
1
objectives
1. Explain how the mechanisms for sour and salty tastes are similar
to each other, and how these differ from the mechanisms
responsible for sweet and bitter tastes.
2. Explain how odorant molecules stimulate the olfactory receptors.
Taste and Smell
The receptors for taste and smell respond to molecules that
are dissolved in fluid; hence, they are classified as
chemoreceptors.
Although there are only four basic modalities of taste, they
combine in various ways and are influenced by the sense of
smell, thus permitting a wide variety of different sensory
experiences.
Chemoreceptors that respond to chemical changes in the internal
environment are called interoceptors; those that respond to
chemical
changes
in
the
external
environment
are
exteroceptors. Included in the latter category are taste
(gustatory) receptors, which respond to chemicals dissolved in
food or drink, and smell (olfactory) receptors, which respond to
gaseous molecules in the air. This distinction is somewhat
arbitrary, however, because odorant molecules in air must first
dissolve in fluid within the olfactory mucosa before the sense of
smell can be stimulated. Also, the sense of olfaction strongly
influences the sense of taste, as can easily be verified by eating
an onion (or almost anything else) with the nostrils pinched
together.
Taste
Gustation, the sense of taste, is evoked by receptors that consist
of barrel-shaped taste buds (fig.). Located primarily on the
dorsal surface of the tongue, each taste bud consists of 50 to 100
specialized epithelial cells with long microvilli that extend
through a pore in the taste bud to the external environment,
where they are bathed in saliva. Although these sensory
epithelial cells are not neurons, they behave like neurons; they
become depolarized when stimulated appropriately, produce

2
action potentials, and release neurotransmitters that stimulate
sensory neurons associated with the taste buds.
Taste buds in the anterior two-thirds of the tongue are
innervated by the facial nerve (VII), and those in the posterior
third of the tongue by the glossopharyngeal nerve (IX).
Dendritic endings of the facial nerve (VII) are located around
the taste buds and relay sensations of touch and temperature.
Taste sensations are passed to the medulla oblongata, where the
neurons synapse with second-order neurons that project to the
thalamus. From here, third-order neurons project to the area of
the postcentral gyrus of the cerebral cortex that is devoted to
sensations from the tongue.
The specialized epithelial cells of the taste bud are known
as taste cells. The different categories of taste are produced by
different chemicals that come into contact with the microvilli of
these cells (figure). Four different categories of taste are traditionally
recognized: salty, sour, sweet, and bitter. There may also be a fifth
category of taste, termed umami (a Japanese term related to a meaty
flavor), for the amino acid glutamate (and stimulated by the flavor-
enhancer monosodium glutamate). Although scientists long
believed that different regions of the tongue were specialized for
different tastes, this is no longer believed to be true. Indeed, is seems
that each taste bud contains taste cells responsive to each of the
different taste categories! It also appears that a given sensory neuron
may be stimulated by more than one taste cell in a number of
different taste buds, and so one sensory fiber may not transmit
information specific for only one category of taste. The brain
interprets the pattern of stimulation of these sensory neurons,
together with the nuances provided by the sense of smell, as the
complex tastes that we are capable of perceiving.
The salty taste of food is due to the presence of sodium
ions (Na
), or some other cations, which activate specific receptor
cells for the salty taste. Different substances taste salty to the degree
that they activate these particular receptor cells. The Na
passes into
the sensitive receptor cells through channels in the apical
membranes. This depolarizes the cells, causing them to release their
transmitter. The anion associated with the Na
, however, modifies
the perceived saltiness to a surprising degree:
NaCl tastes much saltier than other sodium salts (such as

3
sodium acetate). There is evidence to suggest that the anions can
pass through the tight junctions between the receptor cells, and that
the Cl
–
anion passes through this barrier more readily than the other
anions. This is presumably related to the ability of Cl
–
to impart a
saltier taste to the Na
than do the other anions.
Sour taste, like salty taste, is produced by ion movement
through membrane channels. Sour taste, however, is due to the
presence of hydrogen ions (H
); all acids therefore taste sour. In
contrast to the salty and sour tastes, the sweet and bitter tastes are
produced by interaction of taste molecules with specific membrane
receptor proteins.
Most organic molecules, particularly sugars, taste sweet to
varying degrees. Bitter taste is evoked by quinine and seemingly
unrelated molecules. It is the most acute taste sensation and is
generally associated with toxic molecules (although not all toxins
taste bitter). Both sweet and bitter sensations are mediated by
receptors that are coupled to G-proteins .The particular type of G-
protein involved in taste has recently been identified and termed
gustducin. This term is used to emphasize the similarity to a related
group of G-proteins, of a type called transducin, associated with the
photoreceptors in the eye. Dissociation of the gustducin G-protein
subunit activates second-messenger systems,
leading to depolarization of the receptor cell (fig.). The stimulated
receptor cell, in turn, activates an associated sensory neuron that
transmits impulses to the brain, where they are interpreted as the
corresponding taste perception.
Although all sweet and bitter taste receptors act via G-proteins, the
second-messenger systems activated by the
G-proteins depend on the molecule tasted. In the case of the
sweet taste of sugars, for example, the G-proteins activate
adenylate cyclase, producing cyclic AMP (cAMP;
The cAMP, in turn, produces depolarization by closing
K
channels that were previously open. On the other hand, the
sweet taste of the amino acids phenylalanine and tryptophan,
as well as of the artificial sweeteners saccharin and cyclamate, may
enlist different second-messenger systems. These involve the
activation of a membrane enzyme that produces the second
messengers inositol triphosphate (IP
3
) and diacylglycerol (DAG).
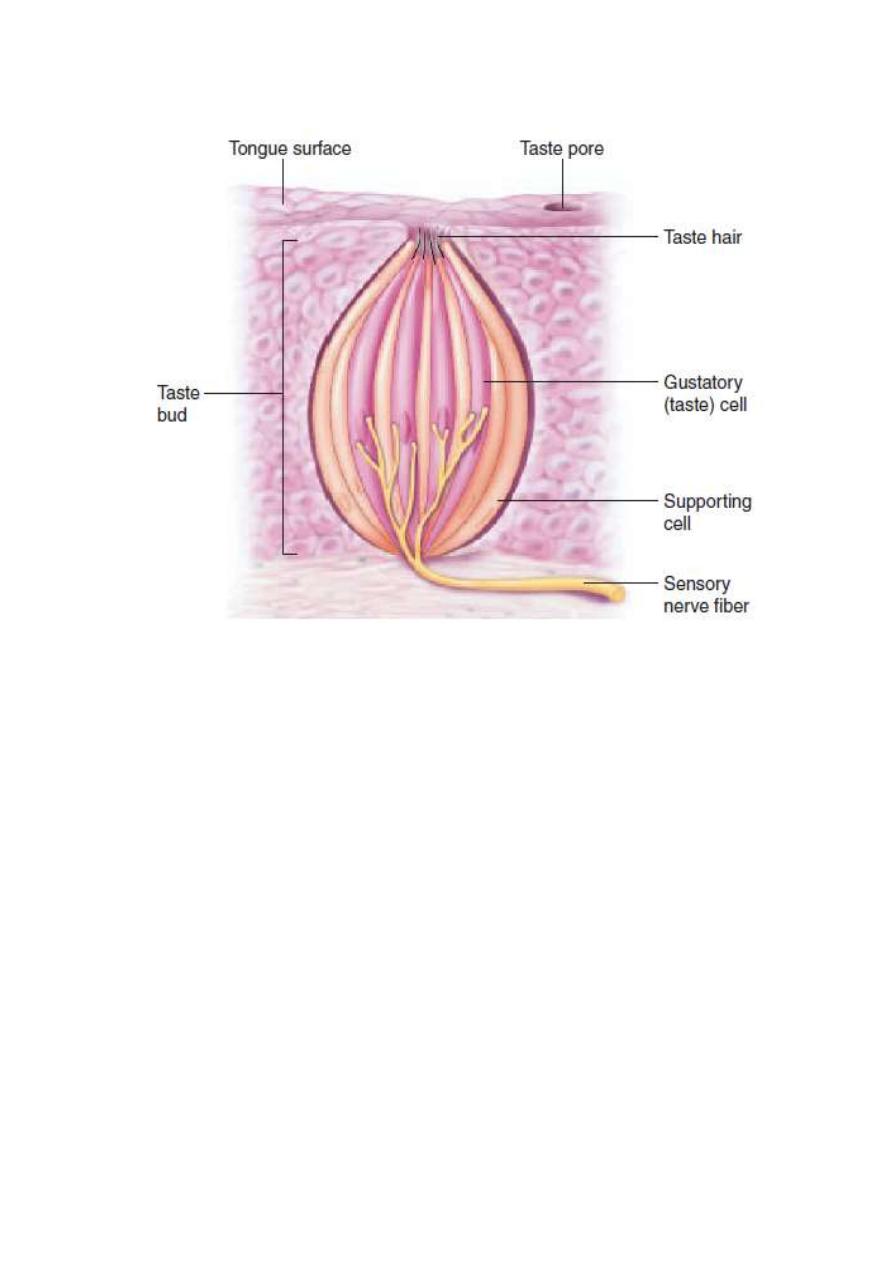
4
Figure
A taste bud. Chemicals dissolved in the fluid at the pore bind to
receptor proteins in the microvilli of the sensory cells. This ultimately
leads to the release of neurotransmitter, which activates the associated
sensory neuron.
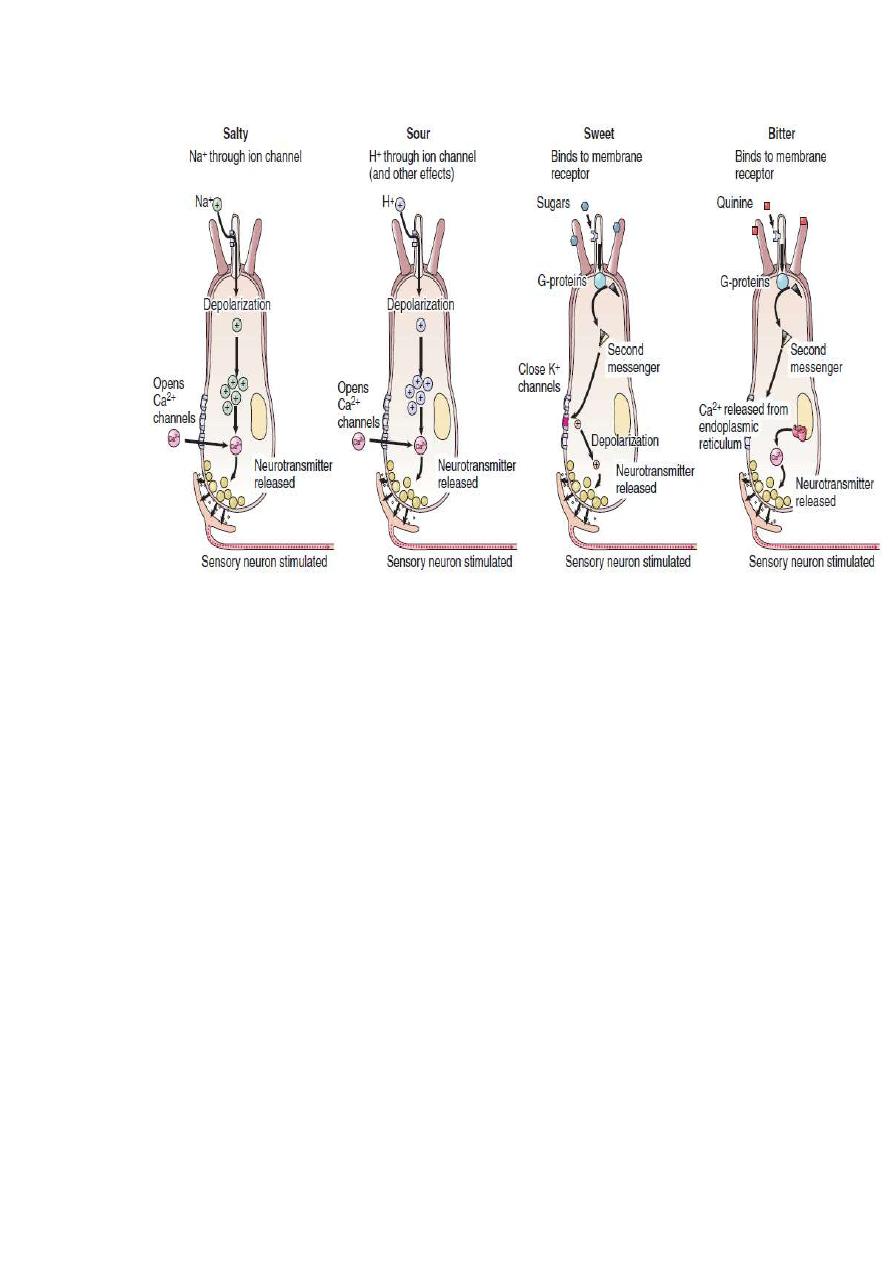
5
Figure
The four major categories of taste. Each category of taste
activates specific taste cells by different means. Notice that taste cells for
salty and sour are depolarized by ions (Na
and H
, respectively) in the
food, whereas taste cells for sweet and bitter are depolarized by sugars and
quinine, respectively, by means of G-protein-coupled receptors and the
actions of second messengers
.
Adenylate Cyclase
–Cyclic AMP Second-Messenger System
Cyclic adenosine monophosphate (abbreviated cAMP)
was the first
“second messenger” to be discovered and is the best understood.
When epinephrine and norepinephrine bind to their
-adrenergic
receptors the effects of these hormones are due to cAMP production
within the target cells. It was later discovered that the effects of
many (but not all) polypeptide and glycoprotein hormones are also
mediated by cAMP.
When one of these hormones binds to its receptor protein, it

6
causes the dissociation of a subunit from the complex of G-proteins
This G-protein subunit moves through the membrane until it reaches
the enzyme adenylate (or adenylyl) cyclase (fig below). The G-
protein subunit then binds to and activates this enzyme, which
catalyzes the following reaction within the cytoplasm of the cell:
ATP
cAMP
PP
i
Adenosine triphosphate (ATP) is thus converted into cyclic AMP
(cAMP) and two inorganic phosphates (pyrophosphate, bbreviated PP
i
).
As a result of the interaction of the hormone with its receptor and the
activation of adenylate cyclase,
therefore, the intracellular concentration
of cAMP is increased.
Cyclic AMP activates a previously inactive enzyme in the cytoplasm
called protein kinase. The inactive form of this enzyme
consists of two subunits: a catalytic subunit and an inhibitory subunit.
The enzyme is produced in an inactive form and becomes
active only when cAMP attaches to the inhibitory subunit.
Binding of cAMP to the inhibitory subunit causes it to dissociate from the
catalytic subunit, which then becomes active.
In summary, the hormone
—acting through an increase in cAMP
production
—causes an increase in protein kinase enzyme activity
within its target cells. Active protein kinase catalyzes the
phosphorylation of (attachment of phosphate groups to) different
proteins in the target cells. This causes some enzymes to become
activated and others to become inactivated. Cyclic AMP, acting
through protein kinase, thus modulates the activity of enzymes that
are already present in the target cell. This alters the metabolism of the
target tissue in a manner characteristic of the actions of that specific
hormone.
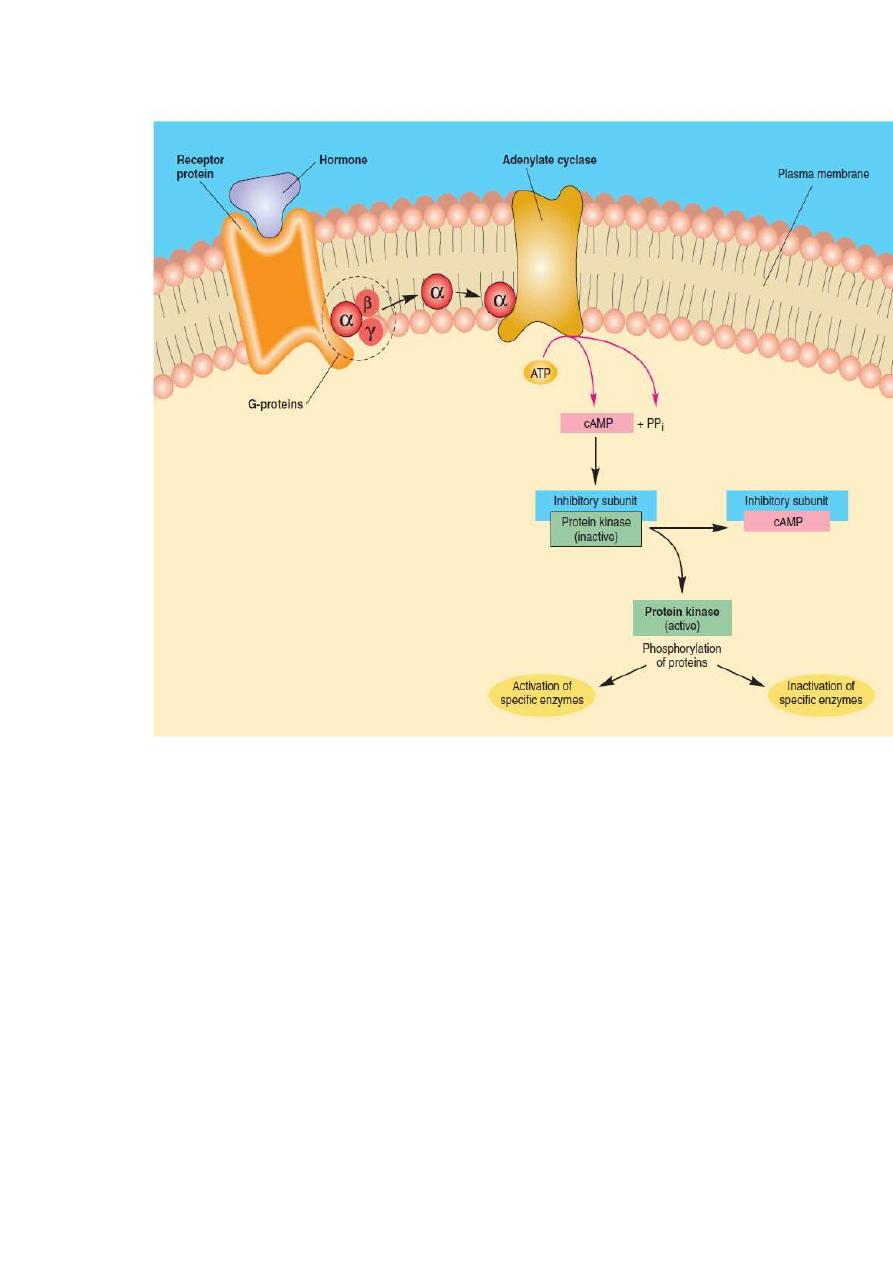
7
The adenylate cyclase-cyclic AMP second-messenger system. The
hormone causes the production of cAMP within the target cell cytoplasm,
and cAMP activates protein kinase. The activated protein kinase then
causes the activation or inactivation of a number of specific enzymes.
These changes lead to the characteristic effects of the hormone on the
target cell.

8
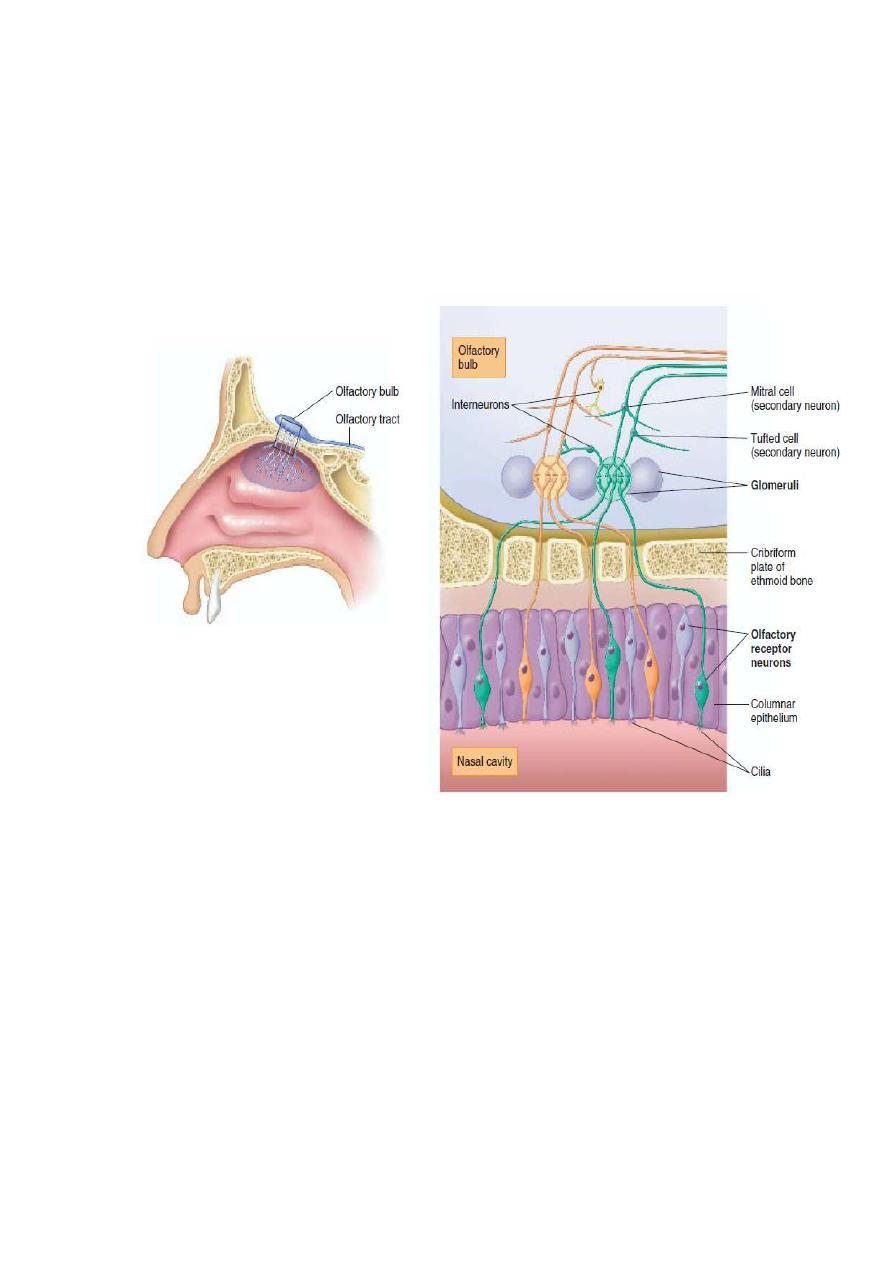
Smell
The receptors responsible for olfaction, the sense of smell, are located in
the olfactory epithelium. The olfactory apparatus consists of receptor
cells (which are bipolar neurons), supporting (sustentacular) cells, and
basal (stem) cells. The basal cells generate new receptor cells every 1 to 2
months to replace the neurons damaged by exposure to the environment.
The supporting cells are epithelial
epithelial cells rich in enzymes that
oxidize hydrophobic, volatile odorants, thereby making these molecules
less lipid-soluble and thus less able to penetrate membranes and enter the
brain.
Each bipolar sensory neuron has one dendrite that projects
into the nasal
cavity, where it terminates in a knob containing cilia The bipolar sensory
neuron also has a single unmyelinated axon that projects through holes in the
cribriform plate of the ethmoid bone into the olfactory bulb of the cerebrum,
where it synapses with second-order neurons. Therefore, unlike other sensory
modalities that are relayed to the cerebrum from the thalamus, the sense of
smell is transmitted directly to the cerebral cortex. The processing of olfactory
information begins in the olfactory bulb, where the bipolar sensory neurons
synapse with neurons located in spherically shaped arrangements called
glomeruli Evidence suggests that each glomerulus receives input from one
type of olfactory receptor. The smell of a flower, which releases many different
molecular odorants, may be identified by the pattern of excitation it produces in
the glomeruli of the olfactory bulb. Identification of an odor is improved by
lateral inhibition in the olfactory bulb, which appears to involve
dendrodendritic synapses between neurons of adjacent glomeruli.
Neurons in the olfactory bulb project to the olfactory cortex in the medial
temporal lobes, and to the associated hippocampus and amygdaloid nuclei.
These structures are part of the limbic system, which was described in chapter
8 as having important roles in both emotion and memory. The human
amygdala, in particular, has been implicated in the emotional responses to
olfactory stimulation.Perhaps this explains why the smell of a particular odor
can so powerfully evoke emotionally charged memories. The molecular basis
of olfaction is complex. At least in some cases, odorant molecules bind to
receptors and act
through
G-proteins to increase the cyclic AMP within the cell.
This, in turn, opens membrane channels and causes the depolarization of the generator
potential, which then stimulates the production of action potentials. Up to fifty G-
proteins may be associated with a single receptor protein. Dissociation of these G-
proteins releases many
G-protein subunits, thereby amplifying the effect
many times. This amplification could account for the extreme
sensitivity of the sense of smell: the human nose can detect a billionth of
an ounce of perfume in air. Even at that, our sense of
smell is not nearly as keen as that of many other mammals.
A family of genes that codes for the olfactory receptor proteins has been
discovered. This is a large family that may include as many as a thousand
9
genes. The large number may reflect the mportance of the sense of smell
to mammals in general. Even a thousand different genes coding for a
thousand different receptor proteins, however, cannot account for the fact
that humans can distinguish up to 10,000 different odors. Clearly, the
brain must integrate the signals from several sensory neurons that have
different olfactory receptor proteins and then interpret the pattern as a
characteristic
“fingerprint” for a particular odor.
Figure
:The neural pathway for olfaction. The olfactory epithelium contains receptor
neurons that synapse with neurons in the olfactory bulb of
the cerebral cortex. The
synapses occur in rounded structures called glomeruli. Secondary neurons, known as
tufted cells and mitral cells, transmit impulses from the olfactory bulb to the olfactory
cortex in the medial temporal lobes. Notice that each glomerulus receives input from
only one type of olfactory receptor, regardless of where those receptors are located in
the olfactory epithelium.
