Maintaining patients on anticoagulants: how to do it
د. حسين محمد جمعةاختصاصي الامراض الباطنة
البورد العربي
كلية طب الموصل
2010
Anticoagulant drugs
Anticoagulant drugs can be divided into two categories: oral and injectable.Oral anticoagulants
Oral anticoagulants interfere with cyclical conversion of vitamin K to reduce the biological activity of clotting factors II, VII, IX, and X and the anticoagulant proteins C and S.
Vitamin K antagonists are the only type of oral anticoagulant available, but it is likely that other agents (such as direct thrombin inhibitors) could become licensed for clinical use.
Vitamin K antagonists comprise the:
Coumarins (such as warfarin and acenocoumarol)Indanediones (such as phenindione).
Because of their mechanism of action, the anticoagulant effects of warfarin can be overcome by small doses of vitamin K.
The expected advantages of thrombin inhibitors are that their pharmacokinetics are predictable and they can be taken orally without regular monitoring of the INR and the activated partial thrombin time (APTT).
A disadvantage is the absence of an antidote that can be used to treat acute bleeding.
Ximelagatran was the first member of this drug class. Ximelagatran was generally well tolerated in trials, but a small proportion (5-6%) of patients developed elevated liver enzyme levels, which prompted the Food and Drug Administration in the US to reject an initial application for approval in 2004. Further development of ximelagatran was stopped in 2006 after it was found that liver damage could develop after withdrawing the drug.
Injectable anticoagulants
Injectable anticoagulants comprise:• Heparins
• Heparinoids
• Fondaparinux
• Hirudins.
The main mechanism for the anticoagulant activity of standard or unfractionated heparin is binding to antithrombin III, which catalyses the inactivation of factors IIa, Xa, IXa, and XIIa. Thrombin and factor Xa are most sensitive to the effects of heparin.
Low molecular weight heparins exert their effects almost entirely through inhibiting factor Xa.
Thrombin and factor Xa are most sensitive to the effects of heparin.
Antithrombin III, which catalyses the inactivation of factors IIa, Xa, IXa, and XIIa.Low molecular weight heparins exert their effects almost entirely through inhibiting factor Xa.
Clinical indications
The most common clinical indications for oral anticoagulation are:• Atrial fibrillation
• Treating and preventing venous thromboembolism
• Heart valve prostheses.
Warfarin
Oral anticoagulants (also known as vitamin K antagonists) interfere with cyclical conversion of vitamin K to reduce the biological activity of clotting factors II, VII, IX, and X and the anticoagulant proteins C and S. Vitamin K antagonists comprise:Coumarins, such as warfarin and acenocoumarol
Indanediones, such as phenindione.
Because of this mechanism of action, the anticoagulant effects of warfarin can be overcome by small doses of vitamin K.
Warfarin is the most commonly used oral anticoagulant in the UK. Because oral anticoagulants act by interfering with the activation of clotting factors, it takes several days before the clotting factors already present are degraded and the full effects of a dose are seen.
Moreover, in the early stages of anticoagulation a temporary hypercoagulable state can be induced by the rapid depletion of the natural anticoagulant protein C. Half lives of vitamin K dependent clotting factors are given in Table 1.
The mean plasma half life of warfarin is 35-40 hours and the duration of effect is two to five days.
The antithrombotic effect of warfarin depends on the clearance of prothrombin, which has a half life of around 50 hours in patients with normal hepatic function.
So the full effects of starting warfarin or of increasing the dose are not seen for five days.
Newly anticoagulated patients
Rapid induction of anticoagulation (for venous thromboembolism) involves giving a loading dose of warfarin to reach the desired degree of anticoagulation as quickly as possible.
Ideally, patients who have started warfarin in hospital will have been stabilised before discharge and the treatment will have been fully explained to them before they leave hospital. Points at which counselling should take place are advised in the NPSA patient safety alert Safer use of anticoagulant therapy.
Starting treatment: rapid anticoagulation
The goal of rapid anticoagulation is to achieve the target INR as quickly as possible. It takes five to 10 days for the full effects of warfarin to be seen and in the early stages a hypercoagulable state may be induced as a result of the rapid depletion of proteins C and S. For this reason, rapid anticoagulation is achieved using heparin. Heparin and warfarin are started together and heparin should continue until the INR has been in the therapeutic range for two consecutive days. This will usually be at least four or five days after starting.In many institutions the target values are also reproduced on paper prescribing documents or in computerised prescribing systems. The target values are evidence based and must be used. There is ample evidence that failing to aim for and maintain the correct target INR is associated with adverse events.
Starting warfarin therapy: rapid anticoagulation
For rapid anticoagulation you must give a loading dose of warfarin starting at the same time as the heparin. Two dosing algorithms have been validated. The algorithms start from the basis that a healthy person would need 20-30 mg of warfarin over three days as a loading dose.The dose is then reduced to take account of factors that increase sensitivity to the effects of warfarin :
Body weight less than 50 kg
Low serum albumin
Age older than 65
Raised baseline INR
Interacting drugs (especially drugs that inhibit the metabolism of warfarin, such as metronidazole and erythromycin)
Liver disease
Heart failure.
You should start patients older than 60 on a dose of about 5 mg. A warfarin induction protocol is given in Table 6.
Introducing anticoagulation in the non-acute environment (for example for thromboprophylaxis in people with atrial fibrillation) should be started slowly because this avoids the risk of overanticoagulation and bleeding, which can occur with rapid anticoagulation. It also avoids the theoretical risk of evoking a paradoxical prothrombotic state.
Starting warfarin: slow induction
Patients with atrial fibrillation are at increased risk of thromboembolism and stroke,are likely to benefit from warfarin and in these groups slow induction of anticoagulation is suitable. One advantage of this approach is that the potential hypercoagulability seen with rapid anticoagulation does not occur. Several schemes have been shown to be safe and effective (that is they produce stable INRs within two to four weeks and few episodes of overanticoagulation). The rapid methods expose the patient to the risk of hypercoagulation and to the risk of bleeding if the INR rises too high.One way to start treatment is to give 1 mg of warfarin daily and then measure the INR after one week. You can then adjust the dose using a recognised algorithm and test after another week and so on until the therapeutic range is reached. After this you can increase the monitoring intervals.
Many hospitals have a scheme built into paper prescribing documents or computerised prescribing systems. Slow induction, however, is often undertaken in primary care and computerised decision support systems are ideal in this situation.
Table 1. Half lives of the vitamin K dependent clotting factors
FactorHalf life (hours)
II (prothrombin)
48-72
VII
2-6
IX
18-30
X
32-60
Protein C
6
Protein S
42
If the baseline INR is greater than 1.4 screen the patient for coagulopathies
There is no standard loading regimen for warfarin that is suitable for all patients. Published loading protocols recommend an initial loading dose dependent on the patient's age and weight and results from baseline measurements
Introducing anticoagulation in the non-acute environment (such as for atrial fibrillation) should be started at low doses of 1 mg because this avoids the risk of overanticoagulation and bleeding that can occur with rapid anticoagulation.
Target INR for different clinical indications
IndicationTarget INR
Duration of anticoagulation
Pulmonary embolus
2.5
Six months
Proximal deep vein thrombosis
2.5
Six months*
Calf vein thrombus
2.5
Three months
Recurrence of venous thromboembolism when no longer on warfarin
2.5
Consider long term
Recurrence of venous thromboembolism while on warfarin
3.5
Consider long term
Antiphospholipid syndrome
2.5
Consider long term
Atrial fibrillation
2.5
Long term
Cardioversion
2.5 or 3.0
Three weeks before and four weeks after procedure
Mural thrombus
2.5
Three months
Cardiomyopathy
2.5
Long term
Mechanical prosthetic heart valve
3.0 or 2.5
Long term
*Shortening treatment to three months is recommended if circumstances indicate that the risk:benefit ratio favours this, for example if a reversible precipitating factor was present and there are risk factors for bleeding (such as age >65 years).
Table 2. Clinical indications for oral anticoagulation and target INR values
Indication
Target INRPulmonary embolus
2.5
Proximal deep vein thrombosis
2.5
Calf vein thrombus
2.5
Recurrence of venous thromboembolism when no longer on warfarin therapy
2.5
Recurrence of venous thromboembolism while on warfarin therapy
3.5
Symptomatic inherited thrombophilia
2.5
Antiphospholipid syndrome
2.5
Non-rheumatic atrial fibrillation
2.5
Atrial fibrillation due to rheumatic heart disease, congenital heart disease, and thyrotoxicosis
2.5
Cardioversion
2.5 or 3.0
Mural thrombus
2.5
Cardiomyopathy
2.5
Mechanical prosthetic heart valve: aortic
3.0 or 2.5
Mechanical prosthetic heart valve: mitral
3.5 or 3.0
Bioprosthetic valve
2.5 if anticoagulated
• Target INR values are given in Table 2.
• Patient self management
• The ready availability and reliability of near patient testing devices for INR measurement has made self management of oral anticoagulation a feasible proposition for suitably motivated and counselled patients.
• A randomised controlled trial of 617 patients in the UK showed that self management of oral anticoagulation is as effective as routine care (in this case a mixture of hospital and primary care clinics).
Patients measured their INR values every two weeks (or every week if there were dose changes) and adjusted their doses. There were no significant differences in the time spent in the therapeutic range or the numbers of side effects. In addition, patients who had poor control of their INR values before the study improved in the intervention group. In another study, the cost of self management was £350 per year compared with about £100 for routine care.
In self management schemes patients are trained in the theoretical and practical aspects of anticoagulation and are provided with an approved near patient testing device and a personalised dose adjustment schedule. Patients remain in contact with a named doctor who takes clinical responsibility for their care. Patients must attend a clinic every three months. Recommendations for patients undertaking self management of oral anticoagulation have been published.
The dose of warfarin may need to be reduced in patients:
• With liver dysfunction• With heart failure
• With hyperthyroidism
• Starting some drugs (for example those that inhibit warfarin metabolism (check details in the British National Formulary)) or discontinuing drugs that increase warfarin metabolism
With pyrexia.
The dose of warfarin may need to be increased in patients:
• With hypothyroidism
• Taking remedies containing vitamin K, for example some herbal remedies and enteral feeds
• Starting some drugs (for example those that increase warfarin metabolism) or stopping drugs that inhibit warfarin metabolism.
The reasons for overanticoagulation were:
• Poor concordance (31%)• Influence of other medications (17%)
• Congestive heart failure (28%).
The authors concluded that admission due to bleeding with warfarin could have been avoided with better control.
Guidelines for managing anticoagulation have been produced by the British Committee for Standards in Haematology of the British Society for Haematology and by the American College of Chest Physicians.
Learning bite
When adding another drug onto warfarin, you should always consider the risk of interaction. Interactions are common but not all drugs interact with warfarin. For example there is a low risk of interaction with amoxicillin. In this circumstance there is no reason to change the dose of warfarin.
You should also remember that diseases as well as drugs can interact with warfarin. For example the dose of warfarin may need to be increased in patients with hypothyroidism.
•
• Dose adjustment• Dosing algorithms
• Dosing algorithms have been designed to help those responsible for dosing to take into account all the factors that could influence dose requirements. The algorithm used may depend on local protocols.
Supply of doses
There is wide variation in the supply and dosing methods used for warfarin tablets in NHS organisations, and this can be confusing for patients, carers, and healthcare professionals. Patients and carers have informed the NPSA that they would prefer warfarin regimens to have the following characteristics:Use the least number of tablets each day
Use constant daily dosing rather than alternate day dosing
Use whole tablets only (patients find it difficult to break tablets in half and would rather use 0.5 mg tablets when necessary).
The NPSA recommends that NHS organisations should review their local regimens to incorporate these characteristics. All strengths of warfarin tablets should be available to best meet the needs of patients.
Doses must always be expressed as "mg" rather than "number of tablets."
Particular care is recommended for patients who need to administer one or more loading doses on discharge from hospital and in ambulatory care settings. T
hese patients should be supplied only with sufficient warfarin 5 mg tablets to administer their prescribed loading doses and maintenance supplies of 1 mg and 3 mg tablets.
Safe practice tips
Warfarin tablet strengths Warfarin is available as 1 mg (brown) tablets, 3 mg (blue) tablets, and 5 mg (pink) tablets. In some centres newly anticoagulated patients are supplied with all three strengths so they can make up the dose required. Others use only 3 mg tablets and adjust the dose in increments of 1.5 mg. Patients whose vision is impaired should be prescribed or dispensed only 1 mg tablets to avoid the risk of mix ups.
Using 0.5 mg and 5 mg tablets A 0.5 mg (white) warfarin tablet is also available but is not used in all centres. Some clinics are reluctant to use both 0.5 mg and 5 mg warfarin tablets in case there is confusion and accidental overdose. A small number of patients require large doses of warfarin and for these the 5 mg tablets may be most convenient.
For rapid and complete reversal within 10-15 minutes give prothrombin complex concentrates (immediate replacement of vitamin K dependent coagulation factors) plus intravenous vitamin K.
Fresh frozen plasma immediately replaces vitamin K dependent coagulation factors, but the correction of the coagulopathy is partial.
Intravenous vitamin K alone would not bring the INR back to normal as quickly as the other agents
•
• Recording INR results and communicating changes to the patient• Regardless of the source of the INR results, it is important that a continuous record of a patient's results is held both in their medical record and in the patient held anticoagulant record. Results also need to be entered into the computer if you are using a computerised dosing support system.
• When the dose needs to be changed you should ideally explain this to the patient face to face. If you are able to do this in the clinic you can also explore the reasons for an unusual result (such as temporary antibiotic treatment, binge drinking, or non-concordance) and to decide on an appropriate course of action.
•
Learning bite
• It is important to remember that there is no maximum daily dose of warfarin. You should use the dose necessary to keep the INR in the target range.• In patients taking warfarin
• cranberry juice can cause the INR to rise and therefore cause bleeding
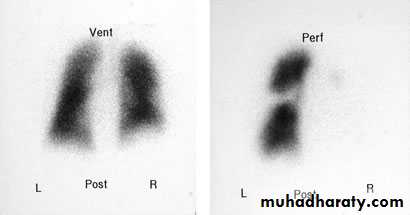
This scan shows a high probability of a pulmonary embolus. There is a marked mismatch affecting the right lung, with good ventilation but little perfusion.
When anticoagulated patients are discharged from hospital you should send complete information about their anticoagulant treatment to the long term healthcare provider so that it can be continued safely. Experience has shown that information provided to long term healthcare providers is often incomplete and this has led to dosing errors and adverse events.
Clinical tips
Before starting anticoagulants explain the proposed therapy with the patient. Assess the clinical, cognitive, and social status of the patient to ensure they are willing and able to take the drug as intendedIf using low molecular weight heparin or unfractionated heparin, continue treatment for at least five days and until the international normalised ratio (INR) is greater than 2 for two successive days.
If treatment with heparin is needed for more than five days check the platelet count.
• Special situations
• Pregnancy• Oral anticoagulants are contraindicated during pregnancy. Long term treatment with low molecular weight heparin is therefore used when deep vein thrombosis occurs.
• Surgery
• Unless there is a very high risk of thromboembolism, anticoagulation should be temporarily stopped in preparation for surgery.
• Dental surgery
• You do not need to stop anticoagulation for dental surgery provided the INR is <3.
Patients with a contraindication to anticoagulation
Vena cava filters can be used to prevent pulmonary embolus in patients with venous thromboembolism who have a contraindication to anticoagulation. These filters are small cone shaped devices. The filters are inserted as a radiological procedure through a vein in the groin or neck and then guided to the vena cava.
Prescribing for patients undergoing cardioversion, dental treatment, endoscopy, or surgery
You should follow the latest recommendations from the British Committee for Standards in Haematology.Cardioversion
A target INR of 2.5 is recommended for three weeks before and four weeks after cardioversion. To minimise cancellations due to low INRs on the day of the procedure a higher target INR, for example 3.0, can be used before the procedure.
Safe practice tip
The practice of starting all patients on a three day dose regimen of 10 mg, 10 mg, 5 mg, and taking an INR on day four is unsafe.A warfarin induction protocol
DayINR
Dose
1
<1.4 1.5-2.0 and patients >60 years >2.0
10 mg 5 mg None
2
No test required
10 mg 5 mg (if INR >1.4 on day 1 and patients >60 years)
3
<2.0 2.0-2.1 2.2-2.5 2.6-2.9 3.0-3.3 3.4-4.0 >4
10 mg 5 mg 4 mg 3 mg 2 mg 1 mg None
4
<1.4 1.4-1.5 1.6-1.7 1.8-1.9 2.0-2.3 2.4-3.0 3.1-4.0 4.1-4.5 >4.5
>8 mg 8 mg 7 mg 6 mg 5 mg 4 mg 3 mg Miss 1 day then 2 mg Miss 2 days then 1 mg
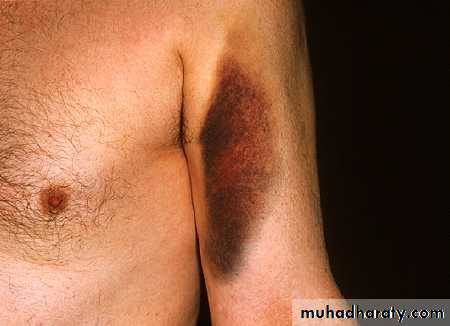
Harms caused by warfarin
There were 600 patient safety incidents of harm or near harm associated with the use of anticoagulants in the UK between 1990 and 2002 Of these, 20% (120) resulted in the death of the patient. During this period the Medical Defence Union logged 79 reports of deaths due to warfarin, 60 of which occurred in primary care. The most common causes for these incidents were:
Inadequate laboratory monitoring
Drug interactions involving non-steroidal anti-inflammatory drugs (NSAIDs).
A study in 2004 showed that warfarin and NSAIDs were the most frequent cause of drug related morbidity accounting for hospital admission. A study of acute admissions carried out in 2002 showed that of 103 patients taking anticoagulants, 29 were over anticoagulated and 17 were admitted with bleeding symptoms .
Between 1990 and 2002 some 600 patient safety incidents of harm or near harm associated with the use of anticoagulants were reported to medical and pharmacy defence associations and the NHS Litigation Authority. Of these, 20% (120) resulted in the death of the patient.
Anticoagulants are consistently associated with adverse incidents in secondary care. The process of starting anticoagulation is particularly risky. One reason for this may be the use of fixed loading doses where clinicians have not taken into account the influence of:
• Age
• Concurrent illnesses
• Interacting drugs.
Unsafe use of anticoagulants is a frequently reported cause of medication incidents, which may lead to patient harm and death
These incidents can be avoided by:
Assessing patients carefully before starting anticoagulation therapy (this includes baseline blood tests)
Adequately documenting anticoagulation therapy in the medical record at the start of therapy
Discussing anticoagulation therapy with the patient and providing them with adequate verbal and written information
Carefully following guidelines for starting and monitoring therapy
Ensuring that all essential information is provided to the patient, anticoagulant clinic, and primary care team
The risk of major bleeding is greatest during the first three months of treatment with oral anticoagulants.
Introducing anticoagulation safely is a complex procedure that, in the presence of thrombosis, involves initial anticoagulation with fast acting heparin followed by the introduction of slower acting oral anticoagulants. The challenge is to ensure the patient is neither undertreated nor overtreated.
• Maintaining patients on anticoagulants: how to do it
• Prescribing interacting medicines• Numerous drugs interact with warfarin and the British National Formulary contains a useful list.
• Warfarin is metabolised by cytochrome p450 2C9 (CYP2C9). Patients with liver disease or those taking drugs that inhibit the activity of CYP2C9 (for example macrolide antibiotics or quinolones) will require less warfarin. Patients taking drugs that accelerate the metabolism of warfarin (for example rifampicin, barbiturates, and carbamazepine) will require more warfarin.
interactions with azole antibiotics, macrolides, quinolones, non-steroidal anti-inflammatory drugs, including selective cyclo-oxygenase-2 inhibitors, selective serotonin reuptake inhibitors, omeprazole, lipid-lowering agents, amiodarone, and fluorouracil, suggests that coadministration with warfarin should be avoided or closely monitored."
As a general rule the most common medicines causing clinically significant drug interactions with warfarin include NSAIDs, antibiotics, and amiodarone.
Recommended action following a high INR or bleeding
INR/bleedingAction
3.0 <INR <6.0 (target 2.5) or 4.0 <INR <6.0 (target 3.5)
Reduce or stop warfarin Restart when INR <5.0
6.0 <INR <8.0
Stop warfarin Restart when INR <5.0
INR >8.0, no bleeding or minor bleeding
Stop warfarin Restart when INR <5.0 If other risk factors for bleeding, give vitamin K 0.5-2.5 mg orally
Major bleeding
Stop warfarin Admit to hospital Give factor concentrate (prothrombin complex concentrate) plus intravenous vitamin K
• Managing raised INR with and without bleeding
• Table 7 lists the recommended action with a high INR or bleeding.
The following advice on managing patients with a high INR is taken from the British National Formulary.
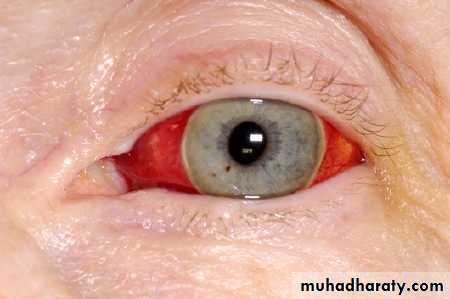
The main side effect of all oral anticoagulants is haemorrhage. Checking the INR and omitting doses when appropriate is essential; if the anticoagulant is stopped but not reversed, you should measure the INR every two to three days later to ensure it is falling. The recommendations in Table 3 (which take into account the British Society for Haematology guidelines) are based on the result of the INR and whether there is major or minor bleeding. The recommendations apply to patients taking warfarin.
Table 3. Recommendations for avoiding haemorrhage
Major bleedingStop warfarin; give phytomenadione (vitamin K1) 5-10 mg by slow intravenous injection; give prothrombin complex concentrate (factors II, VII, IX, and X) 30-50 units/kg or (if no concentrate available) fresh frozen plasma 15 ml/kg
INR >8.0, no bleeding or minor bleeding
Stop warfarin, restart when INR <5.0; if there are other risk factors for bleeding give phytomenadione (vitamin K1) 500 µg by slow intravenous injection or 5 mg by mouth (for partial reversal of anticoagulation give smaller oral doses of phytomenadione for example 0.5-2.5 mg using the intravenous preparation orally); repeat dose of phytomenadione if INR still too high after 24 hours
INR 6.0-8.0, no bleeding or minor bleeding
Stop warfarin, restart when INR <5.0
INR <6.0 but more than 0.5 units above target value
Reduce dose or stop warfarin, restart when INR <5.0
Unexpected bleeding at therapeutic levels
Always investigate the possibility of an underlying cause, for example unsuspected gastrointestinal tract pathology
Dose adjustment
In one study, the use of computerised decision support in oral anticoagulation compared with usual care increased the time spent in the target INR range. It is not known whether this improved laboratory outcome will translate into improved clinical outcomes in the long term.Audit
Computerised decision support systems quickly amass a wealth of data that can be used for routine audit of the anticoagulant service. Routine audit of clinical management together with a review of overanticoagulated patients should be an integral part of the service. An understanding of how the service performs is essential for benchmarking against comparable services and as a basis for improvements.
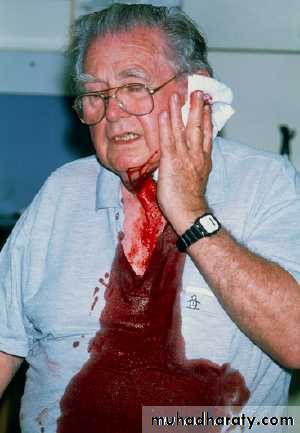
Harms caused by warfarin
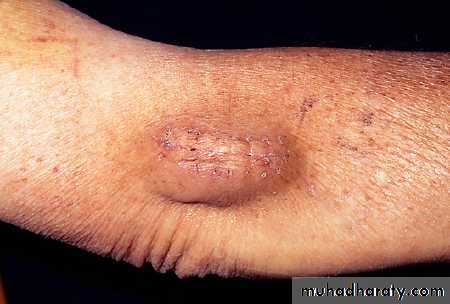
This man had a squamous cell carcinoma removed from his ear two days ago. He restarted warfarin the next day. He was prescribed warfarin to treat atrial fibrillation.
Computerised dosing support systems
Computerised dosing systems have several important advantages. For example, they can:Adjust doses more consistently than humans
Track and recall patients
Generate reminders about dates for stopping anticoagulation
Enable audits of clinical performance.
Table 2. Warfarin: maximum recall periods during maintenance therapy
INR
ActionOne high INR
Recall in seven to 14 days (stop treatment for one to three days) (maximum one week in prosthetic valve patients)
One low INR
Recall in seven to 14 days
One therapeutic INR
Recall in four weeks
Two therapeutic INRs
Recall in six weeks
Three therapeutic INRs
Recall in eight weeks, except patients with a prosthetic valve
Four therapeutic INRs
Recall in 10 weeks, except patients with a prosthetic valve
Five therapeutic INRs
Recall in 12 weeks, except patients with a prosthetic valve
Patients with a prosthetic heart valve seen after discharge from hospital may need more frequent INR monitoring in the first few weeks.
Proportion of time within range
For each patient the proportion of time spent in the therapeutic range is computed using a validated formula.17 If a patient's INR is below the range on one occasion and above it a few days later, although the individual results are out of range the patient, logically, must have spent some of the intervening time in range. This measure, therefore, reflects the overall effectiveness of the clinic procedures.Other useful performance measures that may be available from computerised dosing support systems include:
Frequency of review
Frequency of side effects
Proportion of appointments that have been missed
Patient satisfaction.
Ongoing monitoring
Once a patient has a stable INR you can progressively lengthen the recall interval. This is built into many computerised dosing support systems. Table 2 gives a suggested schedule.
Safe practice tip
High citrate concentrations will give spuriously high INRs so you must take care not to underfill the sample bottle or to pour two small samples into one bottle to make up the volume.Safe practice tip
The practice of prescribing fraction doses of warfarin for induction (that is 3.5 mg) is unnecessary and requires warfarin tablets to be halved. Although 0.5 mg tablets are marketed they are not usually available in practice. Halving tablets leads to inaccurate doses.Surgery
Unless there is a high risk of thromboembolism you should discontinue anticoagulation temporarily in preparation for surgery.Dental treatment
Patients whose INR is in therapeutic range (<4.0) do not need to stop anticoagulation for dental extraction. Oral tranexamic acid mouthwash can prevent bleeding after dental extraction.
Endoscopy without biopsy
Patients whose INR is in the therapeutic range (<3.0) do not need to stop anticoagulation for endoscopy.
• Dr P Marazzi/SPL
•
Discontinuing anticoagulant therapy
Providing there is no clinical reason to extend the period of anticoagulation, you can safely stop warfarin abruptly when the date for discontinuation is reached. There is no need to taper the dose.Audit standards have been proposed by the British Committee for Standards in Haematology:
Provide adequate data for safe transfer of care
Provide anticoagulant cards for patients on hospital discharge
Ensure patients are made aware of the need for anticoagulation and the possible side effects of treatment
Ensure hospital notes contain information that the patient is currently on warfarin
Provide follow up arrangements for patients who do not attend appointments
Achieve 50% of INRs within 0.5 INR units of target and 80% within 0.75 INR units of target.
Point prevalence
This is simply the proportion of patients with a therapeutic INR at a given time or in a given time, also referred to as "last look in the book." The BCSH guidelines suggest that 50% of patients should be within 0.5 of their target INR at any time, or 75% within 0.75 of target.Proportion of tests within range
The denominator here is the number of tests rather than the number of patients, with more unstable patients contributing a greater proportion of results.
Injectable anticoagulants
Injectable anticoagulants comprise:• Heparins
• Heparinoids
• Fondaparinux
• Hirudins.
The main mechanism for the anticoagulant activity of standard heparin is binding to antithrombin III, which catalyses the inactivation of factors IIa, Xa, IXa, and XIIa. Thrombin and factor Xa are most sensitive to the effects of heparin.
LMWH exert their effects almost entirely through inhibiting factor Xa.
Heparinoids, such as danaparoid, have similar activity to that of LMWH , but contain no heparin or heparin fragments. Danaparoid is indicated only for treating venous thromboembolism in patients with a history of heparin induced thrombocytopenia.
Fondaparinux is licensed for prophylaxis of VTE in medical patients and in patients undergoing major orthopaedic surgery of the legs. It is also licensed for treating deep vein thrombosis and for pulmonary embolism.
Hirudins are polypeptides that inhibit factor IIa (prothrombin)
therefore be classed as direct thrombin inhibitors.Two products are available in the UK:
• Lepirudin is licensed for anticoagulation of people with heparin induced thrombocytopenia type II• Bivalirudin is licensed for patients undergoing PCI.
Dose of unfractionated heparin
An initial bolus dose of 5000 units (or 75 units/kg body weight) should be followed by a continuous intravenous infusion of 18 units/kg/hour with adjustment of dose according to at least once daily APTT measurement, with repeat measurement 2-4 hours after any dose adjustment. Alternatively, you can give an equivalent daily dose by two subcutaneous injections.Treatment with unfractionated heparin is monitored using the APTT. The target is a ratio of 1.5-2.5. The APTT is generally insensitive to low molecular weight heparins and cannot be used for monitoring.
Antifactor Xa monitoring is not routinely undertaken because it provides an incomplete picture of the anticoagulant effect and is poorly predictive of antithrombotic efficacy and risk of haemorrhage.
Before starting, you should test the INR, APTT, platelets, and liver function. If the baseline INR is greater than 1.4, you should screen the patient for coagulopathies.
Stopping heparin
You should stop heparin once the INR has been in the therapeutic range for two consecutive days. This is likely to be at least four days after starting warfarin.Monitoring unfractionated heparin
Unfractionated heparin requires monitoring by the APTT. APTT ratios below 1.5 are ineffective and ratios of above 2.5 are associated with increased risk of bleeding. Using protocols for dose adjustment according to APTT ratios helps you achieve therapeutic targets.
Table 5. Sample protocol for unfractionated heparin
APTT ratioAction if there is no bleeding
1.0-1.2
Give IV bolus of 40 units/kg Increase 24 hour dose by 4000 units
1.3-1.4
Increase 24 hour dose by 3000 units
1.5-2.5
No change to daily dose
2.6-3.0
Reduce 24 hour dose by 1000 units
3.1-4.0
Reduce 24 hour dose by 2000 units
4.1-5.0
Stop infusion for 30 minutes Reduce 24 hour dose by 5000 units
>5.1
Stop infusion for 1 hour Reduce 24 hour dose by 6000 units
Safe practice tip
It is safe practice to standardise on one strength of heparin infusion to be administered via a syringe driver pump and to adjust the rate of administration of this standard infusion to adjust the daily dosage.
Heparin concentrate injections are available as 5000 and 25 000 units/ml products. They must be diluted before use. Preparations are also available as 1000 units/ml ready diluted in 10 ml and 20 ml that can be drawn up into a 50 ml syringe and administered by a syringe driver.
In patients with renal failure (creatinine clearance <30 ml/min) there is a danger that low molecular weight heparin will accumulate (it is predominantly excreted by the kidneys). In this situation, unfractionated heparin is the treatment of choice. Alternatively, if you give low molecular weight heparin you must reduce the dose and monitor the patient closely
Monitoring low molecular weight heparin
Because there is a risk of antibody mediated thrombocytopenia you should monitor platelet levels. Thrombocytopenia, should it occur, usually appears between day 5 and 21 of treatment. If the platelet count is significantly reduced (30-50% of the initial value) you must stop therapy immediately and consider alternative therapy.The anticoagulant effects of low molecular weight heparin are only partially reversible with protamine. The anticoagulant effects of unfractionated heparin can be reversed by protamine given by intravenous injection. A dose of 1 mg of protamine neutralises 80-100 units of unfractionated heparin when administered within 15 minutes of the heparin dose. Less is needed if protamine is given after a longer period because of the short half life of intravenous unfractionated heparin
Heparin can suppress adrenal secretions of aldosterone and lead to hyperkalaemia, particularly in patients with:
Diabetes mellitus
Chronic renal failure
Pre-existing metabolic acidosis
A raised plasma potassium.
The risk of hyperkalaemia appears to increase with the duration of therapy, but it is usually reversible. You should measure plasma potassium in at-risk patients before starting heparin therapy and you should monitor this regularly.
Heparin (including low molecular weight heparin) can cause hyperkalaemia by inhibiting aldosterone secretion. Patients with chronic renal failure may be more susceptible.
Prolonged treatment with heparin may cause osteoporosis.
Heparin is not known to cause hyponatraemia.
Heparin can cause an immune mediated thrombocytopenia
In patients with renal failure (creatinine clearance <30 ml/min) there is a danger of accumulating low molecular weight heparin (it is predominantly excreted by the kidneys). In this situation unfractionated heparin is the treatment of choice. Alternatively, if you give low molecular weight heparin you must reduce the dose and monitor the patient closely.
Starting unfractionated heparin
Unfractionated heparin is still used. There are limited data for the use of low molecular weight heparin in patients with massive deep vein thrombosis and pulmonary embolism and some clinicians consider unfractionated heparin the treatment of choice because of its rapid effect and because of clinical experiencePharmacokinetics of heparins
At usual intravenous doses the half life of unfractionated heparin is 45-60 minutes. The bioavailability of subcutaneous unfractionated heparin is less than 50%.Low molecular weight heparins have a half life of about four hours. They are 90-100% bioavailable after subcutaneous injection.
Discussing proposed therapy with the patient
Patients need a clear explanation of the proposed treatment and the implications it will have for their lifestyle. For example, they need to understand that monitoring is essential, not optional. Surveys have shown that many patients could not describe what their anticoagulants do and were unaware that an INR value outside the target range posed a problem.Points that need to be covered are:
The purpose of anticoagulation - what it will achieve and how long it is needed
How it will operate in practice
An individual dose is adjusted to achieve the desired level of anticoagulation
The dose is made up of a number of tablets of standard strengths (different colours)
The process is monitored regularly
Implications for the patient - they must be committed to:
Attend for regular blood testsChange the dose when advised
Monitor for bruising and bleeding
Keep the anticoagulation record safely and ensure the dosing and INR records are kept up to date
Avoid medicines containing aspirin
Not start or stop other drugs (including over the counter medicines) without first discussing it with their doctor or pharmacist
Avoid binge drinking
Tell anyone involved in treating them that they are taking anticoagulants.
In some circumstances it may be possible for the patient to monitor their own clotting. This depends on whether there is an established patient self monitoring scheme with appropriate training and back up facilities.
Selecting an appropriate product and dose
Bemiparin, dalteparin, enoxaparin, tinzaparin, and fondaparinux are all licensed for treating venous thromboembolism. In practice there is most experience with dalteparin, enoxaparin, and tinzaparin and many hospitals have opted for a single product. In some cases standardised prescribing regimens have been developed, either on paper or as part of computerised prescribing.Safe practice tip
It is essential to calculate carefully both the dose required and the volume of injection needed to deliver the dose. There have been many reports of accidental overdoses due to errors with one or both of these steps.Doses can be expressed as mg or units. It is best to use only one type of dose expression: whatever is used routinely in your practice.
If doses are expressed in units, always write "units" in full. Never abbreviate to "U" or "IU" because these may be read as additional zeros and cause tenfold overdoses.
There are minor differences between the different types of low molecular weight heparins. For practical purposes it makes sense to use the local formulary product of choice.
Doses of low molecular weight heparins are given in Table 3.
Table 3. Low molecular weight heparins and doses
ProductDose (mg)
Dose (units)
Tinzaparin
175 units/kg, once daily
Enoxaparin
1.5 mg/kg, once daily
150 units/kg, once daily
Dalteparin
200 units/kg, once daily
No dose adjustments are necessary in elderly people unless they have renal failure (GFR <30 ml/min). No dose adjustments are necessary for patients with obesity or low body weight.
Safe practice tip
In patients with renal failure (glomerular filtration rate (GFR) <30 ml/min) there is a danger of accumulation because low molecular weight heparin is predominantly excreted by the kidneys. In this situation unfractionated heparin is the treatment of choice. Alternatively, if low molecular weight heparin is administered you must reduce the dose and monitor the patient for bleeding.Starting low molecular weight heparin
Subcutaneous low molecular weight heparin is now considered the treatment of choice for venous thromboembolism and acute coronary syndromes (in patients with normal renal function).
Worked example 1
A 50 year old man has a deep vein thrombosis. He weighs 55 kg. What dose of enoxaparin should you give and how should you give it?A 55 kg patient requires a dose of 83 mg or 0.85 ml from the 100 mg in 1 ml prefilled syringe (note the prescribed volume is 0.85 ml because the syringes are only sufficiently accurate to measure changes in dose volume of 0.05 ml).
You must eliminate a volume of 0.15 ml from the syringe before giving the dose.
Worked example 2
A 55 year old man with renal impairment has a large pulmonary embolism. He weighs 70 kg. A bolus intravenous injection of 5000 units of sodium heparin has already been given. A prescription for heparin is needed. What should you prescribe? The hourly rate for sodium heparin is 18 units x body weight in kg.The hourly dose of sodium heparin = 18 x 70 = 1260 units/hour.
If a 1000 unit per ml infusion is used, the rate of infusion to be entered into the syringe driver is calculated by dividing the hourly rate by 1000:
Rate of infusion = 1260/1000 = 1.3 ml/hour.
The next day the APTT ratio is 1.3. What should you do?
Using the sample protocol in Table 5, you should increase the daily dose by 3000 units.The previous daily dose was 30 240 units/24 hours.
The new daily dose = 33 240 units/24 hours.
The dose/hour = 1385 units/hour = 1.4 ml/hour.
Five days after starting treatment the APTT ratio is 4.2. What should you do next?
Using the sample protocol in Table 5 you should adjust the APTT ratio by stopping the infusion for 30 minutes and reducing the 24 hour dose by 5000 units.
The previous daily dose was 33 240 units/24 hours.
The new daily dose = 28 240/24 hours.
The dose/hour = 1177 units/hour = 1.2 ml/hour.
Worked example 3
A 75 year old man has a deep vein thrombosis. His baseline INR is 1.1. What dose of warfarin does he need on days one and two?
Because the patient is older than 60 it is recommended that the daily dose of warfarin on days one and two is 5 mg.
What dose of warfarin is needed for the same patient if the INR result on day three is 3.7?
Using the algorithm in Table 6 the recommended dose on day three is 1 mg.
Reversing anticoagulant effects
The anticoagulant effects of warfarin can be reversed by oral or intravenous vitamin K. Large doses of vitamin K can render a patient "warfarin resistant" for a week or more.10The anticoagulant effects of unfractionated heparin can be reversed by protamine given by intravenous injection. A dose of 1 mg of protamine neutralises 80-100 units of unfractionated heparin when given within 15 minutes of the heparin. Less protamine is needed if it is given after a longer period because of the short half life of intravenous heparin.
The anticoagulant effects of low molecular weight heparin are only partially reversible with protamine.15 There is anecdotal evidence of clinical benefits of using protamine in bleeding patients.
Contraindications
Anticoagulation is contraindicated in a number of situations where the risks of harm are likely to outweigh the benefits of treatment. But there are many situations where contraindications are relative rather than absolute.Absolute contraindications to anticoagulation are:
Potential bleeding lesionsActive peptic ulcer, oesophageal varices, aneurysm, and proliferative retinopathy
Recent organ biopsy
Recent trauma or surgery to the head, orbit, or spine
Recent stroke
Confirmed intracranial or intraspinal bleed
Uncontrolled hypertension
Infective endocarditis.
A history of heparin-induced thrombocytopenia or thrombosis is an absolute contraindication for using heparin.
Homozygous protein C deficiency (risk of skin necrosis) and a history of warfarin related skin necrosis are absolute contraindications to warfarin.
Relative contraindications to anticoagulation are:
History of gastrointestinal bleeding
Liver disease
Renal failure
Alcoholism
Mental impairment
Thrombocytopenia
Coagulation disorders
Interacting drugs, in particular non-steroidal anti-inflammatory drugs
Poor concordance
Poor attendance for regular blood tests
Many people are involved in the management of anticoagulation and it is important to ensure that the appropriate information is available to all parties. In some hospitals a formal multidisciplinary team is responsible for anticoagulation. The following people may be relevant to the process of starting anticoagulation:
Consultant haematologist
Junior doctor
Specialist nurse
Phlebotomist
Pharmacist
Pharmacy technician
Biomedical scientist (haematology).
The anticoagulation team