1
NUTRITIONAL SUPPORT OF THE SURGICAL PATIENT
DR. N P LATCHMANAN
COMMENTATORS : DR V. PADAYACHY, P REDDY
MODERATOR : PROF. AA HAFFEJEE
INTRODUCTION
While most patients undergoing elective operations easily tolerate a brief period of
perioperative starvation, as many as 50 per cent of hospital patients may be nutritionally
compromised, depending on the definition. Nutritional status directly impacts on patient
care and adequate attention to nutritional issues can help to minimize morbidity and
complications
Steps to Optimal Nutrition Support
The five steps to optimal nutrition support are:
1) Begin when the benefits are likely to exceed the risk,
2) Set protein and calorie goals,
3) Choose and establish a method for administering the nutrients, enteral (site and
route) or parenteral (peripheral or central),
4) Choose or design a formula suitable for the particular patient, and
5) Monitor the patient for adequacy of nutrient intake and to avoid or minimize
complications.
NUTRITIONAL REQUIREMENTS
Protein
Protein is perhaps the most important nutrient. Although protein can be degraded and
used for gluconeogenesis, this yields only one-fourth of the energy required for protein
synthesis and is thus a wasteful process. A primary goal in nutritional support is to
provide adequate non-protein sources of fuel so that protein catabolism is minimized.

2
Protein requirements
The average normal protein requirement of 'high biologic value protein' is 0.8 g/kg or 56
to 60 g/day. And may increase to 1-2g/kg/day in stressed patients.
Assuming an adequate non-protein energy supply, most amino acids can be recycled. In
this fashion, only small amounts of essential amino acids are needed in order to maintain
nitrogen equilibrium. In adults, 19 to 20 per cent of protein intake should be essential
amino acids. This percentage should increase with depletion or injury.
Amino Acids
Humans cannot synthesize the essential amino acids. Thus, they must be obtained from
the diet. In addition, several other amino acids are conditionally indispensable, as their
low synthetic rates may be exceeded by increased requirements, especially in infants..
The semi-essential amino acid glutamine has recently received a great deal of attention.
Glutamine is abundant in the circulation and serves as a precursor for other amino acids
and proteins. Glutamine also serves as the major energy substrate for the intestinal
mucosa, as a nitrogen transporter between organs, and as an important route of ammonia
detoxification during acidemia. Enteral glutamine supplementation may promote small
bowel adaptation and lead to increased intestinal mucosa villous height and enterocyte
protein content, but this effect is not universally seen with parenteral glutamine
Nitrogen losses and balance
One gram of nitrogen equals 6.25 g of protein.
Obligate nitrogen losses are 56 to 57 mg/kg per day.
N
loss
= 24 h urinary urea nitrogen + 4 g/day (fecal and non-urinaryloss)
Calories
There are three major sources of energy: protein, fat, and carbohydrates. Of normal daily
energy expenditure, 85 per cent is from fat and carbohydrates, and 15 per cent from
protein.
Protein as an energy source
Of the 15 per cent of normal daily energy expenditure supplied by protein, approximately
50 per cent of this is through direct oxidation of branched chain amino acids to high-
energy phosphate. The remainder is via gluconeogenesis. Protein breakdown yields
4 kcal/g

3
Carbohydrates as an energy source
Glucose yields about 4 kcal/g through glycolysis and the tricarboxylic acid cycle.
Glycogen breakdown yields only 1 to 2 kcal/g. Most carbohydrates in parenteral nutrition
are supplied in the form of dextrose, which provides 3.4 kcal/g.
Glycogen stores, however, are exhausted within 24 h of initiation of a fast, and the body
then becomes dependent on gluconeogenesis as a source of glucose.
At least 400 cal/day in the form of exogenously administered glucose are required to
minimize proteolysis.
Fat as an energy source
Fat about 9 kcal/g. After lipolysis, free fatty acids are released into the circulation. Free
fatty acids actually circulate bound non-covalently to albumin. Nearly all tissues, with the
notable exception of the brain, can utilize fatty acids as an energy source
Determination of caloric needs
Caloric needs are related to the metabolic rate, which in turn is demonstrated by the
formula:
metabolic rate = 4.83 × Vo
2
where Vo
2
is oxygen consumption in liters per unit of time. Furthermore, the respiratory
quotient (RQ), a ratio of carbon dioxide produced to oxygen consumed, can be used to
estimate utilization of the various caloric sources by the following formula:
RQ = Vco
2
/ Vo
2
An RQ of 1 is consistent with pure carbohydrate utilization.
An RQ of 0.7 is consistent with utilization of fat.
An RQ of less than 0.7 indicates ketogenesis.
In clinical practice, and RQ of greater than 1 is uncommon, but this may indicate
overfeeding.
An RQ of between 0.8 and 1, indicating mixed substrate utilization, is desirable.
The basal energy expenditure (BEE) can be estimated from the

4
Harris–Benedict equation:
BEE (men) = 66.47 + [13.75 × W] + [5 × H] – [6.76 × A]
BEE (women) = 655.1 + [9.56 × W] + [1.85 × H] – [4.68 × A]
where W is weight in kilograms, H is height in centimetres, and A is age in years.
The additional caloric needs due to illness or other metabolic stress can then be calculated
by multiplying the BEE by an injury factor:
minor operation = 1.2 (20 per cent increase)
skeletal trauma = 1.35 (35 per cent increase)
major sepsis = 1.6 (60 per cent increase)
severe thermal injury = 2.10 (110 per cent increase)
This estimate can be further refined to account for activity by multiplication by 1.2 if the
patient is confined to bed and 1.3 if the patient is not confined to bed. Therefore, caloric
needs equal:
BEE × injury factor × activity factor
Calorie to nitrogen ratio
Non protein energy is usually required at 30-35 kcal/kg/day
A non pretein energy : Nitrogen ratio of 100 – 200 is required to prevent protein
breakdown for energy
5
NUTRITIONAL ASSESSMENT
PARAMETERS
1. Clinical:
General appearance (oedema, ascites, cahexia, obesity, skin changes)
2. Anthropometry
• Midarm muscle circumference: < 23cm (male) & < 22cm (female) indicates
malnutrition.
• Triceps skinfold thickness: < 10mm (male) & < 13mm (female) indicates
malnutrition.
• Handgrip dynometry .
Body Mass Index (kg/m
2
)
=
18.5-25
=
normal
=
< 15
=
significant increase in morbidity
= <
18.5
=
associated
with longer hospital stay
=
> 30
=
obesity, increased incidence of heart disease,
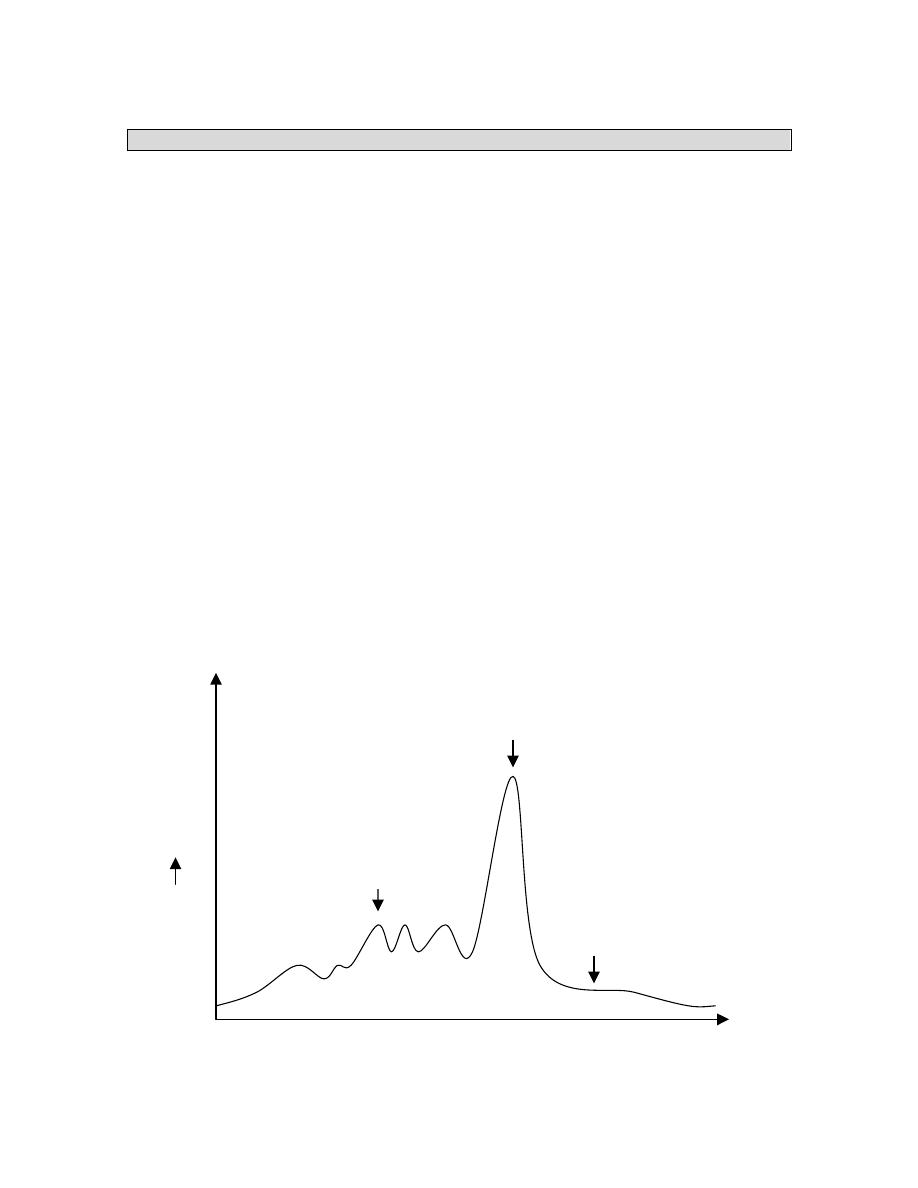
3 Biochemical Markers
i) Plasma
Proteins
OPTICAL
DENSITY
MIGRATION DISTANCE
Transferrin
8 day ½ life
Albumin
20 day ½ life
Transthyretin
Pre albumin
2 day ½ life

6
Serum Albumin Concentration: Good Predictor of Outcome, Poor Marker for
Nutrition Status
Serum albumin is an exceedingly poor indicator of nutrition status because it is very
insensitive and nonspecific. Infections and other inflammatory conditions which elevate
inflammatory cytokines cause a decrease in albumin synthesis and also cause capillary
leak resulting in an increased volume of distribution of albumin. Consequently, in sepsis
and burns, serum albumin concentration may decrease, more than 1 g/dl in 24 hours. In
contrast to its poor performance as an indicator of nutrition status, many studies have
documented that serum albumin concentration is a good predictor of morbidity and
mortality in many conditions.
Serum Proteins and Nutrition Status
Serum (visceral) proteins with a short half-life such as transferrin (8 days) and
transthyretin (prealbumin) (2 days) respond more quickly to declining or improving
nutrition status than does albumin, which has a half-life of 20 days. However, all three
are negative acute phase reactants, that is, the serum levels decrease rapidly when
catabolic inflammatory cytokines are released by infection or trauma. Also, the
transferrin level is increased by iron deficiency while the transthyretin level is increased
by renal failure and decreased by vitamin A deficiency. If these confounding factors are
kept in mind, transthyretin measurements and to a lesser extent transferrin measurements,
can be useful tools for assessing nutrition status. An undernourished patient with low
serum transthyretin will show a significant increase in transthyretin concentration after as
little as one week of adequate protein and calorie intake. Note: the quantity of
transthyretin in normal serum is too small to produce a distinct protein peak on serum
protein electrophoresis.
Fibronectin: opsonic glycoprotein (mw 440 000). Depletion correlates with
reticuloendothelial phagocyte clearance depression.
Creatinine - Height Index: is the ratio of 24 hour urine creatinine excreted compared
with height matched controls of the same sex. Expressed as a % and an index of 100%
indicates normal muscle mass, provided there is normal excretion of creatinine.
Immunological Markers: Total lymphocyte count < 1500 cells/pJ associated with
severe malnutrition. Clinical trials suggest that impaired delayed cutaneous
hypersensitivity is present in severe malnutrition and with poor clinical outcome.
Indirect Calorimetry and body composition analysis: are helpful when nutritional
requirements are difficult to estimate. Routine use cannot be advocated because they are
expensive, technically demanding, and not demonstrated to effectively predict clinical
outcome.
7
Multifactorial Prognostic Indices
a. Prognostic Nutritional Index: uses serum albumin and transferrin levels TSF and
delayed hypersensitivity skin test reactivity to predict the risk of operative morbidity and
mortality in relation to nutrition status. PNI 50% indicates high risk patients.
b. Prognostic Inflammatory & Nutritional Index: uses markers of inflammatory response
ie a - 1 acid glycoprotein & c-reactive protein, in combination with nutrition assessment
parameters (alb. & prealb.) to predict infectious complications and death.
c. Nutritional risk index: used in Veterans - Administration - cooperative group study of
preoperative morbidity and mortality using serum albumin and the ratio of current weight
to usual weight.
Dynamic Nutritional Assessment
Predictors of Poor Clinical Outcome
A number of measures initially designed and used to assess nutrition status correlate with
outcome for patients in acute hospitals and chronic healthcare institutions. However, it
remains unknown how much, if any, of the correlation between the measurements and
outcomes is a result of nutrition status as opposed to severity of illness or other factors.
The Prognostic Nutritional Index (PNI) % = 158 - 16.8 (ALB) - 0.78 (TSF)- 0.20 TFN -
5.8 DH where PNI is an estimate of postoperative risk of complication, ALB is serum
albumin concentration (g/100 ml), TSF is triceps skinfold thickness (mm), TFN is serum
transferrin concentration (g/100 ml) and DH is delayed cutaneous hypersensitivity to any
of three recall antigens (0, nonreactive:
1, =5 mm induration: 2, > 5 mm induration).
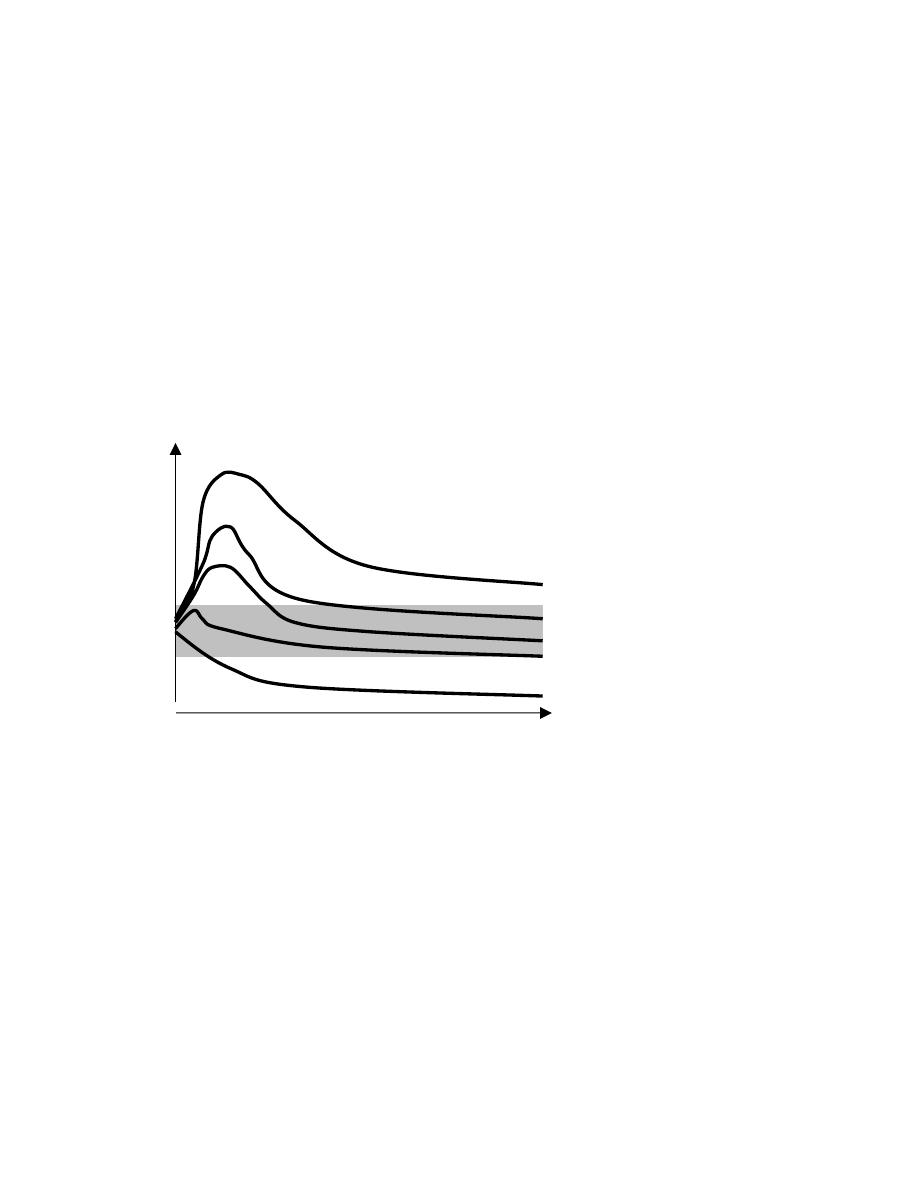
Major burn
Sepsis
Skeletal trauma
Elec. op
Starvation
0
25
50
Days
Resting
Energy
Expenditure
% of normal
50%
200%
8
NUTRITIONAL SUPPORT
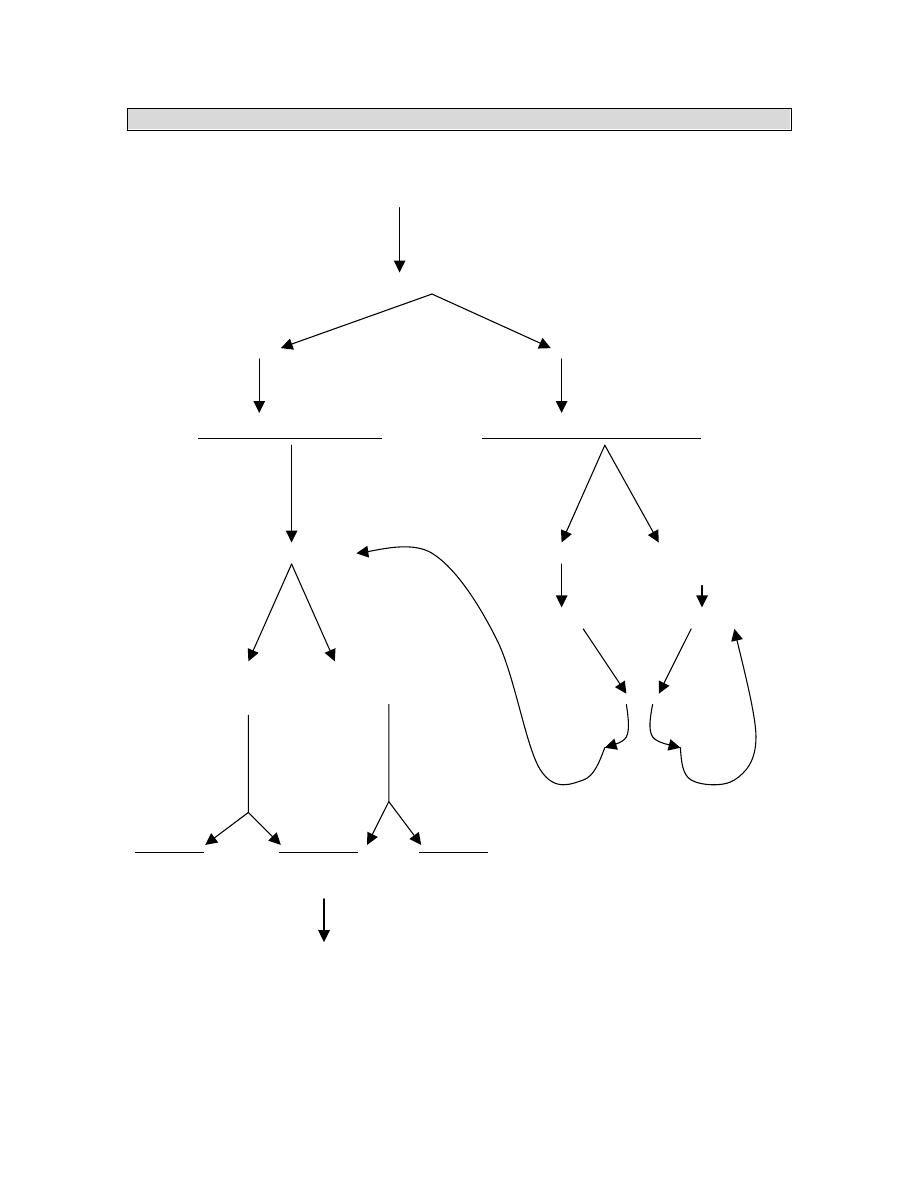
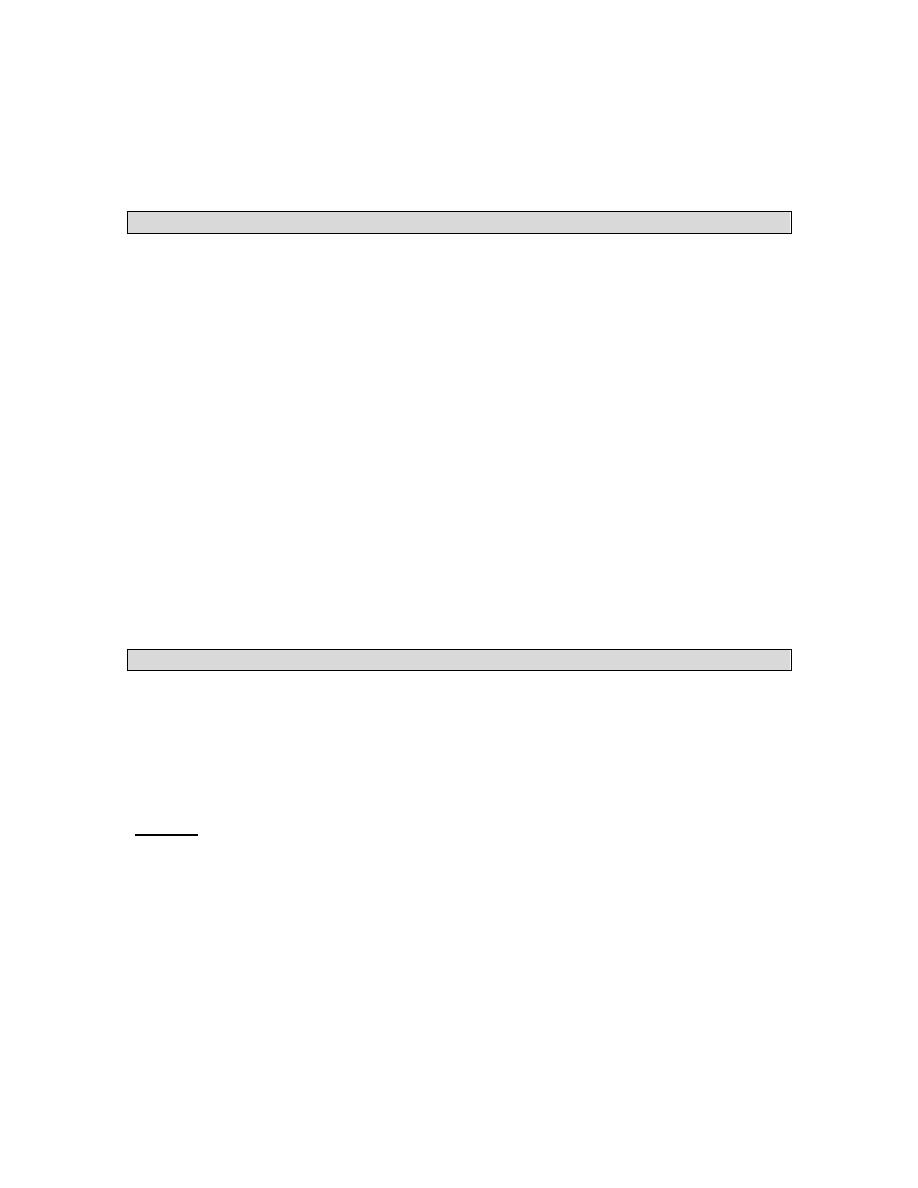
ROUTES TO DELIVER NUTRITIONAL SUPPORT
Nutrition assessment
Decision to initiate specialised nutrition support
FUNCTIONAL GI TRACT
YES NO (Obstruction, peritonitis,
intractable vomiting, acute
pancreatitis, short bowel
syndrome, ileus)
ENTERAL NUTRITION PARENTERAL NUTRITION
Long term Short term
Gastrostomy Nasogastric
Jejunostomy Nsoduodenal
Nasojejunal
GI Function Short term Long term or
fluid restriction.
Normal Compromised Peripheral PN
*
Central; PN
*
Intact Defined
Nutrients
3
formula
2
GI function returns
YES NO
NUTRIENT
TOLERANCE
Adequate
Inadequate
Adequate
Progress
PN
Progress to more
to oral feedings Supplementation complex diet and oral
Feedings
as
tolerated
Progress to total
Enteral feedings

9
Enteral vs. Parenteral
Compared to parenteral nutrition, enteral nutrition is less costly and has a more complete
nutrient profile. The complication rate, including the likelihood of infection, is probably
less for enteral than for parenteral nutrition although that is not well documented. On the
other hand, parenteral nutrition is easier to administer, better accepted by patients, and
provides more reliable delivery of nutrients. Taken together, the benefits of enteral
nutrition outweigh those of parenteral when both are possible.
ENTERAL NUTRITION
ADVANTAGES
1. Maintains git integrity and positive effect on immunity of small intestine
2. Enhanced utilization of nutrients
3. More efficient plasma insulin response
4. Ease and safety of administration
5. Less cost than TPN
6. Mechanical, infectious and metabolic complications less severe than with TPN.
INDICATIONS
Any condition which requires nutritional support and in which the GIT is functional.
CONTRAINDICATIONS
1. Generalized
peritonitis
2. Shock
3. Complete intestinal obstruction
4. Intractable vomiting/severe diarrhoea
5. Paralytic
ileus
6. Severe git bleeding
7. High
output
fistula
8. Early stages of short bowel syndrome
9. Acute severe pancreatitis.
Short-term supplementation
These are patients in whom the anticipated need for nutritional support is for a relatively
short period, often less than 6 weeks. A variety of commercially available feeding tubes
can be used to access the stomach or small intestine. For patient comfort, soft small bore
(7 to 9 French) tubes should be used. These tubes may be placed nasogastrically if
adequate gastric emptying and an intact gag reflex is present. They may also be placed
nasoenterically in patients with a higher risk of aspiration

10
Long-term supplementation
Patients in whom the anticipated need for nutritional support is greater than 6 weeks may
benefit from more permanent enteral access.
Gastrostomy tubes may be placed either operatively or percutaneously with endoscopic
guidance.
This route of feeding requires that gastric emptying is present and is contraindicated by
evidence of gastroesophageal reflux and absence of a gag reflex. One advantage of a
gastrostomy tube is that feedings may be administered either continuously or as
intermittent boluses, thus potentially simplifying care, especially in the outpatient setting.
Jejunostomy tubes are commonly placed operatively, either via laparotomy or with
laparoscopic assistance. They may either be permanent (end Roux-en-Y type) or
temporary (Witzel).
In selected cases, a jejunostomy may be placed endoscopically or using the needle
catheter technique. A small bore feeding tube may also be passed through an existing
gastrostomy tube to create a functional jejunostomy. Jejunostomy feedings are given
continuously, rather than as boluses.
Complications Of Enteral Nutrition
17
1. Gastro-intestinal : diarrhoea, vomiting, bloating, abdominal cramps.
2. Metabolic : glucose intolerance, excess CO
2
production, electrolyte
imbalances
3. Mechanical : Blocked tube, tube dislodgement, nasopharyngeal discomfort,
nasal erosions and necrosis (esp. children)
Complications of surgery ( gastrostomy; jejunostomy)
Perforation
Haemorrhage
Wound
infection
Bowel
obstruction/necrosis
Stomal
leakage
4. Infections : Aspiration pneumonia,
contaminated feeds - gastroenteritis

11
Principles Of Administration
1. Choosing the appropriate feed.
2. Most patients tolerate a polymeric feed.
3. In the presence of malabsorption - semi elemental formula used. Single nutrient
deficiencies - modular feed used.
4. Rate of administration: start slowly (approx. 20ml/hr) and increase to 80ml/hr within
48hrs. If poorly tolerated, reduce rate or discontinue feed and recommence slowly
once mechanical obstruction excluded.
5. Early feeding ( 48 hrs) has shown to prevent gut mucosal atrophy, peserve mucosal
integrity - reduced bacterial and endotoxin translocation, ensure maintenance of
normal gut flora - reduces gram negative proliferation and improves the status of the
gut immune system.
Products
Many formulas have been developed for enteral supplementation. These vary in
osmolarity, caloric content, protein complexity and density, and fat content. Typical
formulas contain 1 to 2 kcal/ml and between 30 and 60 g of protein per liter
COMMERCIALLY AVAILABLE PRODUCTS
Formulae
(1000ml)
Kcal CHO
(g)
FAT
(g)
PROT
(g)
Kcal:
gN
OSM
mosmol/kg
USE
Ensure 1100
162
37,2
39,7
150:1
480
Osmolite 1060
145 37,6 37,2 178:1
300
Nutrison 1000
122,5
38,9
40
131:1
290
Alitraq
1000 165
15,5 52,5
120:1 575
Impaired GI function. Rich in
Glutamine (27g/100g)
Jevity 1060
151,7
35,9
44,4
150:1
300
Paediasure 1000 109,7 49,7 30
208:1 310
Peptison 1000
187,5
10 40
131:1
470
Glucerna 1000
33,3 50 16,7 150:1
375
Suplena 2000
255,2
95,6
30 418:1
600
Advera 1280
215,8
22,8
60 133:1
680
12
Early Enteral Feeding
A compelling clinical and scientific rationale appears to support the use of enteral feeding
as opposed to bowel rest or total parenteral nutrition (TPN) in patients after trauma,
including major surgery.
1. Aggressive early enteral feeding has been shown to decrease sepsis and improve
outcome in critically ill patients after major trauma.
2. In individual studies and in meta-analysis, patients fed via total enteral nutrition
(TEN) after abdominal surgery for trauma experienced fewer septic complications
than patients fed via the parenteral route.
3. Third, the use of perioperative TPN in the perioperative period is highly
controversial, with several studies reporting an increase in infective complications
in subsets of patients.
4. The cost of TEN is considerably less than TPN.
Immuno modulating drugs
(Discussed later under Management of the critically ill patient)
OXEPA
OXEPA is a low-carbohydrate, calorically dense enteral nutrition product designed for
the dietary management of critically ill patients on mechanical ventilation. It contains
eicosapentaenoic acid (EPA) (from sardine oil), gamma-linolenic acid (GLA) (from
borage oil), and antioxidants. OXEPA can be used as a sole source of nutrition for tube
feeding.
•
For critically ill patients on mechanical ventilation
•
For critically ill patients with LUNG INJURY, such as: pneumonia; sepsis; chest
injury; multiple trauma; burns; shock and hypoperfusion; aspiration or near-
drowning; cardiopulmonary bypass; or hyperfusion-associated lung injury.

13
Features :
•
Complete, balanced nutrition for tube-feeding patients
•
Unique patented oil blend-contains EPA from sardine oil and GLA from borage
oil
•
Contains 25% of fat as MCTs for improved fat absorption
•
Fortified with elevated levels of the antioxidants all-natural vitamin E, beta-
carotene, and vitamin C
•
1.5 Cal/mL, 355 Cal/8 fl oz, and a moderate osmolality of 493 mosm/kg H20
•
Caloric density is high to minimize the volume required to meet energy needs
•
Meets 100% of RDI for 24 key vitamins and minerals in 1420 Calories
(four 8-fl-oz cans)
•
Lactose- and gluten-free
PARENTERAL NUTRITION
If enteral nutrition is not possible in the malnourished or at-risk patient, the parenteral
route must be utilized. Parenteral nutrition may be used for either primary or supportive
therapy
Indications for Parenteral Nutrition
Primary therapy
Gastrointestinal fistula
Short bowel syndrome
Acute renal failure
Hepatic insufficiency
Inflammatory bowel disease Efficacy not established
Secondary therapy
Radiation enteritis/chemotherapy toxicity
Hyperemesis gravidarum
Prolonged ileus
Preoperative therapy
Efficacy not established
Cardiac cachexia
Efficacy not established
Pancreatitis insufficiency
Efficacy not established
Cancer
Efficacy not established
Sepsis
Efficacy not established

14
TPN Formula Composition
Components of TPN include dextrose; lipids; amino acids; electrolytes including
phosphorus, potassium, sodium, magnesium, calcium, chloride, and acetate; vitamins and
trace elements.
Typical vitamin amounts administered daily in TPN are:
vitamin A 3,300 IU, vitamin D 200 IU, ascorbic acid 100 mg, folic acid 400 mcg, niacin
40 mg, riboflavin 3.6 mg, thiamin 3 mg, pyridoxine 4 mg, cyanocobalamin 5 mcg,
pantothenic acid 15 mg, biotin 60 mcg, and vitamin E 10 IU.
These approximate amounts are present in commercial mixtures. Vitamin K 1 mg daily or
5-10 mg weekly is often added separately.
A typical trace element cocktail provides approximately the following per day: zinc
5 mg, copper 1 mg, manganese 500 mcg, chromium 10 mcg, and selenium 60 mcg.
Molybdenum may also be included.
NUTRIENT MIXTURES
Standard solutions
-
Peripheral - Vamin 750 ml + 20 % intralipid + fluids
-
Central - Synthamin 14 - 14g N2
-
Intralipid 10% - soybean oil + egg yolk phospholipids + glycerol 500mls.
-
Glucose 50% - 500mls.
-
Maintelyte - 10% glucose + electrolytes - 1
l
-
Soluvit - water soluble vitamins
-
Vitalipid - fat soluble vitamins
-
Addamel - essential micronutrients.
All mixed into 3
l bags.
15
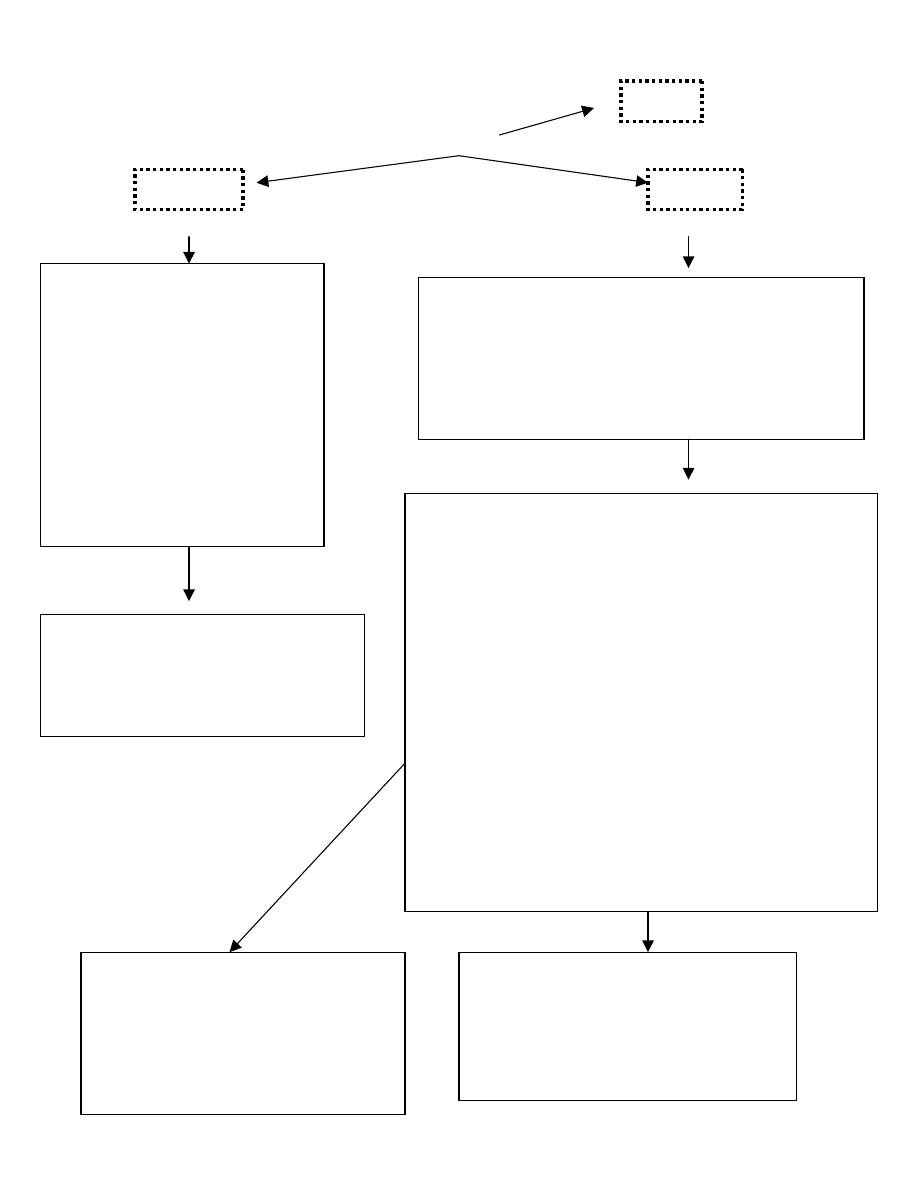
Routes
800 mOsm/L range
2,000 mOsm/L range
Peripheral
Central
1. Needs good peripheral veins
2. Limited to 2 major veins
3. Sites have to be changed
every 24 hrs.
4. Prone to phlebitis if
osmolarity >900 mOsm/L
5. In order to provide adequate
calories in a reasonable
volume with a tolerable
osmolality, lipid provides
50% to 70% of the
nonprotein calories in
peripheral TPN
1. Usually via the infraclavicular route (subclavian
vein) using a silicone, double-lumen catheter.
2. strict aseptic conditions.
3. Occlusive
hydrocolloie dressings are most
suitable. The dressings and administration set
must be changed every third day.
Risk minimized by:
1. hydrocortisone to each litre of solution
2. adding 1% sodium bicarbonate
3. change site daily
4. run fluids over 12-16 hours
Complications Complications
Catheter related
(i)
Mechanical : central vein thrombosis, catheter
embolism, haemo-pneumo
thorax,
haemopericardium, air-embolism, tracheal
puncture, arterial laceration, brachial plexus
injury.
(ii)
Sepsis : Staphylococcus epidermidis,
candida albicans
(iii)
Blockage : Use of heparin and choice of
catheter (silicone/polyurethane)
Metabolic : Hyperglycaemia, electrolyte and acid
base
abnormalities,
trace
element
and vitamin deficiencies.
Electrolyte abnormalities
Hypoglycaemia
Hepatic function changes: Cholestasis, elevated
liver enzymes and hepatomegaly
GI changes
Atrophy of intestinal mucosa
PREVENTING HYPERGLYCAEMIA
Begin the infusion at 40 ml/h of 25 per
cent glucose based solution and
increasing the infusion rate by 20 ml/h
every 24 h, depending on glucose
tolerance.
PREVENTING TPN ASSOCIATED
LIVER DISEASE
Decrease dextrose content < 40 kcal/kg/day
Decrease fat content < 1 g / kg / day
Adequate protein intake < 1-1.5g/kg/day
PICC
16
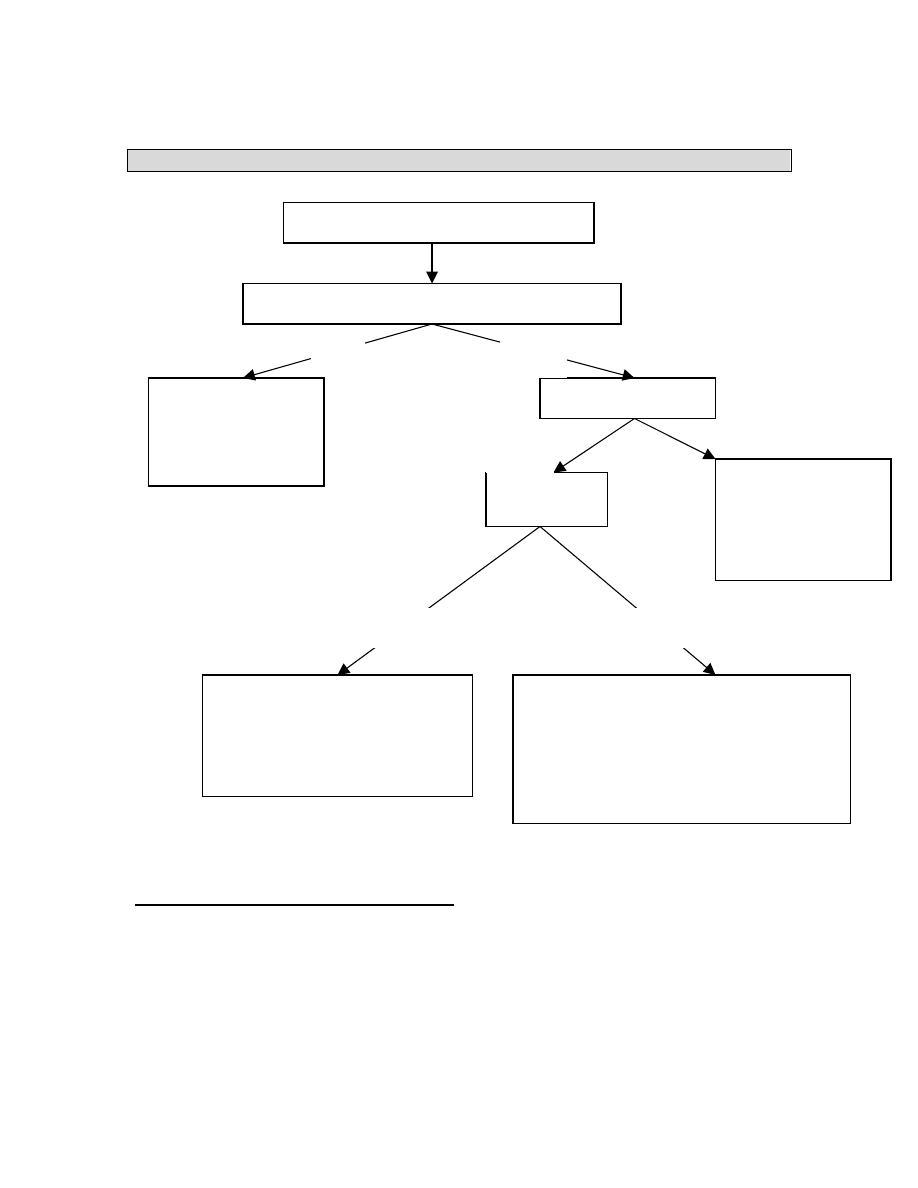
MANAGEMENT OF CATHETER RELATED SEPSIS
PICC (Peripherally inserted central line)
A cubital fossa PICC provides a safe effective means of administering intravenous
therapy.
A PICC can be expected to last two months without complication.
Complications that do occur are most likely in the first week.
The most common complication resulting in line removal was phlebitis.
Line-related sepsis is a very rare event.
Pyrexia > 38
°C / Swinging temperature
Does the patient still require nutritional support
Remove central line
catheter for culture
If pyrexia persists –
full septic screen.
Is the patient stable?
NO
YES
Stop TPN.
Resuscitate.
Broad spectrum
antibiotics.
Full septic screen
Check blood
cultures.
NO
YES
Remove central line (MCS)
Start PPN
Appropriate antibiotic.
Await 3 neg blood cultures before
reinserting CVP
Clinical evaluation to
exclude obvious source
eg intra-abdominal
sepsis (U/S or CT useful)
Full septic screen: Rail-road CVP – MCS
Body fluids / secretions – MCS
POSITIVE
NEGATIVE

17
This means that the PICC compares favourably with any other means of venous access
4
and is the method of choice for most intermediate or longer term intravenous therapy.
NUTRITIONAL SUPPORT IN CLINICALLY RELEVANT SITUATIONS
A. BURNS
• Appropriate nutritional support contribute significantly to improve patients survival.
• Helps to maintain body weight, promote wound healing, combat infection, preserve
body nutrient reserves and replace visceral and somatic proteins
• Early enteral nutrition route unless non-functioning gut → then parenteral nutrition.
• Carbohydrates 50%, protein 20% and lipid 30%.
• Recent evidence that omega-3-fatty acids results in increased feeding tolerance.
• Arginine supplementation improves CMI and wound healing and is a conditionally
indispensable a/acid for maintaining body protein homeostasis and nutrition in
severely burned patients.
B. PANCREATITIS
Estimating the severity of pancreatitis is important.
Most acute pancreatitis is mild, self-limiting and resolves within 5-7 days, hence no
need for nutritional support.
Severe pancreatitis induces a hypercatabolic state resulting in rapid weight loss and
increased morbidity and mortality.
Chronic pancreatitis results in malnutrition. Therefore the aim is to treat the
malnutrition and to avoid the development of malnutrition.
In severe pancreatitis the best route of nutrition is the nasojejunal feeding with a
semi-elemental low triglyceride diet. The outcome s are similar than those patients
receiving TPN. If enteral feeding increases pain. Ascites or fistula output then
discontinue.
Pancreatic fistula: Low output ----enteral feed
High output ---TPN
Pancreatic Ascites: TPN + somatostatin
C. ENTEROCUTANEOUS FISTULA
-
TPN has been shown to have no effect on mortality but does influence
closure as shown in local studies.
-
high output > 500 ml - TPN + somatostatin
-
definitive closure if no decrease in 6 weeks , patient afebrile.
-
Low output < 500 ml - enteral feed.

18
D. CRITICALLY ILL PATIENT
Have increased metabolic demands (20-60% above basal needs). Goal is to
maintain and not to replete. Initial /calorie delivery 25 Kcal/kg/day and 1.5-2.0g
protein/kg/day.
Associated complications
1. Hepatic
dysfunction
Aetiology : TPN, hepatic hypoperfusion, drug induced liver disease
Appropriate nutrition ( prevention of hepatic encephalopathy)
Reducing the gastro-intestinal protein load by restricting dietary protein
intake, preventing gastrointestinal bleeding, and encouraging intestinal
emptying with agents such as lactulose. In stage I and II encephalopathy,
protein intake should be restricted to fewer than 40 g/day, and this should
be further reduced to fewer than 20 g/day in patients with stage III and IV
encephalopathy. Caloric intake, preferably as dextrose, should be
maintained in excess of 2000 cal (8.4 kJ)/day. Patients with liver damage
may be lipid intolerant, and the presence of lipaemic serum requires a
reduction of lipid intake.
2. Acute pancreatitis (discussed above)
3. Stress ulcer prophylaxis : Critically ill surgical patients are at risk from
gastrointestinal stress ulceration. Ulcers are most often sited in the fundus of the
stomach or the first part of the duodenum. They are small, multiple, superficial,
well demarcated, and usually without surrounding oedema. Stress ulcer bleeding
is less common in patients who are receiving enteral nutrition; this could reflect a
lower severity of illness in view of the maintenance of gastrointestinal absorptive
function or be a direct protective effect of nutrients in the stomach and
gastrointestinal tract.
Immunonutrition :
Intended for critically ill patients at risk for infections and for use in the
immediate postoperative period. They contain increased protein,
immunostimulatory amino acids, and lipids, and may decrease infectious
complications.
Immune enhancing formulas include arginine, glutamine, nucleic acids and
omega-3 fatty acids.
L-arginine
and
I-glutamine: -
stimulates host defenses
- modulates
tumour
metabolism

19
- increases
wound
healing
- decreases
nitrogen
losses
after
trauma
L-Glutamine: - maintains integrity of intestinal barrier function in preventing
translocation of bacteria and endotoxins from bowel lumen into systemic
circulation. Combination of nutrients have shown to enhance host defences
significantly as reported by Cerr et al. Benefits of immunonutrition were
reduction in hospital stay and decrease infections but has no effect on
mortality as shown by Beale et al. In another study by Galban et al. has
shown a significant reduction in mortality in patients on Immunonutrition.
Essential Fatty Acids: Acts as an intracellular messenger and plays a
regulatory role in the metabolic process. Maintains cell structure and
function. Administered enterally or parenterally via central or peripheral vein.
Eg: Omega – 3 and Omega 6 fatty acids: enhances cell- mediated immunity
and has shown to improve histological appearance of IBD. Improves
immunocompetence, has antithrombotic effects which prolongs bleeding time.
Long and Medium Chain Triglycerides: a 50:50 mixture of LCT & MCT
has shown to have greater physiochemical stability. Resulting in more oxygen
radicals being produced LCT impairs the RES while MCT stimulates it. Has a
trophic effect on the bowel, reducing bile output and decrease the risk of
hepatic dysfunction.
Branched Chain Amino Acids: Leucine, isoleucine and valine metabolized
in skeletal muscle and are important oxidative fuels. Leucine rich amino acid
stimulates resting energy expendature and thermogenesis by up to 20%.
INTENSIVE INSULIN THERAPY
Hyperglycemia associated with insulin resistance is common
in critically ill patients, even
those who have not previously
had diabetes.
In diabetic patients
with acute myocardial infarction, therapy to maintain blood
glucose at
a level below 215 mg per deciliter (11.9 mmol per
liter) improves the long-term outcome.
In nondiabetic
patients with protracted critical illnesses, high serum levels
of insulin-like
growth factor–binding protein 1, which
reflect an impaired response of hepatocytes to
insulin, increase
the risk of death.
In a study by
Van den Berghe et al,
2001 showed that intensive insulin therapy to
maintain blood glucose at or below 110 mg per deciliter reduces morbidity and mortality
among critically ill patients in the surgical intensive care unit.

20
1. HEAD INJURY PATIENTS
-
The metabolic response to trauma is usually a sustained hypermetabolic state
with a peak hormonometabolic response at 3 - 5 days.
-
The exact mechanisms involved are unknown with the following postulates :
a. Damage to the cardioregulatory pons with autonomic hyperactivity and
increased catecholamines release.
b. Damage to the blood brain barrier with resultant catecholamine accumulation.
c. Steroid therapy .
d. Raised intracranial pressure may result in gastric hypersecretion.
This effectively leads to a basal metabolic rate that may be up to 140% of
normal. Nitrogen excretion may be increased up to 7 times which depends on
intake , muscle mass , muscle use and steroid therapy. Hyperglycaemia develops due
to the mechanisms mentioned and the overall response to trauma. This tends to
produce and worsen intracellular lactic acidosis , may result in increased pCO2
with further increase in intracranial pressure.
Requirements :
i)
40 - 50 kcal/kg/day , indirect calorimetry is the standard for determining the
requirements.
ii)
protein 1,5 - 2,0 g/kg/day although levels of 2 - 2,5 g/kg/day are now being
given.
iii)
The caloric needs are the basal energy expenditure multiplied by 1,4
iv)
Non protein energy : nitrogen is 80 : 1 initially then up to 130 : 1.
Route : enteral feeds should be aimed for early on ie within 48 hrs.
Other options include percutaneous endoscopic jejunstomy or gastrostomy
with long term requirements.
The standard indications for parentral therapy apply.
SHORT BOWEL SYNDROME
A number of conditions require surgical resection or bypass of intestine resulting in a
short bowel. The pathophysiologic consequence of a short bowel is malabsorption.
Malabsorption due to a short bowel and its clinical consequences is referred to as the
short bowel syndrome. Severity is determined by the amount of intestine resected, the
site resected and the ability of the intestine to undergo adaptive hyperplasia. A short
bowel is one of the causes of intestinal failure.
Other causes of intestinal failure include motility disorders (visceral myopathy, visceral
neuropathy, scleroderma, amyloidosis), refractory celiac disease and radiation enteritis.
In adults, the length of the small intestine when measured at autopsies is on average 600
cm (20 feet). The length of the colon is about 150 cm (5 feet). There is no anatomic
distinction that demarcates jejunum from ileum. The proximal 2/5 of small intestine is
21
usually accepted as jejunum and the distal 3/5 as ileum. Most dietary carbohydrates, fats,
proteins, vitamins, minerals, and trace elements are absorbed within the first 2/3 of the
small intestine.
Most iron is absorbed in the duodenum; folate in the proximal jejunum. Vitamin B12
(cobalamin) and bile salts are only absorbed in the distal ileum. Water and electrolytes
are absorbed throughout the small intestine and colon. A small amount of carbohydrate
escapes absorption in the small bowel and enters the colon. This is important for colonic
health. Bacteria in the colon metabolize carbohydrate, soluble fiber and some
unabsorbed fats to short chain fatty acids which are the preferred energy source for
colonocytes. Short chain fatty acids are absorbed in the colon along with sodium and
water, thus promoting caloric salvage and fluid absorption.
Small feedings should be started as soon as possible because nutrients are important in
stimulating adaptive hyperplasia in the intestine. Only a small amount of calories (about
500-750 kcal of a soft normal diet) should be given orally to start and then increased as
tolerated. Oral fluids should be limited and given separately from food. Feeding
stimulates digestive juices and may increase intestinal fluid losses. Intestinal fluid and
electrolyte losses should be measured and replaced daily.
There are a number of ways to enhance calorie absorption for optimal weight in those
with a short bowel. Frequent small meals and slowing transit enhances absorption by
maximizing the contact time for nutrient absorption. Medium chain triglycerides
(MCTs)are water soluble and can diffuse across the epithelium intact or as medium chain
fatty acids. Administration of excess amounts of MCTs should be avoided because
unabsorbed MCTs in the intestinal lumen cause an osmotic diarrhea. In those with >100
cm of ileum resected, exogenous bile salts (e.g. ox bile, cholylsarcosine) improves fat
absorption; ursodeoxycholic acid does not.
CONCLUSION
Nutritional support of the surgical patient is an extremely important part of the total care
of the patient, unfortunately in our hospital setting it is given little importance to the
average patient and only reserved for the acutely ill patient. Early assessment of the
patient and initiation of nutritional support will yield excellent results and reduce
morbidity and mortality.
22
REFERENCES
1. Haffejee AA . Surgical nutrition in Mieny and Mennen eds. Principles of
Surgical Patient care. Pretoria Academica 1990 : 1 - 16.
2. Van den Berghe M.D., Ph.D., Pieter Wouters, M.Sc., Frank Weekers, M.D., Charles
Verwaest . Intensive insulin therapy in the critically ill patient. NEJM 2001; 345 :
1359 – 67.
3. Downs JH , Haffejee AA . Nutritional assessment in the Critically Ill . Curr
Opinion Clin. Nutr. Metabolic Care 1990 ; 1 : 275 - 279.
4. Griffiths R D. Glutamine enriched TPN formula. Nutrition 1997; 13 : 295 – 302.
5. Bissetty T V. Nutritional support of the Surgical patient. Surgical Seminar 2003.
6. Naidoo R . Nutritional support of the Surgical patient. Surgical Seminar 2001.
7. Alli MO. Nutritional support of the Surgical patient. Surgical Seminar 1999.
8. Moodley S. Nutritional support of the Surgical patient. Surgical seminar 1997.
9. Alan L. Miller, ND Therapeutic Considerations of L-Glutamine: A Review of the
Literature. Glutamine physiology, biochemistry, and nutrition in critical illness.
Austin, TX: R.G. Landes Co.; 1992.
10. ASPEN Board of Directors . Rationale of Adult nutritional support guidelines.
JPEN 1993 ; 17 ( 4 ) : 58 - 68.
11. Jeejeebhoy K , Detsky A . Assessment of nutritional status. JPEN 1990 ;14
(supplemental ) :193 -195.
12. Klein S , Kinney J . Nutrition support in clinical practise : review of published
data and recommendations for future research directions. JPEN 1997 ; 21 ( 3 ) :
133 - 155.
13. Gibbs J , Cull W. Pre - operative albumin as a predictor of operative mortality
and morbidity. Arch Surg 1999 ; 134 : 36 - 42.
14. Tellado - Rodrigues J , Garcia J. Nae / Ke Ratio is a better indicator of
nutritional status than standard anthropometric and biochemical indices. Surg
Forum 1988 ; 38 : 56 - 58.
15. Sacks G , Poole G. Continuous versus intermittent nasogastric enteral feeding in
trauma patients. JPEN 2000 ; 24 ( 1 ) : 15 - 20.
16. Kealer A , Swails W. Early enteral feeding in post surgical cancer patients.
Ann Surg 1996 ; 223 ( 3 ) : 316 - 333.
17. Ling E , Caro C. Randomised trial of safety and efficacy of immediate post
operative enteral feeding in patients undergoing gastrointestinal resection. BMJ
1996 ; 312 ( 6 ) : 332 - 335.
18. Payne J , Whanwaya H. First choice for TPN : the peripheral route. JPEN 1993 ;
17 ( 5 ) : 468 - 478.
19. Alexander WJ. Immunoenhancement via enteral nutrition. Arch Surg 1993 ; 128
: 1242 - 1244.
20. Berard MP , Zazzo JF. Total parentral nutrition enriched with arginine and
glutamate generates glutamine and limits protein catabolism in surgical patients
hospitalized in intensive care units. Crit Care Med 2000 ; 28 ( 11 ) : 3637 - 3644.
21. Wu Y , Fukatsu K. Glutamine enriched TPN maintains intestinal IL 4 and
mucosal IgA. JPEN 2000 ; 24 ( 1 ) : 46 - 52.

23
22. Loughran S , Hataneka T. Leucine rich amino acid solution prevents operative
hypothermia under general anaesthesia. JPEN 2000 ; 24 ( 1 ) : 67 - 69.
23. Heys SD , Gardner E. Nutrients and the surgical patient : current and potential
therapeutic applications to clinical practise. J R Coll Surg Edin 1999 ; 44 : 283
- 293.
24. Driscoll DF , Bacon MN. Physicochemical stability of 2 types of IV lipid
emulsion as total nutrient admixture. JPEN 2000 ; 24 ( 1 ) : 15 - 22.
25. Naber A , Kruimel I. With MCT , higher and faster oxygen radical production
by stimulated polymorphs occurs. JPEN 2000 ; 24 ( 2 ) : 107 - 112.
26. Rothstein R , Rombeau J. Nutrient pharmacotherapy for gut mucosal diseases.
Gastroenterology clinics N America 1998 ; 27 ( 22 ) : 387 - 399.
27. Kalfarentzos F , Kehagias J. Enteral versus parentral nutrition in acute
pancreatitis. BJS 1997 ; 84 : 1665 - 1669.
28. Lob D , Naemon A. Evolution of nutritional support in acute pancreatitis. BJS
2000 ; 87 : 695 - 707.
29. Madiba TE , Haffejee AA. Nutritional support in the management of external
pancreatic fistulas. SAJS 1995 ; 33 ( 2 ) : 81 - 84.
30. Norton J , Linda G. Intolerance to enteral feeding in brain injured patients. J
Neurosurg 1988 ; 68 : 62 - 66.
31. Borzotta A , Paxton J. Enteral versus parentral nutrition after severe closed
head injury. J Trauma 1994 ; 37 ( 3 ) : 459 - 468.
32. Heyland DK, Dhaliwal R, Drover JW, Gramlich L, Dodek P, and the Canadian
Critical Care Clinical Practice Guidelines Committee. Canadian clinical practice
guidelines for nutrition support in mechanically ventilated, critically ill adult patients.
JPEN. 2003;27:355-373.
24
APPENDIX
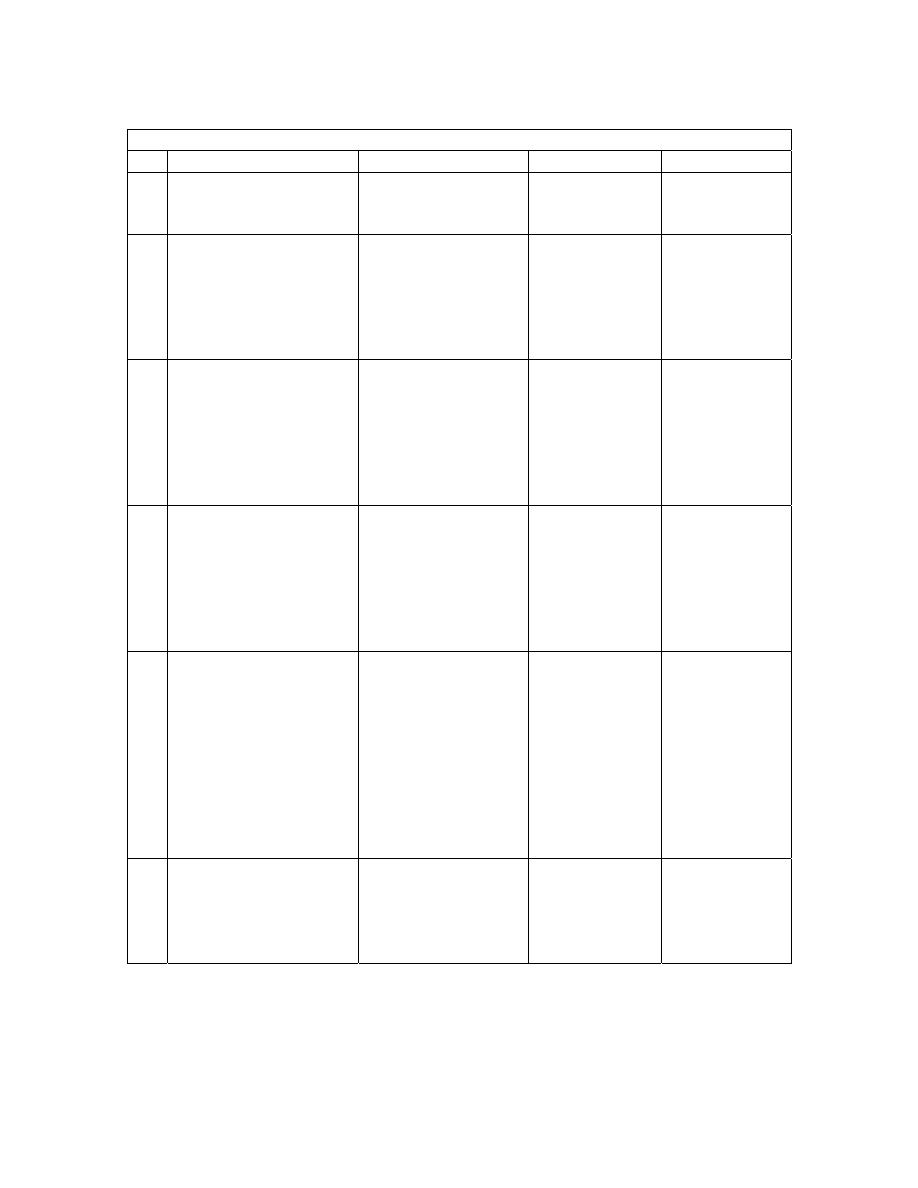
KING EDWARD VIII HOSPITAL (ICU) ENTERAL FEEDING PROTOCOL
Protocol 1
Protocol 2
Protocol 3
Protocol 4
Haemodynamically
stable
Hypoalbinaemia Diarrhoea
/
intolerance
Diabetic/
glucose
intolerance
Day 1
Jevity (500 ml) 20ml/hr
Protein = 22,2g
energy = 530Kcal
Jevity (500ml)
20ml/hr
Protein = 22,2g
Energy = 1060 Kcal
Jevity (500ml)
20ml/hr
Protein = 22,2g
Energy = 1060
Kcal
Jevity (500ml)
20ml/hr
Protein = 22,2g
Energy = 1060
Kcal
Day 2
Jevity (1000ml)
40ml/hr
Protein = 44,3g
Energy 1060Kcal
Jevity (1000ml)
40ml/hr
Protein = 44,3g
Energy = 1060 Kcal
Jevity (1000ml)
40ml/hr
Protein = 44,3g
Energy = 1060
Kcal
Jevity
(1000ml)
40ml/hr
Protein = 44,3g
Energy = 1060
Kcal
Day 3
Jevity (1500ml)
60ml/hr
Protein = 66,5g
Energy 1590Kcal
Jevity (1500ml)
60ml/hr
Protein = 66,5g
Energy = 1590 Kcal
Jevity (1500ml)
60ml/hr
Protein = 66,5g
Energy = 1590
Kcal
Jevity
(1500ml)
60ml/hr
Protein = 66,5g
Energy = 1590
Kcal
Day 4
Jevity (2000 ml) 80 ml /
hr
Protein – 88,6g
Energy 2120Kcal
IL Jevity + IL Jevity
plus 80ml/hr
Protein = 99,8g
Energy = 2260 Kcal
or for poor wound
healing operative 60
ml/hr
Protein = 99,9g
Energy = 1950 Kcal
Jevity (2000
ml) 80 ml / hr
Protein – 88,6g
Energy
2120Kcal
Jevity (2000
ml) 80 ml / hr
Protein – 88,6g
Energy
2120Kcal
Long term
As above
Once nutritionally
repleted, return to
jevity 2L/day
(80ml/hr)
If still poor
tolerance, try
pepttsorb
(semi-elemental
feed)
As above
25
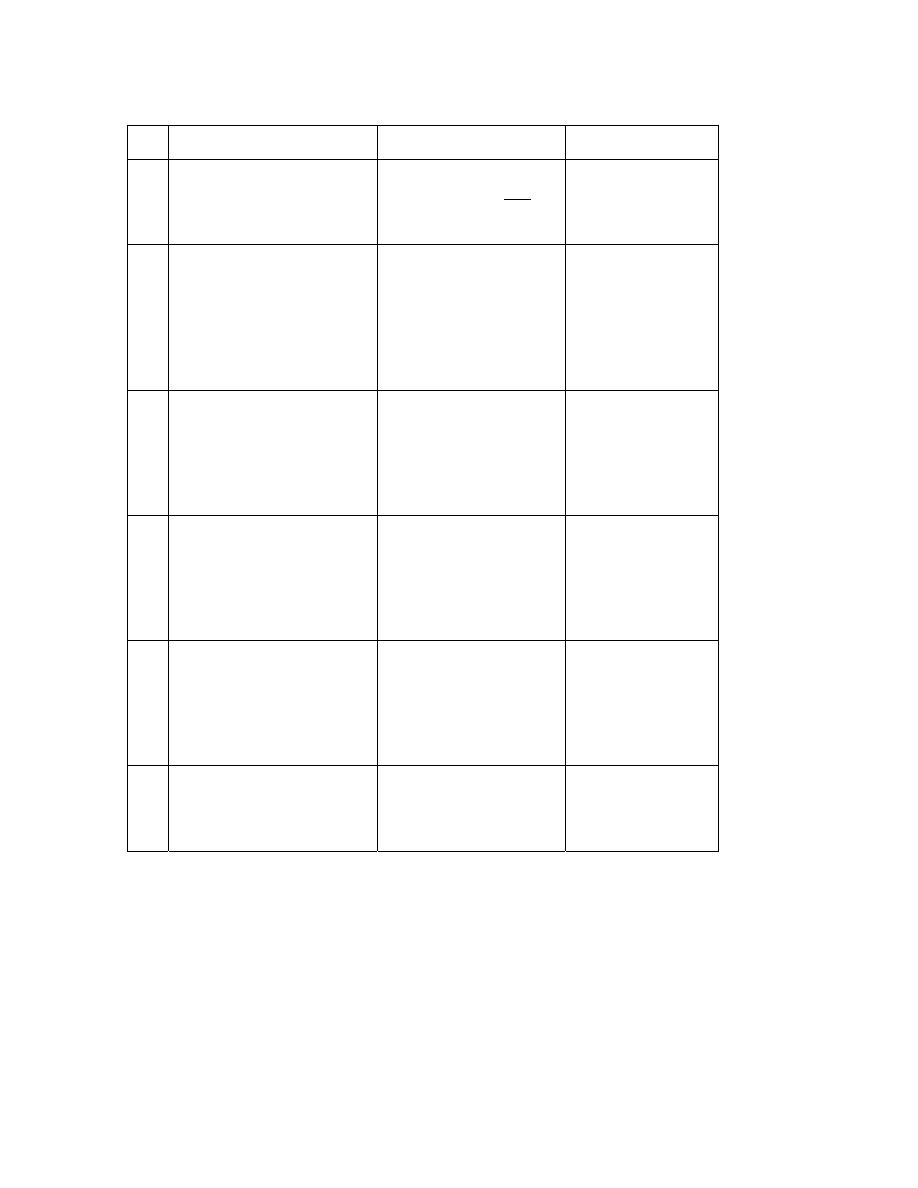
PROTOCOL 5
PROTOCOL 6
PROTOCOL 7
Renal failure
(Acute/Chronic)
On haemo-or peritoneal
dialysis
Renal failure
(acute/chronic ) Not on
haemo or peritoneal
dialysis
Hepatic failure (C)
Encephalopathy
Day 1
Urine output> 1000ml/day
= 2L osmolite (80ml/hr)
Protein = 74g Energy =
2124Kcal
Urine output < 1000ml
Suplena 1175 ml (40
ml/hr = max rate)
Suplena (1175ml/dy)
40ml/hr (max rate)
Protein = 35,3g
Energy = 2350Kcal
Osmolite (500 ml)
20ml/hr
Protein = 18,5g
Energy = 531Kcal
Day 2
As Above
As Above
Osmolite (1000
ml)
40ml/hr
Protein = 37g
Energy =
1062Kcal
Day 3
As Above
As Above
Osmolite (1500
ml)
60ml/hr
Protein = 55,5g
Energy =
1593Kcal
Day 4
As Above
As Above
Osmolite (2000
ml)
80ml/hr
Protein = 74g
Energy =
2124Kcal
Long-
ter
m
As Above
As Above
NB 1. Periative and Stresson Multifibre are contraindicated for hypotensive and severe
head patients (both products are rich in arginine which forms nitric oxide which is a
vasodilator, however arginine is very good for wound healing.
Ideally 80 ml/hr should be achieved in 3 days, but otherwise by day 5. if a patient does
not tolerate > 40ml/hr, consider supplementing the nutritional intake with peripheral
TPN.
Peptic (semi-elemental feed) is very high in carbohydrate (carb = 352g) – need to monitor
Dm and glucose intolerant pts. (2L Pepti = protein = 80g; Energy = 2000 Kcal)
26
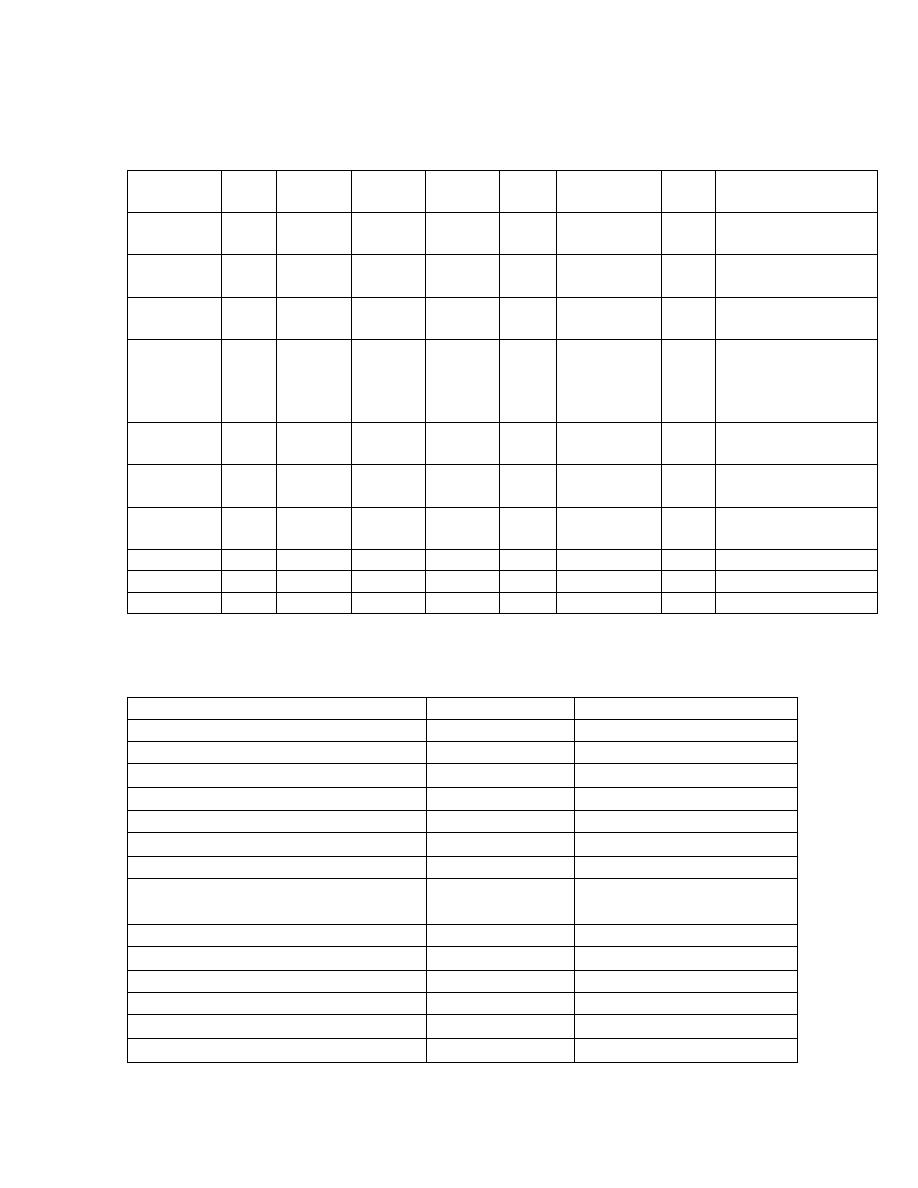
COMMERCIALLY AVAILABLE PRODUCTS
Formulae
(1000 ml)
Kcal CHO
(g)
FAT
(g)
PROT
(g)
Kcal
: gN
OSM
mosmol/kg
R
cost
Use
Ensure 1100
162 37,2 39,7 150:1 480
10,7
5
Osmolite
1060
145 37,6 37,2 178:1
300
22,3
5
Nutrison 1000
122,5 38,9 40
131:1
290
17,6
8
Alitraq 1000
165 15,5 52,5 120:1 575
18,5
5
Impaired GI
function rich in
glutamine
(27g/100g)
Jevity 1060
151,7
35,9 44,4 150:1
300
24,6
5
Paediasure 1000 109,7
49,7
30
208:1
310
24,3
0
Peptison
1000
187,5
10 40 131:1
470
44,4
5
Glucerna
1000
33,3 50 16,7 150:1
375
7,98
Suplena 2000
255,2 95,6 30
418:1
600
7,98
Advera 1280
215,8 22,8 60 133:1
680
9,35
Examples of immunofeeds
IMPACT
IMMUNO-AID
Calories (kcal)
1000
1000
Protein (g)
56
37
Arginine (g)
12.5
14
Glutamine
9.0
Branch-chain amino acids (g)
20
Nucleic acids (g)
1.23
1.0
Fat (g)
27.8
22.0
Omega-3 polyunsaturated fatty acids
(%)
10.5 4.5
Osmolality (mosm/kg)
300
460
Vitamin C (mg)
67
60
Iron (mg)
12
9
Zinc (mg)
15
26
Selenium (
µg)
46 100
Copper (mg)
1.7
2

27
