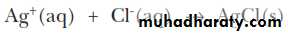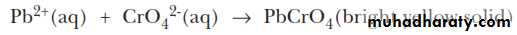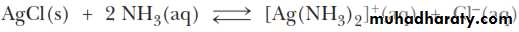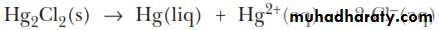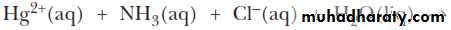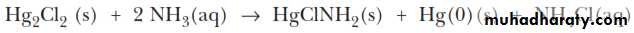ANALYSIS OF THE
SILVER GROUP CATIONSAg+ Hg22+ Pb2+
Analysis of a Mixture of CationsANALYSIS OF THE SILVER GROUP CATIONS
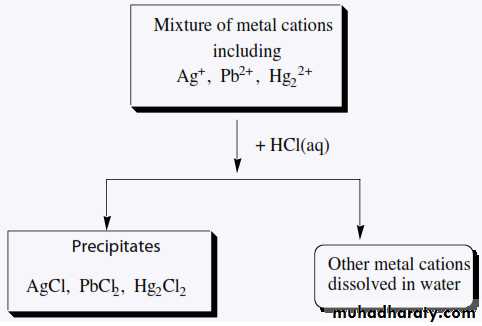
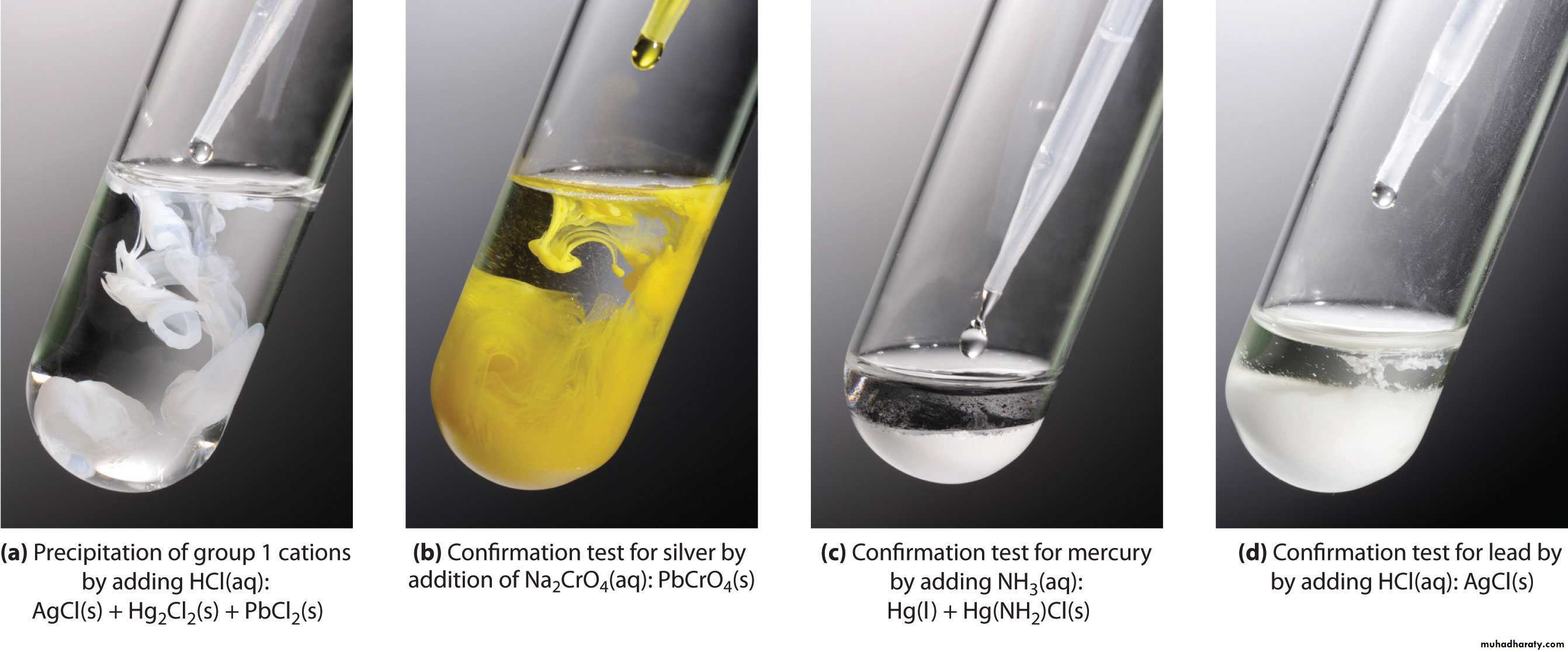
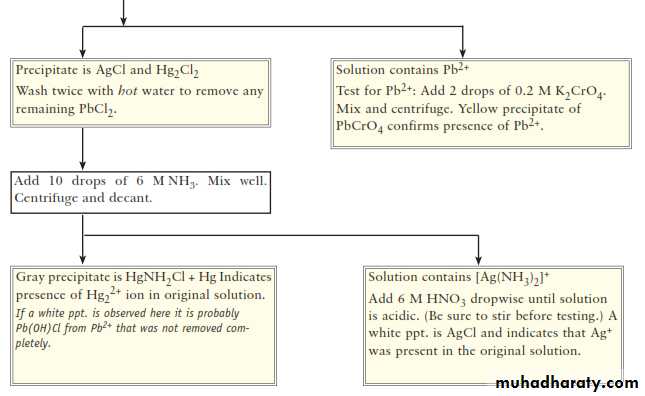
CHEMISTRY OF THE SILVER GROUP CATIONSSilver(I), lead(II), and mercury(I) are grouped together in qualitative analysis schemes because they are the only common metal cations that form insoluble precipitates with chloride ion. For example
ANALYSIS OF THE SILVER GROUP CATIONS
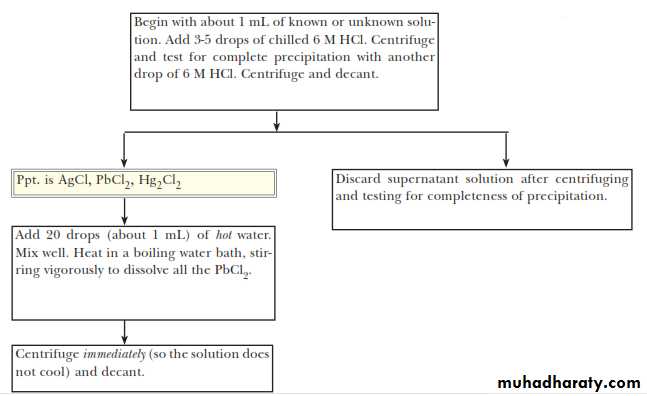
This means that, in a mixture of metal cations, these three metal Ions can be separated from all others by Precipitating them. Their insoluble chlorides, usually with 6 M HCl.ANALYSIS OF THE SILVER GROUP CATIONS
ANALYSIS OF THE SILVER GROUP CATIONS
ANALYSIS OF THE SILVER GROUP CATIONS
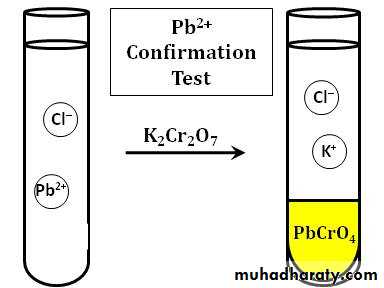
The first step in separating the three chlorides is to treat the solid mixture with hot water to selectively dissolve PbCl2and to leave AgCl and HgCl2 as a solid mixture.The Pb2+ ion and the chromate ion, CrO42-, combine to form the bright yellow, insoluble solid lead(II) chromate, PbCrO4
ANALYSIS OF THE SILVER GROUP CATIONS
ANALYSIS OF THE SILVER GROUP CATIONS
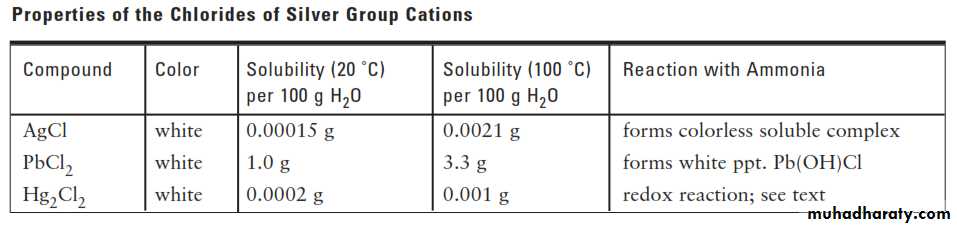
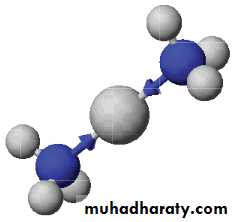
We take advantage of the tendency of transition metal ions such as Ag+ to form water-soluble complex ions with ammonia, NH3. Here the ammonia uses the lone pair of electrons of the N atom to form a bond with the Ag+ ion.ANALYSIS OF THE SILVER GROUP CATIONS
Mercury(I) chloride can undergo what is known as a disproportionation reaction. That is, the mercury(I) ion is both oxidized [to mercury(II), Hg2+] and reduced (to metallic mercury).ANALYSIS OF THE SILVER GROUP CATIONS
ANALYSIS OF THE SILVER GROUP CATIONS
ANALYSIS OF THE SILVER GROUP CATIONS
ANALYSIS OF THE SILVER GROUP CATIONS
Tools of the test1- Test tube
ANALYSIS OF THE SILVER GROUP CATIONS
ANALYSIS OF THE SILVER GROUP CATIONS
2- DropperANALYSIS OF THE SILVER GROUP CATIONS
3- Washing bottleANALYSIS OF THE SILVER GROUP CATIONS
4- Centrifuge