IONIZING RADIATION
Dr. Nadia Aziz NasirC.A.B.C.M
Department of Community Medicine
Baghdad Medical college
Objectives
Define Radiation & Ionizing RadiationDescribe types & sources of Ionizing Radiation
Understand measurement of Ionizing Radiation
Describe biological & clinical effects
Describe preventive measures
Radiation
Definition: Energy emitted from a source e.g.heat or light from the sun
microwaves from an oven
X rays from an X-ray tube
gamma rays from radioactive elements
Ionizing Radiation
Radiation with enough energy so that during an interaction with an atom, it can remove tightly bound electrons from the orbit of an atom, causing the atom to become charged or ionized.
Occurs in two forms - waves or particles.
Ionizing Radiation
Forms of electromagnetic radiation differ only in frequency and wave length.Heat waves
Radio waves
Infrared light
Visible light
Ultraviolet light
X rays
Gamma rays
Ionizing Radiation
Longer wave length, lower frequency waves (heat and radio) have less energy than shorter wave length, higher frequency waves (X and gamma rays).Not all electromagnetic (EM) radiation is ionizing.
Only the high frequency portion of the electromagnetic spectrum which includes X rays
Ionizing Radiation
Ionizing Radiation occurs in two forms :waves
particles.
Ionizing Radiation
Waves
Most of the types of electromagnetic radiation (e.g. visible light, radio waves) exhibit “wave-like” behavior in their interaction with matter.
Photons are chargeless bundles of energy that travel in a vacuum at the velocity of light, which is 300 000 km/sec.
Ionizing Radiation
ParticulateParticulate radiation, consisting of atomic or subatomic particles (electrons, protons, etc.) which carry energy in the form of kinetic energy.
• Alpha particles and beta particles are considered directly ionizing because they carry a charge and can, therefore, interact directly with atomic electrons
Ionizing Radiation
2- Electromagnetic type of ionizing radiation includes gamma and X rays. These are indirectly ionizing because they are electrically neutral (as are all electromagnetic radiations) and do not interact with atomic electronsIonizing Radiation
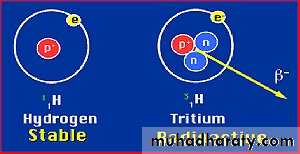
Atoms, in their normal state, are electrically neutral because the negative charge of electrons outside the nucleus equals the positive charge of the nucleus.Isotopes
Are atoms with the same number of protons and different number of neutrons. An isotope may be one or more forms of the same element having the same atomic number, differing mass numbers.Isotopes
The different forms of an element may be stable or unstable (radioactive).Since they are forms of the same element, they possess identical chemical and biological
properties.
Isotopes
Activity of a radioisotopeIs measured by how many atoms undergo radioactive decay per a unit of time.
Only the amount of energy of ionizing radiation that absorbed by the human body can cause harm to health.
Measures of Ionizing Radiation
The international (SI) unit of measure for absorbed dose is the gray (Gy)The gray is defined as 1 joule of energy deposited in 1 kilogram of mass.
The old unit of measure for this is the rad, which stands for "radiation absorbed dose."
1 Gy = 100 rad.
Measures of Ionizing Radiation
Equivalent dose : the biological effect depends on:
The amount of the absorbed dose
The intensity of ionization in living cells caused by different type of radiations.
The unit of equivalent dose is the sievert (Sv).
The old unit of measure is the rem. 1 Sv = 100 rem.
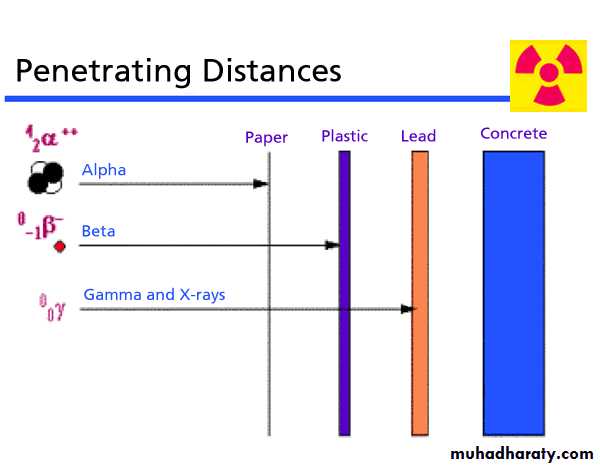
Alpha particles
Are identical to a helium nucleus.They are a highly ionizing form of particle radiation
have low penetration depth.
They can be stopped by a few centimeters of air, or by the skin.
Beta particles
Are high-energy & high-speed electronsEmitted by certain types of radioactive nuclei, such as potassium-40.
The production of beta particles is termed beta decay.
Gamma radiation
It’s a type of electromagnetic radiation of extremely high frequency.Shielding from gamma rays requires large amounts of mass, in contrast to alpha particles which can be blocked by paper or skin, and beta particles which can be shielded by foil.
Lead shield is better as a gamma shield, than an equal mass of another shielding material such as aluminum, concrete, water or soil.
X-rays
Due to their penetrating ability, X-rays are widely used to image the inside of objects, e.g. in medical radiography and airport security
Sources of Radiation
Large proportion of the average annual radiation dose received by people results from natural environmental sources (air, water, food & soil).Each member of the world population is exposed, on average, to 2.4 mSv/yr of ionizing radiation from natural sources.
In some areas, the natural radiation dose may be 5 to 10-times higher to large number of people
Sources of Radiation
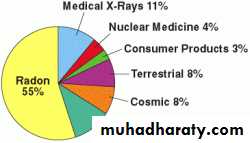
a- cosmic rays, which originate in outer space are higher in mountains & much higher at aircraft altitudes.b- Terrestrial radiation, which emanate from thorium, uranium, radium & other radioactive constituents of the earth crust
Sources of Radiation
c- internal radiation, which is emitted by the potassium-40, carbon-14, radium and other radionuclides normally present in living cellsd- radon & its daughter elements, which are inhaled in indoor air.
Sources of Radiation
On average, 80% of the annual dose that a person receives is due to naturally occurring terrestrial and cosmic radiation.
Human exposure to radiation also comes from human-made sources ranging from nuclear power generation to medical uses of radiation diagnosis or treatment.
The most common human-made sources of ionizing radiation are X-ray machines and other medical devices.
Sources of Radiation
Radiation exposure
may be1- Instantaneous (atomic bomb)
2-chronic (uranium miners)
3-fractionated (radiotherapy)
4-partial-body
Radiation exposure
The whole-body exposure is more harmful than partial-body exposure for a given dose.Radioisotopes decay with time into stable elements and have physical half- lives of various lengths, from fractions of a second to millions of years.
Biological & clinical effects
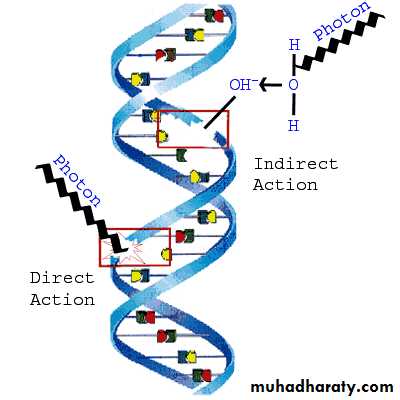
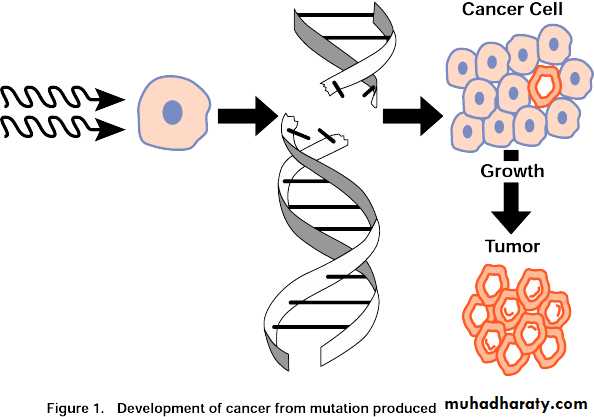
Atoms or molecules that become ionized attain stability again by forming substances that may alter molecular processes within a cell or its environment.Ionizing radiation, in colliding with a cell, can cause changes in its constituents, including deoxyribonucleic acid(DNA).
Such damage, if unrepaired, may disable or kill the cell.
Biological & clinical effects
The most sensitive: Blood forming organs
Reproductive organs
Skin
Bone and teeth
Muscle
Least sensitive: nervous system
Biological & clinical effects
Developing embryo is most sensitive to radiation during the early stages of differentiation, and an embryo/fetus is more sensitive to radiation exposure in the first trimester than in later trimesters.Acute Effects
All ionizing radiation causes similar damage at a cellular levelAlpha and Beta particles cause only localized damage, e.g. radiation burns to the skin.
Gamma rays are more penetrating, causing diffuse damage throughout the body (e.g. radiation sickness, cell's DNA damage, cell death& increasing incidence of cancer).
Acute radiation syndrome
The onset and type of symptoms depends on the radiation exposure.smaller doses result in gastrointestinal effects such as nausea and vomiting and symptoms related to falling blood counts such as infection and bleeding.
larger doses can result in neurological effects and rapid death.
Delayed effects
Largely are due to
1- Mutagenesis
2- Teratogenesis
3- Carcinogenesis
Mutagenesis
Ionizing radiation is a germ- cell mutagen &causes chromosome breaks in somatic cells.Teratogenesis
Intrauterine exposure to ionizing radiation may cause small head size alone or with severe mental retardation.Susceptibility to severe mental retardation is greatest at 8 to 15 weeks of gestational age.
Carcinogenesis
The maternal exposure to diagnostic x-rays during pregnancy was associated with 1.5 fold excess of almost every type of cancer in children younger than 10 years.Its believed that leukemia is associated with in utero exposure to radiation and an increase in thyroid cancer occurred in children exposed to the atomic bomb
Radon
Accounts for 55% of background radiation. Radon gas comes from radioactive decay of radium, a product of ubiquitous uranium deposits in rocks & soil.Radon enters homes through cracks in the foundation & granite walls. Thus radiation exposures in basement may be higher than those on the first floor level.
Prevention of exposure
External Radiation
The risk of cancer associated with most diagnostic radiation is low, and use of radiation should not be restricted when needed for correct diagnosis.
Limitation of radiation, shielding sensitive body parts such as the thyroid, and ensuring a non pregnant state are components of good medical practice.
Prevention of exposure
External RadiationComputed tomography(CT) scan require radiation exposures that induce cancer later in life, so more active reduction in CT exposure setting was recommended.
Prevention of exposure
RADONRadon exposure can be reduced by
1- Increasing ventilation
2- Reducing the influx of radon in the home, by sealing cracks in the foundation, creating negative pressure under the basement floor, and prohibiting the use of building materials containing excessive radium.
Prevention of exposure
Fallout(Internal Emitters)Radioiodines are expected to be released after a malfunction or terrorist event occurring at a nuclear power plant or after the detonation of a nuclear weapon.
Potassium iodide (KI) should be administered promptly to protect the thyroid from radioiodines.