First Class/Practical Medical Chemist
Page 1 of 9
By : Dr. Tamathir Abass
Carbohydrates
By : Dr.Tamathir Abbas
Disaccharide
Defination
• Disaccharides are those sugars which yield two molecules of the
same or different molecules of monosaccharide on hydrolysis.
• General formula:- C
n
(H
2
O)
n-1
• Disaccharides are sweet in taste and soluble in water. Generally
they are not diffusible through the cell membrane. Hence they have
to be digested.
There are two different types of disaccharides: Reducing disaccharides,
Maltose is an example of reducing disaccharides. and Non-reducing
disaccharides. Sucrose is an example of non-reducing disaccharides.
Important Disaccharides are:-
• Maltose
• Lactose
• Sucrose
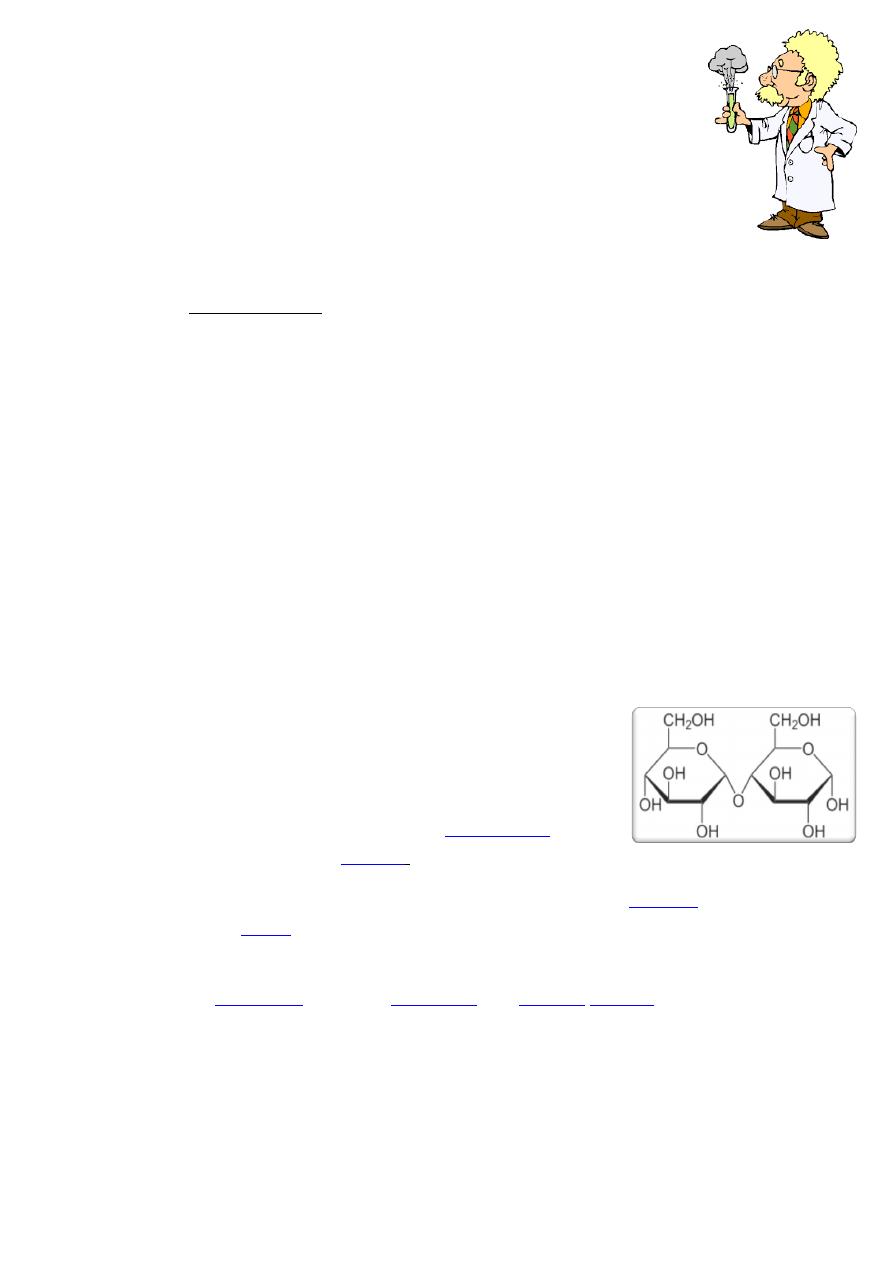
Maltose
Maltose is the disaccharide produced when
Maltose can be broken down into two glucose molecules
. In living
, the
this very rapidly.
It has reducing properties as it has one aldehyde free.
First Class/Practical Medical Chemist
Page 2 of 9
By : Dr. Tamathir Abass
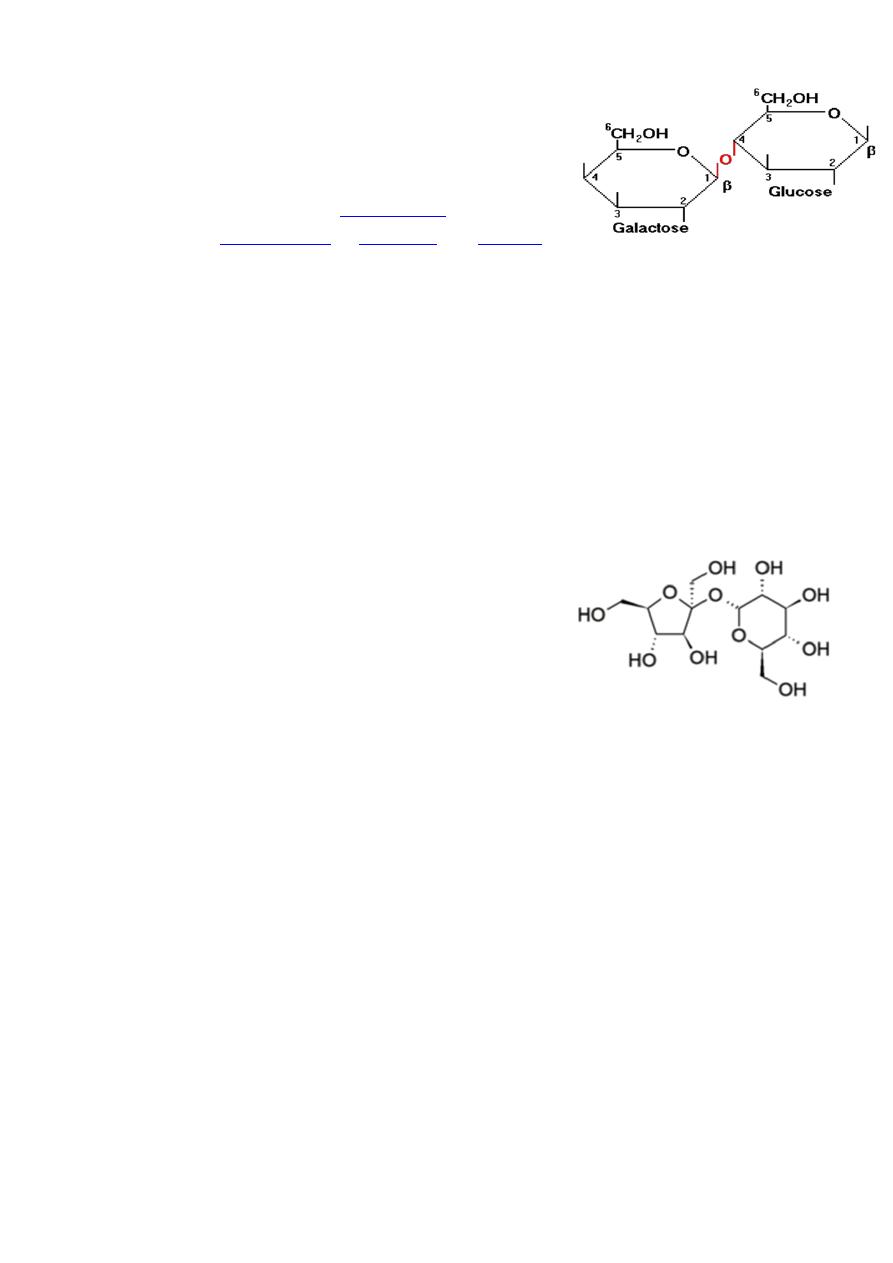
Lactose
Lactose
is
from
Lactose is milk sugar and found in appreciable quantities in milk to
the extent of about 5%.
Specific enzyme which hydolyses is lactase present in intestinal
juice.
It is dextrorotatory.
Like maltose, it also has reducing properties as it has one aldehyde
free.
Sucrose
Sucrose is commonly known as table sugar.
It is a white, odorless, crystalline powder with a sweet taste, it is
best known for its role in human nutrition.
The molecule is a disaccharide derived from glucose and fructose
with the molecular formula C
12
H
22
O
11
.
It is very soluble and very sweet.
The specific enzyme which hydrolyses sucrose is sucrase present in
intestinal juice.
As both aldehyde & ketone groups are linked together it does not
have reducing properties.

First Class/Practical Medical Chemist
Page 3 of 9
By : Dr. Tamathir Abass
Introduction
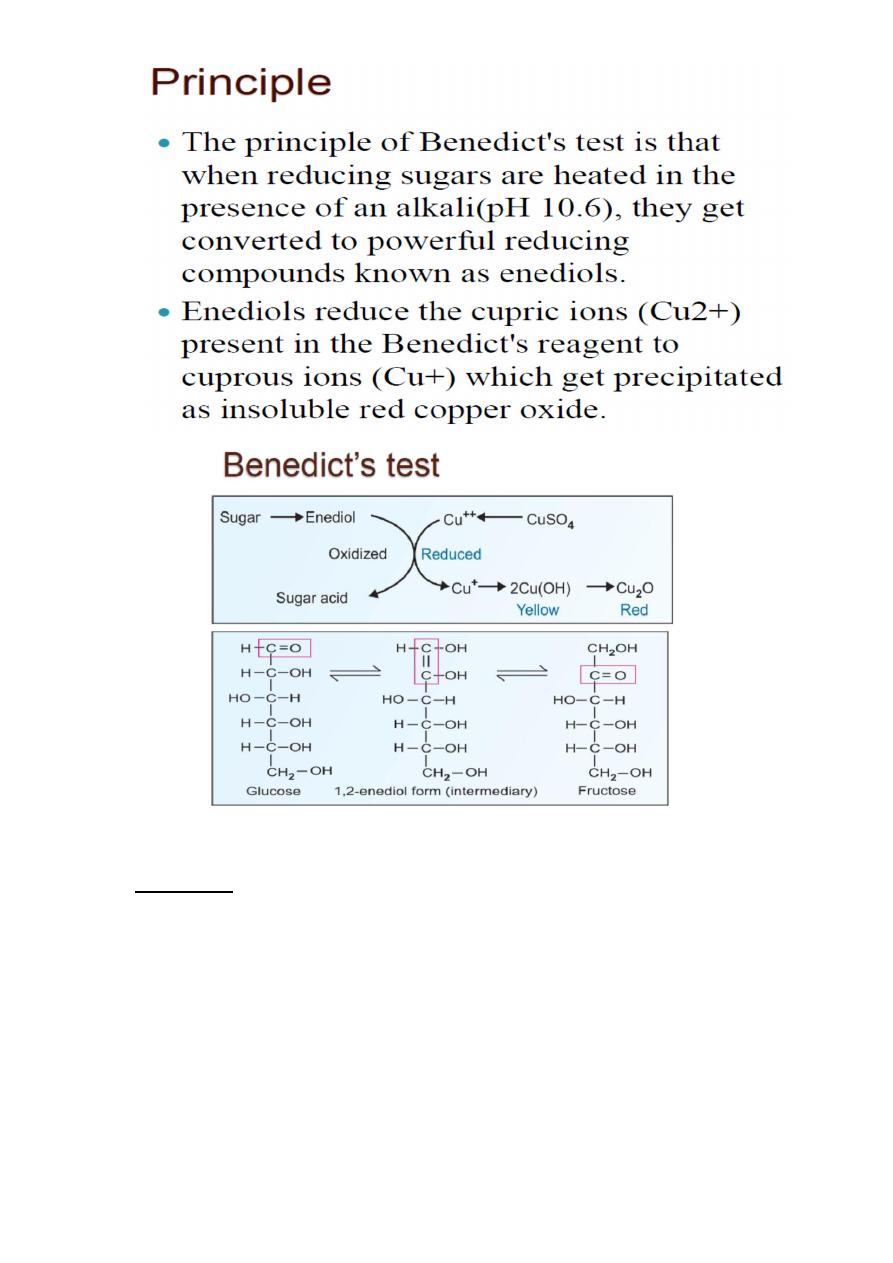
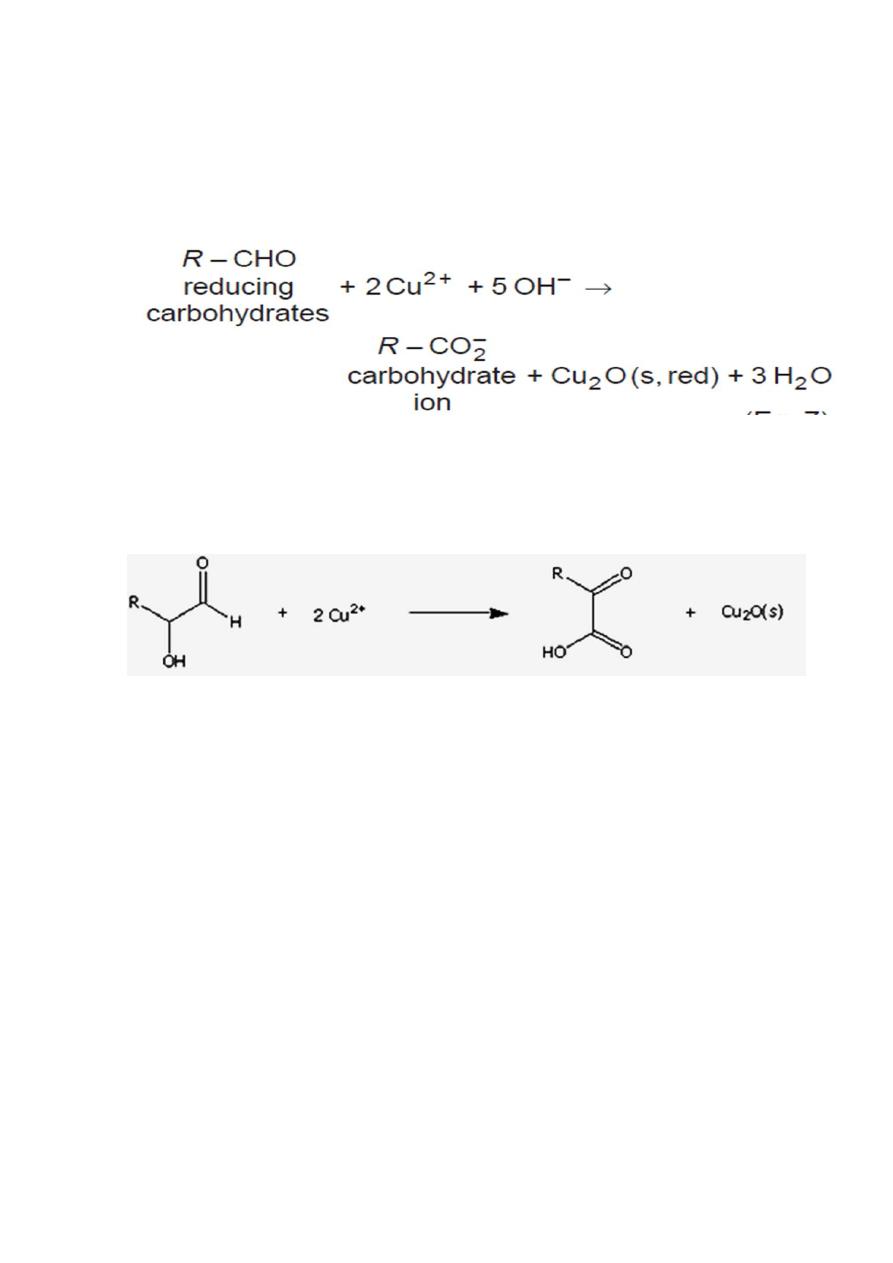
“Reducing sugars are oxidized by copper (II) ions in two other saccharide
test solutions: Benedict's reagent, a mildly basic solution and
Barfoed's reagent, a mildly acidic solution. The presence of red copper
(I) oxide precipitate indicates that the saccharide has reduced the copper
(II) ions.”
Benedict’s Test
General test for reducing sugars
Reducing sugars – sugars that has free aldehyde group, can cause
oxidation and so serve as reducing agents. All monosaccharides,
some disaccharides.
Rgt.: copper(II) sulfate, sodium citrate, sodium carbonate, in
mildly basic medium
Procedure: sple. + Benedict‟s rgt., boil for a few minutes (2-3
mins.)
(+) result: Brick-red ppt., intensity of color is α to [reducing sugar]
First Class/Practical Medical Chemist
Page 4 of 9
By : Dr. Tamathir Abass
Procedure
1. Take 3 mL of Benedict‟s solution in a test tube and boil vigorously
for about one minute
2. Add 8 drops of test solution and continue boiling for another 2
minutes.
First Class/Practical Medical Chemist
Page 5 of 9
By : Dr. Tamathir Abass
Results &Discussion
Proposed result :
During a water bath, which is usually 4–10 minutes, the solution
should progress in the
colors of blue (with no glucose present),
green, yellow, orange, red, and then brick red
or brown (with
high glucose present).

First Class/Practical Medical Chemist
Page 6 of 9
By : Dr. Tamathir Abass
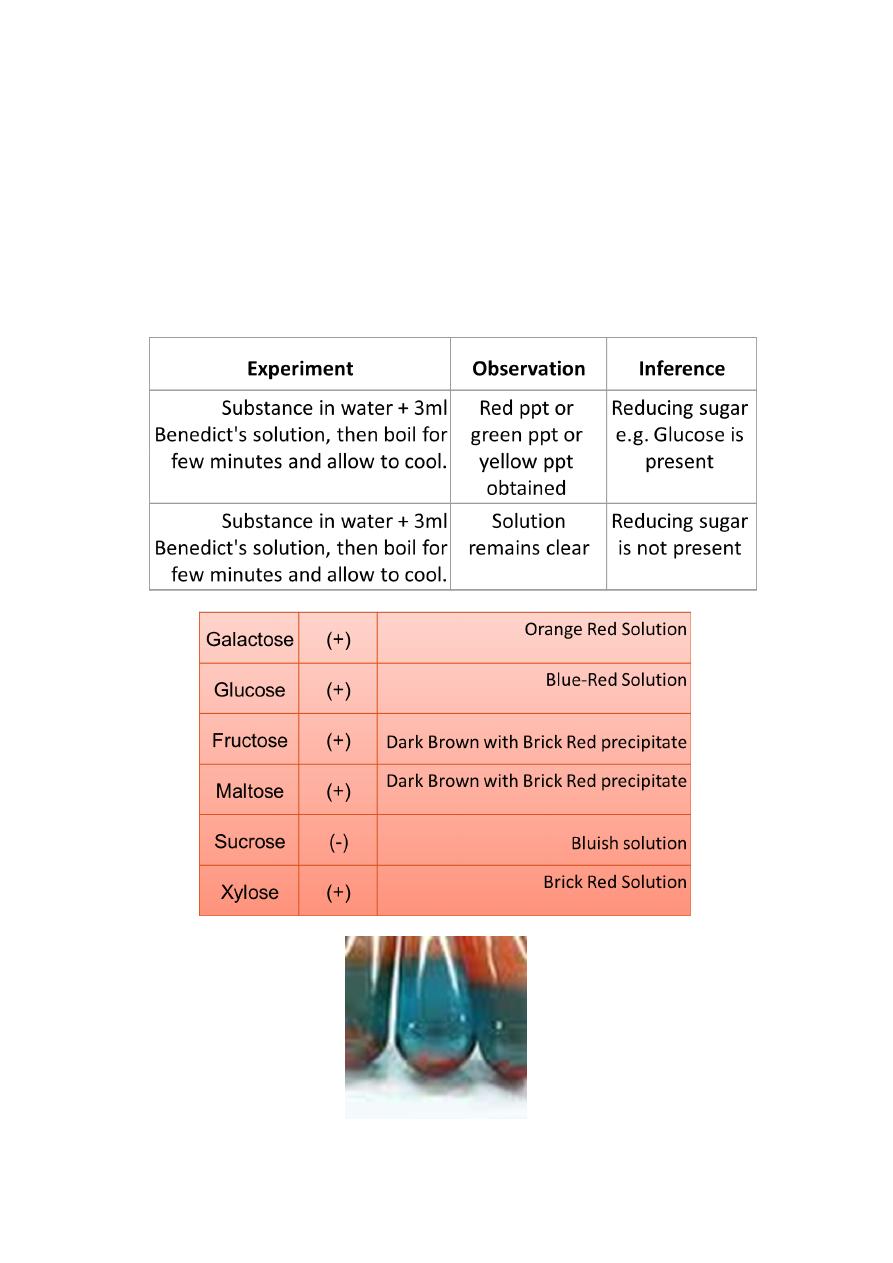
Shows positive test for:
Reducing sugars
Reactions:
• Reducing sugars are oxidized by the copper ion in solution to form
a carboxylic acid and a reddish precipitate of copper (I) oxide.
• ketose fructose is strictly not a reducing sugar and gives a
POSITIVE TEST.
• A colour change would signify the presence of glucose.
• The common disaccharides lactose and maltose are directly
detected by Benedict's reagent, because each contains a glucose
with a free reducing aldehyde moiety, after isomerization.
• Ketose fructose is an alpha-hydroxy-ketone, and gives a positive
test because it is converted to the ldoses glucose and mannose by
the base in the reagent.
• Sucrose contains two sugars (fructose and glucose) joined by
their glycosidic bond in such a way as to prevent the
glucose isomerizing to aldehyde, or the fructose to alpha-hydroxy-
ketone form.
• Sucrose is thus a non-reducing sugar which does not react with
Benedict's reagent.
• Sucrose indirectly produces a positive result with Benedict's
reagent if heated with dilute hydrochloric acid prior to the test,
although after this treatment it is no longer sucrose.
• The acidic conditions and heat break the glycosidic bond in sucrose
through hydrolysis. The products of sucrose decomposition are
glucose and fructose, both of which can be detected by benedict's
reagent, as described above.
First Class/Practical Medical Chemist
Page 7 of 9
By : Dr. Tamathir Abass
Benedict's test uses a mixture of copper (II) sulfate, sodium citrate, and
sodium carbonate in a mildly basic solution.
If the saccharide is a reducing sugar, it will reduce the copper (II) ions to
copper (I) oxide, a red precipitate.
Alkaline solutions of copper are reduced by sugars having a free
aldehyde or ketone group. the citrate will form soluble complex ions
with Cu++, preventing the precipitation of CuCO
3
in alkaline
solutions.
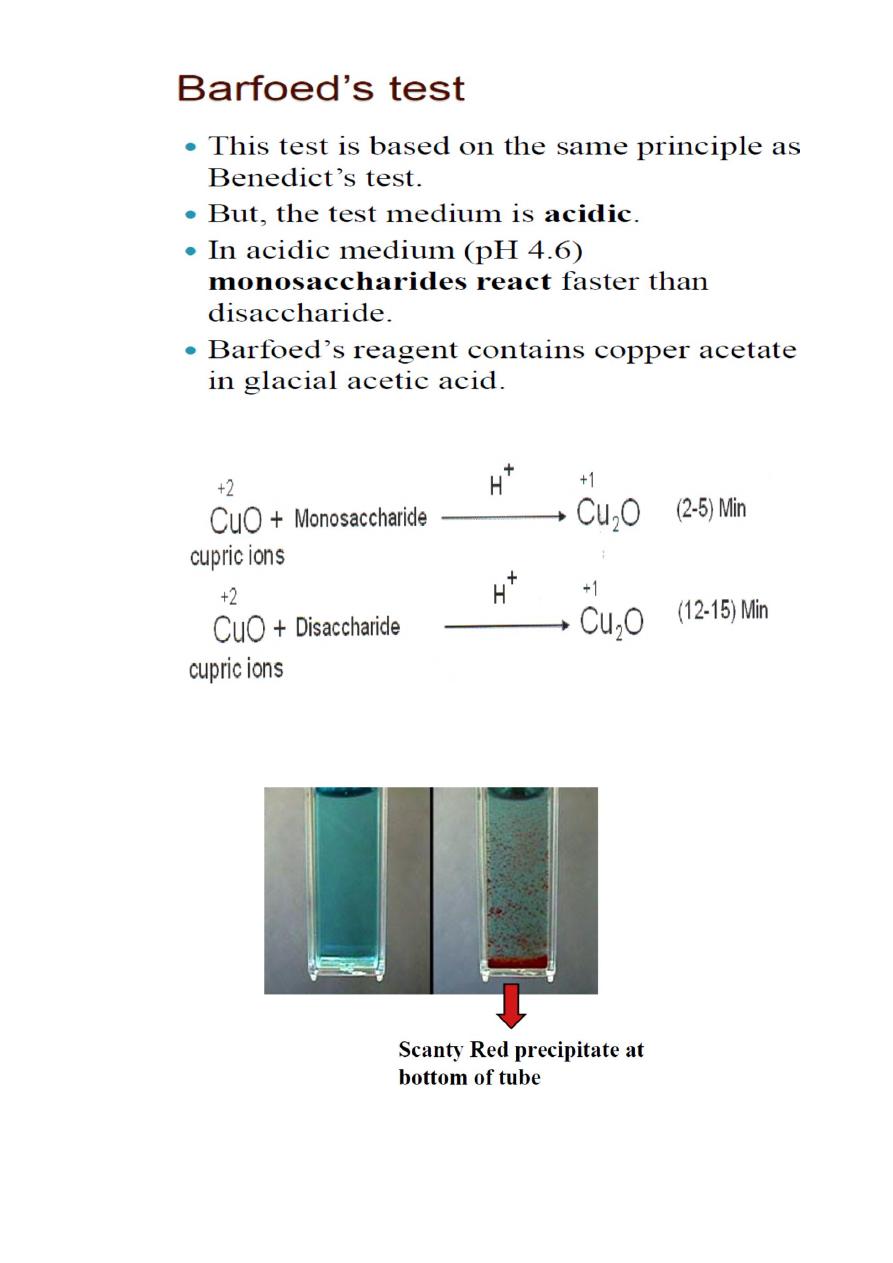
Barfoed’s Test
Test for reducing monosaccharides. Reducing disaccharides also
give a positive test result but a much slower rate, about 10 minutes
of boiling.
Rgt.: copper acetate + H
2
O + lactic acid
Procedure: Barfoed‟s rgt. + saple, boil for 1-2 minutes, cool.
Barfoed’s rgt. Is weakly acidic so only monosaccharides are
sensitive to this test.
First Class/Practical Medical Chemist
Page 8 of 9
By : Dr. Tamathir Abass

First Class/Practical Medical Chemist
Page 9 of 9
By : Dr. Tamathir Abass
Principle: Aldoses and ketoses can reduce cupric ions even in acidic
conditions. This test is used to distinguish reducing mono saccharides
from disaccharides by controlling pH and time of heating. Mono
saccharides react very fast whereas disaccharides react very slowly.
Procedure:
• To 2 ml of Barfoed„s reagent, add 2 ml of carbohydrate solution.
• Keep the test tubes in the boiling water bath for 3 minutes.
• Cool under running water.
• Over-heating should be avoided.
Interpretation:
• The positive reaction indicates the presence of a reducing mono
saccharide.
• On prolonged heating disaccharides can also give this test positive.
• Hence, the solution should be boiled for 3 minutes only.
