
Lec.1&2
IMMUNITY TO INFECTION
: االستاذة المساعدة
د وفاق محمود الوتار
_ Viral Infections
_ Bacterial Infections
_ Fungal infections
_ Protozoan Diseases & diseases Caused by Parasitic Worms (Helminthes)
_ Emerging Infectious Diseases
One of the first and most important features of host innate immunity is the
barrier provided by the epithelial surfaces of the skin and the lining of the
gut. The difficulty of penetrating these epithelial barriers ensures that most
pathogens never gain productive entry into the host. In addition to providing
a physical barrier to infection, the epithelia also produce chemicals that are
useful in preventing infection. The secretion of gastric enzymes by
specialized epithelial cells lowers the pH of the stomach and upper
gastrointestinal tract, and other specialized cells in the gut produce
antibacterialpeptides.
A major feature of innate immunity is the presence of the normal gut flora,
which can competitively inhibit the binding of pathogens to gut epithelial
cells. Innate responses can also block the establishment of infection. For
example, the cell walls of some gram-positive bacteria contain a
peptidoglycanthat activates the alternative complement pathway,resulting
in the generation of C3b, which opsonizes bacteria and enhances
phagocytosis. Some bacteria produce endotoxins such as LPS, which

stimulate the production of cytokines such as TNF-_, IL-1, and IL-6 by
macrophages or endothelial cells. These cytokines can activate
macrophages. Phagocytosis of bacteria by macrophages and other
phagocytic cells is another highly effective line of innate defense. However,
some types of bacteria that commonly grow intracellularly have developed
mechanisms that allow them to resist degradation within the phagocyte.
Viruses are well known for the stimulation of innate responses. In particular,
many viruses induce the production of interferons, which can inhibit viral
replication by inducing an antiviral response. Viruses are also controlled by
NK cells. NK cells frequently form the first line of defense against viral
infections. Generally, pathogens use a variety of strategies to escape
destruction by the adaptive immune system. Many pathogens
reduce their own antigenicity either by growing within host cells, where they
are sequestered from immune attack,or by shedding their membrane
antigens. Other pathogens camouflage themselves by mimicking the
surfaces of host cells, either by expressing molecules with amino acid
sequences similar to those of host cell-membrane molecules or by acquiring
a covering of host membrane molecules. Some pathogens are able to
suppress the immune response selectively or to regulate it so that a branch
of the immune system is activated that is ineffective against the pathogen.
Continual variation in surface antigens is another strategy that enables a
pathogen to elude the immune system. This antigenic variation may be due
to the gradual accumulation of mutations, or it may involve an abrupt
change in surface antigens.
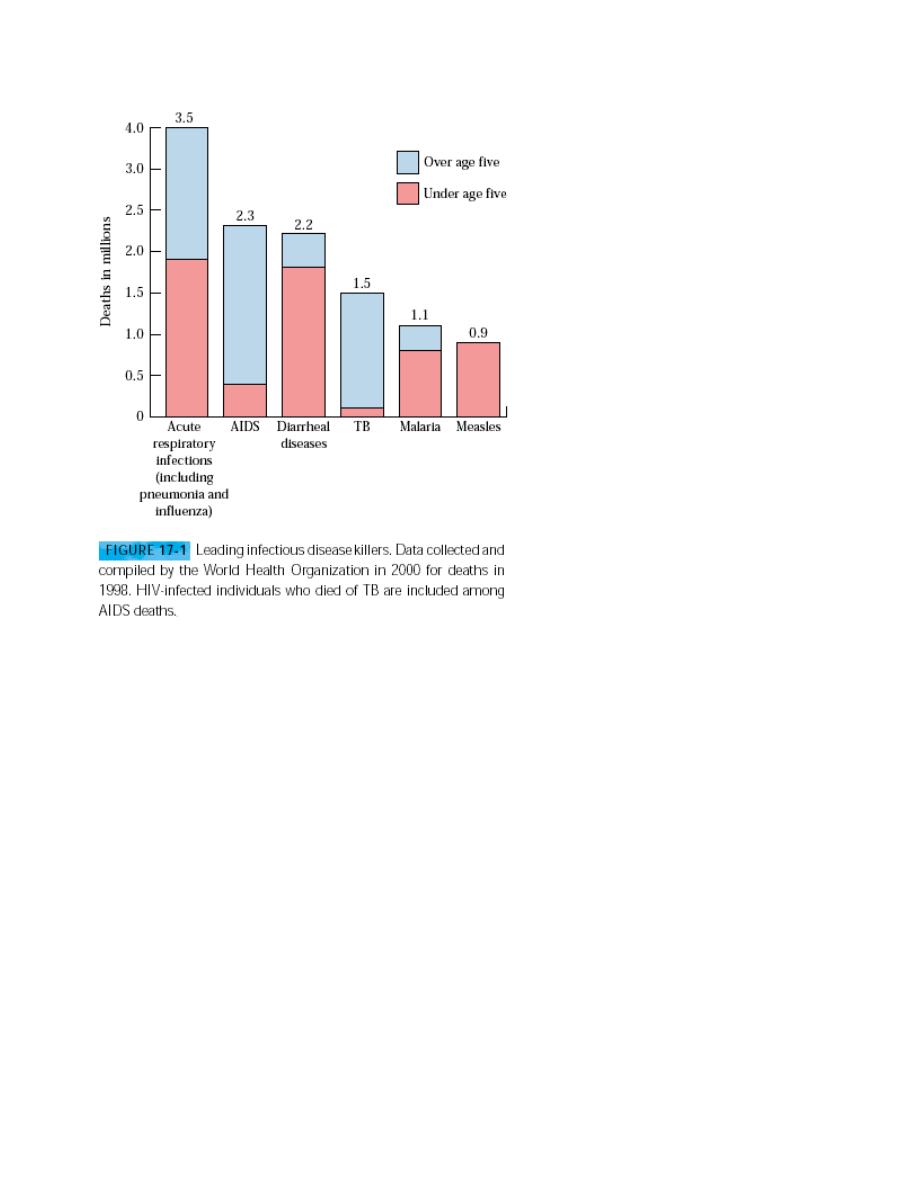
Both innate and adaptive immune responses to pathogens provide critical
defense, but infectious diseases, which have plagued human populations
throughout history, still cause the death of millions each year. Although

widespread use of vaccines and drug therapy has drastically reduce
mortality from infectious diseases in developed countries, such diseases
continue to be the leading cause of death in the Third World. It is estimated
that
over 1 billion
people are infected worldwide, resulting in more than
11
million
deaths every year (Figure 17-1). some of these diseases are
beginning to emerge or re-emerge in developed countries. For
example, some United States troops returned from the Persian
Gulf with leishmaniasis; cholera cases have recentlyincreased worldwide,
with more than 100,000 cases reported in KwaZulu-Natal, South Africa,
during the summer of 2001; and a new drug-resistant strain of
Mycobacterium tuberculosis is spreading at an alarming rate in the United
States. The selected infectious diseases caused by viruses, bacteria,
protozoa, and helminthes ,the four main types of pathogens.
Viral Infections
A number of specific immune effectors mechanisms, together with
nonspecific defense mechanisms, are called into play to eliminate an
infecting virus (Table 17-1). At the same time, the virus acts to subvert one
or more of these mechanisms to prolong its own survival. The outcome of
the infection depends on how effectively the host’s defensive mechanism
resist the virus.
The innate immune response
to viral infection is primarily
through the induction of type I interferons (IFN-alpha_ and IFN-beta_) and
the activation of NK cells. Double stranded RNA (dsRNA) produced during
the viral life cycle can induce the expression of IFN-alpha _ and IFN-beta_ by

the infected cell. Macrophages, monocytes, and fibroblasts also are capable
of synthesizing these cytokines, but the mechanisms that induce the
production of type I interferons in these cells are not completely
understood. IFN-alpha_ and IFN-beta_ can induce an antiviral response or
resistance to viral replication by binding
to the receptor leading to inhibition of viral replication. The binding of IFNs
to NK cells induces lytic activity,making them very effective in killing virally
infected cells. The activity of NK cells is also greatly enhanced by IL-12, a
cytokine that is produced very early in a response to viral infection.
HUMORAL IMMUNE RESPONSE
Many Viruses are Neutralized by Antibodies ,antibodies specific for viral
surface antigens are often crucial in containing the spread of a virus during
acute infection and in protecting against reinfection. Antibodies are
particularly effective in protecting against infection if they are localized at
the site of viral entry into the body. Most viruses express surface receptor
molecules that enable them to initiate infection by binding to specific host-
cell membrane molecules. For example, influenza virus binds to sialic acid
residues in cell membrane glycoproteins and glycolipids; rhinovirus binds to
intercellular adhesion molecules (ICAMs); and Epstein-Barr virus binds to
type 2 complement receptors on B cells. If antibody to the viral receptor is
produced, it can block infection altogether by preventing the binding of viral
particles to host cells. Secretory IgA in mucous secretions plays an important
role in host defense against viruses by blocking viral attachment to mucosal
epithelial cells. The advantage of the attenuated شلل االطفالoral polio vaccine,
is that it induces production of secretory IgA, which effectively blocks
attachment of poliovirus along the gastrointestinal tract. Viral neutralization
by antibody sometimes involves mechanisms that operate after viral

attachment to host cells. also complement can agglutinate viral particles
and function as an opsonizing agent to facilitate Fc- or C3b-receptor–
mediated phagocytosis of the viral particles.
Cell-Mediated Immunity
is Important for Viral Control and Clearance
although antibodies have an important role in containing the spread of a
virus in the acute phases of infection, they are not usually able to eliminate
the virus once infection has occurred—particularly if the virus is capable of
entering a latent state in which its DNA is integrated into host chromosomal
DNA. Once an infection is established, cell-mediated immune mechanisms
are most important in host defense. Ingeneral,CD8+ TC cells and CD4+ TH1
cells are the main components of cell-mediated antiviral defense, although
in some cases CD4+ TC cells have also been implicated .Activated TH1 cells
produce a number of cytokines, including IL-2, IFN-a_,and TNF-b, that
defend against viruses either directly or indirectly.IFN-gamma_ acts directly
by inducing an antiviral state in cells. IL-2 acts indirectly by assisting in the
recruitment of C TL precursors into an effector population. Both IL-2 and
IFN-gamma_ activate NK cells, which play an important role in host
defense during the first days of many viral infections until a specific CTL
response develops. In most viral infections, specific CTL activity arises within
3–4 days after infection, peaks by 7–10 days, and then declines. Within 7–10
days of primary infection, most virions have been eliminated, The viral
specificity of the CTL as well can be demonstrated with IFN-α/β receptor.
How the Viruses Can Evade Host Defense Mechanisms
Despite their restricted genome size, a number of viruses
have been found to encode proteins that interfere at various

levels with specific or nonspecific host defenses. Presumably,
the advantage of such proteins is that they enable viruses to
replicate more effectively amidst host antiviral defenses. As
described above, the induction of IFN-α/β is a major innate defense against
viral infection, but some viruses have developed strategies to evade the
action of both.These include hepatitis C virus, which has been shown to
overcome the antiviral effect of the interferons
.Another mechanism for
evading host responses, utilized in particular by herpes simplex viruses (HSV)
is
inhibition of antigen presentation by infected host cell
by HSV-1 or
HSV-2 thus preventing presentation of viral antigen to CD8+ T cells. This
results in the trapping of empty class I MHC molecules in the endoplasmic
reticulumand effectively shuts down a CD8+ T-cell response to HSV-infected
cells.
The targeting of MHC molecules is not unique to HSV.Other viruses have
been shown to
down-regulate class I MHC expression
shortly after infection.
Two of the best characterized examples, the adenoviruses and
cytomegalovirus(CMV), use distinct molecular mechanisms to reduce
the surface expression of class I MHC molecules, again inhibiting antigen
presentation to CD8+ T cells. Some viruses—CMV, measles virus, and HIV
Antibody-mediated destruction of viruses requires complement activation,
resulting either in direct lysis of the viral particle or opsonization and
elimination of the virus by phagocytic cells. A number of viruses have
strategies for
evading complement-mediated destruction.
Vaccinia virus, for

example, secretes a protein that binds to the C4b complementcomponent,
inhibiting the classical complement
pathway;and herpes simplex viruses
have a glycoprotein componentthat binds to the C3b complement
component, inhibiting both the classical and alternative pathways.
can have
the same effect as an antigenic shift that generates anew subtype.
protection against influenza, but its specificity is strain-specific and is readily
bypassed by
antigenic shift&drift
is responsible for our inabilityto produce
an effective vaccine for colds. Now here is antigenic variation greater than in
the human immunodeficiency virus (HIV), the causative agent of AIDS.
Estimates suggest that HIV accumulates mutations at a rate 65 times faster
than does influenza virus.
.
Bacterial Infections
Immunity to bacterial infections is achieved by means of antibody unless the
bacterium is capable of intracellular growth, in which case delayed-type
hypersensitivity has an important role. Bacteria enter the body either
through a number of natural routes (e.g., the respiratory tract, the
gastrointestinal tract, and the genitourinary tract) or through normally
inaccessible routes opened up by breaks in mucous membranes or skin.
Depending on the number of organisms.
specific immune response.Immune Responses to Extracellular
and Intracellular Bacteria
different levels of host defense are enlisted. If the inoculum size and the
virulence are both low, then localized tissue phagocytes may be able to
eliminate the bacteria with an innate, nonspecific defense. Larger inoculums
or organisms with greater virulence tend to induce an adaptive, specific

immune response.Immune Responses to extracellular and Intracellular
Bacteria Can Differ
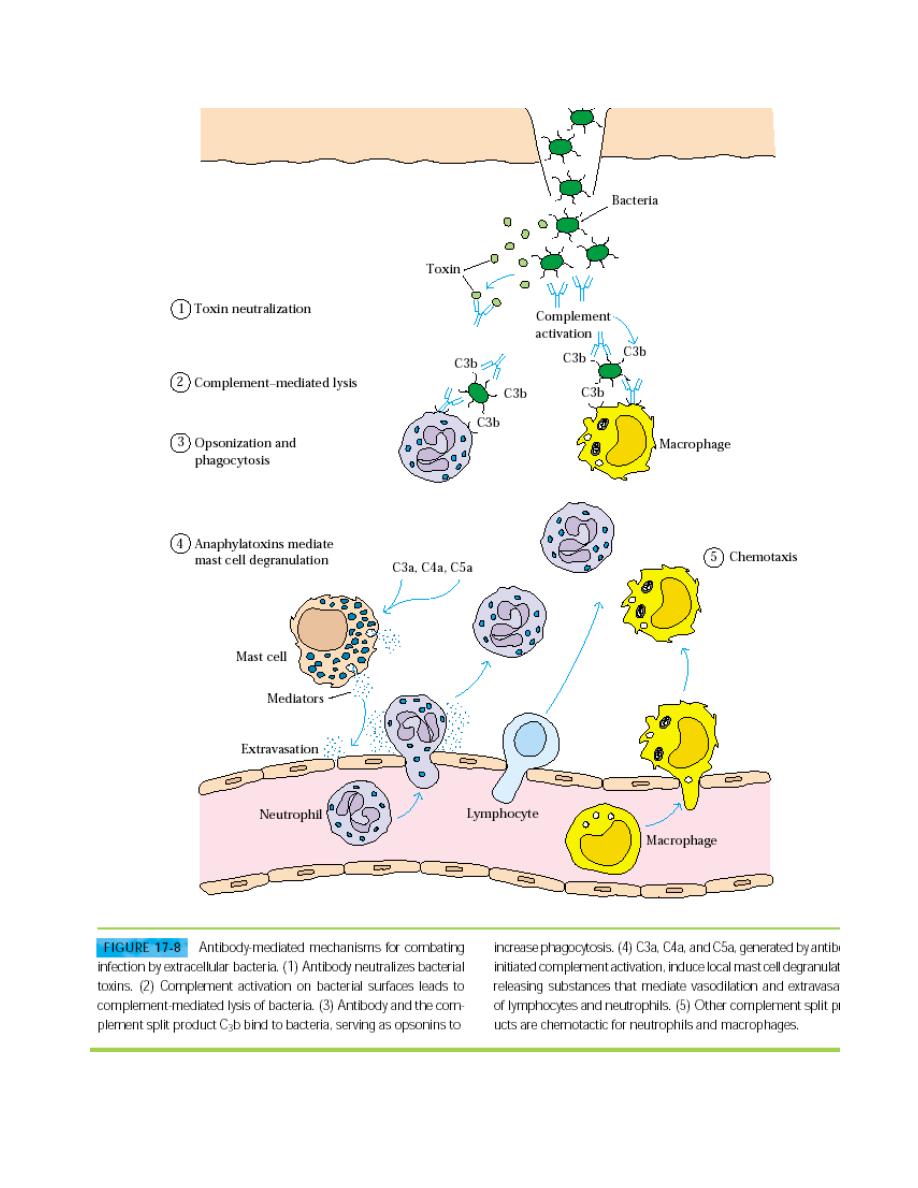
Infection by extracellular bacteria induces production of humoral antibodies,
which are ordinarily secreted by plasma cells in regional lymph nodes and
the submucosa of the respiratory and gastrointestinal tracts. The humoral
immune response is the main protective response against extracellular
bacteria. The antibodies act in several ways to protect the host from the
invading organisms, including removal of the bacteria and inactivation of
bacterial toxins (Figure 17-8).
Extracellular bacteria can be pathogenic because they induce a localized
inflammatory response or because they produce toxins. The toxins,
endotoxin or exotoxin, can be cytotoxic but also may cause pathogenesis in
other ways. An excellent example of this is the toxin produced by diphtheria,
which exerts a toxic effect on the cell by blocking protein synthesis.
Endotoxins, such as lipopolysaccharides (LPS), are generally components of
bacterial cell walls, while exotoxins, such as diphtheria toxin, are secreted by
the bacteria.
Antibody that binds to accessible antigens on the surface of a bacterium can,
together with the C3b component of complement, act as an opsonin that
increases phagocytosis and thus clearance of the bacterium (see Figure 17-
8). In the case of some bacteria—notably, the gram-negative organism
complement activation can lead directly to lysis of the organism. Antibody-
mediated activation of the complement system can also induce localized
production of immune effector molecules that help to develop an amplified
and more effective inflammatory response. For example, the complement
split products C3a, C4a, and C5a act as anaphylatoxins, inducing local mast-
cell degranulation and thus vasodilation and the extravasation of

lymphocytes and neutrophils from the blood into tissue space (see Figure
17-8).
Other complement split products serve as chemotactic factors for
neutrophils and macrophages, thereby contributing to the buildup of
phagocytic cells at the site of infection.Antibody to a bacteria toxin may bind
to the toxin and neutralize it; the antibody-toxin complexes are then cleared
by phagocytic cells in the same manner as any other antigen-antibod
complex.
While innate immunity is not very effective against intracellular bacterial
pathogens, intracellular bacteria can activate NK cells, which, in turn,
provide an early defense against these bacteria. Intracellular bacterial
infections tend to induce a cell-mediated immune response, specifically,
delayed type hypersensitivity. In this response, cytokines secreted by
CD4+ T cells are important—notably IFN-gamma, which activates
macrophages to kill ingested pathogens more effectively .
Bacteria Can Effectively Evade Host Defense Mechanisms
There are four primary steps in bacterial infection:
_ Attachment to host cells
_ Proliferation
_ Invasion of host tissue
_ Toxin-induced damage to host cells
Host-defense mechanisms act at each of these steps, and many bacteria
have evolved ways to circumvent some of these host defenses. Some
bacteria have surface structures or molecules that enhance their ability to

attach to host cells. A number of gram-negative bacteria, for instance, have
pili (long hair like projections), which enable them to attach to the
membrane of the intestinal or genitourinary tract .
Other bacteria, such as Bordetella pertussis, secrete adhesion moleculesthat
attach to both the bacterium and the ciliated epithelial cells of the upper
respiratory tract. Secretory IgA antibodies specific for such bacterial
structures can block bacterial attachment to mucosal epithelial cells and are
the main host defense against bacterial attachment.
However, some bacteria (e.g., Neisseria gonorrhoeae,Haemophilus
influenzae, and Neisseria meningitidis) evade the IgA response by secreting
proteases that cleave secretory IgA at the hinge region; the resulting Fab
and Fc fragments have a shortened half-life in mucous secretions and are
not able to agglutinate microorganisms.
Some bacteria evade the IgA response of the host bychanging these surface
antigens. In N. gonorrhoeae, for example, pilin, the protein component of
the pili, has a highly variable structure ,the continual changes in the pilin
sequence allow the organism to evade neutralizationby IgA.
Some bacteria possess surface structures that serve to inhibit phagocytosis.
A classic example is Streptococcus pneumoniae, whose polysaccharide
capsule prevents phagocytosis very effectively.


Other bacteria, such as Streptococcus pyogenes, a surface protein projection
called the M protein inhibits phagocytosis. Some pathogenic staphylococci
are able to assemble a protective coat from host proteins. These bacteria
secrete a coagulase enzyme that precipitates a fibrin coat around them,
shielding them from phagocytic cells.
Mechanisms for interfering with the complement system help other bacteria
survive. In some gram-negative bacteria, resist complement mediated
lysis. Pseudomonas secretes an enzyme, elastase, that inactivates both the
C3a and C5a anaphylatoxins, thereby diminishing the localized inflammatory
reaction.
A number of bacteria escape host defense mechanisms by their ability to
survive within phagocytic cells. Some, such as Listeria monocytogenes, do
this by escaping from the phagolysosome to the cytoplasm,which is a more
favorable environment for their growth. Other bacteria, such as
Mycobacterium avium, block lysosomal fusion with the phagolysosome;
andsome mycobacteria are resistant to the oxidative attack that takes place
within the phagolysosome.
Immune Responses Can Contribute to Bacterial Pathogenesis
In some cases, disease is caused not by the bacterial pathogen itself but by
the immune response to the pathogen, pathogen-stimulated
overproduction of cytokines leads to the symptoms of bacterial septic shock,
food poisoning, and toxic-shock syndrome. For instance,cell-wall endotoxins
of some gram-negative bacteria activate macrophages, resulting in release

of high levels of IL-1 and TNF-, which can cause septic shock. In
staphylococcalfood poisoning and toxic-shock syndrome, exotoxins
produced by the pathogens function as superantigens, which
can activate all T cells that express T-cell receptors with a particular v-
domain. The resulting overproduction of cytokines by activated TH cells
causes many of the symptoms of these diseases.
The ability of some bacteria to survive intracellularly within infected cells
can result in chronic antigenic activation of CD4+ T cells, leading to tissue
destruction by a cell-mediated response with the characteristics of a
delayed-type hypersensitivityreaction
Cytokines secreted by these activated CD4+ T cells can lead to extensive
accumulation and activation of macrophages, resulting in formation of
agranuloma. The localized concentrations of lysosomal enzymes in these
granulomas can cause extensive tissue necrosis.
Much of the tissue damage seen with M. tuberculosis is due to a cell-
mediated immune response.
Diphtheria (Corynebacterium diphtheriae) may be controlled by
Immunization with Inactivated Toxoid
Diphtheria is the classic example of a bacterial disease caused by a secreted
exotoxin to which immunity can be induced by immunization with an
inactivated toxoid.
The virulence of the organism is due completely to its potent exotoxin. The
toxin causes destruction of the underlying tissue, resulting in the formation
of a tough fibrinous membrane (“pseudomembrane”) composed of
fibrin,white blood cells, and dead respiratory epithelial cells. The membrane

itself can cause suffocation. The exotoxin also is responsible for widespread
systemic manifestations. Pronounced myocardial damage and neurologic
damage (ranging from mild weakness to complete paralysis) are common.
Tuberculosis (Mycobacterium tuberculosis) Is Primarily Controlled by CD4+ T
Cells Tuberculosis is the leading cause of death in the world from a single
infectious agent, killing about 3 million individuals every year ,roughly one-
third of the world’s population, are infected with the causative agent M.
tuberculosis and are at risk of developing the disease. Long thought to have
been eliminated as a
Protozoan Diseases
Protozoans are unicellular eukaryotic organisms. They are responsible for
several serious diseases in humans, including amoebiasis, Chagas’ disease,
African sleeping sickness, malaria, leishmaniasis, and toxoplasmosis.
The type of immune response that develops to protozoan infection and the
effectiveness of the response depend in part on the location of the parasite
within the host. Many protozoans have life-cycle stages in which they are
free within the bloodstream, and it is during these stages that humoral
antibody is most effective.
Many of these same pathogens are also capable of intracellular growth;
during these stages, cell-mediated immune reactions are effective in host
defense. In the development of vaccines for protozoan diseases, the branch
of the immune system that is most likely to confer protection must be
carefully considered.
Malaria
(Plasmodium Species) Infects 600 Million People Worldwide
Malaria is one of the most devastating diseases in the world today, infecting
nearly 10% of the world population and causing 1–2 million deaths every

year.There is speculation that some of the symptoms of malaria may be
caused not by Plasmodium itself but instead by excessive production of
cytokines.
HOST RESPONSE TO PLASMODIUM INFECTION
In regions where malaria is endemic, the immune responseto Plasmodium
infection is poor. The low immune response to Plasmodium among children
can be demonstrated by measuring serum antibody levels to the sporozoite
stage. Only 22% of the children living in endemic areas have detectable
antibodies to the sporozoite stage, whereas 84% of the adults have such
antibodies. Even in adults, the degree of immunity is far from complete,
however, and most people living in endemic regions have lifelonglow-level
Plasmodium infections.
A number of factors may contribute to the low levels of immune
responsiveness to Plasmodium. The maturational changes from sporozoite
to merozoite to gametocyte allow the organism to keep changing its surface
molecules, resulting in continual changes in the antigens seen by the
immune system. The intracellular phases of the life cycle in liver cells and
erythrocytes also reduce the degree of immune activation generated by the
pathogen and allow the organism to multiply while it is shielded from attack.
DESIGN OF MALARIA VACCINES
An effective vaccine for malaria should maximize the most effective immune
defense mechanisms. Unfortunately, little is known of the roles that
humoral and cell-mediated responses play in the development of protective
immunity to this disease.

African Sleeping Sickness
(Trypanosoma Species)
Two species of African trypanosomes, causing meningoencephalitis and
eventually the loss of consciousness. As parasite numbers increase after
infection, an effective humoral antibody response develops to the
glycoprotein coat, called variant surface glycoprotein (VSG), that covers
the trypanosomal surface These antibodies eliminate most of the parasites
from the bloodstream, both by complement-mediated lysis and by
opsonization and Millions of trypanosomes per milliliter of blood
subsequent phagocytosis. However, about 1% of the organisms, which bear
an antigenically different VSG, escape the initial antibody response. These
surviving organisms now begin to proliferate in the bloodstream, and a new
wave of parasitemia is observed. The successive waves of parasitemia reflect
a unique mechanism of antigenic shift by which the trypanosomes can
evade the immune response
Leishmaniasis
Is a Useful Model for Demonstrating Differences in Host Responses
The protozoan parasite Leishmania major provides a powerful and
illustrative example of how host responses can differ between individuals.
These differences can lead to either clearance of the parasite or fatality from
the infection. Leishmania is a protozoan that lives in the phagosomes of
macrophages. Resistance to the infection correlates well with the
production of IFN-gamma_ and the development of a TH1 response.
Elegant studies in mice have demonstrated that strains that are resistant to
Leishmania develop a TH1 response and produce IFN-gamma_ upon

infection. Such strains of mice become highly susceptible to Leishmania-
induced fatality if they lose either IFN-¥_ or the IFN-¥_ receptor, These mice
mount a TH2-type response to Leishmania infection; they produce high
levels of IL-4 and essentially no IFN-¥_. Thus, one difference between an
effective and an ineffective defense against the parasite is the development
of a TH1 response or a TH2 response.
Diseases Caused by ParasiticWorms (Helminths)
Unlike protozoans, which are unicellular and often grow within human cells,
helminths are large, multicellular organisms that reside in humans but do
not ordinarily multiply there and are not intracellular pathogens. Although
helminthes are more accessible to the immune system than protozoans,
most infected individuals carry few of these parasites; for this reason, the
immune system is not strongly engaged and the level of immunity generated
to helminths is often very poor. Parasitic worms are responsible for a wide
variety of diseases in both humans and animals. More than a billion people
are infected with Ascaris, a parasitic roundworm that infects the small
intestine, and more than 300 million people are infected with Schistosoma, a
trematode worm that causes a chronic debilitating infection. Several
helminths are important pathogens of domestic animals and invade humans
ingest contaminated food. These helminths include Taenia, a tapeworm of
cattle and pigs, and Trichinella, the roundworm of pigs that causes
trichinosis.
Several Schistosoma species are responsible for the chronic, debilitating, and
sometimes fatal disease schistosomiasis(formerly known as bilharzia). Three
species, are the major pathogens in humans, infecting individuals in Africa,
the Middle East, South America, the Caribbean, China, Southeast Asia,.A rise
in the incidence of schistosomiasis in recent years has paralleled the

increasing worldwide use of irrigation, which has expanded the habitat of
the freshwater snail that serves as the intermediate host for
schistosomes.the worms survive for up to 20 years. The schistosomules
would appear to be the forms most susceptible to attack, but because they
are motile, they can evade the localized cellular buildup of immune and
inflammatory cells. Adult schistosome worms also have several
uniquemechanisms that protect them from immune defenses. The adult
worm has been shown to decrease the expression of antigens on its outer
membrane and also to enclose itself in a glycolipid and glycoprotein coat
derived from the host, masking the presence of its own antigens. Among the
antigens observed on the adult worm are the host’s own ABO
blood-group antigens and histocompatibility antigens! The immune
response is, of course, diminished by this covering made of the host’s self-
antigens, which must contribute to the lifelong persistence of these
organisms. The relative importance of the humoral and cellmediated
responses in protective immunity to schistosomiasis
is controversial. These manifestations suggest that cytokines produced by a
TH2-like subset are important for the immune response: IL-4, which induces
B cells to class-switch to IgE production; IL-5, which induces bone-marrow
precursors to differentiate into eosinophils; and IL-3, which (along with IL-4)
stimulates growth of mast cells. Degranulation of mast cells releases
mediators that increase the infiltration of such inflammatory cells as
macrophages and eosinophils. The eosinophils express Fc receptors for IgE
and IgG and bind to the antibody-coated parasite. Once bound to the
parasite, an eosinophil can participate in antibody-dependent cell-mediated
cytotoxicity (ADCC), releasing mediators from its granules that damage the
parasite . One eosinophil mediator, called basic protein, is particularly

toxic to helminths. الجدول األخير لالطالع فقط
