COPD: diagnosis and management of exacerbations
د. حسين محمد جمعةاختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2010
Key points
An exacerbation of chronic obstructive pulmonary disease is defined as an acute deterioration of respiratory symptoms that needs medical attention and may need a change in regular treatmentExacerbations are associated with increased mortality, an accelerated decline in lung function, and impaired quality of life
The diagnosis is confirmed by spirometry.
The presence of a postbronchodilator FEV1< 80% of the predicted value in combination with an FEV1/FVC < 70% confirms the presence of airflow limitation that is not fully reversible.There are three cardinal symptoms of an exacerbation
• Increased shortness of breath• Increased cough
• Increased sputum volume or purulence
You should treat exacerbations with antibiotics, bronchodilators, and corticosteroids (ABC)
Respiratory failure is frequently present during exacerbations and you may need to start non-invasive ventilation.
Introduction
Most patients with chronic obstructive pulmonary disease experience exacerbations during the course of their disease. As the disease becomes more severe, the frequency of exacerbations also increases.
In the UK, exacerbations of chronic obstructive pulmonary disease account for up to 10% of all medical admissions, equating to more than 100 000 admissions a year.
Mortality
The mortality rate for an exacerbation of chronic obstructive pulmonary disease is highIt is about 10% in exacerbations associated with retention of CO2 It is about 40% at one year in those needing ventilation.
Definition
Exacerbations are episodes of acute worsening of chronic obstructive pulmonary disease. This is the formal definition:"A worsening of the patient's condition, from the stable state and beyond day to day variations, that is acute in onset and necessitates a change in regular medication in a patient with underlying chronic obstructive pulmonary disease."
Symptoms
Exacerbations are usually defined by a change in a patient's baseline symptoms: there are three cardinal symptoms:Increased shortness of breath
Increased cough
Increased sputum volume or purulence.
To assess the need to treat exacerbations with antibiotics, Anthonisen et al grouped exacerbations into three types, based on these three symptoms:
Type 1: All of the three cardinal symptoms
Type 2: Two cardinal symptoms
Type 3: One cardinal symptom plus one of the following:
An upper respiratory tract infection in the past five days
Fever for which there is no other cause
Increased wheeze or cough
An increase in respiratory rate or heart rate by 20% compared to baseline readings.
But patients may also present with other symptoms such as:
Malaise
Worsening exercise tolerance
Fluid retention
Increased fatigue
Confusion.
Chest pain and fever are rare features of exacerbations and should prompt you to search for other causes, such as myocardial infarction or pneumonia.
Causes
Exacerbations of chronic obstructive pulmonary disease are thought to be caused by an interaction between the host, bacteria, viruses, and pollution.Published data suggest that:
Fifty to seventy per cent of exacerbations are due to respiratory infections
About 10% are due to pollution
In about 30% the cause cannot be identified.
Bacterial infections are thought to cause about 30% of infective exacerbations.
Bronchoscopic studies have found high concentrations of bacteria in the lower airways during severe exacerbations, in around 50% of patientThe organisms which are most commonly isolated include:
Haemophilus influenzae (11%)Streptococcus pneumoniae (10%)
Moraxella catarrhalis (10%)
Haemophilus parainfluenzae (10%)
Pseudomonas aeruginosa (10%).
But an important proportion of these patients also have bacteria colonising their lower airways in the stable phase of the disease. During exacerbations, it is thought that the bacterial burden may increase; and that the patient may acquire strains of the bacteria that are new to them.
Viral infections are thought to cause
20-40% of exacerbations. The most commonly isolated organisms are:Rhinovirus (23%)
Respiratory syncytial virus (11%)
Influenzae, parainfluenzae, adenovirus, and coronavirus (6%).
Making the diagnosis
The presence of cough, sputum, and shortness of breath with a reduction in breath sounds and wheezing usually point to a diagnosis of an exacerbation.Medical history
Ask about the three cardinal symptoms:
Increased shortness of breath
Increased cough
Increased sputum volume or purulence.
Look for the following factors, which are associated with increased severity of an exacerbation:
The magnitude of the reduction of the FEV1
Increased duration of, worsening of, or new symptoms
Number of previous exacerbations and admissions to hospital
Presence of other comorbid conditions
Low socioeconomic status.
Physical examination
A patient with an exacerbation of chronic obstructive pulmonary disease may have the following signs:
Upper airway symptoms (for example, colds and sore throats)
Increased wheeze
Chest tightness
Reduced exercise tolerance
Increased fatigue.
The following signs are features of a severe exacerbation:
Marked shortness of breathTachypnoea
Pursed lip breathing
Use of accessory muscles (sternomastoid and abdominal) at rest
Paradoxical chest wall motion
Acute confusion
cyanosis
New onset peripheral oedema
Haemodynamic instability
Right heart failure
Marked reduction in activities of daily living.
Differential diagnosis of an exacerbation
Ten to thirty per cent of patients with apparent exacerbations of chronic obstructive pulmonary disease do not respond to treatment.
In such patients, you need to look again for other medical conditions that can aggravate symptoms or mimic an exacerbation of chronic obstructive pulmonary disease. These include:
Pneumonia
Pneumothorax
Pleural effusion
Lung cancer
Upper airway obstruction
Rib fracture
Bronchiectasis
Pulmonary embolism
Congestive heart failure
Cardiac arrhythmia.
Learning bite
You may find it difficult to distinguish between a pulmonary embolism and an exacerbation. This is because in advanced chronic obstructive pulmonary disease, right ventricular hypertrophy and large pulmonary arteries are often present, which also support a diagnosis of pulmonary embolism. A low systolic blood pressure and an inability to increase the PaO2 above 8.0 kPa despite high flow oxygen suggest pulmonary embolism.If you suspect that a pulmonary embolism has occurred, you should treat for this as well as for an exacerbation, while you wait for the CT pulmonary angiogram.
Learning bite
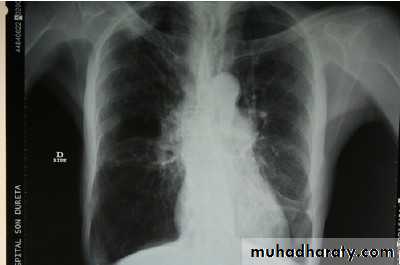
Lung function tests are not accurate during an exacerbation, so their routine use is not recommended.Chest x ray showing signs of hyperinflation
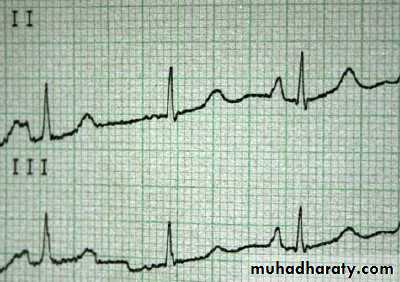
P pulmonale on ECG - indicative of right heart failure
ManagementPharmacological treatment
An ABC approach (Antibiotics, Bronchodilators, and Corticosteroids) is commonly used to treat exacerbations. But the level of scientific evidence for antibiotics is weaker than that for bronchodilators and corticosteroids.
Bronchodilators
First line treatmentThese include:
Short acting beta agonists such as salbutamol or terbutaline
Anticholinergic drugs such as ipratropium bromide.
They reduce symptoms and improve airflow obstruction.
The duration of action of both classes of bronchodilator is between four and six hours, and they are both generally well tolerated. Both bronchodilators may be given concomitantly. But there is minimal evidence to support any additional benefit of concomitant therapy.
A hand held metered dose inhaler with or without a spacer device and nebulisers are equally effective at delivering the bronchodilator. Since the patient does not need to make any effort with a nebuliser, it is often easier to use nebulisers for sicker patients.
In patients with hypercapnia, you should give nebulisers by compressed air.
Second line treatment
Methylxanthines such as aminophyllineThe use of aminophylline in treating exacerbations of chronic obstructive pulmonary disease is controversial, because of its modest benefits and the high incidence of side effects.
Current guidelines suggest adding intravenous aminophylline to standard therapy for patients with moderate to severe exacerbations or those not responding to nebulised bronchodilators.
In patients not taking an oral preparation of theophylline:
Arrange cardiac monitoringGive a loading dose of 5 mg per kg over at least 20 minutes
Follow with a subsequent maintenance infusion at 0.5 mg per kg per hour.
If a patient is already taking a theophylline, omit the loading dose of aminophylline and check plasma levels of theophylline before starting a maintenance infusion at 0.5 mg per kg per hour.
Measure daily plasma theophylline concentration and alter infusion rate to maintain a concentration of 10-20 mg per litre (55-110 µmol/l)
Systemic corticosteroids
Are beneficial in the management of exacerbations of chronic obstructive pulmonary disease. You should consider them in addition to bronchodilators if the patient's baseline FEV1is less than 50%. You can give them to patients at home and in hospital.Steroids:
Shorten recovery time
Improve FEV1
Improve hypoxaemia
May reduce the risk of early relapse, treatment failure, and length of hospital stay.
For a patient at home and in hospital, you should give 30 to 40 mg of oral prednisolone per day for seven to 10 days
You should give 100 to 200 mg of intravenous hydrocortisone or 0.5 mg per kg every 8 hours of methylprednisolone, initially, to severely ill patients or those who are unable to swallow.
You should not give corticosteroids for longer than two weeks as there is no advantage in prolonged therapy.
On discharge:
Give clear instructions to the patient about why, when, and how to stop their corticosteroidsMake the patient aware of the optimum duration of treatment of corticosteroids and the side effects of prolonged therapy - hyperglycaemia, muscle atrophy, osteoporosis, and weight gain.
You should consider giving osteoporosis prophylaxis in patients requiring frequent courses of oral corticosteroids.
Antibiotics
results in a lower risk of treatment failure, mortality, and sputum purulence, in moderate to severe exacerbations.NICE recommends using antibiotics to treat exacerbations associated with a history of more purulent sputum.
The global initiative for chronic obstructive lung disease (GOLD) gives more specific recommendations: that you should give antibiotics to patients with chronic obstructive pulmonary disease:
With three major symptoms (increased shortness of breath, sputum volume, and sputum purulence)
With two major symptoms provided increased sputum purulence is present
Who are critically ill and need ventilatory support.
The oral route is preferred and is cheaper. You should continue antibiotics for
three to 12 days.You should choose empirical antibiotics based on the patterns of local resistance and clinical severity
Group A
Patients with a mild exacerbation of chronic obstructive pulmonary disease who do not have risk factors for a poor outcome. These patients can usually be managed at home.
Group B
Patients admitted to hospital with moderate chronic obstructive pulmonary disease who do not have risk factors for P aeruginosa but have at least one of the following risk factors for a poor outcome:• The presence of a comorbid condition
• Severe chronic obstructive pulmonary disease
• Frequent exacerbations - more than three per year
• Use of antimicrobials within the last three months.
Group C
Patients admitted to hospital with severe chronic obstructive pulmonary disease who have risk factors forP aeruginosa infection:Recent inpatient stay
Frequent courses of antibiotics (≥4 courses in the last year)
Severe exacerbations of chronic obstructive pulmonary disease or bronchial colonisation during a stable period.
Antibiotics for different groups
Group ALikely micro-organisms: Haemophillus influenzae, Streptococcus pneumoniae, M catarrhalis, Chlamydia pneumoniae, viruses
Oral treatment
A beta lactam such as amoxicillin or
A tetracycline
Alternative treatment
A beta lactam with a beta lactamase inhibitor such as co-amoxiclav or
A macrolide such as azithromycin or clarithromycin
Group B
Likely micro-organisms: As for Group A plus the presence of resistant micro-organisms - Beta lactamase producing, penicillin resistant Streptococcus pneumoniae or Enterobacteria such as K pneumoniae, E coli, Proteus.
Oral treatment
A beta lactam with a beta lactamase inhibitor such as co-amoxiclavAlternative oral treatment
A fluoroquinolone such as levofloxacin
Parenteral treatment
A beta lactam with a beta lactamase inhibitor such as co-amoxiclav
A fluoroquinolone such as levofloxacin
Learning bite
Penicillin resistant Streptococcus pneumoniae is unusual in the UK (<1% of isolates), but much higher in certain European countries. If the patient has travelled recently, this would make the likelihood of penicillin resistant S pneumoniae higher.Group C
Likely micro-organisms: As for group B plus P aeruginosaOral treatment
A fluoroquinolone such as ciprofloxacin or high dose levofloxacin
Parenteral treatment
A fluoroquinolone such as ciprofloxacin or high dose levofloxacin
A beta lactam with P aeruginosa activity, such as piperacillin with tazobactam
Learning bite
Levofloxacin, piperacillin with tazobactam, co-amoxiclav, and cephalosporins are commonly associated with Clostridium difficile associated diarrhoea, so you should prescribe them with caution.
Non-pharmacological treatment
This consists of:• Controlled oxygen therapy
• Ventilatory support
• Non-invasive ventilation
• Invasive ventilation.
Controlled oxygen therapy
Is of beneficial value in acute respiratory failure during an exacerbation of chronic obstructive pulmonary disease. It improves PaO2 with small increases in PaCO2 and relieves shortness of breath.During an exacerbation of chronic obstructive pulmonary disease, acute or acute on chronic respiratory failure can be defined as a clinical condition characterised by decreased
PaO2 (<8 kPa) with or without increased PaCO2 (>6.0 kPa).
You should give oxygen through a venturi facemask, to maintain the saturation of arterial blood at more than 90%.
The port size of the venturi valve ensures that the correct proportions of oxygen and entrained air are mixed to obtain a fixed oxygen concentration.
Low flow devices, such as nasal cannulae, are not able to deliver a fixed oxygen concentration as they are dependent on a patient's respiratory rate and tidal volume. They deliver a variable inspired oxygen concentration, which can result in suppression of respiratory drive, carbon dioxide narcosis, and respiratory arrest.
In hypercapnic respiratory failure, you should start at an oxygen concentration of 24%.
You should aim to increase the PaO2 sufficiently to maintain optimal levels above 8.0 kPa without risking detrimental carbon dioxide retention and acidosis.
After giving oxygen for 30 to 60 minutes, you should recheck arterial blood gases, especially in patients with hypercapnic respiratory failure. This will allow you to detect whether loss of hypoxic drive has led to a further rise in PaCO2 and a respiratory acidosis.
Ventilatory support
The aims of ventilatory support in patients with exacerbations of chronic obstructive pulmonary disease who are in acute respiratory failure are:• To decrease mortality and morbidity
• To relieve symptoms.
Ventilatory support includes non-invasive and invasive mechanical ventilation.
Non-invasive ventilation
A cost effective intervention. You should use it where hypercapnic respiratory failure persists despite giving a patient optimal medical therapy and treatment with controlled oxygen.It has been shown to:
• Improve respiratory acidosis - increasing pH and decreasing PaCO2
• Reduce the respiratory rate
• Decrease mortality
• Decrease the need for intubation
• Reduce the length of hospital stay.
The indications for non-invasive ventilation are:
• Moderate to severe dyspnoea with use of accessory muscles and paradoxical abdominal motion• Moderate to severe acidosis (pH ≤7.35) and/or hypercapnia (PaCO2 >6.0 kPa)
• Respiratory rate of more than 25 breaths per minute.
Exclusion criteria
Relative contraindications:
• Extreme obesity
• Cardiovascular instability - hypotension, arrhythmias, myocardial infarction
• Change in mental status; uncooperative patient
• High risk of aspiration
• Viscous or copious secretions.
Absolute contraindications:
• Recent facial or gastro-oesophageal surgery• Craniofacial trauma
• Fixed nasopharyngeal abnormalities
• Burns
• Respiratory arrest.
Noninvasive ventilation (NIV), a form of ventilatory support that avoids airway invasion, has seen increasing use in emergency departments and intensive care units (ICUs) in recent years, based on the results of clinical trials showing improved outcomes in certain types of acute respiratory failure (ARF).[1] NIV usually refers to the provision of inspiratory pressure support plus positive end-expiratory pressure (PEEP) via a nasal or face mask.
Although continuous positive airway pressure (CPAP) does not actively assist inspiration and is not a ventilatory support mode, it is considered a form of NIV here when used as a therapy for ARF. The successful application of NIV requires the training and collaboration of an experienced ICU team, including intensivists, nurses, and respiratory therapists.
Strong evidence from randomized trials supports the use of NIV in the management of ARF to prevent endotracheal intubation in patients with COPD exacerbations or acute cardiogenic pulmonary edema, and in immunocompromised patients, as well as to facilitate extubation in patients with COPD. NIV should be contemplated in patients with postoperative respiratory failure or at high risk for postextubation respiratory failure who are otherwise good candidates for NIV, and as a means of preoxygenating critically ill patients with hypoxemia before intubation.
Non-invasive ventilation (Non-invasive Positive Pressure Ventilation or NIPPV)
This refers to all modalities that assist ventilation without the use of an endotracheal tube. Non-invasive ventilation is primarily aimed at minimizing patient discomfort and the complications associated with invasive ventilation. It is often used in cardiac disease, exacerbations of chronic pulmonary disease, sleep apnea, and neuromuscular diseases. Non-invasive ventilation refers only to the patient interface and not the mode of ventilation used; modes may include spontaneous or control modes and may be either pressure or volume modes.
Some commonly used modes of NIPPV include:
Continuous positive airway pressure (CPAP).Bi-level Positive Airway Pressure (BIPAP). Pressures alternate between Inspiratory Positive Airway Pressure (IPAP) and a lower Expiratory Positive Airway Pressure (EPAP), triggered by patient effort. On many such devices, backup rates may be set, which deliver IPAP pressures even if patients fail to initiate a breath.(Wheatley 2000 et all)
Intermittent positive pressure ventilation (IPPV) via mouthpiece or mask
Biphasic Cuirass Ventilation A form of non-invasive ventilation that uses a cuirass instead of a facemask. Allows active control of both inspiration and exhalation.
Noninvasive mechanical ventilation.
This modality of ventilatory support is applied when endotracheal andnasotracheal ventilation are not needed, using either negative pressure ventilation (nPV) or noninvasiveintermittent positive pressure ventilation (NIPPV).Noninvasive negative pressure ventilation (nPV).The useof tank respirators, cuirass, or poncho ventilation is largelyof historical interest in COPD.Problems with patientcomfort and limited access restrict future use of nPV.When this treatment is used in chronic respiratory failure,some patients develop upper airway obstruction during sleep .
A comparison of domiciliary active and shamnPV in patients with chronic respiratory failure due toCOPD showed no differences in shortness of breath,exercise tolerance, arterial blood gases, respiratorymuscle strength, or quality of life between the twotreatments
The role of NIPPV in chronic respiratory failure remains unsettled, although this is now the standard means of providing noninvasive ventilatory support inother instances of chronic respiratory failure not directlyrelated to COPD.NIPPV can be delivered by different types of ventilators: volume-controlled,
pressure-controlled,bilevel positive airway pressure, or continuous positive airway pressure.
Recent technical improvements havefacilitated the use of NIPPV while reducing the possibilityof air leaking through face or nasal masks.
A study of NIPPV compared to conventional therapy in apopulation with end-stage COPD using a randomized,crossover design for a 3-month period found that the noninvasive approach is not well tolerated and is associated with marginal clinical and functional improvements
Evidence B
Although preliminary studies have suggested that combining NIPPV withlong-term oxygen therapy could be beneficial on certainoutcome variables, based on a 12-month study and a24-month study in stable COPD patients with chronicrespiratory failure, its widespread use cannot beadvocated as yet .However, compared with long-termoxygen therapy alone, the addition of NIPPV has someeffect on carbon dioxide retention and improvedshortness of breat
Arterial blood gases:
In the hospital, measurement of arterial blood gases is essential to assess the severity of an exacerbation.
A PaO2< 8.0 kPa (60 mm Hg) and/or SaO2< 90% with or without PaCO2> 6.7 kPa, (50 mm Hg)when breathing room air indicate respiratory failure.
Inaddition, a PaO2< 6.7 kPa (50 mm Hg), PaCO2> 9.3kPa (70 mm Hg), and pH < 7.30 point toward a life-threat-ening episode that needs critical management.
NIV can be considered in patients with asthma exacerbations, pneumonia, and ALI/ARDS, although the supporting evidence is fairly weak; these and other acutely ill patients should be monitored closely for signs of NIV failure until stabilized. If there are signs of NIV failure, patients should be intubated promptly before a crisis develops. The application of NIV by a trained and experienced ICU team, with careful patient selection, should optimize patient outcomes.
Invasive ventilation
Relatively good survival is achieved with mechanical ventilation, with survival rates of 50-89% and better prognosis if:The patient did not have any other previous comorbid condition
Respiratory failure was due to a potentially reversible cause such as infection
The patient was relatively mobile and not using long term oxygen therapy.
The indications for mechanical ventilation are:
Inability to tolerate, or failure to improve on, or contraindications to non-invasive ventilation• Severe shortness of breath with use of accessory muscles and paradoxical abdominal motion
• Respiratory rate of more than 35 breaths per minute
• Life threatening hypoxaemia
• Severe acidosis (pH <7.25) and/or hypercapnia (PaCO2 >8.0 kPa)
• Respiratory arrest
• Somnolence, impaired mental status
• Cardiovascular complications such as hypotension or shock.
Other management
You should also remember to:
• Monitor fluid balance
• Consider low molecular weight heparin to prevent venous thromboembolism
• Give nutritional support if necessary
• Treat any comorbid condition.
Discharge from hospital
There are insufficient data to establish the optimal duration of stay for a patient with an exacerbation of chronic obstructive pulmonary disease. You can use the following discharge criteria for patients with exacerbations of chronic obstructive pulmonary disease:• Inhaled beta2 agonist therapy is required less than every four hours
• The patient, if previously ambulatory, is able to walk across the room• The patient is able to eat and sleep without frequently being woken by shortness of breath
• The patient has been clinically stable for 12-24 hrs
• The patient or home caregiver understands how to use the medications correctly
• Follow up and home care arrangements have been completed, for example visiting nurse and oxygen delivery
• The patient, family, and physician are confident that the patient can manage successfully at home.
• You should arrange a follow up assessment four to six weeks after discharge.
discharge criteria
Hospital at home and supported discharge services
In recent years, assisted hospital discharge schemes have been developed where selected patients with non acidotic exacerbations of chronic obstructive pulmonary disease can be discharged almost immediately with appropriate nursing and medical backup.These patients have similar rates of hospital readmission and mortality to those receiving standard inpatient care and such schemes save money and increase the availability of inpatient beds.
But, if a patient has an altered mental state or confusion, acute changes on ECG or chest x ray, serious comorbid disease, or poor social conditions, you should not consider them for supported discharge.
You should start non invasive ventilation only if the hypercapnic respiratory failure persists despite optimal medical therapy and treatment with controlled oxygen.
Paradoxical chest wall motion is a sign of severe lung hyperinflation due to air trapping that may occur during severe exacerbations.
Haemoptysis is not one of the three cardinal symptoms of an exacerbation of chronic obstructive pulmonary disease.
Adding the anticholinergic agent tiotropium bromide, approved for the treatment of (COPD), to low-dose, inhaled glucocorticoid therapy is more effective at improving symptoms and lung function than doubling the corticosteroid dose in controlling asthma.
Also, the addition of tiotropium is as effective as the addition of the long-acting β-agonist (LABA) salmeterol to an inhaled corticosteroid, according to study results released by the US National Heart, Lung, and Blood Institute (NHLBI).
European Respiratory Society (ERS) 2010 Annual Congress
September 19, 2010. N Engl J Med
Although there was initially concern about cardiovascular risks with all anticholinergics, safety trials demonstrated that there is no increased cardiovascular risk associated with tiotropium.
However, data indicate that ipratropium is associated with a slight risk for excess cardiovascular mortality.
Both Spiriva (Tiotropium) and Ipratropium (Atrovent) are anticholinergic agents that are used for maintenance treatment of bronchospasm associated with COPD .Spiriva has an additional indication for reducing COPD exacerbations based on the outcomes of the large UPLIFT clinical trial. Both medications are not indicated for acute treatment of a bronchospasm and should never be used as rescue medications. The major difference between the two drugs is that Spiriva is a long acting medication and Ipratropium is a short acting drug. Spiriva is used once daily, while Ipratropium may be used every 4 to 6 hours.
In general Ipratropium is used as-needed for persistent or worsening symptoms. When regular use is needed, long-acting bronchodilator like Spiriva may be more effective and convenient than short-acting agent. Both medications share a similar adverse effect profiles. As anticholinergic drugs they may increase the risk of dry mouth and urinary retention. Both medications have a warning regarding the possible increase in intra-ocular pressure and increased risk of narrow angle glaucoma. It is recommended to avoid the contact between these medications and the eyes which may be harder to do with a nebulizer treatment.
Recently, some clinical studies raised the concern regarding he use of Ipratropium (Atrovent) and Spiriva (Tiotropium) and an increased risk of negative cardiovascular outcomes. In the case of Spiriva a large 4 year study (UPLIFT) conducted in 490 investigational centers in 37 countries did not show an increased risk of negative cardiovascular outcomes. There is some preliminary evidence that Ipratropium may increase risk of adverse cardiovascular effects, but more research with well designed clinical studies is needed to come to any definite conclusion.
Ipratropium bromide and tiotropium bromide are structural analogues of atropine which have minimal systemic absorption following inhalation because of their quaternary ammonium structure. These anticholinergic drugs are useful bronchodilators in chronic obstructive pulmonary disease. They are rarely indicated in asthma. Bronchodilators provide symptomatic relief and improve health-related quality of life in patients with chronic obstructive pulmonary disease, but they do not influence the decline in lung function. The only measure currently known to halt this decline is stopping cigarette smoking.
Animal studies show that cholinergic innervation is greatest in larger airways and diminishes peripherally. Studies in humans have shown that cholinergic bronchoconstriction occurs mainly in larger airways whereas bronchodilatation induced by beta adrenergic drugs occurs in both large and small airways. The resting bronchomotor tone in normal airways has a cholinergic component, because giving an anticholinergic drug such as atropine causes bronchodilatation while the inhalation of edrophonium, an acetylcholinesterase inhibitor, results in bronchoconstriction.
Muscarinic receptors
The effects of vagal stimulation in the lung are mediated via muscarinic receptors. mediate the mucus secretory response. Cholinergic agonists will stimulate mucus secretion from both submucosal glands and from goblet cells within the epithelium. These goblet cells are a major source of mucus in peripheral airways.The muscarinic receptors on airway smooth muscle belong to the M3 subtype and the presynaptic muscarinic receptors on vagal motor nerve fibres belong to the M2 subtype. These M2 receptors are called autoreceptors because their activation by acetylcholine inhibits further release of acetylcholine from the nerve terminals.
Anticholinergic bronchodilators
AtropineGiving atropine, either systemically or as a nebulised solution, results in bronchodilatation. Inhaled doses of 2.5 mg atropine are associated with adverse effects such as dryness of the mouth, tachycardia, palpitations and blurred vision. With higher inhaled doses, systemic absorption can result in urinary retention (particularly in the elderly), headache and changes in mental status. Atropine is therefore no longer given as a nebulised solution.
Ipratropium bromide is a structural analogue of atropine, with a quaternary nitrogen structure. This structure reduces the ability of the molecule to cross cell membranes. There is, therefore, less systemic absorption with nebulised ipratropium than with nebulised atropine. Ipratropium blocks methacholine-induced bronchoconstriction, and induces bronchodilatation in patients with asthma and patients with (COPD). There are no measurable effects on sputum volume, sputum viscosity or mucociliary clearance with clinically recommended doses of ipratropium.
The maximal bronchodilatation with ipratropium, inhaled from a metered-dose inhaler, occurs with a dose of 40-80 microgram. Although some bronchodilatation is evident soon after inhalation the maximal response occurs 1.5-2.0 hours afterwards. The duration of significant bronchodilatation after a standard dose of ipratropium is 4-6 hours.
Ipratropium cannot be detected in the blood after an inhalation.. Long-term studies have shown no evidence of diminished responsiveness (tachyphylaxis) with regular therapy.
The main adverse effects of ipratropium relate to its anticholinergic activity. Up to 15% of patients will report transient dryness of the mouth and 'scratchiness' in the throat. In some studies up to 30% of patients have reported a bitter taste. These adverse effects rarely lead to patients discontinuing the drug if they perceive that it is helping them. Cardiovascular effects (tachycardia and increased cardiac output), are not seen with the usual doses of ipratropium.
The main clinical indication for ipratropium bromide is the symptomatic relief of breathlessness in patients with COPD. It is rarely required for the treatment of patients with asthma because proper treatment of asthmatic patients with inhaled corticosteroids and long-acting beta agonists provides good control for the majority of patients. The extent of bronchodilatation with ipratropium in patients with COPD is similar to that achieved with inhaled beta agonists. The choice between ipratropium and beta agonists for a patient with COPD is determined by the patient's tolerance of the drug, rather than its efficacy.
Tiotropium bromide is a structural analogue of ipratropium. In vitro studies have shown that tiotropium has a half-life on the M3 receptor of approximately 36 hours, whereas the receptor binding half-life of ipratropium is three hours. The duration of this binding to M3 receptors may explain why a single inhaled dose of tiotropium results in bronchodilatation which lasts for approximately 24 hours. Large-scale clinical trials have shown that tiotropium inhaled once daily increases the forced expiratory volume (FEV1)and quality of life in patients with COPD.
In comparative studies patients took tiotropium once daily, or ipratropium four times daily, for one year. Both drugs improved quality of life, but tiotropium resulted in a higher FEV1 at the end of the dose interval. Tiotropium also lengthened the time to first exacerbation and the time to first hospital admission due to an exacerbation of COPD. The number of patients who need to be treated with tiotropium for one year to prevent one exacerbation is nine, and 23 need to be treated to prevent one admission due to COPD.
There are no long-term studies of tiotropium in asthma so it is not indicated for patients with asthma.
Combination therapy
Anticholinergic drugs and beta adrenoceptor agonists produce bronchodilatation via separate mechanisms so there are theoretical reasons why they may be used in combination. Several studies have shown that the combination of ipratropium with a beta adrenoceptor agonist (either fenoterol or salbutamol) produces greater bronchodilatation than either drug alone.2 None of these studies has investigated whether a higher dose of the single drug(either ipratropium or beta agonist) would have achieved the same result as the combination. However, a higher dose of either drug would carry with it the greater risk of unwanted adverse effects.Both beta agonists and ipratropium are therefore frequently used in combination to treat inpatients with acute exacerbations of COPD. There is also a place for the combination of beta agonists and ipratropium in maintenance therapy for COPD, primarily to minimise the risk of adverse effects with higher doses of either ipratropium or the beta agonist.
Spiriva (tiotropium) is a new anticholinergic inhalation product approved solely for the treatment of chronic obstructive pulmonary disease (COPD). Its efficacy is only marginally better than that of ipratropium (Atrovent). Unlike Atrovent, Spiriva is only administered once daily. Treatment with Spiriva, as with all other drugs for the treatment of COPD, is symptomatic treatment only, and its clinical efficacy is often not very high. As is the case with so many other illnesses, cessation of smoking is the most efficacious treatment.
Spiriva (tiotropium) is a new anticholinergic product marketed in Denmark since 24 June 2002. Spiriva is indicated solely for the maintenance of COPD. Administration is once a day; Atrovent is taken four times daily. Spiriva is marketed as an inhalation powder only. The only efficacious therapy for COPD is if the patient stops smoking, so medical treatment of COPD is symptomatic only. Possible therapy choices are short- and long-acting B2 agonists, theophyllin and anticholinergics, alone or in combination with a B2 agonist. Prophylactic treatment with inhalation steroids can be used for patients with severely reduced pulmonary function (FEV1<1.4), although efficacy is also limited in this case.
Ipratropium blocks muscarinic acetylcholine receptors, without specificity for subtypes, resulting in an increase in the formation of cyclic guanosine monophosphate (cGMP). Most likely due to actions of cGMP on intracellular calcium, this results in decreased contractility of smooth muscle in the lung, inhibiting bronchoconstriction and mucus secretion. It is a nonselective muscarinicantagonist, and does not diffuse into the blood, which prevents systemic side effects. Ipratropium is a derivative of atropine[4] but is a quaternary amine and therefore does not cross the blood-brain barrier, which prevents central side effects (anticholinergic syndrome). Ipratropium is considered a short-acting bronchodilator
Tiotropium bromide (INN) is a long-acting, 24 hour, anticholinergicbronchodilator used in the management of chronic obstructive pulmonary disease (COPD). Tiotropium is used for maintenance treatment of chronic obstructive pulmonary disease (COPD) which includes chronic bronchitis and emphysema.[1] It is not however used for acute exacerbations.[