
Physiology of
Blood
Hemoglobin
By prof. Israa f. jaafar

Learning objectives
•
Describe Hemoglobin structure and
function
•
Identify types of hemoglobin
•
To delineate the abnormal types of Hbs
•
To describe the synthesis and destruction
of Hb
Structure of Hb
* 4 Heme Molecules =
* 4 Oxygen Molecules
*Oxygenated Hemoglobin
Bright Red (systemic)
*Deoxygenated Hemoglobin
Blue (venous circulation)
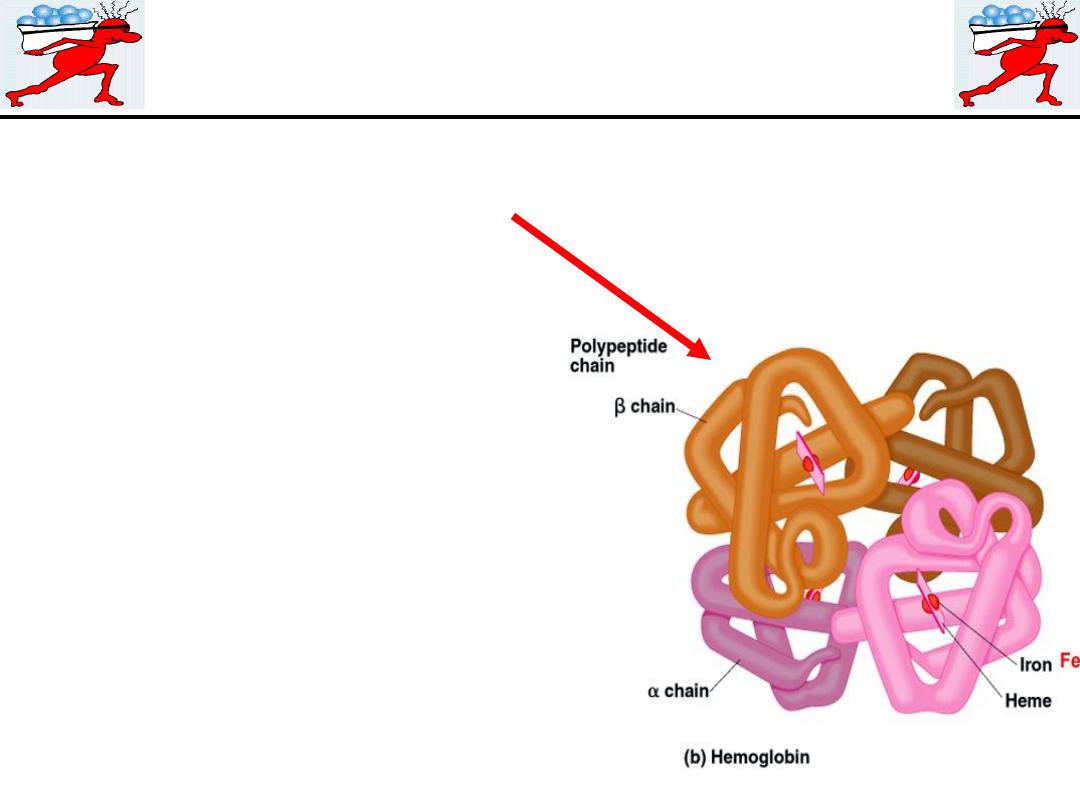
HEMOGLOBIN
Hemoglobin Structure
•
Complex quaternary structure
Figure 19–3

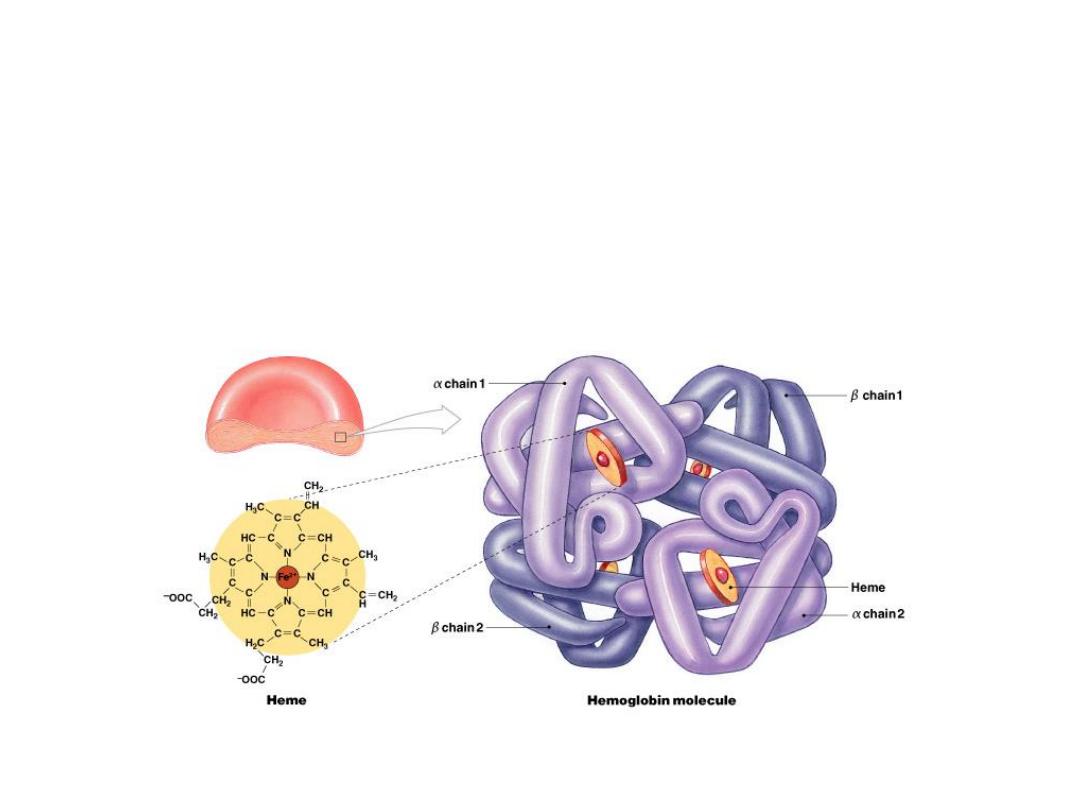
Hemoglobin is the red, oxygen-carrying pigment in the
red blood cells. its molecular weight is 64,450
Hemoglobin is a globular molecule made up of four subunits
Each subunit contains a heme moiety conjugated to a polypeptide.
Heme is an iron-containing porphyrin derivative The polypeptides
are referred to collectively as the globin portion of the hemoglobin
molecule.
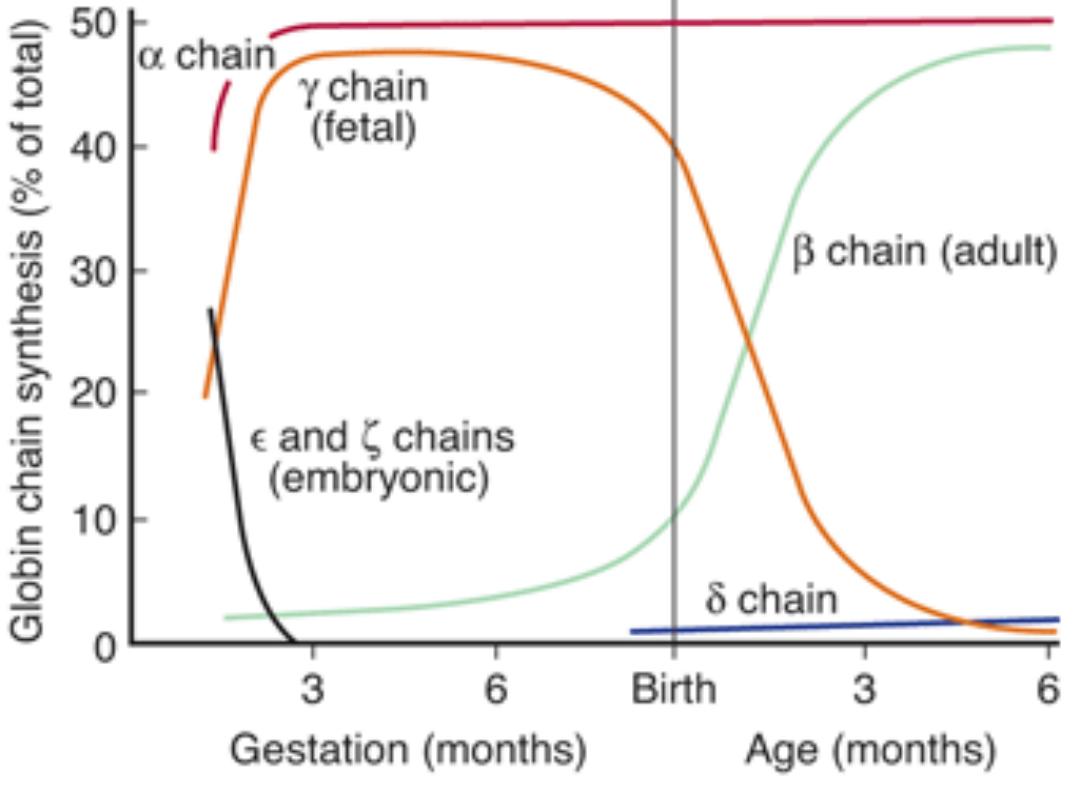
ADULT Hb
In normal adult human
hemoglobin (hemoglobin A): (α
2
β
2
).
the two polypeptides α chains, contains 141 amino acid residues,
and β
chains, each of which contains 146 amino acid residues.
hemoglobin A
2
2.5% of the hemoglobin is
(α
2
δ
2
).
, in which β
chains are replaced by δ chains which also contain 146 amino acid
residues, but 10 individual residues differ from those in the β chains
Hb1c
has a glucose attached to the terminal valine in each chain
and it increases in the blood of patients with poorly controlled
diabetes mellitus

Fetal hemoglobin (hemoglobin F) ( β
2
γ
2
):
Its structure is similar to that of hemoglobin A except
that the β chains are replaced by γ chains; The γ
chains also contain 146 amino acid residues but have 37
that differ from those in the β chain.
Fetal hemoglobin is normally replaced by adult
hemoglobin soon after birth. In the body, its O
2
content
at a given P
O
2
is greater than that of adult hemoglobin
because it binds 2,3-BPG less avidly.
Hemoglobin F is critical to facilitate movement of O
2
from the maternal to the fetal circulation, particularly
at later stages of gestation where oxygen demand
increases
In young embryos there are, in addition, Gower 1
hemoglobin, and Gower 2 hemoglobin.

Reactions of Hemoglobin
1.Hemoglobin binds O
2
to form oxyhemoglobin
, O
2
attaching to the Fe
2+
in the
heme. The affinity of Hb for O
2
is affected by pH, temperature, and the
concentration in the red cells of 2,3-bisphosphoglycerate (2,3-BPG). 2,3-BPG
and H
+
compete with O
2
for binding to deoxygenated hemoglobin, decreasing
the affinity of Hb for O
2
.
2.When blood isexposed to oxidizing agents the ferrous iron (Fe
2+
) is converted
to ferric iron (Fe
3+
),
forming methemoglobin.Methemoglobin is dark-colored,
and when it is present in large quantities in the circulation, it causes a dusky
discoloration of the skin resembling cyanosis.
Some oxidation of hemoglobin to methemoglobin occurs normally, but an
enzyme system in the red cells, the dihydronicotinamide adenine dinucleotide
(NADH)-methemoglobinreductase system, converts methemoglobin back to
hemoglobin. Congenital absence of this system is one cause of hereditary
methemoglobinemia.
3.Carbon monoxide reacts with hemoglobin to form carbon
monoxyhemoglobin (carboxyhemoglobin).
The affinity of hemoglobin for O
2
is
much lower than its affinity for carbon monoxide, which consequently
displaces O
2
on hemoglobin, reducing the oxygen-carrying capacity of blood .

Fetal Hemoglobin
•
Strong form of hemoglobin found in embryos
•
Takes oxygen from mother’s hemoglobin
Carbaminohemoglobin
•
With low oxygen (peripheral capillaries):
–
hemoglobin releases oxygen
–
binds carbon dioxide and carries it to lungs

hemoglobinopathies
,
in which abnormal globin polypeptide chains
are produced, and the
thalassemias
and related disorders, in which the
chains are normal in structure but produced in decreased amounts or
absent because of defects in the regulatory portion of the globin genes.
Mutant genes that cause the production of abnormal hemoglobins are
widespread, and over 1000 abnormal hemoglobins have been described
in humans. In one of the most common examples,
hemoglobin S,
the
chains are normal but the chains have a single substitution of a valine
residue for one glutamic acid, leading to sickle cell anemia . When an
abnormal gene inherited from one parent dictates formation of an
abnormal hemoglobin (ie, when the individual is heterozygous), half the
circulating hemoglobin is abnormal and half is normal. When identical
abnormal genes are inherited from both parents, the individual is
homozygous and all the hemoglobin is abnormal. It is theoretically
possible to inherit two different abnormal hemoglobins, one from the
father and one from the mother.

Hemoglobin Structure
•
4 globular protein subunits:
–
each with 1 molecule of
heme
–
each heme contains 1 iron ion
•
Iron ions easily:
–
associate with oxygen (
oxyhemoglobin
)
–
or dissociate from oxygen (
deoxyhemoglobin
)

Synthesis of Hemoglobin
The average normal hemoglobin content of
blood is 16 g/dL in men and 14 g/dL in
women, all of it in red cells. In the body of
a 70-kg man, there are about 900 g of
hemoglobin, and 0.3 g of hemoglobin is
destroyed and 0.3 g synthesized every hour
The heme portion of the hemoglobin
molecule is synthesized from glycine and
succinyl-CoA.

Hemoglobin Recycling
•
Phagocytes break hemoglobin into
components:
–
globular proteins to amino acids
–
heme to
biliverdin
–
Iron
Iron Recycling
•
To transport proteins (
transferrin
)
•
To storage proteins (
feritin
and
hemosiderin

Breakdown of Biliverdin
•
Biliverdin
(green) is converted to
bilirubin
(yellow)
•
Bilirubin is:
–
excreted by liver (bile)
–
jaundice
is caused by bilirubin buildup
–
converted by intestinal bacteria to
urobilins
and
stercobilins

Iron
In adults, the amount of iron lost from the body is relatively small. The
losses are generally unregulated, and total body stores of iron are
regulated by changes in the rate of absorbtion from the intestine.
Men lose about 0.6 mg/d, largely in the stools. Women losses twice this
value because of the additional iron lost during menstruation.
The average daily iron intake in the United States and Europe is about 20
mg, but the amount absorbed is equal only to the losses. the amount of
iron absorbed is about 3–6% of the amount ingested.
dietary factors affect the availability of iron for absorption;
1. phytic acid found in cereals reacts with iron to form insoluble
compounds in the intestine, as do phosphates and oxalates.
2.Most of the iron in the diet is in the ferric (Fe
3+
) form, whereas it is the
ferrous (Fe
2+
) form that is absorbed. Fe
3+
reductase activity is associated
with the iron transporter in the brush borders of the enterocytes .
Gastric secretions dissolve the iron and permit it to form soluble
complexes with ascorbic acid and other substances that aid its reduction
to the Fe
2+
form.

Almost all iron absorption occurs in the duodenum
.
Some is stored in
ferritin,
and the remainder is transported out of the
enterocytes by a basolateral transporter named ferroportin 1 it
facilitates basolateral transport. In the plasma, Fe
2+
is converted to
Fe
3+
and bound to the iron transport protein
transferrin
. transferrin is
about 35% saturated with iron, and the normal plasma iron level is
about 130 g/dL in men, and 110 g/dL in women.
70% of iron in the body is in hemoglobin, 3% in myoglobin, and the
rest in ferritin, which is present in enterocytes, and other cells.
Ferritin molecules in lysosomal membranes may aggregate in
deposits that contain as much as 50% iron. These deposits are called
hemosiderin.
Intestinal absorption of iron is regulated by three factors:
1. recent dietary intake of iron.
2. the state of the iron stores in the body.
3. the state of erythropoiesis in the bone marrow.

Disorders of Iron uptake
Iron deficiency causes anemia. Conversely, iron overload
causes hemosiderin to accumulate in the tissues,
producing
hemosiderosis.
Large
amounts
of
hemosiderin
can
damage
tissues,
causing
hemochromatosis. This syndrome is characterized by
pigmentation of the skin, pancreatic damage with
diabetes ("bronze diabetes"), cirrhosis of the liver, a high
incidence of hepatic carcinoma, and gonadal atrophy.
If the abnormality is diagnosed before excessive amounts
of iron accumulate in the tissues, life expectancy can be
prolonged by repeated withdrawal of blood.
