Sunday 15 / 3 / 2015
©Ali Kareem 2014-2015
Name
:
______________________________
Class
:
_______________________________
مكتب اشور لالستنساخ
ANTI-VIRAL DRUGS
Lecture 7
Total lectures NO. 45
Dr. Mohammed Rashad

2
Antiviral Drugs
Dr. Mohammed Rashad
I. OVERVIEW
• Viruses are obligate intracellular parasites.
• They lack both a cell wall and a cell membrane, and they do not
carry out metabolic processes.
• Viral reproduction uses much of the host’s metabolic machinery,
and few drugs are selective enough to prevent viral replication
without injury to the host.
• II. TREATMENT OF RESPIRATORY VIRUS INFECTIONS
A.Neuraminidase inhibitors
• Orthomyxoviruses that cause influenza contain the enzyme neura-
minidase, which is essential to the life cycle of the virus.
• Viral neuraminidase can be selectively inhibited by the sialic acid
analogs,oseltamivirand zanamivir.
• Oseltamivir and zanamivir are effective against both Type A and
Type B influenza viruses.
• They do not interfere with the immune response to influenza A
vaccine.
• Administered prior to exposure, neuram inidase inhibitors prevent
infection, and, when administered within the first 24 to 48hours
after the onset of infection, they have a modest effect on the
intensity and duration of symptoms.
1. Mode of action:
• Oseltamivir and zanamivir analogs of the sialic acid substrate and
serve as inhibitors of the enzyme activity.
2. Pharmacokinetics:
• Oseltamivir is an orally active prodrug that is rapidly hydrolyzed by
the liver to its active form.

3
• Zanamivir is not active orally and is either inhaled or administered
intranasally.
• Both drugs are eliminated unchanged in the urine.
3. Adverse effects:
• The most common side effects of oseltamivir are gastrointestinal
(GI) discomfort and nausea.
• Zanamivir should be avoided in individuals with severe reactive
asthma or chronic obstructive respiratory disease, because
bronchospasm may occur with the risk of fatality.
B. Inhibitors of viral uncoating
• Amantadine and rimantadine
• is limited to influenza A infections(equally effective in both
treatment and prevention
• these drugsare 70 to 90 percent effective in preventing infection if
treatment is begun at the time of, or prior to, exposure to the
virus.
• Also, both drugsreduce the duration and severity of systemic
symptoms if started within the first 48 hours after exposure to the
virus.
1. Mode of action:
• Amantadine and rimantadine is to block the viral membrane
matrix protein,M2, which functions as a channel for hydrogen
ions.
• This channel is required for the fusion of the viral membrane with
the cell membrane that ultimately forms the endosome.
2. Pharmacokinetics:
• Both drugs are well absorbed orally.

4
• Amantadine penetrates into the central nervous system (CNS),
whereas rimantadine does not cross the blood-brain barrier.
3. Adverse effects:
• Minor neurologic symptoms include insomnia,dizziness, and
ataxia.
• More serious side effects, for example, hallucinations and seizures
(caution in patients with psychiatric problems)
• Rimantadine causes fewer CNS reactions
C. Ribavirin
• Ribavirin is a synthetic guanosine analog.
• Ribavirin is used in treating infants and young children with severe
RSV infections.
• Ribavirinis also effective in chronic hepatitis C infections when
used in combination with interferon-α.Ribavirin may reduce the
mortality and viremia of Lassa fever.
1. Pharmacokinetics:
• Ribavirin is effective orally and intravenously.
• Anaerosol is used in certain respiratory viral conditions such as
thetreatment of RSV infection.
2. Adverse effects:
• dose-dependent transient anemia.
• Elevated bilirubin has been reported.
• The aerosol may be safer, although respiratory function in infants
can deteriorate quickly after initiation of aerosol treatment.

5
III. TREATMENT OF HEPATIC VIRAL INFECTIONS
• hepatitis B and hepatitis C are the most common causes of chronic
hepatitis, cirrhosis, and hepatocellular carcinoma.
• Chronic hepatitis B may be treated with peginterferon α-2a, which
is injected subcutaneously once weekly. [Note: Interferon-α2b
injected intramuscularly or subcutaneously three times weekly]
• Oral therapy includes lamivudine, adefovir, entecavir,tenofovir, or
telbivudine.
• In the treatment of chronic hepatitis C, the preferred treatment is
the combination of peginterferon-α-2a or peginterferon-α-2b plus
ribavirin.
A.Interferon
• Interferon is a family of naturally occurring, inducible
glycoproteins that interfere with the ability of viruses to infect
cells.
• The interferons are synthesized by recombinant DNA technology.
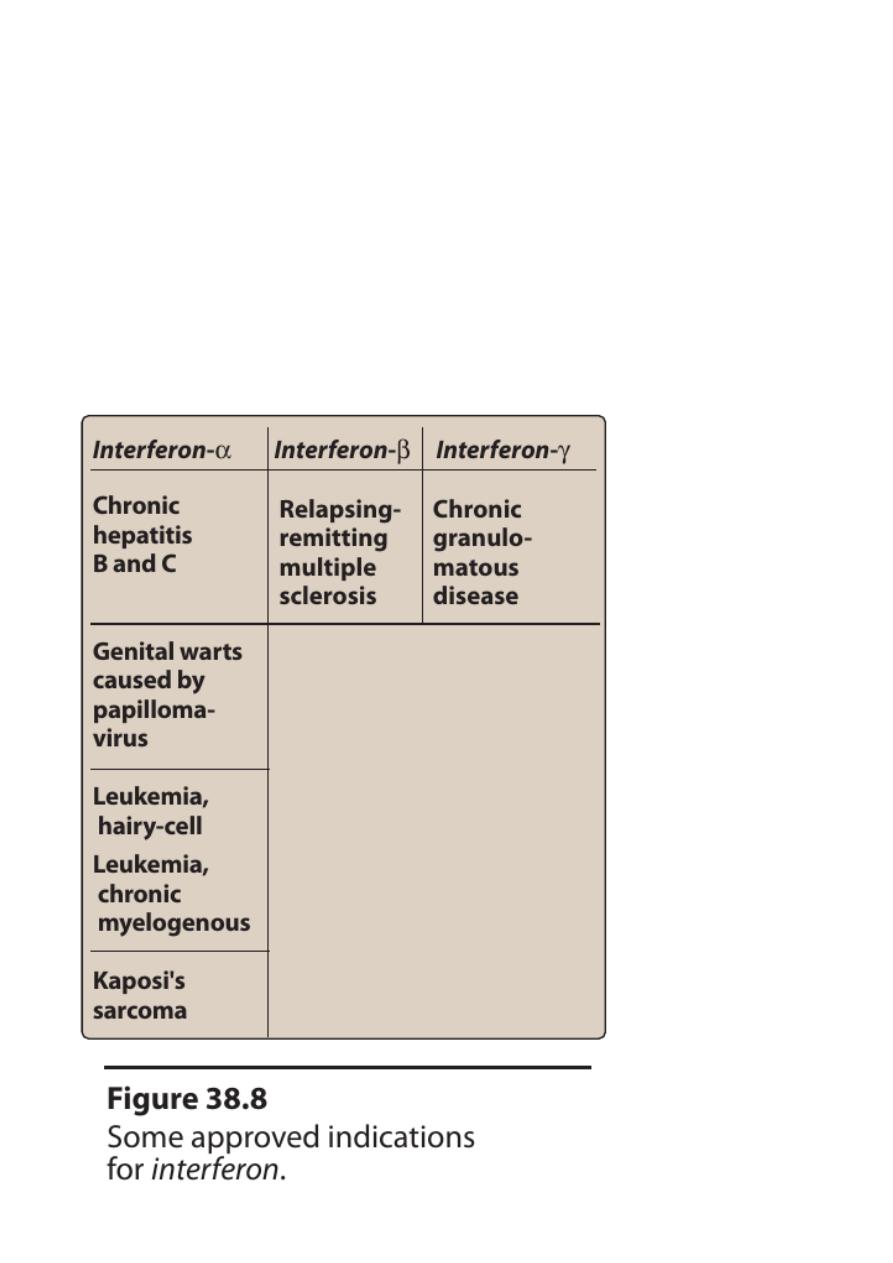
• At least three types of interferons exist, α, β, and γ.
One of the 15 interferon-α glycoproteins, interferon-α-2b, has been
approved for:
• treatment of hepatitis B and C
• condylomataacuminate
• hairy cell leukemia
• Kaposi sarcoma.
• Interferon-β has some effectivenessin the treatment of multiple
sclerosis. In so-called “pegylated” formulations. The larger
molecular size delays absorption, lengthens the duration of action,
and decreases its clearance.

6
1.Mode of action:
• induction of host cell enzymes that inhibit viral RNA translation,
leading to the degradation of viral mRNA and tRNA.
2.Pharmacokinetics:
• Interferon is not active orally
• but it may be administered intralesionally, subcutaneously, or
intravenously.
3.Adverse effects:
• flu-like symptoms on injection, such as fever, chills, myalgias,
arthralgias, and GI disturbances.
• Fatigue and mental depression are common.
• dose-limiting toxicities are:
• bone marrow suppression including granuloytopenia
• neurotoxicity characterized by somnolence and behavioral
disturbances
• severe fatigue and weight loss
• autoimmune disorders suchas thyroiditis
• and, rarely, cardiovascular problems such as congestive heart
failure.
• Acute hypersensitivity reactions and hepatic failure are rare.
B.Lamivudine
• This cytosine analog is an inhibitor of:
• hepatitis B virus (HBV) DNA polymerase
• human immunodeficiency virus (HIV) reverse transcriptase

7
C.Adefovir
• Adefovir is a nucleotide analog
• As with other agents, discontinuation of adefovir results in severe
exacerbation of hepatitis in about 25 percent of patients.
• The drug should be used cautiously in patients with existing renal
dysfunction.
D.Entecavir
• Entecavir is a guanosine analog approved for the treatment of
HBV infections.
• Entecavir has been shown to be effective against lamivudine-
resistant strains of HBV.
• Liver inflammation and scarring are improved.
E.Telbivudine
• Telbivudine is a thymidine analog that can be used in the
treatment of HBV.
• Unlike lamivudine and adefovir, telbivudine is not active against
HIV or other viruses.
• The dose must be adjusted in renal failure.
IV. TREATMENT OF HERPESVIRUS INFECTIONS
A. Acyclovir
• Acyclovir (acycloguanosine) is the prototypic antiherpetic
therapeutic agent.It has a greater specificity than vidarabine
against herpesviruses:
• Herpes simplex virus (HSV)
• varicella-zoster virus (VZV)
• Epstein-Barr virus

8
• It is the treatment of choice in HSV encephalitis.
• The most common use of acyclovir is in therapy for genital herpes
infections.
• It is also given prophylactically before bone marrow and after
heart transplants.
1. Mode of action:
• Acyclovir triphosphate competes with deoxyguanosine
triphosphate as a substrate for viral DNA polymerase and is itself
incorporated into the viral DNA, causing premature DNA-chain
termination.
• The drug is less effective against the host enzyme.
2. Pharmacokinetics:
• Administration of acyclovir can be by an intravenous (IV), oral, or
topical route.
• The drug distributes well throughout the body,including the
cerebrospinal fluid (CSF).
• Excretion into the urine occurs both by glomerular filtration and
by tubular secretion.
• Acyclovir accumulates in patients with renal failure.
3. Adverse effects:
• local irritation may occur from topical application
• headache, diarrhea, nausea, and vomiting may result after oral
administration.
• Transient renal dysfunction may occur at high doses or in a
dehydrated patient
• High-dose valacyclovir can cause GI problems and
thromboticthrombocytopenic purpura in patients with AIDS.

9
B. Cidofovir
• Cidofovir is approved for treatment of CMV-induce dretinitis in
patients with AIDS.
• Cidofovir is a nucleotide analog of cytosine (It inhibits viral DNA
synthesis)
• Cidofoviris available for intravitreal (injection into the eye’s
vitreous humor) and topical administration.
• Cidofovir produces significant toxicity to the kidney
• Neutropenia, metabolic acidosis.
• Probenecid must be co-administered with cidofovirto reduce the
risk of nephrotoxicity.
C. Ganciclovir
• Ganciclovir is an analog of acyclovir that has 8 to20 times greater
activity against CMV
• It is currently available for treatment of CMV retinitis in
immunocompromised patients and for CMV prophylaxis in
transplant patients.
1. Mode of action:
• Like acyclovir, ganciclovir is activated through conversion to the
nucleoside triphosphate.
• The nucleotide competitively inhibits viral DNA polymerase and
incorporated into the DNA, thereby decreasing the rate of chain
elongation.
2. Pharmacokinetics:
• Ganciclovir is administered IV and distributes throughout the
body, including the CSF.
• Excretion into the urineoccurs through glomerular filtration and
tubular secretion.

01
• Valganciclovir, like valacyclovir, valganciclovir has high oral
bioavailability, because rapid hydrolysis after oraladministration
leads to high levels of ganciclovir.
3. Adverse effects:
• severe, dose-dependentneutropenia.
Ganciclovir is carcinogenic as well as embryotoxic and teratogenicin
experimental animals.
D.Vidarabine (ara-A)
• Vidarabine is active against HSV‐0,HSV-2, and VZV
• its use is limited to treatment of immunocompromised patients
with herpetic and vaccinial keratitis and in HSV
keratoconjunctivitis. [Note: Vidarabine is only available as an
ophthalmic ointment.]
E.Trifluridine
• It is structurally very similar to thymidine.
• Trifluridineis active against HSV-1, HSV-2, and vaccinia virus.
• It is the drug of choice for treatment of HSV
• Because the triphosphate form of trifluridine can also incorporate
to some degree into cellular DNA, thedrug is considered to be too
toxic for systemic use.
• Therefore, the use of trifluridine is restricted to topical application
as a solution to the eye.
• A short half-life of approximately 12 minutes necessitates that the
drug be applied frequently.
• Side effects include a transient irritation of the eye and palpebral
(eyelid) edema.
V. OVERVIEW OF THE TREATMENT FOR HIV INFECTION

00
Prior to approval of zidovudine in 1987
• treatment of HIV infections focused on decreasing the occurrence
of opportunistic infections that caused a high degree of morbidity
and mortality in AIDS patients rather than on inhibiting HIV itself.
Today
• highly active regimen is employed that uses combinations of drugs
to suppress replication of HIV and restore the number of CD4+
cells and immunocompetence to the host.
This multidrug regimen is commonly referred to as “highly active
antiretroviral therapy,” or HAART.
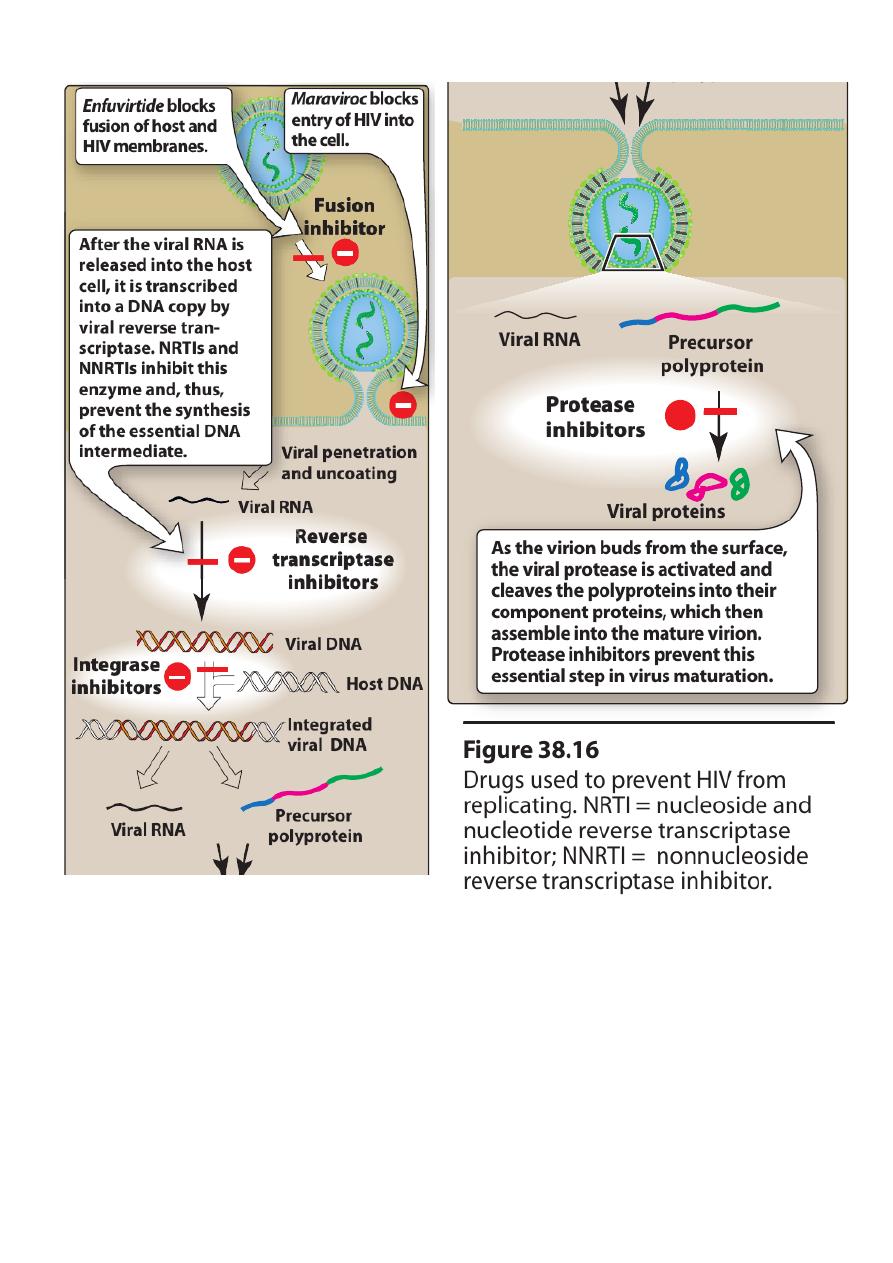
There are five classes of antiretroviral drugs
• each of which targets one of four viral processes.
• nucleoside and nucleotide reverse transcriptase inhibitors (NRTIs)
• nonnucleoside reverse transcriptase inhibitors (NNRTIs),
• protease inhibitors
• entry inhibitors
• integrase inhibitors
• The current recommendation for primary therapy
• administer two NRTIs with either a protease inhibitor, an NNRTI,
or an integrase inhibitor.
• Selection ofthe appropriate combination is based on
• avoiding the use of two agents of the same nucleoside analog
• avoiding overlapping toxicities
• patient factors, such as disease symptoms and concurrent
illnesses
• impact of drug interactions
02
• ease of adherence to a frequently complex administration
regimen.
• The goals of therapy:
• maximally and durably suppress viral load replication
• restore and preserve immunologic function
• reduce HIV-related morbidity and mortality
• improve quality of life.
03
Done by
Ali Kareem
