
1
Histology
Prof. Dr. Faraid
Lec.5
LIVER
The liver is located in the peritoneal cavity below the diaphragm.
The liver is the largest gland in the body and is the second largest organ
(the largest is the skin), weighing about 1.5 Kg in the adult. The liver
produces bile and plays a major role in lipid, carbohydrate, and protein
metabolism. The liver inactivates and metabolizes many toxic
substances and drugs. It also participates in iron metabolism and the
synthesis of blood proteins and the factors necessary for blood
coagulation. Elimination of toxic substances occurs in the bile, an
exocrine secretion of the liver that is important for lipid digestion.
The liver is covered by a thin connective tissue capsule (Glisson's
capsule) that becomes thicker at the hilum (porta hepatis) where the
portal vein and the hepatic artery enter the organ and where the right
and the left hepatic ducts and lymphatics exit. Trabeculae arise from the
capsule subdivide the liver into lobes and lobules. In man the trabeculae
are very incomplete.
The structural units of the liver are called liver lobules. In certain
animals (eg. pigs) these lobules are separated from each other and
sharply delimited by a layer of connective tissue. This is not the case in
humans, making it difficult to establish the exact limits between
different lobules.
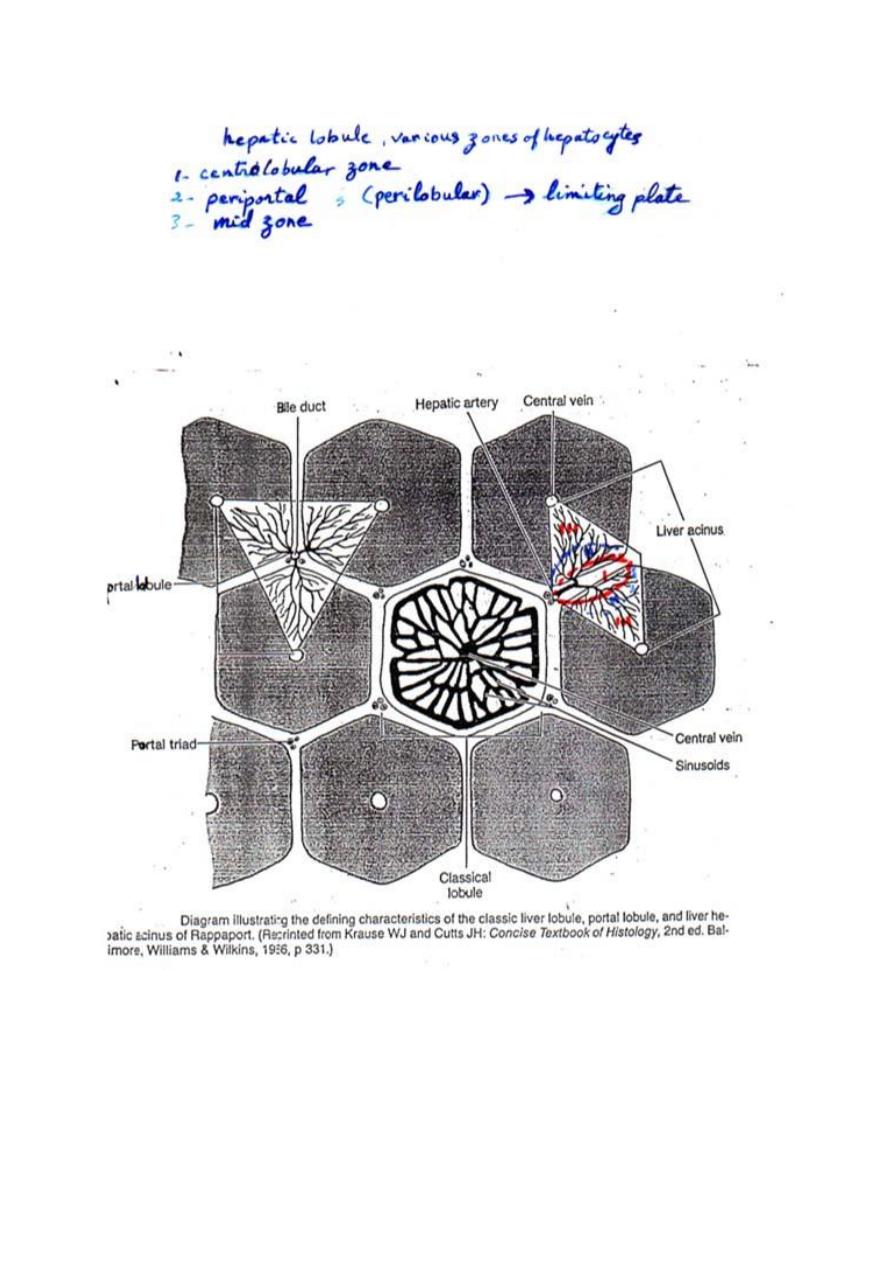
Liver Lobules
:
1. Classic Liver Lobule: (this is based on the direction of the blood
flow)
It is a hexagonal mass of tissue primarily composed of plates of
hepatocytes which radiate from the region of the central vein (present in
2
the center) toward the periphery. The plates are separated by hepatic
sinusoids. At the corners of the lobule, there is the portal canals [portal
areas, portal tracts, portal spaces or portal triad].Each portal canal
contains: a portal venule (a branch of portal vein), a hepatic arteriole (a
branch of hepatic artery), bile duct and lymphatic vessels. These
structures are surrounded by loose connective tissue.
The arterial and venous blood flows centripetally in each lobule (from
the portal areas to drain in the central vein), whereas the bile flows
centrifugally towards the portal areas.
In the lobule, various zones of hepatocytes can be identified. These are
the centrolobular, periportal, and mid zones.
1. The centrolobular zone: is composed of hepatocytes surrounding
the central vein, and is the zone most distant from the oxygenated
arterial blood supply.
2. The periportal (perilobular zone): at the periphery of the lobule, is
closely related to the portal tracts. The outermost layer of
periportal hepatocytes immediately adjacent to the portal tract is
called the limiting plate; it is the first group of hepatocytes to be
damaged in liver disorders that primarily involve the portal tracts.
3. The mid zone: is the zone of hepatocytes between the
centrolobular and periportal zones.
2. Portal Lobule: (this is based mainly on the direction of bile flow)
It is a triangular region with a portal triad at its center and a central vein
at each of its three corners. It contains portions of three adjacent classic
liver lobules all of which drain bile into one portal canal.
3. Hepatic Acinus (of Rappaport): (it is based on changes in oxygen,
nutrient, and toxin content as blood flowing through the sinusoids is
acted on by hepatocytes)
It is that region which is supplied by a terminal branch of the portal vein
and hepatic artery and drained by a terminal branch of the bile duct. It is
3
a diamond-shaped region, contains portions (triangular sections) of two
adjacent classic liver lobules (whose apices are the central veins).
In relation to their proximity to the distributing vessels, cells in the
hepatic acinus can be subdivided into three zones. Cells in zone I would
be those closest to the distributing vessels, whereas zone III is closer to
the central vein. Zone II is intermediate between zone I and III. Blood in
zone I sinusoids has higher oxygen, nutrient, and toxin concentrations
than in the other zones. As the blood flows toward the central vein,
these substances are gradually removed by hepatocytes. Zone I
hepatocytes thus have a higher metabolic rate and larger glycogen and
lipid stores. They are also more susceptible to damage by blood-borne
toxins, and their energy stores are the first to be depleted during fasting.
This explains regional histopathologic differences in patients with liver
damage.
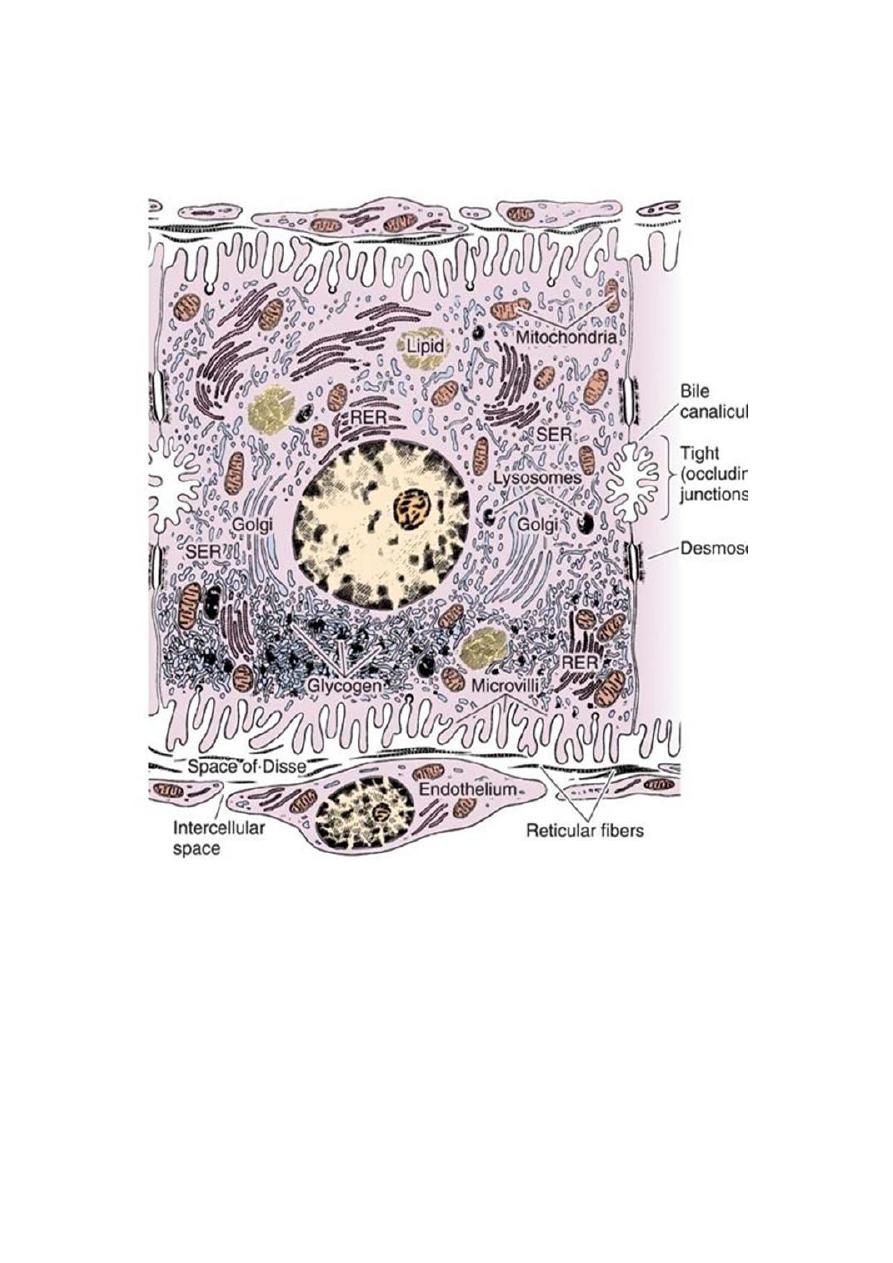
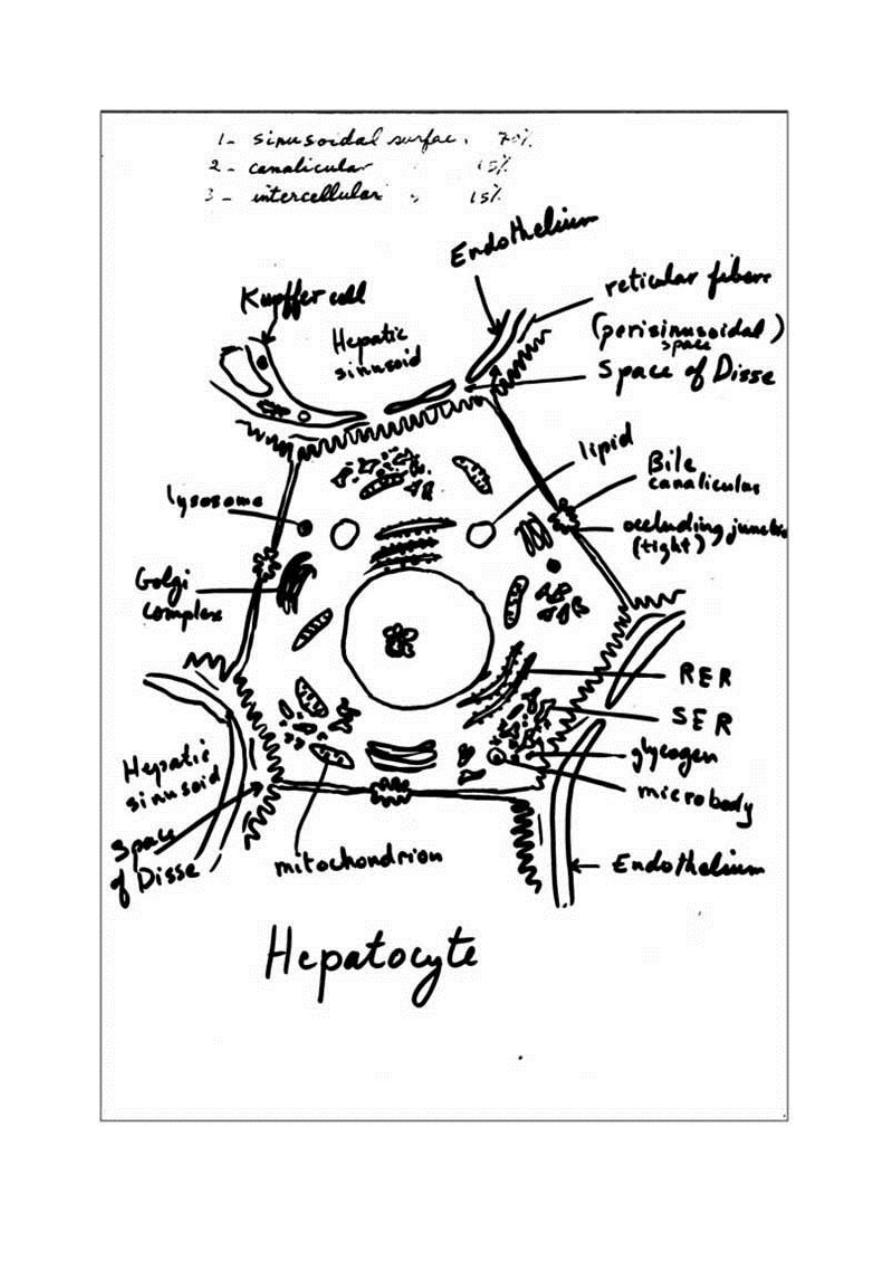
Hepatocytes:
They are the main cells of the lobules. They are epithelial cells (derived
from embryonic endoderm).
They are arranged in plates that anastomose freely and separated from
each other by spaces filled by hepatic sinusoids and are disposed
radially around the central vein. Hepatocytes are large polyherdral cells
(20-30 µm in diameter). Hepatocytes have three important surfaces:
1- Sinusoidal surfaces: are separated from the sinusoidal vessel by
the space of Disse. They account for approximately 70% of the
total hepatocyte surface. They are covered by short microvilli,
which protrude into the space of Disse. The sinusoidal surface is
the site where material is transferred between the sinusoids and the
hepatocyte.
2- Canalicular surfaces: are the surfaces across which bile drains
from the hepatocytes into the canaliculi. They account for
approximately 15% of the hepatocyte surface.

4
3- The intercellular surfaces: are the surfaces between adjacent
hepatocytes that are not in contact with sinusoids or canaliculi.
They account for about 15% of the hepatocyte surface.
Hepatocytes usually contain one round, central nucleus; some are
binucleated (25%). Occasionally nuclei are polyploid (more than one set
of chromosomes). The cytoplasm of the hepatocytes is typically fairly
acidophilic (many mitochondria and some SER), with areas of
basophilia (rough endoplasmic reticulum). The hepatocytes are unusual
in that they possess abundant RER and SER in the same cells. The RER
is associated with protein synthesis such as: albumin, fibrinogen,
globulin, prothrombin. The SER is associated with steroid metabolism
and also is responsible for the processes of oxidation, methylation, and
conjugation required for inactivation or detoxification of various
substances before their excretion from the body.
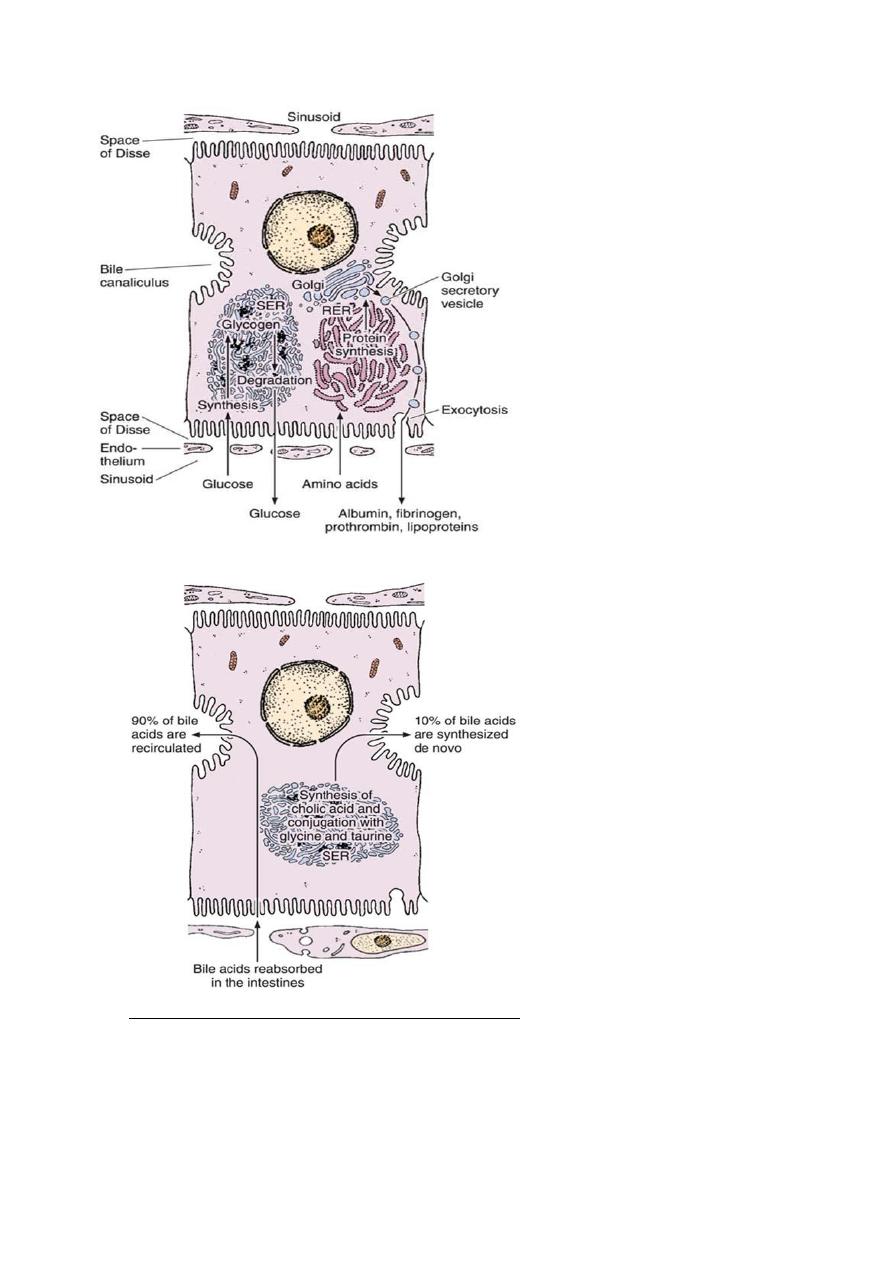
One of the main processes occurring in the SER is the conjugation of
water-insoluble toxic bilirubin with glucuronide by glucuronyl-
transferase activity to form a water-soluble nontoxic bilirubin
glucuronide. This conjugate is excreted by hepatocytes into the bile.
When bilirubin or bilirubin glucuronide is not excreted, various diseases
characterized by jaundice can result.
Each hepatocyte has approximately 2000 mitochondria. Golgi
complexes are also numerous up to 50 per cell. The functions of this
organelle include the formation of lysosomes and the secretion of
plasma proteins (eg. albumin, proteins of the complement system),
glycoproteins (eg. transferrin), and lipoproteins (eg. very low-density
lipoproteins). Hepatocytes possess lysosomes which are important in the
turnover and degradation of interacellular organelles. Peroxisomes are
abundant. Lipid droplet and glycogen are found in varying amounts
depending upon the functional state of the cells. The glycogen appears
in the electron microscope as coarse, electron dense granules that
frequently collect close to the SER.
5
The hepatocyte is probably the most versatile cell in the body. It is a
cell with both endocrine and exocrine functions. Endocrine secretion
involves the production and release of several plasma proteins. Unlike
other protein synthesizing and secreting cells of the body, the
hepatocytes are unusual in that they lack protein storage granules.
Exocrine secretion involves the production and release of bile.
Hepatocytes located at different distances from the portal triads show
differences in structural, histochemical, and biochemical characteristics.
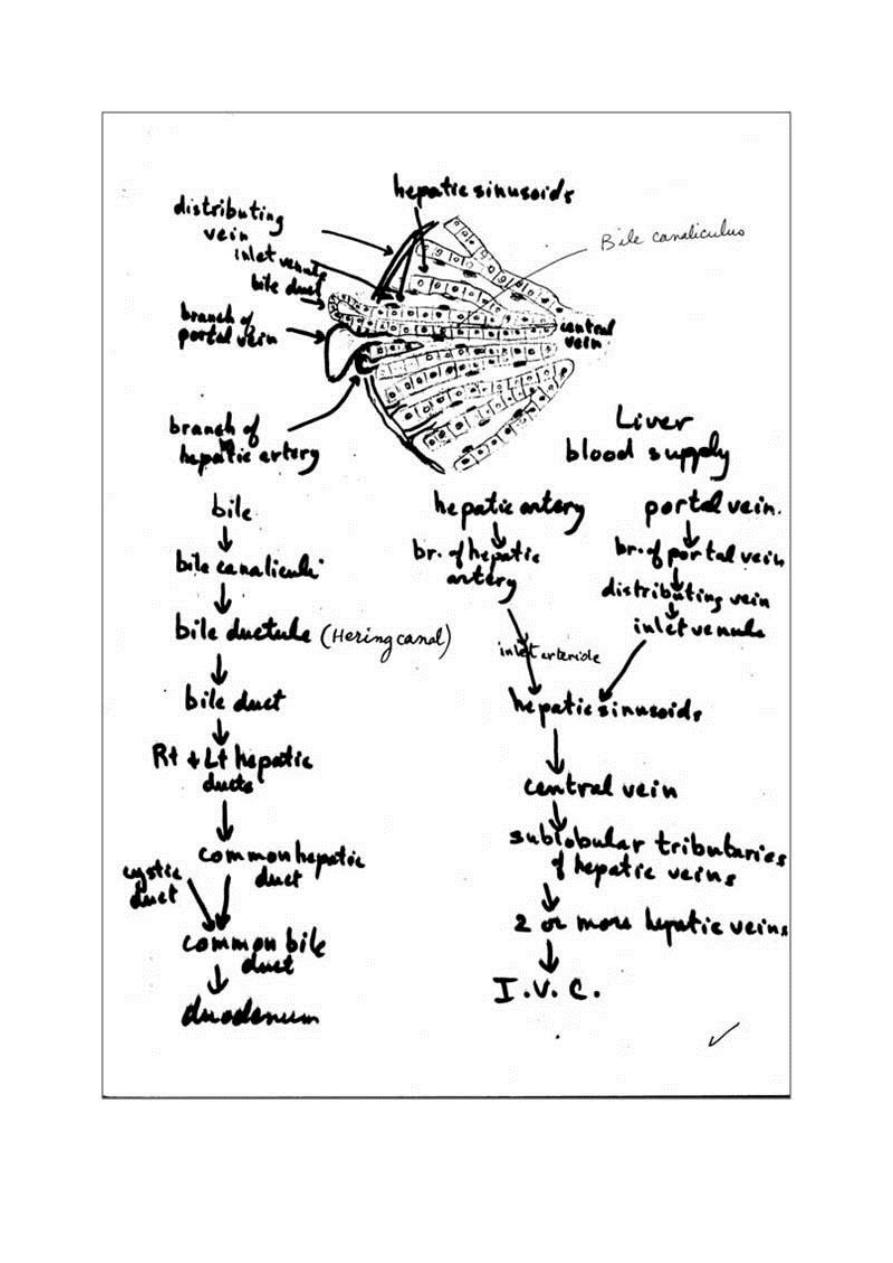
Bile canaliculi: are the first portions of the bile duct system. They are
tubular spaces 1-2 µm in diameter, limited only by the plasma
membranes of 2 adjacent hepatocytes and have a small number of
microvilli in their interiors. The cell membranes near these canaliculi
are sealed together by tight junctions. The canaliculi drain outwards in
the direction of the portal canal. The bile flow therefore progresses in a
direction opposite to that of the blood, i.e., from the center of the lobule
to its periphery. At the periphery, bile enters the bile ductules (or
Hering's canals), lined with simple cuboidal epithelium. After a short
distance, the ductules cross the limiting hepatocytes of the lobule and
end in the bile ducts in the portal spaces. Bile ducts are lined by simple
cuboidal or columnar epithelium and have a distinct connective tissue
sheath. They gradually enlarge and fuse, forming right and left hepatic
ducts, which leave the liver, unite to form a common hepatic duct. The
hepatic duct after receiving the cystic duct from the gallbladder
continues to the duodenum as the common bile duct. The hepatic, cystic,
and common bile ducts are lined with simple columnar epithelium.
Hepatic sinusoids: are vessels that arise at the periphery of a lobule and
run between adjacent plates of hepatocytes. They receive blood from the
vessels in the portal areas and deliver it to the central vein. Sinusoids are
larger than capillaries, more irregular in shape and their lining cells are
directly related to the epithelial cells with no intervening connective
tissue.The lining of the sinusoids consists of a discontinuous layer of
fenestrated endothelium also contain Kupffer cells in their endothelial
lining. They lack basal lamina.

6
Kupffer cells: are mononuclear phagocytic cells (fixed macrophages)
derived from blood monocytes and located in the lining of hepatic
sinusoids. They are large cells with several processes, and exhibit an
irregular or stellate outline. Their main functions are to metabolize aged
erythrocytes,
digest
hemoglobin,
secrete
proteins
related
to
immunologic processes, and destroy bacteria that enter the portal blood
through the large intestine.
Space of Disse: is the perisinusoidal space located between hepatocytes
and the endothelium of hepatic sinusoids. It contains microvilli of the
hepatocytes, plasma, reticular fibers, and fat-storing cells (Ito cells).
Blood plasma enters this space through openings between the
endothelial cells that are too small for blood cells to pass. Blood-borne
substances thus directly contact the microvilli of hepatocytes. These
cells absorb nutrients, oxygen, and toxins from, and release endocrine
secretions into, these spaces.
It functions in the exchange of material between the bloodstream and
hepatocytes, which do not directly contact the blood stream.
Fat-storing (Ito) cells: are stellate cells lie in the space of Disse and
have the ability to accumulate lipid droplets. They are the main source
of vitamin A storage in the body and also play a role in wound healing
(hepatic fibrogenesis).
Blood supply of the liver:
The liver is unusual in that it receives blood from two sources:70- 80%
of the blood derives from the portal vein, which carries oxygen-poor,
nutrient-rich blood from the abdominal viscera, and 20-30% derives
from the hepatic artery, which supplies oxygen-rich blood.
7
Portal vein system:
The portal vein branches repeatedly and sends small portal venules to
the portal spaces. The portal venules branch into the distributing veins
that run around the periphery of the lobule. From the distributing veins,
small inlet venules empty into the sinusoids. The sinusoids run radially,
converging in the center of the lobule to form the central, or
centrolobular, vein. The central vein has thin walls consisting only of
endothelial cells supported by a sparse population of collagen fibers. As
the central vein progresses along the lobule, it receives more and more
sinusoids and gradually increases in diameter. At its end, it leaves the
lobule at its base by merging with the larger sublobular vein. The
sublobular veins gradually converge and fuse, forming the two or more
large hepatic veins that empty into the inferior vena cava.
The portal system conveys blood from the pancreas and spleen and
blood containing nutrients absorbed in the intestines. The portal vein is
formed by the junction of mesenteric and splenic veins.
Arterial system:
The hepatic artery branches repeatedly and forms the interlobular
arteries (hepatic arterioles). Some of these arteries irrigate the structures
of the portal canal, and others form arterioles (inlet arterioles) that end
directly in the sinusoids, thus providing a mixture of arterial and portal
venous blood in the sinusoids. The hepatic artery is a branch of the
celiac artery of the abdominal aorta.
Blood flows from the periphery to the center of the liver lobule.
Consequently, oxygen and metabolites, as well as all other toxic or
nontoxic substances absorbed in the intestines, reach the peripheral cells
first and then reach the central cells of the lobule. This direction of
blood flow explains why the behavior of perilobular cells differs from
that of the centrolobular cells. This is particularly evident in pathologic

8
specimens, where changes are seen in either the central cells or the
peripheral cells of the lobule.
Liver Regeneration:
Despite its low rate of cell renewal, the liver has an extraordinary
capacity for regeneration. The process of regeneration is probably
controlled by circulating substances called chalones, which inhibit the
mitotic division of certain cell types. When a tissue is injured or
partially removed, the number of chalones it produces decreases;
consequently, a burst of mitotic activity occurs in this tissue. As
regeneration proceeds, the quantity of chalones produced increases and
mitotic activity decreases. This process is self-regulating. The
regenerated liver tissue is usually similar to the removed tissue. If there
is continuous or repeated damage to hepatocytes over a long period, the
multiplication of liver cells is followed by a pronounced increase in the
amount of connective tissue. The excess of connective tissue results in
disorganization of the liver structure, a condition known as cirrhosis, is
a progressive and irreversible process, causes liver failure, and is usually
fatal.
Cirrhosis is a consequence of any sustained progressive injury to
hepatocytes produced by several agents, such as ethanol, drugs or other
chemicals, hepatitis virus, and autoimmune liver disease.
9
11
Ultrastructure of a hepatocyte. RER, rough endoplasmic reticulum;
SER, smooth endoplasmic reticulum. x10,000
11
12
Protein synthesis and carbohydrate
storage in the liver. Carbohydrate is
stored as glycogen, usually associated
with the smooth endoplasmic reticulum
(SER). When glucose is needed, glycogen
is degraded. In several diseases, glycogen
degradation is depressed, resulting in
abnormal intracellular accumulations of
glycogen. Proteins produced by
hepatocytes are synthesized in the rough
endoplasmic reticulum (RER), which
explains why hepatocyte lesions or
starvation lead to a decrease in the
amounts of albumin, fibrinogen, and
prothrombin in a patient’s blood. The
impairment of protein synthesis leads to
several complications, since most of these
proteins are carriers, important for the
blood’s osmotic pressure and for
coagulation.
Mechanism of secretion of bile acids. About 90% of bile acids are derived from the
intestinal epithelium and transported to the liver. The remaining 10% are synthesized in
the liver by the conjugation of cholic acid with the amino acids glycine and taurine. This
process occurs in the smooth endoplasmic reticulum (SER).
13
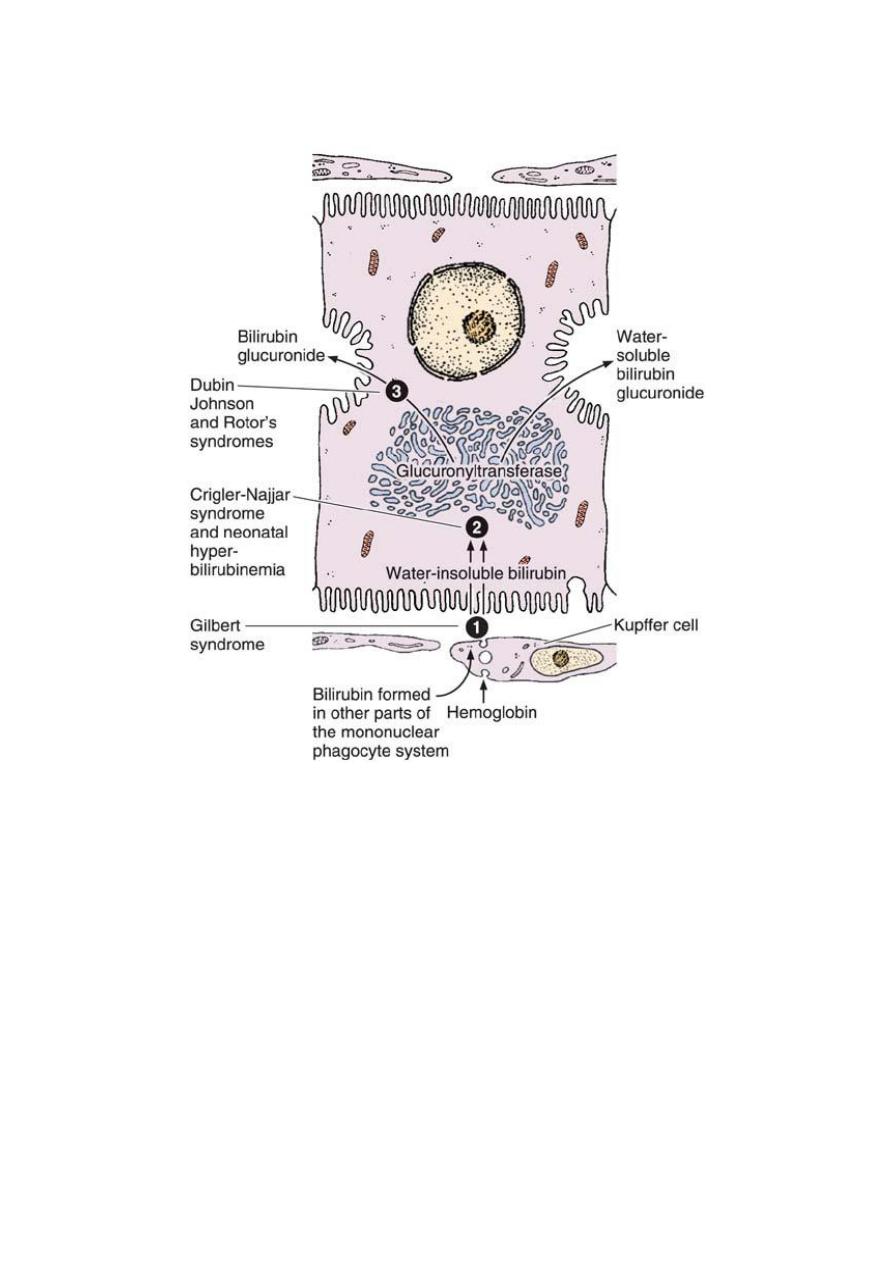
The secretion of bilirubin. The water-insoluble form of bilirubin is derived from the
metabolism of hemoglobin in macrophages. Glucuronyltransferase activity in the
hepatocytes causes bilirubin to be conjugated with glucuronide in the smooth endoplasmic
reticulum, forming a water-soluble compound. When bile secretion is blocked, the yellow
bilirubin or bilirubin glucuronide is not excreted; it accumulates in the blood, and
jaundice results. Several defective processes in the hepatocytes can cause diseases that
produce jaundice: a defect in the capacity of the cell to trap and absorb bilirubin (1), the
inability of the cell to conjugate bilirubin because of a deficiency in glucuronyltransferase
(2), or problems in the transfer and excretion of bilirubin glucuronide into the bile
canaliculi (3). One of the most frequent causes of jaundice, however–unrelated to
hepatocyte activity–is the obstruction of bile flow as a result of gallstones or tumors of the
pancreas.
14
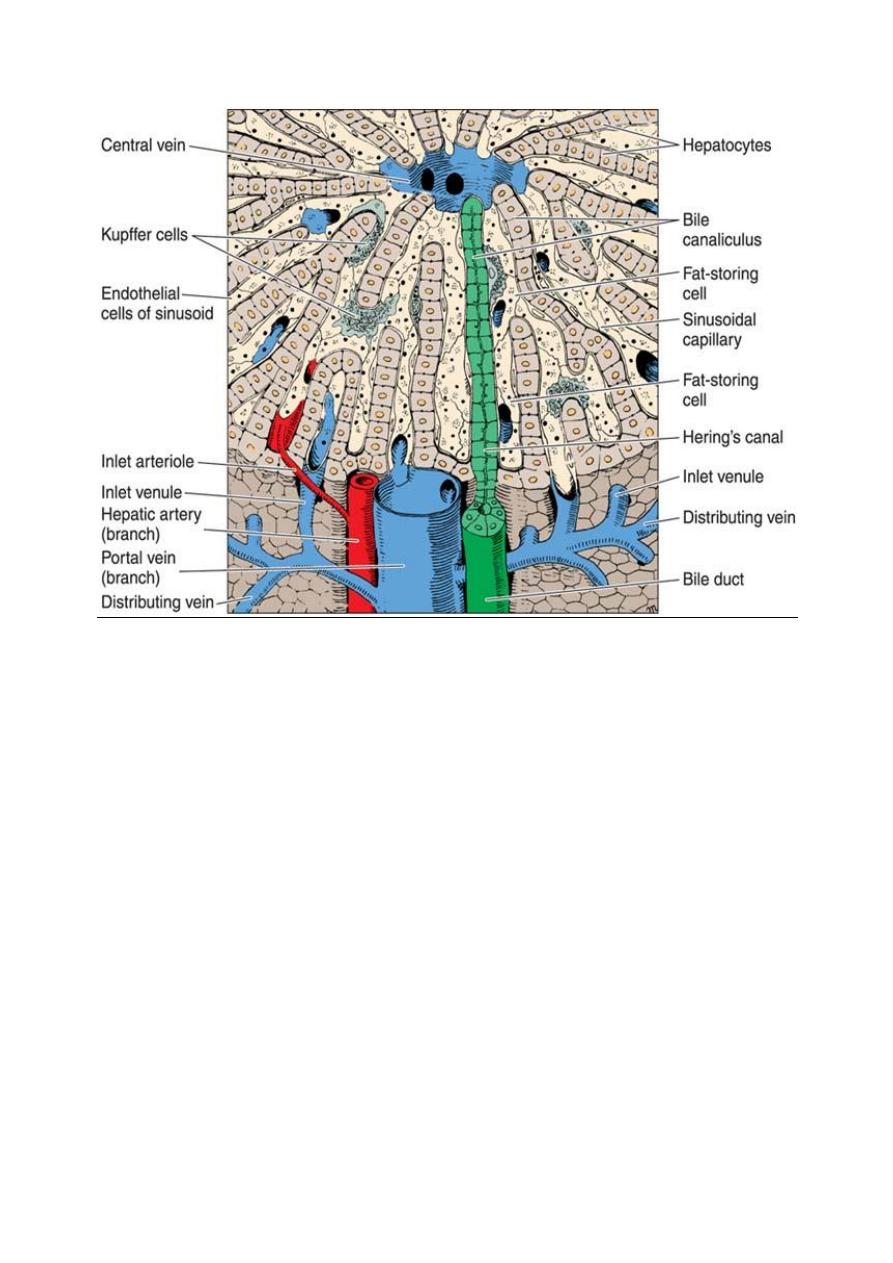
Three-dimensional aspect of the normal liver. In the upper center is the
central vein; in the lower center is the portal vein. Note the bile canaliculus,
liver plates, Hering’s canal, Kupffer cells, sinusoid, fat-storing cell, and
sinusoid endothelial cells. (Courtesy of M Muto.)
15
16
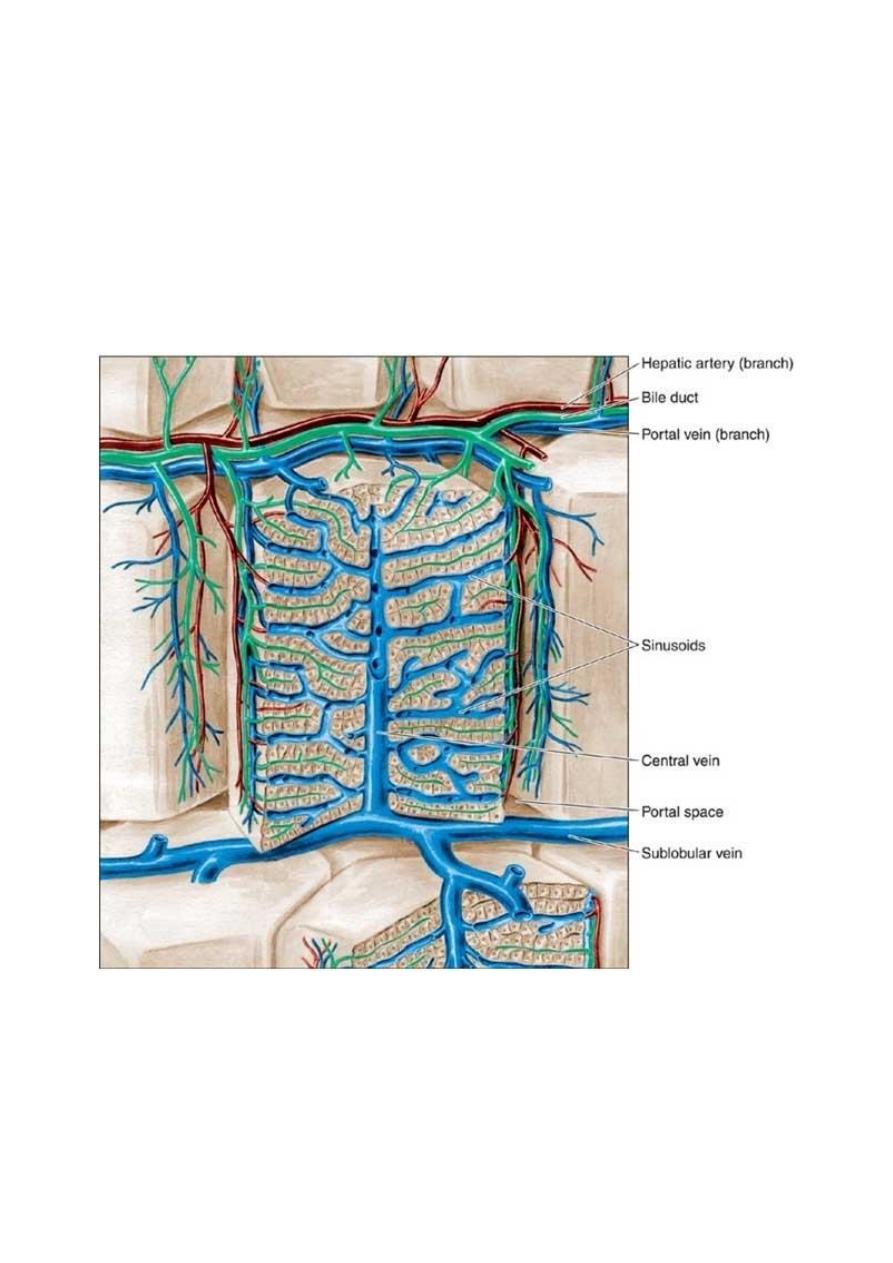
Schematic drawing of the structure of the liver. The liver lobule in the center is
surrounded by the portal space (dilated here for clarity). Arteries, veins, and bile ducts
occupy the portal spaces. Nerves, connective tissue, and lymphatic vessels are also present
but are (again, for clarity) not shown in this illustration. In the lobule, note the radial
disposition of the plates formed by hepatocytes; the sinusoidal capillaries separate the
plates. The bile canaliculi can be seen between the hepatocytes. The sublobular
(intercalated) veins drain blood from the lobules. (Redrawn and reproduced, with
permission, from Bourne G: An Introduction to Functional Histology. Churchill, 1953.
