
Antineoplastic Agents
Lecture 4
ANTIBIOTICS AND NATURAL PRODUCTS

ANTIBIOTICS AND NATURAL PRODUCTS
Are derived from natural sources, several of these being
obtained from microbial sources (antibiotics). Many of the
antineoplastic antibiotics are produced by the soil fungus
Streptomyces. Both the antibiotic and natural product
classes have multiple inhibitory effects on cell growth;
however, they primarily act to disrupt DNA function and cell
division.
There are several mechanisms by which these agents target
DNA, including:
1. intercalation
2.alkylation
3.Strand breakage either directly or as a result of enzyme
inhibition.
2

Intercalation is a process by which a planar molecule of
the appropriate size inserts itself between adjacent
base pairs of DNA and causing a local unwinding that
may disrupt the normal template function of DNA.
The interaction of the intercalator between adjacent
base pairs occurs by:
Overlap of p-orbitals of the intercalator and the base
pairs. The p-orbitals of the intercalation species are
provided by a combination of aromatic and conjugated
systems that impart the planarity required for
intercalation.
3

The drug–DNA interaction is further stabilized by side
chains attached to the intercalation species. The side
chains often include a cationic moiety, which may form
ionic bonds with the anionic phosphate backbone.
Alternative modes of stabilization may occur through a
combination of van der Waals interaction or hydrogen
bonds.
.
The overall result of these interactions is to cause
1. A local shape distortion of the DNA leading to the
inhibition of normal DNA function.
2. Inhibition of topoisomerase enzymes.
4

Topoisomerase enzymes are responsible for the
unwinding and relaxation of DNA so that transcription
may occur.
types of topoisomerase enzymes
1. Topoisomerase I makes a single-strand break in DNA
and subsequently allows the other strand to spin,
relieving any tension associated with the packing
process and subsequently reseals the broken strand.
2. Topoisomerase II makes double-strand breaks in DNA
allowing an intact chain to pass through and then
subsequently reseals the double-strand break.
5

Several natural products that are capable of disrupting the
formation and function of the mitotic spindle. These
include the epipodophyllotoxins, the taxanes, and the
vinca alkaloids. The mitotic spindle forms during the M
phase of the cell cycle and is responsible for moving the
replicated DNA to opposite ends of the cell in preparation
for cell division.
6
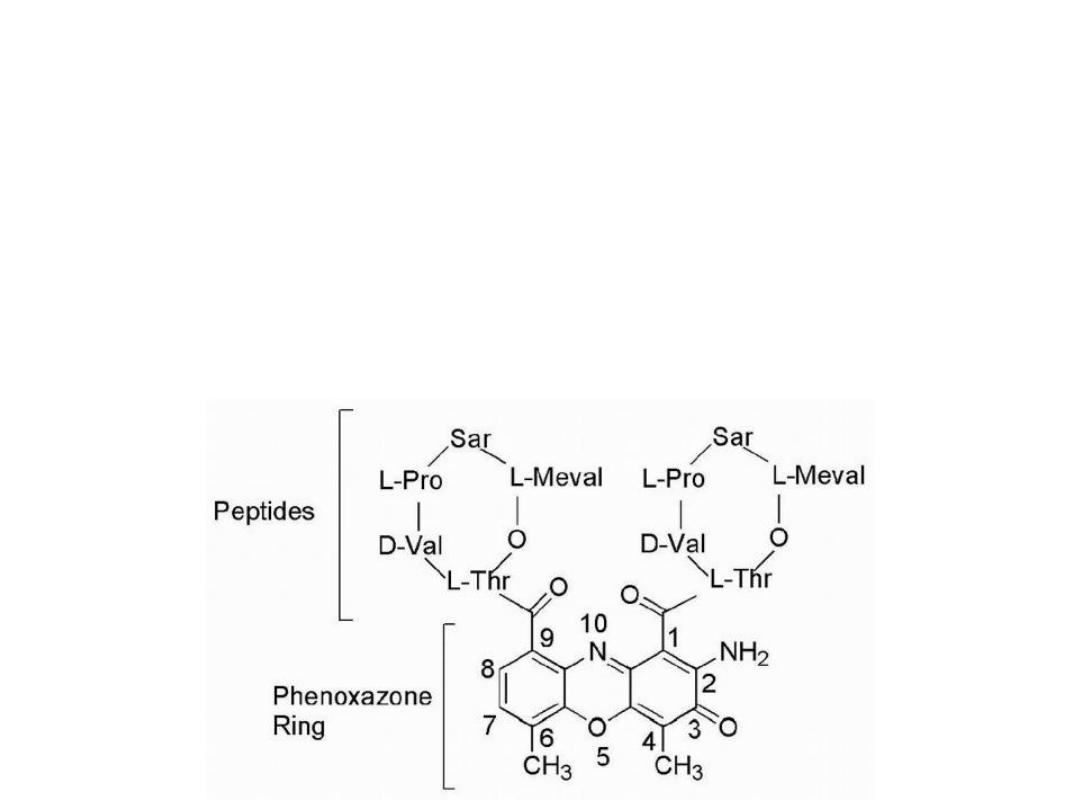
Actinomycins
Are a group of compounds that are isolated from various
species of Streptomyces, all of which contain the same
phenoxazone chromophore but differ in the attached
peptide portion.
From this group emerged actinomycin D, which is known
as dactinomycin
7

Dactinomycin it contains identical pentapeptides bound
through an amide linkage utilizing the amino group of L-
threonine with carbonyls at positions 1 and 9.
Dactinomycin binds non-covalently to double-stranded
DNA by partial intercalation between adjacent guanine
cytosine bases resulting in inhibition of DNA function.
8
The preference for GpC base pairs is thought to be partly
related to the formation of a hydrogen bond between the
2-amino groups of guanine and the carbonyls of the L-
threonine residues
.
9

1. The primary effect is the inhibition of DNA-directed
RNA synthesis ( specifically RNA polymerase).
Mechanism of action
10
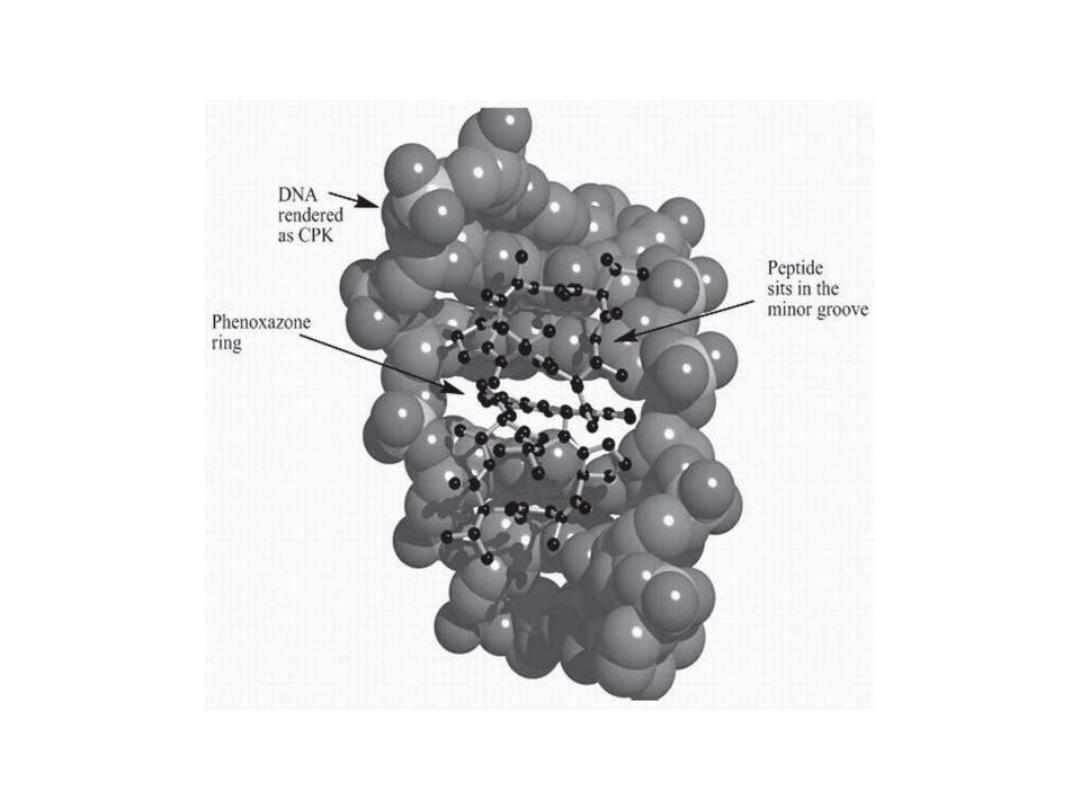
The planar phenoxazone ring, which facilitates
intercalation between DNA base pairs. The peptide
loops are located within the minor groove and provide
for additional interactions.

2. Inhibition of DNA synthesis, and the agent is considered
cell cycle specific for the G1 and S phases.
3. Inhibition of topoismerase II.
Resistance
Resistance is caused by:
1. Decreased ability of tumor cells to take up the drug
2. P-glycoprotein (Pgp)-mediated efflux.
11
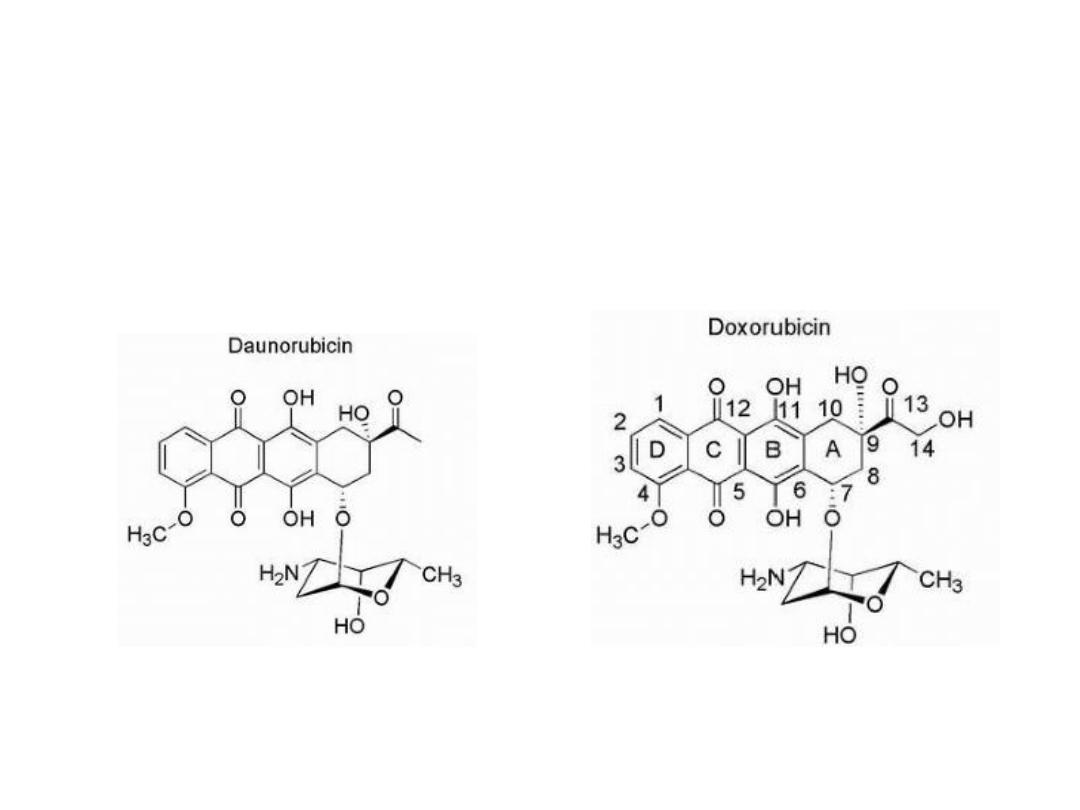
Anthracyclines
Are characterized by a planar oxidized anthracene nucleus
fused to a cyclohexane ring that is subsequently connected
via a glycosidic linkage to an amino sugar.
12

Mechanism of action
The accepted mechanism involves intercalation followed by
inhibition of topoisomerase II resulting in strand breakage
leading to apoptosis
The anthracyclines are considered specific for the S phase of
the cell cycle.
13

In the case of doxorubicin and daunorubicin, specificity for
GpC is provided by the hydrogen bonding between O-9 of
the anthracycline and N-2 and N-3 of guanine.
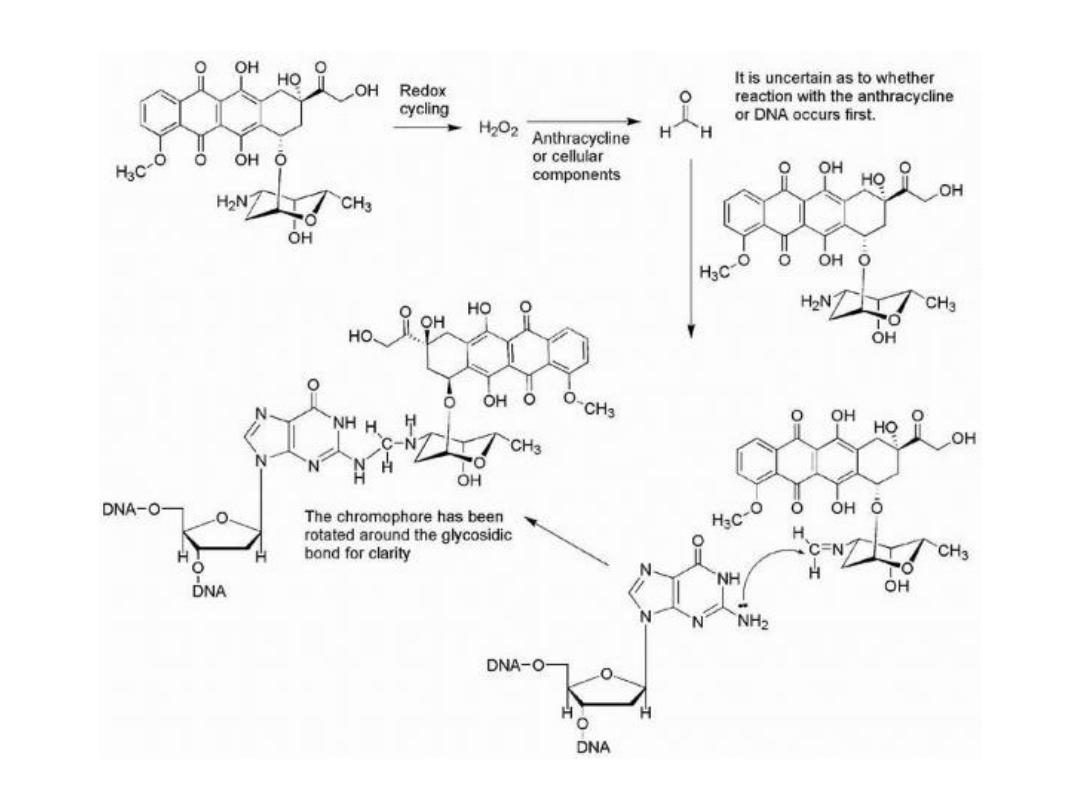
The formation of covalent bonds between anthracyclines and
DNA also is supported by several studies in which
formaldehyde is produced by oxidation of cellular
components or other anthracycline molecules. The
generated formaldehyde may then form a methylene bridge
between the 4′-amino group of the anthracycline and the 2-
amino group of guanine in DNA
14
15

Anthracyclines produce several adverse effects that are
typical for antineoplastics, cardiotoxicity is a special
concern with this class of agents. The associated
cardiomyopathies and congestive heart failure (CHF) have
been related to the ability of these compounds to undergo
redox cycling and give free radicals.
Resistance
- Decreased expression of topoisomerase II.
- Mutations in this enzyme that decrease binding of the
drug.
- P-glycoprotein (Pgp)-mediated efflux.
16
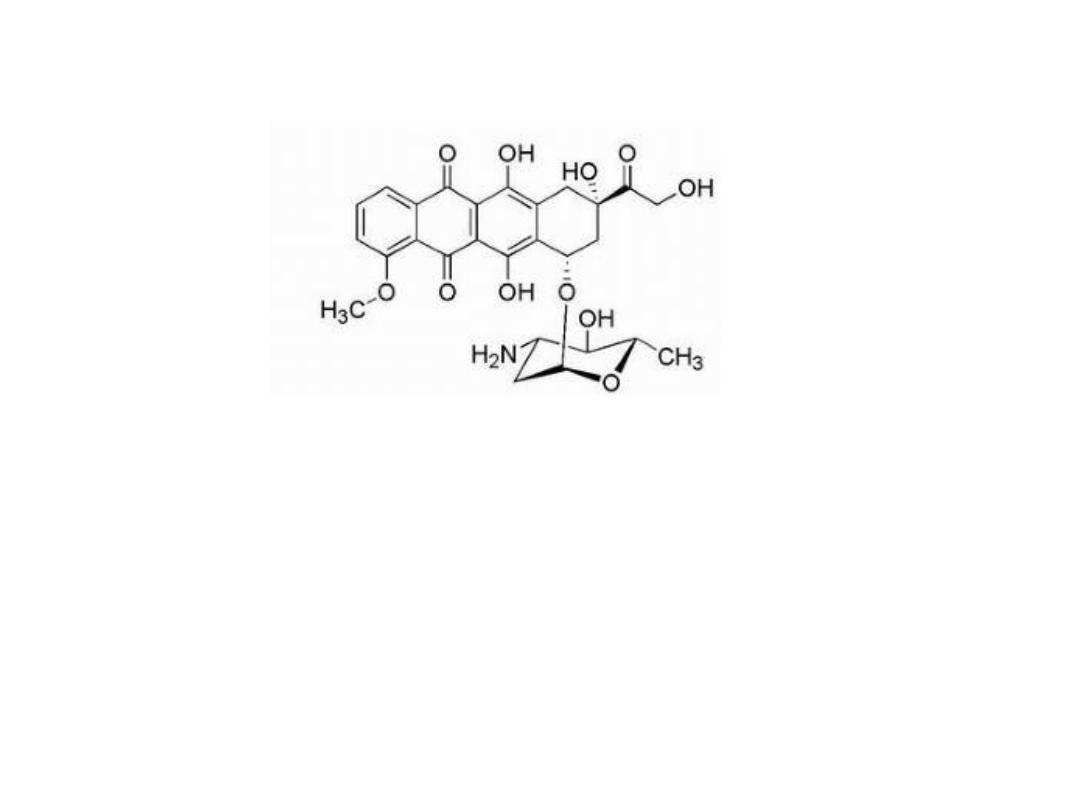
EPIRUBRICIN HYDROCHLORIDE
Epirubicin is the 3′-epimer of doxorubicin, which exhibits a lower
level of cardiotoxicity compared with doxorubicin. The reduced
cardiotoxicity has been attributed to the epimerization of 3′-OH,
which places this -OH function in an equatorial position resulting
in increased glucuronidation, faster clearance, and decreased
metabolic reduction to epirubicinol, the C-13 alcohol (compared
with
doxorubicin).
The
glucuronide,
which
forms
via
glucuronidation of the 3′-alcohol
17

Epipodophyllotoxins
Are semisynthetic derivatives of podophyllotoxin
Etoposide acts on topoisomerase II.
The agents are considered cell cycle specific and act in
the late S and G2 phases of the cell cycle.
18
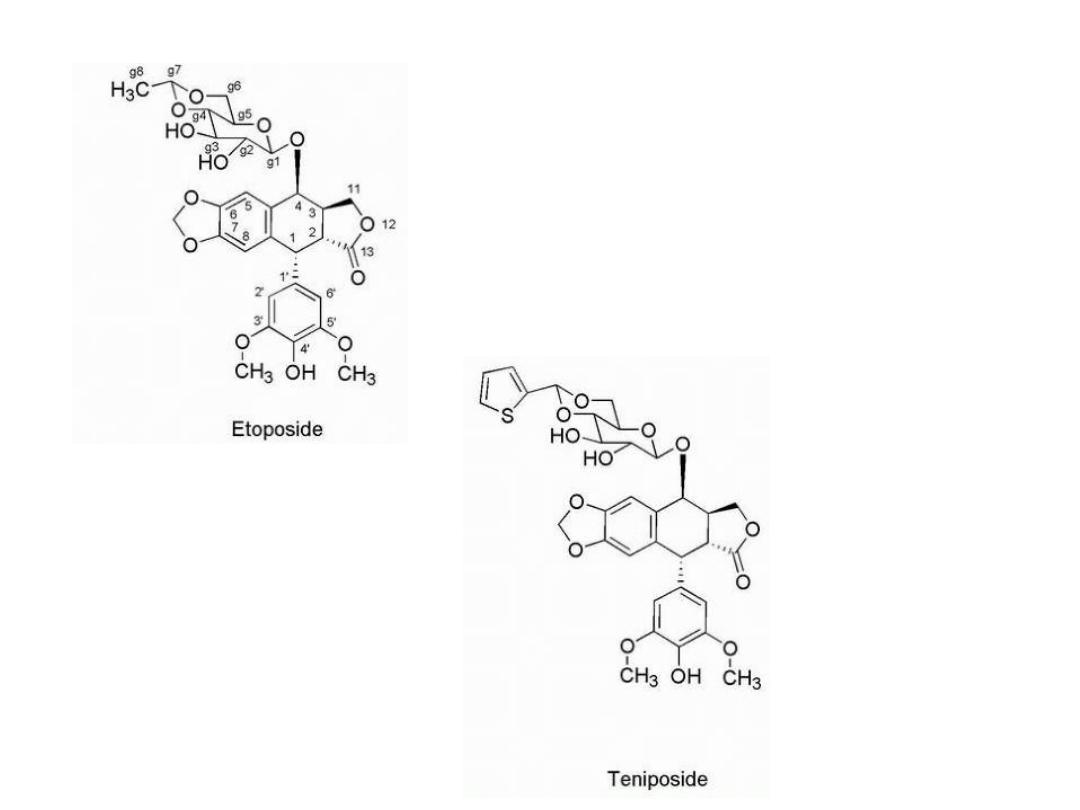
Replacement of the glycosidic 8-methyl
group with thiophene gives tenoposide,
which is 10-fold more potent than
etoposide. The glycosidic moiety is not
an absolute requirement for activity,
and other more active compounds are
known in which it has been replaced.
19

The 4′-OH group is important for the activity of the
compounds, and loss of this functionality results in greatly
reduced levels of strand breaks. Removal of one of the
adjacent methoxy groups by oxidative-O-dealkylation gives
the catechol analogs, which are more potent than the
parent molecules. The catechol analogs may be further
oxidized to give the quinones, which are also more active
than the parent
20

1. Increased efflux by Pgp.
2. Topoisomerase II levels may decrease or develop altered
binding sites with lower affinity for these agents.
3. Increased DNA repair mechanisms.
Resistance
21
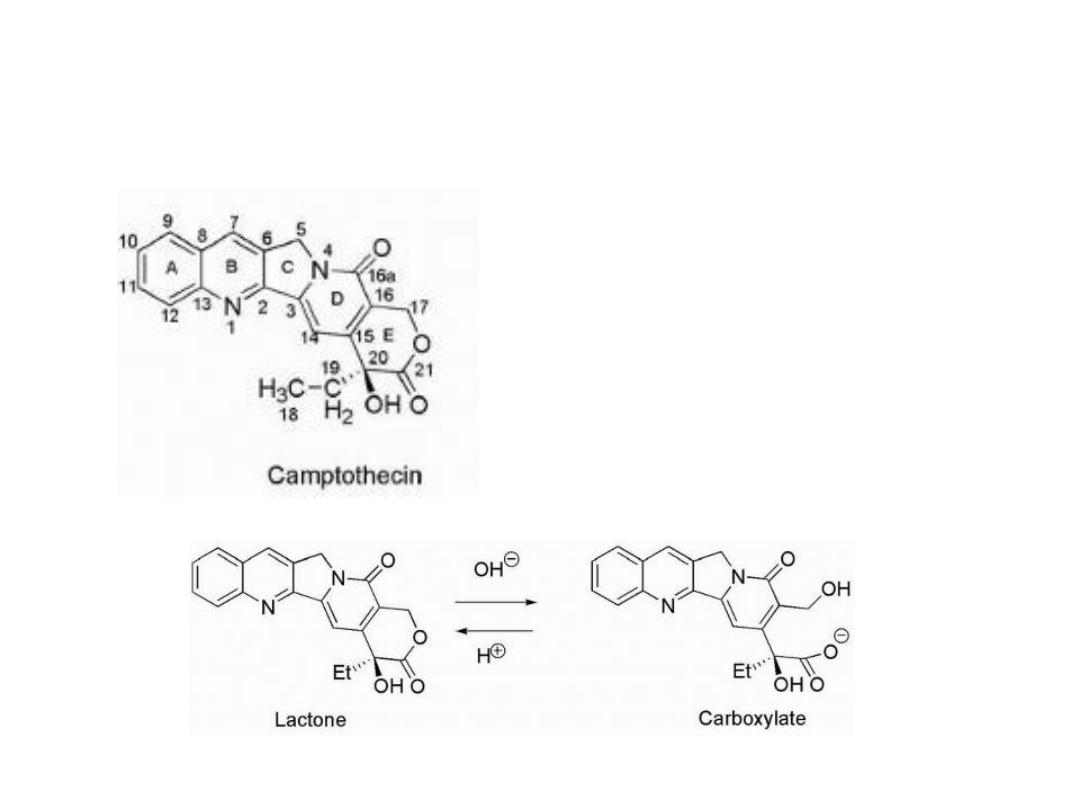
Camptothecins
Are inhibitors of topoisomerase I
Camptothecin had low water
solubility, and to overcome this:
1- The sodium salt had been
prepared and used during the
trials. This was accomplished by
hydrolysis of the E-ring lactone to
give the carboxylate salt
22
The resulting ring-opened material was 10 times less active
and more toxic.
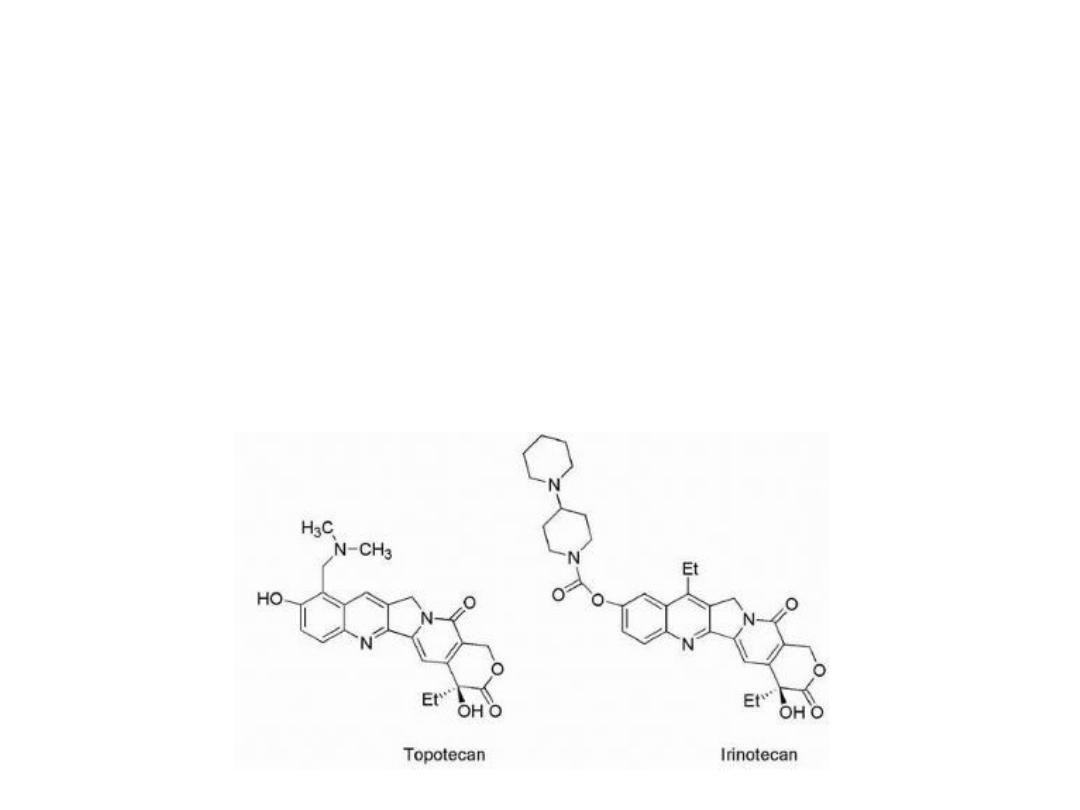
2- The incorporation of side chains containing basic amines
led to the more water-soluble derivatives, topotecan and
irinotecan. These agents could be administered as the
lactone giving better clinical results.
23

The camptothecin analogs bind to the enzyme DNA
complex after strand cleavage has occurred, the binding
site for this intercalation is only formed after the enzyme
is bound to DNA, and once this site is occupied by the
drug, it prevents the realignment necessary for resealing
of the initial strand break.
24
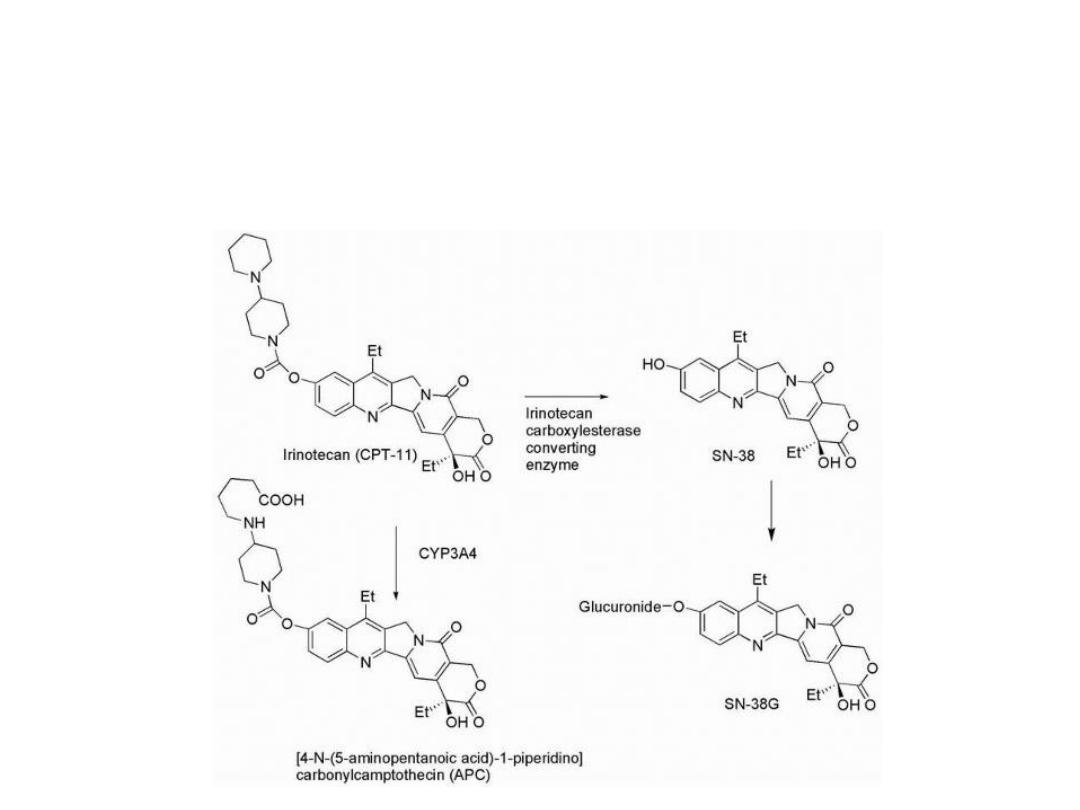
Irinotecan undergoes hydrolysis of its carbamate
moiety by irinotecan-converting enzyme to give SN-38,
which is 1,000 times more potent than the parent
compound
25

Further metabolism involves the glucuronidation of the
resulting phenolic function of SN-38 to give SN-38G,
which is inactive.
An additional metabolite forms as a result of CYP3A4-
mediated conversion to (APC), which is 100 times less
active than SN-38.
26
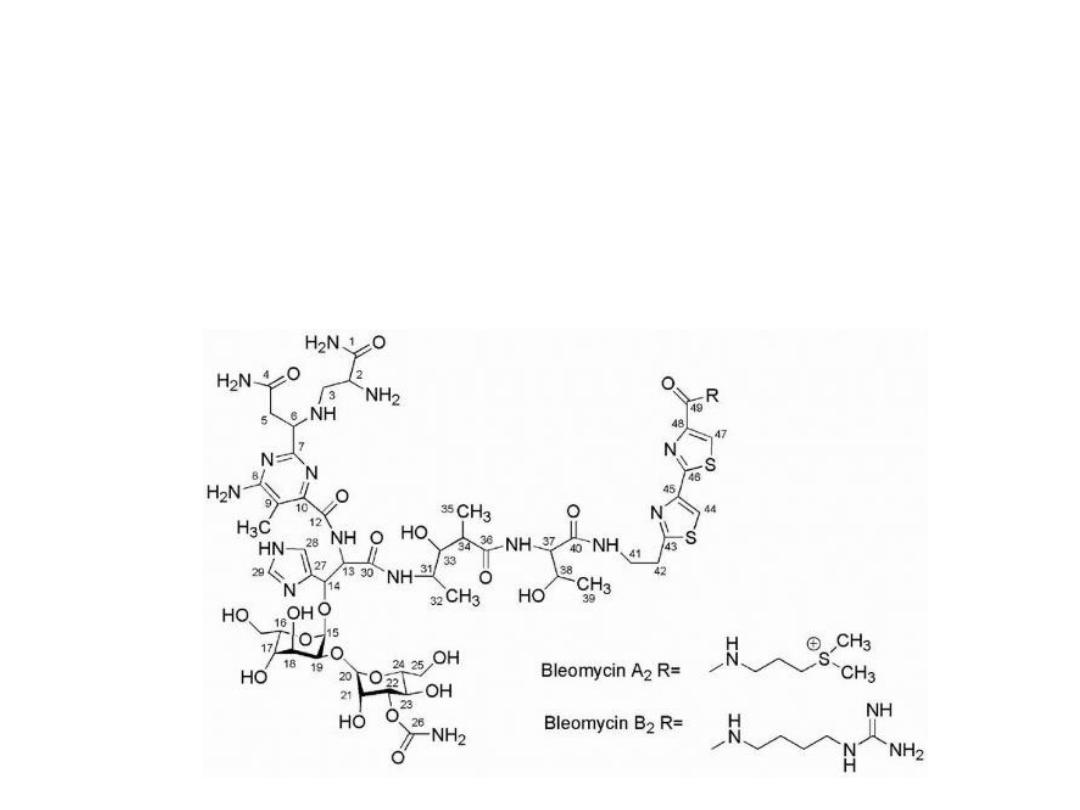
Bleomycin
Is a glycopeptide antibiotic complex, At least 13 different
fractions of bleomycin have been isolated with the clinically
used product (Blenoxane) being a mixture of predominantly
A2 (55%-70%) and B2 (25%-32%) fractions. A2 appears to
possess the greatest antineoplastic activity.
27

-Intercalates with DNA
- Destruction of DNA via cytotoxic free radicals formed by
Fe2+-chelated bleomycin.
Mechanism of action
28

Hydrolysis of the N-terminal amide to the carboxylic acid
increase the pKa of the amine at C-2 from 7.3 to 9.4,
resulting in a greater degree of ionization and decreased
binding to DNA.
The enzyme responsible for this conversion is known as
bleomycin hydrolase. Tumor cells that are resistant to
bleomycin may contain high levels of this enzyme.
Resistance
29
