
BioChemistry
Dr.Munaf 4
Lecture 4
Glycogen Metabolism
Objectives:
1- Describe glycogen synthesis
(Glycogenesis) and degradation ( glycogenolysis)
and their control.
2- Outline hormonal regulation
(epinephrine , glucagon ) at cell surface.
MSD

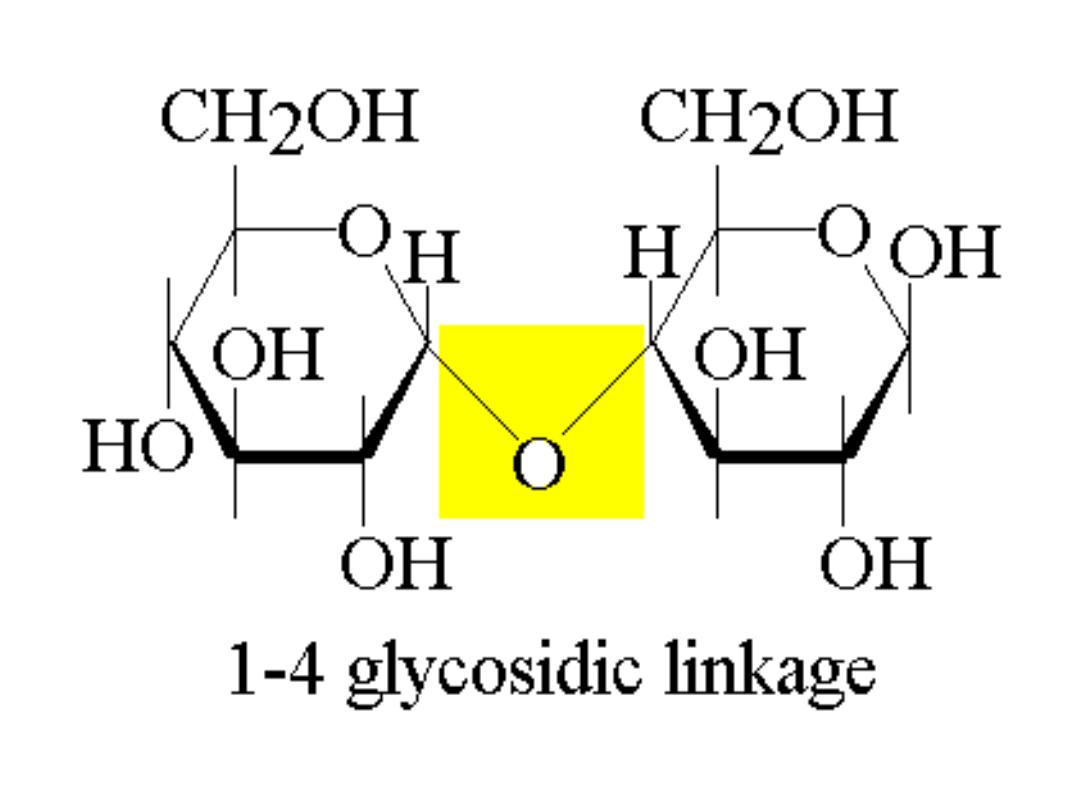
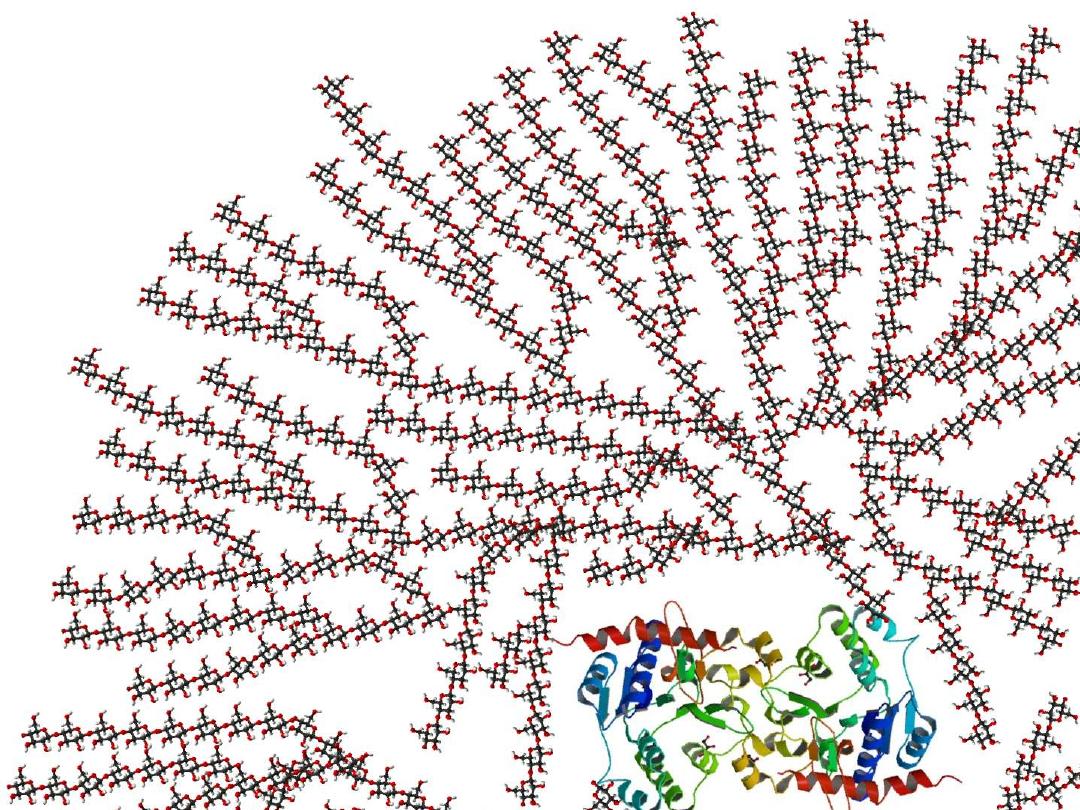
●Glycogen is a storage form of Glc, highly
branched very large Glc polymer linked by
α-1,4 glycosidic linkage ( bond ) and branches by
α-1,6 glycosidic bond at every several Glc
residues , found in cytosol as granules & its
major sites are muscle & liver ( concentration is
higher in liver than muscle but amount is higher
in muscle than liver ).
●Liver glycogen release Glc into blood but not
the muscle glycogen because of the absence of
Glc6Pase
enzyme in the muscle.The duration of
liver glycogen exhaustion is about 12 hours ( i.e.
enough for ≈ 12 hrs) then gluconeogenesis
starts.
MSD

Synthesis ( Glycogenesis )
● It starts with Glc6P that converts into Glc1P
which reacts with UTP to be pyrophosphorylated
into UDP-Glc by
pyrophosphorylase
enzyme.
UDP-Glc , a high energy compound starts
adding Glc residue to a preexisting glycogen
chain ( Glycogen primer ) formed on a protein
primer known as
Glycogenin.
UDP is released
after addition & Glc is added successively in the
1
→ 4 position ( 1,4 glycosidic bond ) by
Glycogen Synthase
( straight chain molecule
known as Amylose chain is formed ).
msd

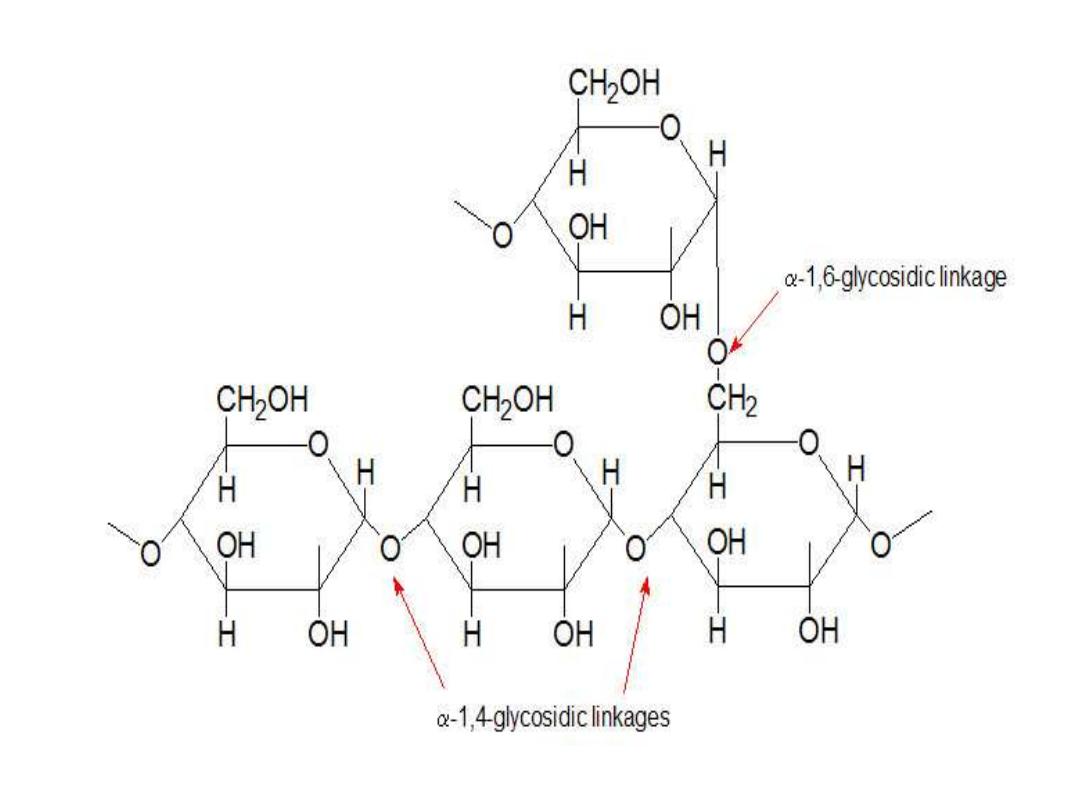
● When segments of Amylose chain are at least
of 11 Glc residues in length away from core, 7
Glc residues are transferred by the
Branching
enzyme glucosyl-4:6 transferase
onto C-6 OH of
the same Amylose chain thus forming a branch .
This 7Glc
– residues segment is elongated by
additive Glc by the
Glycogen Synthase
onto C-4
OH until it becomes 11 residues or more ( i.e. a
new Amylose chain is formed ).
Another transfer of 7-residues segment of this
new Amylose chain is made & a branch is formed
on C-6 OH of a neighboring chain. This process
goes on until the whole branched Glycogen
structure if formed.
msd

Degradation ( Glycogenolysis)
1- A phosphorylitic cleavage (removeal) of Glc
of the terminal ( outermost chains ) α-1,4
glycosidic bond of Glycogen by
Glycogen
Phosphorylase
to give
Glc1-P .Removal goes on sequentially until
about 4 Glc
–residues remain.
2- Removal of branch chains:
This is catalyzed by the
Debranching
enzyme
system ; it has 2 enzymatic
activities.
MSD

a-
α ( 1,4→1,4 ) glucantransferase
(
glucosyl transferase
) . In this step 3 Glc-
residues from a branch are transferred onto
another chain terminus,leaving a single
residue on C-6 branch point.
b-
α- 1,6 glucosidase
In this step a single residue on C-6 is
removed to give a free Glc molecule.
3- In lysosomes another enzyme
α-1,4
glucosidase
is involved in glycogen
degradation to give Glc.
MSD

● Regulation:
1- Hormonal
Glucagon(liver) and epinephrine(liver &
muscle) stimulates glycogenolysis & inhibit
glycogenesis while insulin stimulates glycogenesis in
both liver & muscle .
2- Covalent modification i.e. Phosphorylation or
dephosphorylation by cAMP.
Allosteric mechanisms & covalent modification
by reversible phosphorylation of enzyme protein in
response to hormone action. cAMP formed from ATP
by
Adenylate cyclase
at the inner surface of cell
membranes in response to hormones such as
epinephrine & glucagon. cAMP is hydrolyzed by
MSD

Phophodiesterase
to AMP , so ending hormone
action and insulin increase the activity of this
enzyme in the liver.
Phosphorylated enzyme can be dephosphorylated by
a
phosphatase
enzyme e.g.removal of P from
Phosphorylase a
( active )
→
Phosphorylase b
(
inactive ).
☻Genetic defects ( inborn errors of glycogen
metabolism)
Glycogen Storage Diseases ( GSDs)are
inherited disorders ( more than 10 characterized
by deposition of an abnormally type or quantity of
glycogen in tissues, or failure to mobilize
glycogen e.g. vonGierke
̛s disease –Type I. MSD
Effect of Epinephrine and /or glucagon regulation at cell
membrane receptors
Epinephrine
↓
Adenylate Cyclase ( Ia )
→
Adenylate Cyclase ( a )
↓
ATP
→ cAMP → AMP
↓
Protein Kinase ( Ia )
→
Protein Kinase ( a )
( Ia is for inactive & a for active ).
After activation of
protein kinase
, there are 2 pathways :
1-
Protein Kinase ( a )
↓
Glycogen Synthase I
→
Glycogen Synthase D
active inactive
dephosphorylated phosphorylated
MSD
↓

Glycogen synthase D
( phosphorylated & inactive )
↓inhibits
UDP-Glc → Glycogen ( glycogenesis stops )
……………………………………………………………………
2-
Protein kinase ( a )
↓
Phosphorylase kinase
→
Phosphorylase kinase
dephosphorylated phosphorylated
inactive active
↓ Ca+²
Phosphorylase b
→
Phosphorylase a
inactive( dephospho.) active ( phospho.)
↓ stimulates
Glycogen → Glc-1P
( glycogenolysis starts)
MSD
