Purine and Pyrimidine Metabolism
Nucleotides are needed for DNA and RNA synthesis (DNA replication and transcription) and for energy transfer. Nucleoside triphosphates (ATP and GTP) provide energy for reactions that would otherwise be extremely unfavorable in the cell. Ribose 5-phosphate for nucleotide synthesis is derived from the hexose monophosphate shunt and is activated by the addition of pyrophosphate from ATP, forming phosphoribosyl pyrophosphate (PRPP) using PRPP synthetase.Cells synthesize nucleotides in two ways, de novo synthesis and salvage pathways . In de novo synthesis, which occurs predominantly in the liver, purines and pyrimidines are synthesized from smaller precursors, and PRPP is added to the pathway at some point. In the salvage pathways, preformed purine and pyrimidine bases can be converted into nucleotides by salvage enzymes distinct from those of de novo synthesis. Purine and pyrimidine bases for salvage enzymes may arise from:
Synthesis in the liver and transport to other tissues
Digestion of endogenous nucleic acids (cell death, RNA turnover)
In many cells, the capacity for de novo synthesis to supply purines and pyrimidines is insufficient, and the salvage pathway is essential for adequate nucleotide synthesis. In patients with Lesch-Nyhan disease, an enzyme for purine salvage (hypoxanthine guanine phosphoribosyl pyrophosphate transferase, HPRT) is absent. People with this genetic deficiency have CNS deterioration, mental retardation, and spastic cerebral palsy associated with compulsive self-mutilation. Cells in the basal ganglia of the brain (fine motor control) normally have very high HPRT activity. These patients also all have hyperuricemia because purines cannot be salvaged.
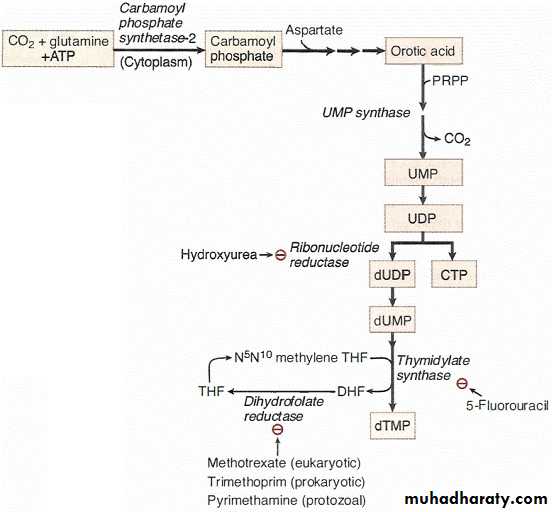
PYRIMIDINE SYNTHESIS
Pyrimidines are synthesized de nova in the cytoplasm from aspartate, C02, and
glutamine. Synthesis involves a cytoplasmic carbamoyl phosphate synthetase that differs from the mitochondrial enzyme with the same name used in the urea cycle.Orotic Aciduria
Several days after birth , an infant was observed to have severe anemia,
which was found to be megaloblastic. There was no evidence of hepatomegaly or splenomegaly. The pediatrician started the newborn on a
bottle-fed regimen containing folate, vitamin B12, vitamin B6, and iron. One week later, the infant's condition did not improve. The pediatrician noted that the infant's urine contained a crystalline residue, which was analyzed and determined to be orotic acid. Laboratory tests indicated no evidence of hyperammonemia. The infant was given a formula that contained uridine. Shortly thereafter, the infant's condition improved significantly.
Orotic aciduria is an autosomal recessive disorder caused by a defect in uridine
monophosphate (UMP) synthase. This enzyme contains two activities, orotate
phosphoribosyl transferase and orotidine decarboxylase. The lack of pyrimidines impairs nucleic acid synthesis needed for hematopoiesis, explaining the megaloblastic anemia in the affected infants. Orotic acid accumulates and spills into the urine, resulting in orotic acid crystals and orotic acid urinary obstruction . The presence of orotic acid in urine might suggest that the defect could be ornithine transcarbamylase (OTC) deficiency, but the lack of hyperammonemia rules out a defect in the urea cycle. Uridine administration relieves the symptoms by bypassing the defect in the pyrimidine pathway. Uridine is salvaged to UMP, which feedback-inhibits carbamoyl phosphate synthase-2, preventing orotic acid formation.
Note:------------------------------------------------
Two Orotic Acidurias
1 . Hyperammonemia but No megaloblastic anemia
• Pathway: Urea cycle
• Enzyme deficient: OTC (ornithine transcarbamylase)
2 . Megaloblastic anemia No hyperammonemia
• Pathway: Pyrimidine synthesis
• Enzyme deficient: UMP synthase
Folate deficiency: megaloblastic anemia, but no orotic aciduria
--------------------------------
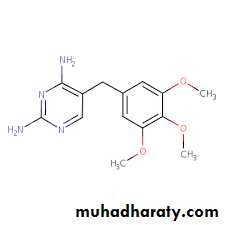
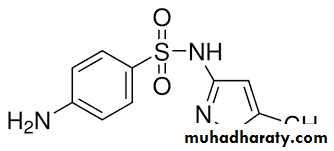
Bridge to Pharmacology
Cotrimoxazole contains the synergistic antibiotics sulfamethoxazole and trimethoprim, which inhibit different steps in the prokaryotic synthesis of tetrahydrofolate THF.
sulfamethoxazole trimethoprim
-----------------------------------------------------------
The primary end product of pyrimidine synthesis is UMP. In the conversion of UMP to dTMP, three important enzymes are ribonucleotide reductase, thymidylate synthase, and dihydrofolate reductase. All three enzymes are targets of antineoplastic drugs .
Enzyme
Functiondrug
Ribonucleotide reductase
Reduce all NDP to dNDP for DNA synthesis
Hydroxyurea(S phase)
Thymidylate synthase
Methylate dUMP to dTMP
Need for THF
5-fluorouracil(S phase)
Dihydrofolate reductase
Convert DHF to THF
Methotrixate(eukaryotic)
Trimethoprim(prokaryotic)
Pyrimethamine(protozoal)
Ribonucleotide Reductase
Ribonucleotide reductase is required for the formation of the deoxyribonucleotides for DNA synthesis.
• All four nucleotide substrates must be diphosphates.
• dADP and dATP strongly inhibit ribonucleotide reductase.
• Hydroxyurea, an anticancer drug, blocks DNA synthesis indirectly by
inhibiting ribonucleotide reductase.
PYRIMIDINE CATABOLISM
Pyrimidines may be completely catabolized (NH4+ is produced) or recycled bypyrimidine salvage enzymes.
PURINE SYNTHESIS
Purines are synthesized de novo beginning with PRPP. The most important enzyme is PRPP amidotransferase, which catalyzes the first and rate-limiting reaction of the pathway. It is inhibited by the three purine nucleotide end products AMP, GMP, and IMP.
The drugs allopurinol (used for gout) and 6-mercaptopurine (antineoplastic) also
inhibit PRPP amidotransferase. These drugs are purine analogs that must be converted to their respective nucleotides by HGPRT within cells.
Also note that:
• The amino acids glycine, aspartate, and glutamine are used in purine synthesis.• Tetrahydrofolate is required for synthesis of all the purines.
• Inosine monophosphate (contains the purine base hypoxanthine) is the precursor for AMP and GMP.
PURINE CATABOLISM AND THE SALVAGE ENZYME HGPRT
Excess purine nucleotides or those released from DNA and RNA by nucleases are
catabolized first to nucleosides (loss of P) and then to free purine bases (release of ribose or deoxyribose). Excess nucleoside monophosphates may accumulate when:
• RNA is normally digested by nucleases (mRNAs and other types of RNAs are continuously turned over in normal cells).
• Dying cells release DNA and RNA, which is digested by nucleases.
• The concentration of free Pi decreases as it may in galactosemia, hereditary
fructose intolerance, and glucose-6-phosphatase deficiency. Salvage enzymes recycle normally about 90% of these purines, and 10% are converted to uric acid and excreted in urine. When purine catabolism is increased significantly, a person is at risk for developing hyperuricemia and potentially gout
Adenosine Deaminase Deficiency
Adenosine deaminase (ADA) deficiency, an autosomal recessive disorder, produces severe combined immunodeficiency (SCID). Lacking both B-cell and Tcell function, children are multiply infected with many organisms (Pneumocystiscarinii, Candida) and do not survive without treatment. Enzyme replacement
therapy and bone marrow transplantation may be used. High levels of dATP accumulate in red cells of ADA patients and inhibit ribonucleotide reductase, thereby inhibiting the production of other essential deoxynucleotide precursors for DNA synthesis. Although it is believed that the impaired DNA synthesis contributes to dysfunction of T cells and B cells, it is not known why the main effects are limited to these cell types.
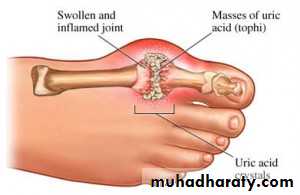
Hyperuricemia and Gout
Hyperuricemia may be produced by overproduction of uric acid or under excretion of uric acid by the kidneys. Hyperuricemia may progress to acute and chronic gouty arthritis if uric acid (monosodium urate) is deposited in joints and surrounding soft tissue, where it causes inflammation. Uric acid is produced from excess endogenous purines, and is also produced from dietary purines (digestion of nucleic acid in the intestine) by intestinal epithelia. Both sources of uric acid are transported in the blood to the kidneys for excretion in urine.
Allopurinol inhibits xanthine oxidase and also can reduce purine synthesis by inhibiting PRPP amidotransferase (provided HGPRT is active). Hyperuricemia and gout often accompany the following conditions:
• Lesch-Nyhan syndrome (no purine salvage)
• Partial deficiency of HGPRT
• Alcoholism (lactate and urate compete for same transport system in the
kidney)
• Glucose 6-phosphatase deficiency
• Hereditary fructose intolerance
• Galactose I -phosphate uridyl transferase deficiency (galactosemia)
In the last two diseases, phosphorylated sugars accumulate, decreasing the available Pi and increasing AMP (which cannot be phosphorylated to ADP and ATP). The excess AMP is converted to uric acid.
Lesch-Nyhan Syndrome
Lesch-Nyhan syndrome is an X-linked recessive condition involving:
• Near-complete deficiency of HGPRT activity
• Mental retardation
• Spastic cerebral palsy with compulsive biting of hands and lips.
• Hyperuricemia.
• Death often in first decade.
Over 100 distinct mutations of the HGPRT gene located on the X chromosome
have been reported to give rise to Lesch-Nyhan syndrome. These mutations include complete deletions of the gene, point mutations that result in an increased Km for hypoxanthine and guanine for the enzyme, and mutations that cause the encoded enzyme to have a short half-life.
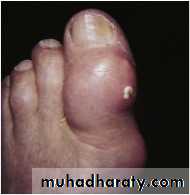
Gout
Acute gouty arthritis, seen most commonly in males, results from precipitation of monosodium urate crystals in joints. The crystals, identified as negatively birefringent and needle-shaped, initiate neutrophil mediated and acute inflammation, often first affecting the big toe. Chronic gout may manifest over time as tophi (deposits of monosodium urate) develop in soft tissue around joints, leading to chronic inflammation involving granulomas.
• Acute attacks of gout are treated with indomethacin to reduce the inflammation.
• Chronic hyperuricemia, because of underexcretion, is treated with a uricosuric drug (probenecid).
• Overproduction of uric acid and chronic gout are treated with allopurinol.
Lesch-Nyhan syndrome
The parents of a 9-month-old male infant were concerned that their son appeared generally weak, had difficulty moving his arms and legs, repeatedly bit his lips, and frequently seemed to be in pain. The infant was brought to the pediatrician. The parents mentioned that since the baby was born, they often noticed tiny, orange-colored particles when they changed the infant's diapers. Laboratory analysis of uric acid in urine was normalized to the urinary creatinine in the infant, and it was found that the amount was 3 times greater than the normal range. One of the earliest signs of Lesch-Nyhan syndrome is the appearance of orange crystals in diapers. They are needle-shaped sodium urate crystals. Without the salvaging of hypoxanthine and guanine by HGPRT, the purines are shunted toward the excretion pathway. This is compounded by the lack of regulatory control of the PRPP amidotransferase in the purine synthesis pathway, resulting in the synthesis of even more purines in the body. The large amounts of urate will cause crippling, gouty arthritis and urate nephropathy. Renal failure is usually the cause of death.
Treatment with allopurinol will ease the amount of urate deposits formed.