1
Forth stage
Medicine
Lec-1
د.خالد نافع
20/4/2016
Diabetes Mellitus
Diabetes mellitus is a clinical syndrome characterized by an increase in plasma blood
glucose (hyperglycaemia).
Diabetes has many causes but is most commonly due to type 1 or type 2 diabetes.
Type 1 diabetes: is caused by autoimmune destruction of insulin-producing cells (B cells) in
the pancreas, resulting in absolute insulin deficiency
Type 2 diabetes: is characterised by resistance to the action of insulin and an inability to
produce sufficient insulin to overcome this ‘insulin resistance.
**

2
Globally, diabetes caused 4.6 million deaths in 2011, and health-care expenditure
attributed to diabetes was estimated to be at least US$465 billion, or 11% of total health-
care expenditure.
The incidence of diabetes is rising. Globally, it is estimated that 366 million people had
diabetes in 2011 (approximately 8.3% of the world population, or 3 new cases every 10
seconds), and this figure is expected to reach 552 million by 2030.
Type 2
Environmental factors such as greater longevity, obesity, unsatisfactory diet, sedentary
lifestyle,increasing urbanisation and economic development .
Aetiology and pathogenesis of diabetes:
In both of the common types of diabetes, environmental factors interact with genetic
susceptibility to determine which people develop the clinical syndrome, and the timing of
its onset.
Type 1 diabetes
Type 1 diabetes is a T cell-mediated autoimmune disease involving destruction of the
insulin-secreting β cells in the pancreatic islets
80–90% of the functional capacity
Type 1 diabetes is associated with other autoimmune disorders
Genetic factors
Account for about one-third of the susceptibility to type 1 diabetes, the inheritance of
which is polygenic .
Environmental predisposition
The concordance rate between monozygotic twins is less than 40% , and wide
geographic and seasonal variations in incidence.
Direct toxicity to B cells or by stimulating an autoimmune reaction directed
against B cells.
Potential candidates fall into three main categories: viruses, specific drugs or
chemicals, and dietary constituents.
Type 2 diabetes
1. Thought to be caused by resistance to insulin action.
2. obesity is a major cause
3. Inactivity
3
Environmental and other risk factors
Diet and obesity
Tenfold in people with a body mass index (BMI) of more than 30 kg/m2
Age
Type 2 diabetes is more common in the middle-aged and elderly .
In the UK, it affects 10% of the population over 65, and over 70% of all cases of
diabetes occur after the age of 50 years.
Aetiological classification of diabetes mellitus
Type 1 diabetes
• Immune-mediated
• Idiopathic
Type 2 diabetes
Other specific types
1. Genetic defects of β-cell function
• Genetic defects of insulin action (e.g. leprechaunism, lipodystrophies)
2. Pancreatic disease (e.g. pancreatitis, pancreatectomy, neoplastic disease, cystic
fibrosis, haemochromatosis, fibrocalculous pancreatopathy
3. Excess endogenous production of hormonal antagonists to insulin, e.g.
Growth hormone – acromegaly,Glucocorticoids – Cushing’s syndrome
Glucagon – glucagonom, ,Catecholamines – phaeochromocytoma
Thyroid hormones – thyrotoxicosis
4. Drug-induced (e.g. corticosteroids, thiazide diuretics, phenytoin)
5. Uncommon forms of immune-mediated diabetes (e.g. IPEX (immunodysregulation
polyendocrinopathy X) syndrome)
6. Associated with genetic syndromes (e.g. Down’s syndrome; Klinefelter’s syndrome;
Turner’s syndrome; DIDMOAD (Wolfram’s syndrome)
7. optic atrophy, nerve deafness; Friedreich’s ataxia; myotonic dystrophy)
8. Gestational diabetes
4
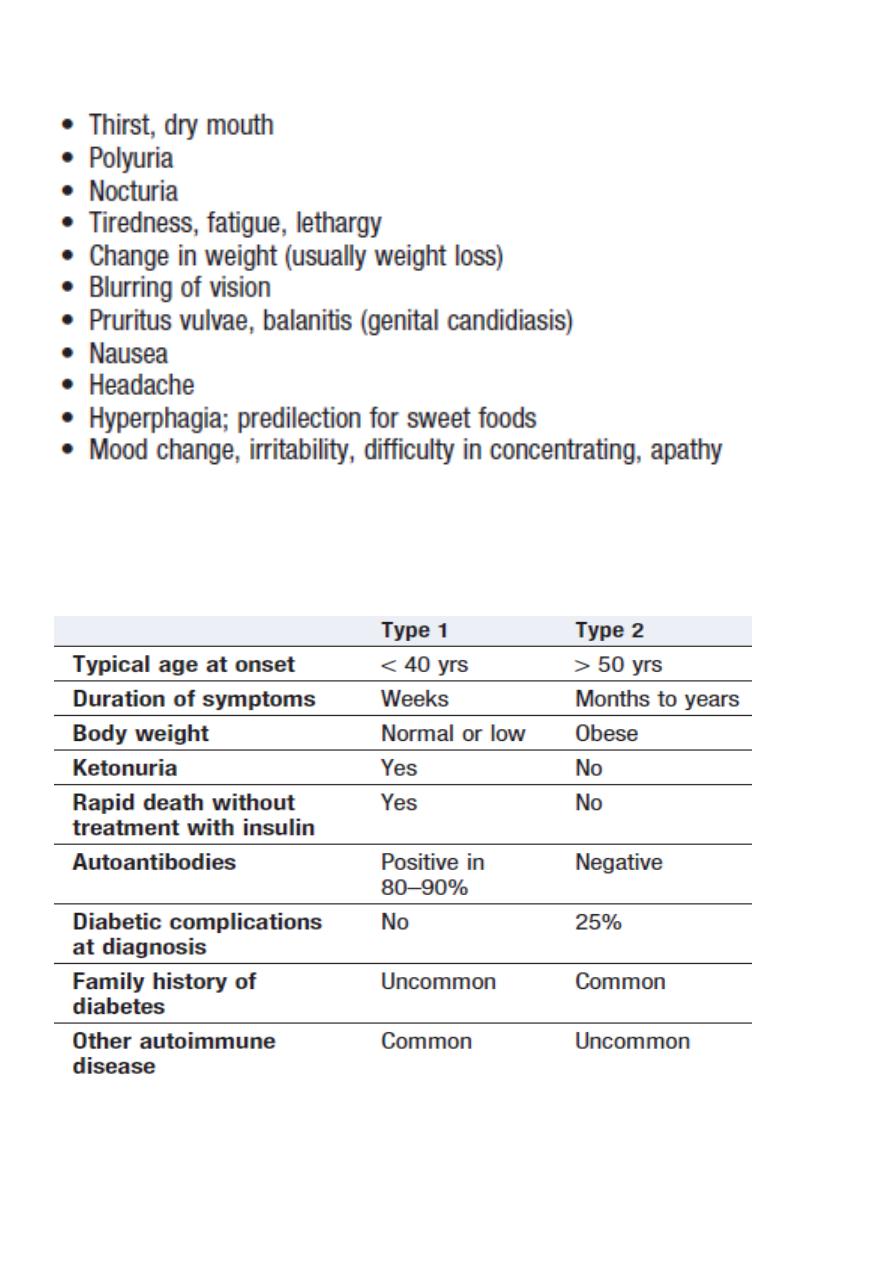
Symptoms of hyperglycaemia
Classical features of type 1 and type 2 diabetes
5
Investigation:
To make the diagnosis of diabetes, the blood glucose concentration should be estimated
using an accurate laboratory method rather than a portable technique.
Glucose concentrations are lower in venous than arterial or capillary (fingerprick) blood.
Whole blood glucose concentrations are lower than plasma concentrations because red
blood cells contain relatively little glucose.
Venous plasma values are usually the most reliable for diagnostic purposes
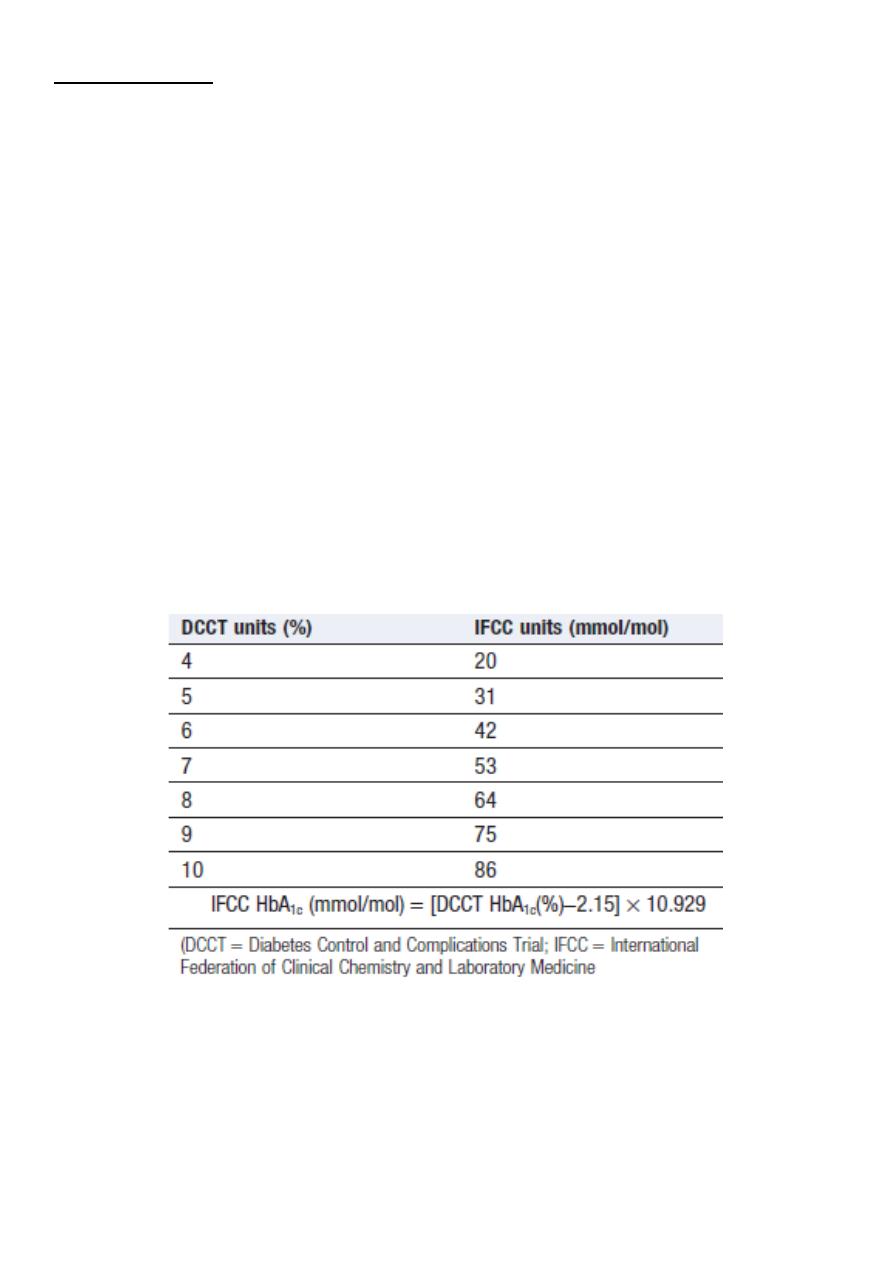
Glycated haemoglobin
In diabetes, the slow non-enzymatic covalent attachment of glucose to haemoglobin
(glycation) increases the amount in the HbA1 (HbA1c) fraction relative to nonglycated adult
haemoglobin (HbA0).
Although HbA1c concentration reflects the integrated blood glucose control over the
lifespan of erythrocytes (120 days), HbA1c is most sensitive to changes in glycaemic control
occurring in the month before measurement.
Conversion between DCCT and IFCC units
for HbA1c

6
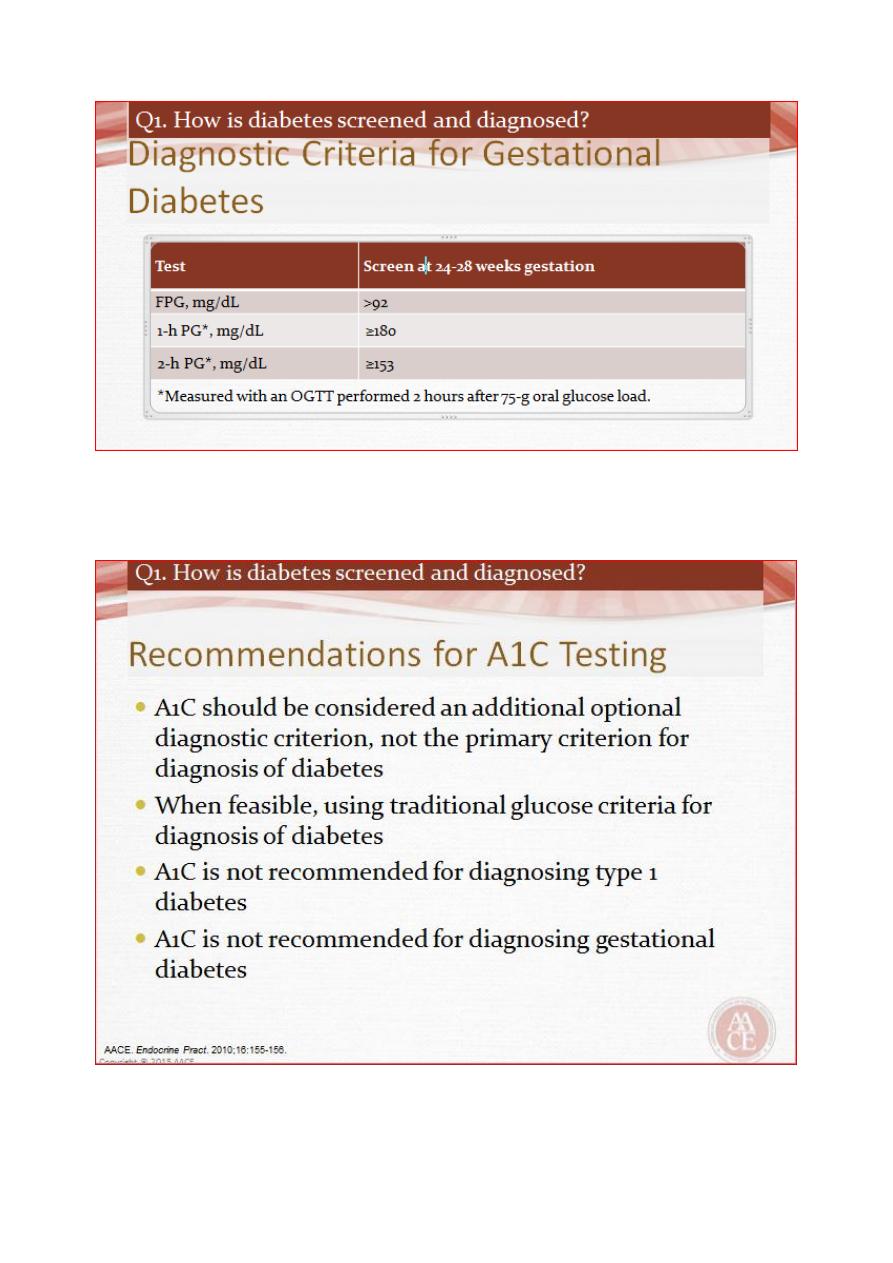
Establishing the diagnosis of diabetes
Glycaemia can be classified into three categories:
1. Normal
2. impaired (pre-diabetes) and
3. diabetes
The glucose cut-off that defines diabetes is based upon the level above which there is a
significant risk of microvascular complications (retinopathy, nephropathy, neuropathy).
People categorised as having pre-diabetes have blood glucose levels that carry a negligible
risk of microvascular complications but are at increased risk of developing diabetes. Also,
people with pre-diabetes have increased risk of cardiovascular disease (myocardial
infarction, stroke and peripheral vascular disease).
Diagnosis of diabetes and pre-diabetes
Diabetes is confirmed by either:
• Plasma glucose in random sample or 2 hrs after a 75 g glucose load ≥ 11.1 (200 mg/dL) or
• Fasting plasma glucose ≥ 7.0 mmol/L (126 mg/dL)
In asymptomatic patients, two diagnostic tests are required to confirm diabetes.
Pre-diabetes’ is classified as:
• Impaired fasting glucose = fasting plasma glucose ≥ 6.0 (108 mg/dL) and < 7.0 mmol/L
(126 mg/dL)
• Impaired glucose tolerance = fasting plasma glucose< 7.0 mmol/L (126 m g/dL) and 2-hr
glucose after 75 g oral glucose drink 7.8–11.1 mmol/L (140–200 mg/dL)

7
**
**
8
**
**

9
**
How to perform an oral glucose tolerance test (OGTT)
Which patients to test
a. Fasting plasma glucose 6.1–7.0 mmol/L (110–126 mg/dL)
b. Uncertainty about the diagnosis of diabetes
Preparation before the test
Unrestricted carbohydrate diet for 3 days
Fasted overnight for at least 8 hrs
Rest for 30 mins
Remain seated for the duration of the test, with no smoking
a. Sampling
b. Measure plasma glucose before and 2 hrs after a 75 g oral glucose drink

10
Forth stage
Medicine
Lec-2
د.خالد نافع
20/4/2016
Management of Diabetes Mellitus
The aims of management are to improve symptoms of hyperglycaemia and to minimize
the risks of long-term microvascular and macrovascular complications.
Treatment methods for diabetes include:
1. Dietary/lifestyle modification,
2. Oral anti-diabetic drugs and
3. Injected therapies.(insulin)
Blood glucose targets vary according to individual circumstances, but, in general,
Pre-meal values between 4 and 7 mmol/L (72 and 126 mg/dL) and 2-hour post-meal values
between 4 and 8 mmol/L represent optimal control.
HbA1c target depends on the individual patient:
Early on in diabetes (i.e. patients managed by diet or one or two oral agents), a target of 48
mmol/mol (6.5%) or less may be appropriate. However, a higher target of 58 mmol/mol
(7.5%) may be more appropriate in older patients with pre-existing cardiovascular disease,
or those treated with insulin and therefore at risk of hypoglycaemia.
**

11
1. Dietary management of diabetes
Aims of dietary management:
Achieve good glycaemic control
Reduce hyperglycaemia and avoid hypoglycaemia
Assist with weight management:
1. Weight maintenance for type 1 diabetes and non-obese type 2 diabetes
2. Weight loss for overweight and obese type 2 diabetes
Reduce the risk of micro- and macrovascular complications
1. Ensure adequate nutritional intake
2. Avoid ‘atherogenic’ diets or those that aggravate complications, e.g. high protein
intake in nephropathy
Dietary constituents and recommended % of energy intake
Carbohydrate: 45–60%
Sucrose: up to 10%
• Fat (total): < 35%
• Protein: 10–15% (do not exceed 1 g/kg body weight/day)
• Fruit/vegetables: 5 portions daily

12
**
13
**
**

14
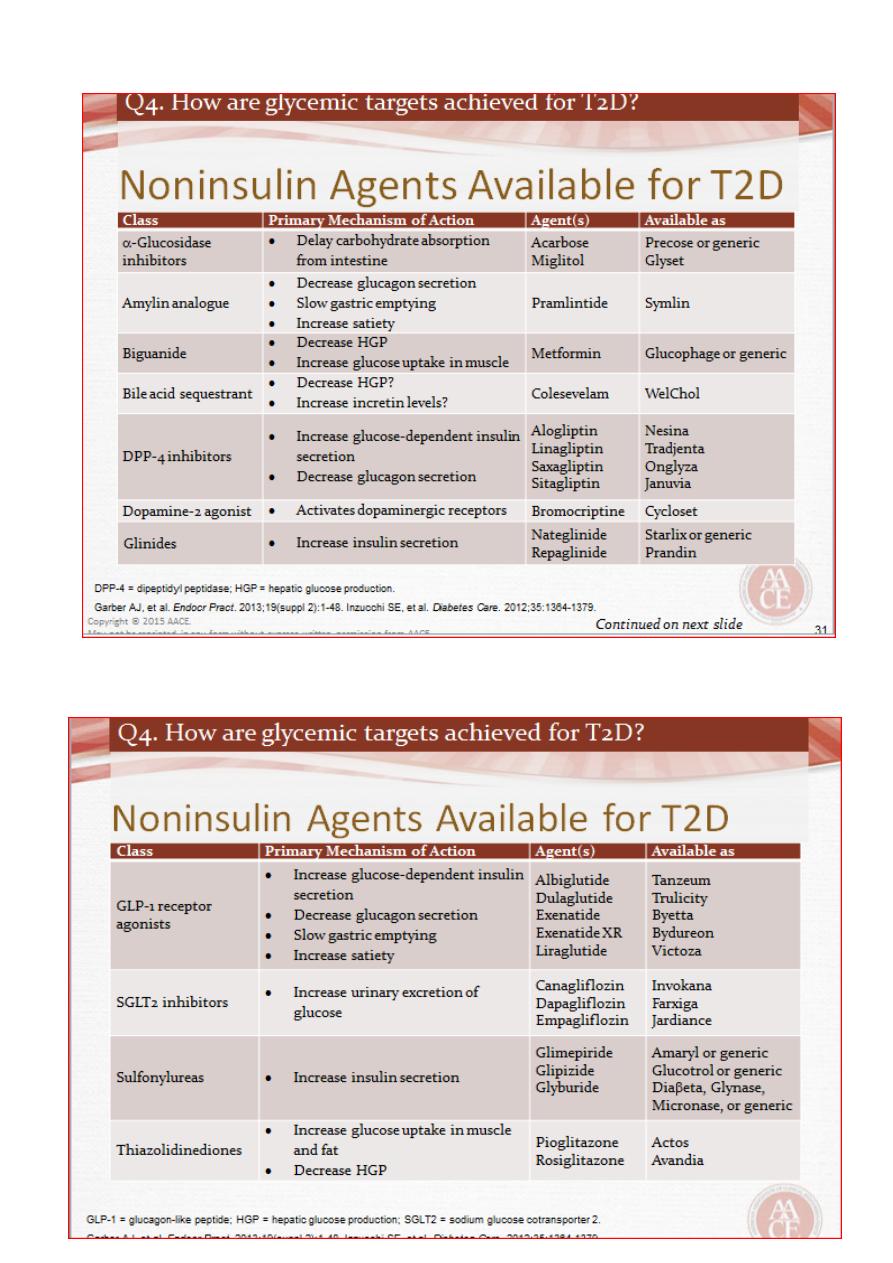
2. Oral hypoglycemic Agents
A. Biguanides(Metformin)
It is employed as first-line therapy in all patients who tolerate it, and its use is
maintained when additional agents are added as glycaemia deteriorates .
Metformin is usually introduced at low dose (500 mg twice daily) to minimize the risk of
gastrointestinal side effects.
The usual maintenance dose is 1 g twice daily.
Metformin reduces hepatic glucose production,
may also increase insulin-mediated glucose
Uptake, and has effects on gut glucose uptake and utilization.
B. Sulphonylureas:
Promote pancreatic B-cell insulin secretion.
Sulphonylureas are an effective therapy for lowering blood glucose and are often used
as an add-on to metformin, if glycaemia is inadequately controlled on metformin alone
The main adverse effects of sulphonylureas are weight gain and hypoglycaemia.
Glibenclamide, gliclazide ,glimepiride and glipizide.
C. Alpha-glucosidase inhibitors
The Alpha -glucosidase inhibitors delay carbohydrate absorption in the gut by inhibiting
disaccharidases.
Acarbose and miglitol are available and are taken with each meal.
Both lower post-prandial blood glucose and modestly improve overall glycaemic
control.
D. Thiazolidinediones
Enhance the actions of endogenous insulin, in part directly (in the adipose cells) and
in part indirectly (by altering release of ‘adipokines’, such as adiponectin, which alter
insulin sensitivityin the liver)
Pioglitazone can be very effective at lowering blood
Glucose in some patients and appears more effective in insulin-resistant patients.
15
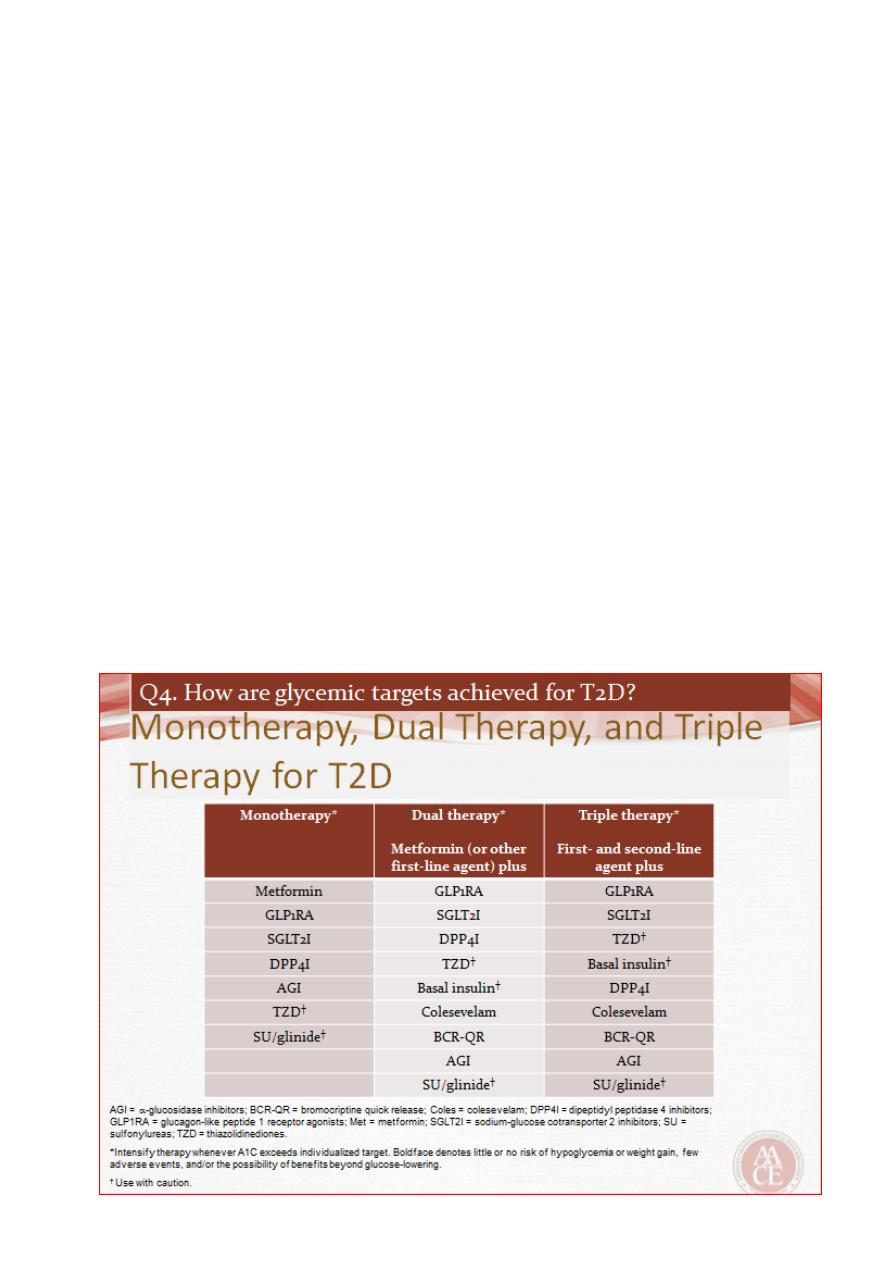
Incretin-based therapies: DPP-4 inhibitors and GLP-1 analogues
The incretin effect is the augmentation of insulin secretion seen when a glucose
stimulus is given orally rather than intravenously, and reflects the release of incretin
peptides from the gut .
The incretin hormones are primarily glucagon-like peptide 1 (GLP-1) and gastric
inhibitory polypeptide (GIP). These are rapidly broken down by the peptidase DPP-4
(dipeptidyl peptidase 4).
Sitagliptin; others now available include vildagliptin, saxagliptin and linagliptin.
GLP-1 receptor agonists have to be given by subcutaneous injection
Delays gastric emptying and, at the level of the hypothalamus, decreases appetite.
Exenatide (twice daily), exenatide MR (once weekly) and liraglutide (once daily)..
SGLT2 inhibitors
Glucose is filtered freely in the renal glomeruli and reabsorbed in the proximal tubules.
SGLT2 is involved in reabsorption of glucose. Inhibition results in approximately 25% of
the filtered glucose not being reabsorbed, with consequent glycosuria.
Dapagliflozin
**
16
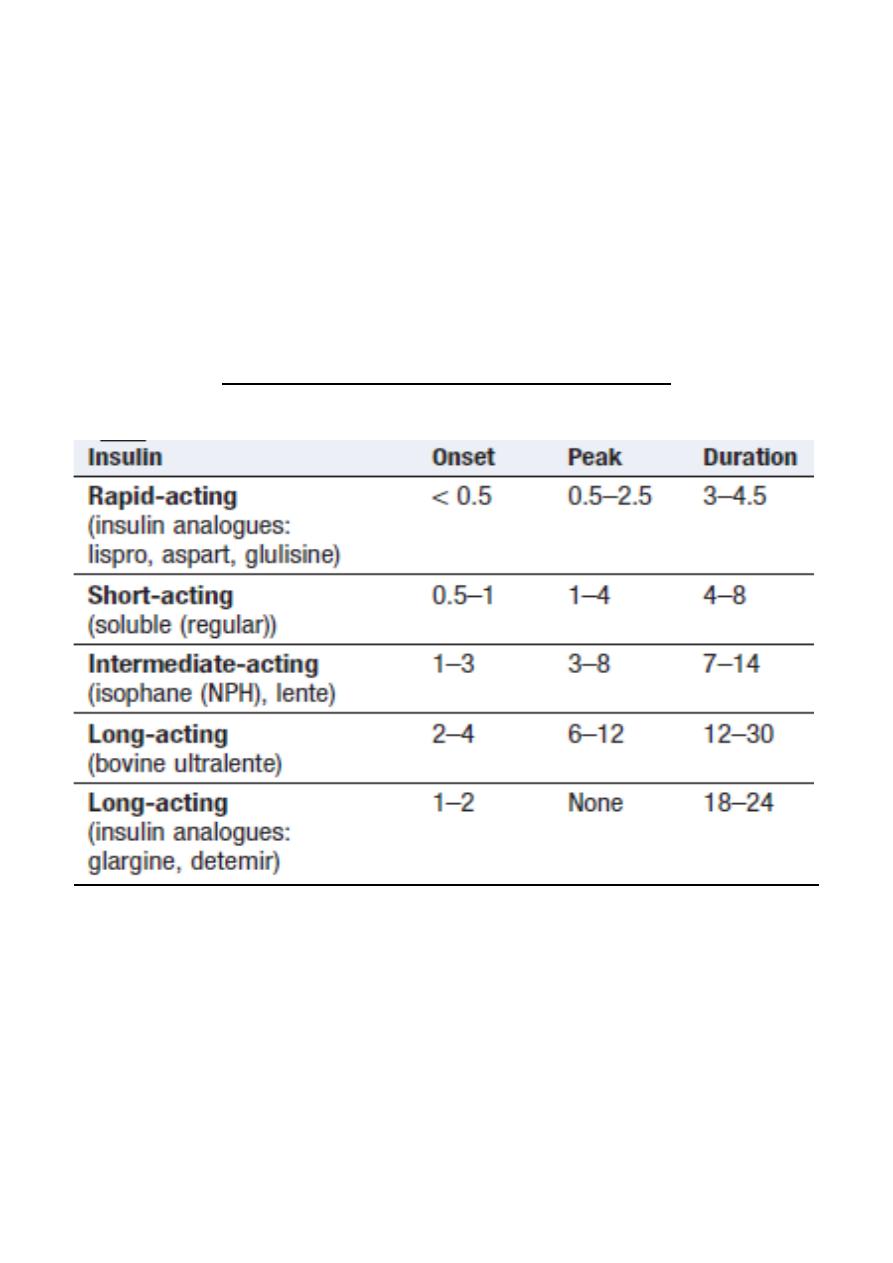
3. Insulin therapy
Insulin was discovered in 1921
Until the 1980s, insulin was obtained by extraction
and purification from pancreata of cows and pigs
Recombinant DNA technology enabled large-scale production of human insulin.
More recently, the amino acid sequence of insulin has been altered to produce
analogues of insulin, which differ in their rate of absorption from the site of injection.
Duration of action (in hours) of insulin preparations

17
Insulin dosing regimens
The choice of regimen depends on the desired degree of glycaemic control, the
severity of underlying insulin deficiency, the patient’s lifestyle, and his or her ability to
adjust the insulin dose.
Most people with type 1 diabetes require two or more injections of insulin daily.
In type 2 diabetes, insulin is usually initiated as a once-daily long acting insulin, either
alone or in combination with oral hypoglycaemic agents.
Twice-daily administration of a short-acting and intermediate-acting insulin (usually
soluble and isophane insulins), given in combination before breakfast and the evening
meal, is the simplest regimen and is still used commonly in many countries.
Initially, two-thirds of the total daily requirement of insulin is given in the morning in a
ratio of short-acting to intermediate-acting of 1 : 2, and the remaining third is given in
the evening.
Multiple injection regimens (intensive insulin therapy) are popular, with short-acting
insulin being taken before each meal, and intermediate- or long-acting insulin being
injected once or twice daily (basal-bolus regimen).
This type of regimen allows greater freedom with regard to meal timing and more
variable day-today physical activity.
Side-effects of insulin therapy

18
Alternative insulin therapies
1. Open-loop’ systems are battery-powered portable pumps providing continuous
subcutaneous (CSII), intraperitoneal or intravenous infusion of insulin.
2. Artificial Pancreas uses glucose sensors to communicate wirelessly with the insulin
pump, which would automatically adjust its rate.
3. Alternative routes of insulin delivery have been investigated. Clinical trials with
intrapulmonary (inhalation), transdermal and oral insulins are ongoing but as yet
none has proven commercially viable.
4. Whole pancreas transplantation :
Transplantation of isolated pancreatic islets ,Stem cells
19
Forth stage
Medicine
Lec-3
د.خالد نافع
20/4/2016
Diabetic ketoacidosis
A medical emergency and remains a serious cause of morbidity, principally in people
with type 1 diabetes.
Mortality is low in the UK (approximately 2%) but remains high in developing countries
and among non-hospitalized patients.
Mortality in DKA is most commonly caused in children and adolescents by cerebral
oedema and in adults by hypokalemia, acute respiratory distress syndrome and
comorbid conditions such as acute myocardial infarction, sepsis or pneumonia.
DKA is characteristic of type 1 diabetes and is often the presenting problem in newly
diagnosed patients.
DKA may be precipitated by an intercurrent illness because of failure to increase
insulin dose appropriately to compensate for the stress response.
The hyperglycaemia causes a profound osmotic diuresis leading to dehydration and
electrolyte loss, particularly of sodium and potassium.
Potassium loss is exacerbated by secondary hyperaldosteronism as a result of reduced
renal perfusion.
Ketosis results from insulin deficiency, exacerbated by elevated catecholamines and
other stress hormones, leading to unrestrained Lipolysis and supply of FFAs for hepatic
ketogenesis.
When this exceeds the capacity to metabolize acidic ketones, these accumulate in
blood. The resulting metabolic acidosis forces hydrogen ions into cells, displacing
potassium ions.
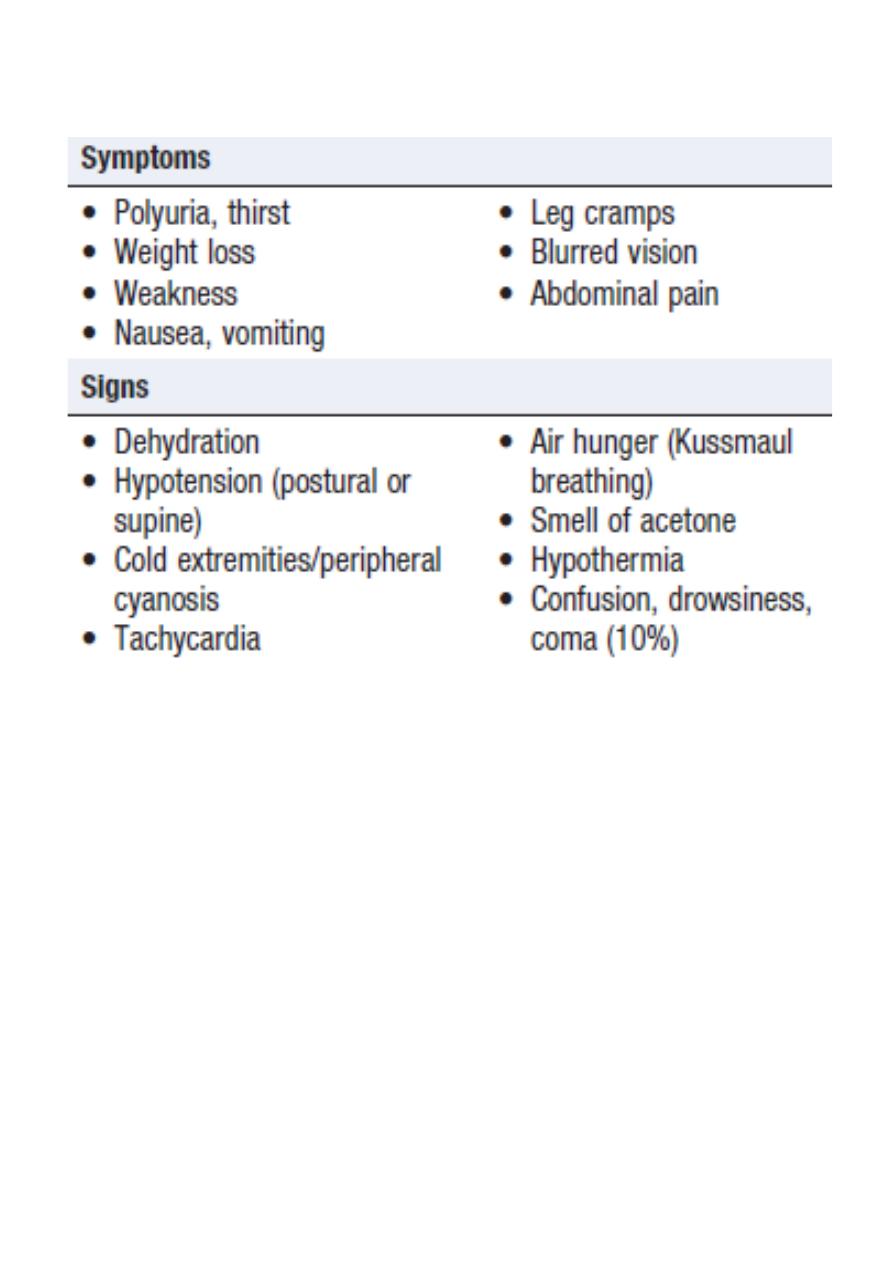
Hyperketonaemia (≥ 3 mmol/L) and ketonuria (more than 2+ on standard urine
sticks)
Hyperglycaemia (blood glucose ≥ 11 mmol/L (~200 mg/dL))
Metabolic acidosis (venous bicarbonate < 15 mmol/L and/or venous pH < 7.3).
Every patient in DKA is potassium-depleted, but the plasma concentration of
potassium gives very little indication of the total body deficit.
Plasma potassium may even be raised initially due to disproportionate loss of water,
catabolism of protein and glycogen, and displacement of potassium from the
intracellular compartment by H+ ions.
20
Clinical features of diabetic ketoacidosis
Investigations
• Venous blood: for urea and electrolytes, glucose and bicarbonate (severe acidosis is
indicated by a venous plasma bicarbonate < 12 mmol/L).
• Urine or blood analysis for ketones
• ECG.
• Infection screen

21
Management
1. Insulin
2. Fluid replacement
3. Potassium
4. Bicarbonate
1. Insulin
A fixed-rate intravenous insulin infusion of 0.1 U/ kg body weight/hr is recommended.
Exceptionally, if intravenous administration is not feasible, soluble insulin can be given by
intramuscular injection (loading dose of 10–20 U, followed by 5 U hourly), or a fast-acting
insulin analogue can be given hourly by subcutaneous injection (initially 0.3 U/kg body
weight, then 0.1 U/kg hourly).
The blood glucose concentration should fall by 3–6 mmol/L (approximately 55–110 mg/dL)
per hour.
When the blood glucose has fallen, 10% dextrose infusion is introduced and insulin
infusion continued to encourage glucose uptake into cells and restoration of normal
metabolism.
2. Fluid replacement
Rapid fluid replacement in the first few hours is usually recommended.
Caution is recommended in children and young adults because of the risk of cerebral
oedema. Most current guidelines favour correction of the extracellular fluid deficit with
isotonic saline (0.9% sodium chloride).
3. Potassium
Potassium replacement is not usually recommended with the initial liter of fluid because
pre renal failure may be present secondary to dehydration.
Treatment with 0.9% sodium chloride with potassium chloride 40 mmol/L is recommended
if the serum potassium is below 5.5 mmol/L and the patient is passing urine .
If the potassium falls below 3.5 mmol/L, the potassium replacement regimen needs to be
reviewed. Cardiac rhythm should be monitored in severe DKA because of the risk of
electrolyte-induced cardiac arrhythmia.

22
4. Bicarbonate
Adequate fluid and insulin replacement should resolve the acidosis.
The use of intravenous bicarbonate therapy is currently not recommended.
Acidosis may reflect an adaptive response, improving oxygen delivery to the tissues, and so
excessive bicarbonate may induce a paradoxical increase in cerebrospinal fluid acidosis and
has been implicated in the pathogenesis of cerebral oedema in children and young adults.
Management of diabetic ketoacidosis

23
Hyperglycaemic hyperosmolar state
Characterized by:
1. Severe hyperglycaemia (> 30 mmol/L (600 mg/ dL)),
2. hyperosmolality (serum osmolality > 320 mOsm/ kg), and
3. Dehydration in the absence of significant hyperketonaemia (< 3 mmol/L) or acidosis (pH
> 7.3, bicarbonate > 15 mmol/L).
There is glycosuria, leading to an osmotic diuresis, with loss of water, sodium, potassium
and other electrolytes.
However, in HHS, hyperglycaemia usually develops over a longer period (a few days to
weeks), causing more profound hyperglycaemia and dehydration (fluid loss may be 10–22
litres in a person weighing 100 kg).
Common precipitating factors include
1. Infection,
2. Myocardial infarction,
3. Cerebrovascular events Drug therapy (e.g. corticosteroids).
Poor prognostic signs
1. Hypothermia,
2. Hypotension (systolic blood pressure < 90 mmHg),
3. Tachy- or bradycardia,
4. Severe hypernatraemia (sodium > 160 mmol/L), Serum osmolality > 360 mOsm/kg,
5. The presence of other serious comorbidities.
Principles of management of hyperglycaemic hyperosmolar state

24
Forth stage
Medicine
Lec-4
د.خالد نافع
20/4/2016
Hypoglycaemia
Hypoglycaemia: (blood glucose < 3.5 mmol/L (63 mg/dL))
In diabetes results in most circumstances from insulin therapy, less frequently from use
of oral insulin secretagogues such as sulphonylurea drugs, and rarely with other anti-
diabetic drugs.
In health, if blood glucose falls,:
1. Endogenous insulin release from pancreatic B cells is suppressed;
2. release of glucagon from pancreatic α cells is increased; and
3. The autonomic Nervous system is activated, with release of catecholamines both
systemically and within the tissues.
4. In addition, stress hormones, such as cortisol and growth hormone, are increased in
the blood. These actions
5. Reduce whole-body glucose uptake and increase hepatic glucose production,
maintaining a glucose supply to the brain.
People with type 1 diabetes cannot regulate
1. Insulin once it is injected subcutaneously, and so it continues to act, despite
developing hypoglycaemia.
2. In addition, within 5 years of diagnosis, most patients will have lost their ability to
release glucagon specifically during hypoglycaemia.
3. This is thought to result mainly from loss of α-cell regulation by β cells. These two
primary defects mean that hypoglycaemia occurs much more frequently in
people with type 1 and longer duration type 2 diabetes.
Most common symptoms of hypoglycaemia

25
Causes of hypoglycaemia
Risk factors for severe hypoglycaemia

26
Nocturnal hypoglycaemia in patients with type 1 diabetes is common but often
undetected, as hypoglycaemia does not usually waken a person from sleep.
Patients may describe poor quality of sleep, morning headaches and vivid dreams or
nightmares, or a partner may observe profuse sweating, restlessness, twitching or even
seizures.
Exercise-induced hypoglycaemia occurs in people with well-controlled, insulin-treated
diabetes because of hyperinsulinaemia. Suppression of endogenous insulin secretion to
allow increased hepatic glucose production to meet the increased metabolic demand is
key to the normal physiological response to exercise.
Emergency treatment of hypoglycaemia
27
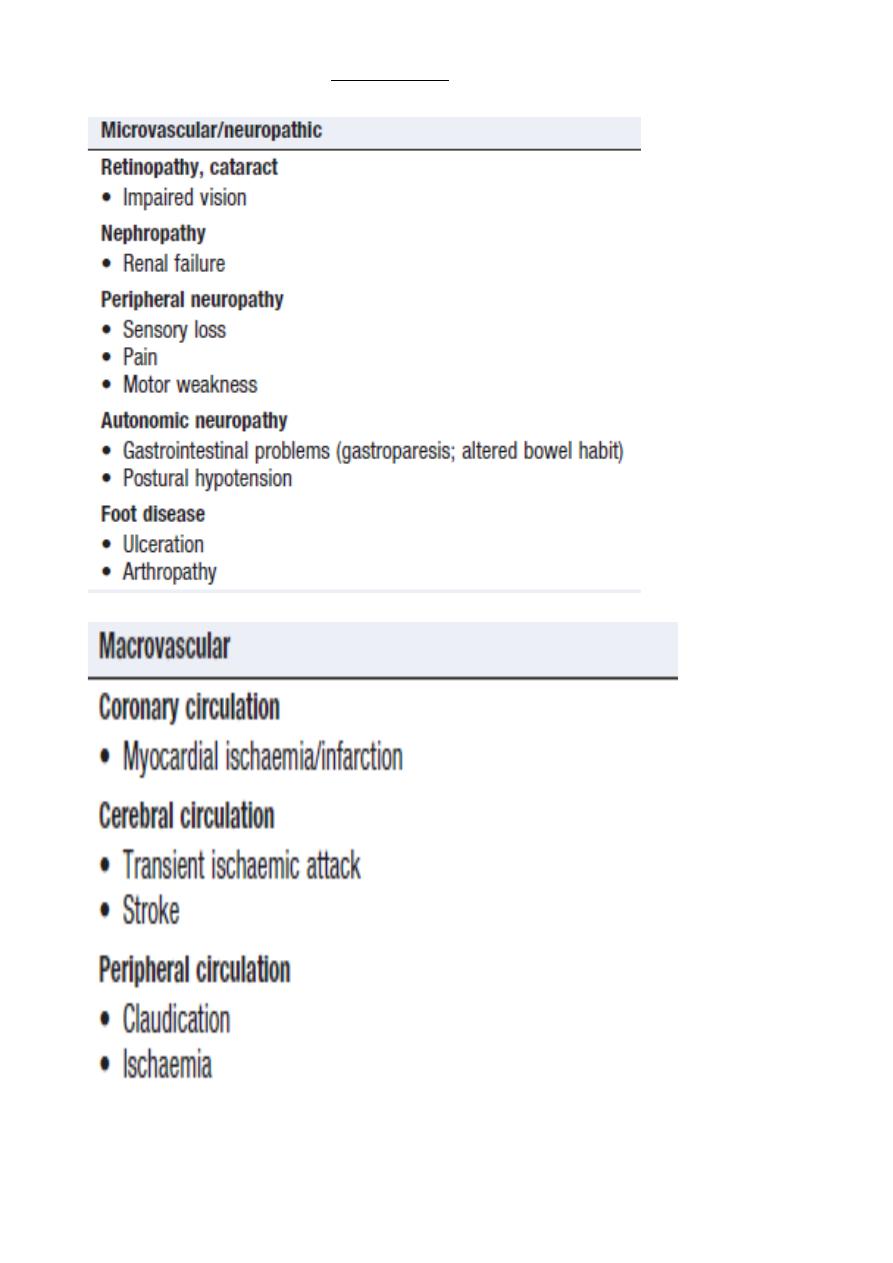
COMPLICATIONS OF DIABETES
28
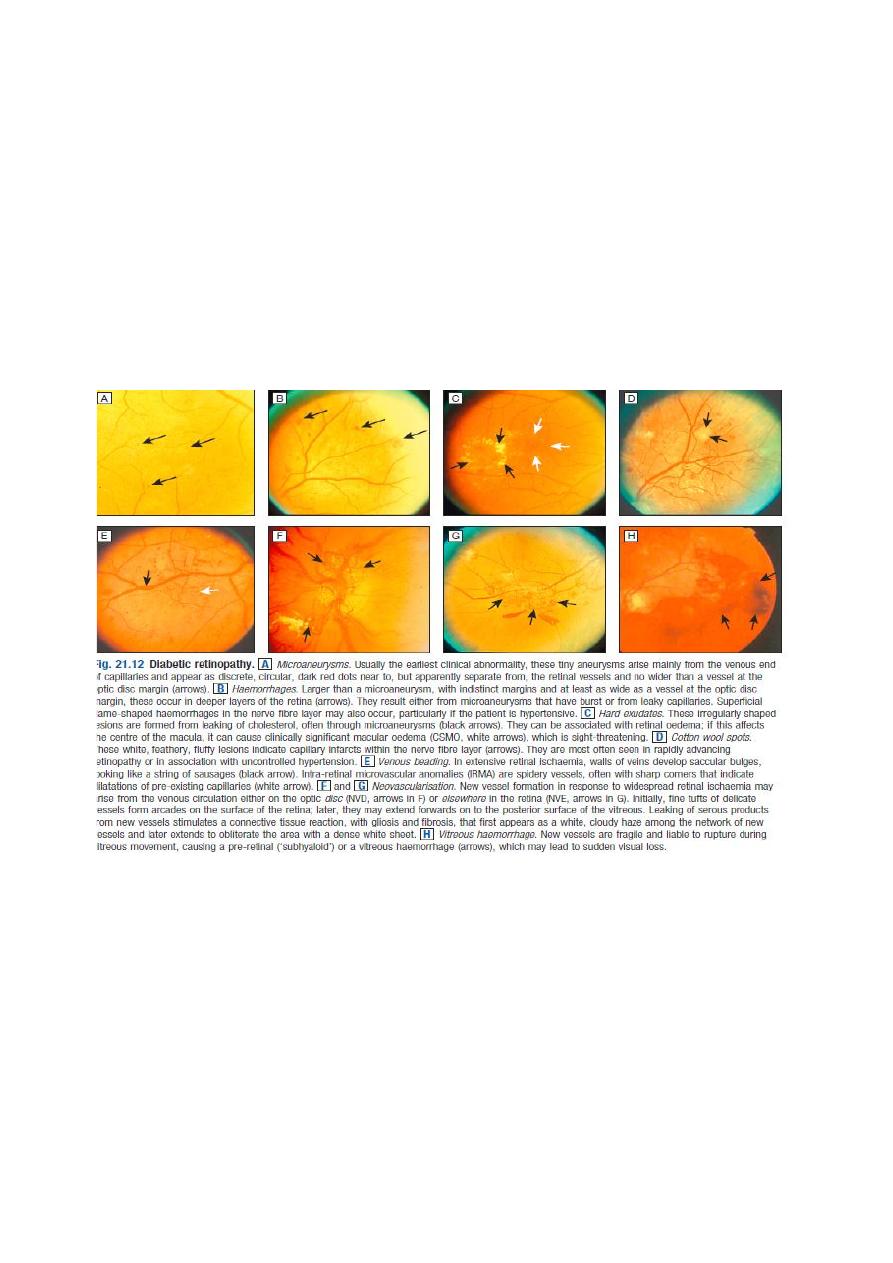
Diabetic retinopathy
Microaneurysms and retinal haemorrhages.
Cotton wool spots,
Venous beading and
Intra-retinal microvascular abnormalities.
The disease may then progress to proliferative DR, which is characterised by
Growth of new blood vessels on the retina or optic disc .The new vessels are abnormal
and often bleed, leading to vitreous haemorrhage, subsequent fibrosis and scarring,
and finally tractional retinal detachment.
**
Diabetic nephropathy
Among the most common causes of end-stage renal failure in developed countries.
About 30% of patients with type 1 diabetes have developed diabetic nephropathy 20 years
after diagnosis, but the risk after this time falls to less than 1% per year
29
Risk factors for diabetic nephropathy
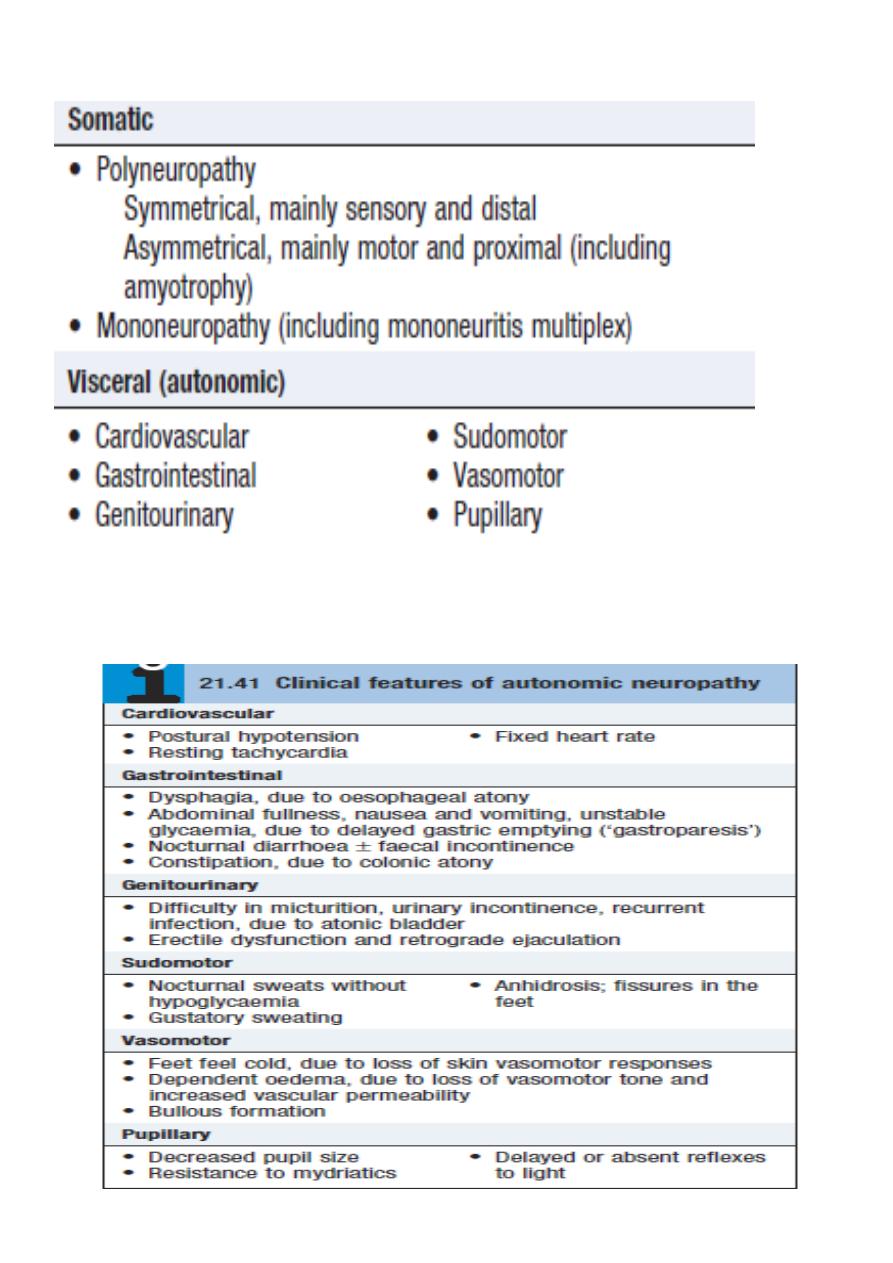
Natural history of diabetic nephropathy (Imp.)
30
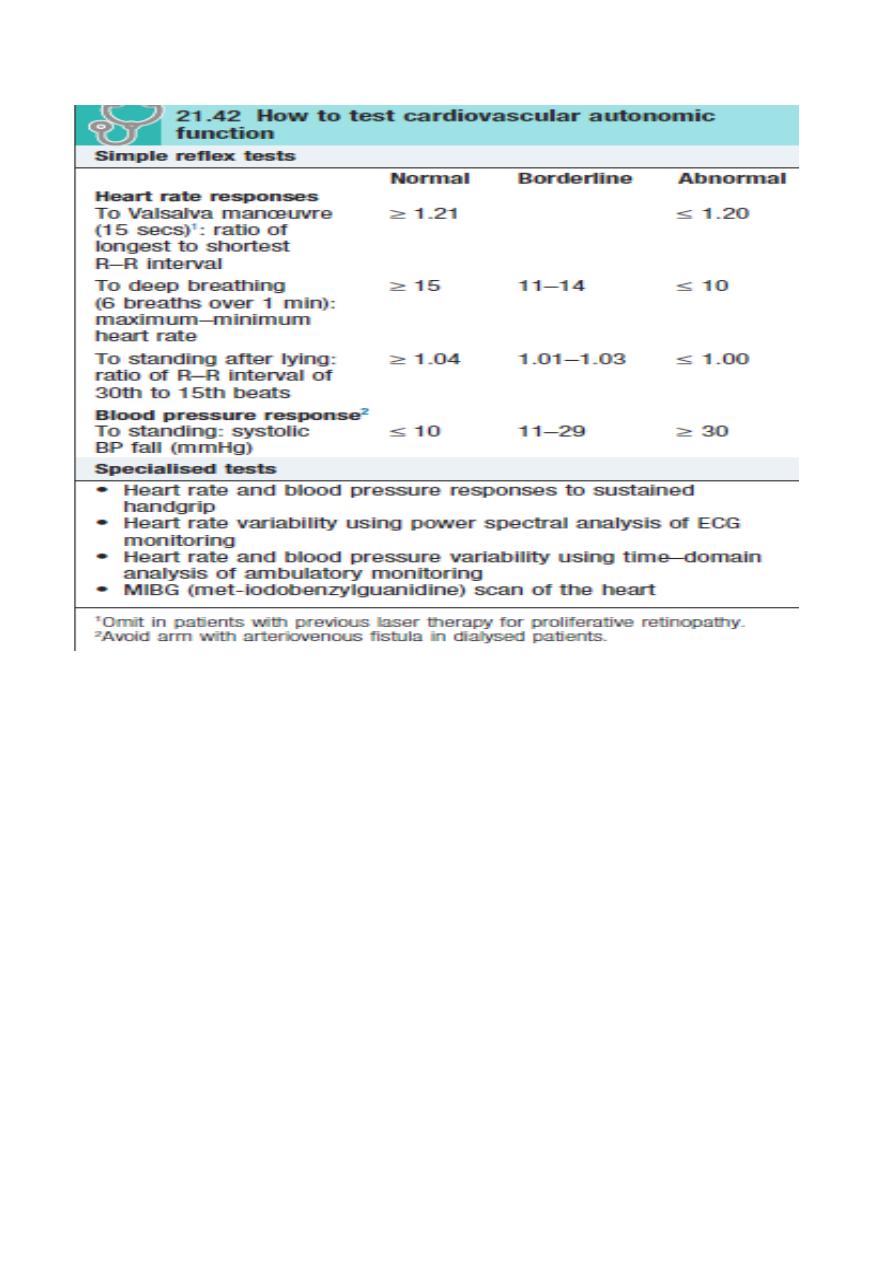
Diabetic neuropathy Classification
**
31
**

32
**
Preventing diabetes complications
1. Glycaemic control
2. Control of other risk factors
3. Management of blood pressure
4. Management of dyslipidaemia
33
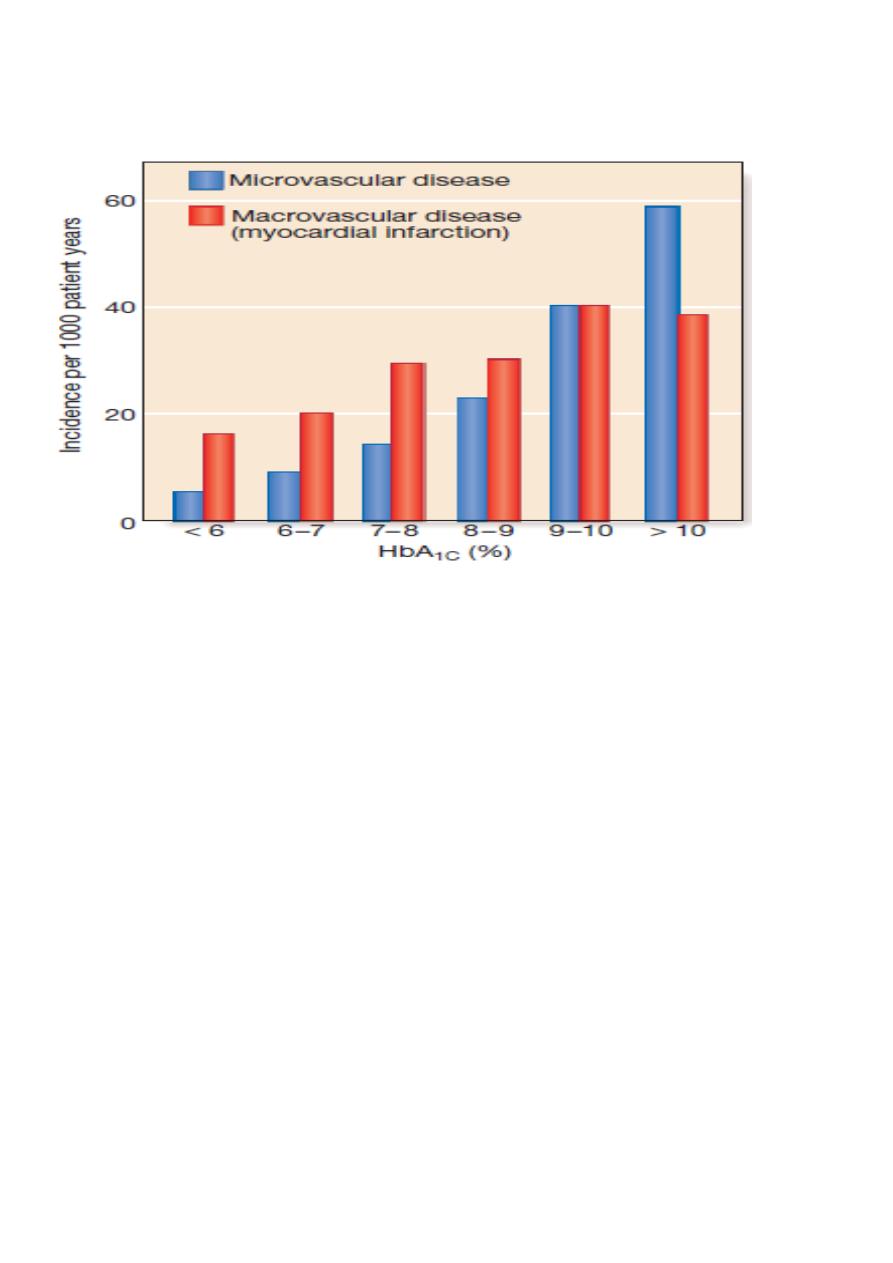
Association between HbA1c and risk of diabetes complications
**
A.LY
