Sunday 12/ 4 / 2015
©Ali Kareem 2014-2015
Name
:
______________________________
Class
:
_______________________________
مكتب اشور لالستنساخ
ANTI-DIABETIC DRUGS
Lecture 9
Total lectures NO. 53
Dr. Mohammed Rashad

2
Insulin and Other Glucose-LoweringDrugs
I. OVERVIEW
• The pancreas is both an endocrine gland that produces the
peptide hormones insulin, glucagon, and somatostatin and an
exocrine gland that produces digestive enzymes.
• Hyperinsulinemia cause severe hypoglycemia.
• Diabetes mellitus can cause serious hyperglycemia.
retinopathy,nephropathy, neuropathy, and cardiovascular
complications may result.
• Administration of insulin or glucose-lowering agents can prevent
morbidity and reduce mortality
II. DIABETES MELLITUS
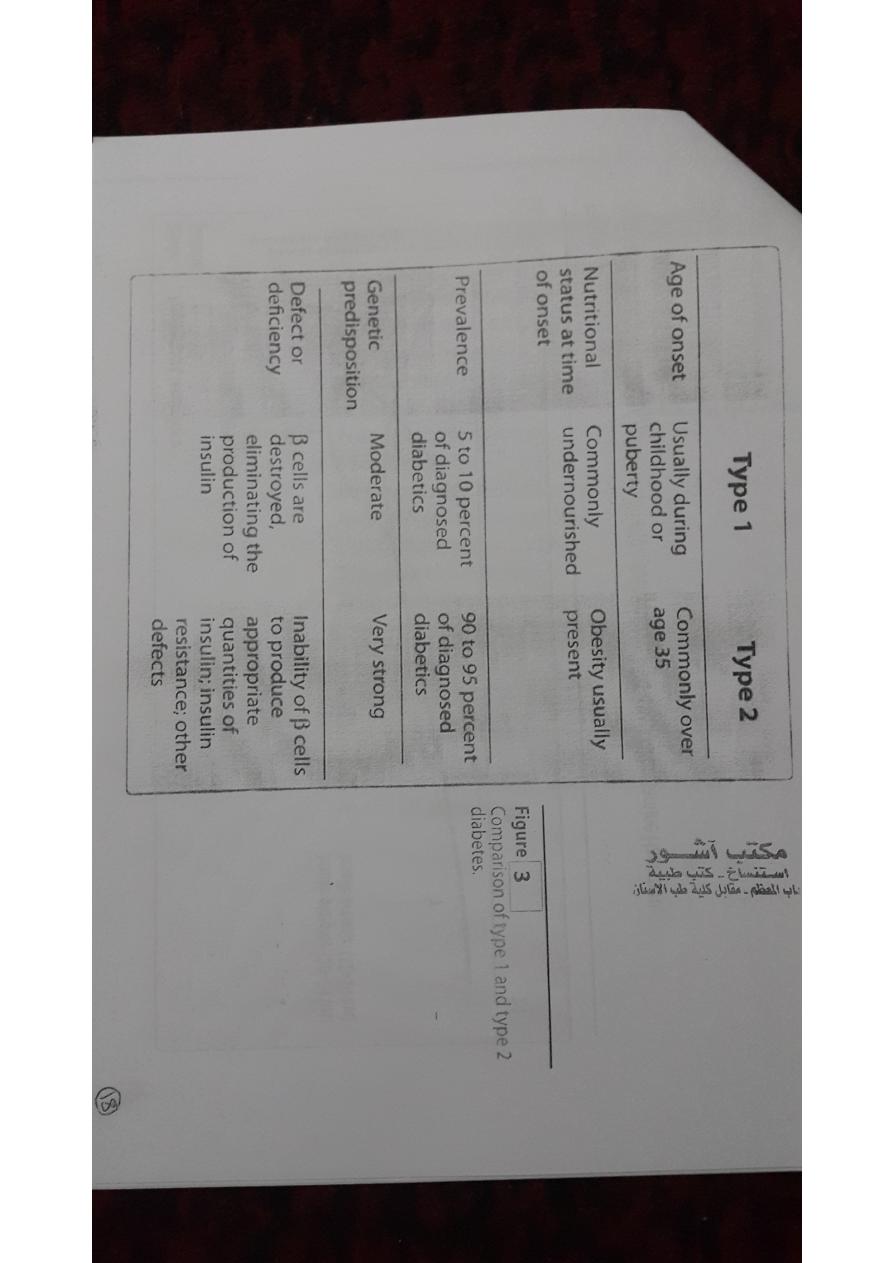
The American Diabetes Association (ADA) recognizes four clinical
classifications of diabetes:
• type 1 diabetes (formerly, insulin dependent diabetes mellitus)
• type 2 diabetes (formerly, non-insulin dependent diabetes
mellitus)
• gestational diabetes
• diabetes due to other causes (for example, genetic defects or
medications).
Gestationaldiabetes
• defined as carbohydrate intolerance
• Diet, exercise, and/or insulin administration are effective.
A.Type 1 diabetes
• Type 1 diabetic shows classic symptoms (polydipsia, polyphagia,
polyuria, and weight loss).

3
• Type 1 diabetics require exogenous insulin to avoid the catabolic
state that results from hyperglycemia and life-threatening
ketoacidosis.
Treatment:
• exogenous (injected) insulin to control hyperglycemia, avoid
ketoacidosis, and maintain acceptable levels of glycosylated
hemoglobin (HbA1c).
• The goal is to maintain blood glucose concentrations as close to
normal as possible and to avoid long-term complications.
• Continuous subcutaneous insulin infusion(also called the insulin
pump).
• Other methods of insulin delivery (transdermal, buccal, and
intranasal, are currently under investigation).
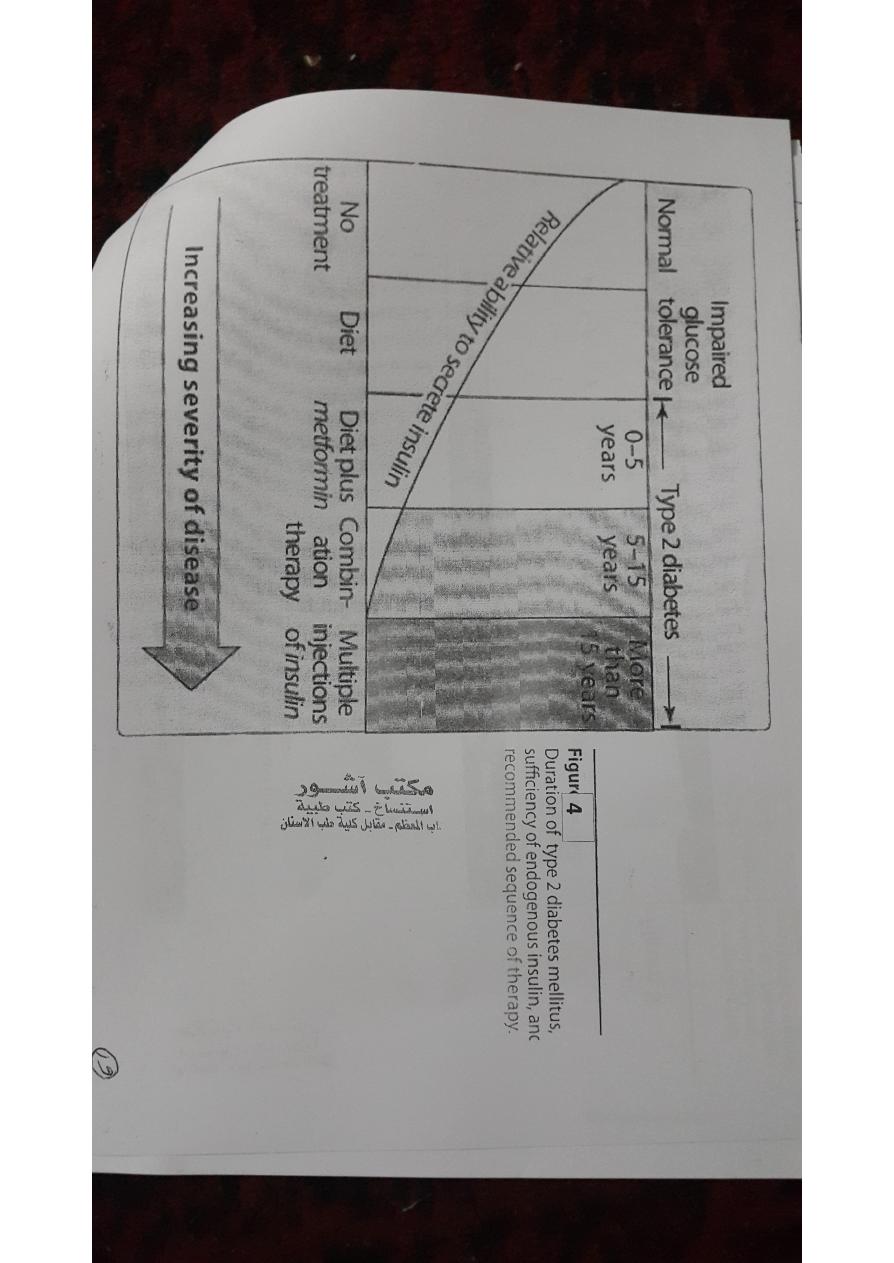
B.Type 2 diabetes
• Most diabetic patients have type 2 disease.
• Type 2 diabetes is influenced by genetic factors, aging, obesity,
and peripheral insulin resistance, rather than by autoimmune
processes or viruses
1.Cause: In type 2 diabetes
• Type 2 diabetes is frequently accompanied by the lack of
sensitivity of target organs to either endogenous or exogenous
insulin.
• This resistance to insulin is considered to be a major cause of this
type of diabetes.
2.Treatment:
• The goal is to maintain blood glucose concentrations within
normal limits and to prevent the development of long-term
complications.

4
• Weight reduction, exercise, and dietary modification decrease
insulin resistance and correct the hyperglycemia in some patients.
• However, most patients are dependent on oral glucose-lowering
agents.
As the disease progresses,β-cell function declines and insulin therapy is
often required.
III. INSULIN AND ITS ANALOGS
• Insulin is a polypeptide hormone consisting of two peptide
chainsthat are connected by disulfide bonds.
• It is synthesized as a precursor (proinsulin) that undergoes
proteolytic cleavage to form insulin and C-peptide.
• Measurement of circulating C-peptide provides a better index of
insulin levels.
A.Insulin secretion
• Secretion is most commonly triggered by high blood glucose,
which is taken up by the glucose transporter into the β cells of the
pancreas.
• There, it is phosphorylated by glucokinase, which acts as a glucose
sensor.
• The products of glucose metabolism enter the mitochondrial
respiratory chain and generate adenosine triphosphate (ATP).
• The rise in ATP levels causes a block of K+ channels, leading to
membrane depolarization and an influx of Ca2+.
• The increase in intracellular Ca2+ causes pulsatile insulin
exocytosis.
• The sulfonylureas and glinides owe their hypoglycemic effect to
the inhibition of K+ channels.
B. Sources of insulin

5
• Human insulin is produced by recombinant DNA technology using
special strains of Escherichia coli or yeast.
• Modifications of the amino acid sequence of human insulin have
produced insulins with different pharmacokinetic properties.
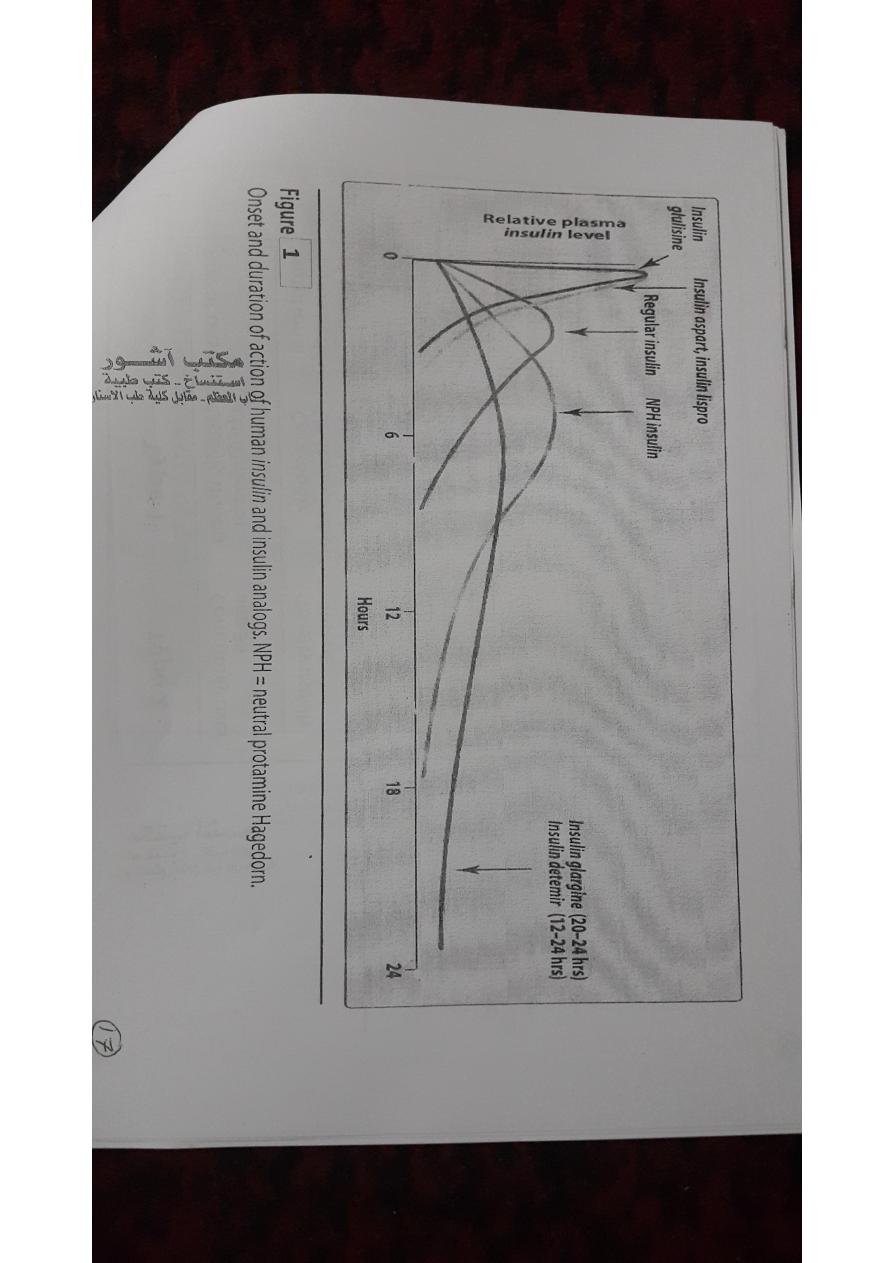
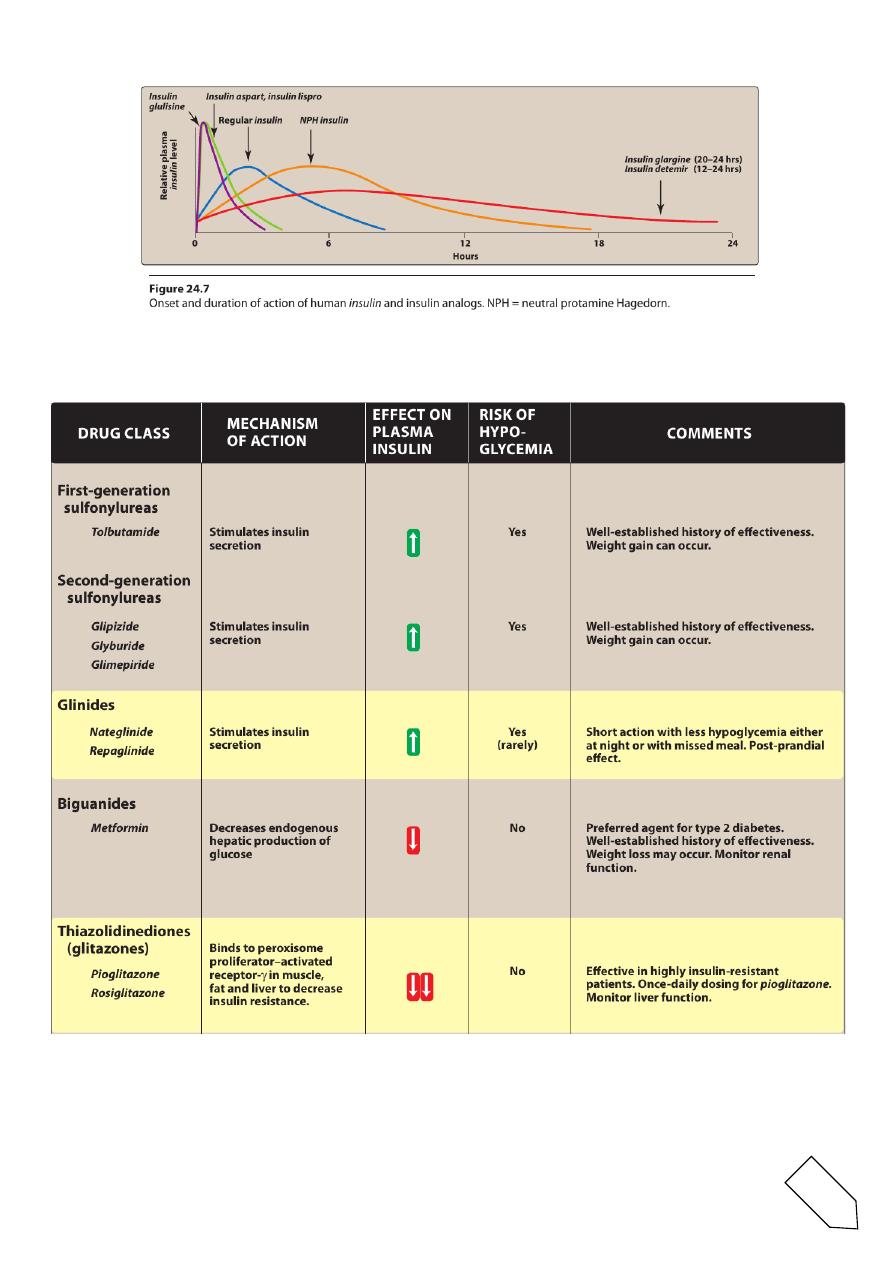
• For example, three such insulins, lispro, aspart,and glulisine, have
a faster onset and shorter duration of action than regular insulin.
• glargine and detemir are long-acting insulins and show prolonged,
flat levels of the hormone following injection.
C. Insulin administration
• insulin is administered by subcutaneous injection.
• In a hyperglycemic emergency, regular insulin is injected
intravenously (IV).
• Insulin preparations vary in their onset ofactivity and in duration
of activity.
• This is due to amino acid sequence.
• Dose, site of injection, blood supply, temperature, and physical.
• Insulin is inactivated by insulin protease in the liver and kidney.
D. Adverse reactions to insulin
• The symptoms of hypoglycemia are the most serious and common
adverse.
• Long term diabetic patients commonly do not produce adequate
amounts ofthe counter-regulatory hormones (glucagon,
epinephrine, cortisol, and growth hormone).
• Other adverse reactions include weight gain, lipodystrophy (less
common with human insulin), allergic reactions, and local
injection site reactions.
IV. INSULIN PREPARATIONS AND TREATMENT

6
A.Rapid-acting and short-acting insulin preparations
• Four preparations fall into this category: regular insulin, insulin
lispro,insulin aspart, and insulin glulisine.
• Regular insulin is a short-acting,soluble, crystalline zinc insulin
given subcutaneously (or IV in emergencies).
• Regular insulin, insulin lispro, and insulin aspart are pregnancy
category B.
• The lispro,aspart, and glulisine forms are classified as rapid-acting
insulins.
• Peak levels of insulin lispro are seen at 30 to 90minutes with 50 to
120 minutes for regular insulin.
• They are administered to mimic the prandial (mealtime) release of
insulin, and they are used with a longer-acting insulin.
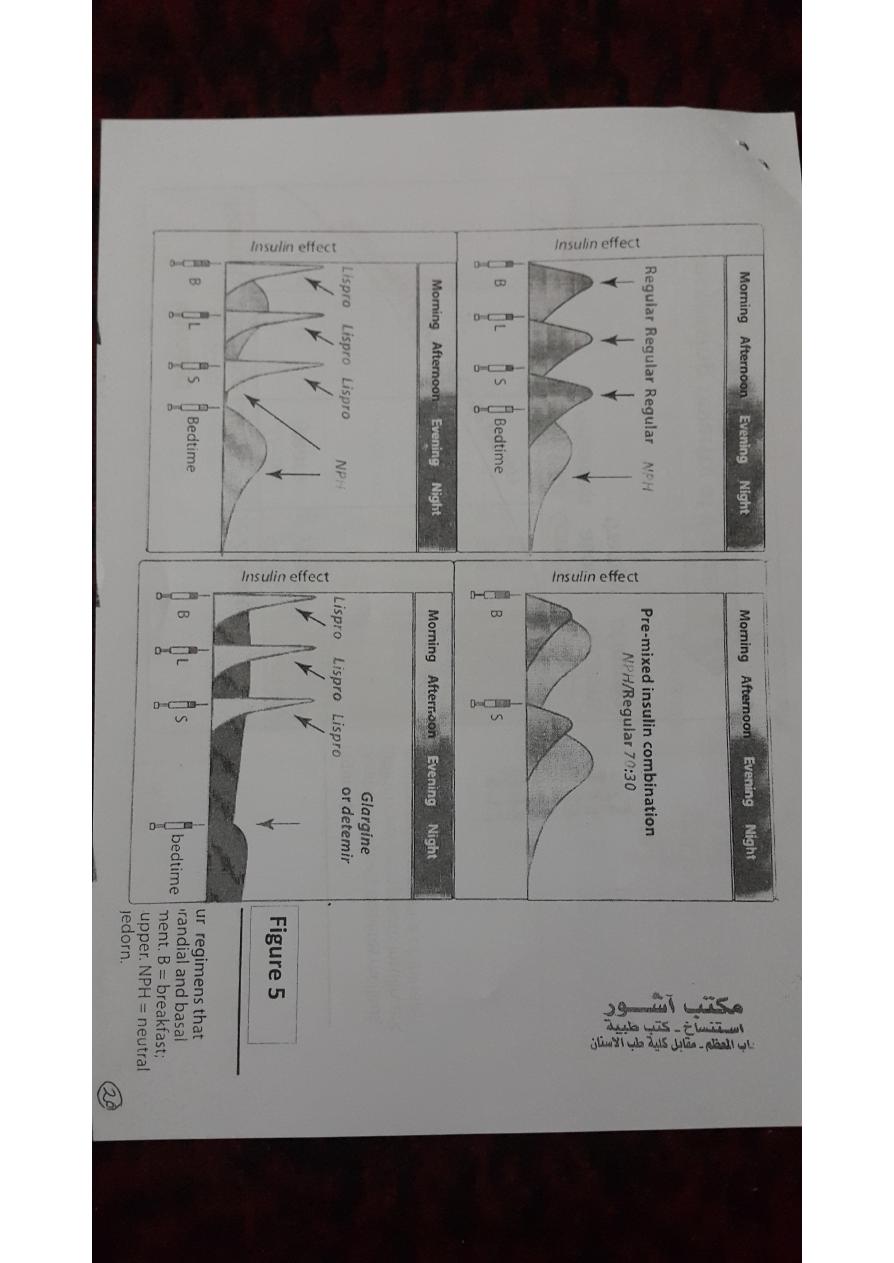
B.Intermediate-acting insulin
• Neutral protamine Hagedorn (NPH) insulin is a suspension of
crystalline zinc insulin with the positively charged polypeptide
protamine (isophane).
• NPH insulin is useful in treating all forms of diabetes except
diabetic ketoacidosis and emergency hyperglycemia.
• It is used for basal control and is usually given along with rapid- or
short-acting insulin for mealtime control.
• neutral protamine lispro (NPL) insulinis used only in combination
with insulin lispro.
• C.Long-acting insulin preparations
1.Insulin glargine:
• It is slower in onset than NPH insulin and has a flat, prolonged
hypoglycemic effect withno peak.
2.Insulin detemir:

7
• Insulin detemir has a fatty-acid side chain results in long-acting
properties
• Neither insulin detemir nor insulin glargine should be mixed in the
same syringe with other insulins.
D.Insulin combinations
• 70-percent NPH insulin plus 30-percent regular insulin
• 50 percentof each of these
75-percent NPL insulin plus 25-percent insulin lispro
E.Standard treatment versus intensive treatment
• standard treatment: injection of insulin twice daily.
• intensive treatment: injections of insulin (three or more times
daily).
• The frequency of hypoglycemic episodes, coma, and seizures is
higher with intensive treatment regimens.
• patients on intensive therapy show a significant reduction in such
long-term complications (retinopathy, nephropathy, and
neuropathy) compared to standard care.
• Intensive therapy does not reduce the macrovascular
complications of diabetes.
V. SYNTHETIC AMYLIN ANALOG
• Pramlintide is a synthetic amylin analog that is indicated asan
adjunct to meal time insulin therapy in patients with type 1 and
type 2 diabetes.
• pramlintide delays gastric emptying, decreases postprandial
glucagon secretion, and improves satiety.
• Pramlintide is administered by subcutaneous injection
immediately prior to meals.

8
• the dose of rapid- or short-acting insulin should be decreased by
50 percent prior to meals.
• Pramlintide may not be mixed with any insulin preparation.
• Adverse effects are mainly gastrointestinal and consist of nausea,
anorexia, and vomiting.
VI. ORAL AGENTS: INSULIN SECRETAGOGUES
These agents are useful in the treatment of patients who have type 2
diabetes but who cannot be managed by diet alone
A.Sulfonylureas
• These agents are classified as insulin secretagogues, because they
promote insulin.
• The primary drugs used today are the second-generation drugs
glyburide, glipizide, and glimepiride.
1.Mechanism of action:
• stimulation of insulin release by blocking the ATP-sensitive K+
channels, resulting in depolarization and Ca2+influx
• reduction in hepatic glucose production
• increase in peripheral insulin sensitivity.
2. Pharmacokinetics and fate:
• Given orally, these drugs bind to serum proteins, are metabolized
by the liver, and are excreted by the liver or kidney.
• The duration of action ranges from 12 to 24 hours.
3. Adverse effects:
• Weight gain, hyperinsulinemia, and hypoglycemia.These drugs
should be used with caution in patients with hepatic orrenal
insufficiency

9
• Glyburide has minimal transfer across the placenta and may be a
reasonably safe alternative to insulin therapy for diabetes in
pregnancy.
B. Glinides
• This class of agents includes repaglinide and nateglinide.
• Although they are not sulfonylureas, they have common actions.
1. Mechanism of action:
• They bind to a distinct site on the sulfonylurea receptor of ATP-
sensitive potassium channels
• However, in contrast to the sulfonylureas, the glinides have a
rapid onset and a short duration of action.
• They are particularly effective in the early release of insulin that
occurs after a meal and are categorized as postprandial glucose
regulators.
2. Pharmacokinetics and fate:
• These drugs are well absorbed orally.
• Both glinides are metabolized to inactive products by the liver and
are excreted through the bile.
3. Adverse effects:
• hypoglycemia,lower than that with the sulfonylureas.
• Repaglinidecauses severe hypoglycemia in patients taking the
lipid-lowering drug gemfibrozil.
• Weight gain is less of a problem.
These agents must be used with caution in hepatic impairment
VII. ORAL AGENTS: INSULIN SENSITIZERS
• the biguanides and thiazolidinediones improve insulin action.

10
• These agents improve target-cell response to insulin without
increasing insulin secretion.
A. Biguanides
• Metformin increases glucose uptake and use by target tissues,
thereby decreasing insulin resistance.
• Metformin does not promote insulin secretion. Therefore, the risk
of hypoglycemia is far less than that with sulfonylurea agents.
1. Mechanism of action:
• The main mechanism of action of metforminis:
• reduction of hepatic glucose output by inhibiting
hepaticgluconeogenesis.
• Metformin slows intestinal absorption of sugars
• improves peripheral glucose uptake and utilization.
• An important property of this drugis its ability to modestly reduce
hyperlipidemia.
• Metformin as the drug of choice for newly diagnosed type 2
diabetics.
2. Pharmacokinetics and fate:
• Metformin is well absorbed orally, is not bound to serum proteins,
and is not metabolized.
• Excretion is via the urine.
3. Adverse effects:
• These are largely gastrointestinal.
• Metformin is contraindicated in renal and/or hepatic disease and
in diabetic ketoacidosis.

11
• It should be discontinued in acute myocardial infarction,
exacerbation of congestive heart failure, and severe infection.
• Metformin should be used with caution in patients older than age
80 years and in those with a history of congestive heart failure or
alcohol abuse.
• Metformin should be temporarily discontinued in patients
undergoing IV radiographic contrast agents.
• Rarely, potentially fatal lactic acidosis.
Long-term use may interfere with vitamin B12 absorption
4.Other uses:
• the treatment of type 2 diabetes
• metformin is effective in the treatment of polycystic ovary disease
(lower insulin resistance) resulting in ovulation and possibly
pregnancy.
B.Thiazolidinediones (glitazones)
• Troglitazone was the first of these to be approved for the
treatment of type 2 diabetesbut was withdrawn after a number of
deaths from hepatotoxicity.
The two members of this class currently available are pioglitazone and
rosiglitazone.
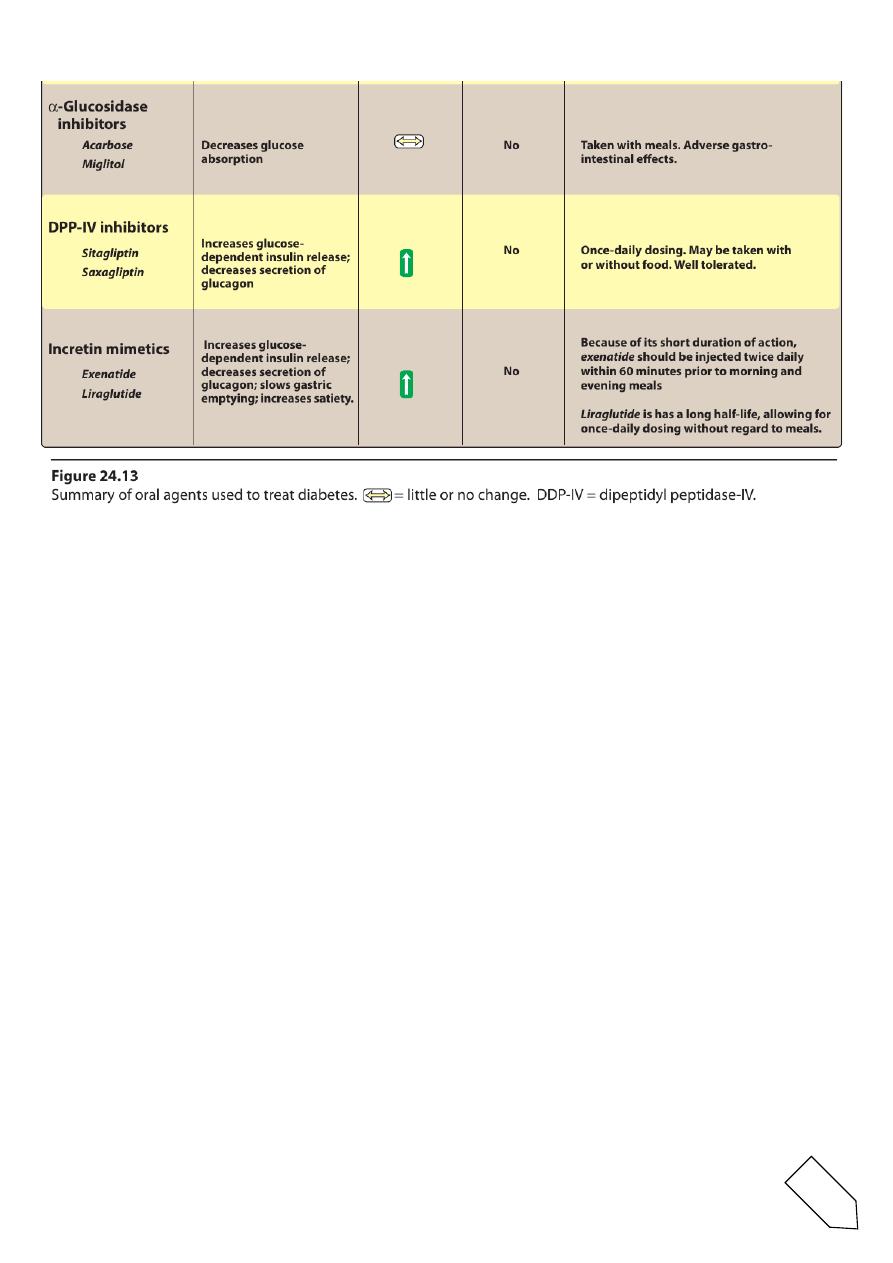
VIII. ORAL AGENTS: α-GLUCOSIDASE INHIBITORS
Acarbose and miglitol
are orally active drugs used for the treatment of patients with type 2
diabetes.
A.Mechanism of action
• They act by delaying the digestion of carbohydrates (reversibly
inhibiting membrane-bound α-glucosidase in the intestinal brush
border).

12
• This enzyme is responsible for the hydrolysis of oligosaccharides
to glucose and other sugars.
• Acarbose also inhibits pancreatic α-amylase,thereby interfering
with the breakdown of starch to oligosaccharides.
• these drugs neither stimulate insulin release nor increase insulin
action in target tissues.
B.Pharmacokinetics and fate
• Acarbose is poorly absorbed. It is metabolized primarily by
intestinal bacteria, and some of the metabolites are absorbed and
excreted into the urine.
C.Adverse effects
• The major side effects are flatulence, diarrhea, and abdominal
cramping.
• Patients with inflammatory bowel disease, colonic ulceration, or
intestinal obstruction should not use these drugs.
IX. ORAL AGENTS: DIPEPTIDYL PEPTIDASE-IV INHIBITORS
Sitagliptin and saxagliptin
• orally active dipeptidyl peptidase-IV (DPP-IV) inhibitors used for
the treatment of patients with type 2 diabetes.
A.Mechanism of action
• These drugs inhibit the enzyme DPP-IV, which is responsible for
the inactivation of incretin hormones.
• Prolonging the activity of incretin hormones results in increased
insulinrelease and a reduction in secretionof glucagon.
• DPP-IV inhibitors may be used as monotherapy or in combination
with a sulfonylurea, metformin, glitazones, or insulin.
B.Pharmacokinetics and fate

13
• The DPP-IV inhibitors are well absorbed after oral administration.
• The majority of sitagliptin is excreted unchanged in the urine.
Saxagliptin is metabolized via CYP4503A4/5 to an active
metabolite.
• The primary route of elimination for saxagliptin and the
metabolite is renal.
• Dosage adjustments for both DPPIV inhibitors are recommended
for patients with renal dysfunction.
C.Adverse effects
• nasopharyngitis and headache are the most common adverse
• Pancreatitis has occurred with use of sitagliptin.
• ketoconazole, and clarithromycin, may increase levels of
saxagliptin.
X. INCRETIN MIMETICS
• Oral glucose results in a higher secretion of insulin than occurs
when an equal load of glucose is given IV.
• This effect is referred to as the “incretin effect” and is markedly
reduced in type 2 diabetes.
• The incretin effect occurs because the gut releases incretin
hormones, notably GLP-1 and glucose dependent insulinotropic
polypeptide, in response to a meal.
• Incretin hormones are responsible for 60 to 70 percent of
postprandial insulin secretion.
Exenatide and liraglutide
• are injectable incretin mimetics used for the treatment of patients
with type 2 diabetes.
• These agents are used as adjunct therapy in patients on a
sulfonylurea, metformin, a glitazone, or a combination.

14
A.Mechanism of action
• The incretinm imetics are analogs of GLP-1 acting as GLP-1
receptor agonists.
• These agents:
• improve glucose-dependent insulin secretion
• slow gastric emptying time
• decrease food intake
• decrease postprandial glucagon secretion
• promote β-cell proliferation.
• Consequently, weight gain and postprandial hyperglycemia are
reduced, and HbA1c levels decline.
B.Pharmacokinetics and fate
• exenatide and liraglutide must be administered subcutaneously.
• Liraglutide is highly protein bound and has a long half life, allowing
for once-daily dosing without regard to meals.
• Exenatide is eliminated mainly via glomerular filtration and has a
much shorter halflife (injected twice daily within 60 minutes prior
to morning and evening meals).
C.Adverse effects
• the main adverse effects (nausea, vomiting, diarrhea, and
constipation).
• patients may form antibodies to these agents.
• Exenatide and liraglutide have been associated with pancreatitis.
Liraglutide causes thyroid C-cell tumors in rodents
15
16
Done by
Ali Kareem