
Medicine
Dr. Ali
“ Chronic Kidney Diseases ”
Total Lec: 47

Chronic kidney disease (CKD)
Dr. ALI A. ALLAWI
Nephrologist and Transplant specialist
Internist
CABMS (NEPHROLOGY) , FICMS (NEPHROLOGY) FICMS (MEDICINE)
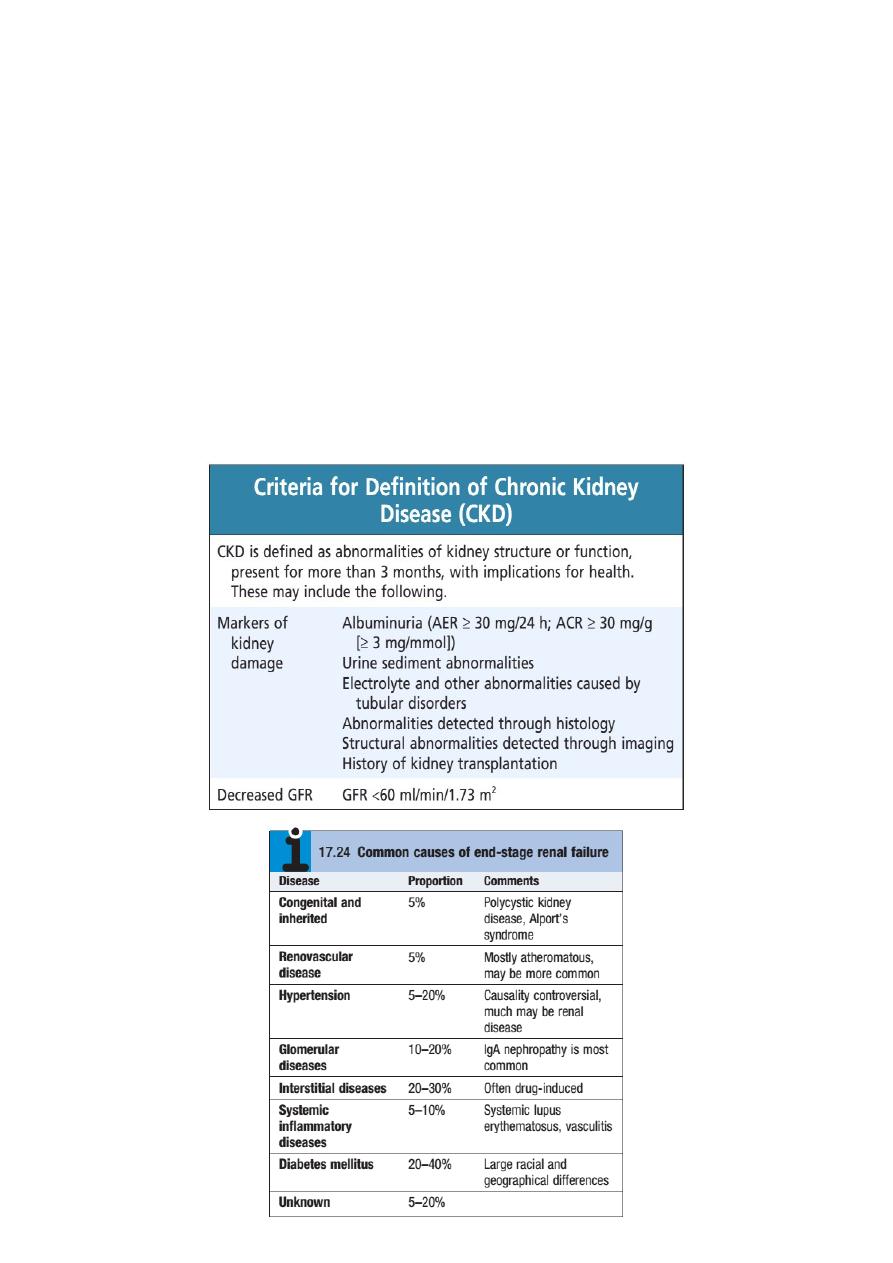
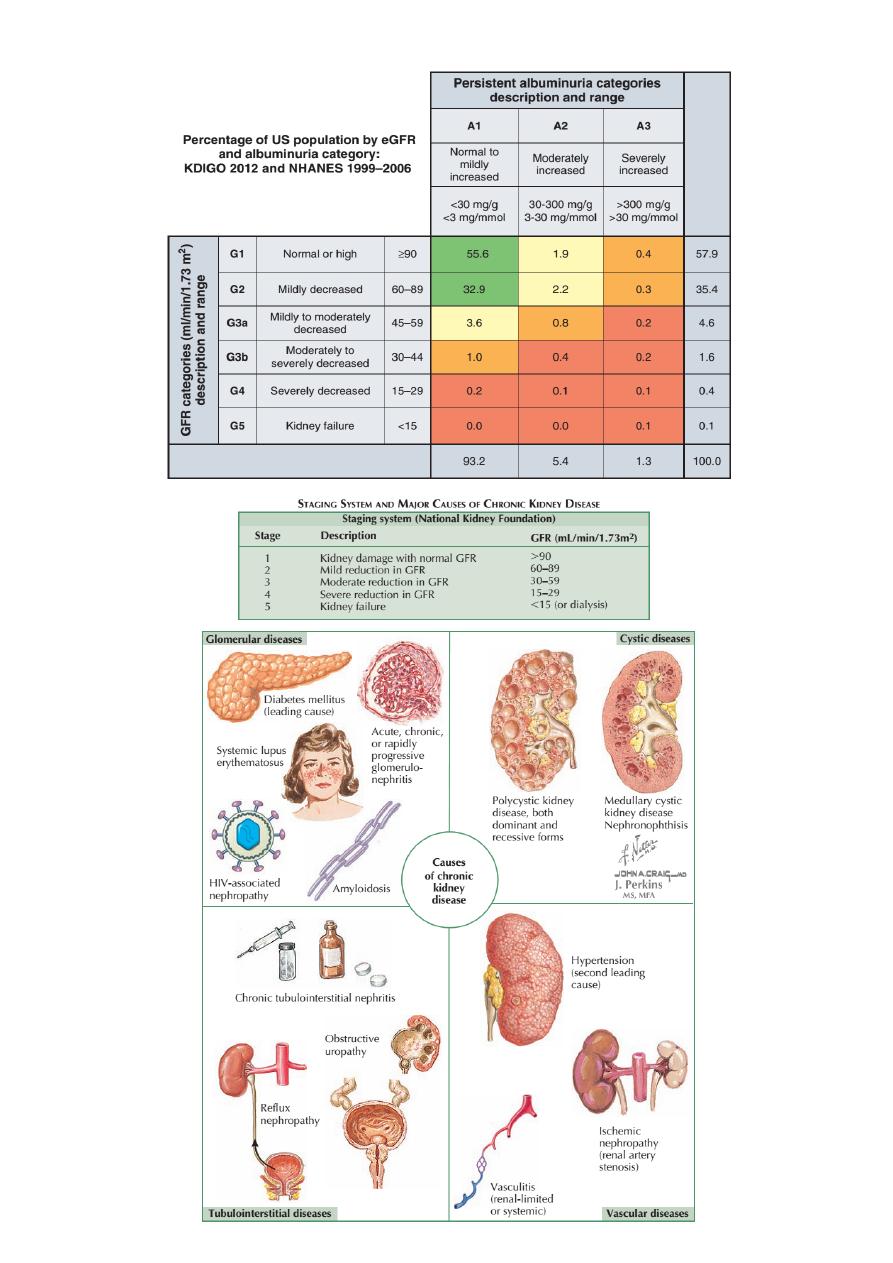
Chronic kidney disease (CKD)
Previously termed chronic renal failure, refers to an irreversible deterioration
in renal function which usually develops over a period of years. Initially, it is
manifest only as a biochemical abnormality but, eventually, loss of the excretory,
metabolic and endocrine functions of the kidney leads to the clinical symptoms
and signs of renal failure, collectively referred to as uraemia.
of
1
24
of
2
24
Clinical approach to the patient with CKD or any other form of renal disease
History
Particular attention should be paid to:
• Duration of symptoms
• Drug ingestion, including non-steroidal anti-inflammatory agents, analgesic and
other medications, and herbal remedies
• Previous medical and surgical history, e.g. previous chemotherapy, multisystem
diseases such as SLE, malaria
• Previous occasions on which urinalysis or measurement of urea and creatinine
might have been performed, e.g. pre-employment or insurance medical
examinations, new patient checks
• Family history of renal disease.
Symptoms
The early stages of CKD are often completely asymptomatic, despite the
accumulation of numerous metabolites. Serum urea and creatinine concentrations
are measured in CKD, since methods for their determination are available and a
rough correlation exists between urea and creatinine concentrations and
symptoms. These substances are, however, in themselves not particularly toxic.
Such metabolites must be products of protein catabolism (since dietary
protein restriction may reverse symptoms associated with CKD) and many of them
must be of relatively small molecular size (since haemodialysis employing
membranes which allow through only relatively small molecules improves
symptoms). Symptoms are common when the serum urea concentration exceeds 40
mmol/L, but many patients develop uraemic symptoms at lower levels of serum
urea. Symptoms include:
• Loss of appetite, Malaise, loss of energy, Insomnia
• Nocturia and polyuria due to impaired concentrating ability
• Itching
• Nausea, vomiting and diarrhoea
• Paraesthesiae due to polyneuropathy
• Restless legs’ syndrome (overwhelming need to frequently alter position of lower
limbs)
• Bone pain due to metabolic bone disease
• Paraesthesiae and tetany due to hypocalcaemia
• Symptoms due to salt and water retention – peripheral or pulmonary oedema
• Symptoms due to anaemia
of
3
24
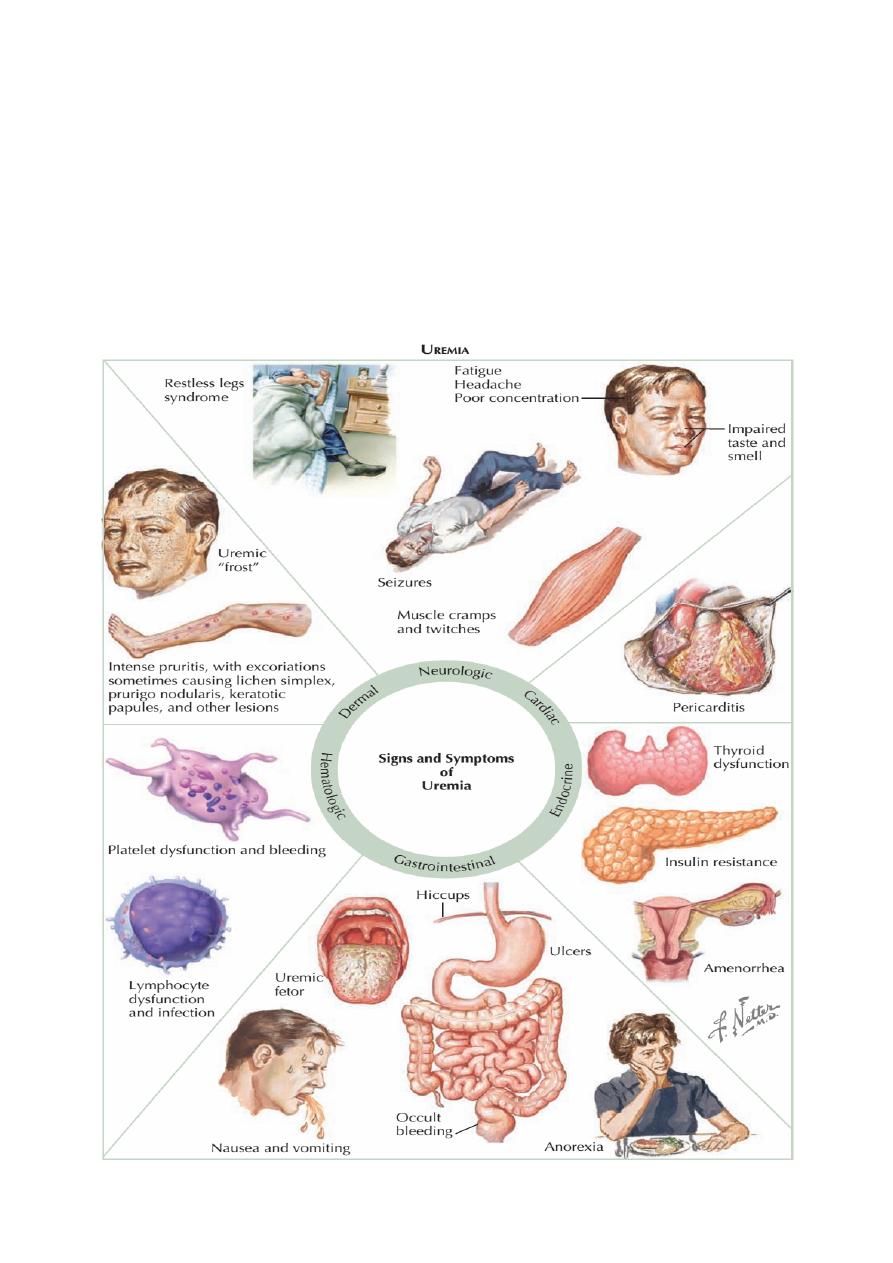
• Amenorrhoea in women; erectile dysfunction in men. In more advanced uraemia
CKD stage 5, these symptoms become more severe and CNS symptoms are
common:
✴
Mental slowing, clouding of consciousness and seizures, Myoclonic twitching.
✴
Severe depression of glomerular filtration can result in oliguria. This can occur
with either acute kidney injury or in the terminal stages of CKD. However, even
if the GFR is profoundly depressed, failure of tubular reabsorption may lead to
very high urine volumes; the urine output is therefore not a useful guide to
renal function.
of
4
24
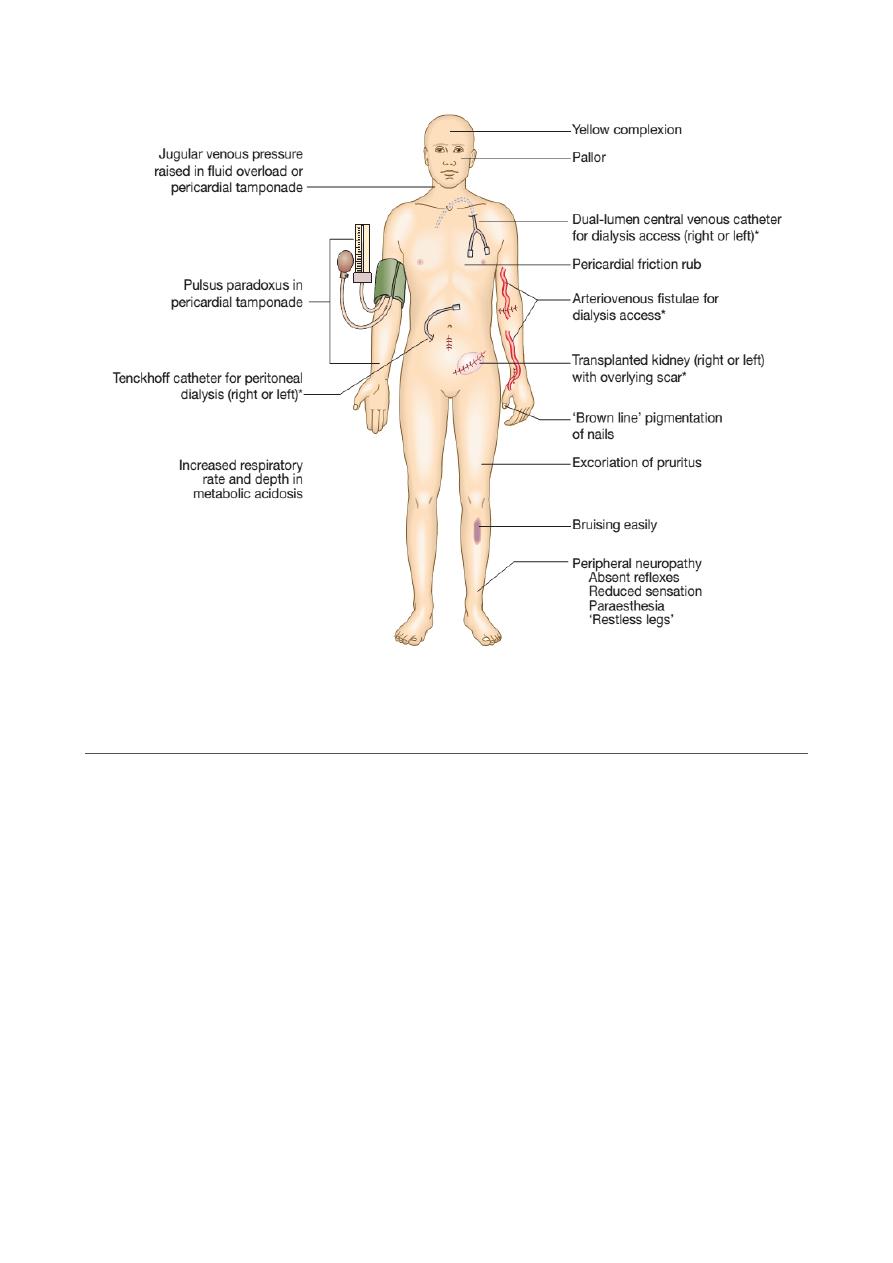
Investigations
The recommended investigations aims are:
•
to identify the underlying cause where possible, since this may influence the
treatment
•
to identify reversible factors that may worsen renal function, such as
hypertension, urinary tract obstruction, nephrotoxic drugs, and salt and water
depletion
•
to screen for complications of CKD, such as anaemia and renal osteodystrophy
•
to screen for cardiovascular risk factors.
Referral to a nephrologist is appropriate for patients with potentially
treatable underlying disease and those who are likely to progress to ESRD.
of
5
24
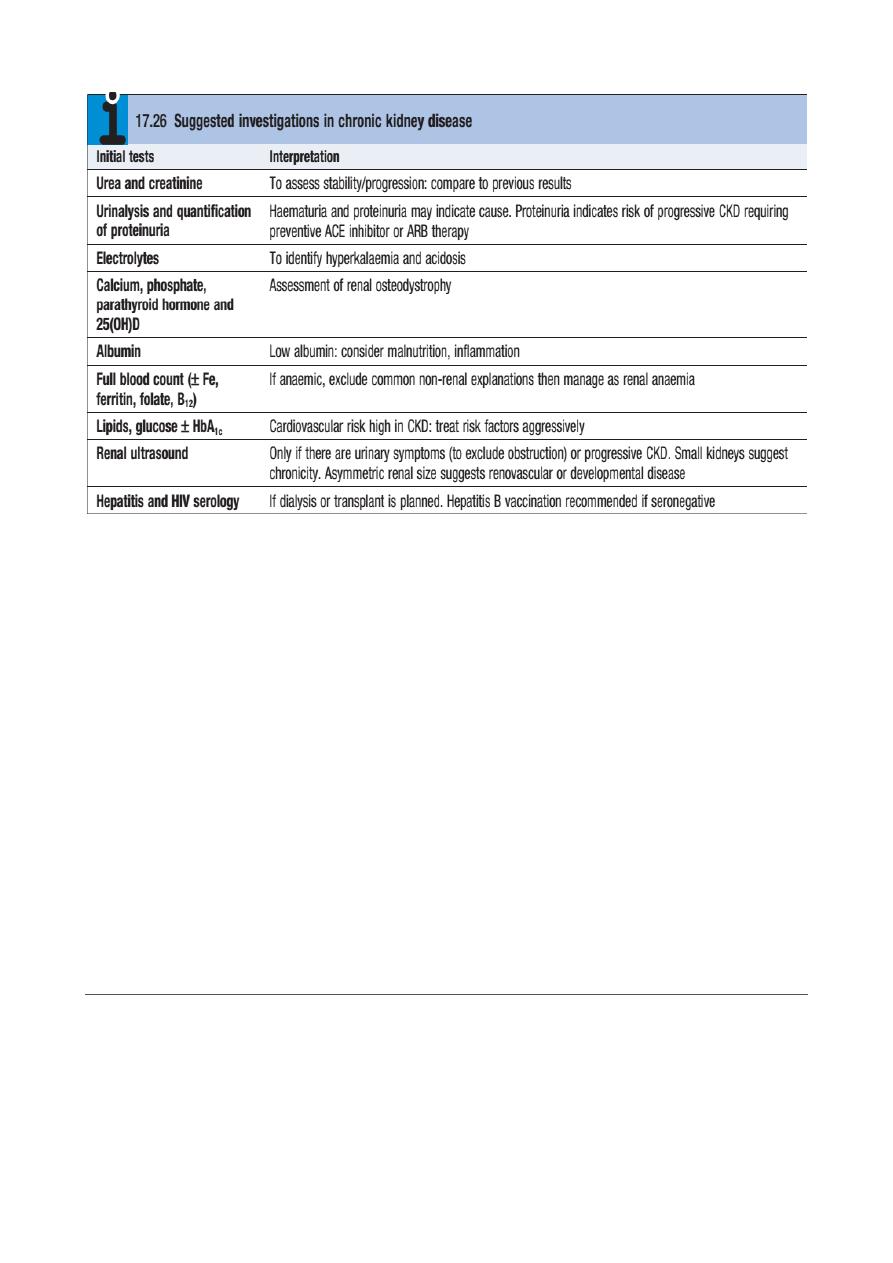
✦
Kidney Biopsy: In the patient with bilaterally small kidneys, renal biopsy is not
advised because (1) it is technically difficult and has a greater likelihood of
causing bleeding and other adverse consequences, (2( there is usually so much
scarring that the underlying disease may not be apparent, and (3) the window
of opportunity to render disease-specific therapy has passed.
✦
Other contraindications to renal biopsy include uncontrolled hypertension,
active urinary tract infection, bleeding diathesis (including ongoing
anticoagulation), and severe obesity. Ultrasound-guided percutaneous biopsy
is the favored approach, but a surgical or laparoscopic approach can be
considered, especially in the patient with a single kidney where direct
visualization and control of bleeding are crucial.
✦
In the CKD patient in whom a kidney biopsy is indicated (e.g., suspicion of a
concomitant or superimposed active process such as interstitial nephritis or in
the face of accelerated loss of GFR), the bleeding time should be measured,
and if increased, desmopressin should be administered immediately prior to
the procedure.
Complications of chronic kidney disease
A. Anaemia
Several factors have been implicated:
• Erythropoietin deficiency (the most significant)
of
6
24
• Toxic effects of uraemia on marrow precursor cells and retaining of Bone
marrow toxins in CKD
• Reduced red cell survival
• Bone marrow fibrosis secondary to hyperparathyroidism
• Haematinic deficiency – iron, vitamin B12, folate
• Reduced intake, absorption and utilisation of dietary iron
• Increased red-cell destruction, Abnormal red-cell membranes causing increased
osmotic fragility . Increased red-cell destruction may occur during haemodialysis
owing to mechanical, oxidant and thermal damage.
• Increased blood loss – occult gastrointestinal bleeding, blood sampling, blood
loss during haemodialysis, capillary fragility, or because of platelet dysfunction
• ACE inhibitors (may cause anaemia in CKD, probably by interfering with the
control of endogenous erythropoietin release).
of
7
24
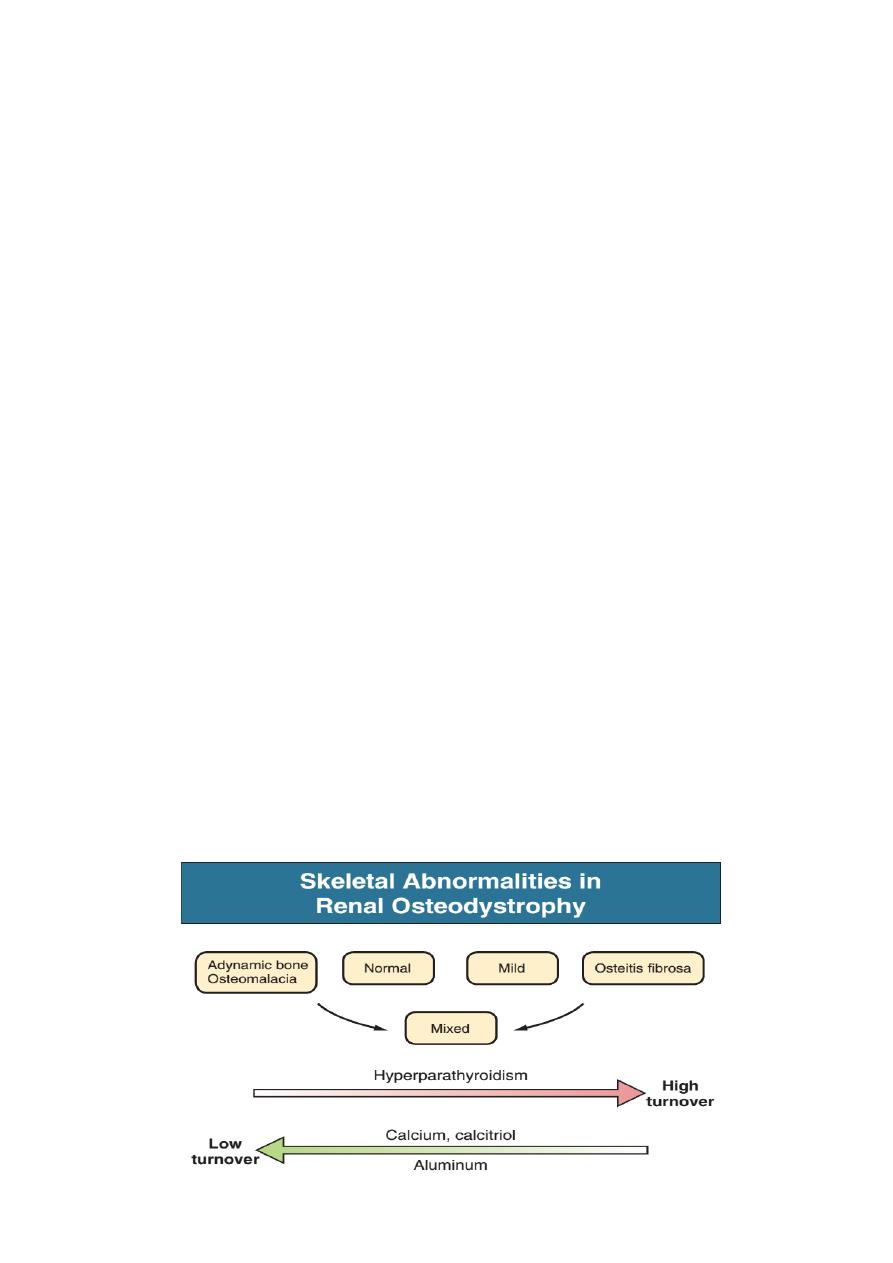
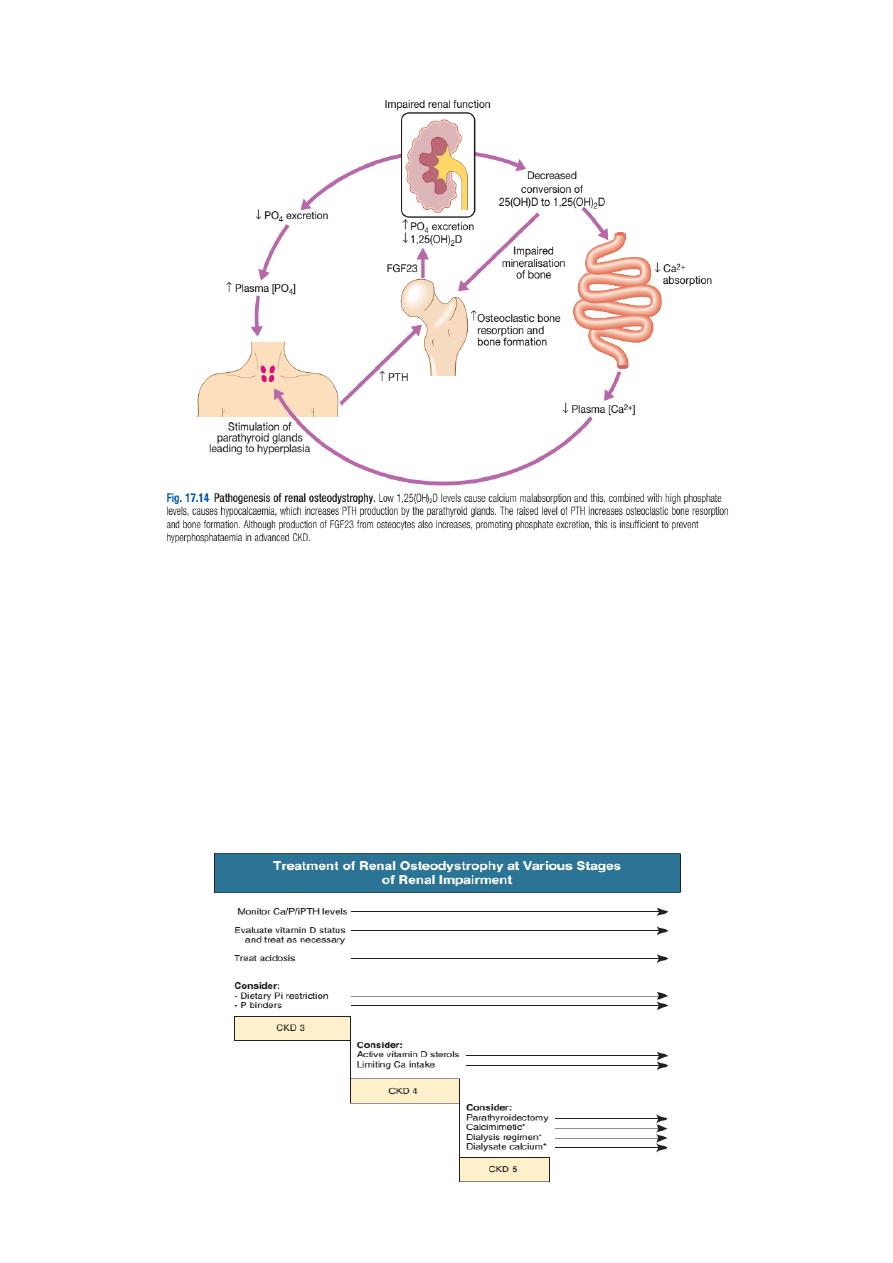
B. Chronic kidney disease-mineral and bone disorder (CKD-MBD)
Disturbances of mineral metabolism are common. The spectrum of disorders
includes abnormal concentrations of serum calcium, phosphate, and magnesium
and disorders of parathyroid hormone (PTH), fibroblast growth factor 23 (FGF-23),
and vitamin D metabolism.
These abnormalities as well as other factors related to the uremic state affect
the skeleton and result in the complex disorders of bone known as renal
osteodystrophy; it is now recommended that this term be used exclusively to
define the bone disease associated with CKD. The clinical, biochemical, and
imaging abnormalities heretofore identified as correlates of renal osteodystrophy
should be defined more broadly as a clinical entity or syndrome called chronic
kidney disease–mineral and bone disorder (CKD-MBD). The spectrum of skeletal
abnormalities seen in renal osteodystrophy includes the following :
‣
Osteitis fibrosa, a manifestation of hyperparathyroidism characterized by
increased osteoclast and osteoblast activity, peritrabecular fibrosis, and
increased bone turnover.
‣
Osteomalacia, a manifestation of defective mineralization of
newly formed osteoid most often caused by aluminum deposition;
bone turnover is decreased.
‣
Adynamic bone disease (ABD), a condition characterized by
abnormally low bone turnover in which both bone formation and resorption are
depressed (in the absence of aluminium bone disease or overtreatment with
vitamin D) is also seen.
‣
Osteopenia or osteoporosis.
‣
Combinations of these abnormalities termed mixed renal
osteodystrophy.
‣
Other abnormalities with skeletal manifestations (e.g., chronic
acidosis, β2-microglobulin amyloidosis).
of
8
24
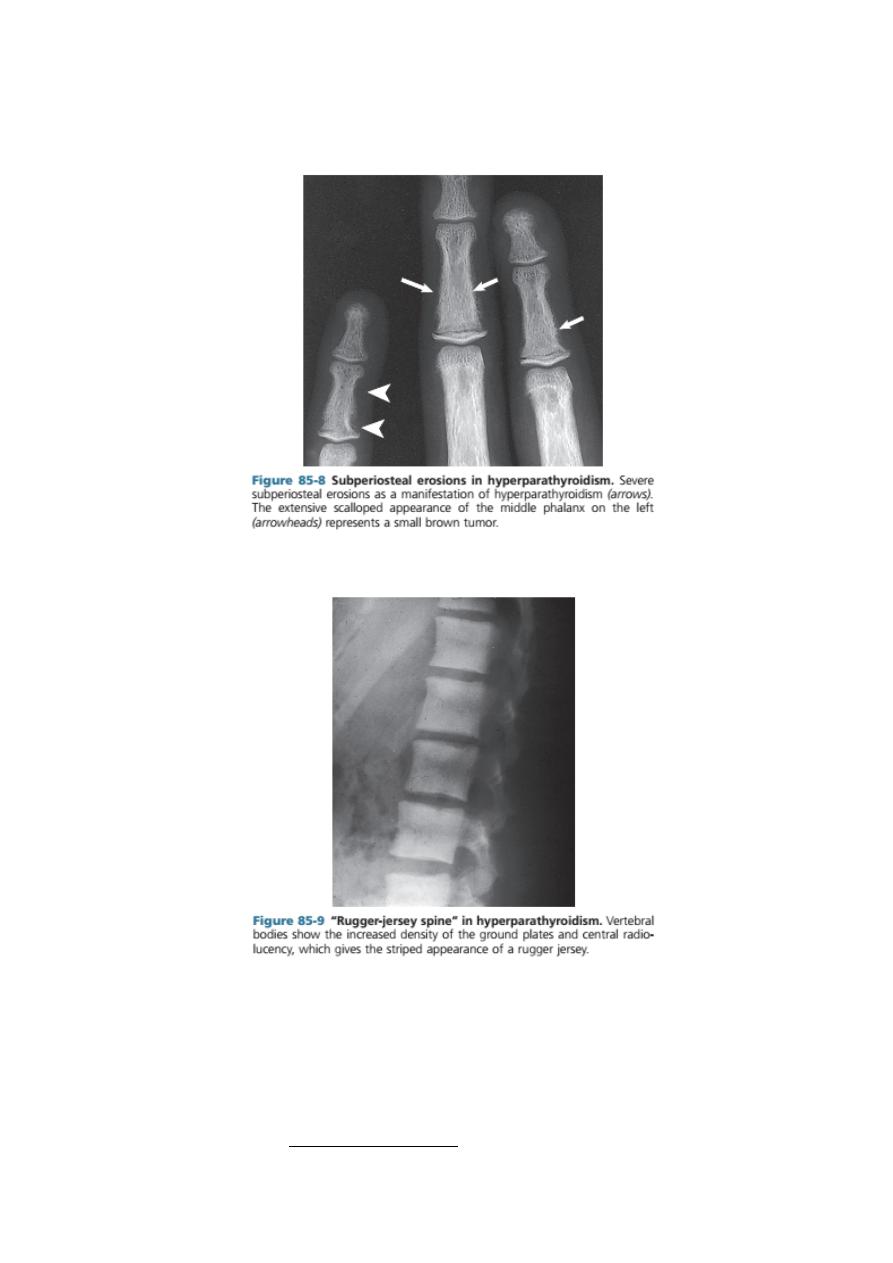
Radiologically,
(
a part of menial and bon disorder )
- Digital subperiosteal erosions and ‘pepperpot skull’ are seen.
- Longstanding secondary hyperparathyroidism ultimately leads to hyperplasia of
the glands with autonomous or tertiary hyperparathyroidism in which
hypercalcaemia is present. Serum alkaline phosphatase concentration is raised in
both secondary and tertiary hyperparathyroidism. Longstanding parathyroid
hormone excess is also thought to cause increased bone density (osteosclerosis)
seen particularly in the spine where alternating bands of sclerotic and porotic
bone give rise to a characteristic ‘rugger jersey’ appearance on X-ray.
of
9
24
C. Immune dysfunction
Cellular and humoral immunity is impaired in advanced CKD and there is
increased susceptibility to infections, the second most common cause of death in
dialysis patients, after cardiovascular disease.
D. Haematological
- There is an increased bleeding tendency in advanced CKD, which manifests as
cutaneous ecchymoses and mucosal bleeds.
of
10
24

- Platelet function is impaired and bleeding time prolonged. Adequate dialysis
partially corrects the bleeding tendency in those with severe uraemia, but these
patients are at significantly increased risk of complications from all
anticoagulants, including those that are required during dialysis.
- Anaemia is common and is due in part to reduced erythropoietin production.
Haemoglobin can be as low as 5-7 g/dL in CKD stage 5, although it is often less
severe or absent in patients with polycystic kidney disease.
E. Electrolyte abnormalities
Patients with CKD often develop electrolyte abnormalities like hyperkalemia
and metabolic acidosis. Fluid retention is common in advanced CKD , Conversely,
some patients with tubulointerstitial disease can develop ‘saltwasting’ disease
and may require a high sodium and water intake, including supplements of sodium
salts, to prevent fluid depletion and worsening of renal function. Acidosis is
common. Although it is usually asymptomatic, it may be associated with increased
tissue catabolism and decreased protein synthesis, and may exacerbate bone
disease and the rate of decline in renal function.
F. Neurological and muscle function
- Generalised myopathy may occur due to a combination of poor nutrition,
hyperparathyroidism, vitamin D deficiency and disorders of electrolyte
metabolism.
- Muscle cramps are common.
- The ‘restless leg syndrome’, in which the patient’s legs are jumpy during the
night, may be troublesome. Symptoms may improve with the correction of
anaemia by erythropoietin. Clonazepam is sometimes useful. Renal
transplantation cures the problem.
- Both sensory and motor neuropathy can arise, presenting as paraesthesia and
foot drop, respectively, but appear late during the course of CKD. They are now
unusual, given the widespread availability of RRT; if present, they can often
improve once dialysis is established.
- Asterixis, fits, tremor and myoclonus are features of severe uraemia.
- Dialysis Disequilibrium Syndrome: Rapid correction of severe uraemia by
haemodialysis leads to dialysis disequilibrium owing to osmotic cerebral
swelling.This can be avoided by correcting uraemia gradually by use of
volumetric-controlled hemodialysis machines, bicarbonate dialysate, sodium
modeling, earlier recognition of uremic states, and stepped initiation of dialysis
(short initial treatment times with lower blood pump speeds). In addition, short
and more frequent dialysis treatments are recommended with use of small
surface area dialyzers and reduced blood flow rates.
- Seizures: convulsions in a uraemic patient are much more commonly due to other
causes such as accelerated hypertension, thrombotic thrombocytopenic purpura
or drug accumulation.
of
11
24
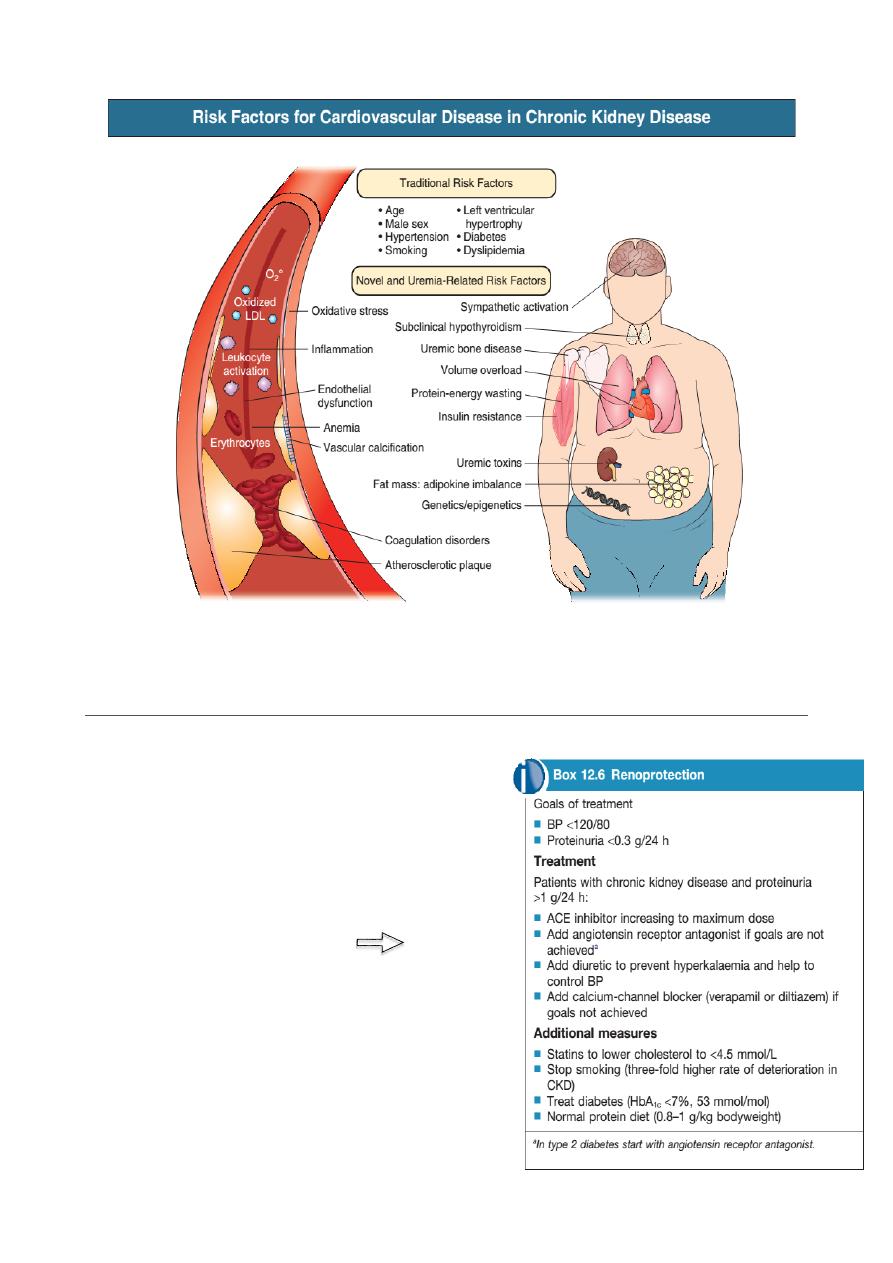
G. Cardiovascular disease
‣ The risk of cardiovascular disease is substantially increased in patients with CKD
stage 3 or worse (GFR < 60 mL/min/1.73 m2) and those with proteinuria or
microalbuminuria.
‣ Left ventricular hypertrophy may occur, secondary to hypertension, and may
account for the increased risk of sudden death (presumed to be caused by
dysrhythmias) in this patient group.
‣ Pericarditis may complicate untreated or inadequately treated ESRD and cause
pericardial tamponade or constrictive pericarditis. This is common and occurs in
two clinical settings:
‣ Uraemic pericarditis is a feature of severe, pre-terminal uraemia or of
underdialysis. Haemorrhagic pericardial effusion and atrial arrhythmias are often
associated. There is a danger of pericardial tamponade, and anticoagulants
should be used with caution. Pericarditis usually resolves with intensive dialysis.
‣ Dialysis pericarditis occurs as a result of an intercurrent illness or surgery in a
patient receiving apparently adequate dialysis.
of
12
24
Management of chronic kidney disease
The underlying cause of CKD should be
treated aggressively wherever possible.
1. Renoprotection
The multidrug approach to chronic
nephropathies has been formalized in an
international protocol beside:
of
13
24
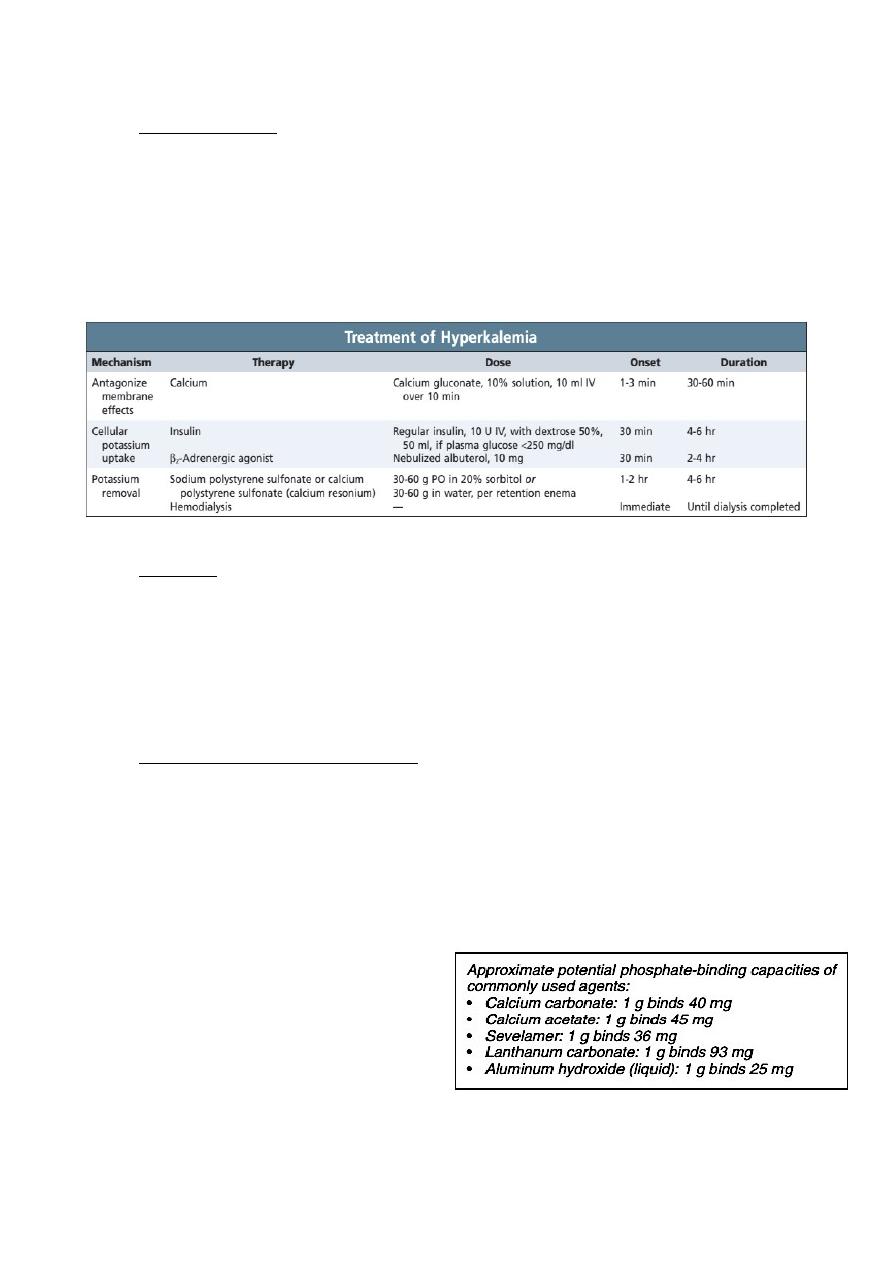
2. Correction of complications
Hyperkalaemia : Hyperkalaemia often responds to dietary restriction of
potassium intake. Drugs like ACEi/ARBs and others which cause potassium
retention should be stopped. Occasionally, it may be necessary to prescribe ion-
exchange resins to remove potassium in the gastrointestinal tract such as sodium
polystyrene sulfonate (Kayexalate) or calcium polystyrene sulfonate (calcium
resonium). Emergency treatment of severe hyperkalaemia is mandatory in CKD
patients.
Acidosis: Correction of acidosis helps to correct hyperkalaemia in CKD, and
may also decrease muscle catabolism. The plasma bicarbonate should be
maintained above 22 mmol/L by giving sodium bicarbonate supplements (starting
dose of 1 g 3 times daily, increasing as required). If the increased sodium intake
induces hypertension or oedema, calcium carbonate (up to 3 g daily) may be used
as an alternative, since this has the advantage of also binding dietary phosphate.
Renal bone disease (CKD MBD): treatment should be initiated with
‣ Active vitamin D metabolites (either 1αhydroxyvitamin D or 1,25
dihydroxyvitamin D) in patients who are found to have hypocalcaemia or serum
PTH levels more than twice the upper limit of normal. The dose should be
adjusted to try to reduce PTH levels to between 2 and 4 times the upper limit of
normal to avoid over suppression of bone turnover and adynamic bone disease,
but care must be exercised in order to avoid hypercalcaemia.
‣ Hypocalcaemia and hyperphosphataemia should be treated aggressively.
‣ Hyperphosphataemia should be treated
by dietary restriction of foods with high
phosphate content (milk, cheese, eggs
and protein rich foods) and by the use
of phosphate binding drugs. Various
drugs are available, including calcium
carbonate, aluminium hydroxide,
lanthanum carbonate and polymerbased phosphate binders such as sevelamer.
The aim is to maintain serum phosphate values at 1.8 mmol/L (5.6 mg/dL) or
of
14
24

below if possible, but many of these drugs are difficult to take and compliance
may be a problem.
‣ Parathyroidectomy may be required for the treatment of tertiary
hyperparathyroidism. An alternative is to employ calcimimetic agents, such as
cinacalcet, which bind to the calcium sensing receptor and reduce PTH secretion.
They have a place if parathyroidectomy is unsuccessful or not possible.
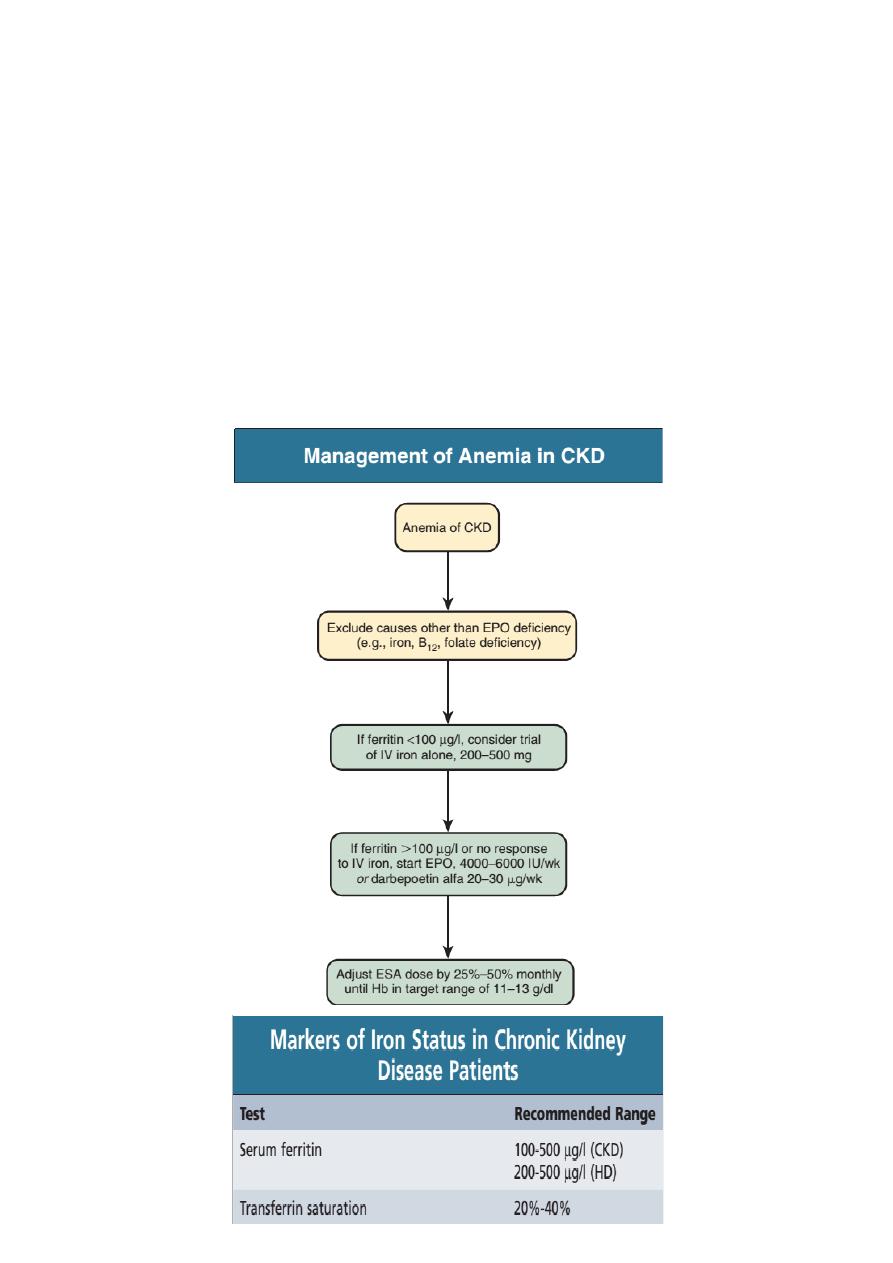
Treatment of Anemia
1. Correction of iron deficiency anemia
2. Erythropoiesis-Stimulating Agents such as Epoetin alpha (Eprex) , Epoetin
Beta (Mircera), Darbepoetin and Peginesatide (previously called Hematide) is an
EPO-mimetic peptide.
3. Renal replacement therapy (RRT)
((=
dialysis or kidney transplantation))
The aim of all renal replacement techniques is to mimic the excretory
functions of the normal kidney, including excretion of nitrogenous wastes,
maintenance of normal electrolyte concentrations, and maintenance of a normal
extracellular volume. At the present time, the average eGFR at the time of initiating
RRT in the UK is about 8 mL/min/1.73 m2 but there is wide variation. Since there is
no evidence that early initiation of RRT improves outcome, the overall aim is to
commence RRT by the time symptoms of CKD have started to appear but before
serious complications have occurred.
of
15
24

Renal transplantation offers the best chance of long term survival in ESRD,
and is the most cost effective treatment. Transplantation can restore normal kidney
function and correct all the metabolic abnormalities of CKD but requires long term
immunosuppression with its attendant risks. All patients with ESRD should be
considered for transplantation, unless there are contraindications.
of
16
24
Dialysis
Dr. ALI A. ALLAWI
Nephrologist and Transplant specialist
Internist
CABMS (NEPHROLOGY) , FICMS (NEPHROLOGY) FICMS (MEDICINE)
Haemodialysis
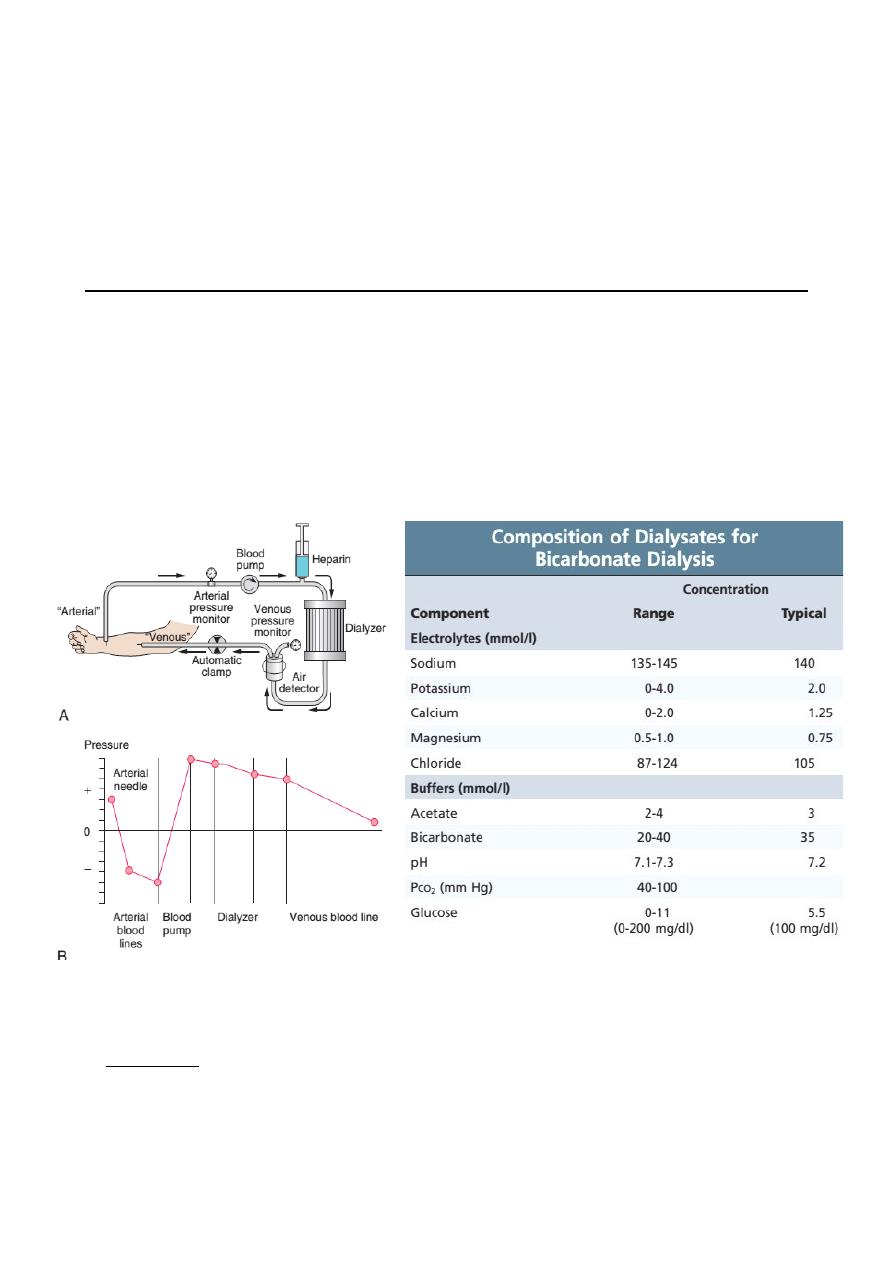
Basic principles
In haemodialysis, blood from the patient is pumped through an array of
semipermeable membranes (the dialyser, also called an hemofilter ), which bring
the blood into close contact with dialysate, flowing countercurrent to the blood.
The plasma biochemistry changes towards that of the dialysate owing to diffusion
of molecules down their concentration gradients.
Access for haemodialysis
Adequate dialysis requires a blood flow of at least 200 mL/min.
‣ AV Fistula : The most reliable long-term way of achieving this is surgical
construction of an arteriovenous fistula , using the radial or brachial artery and
the cephalic vein. This results in distension of the vein and thickening
(‘arterialization of veins’) of its wall, so that after 6–8 weeks large-bore needles
may be inserted to take blood to and from the dialysis machine.
of
17
24

‣ AV Graft: In patients with poor-quality veins or arterial disease (e.g. diabetes
mellitus), arteriovenous polytetrafluoroethylene (PTFE) grafts are used for access.
However, these grafts have a very high incidence of thrombosis and 2-year graft
patency is only 50–60%. Dipyridamole or fish oils improve graft patency but
warfarin, aspirin and clopidogrel do not and are associated with a high incidence
of complications.
‣ Double lumen catheter : If dialysis is needed immediately, a large-bore double
lumen catheter may be inserted into a central vein usually the jugular, femoral or
subclavian. The internal jugular vein route is preferred.
Haemodialysis in AKI
Haemodialysis offers the best rate of small solute clearance in AKI, as
compared with other techniques such as haemofiltration, but should be started
gradually because of the risk of confusion and convulsions due to cerebral oedema
(dialysis disequilibrium). Typically, 1–2 hours of dialysis is prescribed initially but,
subsequently, patients with AKI who are haemodynamically stable can be treated
by 4–5 hours of haemodialysis on alternate days, or 2–3 hours every day. During
dialysis, it is standard practice to anticoagulate patients with heparin but the dose
may be reduced if there is a bleeding risk. Epoprostenol can be used as an
alternative but carries a risk of hypotension.
of
18
24
Haemodialysis in CKD
In CKD, vascular access for haemodialysis is gained by formation of an
arteriovenous fistula, usually in the forearm, up to a year before dialysis is
contemplated. After 4–6 weeks, increased pressure transmitted from the artery to
the vein leading from the fistula causes distension and thickening of the vessel wall
(arterialisation of vein). Largebore dual lumen catheter can then be inserted into the
vein to provide access for each haemodialysis treatment. Preservation of arm veins
is thus very important in patients with progressive renal disease who may require
haemodialysis in the future. Haemodialysis is usually carried out for 3–5 hours three
times weekly, in an outpatient dialysis unit. The intensity and frequency of dialysis
should be adjusted to achieve a reduction in urea during dialysis (urea reduction
ratio) of over 65%. Most patients notice an improvement in symptoms during the
first 6 weeks of treatment. Plasma urea and creatinine are lowered by each
treatment but do not return to normal.
of
19
24
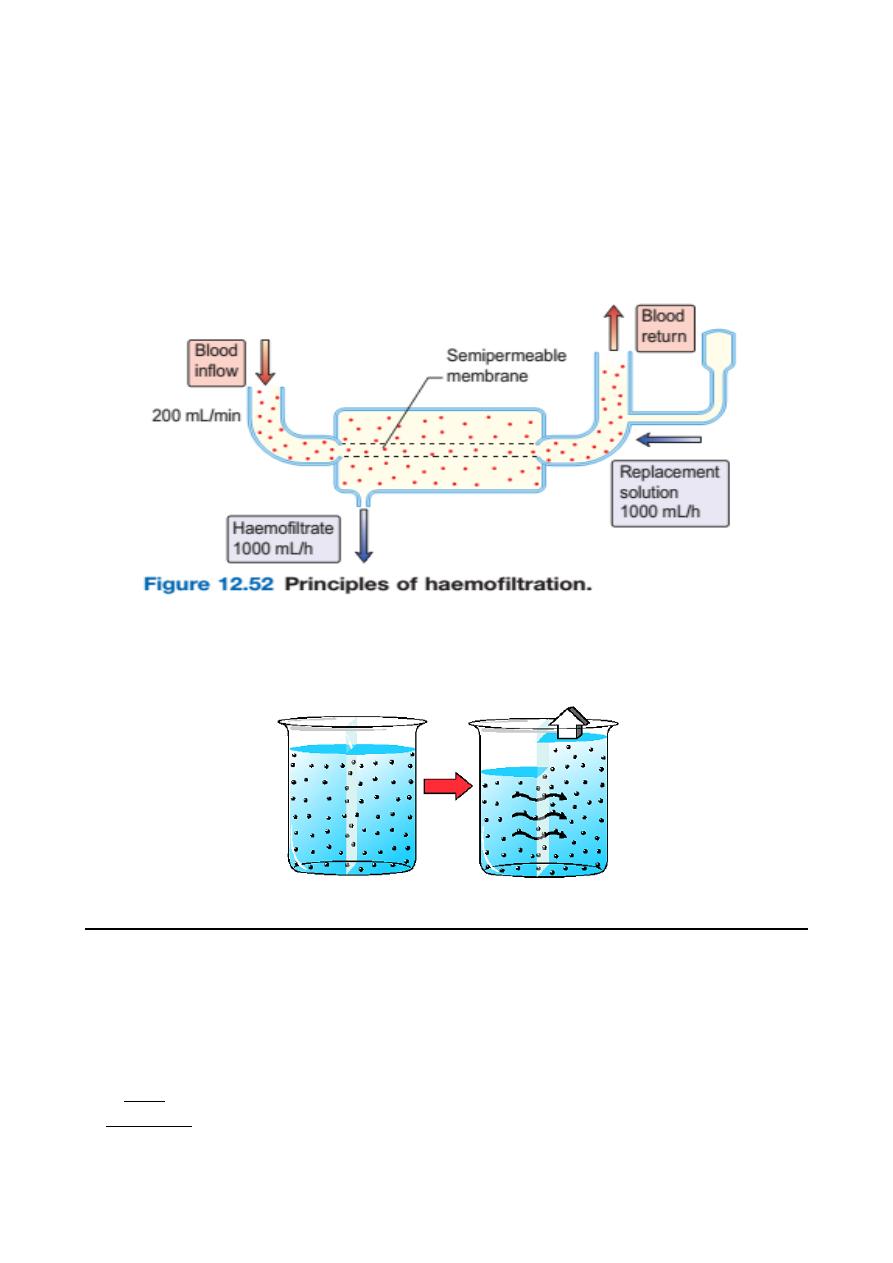
Haemofiltration
This involves removal of plasma water and its dissolved constituents (e.g. urea,
phosphate) by convective flow across a high-flux semipermeable membrane, and
replacing it with a solution of the desired biochemical composition.
Peritoneal dialysis
Peritoneal dialysis utilizes the peritoneal membrane as a semipermeable
membrane, avoiding the need for extracorporeal circulation of blood. This is a very
simple technology treatment compared to haemodialysis. The principles are
simple:
‣ A tube is placed into the peritoneal cavity through the anterior abdominal wall.
‣ Dialysate is run into the peritoneal cavity, usually under gravity.
of
20
24
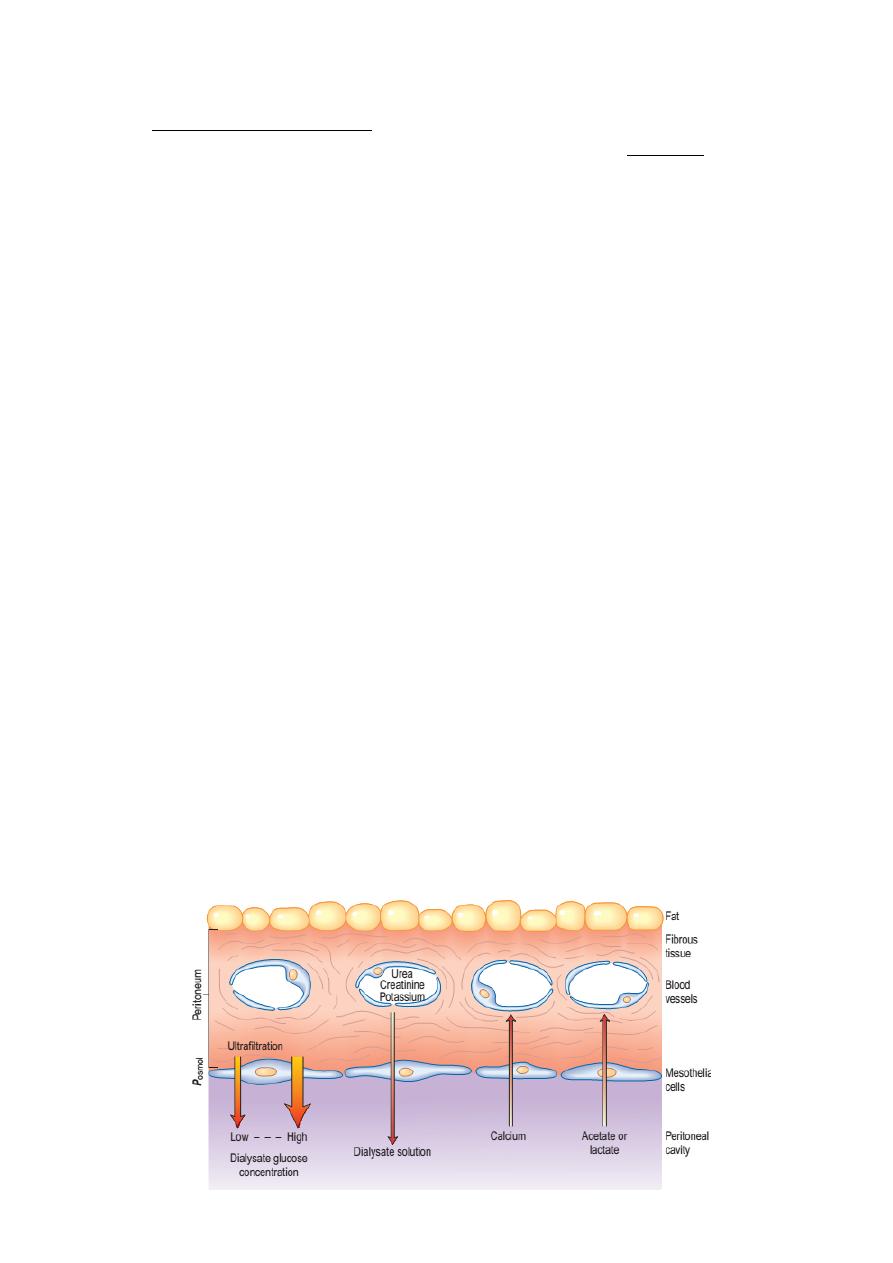
‣ Urea, creatinine, phosphate and other uraemic toxins pass into the dialyse down
their concentration gradients.
‣ Water (with solutes) is attracted into the peritoneal cavity by osmosis, depending
on the osmolarity of the dialysate. This is determined by the glucose or polymer
(icodextrin) content of the dialysate .
‣ The fluid is changed regularly to repeat the process.
Chronic peritoneal dialysis
Requires insertion of a soft catheter, with its tip in the pelvis, exiting the
peritoneal cavity in the midline and lying in a skin tunnel with an exit site in the
lateral abdominal wall .This form of dialysis can be adapted in several ways.
Continuous ambulatory peritoneal dialysis (CAPD)
Dialysate is present within the peritoneal cavity continuously, except when
dialysate is being exchanged. Dialysate exchanges are performed three to five
times a day, using a sterile no-touch technique to connect 1.5–3 L bags of dialysate
to the peritoneal catheter; each exchange takes 20–40 min. This is the technique
most often used for maintenance peritoneal dialysis in
patients with ESKD.
Automated peritoneal dialysis (APD)
Also called Nightly intermittent peritoneal dialysis (NIPD). An automated
device (cycler) is used to perform exchanges each night while the patient is asleep.
Sometimes dialysate is left in the peritoneal cavity during the day in addition, to
increase the time during which biochemical exchange is occurring.
Few trials have demonstrated superiority of APD over CAPD with regard to
complications such as peritonitis, fluid status and in anuric patients.
Tidal dialysis: A residual volume is left within the peritoneal cavity with continuous
cycling of smaller volumes in and out.
of
21
24
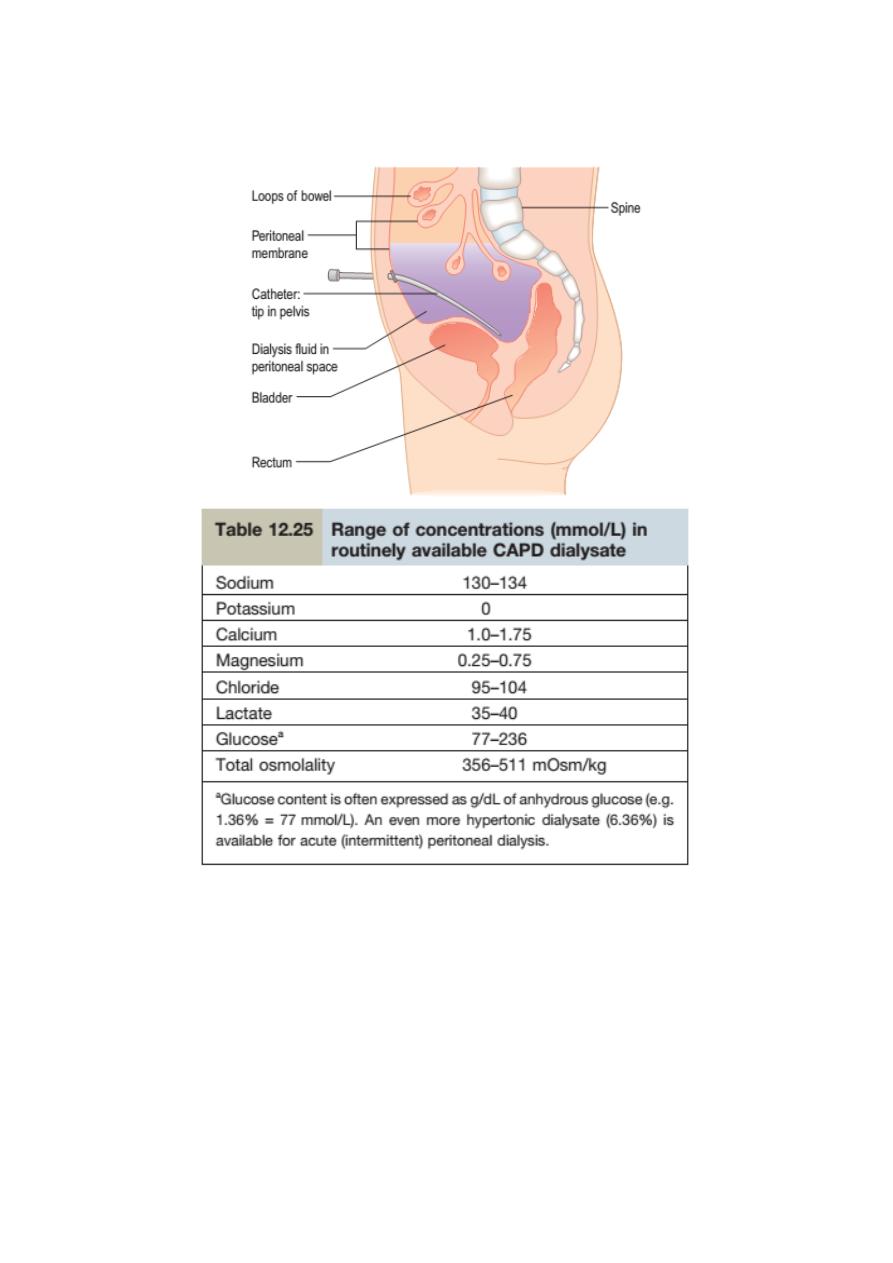
Acute Peritoneal Dialysis Prescription
Acute peritoneal dialysis is usually performed manually but can be done with
the assistance of a cycler (automated machine) . It avoids the potential problems
related to vascular access (hemorrhage, air embolism, thrombosis, infection) and
does not require anticoagulation.
The gradual but continuous nature of the procedure results in effective
removal of fluid and solute with less hemodynamic instability.
Acute peritoneal dialysis is most often used in the setting of
‣ acute kidney injury(AKI ),
‣ it is also beneficial in the control of volume overload states in patients with
cardiovascular compromise, such as those with congestive heart failure, and
of
22
24

‣ in the treatment of hypothermia or
‣ hemorrhagic pancreatitis (where peritoneal lavage may be beneficial).
‣ It is most beneficial in the treatment of hemodynamically unstable patients or
patients in whom vascular access is problematic.
This is the combined time required for inflow, dwell, and drain. To maximize
dialysis efficiency in acute peritoneal dialysis, the exchange time most commonly
used is 1 hour
• Inflow time: Inflow is by gravity and usually requires about 10 minutes. It may be
prolonged due to kinking of the tubing or increased inflow resistance by intra-
abdominal tissues in close proximity to the catheter tip. Cold dialysate solution
also results in discomfort, and for this reason, the solution should be warmed to
37°C before infusion.
• Dwell time: The dwell period is the time during which the total exchange volume
is present in the peritoneal cavity (i.e., the time from the end of inflow to the
beginning of outflow).
Standard dwell period. When initiating peritoneal dialysis in acutely ill and
catabolic patients, the usual dwell time is 30 minutes to achieve an exchange time
of 60 minutes. With a 2-L exchange volume, 48 L of fluid will thus be exchanged
daily.
• Outflow time: Outflow of spent dialysate is by gravity and usually requires 20-30
minutes. If outflow continues to be poor, then outflow obstruction should be
managed . Pain during outflow is unusual, but localized pain may occasionally be
noted at the end of a drain due to a siphoning effect of the catheter on the
peritoneum.
of
23
24
of
24
24
