Antiviral Drugs
1General principles:
Viruses are parasitic, i.e. they utilize:Host metabolic enzymes
Host ribosome for protein synthesis
Structure of viruses:
Nucleic acid “Core”: DNA or RNAOften contain crucial virus-specific “Enzymes”
Surrounded by “Protein”: “Capsid”
… and sometimes an outer lipid “Envelope”
Complete viral particle = “Virion”
“ Often visible by electron microscopy”2
HIV-1
3General principles:
DNA viruses:Based on viral genomic ds-DNA
Life cycle of a generic DNA virus:Virion often contains specialized enzymes
viral DNA/RNA polymerases etc.4
General principles
RNA viruses:Based on viral genomic ss-RNA
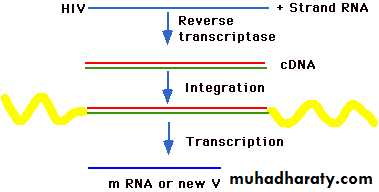
Example HIV-1:
HIV virion contains enzymes:
Reverse transcriptase
Integrases
Proteases
But note: NOT all RNA viruses are retroviruses
(e.g. influenza)5
6
7
DNA-based viruses Resultant disease
Herpes simplex types 1, 2 herpes (skin); encephalitis (brain)Varicella zoster chickenpox (children)
Herpes zoster shingles (adult)
Human papillomavirus warts (plantar, genital), cancer
Epstein-Barr virus Mononucleosis
Burkitt’s lymphoma;
nasopharyngeal carcinoma
Poxvirus smallpox; chickenpox
8
RNA-based viruses Resultant disease
HIV-1, HIV-2 HIV; AIDS
Rhinovirus respiratory/GI infections
(“common cold”)
Hepatitis A, B, C viruses Hepatitis
Influenza A, B, C viruses Influenza A, B, C
9
Approaches to treat viral diseases:
As viruses are intracellular parasites (utilizing host machinery), there are “Very few unique targets” in virusesThis distinguishes viruses from other infectious organisms:
(Bacteria, protozoa, fungi)Challenges in designing anti-viral treatments:
1-Host cell must be Immune to treatment.(to limit off-target toxicity)
2-Viral infection disease symptoms often associated with latency period.
10General anti-viral strategies are to inhibit:
1-Viral “Attachment” to host cell, penetration, and uncoating.
2-Viral “Enzymes”:DNA/RNA polymerases, etc
Reverse transcriptase, proteases, etc.
3-Host “Expression” of viral proteins.
4-”Assembly” of viral proteins.
5-”Release” of virus from cell surface membranes.
11
12
General anti-viral approaches:
1-Targeting influenza virus2-Specifically targeting DNA viruses (e.g. HSV)
3-Specifically targeting RNA viruses (e.g. HIV)
13
Antiviral drugs for influenza:
Are effective for both early treatment and Chemoprophylaxis of influenza infections.Likely the only virus-specific interventions available during the initial pandemic response, as a suitable vaccine is unlikely to be available for at least 6-8 months.
14
Classes of Influenza Antiviral Drugs
Adamantanes (M2 inhibitors)Amantadine
Rimantadine
Neuraminidase inhibitors
OseltamivirZanamivir
15
Amantadine, Rimantadine
Chemically relatedOrally administered (100 mg tablets and syrup for children)
Activity against Influenza A viruses only, through inhibiting replicationHave comparable antiviral and clinical activities when used for prophylaxis or treatment
1617
• 1-Interfere with the function of the transmembrane domain of the M2 protein of influenza A viruses.
• 2-Interfere with virus assembly during replication of influenza A viruses.
• 3-Decrease the release of influenza A viral particles into the host cell.
Amantadine, Rimantadine
Mechanism of Action:
18
• Decreases length of illness due to influenza A by about 1 day.
• Reduces shedding of influenza A viruses
• Must be started within 2 days of illness
• Placebo-controlled studies:
• both reduced fever, symptom severity, and time to resumption of normal activities
Amantadine, RimantadineTreatment (3-5 days)
19
• Well-absorbed, half-life 12-16 hours
• Excreted largely unchanged in the urine by glomerular filtration and tubular secretion
• Has the narrowest toxic to therapeutic ratio of available antivirals
• Dose adjustments required for relatively small decreases in renal function
• creatinine clearance <50-80 ml/min
• including those typically observed with aging
Amantadine Metabolism
20
• Commonly associated with dose-related minor CNS side effects
• anxiousness, difficulty concentrating, insomnia, lightheadedness
• Less often with severe CNS toxicities
• delirium, hallucinosis, acute psychosis, seizures, coma
• most often in older persons and those with pre-existing renal insufficiency, seizure disorders, or psychiatric illness
Amantadine Adverse Effects
21
• Rapid development of resistance to amantadine and rimantadine in 30% of treated patients (can develop in 2-5 days).
• Cross-resistance: viruses resistant to amantadine are also resistant to rimantadine
Amantadine, Rimantadine Resistance
22
Newer medications: became available in 1999
Have activity against both influenza A and influenza B virusesChemically related, but have different routes of administration
Neuraminidase Inhibitors Oseltamivir, Zanamivir23
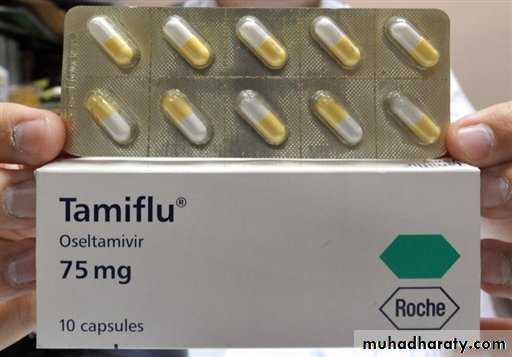
Oseltamivir (Tamiflu)
Zanamivir (Relenza)
24
25
26
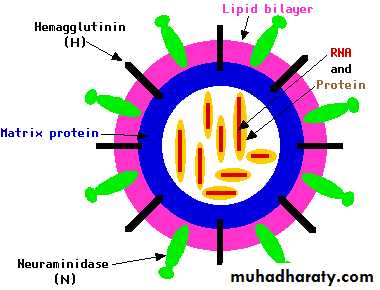
Block the active site of Neuraminidase, present in all influenza A and B viruses
Reduce the number of viral particles released from infected cells
Oseltamivir, ZanamivirMechanism of Action
27
28
Orally administered: 75 mg capsules and syrup for children
Approved for treatment and prophylaxis of influenza A and B
Treatment 1 yearChemoprophylaxis 13 years
Oseltamivir
29
Inhalational delivery of dry powered drug (5 mg per package) in a lactose carrier.
A proprietary device is used to deliver drug (Diskhaler)
Approved for treatment ofinfluenza A & B
Among those aged 7 years
Zanamivir
30Treatment
Must be administered < 48 hours after onset of illness
Reduce symptoms and decrease length of illness due to influenza A & B virus infections by approximately 1 dayDecrease viral shedding
Oseltamivir, ZanamivirTreatment: 5 Days
31
Prophylaxis
Oseltamivir and zanamivir are both approximately 80% effective in preventing illness.
Can prevent influenza in family members after one family member in the home has influenza.Uncontrolled studies of both oseltamivir and zanamivir report termination of nursing home outbreaks that continued despite the use of amantadine
Oseltamivir, Zanamivir
32
• Mild-to-moderate nausea/vomiting in 10-15% of adults; symptoms are not usually dose-limiting
• Fewer GI symptoms if given with food
• Only 1-2% stop because of adverse events
• Headache reported in older adults
• Cases of hypersensitivity reactions, rash, hepatotoxicity, and thrombocytopenia reported rarely
Oseltamivir Adverse Effects
33
Gastrointestinal (nausea, diarrhea)
HeadacheCough
Use in influenza-infected persons with pre-existing lower airway tract disease associated infrequently with bronchospasm
Rarely with a severe or fatal outcome
Not recommended in those with obstructive lung disease
Zanamivir
Adverse Effects
34
Amantadine:
Ages 1-9 years: 5 mg/kg/day divided twice daily
(not to exceed 150 mg per day)
Ages 10-64 years: 100 mg twice daily
Ages 65 years: 100 mg per day
Rimantadine:
Ages 1-9 years: 5 mg/kg/day divided twice daily(not to exceed 150 mg
Ages 10-64 years: 100 mg twice daily
Ages 65 years: 100 mg per day
Prophylaxis of Influenza A
35Oseltamivir:
Age 13 years: 75 mg per dayZanamivir
Prophylaxis of Influenza A & B
Specifically Targeting DNA viruses (HSV)Herpes simplex virus (HSV)
Cause of several painful skin/eye infectionsThe two most common types:
HSV-1: orofacial (cold sores on the mouth and lips)HSV-2: genital herpes
Both types:
can have dormancy periods (often for several year periods)
are infectious, but the potential is greatest during an outbreak
currently incurable but generally not fatal
Neonatal HSV (transmission from mother to child)
36
37
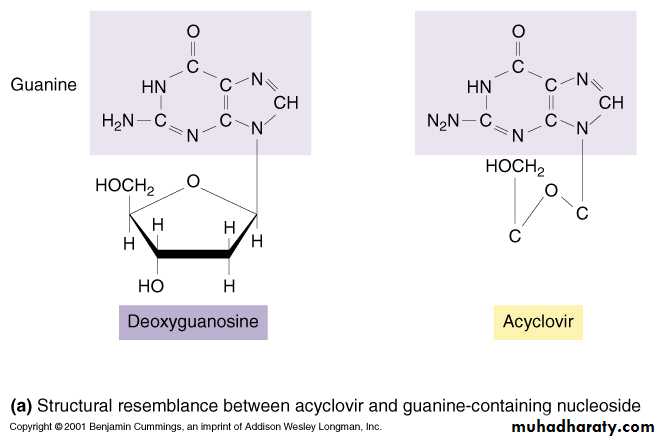
• Acyclovir
• Valacyclovir
• Famciclovir
• Penciclovir
• Idoxuridine
• These are Nucleoside derivatives
• Mechanism of Action:
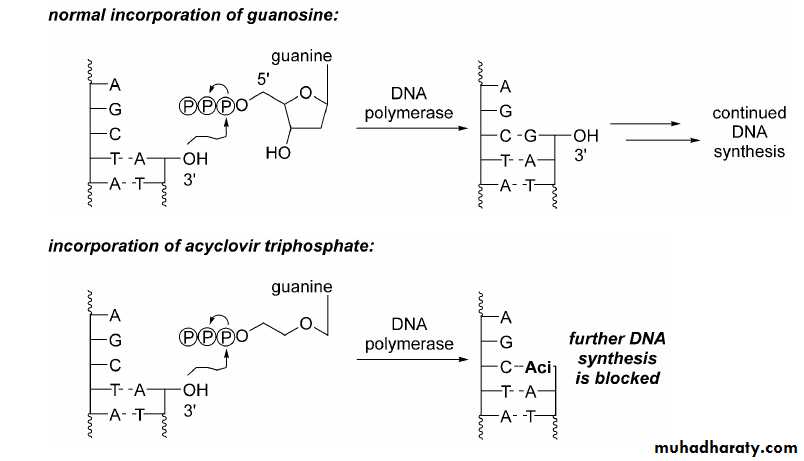
• Competitive inhibition of deoxy GTP for viral DNA polymerase
• Chain termination of viral DNA after incorporation
• Resistance mutants can develop
Antiherpes Agents
38
Two types of nucleotides:
Purine nucleotides
GuanineAdenine
Pyrimidine nucleotides
Thymine
Cytosine
Specifically Targeting DNA viruses (HSV)
Acyclovir: Mechanism of action
Step 1: Activation39
…so will “normal” (non-infected) cells be sensitive to this drug?
40Acyclovir: Mechanism of action
Step 2: incorporation into growing DNA chain41
42
Note similarity to 2-deoxythymidine: with iodxouridine base
4344
• Spectrum of Activity :
• HSV-1, HSV-2, VZV
• Oral, intravenous, topical formulation (Acyclovir)
• Excretion primarily by glomerular filtration and tubular secretion.
• Good tissue penetration 50 - 100%; CSF - 50% (Acyclovir)
Antiherpes Agents
45
• Clinical Uses:
• Genital herpes treatment and suppression varicella-Zoster
• Anti-CMV prophylaxis in organ transplants
• Herpes encephalitis
Antiherpes Agents
46
• Adverse Effects:
GIT disturbances (orally).
IV use →local inflammation if there is extravasationsrenal dysfunction (high doses or dehydrated patients) due to crystalluria
(adequate hydration + slow infusion rate).
Topically on the eye →stinging sensation.
Headache & CNS effects (confusion, lethargy & tremors).Antiherpes Agents
47
• 1-Ganciclovir
• 2-Foscarnet
• 3-Cidofovir
• 4-Valganciclovir
Anti-cytomegalovirus Agents
Drugs used to treat HIV viruses
Anti-Retroviral Drugs
48
49
50
Classes if anti retroviral drugs:
1-Neucloeside reverse transcriptase inhibitors. (NRTIs)2- Non-Nucleoside reverse transcriptase inhibitors. (NNRTIs)
3-Protease inhibitors. (PI)51
52
Nucleoside Reverse Transcriptase Inhibitors (NRTIs)
Lamivudine
ZidovudineDidanosine
Stavudine
53
• These are Nucleoside derivatives
• Competitive inhibitors of reverse transcriptase
• Incorporated in DNA leading to chain termination
• Effective against HIV-1 & HIV-2
• “Resistance develops with monotherapy”
54
• Clinical Uses:
• HIV-infection with features of AIDS.
• Prophylaxis for contacts (Zidovudine)
• Prevent maternal to fetal transmission (Zidovudine).
55
• Adverse Effects:
• Zidovudine:
• Myelosuppression, Anemia, Neurpenia, GI intolerance, Headaches, Insomnia, Myopathy, Lactic Acidosis, Hepatotoxicity.
• Didanosine:
• Pancreatitis, Peripheral Neuropathy, Diarrhoea, Hyperuricemia
• Stavudine:
• Peripheral Neuropathy
56
• Nevirapine
• Delavirdine
• Efavirenz
• Reverse transcriptase inhibitors
• Inhibit RNA- and DNA- dependent DNA polymerases
Non-nucleoside Reverse Transcriptase Inhibitors (NNRTIs)
57
• Adverse Effects:
• Severe skin rashes,
• Hepatitis,
• Nausea,
• Headache.
58
• Saquinavir
• Ritonavir
• Indinavir
• Nelfinavir
• Inhibitors of viral protease which are essential for production of mature infectious virions
• Resistance and cross-resistance develops
Protease Inhibitors (PI)
59
• Good Oral bioavailability
• Metabolized by CYP450 isoenzymes like CYP3A4, CYP2D6
• “Drug - drug interactions common’
• Uses:
• Antiretroviral therapy in combination with NRTIs and NNRITs.
60
• Altered body Fat distribution (buffalo lump and truncal obesity, facial and peripheral atrophy).
• Insulin resistance.
• Hyperlipidemia.
• GI intolerance.
• Nephrolithiasis (Indinavir)
Protease InhibitorsAdverse Effects
61
Treatment of AIDS
The most effective combinations so called
highly active anti- retroviral therapy
(HAART)
This comprises
1-Two nucleoside reverse transcriptase inhibitors (NRTIS).
2- With either:
Non-nucleoside reverse transcriptase inhibitor (NNRTI)
Or
Protease inhibitor (PI).
HAART
This combination produces profound suppression of viral replication and results in useful restoration of immune system.Treatment of AIDS
62Anti-Malarial Drugs
63Life cycle of malarial parasite
64
65
1-Treatment of malaria:
A- initial treatment.B- prevention of relapse(radical cure).
2-Prevention of malaria (chemoprophylaxis).
Drugs used in malaria66
• Quinine
• Chloroquine• Sulfadoxine + pyrimethamine (fansidar)
• Primaquine
• Mefloquine
• Proguanil
Therapeutic options
67
Quinine 600 mg 3 times daily for 3-5 days
Reduce to twice if toxicity appears.
Followed by single dose of sulfadoxine 1.5gm with pyrimethamine 75 mg(Fansidar)
(3 tablets)
Or
Doxycycline 100 mg daily for 7 days
Treatment of plasmodium falciparum
68
Chloroquine 150 mg tab.
600 mg initially300 mg after 6 hours
150 mg 12 hourly for 2 days
10 tablets
Plasmodium vivax , ovale ,malariae69
Primaquine 15 mg daily for 14 days
Haemolysis in patients with G6PD deficiency
Methaemoglobinaemia leading to cyanosis is more common but
“Not dangerous”
Prevention of relapse
70
When should we start the prophylaxis?
chemoprophylaxis71
1 week before entering
4 weeks after leavingThe endemic area
Malaria prophylaxis72
1-Chloroquine resistance absent or low:
Chloroquine tablet 150 mg 2 tab. weeklyOr
Proguanil 100 mg tab daily
Malaria prophylaxis73
2-Chloroquine resistance is high
Mefloquine 250 mg week(neuro psychiatric symptoms conductive disorders)
Or
Doxycyclin 100 mg dailyMalaria prophylaxis
74
Half life = 50 days
Safe in short treatmentpruritus headache nausea ….etc
Adverse effects in long term:
Corneal effect
Retinal effects
Chloroquine Adverse reactions
75
Corneal deposits:
Asymptomatic.Halos around light.
Photophobia.
Reversible on stopping the treatment
Corneal effects
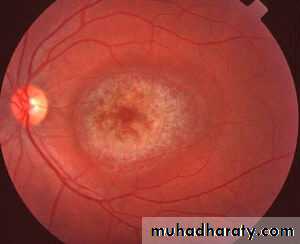
76More serous
IrreversibleEarly visual field defects.
Late macular pigmentation.
Ring of pigment
“Bull’s eye macula”
Scotomas, photophobia, defects in vision
Blindness
Acute Overdose is rapidly fatal
Retinal toxicity
77
78
Ophthalmoscope
7980
Hale life = 9 hours but increase to 18 hours in malaria
Adverse effects:Tinnitus
Reduced auditory acuityHeadache blurred vision nausea diarrhea
“Acute cinchonism”
Quinine, Quinidine, Salicylate
Hypoglycemia
Quinine:
Hypotension
Disturbed AV conductionCardiac arrest
Acute overdose of Quinine