
Prof. Dr. Malak A. Al –yawer

At the end of this lecture, the medical
student will be able to
State the stem cell theory of hematopoiesis.
Compare between stem cells, progenitor cells, blast cells
and mature cells
Discuss factors involved in the stimulation and
regulation of hematopoietic activity.
Review maturation stages of erythrocytes
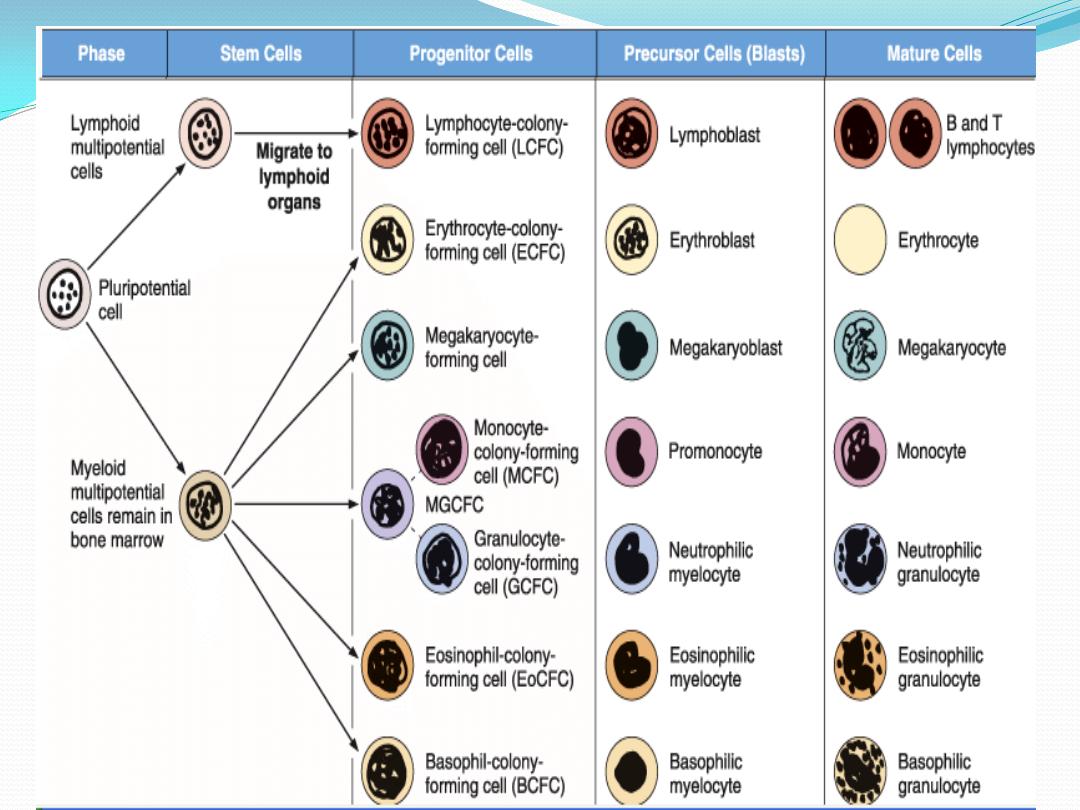

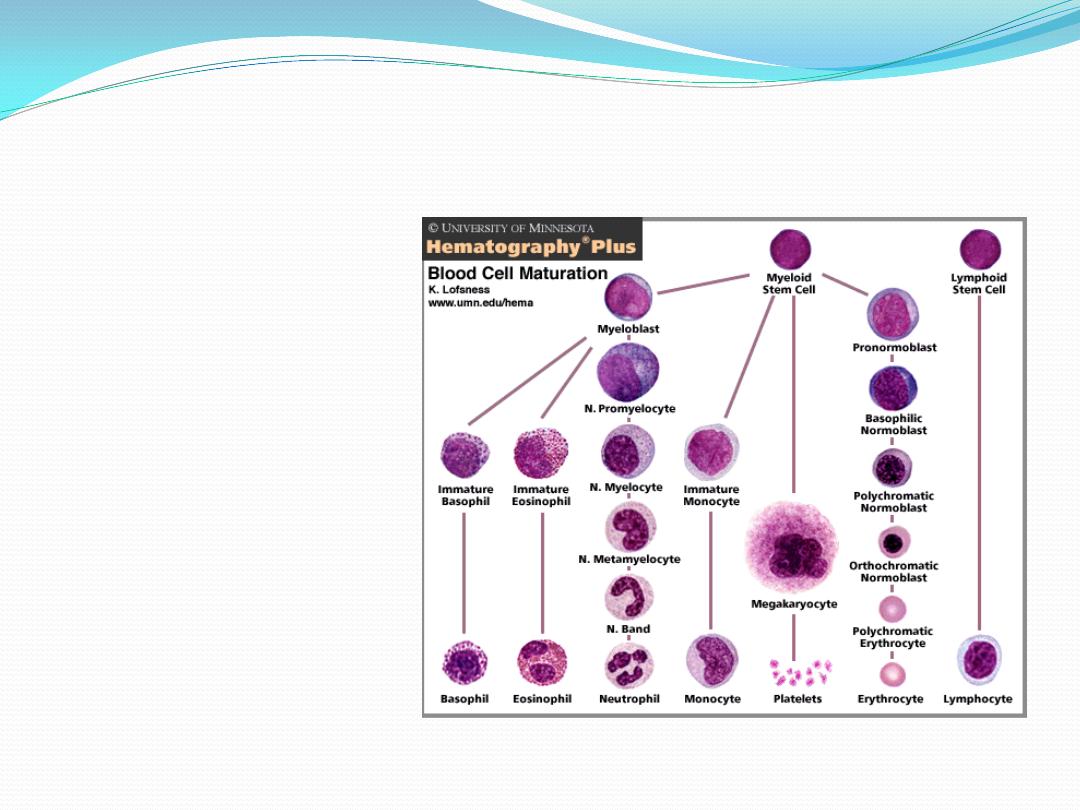
Hematopoiesis
takes place in the extravascular compartment
The currently accepted theory on how this process
works is called the
monophyletic theory
which
simply means that a single type of stem cells gives rise
to all mature blood cells in the body.
This stem cells is called the
pluripotential stem
cells
.
pleuripotential stem
cell
It is called a
pleuripotential
stem cell because
it can porduce all
blood cell types
.
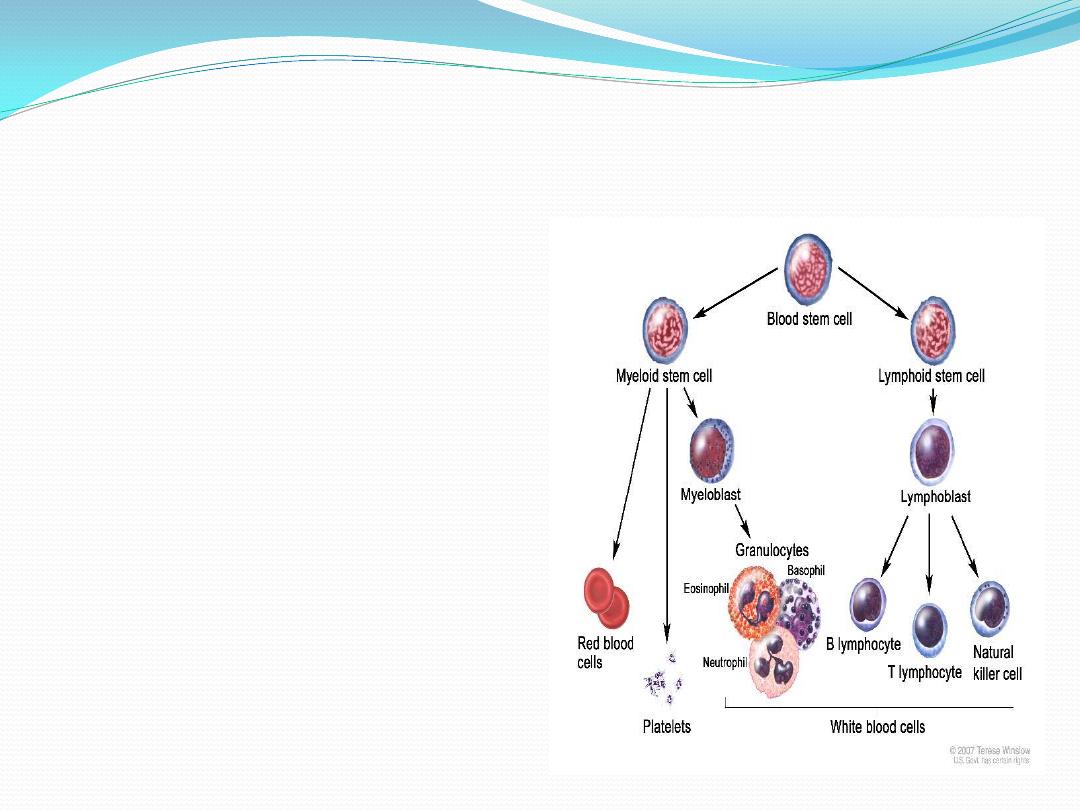
Pleuripotential stem cell
proliferate and form
1.
lymphoid multipotential
cells
: one cell lineage that
will become lymphocytes .
And
2.
myeloid multipotential
cells
: another lineage that
will form the myeloid cells
( granulocytes, monocytes,
erythrocytes and
megakaryocytes)
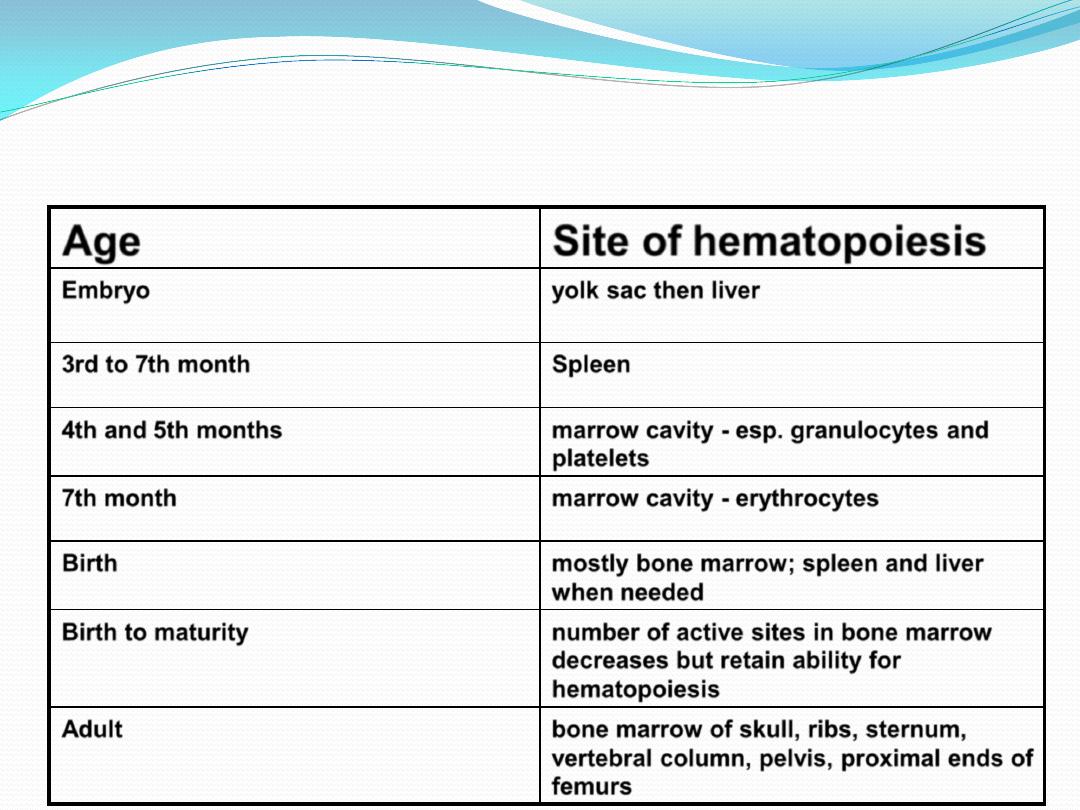
SITES OF HEMATOPOIESIS
Site of hematopoiesis
Age
yolk sac then liver
Embryo
Spleen
3rd to 7th month
marrow cavity - esp. granulocytes and
platelets
4th and 5th months
marrow cavity - erythrocytes
7th month
mostly bone marrow; spleen and liver
when needed
Birth
number of active sites in bone marrow
decreases but retain ability for
hematopoiesis
Birth to maturity
bone marrow of skull, ribs, sternum,
vertebral column, pelvis, proximal ends of
femurs
Adult

Hematopoiesis depends on
favorable
microenvironmental
conditions
and
the presence of
growth factors
.
The microenvironmental
conditions are furnished by cells
of the stroma of hematopoietic
organs, which produce an
adequate extracellular matrix.
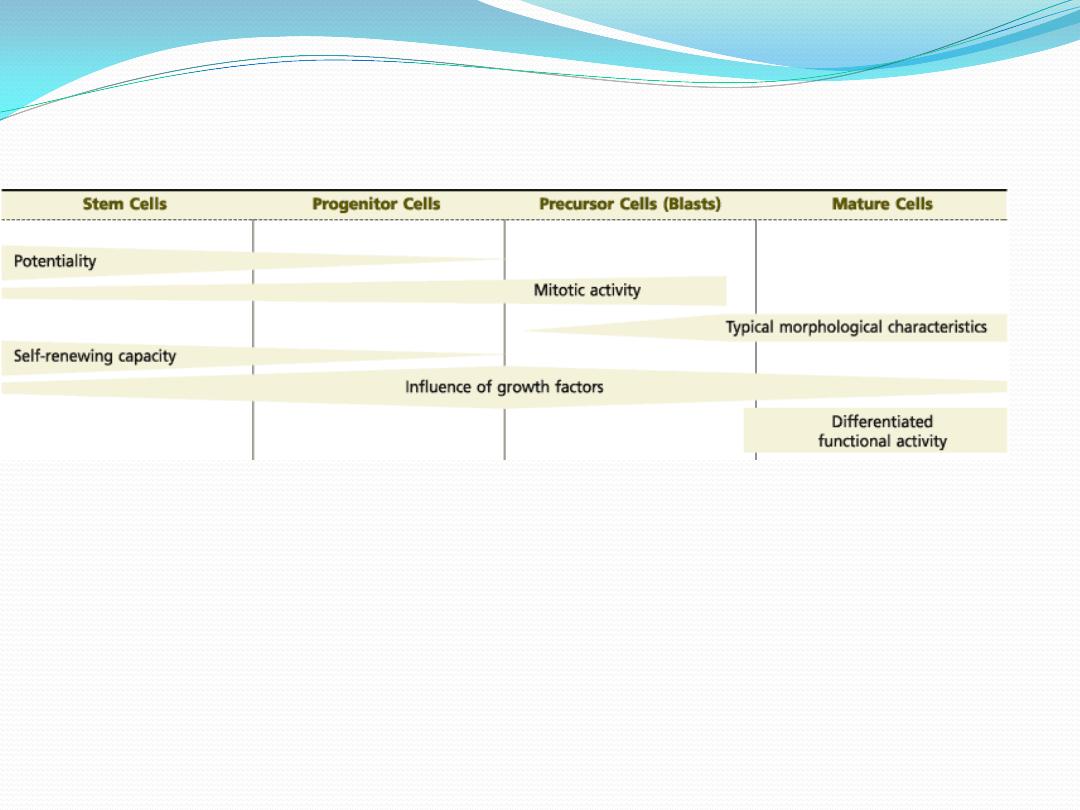
A general view of hematopoiesis
shows that
both the potential for differentiation and the self-renewing capacity of the initial cells
gradually decrease.
the mitotic response to growth factors gradually increases, attaining its maximum in the
middle of the process.
From that point on, mitotic activity decreases, morphological characteristics and functional
activity develop, and mature cells are formed
stem cells:
1.
This cell can produce all
blood cell types
2.
Low mitotic activity
3.
Self renewing
4.
Scarce in the bone marrow
5.
Cannot be
morphologically
distinguished ( resemble
large lymphocyte )
Progenitor cells :
1.
Could be unipotential or
bipotential
2.
High mitotic activity
3.
Self renewing
4.
Common in marrow and
lymphoid organs
5.
Cannot be
morphologically
distinguished ( resemble
large lymphocyte )
Precursor cells(blast ):
1.
Monopotential cells
2.
High mitotic activity
3.
Not self renewing
4.
Common in marrow
and lymphoid organs
5.
Beginning of
morphologic
differentiation
Mature cells :
1.
No mitotic activity
2.
Abundant in the blood
and haematopoietic
organs
3.
Clear morphologic
differentiation
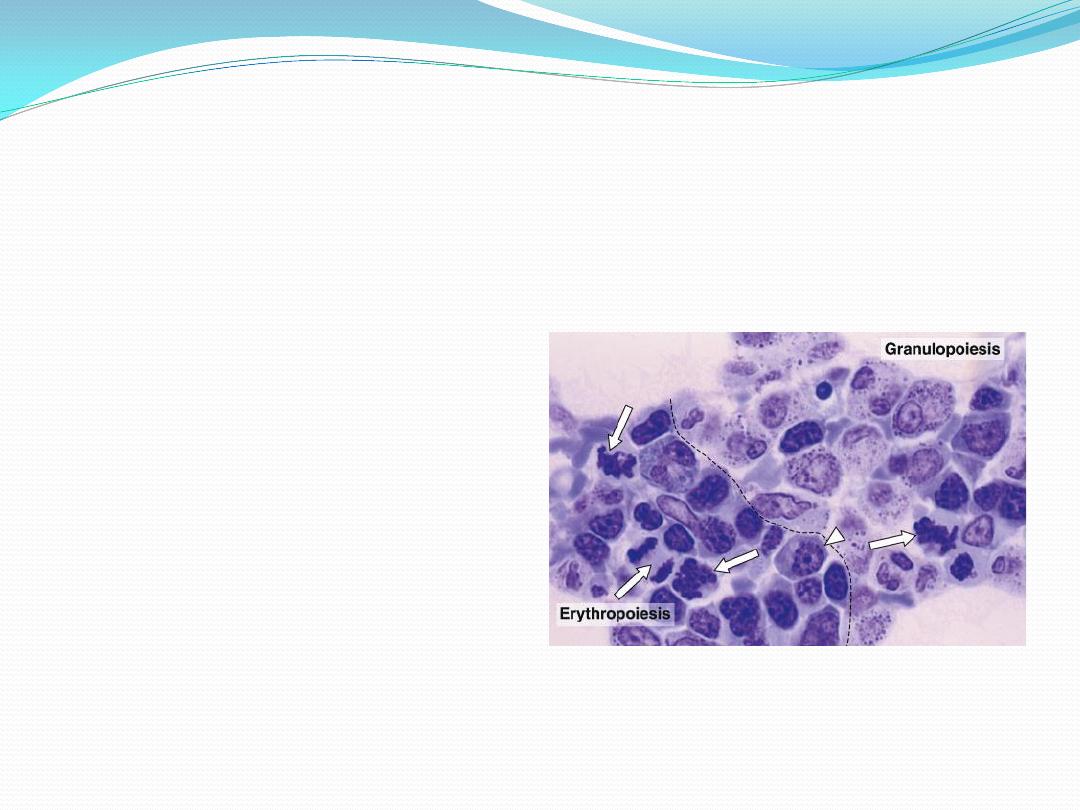
Hematopoiesis
is a compartmentalized process within the hematopoietic
tissue
erythropoiesis
taking place
in distinct anatomical
units (erythroblastic
islands) ;
granulopoiesis
occurs in
less distinct foci
megakaryopoiesis
occurs
adjacent to the sinus
endothelium .
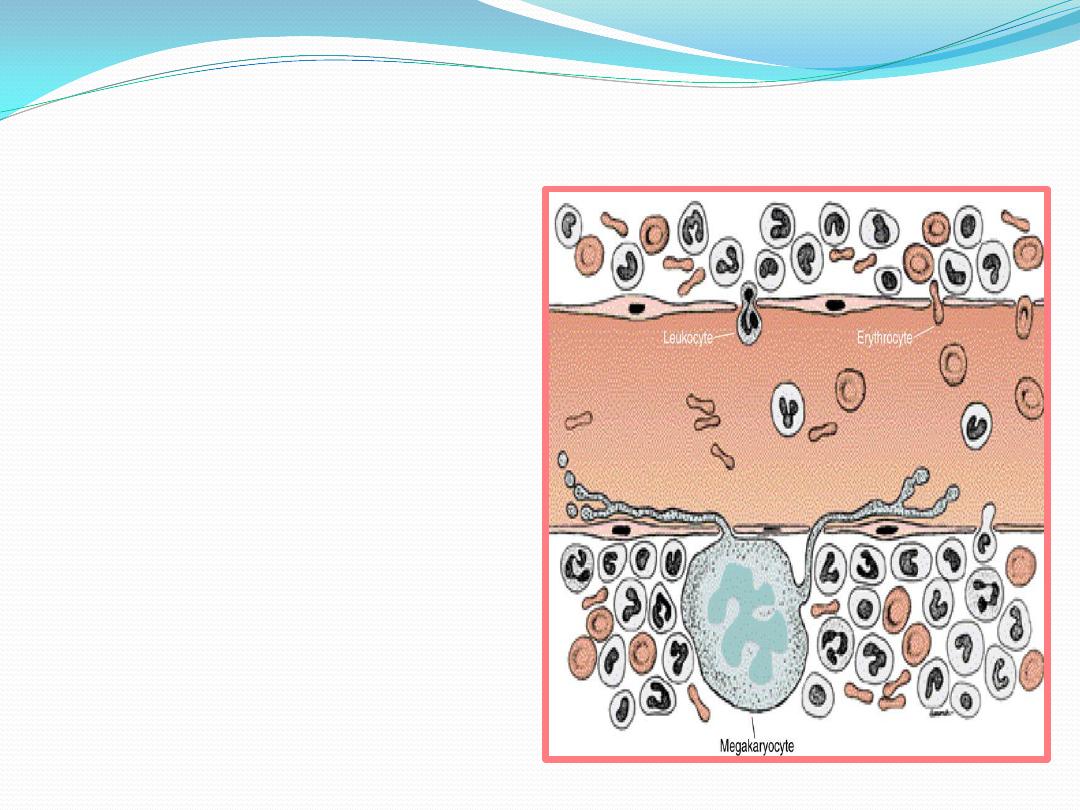
Upon
maturation, the hematopoietic cells, regulated by the reticular cells,
traverse
the wall of the venous sinuses to enter the bloodstream
Leukocytes
, after the action of
releasing substances, cross the
wall of the sinusoid
by their own
activity
.
Because
erythrocytes
(unlike
leukocytes) do not have sufficient
motility to cross the wall of the
sinusoid, they are believed to
enter the sinusoid by
a pressure
gradient that exists across its wall
.
Megakaryocytes
form thin
processes
(proplatelet processes)
that cross the wall of the sinusoid
and fragment at their tips,
liberating the
platelets
.
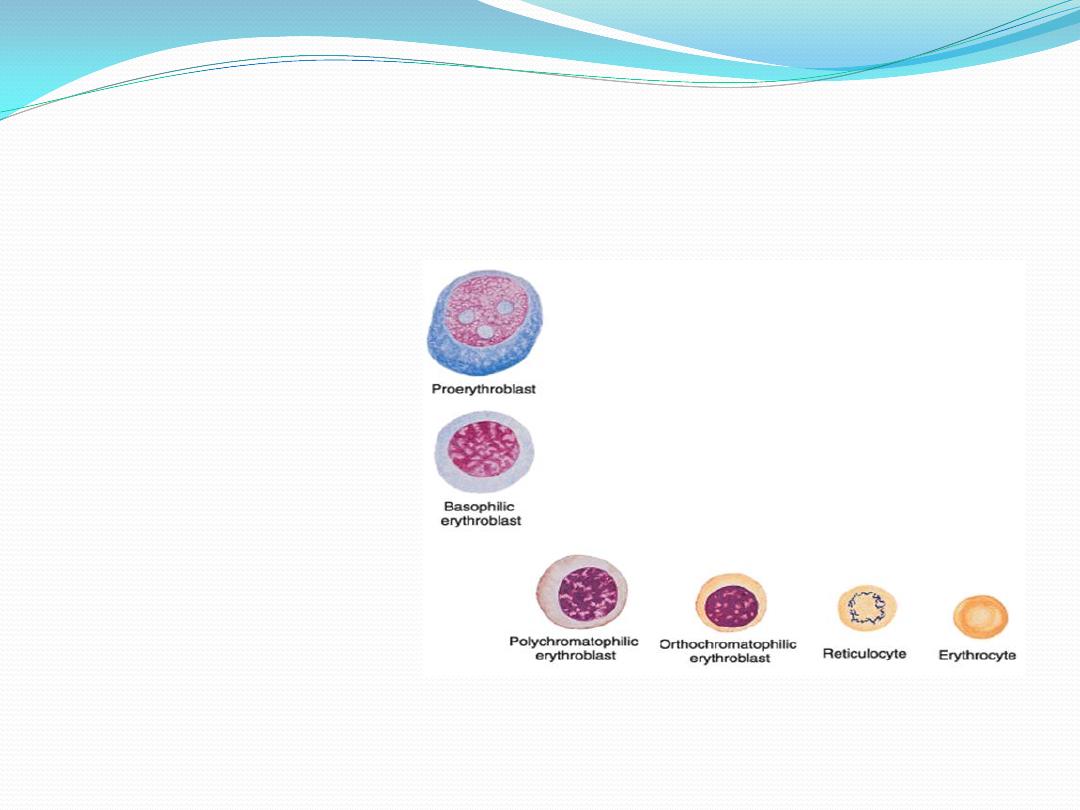
Maturation of erythrocytes
•
Regulated
mainly by
erythropoietin
released by the
kidneys;
•
also influenced
by androgens
Maturation of erythrocytes
1.
pluripotential cell
2.
Myeloid multipotential cell
3.
Erythrocyte – colony forming cell
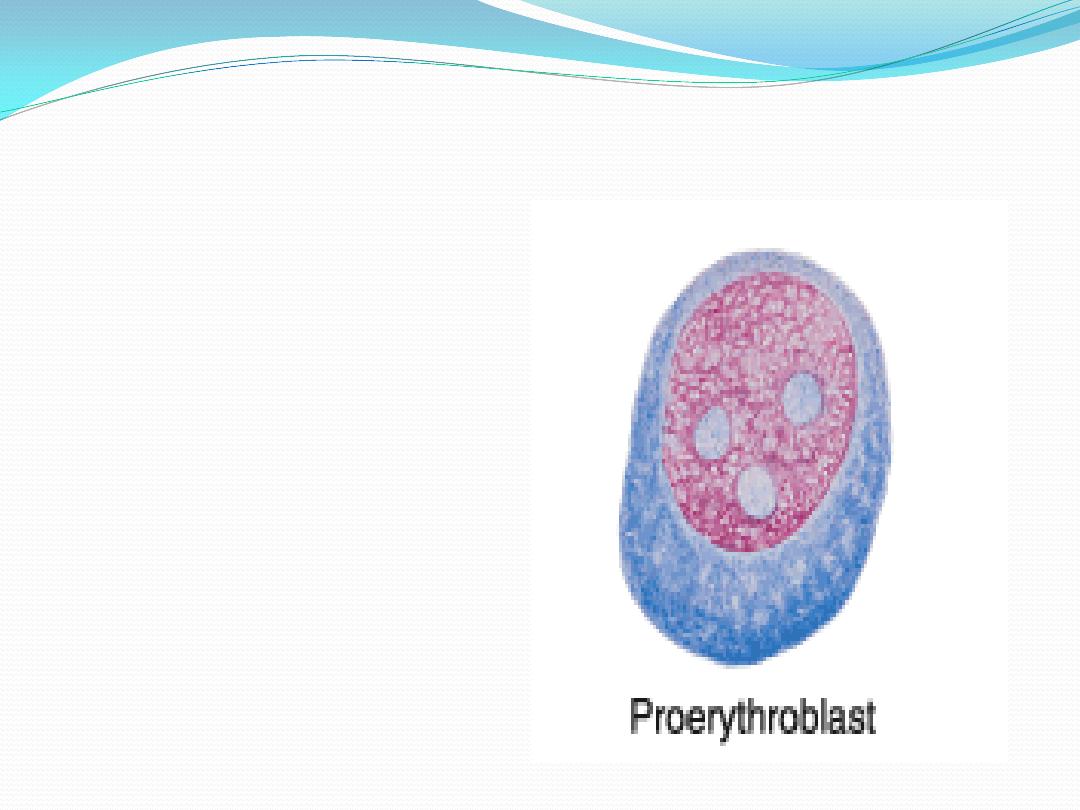
4.
Proerythroblast
(
pronormoblasts
)
:
large cell , rounded nucleus
coarse chromatin , visible
nucleoli, intense basophilia
of the cytoplasm
Maturation of erythrocytes
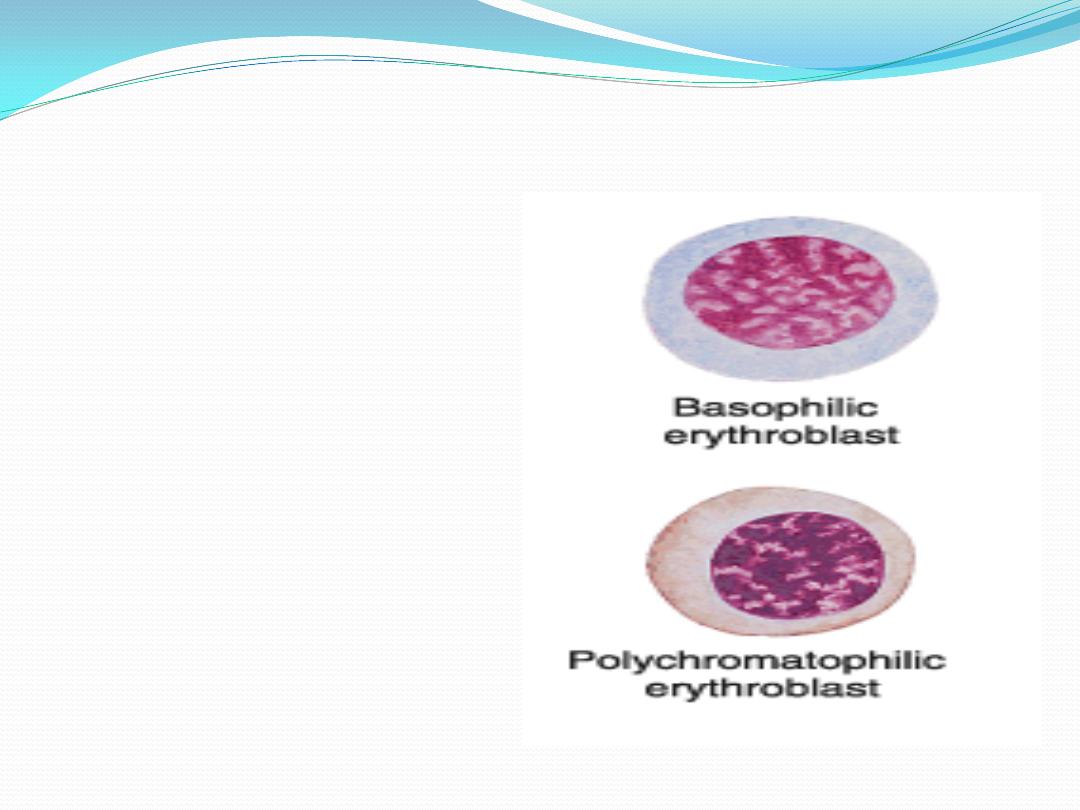
5.
Basophilic erythroblast
(
basophilic normoblasts
)
:
condensed nucleus , no
visible nucleoli , strongly
basophilic cytoplasm
because of free ribosomes
and polyribosomes.
6.
Polychromatophilic erythroblast
(
polychromatophilic normoblasts
)
:
mixed color cytoplasm
purplish blue to grey
Maturation of erythrocytes
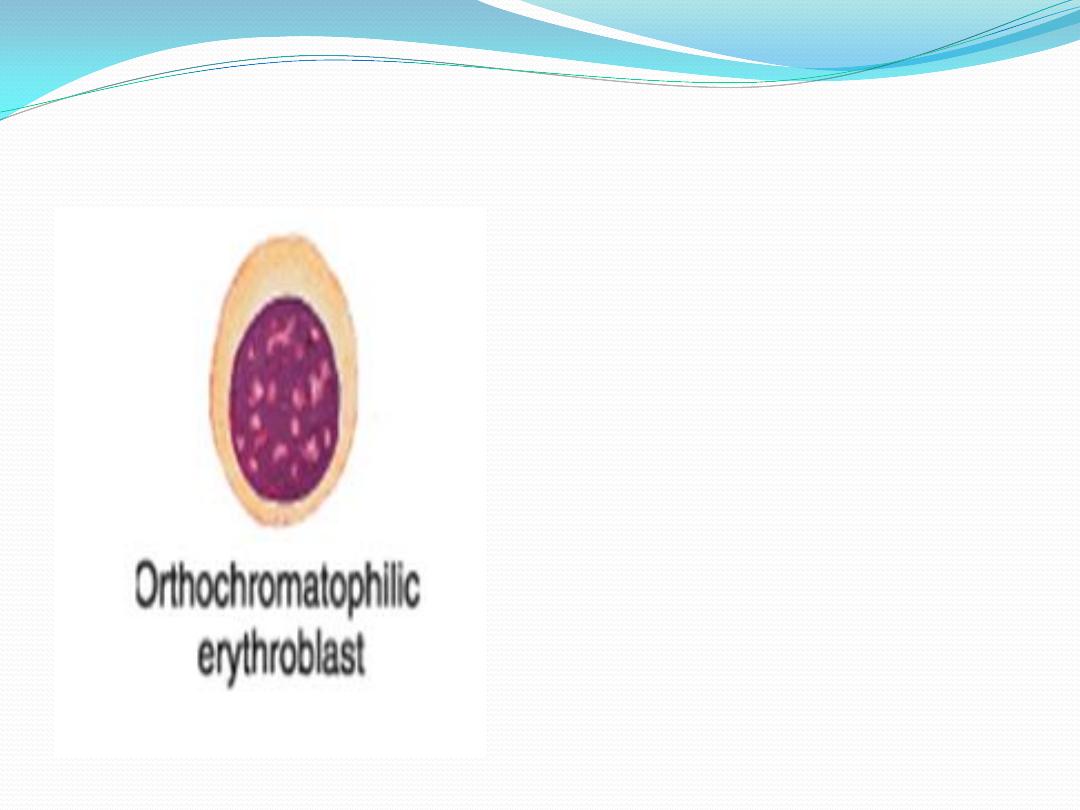
7.
Orthochromatophilic
erythroblast
: the amount of
haemoglobin is the same as that
of erythrocyte . Nucleus with
dense and compact chromatin ----
-----pyknotic --------- extruded
from the cell with a thin rim of
cytoplasm and plasma membrane
8.
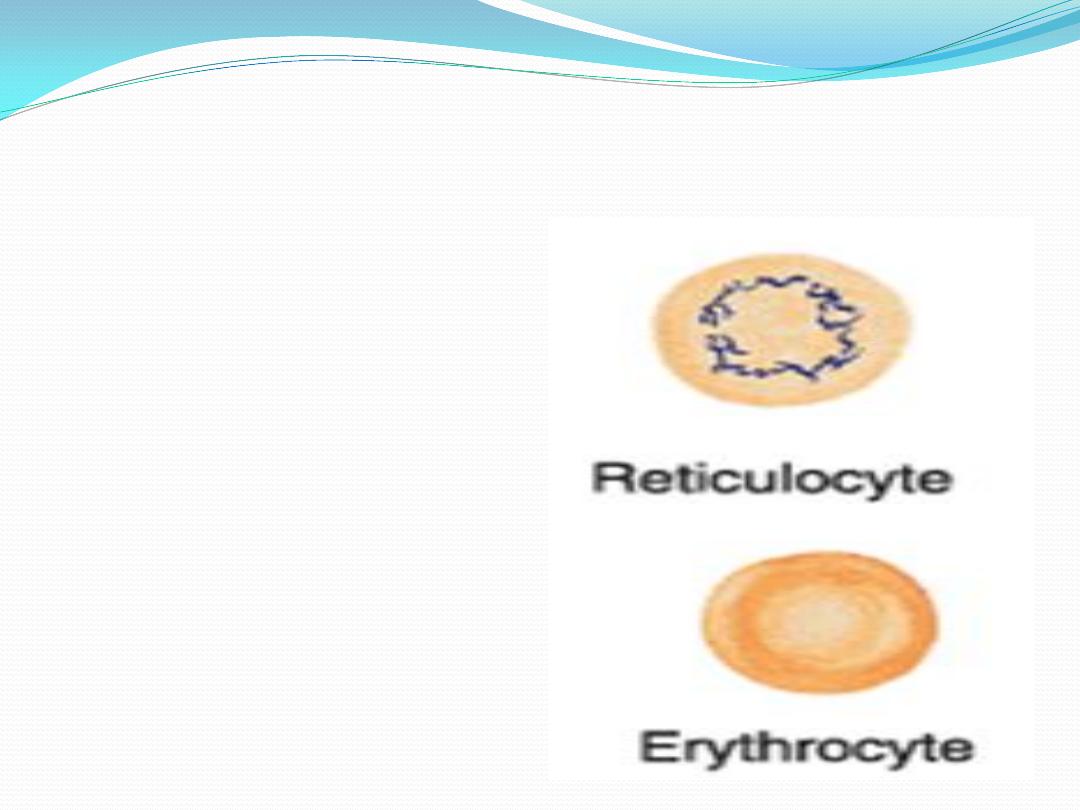
Reticulocyte:
youngest
erythrocyte containing a delicate
reticulum
the clumped ribosomes
responsible for the distinctive
staining of the reticulocytes are
degraded within 24 hours
9.
Erythrocyte
: anucleated and
biconcave in peripheral blood
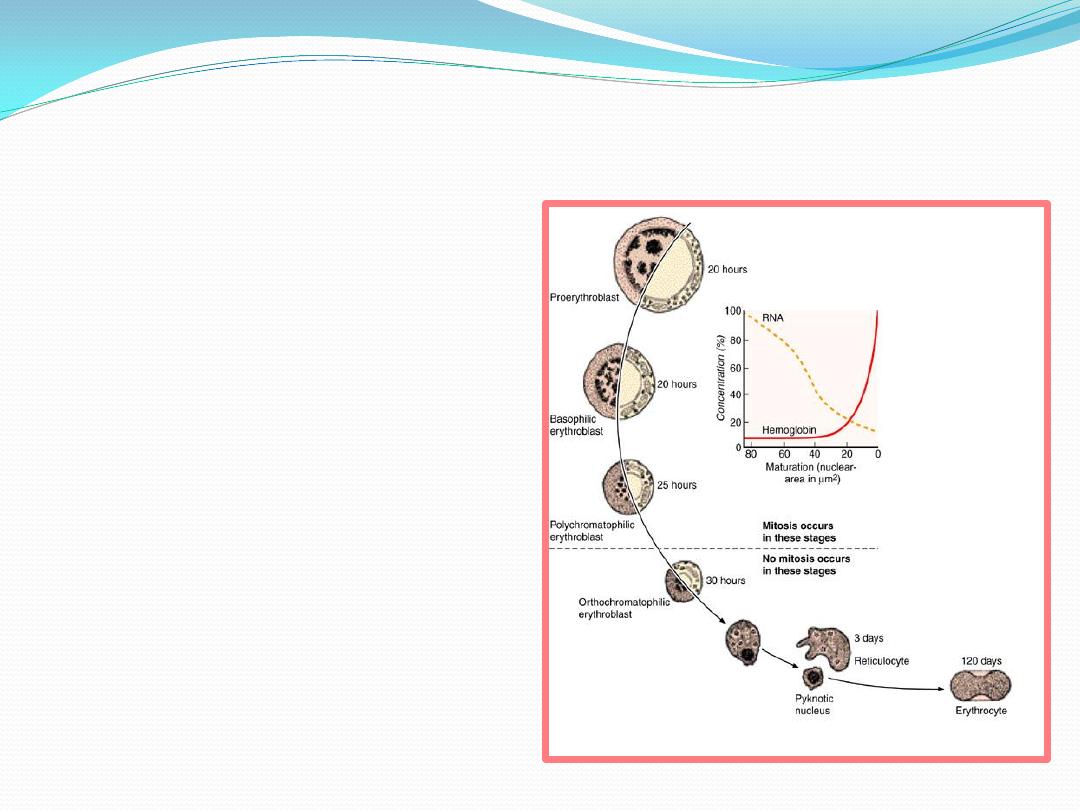
Several major changes take place during
maturation of erythrocyte
1.
cell volume
decreases
2.
nucleoli
diminish in size until
they become invisible
3.
nuclear diameter
decrease and
chromatin
increase until the
nucleus become pyknotic and
extruded from the cell
4. gradual decrease in the number of
polyribosomes (
basophilia
)with
a simultaneous increase in the
amount of haemoglobin(
acidophilic protein
)
5.
mitochondria
and other
organelles
gradually disapear
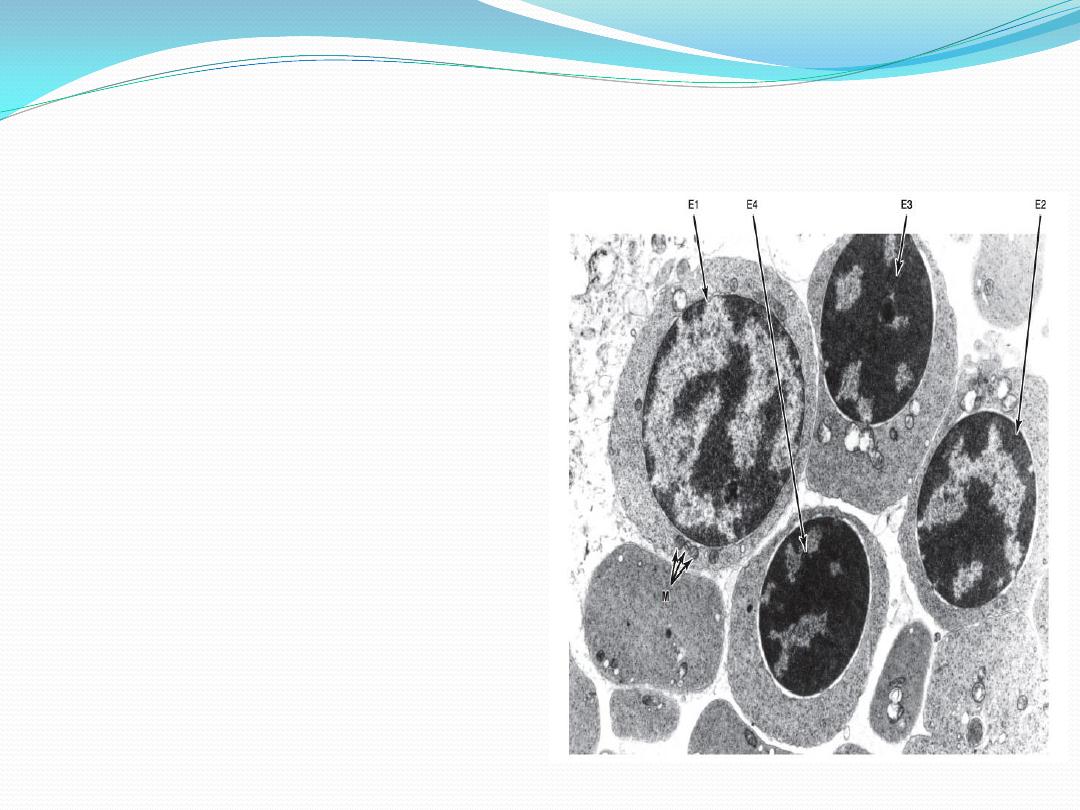
Electron micrograph of red bone
marrow
Four erythroblasts in
successive stages of maturation
are seen (E1, E2, E3, and E4). As
the cell matures, its chromatin
becomes gradually condensed,
the accumulation of hemoglobin
increases the electron density of
the cytoplasm, and the
mitochondria (M) decrease in
number.
