
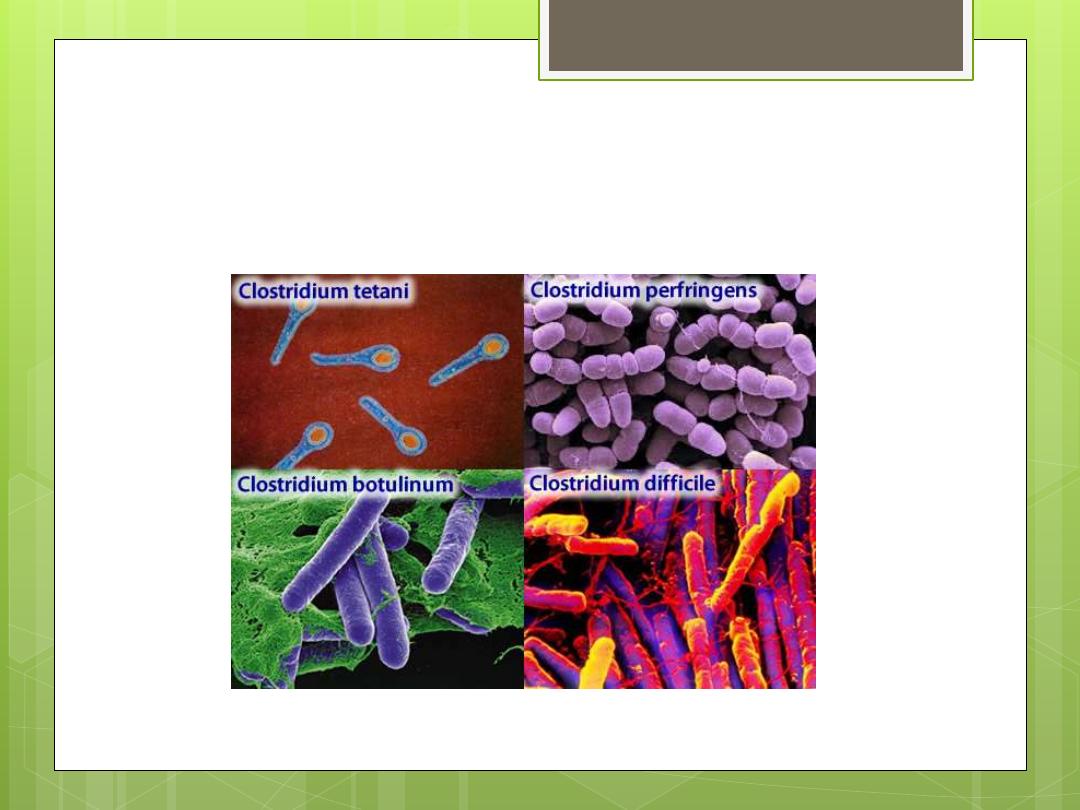
Clostridium
(anaerobic bacilli)

Clostridium (anaerobic bacilli)
Most Clostridium species decompose
proteins or form toxins and some do both.
Their natural habitat is the soil or intestinal
tract as saprophytes. The important
pathogenic species are:
Clostridium botulinum: Causes botulism
Clostridium tetani: Causes tetanus
Clostridium perfringens: Causes gas
gangrene

Morphology
Large anaerobic gram positive motile
rods.
The spore is usually wider than the rods.
Spores are placed centrally, terminally, or
subterminally according to the genus.

Culture
Anaerobic culture conditions are
established by one of the following:
Agar plates or culture tubes are placed in
air tight jar from which air is removed and
replaced by N and CO2.
Fluid media contain either:
Fresh animal tissue (chopped meat)
Reducing agent (Thioglycolate)
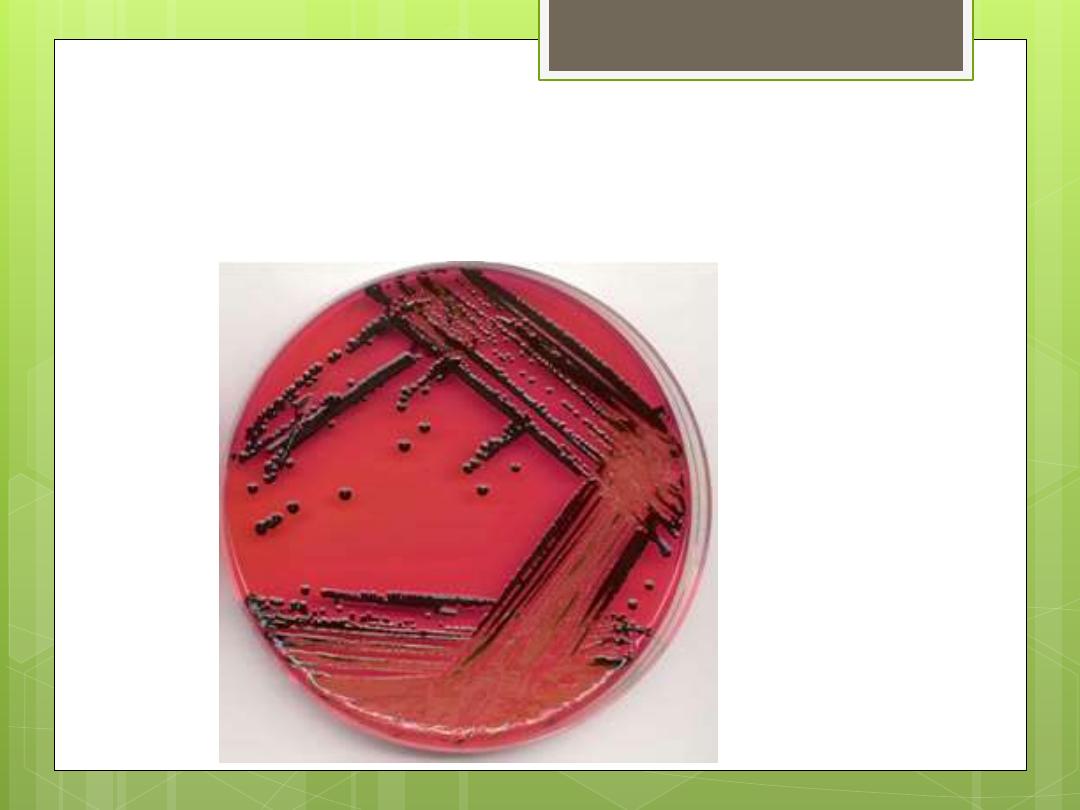

Colony forms
Clostridium perfringens: Large raised colonies with
entire margins.
Clostridium tetani : Smaller colonies with fine
filaments.
Most Clostridia produce a zone of hemolysis on blood
agar.
Growth characteristics of anaerobic microorganisms
are:
1. Unable to utilize O2 as the final oxygen acceptor.
2. Lack of cytochrome and cytochrome oxidase.
3. Unable to break down hydrogen peroxide H2O2
because they lack catalase of peroxidase so H2O2 will
accumulate to toxic conc. in the presence of O2.

Colony forms
Clostridium botulinum
It causes botulism, infant’s botulism, and
rarely wound infection. It is found in soil
and animal feces. The spores are
subterminal highly resistant to heat. They
resist boiling 3-5 hours. This resistance is
diminished at acidic pH and salt. It
produces toxin during life and autolysis of
bacteria.

Toxin
Types of Clostridium botulinum: They are from A – H
according to the type of toxin produced.
Types A, B, and E are the most commonly associated with
illness
Toxins of types A, B, and E have the following
characteristics:
They are among the most highly toxic substances known.
They are neurotoxic proteins (MW = 150 000)
Lethal dose for human is 1 – 2 mg
They are destroyed by heating for 20 minutes at 100 oC
Toxin production is under the control of a viral gene
(Bacteriophage yielded from toxigenic strain and it may infect
non-toxigenic strain and convert it to toxigenic).

Action of botulism toxin
It is a neurotoxic protein. All of its types (A, B,
and E) are made of heavy and light chains
linked by disulfide bonds. The heavy chain is
thought to bind the toxin to the motor nerve
end plate. The light chain blocks the
calcium-mediated release of acetyl
choline. The toxin acts by blocking the
release of acetyl choline at synapses and
neuromuscular junctions causing flaccid
paralysis.

Pathogenesis
Botulism is intoxication. It results from ingestion of
food in which Clostridium botulinum spores
germinate and produce toxins under anaerobic
conditions. These foods are spiced, smoked,
vacuum-packed, or canned alkaline foods (if
eaten without smoking). The toxin acts by
blocking the release of acetyl choline at
synapses and neuromuscular junctions causing
flaccid paralysis. Patients who recover don’t
develop an antitoxin in the blood.

Symptoms
within 18 – 24 hours
Visual disturbances
Inability to swallow
Speech difficulty
Respiratory paralysis or cardiac arrest
Infant botulism …
It may result from honey feeding and cause
signs of paralysis or sudden death.

Lab diagnosis
Toxin can be detected in the patient serum
and left over food.
1. Mice are injected with the specimen and
then neutralized by injections of antitoxin.
2. Culture of food remains of its growth test
for toxin production.
3. Toxin is tested by hemoagglutination or
radioimmunoassay (RIS).

Treatment
IV administration of antitoxin (trivalent
antitoxin of types A, B, and E).
Adequate ventilation by mechanical
respirator. This will reduce mortality form
65% to 25%
Infant botulism is recovered with
supportive therapy alone.

Control
1.
Boiling of home-canned food for 20 minutes to
destroy the toxin.
2.
Strict regulation of commercial canning
3.
Avoiding swelled canned food or that with
suspected appearance or odor.
4.
Clostridium botulinum is widely distributed in soil
and contaminated fruits and
5.
vegetables
6.
. Inadequate precautions in processing and
handling of a certain food
7.
will allow this organism to grow and produce one
of the most powerful exotoxins known.

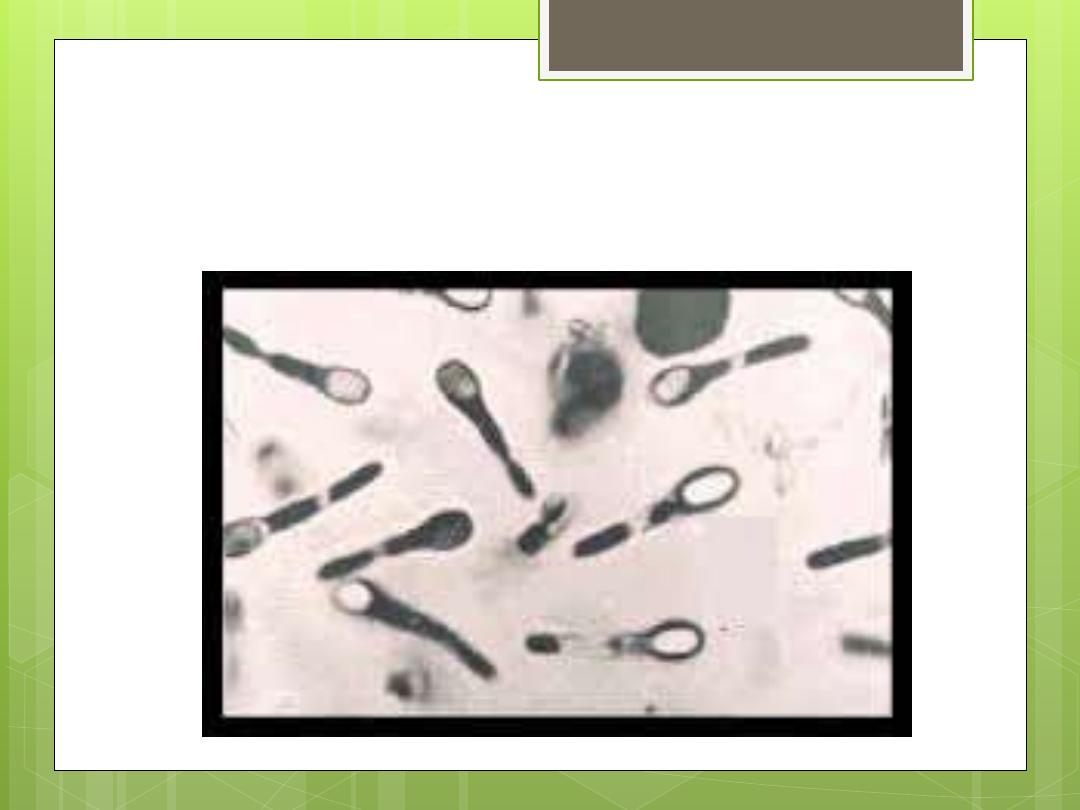
tetani
Clostridium
It causes tetanus, uterus, and tetanus
neonatrum. It is distributed in soil and feces
of animals. The spores are located at one
end of the bacilli (drum-stick). It is
differentiated into several types according
to their specific flagellum antigens.

Toxin
Vegetative cells of Clostridum tetani produce
tetanospasmin that has the following properties:
1.
It is a polypeptide in nature.
2.
Its production is under the control of a
plasmid gene.
3.
The proteolytic enzymes split this toxin into
two fragments of increased toxicity.
4.
It contains 2 * 107 mouse lethal dose / mg
5.
It acts upon CNS

Mode of action
1.
It inhibits the release of acetyl choline
thus it interferes with neuromuscular
transmission.
2.
2. Inhibition of postsynaptic spinal
neurons by blocking the release of an
inhibiting mediator.

Pathogenesis
Clostridium tetani is not an invasive organism. The
infection remains strictly localized in the area of dead
tissue (into which the spores have been introduced).
Germination of spores to vegetative organisms that
produce toxin is aided by:
1.
Necrotic tissue
2.
Calcium salts
3.
Associated pyogenic infections
4.
All aid in the establishment of low oxidation –
reduction potent.
Tetanospasmin released from vegetative cells will
reach the CNS via the blood and result in
generalized muscular spasm

Clinical findings of tetanus
Duration is 4 to 5 days – many weeks. There is
muscular contraction of the voluntary muscles (1st
area of infection) then the muscles of the jaw
(Lock-Jaw disease). Later, other voluntary muscles
are involved causing generalized spasm resulting
in respiratory paralysis and cardiac failure which
lead to death (50%).
Uterus tetanus: Follows septic abortion.
Tetanus neonatrum: Follows contamination of the
umbilical cord of newborns when it is cut by
contaminated instument .

Prevention
1.
Active immunization with toxoid
(detoxified toxin) to stimulate Ab.
2.
Proper care of wound (Remove the
necrotic tissue).
3.
Prophylactic use of antitoxin.
4.
Administration of penicillin (to inhibit
Clostridium and pyogenic bacteria).
5.
Treatment with antitoxin in tetanus
neonatrum is life saving.

Control
Active immunization of children with tetanus
toxoid 3 injections:
1.
In the 1st year
2.
Booster injection at entry to school
3.
Boosters are spaced 7-10 years
Usually in young children: In immunization,
tetanus toxoid is combined with diphtheria
toxoid and Pertussis vaccine (DTP).

Lab diagnosis
Diagnosis rests on clinical pictures.
1.
Anaerobic culture of necrotic tissue.
2.
Growth is tested for toxin production.
3.
Neutralization of the toxin produced with
specific antitoxin.

Clostridium perfringens
It produces invasive infection. It is
responsible for 90% of myonecrosis and gas
gangrene cases infecting contaminated
wounds (e.g. compounds fracture and post
partum uterus). There are other 30 species
of Clostridium which cause the rest 10% of
infection.

Morphology
They are found in the soil and the intestine
of man and animals. They are anaerobic
large G+ve rods. They produce subterminal
non-bulging spores (rarely produce spores
in laboratory media). They produce capsule
in the patient’s tissue.
Clostridium perfringens also causes profuse
diarrhea (food poisoning).

Toxin
There are 5 types of Clostridium perfringens (A, B, C,
D, and E). They are differentiated on the basis of
production of 4 major toxins (α, B, E, and Iota).
α toxin is responsible for severe toxemia in gas
gangrene and has the following properties:
1.
It is lethal for lab animals.
2.
It is Ca+2, Mg+2 – dependent lecithinase.
3.
Causes lysis of RBCs.
4.
Produced by all types of Clostridium prefringens.
5.
It splits lecithin of cytoplasmid membrane →
Phosphorylendin + Diglyceride
6.
It has necrotizing and hemolytic effect.

Enzymes
Clostridium perfringens produce enzymes
that digest subcutaneous tissue and
muscles.
1.
DNAase
2.
Hyaluronidase
3.
Collagenase

Pathogenesis
1.
Gas gangrene:
The spores reach traumatized tissue from soil or intestine
of patients. The spores will germinate to vegetative
cells. Vegetative cells will multiply and ferment
carbohydrates of tissue producing CO2 gas. Distention
of tissue and interference with blood supply together
with secretions of necrotizing toxins and enzymes →
spread of infection and necrosis of tissue.
The necrosis extends → bacterial growth, hemolytic
anaemia, and severe toxemia and death.
In gas gangrene, a mixed infection is the rule (Clostridia
+ G+ve cocci + G-ve bacilli).

Pathogenesis
1.
Uterine gas gangrene: May follow
instrumental abortion because
Clostridium perfringens is present in the
genital tract of 5% of women.
2.
Clostridial bacteremia is frequent in
patients with neoplasms.
3.
Food poisoning due to enterotoxin.

Clinical Findings
The infection spread from a contaminated
wound in 1 -3 days to produce:
1.
Crepitation in the subcutaneous tissue.
2.
Foul smelling discharge.
3.
Necrosis.
4.
Fever.
5.
Hemolysis f.
Toxemia
6.
Shock h. Death

Treatment
Immediate surgical debridement of all
dead tissue.
2. Administration of antibiotics
(ampicillin).
3. Polyvalent antitoxin could be used.
4. Hyperbaric oxygen detoxifies the
patient rapidly.

Lab diagnosis
Specimen: Tissue form wounds, pus, and deep swabs.
BGram stain.
Culture on:
Chopped meat and glucose media.
Thioglycolate media.
Blood agar media
Incubated anaerobicly
Action on milk.
Biochemical shirt (Sugar from)
F. Lecithin’s activity
Test for toxin production

Claustridium perfringens food
poisoning
Usually it follows the ingestion of large no. of
Clostridium perfringens that have grown in
warmed meat dishes. The toxin is formed when
Clostridium sporulate in gut with the onset of
diarrhea. There is no vomiting or fever in 6 – 18
hours. It lasts only 1 – 2 days. It is self limited.
Toxin is heat labile enterotoxin that has a
mechanism of action which resembles that of E.
coli.
