Subfertility
Dr. Ruaa Abdul jabbar Al najmawiDefinition: is a failure to conceive after 12 months of regular unprotected intercourse, a delay in conception is one of the commonest reasons that a woman will consult her doctor.
The incidence: subfertility is thought to affect about one in seven couples. Types of subfertility may be primary in couples that have never conceived together, or secondary in couples that have previously conceived together (although either partner may have conceived in a different relationship). The approach to fertility investigation and management should always be couple-centered.
Natural conception:
A healthy couple having frequent intercourse have about an 18–20% chance of conceiving in a single menstrual cycle. There is a cumulative increase in pregnancy rates over time as couples try for conception. Within 6 months 70% of couples will have conceived, after 12 months 80% and after 24 months 90% of couples will achieve a pregnancy. Couples having intercourse three times a week are three times more likely to conceive than couples having intercourse once a week. Increased frequency of intercourse should be encouraged in the periovulatory period. Eggs are thought to be fertilizable for about 12–24 hours postovulation, while sperm can survive in the female reproductive tract for up to 72 hours. Ovulation usually occurs about 14 days prior to menstruation, with the luteal phase being relatively stable at this length. The ‘fertile window’ for women will, therefore, be different depending on the average length of their menstrual cycle (e.g. for a woman with a 28-day menstrual cycle, her optimal fertile window will be between days 12 and 15).
Factors affecting fertility:
Female age: the most important factor affecting fertility , which is related to a decline in the quality and quantity of eggs. Female fertility tends to fall sharply over the age of 36, with a further dip after the age of 40, however, there is a considerable variation.Male age: is also an important factor; semen quality tends to fall over the age of 50, while frequency of intercourse tends to fall in men over the age of 40.
Frequency & timing of sexual intercourse :
External factors : include the following
Smoking: There is now strong evidence that smoking can decrease the quality & quantity of eggs & sperm.
Body mass index (BMI): with male and female BMI at both the high and low extremes associated with a reduced chance of conceiving.
Stress can have direct influence on the hypothalamic–pituitary–ovarian (HPO) axis, interfering with regular ovulation, & may indirectly reduce conception by reducing libido & frequency of intercourse.
Causes of subfertility:.
Female subfertility: Ovulatory disorders, tubal damage & uterine disorders are the most common causes with endometrial pathology, specific gamete or embryo defects & endometriosis contributory, smoking, medical conditions can reduce the chance of conception. Ovulatory disorders:
polycystic ovary syndrome (PCOS): is the commonest cause of problems with ovulation, women with PCOS suffer from oligomenorrhoea due to anovulation & due to hormonal treatments taken by these women to regulate their periods or help hirsutism (e.g. the combined oral contraceptive pill).
hypothalamic disorders secondary to low BMI, extreme exercise & extreme stress,
Pituitary disorders : e.g: hyperprolactinaemia,
ovarian disorders: premature ovarian failure, low ovarian reserve,
endocrine disorders: e.g: thyroid gland dysfunction
general medical disorders e.g diabetes, renal impairment...
Tubal problems:
Tubal blockage is usually associated with inflammatory processes in the pelvis such as
Pelvic inflammatory disease (PID): Chlamydial infections in particular can produce significant degrees of tubal damage, often resulting in a hydrosalpinx.
Endometriosis & Previous pelvic or abdominal surgery can result in postoperative scar tissue or adhesions that can also compromise tubal patency and function. The Fallopian tube is not a passive conduit – normal tubal function requires both patency and a healthy anatomy and physiology for gamete and embryo transport.
Uterine problems:
Fibroids can interfere with fertility, but their impact depends on their size & location. there is good evidence that submucos fibroids have a direct impact on embryo implantation , intramural fibroids may reduce fertility if they are large (>5 cm). Subserosal fibroids have very little impact if present in isolation.
Endometrial polyps can reduce the chance of implantation, although this tends not to be absolute.
Endometrial scarring (Asherman’s syndrome) from surgery or infection can be associated with lighter periods and a significantly reduced chance of conception.
Male factor:
Orchitis: spermatogonial cells that produce the sperm can be damaged by inflammation or the epididymis that stores mature sperm can also be damaged.
Iatrogenic influences such as pelvic radiotherapy or surgery for undescended or tortted testes
Medical conditions such as diabetes & certain occupations involving contact with chemicals or radiation.
Erectile difficulties or problems with ejaculation: Occasionally, sperm production may be normal but there are erectile difficulties or problems with ejaculation.
Genetic causes include aneuploidy of sex chromosomes (Klinefelter XXY the commonest) or structural abnormalities of the autosomes, e.g balanced translocations, Microdeletions of the Y chromosome are associated with low sperm counts & motility.
Unexplained subfertility:
Is subfertility that is idiopathic, the cause remain unknown even after subfertility work up , the possible causes are likely to be present but not detected by current methods, e.g: the ovum is not released at the optimal time for fertilization, sperm may not be able to reach the egg, fertilization fail to occur or implantation failure, about 10% of sub fertile couples have unexplained subfertility, the prognosis depend on female age, duration of subfertility, being primary or secondary,& the percentage of motile sperms.
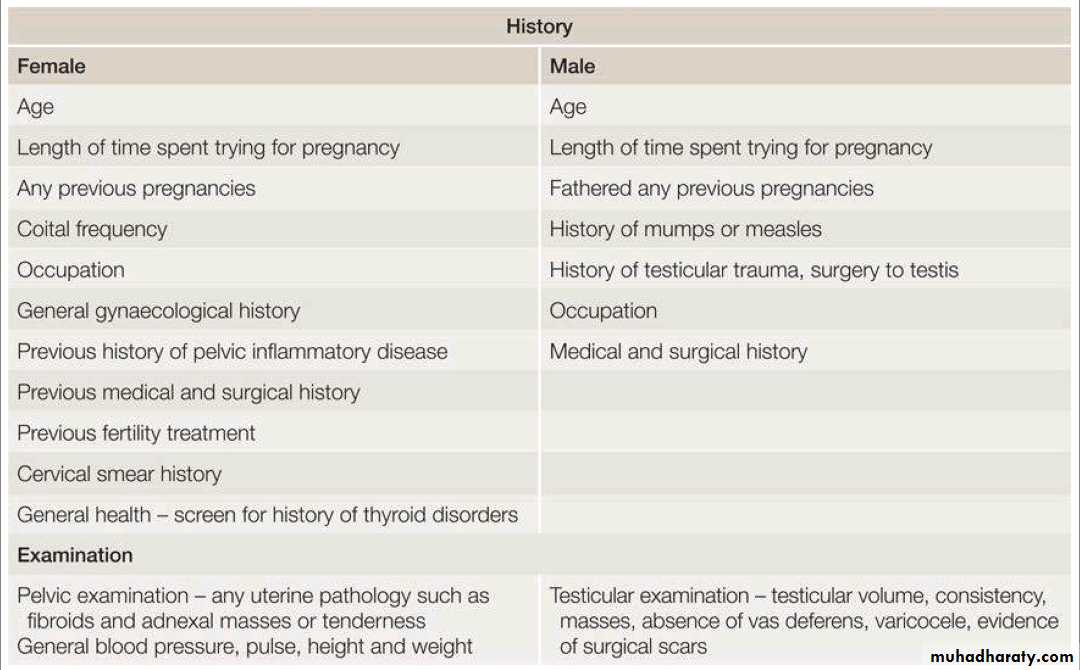
Approach to subfertile couple: History & examination:
A thorough & detailed history must be taken from patients presenting with subfertility Table 1. In many clinics a template is employed to ensure all these features are covered.
Investigations:
It is usually preferable to diagnose a cause of subfertility so that an appropriate treatment can be targeted to a specific pathology. Most authorities suggest that investigations are commenced in a couple that have not conceived after 1 year of regular unprotected intercourse. Investigations can be commenced earlier if the couple have a history of predisposing factors such as amenorrhoea, oligomenorrhoea, PID, women with low ovarian reserve or known male factor subfertility.Female investigations
Blood hormone profile:
In a woman with a regular menstrual cycle this should include early follicular phase :
follicular-stimulating hormone (FSH),
oestradiol
luteinizing hormone (LH).
Anti-Müllerian hormone (AMH).
A mid-luteal progesterone measurement should be taken to confirm ovulation.
In women with an irregular menstrual cycle:
thyroid function.
Prolactin.
testosterone.
Transvaginal ultrasound (TVUSS): for assessment of pelvic anatomy, including uterine size and shape, the presence of any fibroids, ovarian size, position and morphology, with antral follicle count (AFC), pathology such as hydrosalpinges and endometriotic cysts can be detected, and access to the ovaries for ART can be assessed.
Measurement of ovarian reserve: The ovarian reserve can help to predict the response to ovarian stimulation in ART.
• AFC seen on transvaginal ultrasound is a good indicator of ovarian reserve (<4 predicting low response, >16 high response).
• Antimullarian hormonAMH is produced in the granulosa cells of ovarian follicles and does not change in response to gonadotrophins during the menstrual cycle.
Tubal assessment: Tubal patency and assessment of the uterine cavity are traditionally investigated by
hysterosalpingography (HSG) using X-ray.
hysterocontrast synography (HyCoSy) using ultrasound.
Three dimensions (3D) hysterocontrast synography.
laparoscopy & hysteroscopy: patients deemed at high risk of pelvic pathology could benefit from laproscope as a dual diagnostic & potentially therapeutic procedure Currently there is as yet no effective test to check for tubal function.
5. Chlamydia testing: should be offered prior to any uterine instrumentation. 6. Screening for (HIV) & HBV & HCV If ART is to be offered.
Male investigations:
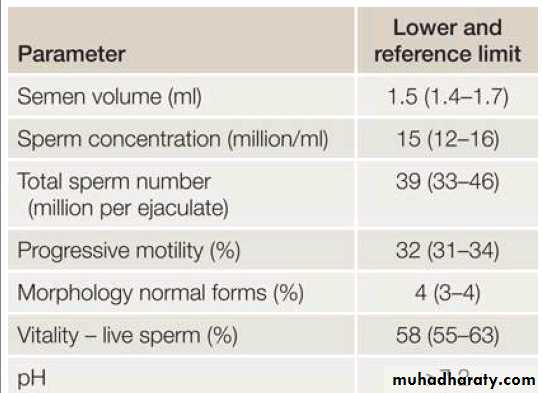
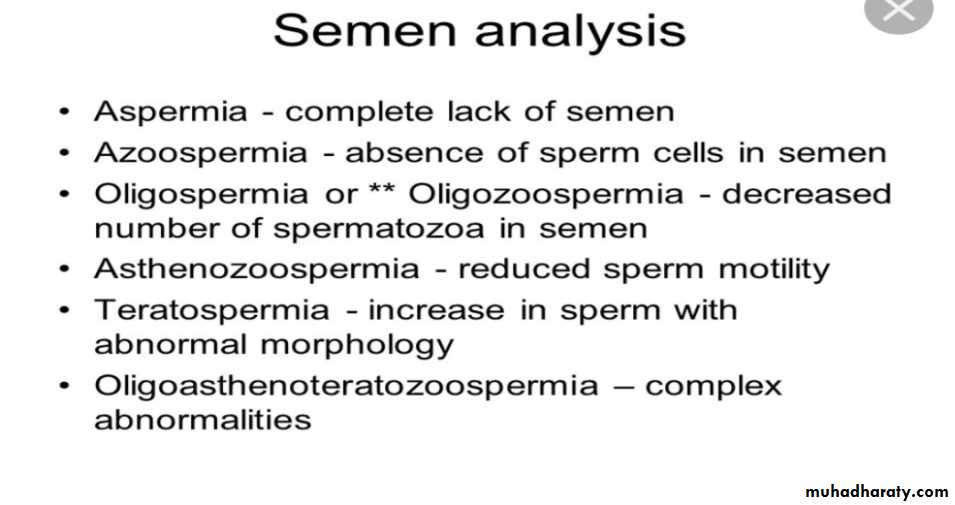
Semen fluid analysis: the only routine investigation on the male side is a (SFA). Most centers recommend between a 2-4day abstinence from ejaculation before providing the semen sample. if the initial SFA is abnormal it should be repeated 3 months later, to allow adequate time for spermatogenesis.
Hormone profile including FSH, LH & testosterone for men with a very low sperm count or azoospermia.
Karyotype & cystic fibrosis screen is also recommended for patients with low sperm count or azospermia.
Table .2 WHO parameters for semen analysis – 5th centile
Management:
Management may be expectant, medical, surgical or a combination of these. Fertility treatment should be individualized to optimize the treatment result.Lifestyle modification:
• Healthy diet.
• Stop smoking/recreational drugs.
• Reduce alcohol consumption.
• Regular exercise.
• Folic acid.
• Avoid timed intercourse (every 2–3 days).
• Avoid ovulation induction kits/basal temperature measurements (no evidence of success, & stressful).
Ovulation induction:
PCOS is responsible for 75–80% of anovulatory subfertility, ovulation induction (OI) is usually the 1st line of management as long as there is tubal patency & normal semen analysis
Antioestrogens (e.g. clomifene citrate 50 mg days 2–6): binds to oestrogen receptors in the hypothalamus & pituitary, this blocks the normal feedback loops of oestrogen & results in a surge of gonadotrophin release, stimulating the ovary to recruit more follicles for maturation. Approximately 70% of women on clomiphene will ovulate & approximately one-half of these will be pregnant within 6 months of trying. There is a risk of multiple pregnancies (12%) and therefore women on clomiphene should be monitored by ultrasound scans. In clomiphene-resistant women, alternative strategies include :
1.Augmentation with metformin.
2.Use of aromatase inhibitors: e.g letrozol ( not licensed for this indication in some countries) 3.Injectable gonadotrophins: expensive, with multiple pregnancy risk, need ultrasound monitoring (abandon cycle if over response)
4.Laparoscopic ovarian drilling (LOD): aims to restore ovulation in patients with PCOS its effect lasts 12–18mths if successful.
In cases of hyperprolactenemic anovulation dopamine agonist are used e.g: bromocriptine, or cabergoline.
In women with anovulation of hypothalamic origin,correction of weight abnormalities if present.
Patients with premature ovarian failure will have poor response to OI agents.
Surgery:
Most fertility surgery is currently performed using minimal access techniques such as laparoscopy or hysteroscopy, Surgery to treat subfertility can be helpful in different scenarios:
Investigation of infertility and tubal potency testing by minimal access surgery (MAS) is undertaken if the patient is symptomatic or if specific therapeutic treatment is planned.
Laparoscopic ablation of endometriosis can help improve natural conception rates.
adjunct to ART. For example: removal of hydrosalpinges is associated with a significant improvement in in-vitro fertilization (IVF) success rates.
Some practitioners still recommend a more traditional open laparotomy approach for very large uterine fibroids or for the use of tubal microsurgery in reversal of sterilization, proximal or distal tubal microsurgery.
Submucosal fibroids, endometrial polyps, Asherman syndrome and some congenital uterine anomalies, such as a septum, are usually managed hysteroscopically.
Intrauterine insemination:
Intrauterine insemination (IUI) is performed by introducing a small sample of prepared sperm into the uterine cavity with a fine uterine catheter. Indications of IUI: 1. Mild endometriosis.
2. Mild male factor subfertility.
3. Unexplained subfertility.
4. Coital difficulty.
The success rate is (10% - 20%) per treatment cycle. This process may be preceded by :
several days stimulation with subcutaneous injections daily of exogenous FSH, with the aim of stimulating the ovaries to produce 2–3 mature follicles (stimulated IUI).
Follicular tracking with ultrasound is essential to avoid over- or under stimulation.
Triggering of ovulation (& therefore the timing of the insemination) is achieved with a subcutaneous injection of human chorionic gonadotrophin (hCG).This mimics endogenous LH surge, due to crossover of the alpha-subunits of the two hormones.
In-vitro fertilization:
IVF was originally designed for couples with tubal factor subfertility. Steptoe and Edwards performed the first successful case in 1978. There are now over 5 million babies worldwide as a result of IVF. IVF is now used for almost all cases of subfertility including tubal disease, endometriosis, failed ovulation induction, failed IUI or where donor eggs are needed.
Originally, IVF was performed in the natural menstrual cycle, but the use of gonadotrophin-controlled ovarian stimulation made IVF a much more efficient process. It can be performed with different protocols & medications, but the principal steps of IVF are as follows.
Pituitary down-regulation: to prevent endogenous LH surges & premature ovulation. Gonadotrphin releasing hormon (GnRH) agonist is used to block the FSH and LH release from the pituitary. Newer approaches involving the use of GnRH antagonists can shorten treatment time and reduce the incidence of ovarian hyperstimulation syndrome.
Controlled ovarian stimulation: using daily subcutaneous doses of gonadotrophin medications, which cause multiple follicle recruitment. Close monitoring with transvaginal ultrasound TVUSS predicts the number of follicles & the timing of the egg collection. Ideally, around 15 follicles are recruited.
Inhibition of premature ovulation: feedback from rising oestradiol associated with follicular development lead to an LH surge, resulting in final oocyte maturation & ovulation. In IVF this is blocked to allow scheduling of egg collection. This is traditionally done by the administration of GnRH agonists, or with a newer shorter GnRH antagonist protocol.
hCG trigger: hCG is used as a surrogate for the endogenous LH surge. It causes final maturation of the egg and allows scheduling of the egg collection procedure.
Egg collection: this procedure is usually performed about 37 hours post hCG trigger. Under anaesthesia a needle is inserted into the ovaries under TVUSS control, and follicular fluid is aspirated from each follicle that contains an oocyte, which is collected by the embryologist into the laboratory.
Fertilization: fertilization is performed using prepared sperm. conventional IVF fertilization involves the insemination of around 100,000 sperm in a petri-dish with an egg. In cases of poor sperm parameters or in cases of previous poor fertilization, individual sperm can be isolated & directly injected into the cytoplasm of the oocyte (intracytoplasmic sperm injection, ICSI), Fertilization is checked the next morning & is usually in the region of 60% for IVF & 70% for ICSI.
Embryo culture: embryos are incubated under strict conditions of temperature, pH, humidity & oxygen concentration. They may be transferred back into the uterus after 2, 3 or 5 days of development. Embryos reaching the blastocyst stage on day 5 of development usually exhibit the best chance of implantation.
Embryo transfer: embryos are transferred into the uterus using a soft plastic catheter. It is usually performed under transabdominal ultrasound control to ensure correct placement of the embryos.
Embryo cryopreservation: spare embryos of good quality may be cryopreserved for future use.
Luteal phase support: the use of gonadotrophin agonists or antagonists to prevent a premature LH surge will lead to a reduction in the ability of the corpus luteum to produce progesterone. Patients are therefore supplemented with progesterone following the egg collection. A pregnancy test is performed around 14 days after embryo transfer.
ART success rates
IVF success rates are sensitive to female age. In young patients under the age of 35 success rates can be as high as 40–45% from a single cycle, while in women over the age of 40 they will fall below 15%. Undergoing IVF does not preclude the patient from the normal complications of pregnancy, such as miscarriage or ectopic pregnancies.
Ovarian hyperstimulation syndrome:
The most significant risk of IVF treatment is ovarian hyperstimulation syndrome (OHSS), occurring in 1–3% of cases. Patients with severe OHSS present with ascites, enlarged multifollicular ovaries, pulmonary oedema & coagulopathy. These patients need to be admitted to hospital and managed under strict protocols under the care of specialist teams. The use of low-dose stimulation, ultrasound monitoring, GnRH antagonist protocols, and a more liberal freezing policy have significantly reduced the incidence of this very serious condition.
Pre-implantation genetic diagnosis
Couples who carry a genetic disease (but who are fertile) may choose to use IVF and pre-implantation genetic diagnosis (PGD) to avoid an affected pregnancy. These patients may previously have had affected children or terminations for an affected fetus. IVF will create multiple embryos, these embryos can then be genetically tested for the relevant disease, only embryos free of the disease are transferred into the uterus. PGD has now been used worldwide for most monogenic diseases, as well as for translocations.