such as pyridium
Schematic drawing of the glomerular barrier. Podo = podocytes; GBM = glomerular basement membrane; Endo = fenestrated endothelial cells; ESL = endothelial cell surface layer (often referred to as the glycocalyx). Primary urine is formed through the filtration of plasma fluid across the glomerular barrier (arrows); in humans, the glomerular filtration rate (GFR) is 125 mL/min. The plasma flow rate (Qp) is close to 700 mL/min, with the filtration fraction being 20%. The concentration of albumin in serum is 40 g/L, while the estimated concentration of albumin in primary urine is 4 mg/L, or 0.1% of its concentration in plasma.
ACE inhibitors and angiotensin-receptor blockers have an established antiproteinuric effect that appears to be unrelated to their other diverse effects, including control of hypertension. The conventional explanation for the mechanism of the antiproteinuric effect is that selective vasodilatation of the efferent arteriole lowers the filtration pressure and, hence, the amount of albumin in the ultrafiltrate. This explanation is based on the concept that chronic kidney disease is attended by increased intraglomerular pressure and hyperfiltration as an adaptation to a decline in the number of intact nephrons. However, this does not provide a complete explanation because other medications that do not affect intraglomerular pressure also reduce albuminuria (eg, nondihydropyridine calcium channel blockers).
Skin examination: Chronic kidney disease
can cause any of the following:• Xerosis due to sebaceous and eccrine sweat gland atrophy
• Pallor due to anemia
• Hyperpigmentation due to melanin deposition
• Sallow or yellow-brown skin due to urochrome deposition
• Petechiae or ecchymoses due to platelet dysfunction
• Uremic frost, the deposition of white-to-tan urea crystals on the skin after sweat evaporation, is rare.
• kidneys are two bean-shaped organs, each about the size of fist. weigh about 0.5 percent of your total body weight. Although the kidneys are small organs by weight, they receive a huge amount -- 20 percent -- of the blood pumped by the heart. Each kidney is approximately 4 inches long, 2.5 inches wide, and 1.5 inches thick.
• Regulate the composition of your blood
• Keep the concentrations of various ions and other important substances constant
• Keep the volume of water in your body constant
• Remove wastes from your body (urea, ammonia, drugs, toxic substances
• Keep the acid/base concentration of your blood constant
• Help regulate your blood pressure
• Stimulate the making of red blood cells
• Maintain your body's calcium levels
Nephrotic Syndrome
Nephrotic syndrome is kidney disease with proteinuria, hypoalbuminemia, and edema. Nephrotic range proteinuria is 3 grams per day or more. On a single, "spot" urine collection, it is 2 grams of protein per gram of urine creatinine.The concentration of albumin in serum is 40 g/L, while the estimated concentration of albumin in primary urine is 4 mg/L, or 0.1% of its concentration in plasma (In a healthy individual, less than 0.1% of plasma albumin may traverse the glomerular filtration barrier(.Unless there is associated kidney disease, , there will be little or no proteinuria in cirrhosis.
• There are many specific causes of nephrotic syndrome.
• These include kidney diseases such as minimal-change nephropathy, focal glomerulosclerosis, and membranous nephropathy.• Nephrotic syndrome can also result from systemic diseases that affect other organs in addition to the kidneys, such as diabetes, amyloidosis, and lupus erythematosus.
Classification
Nephrotic syndrome can be primary, being a disease specific to the kidneys, or it can be secondary, being a renal manifestation of a systemic general illness. In all cases, injury to glomeruli is an essential feature.
• Primary causes of nephrotic syndrome include, in approximate order of frequency:
• Minimal-change nephropathy• Focal glomerulosclerosis
• Membranous nephropathy
• Hereditary nephropathies
• Secondary causes include, again in order of approximate frequency:
• Diabetes mellitus• Lupus erythematosus
• Amyloidosis and paraproteinemias
• Viral infections (eg, hepatitis B, hepatitis C, human immunodeficiency virus )HIV(
• Preeclampsia
• 6. The association of membranous nephropathy with cancer is a clinical dilemma. This association presumably results from immune complex injury to the glomerulus caused by cancer antigens.
• Nephrotic-range proteinuria may occur in other kidney diseases, such as IgA nephropathy. Nephrotic syndrome may occur in persons with sickle cell disease. Membranous nephropathy may complicate bone marrow transplantation, in association with graft versus host disease.
• Kidney diseases that affect tubules and interstitium, such as interstitial nephritis, will not cause nephrotic syndrome.
According to the International Study of Kidney Diseases in Childhood (ISKDC),
• 84.5% of all children with primary nephrotic syndrome have minimal-change nephrotic syndrome• 9.5% have focal segmental glomerulosclerosis
• 3.5% have membranous nephropathy or another cause of the disease.
• 2.5% have mesangial proliferation
The connection of nephrotic syndrome to quartan malaria is not well-established. It is possible that the perceived association between nephrotic syndrome and parasitic infections was coincidental .
Laboratory Studies
UrinalysisNephrotic range proteinuria will be apparent by 3+ or 4+ readings on the dipstick, or by semiquantitative testing by sulfosalicylic acid. A 3+ reading is 300 mg/dL of urinary protein or more, which is 3 g/L or more and thus in the nephrotic range.
Glucosuria points to diabetes.
The urine sediment exam may show cells and/or casts.
Waxy casts mark proteinuric renal disease. By use of a polarizing microscope, one can see oval fat bodies and also fatty casts. These point to the nephrotic syndrome. They occur because of glomerular filtration of lipoproteins, the uptake of these by the tubular cells that then fall off into the urine. Viewed by polarizer, the oval fat bodies and fatty casts cause a "Maltese cross" appearance.
The presence of more than 2 red blood cells (RBCs) per high power field is indicative of microhematuria. Microhematuria may occur in membranous nephropathy but not in minimal-change nephropathy. Glomerular disease may allow RBCs to traverse the damaged glomerular basement membrane, and the RBCs in the sediment may then be deformed, or dysmorphic. This points to glomerular disease with inflammation and destruction of the normal structures, ie, a nephritis (and thus a nephritic picture). This could occur in, for example, nephrotic syndromes associated with IgA nephropathy or proliferative glomerulonephritis.
More than 2 granular casts in the entire sediment is a biomarker for renal parenchymal disease. Variable-caliber granular casts point to reduced renal function.
Urinary protein is measured by a timed collection or a single, spot collection. A timed collection is typically done over a 24-hour period, starting at 7 am and finishing the next day at the same time. In healthy individuals, there are no more than 150 mg of total protein in a 24-hour urine collection.
The exact type of urine protein is of potential interest. This can be tested by urine protein electrophoresis. Proteinuria that does not include albumin may point to overflow proteinuria that occurs in paraproteinemias, such as multiple myeloma.
proteinuria is "selective" for albumin, being more than 85% composed of albumin.
In the case of selective proteinuria, there could be a charge-selective leak of albumin across the glomerular barrier, perhaps due to reduced negative charges on that barrier, whereasnonselective proteinurias would point to more substantial glomerular injury and perhaps also to lesser response to prednisone treatment.
In children, the serum creatinine level will be lower than it is in adults. The normal adult serum creatinine level is approximately 1 mg/dL, whereas that of a child aged 5 years will be about 0.5 mg/dL. Values higher than this indicate reduced kidney function.
The serum albumin is classically low in nephrotic syndrome, being below its normal range of 3.5-4.5 g/dL. A single-center study showed that when a patient's serum albumin level was normal, rather than low, focal glomerulosclerosis, rather than other conditions, tended to be the cause of nephrotic syndrome.
Imaging Studies
Ultrasonographic scanning can be used to determine whether a patient possesses 2 kidneys and to demonstrate their echogenicity. Individuals with a single kidney may be prone to developing focal glomerulosclerosis.
Having only 1 kidney is also a relative contraindication to kidney biopsy. Increased renal echogenicity by ultrasonography is consistent with intrarenal fibrosis, ie, chronic disease with reduced kidney function.
Other Tests
In infants with nephrotic syndrome, genetic testing for the NPHS1 and NPHS2 mutations may be useful. These are mutations of nephrin and podocin, respectively. In children with steroid-resistant nephrotic syndrome, testing for the NPHS2 mutation may be indicated.In adults with nephrotic syndrome, tests for hepatitis B and C, HIV, and even syphilis may be useful. Tests for lupus, including ANA, anti-dsDNA, and complement, may be useful.
Testing for antineutrophil cytoplasmic antibodies (ANCA) is not indicated in typical nephrotic syndrome, because that test is associated with rapidly progressive glomerulonephritis, which presents with a nephritic picture rather than one that is typically nephrotic.
Tests for previous streptococcal infection, such as antistreptolysin O, are not usually indicated for nephrotic syndrome, since postinfectious glomerulonephritis usually causes a nephritic picture, not nephrotic syndrome.
• Procedures
• For childhood nephrotic syndrome, a renal biopsy is indicated for the following:• Congenital nephrotic syndrome
• Children older than 8 years at onset
• Steroid resistance
• Frequent relapses or steroid dependency
• Significant nephritic manifestations
Adult nephrotic syndrome of unknown origin may require a renal biopsy for diagnosis.
A renal biopsy is not indicated in adults when nephrotic syndrome is due to an obvious cause, such as diabetes mellitus. Thus, a subject with diabetes, diabetic retinopathy, and the nephrotic syndrome may well have diabetic nephropathy and not need to undergo kidney biopsy.Abdominal fat-pad biopsy or gingival biopsy May be useful in adult patients to help diagnose either primary or secondary amyloidosis
Staging
There are histopathologic stages for membranous nephropathy but not for other causes of nephrotic syndrome
Mortality/Morbidity
The prognosis may worsen because of(1) an increased incidence of renal failure and the complications secondary to nephrotic syndrome, including thrombotic episodes and infection, or
(2) treatment-related conditions, such as infectious complications of immunosuppressive treatments.
In secondary nephrotic syndromes, morbidity and mortality are related to the primary disease process, such as diabetes or lupus, although in diabetic nephropathy, the magnitude of proteinuria itself relates directly to mortality.
There is a male predominance in the occurrence of nephrotic syndrome, as there is for chronic kidney disease in general. This male overrepresentation is also seen in paraneoplastic membranous nephropathy.
However, lupus nephritis affects mostly women.
The first sign of nephrotic syndrome in children is usually swelling of the face; this is followed by swelling of the entire body
Adults can present with dependent edema
Foamy urine may be a presenting feature
A thrombotic complication, such as deep vein thrombosis of the calf veins or even a pulmonary embolus, may be the first clue indicating nephrotic syndrome.
• Congenital and hereditary focal glomerulosclerosis may result from mutations of genes that code for podocyte proteins, including nephrin, podocin, or the cation channel 6 protein.
Nephrotic Syndrome: Treatment & Medication
• Acute management of childhood nephrotic syndromeWith good parental and patient education and close outpatient follow-up care, hospitalization is not usually necessary.• Hospitalization should be considered if a patient has
• generalized edema severe enough to cause respiratory distress,
• If a patient has tense scrotal or labial edema,
• if he or she has complications (eg, bacterial sepsis, peritonitis, pneumonia, thromboembolism, failure to thrive), or
• if patient or family compliance with treatment is in doubt.
Diuretics will be needed; furosemide (1 mg/kg/d) and spironolactone (2 mg/kg/d) will help when fluid retention is severe, provided no signs of renal failure or volume contraction are evident. Achieving a satisfactory diuresis is difficult when the patient's serum albumin level is less than 1.5 g/dL. Albumin at 1 g/kg may be given, followed by intravenous furosemide. Complications may occur, including pulmonary edema.
Some evidence suggests that albumin may delay the response to steroids and may even induce more frequent relapses, probably by causing severe glomerular epithelial damage. Fluid removal and weight loss remain transient unless proteinuria remits.
With regard to infection, oral penicillin can be prescribed as prophylaxis for children with gross edema. Abdominal paracentesis should be performed if the patient develops signs of peritonitis, and any bacterial infection should be treated promptly. A nonimmune patient with varicella should receive zoster immunoglobulin therapy if exposed to chickenpox, and acyclovir should be given if the patient develops chickenpox.
Acute management of adult nephrotic syndromeThe principles for acute management of adults with nephrotic syndrome are similar to those for children. Diuretics will be needed; furosemide, spironolactone, and even metolazone may be used. Volume depletion may occur with diuretic use, which should be monitored by assessment of symptoms, weight, pulse, and blood pressure.Anticoagulation has been advocated by some for use in preventing thromboembolic complications, but its use in primary prevention is of unproven value.Hypolipidemic agents may be used, but if the nephrotic syndrome cannot be controlled, there will be persistent hyperlipidemia.
In secondary nephrotic syndrome, such as that associated with diabetic nephropathy, angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin II receptor blockers are widely used. These may reduce proteinuria by reducing the systemic blood pressure, by reducing intraglomerular pressure, and also by direct action on podocytes.
Specific treatmentSpecific treatment of nephrotic syndrome depends on the disease's cause. Thus, glucocorticosteroids, such as prednisone, are used for minimal-change nephropathy. Prednisone and cyclophosphamide are useful in some forms of lupus nephritis. Secondary amyloidosis with nephrotic syndrome may respond to anti-inflammatory treatment of the primary disease.
Diet
For patients with nephrotic syndrome, their diet should provide adequate energy (caloric) intake and adequate protein (1-2 g/kg/d). Supplemental dietary protein is of no proven value.
A diet with no added salt will help to limit fluid overload.
Management of hyperlipidemia could be of some importance if the nephrotic state is prolonged.
Fluid restriction per se is not required.
Activity
There are no activity restrictions for patients with nephrotic syndrome. Ongoing activity, rather than bedrest, will reduce the risk of blood clots.Medication
PrednisoneImmunosuppressant for treatment of autoimmune disorders. May decrease inflammation by reversing increased capillary permeability and suppressing PMN activity. May be administered as a single dose in the morning or as divided doses. Studies show that a single dose is equally effective and greatly improves compliance.
Adult
60 mg/m2/d PO, titrate to a maximum 80 mg/m2/d until remission; then, 40 mg/m2/d, titrate to 60 mg/m2 qod for 4 wk
Pediatric
1 to 2 mg/kg/d; taper over 2 wk as symptoms resolve
ImmunomodulatorsThese agents regulate key steps of the immune system.
Cyclophosphamide (Cytoxan)Antineoplastic drug chemically related to nitrogen mustard. Potent immunomodulator that has been used successfully in conditions that require immunosuppression. Highly effective for frequently relapsing steroid-sensitive nephrotic syndrome; half of the children enter a prolonged remission.This drug is used for the time required to induce remission and should be continued thereafter, but probably not for more than a year.
Immunosuppressants
Inhibit key steps that mediate immune reactions.Mycophenolate (CellCept, Myfortic)
Inhibits inosine monophosphate dehydrogenase and suppresses de novo purine synthesis by lymphocytes, thereby inhibiting their proliferation. Inhibits antibody production.
Adult
Mycophenolate mofetil (CellCept): 500 to 1500 mg PO bidMycophenolate sodium (Myfortic): 360 to 720 mg PO bidPediatric
CellCept oral suspension: 600 mg/m² PO bid; 1 g PO bid maximum
Angiotensin-converting Enzyme (ACE) Inhibitors
These agents improve hypertension by inhibiting renin or angiotensin II production.Angiotensin II Receptor AntagonistThese agents inhibit angiotensin II activity by interfering with the binding of formed angiotensin II to its endogenous receptor.
Follow-up
Immunization - Routine immunizations should be delayed until the patient is free of relapses and is off immunosuppression for 3 months.Pneumococcal and influenza vaccines are recommended but are not routinely used, because their efficacy is not established. Children who have received immunosuppressive therapy in the preceding 3 months and are not immune to varicella should receive zoster immunoglobulin if they are exposed to chickenpox or shingles. These patients should also receive acyclovir if they develop chickenpox.
Treatment of relapses of steroid-responsive Most patients experience relapses; relapse rates of 76-97%,.
The first 2 relapses are treated in the same manner as the initial presentation;
frequent relapses are treated with a maintenance dose of prednisone at 0.1-0.5 mg/kg on alternate days for 3-6 months, with the drug then tapered.
Monitoring steroid toxicity - Monitoring every 3 months in the outpatient clinic is necessary to help detect adverse effects and to record growth. Supplemental calcium and vitamin D may attenuate bone loss .Ongoing use and adjustment of diuretics and angiotensin antagonists are necessary according to the amount of edema and proteinuria that a patient has.
Inpatient & Outpatient Medications
immunosuppressive medications - These medications are usually reservedfor steroid-resistant cases in patients who are persistently edematous or for
steroid-dependent patients with significant steroid-related adverse effects.
• Cyclophosphamide may benefit patients who have frequently relapsing steroid-sensitive nephrotic syndrome. Associated complications include bone marrow suppression, hair loss, azoospermia, hemorrhagic cystitis, malignancy, mutations, and infertility.
• Cyclosporine is indicated when relapses occur after cyclophosphamide treatment. Cyclosporine may be preferable in a pubertal male who is at risk of developing cyclophosphamide-induced azoospermia. Cyclosporine is a highly effective maintenance therapy for patients with steroid-sensitive nephrotic syndrome who are able to stop steroids or take lower doses; however, some evidence suggests that although remission is maintained as long as cyclosporine is administered, relapses are frequent when treatment is discontinued. Cyclosporine can be nephrotoxic and can cause hirsutism, hypertension, and gingival hypertrophy.
Deterrence/Prevention
Amniocentesis may show high levels of alpha-fetoprotein when the fetus has congenital nephrotic syndrome of the Finnish type. This may assist in management and counseling.Complications of nephrotic syndrome
1- Infection is a major concern; increased susceptibility to infection with Streptococcus pneumoniae, Haemophilus influenzae,Escherichia coli,and other gram-negative organisms. Varicella infection is also common. The most common infectious complications are bacterial sepsis, cellulitis, pneumonia, and peritonitis. Proposed explanations for these complications include• decreased immunoglobulin levels,
• edema fluid acting as a culture medium,
• protein deficiency
• decreased bactericidal activity of the leukocytes,
• immunosuppressive therapy,
• decreased perfusion of the spleen caused by hypovolemia
• urinary loss of a complement factor (properdin factor B) that opsonizes certain bacteria.
• 2-Hyperlipidemia
• may be considered a typical feature of the nephrotic syndrome, rather than a mere complication. It is related to the hypoproteinemia and low serum oncotic pressure of nephrotic syndrome, which then leads to reactive hepatic protein synthesis, including of lipoproteins. Some of the elevated serum lipoproteins are filtered at the glomerulus, leading to lipiduria and the classical findings of oval fat bodies and fatty casts in the urine sediment.
• Atherosclerotic vascular disease appears to occur in greater frequency in subjects with nephrotic syndrome than in healthy subjects of the same age..
• 3-Hypocalcemia is common in the nephrotic syndrome, but rather than being a true hypocalcemia, it is usually caused by a low serum albumin level. Nonetheless, low bone density and abnormal bone histology are reported in association with nephrotic syndrome. This could be caused by urinary losses of vitamin D – binding proteins, with consequent hypovitaminosis D and, as a result, reduced intestinal calcium absorption.
• 4-Venous thrombosis and pulmonary embolism are well-known complications of the nephrotic syndrome. Hypercoagulability in these subjects appears to derive from urinary loss of anticoagulant proteins, such as anti-thrombin III, compounded by elevations in procoagulant proteins, such as fibrinogen.
A study by Mahmoodi et al of almost 300 patients with nephrotic syndrome confirmed that venous thromboembolism (VTE) was almost 10 times higher in these persons than in the normal population. This high incidence may justify the routine use of preventive anticoagulation treatment during the first 6 months of a persistent nephrotic syndrome.Mahmoodi's study also showed an increased risk of arterial thrombotic events, including coronary and cerebrovascular ones, in nephrotic syndrome. Unlike the risk of VTE, which was related to proteinuria, this arterial risk was related to usual risk factors for arterial disease, such as hypertension, diabetes, smoking, and reduced GFR.
• 5-Acute renal failure may indicate an underlying glomerulonephritis but is more often precipitated by hypovolemia or sepsis. Edema of the kidneys that causes a pressure-mediated reduction in the GFR has also been hypothesized.
6-Hypertension related to fluid retention and reduced kidney function may occur.
7-Failure to thrive may develop in patients with chronic edema, including ascites and pleural effusion. Failure to thrive may be caused by anorexia, hypoproteinemia, increased protein catabolism, or frequent infectious complications. Edema of the gut may cause defective absorption, leading to chronic malnutrition.Adverse treatment effects may occur. Corticosteroids and other immunosuppressive drugs have significant adverse effects.
Prognosis
The prognosis for patients with primary nephrotic syndrome depends on its cause.The prognosis with congenital nephrotic syndrome is bad. Survival beyond several months is possible only with dialysis and kidney transplantation.
Only approximately 20% of patients with focal glomerulosclerosis undergo remission of proteinuria; an additional 10% improve but remain proteinuric. Many patients experience frequent relapses, become steroid-dependent, or become steroid-resistant. End-stage renal disease develops in 25-30% of patients with FSGS by 5 years and in 30-40% of these patients by 10 years.
The prognosis for children with minimal-change
very good.
Most children respond to steroid therapy; still, about 50% of children have 1 or 2 relapses within 5 years and approximately 20% of them continue to relapse 10 years after diagnosis.
Only 30% of children never have a relapse after the initial episode. Approximately 3% of patients who initially respond to steroids become steroid-resistant.
Poor patient response to steroid therapy may predict a poor outcome, and children who present with hematuria and hypertension are more likely to be steroid-resistant and have a poorer prognosis than are those who do not present with these conditions.
In adult nephrotic syndrome, there is a similar variability according to the underlying cause.
In adult minimal-change nephropathy, there is a burden of relapse similar to that of children. However, the long-term prognosis for kidney function in patients with this disease is excellent, with little risk of renal failure.
As noted, the prognosis of membranous nephropathy is good in terms of patient survival, being the same as that of the unaffected population.
In diabetic nephropathy with nephrotic syndrome, there is usually a good response to angiotensin blockade, with reduction of proteinuria to low, sub-nephrotic levels. However, true remission is uncommon.
Cardiovascular morbidity and mortality increase as kidney function declines, and some subjects will eventually need dialysis or a kidney transplant.
In primary amyloidosis, prognosis is not good, even with intensive chemotherapy. In secondary amyloidosis, remission of the underlying cause, such as rheumatoid arthritis, is followed by remission of the amyloidosis and its associated nephrotic syndrome.
Renal tubular acidosis (RTA)
1. failure of the kidneys to acidify the urine.
2.metabolic acidosis also occurs in those with renal insufficiency, the term RTA is reserved for individuals with poor urinary acidification in otherwise well-functioning kidneys.
3.The metabolic acidosis caused by RTA is normal anion gap acidosis.
It is calculated by subtracting the serum concentrations of chloride and bicarbonate (anions) from the concentrations of sodium plus potassium (cations):
= ( [Na+] +[K+] ) - ( [Cl-]+[HCO3-] ) .
However, for daily practice, the potassium is frequently ignored because potassium concentrations, being very low, usually have little effect on the calculated gap. This leaves the following equation:
= ( [Na+] ) - ( [Cl-]+[HCO3-] ) .
The average anion gap for healthy adults is 8-12 mEq/L.
Normal anion gap
The drop in HCO3-is compensated by an increase in Cl- and hence is also known as hyperchloremic acidosis. The HCO3- lost is replaced by a chloride anion, and thus there is a normal anion gap.Gastrointestinal loss of HCO3- i.e. dirrhea causes hypochloraemic alkalosis
Proximal type 2 RTA renal loss of HCO3- failure to recover sufficient bicarbonate ions from the filtrate in the proximal tubule
distal type 1 RTA insufficient secretion of hydrogen ions into the distal tubule.
Renal failure ,hypoaldosteronism,Ingestions ammonium chloride .
Acetazolamide.
hyperalimentation .
Some cases of ketoacidosis,particularly during rehydration with Na+ containing IV solutions.
• Causes of normal anion gap acidosis
• Hyperalimentation• Acetazolamide and other carbonic anhydrase inhibitors
• Renal tubular acidosis.
• Diarrhea
• Fistula between a ureter and the gastrointestinal tract between the pancreas and duodenum
High anion gap
In these conditions, bicarbonate concentrations decrease, in response to the need to buffer the increased presence of acids (as a result of the underlying condition). The bicarbonate is replaced by the unmeasured anion resulting in a high anion gap.Lactic acidosis
Ketoacidosis
Toxins:
Ethylene glycol
Lactic acid
Methanol
Propylene Glycol
Phenformin
Aspirin
Cyanide
Iron
isoniazid
.
Low anion gap
largely associated with hypoalbuminaemia .
Albumin is a negatively charged protein and its loss from the serum results in the retention of other negatively charged ions such as Chloride and Bicarbonate..
In hypoalbuminaemia the anion gap is reduced from between 1.5 and 2.5 mmol/L per g/dL decrease in serum albumin. haemorrhage, nephrotic, intestinal obstruction and liver cirrhosis.
The anion gap is sometimes reduced in multiple myeloma where there is an increase in plasma IgG..
High anion gap
bicarbonate concentrations decrease, replaced by the unmeasured anionLow anion gap
largely associated with hypoalbuminaemia
Normal anion gap
The drop in HCO3- is compensated by an increase in Cl- and hence is also known as hyperchloremic acidosis.
The metabolic acidosis that results from RTA may be caused either by failure to recover sufficient bicarbonate ions from the filtrate in the early portion of the nephron proximal tubule
or by insufficient secretion of hydrogen ions into the latter portions of the nephron distal tubule.
In patients with a normal anion gap the drop in HCO3- is compensated for almost completely by an increase in Cl- and hence is also known as hyperchloremic acidosis. .
Type I-Distal RTA
Is the classical form of RTA, characterized by a failure of acid secretion by the alpha intercalated cells of the cortical collecting duct of the distal nephron. This failure of acid secretion may be due to a number of causes, and it leads to an inability to acidify the urine to a pH of less than 5.3. there is consequently a tendency towards acidemia.Hypokalemia
stone formation (related to alkaline urine hypercalciuria, and low urinary citrate)
Nephrocalcinosis
Bone demineralisation (causing rickets in children and osteomalacia in adults).
The diagnosis of dRTA can be made by the observation of a urinary pH of greater than 5.3 in the face of a systemic acidemia (usually taken to be a serum bicarbonate of 20 mmol/l or less). In the case of an incomplete dRTA, failure to acidify the urine following an oral acid loading challenge is often used as a test. The test usually performed is the short ammonium chloride test, in which ammonium chloride capsules are used as the acid load. More recently, an alternative test using furosemide and fludrocortisone has been described.
• Causes Type I-Distal RTA
• Autoimmune disease. Classically Sjögren's syndrome, but it is also associated with systemic lupus erythematosus, rheumatoid arthritis and even hypergammaglobulinemia. Hypokalaemia is often severe in these cases.• Hereditary causes autosomal dominant fashion in western European cases, or in an autosomal recessive fashion in South East Asian cases.
• Liver cirrhosis.
• Nephrocalcinosis. While it is a consequence of dRTA, it can also be a cause; related to calcium-induced damage of the cortical collecting duct.
• Renal transplantation.
• Sickle cell anemia.
• Toxins, including ifosfamide, toluene, lithium carbonate, and amphotericin B.
• Chronic urinary tract obstruction.
Treatment
It involves correction of the acidemia with oral sodium bicarbonate or sodium citrate. This will correct the acidemia and reverse bone demineralisation.Hypokalemia and urinary stone formation and nephrocalcinosis can be treated with potassium citrate tablets which not only replace potassium but also inhibit calcium excretion and thus do not exacerbate stone disease as sodium bicarbonate or citrate may do.
Type 2-Proximal RTA
caused by a failure of the proximal tubular cells to reabsorb filtered bicarbonate from the urine, leading to urinary bicarbonate wasting and subsequent acidemia.The distal intercalated cells function normally, so the acidemia is less severe than dRTA and the urine can acidify to a pH of less than 5.3. pRTA may be present as a solitary defect, but is usually associated with a more generalised dysfunction of the proximal tubular cells called Fanconi's syndrome where there is also phosphaturia, glycosuria, aminoaciduria, uricosuria and tubular proteinuria. The principal feature of Fanconi's syndrome is bone demineralization (osteomalacia or rickets) due to phosphate wasting.
Causes
Familial disordersCystinosis.
Galactosemia.
Glycogen storage disease type I.
Hereditary fructose intolerance.
Lowe syndrome.
Tyrosinemia .
Wilson's disease.
Acquired disorders
Amyloidosis.
Multiple myeloma.
Paroxysmal nocturnal hemoglobinuria.
Toxins, such as HAART,ifosfamide, lead, and cadmium
Treatment
Again this depends on oral bicarbonate supplementation. However, this will increase urinary bicarbonate wasting and may well promote a bicarbonate diuresis. The amount of bicarbonate given may have to be very large, to stay ahead of the urinary losses. Correction with oral bicarbonate may exacerbate urinary potassium losses and precipitate hypokalemia. As with dRTA, reversal of the chronic acidosis should reverse bone demineralization.
Type 3 RTA-Combined proximal and distal RTA
In some patients, their RTA shares features of both dRTA and pRTA. This rare pattern was observed in the 1960s and 1970s as a transient phenomenon in infants and children with dRTA, possibly in relation with some exogenous factor such as high salt intake, and is no longer observed.This form of RTA has also been referred to as juvenile RTA.Combined dRTA and pRTA is also observed as the result of inherited carbonic anhydrase II deficiency. Mutations in the gene encoding this enzyme give rise to an autosomal recessive syndrome of osteopetrosis,renal tubular acidosis,cerebral calcification, and mental retardation. It is very rare and cases from all over the world have been reported, of which about 70% are from the Magreb region of North Africa,possibly due to the high prevalence of consanguinity there.The kidney problems are treated as described above. There is no treatment for the osteopetrosis or cerebral calcification.
Type 4 RTA (hyperkalemic RTA(
Type 4 RTA is not actually a tubular disorder at all and nor does it have a clinical syndrome similar to the other types of RTA described above. It was included in the classification of renal tubular acidoses as it is associated with a mild (normal anion gap) metabolic acidosis due to a physiological reduction in distal tubular ammonium excretion, which is secondary tohypoaldosteronism. Its cardinal feature is hyperkalemia.
and measured urinary acidification is normal.
Causes
1.Aldosterone deficiency-Primary (rare(Primary adrenal insufficiency
Congenital adrenal hyperplasia
Aldosterone synthase deficiency
2.Hyporeninemic hypoaldosteronism
due to decreased angiotensin 2 production as well as intra-adrenal dysfunction.
diabetic nephropathy
HIV infection
ACE inhibitors
NSAIDs
Cyclosporine
3.Aldosterone resistance
Drugs Amiloride,Spironolactone,Trimethoprim, Pentamidine
Pseudohypoaldosteronism
Treatment
Aldosterone deficiency should be treated with a mineralocorticoid (such as fludrocortisone), as well as possibly a glucocorticoid for cortisol deficiency, if present.
Hyporeninemic hypoaldosteronism is amenable to fludrocortisone treatment, but the accompanying hypertension and edema can prove a problem in these patients, so often a diuretic (such as the thiazide diuretic, bendrofluazide,or a loop diuretic, such as furosemide) is used to control the hyperkalemia.
FDA approved ferumoxytol injection
(Feraheme, AMAG Pharmaceuticals, Inc) for the treatment of iron deficiency anemia in adult patients with chronic kidney disease. superparamagnetic coated with a carbohydrate shell that helps to isolate the bioactive iron from plasma components until the complex enters the macrophages of the liver, spleen, and bone marrow. The iron is released from the complex within macrophage vesicles and then either enters the intracellular iron storage pool (eg, ferritin) or is transferred via plasma transferin to erythroid precursor cells for incorporation into hemoglobin.The safety and efficacy of ferumoxytol in the episodic treatment of iron-deficiency anemia were assessed in three randomized, open-label, controlled clinical trials enrolling approximately 800 patients with CKD. In all three controlled trials, patients were randomized to either ferumoxytol or oral iron. Two trials evaluated patients with non-dialysis dependent CKD and a third assessed patients undergoing hemodialysis. These trials assessed hemoglobin alterations and clinical outcomes over 35 days. Ferumoxytol was administered as two 510 mg IV injections, with most patients receiving their second injection 3 to 8 days after their initial injection.
In all three clinical trials, ferumoxytol administration increased the mean blood hemoglobin concentrations by approximately 1.0 g/dL over the 35 day period, a mean increase that was greater than what was observed in patients receiving oral iron. Patients receiving ferumoxytol also had increases in blood transferrin saturation and ferritin values.
The novel product is expected to be available during the second half of July 2009 and will be marketed in 17-mL single-use vials containing 510 mg of elemental iron. An initial 510-mg intravenous injection, delivered at a rate of up to 1 mL/second (30 mg/second), should be followed by a second 510-mg dose given 3 to 8 days later. Additional 510-mg doses may be administered to patients with persistent or recurrent iron deficiency anemia.
Ferumoxytol does not cause most of the adverse events associated with oral iron replacement therapies. In clinical trials, the most common adverse reactions (incidence ≥ 2%) reported more frequently with ferumoxytol injection vs ferrous fumarate tablets included hypotension (2.5% vs 0.4%) and dizziness (2.6% vs 1.8%). Events reported with decreased frequency included diarrhea (4.0% vs iron tablets, 8.2%), nausea (3.1% vs 7.5%), constipation (2.1% vs 5.7%), and peripheral edema (2.0% vs 3.2%.(
ferumoxytol is currently being developed to treat iron deficiency anemia in women with abnormal uterine bleeding and in patients with cancer and gastrointestinal diseases.
Because of its potential to improve the visualization of blood vessels, ferumoxytol may also be useful as a diagnostic agent for vascular-enhanced magnetic resonance imaging to assess peripheral arterial disease.
Because of the potential for hypersensitivity reactions and hypotension, patients should be observed for at least 30 minutes after each dose. To avoid iron overload and the potential for iatrogenic hemosiderosis, patients should be regularly monitored for hematologic response, with the caveat that laboratory assays performed in the first 24 hours may overestimate serum iron and transferrin bound iron by also measuring the iron in the ferumoxytol complex.
Ferumoxytol is also a blood pool agent which means that, among other things, it is a true intravascular contrast agent that remains in the blood stream for an extended period of time. As a blood pool agent with a long blood half–life as compared to currently approved MRI contrast agents, it may be useful as a contrast agent in a wide range of MRI applications in both cardiology and oncology.
As a superparamagnetic iron oxide, ferumoxytol can alter magnetic resonance imaging studies for up to 3 months after the last dose.
X-ray, computed tomography, positron emission tomography, single photon emission computed tomography, ultrasound, and nuclear imaging are not affected.
The recommended dose of ferumoxytol is an initial 510 mg IV injection followed by a second 510 mg IV injection 3 to 8 days later. The drug is administered as an undiluted IV injection delivered at a rate not exceeding 1.0 mL/sec. For patients receiving hemodialysis, ferumoxytol is administered after the blood pressure has stabilized and the patient has completed at least one hour of hemodialysis. Patients should be monitored for signs and symptoms of hypotension following each ferumoxytol injection.
Diagnosis, Prevention, and Treatment of Catheter-Associated Urinary Tract Infection in Adults:
2009 International Clinical Practice Guidelines
from the Infectious Diseases Society of America
Catheter-associated (CA) bacteriuria is the most common health care–associated infection worldwide and is a result of the widespread use of urinary catheterization,much of which is inappropriate, in hospitals and longterm care facilities (LTCFs). Unfortunately,the catheter literature generally reports on CA asymptomatic bacteriuria (CA-ASB) or CA bacteriuria (used when no distinction is made between CAASB and CA-UTI; such cases are predominantly CAASB),rather than on CA-UTI.
The incidence of bacteriuria associated with indwelling catheterization is 3%–8% per day ,and the duration of catheterization is the most important risk factor for the development of CA-bacteriuria. By 1 month, nearly all patients with an indwelling catheter will be bacteriuric.
Other risk factors associated with CA-bacteriuria include not receiving systemic antimicrobial therapy, female sex, positive urethral meatal culture results, microbial colonization of the drainage bag, catheter insertion outside the operating room, catheter care violations, rapidly fatal underlying illness, older age, diabetes mellitus, and elevated serum creatinine at the time of catheterization .
The most effective way to reduce the incidence of CA-ASB and CA-UTI is to reduce the use of urinary catheterization by restricting its use to patients who have clear indications and by removing the catheter as soon as it is no longer needed.
Method of Diagnosing CA-ASB and CA-UTI
1. CA-UTI in patients with indwelling urethral, indwelling suprapubic, or intermittent catheterization is defined by the presence of symptoms or signs compatible with UTI with no other identified source of infection along with ≥103 colony forming units (cfu)/mL of ≥1 bacterial species in a single catheter urine specimen or in a midstream voided urine specimen from a patient whose urethral, suprapubic, or condom catheter has been removed within the previous 48 h (A-III).2. CA-ASB should not be screened for except in research studies evaluating interventions designed to reduce the incidence of CA-ASB or CA-UTI (A-III) and in selected clinical situations, such as in pregnant women (A-III).
CA-ASB in patients with indwelling urethral, indwelling suprapubic, or intermittent catheterization is defined by the presence of ≥105 cfu/mL of ≥1 bacterial species in a single catheter urine specimen in a patient without symptoms compatible with UTI (A-III).
3.In the catheterized patient, pyuria is not diagnostic of
CA-bacteriuria or CA-UTI (AII).i. The presence, absence, or degree of pyuria should not be used to differentiate CA-ASB from CA-UTI (A-II).ii. Pyuria accompanying CA-ASB should not be interpreted as an indication for antimicrobial treatment (A-II).iii. The absence of pyuria in a symptomatic patient suggests a diagnosis other than CA-UTI (A-III).4.In the catheterized patient, the presence or absence of
odorous or cloudy urine alone should not be used to differentiate CA-ASB from CA-UTI or as an indication for urine culture or antimicrobial therapy (A-III).
Alternatives to Indwelling Urethral Catheterization
In men for whom a urinary catheter is indicated and who have minimal postvoid residual urine, condom catheterization should be considered as an alternative to short-term (A-II) and long-term(B-II) indwelling catheterization to reduce CA-bacteriuria in those who are not cognitively impaired.Intermittent catheterization should be considered as an
alternative to short-term (C-I) or long-term (A-III) indwelling urethral catheterization to reduce CA-bacteriuria and an alternative to short-term (C-III) or long-term (A-III) indwelling urethral catheterization to reduce CA-UTI.Suprapubic catheterization may be considered as an alternative to short-term indwelling urethral catheterization to reduce CA-bacteriuria (B-I) and CA-UTI (C-III).
Data are insufficient to make a recommendation as to whether suprapubic catheterization is preferable to long-term indwelling urethral catheterization for reduction of CA-bacteriuria or CA-UTI.
Data are insufficient to make a recommendation as to whether intermittent catheterization is preferable to suprapubic catheterization for reduction of CA-bacteriuria or CA-UTI
Antimicrobial Coated Catheters. In patients with short-term indwelling urethral catheterization,antimicrobial (silver alloy or antibiotic)–coated urinary catheters may be considered to reduce or delay the onset of CA-bacteriuria (B-II).
Data are insufficient to make a recommendation about
whether use of such catheters reduces CA-UTI in patients with short-term indwelling urethral catheterization. Data are insufficient to make a recommendation as to
whether use of such catheters reduces CA-bacteriuria or CAUTI in patients with long-term catheterization.
Prophylaxis with Systemic Antimicrobials
Systemic antimicrobial prophylaxis should not be routinely used in patients with short-term (A-III) or long-term (A-II) catheterization, including patients who undergo surgical procedures, to reduce CA-bacteriuria or CA-UTI because of concern about selection of antimicrobial resistance.
Methenamine salts may be considered for the reduction of CA-bacteriuria and CA-UTI in patients after gynecologic surgery who are catheterized for no more than 1 week (C-I).
It is reasonable to assume that a similar effect would be seen after other types of surgical procedures.
Catheter Irrigation
Catheter irrigation with antimicrobials should not beused routinely to reduce or eradicate CA-bacteriuria (A-I) or CA-UTI (A-II) in patients with indwelling catheters.
Catheter irrigation with antimicrobials may be considered in selected patients who undergo surgical procedures and short-term catheterization to reduce CA-bacteriuria (C-I).Data are insufficient to make a recommendation about whether bladder irrigation in such patients reduces CA-UTI.Catheter irrigation with normal saline should not be used routinely to reduce CA-bacteriuria, CA-UTI, or obstruction in patients with long-term indwelling catheterization
Routine Catheter Change
Data are insufficient to make a recommendation as towhether routine catheter change (eg, every 2–4 weeks) in patients with functional long-term indwelling urethral or suprapubic catheters reduces the risk of CA-ASB or CA-UTI, even in patients who experience repeated early catheter blockage from encrustation.
Prophylactic Antimicrobials at Time of Catheter Removal or Replacement
Prophylactic antimicrobials, given systemically or by bladder irrigation, should not be administered routinely to patients at the time of catheter placement to reduce CA-UTI (AI)or at the time of catheter removal (B-I) or replacement (AIII) to reduce CA-bacteriuria. Data are insufficient to make a recommendation as to whether administration of prophylactic antimicrobials to such patients reduces bacteremia.
Screening for and Treatment of CA-ASB in Catheterized Patients to Reduce CA-UTI
Screening for and treatment of CA-ASB are not recommended to reduce subsequent CA-bacteriuria or CA-UTI in patients with short-term(A-II) or long-term(A-I) indwelling urethral catheters.Screening for and treatment of CA-ASB are not recommended to reduce subsequent CA-bacteriuria or CA-UTI in patients with neurogenic bladders managed with intermittent catheterization (A-II).Screening for and treatment of CA-ASB are not recommended to reduce subsequent CA-bacteriuria or CA-UTI in other catheterized patients (A-III), except in pregnant women (A-III) and patients who undergo urologic procedures for which visible mucosal bleeding is anticipated (A-III).Urine Culture and Catheter Replacement before Treatment
A urine specimen for culture should be obtained prior
to initiating antimicrobial therapy for presumed CA-UTI because of the wide spectrum of potential infecting organisms
and the increased likelihood of antimicrobial resistance (A-III).
If an indwelling catheter has been in place for 12 weeks
at the onset of CA-UTI and is still indicated, the catheter should be replaced to hasten resolution of symptoms and to reduce the risk of subsequent CA-bacteriuria and CA-UTI (A-I).
The urine culture should be obtained from the freshly placed catheter prior to the initiation of antimicrobial therapyto help guide treatment (A-II).
If use of the catheter can be discontinued, a culture of a voided midstream urine specimen should be obtained prior to the initiation of antimicrobial therapy to help guide treatment
Duration of Treatment
Seven days is the recommended duration of antimicrobialtreatment for patients with CA-UTI who have prompt
resolution of symptoms (A-III), and 10–14 days of treatmentis recommended for those with a delayed response (A-III),regardless of whether the patient remains catheterized or not.A 5-day regimen of levofloxacin may be considered in patients with CA-UTI who are not severely ill (B-III). Data are insufficient to make such a recommendation about other fluoroquinolones.
A 3-day antimicrobial regimen may be considered for
women aged 65 years who develop CA-UTI without upper
urinary tract symptoms after an indwelling catheter has been removed (B-II).
Complications of long-term catheterization
(≥30 days) include,in addition to almost universal bacteriuria,lower and upper CA-UTI,
bacteremia,
frequent febrile episodes,
catheter obstruction,
renal and bladder stone formation associated with urease-producing uropathogens, local genitourinary infections,
fistula formation, incontinence, and bladder cancer.
Bacteriuria in patients with short-term catheters
(<30 days)
is usually caused by a single organism .Escherichia
coli is the most frequent species isolated, although it comprises fewer than one-third of isolates .Other Enterobacteriaceae,such as Klebsiella species, Serratia species, Citrobacter species,and Enterobacter species, nonfermenters such as P. aeruginosa,and gram-positive cocci, including coagulase-negative staphylococci
and Enterococcus species, are also isolated .Funguria,
mostly candiduria, is reported in 3%–32% of patients
catheterized for short periods of time .
In contrast to patients with short-term catheterization, UTIs in patients with long-term (≥30 days) catheterization are usually polymicrobial. In addition to the pathogens isolated from patients with short-term catheterization,
species such as P. mirabilis, Morganella morganii,
and P. stuartii are common .In these patients, new episodes of infection often occur periodically in the presence of existing infection with organisms that may persist for months.
Symptoms and signs suggestive of UTI in a catheterized
usually do not manifest the classic symptoms of dysuria, frequent urination,and urgent urination, although such symptoms may occur inCA-UTI after the catheter has been removed. In addition, patients with neurogenic bladders frequently have absence of sensation in the pelvis, and ascertainment of potential symptoms
of UTI is often difficult. The majority of patients with Ca-bacteriuria lack symptoms referable to the urinary tract.
The foul smell of urine around patients with urine incontinence is thought to be attributable mainly to the production of ammonia from urea by bacterial ureases. Foul-smelling and/or cloudy urine is often interpreted as warranting antimicrobial treatment in catheterized patients with bacteriuria.However, not all individuals with UTI have an unpleasant odor to their urine, and not all urine with an unpleasant odor is indicative of bacteriuria
No studies have demonstrated that odorous or cloudy urine in a catheterized individual, even if these findings are new, has clinical significance. Thus, odorous or cloudy urine should not be used alone to determine the presence of CA-bacteriuria and, in particular, to distinguish CA-ASB from CA-UTI, and alternate interventions, such as improved continence management or hydration, rather than antimicrobial therapy, should be instituted
causes of cloudy urine
Dehydration
Medications and Vitamins
Certain vitamins are water-soluble – meaning they’re excreted in the urine. If you’re taking high doses of B vitamins or vitamin C which are water soluble, it can cause the urine to look cloudy. Likewise, certain medications can do the same thing. Even drinking large amounts of milk can cause urine cloudiness due to the presence of phosphates in the milk.
Infection. The cloudiness usually comes from white blood cells that enter the urine to fight off the infection.
Kidney stones can also cause urine cloudiness.
Cloudy urine can be caused by pus (white blood cells), blood (red blood cells), sperm, bacteria, yeast, crystals, mucus, or a parasite infection, such as trichomoniasis.protein
Indications for Urinary Catheter Use
• Clinically significant urinary retention if medical therapy is not effective and surgical correction is not indicated.• Urinary incontinence for comfort in a terminally ill patient if less invasive measures fail and collecting devices are not an acceptable alternative.
• Need for accurate urine output monitoring in critically ill patients.
• When the patient is unable or unwilling to collect urine (ie, during prolonged surgical procedures with general or spinal anesthesia; selected urologic and gynecologic procedures in the perioperative period).
Indications and limitations of suprapubic catheterization.
Potential advantages of suprapubic catheters in patients who need bladder drainage, compared with indwelling urethral catheters,include lower risk of CA-bacteriuria, reduced risk of urethral trauma and stricture, ability to attempt normal voiding without the need for recatheterization, and less interference with sexual activity.Even though suprapubic catheterization appears to have advantages over indwelling urethral catheterization, it is not commonly used, except perhaps in gynecologic and urologic surgical procedures in some centers. The use of suprapubic catheterization is limited, because catheter insertion is an invasive procedure with risks of bleeding and visceral injury, the patient can still leak through the urethra, and—a problem especially for patients with long-term catheterization—specially trained caregivers are often needed to change the catheters.Further comparisons of intermittent urethral catheterization,suprapubic catheterization (both open surgical and percutaneous insertion techniques), and indwelling urethral catheterization are needed for patients who require long-term bladder drainage.
Indications and limitations of condom catheter use.
condom catheters appear to be associated with lessrisk for CA-bacteriuria than short-term indwelling urethral
catheters in appropriately selected men with low postvoid residual urine volume. There is no standard definition of abnormal residual urine volume, because the association between residual urine volume and UTI is not well established, although studies often define abnormal retention as the presence of >100 mL of urine on ≥2 consecutive occasions. Another potential advantage is that condom catheters cause less urethral trauma.
However, a condom catheter may not be an option in men whose penis is small or whose skin is ulcerated. In addition,condom catheters can lead to penile skin breakdown and scar formation. Men with neurogenic bladders secondary to spinal cord injury should undergo urodynamic testing to assess the safety of using a condom catheter, because assessment of the postvoid residual may not be a reliable indicator of detrusorsphincter dyssynergia, and long-term use of condom catheterization in the presence of dyssynergia may adversely affect renal function.
There is currently no satisfactory external catheter suitable for use by women.
Periodic catheter irrigation
Is intended to prevent catheter obstruction and infection, but little overall benefit has been seen in studies with closed systems . Agents used for continuous or intermittent bladder irrigation include antiseptics (povidone-iodine or chlorhexidine digluconate) and antibiotics (neomycin or polymyxin B sulfate) .nonbacteriuric adult patients who required short-term urinary catheterization to closed drainage with atriple-lumen, neomycin-polymyxin irrigated system or a double-lumen nonirrigated catheter system. There was no significant difference in the proportion (16% vs 18%, respectively) or in the cumulative prevalence of CA-bacteriuria between the 2 groups, but uropathogens in the irrigation group were significantly more resistant to the irrigating antibiotic than were those in the other group.Catheter blockage can result from encrustation formed by urease-producing organisms in the catheter biofilm.Patients with blocked catheters are more often colonized with P. mirabilis and P. stuartii than are patients without blocked catheters .In a randomized cross-over trial involving 32 women with long-term catheterization and bacteriuria in whom 10 weeks of once-daily normal saline irrigation was compared with 10 weeks of no irrigation, the prevalence and species of CA-bacteriuria and the incidence of catheter obstructions and febrile episodes, including those that appeared to be of urinary origin (ie, CA-UTIs), were similar.
catheter irrigation is time consuming, and some studies, at least those with long-term use of antimicrobial irrigating solutions, have shown that irrigation may promote infection due to organisms that are resistant to the antimicrobials.Routine bladder irrigation may also cause irritation of the bladder mucosa.
WHAT ARE THE APPROPRIATE MANAGEMENT STRATEGIES FOR PATIENTS WITH CA-UTI?
A urine specimen for culture should be obtained priorto initiating antimicrobial therapy for presumed CA-UTI because of the wide spectrum of potential infecting organisms and the increased likelihood of antimicrobial resistance (A-III). If an indwelling catheter has been in place for 12 weeks at the onset of CA-UTI and is still indicated, the catheter should be replaced to hasten resolution of symptoms and to reduce the risk of subsequent CA-bacteriuria and CA-UTI (A-I). The urine culture should be obtained from the freshly placed catheter prior to the initiation of antimicrobial therapy
to help guide treatment (A-II). If use of the catheter can be discontinued, a culture of a voided midstream urine specimen should be obtained prior to the initiation of antimicrobial therapy to help guide treatment (A-III).
Seven days is the recommended duration of antimicrobial
treatment for patients with CA-UTI who have prompt
resolution of symptoms (A-III), and 10–14 days of treatment
is recommended for those with a delayed response (A-III),
regardless of whether the patient remains catheterized or not.i. A 5-day regimen of levofloxacin may be considered in
patients with CA-UTI who are not severely ill (B-III). Data are
insufficient to make such a recommendation about other fluoroquinolones. A 3-day antimicrobial regimen may be considered for women aged 65 years who develop CA-UTI without upper urinary tract symptoms after an indwelling catheter has been removed (B-II).
Allopurinol may slow progression of renal disease in patients with chronic kidney disease (CKD), according to the results of a prospective, randomized trial published online June 10 in the Clinical Journal of the American Society of Nephrology.
"Hyperuricemia is associated with hypertension, inflammation, renal disease progression, and cardiovascular disease," write Marian Goicoechea, MD, from Hospital General Universitario Gregorio Marañón in Madrid, Spain, and colleagues. "However, no data are available regarding the effect of allopurinol in patients with chronic kidney disease."
.
Of 113 patients with estimated glomerular filtration rate (eGFR) levels lower than 60 mL/minute, 57 were randomly assigned to receive allopurinol 100 mg/day, and 56 to continue their usual therapy. To determine renal disease progression, cardiovascular events, and hospitalizations from any cause, clinical, biochemical, and inflammatory parameters were measured at baseline and at 6, 12, and 24 months of treatment.
Participants treated with allopurinol had significantly decreased serum uric acid and C-reactive protein levels. After 24 months, eGFR decreased 3.3 ± 1.2 mL/minute per 1.73 m2 in the control group, whereas it increased 1.3 ± 1.3 mL/minute per 1.73 m2 in the allopurinol group. Independent of age, sex, diabetes, C-reactive protein, albuminuria, and use of renin-angiotensin system blockers, allopurinol treatment was associated with slower progression of renal disease.
allopurinol treatment was associated with a 71% reduction in cardiovascular risk.
"Allopurinol decreases C-reactive protein and slows down the progression of renal disease in patients with chronic kidney disease.
Clin J Am Soc Nephrol. June 10, 2010.
pH of urine is normally 5.0 to 6.0
Specific gravity provides a rough measure of urine concentration (osmolality). Normal range is 1.001 to 1.035; values may be low in the elderly or in patients with impaired renal function, who are less able to concentrate urine. It is measured by hydrometer or refractometer or estimated with a dipstick. Accuracy of the dipstick test is controversial, but the test may be sufficient for patients who have calculi and are advised to self-monitor urine concentration to maintain dilute urine. Specific gravity by dipstick may be spuriously elevated when urine pH is < 6 or low when pH is > 7. Hydrometer and refractometer measurements may be elevated by high levels of large molecules (eg, radiopaque contrast agent, albumin, glucose, carbenicillin in the urine.A highly acidic urine pH occurs in:
Acidosis
Uncontrolled diabetes
Diarrhea
Starvation and dehydration
Respiratory diseases in which carbon dioxide retention occurs and acidosis develops
A highly alkaline urine occurs in:
Urinary tract obstruction
Pyloric obstruction
Salicylate intoxication
Renal tubular acidosis
Chronic renal failure
Respiratory diseases that involve hyperventilation
In people who are not vegetarians, the pH of urine tends to be acidic.
A diet rich in citrus fruits, legumes, and vegetables raises the pH and produces urine that is more alkaline.Unfortunately, blood urea nitrogen (BUN) and creatinine will not be raised above the normal range until 60% of total kidney function is lost. Hence, the more accurate Glomerular filtration rate or its approximation of the creatinine clearance are measured whenever renal disease is suspected or careful dosing of nephrotoxic drugs is required.
RBCs < 3/HPF may be normal (< 5/HPF is sometimes normal, eg, after exercise), and any hematuria should be interpreted in clinical context. On microscopic analysis, glomerular RBCs are dysmorphic, with spicules, folding, and blebs; nonglomerular RBCs retain their normal shape.
WBCs < 5/HPF may be normal; special staining can distinguish eosinophils from neutrophils.
Pyuria is defined as > 5 WBCs /HPF in a sample of centrifuged urine.
Sulpha Crystals
Indinavir Crystals
Ca oxalate crystals
Cystine Crystals
Mg ammonium phosphate(struviteCrystals)
Urinary tract infections are one of the most common conditions seen in female patients in general practice. These infections affect 50% of women at least once in their lives, and more than 20% of infections are resistant to trimethoprim and cephalosporins and 50% to amoxicillin.
Autosomal dominant polycystic kidney disease (ADPKD) affects approximately 1 of every 1000 persons in the general population and develops, by means of slowly progressive renal-cyst growth, to end-stage renal disease in over 50% of patients. Hepatic and pancreatic cysts, as well as cerebral and abdominal aneurysms, contribute to ADPKD-associated morbidity and mortality. Arterial hypertension, recurrent urinary tract infection, nephrolithiasis, and abdominal pain are frequently the presenting symptoms.
Astudy shows that among patients with progressive chronic kidney disease, clinical outcomes, including survival, are similar between patients in whom dialysis is initiated early and those for whom dialysis is electively delayed. The results show that with careful clinical management, dialysis may be delayed until either the GFR drops below 7.0 ml per minute or more traditional clinical indicators for the initiation of dialysis are present.
A Randomized, Controlled Trial of Early versus Late Initiation of Dialysis
Lower urinary tract symptoms have been categorised according to the three stages of the bladder cycle: storage (when filling of the bladder occurs), voiding (when the bladder actively expels its contents), and post micturition (immediately after voiding, while returning to the storage stage)
Categories of lower urinary tract symptomsStorage Urgency, frequencyNocturia,Urinary incontinenceVoiding
Hesitancy,StrainingSlow stream,Splitting or sprayingIntermittency,Terminal dribblePost-micturition
Post-micturition dribbleFeeling of incomplete emptying
Tests to detect nitrites and leukocyte esterase have become part of a routine urinalysis. Most species of bacteria that colonize in the urine cause nitrates, which are derived from dietary metabolites, to be converted to nitrites. In healthy people, both the urinary nitrite test and the leukocyte esterase (LE) tests are negative.
A negative nitrite test does not necessarily mean that the urine is free of all bacteria, particularly if there are clinical symptoms, because some bacteria do not produce nitrites.
Urinary Nitrites and Leukocyte Esterase
Optimal results for a urinary nitrite test are obtained by analyzing an early morning. specimen that has been incubating in the bladder for 4 hours or more. A clean catch or mid-stream specimen is important to reduce bacterial contamination.
White cells in the urine usually indicate a urinary tract infection. The leukocyte esterase (LE) test detects esterase, an enzyme released by white blood cells. Positive test results are clinically significant. The LE test is also used to screen for gonorrhea and for amniotic fluid infections.
• Urine culture - clean catch
A clean catch is a method of collecting a urine sample for various tests, including urinalysis, cytology, and urine culture. After cleaning the genital area, urinate a small amount into the toilet bowl to clear the urethra of any contaminants. Then, collect a sample of urine in a clean or sterile container. About 1 - 2 ounces of urine is needed for a test. Remove the container from the urine stream without stopping the flow. You may finish urinating into the toilet bowl. Return the sample to the health care provider.Infants:
A collection device must be attached to the baby to collect the urine.Thoroughly wash the area around the urethra. Open a urine collection bag (a plastic bag with an adhesive paper on one end), and place it on your infant.For boys, the entire penis can be placed in the bag and the adhesive attached to the skin.
For girls, place the bag over the labia. Diaper the infant as usual, covering and securing the bag. Check the baby frequently and remove the bag after the infant has urinated into it. Active infants may displace the bag, so it may take more than one attempt to obtain the specimen. Drain the urine into a container and give it to the health care provider, as directed.
Do not use antiseptics, as they may prevent bacteria from growing during the culture.
Before urinating, boys and men should clean the head of the penis. Girls and women need to wash the area between the vagina "lips" (labia). The health care provider will give you instructions how to do this. You may be given a special clean-catch kit that contains a cleansing solution and sterile wipes. The clean-catch urine method is used to prevent bacteria from the penis or vagina from getting into a urine sample. The clean catch can be used for a routine urinalysis, a urine culture, or other urine tests that require pure urine for accurate results.
Urine collection technique for women
Wash hands with soap and waterSpread labia with 1 hand and hold apart for collection
Use three povidone-iodine swabs to clean area
Wipe down one side, front to back, with one swab
Wipe down other side, front to back, with second swab
Wipe down center, front to back, with last swab
Dry area with sterile gauze
Void into toilet for a few seconds and then collect
Avoid stopping urine flow while positioning cup
Stopping flow increases risk of contaminated sample
Collect in sterile container
Cap and avoid touching inside of container
Urine collection technique for men
Wash hands with soap and waterRetract foreskin if needed
Use povidone-iodine swabs to clean tip of penis
Clean glans penis
Clean urethral opening
Dry area with sterile gauze
Void into toilet for a few seconds and then collect
Collect in sterile container
Avoid stopping urine flow while positioning cup
Stopping flow increases risk of contamination
Cap and avoid touching inside of container
Clean Catch Urine Collection :Midstream Urine Collection, Bag Urine Collection
Urine collection technique for infants (urine bag)
Wash hands with soap and water
Use povidone-iodine swabs to clean as above
Place sterile urine bag over penis or labia
Reclean and replace new urine bag if no urine in 30 min
Empty bag into sterile urine container
The combination of the LE test with the urinary nitrite test provides an excellent screen for predicting a urinary tract infection. A urine sample that tests positive for both nitrate and leukocyte esterase. The urinary nitrite and LE tests are often ordered to screen patients at high-risk for urinary tract infections, including pregnant women, school-age females, elderly patients, and persons with a history of urinary tract infections.
E Coli
Salmonella
Citrobacter
Proteus
Clebsiella
Oral Phosphate Binders in Patients with Kidney Failure
Hyperphosphatemia, a nearly universal complication of kidney failure, is accompanied by hypocalcemia and low serum levels of vitamin D. Without treatment, these deficiencies usually lead to severe secondary hyperparathyroidism, which in turn leads to painful fractures, brown tumors, and generalized osteopenia. Dietary restriction of phosphate has long been the cornerstone of therapy, but this measure is usually not sufficient to control hyperphosphatemia. As a result, oral phosphate binders are used in over 90% of patients with kidney failure.NEJM April 8 2010
Until the mid-1980s, aluminum was the mainstay of phosphate-binding therapy. Oral aluminum was administered at mealtimes to bind dietary phosphate, but this practice was largely abandoned when its use was linked to systemic aluminum toxicity, manifested as encephalopathy, osteomalacia, and anemia.
Calcium-based phosphate binders, either calcium carbonate or calcium acetate, have been used for decades in patients undergoing dialysis, and the two agents appear to have relatively similar phosphate-binding ability per gram of calcium administered. They are the most commonly used phosphate binders in contemporary practice worldwide.
Sevelamer
Sevelamer is an anion-exchange resin, first released as sevelamer hydrochloride, and almost all the clinical studies of sevelamer have used this formulation. Given concerns about metabolic acidosis due to the hydrochloride moiety, however, sevelamer is currently approved by the Food and Drug Administration and marketed as sevelamer carbonate, which appears to have a similar effect on phosphate lowering but has been much less extensively studied.Lanthanum
Lanthanum carbonate is a nonaluminum, noncalcium phosphate-binding agent. Lanthanum and calcium-based phosphate binders appear to be similarly effective in reducing serum phosphate concentrations in patients with end-stage renal disease.Magnesium-Based Phosphate Binders
Although oral magnesium has been used as a phosphate binder for many years, relatively few data are available concerning its efficacy and safety. Serum magnesium levels are higher in patients undergoing dialysis than in persons with normal kidney function, and hypermagnesemia with respiratory arrest has been reported after excessive oral magnesium ingestion in such patients. Accordingly, most contemporary hemodialysis programs severely restrict or avoid the administration of medications that contain magnesium.Suggestions for Management of Hyperphosphatemia
Dietary phosphate restriction effectively reduces serum phosphate levels and should be encouraged for all patients with end-stage renal disease. Although the optimal method of facilitating adherence to a phosphate-restricted diet is unknown, more frequent patient contact with renal dietitians may be beneficial, at least initially. In addition, specific counseling about the need to avoid phosphate-containing food additives leads to a substantial reduction in serum phosphate, as compared with the usual education about foods that are naturally high in phosphate. Even with careful dietary modification, most patients undergoing dialysis will continue to require oral phosphate binders.calcium-based agents to be the first-line phosphate binders for patients undergoing dialysis, since these preparations remain the least expensive and best tolerated option for the treatment of hyperphosphatemia. Although the optimal starting dose has not been studied, many clinicians initially prescribe 200 mg of elemental calcium with each meal for these patients.
Sevelamer and lanthanum are promising, but their superiority to calcium-containing agents has not been proved. Furthermore, they are expensive and are associated with more adverse events. In the absence of their proven clinical benefit as compared with calcium-based agents, in our view sevelamer and lanthanum cannot be recommended as initial therapy. Although the use of these drugs to supplement or replace calcium-based agents in patients with severe vascular calcification has been advocated, this strategy has not been tested in clinical trials.
For patients in whom phosphate levels cannot be controlled with calcium-based agents alone (especially patients with hypercalcemia), short courses of magnesium-based binders are an inexpensive alternative. However, careful monitoring of serum magnesium levels is required (perhaps in conjunction with a lower magnesium concentration in the dialysate), and the long-term safety and efficacy of this approach are unknown. Some physicians may prefer to use sevelamer or lanthanum despite their higher cost.
Agents under Development
A number of new calcium-free phosphate binders are under study. For example, magnesium iron hydroxycarbonate (fermagate) in a dose of 1 g given 3 times a day before meals was associated with reduced serum phosphate levels, but a higher dose (6 g per day) was associated with adverse gastrointestinal events.1
MCI-196 (colestilan), a novel nonmetallic anion-exchange resin (similar to sevelamer), was associated with reductions in phosphate of approximately 0.2 mmol per liter as compared with placebo, but the longest trial in humans to date has been 3 weeks.
Treatment with niacin and nicotinamide, as compared with placebo, is associated with a significant reduction in serum phosphate levels, possibly through direct inhibition of the sodium-dependent phosphate cotransporter Na-Pi-2b in the gastrointestinal tract. All these newer agents can be given once daily and do not need to be taken with meals. MCI-96, niacin, and nicotinamide also lower serum cholesterol levels and may reduce levels of triglyceride-rich lipoproteins, although the clinical importance of these effects is unclear.
Patients with advanced kidney disease often have markedly elevated salivary phosphate concentration, which is independent of food content. Since phosphate in swallowed saliva can be absorbed in the gastrointestinal tract, it may be a target for phosphate binders. A recent study showed that chewing gum containing a novel chitosan compound was effective in lowering serum phosphate levels in parallel with reductions in salivary phosphate levels.
To date, no information is available as to whether any of these newer phosphate binders affect bone histologic features, vascular calcification, hospitalizations, or mortality. Therefore, larger, double-blind studies with longer follow-up and clinical outcomes as end points are required before these agents can be recommended for clinical use.
Some High Phosphorus Foods
MilkCheese
Yogurt
Ice Cream
Beer, Cola, Milk-based Coffee and Chocolate Drinks
Chocolate
Bran
Brown Rice, Wild Rice
Whole Grain Breads, Cereals & Crackers
Corn Tortillas
Pancakes, Waffles, Biscuits
Pizza
Avocado
Nuts, Seeds, Nut butters
Dried Beans & Peas
Corn & Peas
Processed Meats such as: Hot Dogs, Sausage, Turkey Sausage, Bologna
Organ Meats
Sardines
Some Low Phosphorus Foods
Certain brands of Nondairy Creamers, Rice Milk (Unenriched),
Cream Cheese, Sour Cream
Soda-Lemon-lime, Grape, Strawberry, Cranberry Juice
Jellybeans, Hard Candy
Unsalted Popcorn or Pretzels
Jam, Jelly, Honey, Cream Cheese
Margarine, Butter
Corn or Rice Cereals, Refined Wheat Cereals
Cream of Wheat, Cream of Rice, Grits
French, Italian or White Bread
White Rice, Pasta, Couscous
Fresh or Fresh Frozen Meat, Fish and Poultry (compared to processed)
Fruits such as: Apples, Berries, Grapes, Plums, Pineapple, Canned Pears, Peaches, Fruit Cocktail
Vegetables such as: Green Beans, Cabbage, Carrots, Cauliflower, Eggplant, Bell Pepper, Cucumber, Lettuce, Onion, Radish
Early Death by Junk Food? High Levels of Phosphate in Sodas and Processed Foods Accelerate the Aging Process in Mice
ScienceDaily (Apr. 28, 2010) — Here's another reason to kick the soda habit. New research published online in the FASEB Journal shows that high levels of phosphates may add more "pop" to sodas and processed foods than once thought. That's because researchers have found that the high levels of phosphates accelerate signs of aging. High phosphate levels may also increase the prevalence and severity of age-related complications, such as chronic kidney disease and cardiovascular calcification, and can also induce severe muscle and skin atrophy.
Nearly every food contains some phosphorus, so you can't eliminate all phosphorus from your diet. Generally foods high in protein (some meats, dairy products, beans, legumes, nuts and seeds) are higher in phosphorus. Therefore, unless you're receiving kidney dialysis, you'll be asked to eat smaller quantities of them. Whole grains also are higher in phosphorus, so choose refined ones.
Instead of these higher phosphorus foods:
Milk, pudding or yogurt (from animals and from many soy varieties)Hard cheeses or Neufchatel cheese
Ice cream or frozen yogurt
Soups made with (milk, peas, beans, lentils)
Whole grains, including whole-grain breads, crackers, cereal, rice and pasta
Quick breads, biscuits, cornbread, muffins, pancakes or waffles
Peas (fresh green, split, black-eyed), beans (black, garbanzo, lima, kidney, navy, pinto) or lentils
Starchy vegetables: corn, parsnips, pumpkin or sweet potato
Other vegetables: artichokes, asparagus, broccoli, mushrooms, peapods (cooked) or spinach
Organ meats, walleye, pollock or sardines
Fats: cream (including fat-free, half and half), sesame butter (tahini) or sour cream
Chocolate
Cola soft drinks
lower phosphorus foods:
Rice milk (unfortified) or nondairy creamerCream cheese or cottage cheese
Sherbet or frozen fruit pops
Soups made with lower phosphorus ingredients (broth- or water-based with other lower phosphorus ingredients)
Refined grains, including white bread, crackers, cereals, rice and pasta
Refined (white) dinner rolls, bagels, English muffins or croissants
Green peas (canned, frozen), green beans or wax beans
Starchy vegetables: potato, rutabaga or winter squash
Other vegetables: cabbage, beets, carrots, celery, cucumbers, eggplant, lettuce, peppers, onions, tomatoes or summer squash
Beef, pork, lamb, poultry or other fish
Fats: butter, margarine, mayonnaise, salad dressing, shortening or vegetable oils
Hard candy or gumdrops
Lemon-lime soda, ginger ale or root beer
Anti-neutrophil cytoplasmic antibody(ANCAs)
group of autoantibodies, mainly of the IgG type, against antigen in the cytoplasm of neutrophil and monocyte, detected in a number of autoimmune disorders, but are particularly associated with systemic vasculitis, so called ANCA-associated vasculitides.
Two types of ANCA assays are currently in wide use:
• Indirect immunofluorescence assay, using alcohol fixed buffy coat leukocytes
• Enzyme-linked immunosorbent assay (ELISA), using purified specific antigens
Of these two techniques, the immunofluorescence assay is more sensitive and the ELISA more specific.
Associated Conditions
C-ANCA or PR3 (proteinase 3) -ANCA• Wegener's Granulomatosis (Very Strongly associated)
• A.Positive in 75-98% of patients with Wegener's
• B.Sensitivity drops to 30% during remission
• Crescentic Glomerulonephritis (moderately associated)
• Polyarteritis nodosa (Weakly associated)
P-ANCA or MPO-ANCA
• Crescentic Glomerulonephritis (Strongly associated)
• Polyarteritis nodosa (Moderately associated)
• Churg-Strauss Vasculitis (Moderately associated)
• Wegener's Granulomatosis (Weakly associated)
• Henoch-Schonlein Purpura (Possibly associated)
• Temporal Arteritis (Possibly associated
cytoplasmic (cANCA), and perinuclear locations (pANCA).
The target antigens recognized by most cANCA positive sera are proteinase 3 (PR3) whereas that of pANCA is usually myeloperoxidase (MPO), although other neutrophil cytoplasmic antigens such as elastase and lactoferrin have also been recognized. These antigen specific ANCA (PR3-ANCA and MPO-ANCA) can be detected by enzyme-linked immunosorbent assay (ELISA). The results of ANCA positivity obtained by IIF and by ELISA do not always correlate, since some patients can be positive by ELISA and negative by IIF and vice versa. Among patients with WG, cANCA (or PR3-ANCA) are most frequent but are not specific for this disease. Patients with MPA, idiopathic crescentic GN, and CSS are usually positive for pANCA (or MPO-ANCA), but these antibodies are also associated with ulcerative colitis, rheumatoid arthritis and infective endocarditis.
ANCA constitute a useful marker with which it is possible to substantiate a diagnosis of idiopathic crescentic GN and/or systemic vasculitis. It is still debatable whether ANCA are directly involved in the pathogenesis, or are essentially epiphenomena of vasculitis. However, neutrophils and their tissue destructive mediators (proteinases such as elastase and PR3, as well as oxygen radicals) play a crucial role in the tissue destruction and development of crescentic GN and/or vasculitis. Further studies are necessary to define the initial factors that trigger these diseases.
Hyperoxaluria may occur when dietary calcium
is low or oxalate intake is unusually high or(less commonly) when oxalate is overproduced.
Dietary oxalate restriction to less than 100 mg
per day and the avoidance of an intake of ascorbic
acid above 100 mg per day are prudent if hyperoxaluria is present. Foods that are very high
in oxalate include spinach, rhubarb, wheat bran,
chocolate, beets, miso, tahini, and most nuts.
Stress urinary incontinence
the leakage of urine associated with increases in intraabdominal pressure occurs commonly in women and is associated in many cases with markedly reduced quality of life.The International Continence Society defines overactive bladder as “urinary urgency, usually accompanied by frequency and nocturia, with or without urgency urinary incontinence, in the absence of urinary tract infection or other obvious pathology,” and urgency urinary incontinence as “involuntary loss of urine associated with urgency.” A total of 5 to 10% of women have urgency urinary incontinence at least monthly. Overactive bladder without incontinence is more common, affecting 10 to 15% of women. In many studies, voiding of the bladder eight or more times in 24 hours has been used as the threshold for classifying abnormal urinary voiding as overactive bladder.
Idiopathic urgency urinary incontinence
Measurement of residual urine after voiding is indicated in women reporting clinically significant symptoms of obstructive voiding or those in whom invasive therapies are planned, but is not necessary for most patients. Urodynamic testing is generally not necessary before the initiation of conservative treatment.Fluid intake has not been rigorously studied in this context but appears to have a minor role in provoking urinary incontinence. Given the potential adverse effects of severely restricting fluids, limiting fluids should not be recommended unless a voiding diary clearly illustrates that urinary output is very large (e.g., more than 3000 ml per day). Although data are limited, higher caffeine intake has been associated with detrusor overactivity, and a small, randomized trial showed that decreasing caffeine intake reduced the number of incontinence episodes. Weight loss results in significant symptomatic improvement among obese patients with urgency urinary incontinence.
Urinary incontinence affects millions of people. People of all ages suffer from urinary incontinence. The disease is found in about 30% of women aged 30 to 60 years. There are different types of incontinence. Urge incontinence is the most often pharmacologically treated type. The mainly used substances belong to the class of antimuscarinic drugs. Their use is limited by several side effects. Furthermore, in some patients anticholinergic medication is ineffective and antimuscarinics used as single medication do not lead to a sufficient therapeutic effect.
Other possible pharmacological substances for treatment of overactive bladder (detrusor instability) associated with urge and urge incontinence are the selective β-adrenoceptor-agonists which are mainly responsible for the adrenergic mediated relaxation. It depends on the species, which β-adrenoceptor-subtype (the β2- and/or β3-adrenoceptor) mainly mediates the relaxation.
Non selective β-adrenoceptor-agonists exhibit serious cardiovascular side effects like tachycardia or decrease of blood pressure by stimulating β1- and β2-adrenoceptors. These side effects should be decreased when using selective agonists. Additionally, substances whose targets are membrane channels of muscle cells could be interesting for treatment of overactive bladder. This group includes L-type calcium antagonists and potassium channel openers of ATP-sensitive potassium channels or BK channels. Especially the local use of the pharmacologically very potent calcium antagonists could be an interesting therapeutic approach, since systemic cardiovascular side effects were avoided.
After chronic oral treatment with different calcium antagonists effects on the detrusor muscle were reduced or could not be detected, possibly due to an upregulation of 1,4-dihydropyridine-sensitive potassium channels. A very interesting approach is the use of potassium channel openers said to be selective for the urinary bladder. If there is a selectivity for the detrusor muscle, cardiovascular side effects were reduced. Possibly, the local use is a useful application form.
Selective β-adrenoceptor agonists, calcium antagonists and potassium channel openers are pharmacological approaches, which are not yet available for clinical use.
Retrospective studies, did not show a survival benefit with early initiation of dialysis. On the contrary, patients who started dialysis with a relatively low creatinine clearance ("late start") tended to survive longer. At least two recent studies attributed this somewhat unexpected finding to the higher number of coexisting conditions among patients who start dialysis at a higher estimated GFR. In addition, calculation of the estimated GFR is based on the serum creatinine concentration. The calculation of estimated GFR, whether it is based on the commonly used Modification of Diet in Renal Disease (MDRD) equation or the Cockcroft–Gault equation — or some other estimating equation — may be quite inaccurate when kidney function is extremely reduced
NEJM June 27, 2010
Although a low serum creatinine concentration generally indicates a better GFR, a low creatinine concentration may also be caused by decreased muscle mass due to malnutrition or may be induced by overhydration. In addition, the discussion of timing for the initiation of dialysis is confounded by the distinction between the timing of referral to the nephrologist and the timing of initiation of dialysis. Obviously, only patients who are referred to a nephrologist in a timely manner have the opportunity to plan the timing for the initiation of dialysis.
clinical symptoms and patient follow-up are of greater importance in decision making than the estimated GFR. In other words, the presence of symptoms of uremia led to the initiation of dialysis in the majority of the patients in the late-start group.
Cooper et al. report results from the Initiating Dialysis Early and Late (IDEAL) study, a multicenter, randomized, controlled trial in which adult patients who have progressive end-stage renal disease and are already receiving care in nephrology units were assigned to planned initiation of hemodialysis or peritoneal dialysis when the estimated GFR was either 10 to 14 ml per minute per 1.73 m2 (early start) or 5 to 7 ml per minute per 1.73 m2 (late start).
The patients in the two study groups were well matched with respect to baseline characteristics. After a median follow-up period of 3.59 years, patient survival and the frequency of adverse events were not significantly different between the two groups; however, there was a 6-month separation between the groups in the start time of dialysis.
NEJM June 27, 2010
The main conclusion of this important study — that for asymptomatic patients renal-replacement therapy can be delayed by an average of 6 months — should be placed in perspective. An important prerequisite for a "wait and see" policy is careful clinical follow-up of each patient in order to avoid some of the life-threatening complications of uremia that may necessitate immediate renal-replacement therapy. All the patients in the trial had been followed for some time by their respective nephrologists and were well prepared to start dialysis. The study protocol explicitly advocated that the method of dialysis be selected, and a functioning peritoneal or vascular access be prepared, in advance, a policy that permits the immediate initiation of dialysis if the patient becomes symptomatic.
Indeed, few patients in either group started dialysis with the use of a temporary access catheter.
The Initiation of Renal-Replacement Therapy
Conversely, the results of the study also imply that among asymptomatic patients, delaying the start of dialysis until a permanent access has been created does not jeopardize the outcome. Given the results of the study, the use of temporary catheters, with their high risks of infection and stenosis, can probably be avoided, and patients willing to start peritoneal dialysis can avoid temporary hemodialysis.
NEJM June 27, 2010
The evidence that angiotensin-converting–enzyme (ACE) inhibitors and angiotensin-receptor blockers (ARBs) preserve renal function is limited to patients with a high degree of proteinuria. Given the risk of hyperkalemia, ACE inhibitors and ARBs should not be considered preferred antihypertensive agents for patients with stage IV chronic kidney disease who have a minimal degree of proteinuria. For patients with proteinuria, reduction of urinary protein to approximately 500 mg per day appears to be beneficial. An ACE inhibitor or ARB at modest doses does not typically achieve this goal. Thus, the prescription of an ACE inhibitor or an ARB itself does not define optimal treatment for chronic kidney disease.
Stage IV Chronic Kidney Disease
NEJM May 20,2010
GFR to 15 to 29 ml per minute per 1.73 m2.Clinical experience and trial data suggest that targeted reduction of proteinuria, by increasing the dose of an ACE inhibitor or ARB or (arguably) by adding medications, slows the progression of renal disease. In the Renoprotection of Optimal Antiproteinuric Doses (ROAD) trial, a strategy of increasing the dose of benazepril or losartan to achieve optimal antiproteinuric effects, as compared with conventional dosing, reduced the incidence of the doubling of serum creatinine, end-stage renal disease, or death over the course of a median 3.7-year follow-up. In other studies, high doses of lisinopril (80 mg per day) or candesartan (128 mg per day) have been shown to reduce proteinuria more than do standard doses.
Kidney transplantation remains the treatment of choice for most patients with end-stage renal disease, including and especially patients with diabetic kidney disease. Patients with stage IV chronic kidney disease or renal disease that has progressed almost to end-stage disease should undergo counseling and education about the available methods to treat end-stage renal disease. This should include encouragement to identify live kidney donors when appropriate.
Stage IV Chronic Kidney Disease
NEJM May 20,2010
GFR to 15 to 29 ml per minute per 1.73 m2.For patients who opt to receive a transplant from a live kidney donor, evaluation of both the donor and the recipient should be performed at least 6 months before the predicted need for renal-replacement therapy.
Kidney transplantation offers the best outcome, especially when it is performed before hemodialysis is initiated.
The renoprotective benefits of ACE inhibitors or ARBs are not seen in patients with chronic kidney disease who do not have diabetes or proteinuria. Since cardiovascular disease is the most common cause of complications and death among patients with chronic kidney disease, particularly when they also have diabetes, ACE inhibitors or ARBs should be considered for the treatment of hypertension in these patients. The propensity for hyperkalemia increases with declining glomerular filtration rate, and therefore these agents should be used with caution in patients with advanced chronic kidney disease, with or without proteinuria.
However, doses sufficient to lower blood pressure to target levels may not reduce proteinuria optimally, and residual proteinuria is a risk factor for progression. Evidence suggests that more effective blockade of the renin–angiotensin system, rather than simply more effective blood-pressure control, confers maximal renoprotection. Some, but not all, studies show that doses of ACE inhibitors or ARBs above the maximum recommended doses achieve a greater reduction in proteinuria than do conventional doses in patients with chronic kidney disease — both those with diabetes and those without diabetes.
Alternatively, a combination of conventional doses of an ACE inhibitor and an ARB may achieve similar benefits, although randomized trials are lacking to compare treatment with an ACE inhibitor or ARB at doses that are higher than the maximum recommended doses with treatment that consists of a combination of these two agents at conventional doses.
Less than 2% of patients with chronic kidney disease ultimately require renal-replacement therapy. In part, this low rate is explained by the increased risk of death from cardiovascular causes before progression to end-stage renal disease can occur. Cardiovascular disease is the most frequent cause of death among patients with chronic kidney disease
NEJM january 7, 2010
GFR to 15 to 29 ml per minute per 1.73 m2.
Stage IV Chronic Kidney Disease
Cardiovascular Disease in Patients with Chronic Kidney Disease. Cardiovascular disease in patients with advanced chronic kidney disease (CKD) is characterized by left ventricular (LV) hypertrophy, which occurs in large part as a result of hypertension, expansion of extracellular volume (ECV), and anemia. The left ventricular hypertrophy may be accompanied by left ventricular remodeling and fibrosis, and these changes, with or without coronary artery disease, may lead to cardiac failure, myocardial infarction, or sudden death.
Cardiovascular complications associated with chronic kidney disease include angina pectoris, myocardial infarction, heart failure, stroke, peripheral vascular disease, arrhythmias, and sudden death .The risk of each of these conditions increases from early-stage to advanced chronic kidney disease. The increased risk of death and poor outcomes after myocardial infarction in patients with stage III or stage IV chronic kidney disease may be related to the frequent proximity of lesions to the coronary ostia. In laboratory animals, uremia is associated with cardiac fibrosis
In patients with advanced chronic kidney disease, uremic cardiomyopathy is characterized by diastolic dysfunction, heart failure, and left ventricular hypertrophy; these abnormalities, in combination with myocardial ischemia and electrolyte shifts, probably contribute to the high incidence of sudden death.
Potentially reversible causes that may contribute to the decline in GFR in patients with chronic kidney disease should be identified. These include hypovolemia and hypotension; conditions associated with a decreased effective arterial-blood volume, such as cirrhosis and the nephrotic syndrome; obstructive uropathy, urinary tract infection, or occlusive renovascular disease; the use of nonsteroidal antiinflammatory drugs; and severe hypokalemia or hypercalcemia.
Although serum cystatin C has been proposed as a reliable marker for the estimation of the GFR, other factors
besides the GFR may influence cystatin C levels,and this measurement is not used routinely in practice.
Interventions to Slow the Rate
of Progression of Chronic Kidney DiseaseTreatment of Hypertension
Randomized clinical trials and prospective observational
studies have shown that control of systemic hypertension slows the rate of progression of chronic kidney disease both in subjects who have diabetes and in those who do not. ACE inhibitors or angiotensin-receptor blockers (ARBs) are considered to be the first line of antihypertensive therapy for patients with chronic kidney disease,
including those with advanced chronic kidney disease, whether or not they have diabetes. The current recommendation is that blood pressure should be lowered
to less than 130/80 mm Hg in all patients with chronic kidney disease
NEJM january 7, 2010
An early, rapid decline in the GFR may occur if the target blood pressure is achieved abruptly, and in such cases, renal function should be monitored closely until it stabilizes. Concomitant restriction of the intake of dietary salt and use of a loop diuretic are often required to control blood pressure. A high salt intake blunts the effect of antihypertensive medications and the antiproteinuric effects of ACE inhibitors and ARBs. Beta-blockers and dihydropyridine or non–dihydropyridine calcium-channel blockers are also frequently required to control hypertension in patients with advanced chronic kidney disease;dihydropyridine calcium-channel blockers are acceptable treatments as long as patients are receiving ACE inhibitors or ARBs.
Reduction of Proteinuria
Proteinuria is an independent risk factor for progressiverenal structural damage. Prospective studies have shown that reducing albuminuria with the use of antihypertensive medications, in particular ACE inhibitors or ARBs, results in a reduced rate of decline in the GFR both in subjects who have diabetes and in those who do not. Randomized trials in which ARBs were administered in patients with hypertension and type 2 diabetes,
proteinuria, and chronic kidney disease showed that there was a significant reduction in the risk of the progression of chronic kidney disease (risk reduction of 15 to 37%) cardiovascular events, and death.
A reduction in urinary protein excretion to less than 300 to 500 mg per day is associated with a slowing of the progression of chronic kidney disease. An ACE inhibitor or anARB should be the first line of therapy to reduce proteinuria. Dual therapy with an ACE inhibitor and an ARB may be more effective than either agent alone in reducing proteinuria, but data from long-term randomized trials are lacking to assess the effect of combined therapy in slowing the progression of chronic kidney disease. protein restriction reduces proteinuria and the progression of kidney disease,it is recommended that dietary protein be limited to approximately 0.8 to 1.0 g per kilogram of body weight per day.
Glycemic Control
Poorly controlled blood glucose levels are associated with an increased risk of nephropathy and cardiovascular complications. Although datafrom randomized trials suggest that strict control
of blood glucose may prevent the development of
diabetic nephropathy and retard the progression
from microalbuminuria to proteinuria, no randomized trials have assessed the effect of glycemic control on disease progression in patients with advanced chronic kidney disease.
Management of Associated Disorders Mineral and Bone Disorders
Disorders of mineral and bone metabolism arecommon in patients with stage IV chronic kidney
disease and may be associated with increased cardiovascular calcification, potentially contributing
to an increased risk of complications and death.The
decreased renal phosphate excretion, with resultant
increases in serum phosphate levels; furthermore,
there is decreased conversion of vitamin D to its active
form, 1,25-dihydroxyvitamin D (1,25(OH)D3),
resulting in decreased levels of circulating
1,25(OH)D3 and serum calcium and decreased
intestinal calcium absorption.
The hyperphosphatemia,hypocalcemia, and decreased levels of active vitamin D result in increased synthesis and secretion of parathyroid hormone. High phosphate
levels may also increase the production by osteocytes
of the phosphaturic hormone, fibroblast growth factor (FGF-23). FGF-23 inhibits the synthesis of 1,25(OH)D3 and may contribute to high levels of parathyroid hormone. Hyperparathyroidism is present in more than half the patients who have a GFR of less than 60 ml per minute per 1.73 m2 and is independently associated
with increased mortality and an increased prevalence
of cardiovascular disease.
Current guidelines recommend monitoring of serum calcium and phosphate levels (every 3 to 6 months), parathyroid hormone levels (every 6 to 12 months),and bone-specific alkaline phosphatase activity (every 6 to 12 months) in patients with stage IV chronic kidney disease, with the goal of normalizing these values.Patients with persistently elevated parathyroid hormone levels (secondary hyperparathyroidism) should restrict their intake of dietary phosphate and be treated, in most cases, with a phosphate
binder and an active vitamin D analogue if they have normal calcium levels.
Serum phosphate levels should be monitored,
and if they are found to be higher than 4.6 mgper deciliter (1.5 mmol per liter), a phosphate
binder should be prescribed. A low-dose active
vitamin D analogue will help control secondary
hyperparathyroidism. A bicarbonate concentration below 20 mmol per liter and systemic acidemia should be treated with sodium bicarbonate.
Cardiovascular Disease
Given the high risk of cardiovascular disease in patients with chronic kidney disease, attention should be paid to preventing and treating cardiovascular risk factors in these patients.Most patients with chronic kidney disease have dyslipidemia. The guidelines of the Kidney Disease Outcomes Quality Initiative recommend lowering low-density lipoprotein (LDL) cholesterol levels to less than 100 mg per deciliter although the benefit of this policy has not been documented in patients with advanced chronic kidney disease.A post hoc analysis of patients with a decreased estimated GFR who were enrolled in trials of statin therapy showed that treatment with statins reduced cardiovascular events in patients with stage II or stage III chronic kidney disease, but these trials did not include patients with stage IV disease. A recent metaanalysis of randomized, placebo-controlled trials showed that statins reduced lipid levels, cardiovascular events, and proteinuria, but not all-cause mortality, in patients with chronic kidney disease, irrespective of the stage of the disease.
Mechanisms Underlying the Progression from Early-Stage to Advanced Chronic Kidney Disease.
Progressive glomerulosclerosis and interstitial fibrosis result in the progression from early chronic kidney disease to advanced chronic kidney disease. Loss of renal mass results in activation of the renin–angiotensin–aldosterone system (RAAS), systemic hypertension, proteinuria, and hyperlipidemia. These adaptations result in increased inflammation and oxidative stress, with up-regulation of proinflammatory cytokines and growth factors, their receptors, or both; the increased inflammation and oxidativestress stimulate cell hypertrophy and proliferation and inflammatory-cell infiltration.
Virtually every cellular compartment in the
kidney is affected. Some of these early adaptations, such as hemodynamic and hypertrophic responses, become maladaptive and eventuallycontribute to the structural and functional changes in the kidney that are characteristic of advanced chronic kidney disease. CTGF denotes connective tissue growth factor, EGF epidermal growth factor, IGF-I insulin-like growth factor I, MCP-1 monocyte chemotactic
protein 1, NO nitric oxide, PDGF platelet-derived growth factor, RANTES regulated on activation normal T-cell expressed and secreted,TGF-β transforming growth factor β, and VEGF vascular endothelial growth factor.
Mechanisms Underlying the Progression from Early-Stage to Advanced Chronic Kidney Disease.
AnemiaAnemia is common in patients with chronic kidney
disease, especially in those with diabetes and
in those with stage IV disease, more than half of
whom have anemia. Deficient erythropoietin synthesis,iron deficiency, blood loss, and a decreased erythrocyte half-life are the major causes of anemia associated with chronic kidney disease.The use of erythropoiesis-stimulating agents results in a reduced need for blood transfusions among patients with advanced chronic kidney disease. and has also been associated with a reduction in left ventricular hypertrophy.
However,there is increasing evidence that erythropoiesis stimulating agents should be used cautiously.
In randomized trials involving patients with chronic kidney disease, in which target hemoglobin levels higher than 13 g per deciliter were compared with target levels of 10 to 12 g per deciliter, the higher hemoglobin levels led to an increased risk of death, cardiovascular events, stroke ,and hospitalization for congestive heart failure.
An important aspect of the management of anemia in patients with chronic kidney disease is acareful assessment of iron status to ensure that the transferrin saturation is between 20% and 50% and ferritin levels are between 100 and 800 ng per milliliter. Adequate iron supplementation can
be achieved with either oral or parenteral iron.
Electrolyte and Acid–Base Disturbances
The kidney is generally able to compensate for aloss of functioning nephrons and maintain euvolemia,
electrolyte balance, and acid–base balance until the GFR falls below 30 ml per minute per 1.73 m2. When the GFR is below that level, there is impairment in both sodium excretion in response to a sodium load and sodium conservation in response to an acute reduction in sodium intake;in most patients, sodium excretion does not fall
below 20 to 30 mmol per day, at least initially.
A concomitant impairment in the physiological processes that allow for maximal concentration or dilution of the urine confers a predisposition to hyponatremia or hypernatremia in these patients.
Observational studies showed that low haemoglobin concentrations predicted death in dialysis patients—anaemia was thought to cause left ventricular hypertrophy, dilatation, and heart failure. Anaemia became a therapeutic target for the reduction of cardiovascular mortality in people with end stage renal disease.
Current K/DOQI guidelines suggest a protein intake of 0.6-0.75 grams of protein per kilogram of body weight per day (g/kg/d) for patients in stages 1-4 of CKD. In stage 5, when patients are receiving dialysis, increased protein intake is suggested (approx. 1.2 g/kg/d). The average healthy American consumes the amount of protein equivalent to 1.2 g/kg/d. This means that, on the basis of current K/DOQI recommendations, CKD patients in stages 1-4 need dietary counseling to reduce their protein intake by about half.
Note that there is controversy in the literature about protein intake. Some scientists suggest that limitation is not necessary. They also point out a lack of compliance with the low-protein diet. However, most (but not all) literature suggests that decreasing protein intake in CKD stages 1-4 can delay progression into stage 5.
Adequate vitamin D intake can be problematic in CKD patients as well. Kidney failure reduces the production and conversion of vitamin D to active calcitriol 1,25(OH2)D3. CKD patients in stages 2-4 with GFRs of 20-60 mL/min should have serum 25-OH vitamin D checked (not serum 1,25(OH2)D3). If 25-OH vitamin D is < 75 nmol/L, the patient should receive standard vitamin D supplements.
If the patient's GFR is < 20 mL/min, or if he or she is in stage 5, standard vitamin D is no longer effective and the active vitamin 1,25 (OH2)D3 is needed.
According to K/DOQI guidelines, in CKD patients stage 1-4, sodium is restricted to 2000 mg/d, calcium is restricted to 1200 mg/d, and potassium and phosphorus intakes should be correlated with laboratory values.
Fluid intake can be unrestricted assuming normal urine output. Careful monitoring of laboratory values is necessary.
Patients on dialysis (stage 5) are known to lose certain water-soluble vitamins. However, patients in renal failure have decreased excretion of vitamin A, and vitamin A toxicity has been reported in some cases. Therefore, patients on dialysis should receive a multivitamin supplement that avoids excessive vitamin A.
Non-steroidal anti-inflammatory drugs (NSAIDs) prevent the production of prostaglandins, molecules which dilate the afferent arteriole.
NSAIDs could therefore worsen kidney function by decreasing afferent blood flow to the Bowman's capsule.
In the early placebo controlled randomised trial, quality of life benefits were shown when erythropoietins were used to reach a target haemoglobin range of 95 g/l to 100 g/l. A meta-analysis of quality of life outcomes in patients treated to haemoglobin targets above 120 g/l compared with lower targets found that improvements were not clinically important. The lower targets associated with better outcomes in the three large randomised trials in this area were 100 g/l, 113 g/l, and 105-115 g/l. Finally, it is important not to lose sight of the high cost of erythropoietins, and the fact that costs increase non-linearly with higher haemoglobin targets.
Normalisation of haemoglobin concentrations is known to be hazardous, ineffective, and costly in patients with chronic kidney disease, and these new data suggest that the same applies in patients with renal transplants. The important evidence gap concerns lower haemoglobin targets: we lack randomised evidence to help us choose between haemoglobin targets in the range 95 g/l to 120 g/l.
Most patients with chronic kidney disease —with the exception of some patients who also have diabetes and hypoaldosteronism —have near- normal serum potassium levels. However, hyperkalemia may develop in patients with chronic kidney disease after they receive treatment with aldosterone antagonists, ACE inhibitors, or ARBs.
Non–anion-gap metabolic acidosis can develop in patients with chronic kidney disease, primarily owing to a reduction in renal ammonia synthesis and, among patients with advanced chronic kidney disease, a reduction in titratable acid (phosphate) excretion. An increased anion-gap metabolic acidosis due to the retention of organic acids is common in patients with uremia and stage IV kidney disease that is approaching the end stage.In a recent randomized trial, oral sodium bicarbonate supplementation slowed the progression of chronic kidney disease and improved nutritional status, a finding that warrants confirmation in other trials.
Polycystic kidney disease
Autosomal dominant polycystic kidney disease (ADPKD) is a common cause of renal failure, occurring in one person in every 500. Progressive renal damage leads to renal failure, at which point dialysis treatment or transplantation is inevitable. In families with a mutation in the gene coding for the protein polycystin-1, localised on chromosome 16, renal failure occurs at about age 50. In families with a mutation for the gene coding for polycystin-2 on chromosome 4, the disease may have a more lingering course. Hypertension is common in ADPKD. Indeed, antihypertensive agents are currently the only accepted treatment. Other symptoms such as flank pain or fever are related to bleeding or infections of the renal cysts.The many extrarenal manifestations of the disease include early and severe diverticular disease of the colon, mitral valve prolapse, and intracranial aneurysms. The aneurysms, which occur in a subset of families, may lead to cerebral haemorrhage.
Until the 1960s, patients with ADPKD died at a young age because of uraemia or a ruptured intracranial aneurysm. Since then, renal replacement treatment has improved markedly: haemodialysis is much more bearable than it was 30 years ago and the results of (preferably pre-emptive) kidney transplantation have improved greatly. Furthermore, prophylactic, nearly non-invasive coagulation of the aneurysms has become feasible.
As ADPKD is an inherited disease, most patients are conscious of its course and complications in their affected relatives. ADPKD is an autosomal dominant disease, so offspring having a 50% chance of being affected. Though the disease can be confirmed at any age by means of DNA analysis, the diagnosis is generally made by ultrasound in the late teens or early adulthood. Subsequently, many anxious years follow, even though young adult patients usually have no symptoms at all and need no drugs. Slowly, renal function deteriorates, drugs become necessary to treat hypertension and the progressive abnormalities in calcium-phosphate metabolism.
A stage of life with haemodialysis or (pre-emptive) renal transplantation looms for ADPKD patients, more so than for other patients with progressive renal failure, who have not witnessed these dramatic events in their affected relatives when they were still children. Consequently most ADPKD patients are worried about their future. Many feel as if they are in a long tunnel, with renal replacement treatment waiting behind a closed door at its end.
Fundamental research indicates that cyst formation is caused by ciliary dysfunction, followed by accelerated proliferation and dedifferentiation of the epithelium. Growth of the cysts is related to deterioration of renal function. This process may be slowed down by drugs inhibiting fluid and ion transport into the cysts (vasopressin receptor antagonists and basolateral K+ channel inhibitors). Another approach is inhibiting cell proliferation, for instance with rapamycin.
BMJ 2009
Finally, gene therapy may correct inborn errors of metabolism. Studies are underway, but adverse effects of these potent drugs are inevitable. Because they influence different mechanisms, combining some of these agents may effectively slow progression, while allowing dose reduction and hence the incidence of side-effects. So, there may be a bright light at the end of the tunnel: ADPKD could one day become a treatable disease.
BMJ 2009
Assessment and management of non-visible haematuria
Summary pointsThe terms visible haematuria should replace macroscopic or gross haematuria, and non-visible haematuria (both symptomatic and asymptomatic) should replace microscopic haematuria or dipstick positive haematuriaThe test of choice for diagnosing haematuria is urine dipstick analysis
scores of ≥1+ are positiveTransient or spurious causes of haematuria need to be excludedAll patients aged ≥40 with haematuria should be investigated for urological disease
Diagnosis of haematuria
Exclude transient causes, such as urinary tract infection,A urine dipstick test for blood is generally sufficient. It is sensitive when performed on fresh voided urine with no preservatives. A score of 1+ is positive; a trace amount is considered negativeA positive result for haemolysed red blood cells should be treated the same as for non-haemolysed red cellsFurther assessment is warranted in patients with urinary tract symptoms and non-visible haematuria and a score of 1+ on a single blood dipstick testIn patients with asymptomatic non-visible haematuria confirm persistence of blood in at least two out of three dipstick tests
It is not necessary to confirm the dipstick result by microscopy
Transient or spurious non-visible haematuria Transient
Urinary tract infection
Exercise related
Spurious
Menstrual contamination
Sexual intercourse
Foods such as beetroot, blackberries, and rhubarb
Rhabdomyolysis
Drugs such as doxorubicin, chloroquine, and rifampicin ,Chronic lead or mercury poisoning.
Causes of persistent non-visible haematuriaUrological causes Common
Benign prostatic hyperplasiaCancer (bladder, kidney, prostate, ureter)
Calculus disease or nephrolithiasis
Cystitis or pyelonephritis
Prostatitis or urethritis
Schistosoma haematobium infection
Less common
Radiation cystitisUrethral strictures
Tuberculosis
Medullary sponge kidney
Cyclophosphamide induced cystitis
Rare Arteriovenous malformation
Renal artery thrombosis
Polycystic kidney disease
Papillary necrosis of any cause
Loin pain haematuria syndrome
Nephrological causes Common
IgA nephropathy (Berger’s disease)Thin basement membrane disease
Less common
Acute glomerular disease:
Postinfectious glomerulonephritis
Rapidly progressive glomerulonephritis
Systemic lupus nephritis
Vasculitis
Goodpasture’s disease
Henoch-Schönlein purpura syndrome
Haemolytic-uraemic syndrome
Chronic primary glomerulonephritis:
Focal segmental glomerulonephritisMesangio-capillary glomerulonephritis
Membranous nephropathy
Mesangial proliferative glomerulonephritis
Familial causes:
Polycystic kidney disease (autosomal dominant or recessive)
Hereditary nephritis (Alport’s syndrome)
Fabry’s disease
Nail-patella syndrome
How should we test for non-visible haematuria in primary care?
Urine dipstickChemical dipsticks detect haem (intact red cells, free haemoglobin, or free myoglobin); they provide an instant result and are used to detect non-visible haematuria in primary care. Although it is difficult to interpret studies on the efficiency of this test because of inherent design and reporting bias, analysis of pooled data sets indicates that it is a reasonable way to detect non-visible haematuria in primary care. The detection of trace haematuria can be considered negative because the threshold for significance is probably less than three to five red blood cells per high power field. A positive result in a haemolysed sample should be treated the same as in a non-haemolysed sample because we have no evidence that the clinical relevance differs.
Urine microscopyMicroscopy provides an accurate measure of red blood cells when assessed by trained technicians or nephrologists in fresh voided early morning midstream specimens of urine. However, time to analysis affects the integrity of red blood cells. In a prospective multicentre study, red blood cell counts dropped by 5-9% at five hours, 11-28% at 24 hours, and 29-35% at 72 hours. Because immediate microscopy is not feasible in primary care, the accuracy of quantitative red blood cell microscopy is questionable. In general practice, it is therefore not logical, and rarely necessary, to validate dipstick haematuria by urine microscopy.
How can glomerular haematuria be distinguished from urinary tract haematuria?
Urine microscopy for red cell morphology and castsThe detection of red cell casts is virtually pathognomonic for a glomerular pathology. Casts are fragile, however, and can be very rare or absent even when glomerular disease is present. The test therefore has low sensitivity, particularly for routine samples sent from primary or secondary care clinics to the laboratory.The relevance of dysmorphic red cells as indicators of glomerular disease is unclear. Samples often contain a mixture of isomorphic (normal shaped) and dysmorphic cells, and the term dysmorphic includes cells with a variety of different shapes.
In an observational study to evaluate urinary particles, the presence of acanthocytes (erythrocytes with ring form blebs) was significantly associated with glomerular disease, but other shapes did not distinguish renal disease from urological disease. Urine microscopy relies on the examination of fresh urine by a skilled person and is most useful in specialist clinics rather than as a screening test in primary care.
ProteinuriaProteinuria is a marker of glomerular damage, particularly as the amount of protein increases (low amounts can result from tubular disease or dysfunction). The presence of proteinuria and haematuria increases the likelihood that the underlying disease is glomerular and is an indication for a referral to the nephrology department. The threshold for significant proteinuria in the presence of non-visible haematuria is 0.5 g/d and equivalent to a protein:creatinine ratio ≥ 50 mg/mmol or albumin:creatinine ratio ≥ 30 mg/mmol (NICE guidelines).
Benefits of Aspirin Therapy Outweigh Bleeding Risks in Hypertensive Patients With Chronic Kidney Disease
une 1, 2009 (Milan, Italy) — In a hypertensive population with (CKD), the benefit of aspirin therapy in reducing major cardiovascular events outweighs the risk for bleeding and, in fact, might be even greater than the benefit obtained by people with normal kidney function, according to an analysis of participants in the Hypertension Optimal Treatment (HOT) study, presented here at the World Congress of Nephrology, a Joint Meeting of the European Renal Association–European Dialysis and Transplant Association and the International Society of Nephrology.
Renal patients are at great risk for cardiovascular disease but they often do not receive antiplatelet therapy," said principal investigator Meg Jardine, MD, from The George Institute for International Health in Sydney, Australia.
"For the first time we are showing that patients with renal dysfunction derive benefit from aspirin treatment. Major bleeding events may be increased but they are outweighed by the substantial protective benefits," she said.
The study involved 18,597 participants, aged 50 to 80 years, with diastolic blood pressure of 100 to 115 mm Hg and a baseline glomerular filtration rate (GFR) of 60 mL/min per 1.73 m2 or less, including 536 patients with values less than 45 mL/min per 1.73 m2. Subjects were randomized to daily acetylsalicylic acid (75 mg) or placebo.
In the overall HOT population, the use of aspirin reduced major cardiovascular events by 15% (PÂ = .03), reduced myocardial infarction by 36% (PÂ = .02), and increased the risk for major bleeding by 80% (PÂ < .001).
The risk for major cardiovascular events was reduced by 66% (95% confidence interval [CI], 33% - 83%) in those with a baseline GFR of less than 45Â mL/min per 1.73Â m2, by 15% (95% CI, 17% - 39%) in those with a baseline GFR of 45 to 59Â mL/min per 1.73Â m2, and by 9% (95% CI, 9% - 24%) in those with a baseline GFR of 60Â mL/min per 1.73Â m2 or higher (PÂ = .03), Dr. Jardine reported at a late-breaking clinical-trials session.
Mortality was reduced by 49% (95% CI, 6% - 73%) in those with a baseline GFR of less than 45Â mL/min per 1.73Â m2 and by 11% (95% CI, 31% - 40%) in those with a baseline GFR of 45 to 59Â mL/min per 1.73Â m2(PÂ = .04); there was no significant reduction in those with a baseline GFR of 60Â mL/min per 1.73Â m2 or higher.
"This means that for every 1000 persons with a GFR less than 45Â mL/min per 1.73Â m2, aspirin would prevent 76 major cardiovascular events, 40 myocardial infarctions, 40 strokes, 40 cardiovascular deaths, and 54 all-cause deaths," Dr. Jardine concluded, "and would cause 27 excess major bleeds and 11 minor bleeds."
Bleeding Increased but Not Statistically Significant
The risk for major bleeding events was increased with aspirin by:291% (95% CI, 92% - 927%) for those with a baseline GFR of less than 45Â mL/min per 1.73Â m2
171% (95% CI, 74% - 391%) for those with a baseline GFR of 45 to 59Â mL/min per 1.73Â m2
153% (95% CI, 111% - 210%) for those with a baseline GFR of 60Â mL/min per 1.73Â m2 or higher.
But the differences between these groups was not statistically significant (PÂ = .27), she noted, and the absolute numbers were small. "There were so few bleeding events [that] we could not find them statistically significant," she said.
There were no fatal major bleeds caused by aspirin therapy, and only 27 nonfatal major bleeds in the worst renal-function group, which saw the most benefit. Nonfatal major bleeds occurred in 4 patients with a GFR of 45 to 59Â mL/min per 1.73Â m2, and in 5 patients with a GFR of 60Â mL/min per 1.73Â m2 or higher.
"Annual changes in GFR were minor, therefore CKD progression was not affected by aspirin therapy in any GFR group," she added.
Our findings suggest that aspirin should be used more widely in hypertensive people with CKD, particularly those at a lower risk of bleeding events," Dr. Jardine concluded.
Charles Herzog, MD, director of the Cardiovascular Special Studies Center of the US Renal Data System and professor of medicine at the University of Minnesota, Minneapolis, said that to properly study the benefit of aspirin in these patients, a trial of 5,000 to 10,000 patients would be required, which the HOT trial was. He pointed out that the National Kidney Foundation Kidney Disease Outcomes Quality Initiative recommendation is to use aspirin for cardiovascular protection in patients with renal disease.
"I do advocate and prescribe aspirin in these patients," he said, "and I believe that unless they have a history of gastrointestinal bleeding, they should be on this therapy."
World Congress of Nephrology 2009: A Joint Meeting of the European Renal Association–European Dialysis and Transplant Association (ERA-EDTA) and the International Society of Nephrology (ISN): Abstract 766. Presented May 25, 2009.
Testing for bacteriuria is recommended in pregnant women without symptoms suggesting urinary tract infectionas 2-7% have clinically significant bacteriuria. A systematic review of 14 studies and 2302 women concluded that treatment of asymptomatic bacteriuria in pregnancy reduces the incidence of pyelonephritis later in pregnancy. The overall incidence of pyelonephritis in pregnant women with asymptomatic
bacteriuria is 21%, and treatment leads to a reduction in risk of 75%.
BMJ 2011;343
Learning points
Asymptomatic bacteriuria refers to bacteria in the urine at levels often regarded asclinically significant ( >100 000 colony forming units per millilitre of urine of a single type of bacterium ) in patients with no symptoms suggestive of urinary tract infection. It becomes more common with age.Testing for and treating asymptomatic bacteriuria is of established value in pregnant women as it reduces the risk of pyelonephritis later in pregnancy by about 75% . Consider testing for bacteriuria in any patient with clinical features pointing to urinary tract infection (haematuria, dysuria,frequency, urge incontinence, or back pain) or to systemic sepsis without an apparent focus.
If children, non-pregnant adults, or people with diabetes or indwelling urinary catheters lack specific symptoms of urinary tract infection or systemic infection, avoid testing them for and treating bacteriuria.Testing for bacteriuria in patients with stable stress incontinence is not appropriate as bacteriuria is not associated with stress incontinence in older people.If you feel obliged to try treating bacteriuria in a patient with non-specific or equivocal symptoms, urine culture can guide selection of the safest and most narrow spectrum agent possible; ensure careful assessment of clinical and microbiological response.
Clinically significant bacteriuria
The term is used to differentiate laboratory evidence of bacteriuria in the bladder from bacteriuria that probably results from contamination during micturition. Clinically significant bacteriuria is generally accepted as detection of more than 100 000 colony forming units of a single type of bacterium per millilitre of urine.Contamination with normal flora typically results in lower numbers of bacteria per millilitre and/or mixed bacterial species
However, the criterion of more than 100 000 colony forming units per millilitre of urine has limitations, in that numbers
as low as 1000 colony forming units per millilitre may be clinically significant in patients with symptoms of urinary tract infection.
BMJ 2011;343
As many as 90% of men between age 45 and 80 suffer from the lower urinary tract symptoms of BPH. BPH occurs when the gland enlarges as smooth muscle cells and epithelial cells proliferate. This so-called reawakening of an embryonic process leads to symptoms that can awaken affected men from their much needed sleep.
The BPH Guidelines
Medscape Internal Medicine © 2011
The Work-Up
The new guidelines now recognize that BPH symptoms can also affect younger men, so the index patients' age has been lowered from 50 to 45 years old.Here is the recommended workup:
A full physical exam including digital rectal exam and prostate-specific antigen test;
Urinalysis to check for infection;
Serum creatinine measurement is not necessary; and
Charting urinary frequency and volume can be helpful.
For evaluating symptoms, the guidelines recommend the AUA symptom index. This is a series of 7 questions rating symptoms over the previous month on a scale from 0-5, with 0 indicating no symptoms at all and 5 having symptoms almost always.
Questions are as follows:1. Incomplete emptying: Over the past month, how often have you had a sensation of not emptying your bladder completely after you finished urinating?2. Frequency: Over the past month, how often have you had to urinate again less than 2 hours after you finished urinating?
3. Intermittency: Over the past month, how often have you found that you stopped and started again several times when you urinated?4. Urgency: Over the past month, how often have you found it difficult to postpone urination?5. Weak-stream: Over the past month, how often have you had a weak stream?6. Straining: Over the past month, how often have you had to push or strain to begin urination?7. Nocturia: Over the past month or so, how many times did you get up to urinate from the time you went to bed until the time you got up in the morning?
In general, a total index score of less than 8 indicates mild symptoms.
A score of 8 or more indicates moderate to severe symptoms.Not everyone with a high symptom score needs treatment. Although lower urinary tract symptoms alone are generally not life threatening, they can have a significant impact on quality of life. The guidelines, which are very patient focused, also include a BPH impact index questionnaire that can be helpful.
If symptoms don't bother the patient there is no need to treat. Watchful waiting is best as long as there are no complications, such as renal insufficiency, urinary retention, or repeated infections.
Lifestyle changes that may be helpful include the following:
Regulating fluid intake, especially in the evening;Increasing physical activity;
Avoiding too much alcohol; and
Cutting out highly seasoned foods that can irritate the urinary tract.
Treatment Choices
The guidelines also review treatment choices when appropriate. Medications for BPH include alpha-blockers, anticholinergics, and 5-alpha-reductase inhibitors
Alpha blockers. According to the guidelines, alfuzosin, doxazosin, tamsulosin, and terazosin are equally effective clinically and result in significant improvement of symptoms.
Dizziness is the most common side effect. The older alpha-blockers doxazosin and terazosin are less expensive, but the dose has to be titrated due to low blood pressure concerns.
Men planning cataract surgery should not start alpha-blockers until after surgery. Tamsulosin is of particular concern because it showed a 40%-90% risk for intraoperative floppy iris syndrome. Men already on alpha-blockers should be sure their ophthalmologist or eye surgeon is informed prior to surgery.
5-alpha reductase inhibitors and combinations.
5-alpha reductase inhibitors include dutasteride and finasteride.Combining an alpha-blocker with either a 5-alpha reductase inhibitor or an anticholinergic sometimes works best.
June 1, 2009 (Milan, Italy) — In a hypertensive population with chronic kidney disease (CKD), the benefit of aspirin therapy in reducing major cardiovascular events outweighs the risk for bleeding and, in fact, might be even greater than the benefit obtained by people with normal kidney function, according to an analysis of participants in the Hypertension Optimal Treatment (HOT) study, presented here at the World Congress of Nephrology, a Joint Meeting of the European Renal Association–European Dialysis and Transplant Association and the International Society of Nephrology.
"We believe our results are very important. Renal patients are at great risk for cardiovascular disease but they often do not receive antiplatelet therapy," said principal investigator Meg Jardine, MD, from The George Institute for International Health in Sydney, Australia.
"For the first time we are showing that patients with renal dysfunction derive benefit from aspirin treatment. Major bleeding events may be increased but they are outweighed by the substantial protective benefits," she said.
With respect to blood in the urine, or hematuria, it can come from a number of different places within the urinary tract: from the kidney, ureter, bladder, or urethra. From a nephrologist's perspective, I think that the first step is to determine whether that hematuria is kidney or extra kidney, renal or extrarenal in origin. I find that the urine protein is a great tool to do this. Remember that the glomerulus, which would be the source of blood if it were renal in origin, is really just a capillary looped upon itself.
Medscape Internal Medicine © 04/14/2011
If you've got an inflammatory process that puts blood in the urine, what it's really doing is punching a hole in that capillary big enough to drive a red blood cell through. If you can drive a red blood cell through that hole, you can surely drive a molecule of protein through that hole. So, the presence of hematuria along with proteinuria could indicate a glomerular disease, and those inflammatory glomerulopathies can be quite complex, so I would definitely reach out to a nephrologist if you find this. That's a good tool.
Next, and far more frequently, you will find isolated proteinuria. You have to understand what the urinalysis can and cannot tell you. When you get a positive urinalysis for protein, it means that there's substantial proteinuria. That person is putting out a good degree of protein in their urine. If you get a urine that is negative for protein on that little pad on the dipstick, does that mean they're not putting out abnormal amounts of protein? Actually, no it doesn't mean that. It could mean that they still are putting out small amounts of albumin -- microalbuminuria -- or larger amounts of albumin -- macroalbuminuria -- that aren't at a high enough level to trigger positivity on that urinalysis dipstick.
When you're looking at your urinalysis protein pad and you see the presence of protein, your next step is to quantify the problem using a protein-to-creatinine ration. If you don't see protein on the dipstick, you're next step may be to quantify whether this person has any albumin excretion abnormalities, and you would want to send that urine for an albumin-to-creatinine ratio.
Let's talk about how to use these 2 parameters in parallel because you use them in a paradigm that's very similar. I'll use the analogy of blood pressure. When you find an elevated blood pressure, you know you need to treat it. You know you need to get it from its higher level down to the threshold that you would consider good blood pressure control. So, you get your elevated blood pressure, you institute therapy, and you follow up on that blood pressure to make sure that you get it to threshold. Pretty simple.
If you start out with a blood pressure of 160/90 mm Hg, you might start hydrochlorothiazide. You would bring that patient in after some clinically reasonable time; reassess their blood pressure; and if it's where you want it, great. If it's not where you want it, you make another change and you bring them back to reassess.
Urine protein excretion and urine albumin excretion are really the same way. Your goal is normal. They're like golf. High is bad; low is good. Normal for urine protein excretion is somewhere around a protein-to-creatinine ratio of 0.2 or 0.3. The urine albumin-to-creatinine ratio is somewhere around the ratio of 30. Of course, within both of those parameters the lowest that you can possibly get that protein or albumin excretion, the better.
As we get into the series in further detail, we're going to talk about how to affect these 2 parameters, how to bring down a urine protein-to-creatinine ratio to < 0.3, how to bring down a urine albumin-to-creatinine ratio to < 30. But the most important point here that I want to make -- actually, the 2 most important points are: If you get a urine protein that is positive, overt proteinuria and urine protein-to-creatinine ratio. If you get that urine protein, they're sick and it's negative, they still could have abnormalities in their albumin excretion, and this group will actually be the largest group in your practice, so don't miss them.
When you institute therapy, it's just like blood pressure. Institute a therapy, bring them back, and reassess. Try to get that urine protein or urine albumin to as low as possible and to a goal of as near normal as possible.
February 15, 2011 — Evaluation and management of nephrolithiasis are reviewed in an article reported in the February issue of theSouthern Medical Journal.
"New information has become available on the clinical presentation, epidemiologic risk factors, evaluative approach, and outcome of various therapeutic strategies. In this report, we will review the epidemiology and mechanisms of kidney-stone formation and outline management aimed at preventing recurrences," write Zachary Z. Brener, MD, from the Nephrology Division, Department of Medicine, Beth Israel Medical Center in New York, NY, and colleagues. "Improved awareness and education in both the general population and among health-care providers about these modifiable risk factors has the potential to improve general health and decrease morbidity and mortality secondary to renal-stone disease."
Evaluation of nephrolithiasis centers on 24-hour urine collection, with 2 consecutive collections needed while the patient follows his or her usual diet. In addition to a thorough history and physical examination, evaluation should include analysis of the stone to determine its composition. Helical computed tomography (CT) without contrast material is the preferred imaging procedure to evaluate suspected nephrolithiasis.
The Work-Up
The new guidelines now recognize that BPH symptoms can also affect younger men, so the index patients' age has been lowered from 50 to 45 years old.Here is the recommended workup:
A full physical exam including digital rectal exam and prostate-specific antigen test;
Urinalysis to check for infection;
Serum creatinine measurement is not necessary; and
Charting urinary frequency and volume can be helpful.
Medscape Internal Medicine © 5/24/2011
For evaluating symptoms, the guidelines recommend the AUA symptom index. This is a series of 7 questions rating symptoms over the previous month on a scale from 0-5, with 0 indicating no symptoms at all and 5 having symptoms almost always.
Questions are as follows:1. Incomplete emptying: Over the past month, how often have you had a sensation of not emptying your bladder completely after you finished urinating?2. Frequency: Over the past month, how often have you had to urinate again less than 2 hours after you finished urinating?3. Intermittency: Over the past month, how often have you found that you stopped and started again several times when you urinated?
4. Urgency: Over the past month, how often have you found it difficult to postpone urination?5. Weak-stream: Over the past month, how often have you had a weak stream?6. Straining: Over the past month, how often have you had to push or strain to begin urination?7. Nocturia: Over the past month or so, how many times did you get up to urinate from the time you went to bed until the time you got up in the morning?
In general, a total index score of less than 8 indicates mild symptoms. A score of 8 or more indicates moderate to severe symptoms.
Not everyone with a high symptom score needs treatment. Although lower urinary tract symptoms alone are generally not life threatening, they can have a significant impact on quality of life. The guidelines, which are very patient focused, also include a BPH impact index questionnaire that can be helpful. If symptoms don't bother the patient there is no need to treat. Watchful waiting is best as long as there are no complications, such as renal insufficiency, urinary retention, or repeated infections.
Lifestyle changes that may be helpful include the following:
Regulating fluid intake, especially in the evening;Increasing physical activity;
Avoiding too much alcohol; and
Cutting out highly seasoned foods that can irritate the urinary tract.
Treatment Choices
The guidelines also review treatment choices when appropriate. Medications for BPH include alpha-blockers, anticholinergics, and 5-alpha-reductase inhibitorsAlpha blockers. According to the guidelines, alfuzosin, doxazosin, tamsulosin, and terazosin are equally effective clinically and result in significant improvement of symptoms.
Dizziness is the most common side effect. The older alpha-blockers doxazosin and terazosin are less expensive, but the dose has to be titrated due to low blood pressure concerns. Men planning cataract surgery should not start alpha-blockers until after surgery. Tamsulosin is of particular concern because it showed a 40%-90% risk for intraoperative floppy iris syndrome. Men already on alpha-blockers should be sure their ophthalmologist or eye surgeon is informed prior to surgery.
5-alpha reductase inhibitors and combinations.
include dutasteride and finasteride. Combining an alpha-blocker with either a 5-alpha reductase inhibitor or an anticholinergic sometimes works best.
Other alternatives. The guidelines also discuss other alternatives, including surgery and newer treatment modalities including Botox® and sacral neuromodulation.
Hi, I'm Dr. Henry Black. I'm Clinical Professor of Internal Medicine at the New York University School of Medicine, member of the Center for the Prevention of Cardiovascular Disease, and Immediate Past President of the American Society of Hypertension. I sort of identify myself as a lapsed nephrologist. I'd been a nephrologist by training, but now I spend more time with blood pressure and cholesterol and preventive cardiology.
AASKing About Intensive BP Control -- What Needs it?
Medscape Cardiology © 2010
So, I was particularly interested in this study called AASK (African American Study of Kidney Disease)[1] that began in the 1990s, and completed its trial phase around 2001 or 2002. What they did was conduct a follow-up phase, giving us a total of 12 years of observation. The important things about AASK were that it was an NIH [National Institutes of Health]-sponsored study, and they compared 3 drug classes and 2 goals of treatment. Goals of treatment studies are tough to do.
The 3 drug classes were a calcium channel blocker (amlodipine), an ACE [angiotensin-converting enzyme] inhibitor (ramipril) and a beta blocker (metoprolol). The calcium channel blocker was stopped early in the trial because of increased risk. These were patients in whom high proteinurea developed, which they defined as 0.22 g of protein per g of creatinine, which is roughly 300 mg a day.
They also looked at 2 goals. A goal of less than 107 mm Hg for mean blood pressure, which translates into about 140 mm Hg over 90 mm Hg and a mean blood pressure of less than 92 mm Hg, which is about 130 over 80 mm Hg, or even 125 over 75 mm Hg. The nephrologists look at mean blood pressure, although the rest of us really don't. In this cohort study, they took everybody who finished the trial and looked at the 2 different goals. You got what drug you got.
Almost everybody got an ACE or an ARB [angiotensin receptor blocker] and you could add whatever other drugs the patient needed to get to the goal -- this included loop diuretics (primarily Lasix®), clonodine, or minoxidil. They did achieve this goal, showing that it could be done.
The results at the end of an average of 12 years are also very interesting. There was no particular benefit of the aggressive therapy, getting the blood pressure under 140 over 90 mm Hg vs getting under about 125 over 75 mm Hg did not change outcomes. The outcomes were a reduction in renal function by half or end-stage renal disease or death. However, in the subgroup that had proteinurea (300 mg or more), there was clear benefit from the aggressive therapy.
This is very similar to what we saw in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial, where we think a certain goal is important, but we haven't tested it. We go ahead and test it and we find that aggressive therapy isn't necessarily helpful except perhaps for select events, (in ACCORD, it was stroke) or for some individual patient groups. In ASSK, it is black patients who have chronic kidney disease and protineuria as well.
So, as we refine what we do, we have to take clinical trials into context. It has always amused me to hear people give genetics talks about "personalized medicine" as if it is something they invented. They didn't invent it. We've been personalizing, or individualizing, therapy for long time; for example, in looking at demographic characteristics such as age or ethnicity, and then looking at comorbid events. This is 1 more example in which people -- in this case, black patients with reduced renal function who have proteinurea -- are going to benefit by having an aggressive goal.
Those who don't have proteinurea aren't going to benefit. The disturbing thing is that for patients with proteinurea, 80% of them had an event within 10 years if they received standard therapy, and 70% if they were on aggressive therapy. That means most of those individuals, whose glomerular filtration rates were between 20 and 79 mL/minute when they started, are going to end up needing chronic dialysis soon enough. We've got to do something to prevent them from getting to that stage. Thank you very much.
American Journal of Clinical Dermatology
Chronic Pruritus in the Absence of Specific Skin Disease: An Update on Pathophysiology, Diagnosis, and TherapyAm J Clin Dermatol. 2010
Neurophysiology of Itch
Skin-nerve Interactions and ItchThe skin is highly innervated by primary sensory nerves, postganglionic cholinergic parasympathetic and postganglionic sympathetic nerves, resulting in a complex cutaneous afferent/efferent neuronal network. Itch-specific fibers are unmyelinated C fibers, mainly peptidergic, with slow conduction velocity and large innervation territories.[2] They account for about 5% of the afferent C fibers in human skin, respond to histamine and thermal stimuli but are insensitive to mechanical stimuli.
Nerve fibers usually end at the dermal-epidermal junction, but some unmyelinated C fibers also project into the epidermis to the subcorneal layers. Therefore, nerve fibers may reciprocally communicate with all skin cell types. The number of peripheral and central mediators thought to be involved in the generation of an itch sensation is rapidly increasing ( table I ). Opioid receptors, especially μ- and κ-receptors, modulate pruritus perception in the CNS and in the skin. Activation of μ-opioid receptors stimulates itch perception, whereas activation of κ-opioid receptors suppresses itch perception.
Classification of Itch
Two recently proposed classifications of pruritus are based on the neurophysiologic origin of pruritus or the clinical picture and symptoms of the patient. Twycross et al.[16] classified itch into four categories:• pruritoceptive (originating in the skin);
• neuropathic (lesioned neurons themselves generate itch);
• neurogenic (central mediators generate itch without neuronal damage); and
• psychogenic (somatoform).
Psychogenic pruritus should be suspected only after cautious exclusion of other causes. It is advisable that an expert in psychosomatics or psychiatry should confirm the diagnosis of somatoform pruritus independent of any other organic origin. Psychological factors may influence itch perception or can complicate chronic itch even in the absence of a true psychiatric morbidity.
The International Forum for the Study of Itch (IFSI) distinguished three clinical groups of patients: (i) pruritus on primarily inflamed skin; (ii) pruritus on normal skin; and (iii) pruritus with chronic nonspecific secondary scratch lesions.[1] These three groups can be further classified into six subtypes after clinical and laboratory assessment (table II). Pruritus without skin changes has been previously named 'essential pruritus' or 'pruritus sine materia.' It has been recommended that these two definitions should no longer be used because they may generate confusion.[1]
Indeed, most patients with chronic pruritus unrelated to skin diseases have skin lesions secondary to scratching or simply skin dryness. Therefore, it is very important to distinguish a definite skin disease from nonspecific (scratch- or rubbinginduced) skin changes. Pruritus is the most common symptom of most inflammatory skin disorders (e.g. atopic dermatitis, psoriasis, contact dermatitis, urticaria, drug reactions, pemphigoid, dermatitis herpetiformis), parasitic or infectious diseases (e.g. scabies, mycoses, chickenpox), as well as cutaneous T-cell lymphoma.
Subtype
Relevant examplesDermatologic: arising from skin; dry skin and any specific skin disease
Inflammatory: contact dermatitis, atopic dermatitis, asteatotic eczema, nummular eczema, stasis dermatitis, seborrheic dermatitis, urticaria, psoriasis, lichen planus, drug reactions, polymorphous light eruption, mastocytosis, pemphigoid, dermatitis herpetiformis, dermatomyositis
Infections: pediculosis, scabies, parasitic disease, tinea corporis, impetigo, smallpox
Neoplastic: cutaneous T-cell lymphomas
Dermatoses of pregnancy: pruritic urticarial papules and plaques of pregnancy, prurigo of pregnancy, pemphigoid gestationisSystemic: arising from diseases of organs other than the skin, metabolic or other multifactorial disturbances or from drugs
Endocrine and metabolic disorders: chronic renal failure (dialysis), liver diseases with or without cholestasis, thyroid diseases
Infections: HIV, parasites, hepatitis C virus
Hematologic diseases: polycythemia vera, lymphomasTumors: solid organ tumors, carcinoid
Drug-induced pruritus (with or without cholestasis)Neurologic (neurogenic/neuropathic): arising from disorders of the central or peripheral nervous system and possibly also from liver disease
Multiple sclerosis; spinal or cerebral neoplasms, abscesses, or infarcts; phantom itch; postherpetic neuralgia; transverse myelitis; notalgia paresthetica; brachioradial pruritus; meralgia paresthetica; other conditions associated with nerve damage, compression or irritation, such as entrapment neuropathy, radiculopathy, or polyneuropathy (including diabetes mellitus, vitamin B12 deficiency, etc.)
Psychogenic/psychosomatic
Delusion of parasitosis, psychogenic excoriations, somatoform pruritus, associated with psychiatric disorders
Mixed
Coexistence of dermatologic and neurologic itch in HIV-infected patients or in patients with atopic dermatitis, association of uremic itch with skin xerosis, association of Hodgkin disease with potentially misleading paraneoplastic cutaneous manifestations, such as unexplained adult-onset eczema
Other (of unknown origin)
Senile 'idiopathic' pruritus, aquagenic 'idiopathic' pruritus, pruritus in anorexia nervosa
Table II. Etiologic classification of itch according to the International Forum for the Study of Itch
Item
Examples and/or commentsPersonal and family history of cutaneous and extracutaneous disorders
Atopy, hypersensitivity reactions, and systemic diseases
Previous and concomitant drugs
Surgical procedures or recent hospitalizations
Use of volume expanders (hydroxyethyl starch)Travel history
Infections or infestations that are endemic in particular geographic areas
Work and leisure activities
Exposure to irritating or sensitizing substances, fiberglass, mineral wool, biotic agents, etc.
Lifestyle and personal habits
Eating, smoking, alcohol consumption, drug abuse and addiction, sexual history, indoor environment, etc.
History of spinal injury
Arthritis, trauma, or chronic repetitive microtrauma (in presence of a suspected 'neuropathic' itch)
Mental state and personality characteristics
Recent development of other symptoms and/or signs, including but not limited to:
The following conditions should be suspected:
burning, pain, stinging, tingling, paresthesia, hypoesthesia or hyperesthesia, numbness, muscle weakness, cramps
sensory or sensorimotor neuropathy (neuroanatomic damage)
motor deficits
brain lesions
nocturnal sweating, fever, unexplained weight loss
Hodgkin lymphoma
weight loss
malignancies of various origin, HIV infection, malabsorption, hyperthyroidism
fatigue
malignancies, HIV infection, anemia
nausea, anorexia, malaise, fatigue
cholestasis
flushing and diarrhea
carcinoid syndrome
Relevant information to be collected from a patient's history
Diagnostic Work-up for Chronic Pruritus with Nonspecific Skin Signs
The management of pruritus with nonspecific skin signs may be difficult, time consuming, and frustrating for both the patient and the physician. Patients must be informed about the complexity of this symptom and about the likelihood of step-by-step assessments as needed. At first, the characteristics of pruritus (timing, location, severity, relieving and exacerbating factors) should be recorded and a complete physical examination aimed at excluding a dermatologic condition and detecting signs suggestive of a systemic cause should be performed.
The dermatologic examination should carefully evaluate the presence of any cutaneous changes, including minimal lesions, dermographism, complications of scratching, and skin xerosis, the latter being a relevant cause or co-factor of chronic itch. A thorough medical history should be collected with emphasis on drug exposure, travel history (to exclude endemic infections), contact with environmental irritating and sensitizing substances, lifestyle (diet, substance abuse, working activity, hobbies, etc.), concomitant extracutaneous symptoms, prior hospitalizations, or recent use of volume expanders (such as hydroxyethyl starch)[20] [table III].Mental state and personality characteristics should also be investigated.
Uremic Pruritus
Pruritus is present in 15–49% of patients with chronic renal failure and in up to 90% of patients receiving dialysis. The pathogenesis is still obscure but present data point toward a central role of the immune and opioidergic systems. Hemodialysis-related pruritus seems to be induced by an immune system derangement, resulting in a proinflammatory state.An imbalance in the opioidergic system, with hyperactivity of μ-opioid receptors, has also been observed.
Other factors may include calcium-phosphate imbalance, hyperparathyroidism, anemia, higher serum levels of histamine, and peripheral neuropathy. In two-thirds of patients, pruritus is generalized, while in the others it is localized, particularly on the back. In nearly half of patients it appears on a daily basis, whereas in the other half it occurs more rarely. Some patients report pruritus during or soon after dialysis.Pruritus and its severity seem to correlate with duration of dialysis and skin dryness, but without general agreement.
Chronic pruritus is a strong independent predictor of poor quality of life and of severe sleep disturbances in dialysis patients.
Many attempts have been made to relieve this bothersome symptom; however, with generally limited success.[36] Patients may benefit from the regular use of emollients to control skin xerosis. Sedating antihistamines may be helpful, but no randomized controlled trials (RCTs) support their use.[33] Thalidomide, gabapentin, and nalfurafine, a κ-opioid receptor agonist, have proven to be effective in RCTs, but the potential toxicity associated with these drugs limits their use.[37–39]
Some studies demonstrated that UVB is still the treatment of choice in moderate to severe pruritus, but the need for regular sessions of therapy over the dialysis treatment may be impractical for many patients.[40,41] General considerations regarding treatment of uremic pruritus are reported in table IV .
General measures
Regular use of emollients (even in patients who do not have pruritus)Cool environment, loose clothes, avoid wool and artificial fibers
Improvement in the efficiency of the dialysis technique
Correction of the alterations of calcium-phosphorus metabolism
Parathyroidectomy can be considered in the case of secondary hyperparathyroidism
Topical treatments in localized mild pruritus
Emollients
Capsaicina
Phototherapy with UVB
Treatment of choice in moderate to severe uremic pruritus
Broad-band UVB more effective than narrow-band UVB
Systemic approaches in generalized pruritus
Thalidomidea
Gabapentina
Nalfurafine
Treatments for uremic pruritus
Cholestatic Pruritus
Cholestasis refers to a reduction in bile flow, which may be due to extra-hepatic (usually obstructive) or intra-hepatic causes (e.g. primary sclerosing cholangitis, primary biliary cirrhosis, chronic hepatitis, malignant tumors, pregnancy). A large number of drugs may induce cholestasis, with or without liver injury, after weeks to months from the start of treatment.Pruritus can occur in up to 80%of patients with primary biliary cirrhosis. The pathogenesis of cholestasis-associated pruritus remains poorly understood, and may be multifactorial. Peripherally acting pruritogens (bile acids) and altered central neurotransmission have been implicated.
The management of cholestatic pruritus has been extensively reviewed, and current recommendations are summarized in table V . Naloxone, naltrexone, rifampin (rifampicin), colestyramine (cholestyramine), and phenobarbital (phenobarbitone) are recommended as agents of choice by the American guidelines for the treatment of pruritus in primary biliary cirrhosis. However, the available RCTs have been conducted in small numbers of patients, are few in number, and have used heterogeneous methods.
Cumulative evidence from pooled RCTs suggests that rifampin and opioid antagonists demonstrate a reduction in pruritus, whereas there are insufficient data to judge the efficacy of colestyramine.[45] Some evidence also supports the use of the serotonin reuptake inhibitor sertaline[46] and ursodeoxycholic acid,[47] with the latter drug being the mainstay of therapy in primary biliary cirrhosis.
Drug
DosagePossible adverse effects
Notes
First-line medical approach a
Naloxone
0.2 μg/kg/min IV infusion preceded by 0.4mg IV bolusOpioid withdrawal-like syndrome
Transient adverse effects in a significant proportion of patients in RCTs
Naltrexone
Day 1, 25mg PO bid, then 50mg/day PO
Opioid withdrawal-like syndrome, potential hepatotoxicity
Transient adverse effects in a significant proportion of patients in RCTs
Rifampin (rifampicin)
300–600mg/day PO
Hepatotoxicity (controversial data about real risk)
Adverse effects similar to placebo in RCTs
Colestyramine (cholestyramine)
4–16 g/day PO
Constipation, bloating, malabsorption, interaction with many drugs
Adverse effects similar to placebo in RCTs
Phenobarbital (phenobarbitone)
2–5mg/kg/day PO
Sedation
Surgical management of biliary obstruction when indicated
Stenting or removal of gallstones
Other approaches bMedical treatments
Anticholestatic agents: ademetionine (S-adenosylmethionine); ursodeoxycholic acid (not effective in primary biliary cirrhosis-associated pruritus; apparently effective and safe in cholestasis of pregnancy)Cannabinoids (dronabinol)
Antidepressants: sertralineUVB phototherapy
Others: anesthetics: propofol, lidocaine (lignocaine); bright-light therapy towards the eyes; antioxidants; androgens (danazol)Invasive procedure in patients resistant to medical therapies (tried in a small number of cases)
Nasobiliary drainage or partial external diversion of bile; ileal diversion; hemodialysis; charcoal hemoperfusion; plasmapheresis and plasma perfusion; extracorporeal albumin dialysis, with molecular adsorbent recirculating system; plasma separation and anion adsorption
Treatments for pruritus associated with cholestasis
Pruritus Accompanying Systemic Infections Including HIV InfectionPruritus may be a symptom of cutaneous and extracutaneous infection, as well as of intestinal parasitic infestation. In particular, certain viral infections may have a prominent role in chronic pruritus.
Pruritus is one of the most frequent symptoms encountered in HIV infection and can even be the first clinical symptom. It can be isolated or associated with different skin diseases (such as seborrheic dermatitis, atopic dermatitis, psoriasis, eosinophilic folliculitis) or may result from the release of pruritogenic mediators, neurologic disturbances, drug intake, or systemic disorders (lymphoma, infections, infestations).[48] The immune dysregulation can cause the perturbation of cytokine milieu, with a trend towards a predominant T helper-2 response. For this reason, eosinophilia is not an uncommon finding.
An important skin rash is the pruritic papular eruption, which is correlated with the magnitude of immunodeficiency.[49] Some case series showed that the prevalence of prurigo nodularis was higher in patients with CD4-positive cell counts of <200 cells/mm3 and among patients not receiving highly active antiretroviral therapy (HAART), whereas patients with HIV viral loads >55 000 copies/mL had a higher prevalence of 'idiopathic' pruritus.[50]
Some HIV-associated pruritic skin diseases may be ameliorated by HAART. Both psoralen plus UVA (PUVA) and UVB have proven to be successful in HIV-associated pruritus, with UVB preferred overPUVAdue to safety reasons. Other symptomatic treatments have mostly been used in small case series or uncontrolled studies. Some reports support the efficacy of thalidomide for the treatment of prurigo nodularis in HIV-infected patients, who appear, however, particularly prone to developing peripheral neuropathy.[51]
Pruritus has been reported in up to 15% of patients with chronic HCV infection, and may be a presenting symptom. The pathogenesis of HCV-related itch is still obscure. Chronic hepatitis with moderate to severe fibrosis has been suggested to result in low-grade cholestasis, with pruritus resulting from the disappearance of the bile duct. In the absence of cholestasis, itch may be an adverse effect of antiviral therapy, as it happens in up to 29% of patients treated with interferon-α plus ribavirin.[52] Both HIV and HCV infection are associated with prurigo nodularis.[18]
Malignancy-related Pruritus
Pruritus can be present as a part of a paraneoplastic syndrome in association with some solid tumors including lung, colon, breast, stomach, and prostate. In the palliative care setting, pruritus has been estimated to affect 5–27% of patients.[53] The pathogenesis is complex and may involve central and peripheral mechanisms, including the production of pruritogenic substances by the tumor or itch induced by drugs used in palliative care (opioids).[54]Malignancy-related pruritus is usually generalized. In some patients, localization of pruritus correlates with the site of the tumor: carcinomas of the cervix, rectum/sigmoid colon, and prostate may present with pruritus of the vulva, anus, and scrotum, respectively.[55] In these cases, pruritus may derive from direct activation of peripheral nerve fibers at tumor sites.[56] Brain or spinal tumors may manifest with facial or nasal itch, and dermatomal itch, respectively.[57,58]
Pruritus is common in patients with hematologic malignancies. Itch is reported by about 30%of patients with Hodgkin disease, especially those with the nodular sclerosing subtype. It is considered a B symptom, and can precede any identifiable sign of the tumor by up to 5 years.[55] Nearly half of patients with polycythemia vera have pruritus, either spontaneous or soon after contact with water (aquagenic pruritus), especially at high temperature; and in more than 20% of patients it can persist despite adequate disease control.
Other than true itch sensation, aquagenic pruritus is accompanied by stinging or pin-point sensations lasting 10–30 minutes after contact with water, and in most cases is a transient benign disorder. Aquagenic pruritus, which seems to be more common among polycythemia vera JAK2 617V>F homozygous patients, may be the sole marker of the disease and can appear as many as 3–5 years before the true onset of the disease
Aquagenic pruritus has also been described in patients with acute lymphoblastic leukemia, and myelodysplastic syndrome alone or in association with T-cell non-Hodgkin lymphoma.[60] Generalized pruritus has also been reported in patients with other hematologic malignancies, such as chronic lymphocytic leukemia, non-Hodgkin lymphoma, and multiple myeloma.[61,62]
Endocrine Disorders and Iron Deficiency
Pruritus can be associated with thyroid abnormalities (hyperthyroidism more frequently) and with diabetes mellitus. Hypothyroidism, hypoparathyroidism, and pseudohypoparathyroidism can cause pruritus secondary to severe skin dryness.[63] Although the relationship between diabetes and pruritus is still controversial, some reports suggest an increased frequency of vulvar pruritus only in women with poorly controlled diabetes.[63] However, diabetes may cause neuropathy, and consequently can be implicated in neuropathic itch.Iron deficiency is another well known cause of chronic pruritus. High rates of this association have been recently reported, suggesting the opportunity to include ferritin and iron studies among the routine examinations of patients with chronic pruritus of apparently unknown origin.[64] Pathogenic mechanisms remain unknown. Restoring the serum ferritin within the normal range through iron supplementation has been reported to be helpful. In any case, the presence of iron deficiency should lead to the exclusion of any condition responsible for such a deficiency, including malignancies.[64]
Drug-Induced Pruritus
Pruritus occurs in 10–50% of patients receiving intravenous administration of opioids, and in 20–100% of patients when opioids are given by epidural or intraspinal injections. Postulated mechanisms include a direct central effect, as well as histamine and serotonin release.[65] An extensive review of numerous RCTs investigating the therapeutic approach to opioid-induced itch has been recently published.[65] Few drugs can be used to treat established opioid-induced pruritus. Histamine H1 receptor antagonists have little effect.[65]Intravenous nalbuphine and propofol significantly reduced opioid-induced itch in adults undergoing surgery (RCTs). Nalbuphine seems to be more effective than propofol (RCT). Ondansetron diminished postoperative itch and vomiting after opioid administration and was also found to attenuate intrathecal fentanyl-induced pruritus. Prevention of opioid-induced itch can also be obtained with low doses of opioid antagonists (naloxone, naltrexone, and nalmefene), but they may reverse the analgesic effects.
An agonist/antagonist drug such as nalbuphine can reduce pruritus without compromising analgesia.[71] Of note, recent RCTs have shown that premedication with either mirtazapine or gabapentin is capable of preventing pruritus caused by intrathecal morphine.[72,73]
Essentially any other drug may cause an adverse reaction in the skin, which can be associated with pruritus. Pruritic reactions are usually morbilliform or urticarial; however, a growing number of drugs can induce pruritus without any skin rash. No universally accepted method for assessing the causality of an adverse drug reaction has been approved.[76] A congruous temporal sequence between the beginning of drug therapy and onset of itch, improvement after drug withdrawal, and recurrence after drug rechallenge are useful elements.
Drug-induced pruritus is likely to be underestimated in the general population, and especially in elderly patients, and it can be misdiagnosed as senile 'idiopathic' pruritus.Antihypertensive drugs, especially angiotensin converting enzyme inhibitors, can induce pruritus alone, or pruritus associated with angioedema and other pruritic cutaneous disorders (i.e. urticaria, maculopapular and lichenoid eruptions).
In the elderly, drug-induced pruritus is frequently observed in patients taking multiple medications, which may more easily induce adverse effects because of impaired metabolism and/or pharmacologic interactions. Alist of the principal drugs able to induce pruritus without skin changes is given in table VI .
Drug group
ExamplesAntihypertensive drugs ACE inhibitorsa
Angiotensin II antagonists (sartans)a
β-Adrenoceptor antagonists (β-blockers)aCalcium channel blockersa
MethyldopaSildenafila
Antiarrhythmic drugsAmiodaronea
Anticoagulants
Ticlopidinea
Fractionated heparins
Antidiabetic drugsBiguanidesa
Sulfonylurea derivates
Hypolipemic drugs
HMG-CoA reductase inhibitors (statins)
Antibacterials and chemotherapeutics
Penicillinsa
Cephalosporins
MacrolidesaCarbapenemsa
MonobactamsQuinolones
TetracyclinesaLincosamidesa
StreptograminsMetronidazole
Rifampin (rifampicin)
ThiamphenicolTrimethoprim/sulfamethoxazole (cotrimoxazole)a
AntimalarialsPsychotropic drugs
Tricyclic antidepressantsa
Selective serotonin reuptake inhibitors
AntipsychoticsaAntiepileptic drugs
Carbamazepine
Fosphenytoin
OxcarbazepinePhenytoin
Topiramate
CytostaticsChlorambucil
Paclitaxel
TamoxifenCytokines, growth factors, and monoclonal antibodies
Granulocyte-macrophage colony-stimulating factor
Interleukin-2
MatuzumabLapatinib
Epidermal growth factor receptor inhibitorsPlasma volume expanders
Hydroxyethyl starch
Others
Antithyroid agentsa
NSAIDsa
Corticosteroidsa
Sex hormonesaOpioids
Inhibitors of xanthine oxidaseTable VI. Principal drugs able to induce pruritus without skin changes
General Considerations in the Treatment of Pruritus
Removal of the causative agent, whenever possible, and appropriate treatment of the underlying disease is essential. Skin care measures to alleviate skin dryness such as use of moisturizers, reduction in frequency of bathing, humidification of dry indoor environments, prevention of excessive sweating, and avoidance of hot baths, soap, and irritant fabrics are generally recommended.
In particular, the skin of elderly people benefits from regular use of emollients. Improper use of topical medications (e.g. corticosteroids for prolonged periods, possible sensitizing agents such as topical antihistamines and anesthetics) must be avoided.
Targeting Pruritus Elicitation in the Skin
Dermatologic itch must be managed through treatment of the specific skin disorder or skin changes. Irresistible episodes of pruritus can be controlled by application of cold compresses and wet dressings with topical corticosteroids.[78] Topical antipruritics such as camphor, menthol, oatmeal baths, and polidocanol arecommonly used, but evidence fromRCTsis lacking.Oral antihistamines are frequently prescribed in the case of any pruritic condition, sometimes as an ex juvantibus criterion. Sedative and/or anti-inflammatory qualities of certain H1 receptor antagonists are thought to be useful to control itch. However, with the exception of urticaria and mastocytosis, the role of antihistamines for the management of other pruritic disorders is still controversial. Nevertheless, a recent retrospective analysis of 67 patients with chronic pruritic dermatoses or chronic pruritus of unknown origin has shown good antipruritic effects with high-dosage nonsedating H1 receptor antagonists, used as monotherapy or in combination.
Phototherapy with various UV sources, especially UVB, is widely used in patients with chronic pruritus. It has a wide antiinflammatory cutaneous activity, and can offer relief without many of the adverse effects and risks of systemic medications. Its efficacy has been demonstrated in some RCTs.
Some itchy conditions (e.g. notalgia paresthetica, prurigo nodularis, aquagenic pruritus, and also uremic pruritus) may respond to topical capsaicin, a substance binding to the TRPV1 receptor. TRPV1 receptors are nonselective heat-activated cation channels, located in theCNS and in cutaneous nerve fibers. They are involved in the transmission and modulation of itch.
Capsaicin induces desensitization of nerve fibers, inhibition of neuropeptide accumulation, and suppression of painful and pruritic sensations. Topical calcineurin inhibitors (tacrolimus and pimecrolimus), which are approved for treatment of atopic dermatitis, have been found to bind to TRPVl on cutaneous nerve fibers, and to cause an initial release of substance P and calcitonin gene-related peptide from primary afferent nerve fibers. This effect explains the transient burning occurring during the first days of treatment. Topical calcineurin inhibitors are useful for treating the pruritus associated with some dermatoses, such as atopic dermatitis.
Thalidomide, which can be useful in the treatment of prurigo nodularis, actinic prurigo, as well as uremic pruritus, acts as an immunomodulatory drug, a tumor necrosis factor-α inhibitor, and also a peripheral and central nerve depressant, but has an unfavorable safety profile.[39]
Novel approaches to dermatologic itch, which deserve further evaluation, are H4 receptor antagonists, topically applied opioid receptor antagonists, and cannabinoid receptor agonists such as palmitoylethanolamide, stearoylethanolamide, and stearoylisopropylamide.
The treatment of prurigo nodularis is particularly challenging. Phototherapy (UVB, oral PUVA) has been proven to be effective and safe in a RCT, but the need for regular sessions of therapy is impractical for many patients.[83] No RCTs are available with any other drug, including emollients, corticosteroids, topical capsaicin, topical tacrolimus, cyclosporine (ciclosporin), thalidomide, naltrexone, antidepressants, and oral retinoids.[18] Results are variable, but long-term combinations and rotational treatments can be effective.
Targeting Pruritus Elicitation in the CNS
Gabapentin, carbamazepine, and derivatives, commonly used as antiepileptic drugs, are capable of blocking the neuropathic afferent pathway and therefore may be helpful in neuropathic itch. Gabapentin was also recently proven to be well tolerated and effective in the treatment of uremic pruritus and various pruritic disorders.[37,87] Its mechanism of action is unclear and has been hypothesized to be both central and peripheral.
Gabapentin and pregabalin inhibit release of calcitonin gene-related peptide from primary afferent neurons through an increase of GABA in the spinal cord.[87] Compared with gabapentin, pregabalin is characterized by a more rapid response, but evidence from RCTs in the treatment of pruritus is lacking.[88]
Opioid receptor antagonists, such as naloxone and naltrexone, have an important influence on the neurogenic component of itch by inhibiting itch transmission. Systemically administered opioid receptor antagonists showed antipruritic effects not only in hepatogenic pruritus, but also in hydroxyethyl starchinduced pruritus and various pruritic skin diseases, such as atopic dermatitis, cutaneous lymphoma, and prurigo nodularis.[89,90]
However, adverse effects and costs lead to considering these drugs as a second-line approach to chronic pruritus. Butorphanol possesses both κ-agonist activity and μ-antagonist activity, and has been successfully used in small case series of patients with intractable pruritus.[91]
Antidepressants directly influence central pruritus perception by as-yet unknown mechanisms. It is speculated that they interfere in the neuronal reuptake of neurotransmitters, such as serotonin and norepinephrine, and thereby reduce pruritus perception.
Accordingly, tricyclic (e.g. amitriptyline, clomipramine, doxepin) and tetracyclic (e.g. mirtazapine) antidepressants have been employed with some success in chronic pruritus and prurigo nodularis.[18,92,93] Doxepin and mirtazapine have additional antihistaminic effects. The selective serotonin reuptake inhibitor paroxetine was reported to have antipruritic effects in polycythaemia vera, psychogenic pruritus, paraneoplastic pruritus, and idiopathic pruritus in a small RCT.[
A recent open-label study supports the usefulness and good tolerability of paroxetine and fluvoxamine in patients with chronic pruritus. Paroxetine may produce a variety of cutaneous and noncutaneous adverse effects requiring adequate monitoring of the patient.
A list of the most frequently used systemic therapies for pruritus, other than antihistamines, is given in table VII .
Medication
DosageIndication
Adverse effects
Fluvoxamine
25mg/day PO for 3 days then 50–100mg/day PO as needed
Various pruritic diseases95
Drowsiness, vertigo, fatigue, headache, sexual dysfunction, nausea, vomiting
Gabapentin
300–1800mg/day PO; in dialysis patients, 100–300 mg PO after every dialysis session may be sufficient
Neuropathic,25 uremic itcha 36,37,87
Drowsiness, constipation
Mirtazapine
15–45 mg/day PO
Generalized pruritus93
Drowsiness, increased weight and appetite, dry mouth
Naloxone
0.2 μg/kg/min IV infusion daily preceded by 0.4mg IV bolus over 24 hours
Cholestatic itcha 44,45
Hepatotoxicity, nausea and vomiting, difficulty sleeping, reversal of analgesia
Naltrexone
Day 1, 25mg bid, then 50mg/day PO
Cholestatic itcha 44,45
Hepatotoxicity, nausea and vomiting, difficulty sleeping, reversal of analgesia
Paroxetine
20mg/day PO
Malignancy-related pruritusa,94 other pruritic diseases95
Insomnia, sexual dysfunction
Phototherapy: UVA plus psoralen, UVB
Must be individually determined
Prurigo nodularisa,82,83 uremic pruritusa40,41
Burns, photodamage, skin cancer
Thalidomide
100–200mg/day PO
Prurigo nodularis, uremic pruritusa 39
Teratogenic, peripheral neuropathy, drowsiness, constipation
Table VII. Principal systemic therapies for pruritus other than antihistamines
Conclusions
Pruritus is a very common and sometimes disabling symptom. Pruritus not associated with skin disorders or specific skin changes may be a symptom indicating the presence of an important disease. The diagnostic approach to patients with chronic itch may be complex and require multi-disciplinary interactions. New studies are shedding light on the complex mechanisms that induce chronic itch, and are revealing that multiple mediators may be involved in complex interactive pathways. The management of chronic severe itch is difficult and to be successful may require combination therapy for prolonged time periods.
CHRONIC RENAL FAILURE
Chronic kidney failure is a gradual loss of your kidneys' filtering ability, usually due to high blood pressure or diabetes. When kidney function is seriously impaired, dangerous levels of fluid and waste can quickly accumulate in the body. Chronic loss of function causes generalized wasting (shrinking in size) and progressive scarring within all parts of the kidneys. In time, overall scarring obscures the site of the initial damage. Yet, it is not until over 70% of the normal combined function of both kidneys is lost that most patients begin to experience symptoms of kidney failure.CausesDiabetes and hypertension are the two most common causes. Other conditions which responsible for the development of it are given below-1. Pre- renal-a. Decreased Cardiac outputb. Chronic liver failurec. AtherosclerosisAll these conditions are responsible for continuous hypoperfusion (low blood flow) of the kidneys, leading to kidney atrophy (shrinking), loss of nephron function, and chronic renal failure (CRF).2. Renal-
Chronic renal failure caused by changes within the kidneys, is called renal CRF, and is broadly categorized as follows: Diabetic nephropathy, kidney disease associated with diabetes; the most common cause of CRF
Hypertension nephrosclerosis, a condition that occurs with increased frequency in African Americans; the second leading cause of CRF
Chronic glomerular nephritis, a condition caused by diseases that affect the glomeruli and bring about progressive dysfunction
Chronic interstitial nephritis, a condition caused by disorders that ultimately lead to progressive scarring of the interstitium .Renal vascular CRF, large vessel abnormalities such as renal artery stenosis (narrowing of the large arteries that supply the kidneys)
Vasculitis, inflammation of the small blood vessels
Cystic kidney disease, kidney disease distinguished by multiple cysts (lined cavities or sacs)
Hereditary diseases of the kidney, such as Alport's syndrome (hereditary nephritis)
3. Post- renalInterference with the normal flow of urine can produce backpressure within the kidneys, can damage nephrons, and lead to obstructive uropathy, a disease of the urinary tract. Abnormalities that may hamper urine flow and cause post-renal CRF include the following: Bladder outlet obstruction due to an enlarged prostate gland or bladder stone
Neurogenic bladder, an overdistended bladder caused by impaired communicator nerve fibers from the bladder to the spinal cord
Kidney stones in both ureters, the tubes that pass urine from each kidney to the bladder
Obstruction of the tubules,the end channels of the renal nephrons
Retroperitoneal fibrosis, the formation of fiberlike tissue behind the peritoneum, the membrane that lines the abdominal cavity
Vesicoureteral reflux (VUR), the backward flow of urine from the bladder into a ureter.
Risk FactorsDiabetes, which is the most common include:
High blood pressure
Sickle cell disease
Lupus erythematosus
Atherosclerosis
Chronic glomerulonephritis
Kidney disease present at birth (congenital)
Bladder outlet obstruction
Overexposure to toxins and to some medications
Family history of kidney disease
Age 60 or older
Signs and SymptomsThe early symptoms of chronic kidney disease often occur with other illnesses, as well. These symptoms may be the only signs of kidney disease until the condition is more advanced.
Symptoms may include:General ill feeling and fatigue
Generalized itching (pruritus) and dry skinHeadaches
Weight loss without trying to lose weight
Appetite loss
Nausea Abnormally dark or light skin
Bone pain
nervous system symptoms
>>Drowsiness and confusion >>Problems concentrating or thinking>>Numbness in the hands, feet, or other areas>>Muscle twitching or cramps
Breath odor
Easy bruising, bleeding, or blood in the stool
Excessive thirst
Frequent hiccups
Low level of sexual interest and impotence
Menstrual periods stop (amenorrhea)
Sleep problems, such as insomnia, restless leg syndrome, and obstructive sleep apnea
Swelling of the feet and hands (edema)
Vomiting, typically in the morning
Stages in chronic renal failure1. Stage 1 CKDSlightly diminished function; Kidney damage with normal or relatively high GFR (>90 mL/min/1.73 m2). Kidney damage is defined as pathologic abnormalities or markers of damage, including abnormalities in blood or urine test or imaging studies.2. Stage 2 CKDMild reduction in GFR (60-89 mL/min/1.73 m2) with kidney damage. Kidney damage is defined as pathologic abnormalities or markers of damage, including abnormalities in blood or urine test or imaging studies.3. Stage 3 CKDModerate reduction in GFR (30-59 mL/min/1.73 m2).[1] British guidelines distinguish between stage 3A (GFR 45-59) and stage 3B (GFR 30-44) for purposes of screening and referral.4. Stage 4 CKDSevere reduction in GFR (15-29 mL/min/1.73 m2)[1] Preparation for renal replacement therapy.5. Stage 5 CKDEstablished kidney failure (GFR <15 mL/min/1.73 m2, or permanent renal replacement therapy (RRT).
ComplicationsFluid retention, which could lead to swollen tissues, congestive heart failure or pulmonary edema
hyperkalemia
Cardiovascular disease
Weak bones that fracture easily
Anemia
Stomach ulcers
Dry skin, changes in skin color
Insomnia
Decreased sex drive or impotence
Damage to your central nervous system
Decreased immune response
Pericarditis,
Irreversible damage to your kidneys (end-stage kidney disease), requiring either dialysis or transplant for survival.
InvestigationsHigh blood pressure is almost always present during all stages of chronic kidney disease. A neurologic examination may show signs of nerve damage. The health care provider may hear abnormal heart or lung sounds with a stethoscope.A urinalysis may show protein or other changes. These changes may appear 6 months to 10 or more years before symptoms appear.
Tests that check how well the kidneys are working Creatinine levels
Blood Urea Nitrogen levelCreatinine clearance
Chronic kidney disease changes the results of several other tests. Every patient needs to have the following checked regularly, as often as every 2 - 3 months when kidney disease gets worse:Potassium
Sodium
Albumin
Phosphorous
Calcium
Cholesterol
Magnesium
Complete blood count (CBC)
Electrolytes
Causes of chronic kidney disease may be seen on:Abdominal CT scan
Abdominal MRI
Abdominal ultrasound
Renal scan
This disease may also change the results of the following tests:Erythropoietin
PTH
Bone density test
TreatmentThe goal of therapy is to slow down or halt the otherwise relentless progression of CKD to stage 5. Control of blood pressure and treatment of the original disease, whenever feasible, are the broad principles of management.n some cases, dietary modifications have been proven to slow and even reverse further progression.
Diet for chronic kidney diseaseNeed to limit fluids.
Intake of low-protein diet.Restriction of salt, potassium, phosphorous, and other electrolytes.
Other tips for protecting the kidneys and preventing heart disease and stroke:Do not smoke.
Eat meals that are low in fat and cholesterol
Get regular exercise
Take drugs to lower your cholesterol.
Keep your blood sugar under control.
Haemodialysis:This should be started when the symptoms of uremia have become troublesome, despite adequate medical treatment, preferably before the patient develop serious consequences of uremia. First an arteriovenous fistula is created in the forearm, this results in distention and thickening of the vein wall which allows the repetitive insertion of needles for vascular access for heamodialysis. This is carried out for 3-5 hours 3 times weekly. Most patients notice a gradual reduction of their uremic symptoms during the first 6 weeks of treatment. They can lead relatively normal and active lives, and prolonged survival in excess of 20 years is now regularly reported.
Role of HomoeopathyHomoeopathy does not recognise kidneys as a mere organ of excretion or selective filtration but always recognises it in relation to the individual as a whole. Kidneys have a generalised function--the fluids coming to it and going from it influence every organ, tissue and cell of our body. Kidney function influences the complete vital economy of our body.Homoeopathic medicines does wonder in preventing the progression of disease, haemodialysis, also in reducing the number of dialysis in patient and renal transplantation.
The nephropathy that develops in type 2 DM differs from that of type 1 DM in the following respects:
• Microalbuminuria or macroalbuminuria may be present when type 2 DM is diagnosed, reflecting its long asymptomatic period;
• Hypertension more commonly accompanies microalbuminuria or macroalbuminuria in type 2 DM;
• Microalbuminuria may be less predictive of diabetic nephropathy and progression to macroalbuminuria in type 2 DM.
• Finally, it should be noted that albuminuria in type 2 DM may be secondary to factors unrelated to DM, such as hypertension, congestive heart failure, prostate disease, or infection.
The nephropathy that develops in type 2 DM differs from that of type 1 DM in the following respects:
• Microalbuminuria or macroalbuminuria may be present when type 2 DM is diagnosed, reflecting its long asymptomatic period;
• Hypertension more commonly accompanies microalbuminuria or macroalbuminuria in type 2 DM;
• Microalbuminuria may be less predictive of diabetic nephropathy and progression to macroalbuminuria in type 2 DM.
• Finally, it should be noted that albuminuria in type 2 DM may be secondary to factors unrelated to DM, such as hypertension, congestive heart failure, prostate disease, or infection.
• The kidneys have a special job to do , ie filtering the toxic molecules. The nephrons of the kidneys are probably the most “high – tech” cells in human body (Of course ,next only to brain cells ) .The vascular tuft of glomerulus located within the bowman’s capsule is perfused by afferent arteriole and drained by efferent arteriole .
• The entry of blood into glomerulus is regulated both by afferent and efferent arteriolar tone .These two micro-circulaoty units are under the sensitive control of both neural and humoral signals. Glomerular circulation is meticulously regulated by renal juxta glomerular apparatus.It modulates the glomerular blood flow by secreting renin which acts through Anigiotensin 2 on the efferent arteriole .
• The tone of the efferent arteriole is thought to be the single important factor in this servo control mechanism.
What happens in bilateral renal arterial stenosis ?
When there is bilateral renal arterial stenosis the effective renal blood flow is not significantly reduced , but maintained at the cost of increasing the efferent arteriolar constrictor tone.It is like a check valve at the exit point of a dam , which is partially closed to maintain the adequate pressure head (Here , intra-glomerular pressure head )What happens when ACEI are introduced ?
Once ACE inhibitors administered , the efferent arteriolar tone is removed , this triggers the intra glomerular pressure to drop suddenly and filtration pressure reduces .Note: ACEI does not reduce the renal blood flow directly but the glomerular perfusion pressure drops hence precipitating acute renal function deterioration.
What is your comment about the reno-protective effects of ACEI ?
The medical science’s most crucial moments are , when we confront , two diagonally opposite views are being debated and both suggest , there is definite benefit for the patient ! Cardiologists and nephrologists were always made to believe , that ACEI are unfriendly to kidneys .But ,we now have evidence , ACEI is not an untouchable molecule in renal dysfunction.
Lookin at a long term perspective , AT 2 increases the intra -glomerular hypertension and ACEI inhibitors reduce it.This protects the nephrons from hyper-filtration mode , that accelerates the glomerular injury . So , the current thinking seems to suggest ACEI has a definite role in arresting the progress of renal cell injury .
The only issue for ACEI is , it should not be continued if an ARF like picture sets in. (Acute deterioration ). Otherwise , in CRF at any basal level of serum creatinine , ACEI can be continued . Some think even an increase by few mg of creatinine can be allowed .
So the following can be a working guideline
ACEI can be started or continued at any level of creatinine in stable CKD with or without dialysisBut ,ACEIs need to be stopped in all of the following
• Acute renal failures
• Acute on chronic renal failure
• Accelerated elevation of creatinine (As in bilateral renal artery stenosis)
How much elevation of creatinine is allowed in CKD with ACEI ?
This is not answered yet .
Diabetes mellitus and angiotensin-converting enzyme inhibitors
Angiotensin-converting enzyme (ACE) inhibitors were the first class of antihypertensive drugs shown to reduce the vascular complications in those with diabetes independent of a reduction in blood pressure. The reno-protective effects of ACE inhibitors were not only seen in those with overt nephropathy (macroalbuminuria), but also extend to those with incipient nephropathy (microalbuminuria), even in the absence of hypertension.A slow deterioration in renal function is not a contraindication to the use of ACE inhibitors in patients with renal insufficiency, but a rapid progressive rise in serum creatinine following initiation of ACE inhibitors should trigger immediate discontinuation of the agent and further evaluation of the patient for advanced renovascular disease.
Several of the reno-protective effects of ACE inhibitors have been related to the increase in kinins that occurs with these agents, which is also responsible for some of the side-effects associated with ACE inhibitor therapy such as a dry cough. Renal protection is related to the antihypertensive effects of ACE inhibitors in both normal and hypertensive patients: renal vasodilatation results in increased renal blood flow and dilatation of the efferent arterioles.
The history of ACE inhibitors In 1954, Skeggs and coworkers started to recognize substrates participating in the renin–angiotensin system. In 1956, they purified the enzyme responsible for the conversion of inactive angiotensin I to the active vasoconstrictor angiotensin II in the presence of chloride ions from horse plasma.
In 1965, Ferreira showed that a non-toxic ethanol extract of the venom of the Brazilian viper Bothrops jararaca potentiated the smooth muscle contraction, hypotension and increased capillary permeability induced by bradykinin. However, some years passed before it became clear that ACE was the same as bradykininase and was inhibited by bradykinin-potentiating factor (BPF).
In 1968, Bakhle reported that BPF was a potent inhibitor of ACE from dog lung homogenate, and the long delayed purification of the active components of BPF was initiated by two groups – the first led by Ferreira in 1970 and the second led by Ondetti in 1971. Structure–activity correlation amongst the analogues of BPF suggested that these snake venom peptide inhibitors compete with substrates for binding to the active site of ACE.
By early 1974, the efficacy of ACE inhibitors as antihypertensive drugs had been demonstrated, but they were not yet available in an oral form for use in chronic hypertension. In the early 1980s, Squibb succeeded in developing an oral form known as captopril (Capoten) and received approval from the USA Food and Drug Administration for this drug. Since that time a number of other ACE inhibitors have been developed with differing pharmacokinetic qualities.
More recently, the development of the orally active angiotensin-receptor blockers (ARBs) has added an alternative way to inhibit the effects of angiotensin II. The superiority of ACE inhibitors in preventing the aggregate of major cardiovascular events has been demonstrated in two trials: one in which these agents were compared with diuretics/beta blockers and the other against a calcium antagonist.
The renin–angiotensin system The renin–angiotensin system is located mostly in the kidney and plays a major role in the homeostatic regulation of blood pressure, fluid and electrolytes. It is composed of both functional and anatomical aspects.
Functional aspects The functions of the renin-angiotensin system are mediated by a number of hormones and enzymes. Renin Renin is a glycoprotein synthesized as a long preprohormone composed of 406 amino acid residues. Active renin contains 340 amino acid residues and is exclusively produced by the kidneys, being formed in the secretory granules of the juxtaglomerular cells. It converts the plasma protein angiotensinogen to angiotensin I.
Angiotensinogen Angiotensinogen is a protein synthesized in the liver. It is composed of 453 amino acid residues with a characteristic 32 amino acid signal sequence that is removed in the endoplasmic reticulum. ACEACE (also known as kininase II) is a dipeptidyl carboxpeptidase that converts angiotensin I to angiotensin II. It is located mainly in endothelial cells. Bradykinin, one of the vasodilator hormones, is inactivated by the same enzyme. Most of the conversion of angiotensin I to angiotensin II occurs as the blood passes through the lungs, though it also takes place in many other parts of the body
Angiotensin I, II and III Angiotensin I is a physiologically inactive decapeptide that is produced by the action of renin on angiotensinogen. Angiotensin II is a physiologically active octapeptide known previously as hypertensin or angiotonin. It is rapidly metabolized in the circulation, having a half-life of 1-2 min. Angiotensin III is a physiologically active heptapeptide resulting from the metabolism of angiotensin II.
Anatomical aspects Angiotensin II receptorsThere are at least two classes of angiotensin II receptors (AT). The main type is angiotensin receptor-1 (AT1). The gene for this receptor is located on chromosome 3. AT2 is less important than AT1; its gene is located on chromosome X. The effect of these receptors differs from tissue to tissue. For example, the AT1 receptors in arterioles and those in the adrenal cortex are regulated in opposite directions: an excess of angiotensin II will downregulate the vascular receptors but upregulate the adrenal cortical receptors, making the gland more sensitive to aldosterone-stimulating hormone.
The AT1 receptor is classified into two subtypes. AT1A is located mainly in the blood vessel walls, the brain and other organs and mediates most of the known effects of angiotensin.20 AT1B is found in the anterior pituitary and adrenal cortex. AT2 receptors are more plentiful in fetal and neonatal life, but they persist in the brain and other organs in adults. AT2 receptors are important in fetal kidney development, modulation of pressure natriuresis, angiotensin II-induced renal production of nitric oxide, and the renal conversion of prostaglandin E2 to prostaglandin F2a.21 In addition, experimental evidence suggests that AT2 receptors may counterbalance some of the effects mediated by AT1 receptors.
The juxtaglomerular apparatus Renin is produced by the juxtaglomerular cells, which are epitheloid cells located in the media of the afferent arterioles as they enter the glomeruli. Renin is also found in the granular Lacis cells located at the junction between the afferent and efferent arterioles. The macula densa is a modified region of tubular epithelium located at the beginning of the distal convoluted tubule close to the juxtaglomerular cells. The juxtaglomerular cells together with the macula densa cells are known as the juxtaglomerular apparatus.
The renin–angiotensin system in diabetes mellitus There is increased stimulation of the sympathetic nervous system in diabetics compared with non-diabetics due to their need to increase the secretion of insulin from beta cells through stimulation of b2 receptors and to dilate the renal arterioles through stimulation of b1 and b2 receptors.
Dilatation of the renal arterioles occurs as a response to the pathological changes that develop in the kidney in diabetes. These vascular and interstitial changes eventually lead to deteriorating renal function. The development of diabetic nephropathy stimulates the renin–angiotensin system. Poor diabetic control will further increase this stimulation.
It has also been shown that angiotensin may be present in abundance in some other tissues, including adipose tissue. In 1987, angiotensin mRNA was found in periaortal brown adipose tissue and in cells found within the rat aorta wall. Other studies have demonstrated evidence for the existence of an intrinsic angiotensin-generating system in the pancreas. Recent epidemiological data have shown that administration of ACE inhibitors in hypertensive patients may have a protective role in preventing the occurrence of diabetes. This epidemiological data may explain why some antidiabetic drugs such as thiazoladinedione can decrease blood pressure in obese diabetics.
Clinical recommendations concerning ACE inhibitors The clinical recommendations and guidelines of many medical and diabetic societies and associations include the use of ACE inhibitors in diabetes. The European Society of Hypertension – European Society of Cardiology guidelines for the management of arterial hypertension states that ACE inhibitors are indicated in the following conditions:
congestive heart failure/left ventricular dysfunction
post-myocardial infarction
non-diabetic nephropathy
type 1 diabetic nephropathy/proteinuria.
However, they recommend the use of an ARB in the following conditions:
type 2 diabetic nephropathydiabetic microalbuminuria
proteinuria
left ventricular hypertrophy
ACE inhibitor-induced cough.
In the Canadian Hypertension Education Program recommendations, ACE inhibitors are recommended as part of the initial therapy for the following conditions: diabetes mellitus with or without nephropathy
angina
post-myocardial infarction
heart failure
post-cerebrovascular accident or transient ischaemic attack
renal disease
left ventricular hypertrophy.
The American Diabetes Association recommends that the use of an ACE inhibitor should be considered in all diabetic patients older than 55 years with or without hypertension, but with another cardiovascular risk factor (a history of cardiovascular disease, dyslipidaemia, microalbuminuria or smoking). A combination of an ACE inhibitor and an ARB can be used in the treatment of albuminuria and diabetic nephropathy.
The seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure recommends the use of ACE inhibitors in the following conditions:
hypertension with acute coronary syndromes such as unstable angina or myocardial infarction
post-myocardial infarction
heart failure
diabetic hypertension
chronic kidney disease (a limited increase in serum creatinine of as much as 35% above the baseline with ACE inhibitors or ARBs is acceptable unless hyperkalaemia develops)
cerebrovascular disease.
Clinical trials assessing the use of ACE inhibitors in diabetes In the following trials, patients with type 2 diabetes mellitus were randomized to receive ACE inhibitors as initial therapy and the outcome compared with that in patients receiving other antihypertensive drugs:
the UK Prospective Diabetes Study,27 which compared the effect of captopril versus atenolol
the MICRO-HOPE Diabetic substudy,28 part of the larger Heart Outcome Prevention Evaluation (HOPE) study, which compared the use of ramipril versus placebo
the Appropriate Blood Pressure Control in Diabetes (ABCD) trial,29 which compared the use of enalapril versus nisoldipine
the Captopril Prevention Project (CAPPP),30 which compared the use of captopril versus diuretic or b-blockers
the Fosinopril versus Amlodipine Cardiovascular Events Trial (FACET),31 which compared the use of fosinopril versus amlodipine.
Management of hypertension in African Americans All antihypertensive drug classes can be used by African Americans to lower their blood pressure; in terms of efficacy, there is no rationale for avoiding certain classes of agents on the grounds of race. However, when prescribing ACE inhibitors the clinician should bear in mind that African Americans appear to be at increased risk for ACE inhibitor-associated angio-oedema and cough compared with Caucasians.
Conclusion ACE inhibitors should be recommended for use in all diabetic patients, especially those with type 2 diabetes mellitus. They are useful not only as antihypertensive drugs but also provide renal protection.
Renal effects of ACE inhibitors in hypertension
INTRODUCTIONThe effect of angiotensin converting enzyme (ACE) inhibitors on renal function in the hypertensive patient is related both to the glomerular actions of angiotensin II and to the mechanism of autoregulation of the glomerular filtration rate (GFR) [1]. Angiotensin II constricts both the afferent and efferent arterioles, but preferentially increases efferent resistance [2]. At least three factors may contribute to this response:
The efferent arteriole has a smaller diameter in the basal state; as a result, further constriction at this site will produce a greater increase in resistance than at the afferent arteriole [2].
Angiotensin II stimulates the release of the vasodilator nitric oxide from the afferent arteriole, thereby minimizing constriction at this site .
Angiotensin II minimizes vasoconstriction at the afferent arteriole via the stimulation of angiotensin II type 2 receptors, which results in vasodilation through a cytochrome P-450 dependent pathway .
The net effect of the more prominent increase in efferent tone is that the intraglomerular pressure is stable or increased, thereby tending to maintain or even raise the GFR. In addition to these arteriolar actions, angiotensin II constricts the mesangial cells, an effect that tends to lower the GFR by decreasing the surface area available for filtration.