1
Phenol Synthesis
Diazotization
of aniline
OH

Phenols
2
Phenols are compounds with an
–OH group attached to an aromatic
carbon. Although they share the same functional group with
alcohols, where the
–OH group is attached to an aliphatic carbon, the
chemistry of phenols is very different from that of alcohols.
It is the simplest member of a family of compounds .
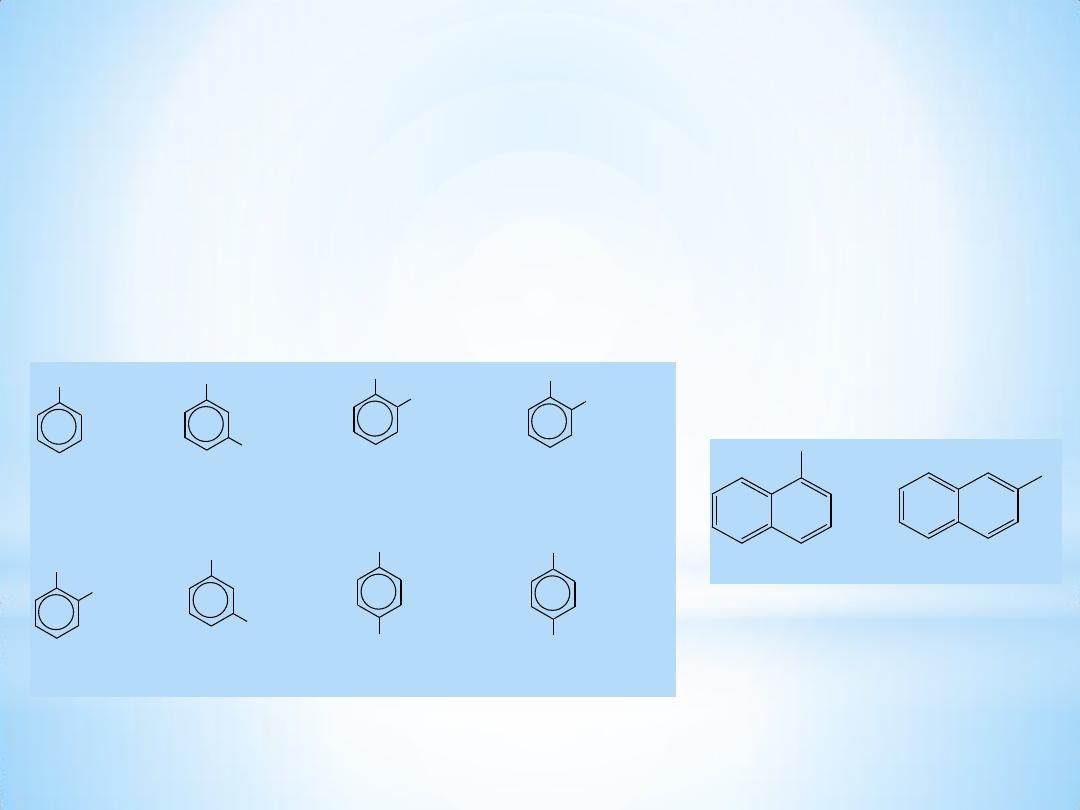
3
OH
phenol
OH
Br
m-bromophenol
CH
3
OH
o-cresol
OH
COOH
salicylic acid
OH
OH
OH
OH
OH
OH
catechol
resorcinol
hydroquinone
COOH
OH
p-hydroxybenzoic acid
OH
OH
1- Naphthol
2- Naphthol
Nomenclature
Phenols are usually named as substituted phenols. The
methylphenols are given the special name, cresols. Some other
phenols are named as hydroxy compounds.

4
physical properties
•colorless –pink needle-like crystals .
•Freely soluble in organic solvents , slightly soluble in water (9 g/100ml)
because of intermolecular hydrogen bonding between phenol and water
molecules .
•Have high B.P (180-183
o
C) because of intermolecular hydrogen
bonding between phenol molecules , M.P=42
o
C .
•phenols are weak acid , more acidic than alcohol and water but weaker
than carboxylic acids .
Intramolecular hydrogen bonding is possible in some ortho-substituted
phenols. This intramolecular hydrogen bonding reduces water
solubility and increases volatility. Thus, o-nitrophenol is steam
distillable while the isomeric p-nitrophenol is not.
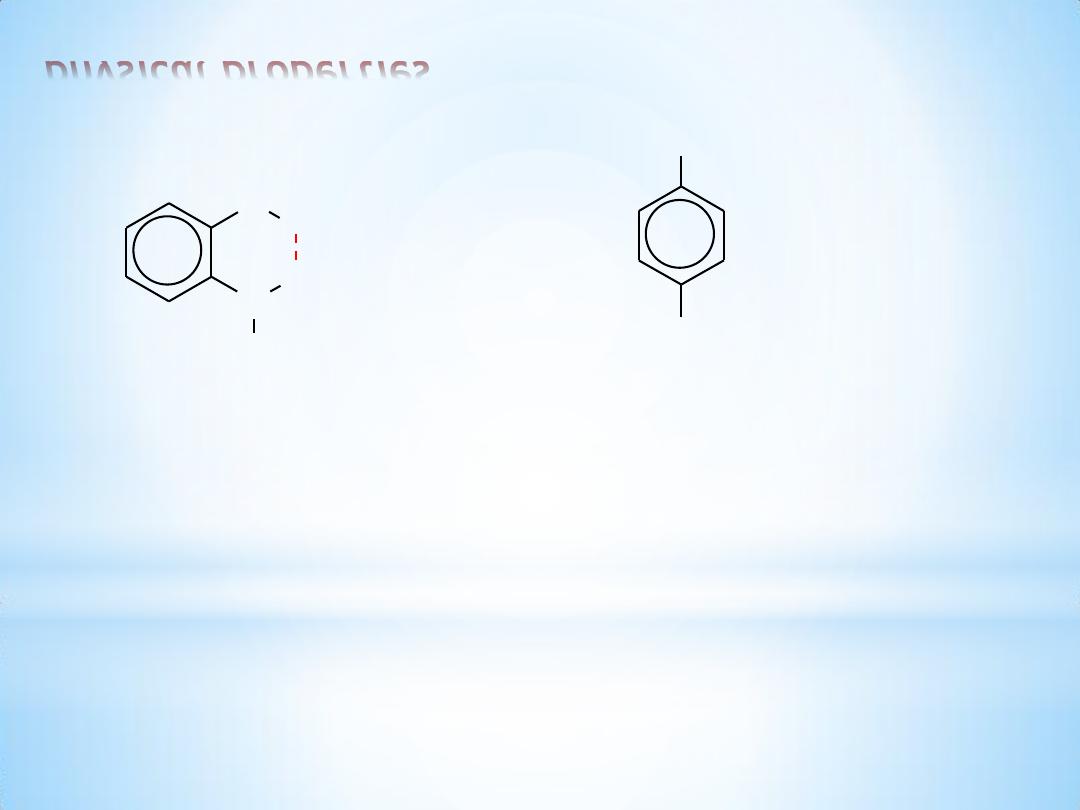
5
physical properties
N
O
H
O
O
o-nitrophenol
bp 100
o
C at 100 mm
0.2 g / 100 mL water
volatile with steam
OH
NO
2
p-nitrophenol
bp decomposes
1.69 g / 100 mL water
non-volatile with steam
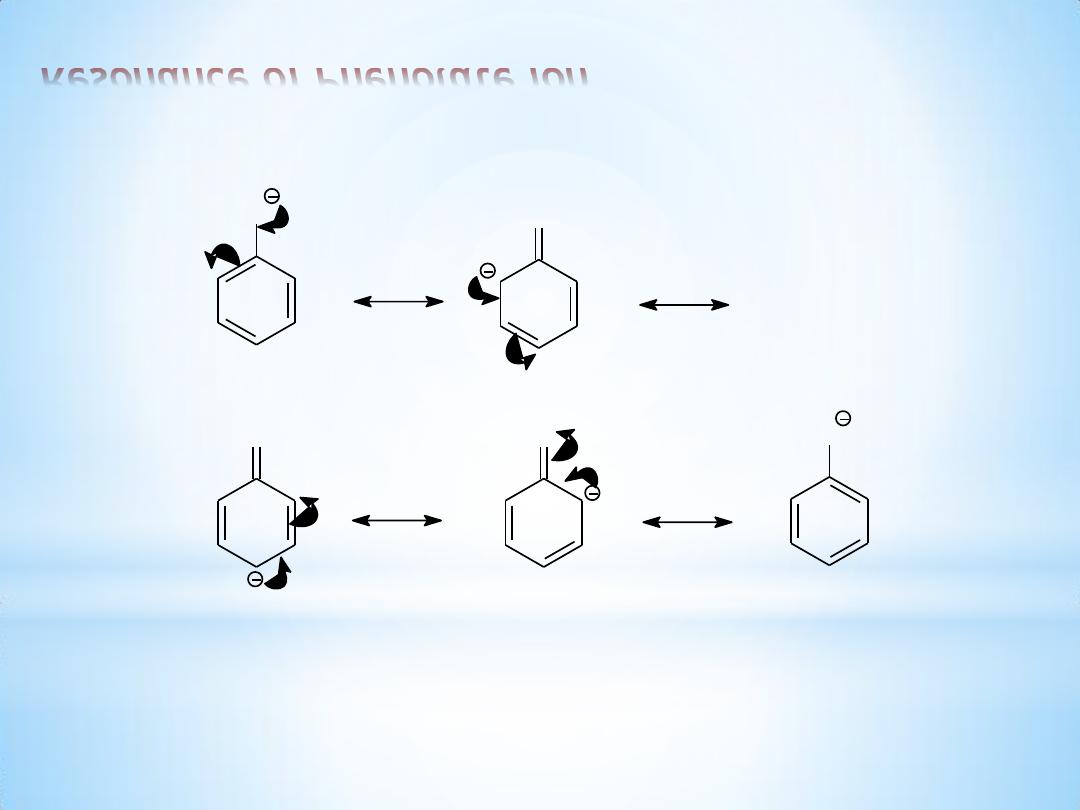
6
Resonance of Phenolate ion
O
O
O
O
O
7
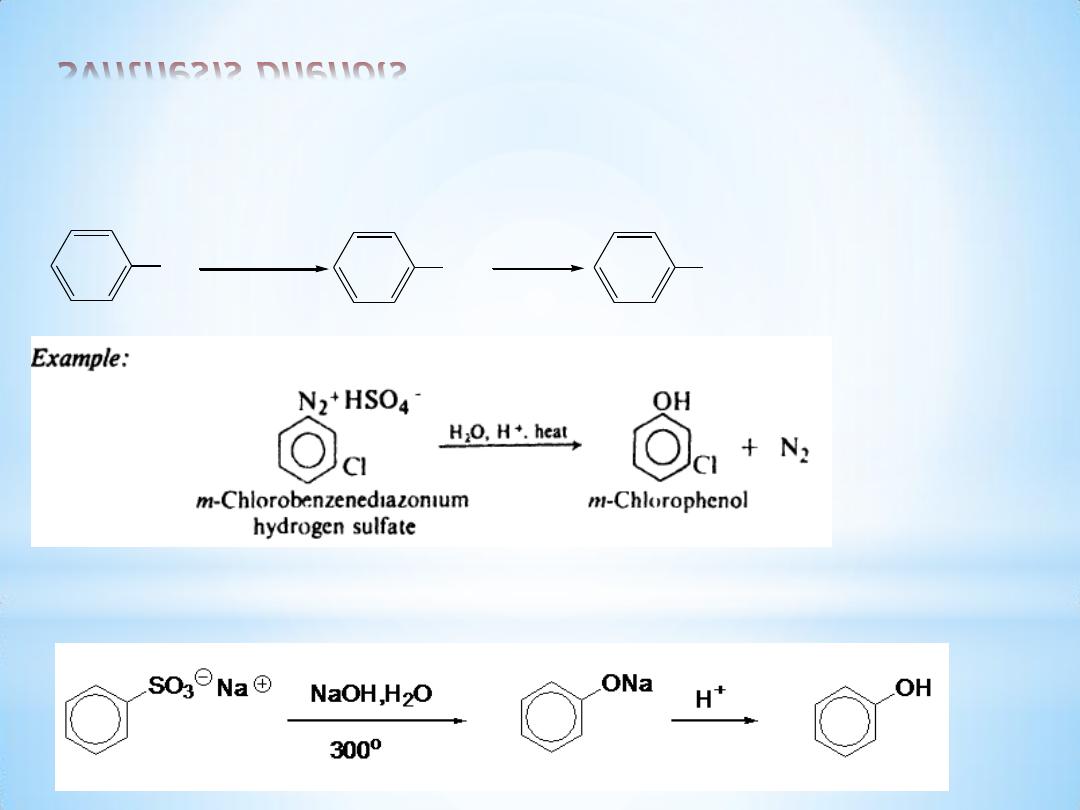
Synthesis phenols
NH
2
NaNO
2
, H
2
SO
4
H
2
O
N
2
+
Cl
-
H
+
H
2
O
OH
(1) From diazonium salts
(2) Alkali fusion of sulfonates
8
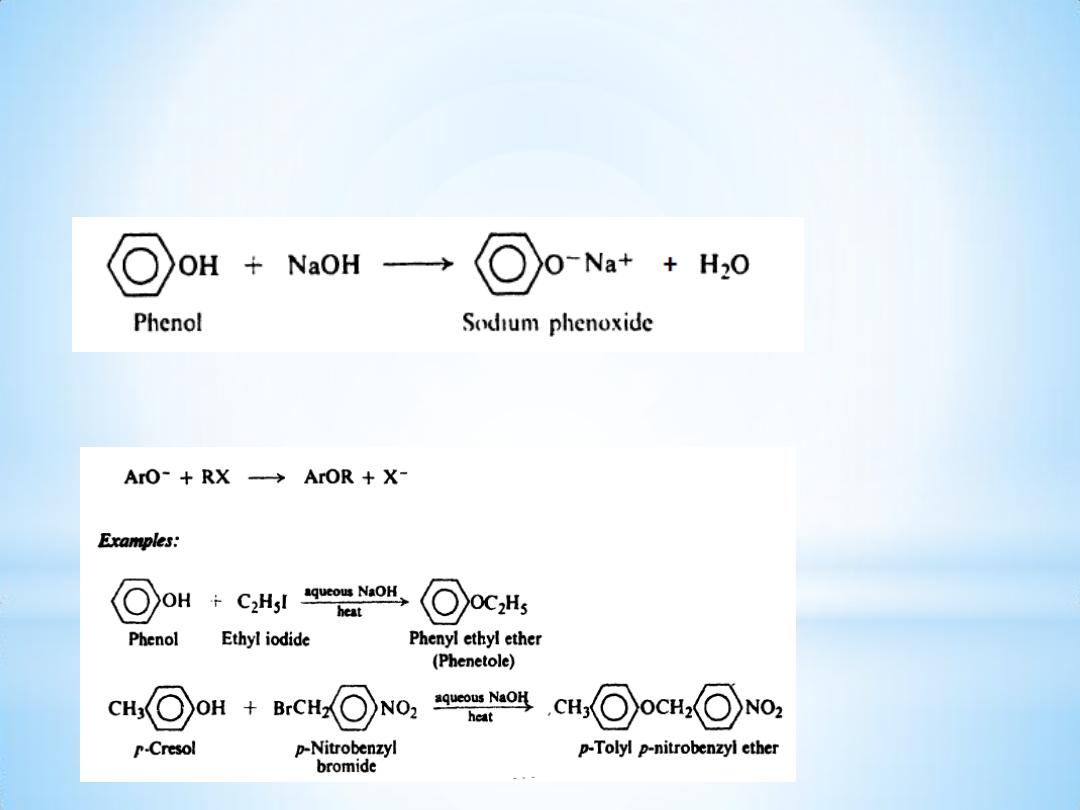
Reactions
(1) Acidity. Salt formation
(2) Ether formation. Williamson synthesis
9
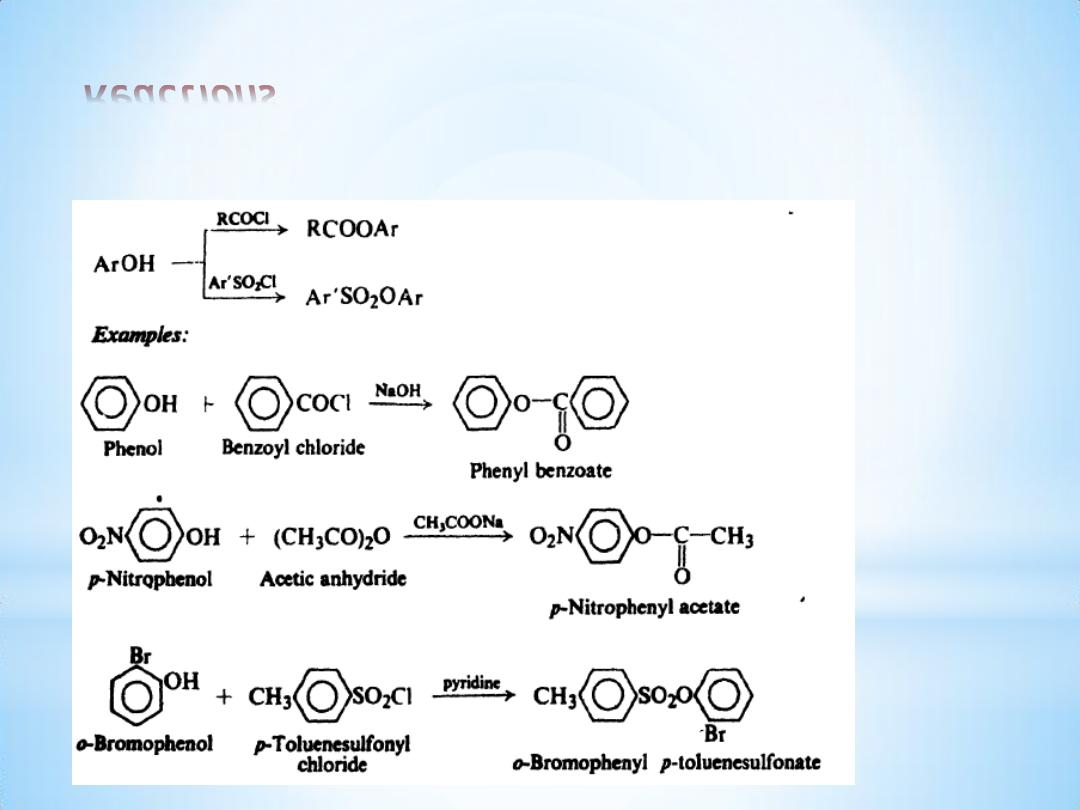
Reactions
(3) Ester formation
10
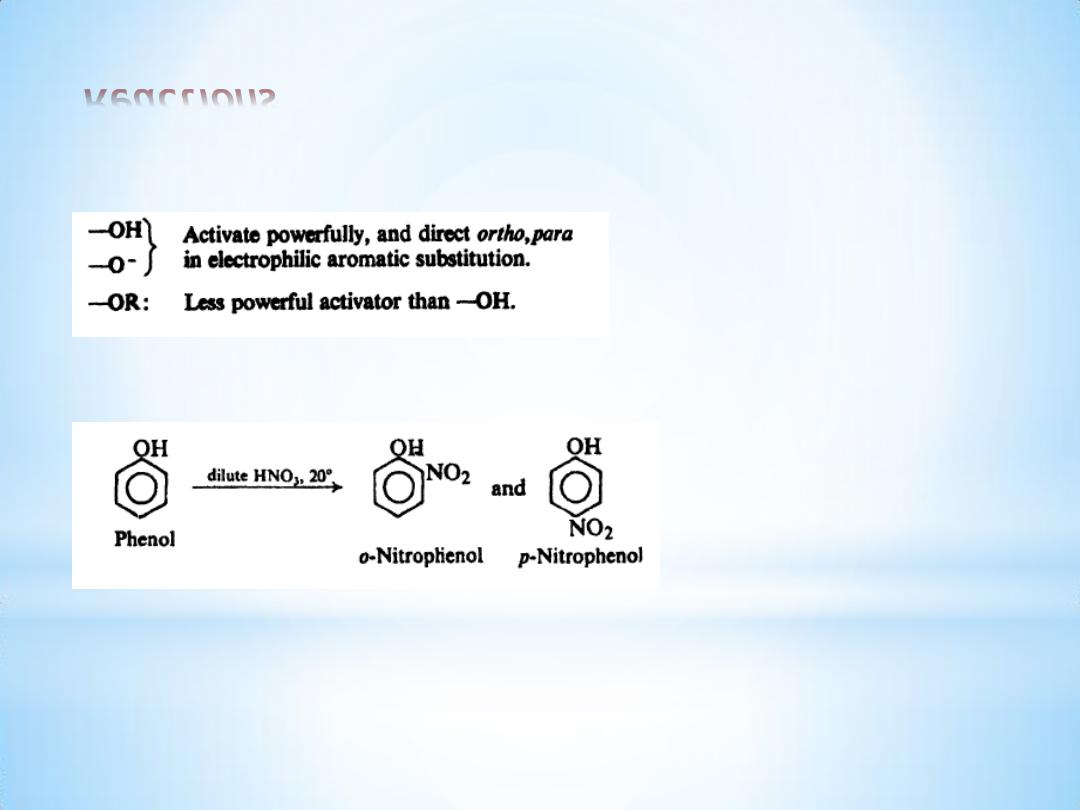
Reactions
(3) Ring substitution.
(a) Nitration.
11
Reactions
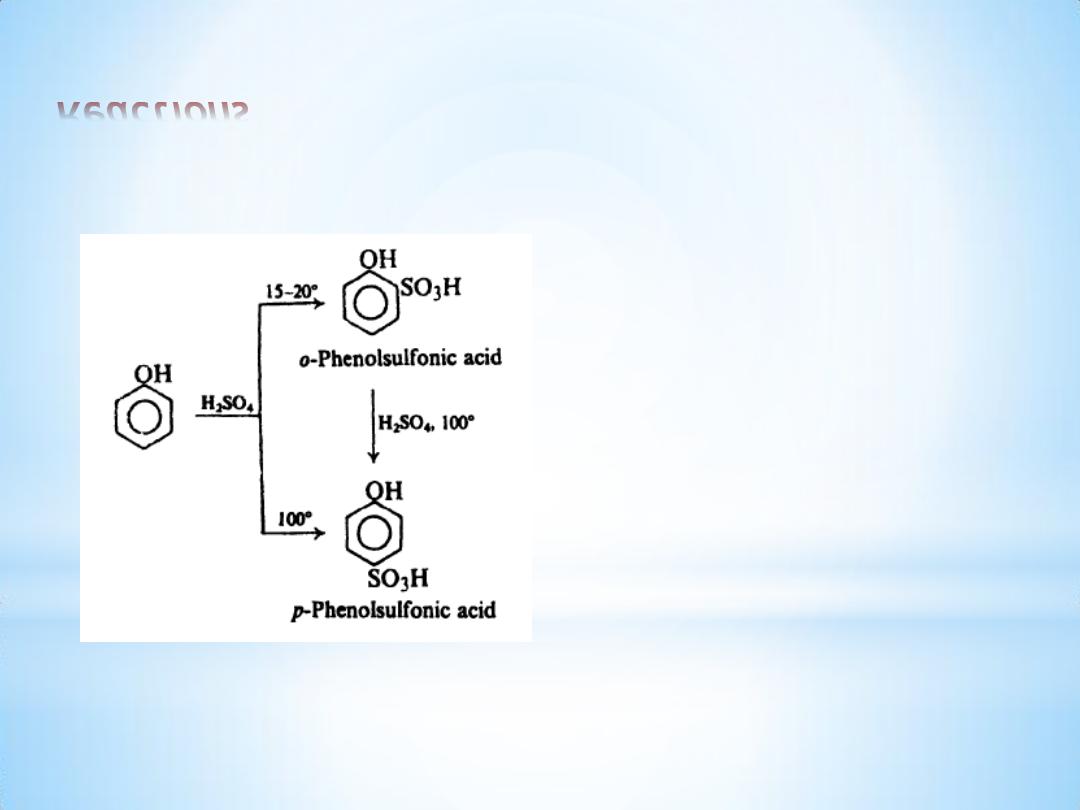
(b) Sulfonation
12
Reactions
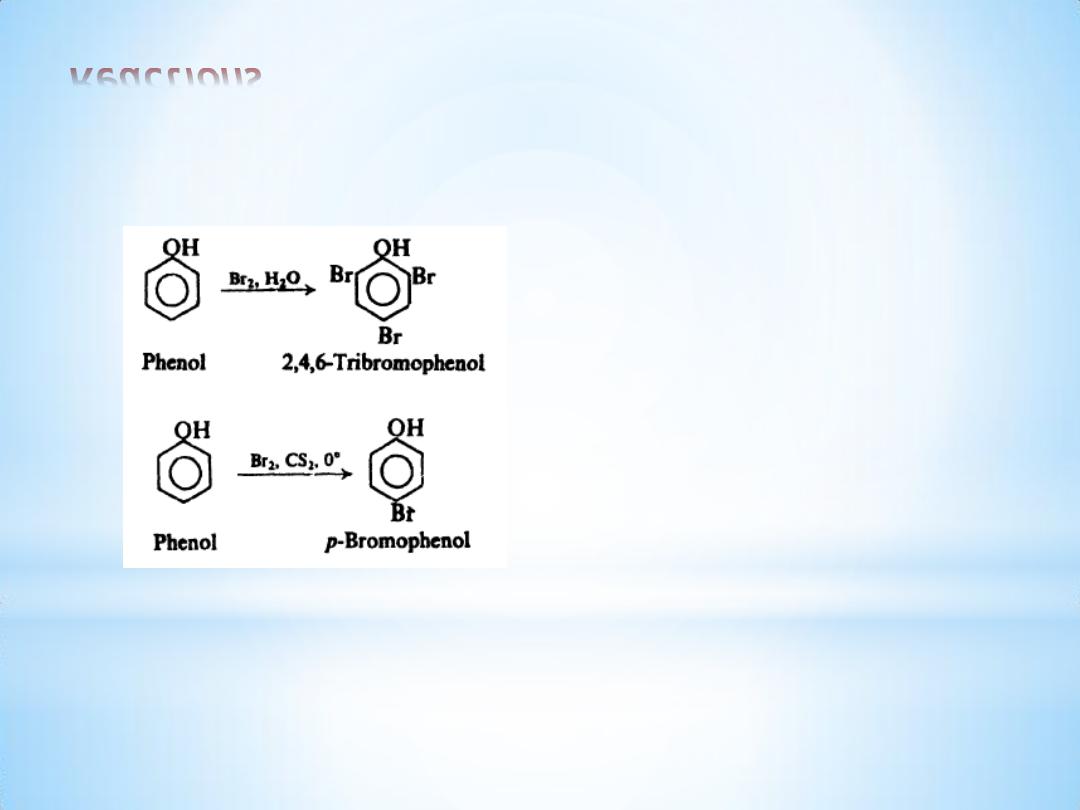
(c) Halogenation
13
Reactions
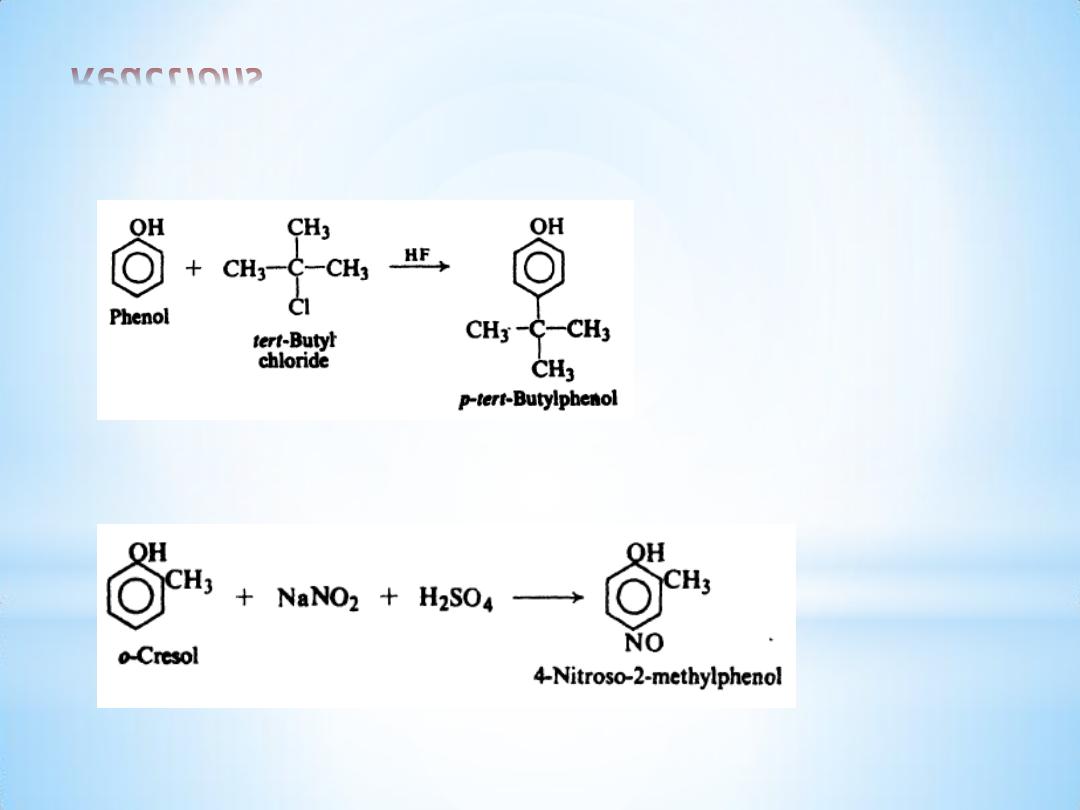
(d) Fiiedel-Crafts alkylation
(e) Nitrosation.
14
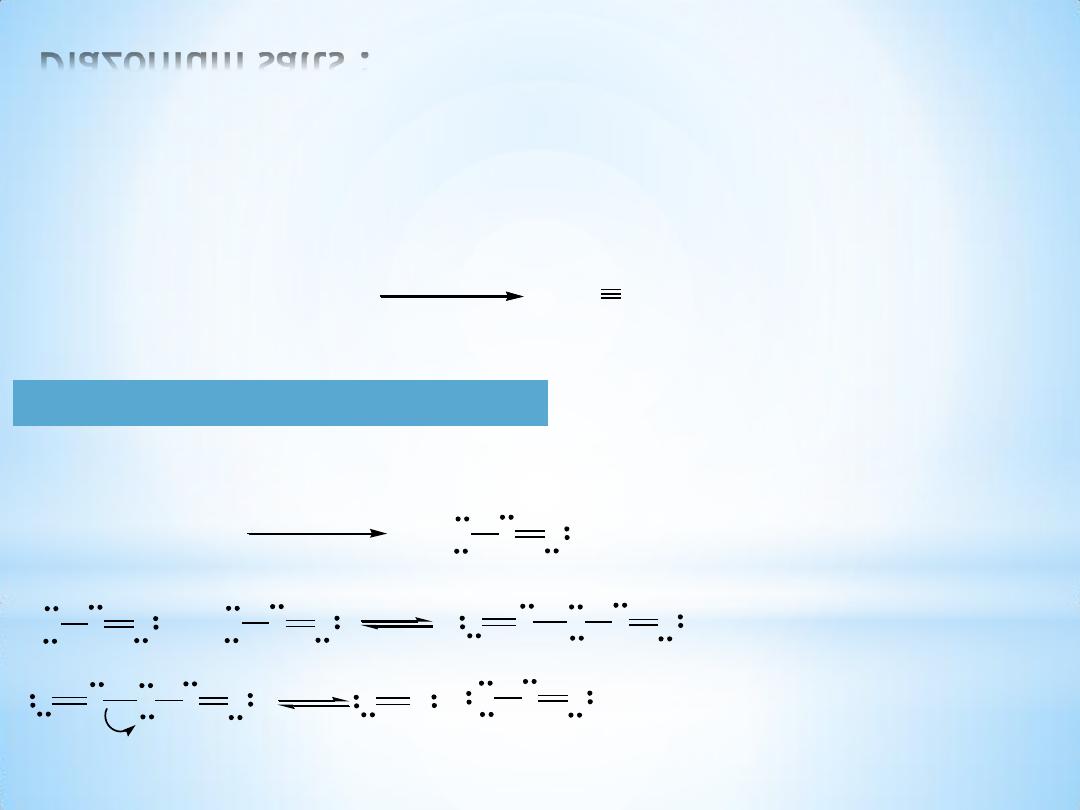
Diazonium salts :
Ar-NH
2
+
HONO +
H
2
SO
4
0-5
O
C
aq. solution
Ar-N
N
+
OSO
3
H
-
+ 2H
2
O
NaNO
2
+
H
2
SO
4
0-5
O
C
HO
N
O
+
Na
2
SO
4
Nitrous acid
HO
N
O
HO
N
O
+
O
N
O
N
O
+ H
2
O
O
N
O
N
O
N
O
+
O
N
O
+
-
Nitrosonium ion
Sodium nitirte
They can be formed by treating an aromatic 1o- amine with HNO
2
(obtained from a mixture of NaNO
2
and H
2
SO
4
at 0
o
C .
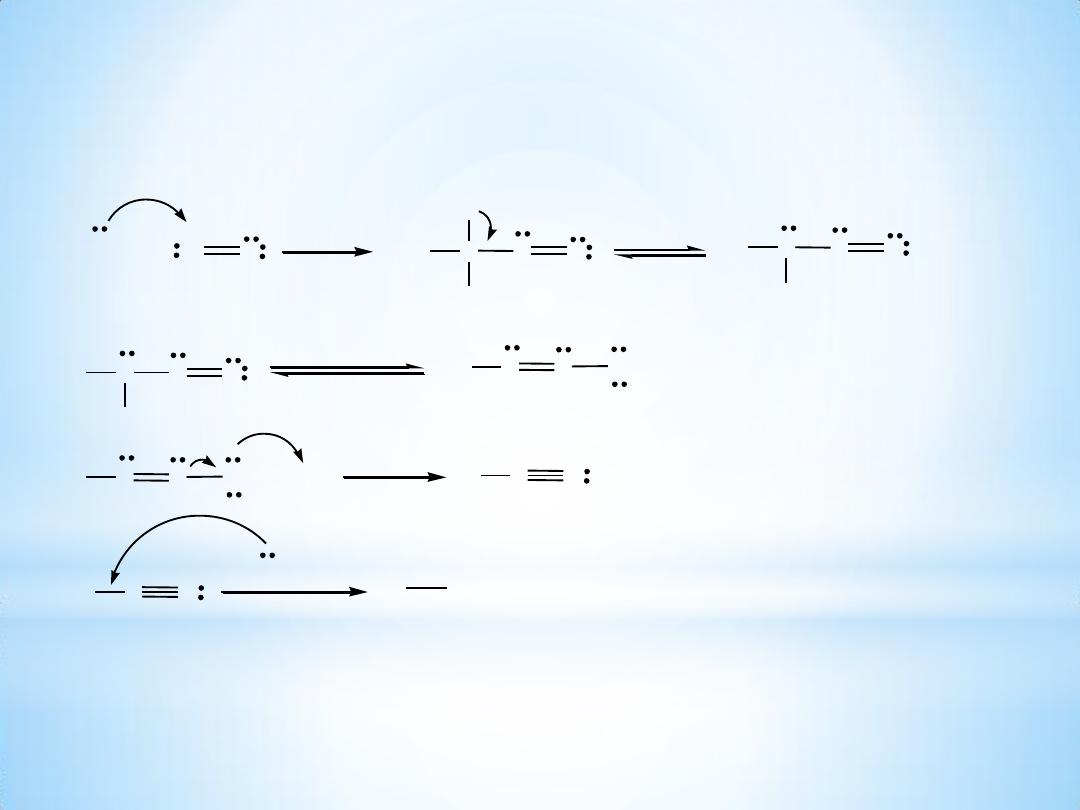
Mechanism of diazotization :
1. Nitrous acid
Ar-NH
2
+
HONO +
H
2
SO
4
0-5
O
C
aq. solution
Ar-N
N
+
OSO
3
H
-
+ 2H
2
O
They can be formed by treating an aromatic 1o- amine with HNO
2
(obtained from a mixture of NaNO
2
and H
2
SO
4
at 0
o
C .
15
2- Nucleophilic attack of 1- amine on the nitrosonium ion :
Ar-NH
2
N
O
+
+
Ar
N
H
H
N
O
+
Ar
N
H
N
O
+
H
+
Ar
N
H
N
O
Tautomerism
Ar
N
N
OH
Ar
N
N
OH
+
H
+
Ar
N
N
+
+ H
2
O
Ar
N
N
+
HO-H
Ar
OH
+
N
2
+
H
+

16
Continuously
Procedure :
1. To a (50 ml) of H
2
O , add (7.5 ml) of H
2
SO
4
and then
add (7.5 ml) of aniline , with stirring .
2. Cool the solution by using ice
– bath with stirring.
3. In another beaker , dissolve (5.5 gm) of sodium nitrite in
(25 ml) of H
2
O .
4. Transfer (22 ml ) of sodium nitrite solution to a separator
funnel .
5. Add drop wisely the solution of sodium nitrite to the
aniline sulfate with keeping temperature at ( 0
o
C)
and
shaking
17
NH
2
OH
Wt. (aniline)
Wt. (phenole)
M.wt. (aniline)
M.wt. (phenole)
Wt. (phenole) =
Wt. (aniline) x M.wt. (phenole)
M.wt. (aniline)
= no. of moles (aniline) x M.wt. (phenole)
Theoretically wt . of phenole = no. of moles (aniline) x M.wt. (phenole)
Percent Yeild % =
practical wt.
Theoretically wt .
x 100
Calculations :
