
1
Third stage
Surgery
Lec-1-2
.د
ب
سام
1/1/2014
Fluid & Electrolytes balance
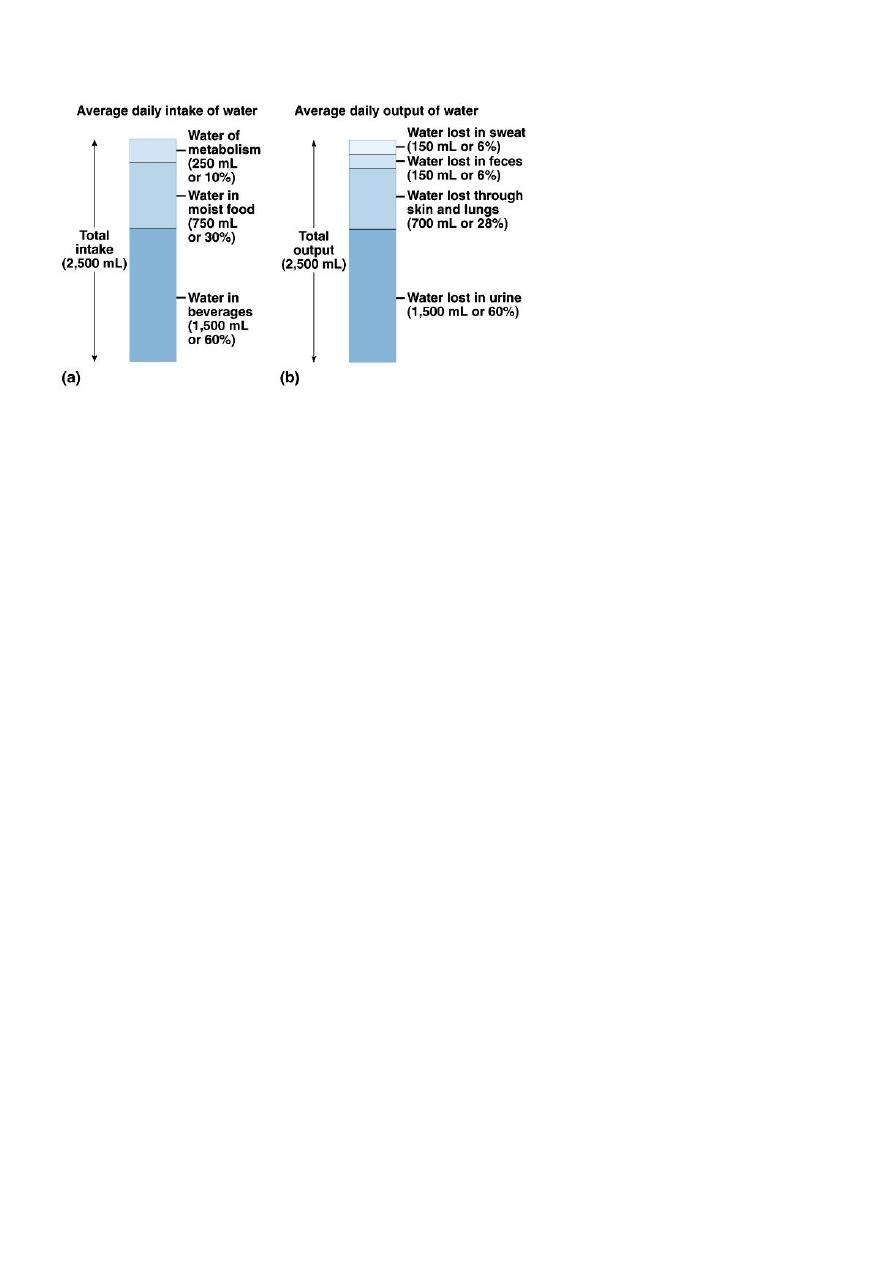
Functions of Fluids
Move electrolytes and oxygen into and out of cells as needed.
Aid digestion.
Cleanse the body of waste.
Regulate body temperature.
Lubricate joints and mucous membranes.
Compartments of Body and Distribution of Water by Weight
60 % Water
o Plasma 5%
o Interstitial 15%
o Intracellular 40%
40% Solids
o fat, protein, carbohydrates, minerals
Total Body Water
Approximately 60% of body weight (males)
Approximately 50% of body weight (females)
Approximately 75% in newborns (less body fat)
Obese/elderly decrease above by 10%
To calculate TBW (L) = Current wt (kg) x 60%
• Fluid compartments are separated by membranes that are freely permeable to water
– but impermeable to solutes.
• Movement of fluids is due to:
– hydrostatic pressure differentials
– osmotic pressure differentials
2
Fluid Balance
Solute Homeostasis
• Electrolytes – charged particles
– Cations – positively charged ions
• Na
+
, K
+
, Ca
++
, H
+
– Anions – negatively charged ions
• Cl
-
, HCO
3
-
, PO
4
3-
• Non-electrolytes - Uncharged particles
• Proteins, urea, glucose, O
2
, CO
2
Giving IVF
• Normal body fluid and composition
• Daily requirement of the body
• Changes and losses
•
Fluids to be given
Daily requirement
• Size : 1.5 l H2O/M2 of the surface area24 hours
• Weight : 35ml/Kg/24 hours

3
Intravenous Fluids
Crystalloids
• Hypertonic
• Isotonic
• Hypotonic
Colloids
• Albumin
• Dextrans
Crystalloids
NS:
154 Na+
154 K+
5% dextrose
278mmol/l dextrose
GS (0.18%saline, 4%dextrose) 30 Na 30Cl- 222 mmol/l dextrose
Hartman’s
131 Na+
5 K+ 29mmol/l HCO3
111 mmol/l CL-
2mmol/l Ca++
Ringer’s
147 Na
4 K+
156 Cl-
2.2 mmol/l Ca++
Colloids
Synthetic, commercially prepared volume expanders containing polysaccharide molecules
(sugar & starch)
Albumin (5% and 25%)
10% Dextran 40 and 6% Dextran 70
Prescribing fluid regime
• It depend on :
• Basal requirements
• Continuous abnormal losses over and above basal requirement
• Pre-existing dehydration and electrolyte loss

4
1. Basal requirement
• 2000 cc 5% GW + 500 cc 0.9% saline
• 2000 cc 5% GW + 500 cc hartman’s
• 2500( 4% GW 0.18% saline)
• Adding to this K+ 1mmol/kg
• Each 1 cc contains 2 meq of K+
2. Replacing ONGOING LOSS
FROM:
o Nasogastric tube
o Drains
o Evaporation during operation
o Blood loss
o Paracentesis
o Thoracocentesis
3. Pre-existing dehydration
A. Assessment:
o history:(thirst, intake out put, check the chart)
o examination: (mucus mm, eye, skin, UOP, HR, BP, capillary refilling, confusion)
o haematocrit, S.albumin. B. urea, S.electrolyte
o Central venous pressure(3-8 mmH2O), PCWP (5-12 mmHg)
B. correction:
o identify the compartment the fluid has been lost from(bowel losses come from ECF,
pure water loss from TBW, protein containing fluid from plasma)
o fluid replacement should be similar to that which has been lost
Electrolyte balance
Na
+
(Sodium)
o Predominant extracellular cation
o 136 -145 mEq / L
o Pairs with Cl- , HCO3- to neutralize charge
o Most important ion in water balance
5
o Important in nerve and muscle function
• Reabsorption in renal tubule regulated by:
o Aldosterone
o Renin/angiotensin
o Atrial Natriuretic Peptide (ANP)
K + (Potassium)
o Major intracellular cation
o 150- 160 mEq/ L
o Regulates resting membrane potential
o Regulates fluid, ion balance inside cell
• Regulation in kidney through:
o Aldosterone
o Insulin
Cl ˉ (Chloride)
o Major extracellular anion
o 105 mEq/ L
o Regulates tonicity
o Reabsorbed in the kidney with sodium
• Regulation in kidney through:
o Reabsorption with sodium
o Reciprocal relationship with bicarbonate
Hypernatremia
o Plasma Na+ > 145 mEq / L
o Due to ↑ Na + or ↓ water
o Water moves from ICF → ECF
o Cells dehydrate
Due to:
o Excess Na intake (hypertonic IV solution)
o Excess Na retention (oversecretion of aldosterone)
o Loss of pure water
Long term sweating with chronic fever
Respiratory infection → water vapor loss
Diabetes (mellitus or insipidus) – polyuria
o Insufficient intake of water (hypodipsia)

6
Clinical manifestations of Hypernatremia:
o Thirst
o Lethargy
o Irritability
o Seizures
o Fever
o Oliguria
Treatment of Hypernatremia:
o Calculate the free water deficit:
0.6 x wt (kg) x (patient’s sodium/140 - 1)
o Correct the free water deficit at a rate of 1mEq/L/hr
o Check serum Na every 4hr
o Use isotonic salt-free IV fluid
Hyponatremia
Symptoms and signs
o Anorexia
o Headache
o Nausea
o Emesis
o Impaired response to verbal stimuli
o Impaired response to painful stimuli
o oliguria
o Bradycardia
o Hypertension or hypotension
o Altered temperature regulation
o Hypotension
o Renal failure as consequence of hypotension
o Tachycardia
o Weakness
o Muscular cramps
Hypovolemic hyponatremia
o Renal losses caused by diuretic excess, osmotic diuresis, salt-wasting nephropathy,
adrenal insufficiency, proximal renal tubular acidosis, metabolic alkalosis, and
pseudohypoaldosteronism result in a urine sodium concentration greater than 20
mEq/L
o Extrarenal losses caused by vomiting, diarrhea, sweat, and third spacing result in a
urine sodium concentration less than 20 mEq/L

7
o Rx: Volume resuscitation with NS
Normovolemic hyponatremia
o When hyponatremia is caused by SIADH, reset osmostat, glucocorticoid deficiency,
hypothyroidism, or water intoxication, urine sodium concentration is greater than 20
mEq/L
o Rx: Fluid restriction, Correct endocrine abnormality
Hypervolemic hyponatremia
o If hyponatremia is caused by an edema-forming state (eg, congestive heart failure,
cirrhosis, nephrotic syndrome), urine sodium concentration is less than 20 mEq/L
o If hyponatremia is caused by acute or chronic renal failure, urine sodium concentration
is greater than 40 mEq/L
o Rx: Correct underlying state
Treatment of Hyponatremia
o Correct serum Na by 1mEq/L/hr
o Check serum Na q4hr
o Use 3% saline in severe hyponatremia
o Goal is serum Na 130
o Avoid too rapid correction:
– Central pontine myelinolysis
– Flash pulmonary edema
Acute Hyponatremia
o Na < 120 and duration < 48 hrs
o Etiology:
– Postoperative
– Exercise with hypotonic fluid replacement
– Drugs - Ecstasy
o Treat aggressively using 3% saline to raise Na by 5mm/L in one hour
o Beware rapid drop in vasopressin levels
Hypochloremia
o Most commonly from gastric losses
– Emesis, gastric suctioning, EC fistula
o Often presents as a contraction alkalosis with paradoxical aciduria (Na+ retained and
H+ wasted in the kidney)
o Rx: resuscitation with normal saline

8
Hyperchloremia
o Most commonly from over-resuscitation with normal saline
o Often presents as a hyperchloremic acidemia with paradoxical alkaluria (H+ retained
and Na+ wasted in the kidney)
o Rx: stop normal saline and replace with hypotonic crystalloid
Hypokalemia
o Serum K+ < 3.5 mEq /L
o Beware if diabetic
– Insulin pushes K+ into cells
– Ketoacidosis – H+ replaces K+, which is lost in urine
o β – adrenergic drugs or epinephrine
Causes of Hypokalemia
o Decreased intake of K+
o Increased K+ loss
– Chronic diuretics
– Severe vomiting/diarrhea
– Acid/base imbalance
– Trauma and stress
– Increased aldosterone
– Redistribution between ICF and ECF
Clinical manifestations of Hypokalemia
o Neuromuscular disorders
o Weakness, flaccid paralysis, respiratory arrest, constipation
o Dysrhythmias, appearance of U wave
o Postural hypotension
o Cardiac arrest
Rx- Increase K+ intake, but slowly, preferably by foods
Hyperkalemia
o Serum K+ > 5.5 mEq / L
o Check for renal disease
o Massive cellular trauma
o Insulin deficiency
o Addison’s disease
o Potassium sparing diuretics

9
o Decreased blood pH
o Exercise pushes K+ out of cells
Clinical manifestations of hyperkalemia:
o Early – hyperactive muscles , paresthesia
o Late - muscle weakness, flaccid paralysis
o Peaked T-waves
o Dysrhythmias
o Bradycardia, heart block, cardiac arrest
Management:
o 10% Calcium Gluconate or Calcium Chloride
o Insulin (0.1U/kg/hr) and IV Glucose
o Frusemide 1mg/kg (if renal function is normal)
o Hemodialysis
Calcium Imbalances
o Most in ECF
o Regulated by:
– Parathyroid hormone
↑Blood Ca
++
by stimulating osteoclasts
↑GI absorption and renal retention
– Calcitonin from the thyroid gland
Promotes bone formation
↑ renal excretion
Hypercalcemia
Results from:
o Hyperparathyroidism
o Hypothyroid states
o Renal disease
o Excessive intake of vitamin D
o Milk-alkali syndrome
o Certain drugs
o Malignant tumors – hypercalcemia of malignancy
– Tumor products promote bone breakdown
– Tumor growth in bone causing Ca++ release
10
Effects:
o Many nonspecific – fatigue, weakness, lethargy
o Increases formation of kidney stones and pancreatic stones
o Muscle cramps
o Bradycardia, cardiac arrest
o GI activity also common
– Nausea, abdominal cramps
– Diarrhea / constipation
Treatment:
o Duiresis
o Calcitonin , mithramycine and corticosteroid.
o Specific treatment of parathormone producing focus.
Hypocalcemia
o Hyperactive neuromuscular reflexes and tetany differentiate it from hypercalcemia
o Convulsions in severe cases
Caused by:
o Renal failure
o Lack of vitamin D
o Suppression of parathyroid function
o Hypersecretion of calcitonin
o Malabsorption states
o Abnormal intestinal acidity and acid/ base bal.
o Widespread infection or peritoneal inflammation
Diagnosis:
o Chvostek’s sign
o Trousseau’s sign
Treatment
o IV calcium for acute
o Oral calcium and vitamin D for chronic

11
Water homeostasis
acid and base regulation
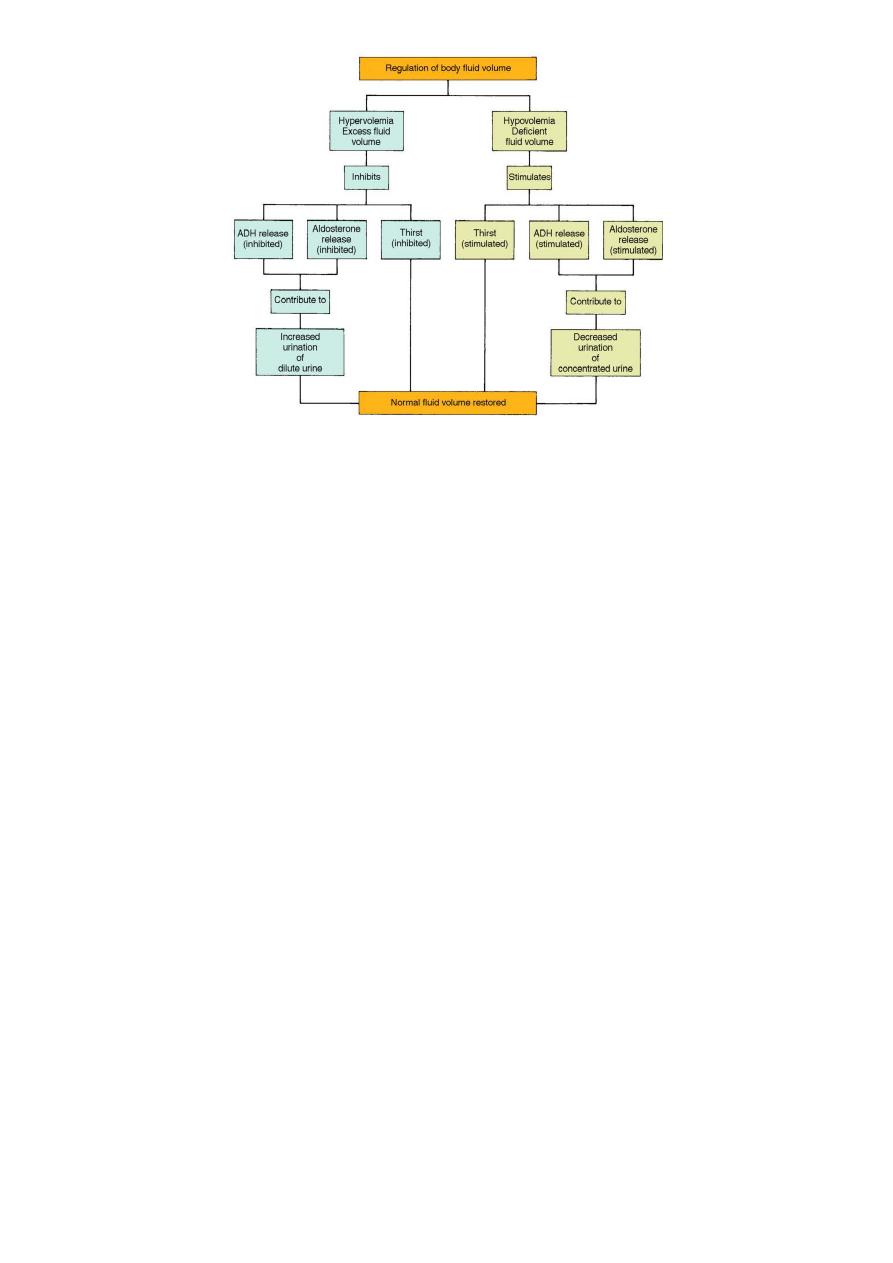
Regulation of body water
Any of the following:
• Decreased amount of water in body
• Increased amount of Na+ in the body
• Increased blood osmolality
• Decreased circulating blood volume
Results in:
• Stimulation of osmoreceptors in hypothalamus
• Release of ADH from the posterior pituitary
• Increased thirst
And thus: water consumption and conservation
Transport of Water and Fluids
Osmolality: concentration of a solution determined by the number of dissolved
particles per kilogram of water. Osmolality controls water movement and distribution
in body fluid compartments
Diffusion: the random movement of particles in all directions through a solution
Active transport: movement of solutes across membranes; requires expenditure of
energy
Filtration: transfer of water and solutes through a membrane from a region of high
pressure to a region of low pressure
Osmosis: movement of water across a membrane from a less concentrated solution to
a more concentrated solution
12
Fluid shifting:
1st space shifting- normal distribution of fluid in both the ECF compartment and ICF
compartment.
2nd space shifting- excess accumulation of interstitial fluid (edema)
3rd space shifting- fluid accumulation in areas that are normally have no or little
amounts of fluids (ascites)
Water depletion
This occur due to:
Insufficient intake of water and electrolytes:
Impaired thirst mechanism
Inability to swallow fluids
Excessive fluid loss through secretions or excretions:
Potent diuretic therapy
Diabetes insipidus
Fluid losses from GI tract
Excessive sweating
Clinical signs and symptoms:
Intense thirst
Acute weight loss
Decreased skin turgor, Dry mucous membranes, Rough, dry tongue
Changes in behavior :agitation, restlessness, weakness

13
Flat neck veins in supine position
Weak thready pulse
Orthostatic hypotension
oliguria
Treatment:
Since a fluid volume deficit decreases blood flow to kidneys, treatment must begin
promptly to prevent damage to kidneys.
If fluids cannot be ingested, isotonic IV fluids (.9% NaCL and D5W) are given
initially. Electrolytes are added to IV solution if adequate renal function is present
(Lactated Ringer solution)
Water excess (intoxication)
Overloading body with sodium:
Excessive administration of IV fluids, especially hypertonic solutions
Altered homeostatic regulation of sodium and water:
Chronic renal failure
Congestive heart failure
Excessive corticosteroid therapy
Syndrome of inappropriate secretion of ADH (SIADH)
Clinical signs and symptoms:
Acute weight gain
Peripheral edema
Shortness of breath ( rales in lungs)
Changes in behavior : confusion, lethargy, weakness
Distended neck veins
Full, bounding pulse
Elevated BP
Treatment:
Fluid restriction
Dietary Na+ restriction
Diuretic therapy
14
Volume Abnormalities
Edema
The accumulation of fluid within the interstitial space
Results in:
increased distance for diffusion
impaired blood flow
slower healing
increased risk of infection
pressure sores over bony prominences
impaired organ function (brain, liver, gut, kidney)
Causes:
increased hydrostatic pressure venous obstruction, lymphedema, CHF, renal
failure
lowered plasma osmotic pressure (protein loss) liver failure, malnutrition, burns
increased capillary membrane permeability Inflammation, SIRS, sepsis
Acid–Base Balance
Body produces large amounts of acid from normal metabolic processes, such as
breakdown of proteins and glucose or oxidation of fat
Body fluids remain slightly alkaline
pH is maintained within a narrow range: 7.38 to 7.42
Regulatory mechanisms maintain pH
Neutralize and eliminate the acids as soon as they are produced to maintain normal
pH
– Blood buffers: resist pH change
– Lungs: control carbonic acid (H
2
CO
3
)concentration
– Kidneys: control bicarbonate concentration
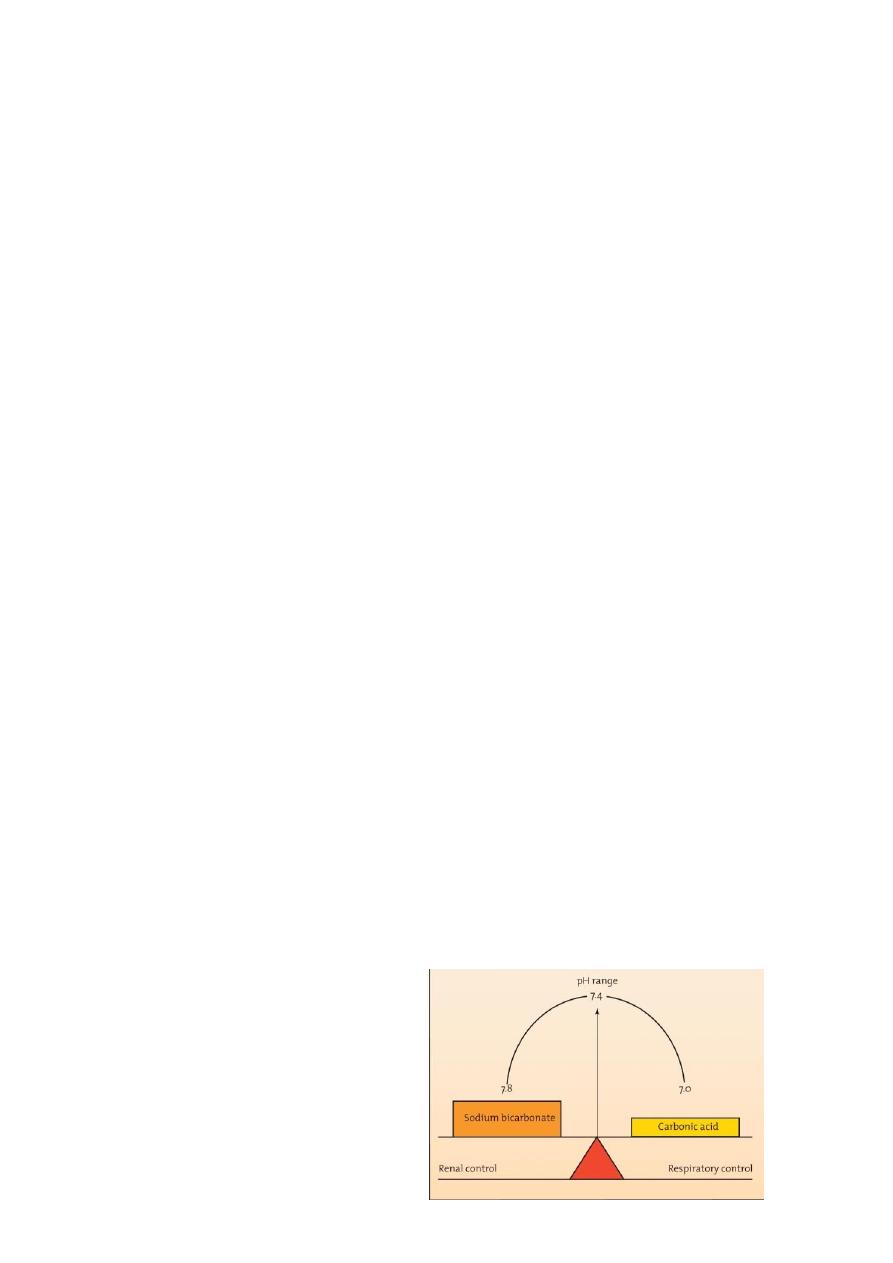
“Board-and-fulcrum” concept of normal
bicarbonate-carbonic acid relationships

15
Blood Buffer System
Respiratory control of carbonic acid
– Carbonic acid (H2CO3): dissolved as CO2 in plasma
– Hyperventilation: lowers CO2 and H2CO3 in plasma
– Decreased or inadequate ventilation: raises CO2 and H2CO3 in plasma
Renal control of bicarbonate concentration
– Kidneys selectively reabsorb filtered bicarbonate
– Kidneys can manufacture bicarbonate to replace amounts lost in buffering acids
from metabolic processes
In any buffer system
– pH depends on ratio of bicarbonate to H2CO3
– Normal ratio: 20 parts Na bicarbonate: 1 part H2CO3
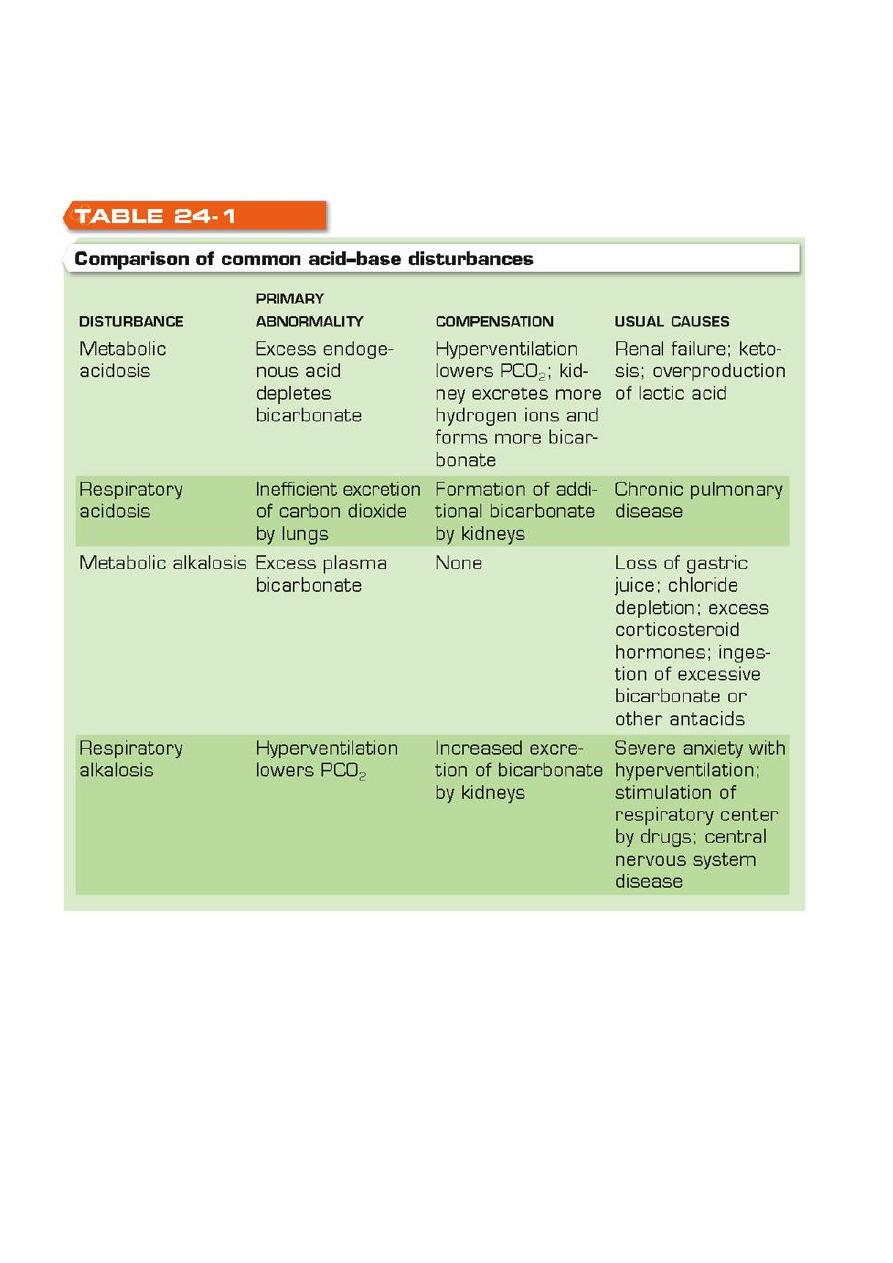
Classification of Acid–Base Disturbances
Metabolic acidosis: increased endogenous acid generated
– Amount of acid generated exceeds body’s buffering capacity
– Excess acid is neutralized by bicarbonate
– Bicarbonate in plasma falls from being consumed in neutralizing excess acid
– Uremia, ketosis, lactic acidosis
Compensation: by hyperventilation to lower PCO2 and increased bicarbonate
production in kidneys
Metabolic acidosis
Result from primary disturbance of a decreased HCO3 or increased H+ leading to
decrease in pH and a compensatory decrease in PaCO2
Causes:
– renal glomerular failure
– salicylate overdose
– Ketoacidosis (diabetic or alcoholic)
– renal tubular acidosis
– ureterosigmoidostomy
– excess acid intake (TPN)
– bicarbonate loss(diarrhea, fistula, proximal renal tubular acidosis)
Metabolic alkalosis
Result from primary disturbance of an increase in HCO3 or decrease in H+ leading to
increase in pH and a compensatory increase in PaCO2

16
Causes:
– excess alkaline intake
• alkali abuse
• over treatment of acidosis
– excessive loss of acid
• vomiting in pyloric stenosis
– increase urinary acidification
• diuretics
• excess aldosterone
• hypokalaemia
Respiratory acidosis
Primary disturbance of increase PCO2 leading to decrease pH and compensatory
increase in HCO3
causes:
– depression of respiratory centre
• CVA
• tumors
• drugs(narcotic,sedative)
• encephalitis
– decreased chest wall movement
• neuromuscular disorder(MG)
• trauma, surgery
• ankylosing spondylitis
– pulmonary disease(type 2 respiratory failure)
• COAD
• pneumonia
Respiratory alkalosis
Primary disturbance of a decreased PaCO2 leading to an increase in pH and a
compensatory decrease in HCO3
causes:
– stimulation of respiratory centre
• CVA,encephalitis
• htpermetabolic state(fever, sepsis, thyrotoxicosis)
• excersize
• hypoxia
– exess mechanical ventilation(by patient or ventilator)
• anxiety
• drug(aspirin)
17
Treatment of acid base disturbances
Treat the underlying cause
Give sodium bicarbonate(8.4%) in metabolic acidosis due to uraemia, diarrhea and
renal tubular acidosis but in other conditions is controversial.
Diagnostic Evaluation of Acid–Base Balance
Clinical evaluation: determination of concentration of bicarbonate in plasma as an
index of patient’s overall status
Laboratory studies
– Blood pH
– PCO
2
– Bicarbonate
