
Nephrolithiasis
Dr. Montadhar Almadani

1. Calcium oxalate (dihydrate and monohydrate):
70%.
2. Calcium phosphate (hydroxyapatite): 20%.
Composition of Renal Stones

3. Mixed calcium oxalate and calcium phosphate: 11% to 31%.
4. Uric acid: 8%.
5. Magnesium ammonium phosphate (struvite): 6%.
6. Cystine: 2%.
7. Miscellaneous: xanthine, silicates, and drug metabo- lites, such as
indinavir (radiolucent on x-ray and CT scan).

Pathogenesis and
Physiochemical Properties
GENETICS
1. Idiopathic hypercalciuria
a. Polygenic
b. Calcium salt stones
c. Rare nephrocalcinosis
d. Rare risk of end-stage renal
disease

2. Primary hyperoxaluria types 1, 2, and 3
a. Autosomal recessive
b. Pure monohydrate calcium oxalate stones (whewellite)
c. Nephrocalcinosis
d. Risk of end-stage renal disease
3. Distal renal tubular acidosis (RTA)
a. Autosomal recessive or dominant
b. Apatite stones
c. Nephrocalcinosis
d. Risk of end-stage renal disease

4. Cystinuria
a. Autosomal recessive associated with a defect on chromosome 2
b. Cystine stones
c. No nephrocalcinosis
d. Risk of end-stage renal disease
5. Lesch-Nyhan syndrome (HGPRT deficiency)
a. X-linked recessive
b. Uric acid stones
c. No nephrocalcinosis
d. Risk of end-stage renal disease

ENVIRONMENTAL
•
1. Dietary factors
•
a. Normal dietary calcium intake is associated with a reduced risk of calcium
stones secondary to binding of intestinal oxalate.
•
b. Increased calcium and vitamin D supplementation may in- crease the risk
of calcium stones.
•
c. Increased dietary sodium intake is associated with an in- creased risk of
calcium and sodium urinary excretion, which leads to increased calcium
stones.
•
d. Increased dietary animal protein intake may lead to in- creased uric acid
and calcium stones.
•
e. Increased water intake is associated with a reduced risk of all types of
kidney stones.

•
2. Obesity
•
a. Obesity and weight gain are associated with an increased risk of
developing kidney stones.
•
3. Diabetes
•
a. Diabetes is a risk factor for the development of kidney stones.
•
b. Insulin resistance may lead to altered acidification of the urine and
increased urinary calcium excretion.

4. Geographical factors
a. The highest risk of developing kidney stones is in
the south- eastern United States; the lowest risk is
in the northwestern United States.
b. Stone incidence peaks approximately 1 to 2
months after highest annual temperature.

PATHOPHYSIOLOGY OF STONE
FORMATION
1. Idiopathic calcium oxalate
a. Approximately 70% to 80% of incident stones are
calcium oxalate.
b. Initial event is precipitation of calcium phosphate on the
renal papilla as Randall plaques, which serve as a nucleus
for calcium oxalate precipitation and stone formation.
c. Calcium oxalate stones preferentially develop in acidic
urine (pH less than 6.0).
d. Development depends on supersaturation of both
calcium and oxalate within the urine.

2. Idiopathic hypercalciuria
a. Identified in 30% to 60% of calcium oxalate stone
formers and in 5% to 10% of nonstone formers
b. The upper limit of normal for urinary calcium excretion is
250 mg/day for women and 300 mg/day for men.
c. Need to exclude hypercalcemia, vitamin D excess,
hyper- thyroidism, sarcoidosis, and neoplasm.
d. Diagnosed via exclusion in patients with a normal serum
calcium but elevated urinary calcium on a random diet.

3. Absorptive hypercalciuria
a. Increased jejunal absorption of calcium possibly caused by elevated
calcitriol (1,25 dihydroxy vitamin D 3 ) levels and increased vitamin D
receptor expression.
b. Divided into type I, II and III, depending on whether uri- nary calcium
levels can be affected by calcium in the diet (type I and II) or renal
phosphate leak leading to increased calcium absorption (type III).
c. Increased calcium absorption leads to a higher filtered load of calcium
delivered to the renal tubule.
d. The treatment for each subtype is generally the same, so de- termining
which type (often requiring inpatient evaluation) is no longer necessary.
e. Normal serum calcium

4. Renal hypercalciuria
a. Impaired proximal tubular reabsorption of
calcium leads to renal calcium wasting.
b. Normal serum calcium; hypercalciuria persists
despite a calcium restricted diet.
c. Distinguished from primary hyperparathyroidism
by normal serum calcium levels and secondary
hyperparathyroidism.

5. Resorptive hypercalciuria
a. Primary hyperparathyroidism is the underlying
mechanism.
b. Increased PTH levels cause bone resorption and
intestinal calcium absorption, which leads to
elevated serum calcium that exceeds the
reabsorptive capacity of the renal tubule.
c. Normal to slightly elevated serum calcium

6. Hypercalcemic hypercalciuria
a. Primary hyperparathyroidism, hyperthyroidism,
sarcoid- osis, vitamin D excess, milk alkali
syndrome, immobiliza- tion, and malignancy

7. Hyperoxaluria
a. The upper limit of normal for urinary oxalate excretion is 45 mg/d in
women and 55 mg/d in men.
b. Acts as a potent inhibitor of stone formation by complexing with calcium
c. Dietary hyperoxaluria is related to increased consumption of oxalate-rich
foods, and/or a low-calcium diet, which by reducing the availability of
intestinal calcium to complex to oxalate, allows an increased rate of free
oxalate absorption by the gut.
d. Enteric hyperoxaluria can be caused by small bowel disease or loss,
exocrine pancreatic insufficiency, or diarrhea, all of which reduce small
bowel fat absorption, leading to an increase in fat complexing with calcium,
and thereby facili- tating free oxalate absorption by the colon.
e. Primary hyperoxaluria is a genetic disorder in one of two genes, which
results in increased production or urinary ex- cretion of oxalate.

8. Hypocitraturia
a. The lower limit of normal is less than 500 mg/d for women and 350
mg/d for men.
b. Acts as an inhibitor of stone formation by complexing with calcium.
c. Citrate is regulated by tubular reabsorption, and reab- sorption varies
with urinary pH. In acidic conditions, tubular reabsorption is enhanced,
which lowers urinary citrate levels.
d. Diseases that cause acidosis, such as chronic diarrhea or distal RTA,
cause lower urinary citrate levels. Thiazide therapy can also reduce
citrate levels via potassium depletion.
e. In the majority of patients with hypocitraturia, no etiol- ogy is identified,
and these patients are classified as having idiopathic hypocitraturia.

9. Hyperuricosuria/uric acid stones
a. Approximately 5% to 10% of incident stones are uric acid.
b. The upper limit of normal is greater than 750 mg/d for women and 800 mg/d for
men.
c. Uric acid is a promoter for calcium oxalate stone forma- tion by serving as a
nucleus for crystal generation and also by reducing the solubility of calcium oxalate.
d. A low urinary pH is critical for uric acid stone forma- tion. At a urinary pH less
than 5.5, uric acid exists in its insoluble undissociated form, which facilitates uric
acid stone formation. As the urinary pH increases, the dissociated monosodium
urate crystals are predominant and serve as a nucleus for calcium-containing stone
formation.
e. Increased uric acid production is common in patients with a high dietary intake of
animal protein, in myeloprolifera- tive disorders, and in gout. However, uric acid
stone for- mation is also common in patients with diabetes and the metabolic
syndrome presumably caused by insulin resis- tance, which impairs renal ammonia
excretion necessary for urinary alkalization.

10. Cystinuria
a. In both men and women, urinary cystine excretion exceeds 350 mg/
d.
b. Caused by autosomal recessive disorder involving the SLC3A1
amino acid transporter gene on chromosome 2
c. The dibasic amino acid transporter, which is located within the
tubular epithelium, facilitates reabsorption of dibasic amino acids, such
as cystine, ornithine, lysine, and arginine, (COLA). A defect in this
enzyme leads to de- creased cystine reabsorption and increased
urinary excre- tion of cystine.
d. Cystine solubility rises with increasing pH and urinary volume.
e. Positive urine cyanide-nitroprusside colorimetric reaction is a
qualitative screen.

11. Calcium phosphate stones
a. Approximately 12% to 30% of incident stones are
cal- cium phosphateb.
B
b. Calcium phosphate stones preferentially develop in
alka- line urine (pH greater than 7.5).
c. Calcium phosphate stones can be present as either
apatite or brushite (calcium phosphate monohydrate).
d. Overalkalinization with potassium citrate for
hypercalci- uria can sometimes lead to calcium
phosphate stones.

12. Struvite stones/triple phosphate/infection stones
a. Approximately 5% of incident stones are struvite.
b. Struvite stones are composed of magnesium ammonium phosphate and
calcium phosphate. They may also contain a nidus of another stone composition.
c. Often grow to encompass large areas in the collection sys- tem or staghorn
calculi.
d. Urinary tract infections (UTIs) with urease splitting organisms, which include
Proteus spp., Klebsiella spp., Staphylococcus aureus, Pseudomonas spp., and
Ureaplasma spp., are required to split urea into ammonia, bicarbonate, and
carbonate.
e. A urinary pH greater than 7.2 is required for struvite stone formation.
f. Conditions that predispose to urinary tract infections in- crease the likelihood of
struvite stone formation. Struvite stones are common in patients with spinal cord
injuries and neurogenic bladders.

Clinical Manifestations of Nephrolithiasis

a. Asymptomatic kidney stones are found in 8% to
10% of screen- ing populations undergoing a CT
scan for unrelated reasons.
b. Pain is the most common presenting symptom in
the major- ity of patients.
1) The stone produces ureteral spasms and
obstructs the flow of urine, which causes a resultant
distention of the ureter, pyelocalyceal system, and
ultimately the renal capsule to produce pain.

2) Renal colic is characterized by a sudden onset
of severe flank pain, which often lasts 20 to 60
minutes. The pain is paroxysmal, and patients are
often restless and unable to get comfortable.

3) Three main sites of anatomic narrowing or
obstruction are within the ureter: the ureteropelvic
junction, the lumbar ureter at the crossing of the
iliac vessels, and the ureterovesical junction.
4) The location of pain generally correlates with
these sites of anatomic narrowing: the
ureteropelvic junction pro- duces classic flank pain,
the midureter at the level of the iliac vessels
produces generalized lower abdominal dis-
comfort, and the ureterovesical junction produces
groin or referred testes/labia majora pain.

c. Associated nausea and vomiting are frequent.
Fever and chills are common with concomitant UTI.
d. Dysuria or strangury, which is the desire to void
but with urgency, frequency, straining, and small
voided vol- umes, is possible with stones located
at the ureterovesical junction.

DIFFERENTIAL DIAGNOSIS
Pyelonephritis: Fever with associated flank pain
Musculoskeletal pain: Pain with movement
Appendicitis: Right lower quadrant tenderness at McBurney point
Cholecystitis: Right upper quadrant tenderness with Murphy sign
Colitis/diverticulitis: Left lower quadrant tenderness with GI
symptoms
Testicular torsion: Abnormal testicular exam with high-riding testicle
Ovarian torsion/ruptured ovarian cyst: Adnexal tenderness

Evaluation of Patients with Nephrolithiasis
1. General considerations
a. All patients in the acute phase of renal colic should have a
history and physical, a urinalysis, a urine culture if uri- nalysis
demonstrates bacteruria or nitrites, and a serum cre- atinine. If
the patient presents with fever, then a complete blood count
should also be included.

b. All patients with a first stone episode should
undergo a ba- sic evaluation with a medical
history including family his- tory, dietary history,
and medications; physical exam and ultrasound;
blood analysis with creatinine, calcium, and uric
acid; urinalysis and culture; and stone analysis.
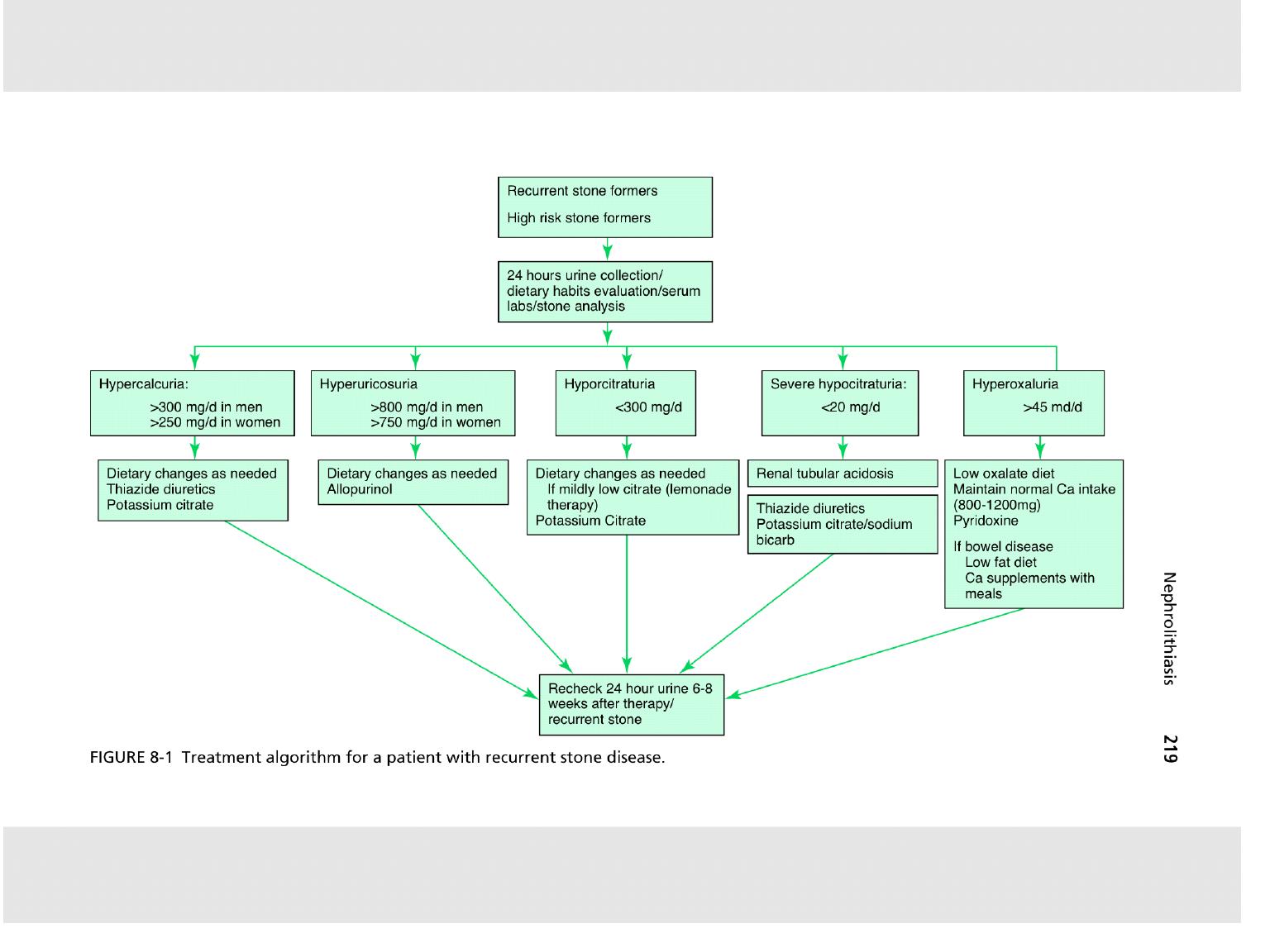

c. Patients at high risk include those with a family
history of nephrolithiasis, recurrent stone formation,
large stone bur- den, residual stone fragments after
therapy, solitary kidney, metabolic, or genetic
abnormalities known to predispose to stone formation,
stones other than calcium oxalate, and children given
a higher rate of an underlying metabolic, anatomic,
and/or functional voiding abnormality. These pa- tients
they should undergo the basic evaluation, plus two
24-hour urine collections, at least 4 weeks following
the acute stone episode. Further therapy will be
guided by the stone analysis and 24-hour urine
collections.

2. Medical history
a. General medical history is mandatory in all stone formers.
b. Past medical history with a specific focus on diseases
known to contribute to stone formation, including
inflammatory bowel disease, previous bowel resection, or
gastric bypass, hyperparathyroidism, hyperthyroidism, RTA,
and gout.
c. Family history is of particular importance because a
positive family history is a risk factor for incident stone
formation and recurrence.

d. Review medications for drugs known to increase
stone for- mation, such as acetazolamide, ascorbic
acid, corticoste- roids, calcium-containing
antacids, triamterene, acyclovir, and indinavir.
e. Dietary history can also be relevant, especially in
those with high- or low-calcium diets, diets high in
animal protein, and diets with significant sodium
intake.

3. Physical exam
a. May provide clues to underlying systemic
diseases.

4. Laboratory evaluation
a. Urinalysis
1) Specific gravity may indicate relative hydration
status.
2) Calcium oxalate stones preferentially form in a rela-
tively acidic pH (less than 6.0), whereas calcium phos-
phate stones preferentially form in a relatively alkaline
pH (greater than 7.5). A low pH (less than 5.5) is man-
datory for uric acid stone formation. A high pH (greater
than 7.2) is critical for struvite stone formation. A pH
constantly greater than 5.8 may suggest an RTA.

3) Microscopy may reveal red blood cells, white
blood cells (WBCs), and bacteria.
4) Crystalluria can define stone type: Hexagonal
crystals are cystine, coffin lid crystals are calcium
phosphate, and rhomboidal crystals are uric acid.

b. Urine culture is mandatory if microscopy reveals
bacte- riuria, if struvite stones are suspected, or if
symptoms or signs of infection are present.
c. Electrolytes
1) Calcium (ionized or calcium with albumin): Elevated
calcium may suggest hyperparathyroidism, and a para-
thyroid hormone (PTH) blood test should be done.
2) Uric Acid: Elevated uric acid is common in gout and,
in conjunction with a radiolucent stone, is suggestive of
uric acid nephrolithiasis.

d. A complete blood count (CBC) may show mild
peripheral leukocytosis. WBC counts higher than
15,000/mm 3 may suggest an active infection.
e. Ammonium chloride load test: Identifies distal or
Type I RTA. Oral ammonium chloride load (0.1 gm/
kg body weight) given over 30 minutes will not
raise the urinary pH greater than 5.4 if a Type I RTA
is present.

f. Sodium nitroprusside test: Identifies cystinuria.
Addition of sodium nitroprusside to urine with
cystine concentration higher than 75 mg/L alters
the urine color to purple-red.

g. 24-Hour urine collection: Typically one to two 24-
hour urine collections are obtained at least 6 weeks
after an acute stone event or following initiation of
medical therapy or dietary modification. Collection is
done to determine total urine volume, pH, creatinine,
calcium, oxalate, uric acid, citrate, magnesium,
sodium, potassium, phosphorus, sulfate, urea, and
ammonia. Cystine is also determined if a cystine
screening test is positive. Supersaturation indices
are also calculated. An adequate collection is
determined by total creatinine, which is 15-19 mg/kg
for women and 20-24 mg/kg for men.

h. Stone analysis: Performed either with infrared
spec- troscopy or x-ray diffraction. It provides
information about the underlying metabolic,
genetic, or dietary abnormality.

5. Imaging considerations
a. A noncontrast CT scan is the recommended
initial imaging modality for an acute stone episode.
A noncontrast CT scan has a sensitivity of 98%
and a specificity of 97% in detect- ing ureteral
calculi. A low-dose noncontrast CT scan (less than
4 mSv) is preferred in patients with a body mass
index (BMI) less than 30. When a ureteral stone is
visualized on a noncontrast CT scan

b. A renal bladder ultrasound is the recommended
initial im- aging modality in both children and
pregnant patients in order to limit ionizing radiation.
Ultrasonography has a median sensitivity of 61%
and a specificity of 97%. If ultra- sonography is
equivocal, and the clinical suspicion is high for
nephrolithiasis, then a low-dose noncontrast CT
scan may be performed in both children and
pregnant patients.

c. Plain film of the kidneys, ureters, and bladder
(KUB) is also routinely used. Conventional
radiography with a KUB has a median sensitivity of
57% and a specificity of 76%. Pure uric acid,
cystine, indinavir, and xanthine stones are radio-
lucent, and are not visible on KUB.

intravenous pyelography (IVP) was commonly
utilized for diagnosis of stone disease because it
could read- ily identify radiolucent stones and
define calyceal anatomy. It has a median sensitivity
of 70% and a specificity of 95%. However, CT has
largely replaced IVP.

e. Magnetic resonance imaging is generally not
performed for urolithiasis because of cost, low
sensitivity, and time needed to acquire images.

f. A combination of ultrasonography and KUB is recom-
mended for monitoring patients with known radiopaqueureteral
calculi on medical expulsion therapy because this limits costs
and radiation exposure. Those with radiolucent stones will
require a low-dose noncontrast CT scan.

Management of Nephrolithiasis
MEDICAL THERAPY

1. All stone formers
a. High fluid intake of 2.5-3.0 L/day with urine volume greater than 2 L/
day
b. Normal calcium diet of 800-1200 mg/day, preferably not through
supplements. Avoid excess calcium supplementa- tion; however calcium
citrate is preferred if indicated.
c. Limit sodium to 4-5 g/day.
d. Limit animal protein to 0.8-1.0 g/kg/day.
e. Limit oxalate-rich foods.
f. Maintain a normal BMI and physical activity.
g. Targeted therapy depending on underlying metabolic ab- normality
and/or 24-hour urine collection results

2. Calcium oxalate stones
a. Dietary hyperoxaluria: Limit oxalate-rich foods.
b. Enteric hyperoxaluria: Limit oxalate-rich foods
and cal- cium supplementation with greater than
500 mg/day.
c. Primary hyperoxaluria: Pyridoxine can decrease
endog- enous production of oxalate. The dose is
100-800 mg/day.

d. Hypocitraturia: Potassium citrate both raises the
uri- nary pH out of the stone-forming range and
restores the normal urinary citrate concentration.
Sodium bicarbonate may also be used, if unable to
tolerate potassium supple- mentation.
e. Hypercalciuria: Thiazide diuretics, which inhibit a
sodium- chloride co-transporter, therefore
enhancing distal tubular sodium reabsorption via
the sodium-calcium co-transporter to promote
tubular calcium reabsorption. Thiazides de- crease
urinary calcium by as much as 150 mg/day.

3. Calcium phosphate stones
a. Primary hyperparathyroidism: Requires parathyroidectomy.
b. Distal RTA (Type I): Potassium citrate or sodium bicarbon- ate to
restore the natural pH balance.
4. Struvite/infection stones
a. Total stone removal because each fragment harbors urease-
producing bacteria and serves as a nidus for further stone growth.
b. Appropriate antibiotic therapy to eradicate the urease-pro- ducing
bacteria
c. Restoration of normal pH with urinary acidification with L-methionine
or inhibition of urease enzyme with acetohy- droxamic acid

5. Uric acid stones
a. Low animal protein diet
b. Specific therapy depends on 24-hour urine collection
results.
c. Alkalinization of the urine with potassium citrate or so-
dium bicarbonate for stone dissolution is possible with a pH
of 7.0 to 7.2 and for maintenance of a stone-free state with a
pH of 6.2 to 6.8.
d. Hyperuricosuria (with or without hyperuricemia): Allopurinol
at 100-300 mg/day, which inhibits xanthine oxidase to reduce
uric acid production.

6. Cystine stones
a. Increase daily fluid intake to 3.5 to 4.0 L/day.
b. Specific therapy depends on 24-hour urine
collection results.
c. Alkalinization of the urine with potassium citrate
or so- dium bicarbonate above a pH of 7.5 to
improve solubility of cystine threefold

d. D-penicillamine is a chelating agent that forms a
disulbond with cysteine to produce a more soluble
compound, thereby preventing the formation of
cysteine into the insoluble, stone forming, cystine.
Alpha-mercaptopropionyl-glycine (tiopronin) is the
preferred alternative to D-penicillamine, as it has a
better safety and efficacy profile. Alpha-
mercaptopropionyl-glycine reduces the disulfide
bond of cystine to form the more soluble cysteine,
again reducing stone formation. Lastly, captopril is
an angiotensin-converting enzyme inhibitor, which
can reduce cystine, but its role in therapy is not yet
well defined.

ACUTE RENAL COLIC

1. General considerations
a. Primary considerations include symptomatic control
with analgesics, antiemetics, and adequate hydration.
b. First-line analgesia is generally a nonsteroidal
antiinflam- matory, such as ketorolac.
c. Patients should be instructed to sieve their urine for
collec- tion of stone fragments.
d. Spontaneous stone passage occurs in 80% of patients
with sizes less than 4 mm. With sizes greater than 10
mm, there is a low probability of spontaneous passage.

e. Referral to a urologist is necessary with
persistent pain, high- grade obstruction, bilateral
obstruction, presence of infection, solitary kidney,
abnormal anatomy, failure of conservative
management, large stone burden, pregnancy, or in
children.

2. Medical expulsive therapy
a. Alpha-blockers, such as tamsulosin, and calcium
channel blockers (nifedipine) or steroids can facilitate
stone passage via ureteral smooth muscle relaxation.

d. Medical expulsive therapy (MET) is acceptable in
patients with ureteral calculi less than 10 mm who
have well- controlled pain, no evidence of infection,
adequate renal function, and no other
contraindications to the therapy.

SURGICAL THERAPY
1. Shock wave lithotripsy (SWL):

a. Shock waves are high-energy focused-pressure
waves that can travel in air or water. When passing
through two dif- ferent mediums of different
acoustic impedance, energy is released, which
results in the fragmentation of stones. Shock waves
travel harmlessly through substances of the same
acoustic density. Because water and body tissues
have the same density, shock waves can travel
safely through skin and internal tissues. The stone
is a different acoustic density and, when the shock
waves hit it, they shatter and pulverize it. Urinary
stones are thus fragmented, facilitating in their
spon- taneous passage.

b. Treatment success depends on stone size,
location, composi- tion, hardness, and body
habitus. For renal stones, upper or middle polar
stones are ideally treated with SWL, whereas lower
pole stones have a clearance rate as low as 35%.

c. Ideally all stones less than 1 cm in any location
in the kid- ney can be treated with SWL.

e. Contraindications of SWL include (absolute)
pregnancy, bleeding diathesis, and obstruction
below the level of the stone; and (relative) calcified
arteries and/or aneurysms and cardiac pacemaker.

g. Complications of SWL include skin bruising,
subscapular and perinephric hemorrhage,
pancreatitis, urosepsis, and Steinstrasse (“street of
stone,” which may accumulate in the ureter and
cause obstruction).

2. Percutaneous nephrolithotomy (PCNL)

a. The technique is establishment of access at a
lower pole ca- lyx, dilation of the tract with a
balloon dilator or Amplatz dilators under
fluoroscopy, and stone removal with grasp- ers or
its fragmentation using electrohydraulic, ultrasonic,
or laser lithotripsy. A nephrostomy tube or ureteral
stent is left for drainage.

d. Additional candidates for PCNL include cystine
calculi, which are large volume and resistant to
SWL, and anatomic abnormalities, such as those
with ureteropelvic junction (UPJ) obstruction,
caliceal diverticula, obstructed infundib- ula
(hydrocalyx), ureteral obstruction, malformed
kidneys (e.g., horseshoe and pelvic), and
obstructive or large adja- cent renal cysts.

e. Contraindications of PCNL include uncontrolled
bleeding diathesis, untreated urinary tract infection
(UTI), and in- ability to obtain optimal access for
PCNL because of obe- sity, splenomegaly, or
interposition of colon.

f. Complications of PCNL include hemorrhage (5% to
12%), perforation, and extravasation (5.4% to 26%),
damage to adjacent organs (1%), ureteral obstruction
(1.7% to 4.9%), and infection/urosepsis (3%).

3. Retrograde intrarenal surgery (ureteroscopy
[URS])
a. Instrumentation includes both rigid and flexible
uretero- scopes. Rigid ureteroscopes are ideally
suited for access to the distal ureter but can be
utilized up to the proximal ureter. Flexible
ureteroscopes are ideally suited for ureteral and
intrarenal access.

c. URS may be safely performed in patients with
morbid obe- sity, pregnancy, and bleeding
diathesis.
d. Complications include failure to retrieve the
stone, muco- sal abrasions, false passages,
ureteral perforation, complete ureteral avulsion, and
ureteral stricture.

4. Open/laparoscopic/robotic surgery
a. Since the introduction of minimally invasive techniques
such as SWL, URS, and PCNL, open surgery has been re-
duced to rates of 1% to 5%.
b. Indications for open stone surgery include complex stone
burden, treatment failure with endoscopic techniques, ana-
tomic abnormalities, and a nonfunctioning kidney.
c. Laparoscopic or robotic surgery can be used in place of
open techniques, but because of the complexity and rarity of
these procedures, they are generally referred to centers of
excellence.
