
Trace Elements

Trace elements
TEs
a
re expressed in µg/dl in fluids and
mg/kg
in
tissues.
The
ultraTE are expressed
in ng/dl
in fluids
and µg/kg
in
tissues. They
are essentials
when
the sign and
symptoms
induced by an
element deficiency
are
reversed only by adequate supply of
that element.

TEs
are important
or essential for
many
critical
biochemical
processes,
deficiencies are often
associated with decreased activities
of
the
Enzymes
Es
that require TEs for optimal activity.
Function
can
be
restored by dietary replacement,
but must be in care
from toxicity. A chemical element
required in minute quantities by an organism
to
maintain
proper
physical
functioning.

Dose-Effect relationships:
In low intake of an element the biological function of
humans
decreased (detrimental
effects),
with
continues
supply
or
intake of
element
the biological functions
improved
with
approaching the plateau region(constant optimal human
function even with increased element concentrations), but
with increased element levels the biological functions
decline (Toxicity region,
which depend
on
element and its
chemical structure in
the
diet).

IRON

Importance of Iron
1. Serve as both electron donor and acceptor in
the Electron Transport Chain.
2. Needed by peroxidase enzymes as catalysts
to convert harmful peroxides into water.
3. Needed in oxidative phosphorylation
(oxidation of nutrients into ATP) by iron-sulfur
proteins.
4. Main Importance:
Oxygen Transport
(incorporated in heme in hemoglobin)

Distribution of Iron
3-5 g of iron is in the body (total)
2-2.5 g of which is in the hemoglobin.
Some (about 130 mg) are in myoglobin (oxygen
carrier in tissues).
Little (about 8 mg) is bound to enzymes like
peroxidases, cytochromes and other enzymes
involved in the Krebs Cycle.
Some are stored in ferritin and hemosiderin.
Little (3-5 mg) is in plasma in transferrin.

Storage Iron and Transferrin
2 forms of storage iron:
Ferritin
– water-soluble complex of ferric salt
(Fe
+3
) and the protein
apoferritin
. Present in blood.
- a
positive
acute phase reactant/protein –
increases in inflammation.
Hemosiderin
– water-insoluble. Present in
tissues.

Storage Iron and Transferrin
Transferrin
– main protein for iron transport.
- a negative acute phase reactant/protein –
decreases in inflammation.
Transferrin + iron =
serum iron
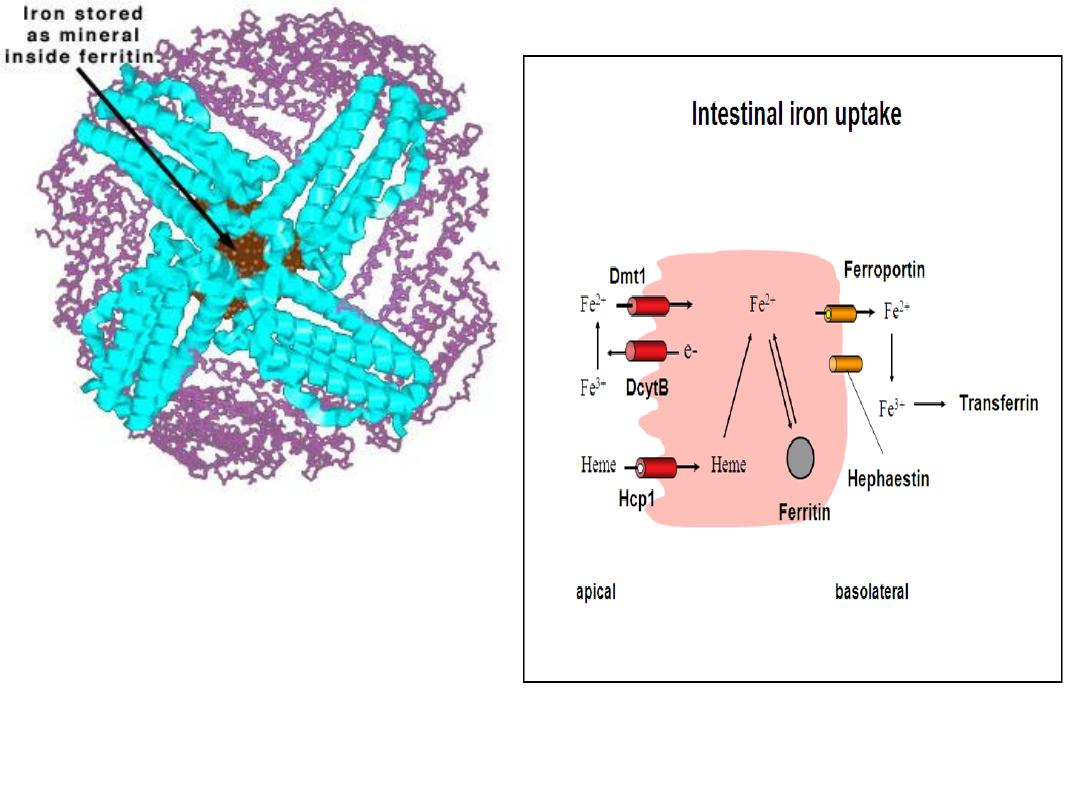
Iron Metabolism
1.Iron (Ferric) from foods is ingested.
2.To be absorbed by the intestinal cells, ferric ions
(Fe
+3
) must be reduced to ferrous ions (Fe
+2
) by
agents like Vitamin C (ascorbate).
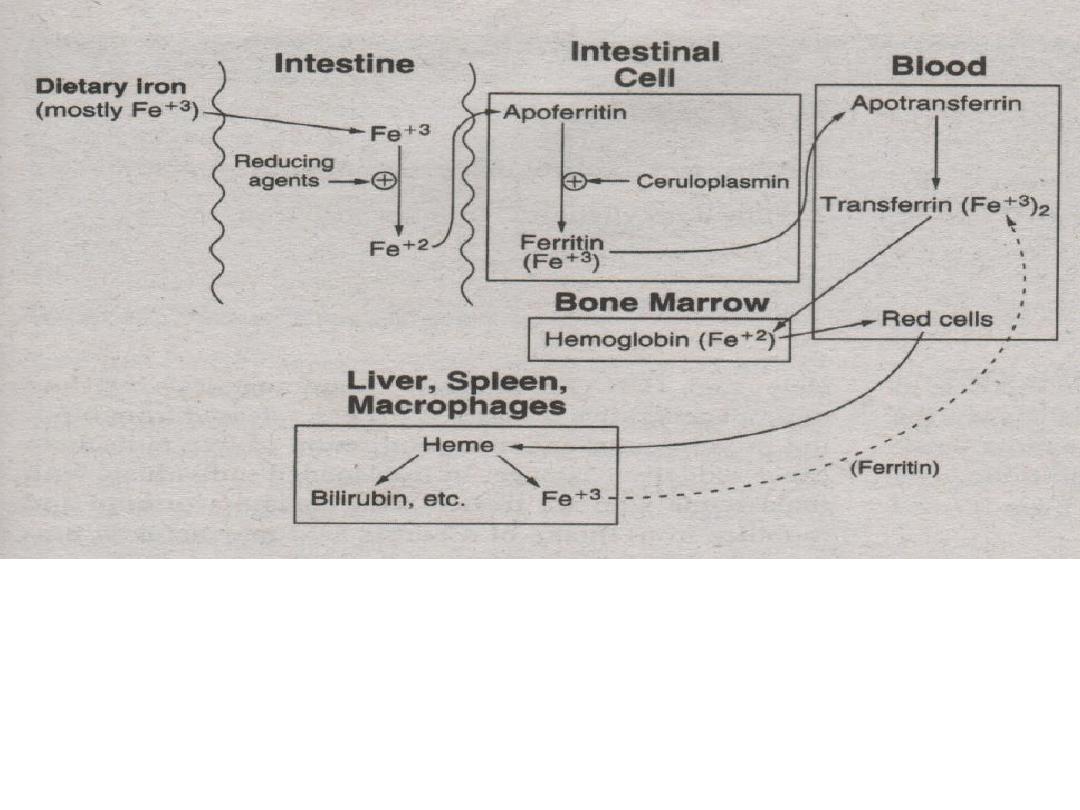
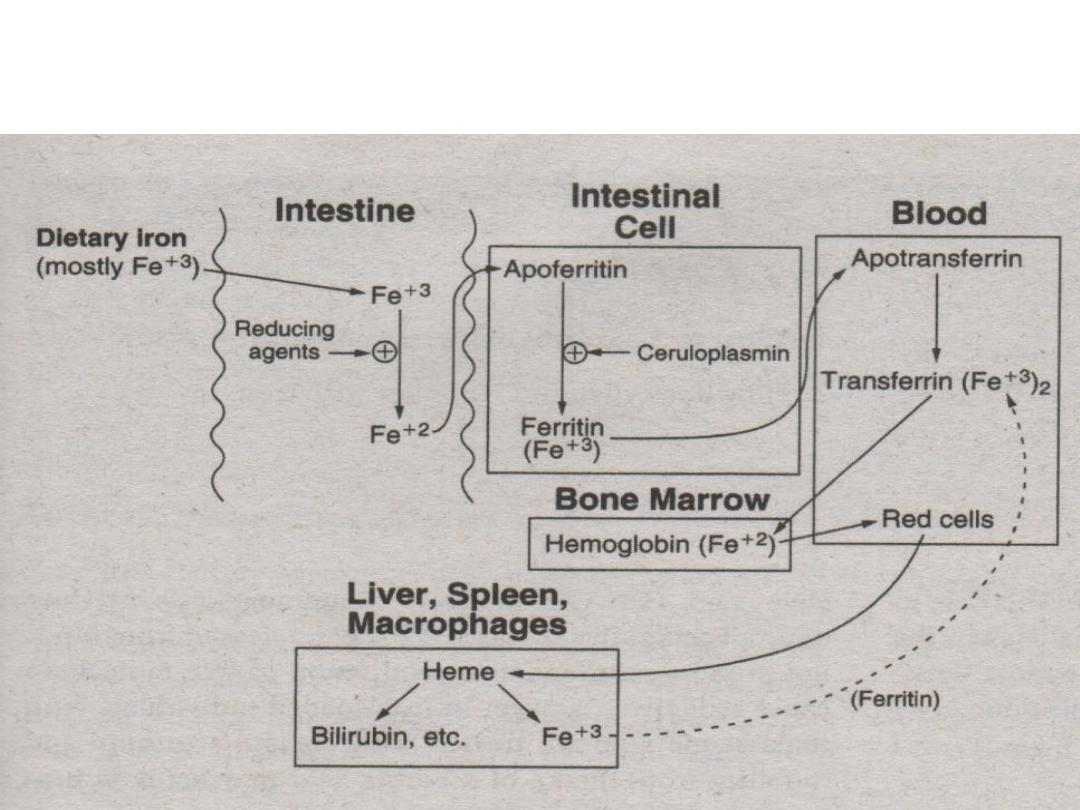
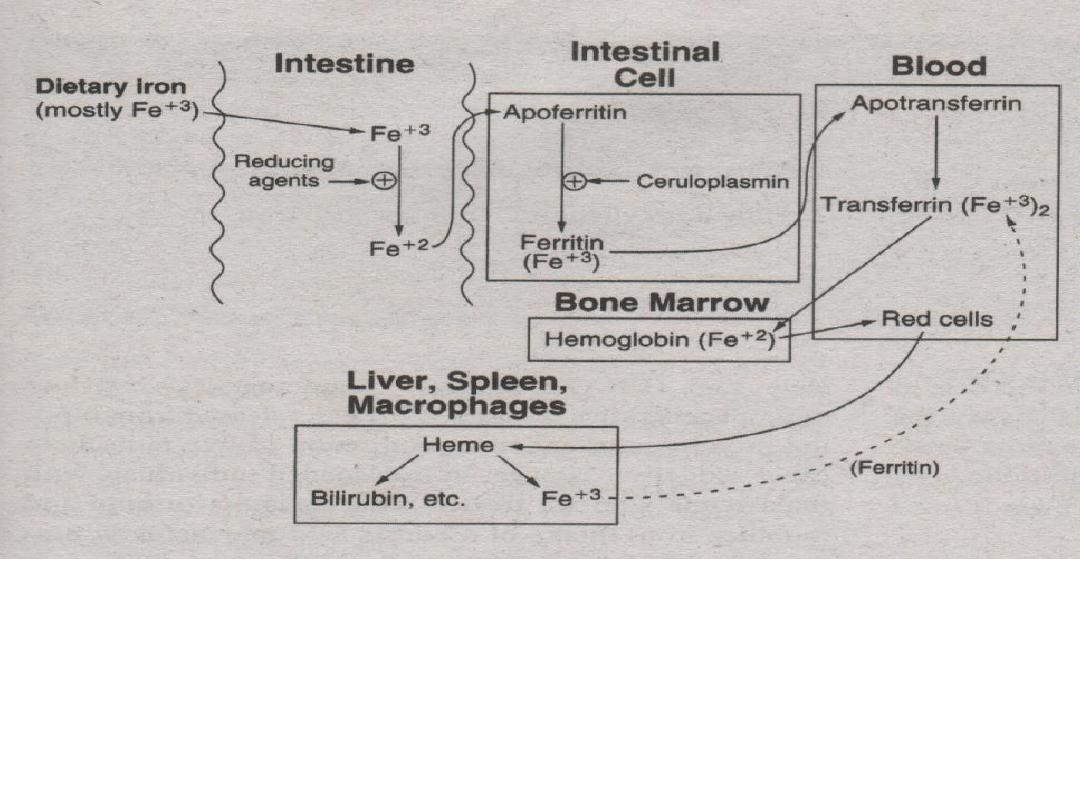
3. Ferrous ions (Fe
+2
) are bound to apoferritin
then oxidized to ferric ions (Fe
+3
) by
ceruloplasmin to become bound as ferritin.
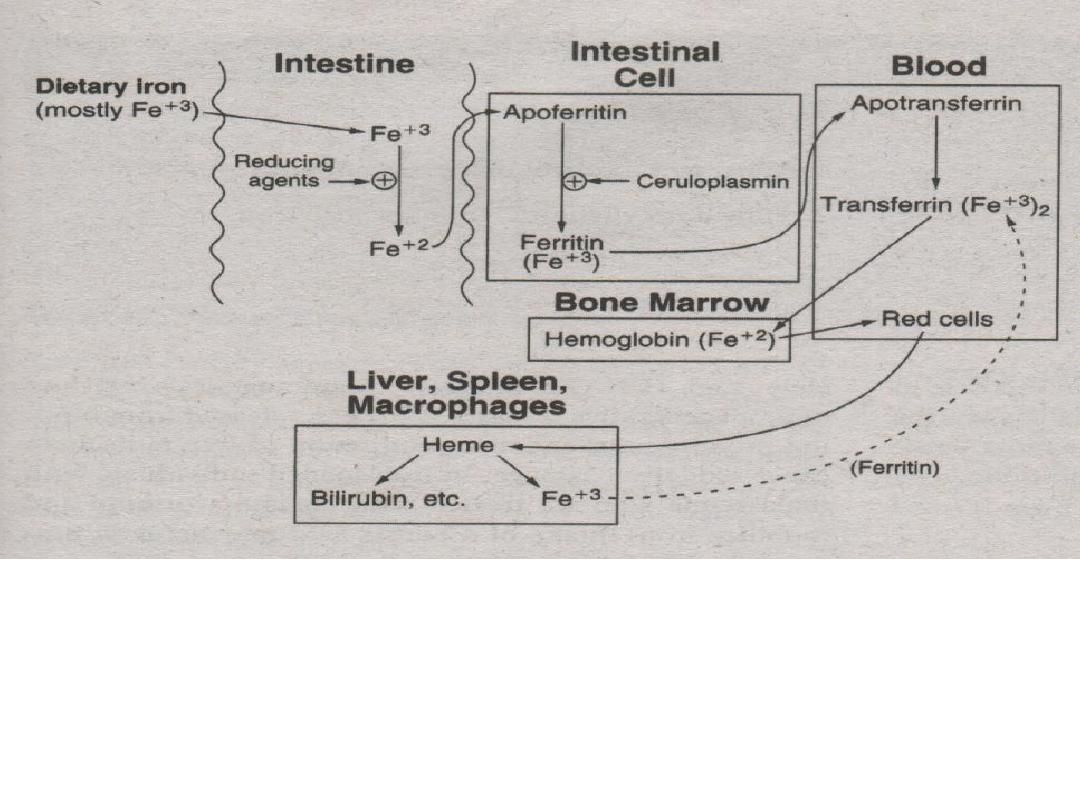
4. Ferritin is carried into blood (plasma) and releases
its ferric ions (Fe
+3
). 2 ferric ions (Fe
+3
) are then
absorbed by the protein apotransferrin to become
transferrin.
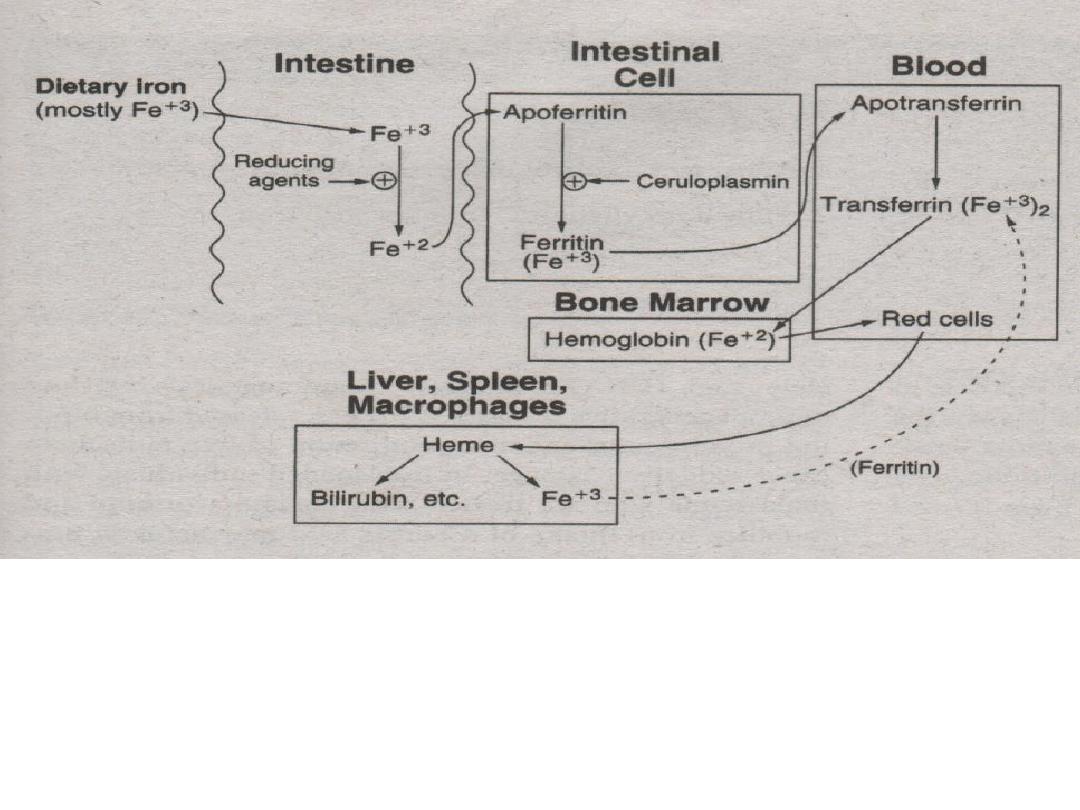
5. Ferric (Fe
+3
) ions are then incorporated into the bone
marrow for hemoglobin production.
* methemoglobin reductase – reduces (Fe
+3
) to (Fe
+2
)
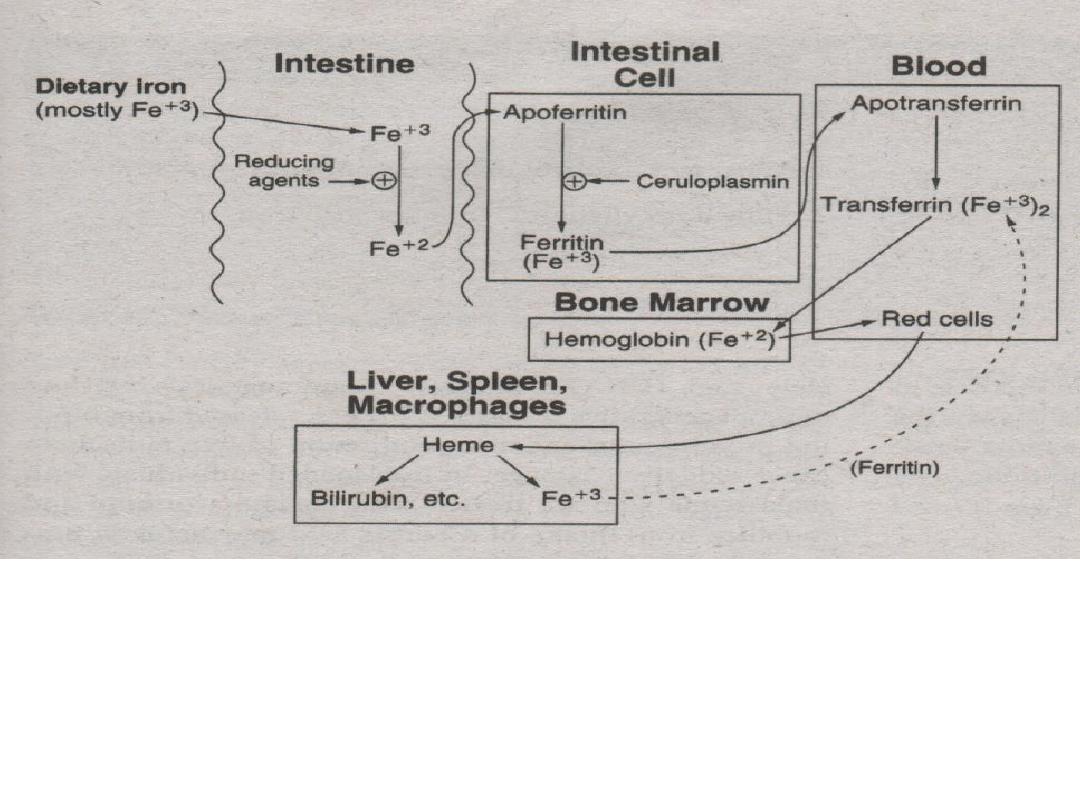
6. RBC’s are degraded by the spleen, liver and
macrophages. Iron leftovers from RBC’s are
carried by transferrin and recycled.
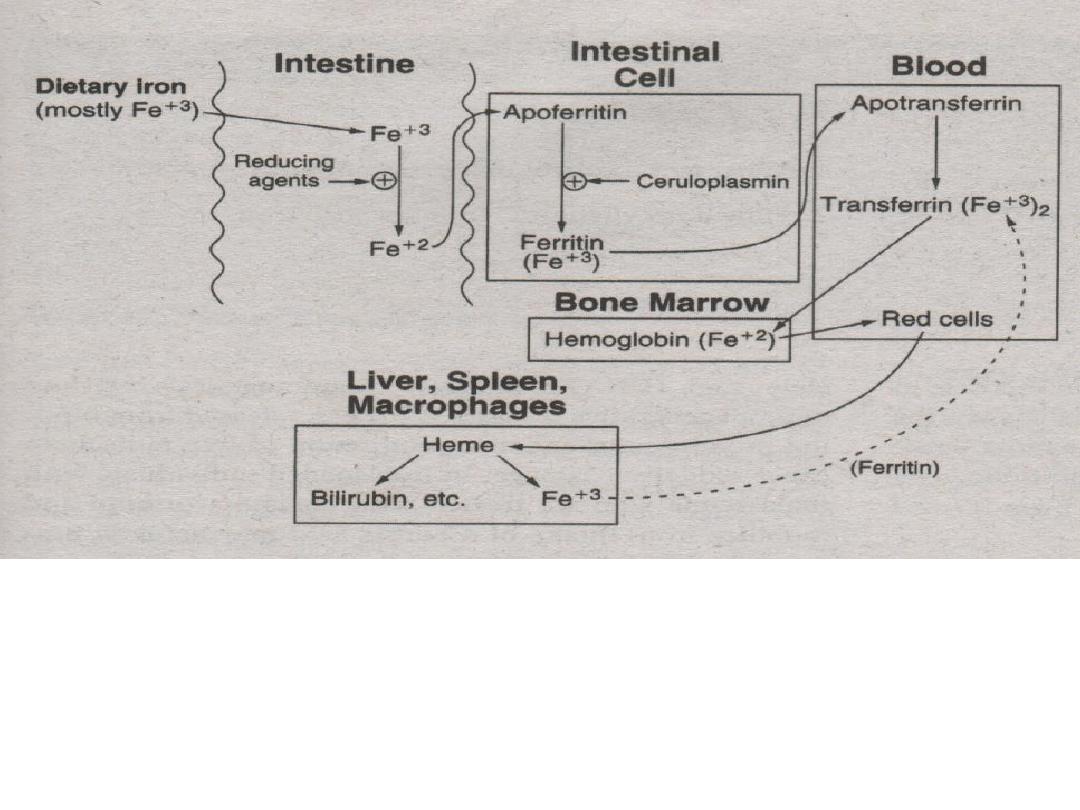
However, some iron is lost and excreted in the feces
or urine. Women lose 20-40 mg of iron due to
menstruation...

Implication
Diagnosis of conditions/diseases
- sort out the diseases
- most common: iron deficiency
anemia
Assess nutritional status of the patient.

Specimen and Patient Preparation
Hemolyzed specimens must be
rejected.
Specimen must be collected as serum:
-
Oxalate, citrate and EDTA
as anticoagulant is
unacceptable – chelators that can bind to iron.
Early morning samples are preferred – diurnal
variation in iron concentration. 25% lower in the evening.
Fasting specimen is required – diet may contain iron.
Patient must not be in iron medication.
Condition
Ferritin Transferrin
TIBC
Serum Iron
Iron Deficiency
Anemia
Thalassemia Major
N /
N
N
N
Hemochromatosis
/ Iron Overload
N or

Iron Deficiency Anemia
- An impaired production disease.
- Exists when there’s an increased need for iron or
when excessive blood loss has reduced the body's
iron reserves.
- Insufficient iron is available for normal
hemoglobin production.
- Most common cause of anemia on the planet,
affecting at least 1/3 of the world's population

Iron Deficiency Anemia
The sequence of events in developing iron deficiency anemia:
Stage 1: Iron Depletion
– when blood loss exceeds
absorption, iron is mobilized from stores, ferritin decreases, iron
absorption increases, and plasma iron-binding capacity
(transferrin) increases.
Stage 2: Iron-Deficient Erythropoiesis
– after iron stores are
depleted, the plasma iron concentration falls, As a result of lack
of iron for heme synthesis, Hb and RBCs will be decreased
gradually.
Stage 3: Iron Deficiency Anemia
–
in addition to the above
abnormalities, microcytic, hypochromic anemia is present.
Condition
Iron
Replete
(normal)
Stage 1
(Iron
Depletion)
Stage 2 (Iron-
Deficient
Erythropoiesis)
Stage 3
(IDA)
Iron
Overload
Ferritin
> 12
< 12
< 12
< 12
> 300
TIBC
300-360
360
390
410
< 300
Serum Iron
65-165
115
< 60
< 40
> 175

Iron Deficiency Anemia
The mechanisms of Iron Deficiency include:
Increased physiologic demand:
Rapid growth of infants and children.
Pregnancy, lactation.
Inadequate intake:
Iron-deficient diet
Inadequate absorption (achlorhydria, decreased
absorptive surface)
Blood loss:
Menstruation
Gastrointestinal bleeding
Hemorrhoids
Regular blood donation
Hemolysis

Iron Deficiency Anemia
Clinical Presentation:
Fatigue, breathlessness and dizziness
– due to
reduced oxygen delivery.
Pica
– persistent compulsive desire of eating substances
like ice, clay, plaster, dirt and even insects.
Disturbances in the gastrointestinal system
Koilonychia
– spooning of nails


Plummer-Vinson Syndrome
Plummer-Vinson syndrome (US)
or
Paterson-Brown Kelly
syndrome (UK)
is a rare disease defined by
severe, long-term
iron
deficiency anemia, which causes swallowing difficulty (dysphagia) due
to web-like membranes of tissue growing in the throat (esophageal
webs).
