1
Fifth stage
Pediatric
Lec-2
.د
خليل
19/10/2016
The cardiovascular system
Acyanotic Congenital Heart Disease
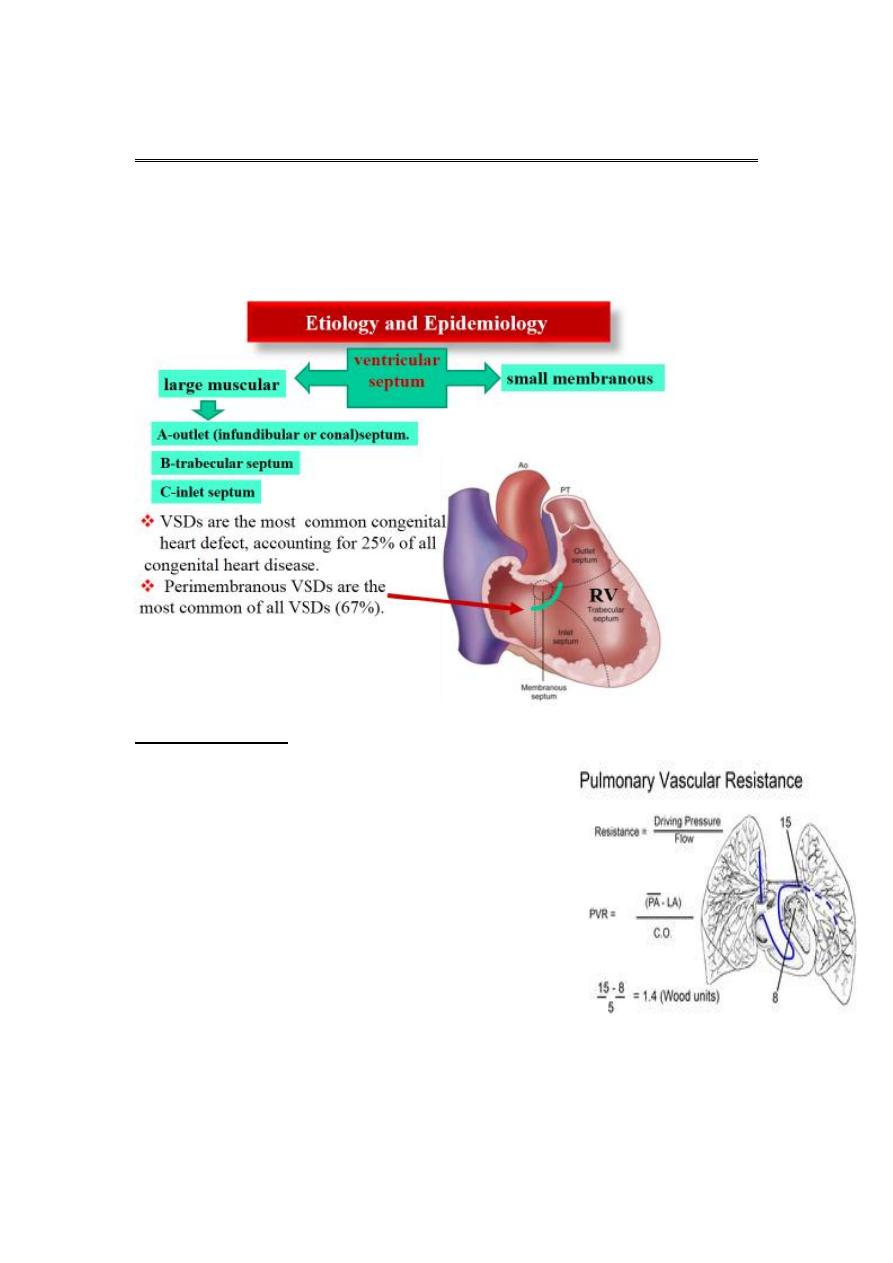
VENTRICULAR SEPTAL DEFECT
Pathophysiology:-
o The amount of flow crossing a VSD
depends on the size of the defect and the
pulmonary vascular resistance.
o Even large VSDs are not symptomatic at
birth because the pulmonary vascular
resistance is normally elevated at this
time.
o As the pulmonary vascular resistance
normally decreases over the first 6to 8
Weeks of life, however,
o the amount of shunt increases, and symptoms may develop.
2
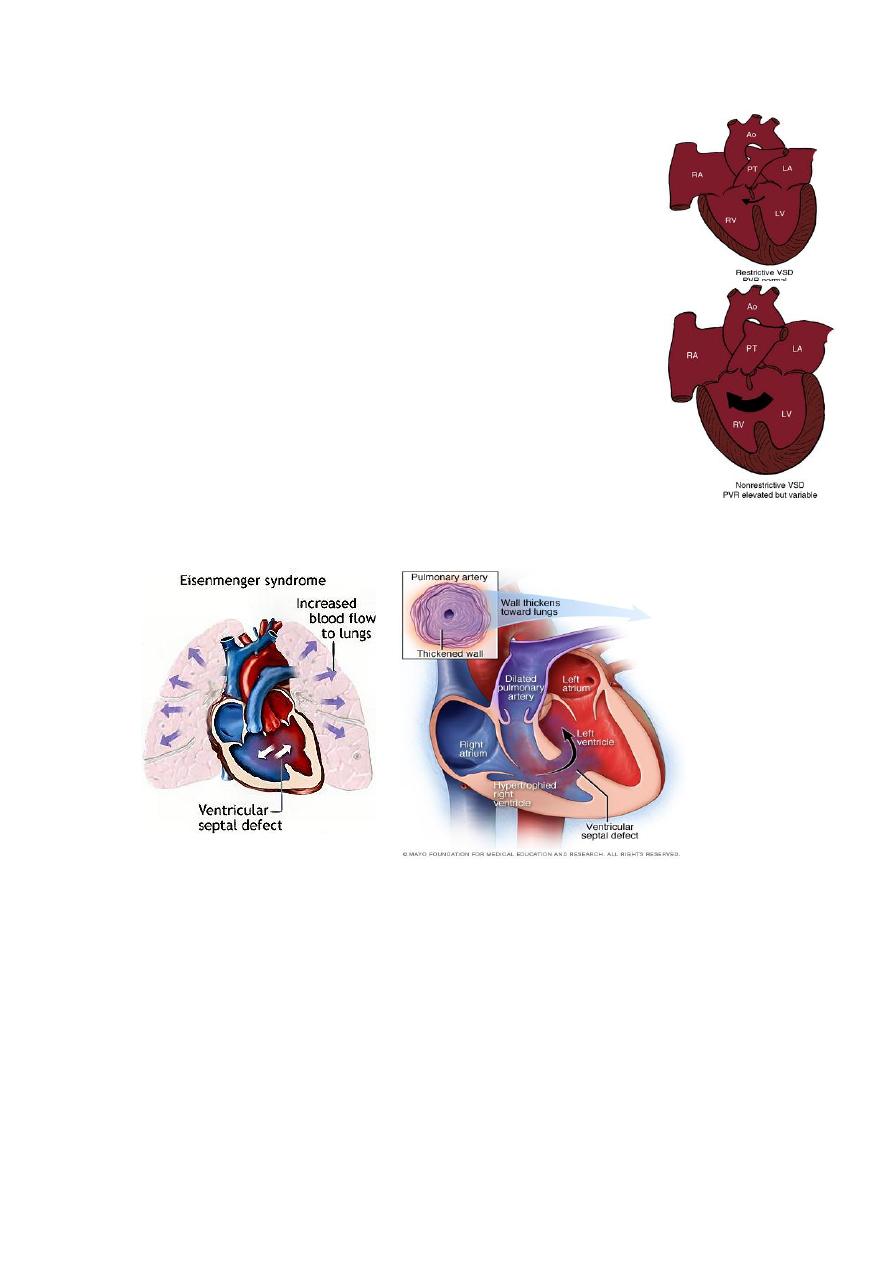
• When a small communication is present (usually <0.5
cm2), the VSD is called restrictive and right
ventricularpressure is normal.
• In large nonrestrictive VSDs (usually >1.0 cm2), right
and
left ventricular pressure is equalized. In these defects,
the direction of shunting and shunt magnitude are
determined by the ratio of pulmonary to systemic vascular
resistance
Chronic left-to-right shunt causes gradual ↑pulmonary
vascular pressure, ↓gradient between ventricles, and ↓
shunt volume, and finally pulmonary hypertension and right
to left shunt, a condition called Eisenmengers’s syndrome
3
Clinical manifestations
o Small VSDs, with little shunt, are often asymptomatic, other than
a loud murmur.
o Moderate to large VSDs result in pulmonary overcirculation and
CHF, presenting as fatigue, diaphoresis with feedings, and poor
growth.
o The typical physical finding with a VSD is a pansystolic murmur
usually heard best at the lower left sternal border. There may be a
thrill in the same region.
Investigations
Electrocardiography
o With a small VSD, the ECG is normal.
o With a moderate VSD, left ventricular hypertrophy (LVH) and
occasional left atrial hypertrophy (LAH) may be seen.
o With a large defect, the ECG shows biventricular hypertrophy
(BVH) with or without LAH .
o If pulmonary vascular obstructive disease develops, the ECG
shows RVH only.
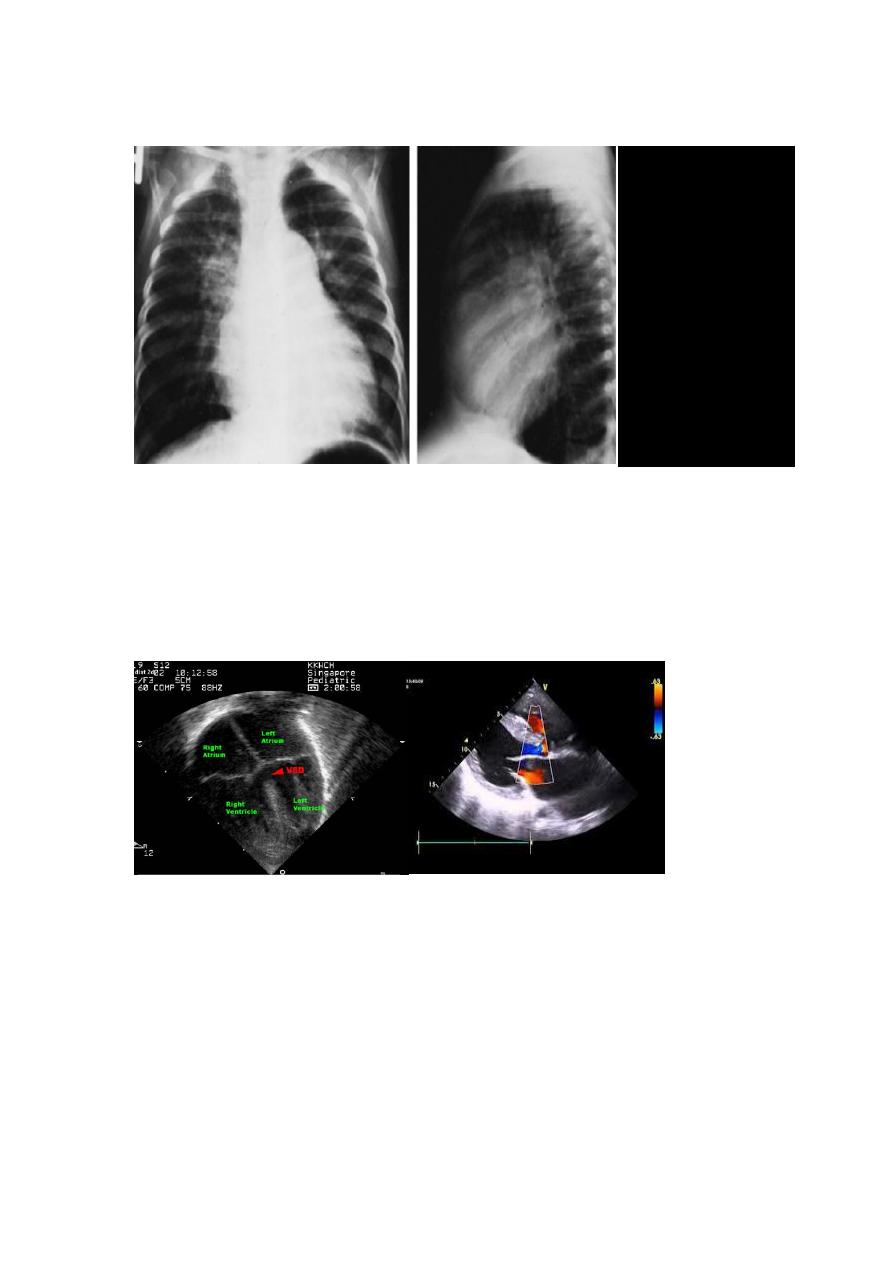
X-ray Studies
With large VSD cardiomegaly of varying degrees is present and involves
the LA, left ventricle (LV), and sometimes RV. Pulmonary vascular
markings increase. The degree of cardiomegaly and the increase in
pulmonary vascular markings directly relate to the magnitude of the Lt
to Rt shunt.
4
Echocardiography.
Two-dimensional and Doppler echo studies can identify the number,
size, and exact location of the defect; estimate PA pressure; identify
other associated defects; and estimate the magnitude of the shunt
.
CXR of 6 years old
child PA and lateral
views showing
cardiac
enlargement and
increased
pulmonary
markings

5
NATURAL HISTORY
o Spontaneous closure occurs in 30% to 40% of patients with
membranous VSDs and muscular VSDs during the first 6 months of
life. It occurs more frequently in small defects.The vast majority of
defects that close do so before the age of 4 yr
o CHF develops in infants with large VSDs but usually not until 6 to 8
weeks of age.
o Pulmonary vascular obstructive disease may begin to develop as
early as 6 to 12 months of age in patients with large VSDs, but the
resulting right-to-left shunt usually does not develop until the
teenage years.
o Repeated chest infections and arrhythmias.
o Infective endocarditis rarely occurs.
Treatment
• Small VSDs usually close spontaneously; if they do not close,
surgical closure may not be required.
• Initial treatment for moderate to large VSDs includes diuretics
and digoxin. Continued poor growth or pulmonary hypertension
despite therapy requires closure of the defect. Most VSDs are
closed in surgery, but some VSDs, especially muscular defects, can
be closed with devices placed at cardiac catheterization
6
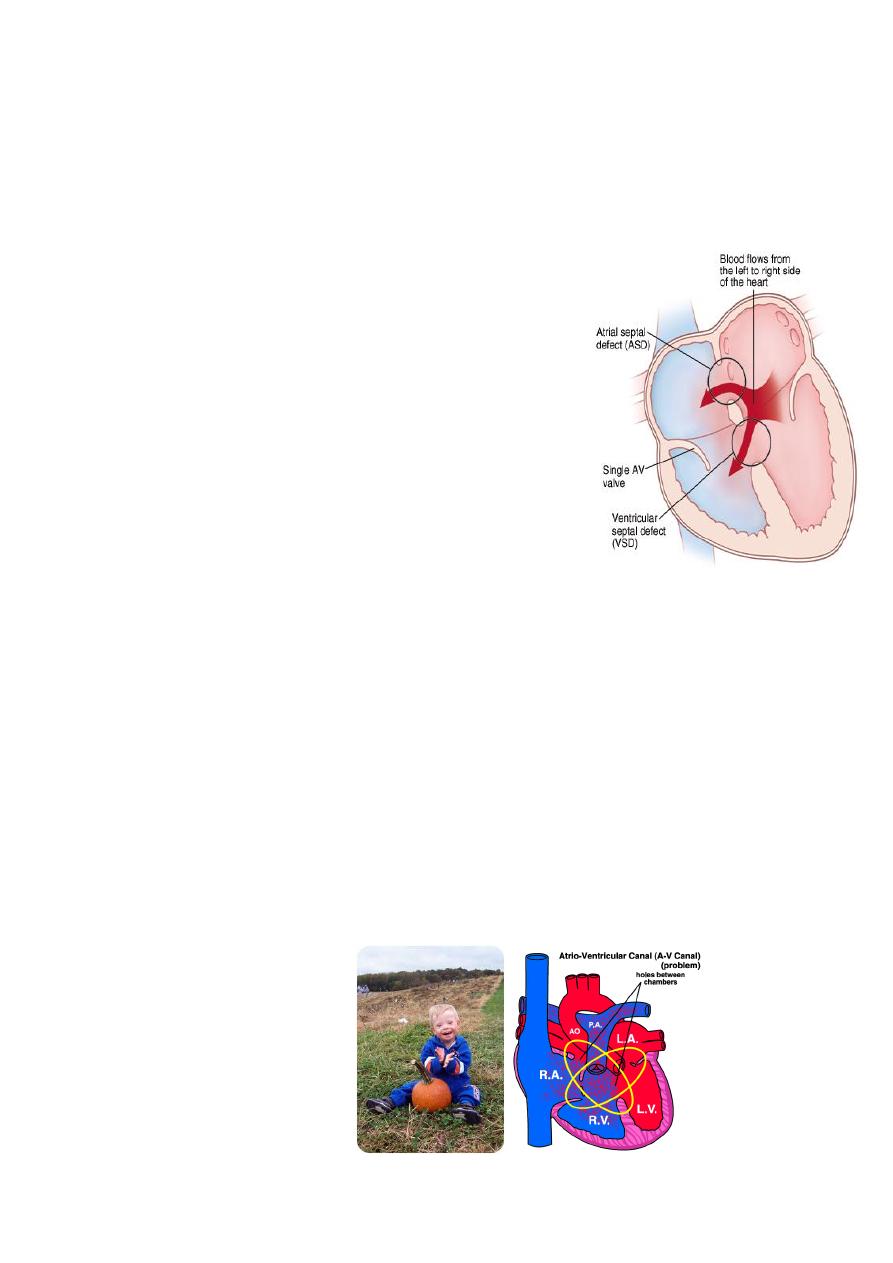
ENDOCARDIAL CUSHION DEFECT (atrioventricular septal
defect ) (AV canal defect)
Etiology and Epidemiology
The defect occurs as the result of abnormal
development of the endocardial cushion tissue,
resulting in failure of the septum to fuse with the
endo-cardial cushion; this results in abnormal AV
valves as well.
The complete defect results in a primum ASD, a
posterior or inlet VSD, and clefts in the anterior
leaflet of the mitral and septal leaflet of the
tricuspid valves. In addition to left-to-right shunting
at both levels, there may be atrioventricular
valvular insufficiency. The partial defect is
presented as ASD primum only.
Clinical manifestation:
The symptoms of CHF usually develop as the pulmonary vascular
resistance decreases over the first 6 to 8 weeks of life.
Growth is usually poor.
Many children with Down’s syndrome have complete endocardial
cushion defects .
Pulmonary hypertension resulting from increased pulmonary
circulation often develops early; this results in a prominent S2 .
7
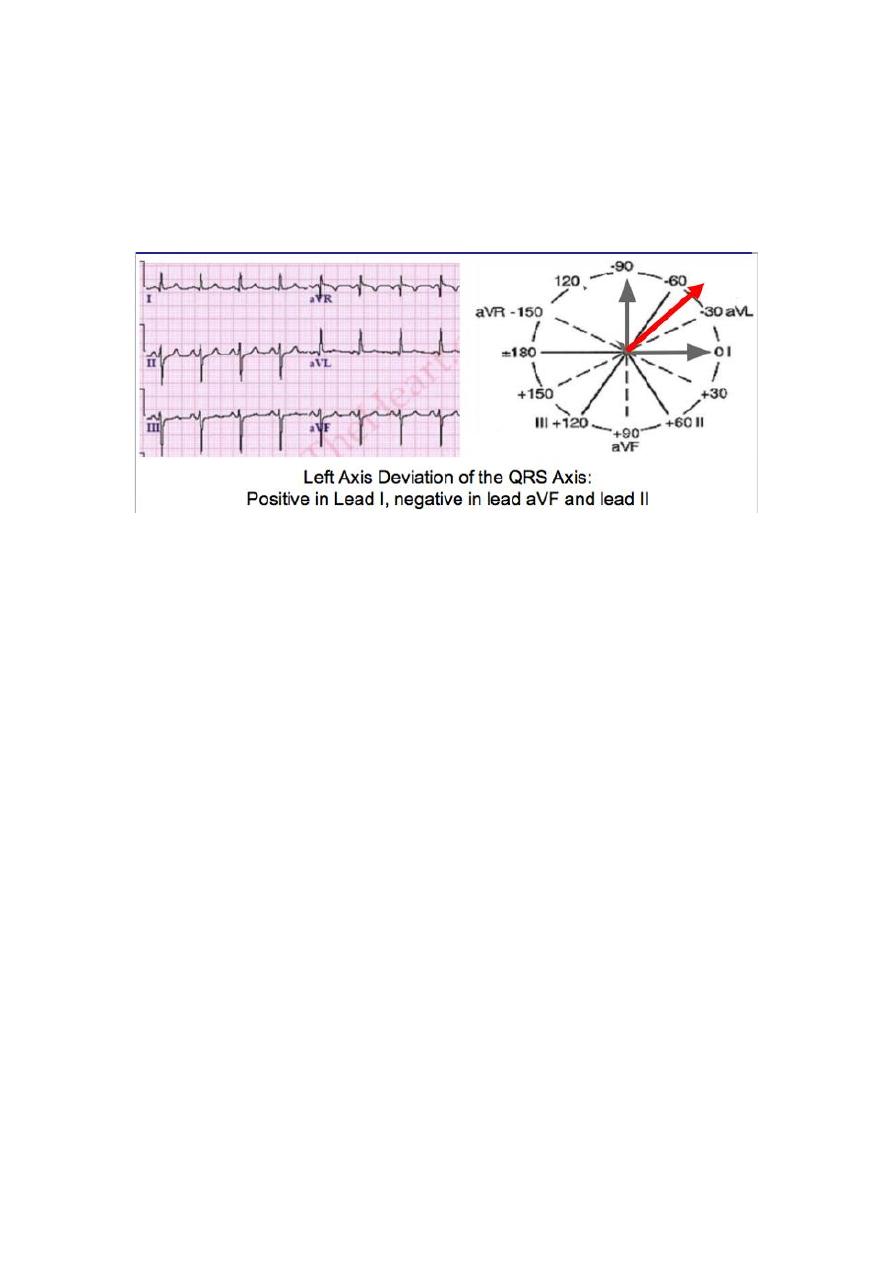
Investigation :
o An ECG reveals left axis deviation and combined ventricular
hypertrophy and may show combined atrial enlargemen
o CXR shows cardiomegaly and increased pulmonary vascular
markings
o Echocaediography is diagnostic and shows the details of the
defect.
Treatment
o Initial management includes digoxin and diuretics for treatment of
CHF .
o Surgical repair of the entire defect ultimately is required,
however .
8
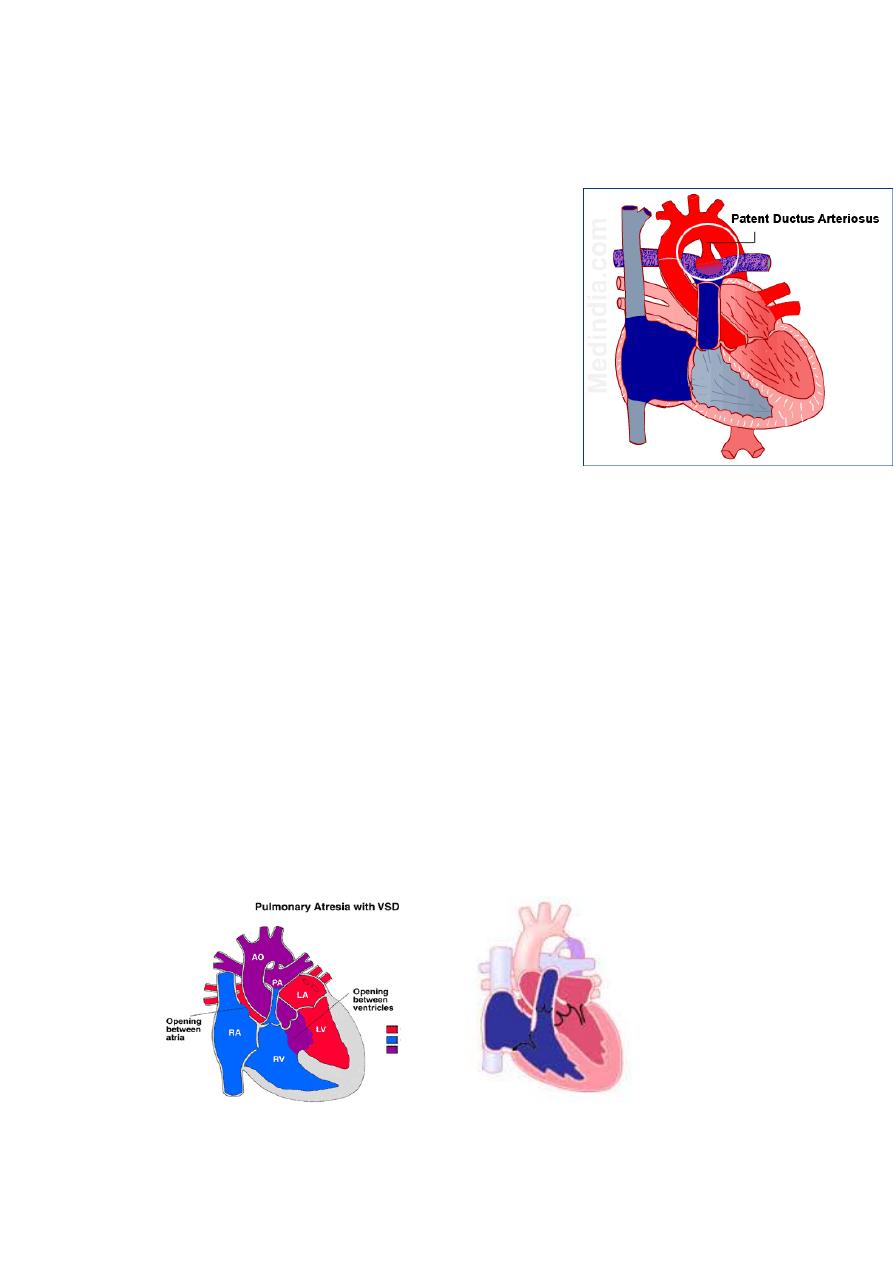
Patent ductus arteriosus
During fetal life, most of the pulmonary
arterial blood is shunted through the ductus
arteriosus into the aorta .
Functional closure of the ductus normally
occurs soon after birth, but if the ductus
remains patent when pulmonary vascular
resistance falls, aortic blood is shunted into
the pulmonary artery.
The aortic end of the ductus is just distal to
the origin of the left subclavian artery, and
the ductus enters the pulmonary artery at
its bifurcation.
Female patients with PDA outnumber males 2 : 1.
Epidemiology
PDA is more common in premature babies and is associated with
maternal rubell infection
PDA is seen in 10% of patients with other congenital heart lesions
and often plays a critical role in providing pulmonary blood flow
when the right ventricular outflow tract is stenotic or atretic or in
providing systemic blood flow in the presence of aortic
coarctation or interruption
9
Pathophysiology
As a result of the higher aortic pressure, blood shunts left to right
through the ductus, from the aorta to the pulmonary artery.
If the PDA is small, pressure within the pulmonary artery, the right
ventricle, and the right atrium is normal.
If the PDA is large pulmonary artery pressure may be elevated to
systemic levels during both systole and diastole. Patients with a
large PDA are at extremely high risk for the development of
pulmonary vascular disease if left unoperated.
Pulse pressure is wide because of runoff of blood into the
pulmonary artery during diastole.
Clinical manifestation:
Symptoms
They are more less similar to VSD
Patients with small PDAs are asymptomatic.
Moderate to larger shunts produce the symptoms of CHF as the
pulmonary vascular resistance decreases over the first 6 to 8
weeks of life.
11
Physical examination
A widened pulse pressure .
A thrill may be palpable with hyperdynamic precordium.
A continuous machine-like murmur can be heard at the left
infraclavicular area, and the murmur radiates along the pulmonary
arteries and is often well heard over the left back.
Investigations
The chest X-ray and ECG are usually normal with small PDA, but if
the PDA is large and symptomatic the features on chest X-ray and
ECG are indistinguishable from those seen in a patient with a large
VSD.
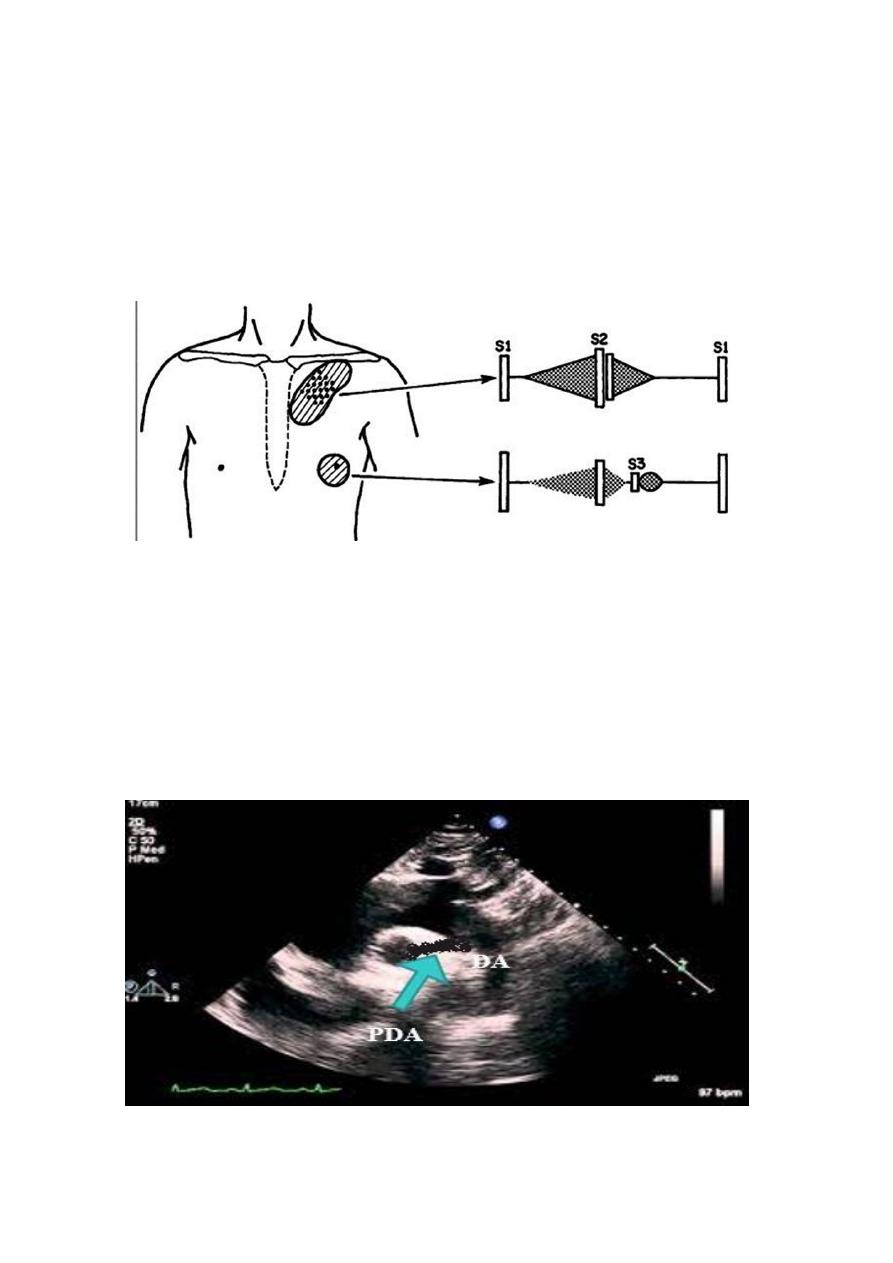
Echocardiography show the PDA and the cardiac chambers.
11
Differential Diagnosis
o Aortopulmonary window,
o truncus arteriosus,
o ventricular septal defect with aortic regurgitation
o , or arteriovenous fistula.
Natural HX;
o Unlike that in premature infants, spontaneous closure of a PDA
does not usually occur in full-term infants and children.
o CHF or recurrent pneumonia or both develop if the shunt is large.
o Pulmonary vascular obstructive disease may develop if a large PDA
with pulmonary hypertension is left untreated.
o Infective endocarditis may occur.
o .Although rare, an aneurysm of PDA may develop and possibly
rupture in adult life.
TREATMENT
o Irrespective of age, patients with PDA require surgical or
transcatheter closure.
