Sunday 19 / 4 / 2015
©Ali Kareem 2014-2015
Name
:
______________________________
Class
:
_______________________________
"Pharmacology
مكتب اشور لالستنساخ
ANTI-CANCER DRUGS
Lecture 11
Total lectures NO. 56
Dr. Samer Matloub

Anticancer Drugs
Page 2
Antibiotics for cancer therapy
They're natural substances that inhibit DNA & RNA synthesis; they behave as both
phase specific (Bleomycin) & phase non specific agents they include:
Dactinomycin, Daunarubicin, Doxorubicin&( Amsacrine which is similar),
Plicamycin & Mitomycin, epirubicin & the related Mitozantrones ( Idarubicin &
Aclarubicin).
The antibiotics depress BM, cause GIT upsets& stomatitis, Alopecia,
cardiomyopathy (Daunarubicin & Doxorubicin), pulmonary fibrosis & skin rashes
(Bleomycin). The effects of some are radiomimetic & the use of radiation causes
additive toxicity.
Dactinomycin (ActinomycinD)
Mechanism of action
: the drug intercalates between guanine cytosine base
pairs of the DNA forming a stable drug DNA complex. At high doses may hinder
DNA synthesis & may cause strand breaks.
Uses:
adjuvant chemotherapy with vincristine & Methotrexate in Rx of Wilm's
tumor, also used in choriocarcinoma & soft tissue sarcoma.
Kinetics:
metabolized in the liver, the drug & its metabolites are excreted in bile &
urine, the drug is given I.V.
Side effects:
B.M. (Bone Marrow) depression, extravasations, GIT upsets,
alopecia & immunosuppression.

Anticancer Drugs
Page 3
Doxorubicin
(Adriamycin)
& Daunorubicin
(Danomycin)
Classified as Anthracycline antibiotics, doxorubicin is the hydroxylated form of
Daunorubicin.
Site of action (Mechanism of action):
1. Intercalation with DNA, the drug inserts non-specifically between base pairs
causing local uncoiling & blocking of DNA & RNA synthesis.
2. Binding to cell membrane altering the function of transport process coupled to
phosphotidyl inositol activation (IP
3
).
3. Generation of O
2
radicals (formation of O
-
2
& H
2
O
2
) causing single strand
breaks in DNA.
Tissues with adequate superoxide dismutase & glutathione peroxidase are
protected while tissues having low dismutase are more liable for injury.
Heart & tumor cells have low SOD (superoxide dismutase), the heart also lacks
catalase (which is responsible for disposition of H
2
O
2
), & thus these drugs will
affect heart cells as well as tumor cells leading to cardiac toxicity.
(cardiomyopathy).
Uses:
1. Doxorubicin is most important & widely used anticancer it is used for: breast
CA, lymphomas, sarcomas, lung CA, stomach CA & thyroid CA.
2. Daunorubicin is used for Acute Myeloid Leukemia (AML).
3. Both drugs are given I.V., inactivated in GIT (not given orally) don't penetrate
CNS, extensively metabolized & excreted mainly in bile.
Adverse effects:
cardiac toxicity (irreversible & dose dependant), alopecia &
infertility.

Anticancer Drugs
Page 4
Bleomycin
Cycle specific causes cells to accumulate in G
2
phase; it causes scission of DNA by
an oxidation process by forming O
-
2
& OH
-
radicals
(superoxide & hydroxyl radicals).
Uses:
1. Testicular CA together with Vinblastin & cisplastin (etoposide maybe used
instead of vinblastin).
2. Head & neck CA. 3. Lymphoma. 4. Hodgkin's disease. 5. Cervical CA.
Given I.M., S.C., I.V. & intraperitoneally. Excreted unchanged by kidney.
Adverse effects:
1. Pulmonary toxicity leads to fatal pulmonary fibrosis.
2. Hypertrophic skin damages: hyper pigmentation of hands, nails & alopecia.
3. High incidence of fever chills.
4. B.M. depression (rare & mild).
Plicamycin (Mithramycin)
Acts through restriction of DNA-directed RNA synthesis, it has specific toxicity to
osteoclast preventing their resorption & also lowers Ca
++
concentration in
hypercalcemia especially those with bone tumors.
Adverse effects:
1.
Coagulopathy, haemorrhage.
2.
Liver & renal toxicity.
3.
B.M. depression.
4.
Nausea & vomiting.

Anticancer Drugs
Page 5
Antimetabolites
Are phase specific, act during S-phase of cell cycle.
Are structurally related to normal cellular components, they generally interfere
with availability of normal purine & pyrimidine, nucleotide precursors by
inhibiting their synthesis or by competing with them in DNA or RNA synthesis.
These include:
1.
A folic acid antagonist: Methotrexate (MTX).
2.
Purine antagonists: mercaptopurine 6-MP, Azothiopurine and
thioguanine, Fludarabine, cladribine.
3.
Pyrimidine antagonists: capecitabine, Fluorouracil (5FU), cytarabine,
& Gemcitabine.
Fludarabine: used in low grade non Hodgkin and CLL.
Cladribine: used as Fludarabine and in hairy cell leukemia
Adverse effect:
1. GIT upset (ulceration, mucositis).
2. B.M. depression.
3. Renal impairment potentiates their toxicity.
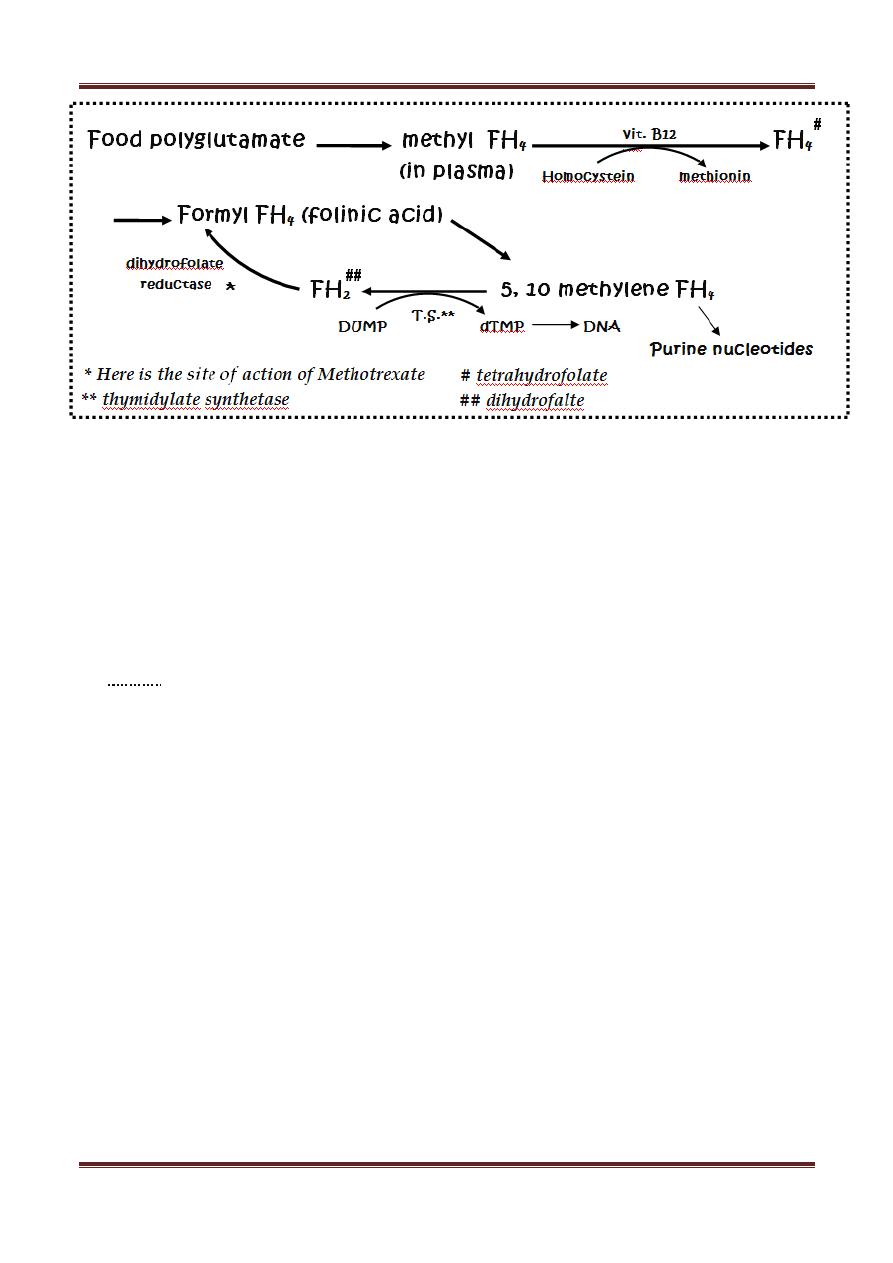
Methotrexate
Mechanism of action:
structurally related to folic acid, it acts as folic acid
antagonist by inhibiting (dihydrofolate reductase) which is responsible for
conversion of folic acid into active form (tetrahydrofolic acid) which is important
in synthesis of amino acids & nucleic acids.
Anticancer Drugs
Page 6
Methotrexate enters cells by active transport process; it has strong affinity to FH
2
reductase. This inhibitory step by MTX can be bypassed by giving FOLINIC acid
(leucovorin) & this is called "leucovorin rescue" which means B.M. rescue.
The consequences of ↓ FH
4
leads to ↓ biosynthesis of thymidilic acid, amino acids
(meth. & serine) & purines (adenine & guanine) → ↓ DNA, RNA synthesis, ↓
protein synthesis → cell death.
Uses:
in combination with other drugs, MTX is effective in :
1. Acute lymphoid leukemia.
2. Lymphoma.
3. Osteogenic sarcoma & choriocarcinoma, here MTX is curative, high doses of
MTX followed by leucovorin to rescue B.M.
4. Head & neck carcinoma.
5. Breast carcinoma.
6. Gastric & bladder CA.
7. Carcinomatous meningitis (low dose).
8. As immunosuppressive agent as a single agent for Rheumatoid arthritis &
psoriasis (also low dose).
Anticancer Drugs
Page 7
Kinetics:
1. Rapidly absorbed from gut.
2. Given I.M., I.V., intrathecally because it poorly penetrates CSF.
3. It's metabolized inside the cells to polyglutamate derivatives; they inhibit FH
2
reductase & remain in cells even in the absence of the extracellular drug (i.e.
the effect of MTX lasts for long time after it's been given).
4. MTX also undergoes hydroxylation (7-OH metabolites) both (MTX+
metabolites) are excreted in urine.
5. 7-OH metabolite has ↓ water solubility → crystal urea, therefore, good
hydroxylation & alkylinization of urine is important to avoid renal toxicity.
Adverse effects:
most frequently are stomatitis, B.M. suppression, alopecia, N &
V (Nausea & Vomiting), diarrhea, erythema, rash & urticaria. Some of these can
be prevented or ↓ by leucovorin (but dose should be minimal to avoid
interference with antitumor action of MTX). Leucovorin is taken by normal cells
more readily than cancer cells.
Other toxicities:
Renal, hepatic, pulmonary & neurologic toxicity (intrathecally
→ meningitis-like picture).
Contraindications (CI):
pregnancy because it causes abortion & it's teratogenic.
6-Mercaptopurine (6-MP)
It's a thiol analog of hypoxanthine. Azothioprine (an Immunosupp.) exerts its
effects after conversion to 6-MP.
Mechanism of action (site of action):
it penetrates target cells & is converted
to the corresponding nucleotide (6-MP ribose phosphate also known as Thio-IMP)
this process is catalyzed by HGPRT (Hypoxanthine Guanine Phospho Ribosyl
Transferase).
This Thio-IMP can feed back to inhibit the 1
st
step of de-novo purine synthesis. In
addition, there'll be dysfunction of DNA & RNA resulting from the incorporation of
guanylate analogs generated from the unnatural nucleotide.
Anticancer Drugs
Page 8
Uses:
for maintenance of remission in ALL (Acute Lymphoid Leukemia), absorption
by the oral route is erratic, doesn't penetrate the CNS. Metabolized in the liver to
thio uric acid by (Xanthine oxidase). Allopurinol therefore; ↑ toxicity of 6-MP. The
metabolite & the drug are excreted in urine.
Adverse effects:
N & V, diarrhoea, B.M. depression, (the chief toxicity)
hepatotoxicity.
6-Thioguanine (6-TG)
Another purine analog, used primarily in Rx of Acute Non Lymphocytic Leukemia in
combination with daunorubicine & cytarabine. Like 6-MP it must be converted to
the corresponding nucleotide from which will inhibit purine synthesis.
6-TG can also be incorporated in the NA & RNA. Very little of 6-TG is metabolized
to Thio uric acid (therefore Allopurinol doesn't potentiate the toxicity of 6-TG).
Other toxicities are similar to 6-MP.
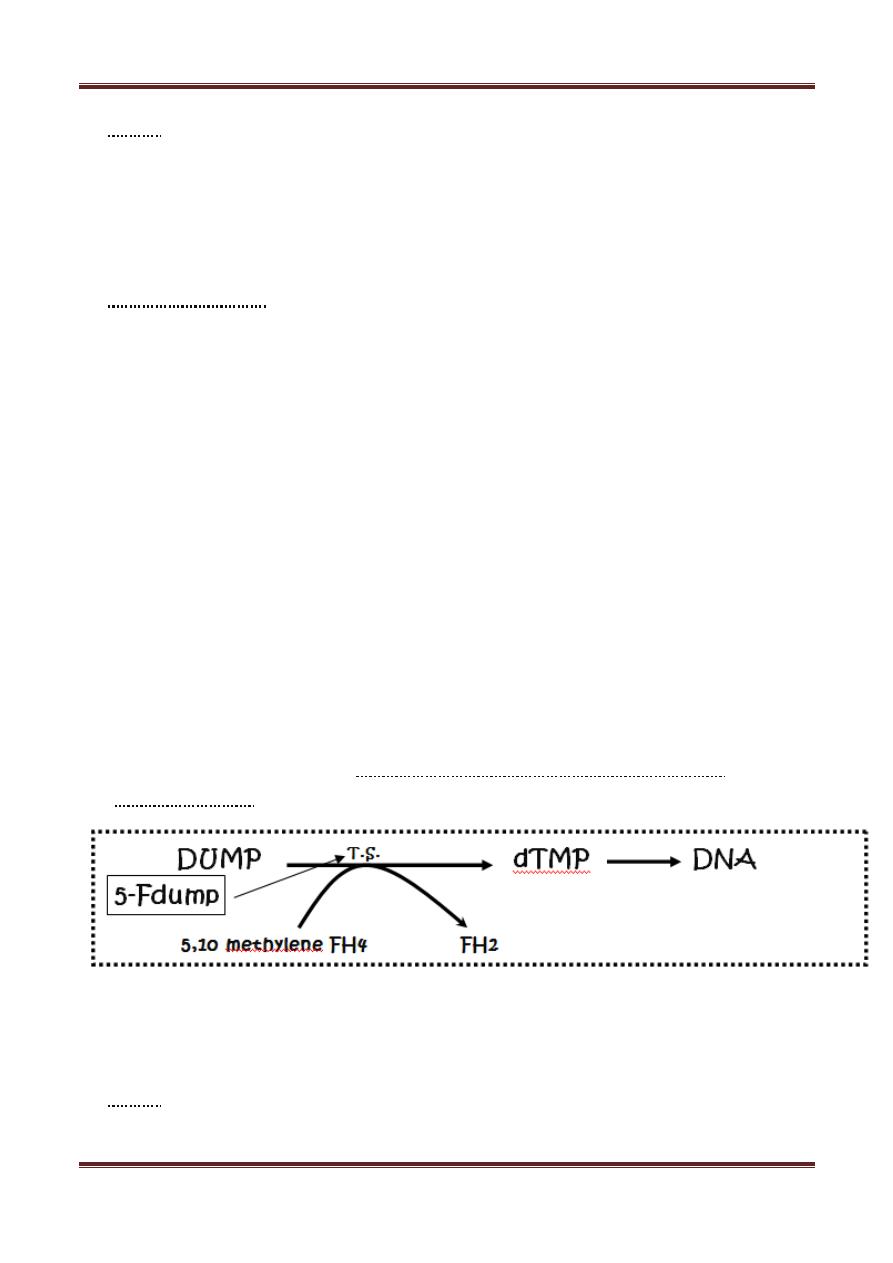
5-Fluorouracil (5-FU)
It's a pyrimidine analog. To be cytotoxic 5 FU is converted to the corresponding
deoxynucleotide (5-F dump) which competes with dump for thymidylate
synthetase (T.S.)
5-F dump acts as pseudosubstrate entrapped with the enzyme that can't proceed
to products. DNA synthesis ↓ because of thymidine lack. 5 FU is also incorporated
in the RNA.
Uses:
solid tumors, colorectal CA, breast & ovarian CA, pancreatic & gastric CA.
Adjuvant therapy with Levamisole improves survival of colon CA.

Anticancer Drugs
Page 9
Adverse effects:
severe toxicity to GIT if given orally, so taken I.V. Penetrates
well to the CSF. Metabolized in the liver to CO
2
. Toxicity includes beside the GIT,
BM depression & hand foot dermopathy.
Cytarabin (Ara-C)
A pyrimidine antagonist, it has to be converted to corresponding nucleotide (Ara-
CTP) in order to be cytotoxic. It is S phase specific & it's also incorporated into
DNA & can terminate chain elongation.
Uses:
AML (Acute Myeloid Leukemia) in combination with 6-TG & Daunorubicin.
Ara-C is ineffective orally, is given I.V., doesn't penetrate to the CSF (can be
injected intrathecally).
Adverse effects:
N & V, diarrhea, severe B.M., suppression (primarily
granulocytopenia) & hepatotoxicity.
Capecitabine:
prodrug, extensively metabolized in the liver to many intermediates that is finally
hydrolyzed by thymidine phosphorylase to fluorouracil (5-FU) in the tumor cell
Uses:
metastatic breast cancer either as a single agent or in combination with
other taxans (docetaxel). Recently approved for use in treatment of metastatic
colo-rectal cancer (combination with oxaliplatin & irinotecan)
Toxicity:
mylosuppression
Gemcitabine:
Approved for pancreatic cancer , non small cell lung cancer, bladder cancer
Main toxicity: mylosuppression
Phosphorylated to triphosphate form, the end result is inhibition of DNA synthesis.

Anticancer Drugs
Page 10
Fludarabine
It's un-natural purine nucleotide, its triphosphate incorporated in both DNA & RNA
decreasing their synthesis & function.
Uses:
CLL (chronic Lymphoid Leukemia) may replace chlorambucil. Hairy cell
leukemia. It's given I.V., causes Myelosuppression (dose-limited toxicity).
Miscellaneous
Procarbazine
Inhibits DNA & RNA synthesis. It's part of the MOPP for Hodgkin's disease. It is
given orally & parenterally, penetrates the CSF, excreted in urine together with its
metabolite.
Adverse effects:
BM depression (Major toxicity), GIT & neurotoxicity. It inhibits
MAO (contraindicated with Tyramine contained food) it induces Disulfiram
reaction with Alcohol. It's both mutagenic & teratogenic (cause non-Lymphocytic
leukemia).
L-Asparaginase
It's derived from bacteria, it catalyses the deamination of Asparagine → Aspartic
acid & ammonia. Neoplastic cells require
an extra source
of asparagine to support
growth & function. The drug will hydrolyze blood Asparagine thus deprives the
tumor cells of their nutrient required for protein synthesis.
Uses:
for ALL in combination with vincristine & prodinsolone. It is given I.V. or I.M.
not given orally (destroyed by gastric acidity).
Toxicity:
hypersensitivity reactions → ↓ clotting factors & liver abnormalities,
also Pancreatitis, coma, seizures due to ammonia toxicity.
