
Corynebacterium
Prof.Dr.Mohammad Alfaham
Microbiology 3
rd
years 2014-2015
Gram stain of Corynebacterium spp. demonstrating "Chinese letters" formations
General Overview:
C. diphtheriae
and related organisms are collectively termed
coryneforms or diphtheroids
Corynebacteria possess capsular (K) and somatic antigens (O)
Morphology & Physiology:
Small, nonmotile, irregularly staining
Gram-positive
rods with club-shaped swelled ends but no spores; may be straight or
slightly curved
Palisade arrangement of cells in short chains ("V" or "Y"
configurations) or in clumps resembling "Chinese letters"
Cells tend to lie parallel to one another (palisades) or at acute
angles (coryneforms), due to their snapping type of division
Vary greatly in dimension, from 0.3 to 1 um in diameter and 1.0 to
8.0 um in length
May also contain inclusion bodies, known as metachromatic
granules, which are composed of inorganic polyphosphates (volutin)
that serve as energy reserves and are not membrane bound
Internal metachromatic granules densely stain ruby red while the
rest of the bacillus stains blue, when stained with an aniline dye such
as toluidine blue O or
Cells appear to be banded or beaded with irregularly staining
granules; may show alternate bands of stained and unstained
material (giving the appearance of septa)
Aerobic or
anaerobic
metabolism (carbohydrates to lactic acid); form acid
but not gas from certain carbohydrates
; Slow growth on enriched medium
positive
Cell wall containing unusual lipids: meso-diaminopimelic acids;
arabino-galactan polymers; short-chain mycolic acids (member of
CMN (Corynebacterium,
Mycobacterium,
Nocardia)group)
Corynebacterium urealyticum
strongly urease positive
Clinical Syndromes:
Determined by site of infection, host immunity, and virulence of
the organism
Corynebacterium diptheriae:
strains cause diphtheria
in humans
Respiratory disease
Initially: sore throat, low-grade
; followed by
adherent pseudomembrane on the tonsils and pharynx
Later stages include localized damage, bleeding,
difficulty in breathing, and
and
peripheral
Complications from systemic spread of exotoxin to
other target organs in the body; eg., heart (concept of
"disease at a distance")
Most mortality from systemic toxin-mediated heart
failure
Cutaneous diphtheria (extra-respiratory disease)
Acquired by skin contact; organism enters through
break in subcutaneous tissue
Chronic non-healing ulcer results
Corynebacterium jeikeium: opportunistic infections (especially in
immunocompromised patients)
Corynebacterium urealyticum
: urinary tract infections (UTI’s);
rare but important
Corynebacterium pseudotuberculosis: subacute relapsing
lymphadenitis
Corynebacterium ulcerans: pharnygitis
Corynebacterium xerosis: bacteremia, skin infections,
pneumonia in immunocompromised hosts (e.g., patients with blood
disorders, bone marrow transplants, intravenous catheters) and
pharyngitis
Corynebacterium pseudodiphtheriticum: endocarditis and
lower-respiratory tract infections
Epidemiology:
Widely distributed in nature; worldwide in occurrence
Only 28 cases reported between 1980-1990 in the U. S. due to
highly successful immunization program
More commonly occurring in other countries
Former Soviet States have had epidemic rise in incidence since
breakup and disruption of immunization program
Human is the only natural host
Corynebacterium diptheriae:
Diphtheria (respiratory or cutaneous) occurs worldwide primarily
in urban areas
Carried assymptomicatically in the oropharynx of immune
individuals
Transmitted by respiratory droplets or skin contact
Corynebacterium jeikeium: carriage on skin of up to 40% of
hospitalized patients (e.g., bone marrow transplants)
Several species form part of the common microbiota of the human
respiratory tract and other mucous membranes, the conjunctiva, and
the skin
Pathogenesis & Immunity:
Non-pathogenic species are called "diphtheroids"; two species
commonly found in humans are
Corynebacterium
xerosis
and
Corynebacterium pseudodiphtheriticum
Pathogenic type species is
Corynebacterium diphtheriae, which
produces a potent
and causes diphtheria in humans
Diptheria A-B exotoxin interrupts peptide formation at the
ribosomal level
Phospholipase D increases vascular permeability, thus
allowing
C. diphtheriae
to spread through tissues of the naso-
pharyngeal area
Toxin Characteristics:
Encoded by
tox
gene introduced by lysogenic
bacteriophage (prophage) in virulent strains of
C.
diphtheriae
63,000 dalton protein toxin consisting of two
fragments, A and B
acts systemically
1. B fragment binds to receptor sites on target
cells and toxin is internalized by receptor-
mediated
2. A fragment blocks protein synthesis by ADP-
ribosylation of elongation factor-2 (EF-2)
Produced in the presence of limiting amounts of
iron;
optimum toxin production
in vitro
occurs in the
presence of 100 mg iron per liter
Used to produce
in DPT and TD vaccines (
Corynebacterium jeikeium: multiple antibiotic resistance
important in opportunistic infections of immunocompromised patients
Corynebacterium urealyticum: urease hydrolyzes urea; release
of NH
4
+
, increase in pH, alkaline urine, renal stones
Laboratory Identification:
Microscopy
shows metachromatic granules
Gram stain shows Gram-positive pleomorphic rods arranged in
perpendicular, parallel, and pallisade formations
Culture
A confirmed diagnosis of diphtheria can only be made by
isolating toxigenic diphtheria bacilli from the primary lesion (in the
throat or elsewhere)
Exudate from the lesion should be inoculated onto blood agar
and selective media:
Three
varieties
of
C. diphtheriae
colonies may be
recognized:
gravis,
intermedius, and
mitis
colonial
morphology:
1. var.
gravis:
large, flat, rough, dark-
gray colonies; not hemolytic; very few
small metachromatic granules; form a
pellicle in broth
2. var.
mitis:
smooth, convex, black,
shiny, entire colonies; hemolytic;
prominent metachromatic granules;
diffuse turbidity in broth
3. var.
intermedius:
dwarf, flat,
umbilicate colony with a black center
and slightly crenated periphery; not
hemolytic; fine granular deposit in
broth
C. diphtheriae
(also
Staphylococcus) produces gray to
black colonies on the tellurite media because the tellurite
is reduced intracellularly to tellurium
Any colonies which appear on the three media should
be stained with toluidine blue O or
Any typical
Corynebacterium
colonies would be
subcultured on a Loeffler's slant, and tested for
toxigenicity, either by the guinea pig virulence test or by
the
Diphtheroids may be distinguished from
C. diphtheriae
by means
of
CTA sugar fermentation reactions
and tests for toxigenicity
In vivo
test:
Guinea pig virulence test
In vitro
test:
(immunodiffusion)
Treatment, Prevention & Control:
Antitoxin
Used for neutralizing
Effective in conjunction with
therapy
Toxoid preparations are used for vaccines as active immunization
for diphtheria
Usually given in conjunction with pertussis and tetanus vaccines
(DPT vaccine) or as a booster with tetanus (TD)
Antibiotics
Penicillin G
Erythromycin if allergic
Distinguishing Characteristics
of Corynebacterium spp.
ORGANISM
CELLULAR
MORPHOLO
GY
HEMOLYS
IS
SUGAR
FERMENTATION TOXI
N
GLUCOS
E
SUCROS
E
C. diphtheriae
Slender
pleomorphic
rods; often club-
shaped; often
banded or
beaded with
irregularly
staining
granules
+
+
-
+
C. pseudodiphtheritic
um
Short rods; no
granules;
clubs rare
-
-
-
-

C. xerosis
Polar staining
rods;
few club forms
-
+
+
-
Description and Significance
Corynebacterium diphtheriae is the etiological agent of diphtheria, an upper respiratory
disease mainly affecting children. The virulence factors (most specifically diphtheria toxin)
have been studied extensively and are well understood.
Genome Structure
The Corynebacterium diphtheriae NCTC 13129 genome is 2,488,625 bp in length and has an
average G-C content of 53.5%. The three Corynebacterium genomes that have been
sequenced are all similar in general content - visit NCBI for more information.
Cell Structure and Metabolism

Notice the 'chinese letter' formations made by groups of theseCorynebacterium glutamicum, as a result of their
style of division.
Corynebacteria are small, generally nonmotile, Gram-positive, non-sporulating (although they
have club-like ends), pleomorphic bacilli. Due to their snapping type of division, cells often lie
in clusters resembling chinese letters. Corynebacteria are chemoorganotrophic, aerobic, or
facultatively anaerobic, and they exhibit a fermentative metabolism (carbohydrates to lactic
acid) under certain conditions. They are fastidious organisms, growing slowly on even an
enriched medium.
Ecology
Many species of Corynebacteria can be isolated from various places such as soil, water,
blood, and human skin. Pathogenic strains of Corynebacteria can infect plants, animals, or
humans. Though humans are now the only known reservoir for the disease. The bacterium is
generally found in temperate zones but may also be found in other parts of the world.
NondiptherialCorynebacteria are ubiquitous in nature, and are commonly found in human
mucous membranes and skin.
Isolation and Cultivation
The best method for isolating and cultivating Corynebacteria is to use sheep blood agar plus
one selective medium as the primary plating media. Selective media commonly used are
Cystine-Tellurite blood agar or Tinsdale medium. The plates should be ready after 18 to 24
hours of incubation at 37 degrees Celsius in a 5% carbon dioxide-enriched atmosphere.
Pathology
Diphtheria was first identified by Hippocrates in the 4th century B.C. The disease plagued
Europe through the 17th, 18th, and 19th centuries. It was spread to America where it reached
epidemic proportions around the middle of the 18th
century. Corynebacterium diphtheriae was identified as the etiological agent of diphtheria by
Klebs in 1883, and was first cultivated in 1884 by Loeffler, who also identified the diphtheria
toxin in the same year.
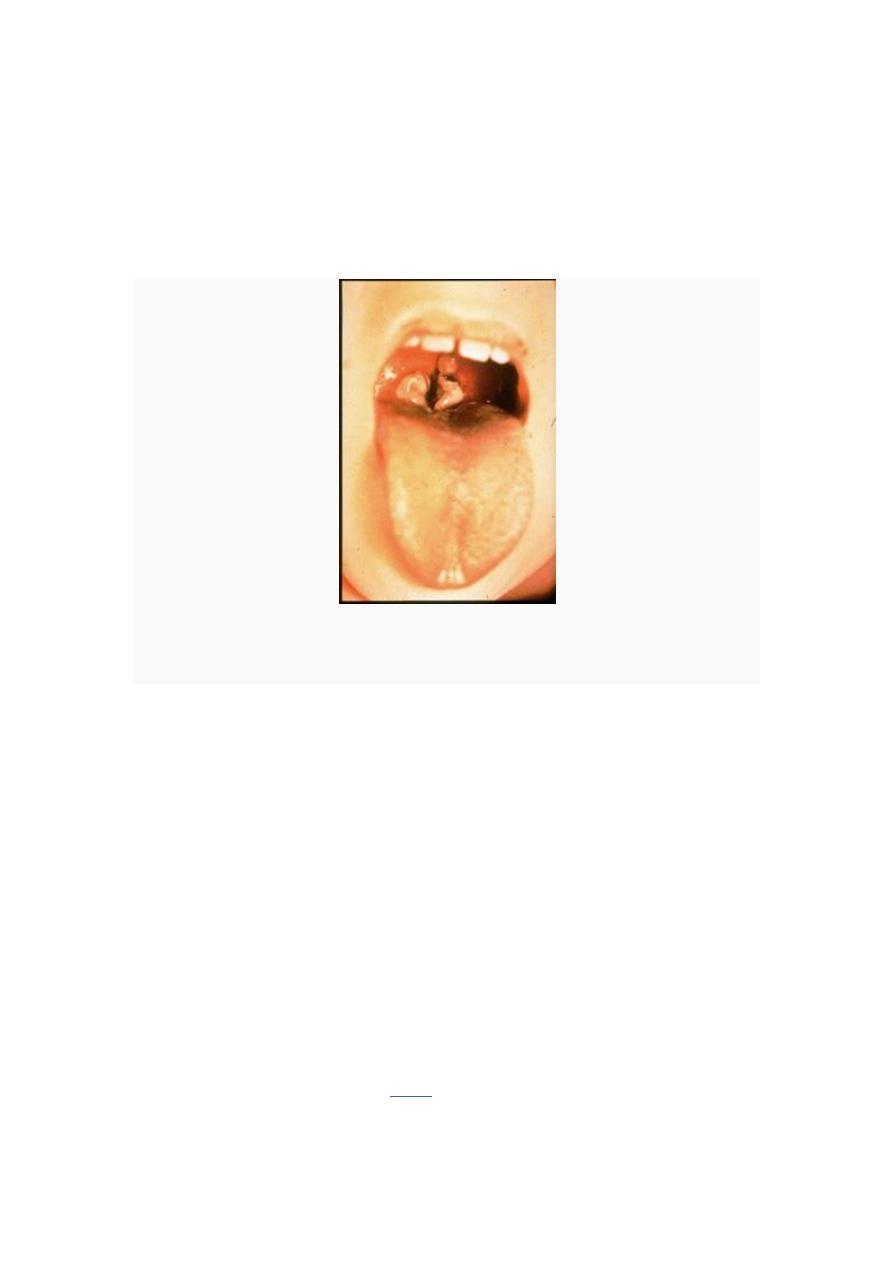
Pseudomembrane at the back of the throat of a child. The membrane can grow and extend further down the
throat, suffocating the child.
Diphtheria is described as "an upper respiratory tract illness characterized by sore throat, low-
grade fever, and an adherent membrane of the tonsil(s), pharynx, and/or nose, The
pathogenesis capabilities of diphtheria are dependent on its ability to colonize the
nasopharyngeal cavity or skin and its ability to produce the diphtheria toxin. C.
diphtheriae usually colonize a local lesion in the upper respiratory tract (although cutaneous
diphtheria can occur as well) where the toxin secreted by the bacteria cases necrotic injury to
epithelial cells. As a result, blood plasma leaks into the area and forms a fibrin network called
a pseudomembrane, which is full of rapidly growing C. diphtheriae cells. At the site of the
lesion the diphtheria toxin is absorbed and disseminated throughout the body via lymph
channels. Most commonly affected areas include heart, muscle, peripheral nerves, adrenal
glands, kidneys, liver, and spleen (rather comprehensive).
The diphtheria toxin works by causing the death of eukaryotic cells and tissues by inhibiting
protein synthesis in the cells. Two key factors aid C. diphtheriae in the production of this
systemic toxin: low extracellular concentrations of iron and the presence of a lysogenic
prophage (talked about in detail in the
section below). The role of iron in C.
diphtheriae cultures is very dramatic, and it is assumed to play the same part in vivo as well.
In iron depleted cultures C. diphtheriae will produce the diphtheria toxin as up to 5% of its

total protein production. It has been found that the tox gene is regulated by a negative control.
A repressor molecule, the product of the DtxR gene, is activated by iron. When activated, the
repressor binds to the tox gene operator and prevents transcription.
There are three different strains of C. diphtheriae which are differentiated by the severity of
the disease they cause in humans. The three strains are gravis, intermedius, and mitis (you
can distinguish the severity of each strain based on its name). The difference in virulence of
the three strains can be attributed to their relative abilities to manufacture the diphtheria toxin
(in both rate and quantity), and their respective growth rates. The mitis strain has a generation
time of about 180 minutes while the gravis strain has a generation time of about 60 minutes.
This faster growth rate may allow colonies to deplete iron supplies in colonized areas faster,
letting them produce toxin in greater quantities sooner.
