Difficult cardiology scenarios for general medical registrars on call
د. حسين محمد جمعةاختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2010
You are a medical registrar working in a district general hospital. You are starting your first of three nights on call. The nearest specialist cardiology centre is over three hours' drive away.You see a 58 year old man in the emergency department. He has a two hour history of retrosternal chest pain and dyspnoea. The pain began while he was lifting heavy objects.He is an ex-smoker with a past medical history of hypertension and hypercholesterolaemia.
His current medications are aspirin, lisinopril, and simvastatin.
On examination his heart rate is 85 beats per minute and regular, his blood pressure is 110/70 mm Hg in both arms, and his oxygen saturation is 96% on air. His chest is clear. There are normal heart sounds and no added murmurs. There is no peripheral oedema.
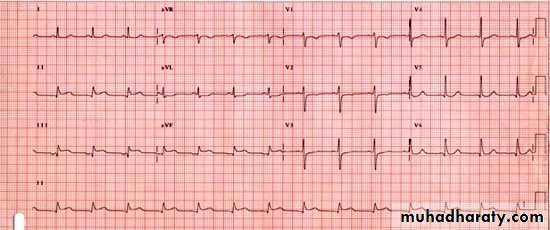
• Figure 1: The initial 12 lead ECG
•Figure 1: The initial 12 lead ECG
What should you do next?Your answer
Correct answer
a.
Transfer this patient urgently to a tertiary centre for primary percutaneous coronary intervention
b.
Thrombolyse the patient with a fibrin specific agent
c.
Treat the patient with intravenous morphine, furosemide, and intravenous glyceryl trinitrate
a : Transfer this patient urgently to a tertiary centre for primary percutaneous coronary intervention
The ECG on admission shows ST elevation in leads II, III, and aVF. This is consistent with an inferior myocardial infarction. Current guidelines recommend primary percutaneous coronary intervention for patients with ST elevation myocardial infarction, but only if the time from initial medical contact to balloon inflation is less than 90 minutes. In this patient's situation, given that the tertiary centre is over three hours' drive away, this is not feasible.
b : Thrombolyse the patient with a fibrin specific agent
For cases of ST elevation myocardial infarction where primary percutaneous coronary intervention is not possible within the recommended time, current guidelines recommend thrombolysis with a fibrin specific agent (alteplase, reteplase, or tenecteplase).c : Treat the patient with intravenous morphine, furosemide, and intravenous glyceryl trinitrate
This is the usual management of acute left ventricular failure. The history and examination are not suggestive of this diagnosis.
Learning bite: the right ventricle
There are a number of anatomical and physiological properties of the right ventricle that differentiate it from the left ventricle:The right ventricle has only 15% of the muscle mass of the left ventricle, but has the same cardiac output. This reflects its role in perfusion of the low pressure pulmonary circulation
Coronary perfusion of the right ventricle occurs biphasically in both systole and diastole. Coronary perfusion of the left ventricle only occurs in diastole
The right ventricle has a lower coronary resistance than the left ventricle, with a tendency for left to right collateral vessel formation.
These differences allow the right ventricle to have a lower oxygen demand and better oxygen delivery, so it is more resilient to ischaemia than the left ventricle.
Learning bite: right ventricular infarction
Is important to recognise because it complicates up to 50% of episodes of inferior myocardial infarction.
Only 10% result in haemodynamic compromise. Patients with right ventricular infarction in addition to inferior myocardial infarction also have a higher rate of morbidity and mortality in hospital (30%) than patients with isolated inferior myocardial infarction (6%) .
The right coronary artery supplies blood to most of the right ventricle, except the anterior wall, which has a dual blood supply from the right and left coronary arteries
Most right ventricular infarctions result from occlusion of the proximal right coronary artery, although in patients with a dominant left sided circulation, occlusion of a left circumflex artery can have the same effect .
Clinical signs
In patients with inferior myocardial infarction the following triad has a high specificity, but a low sensitivity, for the diagnosis of right ventricular infarction:• Raised jugular venous pressure
• Clear lungs
• Hypotension
These signs have a low sensitivity for the diagnosis of right ventricular infarction because conditions such as cardiac tamponade, constrictive pericarditis, and pulmonary embolus share similar clinical features.
You must assess the patient’s hydration status as volume depletion may mask these signs. The key physical signs may only become apparent after volume repletion
Kussmaul’s sign (distension of the jugular vein on inspiration) is an important predictor of right ventricular infarction. It is both highly specific and sensitive.
Learning bite: right sided ECG
You should include a right sided ECG as part of the routine evaluation of all patients with an inferior myocardial infarction, as this will confirm any clinical suspicion of right ventricular ischaemiaST elevation in the right sided leads V3R to V6R has a high sensitivity, specificity, and predictive value for right ventricular infarction.
From the results of numerous studies, a 1 mm ST segment elevation in V4R has been shown to have the highest sensitivity and specificity for right ventricular infarction .
Clinical tip: right precordial chest leads
The sites for the right sided precordial leads are the mirror image of the standard left sided leads
In the fifth right intercostal space, lead V4R is at the mid-clavicular line, lead V5R is at the anterior axillary line, and lead V6R is at the midaxillary line.
Learning bite: echocardiography
extremely useful non-invasive tool to aid you in the diagnosis of right ventricular infarction. The key abnormal echocardiographic features are:• Right ventricular dilation
• Asynchrony of the right ventricular free wall
• Paradoxical movement of the interventricular septum.
• The short axis views have the highest sensitivity (82%) and specificity, ranging from 62% to 93% for haemodynamically significant right ventricular impairment.
• Echocardiography can also rule out pericardial conditions and cardiac tamponade, which are the main differential diagnoses of right ventricular infarction.
• Learning bite: management of right ventricular infarct
• A right ventricular infarct can make the ventricle non-compliant, with subsequent poor contractility. This reduces left ventricular preload and results in poor cardiac output• Initial management should focus on:
• Reperfusion by thrombolysis or primary percutaneous coronary intervention
• Improving blood flow from the right ventricle to the left ventricle
• If, after reperfusion measures, there is hypotension, then use judicious fluid loading to increase right ventricular filling pressure. This should improve left ventricular preload, and therefore cardiac output and systemic blood pressure.
If hypotension persists despite optimising right ventricular filling pressure, then you need to use inotropic support with dobutamine to enhance contraction of the right ventricle, or rescue angioplasty (if available)
Avoid certain drugs that, although used commonly in left ventricular infarction, are poorly tolerated in right ventricular infarction. These include morphine, diuretics, and nitrates; all of which further exacerbate hypotension, as they all reduce right ventricular preload .
Occlusion of the right coronary artery can also cause rate and rhythm disturbance. Common disturbances include:
• Sinus bradycardia - treat with atropine
• Second or third degree heart block - the recommended treatment is transvenous atrioventricular sequential pacing
• Atrial fibrillation - this is best managed with early synchronised direct current cardioversion
Concomitant systolic dysfunction of the left ventricle, when significant, can further compromise right ventricular function by increasing right ventricular afterload. This might happen if the infarct area is large, such as in an inferolateral myocardial infarction that affects a large part of the left ventricle. In this case, it may be worth cautiously trying drugs that reduce afterload, such as sodium nitroprusside and angiotensin converting enzyme inhibitors. This should only be done in patients who are not hypotensive and who do not have early signs of cardiogenic shock (for example, tachycardia).
Intra-aortic counterpulsation devices are also useful in this setting to unload both ventricles and augment coronary perfusion.
In patients with refractory hypotension (cardiogenic shock), you should consider early angioplasty or coronary artery bypass grafting, depending on the extent of coronary disease at angiography, although data in this group of patients are limited.
Certain drugs that are used commonly in left ventricular infarctions are poorly tolerated by patients with right ventricular infarction since they exacerbate hypotension. You should therefore avoid morphine, diuretics (for example, frusemide), and nitrates; all of which exacerbate hypotension.
Learning bite: atrial fibrillation in Wolff-Parkinson-White syndrome
Atrial fibrillation is the second most common arrhythmia in Wolff-Parkinson-White syndrome, occurring in approximately a third of patients ((The most common tachycardia is the atrioventricular re-entrant tachycardia)). Atrial activity predominantly conducts down the accessory pathway, causing ventricular pre-excitation. The ECG will show an irregular rhythm and broad QRS complexes, with delta waves. Some atrial impulses will conduct down the standard pathway intermittently and produce normal QRS complexes. When atrial fibrillation develops in patients with Wolff-Parkinson-White syndrome, there is a small risk of degeneration into ventricular fibrillation and sudden death. If the refractory period of the accessory pathway is short, rapid antegrade conduction can occur to the ventricles. This increases the risk of life threatening arrhythmias, such as ventricular fibrillation.It is preferable to use drugs that reduce conduction through the accessory pathway, such as flecainide, procainamide, and amiodarone, in patients with atrial fibrillation associated with Wolff-Parkinson-White syndrome.
Both verapamil and digoxin are atrioventricular nodal blocking drugs. All atrioventricular nodal blocking drugs are contraindicated in patients with Wolff-Parkinson-White syndrome who are in atrial fibrillation. These drugs will accelerate conduction of the atrial impulses down the accessory pathway, resulting in a fast ventricular response. This may sometimes degenerate into ventricular fibrillation.
Both verapamil and digoxin are atrioventricular nodal blocking drugs. All atrioventricular nodal blocking drugs are contraindicated in patients with Wolff-Parkinson-White syndrome who are in atrial fibrillation. These drugs will accelerate conduction of the atrial impulses down the accessory pathway, resulting in a fast ventricular response. This may sometimes degenerate into ventricular fibrillation.
atrioventricular nodal blocking will accelerate conduction of the atrial impulses down
the accessory pathwayAdelta wave on the QRS complex precludes the diagnosis of Lown-Ganong-Levine syndrome. Lown-Ganong-Levine syndrome is a rare pre-excitation syndrome. It is characterised by a short PR interval (≤120 ms), a normal QRS complex and duration, and the occurrence of supraventricular tachycardia but not atrial fibrillation or atrial flutter. No single structural abnormality has been identified to directly explain the pathophysiology behind Lown-Ganong-Levine syndrome.
This ECG shows a Wolff-Parkinson-White syndrome type A pathways left sided accessory pathway with a short PR interval, positive delta wave, and QRS complex in lead V1, denoting a left sided pathway.
Wolff-Parkinson-White type B pathways have a short PR interval, negative delta wave, and QRS complex in lead V1, denoting a right sided pathway. This patient has a positive delta wave.
Learning bite: Wolff-Parkinson-White syndrome
congenital malformation that results in incomplete separation of the atria from the ventricles during fetal maturation. In Western societies its prevalence is 3 per 1000 of the population. It is characterised by the presence of an accessory pathway between the atria and the ventricles. This pathway provides an alternative route for an atrial impulse to reach, and prematurely depolarise, the ventricular myocardium, hence the term ventricular pre-excitation. Multiple accessory pathways occur in 10% of episodes. The accessory pathways provide the substrate for a re-entrant circuit, allowing the occurrence of paroxysmal supraventricular tachycardia.Definition of Wolff-Parkinson-White syndrome
Patients with Wolff-Parkinson-White syndrome present with paroxysmal supraventricular tachycardia, and an ECG will show the following features:• Short PR interval (<0.12 s)
• Slurred upstroke of QRS complex (delta wave)
• Prolonged QRS complex (>0.12 s).
ECG features of Wolff-Parkinson-White syndrome
In the normal heart, the atrioventricular node slows down electrical depolarisation between the atria and ventricles. This protects the ventricles from fast atrial activity. In patients with Wolff-Parkinson-White syndrome there is accelerated conduction of atrial impulses in sinus rhythm to the non-specialised ventricular myocardium via the accessory pathway. This results in a short PR interval, as it bypasses the slowing effect of the atrioventricular node.Depolarisation of the non-specialised myocardium progresses slowly and causes an initial slurring of the QRS complex. This is known as the “delta wave.” Simultaneous depolarisation via the normal conduction system catches up and normalises the remainder of the QRS complex
Not all patients with Wolff-Parkinson-White exhibit these characteristic ECG features. Some accessory pathways only conduct retrogradely from the ventricles to the atria. In these patients, the resting ECG does not have features of pre-excitation and appears normal. This is termed a “concealed pathway”
• Tachycardias in Wolff-Parkinson-White syndrome
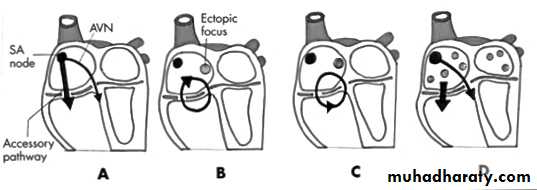
• Figure 8: Diagrammatic representation showing the mechanisms producing tachycardia in Wolff-Parkinson-White syndrome22•
Tachycardias in Wolff-Parkinson-White syndrome
Figure 8: Diagrammatic representation showing the mechanisms producing tachycardia in Wolff-Parkinson-White syndrome
(A) A physiological mechanism of conduction in Wolff-Parkinson-White syndrome with accessory pathway conduction (atrioventricular) resulting in the delta wave
(B) Orthodromic conduction in Wolff-Parkinson-White syndrome occurring down the atrioventricular node and retrogradely up the accessory pathway
(C) Antidromic conduction in Wolff-Parkinson-White syndrome, where the accessory pathway conducts anterogradely (atrioventricular) and the impulse returns to the atria via the atrioventricular node
(D) Mechanism of atrial fibrillation in Wolff-Parkinson-White syndrome
The most common tachycardia in Wolff-Parkinson-White syndrome is the atrioventricular re-entrant tachycardia, which uses the accessory pathway and atrioventricular node as a re-entry circuit. They can be either orthodromic or antidromic:
In orthodromic tachycardia, an atrial ectopic triggers the tachycardia with conduction antegradely down the atrioventricular node to the ventricles, and then retrogradely back up the accessory pathway to the atria. This results in a circus movement, or circular movement, between the atria and ventricles. The QRS complex is narrow, without a delta wave, as ventricular depolarisation has occurred down the normal conduction pathway. Orthodromic tachycardia accounts for most of the tachycardias in patients with symptomatic Wolff-Parkinson-White. The QRS is often followed by P waves, as atrial depolarisation occurs after ventricular depolarisation. This is sometimes difficult to discern and may appear as a distorted part of the ST segment.
In antidromic tachycardia, antegrade conduction occurs down the accessory pathway, and retrogradely up the atrioventricular node to the atria. The QRS complex is broad due to ventricular depolarisation via the non-specialised myocardium, and delta waves are often seen.
The most common tachycardia in Wolff-Parkinson-White syndrome is: the atrioventricular re-entrant tachycardia .
Atrial fibrillation is the second most common arrhythmia .
Learning bite: management of arrhythmias in Wolff-Parkinson-White syndrome
Narrow complex tachycardiasTreatment of narrow complex tachycardias in patients with Wolff-Parkinson-White syndrome is aimed at breaking the re-entrant circuit
In patients who have a normal blood pressure, you can use the vagal manoeuvres, adenosine, and drugs such as verapamil or beta blockers
It is important to ensure that full resuscitation equipment is available, particularly when using adenosine, which can induce fast atrial fibrillation in up to 12% of patients.
Atrial fibrillation
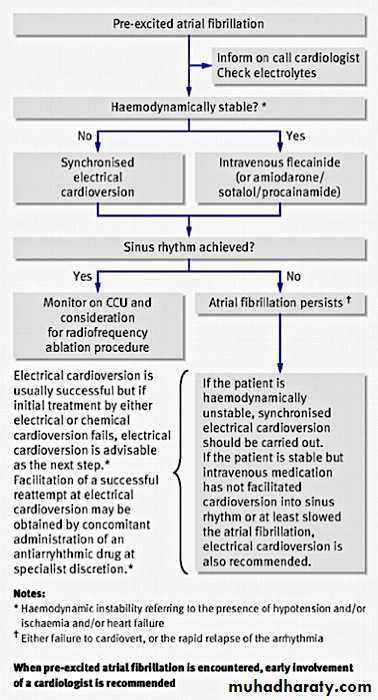
Figure 10: Algorithm for management of pre-excited atrial fibrillation in Wolff-Parkinson-White syndrome26In patients with Wolff-Parkinson-White syndrome who have atrial fibrillation and who are haemodynamically stable, you should first treat them with an intravenous drug. There are some important differences in treatment of atrial fibrillation when compared to patients who do not have Wolff-Parkinson-White syndrome:
Drugs that block the atrioventricular node, such as adenosine, verapamil, digoxin, and beta blockers, are contraindicated in patients with Wolff-Parkinson-White syndrome. These drugs will accelerate conduction of the atrial impulses down the accessory pathway, resulting in a fast ventricular response. This can degenerate into ventricular fibrillation.
It is preferable to use drugs that reduce conduction through the accessory pathway in patients in atrial fibrillation associated with Wolff-Parkinson-White syndrome. These include:
Intravenous flecainide. You should not use this drug if the patient has structural or ischaemic heart disease as it is negatively inotropic and can cause ventricular arrhythmias in this group of patients
Intravenous procainamide
Intravenous amiodarone (given slowly).
Synchronised direct current cardioversion
Direct current cardioversion is the mainstay of treatment for a narrow or wide complex tachycardia in patients who1-have a low blood pressure. It is an effective and safe method of terminating arrhythmias and converting them back into sinus rhythm. It is also useful in patients who
2- have a normal blood pressure where intravenous drugs, such as flecainide, have been unsuccessful.
Long term management
The long term aim of management in patients with Wolff-Parkinson-White syndrome is a definitive cure. This is because:The incidence of sudden cardiac death in patients with Wolff-Parkinson-White ranges from 0.15% to 0.39% over a three to 10 year follow up period
The prevalence of atrial fibrillation in patients with Wolff-Parkinson-White is high and is associated with the risk of pre-excitation atrial fibrillation, which can degenerate into ventricular arrhythmias.
The following are options for long term treatment:
Catheter ablation is first line therapy in patients with symptomatic Wolff-Parkinson-White syndrome, particularly if there is evidence of a fall in blood pressure during episodes. In up to 95% of patients, this procedure is completely curative, although a small percentage of these patients will need further ablation later. The risk of the procedure is 1% to 2% for major complications. These include complete atrioventricular block (needing pacemaker implantation) and cardiac tamponade.
Asymptomatic patients in high risk occupations, such as bus drivers, pilots, and scuba divers, are generally recommended to have catheter ablation, although this remains a controversial procedure because of the small risk of major complications in asymptomatic people.
Using drugs as first line therapy, particularly in patients with very mild and infrequent symptoms. Giving prophylactic drug therapy long term is generally undesirable, particularly if an alternative definitive cure is available.
Learning bite: atrial fibrillation in Wolff-Parkinson-White syndrome
Atrial fibrillation is the second most common arrhythmia in Wolff-Parkinson-White syndrome, occurring in approximately a third of patients. Atrial activity predominantly conducts down the accessory pathway, causing ventricular pre-excitation. The ECG will show an irregular rhythm and broad QRS complexes, with delta waves. Some atrial impulses will conduct down the standard pathway intermittently and produce normal QRS complexes.When atrial fibrillation develops in patients with Wolff-Parkinson-White syndrome, there is a small risk of degeneration into ventricular fibrillation and sudden death. If the refractory period of the accessory pathway is short, rapid antegrade conduction can occur to the ventricles. This increases the risk of life threatening arrhythmias, such as ventricular fibrillation. Drugs such as intravenous flecainide, amiodarone (given slowly), and procainamide are first line drugs in patients who are haemodynamically stable and have a normal blood pressure. If there is haemodynamic instability, indicated by a low systolic blood pressure (<90 mm Hg), then you should use direct current cardioversion.
You see a 37 year old man in the emergency department complaining of palpitations. He has been at a party, where he drank six units of alcohol. He became unwell two hours ago with a sudden onset of palpitations and dizziness. He has no chest pain.
He has no other past medical history and does not take any regular medication. He is a non-smoker, has no allergies, and says that he does not use recreational drugs.
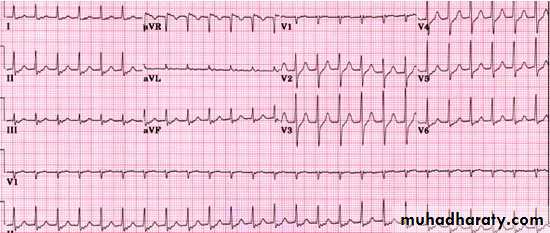
On examination he is tachycardic, with a blood pressure of 125/70 mm Hg. Otherwise his examination is normal. You arrange for him to be put on a cardiac monitor and ask for a 12 lead ECG.
• What should your initial treatment be?
What should your initial treatment be?
The ECG shows a narrow complex tachycardia at a rate of approximately 150 beats a minute. This is consistent with a supraventricular tachycardia. The atrioventricular node is often an intrinsic part of the re-entrant circuit in narrow complex tachycardias. Initial management is therefore aimed at blocking the atrioventricular node. If the patient has a normal blood pressure, then the vagal manoeuvres (carotid sinus massage, the valsalva manoeuvre, and splashing ice cold water over the face) should be your first line of management.
If the vagal manoeuvres are unsuccessful, intravenous adenosine is the second step. Adenosine is an atrioventricular nodal blocking drug with a very short half life. It is given intravenously, and will usually terminate any atrioventricular node dependent tachycardia.
Adenosine is given initially as a 6 mg bolus. If this dosage is ineffective, then it can be increased to 12 mg for most patients, and up to 18 mg can be given in larger patients. Always follow this with a rapid 10 ml flush of saline
Monitor patients continuously and make sure that full resuscitation equipment is available
You should warn the patient of the transient side effects of the drug. These include chest discomfort, flushing, and sweating
Adenosine is contraindicated in patients with severe asthma, patients who have had a cardiac transplant, and patients taking dipyridamole.
Adenosine is contraindicated in
• patients with severe asthma• patients who have had a cardiac transplant, and
• patients taking dipyridamole. as dipyridamole prevents the breakdown of adenosine and can potentiate its effect
Adenosine is contraindicated in patients with severe asthma as it causes bronchospasm. Adenosine is a short acting atrioventricular nodal blocking drug with a half life of 10 to 30 seconds. Its side effects include headache, flushing, chest discomfort, and excess atrial nodal blockade.
Although adenosine is relatively contraindicated in patients taking dipyridamole, as dipyridamole prevents the breakdown of adenosine and can potentiate its effect, it is not contraindicated in patients taking clopidogrel.
Intravenous amiodarone has a wide spectrum of antiarrhythmic activity, and is useful in both atrial and ventricular arrhythmias. You should use it when first and second line agents such as adenosine and beta blockers, or calcium channel blockers, have failed in the management of a narrow complex tachycardia.