ANALYSIS OF THE
SILVER GROUP CATIONS
Ag
+
Pb
2+
Hg
2
2+
Analysis of a Mixture of Cations
O
ne problem often faced in qualitative analysis is to test for one ion in a
mixture of many ions. To find a test for one ion that is not interfered
with by another ion is nearly impossible. Therefore, if one has a mixture of
a large number of ions, the usual approach is to use a chemical method to
separate the mixture into subgroups that consist of just a few ions. Then it
may be possible to test for one particular ion in the presence of just one or
two others. Alternatively, each subgroup of just a few ions may be separated
further so that each ion in the subgroup ends up in a different test tube where
its presence can be confirmed by other chemical tests.
The chemical reactions encountered in qualitative analysis fall conve-
niently into four categories: (i) acid-base (proton transfer), (ii) precipitation,
(iii) complex formation, and (iv) oxidation-reduction (electron transfer).
Precipitation reactions are of particular importance in qualitative analysis (as
you have already seen in the analysis of anions), and they are important in the
silver group. In addition, acid-base, complexation, and even oxidation-reduc-
tion reactions are useful.
The
silver group
of ions — silver(I) (Ag
+
), lead(II) (Pb
2+
), and mercury(I)
(Hg
2
2+
) — is a chemically related subgroup of ions. In this experiment we
want to focus on this small group to show how to use the basic reaction
types—especially precipitation, acid-base, and complex formation—to sepa-
rate one ion from another and to confirm the presence of that ion.
CHEMISTRY OF THE SILVER GROUP CATIONS
Silver(I), lead(II), and mercury(I) are grouped together in qualitative analy-
sis schemes because they are the only common metal cations that form insoluble
precipitates with chloride ion. For example,
Ag
+
(aq) + Cl
-
(aq)
→ AgCl(s)
This means that, in a
mixture of metal cat-
ions, these three metal
ions can be separat-
ed from all others by
precipitating them as
their insoluble chlo-
rides, usually with 6
M HCl.
Once the precipi-
tates of AgCl, PbCl
2
,
and Hg
2
Cl
2
have been
Chemistry 112 Laboratory: Silver Group Analysis
Page 11
Revised: December 2005
Other metal cations
dissolved in water
Precipitates
AgCl, PbCl
2
, Hg
2
Cl
2
+ HCl(aq)
Mixture of metal cations
including
Ag
+
, Pb
2+
, Hg
2
2+
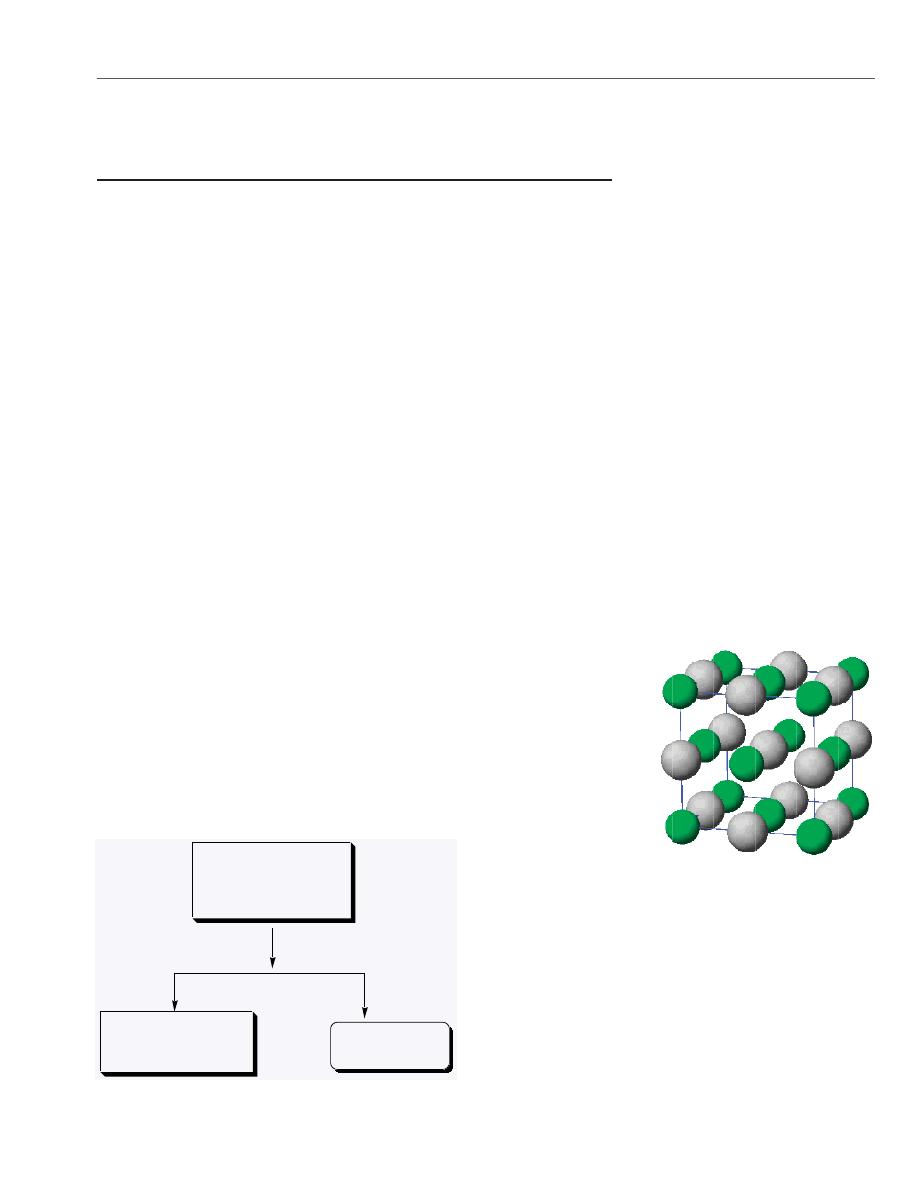
The structure of solid AgCl. Note
that this model of the unit cell of AgCl
has a net of 4 Ag
+
ions and 4 Cl
-
ions.
As you will see in Chapter 13 of
Chemistry & Chemical Reactivity, the
structure can be thought of as a face
centered cubic lattice of Cl
-
ions with
Ag
+
ions in the octahedral holes (like
NaCl). See the Models folder on the
General Chemistry Interactive CD-
ROM.
isolated from the solution containing the other metal cations, the three insol-
uble chlorides can be separated from one another by chemical means. To do
this, we exploit differences in the chemistry of the three ions according to the
separation scheme given on a separate sheet and in the table.
As you can see in the table of properties of the three silver group chlorides,
PbCl
2
is by far the most soluble of the three in water. Therefore, the first step
in separating the three chlorides is to treat the solid mixture with hot water
to selectively dissolve PbCl
2
and to leave AgCl and Hg
2
Cl
2
as a solid mixture.
Once Pb
2+
is back in aqueous solution, the presence of this ion in this solu-
tion can be confirmed by the addition of potassium chromate, K
2
CrO
4
. The
Pb
2+
ion and the chromate ion, CrO
4
2-
, combine to form the bright yellow,
insoluble solid lead(II) chromate, PbCrO
4
.
Pb
2+
(aq) + CrO
4
2-
(aq)
→ PbCrO
4
(bright yellow solid)
Silver(I) and mercury(I) chlorides are too insoluble in water to be redis-
solved in water, even when the water is boiled. Therefore, we turn to another
useful trick for dissolving precipitates: we take advantage of the tendency of
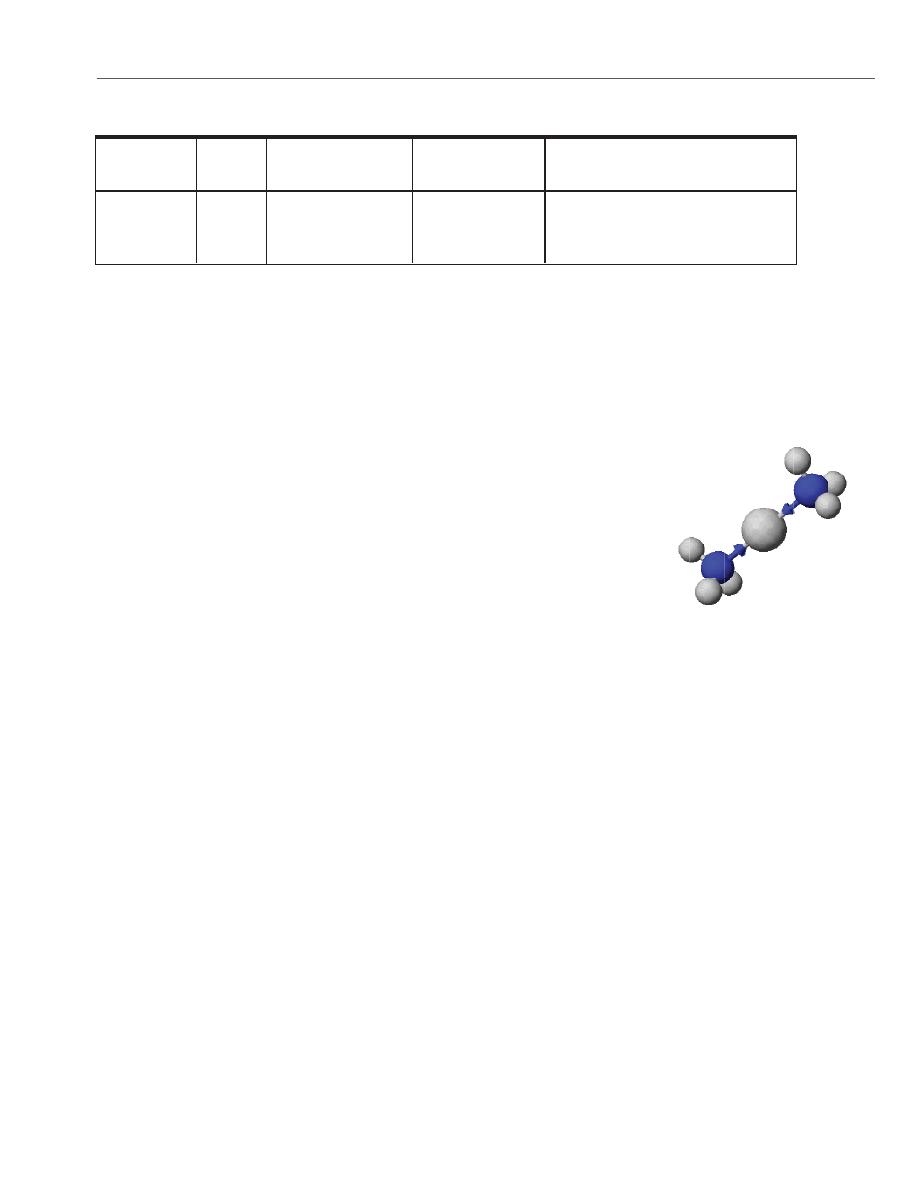
transition metal ions such as Ag+ to form water-soluble complex ions with
ammonia, NH3. Here the ammonia uses the lone pair of electrons of the N
atom to form a bond with the Ag+ ion.
[H
3
N :
→ Ag
+
← : NH
3
] , a water-soluble complex ion
When a large enough concentration of NH
3
is added to an insoluble precipi-
tate of AgCl, the ammonia binds to the Ag+ ion and forms the complex ion.
The net result is that the AgCl dissolves.
AgCl(s) + 2 NH
3
(aq) Æ [Ag(NH
3
)
2
]
+
(aq) + Cl
–
(aq)
In this way, the silver(I) ion is separated from the mercury(I) ion, as noted on
the attached separation scheme.
When relatively concentrated ammonia is added to the mixture of sol-
ids AgCl and Hg
2
Cl
2
, the silver chloride precipitate is dissolved. However,
Hg
2
Cl
2
also reacts with NH
3
, but in a different manner. Mercury(I) chloride
can undergo what is known as a disproportionation reaction. That is, the
mercury(I) ion is both oxidized [to mercury(II), Hg
2+
] and reduced (to
metallic mercury).
Hg
2
Cl
2
(s)
→ Hg(liq) + Hg
2+
(aq) + 2 Cl
–
(aq)
This reaction is induced by the presence of ammonia. In addition, when
ammonia and Cl
–
ion are present, the Hg
2+
ion forms a rather strange com-
pound, an amido salt HgClNH
2
. The latter is an insoluble white solid.
Hg
2+
(aq) + NH
3
(aq) + Cl
–
(aq) + H
2
O(liq)
→
HgClNH
2
(s) + H
3
O
+
(aq)
Chemistry 112 Laboratory: Silver Group Analysis
Page 12
Revised: December 2005
Compound
Color
Solubility (20 ˚C)
Solubility (100 ˚C)
Reaction with Ammonia
per 100 g H
2
O
per 100 g H
2
O
AgCl
white
0.00015 g
0.0021 g
forms colorless soluble complex
PbCl
2
white
1.0 g
3.3 g
forms white ppt. Pb(OH)Cl
Hg
2
Cl
2
white
0.0002 g
0.001 g
redox reaction; see text
The reaction of aqueous ammonia
with AgCl is illustrated and described
on page 779 of Chemistry & Chemical
Reactivity (6e).
Properties of the Chlorides of Silver Group Cations
The silver-ammonia complex ion,
Ag(NH
3
)
2
+
.

Therefore, when NH
3
is added to the AgCl/Hg
2
Cl
2
mixture the AgCl dis-
solves, as described above, and the Hg
2
Cl
2
turns into black or gray finely-
divided mercury metal and the white insoluble solid HgClNH
2
. The net reac-
tion for Hg
2
Cl
2
is
Hg
2
Cl
2
(s) + 2 NH
3
(aq)
→ HgClNH
2
(s) + Hg(0)(s) + NH
4
Cl(aq)
Notice that a second molecule of the base NH
3
is used to “collect” the H
+
, an
acid, that is produced when Hg
2+
reacts with NH
3
; the product is of course
the salt NH
4
Cl. The importance of this reaction is that it provides confirma-
tion of the presence of the mercury(I) ion, Hg
2
2+
, in a solution of unknown
composition.
If we had started with a mixture of AgCl, PbCl
2
, and Hg
2
Cl
2
, we have now
reached the point where the Pb
2+
ion has been separated and identified, the
Hg
2
Cl
2
has been converted to Hg(0) and HgClNH
2
, and the silver(I) ion is
in solution in the form of the complex ion [Ag(NH
3
)
2
]
+
(see the Separation
Scheme attached to this experiment). To prove that silver is present, we make
the solution containing the complex ion acidic with nitric acid. The acid (H
+
)
reacts with the base (NH
3
) to form the very stable ammonium ion. Thus, the
NH
3
is no longer bound to Ag
+
. Because Cl
–
is still present in solution, the
Ag
+
ion and the Cl
–
can once again combine to form the insoluble precipitate
AgCl.
[Ag(NH
3
)
2
]
+
(aq) + 2 H
3
O
+
(aq) + Cl
–
(aq)
→
AgCl(s) + 2 NH
4
+
(aq) + 2 H
2
O(liq)
A white precipitate at this point confirms that Ag
+
was in the original solu-
tion.
THE SILVER GROUP LABORATORY EXPERIMENT
To understand the chemistry of the silver group, you will first take a solution
that contains all three of the ions, the known solution, and perform the sepa-
ration given on the attached separation scheme. As indicated on the scheme,
begin with about 1 mL of the solution; follow the directions given on the
scheme and in the accompanying notes.
When you have completed separating and identifying the ions of the
known solution, and recording your observations, you are ready for an
unknown. Obtain the unknown from your instructor and determine the con-
tents of the solution, again being certain to write down all of your observa-
tions. Summarize your results in your notebook as follows:
Cations probably present _______________________
Cations probably absent _______________________
Show your instructor your results before leaving the laboratory, and they will be checked
for you.
WRITING UP THE NOTEBOOK
As you went through the experiment you described your observations and
intermediate conclusions. There is a final portion to the experimental write-
up that can be done outside of the laboratory. This consists of writing bal-
Chemistry 112 Laboratory: Silver Group Analysis
Page 13
Revised: December 2005
Be sure to record your observations at
each stage in your notebook.

anced equations for at least some of the reactions you observ ed and answering
some other questions regarding the experiment. Your grade on the laboratory
book depends on the overall quality of your write-up and on your answers to
the final questions.
For this experiment, write answers to the following questions in your labo-
ratory book.
1.
Write the balanced, net ionic equation for the reaction occurring when
(a)
Cl
-
is added to a solution containing Pb
2+
(b)
Cl
-
is added to a solution containing Hg
2
2+
2.
Write a balanced equation for the reaction that occurs on adding excess
aqueous ammonia to AgCl.
Chemistry 112 Laboratory: Silver Group Analysis
Page 14
Revised: December 2005
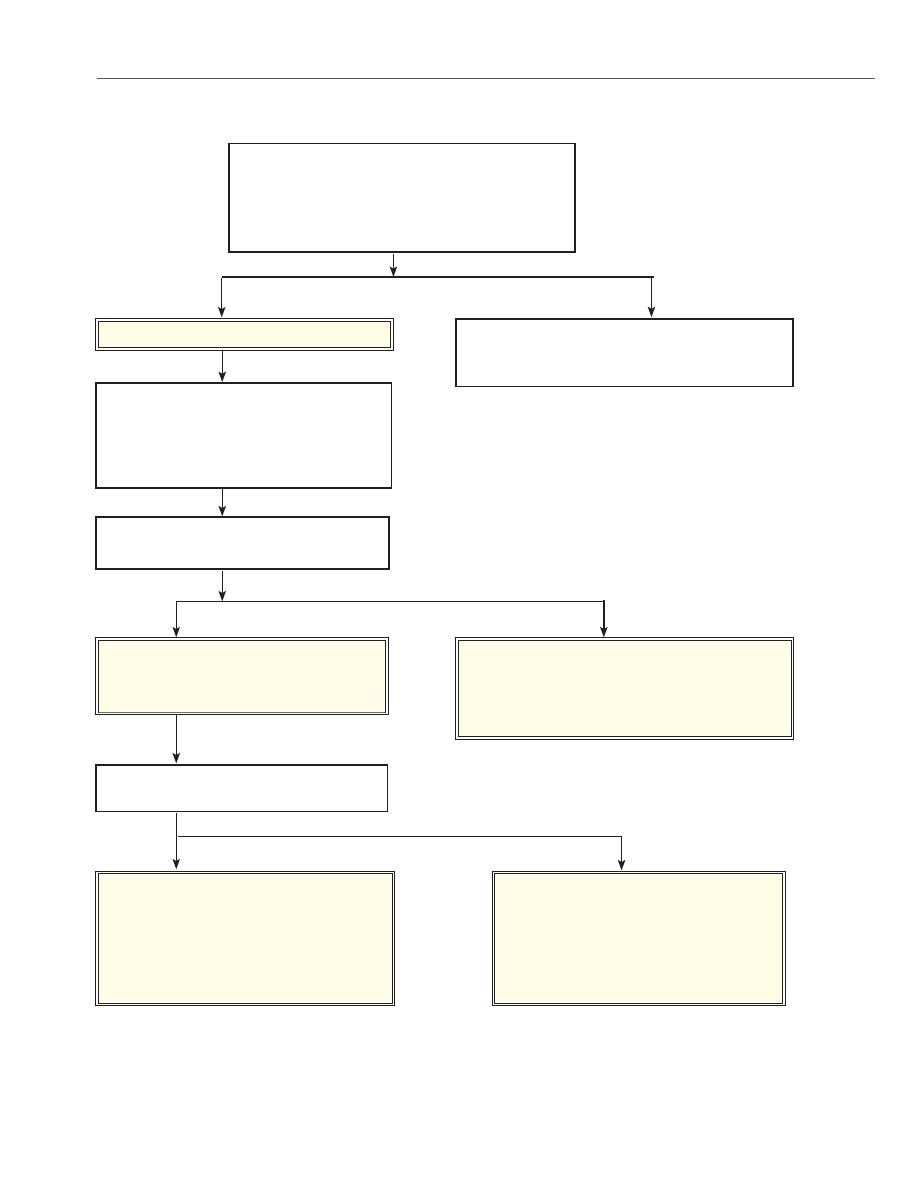
Chemistry 112 Laboratory: Silver Group Analysis
Page 15
Revised: December 2005
Begin with about 1 mL of known or unknown solu-
tion. Add 3-5 drops of chilled 6 M HCl. Centrifuge
and test for complete precipitation with another
drop of 6 M HCl. Centrifuge and decant.
Ppt. is AgCl, PbCl
2
, Hg
2
Cl
2
Discard supernatant solution after centrifuging
and testing for completeness of precipitation.
Add 20 drops (about 1 mL) of hot water.
Mix well. Heat in a boiling water bath, stir-
ring vigorously to dissolve all the PbCl
2
.
Centrifuge immediately (so the solution does
not cool) and decant.
Precipitate is AgCl and Hg
2
Cl
2
Wash twice with hot water to remove any
remaining PbCl
2
.
Solution contains Pb
2+
Test for Pb
2+
: Add 2 drops of 0.2 M K
2
CrO
4
.
Mix and centrifuge. Yellow precipitate of
PbCrO
4
confirms presence of Pb
2+
.
Add 10 drops of 6 M NH
3
. Mix well.
Centrifuge and decant.
Gray precipitate is HgNH
2
Cl + Hg Indicates
presence of Hg
2
2+
ion in original solution.
If a white ppt. is observed here it is probably
Pb(OH)Cl from Pb
2+
that was not removed com-
pletely.
Solution contains [Ag(NH
3
)
2
]
+
Add 6 M HNO
3
dropwise until solution
is acidic. (Be sure to stir before testing.) A
white ppt. is AgCl and indicates that Ag
+
was present in the original solution.
Scheme for Analysis of
Silver Group Cations

NOTES OF SILVER GROUP ANALYSIS
1.
Some observations that may be made early in the analysis often turn out to
be useful later. For example, AgCl acquires a slight purplish tint on stand-
ing in light for a while. Also, the crystals of PbCl
2
look more like snow than
the denser solid, AgCl.
2.
In separating these ions from others, it is important to add enough HCl
to get complete precipitation. Cold HCl is used because this gives a more
complete precipitation of the more soluble salt PbCl
2
. An excess of HCl
must be avoided because soluble complex ions such as AgCl
2
-
or PbCl
4
2-
may be formed.
3.
PbCl
2
may be slow to dissolve on heating. Stir well and make every effort
to make sure it is completely dissolved. Then you should centrifuge and
decant as quickly as possible, before the solution has a chance to cool and
reprecipitate the PbCl
2
.
4.
If the Pb
2+
ion is not completely separated from AgCl and Hg
2
Cl
2
, addi-
tion of NH
3
will produce an insoluble white basic salt, Pb(OH)Cl. This is
soluble in HNO
3
so it should not interfere with the confirming test for
silver, but it should not be confused with the white salt HgClNH
2
in the
confirmation of Hg
2
2+
.
5.
If the solution is quite basic from the addition of NH
3
, a substantial
amount of HNO
3
may be needed to precipitate AgCl. Stir the solution well
before testing for acidity. Remember that blue litmus paper turns red in acid.
6.
The yellow K
2
CrO
4
solution should not be mistaken for the yellow PbCrO
4
precipitate. Centrifuge before concluding that Pb
2+
is present or absent.
Chemistry 112 Laboratory: Silver Group Analysis
Page 16
Revised: December 2005
