Acute coronary syndromes
د. حسين محمد جمعةاختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2010
Key points
The term acute coronary syndrome describes the clinical features caused by disruption of a plaque in one of the coronary arteries, which is then complicated by intraluminal thrombosis and distal embolisationThe severity of the syndrome is determined by the volume of myocardium affected and the extent of collateral perfusion
Myocardial infarction with ST elevation
Emergency primary percutaneous coronary intervention is the best method of reperfusion. When this is unavailable, thrombolysis plus anti-platelet therapy (aspirin plus clopidogrel) substantially improves patients' outcome and survival.Myocardial infarction without ST elevation
The risks of acute coronary syndrome without ST elevation have been underestimated. One in eight patients will die within six months and one in five will need to be readmitted to hospital as an emergencyThe following treatments improve outcome
• Antiplatelet therapy (aspirin and a thienopyridine such as clopidogrel) and
• Antithrombin therapy: this may be a form of heparin (low molecular weight heparin or unfractionated heparin) or a factor Xa inhibitor (fondaparinux) or a direct thrombin inhibitor (bivalirudin)
• In patients at moderate and high risk of complications, angiography followed by percutaneous coronary intervention or coronary artery bypass surgery can reduce the risk of death, myocardial infarction, and refractory angina.
The following features indicate an increased risk
• ST depression on an electrocardiogram
• Continued or recurrent symptoms of ischaemia
• Rise in cardiac enzymes or troponins
• Ischaemia associated with hypotension, arrhythmia, or heart failure
Risk is frequently underestimated in clinical practice and the European Society of Cardiology and the ACC/AHA Guideline recommend using a risk score (GRACE Score or TIMI risk score).
Glycoprotein IIb/IIIa inhibitors reduce complications, especially in patients who need an interventional procedure.
Clinical tip
Recent advances in treatments for patients with acute coronary syndrome reduce the risk of:• Serious cardiac complications
• Rehospitalisation
• Infarction
• Death.
But to achieve these improvements in outcome you need to set up prompt and effective triage systems and consistently apply evidence based treatments.
• Introduction
• The term acute coronary syndrome describes a spectrum of clinical conditions including:• Myocardial infarction with ST elevation
• Myocardial infarction without ST elevation
• Unstable angina (acute coronary syndrome without release of cardiac enzymes or other markers).
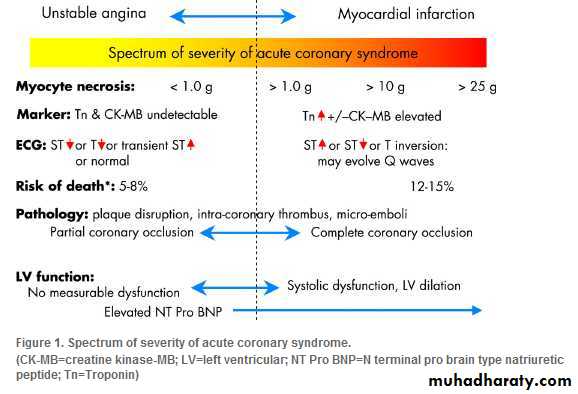
Acute coronary syndrome ranges from:
Unstable angina without detectable necrosis of myocytes toExtensive myocardial infarction.
Unstable angina is characterised by:
The clinical syndrome
Undetectable markers (troponin and creatine kinase-MB)
ECG changes (typically ST depression, T wave inversion, or transient ST elevation).
The risk of death from hospitalisation is around 5-8% in the following six months.
The risk of death from
Unstable angina is around 5-8% in the following six months.Myocardial infarction without ST elevation
One in eight patients will die within six monthsFor patients who are admitted to hospital with an acute myocardial infarction, the subsequent risk of death is 12-15% in the following six months.
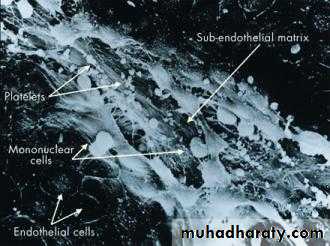
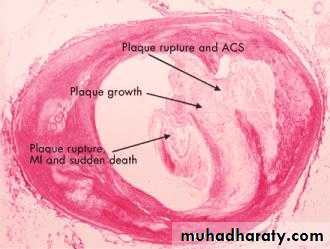
• An acute coronary syndrome is caused by disruption of a vulnerable plaque, complicated by intraluminal thrombosis, embolisation, and varying degrees of obstruction to perfusion (Figures 2 and 3).
• Figure 2. The endothelial lining of a coronary artery with disruption of the endothelium revealing the subendothelial matrix. Platelets are attached to the disrupted endothelium and monocytes are invading beneath it.
•
• Figure 3. Cross section of a coronary artery of a patient with extensive atheroma. A large plaque that was full of lipids has ruptured, resulting in an acute coronary syndrome. The acute coronary syndrome resolved and the plaque reorganised. Subsequent growth of the plaque encroached on the lumen. After a further rupture the patient experienced a myocardial infarction and subsequently died. The coronary artery was partially occluded at the time of the postmortem examination.
•
The clinical features depend on the:
• Severity of obstruction of the coronary artery• Volume of myocardium affected.
Patients with complete occlusion may develop a myocardial infarction with ST elevation if the lesion occludes an artery supplying a substantial volume of myocardium. But the same occlusion in the presence of extensive collateral arteries may show itself as infarction without ST elevation. Similarly, incomplete occlusion at the site of a disrupted plaque may produce ischaemia or microinfarction, depending on the volume of myocardium affected and the extent of distal embolisation.
Sensitive and specific markers of injury to myocytes (troponins) can detect smaller volumes of infarction than can conventional cardiac enzymes. But other causes of necrosis of myocytes (for example myocarditis) may also result in elevation of troponins.
Establishing a working diagnosis
Triage of patients into acute coronary syndrome with ST elevation or acute coronary syndrome without ST elevation is based on the presence of a typical clinical syndrome plus electrocardiographic changes.Remember, if you check troponin levels less than 4-6 hours from the onset of ischaemia, they may not yet be elevated (time delay for release from myocytes).
If patients don't have ST elevation on their ECG, you can diagnose an acute coronary syndrome by the presence of:
A clinical syndrome of acute ischaemia with either pain at rest or a worsening pattern of pain on minimal exertion plus
Evidence of acute ischaemic injury from ECG changes, or rises in troponins, or both.
The predictive accuracy of ST elevation for a final diagnosis of myocardial infarction is very high. But less than 50% of patients with a myocardial infarction without ST elevation are suspected as having an infarction on initial presentation. Within the spectrum of acute coronary syndromes, patients with a myocardial infarction without ST elevation present the most difficult diagnostic challenge.
In patients with a myocardial infarction without ST elevation, the ECG changes may consist of:
ST depression or T wave inversion.
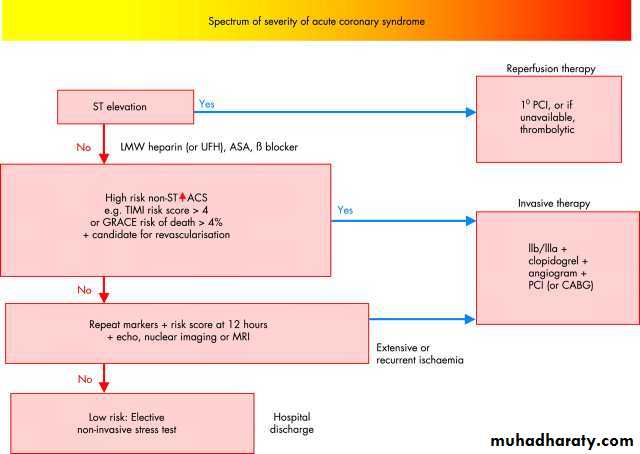
You can distinguish a myocardial infarction without ST elevation from unstable angina on the basis of the presence or absence of a rise in troponins or enzymes (creatine kinase or CK-MB) (you should repeat the assay at 12 hours after presentation to detect those with evolving infarction). How you treat patients with an acute coronary syndrome without ST elevation depends on whether high risk features are present (Figure 4).
GRACE=Global Registry of Acute Coronary Events; TIMI=Thrombolysis In Myocardial Infarction classification)
In the presence of a typical clinical syndrome of acute myocardial infarction and persistent ST elevation, patients should proceed to reperfusion therapy with primary percutaneous coronary intervention or, if this is unavailable, thrombolytic treatment.
The remaining patients have an acute coronary syndrome without ST elevation. Those with raised enzymes or troponins have a myocardial infarction without ST elevation and those with undetectable markers have unstable angina. If higher risk features are present (or high GRACE or TIMI risk score), you should consider invasive treatment (angiography and percutaneous coronary intervention) combined with a glycoprotein IIb/IIIa inhibitor and clopidogrel.
In the absence of high risk features you should repeat estimations of markers at 12 hours. Patients at low risk should undergo elective non-invasive stress testing to determine whether stable but obstructive coronary artery disease is present. You can consider early discharge for patients at low risk of complications.
The revised definition of acute myocardial infarction
The new (and more sensitive definition) of myocardial infarction requires:A typical clinical syndrome plus
A rise and fall in troponins (or creatine kinase-MB) to values greater than 99% of a normal reference population.
As a result of this more sensitive definition about 25% of those that would previously have been classified as having unstable angina now fulfil the criteria for myocardial infarction.
Acute management and early risk stratification as a guide to treatment
Biomarkers of injury to myocytes may be elevated when ischaemia has been present for at least 4-6 hours or if preceding myocardial injury has taken place. An elevated biomarker at presentation signifies an increased risk of death in myocardial infarction with or without ST elevation. But the absence of such elevation may simply reflect the lag before sufficient concentrations of the marker are detectable.
A repeat assay is required following an initially negative measurement. But you shouldn't delay urgent management while waiting for the results.
Immediately after presentation you should ask yourself a series of questions:
Does this patient have an acute coronary syndrome (based on clinical and ECG features)?Is this patient a candidate for emergency reperfusion (do they have ST elevation or a true posterior myocardial infarction or left bundle branch block)?
If they have had an acute coronary syndrome without ST elevation, are they at high or low risk of complications (Table 1)?
Table 1. High risk features in patients with an acute coronary syndrome without ST elevation
High risk features in the early triage of patients:
• Continued or recurrent ischaemia at rest
• Ischaemia complicated by cardiac failure, arrhythmia, or mechanical complications
• Ischaemia with ST depression, or troponin elevation, or both
• Dynamic ST changes
Markers of pre-existing longer term risk:
AgePrior myocardial infarction, coronary artery bypass graft, diabetes, congestive heart failure, or hypertension
Biological markers of renal dysfunction (raised creatinine or reduced creatinine clearance)
Elevated inflammatory markers (C-reactive protein, interleukin 6, CD40 ligand)
Left ventricular dysfunction:
Invasive or non-invasive markers of left ventricular dysfunction or mechanical complication
Biological markers of left ventricular dysfunction (raised N terminal pro brain type natriuretic peptide)
Extent of coronary artery disease on angiography
General measures
The underlying principles are to:Relieve pain
Maintain adequate arterial oxygen concentration
Relieve ischaemia.
If the patient has heart failure or shock you may need to start assisted ventilation with positive end expiratory pressures.
Reperfusion of the myocardium is crucial in patients with acute ST elevation (or left bundle branch block or posterior myocardial infarction).
Haemodynamic support may be necessary in patients with hypotension or cardiogenic shock (this may include intra-aortic balloon pumping to stabilise the patient for percutaneous coronary intervention). You may need to institute specific measures to control hypertension, to reduce stress on the myocardial wall, and to treat acute heart failure and mechanical complications.
Recent advances in treatments are described in the following sections, but established therapies are summarised only briefly.
Managing patients with acute coronary syndrome and ST elevation
The aims of acute management are to:Rapidly establish a working diagnosis
Treat acute complications including cardiac arrest
Provide prompt relief of pain and maintain adequate arterial oxygen concentrations
Start reperfusion therapy
Treat complications
Assess the risk of longer term complications and to start measures of secondary prevention.
You should make a working diagnosis of an acute myocardial infarction if the patient has typical clinical symptoms and specific ECG changes, such as:
ST elevation of ≥0.2 mV in leads V1-V3 or ≥0.1 mV in other leads
ST depression consistent with a posterior myocardial infarction.
Established myocardial infarction (either old or recent) may be defined by the presence of Q waves of ≥0.03 s in leads V1-V6 or II, aVL, aVF.
You should make a working diagnosis of an acute myocardial infarction if the patient has typical clinical symptoms and specific ECG changes, such as:
• ST elevation of ≥0.2 mV in leads V1-V3 or ≥0.1 mV in other leads
• ST depression consistent with a posterior myocardial infarction.
• Established myocardial infarction (either old or recent) may be defined by the presence of Q waves of ≥0.03 s in leads V1-V6 or II, aVL, aVF.
Reperfusion therapy
Prompt and effective reperfusion therapy is the cornerstone of treatment and is the only widely applicable acute treatment to reduce the size of the infarct and to reduce the risk of major complications.Meta-analysis of the trials of primary angioplasty versus fibrinolysis have shown that primary percutaneous coronary intervention is more effective at preventing major complications including death and stroke.
Fibrinolysis
In trials of fibrinolysis versus control, more than 150 000 patients have been randomised and the overall benefit is around 50 lives saved per 1000 patients treated with a thrombolytic agent and aspirin.Any delays are critically important, especially within the first four hours following the onset of symptoms. Overall, there are about 1.6 deaths more per hour of delay, per 1000 patients treated. Within the first two hours the reduction in mortality is twice as large as beyond two hours.
The European guidelines for the management of patients with clear cut changes of acute infarction state that patients should receive:
A primary percutaneous coronary intervention within 60 minutes of arrival (that is the balloon should be inflated within 60 minutes).
If primary percutaneous coronary intervention cannot be provided within 60 minutes of arrival, thrombolysis should be given within 20 minutes of arrival in hospital (or pre-hospital thrombolysis).
Limitations and hazards of fibrinolysis
The major limitation of thrombolysis is that reperfusion is1. gradual or incomplete and inadequate in a significant proportion of patients. This proportion may range from:
About 40% with streptokinase to 20-30% with tissue plasminogen activator (tPA) or tenecteplase (TNK).
2. The principal hazard is intracranial haemorrhage: overall about four extra strokes occur per 1000 patients treated and of these two are fatal.
Overall, the use of accelerated tissue plasminogen activator (alteplase) results in 10 fewer deaths per 1000 patients treated, but at the risk of three additional strokes, compared with streptokinase treatment.
Single bolus agents provide logistical advantages and both reteplase-plasminogen activator or weight adjusted tenecteplase have equivalent efficacy to accelerated tissue plasminogen activator. Tenecteplase causes fewer non-cerebral bleeds and patients need fewer blood transfusions. Bolus agents minimise delays in the pre-hospital setting and in accident and emergency departments.
Fibrinolysis combined with newer antithrombotic agents
aspirin produced similar benefits to streptokinase in the large ISIS-2 trial, and together the benefits were additive. Adding clopidogrel (on top of aspirin) produces additional benefits in achieving an open artery (CLARTIY study) and reducing mortality, in the very large Chinese study of 45,000 patients (COMMIT). This did not produce an excess of major bleeding or strokes.The combination of glycoprotein IIb/IIIa inhibitors and fibrinolysis has been tested in seven trials, including two large trials (ASSENT 3 and GUSTO V) involving more than 30 000 patients.
There was no overall advantage for combining the lytic agent with a glycoprotein IIb/IIIa inhibitor
In contrast, there is benefit for glycoprotein IIb/IIIa inhibitors with primary PCI
Primary percutaneous coronary intervention
Trials comparing thrombolysis with primary percutaneous coronary intervention and a meta-analysis of these trials have demonstrated that if primary PCI can be administered promptly and by an experienced team, it is superior to fibrinolytic therapy and doesn't cause excess cerebral and systemic bleeding.Lower mortality rates have been reported among patients undergoing primary percutaneous coronary intervention in centres with a high volume of percutaneous coronary intervention procedures, compared with low volume centres.
The DANAMI 2 study suggests that for transfer times of two hours or less from a community hospital to the start of percutaneous coronary intervention, a significant reduction in death, reinfarction, and stroke is observed, compared with thrombolysis.
In summary, a series of studies has demonstrated more effective restoration of patency with PCI , compared with fibrinolysis.
PCI also results in:
• Less reocclusion
• Improved left ventricular function
• Better clinical outcomes, including a lower risk of stroke than fibrinolysis.
PCI is therefore the preferred strategy for patients with acute myocardial infarction with ST elevation if a skilled team can provide the intervention within 60 minutes of arrival in hospital.
Percutaneous coronary intervention combined with fibrinolysis
Trials have investigated "facilitated percutaneous coronary intervention," where patients receive first the fibrinolytic agent and then percutaneous coronary intervention, but these have failed to show benefit and may even result in more complications than primary percutaneous coronary intervention (ASSENT-4).Percutaneous coronary intervention combined with a glycoprotein IIb/IIIa inhibitor (abciximab)
The combination of glycoprotein IIb/IIIa inhibitors and primary percutaneous coronary intervention has been tested in several studies (RAPPORT, ISAR 2, CADILLAC, ADMIRAL, and ACE trials, n=3666).13
Pooled analysis of these studies suggests that glycoprotein IIb/IIIa inhibitors reduce adverse outcomes (death and myocardial infarction) in patients who undergo a primary percutaneous coronary intervention (without a lytic agent).
Rates of death or further myocardial infarction at 30 days were:
3.2% in the group receiving percutaneous coronary intervention with abciximab4.8% in the group receiving percutaneous coronary intervention without abciximab.
Although individual trials do not provide conclusive evidence for reducing death or myocardial infarction, they do all show that patients who received percutaneous coronary intervention with abciximab need fewer revascularisations. And the pooled analysis of trials suggests a clear benefit in reducing rates of death and further myocardial infarction when primary percutaneous coronary intervention is combined with abciximab.
Rescue percutaneous coronary intervention
Rescue percutaneous coronary intervention refers to a percutaneous coronary intervention procedure performed in patients without evidence of a response to thrombolysis (less than 50% resolution of ST elevation).
Studies have demonstrated success of percutaneous coronary intervention in achieving coronary patency and flow. and have improved clinical outcomes compared with no therapy or repeat thrombolysis (REACT Trial).
Shortfall in the provision of reperfusion therapy
Despite the clearly demonstrated survival and clinical advantages of prompt reperfusion therapy, around one third of patients do not receive any form of reperfusion (despite presenting with ST elevation within 12 hours of onset of symptoms and without contraindications to reperfusion). A similar frequency occurs in the US (33%); Europe (29%); Australia, New Zealand, and Canada (29%); and Argentina and Brazil (28%).16Specific factors predict which patients are unlikely to undergo reperfusion. These include patients with:
Previous coronary artery bypass graft
Diabetes
A presentation with heart failure
A presentation without chest pain
Age older than 75.
The proportion of patients who receive a primary percutaneous coronary intervention varies widely across geographic regions, but most patients receive fibrinolysis.
What if the patient has symptoms of infarction but non-diagnostic ECG changes?
During the early evolution of infarction the ECG may be abnormal but may not have significant ST elevation. Typical changes may evolve over minutes or hours and it is critically important to institute continuous ST monitoring or perform repeat ECGs to ensure you detect this evolution promptly.In patients with symptoms suggestive of infarction but without diagnostic ECG changes, consider alternative diagnoses (including aortic dissection, gastro-oesophageal disease, and musculoskeletal and mediastinal conditions).
What if the patient has ECG changes without typical symptoms?
Certain groups of patients, including elderly people and those with diabetes, may present without typical symptoms of myocardial infarction. They may present with evolving ECG changes and haemodynamic or mechanical complications. Their treatment should be similar to patients with a typical painful infarction.Managing patients with an acute coronary syndrome without ST elevation
General features
Patients with an acute coronary syndrome without ST elevation are at risk of major cardiovascular complications and death, and the extent of this risk depends on acute risk of coronary occlusion and pre-existing risk factors.
Elevated troponin is a marker of increased risk, but should not be used in isolation, it should be used in conjunction with risk scores (GRACE or TIMI).
Extensive evidence shows that elevated troponins are powerful and independent predictors of thrombotic complications, including myocardial infarction and death. Evidence from trials of percutaneous coronary intervention suggests that troponins can be used as part of the measures to identify patients at higher risk and also those patients who are likely to gain from interventional procedures.
Newer generation troponin assays have higher sensitivity and diagnostic accuracy. With these assays very minor increases in troponins can detect myocyte necrosis in as little as 1 g of myocardium. Even these minor increases predict a higher risk of cardiac complications and death.
Table 2. Independent predictors of death in patients presenting with acute coronary syndromes
• Age (a continuous variable)
• Degree of heart failure
• Low arterial blood pressure
• ST deviation on ECG
• Cardiac arrest
• Elevated creatinine
• Elevated creatine kinase-MB or troponin
• Increased heart rate
Certain markers reflect an upregulation of the inflammatory and thrombotic systems. They are not yet widely applied in practice. They include:
• High sensitivity C-reactive protein
• Interleukin 6
• CD40 ligand
• Platelet-monocyte complexes.
Elevation of both high sensitivity C-reactive protein and troponin signifies a substantially higher risk of death (around 14% at one year) than either marker alone. If both markers are normal then the patient is at very low risk of future cardiac events (<2%).
Importantly, whereas troponins predict the hazard of acute events (including acute myocardial infarction and death), high sensitivity C-reactive protein on presentation does not independently predict the risk of death during hospitalisation, but it is a powerful predictor of death in the following one or two years.
Impaired cardiac muscle function, manifested by the presence of heart failure, is a powerful and independent predictor of death.
Subtle evidence of myocardial dysfunction in acute coronary syndrome (suggested by a raised brain type natriuretic peptide) is a powerful predictor of death and of heart failure.
Although overt renal dysfunction has been recognised as an adverse marker, more subtle elevations of creatinine or reductions in creatinine clearance have recently been demonstrated to be independent predictors of death, even in the absence of previously recognised renal dysfunction.
Markers of ischaemia and intravascular thrombosis
Recurrent ischaemia, at rest, is a powerful indicator of higher risk, especially when accompanied by dynamic ST changes.ST elevation and ST depression are well recognised electrocardiographic markers of risk; however, it has recently been demonstrated that ST deviation (up or down) conveys similar risk for death whether this deviation is upwards or downwards (having controlled for the other elements of baseline risk).
Antiplatelet treatment
The most recent update of the Antithrombotic Trialists' Collaboration, based on 287 studies in 135 000 patients, demonstrates a highly significant reduction in the risk of myocardial infarction and stroke and vascular death as a result of antiplatelet treatment (principally aspirin) versus control.Overall, the event rates were 13.2% in control patients and 10.7% in those treated with antiplatelet therapy, a 22% relative risk reduction. In patients with acute myocardial infarction, and in other high risk patients, the absolute and relative risk reductions were greater.
Therefore, abundant evidence supports the use of aspirin in patients with an acute coronary syndrome. Additional antiplatelet treatment needs evidence of benefit on top of aspirin, rather than as an alternative to aspirin. Recent data suggest that bleeding risk doubles for maintenance aspirin doses above versus below 100 mg daily, with no improved efficacy.
Thienopyridines (adenosine diphosphate (ADP) antagonists)
Thienopyridines inhibit ADP-mediated platelet aggregation. Although initial studies were conducted with ticlopidine, this drug has been superseded by clopidogrel because it is safer.The CURE trial tested clopidogrel in 12 562 patients with non-ST elevation acute coronary syndrome on top of background treatment and aspirin. A 2.1% absolute risk reduction (20% relative risk reduction, p <0.0001) occurred in the frequency of non-fatal myocardial infarction, stroke, or cardiovascular death. The treatment effect was evident within the first 24 hours of starting therapy and although the absolute benefits were greatest in the first three months of treatment, the relative risk reduction was the same beyond three months.
Around 1% more patients experienced major bleeding, but there was no significant excess of life threatening bleeding or haemorrhagic strokes.
Nevertheless, in view of the irreversible nature of the ADP antagonism, guidelines suggest that clopidogrel should be withheld for five days before coronary artery bypass grafts.
In candidates for a very urgent coronary artery bypass graft, reversible glycoprotein IIb/IIIa inhibitors (eptifibatide or tirofiban) can be used before surgery.
In patients with an acute coronary syndrome without ST elevation, the European Society of Cardiology guidelines recommend around twelve months' treatment with clopidogrel (2007 Guidelines).
Thienopyridines reduce the risk of stent occlusion and are now part of standard treatment in all patients undergoing elective percutaneous coronary intervention. Recent observational studies have demonstrated a small but continuing risk of stent thrombosis (about 0.6% per annum) and this may be higher with drug eluting stents.
With drug eluting stents, at least 12 months of clopidogrel and aspirin are needed but, beyond this, robust randomised trial evidence is needed to guide duration of treatment.
In all instances the possible benefits versus risks of longer term dual anti-platelet therapy need to be considered (about 1% excess major bleeds). A long term trial of aspirin plus clopidogrel has been conducted in patients with a range of vascular risks and this did not produce overall benefit (CHARISMA). However, there are subsidiary analyses that suggest that patients with prior ischaemic events (for example myocardial infarction, stroke) may benefit - but this requires further testing.
Newer thienopyridines (for example prasugrel) are being tested against clopidogrel and these have more rapid onset of action and greater inhibition of platelet aggregation than clopidogrel. The balance of risks versus benefits needs to be defined (TRITON Study AHA 2007).
Glycoprotein IIb/IIIa receptor antagonists
Platelet aggregation involves the glycoprotein IIb/IIIa receptor linked to fibrinogen or von Willebrand factor. Intravenous glycoprotein IIb/IIIa receptor antagonists have been extensively tested in patients with acute coronary syndrome without ST elevation. In a meta-analysis of all the major randomised trials the absolute risk reduction for death or myocardial infarction at 30 days was 1% (11.8% control versus 10.8% with glycoprotein IIb/IIIa).The absolute treatment benefit was largest in high risk patients - in particular, those with evidence of troponin release or those undergoing acute percutaneous coronary intervention.Among those without troponin elevation or who didn't undergo a percutaneous coronary intervention, no significant benefits were observed with glycoprotein IIb/IIIa administration.
In lower risk patients both thienopyridines and glycoprotein IIb/IIIa inhibitors may not be needed. There is no evidence to support the use of oral glycoprotein IIb/IIIa antagonists following acute coronary syndrome. Oral glycoprotein IIb/IIIa inhibitors may cause an increased risk of death.
Table 3. Evidence for treatment of acute coronary syndrome
Aspirin remains the cornerstone of antiplatelet treatment in patients with acute coronary syndromeRobust evidence supports the use of clopidogrel in patients presenting with acute coronary syndrome without ST elevation (at least up to nine months)
Extensive evidence supports the use of intravenous glycoprotein IIb/IIIa inhibitors in high risk patients with acute coronary syndrome, especially those undergoing acute percutaneous coronary intervention
Antithrombin treatment
Thrombin is a highly potent stimulus not only of the generation of fibrin, but also platelet activation. In addition it leads to:• Monocyte chemotaxis
• Mitogenesis
• Increased permeability of the vascular wall
• Secretion of cytokines and growth factors from smooth muscle cells.
Effective antithrombotic treatment requires inhibition of platelet function and inhibition of thrombin. Unfractionated heparin has been widely used, but it suffers from practical difficulties in maintaining antithrombin activity within the therapeutic range (influenced by acute phase proteins and the binding to antithrombins).
Nevertheless, there is clear evidence that a form of heparin, either unfractionated or low molecular weight heparin, is superior to placebo in patients with acute coronary syndrome. Heparin reduces the absolute rates of death or myocardial infarction by about 3%.
A meta-analysis of the two trials of enoxaparin (ESSENCE and TIMI 11B) has demonstrated a significant reduction in death or myocardial infarction (odds ratio 0.82, 95% CI 0.69 to 0.97). Both the European and North American guidelines on acute coronary syndrome suggest that enoxaparin is the preferred antithrombin.
Regarding safety, there is a similar safety profile for low molecular weight heparin and unfractionated heparin in the presence or absence of glycoprotein IIb/IIIa inhibitors. A randomised trial of enoxaparin versus unfractionated heparin suggested similar efficacy for enoxaparin in patients treated with glycoprotein IIb/IIIa inhibitors.
The large scale SYNERGY trial suggests that enoxaparin has similar efficacy to unfractionated heparin in patients undergoing percutaneous coronary intervention, but the bleeding risks appear slightly higher.
Direct antithrombins may provide significant advantages over the indirect inhibitors (unfractionated and low molecular weight heparin). Lower rates of bleeding complications have been observed in patients undergoing percutaneous coronary intervention with bivalirudin versus heparin. The ACUITY trial has shown that bivalirudin may be used in place of heparin or low molecular weight heparin plus glycoprotein IIb/IIIa inhibitors, with similar efficacy but significantly reduced bleeding risks (ACUITY Trial). However this is limited to patients undergoing interventional procedures.
Low molecular weight heparins partially inhibit factor Xa of the coagulation cascade, but newer specific inhibitors of Xa have been developed - for example, the pentasaccharide, fondaparinux. Such agents inhibit thrombin generation as distinct from thrombin activity. The OASIS 5 trial tested fondaparinux against enoxaparin in non-ST elevation acute coronary syndrome and demonstrated similar efficacy at nine days (the primary endpoint) but significantly fewer later deaths and a halving of major bleeding. For patients going on to percutaneous coronary intervention, additional anti-thrombotic therapy is required to prevent catheter thrombosis (for example unfractionated heparin). The 2007 ESC guidelines recommend fondaparinux in patients in whom there is no planned percutaneous coronary intervention.
Intervention (invasive treatment) in patients with acute coronary syndrome without ST elevation
Among patients presenting with acute coronary syndrome, but without persistent ST elevation, current guidelines recommend angiography and glycoprotein IIb/IIIa inhibitors for those identified to be at higher risk.
The current US guidelines (AHA/ACC 2007) suggest that either an interventional or a conservative strategy is appropriate in patients at moderate or low risk, but invasive strategies are recommended for those at higher risk. Both the ESC and ACC/AHA 2007 guidelines do not recommend intervention in low risk patients.
Based on a meta-analysis of all of the trials of intervention in acute coronary syndrome there is a reduction in death or myocardial infarction with the interventional strategy (risk ratio 0.88, 95% CI 0.78 to 0.99). However, significant heterogeneity exists among the trials, and the older trials did not employ modern interventional techniques nor stents or modern antiplatelet and antithrombin therapy.
The FRISC 2 trial independently demonstrated an impact on death or on death and myocardial infarction (risk ratio 0.78, 95% CI 0.62 to 0.98). Similarly, the TACTICS TIMI 18 trial demonstrated a reduction in the composite of death, myocardial infarction, and rehospitalisation for acute coronary syndrome as a result of the interventional strategy (in patients treated with tirofiban).
RITA 3 examined whether an interventional strategy was superior in patients at moderate risk and demonstrated a significant reduction in the composite end point of death, myocardial infarction, or refractory angina (from 14.5% to 9.6%, risk ratio 0.66, 95% CI 0.51 to 0.85). The major part of the benefit was a reduction in refractory angina, but the curves for death and myocardial infarction progressively separated in favour of the intervention strategy over prolonged follow up.
The ICTUS trial has not shown a benefit for systematic early intervention, in the presence of aggressive pharmacologic therapy and stress testing and angiography for those with induced ischaemia (ICTUS). Nevertheless, the pooled analysis of all intervention trials still shows a benefit with about 12% lower rates of death or myocardial infarction.
The benefits of intervention are related to the risk status of the patient. Long term outcomes in RITA 3 and FRISC 2 show most benefit in higher risk patients. A risk score can be applied (for example TIMI risk score of >3 points).
An integrated approach to the management of acute coronary syndrome
The risks of acute coronary syndrome with ST elevation are maximal in the minutes and hours after coronary occlusion. However, the risks in acute coronary syndrome without ST elevation are maximal in the first three months, but continue over the longer term (three to four years) as a result of further thrombotic events.
For all patients with acute coronary syndrome the maximum risk of coronary events occurs in the first three months after presentation and treatments are designed to minimise this risk. For patients with suspected myocardial infarction with ST elevation, emergency management requires access to resuscitation facilities and prompt reperfusion. Current evidence suggests that primary percutaneous coronary intervention is superior to thrombolysis provided that it can be delivered promptly and by expert hands.
The limited availability of interventional facilities in many countries, especially in rural areas, suggests that a substantial proportion of patients will need reperfusion with thrombolytic treatment, at least over the next decade. This can be administered in the prehospital setting (provided that adequate diagnostic and resuscitation facilities are available at the point of first patient contact), or at the latest should be administered immediately after arrival in the accident and emergency department.
Most benefit is gained from reperfusion within the first 1-2 hours after onset of symptoms.
Differences between lytic regimens are modest compared with the difference between providing and not providing any reperfusion (in around one third of eligible patients). Therefore, the urgent priority for acute coronary syndrome with ST elevation is to provide prompt and effective reperfusion for all those eligible. Substantial evidence demonstrates that this approach will reduce acute complications, heart failure, and death.
In the remainder of patients without ST elevation, the first priority is to establish a working diagnosis based on the clinical syndrome, ECG, and markers of necrosis. These patients can present a diagnostic challenge.
Pharmacological treatment aims to:
• Reduce ischaemia• Inhibit platelet aggregation
• Inhibit thrombin generation.
High risk patients need combination therapy with glycoprotein IIb/IIIa inhibitors and angiography with a view to percutaneous coronary intervention or surgical revascularisation. In lower risk patients, repeat ECGs or ST monitoring will detect evolving infarction and subsequent measurements of troponin elevation will reflect an increased risk. Current international guidelines recommend that all patients should have a risk score measured at presentation and again at about 24 hours. This is especially important in those not obviously at high risk using clinical parameters (for example ongoing ischaemia and ST depression).
In the presence of a low risk score, absence of evolving changes (for example ECG signs of ischaemia or elevation of troponin), the patient is at low thrombotic risk, but may have important underlying coronary disease. Elective stress testing is needed to determine whether stable but obstructive coronary artery disease is present.
Learning bite: the right ventricle
There are a number of anatomical and physiological properties of the right ventricle that differentiate it from the left ventricle:The right ventricle has only 15% of the muscle mass of the left ventricle, but has the same cardiac output. This reflects its role in perfusion of the low pressure pulmonary circulation
Coronary perfusion of the right ventricle occurs biphasically in both systole and diastole. Coronary perfusion of the left ventricle only occurs in diastole
The right ventricle has a lower coronary resistance than the left ventricle, with a tendency for left to right collateral vessel formation.
These differences allow the right ventricle to have a lower oxygen demand and better oxygen delivery, so it is more resilient to ischaemia than the left ventricle.